Abstract
In a rabbit model of Streptococcus pneumoniae meningitis single doses of 10 and 2.5 mg of the glycopeptide LY333328 per kg of body weight reduced bacterial titers in cerebrospinal fluid (CSF) almost as rapidly as ceftriaxone at 10 mg/kg/h (changes in log CFU, −0.29 ± 0.21 and −0.26 ± 0.22 versus −0.34 ± 0.15/ml/h). A dose of 1 mg/kg was bacteriostatic (change in log CFU, 0.01 ± 0.11/ml/h). In two animals receiving LY333328 at a dose of 40 mg/kg the bacterial titers were reduced by 0.54 and 0.51 log CFU/ml/h. The penetration of CSF by LY333328 was 1 to 5%. The concentrations of lipoteichoic and teichoic acids in CSF and neuronal damage were similar in ceftriaxone- and LY333328-treated animals.
Since the first description of a penicillin-resistant pneumococcal strain in 1967 in Australia (6), penicillin-resistant pneumococci have spread worldwide (1). Furthermore, reduced sensitivity of Streptococcus pneumoniae isolates to cephalosporins and other β-lactam antibiotics (7) resulting in clinical failures of cefotaxime and ceftriaxone in the treatment of meningitis has increased in frequency (2, 4).
Vancomycin is active against penicillin-resistant pneumococci. Although bactericidal concentrations of vancomycin were achieved in cerebrospinal fluid (CSF) in children with bacterial meningitis (9), clinical failures in adults have been reported probably because of a diminished blood-brain barrier penetration during adjunctive steroid therapy (19). This emphasizes the need for compounds either with a higher activity or with an improved CSF penetration compared to vancomycin for treatment of bacterial meningitis. LY333328 diphosphate is a semisynthetic glycopeptide inhibiting the synthesis of peptidoglycan in gram-positive bacteria. It is highly active against many resistant organisms, including methicillin- and teicoplanin-resistant staphylococci, vancomycin-resistant enterococci, and penicillin-resistant and multiresistant pneumococci (3, 5, 13). In a search for new therapeutic agents, we investigated its activity and its influence on the release of proinflammatory bacterial compounds and on neuronal damage in the rabbit model of S. pneumoniae meningitis with a strain susceptible to penicillin.
In vitro antimicrobial activity.
S. pneumoniae type 3 (MIC and minimal bactericidal concentration [MBC] of LY333328, 0.015 and 0.03 μg/ml, respectively; MIC and MBC of ceftriaxone, 0.03 and 0.06 μg/ml, respectively; MIC and MBC of penicillin, <0.1 μg/ml) (gift of M. G. Täuber, Department of Medical Microbiology, University of Bern, Bern, Switzerland) was grown, centrifuged, and resuspended in fresh tryptic soy broth to a final concentration of 6.83 ± 0.16 log CFU/ml (n = 5). Control cultures were grown after resuspension without antibiotics. Ceftriaxone and LY333328 (final concentration, 10 μg/ml) were added to 15-ml aliquots, and bacterial counts were determined at 0, 1, 3, 6, 9, and 12 h.
Rabbit model.
Meningitis was induced by intracisternal injection of approximately 106 CFU of S. pneumoniae. Blood (3 ml) and CSF (300 μl) were drawn before and at 12, 14, 17, 20, and 24 h after infection. Twelve hours after infection, therapy was initiated. Rabbits were treated with a single dose of LY333328 (1 [n = 5], 2.5 [n = 5], 10 [n = 10], or 40 [n = 2] mg/kg of body weight). LY333328 was dissolved in 5% glucose and infused over 30 min. Control animals received a bolus of 20 mg of ceftriaxone (Rocephin; Hoffmann-La Roche, Grenzach-Wyhlen, Germany) per kg followed by a continuous infusion of 10 mg/kg/h over 12 h (n = 12). Ceftriaxone was administered continuously because of its relatively short half-life in rabbits.
Sample processing.
CSF white blood cells were quantified in a Fuchs-Rosenthal hemocytometer. Pneumococcal titers in CSF were counted by plating 10-μl undiluted samples and serial 10-fold dilutions on blood agar plates. To avoid carryover effects, 300-μl samples of 1:100 dilutions were plated on blood agar plates.
