Abstract
Nanotechnology involves developing, characterising, and applying structures ranging in size from 1 to 100 nm. As a key advanced technology, it has contributed to a substantial impact across engineering, medicine, agriculture and food. With regards to their application in food, nanomaterials posses the ability to lead the quantitative and qualitative development of high-quality, healthier, and safer foods by outperforming traditional food processing technologies for increasing shelf life and preventing contaminations. Although rapid progress has been made in nanotechnology in food products, the toxicity of nanoparticles and nanomaterials is not very well known. As a result, nanomaterials are potentially toxic, therefore, considering the constantly increasing employment in food science, they need to be further characterised, and their use must be better regulated. We may face a crisis of nanotoxicity if the molecular mechanisms by which nanoparticles and nanomaterials interact with food and within living organisms is not fully understood. Food safety can be guaranteed only if we are thoroughly aware of nanomaterial properties and potential toxicity. Therefore, it is urgently necessary to have in the food sector a regulatory system capable of managing nanofood risks and nanotechnology, considering the health effects of food processing techniques based on nanotechnology. This present review discusses the impact and role nanotechnology play in food science. The specific application of Nanomaterials in food science, their advantages and disadvantages, the potential risk for human health and the analysis to detect nanocomponents are also highlighted.
Keywords: Nanotechnology, Food production, Safety, Packaging, Toxicity, Health
Graphical abstract
Highlights
-
•
Nanotechnology can be used in food and feed processing at any level.
-
•
Nanocomposite materials have been used in active packaging to prevent the passage of oxygen, carbon dioxide, and moisture into the food.
-
•
Nanomaterials must be used with caution because they have the potential to cause toxic effects.
-
•
Nanomaterials can travel deeper into the nucleus of cells and damaging the DNA.
-
•
Analytical methods are required to reliably detect and characterize nanoparticles.
1. Introduction
In nanotechnology, structures with sizes varying from 1 to 100 nm are created, characterised and applied. Materials comprising aggregates, fragments, or filaments which are smaller than 100 nm are now classified as nanomaterials. The nanoscale alteration and fabrication of materials results in small particles with high surface area to volume ratio. Optical, mechanical, electrical and practical features of matter have improved and are the reason for the effective present and the future applications of this interdisciplinary modern technology (Naseer et al., 2018). Fundamental factors controlling the special properties of nanomaterials are the size of the building blocks, their distribution, the chemical composition of component phases, the number of grain boundaries or interfaces, and their interactions. These manufactured or naturally occurring materials in the twenty-first century are sometimes be referred to as “magic bullets” because their potential to be targeted to transmit a particular message and thus have much promise in a variety of applications across all major scientific fields such as physics, chemistry, medicine, engineering, as well as food science, including food manufacturing and storage (Naseer et al., 2018).
With the increasing world population, environmental risks, global climate change, energy shortages, and arable land shrinking, new technologies are crucial to increasing and enhancing food production and quality. Nanotechnology can be used in food and feed processing at any level, including manufacturing, packaging (Nile et al., 2020; Primozic et al., 2021), storage, value addition (Bajpai et al., 2018), and transportation (Shafique and Luo, 2019). Nanotechnology has several positive impacts in the food industry, including reduced prices (Tran et al., 2017), reduced pollution risks (Nile et al., 2020), disease prevention to mitigate losses (Naseer et al., 2018), and improved agricultural management practices (Shang et al., 2019).
Food technology encompasses every of the unit operations which take place from the farm to the fork. The advantages of this advanced technology in the farm include the utilisation of lightweight smart equipment and the development of nanoprocessor chips. Good agricultural output is dependent on the proper use of fertilizers, insecticides, and fungicides, as well as their excessive usage, which endangers human health and the environment. The controlled delivery of nanofertilizers and encapsulated insecticides and herbicides provides the benefits of controlled release while preventing extreme amounts of these chemicals from being discharged into the soil. For instance, nanosensors have been developed in food packaging as a result of nanotechnology combined with information technology. The use of nanosensors in conjunction with intelligent systems of packaging (active and smart packaging) is a very sensitive and fast method for detecting food pathogens, heavy metals, contaminants and maintaining food safety. Nanosensors are useful in the storage, packaging, and transportation of food products because they are able to sense and signal information about quality, freshness, chemical, physical, and microbiological changes, all of which are essential parameters in preventing food deterioration from dust, moisture, light, off-flavours, and off-odours (Durán and Marcato, 2013).
Nanotechnology allows the modification of the structure, properties, and interactions between different food materials allowing the development of novel foods with enhanced taste, texture, colour, freshness and stability (Yu et al., 2018). However, the analysis of the negative effects of nanoparticles has exploded in the last ten years (Mcclements and Xiao, 2017). Most of the experiments published are in vitro studies in which a certain amount of nanomaterial is dosed to living cells and the effects monitored (Naseer et al., 2018). Several in vitro approaches to studying cell toxicity by nanoparticles include proliferation, apoptosis, necrosis, and oxidative stress assays (Kumar et al., 2017). Biodistribution, clearance, hematology, serum chemistry, and histopathology are among the approaches used to measure in vivo toxicity (Kumar et al., 2017). There are numerous studies about the toxicity of nanomaterial and the potential risk for human health (Sahu and Hayes, 2017; Roberto and Christofoletti, 2019). Due to their size, nanomaterials building blocks can readily pass through the cell membrane to accumulate in the cytosol, affecting the cell viability (Ou et al., 2016). Moreover, nanomaterials can travel deeper into the nucleus of cells and damaging the DNA leading to DNA breaks or mutations that can be responsible for cancer (Singh et al., 2017a).
Nanotechnology has accelerated research and technology advancement across all scientific fields, including food sciences. This present review aims to discuss the role and how nanotechnology has impacted food science, particularly in processing food and food packaging. The specific application of nanomaterials in food science, the advantages and disadvantages of nanomaterials, the analysis to detect nanocomponents and the potential risk for human health are also highlighted.
2. Nanotechnology in food sciences
Food produced employing nanotechnology in the production, processing, storage, and packaging of food is referred to as nanofood. Nanotechnology is being employed commercially in food production. It has the ability to be used in every step in food manufacturing, packaging, and monitoring (Fig. 1).
Fig. 1.
Nanotechnology applications in food sciences.
Many foods use nanoparticles to enhance flow characteristics, flavour, stability, and colour during processing and extend shelf life of food. For example, anticaking agents such as aluminosilicate compounds are often employed in powdered or granulated processed food. In contrast, anatase titanium dioxide is a frequent food brightener and whitener ingredient found in confectionery, sauces, and cheeses (Table 1) (Samal, 2017). SiO2 is primarily used in non-food and food products to thicken pastes, used as an anticaking agent in powdered products to retain flow characteristics, and as a carrier of perfumes or flavours. It is widely used in food products and is approved by the European Union (E551) as a food additive (He et al., 2019). Flavours, as one of the most important components of the food system, deliver sensory perception of smell and taste to improve the whole eating experience. Nanoencapsulation technologies have been extensively employed to improve flavour release and retention, and also to create culinary balance (Nakagawa, 2014). SiO2 nanoparticles have also been found to act as flavour and fragrance carriers in food and non-food products (Dekkers et al., 2011).
Table 1.
