Abstract
Living microtissues are used in a multitude of applications as they more closely resemble native tissue physiology, as compared to 2D cultures. Microtissues are typically composed of a combination of cells and materials in varying combinations, which are dictated by the applications’ design requirements. Their applications range wide, from fundamental biological research such as differentiation studies to industrial applications such as cruelty-free meat production. However, their translation to industrial and clinical settings has been hindered due to the lack of scalability of microtissue production techniques. Continuous microfluidic processes provide an opportunity to overcome this limitation as they offer higher throughput production rates as compared to traditional batch techniques, while maintaining reproducible control over microtissue composition and size. In this review, we provide a comprehensive overview of the current approaches to engineer microtissues with a focus on the advantages of, and need for, the use of continuous processes to produce microtissues in large quantities. Finally, an outlook is provided that outlines the required developments to enable large-scale microtissue fabrication using continuous processes.
Keywords: Tissue engineering, Micromaterials, Microfluidics, Upscaling, Clinical translation, Industrial translation
Highlights
-
•
This review provides an overview of techniques used to create microtissues, and their applications in research and industry.
-
•
Batch production methods are compared to continuous production methods in terms of throughput and translatability.
-
•
Continuous production methods are ideal for many applications related to clinical translation and industrial valorization.
-
•
Optimization of continuous production methods is required to advance translation of microtissues to clinical/industrial use.
1. Introduction
The engineering of living tissues has widespread societal implications including fundamental biological research, animal-free cosmetical and pharmacological screenings, cell-based therapies, and cruelty-free meat production. To endow engineered tissues with improved functions such as mechanical performance or biological responsiveness to physiological stimuli, researchers have pioneered increasingly complex design strategies over the years. Traditionally, engineered tissues were designed in a top-down approach, by seeding cells onto scaffolds or by encapsulating cells in hydrogels. Although great progress has been made with the engineering of top-down designed tissues, these approaches generally lack spatial and temporal control thereby, limiting their ability to mimic the intricate microarchitecture and associated functions of native tissues.
Bottom-up tissue designs have been developed to provide biological and material inspired solutions to mimic the function of tissues at the microscale by offering additional spatial control. These building blocks are dubbed microtissues and are typically created in a size below the critical diffusion limit of nutrients, growth factors, and waste products. By creating non-homogenous assemblies of microtissues, more complex assemblies can be engineered, which include highly tailorable spatially controlled microenvironmental cues that can steer cell fate and tissue organization in a temporally predictable manner. Indeed, as native tissues are inherently non-homogenous, (complex) microtissues represent a prime candidate to recapitulate the body's complexity.
Unleashing the clinical and industrial potential of microtissues is currently hindered by the limited scalability of microtissues’ production process. Target applications such as pharmacological screenings, cruelty-free meat production, and cell-based therapeutical strategies require large amounts of microtissues in order to be of clinical or industrial use. Hence, the present review provides a comprehensive overview of the scalable fabrication, compartmentalization, and applications of living microtissues. Specifically, we advocate for the need of scalability of production processes when considering specific areas of application, and argue for the transition to continuous microfluidic processes to realize large-scale applications. Finally, we address the challenges and limitations that currently hinder the translation of state-of-the-art continuous microfluidic processes and aim to provide an insightful perspective on the developments needed to move towards translatable, scalable, microtissue fabrication strategies.
2. Building microtissues and traditional fabrication methods
2.1. From cells to microtissues
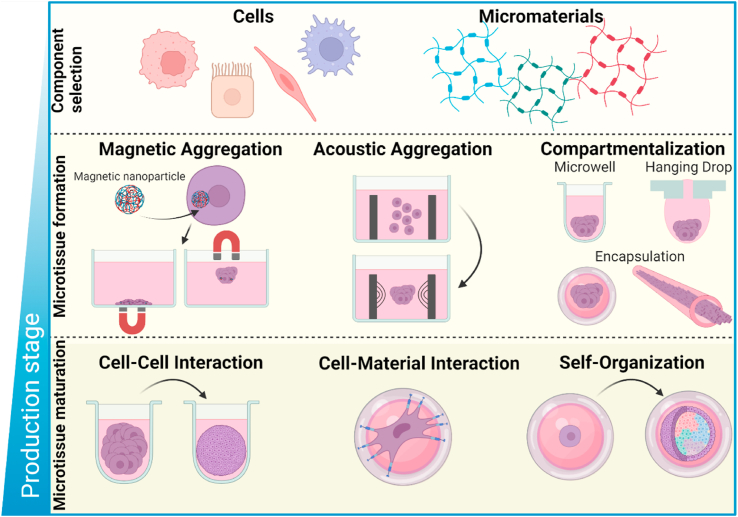
The fabrication of microtissues with well-defined microarchitectural features acts as a corner-stone for modular tissue engineering. Microtissues can be composed of specialized cells and materials, depending on the application, and can be mixed in various combinations, for example cell-material, multi-cell or cell-multi-material. By controlling the cell's microenvironment, cell-cell, cell-material contact and/or aggregation can be steered and adapted to achieve the desired microtissue [1]. The formation of microtissues can be generalized into three main stages; 1) the selection of modular elementary units such as cells, micromaterials or a combination of both; 2) microtissue formation through physical confinement of these base units in order to promote the desired cell aggregation/alignment; 3) microtissue maturation through cell-cell interaction, cell-biomaterial interaction and/or self-organization of cells (e.g., organoid formation by stem cells) (Fig. 1). Carefully choosing the conditions of microtissue formation is of imperative importance: the functionality of many specialized cell types depends on cellular organization and inherent physical cues to faithfully recreate native functions of the microtissue, such as the alignment seen in native muscle fiber, which plays an essential role in maximizing its contractile function [2,3]. This is historically achieved using batch processes such as microwell/microchannel seeding [[4], [5], [6], [7], [8]], hanging drops [[9], [10], [11], [12], [13]], micropatterning [[14], [15], [16]], and cell-sheet engineering [[17], [18], [19]]. All of these batch process-based techniques allow for the fabrication of specialized multi-dimensional microtissue building blocks composed of cells and/or micromaterials such as spheroids, cell fibers, cell sheets, and matured organoids [20]. From these microtissue building blocks, high degrees of complexity can be achieved by assembling cell aggregates/fibers/sheets to macroscale 3D complex constructs such as hierarchically organized structures, woven tissues, and microporous annealed particle scaffolds (MAPs) [21]. In this section, we will overview current batch-processing methods of fabrication of biomaterial-free and biomaterial-based microtissues for tissue engineering.
Fig. 1.
Schematic overview of microtissue production. Formation of microtissues can be generalized into three separate stages. First, cells and/or materials are mixed in varying combinations, forming the basis of the microtissue. Second, microtissues are formed by physically confining cells and/or materials through, for example, magnetic aggregation [22,23], acoustic aggregation [24] or compartmentalization [12,[25], [26], [27]]. Encapsulation is a specific form of compartmentalization where cells are confined within a micromaterial such as a microgel or microfiber. Third, microtissues are matured through cell and/or material interactions. Examples include cell-cell interaction to form spheroids, cell-material interaction to differentiate cells or self-organization of (stem) cells into organoids.
2.2. Fabricating microtissues – cell spheroids
The functionality of many specialized cell types depends on faithfully emulating microtissue-specific native 3D microenvironments. Consequently, cell spheroids have played an essential role in our comprehension of tissue development and homeostasis as they represent a basic and essential tools to understand cell-cell interdependence regarding biological variables such as viability, migration, and differentiation. Cell spheroids typically refer to rounded 3D cell aggregates, which can be composed of a single cell type or a mixture of multiple cell types [28]. Batch processes such as suspension cultures have been widely used to produce cell spheroids. Although these methods often associate with poorly controlled aggregate size and shape inhomogeneity, cell spheroid production in suspension cultures can be optimized by incorporating complementary rotation and spinning methods [[29], [30], [31]]. In contrast, aqueous compartmentalization techniques such as hanging-drop offer more control over the size distribution and yield of individual aggregates, with the added benefit that no specialized equipment or reagents are required [32]. As an example, Frey et al. developed a versatile and reconfigurable hanging drop microfluidic platform for aggregate production that could be used for screening studies [33]. However, this technique only offers very limited throughput as compared to other 3D culturing platforms. Alternatively, microwells, micropatterned gels, U-shaped chips, or microporous membranes have emerged as a valid batch-process technique to produce cell spheroids [34]. These platforms represent a minimal and simplistic approach, that offers accurate control over spheroid size and shape, substantially increased throughputs, and predictable spatial placement of spheroids. Specifically, cell-seeded microwell arrays have emerged as promising 3D substrates for the production of reproducible cell aggregates in amounts that are readily suitable for the screening of a limited amounts of drugs [25,35,36] and performing of in vivo studies using small animal models [7,37]. However, as these conventional batch techniques produce hundreds to thousands of cell spheroids per batch, translation to the industrial/clinical setting has been challenging [38].
Biomaterial-based approaches have also been used to batch produce spheroids as cell-laden hydrogel microcarriers. The development of techniques such as molding [39], two-phase emulsions [40], photolithography, and electrospraying [41,42] have demonstrated consistent spheroid production with easy retrievability, culture, and assembly into complex tissue structures that associated with improved biological functions such as cell adhesion, proliferation, and differentiation [43]. Of note, these approaches also associate with the risk for compromised bioactivity of genes, enzymes, and cell viability due to the use of organic solvents (electrospraying, two-phase emulsions) [44,45] or UV light (photolithography) [46].
Most recently, droplet-based microfluidic systems have been explored to produce microtissues with greater complexity and structural organization at higher throughputs. Microfluidic approaches hold great promise to replicate specialized human tissues in a miniaturized scale, with strict spatiotemporal control over the formation of encapsulated 3D-cell spheroids. To form spheroids, cells are perfused into tailored microfluidic chips, which create specialized microenvironments where cells are physically confined and undergo cell-cell interactions to form compact multicellular structures. In short, these systems allow for the production of microaggregates in a continuous manner in a manner that offers control over chemical concentration gradients, pressure of flow-induced signaling, and pressure and shear stress of encapsulated cells [47,48]. These microfluidic systems can have multiple geometries for the production and culture of encapsulated spheroids in liquid droplets, core-shell microgels, or solid microgels. Although the throughput of microfluidic systems is still insufficient to be considered for all industrial applications, potential solutions such as parallelized microfluidic droplet generators have been developed [49].
2.3. Fabricating microtissues – cell fibers
Cell fibers have received attention as an effective method to create 3D constructs with hierarchical structures [50]. Cell fibers can offer mechanical properties that are desirable to emulate characteristics of native tissues such as skeletal muscle tissue, blood capillaries, and nerve fibers [51,52]. Their surface-to-volume and strength-to-weight ratio provided by their microfibrous nature are frequently leveraged to build-up large scale tissues. These can be used for diverse applications such as living stitches [53,54], extrusion based additive manufacturing [55,56] and weaving [57,58]. Additionally, they may be potentially be used for tissue screening, due to the possibility to tailor cell response through strict control over the fiber's mechanical features [59,60].
A key advantage of microfibers is that they can be endowed with surface patterns in a facile and versatile manner. Indeed, attachment, proliferation, and function of cells can be steered, in addition to chemical modifications such as RGD motifs, via external topographies such as grooves. An elegant example is provided by the work of Kang et al., in which cortical neurons were externally cultured on either grooved or smooth calcium alginate microfibers fabricated by microfluidic spinning. On microgrooved fibers, neurons aligned along the ridges of the grooved surface, while on smooth surfaces neuron cells migrated to the edges and formed networks covering the alginate fibers [61,62]. In another study, scaffold-free engineered microfibers for tendon replacement grafts were created using fibroblast seeded microchannels. The microfibers were subsequently conditioned with uniaxial tensile loads in order to emulate the typical loads applied to tendon tissue [63]. These examples of surface patterning show that specific modulation of cell behavior and fiber thicknesses can be induced by introducing microtopographical changes to micromaterials in an non-invasive and non-chemical manner.
Microfibers are also explored for the production of cell-laden fibers via cell encapsulation, which has been leveraged to provide biomechanical cues to guide growth, migration, and alignment of cells. For example, Hwang et al. demonstrated that microfiber diameter can heavily influence cell orientation. Cell alignment between 10 μm and 242 μm fibers were compared, and more heterogenous orientation of cells in the larger fibers were observed [64]. In addition, fibers can adopt multiple geometries, such as solid [65], core-shell [66,67] and Janus [68,69] conformations to further guide cell behavior.
2.4. Fabricating microtissues – cell sheets
Cell sheets are engineered 2D layers of cells that can be cultured on top of a surface that supports cell growth and possesses adhesive/detaching features. Cell sheets consist of cells, ECM, growth factors, and important surface adhesion moieties that bind to the substrate. Initially, cell sheets were retrieved from monolayered cultured surfaces using proteolytic enzymes that compromised the functionality of cultured cells and cell sheet integrity. This challenge was addressed by the introduction of thermo-responsive polymer surfaces, which were typically based on Poly(N-isopropylacrylamide) (PIPAAm). This polymer surface offers thermally dictated hydrophilic/hydrophobic properties, which enables the harvesting of confluent cell layers via a simple decrease in temperature. In recent years, various alternative biomaterial systems have been reported, which include systems sensitive to ionic strength [70], photonic exposure [71], electric stimulation [72], ultrasonic vibrations [73], magnetic field manipulation [22,23], as well as biochemical treatment through non-proteolytic non-enzymatic incubation [74].