Bacterial titers at 12, 14, 17, 20, and 24 h after infection were subjected to log-linear regression analysis. Neuron-specific enolase concentrations were determined by an immunoluminometric method (LIA-mat NSE Prolifigen; Byk-Sangtec, Dietzenbach, Germany). Lactate was measured enzymatically (Greiner, Flacht, Germany), CSF protein concentration was measured photometrically (BCA Protein Test; Pierce, Rockford, Ill.), and pneumococcal lipoteichoic (LTA) and teichoic acid (TA) levels were measured by enzyme immunoassay (16).
After acidification of serum and CSF and extraction on a C2 cartridge, concentrations of LY333328 were determined by liquid chromatography-mass spectrometry with positive-ion electrospray ionization using a Supelco Discovery C18 column (150 by 2.1 mm; inside diameter, 5 μm) as stationary phase and methanol-water-formic acid (45, 55, and 0.1%, respectively) as mobile phase. Peaks were quantitated by calculating area ratios with calibration standards (BAS Analytics, West Lafayette, Ind.). The detection limit was 0.15 μg/ml in serum and CSF.
In situ tailing of apoptotic neurons.
In deparaffinized and hydrated 1-μm sections, DNA double-strand breaks were stained by in situ tailing, and the density of apoptotic neurons in the granular cell layer of the dentate gyrus in the hippocampal formation was determined by planimetry (20).
Pharmacokinetics and statistics.
The areas under the concentration-time curve from the start of the infusion (0 h = 12 h after infection) until the end of the experiment (12 h = 24 h after infection) in serum (AUCS 0–12h) and CSF (AUCCSF 0–12h) were estimated by the linear trapezoidal rule. Noncompartmental methods were used to estimate clearance, the elimination constant, and volume of distribution.
Leukocyte density, concentrations of protein, lactate, and LTA and TA in CSF, and the density of apoptotic neurons in the dentate gyrus in the groups receiving LY333328 were compared with those for ceftriaxone-treated animals by analysis of variance with correction for multiple testing by the Bonferroni method. The half-maximal dose and the maximum bactericidal rate of LY333328 were estimated by a Lineweaver-Burk plot assuming an increase in the CSF bacterial titers of 0.15 log CFU/ml/h in untreated rabbits.
In vitro results.
At 10 μg/ml LY333328 decreased the bacterial titers of S. pneumoniae rapidly to levels below the detection limit of 102 CFU/ml within 1 h, whereas cultures treated with 10 μg of ceftriaxone/ml were sterile after 12 h. The release of LTA and TA was lower after exposure to LY333328 (141.1 ± 93.5 versus 491.4 ± 298.3 ng/ml at 3 h [P = 0.020], 141.6 ± 89.9 versus 418.0 ± 293.3 ng/ml at 6 h [P = 0.042], 126.2 ± 92.1 versus 465.0 ± 295.8 ng/ml at 9 h [P = 0.035], and 157.8 ± 92.0 versus 425.6 ± 277.1 ng/ml at 12 h [P = 0.051]).
Rabbit model.