Summary of selected nano techniques used by different food industries for food processing and packaging.
| Nanotechniques | Examples | Application | References |
|---|---|---|---|
| Nanoparticles | Titanium dioxide, Silicon dioxide, Silver nanoparticles, Zinc oxide, Inorganic nanoceramic, Polymeric nanoparticles | Food Packaging | Ameta et al. (2020) |
| Nanocomposites | Nanoclay, Nanoencapsulation, Bionanocomposites. | Food Packaging | Pathakoti et al. (2017) |
| Nanoencapsulation | Nanoceuticals, Nanocapsules, Colloidosomes, Nanoliposomes, Nanoceuticals | Food processing | Abdullaeva (2017) |
| Nanoemulsions | Nanoemulsion formulated with β-Carotene. Oil-in-water and Water-in-oil nanoemulsions. | Food processing | Mehmood et al. (2021) |
| Nanosensors | Metal based nanosensors, Nanobiosensors, Nano-smart dust, Nanobarcodes | Food Packaging | Coles and Frewer (2013) |
Nanotechnology techniques can be used to extend the shelf life of items by storing in packaging materials that exude antimicrobials or limit moisture and air exchange with the environment. Protection, tamper resistance as well as specific physical, chemical and biological properties are required for food packaging. Food packaging also displays the product's label, including any nutritional information about the consumed meal (Chellaram et al., 2014). The application of nanotechnology in packaging is classified based on its function. Most nanoparticles used in food packaging possess antimicrobial capabilities, act as antimicrobial polypeptides carriers and protect against microbial deterioration. Through the regulated antimicrobials release from the packaged substance, packaging material which are made of a layer of starch colloids loaded with the antimicrobial agent works as a barrier to bacteria (Nile et al., 2020). Novel nano-antimicrobials materials have demonstrated promising results in preventing food deterioration and thereby prolonging food shelf life. A variety of metal and metal oxide nanoparticles have been proposed as antimicrobials. Their inherent physicochemical features promote the generation of reactive oxygen species, resulting in oxidative stress and consequent bacterial cells damage (Wu et al., 2014). Furthermore, metal ions release at the cell surface, from outside the cell, or within the cell might change cellular function or structure (Krzywoszynska et al., 2020). Nanocomposites based on Metal/metal oxide have therefore been used in food coating and packaging. Silver nanocomposites and nanoparticles are among the most extensively employed nanoparticles in the food industry as antimicrobials (He and Hwang, 2016). Escherichia coli contamination can be reduced by utilising titanium dioxide (TiO2) as a coating agent in packaging material (Chellaram et al., 2014).
2.1. Food processing
Food processing is the preservation of food through procedures and processes to turn food into a consumable or edible state (Pradhan et al., 2015). It is the set of techniques and methods for transforming raw materials into finished and semi-finished products (Monteiro et al., 2010). It consists of the following processes: washing, slicing, cooking, pasteurisation, fermenting, freezing, etc. (European Food Information Council, E, 2017). Food processing also includes the addition of food ingredients to prolong shelf-life (Dwyer et al., 2012; Weaver et al., 2014). Processing additionally includes removing toxins, preventing pathogens, preserving food, and increasing food uniformity for improved marketing and distribution. Processed foods last longer than fresh foods and are preferable for long-distance transport from the producer to the consume (Chellaram et al., 2014). These methods are intended to retain the flavour and food quality while also protecting it from microorganism infestation that causes spoilage (Pradhan et al., 2015)
There are so many health and counterclaims about specific foods as a result of modern food processing. It is not always easy to figure out which foods we can trust. During processing and subsequent storage, nutrients can be lost. Based on the quantity and kind of processing, essential nutrients can deteriorate. Furthermore, whilst plastic containers have transformed our ability to extend the food shelf life while adding minimum load (essential when considering the energy necessary to transport it), significant environmental expenses are concerned with its production and disposal.
Nanotechnology is being used in the food production in produce additives at nanoscale to enhance food colour, texture, and taste (Kessler, 2011). Nanoparticles used as food additives include TiO2 and SiO2, as well as amorphous silica. For example, in the powdered sugar coating on doughnuts, TiO2 is used as a colouring (Uboldi et al., 2012). For improved bioavailability and absorption, nanomaterials are used as additives and components in nutrients and nutritional supplements (e.g., minerals, antimicrobials, antioxidants) (Faridi Esfanjani et al., 2018).
In postharvest processing of food, nanotechnology offers great potential. It enhances food bioavailability, flavour, consistency, and texture, masks an undesirable flavour or odour, and changes particle size, size distribution, surface charge and possible cluster formation (Singh et al., 2017a).
2.1.1. Anticaking agents
Caking of food powders usually occurs during handling, processing, and storage. This phenomenon can help to reduce the functionality and quality of products due to the formation of lumps and agglomerates. Moreover, cacking affects the rehydration and dispersibility of food, favouring the decline of its organoleptic qualities and shortening the shelf-life (Aguilera et al., 1995; Lipasek et al., 2012). The caking process induced in crystalline ingredients from environmental moisture starts with the development of liquid bridges connecting crystals, caused by partial deliquescence or capillary condensation (Salameh and Taylor, 2006; Lipasek et al., 2012). Anticaking compounds can act through various processes, including competing for moisture with the host powder, creating moisture-protective layers on the surface of particles, providing smooth surfaces to decrease inter-particle friction, and inhibiting crystal development (Lipasek et al., 2012). SiO2 is used in non-food and food products in thickening pastes (as an anticaking agent) and maintains the flow characteristics in powdered products (e.g., icing sugar, salts, dried milk, spices, and dry mixes). This has led to its use in food products and thus registered as a food additive E551 in the EU. Synthetic amorphous silica has been used as an anticaking agent for a long time without showing any concern because, until recent times, nanoparticles were considered entirely inert. However, there are numerously studied describing the potential negative effect that nanoparticles and nanomaterials can have within the body at molecular level (Winkler et al., 2017). Currently, there is an active debate about the safety and health issues associated with using such engineered nanoparticles in consumer products. There is a demand to improve the risk assessment that regulates those nanoparticles (Mcclements, 2017). Moreover, aluminium silicate, calcium aluminosilicate, sodium bicarbonate, sodium silicate and many more have also been used as additives in granular and powdered meals to prevent caking (Samal, 2017).
2.1.2. Gelling agents
Nanostructured materials can also be utilised as gelling agents in food processing to improve food texture (Bajpai et al., 2018). Active packaging for chicken fillet and cheese was tested using chitosan nanofiber with a gelatine-based nanocomposite and ZnO nanoparticles (Primozic et al., 2021). Amjadi et al. developed a gelatine-based nanocomposite combining ZnO nanoparticles (ZnONPs) and chitosan nanofiber (CHNF) that exhibits strong antibacterial action against foodborne pathogenic bacteria (Amjadi et al., 2019). They showed the interactions and the good compatibility between gelatine matrix, CHNF (diameter ∼ 28 nm) and ZnONPs (diameter ∼ 30 nm) via Scanning Electron Microscopy (SEM), Differential Scanning Calorimetry (DSC) analyses, and Fourier Transform Infrared spectroscopy (FT-IR). The nanocomposite showed high dense structure making it an efficient mechanical and water barrier. Moreover, they demonstrate that the introduction of CHNF offset the negative effect of ZnONPs on the colour characteristic of gelatine film, and the blended effect between CHNF and ZnONPs improved the antibacterial activity of the nanocomposite (Amjadi et al., 2019). Ahmadi et al. tested the antimicrobial potency of gelatine-based nanocomposite films, containing cellulose nanofibers (CNF) and oxide nanoparticles as a food packaging material against Pseudomonas fluorescens and Staphylococcus aureus inoculated on chicken fillets (Ahmadi et al., 2020). According to the study, using antibacterial film significantly reduced the bacterial count on chicken fillets, particularly against S. aureus.