Harvested cell sheets are highly adhesive owing to their strong cell-cell junctions and deposited ECM composed of adhesion proteins such as integrins. As trypsin only degrades the carboxyl side of lysine or arginine, this matrix is only partially degraded and its integrity remains unaffected. This feature enables cell sheets to readily bind to native tissue and can provide a protective barrier from exogenous agents. Multiple studies have used cell sheets with great success to treat a plethora of different tissues, such as esophagus [75], intestinal/bowel [76,77], cornea [78,79], cardiac tissue [80,81], hepatic tissue [82], and skin [83].
Several studies have focused on the generation of cell sheets as functional units to build up complex tissue structures. Williams et al., for example, used PIPAAm and fibronectin-PDMS grooved stamps to produce and stack hMSCs sheets. This thermo-responsive layer-by-layer approach aimed to mimic physiological vascular tissue organization [84]. In parallel, Okano and collaborators developed a double polymeric hydrolayer surface of hydrophilic poly-acrylamide (PAAm) and PIPAAm aiming to improve cell-sheet detachment. They demonstrated that PAAm could facilitate the hydration and provide water molecules to PIPAAm, which accelerated cell sheet detachment [85]. Another creative example of a novel approach for cell sheet fabrication is given by Zhang and collaborators. In this specific study, the authors managed to label dental-pulp stem cells with Fe3O4 magnetic nanoparticles that were coated with nanoscale graphene oxide in order to organize cell orientation by magnetic fields to form multilayered cell sheets with different patterns [86]. Additionally, the graphene oxide coating provided growth-factor binding features that was used to bind BMP-2, which endowed the cells with a higher osteogenic potential.
Although promising, current cell sheet engineering technologies have inherent limitations to produce complex material-free microtissues, which associate with limited thicknesses (∼60 μm per sheet maximum) [87] and a labor intensive process. Additionally, current techniques display limitations on the vertical integration and combination of distinct tissue structures in layer-by-layer approach [88].
The application of all abovementioned batch techniques, including cell sheets, into the industrial/clinical setting has thus been primarily hampered by the lack of high-throughput production processes; current batch production methods are inefficient due to their highly demanding manual and artisanal labor, poor capability for on-the-fly tuning, and limited temporal and quality control during their production process. These challenges naturally affect the translational application of all batch process to a clinical/industrial setting and highlight the need for the adoption of continuous processes for microtissue fabrication. In contrast with batch techniques, continuous microfluidics offer production features that can omit these drawbacks of batch produced microtissues [89].
3. Continuous methods for scalable production of compartmentalized microtissues
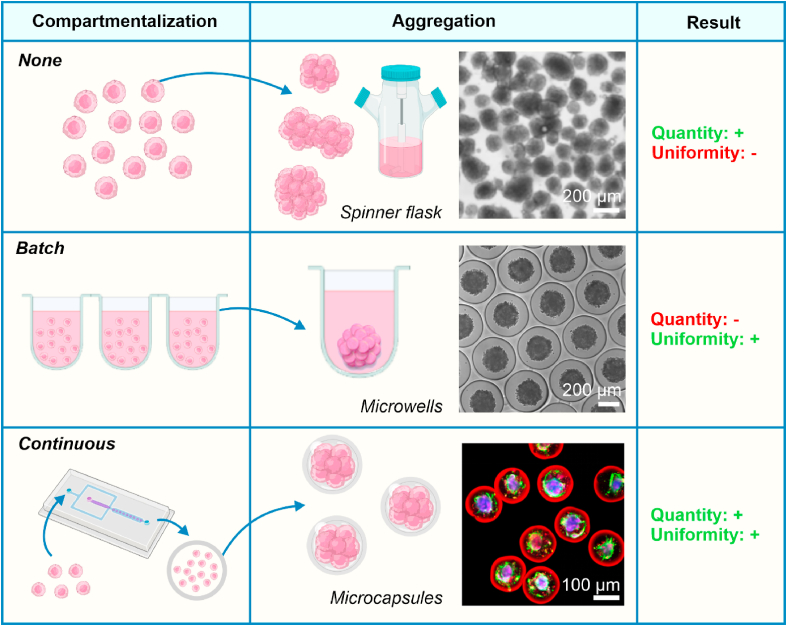
In order to allow for clinical and industrial integration of microtissues, requirements such as a high-throughput production process [115] and minimal batch-to-batch variation [116] have to be met. However, aforementioned conventional batch processes such as low-adherent culture plates [[117], [118], [119], [120]], micropatterning [121,122], microwells [[4], [5], [6], [7], [8]], and hanging-drop techniques [[9], [10], [11], [12], [13]] do not comply with these requirements (Table 1, Fig. 2). Traditional techniques such as cell aggregation in spinner flasks typically produce free-floating microtissues, which leads microtissues to fuse with each other inside large bioreactors used for culture and thus associates with a polydispersity in final microtissue size (Fig. 2) [[123], [124], [125]]. Alternatively, batch-compartmentalization of microtissues using methods such as hanging drop and microwell culture effectively prevents microtissue fusion, but lacks the throughput required for clinical translation. In contrast, microfluidic compartmentalization of microtissues offers a continuous production process that can be elevated to reach high throughput microtissue production [89]. Additionally, the continuous nature of this production process minimizes batch-to-batch variation [126] and, importantly, the encapsulation of cells within micromaterial compartments allows microtissues to be grown in industrial-scale bioreactors by preventing microtissue fusion (Fig. 2). Taken together, these advantages make continuous methods an attractive technique for the clinical and industrial translation of microtissues.
Table 1.
Comparison of non-continuous and continuous production platforms utilizing compartmentalization for microtissue fabrication.
| Technique | Throughput | Dispersity | Microtissue size | Key material limitation | References | |
|---|---|---|---|---|---|---|
| Non-continuous | Microwells | Limited due to 2D nature | Monodisperse | ∼50–300 μm Ø | Surface tension; Hydrophilicity of substrate | [[4], [5], [6], [7]] |
| Hanging-drop | Monodisperse | ∼200 μm Ø | Surface tension; Hydrophilicity of substrate | [[9], [10], [11], [12], [13]] | ||
| Micropatterning | Not applicable | – | Hydrophilicity of substrate | [[14], [15], [16],[90], [91], [92], [93]] | ||
| Cell sheets | ∼5–600 μm thickness | Hydrophilicity of substrate | [[17], [18], [19]] | |||
| Molding | – | Hydrophilicity of substrate | [39,92] | |||
| Photolithography | – | Often depends on UV crosslinking | [41,46] | |||
| Continuous | Droplet microfluidics | ∼10−4 to 10−1 ml/min | Monodisperse | ∼5–800 μm Ø | Surfactant use; Viscosity; | [27,48,[94], [95], [96], [97], [98], [99], [100], [101]] |
| Vibrating jet | ∼10−1 to 100 ml/min | Monodisperse | ∼20–300 μm Ø | Viscosity; need for rapid crosslinking | [102,103] | |
| Jet cutting | ∼10–105 ml/min | Monodisperse | ∼150 μm–1000 μm Ø | Viscosity | [104,105] | |
| Inkjet printing | ∼10−3 to 10−1 ml/min | Monodisperse | ∼30–80 μm Ø | Viscosity | [[106], [107], [108]] | |
| Air-induced spraying | ∼10−1 to 10 ml/min | Polydisperse | ∼5–1000 μm Ø | Viscosity | [[109], [110], [111]] | |
| Electrospraying | ∼103 to 10 ml/min | Polydisperse | ∼5–1000 μm Ø | Organic solvents | [[112], [113], [114]] |
Fig. 2.
Microencapsulation is required to translate microtissues to industrial/clinical scale applications. To expand cells into larger quantities and/or more complex modalities, encapsulation using continuous microfluidics provides an ideal balance between scalability and uniformity. Traditional methods to produce microtissues rely on the culture of cells without compartmentalization (e.g., in spinner flasks) or with batch compartmentalization (e.g., in microwells), leading to low uniformity due to fusion and low throughput, respectively. By compartmentalizing cells in microcapsules using high-throughput microfluidics, microtissues can be produced in large quantities while maintaining microtissue uniformity. Moreover, microtissues produced in this manner can still be cultured in large bioreactors due to the physical barrier provided by the (semi-permeable) microcapsule, preventing microtissue fusion. Figures adapted with permission from [124,137,138].
Microfluidic production of microtissues typically involves two critical steps: (1) dispersion of a liquid cell-laden particle precursor solution into discrete droplets; and (2) solidification of droplets through in situ crosslinking or precipitation. Droplets can be formed via patterning [[90], [91], [92], [93]], molding on/in solid substrates [92], emulsification in an (immiscible) liquid [[127], [128], [129], [130], [131], [132]], or atomization in a gas [102,103,[133], [134], [135], [136]]. Here, emulsification (i.e., liquid-liquid) and atomization (i.e., liquid-air) are continuous droplet formation processes compatible with microfluidics with inherently high throughputs and therefore suitable for scalable production of microtissues.
3.1. Microfluidic micromaterials for microtissue building blocks
In microfluidics, droplet emulsions are typically produced by flowing two immiscible fluids (i.e., a (cell-laden) water-phase and an oil-phase) in a coaxial [127,128], T-junction [129,130], or flow focusing [131,132] configuration, which can be stabilized with surfactants [139]. The discrete aqueous droplets within the emulsion can be solidified into cell-laden micromaterials via a wide range of mechanisms including ionic- [27,140,141], enzymatic- [138], photo- [[142], [143], [144]], or thermal-crosslinking [145]. A wide variety of materials can be chosen, with the key material limitation being viscosity (Table 1). These cell-laden micromaterials can be cultured as individual living microtissues, but can also be assembled into clinically sized, modular, living microtissues.
The most intuitive form of microfluidically produced micromaterials is solid microspheres, where a polymer precursor solution is emulsified into droplets that are fully solidified [[146], [147], [148]]. The introduction of cells into the precursor solution results in either single-cell-laden solid microgels [144,147,149], or multiple-cell-laden solid microgels [[146], [147], [148]], which can in turn be used as building blocks for bottom-up engineered tissues. However, these solid microgel systems hinder cell-cell contact of encapsulated cells, which is known to be an important factor in the benefits of 3D cell culture [150]. Building on the principles utilized in creating solid microspheres, it is possible to create hollow, compartmentalized microgels by using, for example, multi-emulsions [151]. By introduction of cells in the hollow compartment of core-shell microgels, cells are encapsulated in a manner in which cell-cell contact is enabled, which allows for the formation of cellular aggregates and more complex organoids [138,152]. Janus particles are a specific type of compartmentalized structure, typified by a more complex anisotropic morphology and a multicompartmental, but not necessarily core-shell, structure [153]. Their anisotropic properties are often manifested in a chemical or physical manner, but also allow for encapsulation of multiple cell types within a single micromaterial without direct cell-cell contact. Although the aforementioned microfluidically formed microtissue building blocks are all most commonly spherically shaped, non-spherical microparticles can also be produced continuously using microfluidics. Confinement of microfluidic channels allow for the production of disc- or rod-shape microparticles [154], while produced droplets can also be further processed in non-spherical microparticles by arrested coalescence, asymmetric polymer solidification or evaporation-driven clustering [155]. Since non-spherical microwells are known to allow for microtissue polarization [156], these continuously produced non-spherical particles could similarly allow the formation of complex, polarized microtissues, but in a higher-throughput format.
3.2. Microfluidic production methods for micromaterials
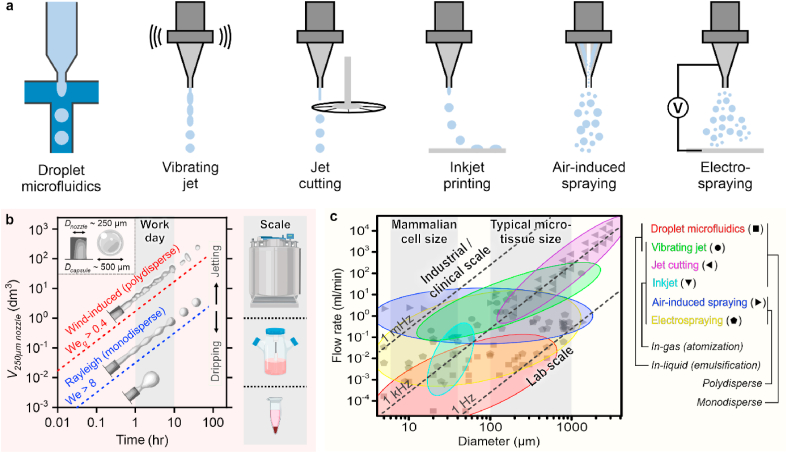
Continuous microfluidic production methods operate in either the dripping regime (Weber number (We) < 8) or jetting regime (We > 8). Techniques in the jetting regime can be further categorized based on the breakup of the jet from a Rayleigh induced breakup regime (Weber gas (Weg) < 0.4) to a wind induced breakup regime (Fig. 3a and b). The throughput as well as monodispersity of the capsules produced using continuous production methods are closely related to these regimes as illustrated by the time it takes to fill the various types of bioreactors on the right y-axis of Fig. 3b. Finally, the desired microtissue size also has an influence on the throughput of various continuous microcapsule production methods (Fig. 3c).