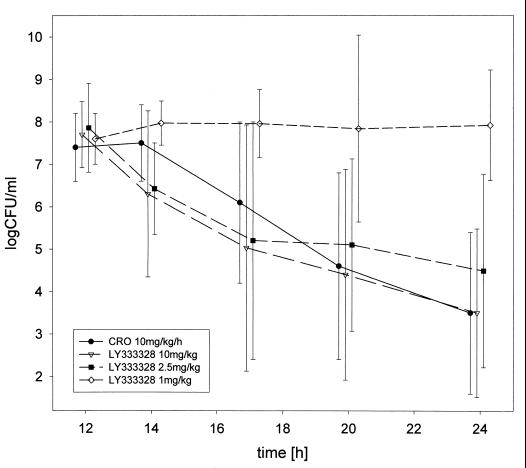
Maximum concentration of LY333328 in serum rose linearly with the dose administered. A plateauing effect, however, appeared to occur at the dose of 40 mg/kg. A similar pattern was observed in an earlier study with LY333328 at very high doses (T. J. Brown, personal communication). Consistently, the apparent volume of distribution (approximately 0.25 liter/kg) in the 1-, 2.5-, and 10-mg/kg-treated animals equalled the extracellular fluid space, whereas it increased to approximately 0.7 liter/kg at a dose of 40 mg/kg. The elimination half-life in serum generally ranged from 5 to 8 h (Table 1). Maximum concentrations in CSF were reached several hours after intravenous administration of LY333328 (8.1 ± 3.8 h for the 10-mg/kg dose and 4.4 ± 1.3 h for the 2.5-mg/kg dose). For this reason, half-life in CSF could not be estimated reliably. The entry into CSF as estimated by AUCCSF 0–12h/AUCS 0–12h in most cases was between 1 and 5% (Table 1). A single 10-mg/kg dose of LY333328 (n = 10) reduced the bacterial titers in CSF almost as rapidly as ceftriaxone (n = 12) (−0.29 ± 0.21 versus −0.34 ± 0.15 log CFU/ml/h). The bactericidal effect was slightly lower for LY333328 at a dose of 2.5 mg/kg (n = 5) (−0.26 ± 0.22 log CFU/ml/h), whereas the bactericidal activities at a dose of 40 mg/kg were −0.54 and −0.51 log CFU/ml/h (n = 2) (all groups statistically not significant versus ceftriaxone). A 1-mg/kg dose of LY333328 (n = 5) was only bacteriostatic (0.01 ± 0.11 CFU/ml/h [P < 0.05 versus ceftriaxone]) (Fig. 1). Plotting 1/effect versus 1/dose and 1/effect versus 1/AUCCSF 0–12h (Lineweaver-Burk plot) yielded estimates of the half-maximal dose (4.6 mg/kg), the half-maximal AUCCSF 0–12h (4.2 mg × h/liter), and the maximum bactericidal rate (−0.68 and −0.58 log CFU/ml/h) of LY333328.
TABLE 1.
| Antibiotic | CmaxS (μg/ml) | CL (ml/min/kg) | t1/2β (h) | V (liter/kg) | AUCS 0–12h (mg × h/liter) | Observed CmaxCSF (μg/ml) | AUCCSF 0–12h (mg × h/liter) | AUCCSF 0–12h/ AUCS 0–12h | Change in log CFU/ml | No. of leukocytes (1/μl) | Protein concn (μg/ml) | Lactate concn (mmol/liter) | NSE concn (μg/ml) | No. of apoptotic neuronsb (1/mm2) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ceftriaxone (n = 12), 20 mg/kg + 10 mg/kg/h | 88.1 ± 19.29 | 3.33 ± 1.7 | ND | ND | 918.5 ± 510.4 | 9.43 ± 5.72 | 60.3 ± 31.4 | 0.072 ± 0.032 | −0.34 ± 0.15 | 2,376 ± 1,562 | 4,183 ± 2,119 | 11.3 ± 5.5 | 153 ± 97 | 141 ± 39 |
| LY333328 | ||||||||||||||
| 1 mg/kg (n = 5) | 3.90 ± 1.51 | 0.58 ± 0.17 | 5.41 ± 0.95 | 0.26 ± 0.06 | 22.8 ± 5.6 | BQL | BQL | ND | 0.01 ± 0.11c | 1,215 ± 1,141 | 7,589 ± 1,995 | 13.6 ± 6.3 | 274 ± 143 | 106 ± 31 |
| 2.5 mg/kg (n = 5) | 10.64 ± 2.34 | 0.35 ± 0.07 | 7.77 ± 2.14 | 0.23 ± 0.05 | 76.1 ± 10.7 | 0.54 ± 0.19 | 3.39 ± 1.33 | 0.045 ± 0.019 | −0.26 ± 0.22 | 1,492 ± 369 | 6,681 ± 3,999 | 13.2 ± 5.6 | 136 ± 99 | 106 ± 29 |
| 10 mg/kg (n = 10) | 42.72 ± 11.92 | 0.41 ± 0.11 | 6.53 ± 1.86 | 0.22 ± 0.07 | 300.1 ± 84.8 | 0.76 ± 0.52 | 5.46 ± 4.28 | 0.018 ± 0.010d | −0.29 ± 0.21 | 2,147 ± 918 | 4,899 ± 2,243 | 14.9 ± 11 | 92 ± 69 | 121 ± 49 |
| 40 mg/kg (n = 2) | 48.77, 53.18 | 0.93, 1.14 | 10.28, 7.41 | 0.74, 0.73 | 409.1, 335.4 | 1.36, 0.54 | 10.04, 2.36 | 0.025, 0.007 | −0.51, −0.54 | 3,260, 3,421 | 5,197, 7,772 | 14.8, 17.6 | ND | ND |
CmaxS and CmaxCSF, maximum concentrations in serum and CSF, respectively; CL, clearance; t1/2β, half-life at β phase; V, volume of distribution; NSE, neuron-specific enolase; ND, not determined; BQL, below quantification limit. AUC ratios were lower in LY333328-treated rabbits. LY333328 at 1 mg/kg was less active than ceftriaxone. Density of leukocytes, concentrations of protein and lactate in CSF, and parameters of neuronal damage showed no significant difference between ceftriaxone- and LY333328-treated animals. Because of the low number of observations, animals treated with 40 mg of LY333328/kg were not included in the statistical analysis.