2.1.3. Antioxidant agents
Antioxidants are a class of molecules that react with free radicals and convert them to harmless substances, thus minimising oxidative stress and playing an important role in the treatment of free radical-induced illnesses. However, antioxidants' activity is restricted due to low absorption, difficulty crossing cell membranes, and breakdown during delivery. As a result, they have a limited availability in the body. To overcome these limitations, antioxidants have been covalently linked or packed into nanoparticles of various origin to improve stability, controlled release, biocompatibility and selective delivery (Khalil et al., 2019). Polymeric nanoparticles are thought to be suitable for encapsulating bioactive compounds like vitamins and flavonoids that are exuded in acidic environments such as the stomach (Singh et al., 2017b). SiO2-gallic acid nanoparticles were also developed and tested as antioxidants, demonstrating their ability to degrade radicals of DPPH (2,2-diphenyl-1-picrylhydrazyl) (Khalil et al., 2019). Browning of fresh-cut fruits can be addressed by using antioxidant treatment option in conjunction with edible coatings, as browning is an unpleasant effect caused by converting phenolic compounds to dark-coloured pigments in the presence of oxygen (air) during marketing and storage (Mendoza-Gómez et al., 2017). However, only a few nanoparticles applications as anti-browning agents have been documented. For example, Fuji apples, a fresh-cut product, had their shelf life extended with nano-ZnO-coated active packaging (Bajpai et al., 2018).
2.1.4. Nanofiltration
Another example of nanotechnology in the food production is the employment of nanofilters, which can be used in removing colour from beetroot juice and retaining its flavour (Nile et al., 2020). Furthermore, nanofilters have been employed to make milk suitable for lactose-intolerant via removing lactose, so that it can be replaced with other sugars (Shah et al., 2015). Nanoscale filters have also been used to remove bacterial species from milk or water without having to boil the water (Nile et al., 2020). Nanomaterials can be used to make nanosieves to filter milk and beer. To avoid foodborne diseases, nanotechnology is used in producing healthier foods that are low in salt, sugar, and fat (Nile et al., 2020).
2.1.5. Nanoemulsions
Nanoemulsions are a colloidal particulate solution with oil-in-water emulsion features, consisting of solid spheres with lipophilic and amorphous surfaces and very small droplet sizes in the range of 10–1000 nm (Jaiswal et al., 2015). The tiny size of nanoemulsions facilitates the formation or presence of a large surface area, that can be critical for significant interaction with diverse bioactive compounds absorbed in the gastrointestinal tract. Furthermore, nanoemulsions digest faster than conventional emulsions because they have greater binding sites for the digestive enzymes lipase and amylase in the gastrointestinal tract (Gasa-Falcon et al., 2020). The nanoemulsion-based technique efficiently increases the bioavailability of biologically active substances since their composition, structures, and characteristics may be controlled (Aswathanarayan and Vittal, 2019).
2.1.6. Nanoencapsulation
Nanoencapsulation is a technique where substances are packed into small structures using nano-emulsification, nano-structuration, or nanocomposites to allow for controlled release of the core. Depending on the application, various nanoencapsulation (liposomes, nanoparticles, micelles, nanospheres, nanoemulsions and nanocochleates) have been used. They can be utilised as nutritional supplements to mask unpleasant flavours, increase bioavailability, and allow for the effective dispersion of insoluble supplements without using emulsifiers or surfactants (Paredes et al., 2016). The use of lipid molecules in nanoencapsulation improved the antioxidant potential by increasing bioavailability and solubility while eliminating unwanted interactions with other food components. Lipid-based nanoencapsulation systems, including nanoliposomes, nanocochleates, and archaeosomes are commonly used (Nile et al., 2020).
Nanoencapsulation has been utilised in improving the shelf life of tomatoes, and this strategy should be expanded to extend the shelf life of other fruits and vegetables (Yadav, 2017). Enriched fruit juices, nanoteas, oat nutritional drinks, nanoceuticals slim shakes, and nanocapsules containing tuna fish oil in bread are just a few examples of nano-processed foods that are commercially available and widely sold in the United States, China, Australia, and Japan (Nile et al., 2020).
2.1.7. Nutraceuticals and bioavailability
Food contains bioactive substances that boost immunity and protect against disease. The potency of most of the food items is low, even though they have higher concentrations of bioactive molecules. This is a result of low solubility, bioavailability, and stability in the gut and poor permeability and retention time in the intestinal tract (Mcclements and Xiao, 2017). Most bioactive molecules, like vitamins, carotenoids, antioxidants, polyphenols, micronutrients, and food ingredients, have low bioavailability, solubility, and stability. However, these can be improved using nanotechnology, and specifically nanoformulations. Nanomaterials have a smaller particle size and a large surface area per unit mass, enhancing bioavailability, biological activity, and solubility of encapsulated food ingredients. To improve the targeted delivery and bioavailability of natural bioactive compounds, nanotechnology-based delivery systems are used (Nile et al., 2020). Nanonutraceuticals are a hybrid of pharmaceuticals and nutrition in which functional foods, bioactive substances, dietary supplements, and herbal products are manufactured using a nanoformulation method (He et al., 2019). Nutraceuticals were delivered using a variety of methods like liposomes, cubosomes, solid lipid nanoparticles (SLNs), microemulsions, monolayers, biopolymeric nanoparticles, microgels, and fibers are used to deliver nanotubes, nanofibers, fullerenes, nanosheets, and nanowhiskers (Nile et al., 2020).
2.2. Food packaging
Food components must be packaged properly to avoid spoilage and degradation due to environmental factors, as well as to maintain protection during storage and transportation. The incorporation of nanoparticles with diverse chemical and physical properties into packaging material has given rise to distinct practical and novel properties (Bumbudsanpharoke and Ko, 2015; Ashfaq et al., 2022). Through using hybrid organic-inorganic structures and inorganic structures as packaging materials, nanocomposites have been created that evoke multiple functions through using nanoclays and layered silicates for food packaging, boost mechanical and barrier properties, and are more stable and biodegradable than traditional packaging materials (Ahari et al., 2021; Ashfaq et al., 2022).
2.2.1. Types of nanoparticles employed in food packaging
Due to the importance of food packaging, many authors have investigated different nanoparticles types recently (Biswas et al., 2020; Carbone et al., 2016; Sharma et al., 2021). Biswas et al., used green route synthesised silver nanoparticles (AgNPs), using Garuga pinnata leaf, a natural, nutritious drink for the tender coconut water (Biswas et al., 2020). This drink is used widely as pharmaceuticals and nutraceuticals, contributing to the fast growth in the functional food industry. Their work found that the interaction of silver nanoparticles (AgNPs) with DNA assists in identifying and addressing the degradation process while considering prevention from microbial attack and making the coconut water a potential functional food entity (Biswas et al., 2020). Sobhan et al. found that a compelling conductive antimicrobial film for smart food packaging was employed when developing silver integrated nanocomposite film based on cellulose nanofiber (CNF) and activated carbon (AC) (Sobhan et al., 2020). Several studies showed that nanoparticles like gold and silver increase the food packaging life as they can inhibit and reduce microbial contamination (Biswas et al., 2020; Mohanta et al., 2015, 2018; Mohanta and Behera, 2014). Toker et al., reported that Zn, Ti, Cu, Au, and Ag are emerging metal nanoparticles with biocidal characteristics used in food packing. De Moura et al. used AgNPs material with hydroxypropyl methylcellulose (HPMC) matrix as a nanoparticle for food packaging because of its importance as a bactericidal agent (De Moura et al., 2012). They used silver nanoparticles with particle size 41 100 nm (De Moura et al., 2012, Toker et al., 2013). Chen and Schluesener; Kumari et al., also investigated Silver nanoparticles as antimicrobial agents (Chen and Schluesener, 2008; Kumari et al., 2009). Sánchez-González et al. reported using polymers that have renewable and biodegradable properties such as polysaccharide HPMC as a food packing nanomaterial (Sánchez-González et al., 2011). They also found the best physicochemical properties in the chitosan and HPMC, with or without bergamot essential oil.