Fig. 3.
Continuous microcapsule production methods and their potential for translation to clinical/industrial settings. a. Schematic representation of various continuous production methods to produce (cell-laden) microcapsules. b. Typical throughputs of continuous production techniques calculated for varying regimes including from dripping; Weber number (We) < 8 to jetting (We > 8) into and from Rayleigh induced break up (gas Weber number (Weg) < 0.4) to wind-induced breakup (Weg > 0.4). A capsule size of 500 μm is chosen for the calculations as a representative example. On the right y-axis, container sizes are indicated to illustrate the required time to fill a specific volume ranging from lab scale (Eppendorf tube) to industrial scale (bioreactor). c. Overview of various continuous production methods and their throughputs (flow rate) as a function of droplet size. Typical mammalian cell size and microtissue sizes are indicated to outline methods of interest. The indicated production regimes are based on data points obtained from the following references: droplet microfluidics [27,[95], [96], [97], [98], [99], [100], [101],104], vibrating jet [102], jet cutting [105,106], inkjet [107,108,176], air-induced spraying [106,[109], [110], [111], [112]], and electrospraying [106,113,114,177]. Figure adapted with permission from [178].
The most common method for continuous droplet formation for production of the aforementioned cell-laden micromaterials is through the use of microfluidic channels within glass [[157], [158], [159]] or transparent elastomers such as polydimethylsiloxane (PDMS) [[160], [161], [162], [163]], which are operated in the dripping regime. These systems have been shown to be successful for the fabrication of microtissues. However, while production in the dripping regime allows for excellent control of monodisperse microparticle formation, their low production rates (<1 ml/min) [164] are inhibitive for clinical and industrial translation (Fig. 3b). Hence, innovations such as parallelization are required in order to achieve commercially relevant throughputs [49,165,166]. Moreover, disadvantages of the single-use and enclosed nature of these systems limits their scalability for the production of microtissues. These disadvantages include the need for advanced lithographic infrastructure to produce the microfluidic systems and their association with significant wastage of (e.g., clogged) microfluidic devices. As an alternative, reusable off-the-shelf microfluidic devices that are manufactured using standard cutting and abrasion methods have been explored to allow widespread adaptation of these water-in-oil systems [142].
Jetting and spraying technologies allow for higher throughputs (up to 100 ml/min) [102], which makes them more suitable for scalable production of cell-laden microcapsules (Fig. 3b) [164]. However, unassisted techniques such as air-induced spraying and electrospraying only offer poor control over resolution and produce polydisperse microparticle populations (Fig. 3b) [167,168]. Therefore, efforts have been made in order to achieve monodisperse microparticles in the jetting regime by for instance jet-cutting [104,105] or by applying microvibrations using piezoelectric elements in liquid-liquid with vibrating jet technology [[169], [170], [171]] and liquid-air configurations with both inkjet [[106], [107], [108]] and vibrating jet technology (Fig. 3a,c) [102,103,172]. Similarly, electrospraying has been utilized by applying (submerged) electromagnetic fields for both liquid-liquid [173,174] and liquid-air configurations (Fig. 3a,c) [[134], [135], [136]].
In contrast to liquid-liquid, liquid-air systems such as sprays or droplets that fly in the air do neither require the use of a potentially sample contaminating immiscible phase (e.g., oil) or surfactants (e.g., amphiphiles) to form droplets while allowing for considerably higher production rates. However, the produced droplets only remain separated until they collide onto their receiving collector, or coalesce in-flight, which necessitates their in-flight stabilization using, for example, extremely rapid (milliseconds) crosslinking mechanisms. In practice, atomization is therefore almost exclusively combined with ionic crosslinking strategies such as alginate with divalent cations [102,103], rapid heating (i.e., spray drying) or cooling (i.e., spray freeze drying) strategies, which is in contrast with lower throughput systems which beside ionic crosslinking materials can utilize materials with slower crosslinking mechanisms such as enzymatic and photo crosslinking [142]. Yet, materials that rely on slower (seconds to minutes) solidification mechanisms such as silk fibroin can still be utilized by leveraging, for example, alginate as a sacrificial structural template in the form of an alginate shell or interpenetrating alginate network [175]. Several complex micromaterial structures have been produced using in-air microfluidics, such as (cell-laden) solid microgels, core-shell microgels, Janus micromaterials and microfiber structures [102,103]. It is important to note that cell-laden micromaterials can be combined with non-cell-laden micromaterials, allowing interesting opportunities to add further functional and/or spatial complexity to influence microtissue formation [21]. In this fashion, a wide array of materials can be processed to yield cell-laden micromaterials of controlled shape and size for complex microtissue formation with high quality and high quantity, allowing for clinical and industrial translation of cell-laden micromaterials. Microfluidic platforms can also be integrated with x-y controlled motors (e.g., inkjet droplet printing), which can be leveraged to engineer hierarchical tissues in an automated fashion [94], or be utilized as handheld printers, for example, for in situ bioprinting [102].
4. Applications of microtissues
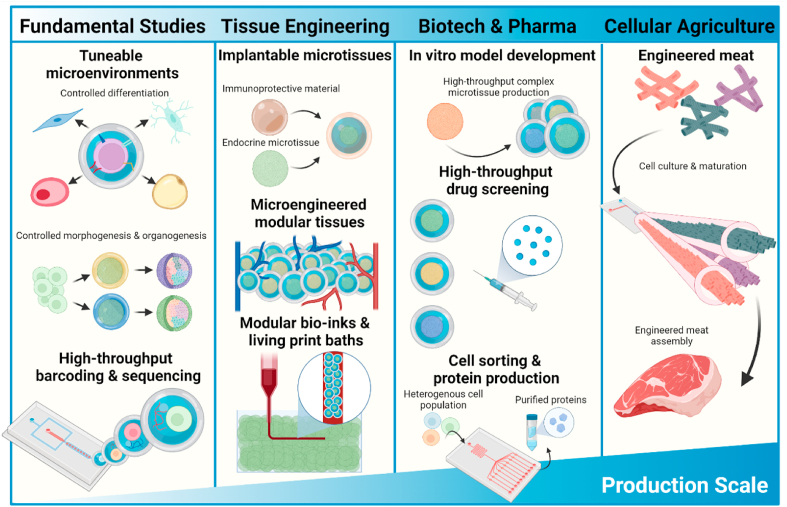
Microtissues have numerous applications in various commercial domains (Fig. 4). Firstly, they can be used as single units to study the fundamentals of microtissue formation, maturation, and homeostasis. Using continuous fabrication processes, microtissues can be tuned on the fly to create large libraries for screening experiments. Second, by combining bottom-up engineered microtissues with top-down production processes, next generation tissues or living implants can be engineered in a modular, hierarchical, and spatially controlled manner. Finally, in recent years, cellular agriculture and engineered meat have garnered a significant amount of attention. The evident ever-growing demand of these applications has highlighted the need for large-scale microtissue production using facile, tunable, and scalable techniques. As applications are a key determinant in the design of microtissues and dictate the criteria for their composition as well as their production methods, this chapter will outline several applications and their associated requirements.
Fig. 4.
Schematic representation of applications of microtissues across various production scales. Applications of complex microtissue range across a wide variety of scales. From building controllable environments for fundamental studies to implantable tissue constructs, high-throughput drug screening platforms and engineered meat. Each application has different requirements for microtissue formation, culture and complexity.
4.1. Fundamental cell studies: high-resolution 3D microenvironments
It is well-known that cells interact intimately with their microenvironment in a reciprocal manner [179]. Microenvironments dynamically dictate cell fate and play an important role in the onset, development, and treatment of diseases such as cancer [180,181]. Indeed, 3D culture systems have repeatedly been demonstrated to more accurately emulate in vivo behavior as compared to the historically dominant 2D culture systems [182]. Moreover, 3D culture systems assembled of a combination of cells and materials allow for a tunable environment with spatially organized microenvironmental cues [183]. The interplay between cells and their microenvironment has been widely explored in vitro and has also been shown to be of importance in vivo in directing regeneration, underlining the importance of the microenvironment for tissue morphogenesis and homeostasis [[184], [185], [186]]. By encapsulating cells in microgels, it becomes possible to precisely tune the cellular microenvironment while minimizing or even avoiding heterogenous cell response due to mass-transport limitations or hindered oxygen diffusion that commonly associate with conventional macro-sized 3D in vitro cultures and 3D cellular spheroids [187,188]. To achieve microenvironments that can steer cell fate or maturation, it is important to develop or adapt tunable materials that can be utilized to control cell-cell interaction and cell-material contact (e.g., material stiffness, topography, and degradation) in a spatial and temporal manner in microtissues.
A typical example of a microenvironmental cue to modulate (stem) cell responses is mechanotransduction through the use of cell-adhesive molecules (CAMs). Microenvironments are endowed with CAMs to enable the stem cell microniche to control, for example, proliferation and differentiation [189]. The physiological microenvironment's dynamic nature strongly regulates how these factors are presented to cells, and is thus important to design temporally controllable microenvironments in vitro [190]. Discrete inducible on-cell crosslinking (DOCKING) has recently been showcased as an example of such a temporally tunable microenvironment. By directly tethering cells to non-adhesive materials, it allowed the transduction of mechanical cues from non-adhesive materials to cells, enabling stem cell programming using material stiffness. Moreover, by modulating the material's stiffness in a temporal manner, the dynamic nature of the natural microenvironment of the cell can be emulated. Using technologies such as DOCKING, it will become possible to study stem-cells and their response to tailored mechanical cues, to steer differentiation and to engineer larger tissues with highly optimized cellular microniches [149]. Another approach to study and direct stem cell fate was reported by Kolb et al. Aggregates of cell lines that could express specific tagged proteins were encapsulated into microgels. The expressed proteins were subsequently chemically captured in the outer layer of the microgel where they could affect the response of cells cultured on the outside of the microgels. In this manner, they were able to create libraries of microniches to screen stem cell fate in response to specific proteins [191].
To gain a better understanding of developmental processes such as organogenesis and morphogenesis, a certain degree of complexity in microtissues is often required. Whether using scaffold-free approaches [192] or compartmentalization using the described techniques, continuous microfluidic techniques enable straightforward creation of large amounts of microtissues, which are necessary for such high throughput screens. To study the mechanisms underlying these processes, sequencing technologies are typically employed. Traditional sequencing technologies struggle to screen single cell populations and identify population distributions in an accurate, high throughput manner and require multiple platforms and techniques to isolate and process cells separately. A recent example of the potential of microgels in cell screening was shown by Lan et al. who leveraged single cell encapsulation to create barcoded, single-cell microgels allowing for high-throughput single cell genome sequencing [193]. By constraining single cells in microgels, cells and genomes were kept separated. The microgel environment additionally served as a microreactor, automating a large part of the process, resulting in less variability due to handling/user bias. By providing a variety of cues to the cells, platforms like these could enable researchers to better understand the cellular mechanisms required for the formation of more complex tissues. An example of this approach was showcased by Klein et al. who screened the heterogenous onset of differentiation by embryonal stem cells in response to withdrawal of leukemia inhibitory factor (LIF). In their work, the use of high-throughput droplet microfluidics was critical to reach the processing scale required to identify small subpopulations. Besides the effects of soluble factors, shape and mechanical properties are known to play important roles in microtissue development [[194], [195], [196]]. In principle, microfluidic compartmentalization allows for the creation of tunable microenvironments that serve as culture vessels for single cells as well as microreactors for downstream processing.
4.2. Tissue engineering: injectable and interconnected microtissues
Owing to their attractive feature of self-assembling and fusing in a bottom-up manner to form complex tissues in a straightforward and scalable manner, the use of microtissues have become commonplace in bioengineering in recent years. Moreover, compartmentalization of microtissues provides an increased degree of modularity and hierarchical structure, control over tissue formation as well a protective layer to shield microtissues from their environment, which may be desirable when assembling them into larger constructs, or for in vivo applications. Individual as well as connected microgel building blocks have found a variety of promising uses in the field of bioengineering, which ranges from minimally invasive therapies to printable bioinks to embedded printing baths.
Microtissues have been used as injectables in cell-based therapies for years, but typically suffer from low retention and post-injection viability. Encapsulation of cells in hydrogels has been used as a tool to address these challenges, but the use of bulk hydrogels also imposes limitations in the diffusion of oxygen and nutrients owing to their typically large size [197]. Compartmentalizing microtissues in micron-sized hydrogels resolves this limitation and has been explored extensively as injectable cell-based therapeuticals. Feng et al. encapsulated bone mesenchymal stem cells in gelatin-hyaluronic acid microgels, which led to improved chondrogenesis as well as post-injection viability and cartilage restoration. Moreover, the stem cells were able to migrate to the shells of the microgels, allowing for in-situ annealing of microtissues through cell-cell contact. This effect prevented unwanted vascularization as well as hypertrophic differentiation, which led to improved in situ cartilage formation [198]. Encapsulation can also play an important role in regulating the immune response post-injection by acting as an immunoprotective semi-permeable shield. This strategy is of particular interest for organs with an endocrine function, such as the pancreas [26] or the adrenal gland [199] under pathological conditions that associate with autoimmune destruction of native tissues. Weaver et al. utilized this approach to inject encapsulated pancreatic islets from an allogeneic source, showing improved implant survival, integration, and retrievability compared to unencapsulated islets, while maintaining the ability to restore normoglycemia in diabetic mice [200]. In addition, immunoprotective biomaterials may further enable the use of allogeneic or xenogeneic cell for a wider set of clinical applications.