In the dentate gyrus of the hippocampus.
P < 0.05 versus ceftriaxone.
P < 0.001 versus ceftriaxone.
FIG. 1.
Bacterial titers in CSF. Antibiotic therapy was initiated 12 h after infection. Only at a dose of 1 mg/kg did LY333328 show significantly less activity than ceftriaxone (CRO) (P < 0.05).
Leukocyte density and concentrations of protein and lactate in CSF at 24 h did not differ between LY333328- and ceftriaxone-treated animals (Table 1). The concentrations of LTA and TA showed no significant difference between ceftriaxone- and LY333328-treated animals at each time point except at 24 h, when animals treated with LY333328 at 1 mg/kg (log 3.99 ± 0.89 ng/ml) contained significantly more LTA in CSF than rabbits treated with ceftriaxone 10 at mg/kg/h (log 2.81 ± 0.72 ng/ml [P < 0.05]) or LY333328 at 10 mg/kg (log 2.86 ± 0.62 [P < 0.05]). Parameters of neuronal damage (20) did not differ significantly among the treatment groups (Table 1). In uninfected animals (n = 4), the density of neuronal apoptosis 24 h after saline injection was 12 ± 4/mm2, and CSF protein and lactate concentrations were 1,021 ± 240 μg/ml and 1.9 ± 0.3 mmol/liter, respectively.
In vivo, a short-duration infusion of LY333328 at doses of 10 and 2.5 mg/kg was slightly less active than a continuous infusion of ceftriaxone (total dose, 140 mg/kg over 12 h) (differences not significant), whereas LY333328-treated cultures in vitro were sterile after 1 h. Probable reasons for the discrepancy between in vitro and in vivo activities are different growth rates and the high binding of LY333328 to serum and CSF proteins. The bactericidal activity of LY333328 in broth was reduced when it was tested in the presence of 90% rabbit serum (8). The binding to serum proteins and the high molecular mass (1,989 Da) resulted in relatively low concentrations in CSF after intravenous administration of LY333328. Consequently, the AUCCSF 0–12h/AUCS 0–12h ratio was lower for LY333328 than for ceftriaxone (Table 1).
The dose and AUCCSF 0–12h of LY333328 estimated to produce half-maximal killing were approximately 5 mg/kg and 4.2 mg × h/liter, respectively. This implies that half-maximal killing occurs at a ratio of the CSF drug concentration to MIC of 20 to 30 and an AUCCSF 0–24h/MIC ratio (10) of approximately 550. In the central compartment, with most quinolones and β-lactam antibiotics the half-maximal effect occurs at lower ratios of the CSF drug concentration and AUCCSF 0–24h to MIC (11, 17). In addition to the slow bacterial replication as a cause of the reduced antibacterial activities of many compounds in the CSF compartment, the relatively high ratios of the CSF drug concentration and AUCCSF 0–24h to MIC necessary to produce half-maximum killing by LY333328 may reflect substantial binding to CSF proteins in meningitis.