2.2.2. Edible and non-edible packaging
Developments in the preparation of nanoparticles which integrate food-safe ingredients have enabled researchers to investigate edible film functional modifications which involve nanoemulsions, nanoparticles, polymeric nanoemulsions, solid lipid nanoparticles, nanofibers, nanotubes, nanocrystals, nanofibers, nanostructured lipid carriers, or blends of inorganic and organic components that are nano-sized. These nanoparticles are typically comprised of protein or polysaccharide known as “nanocomposites,” which are described as mixing materials to generate a blend which enhances the qualities of a component where at least one component is nanoscaled (Mallakpour and Sadaty, 2016). In order to enhance conservation, the invention of nanocomposites allows for the adoption of edible coatings as “temporal distribution systems” which transfer active chemicals from a reticular layer to the food (Liu et al., 2017). Nanomaterials and edible coatings containing nanoparticles outperform traditional packaging materials in terms of food preservation and quality maintenance (Ashfaq et al., 2022).
Nano-coating is now used in packaged foods to create better food packaging. Food coatings are utilised in fine coats or films made of both non-edible and edible materials. The edible film is a potential method for extending food product shelf life. However, coatings formed of a single polymer nanofillers increase the mechanical capabilities, barrier qualities, and the colour of the edible film when compared to simple edible films, but further study is needed to make them economical (Jeevahan and Chandrasekaran, 2019).
Thin edible nano-coatings (∼ 5 nm) can be utilised as moisture and gas barriers in fruits, meat, cheese, vegetables, bakery goods, fast food, and confectionery products. There are a variety of bakery goods available that are coated with edible antibacterial nano-coatings. Nanostructured gelling agents have been used as an edible coating to keep fresh foods fresh for longer periods of time. Examples include edible coatings formulated with gelatine nanoparticles and cellulose nanocrystals, coatings made of nanosilica and chitosan, film made of chitosan and nano-SiO2 and nanolaminate coatings made of lysozyme and alginate (Singh et al., 2017a).
Non-edible packaging in the form of nanocomposites is widely used in packaged food since they are biodegradable and environmentally friendly. Top Screen DS13 is an example of an easily recyclable nanocomposite (Pradhan et al., 2015).
2.2.3. Active and smart packaging
The use of active nanomaterials, for instance, oxygen scavenging materials and antimicrobials are referred to as active packaging. Such nanomaterials are advantageous for interacting directly with food in providing better protection for food products. Some nanomaterials have antimicrobial potentials that can be added to food packaging (Ameta et al., 2020). Nanosilver, nano magnesium oxide, nano-titanium dioxide, carbon nanotubes, nano-copper oxide, and other materials are examples. Active packaging uses packaging materials that interact with the food and environment and plays an active role in prolonging product shelf life. This enables packages to play an active role in the preservation of food (Ameta et al., 2020). Alterations in selective permeation of package materials to various gases are also part of active packaging technology. For active packaging, some nanocomposite materials have been used to prevent the passage of O2, CO2, and H2O into food (Ameta et al., 2020). Nanosensors have also been used in packaging. Metal-based nanosensors (platinum, gold, and palladium) can detect gas production and colour changes in food due to spoilage (Hamad et al., 2018), any change in humidity, heat, light, gas (Pradhan et al., 2015), and toxins like aflatoxin B1 in milk.
Nanosensors are created for smart packaging in order to identify food spoilage and also to release nano-antimicrobials as needed to prolong the shelf life of food products. The use of nanosensors alerts customers to food contamination or food spoilage by detecting pesticides, toxins, and microbial contaminants in products and converts them into observer readable signals like flavour production and colour formation (He et al., 2019). For optimal alarming, the food environment is steadily monitored for oxygen content, temperature, pathogens, and other signs. Nanosensors are also used to establish the shelf life of the food. For example, Au nanoparticle incorporated enzymes are used in the detection of microbes, nanofibrils of perylene-based fluorophores signal and meat rotting by sensing gaseous amines (Chellaram et al., 2014). Others used include nanocomposites of TiO and ZnO for detecting volatile organic compounds. Nanobarcodes are utilised for both labelling and safety measures (Chellaram et al., 2014). The concept of “Smart Packaging” is becoming a reality. Research is being conducted on the development of antigen-specific biomarkers in food packaging and in the incorporation of nanoparticles to make films of nanocomposite polymer. Antigen-specific biomarkers will aid in detecting the presence of the organism responsible for food spoilage. BioSilicon, developed by pSivida in Australia, is utilised in food packaging (Pradhan et al., 2015). It is made of nanopores and is used for food packaging. Its uniqueness comes from the fact that it is made up of nanostructured silicon. Silica is a compound with excellent physicochemical qualities, it is stable, and is heat resistant at high temperatures. Using silica as a filler has the potential to enhance the chemical and physical qualities, performance, and capabilities of the final products. According to prior work by Thuong et al., adding silica filler to natural rubber compounds can improve mechanical properties significantly, with tensile strength increasing by seven times and loss modulus increasing by twenty-five times (Thuong et al., 2020). Bio-silicon and coconut oil have been used to improve the features of starch-based bio foam packaging.
2.2.4. Biobased packaging
Nanotechnology is employed to improve the barrier of plastics, bioactive incorporation, signalling and sensing important food information, changing foil pervasion, developing different barrier characters (mechanical, chemical, thermal, and microbial), and improving heat resistance and mechanical characters (Berekaa, 2015). Using decomposable packaging made of biodegradable plastics will help in reducing pollution in the environment. Various nanocomposites (which are biodegradable polymer) with desired properties have been developed for a broad variety of products (Youssef and El-Sayed, 2018). Starch derivatives, polylactic acid (PLA), polybutylene succinate (PBS), aliphatic polyester polycaprolactone, and polyhydroxybutyrate (PHB) are currently the most commonly used biodegradable nanocomposites for packaging (Ameta et al., 2020).
2.2.5. Migration of nanoparticles
Many researchers have described the migration of nanoparticles in food, and nanoparticles examined in most cases were nanoclay and nanosilver containing polymers. However, ZnO, TiN, and other nanomaterials have been studied. Different authors' conclusions about whether nanoparticles can migrate out of polymers are inconsistent and even conflicting, which could be attributable to flaws in the experiment design (Störmer et al., 2017). This has resulted in the government and public concerns about their health and safety implications. Approval to adopt nanocomposites in packaged foods could be based on their migration tests performance, since nanoparticle migration from nanocomposite system and packaged food system to beverages and foods raises the chances of compromising the customer's health (Cushen et al., 2014). When packaging materials are in contact with foods, metals typically migrate. The migration process has 3 stages: the diffusion of the migrant nanoparticle, dissolution of the nanoparticle, and the dispersion of the nanoparticle in food. Choi et al. discovered that the nanoparticles migration, like AgNPs from baby products, can have a harmful impact on babies' health (Choi et al., 2018). Understanding the migration of nanoparticles is critical for determining the possible health implications of these compounds when they come into contact with food products (De Azeredo, 2013). The rate of migration for nanoparticles can be influenced by the inherent impacts of physicochemical qualities of other dietary components (Bott et al., 2014).
Huang et al. discovered that time and temperature greatly increased the level of silver migration in food modelling solutions (Huang et al., 2011). In two steps, they proposed a probable explanation for this migration phenomena. The initial release of the nanoparticles must be from the encapsulated nanosilver particles on the surface layers of the specimen. The nanosilver was then released via a dual-sorption mechanism, diffusion, and embedding. Farhoodi et al. investigated nanoclay migration from a polyethylene terephthalate stretch blow-molded bottle (Farhoodi et al., 2014). Their findings revealed a link between the concentration of silicon and aluminum in acetic acid solutions and temperature–time. Increase in time and temperature were due to an increase in the level of migration. Lin et al. investigated the impact of particle size on TiO2 migration behaviour (Lin et al., 2014).