In recent years, a new class of microgel based scaffolds termed microporous annealed particle scaffolds (MAPs) has garnered attention. These consist of microgels that are annealed either before or after injection, leading to improved tissue integration compared to traditional nanoporous hydrogels owing to the microporous nature of the construct, which allows for increased cell infiltration [21]. Some examples of the potential of MAPs in regenerative medicine include their use as scaffolds for delivery of cells with improved retention [201], accelerated wound closure [21], improved cartilage regeneration [202] and delivery of therapeutics [203]. Although not widely explored, these scaffolds can also be combined with encapsulated microtissues to engineer large tissues. An example of the potential of this approach was published by Yang et al. who built larger tissues from cell-laden microgels in a bottom up manner [204]. Stem cells were encapsulated in millimetric collagenous microgels using a microfluidic system, which were subsequently allowed to anneal in a mold to form MAPs. Over time, cell-cell contact led to annealing, and cell-ECM interactions also caused the collagen microgels to shrink. Moreover, porous space was found in between the annealed microtissues, allowing for improved media diffusion compared to nanoporous constructs. Although centimeter sized constructs were engineered, the microtissue building blocks in this work were relatively large (>800 μm post shrinkage), which associates with a poor resolution and may still cause oxygenation and nutritional difficulties over long-term culture periods or during implantation.
To improve implant survival, pre-vascularization of engineered tissues is a commonly used approach. Owing to the shear thinning properties of dense microgel and/or microtissue suspensions, it is possible to utilize microtissues as print baths for embedded printing [205,206]. Integration of microtissues with top-down approaches such as 3D printing enables an added degree of spatial organization, allowing for large, highly organized 3D tissues with micro-scale modularity and resolution. Moreover, printing vascular-like channels and pre-vascularizing engineered tissues can improve implant performance and survival. The concept of integrating microtissues with 3D printing has been showcased. For example, Skylar-Scott et al. [206] used spheroids as a print bad and Hinton et al. [207] used microgels as a printing bath. Both of these methods could profit from the benefits of microfluidics, by compartmentalizing microtissues for higher-throughput culture as well as protection during printing to facilitate living modular print baths. Finally, microtissues can also be used as bio-inks instead of baths [208,209]. Combining both ink and bath approaches provides an unprecedented degree of freedom in designing engineered tissues. These printed constructs could then be stabilized through aforementioned approaches such as microgel annealing or tissue fusion. However, to translate such approaches to clinical scales, it is necessary to also scale-up the production throughputs of cell-laden microgels. To this end, Di Carlo et al. created a scalable platform to generate annealable microgels with production rates of ∼25 mL per hour [210]. However, these microgels were not cell-laden, and inclusion of cells would impose additional restrictions on the production method. Ideally, small building blocks (<200 μm) should be used to generate high-resolution bottom-up engineered tissues. Although it is possible to engineer larger microtissue building blocks, additional strategies would be required to circumvent issues related to oxygenation and nutritional limitations within the microtissue, examples include vascularization and oxygen releasing compounds, both of which have been reviewed extensively elsewhere [[211], [212], [213]]. These engineered building blocks can additionally allow for increased control over the hierarchical structure and properties of an engineered tissue, as their modular assembly leads to varying properties (e.g., mechanical properties) over different scales of resolution, which is something that is more difficult to achieve with traditional engineered tissues constructed from nanoporous bulk hydrogels. However, in microfluidic production methods, microgel size is typically inversely related to production speed (Fig. 3c), emphasizing the need for scalable production platforms to engineer high resolution microtissue building blocks. In short, new techniques and protocols to produce cell-laden micromaterials in ultra-high throughput are needed to create clinically-sized, personalized, modular tissues to realize straightforward clinical translation.
4.3. Biotechnology/pharma industry: high-throughput screening of 3D microenvironments
Biotech and biopharma have historically relied on widespread and intensive use of animal testing to develop and verify the safety and efficacy of new pharmaceutical products such as vaccines or drugs. With many governments striving for a reduction in animal testing an abundance of in vitro alternatives have been developed in recent years. A main requirement for such alternatives is that they accurately emulate human physiology. In vitro drug testing has seen significant advances that have enabled the replacement of specific traditional 2D cell culture models with more advanced 3D models, most notably spheroids and organoids, due to the presence of a more physiologically relevant microenvironment. As previously reviewed by Astashkina et al. the microenvironment plays an important role in achieving and maintaining the native phenotype of microtissues, which is required to obtain relevant information from drug toxicity tests [214].
Besides physiological relevance, compatibility of the in vitro model with high-throughput screening methods plays a key role when designing microtissues for drug screening. Production methods need to reliably produce homogenous 3D models with little to no batch-to-batch variation, as variance in size or cellular composition can have detrimental effects on the initial drug response as well as technical reproducibility of results (e.g., size is a limiting factor for optical readouts). Moreover, high throughput screening processes are ideally automized, to reduce variability and error caused by handling and, most importantly, to cost-effectively accelerate the process of identifying promising drugs to be further evaluated.
Compartmentalization using continuous production methods can have an impact on the biopharma and drug screening sector in a variety of ways. First, the throughput rates of microtissue production can lead to accelerated drug screening, as a larger amount/libraries of microtissues can be generated in a substantially shorter timeframe. Second, the cellular composition of microtissues can be controlled in terms of differentiation and maturation through modulating the cell's microenvironment through, for example, choice of microgel material. This allows for increased control over 3D model complexity as opposed to hanging drop or microwell formats, where soluble factors are generally the only method used to control the 3D model. Compartmentalization can thus offer the opportunity to create more (patho)physiologically relevant microenvironments. Third, the microenvironment protects microtissues allowing, for example, for displacement of microtissues using robotics or dispensing systems without incurring microtissue damage thus reducing data bias [215]. Finally, through chemical modification, microgels can be functionalized with biochemical sensors (e.g., biomarkers) allowing for rapid assays without the need for multi-step protocols to analyze microtissues after adding drug compounds [216].
Besides the development of tissue models for drug testing, the bioprocessing field might also benefit from the use of compartmentalization and continuous microfluidic production methods. Mammalian cell lines are used for directed evolution to produce recombinant proteins that are applied in, for example, drug development or as biocatalysts, as other organisms or expression systems (e.g., bacteria) may lack the required environment for some proteins to function properly [217,218]. Two of the major steps in the development of recombinant proteins are selection of the cell lines to produce a population with high expression of the desired protein and downstream protein purification [219]. Microencapsulation of cells could enable higher-throughput screening methods to select desirable clones in heterogenous populations. By including specific protein-immobilizing moieties onto micromaterials, produced proteins could directly be captured inside the microcarrier. In this manner, proteins are concentrated in local small volumes and more easily purified and extracted [220]. Moreover, using in-line fluorescence sorting methods, the high-producing populations could directly be identified and extracted to isolate monoclonal high-producing populations. Finally, cell-cell contact and microenvironmental cues have been implicated as important factors in faster population growth and cloning efficiency. These are issues that can readily be addressed by compartmentalizing cells inside micromaterials to create microtissues and improve directed evolution in the biotech industry [221].
4.4. Cellular agriculture: engineered cruelty-free meat
With global attention focused on improving animal welfare and the environmental impact of the meat industry, cellular agriculture has emerged as a rapidly growing biotechnology field. A well-known example is the lab-grown hamburger presented by the research group of Mark Post in 2013. Fresh muscle was extracted from a cow, and the cells from this piece of tissue were grown in the lab for several weeks to expand the cells. Subsequently, the cells were matured in a ring-like mold to form mature muscle fibers. A large amount of these fibers was then used to combine into an 85 g hamburger [222]. In a more recent example by Furuhashi et al. a pillar based mold was used in conjunction with cells suspended in hydrogels to mature millimeter thick bovine myocytes. This method has scalable modules which theoretically enables the culture of regularly sized steaks [223].
Although the concept of a cultured meat has been shown, the process is still far from optimal. The major roadblock for advancing cellular agriculture is acquiring large quantities of cells in a cost-efficient manner. Traditional cell-culture methods are labor intensive, time consuming, and often demand separate platforms for culture and maturation of cells. Moreover, to engineer a larger diversity of meats, texture and cell types become an important factor as well. The main components typically present in meat are skeletal muscle and fat tissue, which both require different culture and maturation protocols to develop or maintain their respective physiological phenotypes. The amount in which these components and the extracellular matrix they deposit are present in meat greatly determine its texture. A high amount of fat, for example, leads to a more ‘juicy’ texture. These tissues should thus ideally be cultured separately, in large quantities, while maintaining the ability to easily assemble them into diverse combinations when enough cellular material is produced [224].
Microfluidics and cell compartmentalization may allow for the improvement of several steps in cellular agriculture. For example, cells could be compartmentalized and grown in large fibers, such as the meter-long cell laden microfibers prepared by Onoe et al., which enable a less labor-intensive manner of culturing a large amount of cells. Moreover, by culturing cells as fibers, alignment and maturation of tissues may also be achieved, aiding tissue texturization. These microfibers, in this case core-shell alginate fibers, can be removed at the end of the culture process before assembling the cell fibers into desired shapes [57]. By utilizing several types of cell fibers, texture and diversity can be realized to more closely resemble different types of real meat.
The use of microfluidic encapsulation and compartmentalization has the potential to streamline the process of meat culture. By utilizing edible materials to microfluidically engineer culture and maturation platforms for cells, engineering meat could perhaps become a one-step process in the future. However, microfabrication technologies need to be optimized to facilitate a process that is clean, ultra-high-throughput, and highly cytocompatible [225,226]. By choosing edible, FDA approved, human food-grade materials, micromaterials themselves could also provide benefits such as improved texture [226].
5. Future perspectives
The use of continuous microfluidics for the creation of microtissues has surged in recent years. With the introduction of new materials, microfluidic architectures, and new sources of cellular material, increasingly complex microtissues are being developed, which closely resemble their native counterparts. In laboratory settings, an increased use of iPSCs and associated differentiation protocols is anticipated, leading to microtissues that could be used to gain a better understanding of embryogenesis or morphogenesis.
Using patient-derived cells such as iPSCs to create microtissues also allows for the creation of patient-derived libraries to enable personalized drug testing. However, such biopharmaceutical drug screening libraries require the culture of a large amount of cells as well as large-scale microtissue culture. Compartmentalization could accelerate this process by allowing for culture as well microtissue formation in a one-step protocol, as opposed to, for example, microwell culture. To reach the pharmaceutical industry, it is crucial that microfluidic processes achieve higher-throughputs than currently available. Moreover, new processes should be tunable but also clean and automated to streamline the resultant microtissues for downstream utilization, without the need for additional steps, for example, extensive washing steps.
Besides cell-source, innovations in the materials used to compartmentalize and mature microtissues are also required. The compartmentalization of microtissues in microgels with tunable physical, chemical, and architectural properties in a spatiotemporal manner will allow researchers to study which factors play decisive roles in developmental and pathophysiological processes [227]. The development of highly controllable modular microtissue building blocks through continuous production methods will concurrently enable a next generation of tissue engineered constructs, where the nano, micro, and macroenvironments are uncoupled and can be controlled separately, for example, from material functionalization (nanoscale), to controlling microtissue size through compartmentalization (microscale), to bulk properties through the assembly of microtissues into MAPs (macroscale) [144]. This next generation of tissue engineered constructs also includes an inherent porous structure, which can be leveraged to guide and speed up complex biological processes such as vascularization or innervation inside engineered tissues [228]. To this end, scalable production methods should also aim to produce materials with varying aspect ratios, as anisotropic architectures are found in a variety of native tissues and may contribute to improve the fidelity of engineered tissues for both in vitro and in vivo applications [[229], [230], [231]].
With the development of more complex microtissues also comes a need for new media formulations to satisfy the needs of the microtissues that typically contain multiple cell types. Moreover, to scale microtissue formation, media formulations also need to become cheaper and, for applications such as cellular agriculture for lab-grown meat, ideally chemically-defined and animal-product free, for example, avoiding the typically used component fetal bovine serum (FBS).
Finally, it is important to consider the type and degree of complexity that is required for each specific application. Fundamental microtissue formation studies may require systems with highly versatile temporal and/or spatial control, while translational applications are likely to benefit from more limited but more readily translational biomaterial formulations. Similarly, microfluidic production platforms allow for a variety of adaptations based on the requirements of the application. Applications such as tissue engineering as well as lab-grown meat may benefit from the coupling of microfluidic systems to 3D printing platforms to enable one-step bottom-up engineering of high-resolution hierarchical tissues, whereas applications such as drug screening libraries would benefit most from a production process that allows for on-the fly formulation tuning.
In conclusion, continuous microfluidics and compartmentalization have great potential in the scalable production of microtissue formation. However, cleaner, faster, and more scalable microfluidic processes need to be developed to allow for the translation of microtissues towards clinical and industrial applications. Simultaneously, downstream processes need to be expanded to allow for handling and processing of large amounts of microtissues.
