Highlights
-
•
We describe a focal epilepsy patient with shadowboxing-induced reflex seizures.
-
•
We identified a precipitative motion within the shadowboxing by video-EEG.
-
•
Avoiding the motion enabled him to continue boxing free from reflex seizures.
Keywords: Shadowboxing, Reflex epilepsy, Exercise-induced epilepsy, Temporal lobe epilepsy, Premotor cortex
Abstract
Exercise-induced reflex seizures are a rare form of reflex seizures that are exclusively induced by a specific type of exercise. Many patients with exercise-induced reflex seizures exhibit drug-resistance, and are therefore advised to avoid the triggering exercise. Here, we describe a focal epilepsy patient with shadowboxing-induced reflex seizures. His semiology included focal aware seizures with speech and behavioral arrest that evolved to head version to the right, preceded by cephalic aura. We identified a specific motion that induced these seizures during shadowboxing using video-electroencephalographic recording, and the patient was able to continue boxing by avoiding this motion. We speculate that a broad brain network may be the pathological substrate of his exercise-induced reflex seizures. Identification of the specific motion that induces exercise-induced reflex seizures is useful for not only understanding the underlying pathophysiology, but also for minimizing the therapeutic restriction of the exercise.
1. Introduction
Although regular physiological exercise should be encouraged in patients with epilepsy to promote their mental and physical health and suppress their seizure activities [1], it can be a trigger of seizures in patients with exercise-induced epilepsy [2]. In an epidemiological study, Nakken et al. [3] defined exercise-induced seizures as seizures occurring in >50% of training sessions, and showed that 2% of patients with epilepsy had these seizures. Some patients with exercise-induced seizures had their seizures provoked frequently (>80% of training sessions) and exclusively by the same exercise, and were considered to have a rare form of reflex epilepsy [4], [5]. If the specific exercise itself induces seizures with high reproducibility, the seizures can be called exercise-induced reflex seizures, but if seizures tend to appear during or after an unspecified exercise, the seizures cannot be called exercise-induced reflex seizures [6]. However, because there is no clear dichotomy to distinguish them [6], and because it is difficult to identify seizure-provoking factors among the various factors involved in exercise [4], exercise-induced reflex and non-reflex seizures are still treated without distinction. To understand the pathophysiology of exercise-induced reflex epilepsy, it is essential to analyze the motor sequence inducing seizures, and to identify the elements related to seizure induction. However, no previous reports have identified specific motion elements that induce seizures.
Patients with exercise-induced seizures, whether reflex or non-reflex seizures, are advised to avoid the triggering exercise to reduce the risk of seizure [7], [8], [9], although continuing the exercise may be beneficial to their overall mental health [10]. If clinicians can identify the key seizure-triggering motion in the exercise and minimize exercise restriction, patients can continue to maintain their mental and physical health while reducing the occurrence of seizure.
Here, we describe a focal epilepsy patient with shadowboxing-induced reflex seizures. We identified a specific motion that induced seizures during shadowboxing using video-electroencephalographic (EEG) recordings, and avoiding the identified motion enabled the patient to prevent exercise-induced reflex seizures and continue shadowboxing. On the basis of these findings, we discuss the pathophysiology of exercise-induced reflex epilepsy and shadow-boxing.