Hannon et al. carried out research on copper and silver nanoparticles migration from the surface of an antimicrobial nanocoated antimicrobial packaging material to food simulants using ICP–MS and acidic digestion procedures. The findings revealed that 0.82 and 0.46 mg/kg of Cu and Ag respectively, migrated to the food simulants. Cushen et al. used ICP–MS to study the effects of temperature and time on the rate of Cu nanoparticles migration into chicken breast from the polyethylene matrix. They discovered that migration ranges were between 0.024 and 0.042 mg/dm2.
3. Detection of nanomaterials in food
There are various approaches to detect, study and characterise nanoparticles and nanomaterials in food. Among many, microscopy (e.g., AFM, SEM, TEM) and spectroscopy (e.g., Raman, FT-IR, DLS) techniques are the most popular and effective to investigate the food. Following, some examples of these techniques and their application to study nanoparticles and nanomaterials in food science have been discussed.
3.1. Microscopy techniques
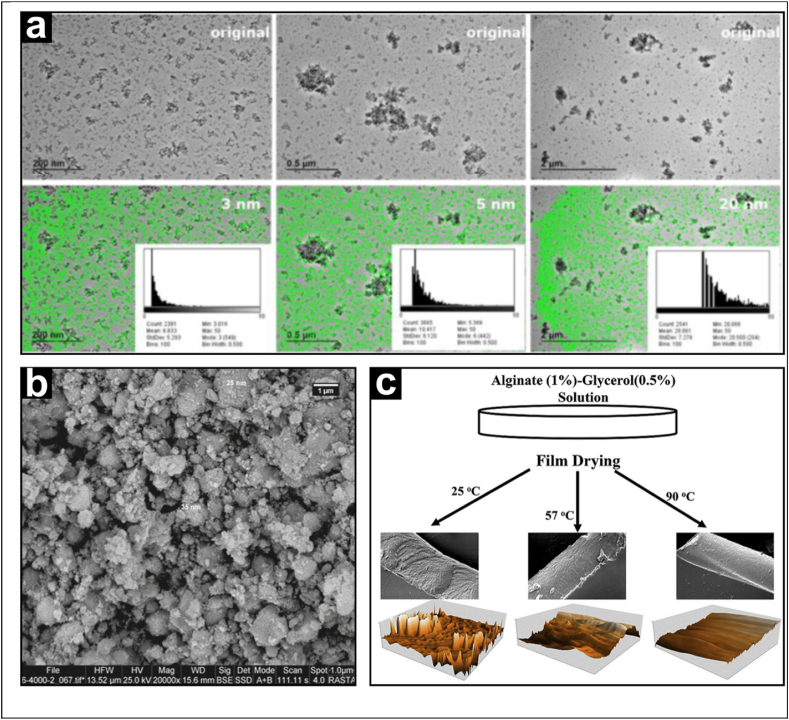
Transmission Electron Microscopy (TEM) is a microscopy technique that relies on the passage of an accelerated electron fascicle via a very thin specimen. The picture contrast is determined by the difference between the absorption coefficients of the several specimen sites. Although TEM shows several disadvantages (e.g., the number of particles that can be analysed is limited to the area of the acquired images; samples are partially damaged during pre-treatments), it provides high-resolution images in the sub-micron scale. Barahona et al. used TEM to examine all dispersions, filtered and unfiltered, for the existence of SiO2 nanoparticles with an equivalent circle diameter of 100 nm or less (Fig. 2a) (Barahona et al., 2016). In studies of polylactide nanoparticles uptake in HeLa cells, heavy-metal labelling of the cells (negative staining approach) increased the contrast and allowed imaging of the nanoparticles using bright-field TEM (Reifarth et al., 2018). The approach of negative staining was initially developed to image bacteria and virus particles, but it can also be used to reveal organic coatings on NPs. Albumin and creatine coatings on gold nanoparticles (AuNPs) have been revealed using this approach (Jain, 2017).
Fig. 2.
(a) Analysis of TEM images of sample E551 2 at various magnifications and minimum ECD thresholds. In each case, the insets indicate the resulting size distributions that were examined under the specified criteria. Reproduced with pemission (Barahona et al., 2016).
(b) SEM pictures of Satureja khuzestanica-produced Ag–TiO2 nanocomposites. Reproduced with pemission (Sallak et al., 2021).
(c) AFM 3D surface images of the films dried at 25°C, 57°C, and 90°C with an air flow rate of 6 L/s. Reproduced with pemission (Bagheri et al., 2019).
Scanning electron microscopy (SEM) is a type of electron microscope which makes images by scanning the surface of specimens with a focused electron beam. The electrons interact with the specimen's atoms, producing a wide range of signals containing information about the specimens' surface topography with nanoscale resolution. (Marshall, 1991). SEM micrographs are very useful for food analysis allowing to study the impacts of engineered nanomaterials on the structure of food, as well as their morphology, composition and location. Sallak et al. performed the biosynthesis on nanoparticles employing plant extracts. The antibacterial, morphological, physical, and mechanical properties of Ag–TiO2 nanocomposites (size: about 30–60 nm) and a corn starch (CS) film containing Satureja khuzestanica essential oil (SEO) were examined in this study. The resulting film could find application in food packaging to extend the shelf life of products. SEM coupled with energy-dispersive X-ray spectroscopy (EDX), was used to study the morphology and the elemental composition of the films (Fig. 2b) (Sallak et al., 2021). Chen et al. created green hard capsules using corn nano-starch and cellulose nanocrystal (CNC). Capsules were produced via dipping and casting methods, and their tensile strength, transparency and gastric juice resistance were characterised. These capsules can find application in the area of medical capsules. SEM was used to characterise the morphology of the capsules (Chen et al., 2021). Yang et al. employed poly(lactic acid) (PLA)/nano-TiO2 films in contact with ethanol solutions to investigate the migration of nano-TiO2. They studied the molecular interactions and the structural changes via X-ray diffraction (XRD) and SEM, respectively. SEM images show that the films' microstructure became rougher after days of exposure to the ethanol as a consequence of the migration of the nano-TiO2 (Yang et al., 2019).
Scanning transmission electron microscopy (STEM) is a technology that combines the SEM and TEM principles and can be done on either type of instrument. STEM, like TEM, needs extremely thin specimens and focuses on electron beams transmitted by the specimen. One of its major strengths over TEM is that it may utilise signals like scattered beam electrons, secondary electrons, characteristic X-rays, and electron energy loss which are not spatially correlated in TEM.
X-ray microscopy (XRM) can image a sample in the aqueous state with a spatial resolution (down to 30 nm, constrained by the X-ray beam focusing optics) without need for sample preparation, such as fixing, sectioning or staining. To allow 3D imaging, X-ray microscopy may be combined with computer tomography (Busse et al., 2019). The scanning transmission X-ray microscopy (STXM) is a variant of the XRM that has also been used, for example, to describe metallic particles of Fe for remediation purposes (Naghdi et al., 2017).
Naghdi et al. (2017) compared the visualisation of natural marine particles and colloids using an environmental scanning electron microscope (ESEM) and a traditional SEM. They discovered that using a traditional SEM to examine river estuary samples yields lower resolution thresholds and clearer images, and additional imaging artefacts due to sample drying. While ESEM samples maintain their morphological structures to some degree without needing the preparation of the sample, imaging and image analysis are more difficult. ESEM imaging was able to produce data on the main structures of the particles in research on natural nanostructures in surface water. In contrast, conventional SEM imaging revealed aggregated colloids and particles (Naghdi et al., 2017).
Scanning Probe Microscopy (SPM) is characterised by a sharp electrode controlled by a 3D aligning component with a sub-nanometric resolution and a laser optical apparatus. The food sample to be examined is about 0.1 nm away. The interaction between the atoms of the sample and the atoms of the probe determines physical magnitudes such as magnetic force, tunnelling current, atomic force, friction force, ionic capacity, etc. SPM uses a precise but straightforward mechanical process based on scanning the specimen surface to determine associated superficial properties (Bruno, 2018). One of the most extensively used types of SPM is atomic force microscopy (AFM) (Wen et al., 2020).