Declaration of interest
The authors declare they have no conflict of interest.
CRediT authorship contribution statement
Maik Schot: Conceptualization, Writing – original draft, Visualization. Nuno Araújo-Gomes: Conceptualization, Writing – original draft, Visualization. Bas van Loo: Conceptualization, Writing – original draft, Visualization. Tom Kamperman: Conceptualization, Writing – original draft, Visualization. Jeroen Leijten: Conceptualization, Supervision, Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
JL acknowledges financial support from Dutch Research Council (Vidi, 17522), European Research Council (Starting Grant, 759425), European Fund for Regional Development (EFRO-00963), and Dutch Arthritis Foundation (17-1-405). Fig. 1, Fig. 4 were created using BioRender software.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Tom Kamperman, Email: t.kamperman@utwente.nl.
Jeroen Leijten, Email: jeroen.leijten@utwente.nl.
References
- 1.Nichol J.W., Khademhosseini A. Modular tissue engineering: engineering biological Tissues from the bottom up. Soft Matter. 2009;5(7):1312–1319. doi: 10.1039/b814285h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Severt S., et al. Mimicking muscle fiber structure and function through electromechanical actuation of electrospun silk fiber bundles. J. Mater. Chem. B. 2017;5(40):8105–8114. doi: 10.1039/c7tb01904a. [DOI] [PubMed] [Google Scholar]
- 3.Yi B., et al. Stiffness of aligned fibers regulates the phenotypic expression of vascular smooth muscle cells. ACS Appl. Mater. Interfaces. 2019;11(7):6867–6880. doi: 10.1021/acsami.9b00293. [DOI] [PubMed] [Google Scholar]
- 4.Sozen B., et al. Self-assembly of embryonic and two extra-embryonic stem cell types into gastrulating embryo-like structures. Nat. Cell Biol. 2018;20(8):979–989. doi: 10.1038/s41556-018-0147-7. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y., et al. Lung cancer organoids analyzed on microwell arrays predict drug responses of patients within a week. Nat. Commun. 2021;12(1):2581. doi: 10.1038/s41467-021-22676-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivron N.C., et al. Blastocyst-like structures generated solely from stem cells. Nature. 2018;557(7703):106–111. doi: 10.1038/s41586-018-0051-0. [DOI] [PubMed] [Google Scholar]
- 7.Moreira Teixeira L.S., et al. High throughput generated micro-aggregates of chondrocytes stimulate cartilage formation in vitro and in vivo. Eur. Cell. Mater. 2012;23:387–399. doi: 10.22203/ecm.v023a30. [DOI] [PubMed] [Google Scholar]
- 8.Branco M.A., et al. Transcriptomic analysis of 3D cardiac differentiation of human induced pluripotent stem cells reveals faster cardiomyocyte maturation compared to 2D culture. Sci. Rep. 2019;9(1):9229. doi: 10.1038/s41598-019-45047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beauchamp P., et al. Development and characterization of a scaffold-free 3D spheroid model of induced pluripotent stem cell-derived human cardiomyocytes. Tissue Eng. C Methods. 2015;21(8):852–861. doi: 10.1089/ten.TEC.2014.0376. [DOI] [PubMed] [Google Scholar]
- 10.Tung Y.-C., et al. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst. 2011;136(3):473–478. doi: 10.1039/c0an00609b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsiao A.Y., et al. Micro-ring structures stabilize microdroplets to enable long term spheroid culture in 384 hanging drop array plates. Biomed. Microdevices. 2012;14(2):313–323. doi: 10.1007/s10544-011-9608-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin B., et al. Surface tension guided hanging-drop: producing controllable 3D spheroid of high-passaged human dermal papilla cells and forming inductive microtissues for hair-follicle regeneration. ACS Appl. Mater. Interfaces. 2016;8(9):5906–5916. doi: 10.1021/acsami.6b00202. [DOI] [PubMed] [Google Scholar]
- 13.Kuo C.-T., et al. Three-dimensional spheroid culture targeting versatile tissue bioassays using a PDMS-based hanging drop array. Sci. Rep. 2017;7(1):4363. doi: 10.1038/s41598-017-04718-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson D.M., Buettner H.M. Schwann cell response to micropatterned laminin surfaces. Tissue Eng. 2001;7(3):247–265. doi: 10.1089/10763270152044125. [DOI] [PubMed] [Google Scholar]
- 15.Park J.A., et al. Freeform micropatterning of living cells into cell culture medium using direct inkjet printing. Sci. Rep. 2017;7(1):14610. doi: 10.1038/s41598-017-14726-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rim N.G., et al. Micropatterned cell sheets as structural building blocks for biomimetic vascular patches. Biomaterials. 2018;181:126–139. doi: 10.1016/j.biomaterials.2018.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao B., et al. In vitro production of human ballooned hepatocytes in a cell sheet-based three-dimensional model. Tissue Eng. 2019;26(1–2):93–101. doi: 10.1089/ten.TEA.2019.0101. [DOI] [PubMed] [Google Scholar]
- 18.Ohashi K., et al. Production of islet cell sheets using cryopreserved islet cells. Transplant. Proc. 2011;43(9):3188–3191. doi: 10.1016/j.transproceed.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 19.Qian Z., et al. Bioactive polydimethylsiloxane surface for optimal human mesenchymal stem cell sheet culture. Bioact. Mater. 2018;3(2):167–173. doi: 10.1016/j.bioactmat.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouyang L., et al. Assembling living building blocks to engineer complex tissues. Adv. Funct. Mater. 2020;30(26):1909009. doi: 10.1002/adfm.201909009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffin D.R., et al. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat. Mater. 2015;14(7):737–744. doi: 10.1038/nmat4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva A.S., et al. Multi-layer pre-vascularized magnetic cell sheets for bone regeneration. Biomaterials. 2020;231:119664. doi: 10.1016/j.biomaterials.2019.119664. [DOI] [PubMed] [Google Scholar]
- 23.Gonçalves A.I., Rodrigues M.T., Gomes M.E. Tissue-engineered magnetic cell sheet patches for advanced strategies in tendon regeneration. Acta Biomater. 2017;63:110–122. doi: 10.1016/j.actbio.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Olofsson K., et al. Acoustic formation of multicellular tumor spheroids enabling on-chip functional and structural imaging. Lab Chip. 2018;18(16):2466–2476. doi: 10.1039/c8lc00537k. [DOI] [PubMed] [Google Scholar]
- 25.Yeh S.I., et al. Development of a simple static microwell array with uniform cell seeding and a chemical concentration gradient. Microfluid. Nanofluidics. 2017;21(5):80. [Google Scholar]
- 26.Jun Y., et al. Microfluidics-generated pancreatic islet microfibers for enhanced immunoprotection. Biomaterials. 2013;34(33):8122–8130. doi: 10.1016/j.biomaterials.2013.07.079. [DOI] [PubMed] [Google Scholar]
- 27.Utech S., et al. Microfluidic generation of monodisperse, structurally homogeneous alginate microgels for cell encapsulation and 3D cell culture. Adv. Healthc. Mater. 2015;4(11):1628–1633. doi: 10.1002/adhm.201500021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edmondson R., et al. Three-dimensional cell culture Systems and their Applications in drug Discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014;12(4):207–218. doi: 10.1089/adt.2014.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammond T.G., et al. Cell spinpods are a simple inexpensive suspension culture device to deliver fluid shear stress to renal proximal tubular cells. Sci. Rep. 2021;11(1):21296. doi: 10.1038/s41598-021-00304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X., et al. A fully defined static suspension culture system for large-scale human embryonic stem cell production. Cell Death Dis. 2018;9(9):892. doi: 10.1038/s41419-018-0863-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carpenedo R.L., Sargent C.Y., McDevitt T.C. Rotary suspension culture enhances the efficiency, yield, and homogeneity of embryoid body differentiation. Stem Cell. 2007;25(9):2224–2234. doi: 10.1634/stemcells.2006-0523. [DOI] [PubMed] [Google Scholar]
- 32.Wang S., et al. Application of hanging drop technique for kidney tissue culture. Kidney Blood Press. Res. 2017;42(2):220–231. doi: 10.1159/000476018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frey O., et al. Reconfigurable microfluidic hanging drop network for multi-tissue interaction and analysis. Nat. Commun. 2014;5(1):4250. doi: 10.1038/ncomms5250. [DOI] [PubMed] [Google Scholar]
- 34.Moshksayan K., et al. Spheroids-on-a-chip: recent advances and design considerations in microfluidic platforms for spheroid formation and culture. Sensor. Actuator. B Chem. 2018;263:151–176. [Google Scholar]
- 35.Wu K.-W., Kuo C.-T., Tu T.-Y. A highly reproducible micro U-well Array plate facilitating high-throughput tumor spheroid Culture and drug assessment. Glob. Challeng. 2021;5(2):2000056. doi: 10.1002/gch2.202000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosaad E.O., et al. The Microwell-mesh: a high-throughput 3D prostate cancer spheroid and drug-testing platform. Sci. Rep. 2018;8(1):253. doi: 10.1038/s41598-017-18050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leijten J., et al. Bioinspired seeding of biomaterials using three dimensional microtissues induces chondrogenic stem cell differentiation and cartilage formation under growth factor free conditions. Sci. Rep. 2016;6(1):36011. doi: 10.1038/srep36011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez-Fernandez T., Tenorio A.J., Leach J.K. Three-dimensional printed stamps for the fabrication of patterned microwells and high-throughput production of homogeneous cell spheroids. 3D Print. Addit. Manuf. 2020;7(3):139–147. doi: 10.1089/3dp.2019.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Svoronos A.A., et al. Micro-mold design controls the 3D morphological evolution of self-assembling multicellular microtissues. Tissue Eng. 2014;20(7–8):1134–1144. doi: 10.1089/ten.tea.2013.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teixeira A.G., et al. Confinement of suspension-cultured Cells in polyethylene glycol/polyethylene oxide-albumin aqueous two-phase systems. Front. Chem. 2019;7:441. doi: 10.3389/fchem.2019.00441. 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cui J., et al. Multicellular Co-Culture in three-dimensional gelatin methacryloyl Hydrogels for liver tissue engineering. Molecules. 2019;24(9):1762. doi: 10.3390/molecules24091762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng Z., et al. 3D Construction of shape-controllable Tissues through self-Bonding of multicellular microcapsules. ACS Appl. Mater. Interfaces. 2019;11(26):22950–22961. doi: 10.1021/acsami.9b05108. [DOI] [PubMed] [Google Scholar]
- 43.Kim S.-Y., et al. Effect of topographical control by a micro-molding process on the activity of human Mesenchymal Stem Cells on alumina ceramics. Biomater. Res. 2015;19(1):1–10. doi: 10.1186/s40824-015-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murasiewicz H., et al. Engineering considerations on the use of liquid/liquid two-phase systems as a cell culture platform. J. Chem. Technol. Biotechnol. 2017;92(7):1690–1698. [Google Scholar]
- 45.Wang J., Jansen J.A., Yang F. Electrospraying: possibilities and challenges of engineering carriers for biomedical applications—a mini review. Front. Chem. 2019;7:258. doi: 10.3389/fchem.2019.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park T.H., Shuler M.L. Integration of cell culture and microfabrication technology. Biotechnol. Prog. 2003;19(2):243–253. doi: 10.1021/bp020143k. [DOI] [PubMed] [Google Scholar]
- 47.Carvalho M.R., et al. Evaluating biomaterial- and microfluidic-based 3D tumor models. Trends Biotechnol. 2015;33(11):667–678. doi: 10.1016/j.tibtech.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Ghadami S., et al. Spiral microchannel with stair-like cross section for size-based particle separation. Microfluid. Nanofluidics. 2017;21(7) [Google Scholar]
- 49.Kamperman T., et al. Engineering 3D parallelized microfluidic droplet generators with equal flow profiles by computational fluid dynamics and stereolithographic printing. Lab Chip. 2020;20(3):490–495. doi: 10.1039/c9lc00980a. [DOI] [PubMed] [Google Scholar]
- 50.Onoe H., Takeuchi S. Cell-laden microfibers for bottom-up tissue engineering. Drug Discov. Today. 2015;20(2):236–246. doi: 10.1016/j.drudis.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 51.Gotti C., et al. Biomimetic hierarchically arranged nanofibrous structures resembling the architecture and the passive mechanical properties of skeletal muscles: a step forward toward artificial muscle. Front. Bioeng. Biotechnol. 2020;8:767. doi: 10.3389/fbioe.2020.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doblado L.R., Martínez-Ramos C., Pradas M.M. Biomaterials for neural tissue engineering. Front. Nanotechnol. 2021;3:21. [Google Scholar]