The 10-mg/kg dose of LY333328 was chosen for the present study to produce concentrations in serum intended for human use; in healthy volunteers, doses of up to 3 mg/kg producing concentrations in plasma of approximately 50 μg/ml at the end of the infusion were tolerated without serious side effects (Eli Lilly & Co., data on file). In the present study, the dose of 40 mg/kg substantially increased the bactericidal activity. The tolerability of such high doses in humans remains to be determined.
Although LY333328 released smaller quantities of LTA and TA from S. pneumoniae than those released by ceftriaxone in vitro, in vivo no significant differences were observed between animals treated with LY333328 at doses of 2.5, 10, and 40 mg/kg and those treated with ceftriaxone. This discrepancy probably originated from the different velocities of bacterial killing by LY333328 in vitro and in the animal model. Consequently, in the CSF compartment the inflammatory reaction and parameters of neuronal damage during treatment with LY333328 were comparable to those observed during ceftriaxone therapy. This suggests that, unlike rifamycins and quinupristin-dalfopristin (12, 14, 18), LY333328 probably does not alter the course of the inflammatory reaction during treatment of S. pneumoniae meningitis. With the bacteriostatic 1-mg/kg dose of LY333328, CSF LTA concentrations at 24 h were higher than those in animals treated with LY333328 at 10 mg/kg or ceftriaxone. This agrees well with previous reports of an increased liberation of proinflammatory bacterial compounds at low antibiotic concentrations both in broth and in CSF (15, 16).
In conclusion, despite low CSF penetration, the extremely low MICs and MBCs against pneumococci and the high in vivo activity in the rabbit model indicate that clinical studies with LY333328 for the treatment of pneumococcal meningitis should be conducted. The selective activity of LY333328 against gram-positive organisms, however, will favor use against penicillin-resistant pneumococci but not in empiric therapy. Since a reduced sensitivity to penicillin does not increase the MIC of LY333328, the compound should be equally active against penicillin-susceptible and -resistant strains, although the in vivo activity of LY333328 against resistant strains in bacterial meningitis remains to be determined.
REFERENCES
- 1.Appelbaum P C. World-wide development of antibiotic resistance in pneumococci. Eur J Clin Microbiol. 1987;6:367–377. doi: 10.1007/BF02013089. [DOI] [PubMed] [Google Scholar]
- 2.Bradley J S, Connor J D. Ceftriaxone failure in meningitis caused by Streptococcus pneumoniae with reduced susceptibility to beta-lactam antibiotics. Pediatr Infect Dis J. 1991;10:871–873. [PubMed] [Google Scholar]
- 3.Fasola E, Spangler S K, Ednie L M, Jacobs M R, Bajaksouzian S, Appelbaum P C. Comparative activities of LY333328, a new glycopeptide, against penicillin-susceptible and -resistant pneumococci. Antimicrob Agents Chemother. 1996;40:2661–2663. doi: 10.1128/aac.40.11.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Figueiredo A M S, Connor J D, Severin A, Vaz Pato M V, Tomasz A. A pneumococcal clinical isolate with high-level resistance to cefotaxime and ceftriaxone. Antimicrob Agents Chemother. 1992;36:886–889. doi: 10.1128/aac.36.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Garrote F, Cercenado E, Alcalá L, Bouza E. In vitro activity of the new glycopeptide LY333328 against multiply resistant gram-positive clinical isolates. Antimicrob Agents Chemother. 1998;42:2452–2455. doi: 10.1128/aac.42.9.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansman D, Bullen M M. A resistant pneumococcus. Lancet. 1967;ii:264–265. doi: 10.1016/s0140-6736(75)91547-0. [DOI] [PubMed] [Google Scholar]