Atomic Force Microscopy (AFM) to produce AFM images, the magnitude of the interaction between the probe and the food sample surface (commonly known as van der Waal's force) is measured as the surface is scanned beneath the probe. AFM gathers image data by “feeling” rather than “looking.” Contact, noncontact, and tapping modes are the three types of operation modes that can be used on various materials. AFM has found widespread application in biology, materials science, chemistry, and, more recently, food science (Venkateshaiah et al., 2020). AFM has been used to research the structural analysis of biomacromolecules of fish oil nanoemulsions with tapping mode. According to Nejadmansouri et al., AFM high resolution at the nanometre level can precisely reflect the microstructural information of nanoemulsion (Nejadmansouri et al., 2016). Tai et al. in their research with egg yolk and soybean lecithin liposomes observed using AFM that adding sterol increased the size of the vesicle compared to the blank liposome. Using non-contact mode, AFM has been used in film studies on pectin films that have been mixed with crystalline nanocellulose. (Tai et al., 2018). The result showed appropriate interaction between pectin matrix and nanocellulose, indicating good dispersion (Chaichi et al., 2017). Bagheri et al., in their film research of glycerol-plasticised alginate films, found that by increasing drying temperature, the samples became denser with a smoother surface (Fig. 2c) (Bagheri et al., 2019). Gilbert et al. also used tapping mode in applying AFM on composite film made by zein and hydroxypropyl methylcellulose nanoparticles used in packaging food; its improved air permeability and packing potential (Gilbert et al., 2017).
3.2. Spectroscopy techniques
Raman spectroscopy is a method for studying rotational, atomic vibrational, and other low-frequency patterns. In chemistry, it is commonly used to identify compounds using a structural fingerprint. The Ramen effect is on the basis of Raman scattering (the inelastic scattering of monochromatic light), which explains the excitation of photons to states of virtual energy as well as the energy lost or gained as a result of light interaction with the vibrational modes of the specific chemical bonds within the specimen (Zhang, 2017).
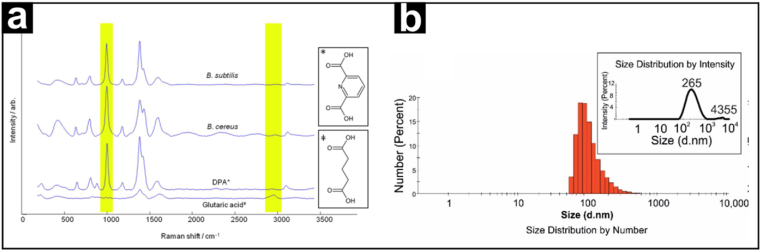
Raman spectroscopy has become a popular approach for characterising many nanomaterials. The primary reason for this is that Raman spectroscopy alone can not determine the composition of each nanoparticle, allowing it to be recognized, but it can also determine the structural information that differentiates different forms of the same nanomaterial type, e.g., the ability to differentiate between multi-walled carbon nanotubes (MWCNTs) and single-walled carbon nanotubes (SWCNTs) (Nebu and Sony, 2017). Raman spectroscopy has been less commonly employed because of its relatively weak signals, interference, fluorescence and high cost of equipment. This has resulted in surface-enhanced Raman spectroscopy (SERS) being developed for a wide range of applications across different fields. This method detects samples in solution and improves selectivity and sensitivity by utilising noble metal nanomaterials to boost the low-concentration single-molecule Raman signal by many orders of magnitude (usually 107 to 1014) (Yin et al., 2020). Cowcher et al. used SERS to mix silver nanoparticle colloids with bacterial solutions to quickly detect Bacillus and other infections. Because of its quick processing speed and great sensitivity, SERS detects Bacillus in food. The results demonstrated that the SERS could identify dipicolinic acid biomarker, which acted as a bacillus marker in vivo, more quickly and efficiently than microscopy. The technique offers the benefits of being simple, readable, and inexpensive (Fig. 3a) (Cowcher et al., 2013).
Fig. 3.
(a) Baseline-corrected SERS spectra of glutaric acid internal standard, pure DPA, and DPA extracted from B. cereus and B. subtilis spores. The DPA ring breathing vibration at 1006 cm−1 and the glutaric acid C–H stretch at 2934 cm−1 used for quantification are highlighted. Chemical structures of DPA and glutaric acid are shown on the right hand side. Reproduced with permission (Cowcher et al., 2013).
(b) Particle size distribution obtained via DLS. Reproduced with permission (Jarzebski et al., 2019).
Fourier Transform Infrared Spectroscopy (FT-IR) is a technology for acquiring emission spectra or infrared absorption from solid, liquid or gas samples. FT-IR is employed in different research fields, including geology, materials, chemistry, biology, and food science, to characterise food and bio-packaging as well as identify contaminants (Rodriguez-Saona, 2011).
FT-IR is based on chemical groups' ability to absorb energy from infrared (IR) light and transfer it to their chemical bonds, resulting in observable vibrational modes like wagging, twisting, rocking, symmetric and anti-symmetric stretching (Griffiths and De Haseth, 2008).
Dynamic light scattering (DLS) is a method used to determine and analyzes light scattering caused by the Brownian movement of particles in a suspension. Through the Stokes-Einstein relationship, DLS may be utilised to characterise particle size distribution, molecular weight, and relaxations in complicated fluids. This is possible because of the distinct chemical and physical properties of nanoparticles (Tosi et al., 2020). DLS has several advantages, in fact, it requires minimal sample preparation, fast results and a relatively inexpensive to set up and run. Jarzebski et al. used pea proteins in combination with lecithin as a co-surfactant to stabilize hempseed oil (HSO)-based water/oil emulsions. DLS, centrifugation, and heat stress tests were used to characterise the system's stability. The study provided evidence suggesting hempseed oil-based emulsions can be used as a possible flavour carrier in food (Fig. 3b) (Jarzebski et al., 2019).
4. Safety and regulation of nanomaterials in food products
Though there has been significant progress in the use of nanoparticles in food nanotechnology, little is known about nanoparticle toxicity. Allergens and heavy metal release are the two main safety concerns when using nanoparticles. Nanoparticles are currently being applied in food products at a rate faster than desired, without the necessary knowledge and regulations, posing a risk to the environment and human health (Ranjan et al., 2014). The application of nanomaterials in food science and the food industry is increasing, they must be used with caution since they possess the potential to induce toxic effects. According to a British Royal Society research, humanity may face a nanotoxicity catastrophe in the future (Amini et al., 2014). We will only be able to find safe and useful food products if we have a thorough understanding of the characteristics of nanomaterials, including solubility, size, composition and surface chemistry. Some of these unique characteristics of nanomaterials make them appealing materials for many applications; however, this could be debatable in the case of food applications, where human health could be at a high-potential risk (Ameta et al., 2020).
Nano-entities have the potential to disrupt a variety of cellular pathways and functional processes (Arora et al., 2012). The changes in the intracellular milieu that may occur as a result of exposure to nanomaterials can have unanticipated effects on the overall functionality of the cellular system, and the fidelity of cell division and DNA replication (Evans et al., 2017). DNA damage has been linked to cellular exposure to some nanomaterials, resulting in genome rearrangements, single and double-stranded breaks, as well as inter/intra-strand breaks. Moreover, the formation of modified bases (5-hydroxy-5-methylhydantoin, thymine glycol, 8-hydroxyguanine) has been documented (Biola-Clier et al., 2017). If left untreated, these various types of DNA mutations can result in gene mutations, chromosomal aberrations, carcinogenesis, apoptosis, or cellular senescence (Broustas and Lieberman, 2014).