2. Case report
The patient was a 26-year-old right-handed man with normal intellect. He had no familial history of epilepsy, including febrile seizures, and no medical history such as head trauma. He experienced his first seizure at 10 years of age when he had a high fever. From the age of 19 years, he began to wake up at midnight, and occasionally had headaches and muscle pains the following morning. His first witnessed nocturnal hyperkinetic seizure occurred at 24 years of age. The witness described that he moved around violently on the bed; however, he had no recollection of the event and simply woke up with the same headache and muscle pain as before. Because similar seizures subsequently occurred once per week, he was admitted to our clinic. Interictal EEG recordings showed intermittent sharp waves distributed from the left frontal to anterior temporal regions (Fig. 1). We diagnosed focal epilepsy and started lacosamide, which immediately reduced his seizure frequency, but did not resolve it completely. We tried perampanel and levetiracetam, but psychiatric adverse events compelled us to abandon these treatments. Finally, by adding lamotrigine and clobazam, his seizures were suppressed for a while. At 25 years of age, he began boxing for daily exercise. However, 10 months later, focal aware seizures emerged during shadowboxing, although he was still taking the three anti-seizure medications. Each seizure started with a cephalic aura followed by speech and behavioral arrest for about 10 seconds without impairment of awareness. The seizures occurred specifically and reproducibly during shadowboxing or no-contact sparring. Meanwhile, exercises without punching, such as rope jumping, jogging and footwork, never triggered seizures. Furthermore, seizures never occurred during practice with a punching bag, full-contact sparring or boxing matches when he did not need to stop the punching motion just before fully stretching the arm. He described that once a seizure had occurred, another seizure did not occur even if he continued the precipitative exercises. His seizures were also induced in excessively nervous situations such as failures in the workplace. Because the number of exercise-induced reflex seizures gradually increased, he was admitted to our hospital for further evaluation. The neurological examination and laboratory data were normal. We carried out 3 days of long-term video-EEG monitoring without withdrawal of anti-seizure medications. Background activity and sleep architecture were normal. There were no paroxysmal findings in interictal EEG recordings. Because controlled punching, in which the punching motion is stopped just before the arm becomes fully stretched, was considered to induce his seizures based on the clinical interview, the patient was asked to perform shadowboxing repeatedly under video-EEG monitoring to confirm the reproducibility and specificity of the seizure-inducing effects and the existence of refractory periods for the seizures. Furthermore, as shown in Table 1, we added some other shadowboxing motions, such as one without punching, one with maximum-intensity punching by fully extending the arms (uncontrolled punching) as in an actual boxing match, and one with the opposite punching hand and standing position to verify the side specificity of the controlled punching. We set each duration of shadowboxing at 3 minutes to match his usual training. When a seizure occurred, we discontinued the trial. We set 10-minute intervals between the trials as sufficient for the patient to catch his breath. We detected the exercise-induced reflex seizures four times only during shadowboxing with right-sided controlled punching (see Fig. 2 and the attached video online). At 10–20 seconds after the initiation of shadowboxing, as soon as he felt a cephalic aura, he showed speech and behavioral arrest. He exhibited phasic head version to the right and right-sided facial jerking without impairment of awareness. Each seizure lasted for 11–18 seconds. The EEG recordings showed moderate amplitude rhythmic theta waves intermittently during versive seizures. He was able to recall almost all names of pictures shown during the seizures as memory tasks. He described that the names came to his mind, but he was unable to say them out loud during the seizures. Once a seizure had occurred, another seizure was less likely to occur, as he described, and even if another seizure did occur, the latency from the exercise initiation to the following seizure was delayed. To further confirm whether a mere forward arm extension without boxing could induce seizures, we asked him to repeat the motion of reaching for an object on the desk as quickly as possible, but no seizures were induced.
Fig. 1.
Interictal epileptiform discharges in the first awake scalp EEG with average reference montage. Note the isolated left temporal sharp wave, with maximum at T3 (A), and left temporal sharp waves, with maximum at T3, overlaying intermittent moderate-amplitude irregular slow waves (B) seen once during a 30-minute outpatient EEG session.
Table 1.
Results of exercise-loaded trials (shadowboxing).
| Day | Trial | Shadowboxing |
Seizures |
|||||
|---|---|---|---|---|---|---|---|---|
| Stance | Punching | Punch laterality | Punch intensity | Induction | Duration (s) | Latency (s) | ||
| 1 | 1 | left-front | + | right | controlled | + | 18 | 11 |
| 2 | left-front | + | right | controlled | − | − | − | |
| 3 | left-front | + | right | controlled | − | − | − | |
| 2 | 1 | left-front | + | right | controlled | + | 14 | 13 |
| 2 | left-front | + | right | controlled | + | 15 | 18 | |
| 3 | left-front | − | − | − | − | − | − | |
| 4 | left-front | + | right | controlled | − | − | − | |
| 3 | 1 | left-front | − | − | − | − | − | − |
| 2 | left-front | + | right | uncontrolled | − | − | − | |
| 3 | left-front | + | right | controlled | + | 11 | 26 | |
| 4 | left-front | + | right | controlled | − | − | − | |
| 4 | 1 | right-front | − | − | − | − | − | − |
| 2 | right-front | + | left | controlled | − | − | − | |
The patient was instructed to continue shadowboxing for 3 minutes in each trial. In the table, “duration” indicates the ictal duration and “latency” indicates the latency from exercise initiation to seizure onset. The trial numbers indicate the order of the trials performed on the same day. Shadowboxing motions without punching means footwork only. We changed the stance of the shadowboxing to match the laterality of the punching. Controlled punching indicates punching in which the patient had to stop the punching motion just before fully stretching the arm as in typical shadowboxing. Uncontrolled punching indicates punching with fully extended arm at maximum intensity as in an actual boxing match. Shadowboxing with controlled punching reproducibly induced his seizures.