- 7.Jetté L P, Lamothe F The Pneumococcus Study Group. Surveillance of invasive Streptococcus pneumoniae infection in Quebec, Canada, from 1984 to 1986: serotype distribution, antimicrobial susceptibility, and clinical characteristics. J Clin Microbiol. 1989;27:1–5. doi: 10.1128/jcm.27.1.1-5.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaatz G W, Seo S M, Aeschlimann J R, Houlihan H H, Mercier R-C, Rybak M J. Efficacy of LY333328 against experimental methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1998;42:981–983. doi: 10.1128/aac.42.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klugman K P, Friedland I R, Bradley J S. Bactericidal activity against cephalosporin-resistant Streptococcus pneumoniae in cerebrospinal fluid of children with acute bacterial meningitis. Antimicrob Agents Chemother. 1995;39:1988–1992. doi: 10.1128/aac.39.9.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li R C, Zhu M, Schentag J J. Achieving an optimal outcome in the treatment of infections. The role of clinical pharmacokinetics and pharmacodynamics of antimicrobials. Clin Pharmacokinet. 1999;37:1–16. doi: 10.2165/00003088-199937010-00001. [DOI] [PubMed] [Google Scholar]
- 11.Nau R, Schmidt T, Kaye K, Froula J L, Täuber M G. Quinolone antibiotics in therapy of experimental pneumococcal meningitis in rabbits. Antimicrob Agents Chemother. 1995;39:593–597. doi: 10.1128/AAC.39.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nau R, Wellmer A, Soto A, Koch K, Schneider O, Schmidt H, Gerber J, Michel U, Brück W. Rifampin reduces early mortality in experimental Streptococcus pneumoniae meningitis. J Infect Dis. 1999;179:1557–1560. doi: 10.1086/314760. [DOI] [PubMed] [Google Scholar]
- 13.Saleh-Mghir A, Lefort A, Petegnief Y, Dautrey S, Vallois J M, Le Guludec D, Carbon C, Fantin B. Activity and diffusion of LY333328 in experimental endocarditis due to vancomycin-resistant Enterococcus faecalis. Antimicrob Agents Chemother. 1999;43:115–120. doi: 10.1128/aac.43.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt H, Zysk G, Reinert R R, Brück W, Stringaris A, Stuertz K, Bartels R, Schaper K, Weinig S, Nau R. Rifabutin for experimental pneumococcal meningitis. Chemotherapy. 1997;43:264–271. doi: 10.1159/000239577. [DOI] [PubMed] [Google Scholar]
- 15.Stuertz K, Schmidt H, Eiffert H, Schwartz P, Mäder M, Nau R. Differential release of lipoteichoic and teichoic acids from Streptococcus pneumoniae as a result of exposure to β-lactam antibiotics, rifamycins, trovafloxacin, and quinupristin-dalfopristin. Antimicrob Agents Chemother. 1998;42:277–281. doi: 10.1128/aac.42.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stuertz K, Schmidt H, Trostdorf F, Eiffert H, Mäder M, Nau R. Lower lipoteichoic and teichoic acid CSF concentrations during treatment of pneumococcal meningitis with non-bacteriolytic antibiotics than with ceftriaxone. Scand J Infect Dis. 1999;31:367–370. doi: 10.1080/00365549950163806. [DOI] [PubMed] [Google Scholar]
- 17.Täuber M G, Doroshow C A, Hackbarth C J, Rusnak M G, Drake T A, Sande M A. Antibacterial activity of beta-lactam antibiotics in experimental meningitis due to Streptococcus pneumoniae. J Infect Dis. 1984;149:568–574. doi: 10.1093/infdis/149.4.568. [DOI] [PubMed] [Google Scholar]
- 18.Trostdorf F, Reinert R R, Schmidt H, Nichterlein T, Stuertz K, Schmitz-Salue M, Sadowski I, Brück W, Nau R. Quinupristin/dalfopristin attenuates the inflammatory response and reduces the concentration of neuron-specific enolase in the cerebrospinal fluid of rabbits with experimental Streptococcus pneumoniae meningitis. J Antimicrob Chemother. 1999;43:87–94. doi: 10.1093/jac/43.1.87. [DOI] [PubMed] [Google Scholar]
- 19.Viladrich P F, Gudiol F, Linares J, Pallares R, Sabate I, Rufi G, Ariza J. Evaluation of vancomycin for therapy of adult pneumococcal meningitis. Antimicrob Agents Chemother. 1991;35:2467–2472. doi: 10.1128/aac.35.12.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zysk G, Brück W, Gerber J, Brück Y, Prange H W, Nau R. Anti-inflammatory treatment influences neuronal apoptotic cell death in the dentate gyrus in experimental pneumococcal meningitis. J Neuropathol Exp Neurol. 1996;55:722–728. doi: 10.1097/00005072-199606000-00006. [DOI] [PubMed] [Google Scholar]