Nanoparticles can cause a variety of diseases when they accumulate or come into contact with cells and their internal components, such as the nucleus, mitochondrion, lipid vesicle, cytoplasm, and membrane (Behzadi et al., 2017).
Taking cognisance of nanoparticle exposure level is essential for determining the form and nature of injuries they could pose to a variety of cells and tissues. Dermal, respiratory, and digestive nanoparticle exposure are the three primary routes of nanoparticle exposure (Sahu and Hayes, 2017). Food and similar materials containing nanoparticles have the potential to enter the human body through any of these routes. Some nanoparticles scatter in the environment during the processing of nanoparticles used in food and other related industries. To preserve workers' welfare, respiratory tract absorption of nanoparticles need to be considered. Nanoparticles found in packaging, pesticides, and fertilisers can enter workers' respiratory systems. The digestive tract is the primary route of nanoparticle absorption. Any nanoparticles that penetrate the respiratory tract make their way through the digestive system through mucociliary clearance (Ameta et al., 2020). From nanoparticle processing to application and use in agriculture, medicine, and other related industries, the skin is the primary point of contact between nanomaterials and humans (Amini et al., 2014).
Toxicity analysis in which mice were exposed to various amounts of aerosolised carbon nanotubes (CNs) showed that these could cause inflammatory disease and destruction in the lungs and develop widespread granulomas (Kobayashi et al., 2017). According to research from similar studies, several nanomaterials interfere with the immune system to cause symptoms ranging from moderate immune system activation to extreme granulomatous changes in the lungs, for example.
Some organs, like the liver, lymph nodes, and spleen absorb nanomaterial significantly faster than others. Thus, when attacking other tissues in the body, a nanomaterial may accumulate in these organs. The sinusoidal wall of the liver, for example, is lined by Kupffer cells (a particular type of macrophage found in the liver and part of the reticuloendothelial system), which are responsible for eliminating contaminants from the blood entering the liver from the gut mesentery.
Depending on their composition and shape, nanoparticles can cause toxicity in cells through different mechanisms. The tendency of inorganic nanoparticles to produce reactive oxygen species (ROS) such as superoxide, singlet oxygen, hydroxyl radicals and hydrogen peroxide is one of the most significant factors contributing to their toxicity (Wu et al., 2014). ROS can destroy organelles, cell membranes, and the nucleus by communicating with proteins, nucleic acids, or lipids. As a result, so many biochemical functions such as DNA replication, gene expression, and ATP formation which are critical to cell viability, might be harmed (Sharma et al., 2014).
Nanoparticle toxicity is also influenced by its solubility. For example, insoluble TiO nanoparticles, are more toxic when compared to soluble (hydrophilic) TiO nanoparticles. Some soluble compounds of nickel have been identified as carcinogens (Grimsrud and Andersen, 2010). Therefore, a good understanding of the biological activities and toxicity of nanoparticles must be put into consideration while using nanotechnology in the food industry and associated industries. That is to say, all aspects of nanoparticle toxicity and environmental behaviour should be studied (Amini et al., 2014).
Food regulations should pay attention to the health impacts of nanotechnology-based food processing systems. To evaluate whether new food safety regulations are required, it is important to investigate the possibility for such items to cause new health risks (Bajpai et al., 2018). There is an urgent need for a regulatory structure capable of regulating any dangers connected to nanofood and the usage of nanotechnologies in the food industry. Governments must also address the broader civil liberties, economic, social, and ethical issues raised by nanotechnology. Public participation in nanotechnology decision-making is critical to ensuring democratic control of these technological advances in the crucial area of agriculture and food (Sodano et al., 2016). Chemicals in the form of nanotubes or nanoparticles must be handled as if they were completely new substances. Before being allowed to be used in any food product, the ingredients in these nanoparticles must undergo full safety assessments by the relevant scientific advisory body. In addition, the inclusion of nanoparticle materials in food products should be disclosed in the ingredients list.
The nanoparticles' large surface area to volume ratio distinguishes them from their natural forms, this could also be the premise of their migration into food and the toxic effect on humans after consumption (Qadri et al., 2018). Nanoparticle toxicity varies according to their nature, concentration, length of exposure, and individual sensitivity (Dimitrijevic et al., 2015). Organic nanoparticles like starch, lipids, proteins, and chitosan are widely known to be non-toxic since they decompose entirely in the gastrointestinal system since they are not bio-persistent (Divya and Jisha, 2017).
Nowhere in the world is there an official regulation for nanomaterials (Blasco and Picó, 2011). Specific nanoparticles have been permitted for food contact materials in a few countries, whereas other legislation assumes bulk and nanomaterials are equally toxic.
In the United States, the Food and Drug Administration (FDA) regulates the use of nanoparticles in food packaging. Nanoparticles are indirect additives and manufacturers are to request pre-market clearance for the use of indirect additives in food. The FDA must reply to a written notification from the manufacturers within 120 days under the Food Contact Notification (FCN) system; otherwise, the product may be commercialized without additional clearance. Other producers who use the same chemical are not eligible for approval (Wagner, 2013).
Nanoparticles in packaged foods are typically controlled in Europe under the European Commission (EC) statutory guideline EC 1935/2004, which says that their usage in packaged foods may well not endanger public health (Article 3). Nanoparticles must be assessed on a case-by-case basis prior to getting placed on the market, this is according to Article 23 of Regulation EC 10/2011. Even when the corresponding bulk substance is already allowed, nanoparticles must be authorized (EEC 89/109). If an unapproved chemical is employed, a migration limit of 0.01 mg/kg using a functional barrier must be met (Article 14, EC 450/2009) (Cushen et al., 2013). Only three nanoparticles are currently approved for use in plastic-packaged foods: silicon dioxide, carbon black and titanium nitride (Wagner, 2013). TiN may not even be noticeable in food, and carbon black must not be used at concentrations greater than 2.5% w/w in packaged foods. There is no specified limit of migration for silicon dioxide (Annex I, EC 10/2011). Pre-market approval is not necessary for products that are Generally Recognized as Safe substances (GRASs). Where a manufacturer provides a scientific risk evaluation of a product in a scientific peer-reviewed journal, the manufacturer may commercialize the chemical without first consulting the FDA. This also applies to products which have already been approved. Historically, the FDA has held that compounds that are chemically similar to approved additives and meet the limits provided in that permission may be utilised without further notifications. It is unclear how many nanoparticles are utilised for FCM under this provision, although some materials, like carbon black, silver, and aluminium, have been reported to be employed in FCMs (Products, 2009).
The European Food Safety Authority (EFSA) produced a guideline in 2011 titled “on the risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain” (Committee, E. S, 2011), indicating what physicochemical data must be provided by the manufacturer. It requires the provision of in vitro distribution, absorption, genotoxicity, metabolism, and excretion test result, and a 90-day oral toxicity assessment with repeated doses. A chemical may be excluded from these standards if data show no migration or full dissolution or degradation (Committee, E. S, 2011). Given the difficulty in correctly measuring and classifying nanomaterials, EFSA does not explain how these standards might be met in a uniform and cost-effective manner.
5. Conclusion
Nanoparticles are attracting a lot of attention in the food industry because of the potential of both organic and inorganic nanoparticles to improve food nutritional attributes, safety, and quality. Alternatively, nanoparticles could behave differently in the body when ingested due to their small size when compared to bulk materials or larger particles commonly used as ingredients in food. More research is needed to better understand how ingested nanoparticles affect consumers and understand the mechanisms and consequences of the emergence of bacterial resistance to nanoparticles. Nanoparticles safety in food must be assessed on regular basis, considering their nature and the characteristics of the food matrix in which they are distributed. Analytical methods are required to identify and characterise nanoparticles and their characteristics in matrices such as soil, air, water, and food and consumer products to which ecosystems and humans are exposed. These methodologies must also be applied to the characterisation of nanoparticle in toxicological and ecotoxicological assessment; only then can an acceptable risk assessment be carried out, and the features of toxic nanoparticle identified, regulated, or utilised in standardised testing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Helen Onyeaka: Conceptualization, Writing – review & editing. Paolo Passaretti: Writing – review & editing. Taghi Miri: Writing – review & editing. Zainab T. Al-Sharify: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to Acknowledge University of Birmingham for its support in the present work.