- 53.Costa-Almeida R., et al. Exploring platelet lysate hydrogel-coated suture threads as biofunctional composite living fibers for cell delivery in tissue repair. Biomed. Mater. 2019;14(3) doi: 10.1088/1748-605X/ab0de6. 034104. [DOI] [PubMed] [Google Scholar]
- 54.Akbari M., et al. Composite living fibers for creating tissue constructs using textile techniques. Adv. Funct. Mater. 2014;24(26):4060–4067. doi: 10.1002/adfm.201303655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grolman J.M., et al. Rapid 3D extrusion of synthetic tumor microenvironments. Adv. Mater. 2015;27(37):5512–5517. doi: 10.1002/adma.201501729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li X., et al. A comparative study of the behavior of neural progenitor cells in extrusion-based in vitro hydrogel models. Biomed. Mater. 2019;14(6) doi: 10.1088/1748-605X/ab3b4b. 065001. [DOI] [PubMed] [Google Scholar]
- 57.Onoe H., et al. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Mater. 2013;12(6):584–590. doi: 10.1038/nmat3606. [DOI] [PubMed] [Google Scholar]
- 58.Moutos F.T., Freed L.E., Guilak F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat. Mater. 2007;6(2):162–167. doi: 10.1038/nmat1822. [DOI] [PubMed] [Google Scholar]
- 59.Calejo I., et al. A textile platform using continuous aligned and textured composite microfibers to engineer tendon-to-bone interface gradient scaffolds. Adv. healthc. mater. 2019;8(15):1900200. doi: 10.1002/adhm.201900200. [DOI] [PubMed] [Google Scholar]
- 60.Guimarães C.F., et al. Engineering polysaccharide-based hydrogel photonic constructs: from multiscale Detection to the Biofabrication of living optical fibers. Adv. Mater. 2021;33(52):2105361. doi: 10.1002/adma.202105361. [DOI] [PubMed] [Google Scholar]
- 61.Kang E., et al. Microfluidic spinning of flat alginate fibers with grooves for cell-aligning scaffolds. Adv. Mater. 2012;24(31):4271–4277. doi: 10.1002/adma.201201232. [DOI] [PubMed] [Google Scholar]
- 62.Kang E., et al. Digitally tunable physicochemical coding of material composition and topography in continuous microfibres. Nat. Mater. 2011;10(11):877–883. doi: 10.1038/nmat3108. [DOI] [PubMed] [Google Scholar]
- 63.Mubyana K., Corr D.T. Cyclic uniaxial tensile strain enhances the mechanical properties of engineered, scaffold-free tendon fibers. Tissue Eng. 2018;24(23–24):1808–1817. doi: 10.1089/ten.TEA.2018.0028. [DOI] [PubMed] [Google Scholar]
- 64.Hwang C.M., et al. Controlled cellular orientation on PLGA microfibers with defined diameters. Biomed. Microdevices. 2009;11(4):739–746. doi: 10.1007/s10544-009-9287-7. [DOI] [PubMed] [Google Scholar]
- 65.Johansson U., et al. Assembly of functionalized silk together with cells to obtain proliferative 3D cultures integrated in a network of ECM-like microfibers. Sci. Rep. 2019;9(1):1–13. doi: 10.1038/s41598-019-42541-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ravikrishnan A., et al. Core–shell Microfibers via bioorthogonal layer-by-layer assembly. ACS Macro Lett. 2020;9(9):1369–1375. doi: 10.1021/acsmacrolett.0c00515. [DOI] [PubMed] [Google Scholar]
- 67.Tian C., Zhang X., Zhao G. Vitrification of stem cell-laden core–shell microfibers with unusually low concentrations of cryoprotective agents. Biomater. sci. 2019;7(3):889–900. doi: 10.1039/c8bm01231h. [DOI] [PubMed] [Google Scholar]
- 68.Akella M., Shabaniverki S., Juárez J.J. Acoustophoretic assembly of millimeter-scale Janus fibers. RSC Adv. 2020;10(1):434–443. doi: 10.1039/c9ra09796a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khang A., et al. Engineering anisotropic biphasic Janus-type polymer nanofiber scaffold networks via centrifugal jet spinning. J. Biomed. Mater. Res. B Appl. Biomater. 2017;105(8):2455–2464. doi: 10.1002/jbm.b.33791. [DOI] [PubMed] [Google Scholar]
- 70.Ma Y., Li Z., Numata K. Synthetic short peptides for rapid fabrication of monolayer cell sheets. ACS Biomater. Sci. Eng. 2016;2(4):697–706. doi: 10.1021/acsbiomaterials.6b00113. [DOI] [PubMed] [Google Scholar]
- 71.Hong Y., et al. Light-induced cell detachment for cell sheet technology. Biomaterials. 2013;34(1):11–18. doi: 10.1016/j.biomaterials.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 72.Guillaume-Gentil O., et al. Polyelectrolyte coatings with a potential for electronic control and cell sheet engineering. Adv. mater. 2008;20(3):560–565. [Google Scholar]
- 73.Imashiro C., et al. Detachment of cell sheets from clinically ubiquitous cell culture vessels by ultrasonic vibration. Sci. Rep. 2020;10(1):1–11. doi: 10.1038/s41598-020-66375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ikeda T., et al. Arginine-mediated dissociation of single cells and cell sheets from a polystyrene culture dish. Biosc. Biotech. Biochem. 2019;83(12):2272–2275. doi: 10.1080/09168451.2019.1659716. [DOI] [PubMed] [Google Scholar]
- 75.Takagi R., et al. Cell sheet technology for regeneration of esophageal mucosa. World J. Gastroenterol.: WJG. 2012;18(37):5145. doi: 10.3748/wjg.v18.i37.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maruya Y., et al. Autologous adipose-derived stem cell sheets enhance the strength of intestinal anastomosis. Regener. ther. 2017;7:24–33. doi: 10.1016/j.reth.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pak S., et al. Endoscopic transplantation of mesenchymal stem cell sheets in experimental colitis in rats. Sci. Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-29617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kobayashi T., et al. Corneal regeneration by transplantation of corneal epithelial cell sheets fabricated with automated cell culture system in rabbit model. Biomaterials. 2013;34(36):9010–9017. doi: 10.1016/j.biomaterials.2013.07.065. [DOI] [PubMed] [Google Scholar]
- 79.Nishida K., et al. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation. 2004;77(3):379–385. doi: 10.1097/01.TP.0000110320.45678.30. [DOI] [PubMed] [Google Scholar]
- 80.Kondoh H., et al. Longer preservation of cardiac performance by sheet-shaped myoblast implantation in dilated cardiomyopathic hamsters. Cardiovasc. Res. 2006;69(2):466–475. doi: 10.1016/j.cardiores.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 81.Hata H., et al. Grafted skeletal myoblast sheets attenuate myocardial remodeling in pacing-induced canine heart failure model. J. Thorac. Cardiovasc. Surg. 2006;132(4):918–924. doi: 10.1016/j.jtcvs.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 82.Itaba N., et al. Hepatic cell sheets engineered from human mesenchymal stem cells with a single small molecule compound IC-2 ameliorate acute liver injury in mice. Regener. ther. 2018;9:45–57. doi: 10.1016/j.reth.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kato Y., et al. Allogeneic transplantation of an adipose-derived stem cell sheet combined with artificial skin accelerates wound healing in a rat wound model of type 2 diabetes and obesity. Diabetes. 2015;64(8):2723–2734. doi: 10.2337/db14-1133. [DOI] [PubMed] [Google Scholar]
- 84.Williams C., et al. Stacking of aligned cell sheets for layer-by-layer control of complex tissue structure. Biomaterials. 2011;32(24):5625–5632. doi: 10.1016/j.biomaterials.2011.04.050. [DOI] [PubMed] [Google Scholar]
- 85.Akiyama Y., et al. Accelerated cell-sheet recovery from a surface successively grafted with polyacrylamide and poly (N-isopropylacrylamide) Acta Biomater. 2014;10(8):3398–3408. doi: 10.1016/j.actbio.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 86.Zhang W., et al. Magnetically controlled growth-factor-immobilized multilayer cell sheets for complex tissue regeneration. Adv. Mater. 2017;29(43):1703795. doi: 10.1002/adma.201703795. [DOI] [PubMed] [Google Scholar]
- 87.Enomoto J., et al. Engineering thick cell sheets by electrochemical desorption of oligopeptides on membrane substrates. Regener. Ther. 2016;3:24–31. doi: 10.1016/j.reth.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zurina I.M., et al. Tissue engineering using a combined cell sheet technology and scaffolding approach. Acta Biomater. 2020;113:63–83. doi: 10.1016/j.actbio.2020.06.016. [DOI] [PubMed] [Google Scholar]
- 89.Whitesides G.M. The origins and the future of microfluidics. Nature. 2006;442(7101):368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 90.Debuisson D., Senez V., Arscott S. SU-8 micropatterning for microfluidic droplet and microparticle focusing. J. Micromech. Microeng. 2011;21(6) 065011. [Google Scholar]
- 91.Kobaku S.P., et al. Patterned superomniphobic-superomniphilic surfaces: templates for site-selective self-assembly. Angew. Chem. Int. 2012;51(40):10109–10113. doi: 10.1002/anie.201202823. Engl. [DOI] [PubMed] [Google Scholar]
- 92.Jackman R.J., et al. Fabricating large arrays of microwells with arbitrary dimensions and filling them using discontinuous dewetting. Anal. Chem. 1998;70(11):2280–2287. doi: 10.1021/ac971295a. [DOI] [PubMed] [Google Scholar]
- 93.Feng W., et al. Single-step Fabrication of high-density microdroplet Arrays of low-surface-tension liquids. Adv. Mater. 2016;28(16):3202–3208. doi: 10.1002/adma.201505972. [DOI] [PubMed] [Google Scholar]
- 94.Gudapati H., Dey M., Ozbolat I. A comprehensive review on droplet-based bioprinting: past, present and future. Biomaterials. 2016;102:20–42. doi: 10.1016/j.biomaterials.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 95.Dendukuri D., Doyle P.S. The synthesis and assembly of polymeric microparticles using microfluidics. Adv. Mater. 2009;21(41):4071–4086. [Google Scholar]
- 96.Femmer T., et al. High-throughput Generation of Emulsions and Microgels in parallelized microfluidic drop-makers Prepared by rapid prototyping. ACS Appl. Mater. Interfaces. 2015;7(23):12635–12638. doi: 10.1021/acsami.5b03969. [DOI] [PubMed] [Google Scholar]
- 97.Tumarkin E., et al. High-throughput combinatorial cell co-culture using microfluidics. Integr. Biol. 2011;3(6):653–662. doi: 10.1039/c1ib00002k. [DOI] [PubMed] [Google Scholar]
- 98.Kemna E.W., et al. High-yield cell ordering and deterministic cell-in-droplet encapsulation using Dean flow in a curved microchannel. Lab Chip. 2012;12(16):2881–2887. doi: 10.1039/c2lc00013j. [DOI] [PubMed] [Google Scholar]
- 99.Yobas L., et al. High-performance flow-focusing geometry for spontaneous generation of monodispersed droplets. Lab Chip. 2006;6(8):1073–1079. doi: 10.1039/b602240e. [DOI] [PubMed] [Google Scholar]
- 100.Liu K., et al. Shape-controlled production of biodegradable calcium alginate gel microparticles using a novel microfluidic device. Langmuir. 2006;22(22):9453–9457. doi: 10.1021/la061729+. [DOI] [PubMed] [Google Scholar]
- 101.Zhang H., et al. Microfluidic production of biopolymer microcapsules with controlled morphology. J. Am. Chem. Soc. 2006;128(37):12205–12210. doi: 10.1021/ja0635682. [DOI] [PubMed] [Google Scholar]
- 102.Visser C.W., et al. In-air microfluidics enables rapid fabrication of emulsions, suspensions, and 3D modular (bio)materials. Sci. Adv. 2018;4(1) doi: 10.1126/sciadv.aao1175. eaao1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kamperman T., et al. Ultrahigh-throughput Production of Monodisperse and multifunctional Janus microparticles Using in-air microfluidics. ACS Appl. Mater. Interfaces. 2018;10(28):23433–23438. doi: 10.1021/acsami.8b05227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin Y.S., et al. Microfluidic synthesis of tail-shaped alginate microparticles using slow sedimentation. Electrophoresis. 2013;34(3):425–431. doi: 10.1002/elps.201200282. [DOI] [PubMed] [Google Scholar]
- 105.Prusse U., et al. Production of spherical beads by JetCutting. Chem. Eng. Technol. 2000;23(12):1105–1110. [Google Scholar]
- 106.Prusse U., et al. Comparison of different technologies for alginate beads production. Chem. Pap. 2008;62(4):364–374. [Google Scholar]
- 107.Xu T., et al. High-throughput production of single-cell microparticles using an inkjet printing technology. J. Manuf. Sci. Eng. Transact. Asme. 2008;130(2) [Google Scholar]
- 108.Nakamura M., et al. Biocompatible inkjet printing technique for designed seeding of individual living cells. Tissue Eng. 2005;11(11–12):1658–1666. doi: 10.1089/ten.2005.11.1658. [DOI] [PubMed] [Google Scholar]
- 109.Kwok K.K., Groves M.J., Burgess D.J. Production of 5-15-mu-M diameter alginate-polylysine Microcapsules by an air-atomization technique. Pharmaceut. Res. 1991;8(3):341–344. doi: 10.1023/a:1015841531372. [DOI] [PubMed] [Google Scholar]
- 110.Cui J.H., et al. Preparation and physical characterization of alginate microparticles using air atomization method. Drug Dev. Ind. Pharm. 2001;27(4):309–319. doi: 10.1081/ddc-100103730. [DOI] [PubMed] [Google Scholar]
- 111.Strasdat B., Bunjes H. Incorporation of lipid nanoparticles into calcium alginate beads and characterization of the encapsulated particles by differential scanning calorimetry. Food Hydrocolloids. 2013;30(2):567–575. [Google Scholar]