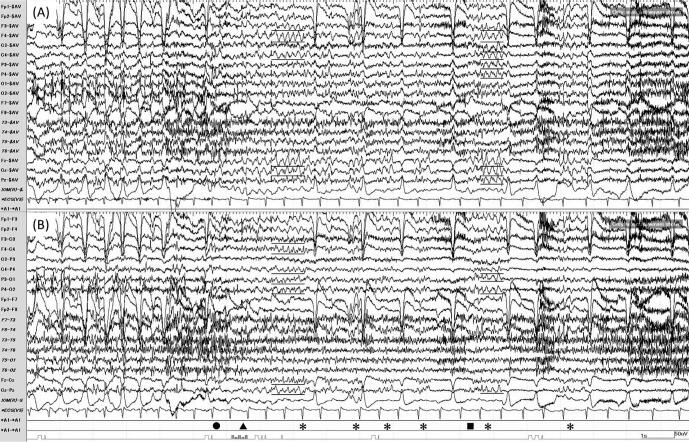
Fig. 2.
Exercise-induced reflex seizure. The EEG of an exercise-induced reflex seizure in Trial 1 on Day 1 is shown. The same EEG is shown with different montages: (A) average reference montage; (B) longitudinal bipolar montage. The patient experienced a cephalic aura (●) at 11 seconds after the onset of shadowboxing. Next, his right hand froze (▲), and moderate amplitude rhythmic theta waves (underlined) appeared intermittently during versive seizures (*). During the seizure, he could not reply to the examiner (■).
Brain magnetic resonance imaging examination showed a left incomplete hippocampal inversion. Slight hypoperfusion in the left temporal lobe was present on brain 123I-iomazenil single-photon emission computed tomography (SPECT) in both the early and delayed states. There was no decrease in fluorodeoxyglucose metabolism on a brain positron emission tomography examination and no interictal epileptic discharges on magnetoencephalography.
From these results, we advised the patient not to perform right-sided force-controlled straight punching during shadowboxing, but we permitted all other exercises including shadowboxing with left-sided force-controlled straight punching, full-contact sparring, and boxing matches, because boxing bolstered his confidence and relieved his anxiety about epilepsy. Based on our advice, he was able to continue boxing and achieve suppression of his exercise-induced reflex seizures.
3. Discussion
We diagnosed a focal epilepsy patient with exercise-induced reflex seizures, and identified right-sided force-controlled straight punching as his seizure precipitant by analyzing video-EEG recordings. Onset symptoms in his early nocturnal seizures were similar to those in the exercise-induced reflex seizures of early-phase focal aware behavioral arrest of the right upper limb and ictal tachycardia masked by exercise, further suggesting his seizure onset zone to be in the vicinity of the left premotor cortex with operculo-insular involvement [11], [12], [13], [14], [15], [16]. However, based on the triggering motion, the seizure onset zone is likely to be a broad pathological network extending from the premotor cortex to the limbic network.
Right-sided straight punching in boxing can be divided into three motions: a forward body translation from an on-guard position, a rotational movement around the trunk long axis, and a forward extension of the right arm [17]. Because the first two movements are also used in footwork for shadowboxing, the seizures are probably mainly induced by the forward extension of the right arm. In addition, punching is an attack with an opponent in mind, and involves emotional factors [18]. The seizures in our patient were consistently induced by force-controlled punching, not by force-released punching or force-controlled arm stretching, indicating involvement of the premotor cortex, which controls the arm stretching [19], and the limbic network, which provides emotional feedback to the premotor cortex [20]. The findings support this concept, including the left anterior temporal interictal epileptic discharges, left temporal hypoperfusion on 123I-iomazenil SPECT, and triggering of seizures in excessively nervous situations without exercise. The presence of a broad pathological network comprising multiple brain areas may underlie the drug-resistant nature of exercise-induced reflex seizures [5].
The International League Against Epilepsy defines reflex seizures as those that are objectively and consistently induced by a specific afferent stimulus or patient activity [21]. Illingworth et al. [6] emphasized that the specificity of the precipitant and the relatively short latency are useful in the diagnosis of reflex seizures. Movement-induced epilepsy is a rare form of reflex epilepsy in which seizures are triggered by simple movement of a single limb. This type of epilepsy has a refractory period after an evoked seizure, and the seizure onset zone is located in the brain area directly related to the movement [7]. The shadowboxing-induced seizures in our patient have all of these characteristics of reflex seizures.