- 112.Hendriks J., et al. Optimizing cell viability in droplet-based cell deposition. Sci. Rep. 2015:5. doi: 10.1038/srep11304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ganan-Calvo A.M., Davila J., Barrero A. Current and droplet size in the electrospraying of liquids. Scaling laws. J. Aerosol. Sci. 1997;28(2):249–275. [Google Scholar]
- 114.Nedović V.A., et al. Optimization of the electrostatic droplet generation process for controlled microbead production: single nozzle system. Chem. Ind. Chem. Eng. Q. 2006;12(1):5. [Google Scholar]
- 115.Menasché P. Cell therapy trials for heart regeneration - lessons learned and future directions. Nat. Rev. Cardiol. 2018;15(11):659–671. doi: 10.1038/s41569-018-0013-0. [DOI] [PubMed] [Google Scholar]
- 116.de Souza N. Organoid variability examined. Nat. Methods. 2017;14(7):655. 655. [Google Scholar]
- 117.Sutherland R.M., et al. A multi-component radiation survival curve Using an in vitro tumour model. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1970;18(5):491–495. doi: 10.1080/09553007014551401. [DOI] [PubMed] [Google Scholar]
- 118.Kelm J.M., et al. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol. Bioeng. 2003;83(2):173–180. doi: 10.1002/bit.10655. [DOI] [PubMed] [Google Scholar]
- 119.Yuhas J.M., et al. A simplified Method for Production and Growth of multicellular tumor spheroids. Cancer Res. 1977;37(10):3639–3643. [PubMed] [Google Scholar]
- 120.Li X., Ootani A., Kuo C. In: Gastrointestinal Physiology and Diseases: Methods and Protocols. Ivanov A.I., editor. Springer New York; New York, NY: 2016. An air–liquid interface culture system for 3D organoid culture of diverse primary gastrointestinal tissues; pp. 33–40. [DOI] [PubMed] [Google Scholar]
- 121.Warmflash A., et al. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat. Methods. 2014;11(8):847–854. doi: 10.1038/nmeth.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nelson C.M., et al. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314(5797):298–300. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yan Y., et al. Derivation of cortical spheroids from human induced pluripotent stem cells in a suspension bioreactor. Tissue Eng. 2018;24(5–6):418–431. doi: 10.1089/ten.TEA.2016.0400. [DOI] [PubMed] [Google Scholar]
- 124.Allen L.M., et al. Serum-free Culture of human mesenchymal stem cell Aggregates in suspension Bioreactors for tissue engineering applications. Stem Cell. Int. 2019:4607461. doi: 10.1155/2019/4607461. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sumi S., et al. A multiple-funnels cell culture insert for the scale-up production of uniform cell spheroids. Regener. Ther. 2017;7:52–60. doi: 10.1016/j.reth.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.van Ballegooie C., et al. Using a microfluidics system to reproducibly synthesize protein nanoparticles: factors contributing to size, homogeneity, and stability. Processes. 2019;7(5):290. [Google Scholar]
- 127.Tran T.M., Cater S., Abate A.R. Coaxial flow focusing in poly(dimethylsiloxane) microfluidic devices. Biomicrofluidics. 2014;8(1):16502. doi: 10.1063/1.4863576. 16502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cramer C., Fischer P., Windhab E.J. Drop formation in a co-flowing ambient fluid. Chem. Eng. Sci. 2004;59(15):3045–3058. [Google Scholar]
- 129.Garstecki P., et al. Formation of droplets and bubbles in a microfluidic T-junction—scaling and mechanism of break-up. Lab Chip. 2006;6(3):437–446. doi: 10.1039/b510841a. [DOI] [PubMed] [Google Scholar]
- 130.Nisisako T., Torii T., Higuchi T. Droplet formation in a microchannel network. Lab Chip. 2002;2(1):24–26. doi: 10.1039/b108740c. [DOI] [PubMed] [Google Scholar]
- 131.Anna S.L., Bontoux N., Stone H.A. Formation of dispersions using “flow focusing” in microchannels. Appl. Phys. Lett. 2003;82(3):364–366. [Google Scholar]
- 132.Theberge A.B., et al. Microdroplets in microfluidics: an evolving platform for discoveries in chemistry and biology. Angew. Chem. Int. Ed. 2010;49(34):5846–5868. doi: 10.1002/anie.200906653. [DOI] [PubMed] [Google Scholar]
- 133.Doméjean H., et al. Controlled production of sub-millimeter liquid core hydrogel capsules for parallelized 3D cell culture. Lab Chip. 2017;17(1):110–119. doi: 10.1039/c6lc00848h. [DOI] [PubMed] [Google Scholar]
- 134.Braghirolli D., et al. Bio-electrospraying of human mesenchymal stem cells: an alternative for tissue engineering. Biomicrofluidics. 2013;7(4) doi: 10.1063/1.4819747. 044130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Song Y., et al. All-aqueous electrosprayed emulsion for templated fabrication of cytocompatible microcapsules. ACS Appl. Mater. Interfaces. 2015;7(25):13925–13933. doi: 10.1021/acsami.5b02708. [DOI] [PubMed] [Google Scholar]
- 136.Zhao S., et al. Coaxial electrospray of liquid core–hydrogel shell microcapsules for encapsulation and miniaturized 3D culture of pluripotent stem cells. Integr. Biol. 2014;6(9):874–884. doi: 10.1039/c4ib00100a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Girgin M.U., et al. Gastruloids generated without exogenous Wnt activation develop anterior neural tissues. Stem Cell Rep. 2021;16(5):1143–1155. doi: 10.1016/j.stemcr.2021.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.van Loo B., et al. Enzymatic outside-in cross-linking enables single-step microcapsule production for high-throughput three-dimensional cell microaggregate formation. Mater. Today Bio. 2020;6:100047. doi: 10.1016/j.mtbio.2020.100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Baret J.-C. Surfactants in droplet-based microfluidics. Lab Chip. 2012;12(3):422–433. doi: 10.1039/c1lc20582j. [DOI] [PubMed] [Google Scholar]
- 140.Lim F., Sun A. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210(4472):908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 141.Tan W.-H., Takeuchi S. Monodisperse alginate hydrogel microbeads for cell encapsulation. Adv. Mater. 2007;19(18):2696–2701. [Google Scholar]
- 142.Kamperman T., et al. On-the-fly exchangeable microfluidic nozzles for facile production of various monodisperse micromaterials. Lab Chip. 2019;19(11):1977–1984. doi: 10.1039/c9lc00054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang S., et al. An in-situ photocrosslinking microfluidic technique to generate non-spherical, cytocompatible, degradable, monodisperse alginate microgels for chondrocyte encapsulation. Biomicrofluidics. 2018;12(1) doi: 10.1063/1.5017644. 014106–014106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kamperman T., et al. Single cell microgel based modular bioinks for uncoupled cellular micro- and macroenvironments. Adv. Healthc. Mater. 2017;6(3):1600913. doi: 10.1002/adhm.201600913. [DOI] [PubMed] [Google Scholar]
- 145.Flueckiger J., Cheung K.C. Microfluidic system for controlled gelation of a thermally reversible hydrogel. IEEE Trans. Biomed. Circuits. Syst. 2009;3(4):195–201. doi: 10.1109/TBCAS.2009.2021657. [DOI] [PubMed] [Google Scholar]
- 146.Henke S., et al. Enzymatic crosslinking of polymer conjugates is superior over ionic or UV crosslinking for the on-chip production of cell-laden microgels. Macromol. Biosci. 2016;16(10):1524–1532. doi: 10.1002/mabi.201600174. [DOI] [PubMed] [Google Scholar]
- 147.Kamperman T., et al. Centering single cells in microgels via delayed crosslinking supports long-term 3D culture by preventing cell escape. Small. 2017;13(22):1603711. doi: 10.1002/smll.201603711. [DOI] [PubMed] [Google Scholar]
- 148.Ma T., et al. High-throughput generation of hyaluronic acid microgels via microfluidics-assisted enzymatic crosslinking and/or Diels–Alder click chemistry for cell encapsulation and delivery. Appl. Mater. Today. 2017;9:49–59. [Google Scholar]
- 149.Kamperman T., et al. Tethering cells via enzymatic oxidative crosslinking enables mechanotransduction in non-cell-adhesive materials. Adv. Mater. 2021;33:2102660. doi: 10.1002/adma.202102660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mueller M., et al. The importance of cell–cell interaction dynamics in bottom-up tissue engineering: concepts of colloidal self-assembly in the fabrication of multicellular architectures. Nano Lett. 2020;20(4):2257–2263. doi: 10.1021/acs.nanolett.9b04160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chu L.-Y., et al. Controllable monodisperse multiple emulsions. Angew. Chem. Int. Ed. 2007;46(47):8970–8974. doi: 10.1002/anie.200701358. [DOI] [PubMed] [Google Scholar]
- 152.Agarwal P., et al. One-step microfluidic generation of pre-hatching embryo-like core–shell microcapsules for miniaturized 3D culture of pluripotent stem cells. Lab Chip. 2013;13(23):4525–4533. doi: 10.1039/c3lc50678a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Seiffert S., Weitz D.A. Microfluidic fabrication of smart microgels from macromolecular precursors. Polymer. 2010;51(25):5883–5889. [Google Scholar]
- 154.Xu S., et al. Generation of monodisperse particles by using microfluidics: control over size, shape, and composition. Angew. Chem. Int. Ed. 2005;44(5):724–728. doi: 10.1002/anie.200462226. [DOI] [PubMed] [Google Scholar]
- 155.Shum H.C., et al. Droplet microfluidics for fabrication of non-spherical particles. Macromol. Rapid Commun. 2010;31(2):108–118. doi: 10.1002/marc.200900590. [DOI] [PubMed] [Google Scholar]
- 156.Samal P., et al. A new microengineered Platform for 4D Tracking of single Cells in a stem-cell-based in vitro morphogenesis model. Adv. Mater. 2020;32(24):1907966. doi: 10.1002/adma.201907966. [DOI] [PubMed] [Google Scholar]
- 157.Utada A.S., et al. Monodisperse double emulsions generated from a microcapillary device. Science. 2005;308(5721):537–541. doi: 10.1126/science.1109164. [DOI] [PubMed] [Google Scholar]
- 158.Lee D., Weitz D.A. Double emulsion-templated nanoparticle Colloidosomes with selective permeability. Adv. Mater. 2008;20(18):3498–3503. [Google Scholar]
- 159.Martinez C.J., et al. A microfluidic Approach to encapsulate living Cells in uniform alginate hydrogel microparticles. Macromol. Biosci. 2012;12(7):946–951. doi: 10.1002/mabi.201100351. [DOI] [PubMed] [Google Scholar]
- 160.Chang F.-C., Su Y.-C. Controlled double emulsification utilizing 3D PDMS microchannels. J. Micromech. Microeng. 2008;18(6) 065018. [Google Scholar]
- 161.Seo M., et al. Microfluidic consecutive flow-focusing droplet generators. Soft Matter. 2007;3(8):986–992. doi: 10.1039/b700687j. [DOI] [PubMed] [Google Scholar]
- 162.Nisisako T., Okushima S., Torii T. Controlled formulation of monodisperse double emulsions in a multiple-phase microfluidic system. Soft Matter. 2005;1(1):23–27. doi: 10.1039/b501972a. [DOI] [PubMed] [Google Scholar]
- 163.Okushima S., et al. Controlled production of monodisperse double emulsions by two-step droplet breakup in microfluidic devices. Langmuir. 2004;20(23):9905–9908. doi: 10.1021/la0480336. [DOI] [PubMed] [Google Scholar]
- 164.Utada A.S., et al. Dripping to jetting transitions in coflowing liquid streams. Phys. Rev. Lett. 2007;99(9) doi: 10.1103/PhysRevLett.99.094502. 094502. [DOI] [PubMed] [Google Scholar]
- 165.Yadavali S., et al. Silicon and glass very large scale microfluidic droplet integration for terascale generation of polymer microparticles. Nat. Commun. 2018;9(1):1222. doi: 10.1038/s41467-018-03515-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nisisako T., Torii T. Microfluidic large-scale integration on a chip for mass production of monodisperse droplets and particles. Lab Chip. 2008;8(2):287–293. doi: 10.1039/b713141k. [DOI] [PubMed] [Google Scholar]
- 167.Alessandri K., et al. A 3D printed microfluidic device for production of functionalized hydrogel microcapsules for culture and differentiation of human Neuronal Stem Cells (hNSC) Lab Chip. 2016;16(9):1593–1604. doi: 10.1039/c6lc00133e. [DOI] [PubMed] [Google Scholar]
- 168.Alessandri K., et al. Cellular capsules as a tool for multicellular spheroid production and for investigating the mechanics of tumor progression in vitro. Proc. Natl. Acad. Sci. Unit. States Am. 2013;110(37):14843–14848. doi: 10.1073/pnas.1309482110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yin Z., et al. Droplet generation in a flow-focusing microfluidic device with external mechanical vibration. Micromachines. 2020;11(8):743. doi: 10.3390/mi11080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ziemecka I., et al. Monodisperse hydrogel microspheres by forced droplet formation in aqueous two-phase systems. Lab Chip. 2011;11(4):620–624. doi: 10.1039/c0lc00375a. [DOI] [PubMed] [Google Scholar]
- 171.Bransky A., et al. A microfluidic droplet generator based on a piezoelectric actuator. Lab Chip. 2009;9(4):516–520. doi: 10.1039/b814810d. [DOI] [PubMed] [Google Scholar]
- 172.Doméjean H., et al. Controlled production of sub-millimeter liquid core hydrogel capsules for parallelized 3D cell culture. Lab Chip. 2017;17(1):110–119. doi: 10.1039/c6lc00848h. [DOI] [PubMed] [Google Scholar]
- 173.Young C.J., Poole-Warren L.A., Martens P.J. Combining submerged electrospray and UV photopolymerization for production of synthetic hydrogel microspheres for cell encapsulation. Biotechnol. Bioeng. 2012;109(6):1561–1570. doi: 10.1002/bit.24430. [DOI] [PubMed] [Google Scholar]
- 174.Marín Á.G., Loscertales I.G., Barrero A. Surface tension effects on submerged electrosprays. Biomicrofluidics. 2012;6(4) doi: 10.1063/1.4762854. 044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Compaan A.M., Christensen K., Huang Y. Inkjet bioprinting of 3D silk fibroin cellular constructs using sacrificial alginate. ACS Biomater. Sci. Eng. 2017;3(8):1519–1526. doi: 10.1021/acsbiomaterials.6b00432. [DOI] [PubMed] [Google Scholar]
- 176.Wijshoff H. The dynamics of the piezo inkjet printhead operation☆. Phys. Rep. 2010;491(4–5):77–177. [Google Scholar]
- 177.Jaworek A. Electrostatic micro- and nanoencapsulation and electroemulsification: a brief review. J. Microencapsul. 2008;25(7):443–468. doi: 10.1080/02652040802049109. [DOI] [PubMed] [Google Scholar]
- 178.Kamperman T., et al. Single-cell microgels: technology, challenges, and applications. Trends Biotechnol. 2018;36(8):850–865. doi: 10.1016/j.tibtech.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 179.Ferreira S.A., et al. Bi-directional cell-pericellular matrix interactions direct stem cell fate. Nat. Commun. 2018;9(1):4049. doi: 10.1038/s41467-018-06183-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bloom A.B., Zaman M.H. Influence of the microenvironment on cell fate determination and migration. Physiol. Genom. 2014;46(9):309–314. doi: 10.1152/physiolgenomics.00170.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ungefroren H., et al. Interaction of tumor cells with the microenvironment. Cell Commun. Signal. 2011;9(1):18. doi: 10.1186/1478-811X-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jensen C., Teng Y. Is it time to start transitioning from 2D to 3D cell culture? Front. Mol. Biosci. 2020;7:33. doi: 10.3389/fmolb.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Leijten J., et al. Spatially and temporally controlled hydrogels for tissue engineering. Mater. Sci. Eng. R Rep. 2017;119:1–35. doi: 10.1016/j.mser.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ballester-Beltrán J., et al. Sensing the difference: the influence of anisotropic cues on cell behavior. Front. Mater. 2015;2:39. [Google Scholar]
- 185.Kaushik G., Leijten J., Khademhosseini A. Concise review: organ engineering: design, technology, and integration. Stem Cell. 2017;35(1):51–60. doi: 10.1002/stem.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Leijten J., et al. Advancing tissue engineering: a tale of nano-, micro-, and macroscale integration. Small. 2016;12(16):2130–2145. doi: 10.1002/smll.201501798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cheng G., Markenscoff P., Zygourakis K. A 3D hybrid Model for tissue growth: the Interplay between cell Population and mass transport dynamics. Biophys. J. 2009;97(2):401–414. doi: 10.1016/j.bpj.2009.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Place T.L., Domann F.E., Case A.J. Limitations of oxygen delivery to cells in culture: an underappreciated problem in basic and translational research. Free Radic. Biol. Med. 2017;113:311–322. doi: 10.1016/j.freeradbiomed.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Abdal Dayem A., et al. The impact of adhesion molecules on the in vitro culture and differentiation of stem cells. Biotechnol. J. 2018;13(2):1700575. doi: 10.1002/biot.201700575. [DOI] [PubMed] [Google Scholar]
- 190.Gattazzo F., Urciuolo A., Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta Gen. Subj. 2014;1840(8):2506–2519. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kolb L., et al. High-throughput stem cell-based phenotypic screening through microniches. Biomater. Sci. 2019;7(8):3471–3479. doi: 10.1039/c8bm01180j. [DOI] [PubMed] [Google Scholar]
- 192.Kelm J.M., et al. Self-assembly of sensory neurons into ganglia-like microtissues. J. Biotechnol. 2006;121(1):86–101. doi: 10.1016/j.jbiotec.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 193.Lan F., et al. Single-cell genome sequencing at ultra-high-throughput with microfluidic droplet barcoding. Nat. Biotechnol. 2017;35(7):640–646. doi: 10.1038/nbt.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jayadev R., Sherwood D.R. Morphogenesis: shaping tissues through extracellular force gradients. Curr. Biol. 2017;27(17):R850–R852. doi: 10.1016/j.cub.2017.07.040. [DOI] [PubMed] [Google Scholar]
- 195.Leferink A., et al. Engineered micro-objects as scaffolding elements in cellular building blocks for bottom-up tissue engineering approaches. Adv. mater. 2014;26(16):2592–2599. doi: 10.1002/adma.201304539. [DOI] [PubMed] [Google Scholar]
- 196.Vrij E., et al. Directed assembly and development of material-free tissues with complex architectures. Adv. mater. 2016;28(21):4032–4039. doi: 10.1002/adma.201505723. [DOI] [PubMed] [Google Scholar]
- 197.Mavris S.M., Hansen L.M. Optimization of oxygen delivery within hydrogels. J. Biomech. Eng. 2021;143(10) doi: 10.1115/1.4051119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Feng Q., et al. Injection and self-assembly of bioinspired stem cell-laden gelatin/hyaluronic acid hybrid microgels promote cartilage repair in vivo. Adv. Funct. Mater. 2019;29(50):1906690. [Google Scholar]
- 199.Balyura M., et al. Transplantation of bovine adrenocortical cells encapsulated in alginate. Proc. Natl. Acad. Sci. Unit. States Am. 2015;112(8):2527. doi: 10.1073/pnas.1500242112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Weaver J.D., et al. Synthetic poly(ethylene glycol)-based microfluidic islet encapsulation reduces graft volume for delivery to highly vascularized and retrievable transplant site. Am. J. Transplant. 2019;19(5):1315–1327. doi: 10.1111/ajt.15168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Koh J., et al. Enhanced in vivo Delivery of stem Cells using microporous annealed particle scaffolds. Small. 2019;15(39) doi: 10.1002/smll.201903147. e1903147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li F., et al. Cartilage tissue formation through assembly of microgels containing mesenchymal stem cells. Acta Biomater. 2018;77:48–62. doi: 10.1016/j.actbio.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 203.Fang J., et al. Injectable drug-releasing microporous annealed particle Scaffolds for treating myocardial infarction. Adv. Funct. Mater. 2020;30(43):2004307. doi: 10.1002/adfm.202004307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yang G., et al. Fabrication of centimeter-sized 3D constructs with patterned endothelial cells through assembly of cell-laden microbeads as a potential bone graft. Acta Biomater. 2021;121:204–213. doi: 10.1016/j.actbio.2020.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bhattacharjee T., et al. Liquid-like solids support cells in 3D. ACS Biomater. Sci. Eng. 2016;2(10):1787–1795. doi: 10.1021/acsbiomaterials.6b00218. [DOI] [PubMed] [Google Scholar]
- 206.Skylar-Scott M.A., et al. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv. 2019;5(9) doi: 10.1126/sciadv.aaw2459. eaaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hinton T.J., et al. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015;1(9) doi: 10.1126/sciadv.1500758. e1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Daly A.C., Davidson M.D., Burdick J.A. 3D bioprinting of high cell-density heterogeneous tissue models through spheroid fusion within self-healing hydrogels. Nat. Commun. 2021;12(1):753. doi: 10.1038/s41467-021-21029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Highley C.B., et al. Jammed microgel inks for 3D printing applications. Adv. Sci. 2019;6(1):1801076. doi: 10.1002/advs.201801076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.de Rutte J.M., Koh J., Di Carlo D. Scalable high-throughput Production of modular Microgels for in situ Assembly of microporous tissue scaffolds. Adv. Funct. Mater. 2019;29(25):1900071. [Google Scholar]
- 211.Zhao X., et al. Review on the vascularization of organoids and organoids-on-a-chip. Front. Bioeng. Biotechnol. 2021:9. doi: 10.3389/fbioe.2021.637048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rahimnejad M., et al. Prevascularized micro-/nano-sized spheroid/bead Aggregates for vascular tissue engineering. Nano-Micro Lett. 2021;13(1):182. doi: 10.1007/s40820-021-00697-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Willemen N.G.A., et al. Oxygen-releasing biomaterials: current Challenges and future applications. Trends Biotechnol. 2021;39(11):1144–1159. doi: 10.1016/j.tibtech.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Astashkina A., Mann B., Grainger D.W. A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol. Ther. 2012;134(1):82–106. doi: 10.1016/j.pharmthera.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 215.Hasturk O., Kaplan D.L. Cell armor for protection against environmental stress: advances, challenges and applications in micro- and nanoencapsulation of mammalian cells. Acta Biomater. 2019;95:3–31. doi: 10.1016/j.actbio.2018.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Battista E., Causa F., Netti P.A. Bioengineering microgels and hydrogel microparticles for sensing biomolecular targets. Gels. 2017;3(2) doi: 10.3390/gels3020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gielen F. In: Single-Cell Microencapsulation for Evolution and Discovery of Biocatalysts, in Handbook of Single Cell Technologies. Santra T.S., Tseng F.-G., editors. Springer Singapore; Singapore: 2020. pp. 1–22. [Google Scholar]
- 218.Hendel S.J., Shoulders M.D. Directed evolution in mammalian cells. Nat. Methods. 2021;18(4):346–357. doi: 10.1038/s41592-021-01090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tripathi N.K., Shrivastava A. Recent developments in bioprocessing of recombinant proteins: expression hosts and process development. Front. Bioeng. Biotechnol. 2019;7(420) doi: 10.3389/fbioe.2019.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Heida T., et al. Cell-free protein Synthesis in bifunctional hyaluronan microgels: a Strategy for in situ Immobilization and Purification of his-tagged proteins. Chem. Syst. Chem. 2020;2(3) e1900058. [Google Scholar]
- 221.Weinguny M., et al. Directed evolution approach to enhance efficiency and speed of outgrowth during single cell subcloning of Chinese Hamster Ovary cells. Comput. Struct. Biotechnol. J. 2020;18:1320–1329. doi: 10.1016/j.csbj.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Post M.J. Cultured beef: medical technology to produce food. J. Sci. Food Agric. 2014;94(6):1039–1041. doi: 10.1002/jsfa.6474. [DOI] [PubMed] [Google Scholar]
- 223.Furuhashi M., et al. Formation of contractile 3D bovine muscle tissue for construction of millimetre-thick cultured steak. npj Sci. Food. 2021;5(1):6. doi: 10.1038/s41538-021-00090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Post M., van der Weele C. In: fifth ed. Lanza R., et al., editors. Academic Press; 2020. Chapter 72-Principles of Tissue Engineering for Food, in Principles of Tissue Engineering; pp. 1355–1368. [Google Scholar]
- 225.Post M.J. Cultured meat from stem cells: challenges and prospects. Meat Sci. 2012;92(3):297–301. doi: 10.1016/j.meatsci.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 226.Bodiou V., Moutsatsou P., Post M.J. Microcarriers for upscaling cultured meat production. Front. Nutr. 2020;7:10. doi: 10.3389/fnut.2020.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kamperman T., et al. Spatiotemporal material functionalization via competitive supramolecular complexation of avidin and biotin analogs. Nat. Commun. 2019;10(1):4347. doi: 10.1038/s41467-019-12390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Nih L.R., et al. Injection of microporous annealing particle (MAP) hydrogels in the stroke cavity reduces gliosis and inflammation and promotes NPC migration to the lesion. Adv. Mater. 2017;29:1606471. doi: 10.1002/adma.201606471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kessel B., et al. 3D Bioprinting of macroporous materials Based on entangled hydrogel microstrands. Adv. Sci. 2020;7(18):2001419. doi: 10.1002/advs.202001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Moysidou C.-M., Barberio C., Owens R.M. Advances in engineering human tissue models. Front. Bioeng. Biotechnol. 2021:8. doi: 10.3389/fbioe.2020.620962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lavrador P., Gaspar V.M., Mano J.F. Engineering mammalian living materials towards clinically relevant therapeutics. EBioMedicine. 2021;74:103717. doi: 10.1016/j.ebiom.2021.103717. [DOI] [PMC free article] [PubMed] [Google Scholar]