To the best of our knowledge, there is no previous case report on shadowboxing-induced reflex seizures. There are several published case reports of epilepsy patients with focal aware behavioral arrest seizure induced by repetitive upper limb movements [14], [22]. Their ictal scalp EEGs showed frontal to central rhythmic waves, and one report identified the premotor cortex as the seizure onset zone by intracranial survey. However, their seizure precipitants were simple movements of a single limb, and were not part of an exercise, which requires complex movements of multiple limbs and cognitive functions such as attention, vigilance and motivation. Furthermore, none of the case reports of epilepsy patients with exercise-induced seizures were intended to identify the seizure-inducing movement in the exercise [4], [5]. Our current finding that a specific movement as well as non-motor and emotional elements induce reflex seizures broadens the concept of movement-induced reflex epilepsy, and extends our understanding of the pathophysiology of refractory exercise-induced seizures [5].
Boxing was indispensable for our patient to gain confidence and alleviate his anxiety about epilepsy. Consequently, depriving him of boxing would diminish his quality of life, even though it would resolve his exercise-induced seizures. For patients with exercise-induced reflex seizures, such as our patient, it is important to break down the exercise into several motions and identify the precipitative movement to minimize exercise restriction. This strategy should help maintain the quality of life of the patient, and may provide insight into the underlying pathophysiology.
4. Conclusion
We described a patient with focal epilepsy who had shadowboxing-induced reflex seizures. By using video-EEG recordings, we were able to identify the specific motion that triggered the exercise-induced reflex seizures. The identification of a specific motion that provokes exercise-induced reflex seizures is useful for not only understanding the pathophysiological basis of the disorder, but also for minimizing restriction of other beneficial exercises.
5. Note
All authors are in agreement with the content of the manuscript.
Ethical statement
The authors confirm that the work was carried out in accordance with the Declaration of Helsinki. The index patient provided clear approval for our publication of his clinical data in this article.
Declaration of conflict of interest
H. Shigeto is supported by a grant from JSPS KAKENHI (Grant No. 19K07964).
N. Isobe is also supported by a grant from JSPS KAKENHI (Grant No. 21K07464). The other authors have no conflicts of interest to disclose.
Acknowledgements
The authors are enormously indebted to the index patient and the nursing and physician staff at each facility in Kyushu University Hospital, including the Brain Center. We would like to appreciate our colleague Rinako Shimada at Kyushu University for advice on English composition. We also thank Alison Sherwin, PhD, and Barry Patel, PhD, from Edanz (https://jp.edanz.com/ac) for editing drafts of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebr.2022.100543.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Cavalcante B.R.R., Improta-Caria A.C., Melo V.H., De Sousa R.A.L. Exercise-linked consequences on epilepsy. Epilepsy Behav. 2021;121 doi: 10.1016/j.yebeh.2021.108079. [DOI] [PubMed] [Google Scholar]
- 2.Carrizosa-Moog J., Ladino L.D., Benjumea-Cuartas V., Orozco-Hernández J.P., Castrillón-Velilla D.M., Rizvi S., et al. Epilepsy, physical activity and sports: A narrative review. Can J Neurosci Sci. 2018;45:624–632. doi: 10.1017/cjn.2018.340. [DOI] [PubMed] [Google Scholar]
- 3.Nakken K.O. Physical exercise in outpatients with epilepsy. Epilepsia. 1999;40:643–651. doi: 10.1111/j.1528-1157.1999.tb05568.x. [DOI] [PubMed] [Google Scholar]
- 4.Strum J.W., Fedi M., Berkovic S.F., Reutens D.C. Exercise-induced temporal lobe epilepsy. Neurology. 2002;59:1246–1248. doi: 10.1212/wnl.59.8.1246. [DOI] [PubMed] [Google Scholar]
- 5.Kamel J.T., Badwy R.A., Cook M.J. Exercise-induced seizures and lateral asymmetry in patients with temporal lobe epilepsy. Epilepsy Behav Case Rep. 2014;2:26–30. doi: 10.1016/j.ebcr.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Illingworth J.L., Ring H. Conceptual distinctions between reflex and nonreflex precipitated seizures in the epilepsies: A systematic review of definitions employed in the research literature. Epilepsia. 2013;54:2036–2047. doi: 10.1111/epi.12340. [DOI] [PubMed] [Google Scholar]
- 7.Striano S., Coppola A., del Gaudio L., Striano P. Reflex seizures and reflex epilepsies: Old models for understanding mechanisms of epileptogenesis. Epilepsy Res. 2012;100:1–11. doi: 10.1016/j.epilepsyres.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Whitty C.W., Lishman W.A., Fitzgibbon J.P. Seizures induced by movement: A form of reflex epilepsy. Lancet. 1964;2:1403–1406. doi: 10.1016/s0140-6736(64)91980-4. [DOI] [PubMed] [Google Scholar]
- 9.Simpson R.K., Jr, Grossman R.G. Seizures after jogging. N Engl J Med. 1989;321:835. doi: 10.1056/NEJM198909213211218. [DOI] [PubMed] [Google Scholar]
- 10.Collard S.S., Ellis-Hill C. How do you exercise with epilepsy? Insight into the barriers and adaptations to successfully exercise with epilepsy. Epilepsy Behav. 2017;70:66–71. doi: 10.1016/j.yebeh.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Gibbs S.A., Proserpio P., Francione S., Mai R., Cardinale F., Sartori I., et al. Clinical features of sleep-related hypermotor epilepsy in relation to the seizure-onset zone: A review of 135 surgically treated cases. Epilepsia. 2019;60:707–717. doi: 10.1111/epi.14690. [DOI] [PubMed] [Google Scholar]
- 12.Rech F., Herbet G., Gaudeau Y., Mézières S., Moureau J.M., Moritz-Gasser S., et al. A probabilistic map of negative motor areas of the upper limb and face: a brain stimulation study. Brain. 2019;142:952–965. doi: 10.1093/brain/awz021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonini F., McGonigal A., Trébuchon A., Gavaret M., Bartolomei F., Giusiano B., et al. Frontal lobe seizures: from clinical semiology to localization. Epilepsia. 2014;55:264–277. doi: 10.1111/epi.12490. [DOI] [PubMed] [Google Scholar]
- 14.Malone S., Miller I., Jakayar P., Resnick T., Bhatia S., Duchowny M. MRI-negative frontal lobe epilepsy with ipsilateral akinesia and reflex activation. Epileptic Disord. 2008;10:349–355. doi: 10.1684/epd.2008.0224. [DOI] [PubMed] [Google Scholar]
- 15.Singh R., Principe A., Tadel F., Hoffmann D., Chabardes S., Minotti L., et al. Mapping the insula with stereo-electroencephalography: the emergence of semiology in insula lobe seizures. Ann Neurol. 2020;88:477–488. doi: 10.1002/ana.25817. [DOI] [PubMed] [Google Scholar]
- 16.Xiao H., Tran T.P., Pétrin M., Boucher O., Mohamed I., Bouthillier A., et al. Reflex operculoinsular seizures. Epileptic Disord. 2016;18:19–25. doi: 10.1684/epd.2016.0801. [DOI] [PubMed] [Google Scholar]
- 17.Whiting W.C., Gregor R.J., Finerman G.A. Kinematic analysis of human upper extremity movements in boxing. Am J Sports Med. 1988;16:130–136. doi: 10.1177/036354658801600207. [DOI] [PubMed] [Google Scholar]
- 18.Milton J., Solodkin A., Hlustik P., Small S.L. The mind of expert performance is cool and focused. Neuroimage. 2007;35:804–813. doi: 10.1016/j.neuroimage.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Chouinard P.A., Paus T. The primary motor and premotor areas of the human cerebral cortex. Neuroscientist. 2006;12:143–152. doi: 10.1177/1073858405284255. [DOI] [PubMed] [Google Scholar]
- 20.Choi E.Y., Ding S.L., Haber S.N. Combinatorial inputs to the ventral striatum from the temporal cortex, frontal cortex, and amygdala, implications for segmenting the striatum. eNeuro. 2017;4:e0392–e417. doi: 10.1523/ENEURO.0392-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blume W.T., Luders H.O., Mizrahi E., Tassinari C., van Emde B.W., Engel J., Jr. Glossary of descriptive terminology for ictal semiology: report of the ILAE task force on classification and terminology. Epilepsia. 2001;42:1212–1218. doi: 10.1046/j.1528-1157.2001.22001.x. [DOI] [PubMed] [Google Scholar]
- 22.Pierelli F., Di Gennaro G., Gherardi M., Spanedda F., Marciani M.G. Movement-induced seizures: a case report. Epilepsia. 1997;38:941–944. doi: 10.1111/j.1528-1157.1997.tb01261.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.