Edited by Quancai Sun
References
- Abdullaeva Z. Nanomaterials in Daily Life. Springer International Publishing AG 2017; 2017. Nanomaterials in Food Industry and Packaging. [Google Scholar]
- Aguilera J., Del Valle J., Karel M. Caking phenomena in amorphous food powders. Trends Food Sci. Technol. 1995;6:149–155. [Google Scholar]
- Ahari H., Anvar A.A., Ataee M., Naeimabadi M. Employing nanosilver, nanocopper, and nanoclays in food packaging production: a systematic review. Coatings. 2021;11 doi: 10.3390/coatings11050509. [DOI] [Google Scholar]
- Ahmadi A., Ahmadi P., Ehsani A. Development of an active packaging system containing zinc oxide nanoparticles for the extension of chicken fillet shelf life. Food Sci. Nutr. 2020;8:5461–5473. doi: 10.1002/fsn3.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameta S.K., Rai A.K., Hiran D., Ameta R., Ameta S.C. Use of nanomaterials in food science. Biog. Nanoparticles Agro Ecosyst. 2020:457–488. doi: 10.1007/978-981-15-2985-6_24. [DOI] [Google Scholar]
- Amini S.M., Gilaki M., Karchani M. Safety of nanotechnology in food industries. Electron. Physician. 2014;6:962–968. doi: 10.14661/2014.962-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amjadi S., Emaminia S., Heyat Davudian S., Pourmohammad S., Hamishehkar H., Roufegarinejad L. Preparation and characterization of gelatin-based nanocomposite containing chitosan nanofiber and ZnO nanoparticles. Carbohydr. Polym. 2019;216:376–384. doi: 10.1016/j.carbpol.2019.03.062. [DOI] [PubMed] [Google Scholar]
- Arora S., Rajwade J.M., Paknikar K.M. Nanotoxicology and in vitro studies: the need of the hour. Toxicol. Appl. Pharmacol. 2012;258:151–165. doi: 10.1016/j.taap.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Ashfaq A., Khursheed N., Fatima S., Anjum Z., Younis K. Application of nanotechnology in food packaging: pros and Cons. J. Agric. Food Res. 2022;7 doi: 10.1016/j.jafr.2022.100270. [DOI] [Google Scholar]
- Aswathanarayan J.B., Vittal R.R. Nanoemulsions and their potential applications in food industry. Front. Sustain. Food Syst. 2019;3 doi: 10.3389/fsufs.2019.00095. [DOI] [Google Scholar]
- Bagheri F., Radi M., Amiri S. Drying conditions highly influence the characteristics of glycerol-plasticized alginate films. Food Hydrocolloids. 2019;90:162–171. doi: 10.1016/j.foodhyd.2018.12.001. [DOI] [Google Scholar]
- Bajpai V.K., Kamle M., Shukla S., Mahato D.K., Chandra P., Hwang S.K., Kumar P., Huh Y.S., Han Y.K. Prospects of using nanotechnology for food preservation, safety, and security. J. Food Drug Anal. 2018;26:1201–1214. doi: 10.1016/j.jfda.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barahona F., Ojea-Jimenez I., Geiss O., Gilliland D., Barrero-Moreno J. Multimethod approach for the detection and characterisation of food-grade synthetic amorphous silica nanoparticles. J. Chromatogr. A. 2016;1432:92–100. doi: 10.1016/j.chroma.2015.12.058. [DOI] [PubMed] [Google Scholar]
- Behzadi S., Serpooshan V., Tao W., Hamaly M.A., Alkawareek M.Y., Dreaden E.C., Brown D., Alkilany A.M., Farokhzad O.C., Mahmoudi M. Cellular uptake of nanoparticles: journey inside the cell. Chem. Soc. Rev. 2017;46:4218–4244. doi: 10.1039/c6cs00636a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berekaa M.M. Nanotechnology in food industry; advances in food processing, packaging and food safety. Int. J. Curr. Microbiol. Appl. Sci. 2015;4:345–357. [Google Scholar]
- Biola-Clier M., Beal D., Caillat S., Libert S., Armand L., Herlin-Boime N., Sauvaigo S., Douki T., Carriere M. Comparison of the DNA damage response in BEAS-2B and A549 cells exposed to titanium dioxide nanoparticles. Mutagenesis. 2017;32:161–172. doi: 10.1093/mutage/gew055. [DOI] [PubMed] [Google Scholar]
- Biswas K., Mohanta Y.K., Kumar V.B., Hashem A., Fathi Abd Allah E., Mohanta D., Mohanta T.K. Nutritional assessment study and role of green silver nanoparticles in shelf-life of coconut endosperm to develop as functional food. Saudi J. Biol. Sci. 2020;27:1280–1288. doi: 10.1016/j.sjbs.2020.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco C., Picó Y. Determining nanomaterials in food. Trac. Trends Anal. Chem. 2011;30:84–99. doi: 10.1016/j.trac.2010.08.010. [DOI] [Google Scholar]
- Bott J., Störmer A., Franz R. A model study into the migration potential of nanoparticles from plastics nanocomposites for food contact. Food Packag. Shelf Life. 2014;2:73–80. doi: 10.1016/j.fpsl.2014.08.001. [DOI] [Google Scholar]
- Broustas C.G., Lieberman H.B. DNA damage response genes and the development of cancer metastasis. Radiat. Res. 2014;181:111–130. doi: 10.1667/RR13515.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno L. Full-field measurement with nanometric accuracy of 3D superficial displacements by digital profile correlation: a powerful tool for mechanics of materials. Mater. Des. 2018;159:170–185. doi: 10.1016/j.matdes.2018.08.052. [DOI] [Google Scholar]
- Bumbudsanpharoke N., Ko S. Nano-food packaging: an overview of market, migration research, and safety regulations. J. Food Sci. 2015;80,(R910–23) doi: 10.1111/1750-3841.12861. [DOI] [PubMed] [Google Scholar]
- Busse M., Muller M., Kimm M.A., Ferstl S., Allner S., Achterhold K., Herzen J., Pfeiffer F. 3D imaging of soft-tissue samples using an X-ray specific staining method and nanoscopic computed tomography. J. Vis. Exp. 2019;1–8 doi: 10.3791/60251. [DOI] [PubMed] [Google Scholar]
- Carbone M., Donia D.T., Sabbatella G., Antiochia R. Silver nanoparticles in polymeric matrices for fresh food packaging. J. King Saud Univ. Sci. 2016;28:273–279. doi: 10.1016/j.jksus.2016.05.004. [DOI] [Google Scholar]
- Chaichi M., Hashemi M., Badii F., Mohammadi A. Preparation and characterization of a novel bionanocomposite edible film based on pectin and crystalline nanocellulose. Carbohydr. Polym. 2017;157:167–175. doi: 10.1016/j.carbpol.2016.09.062. [DOI] [PubMed] [Google Scholar]
- Chellaram C., Murugaboopathi G., John A.A., Sivakumar R., Ganesan S., Krithika S., Priya G. Significance of nanotechnology in food industry. APCBEE Proc. 2014;8:109–113. doi: 10.1016/j.apcbee.2014.03.010. [DOI] [Google Scholar]
- Chen X., Schluesener H.J. Nanosilver: a nanoproduct in medical application. Toxicol. Lett. 2008;176:1–12. doi: 10.1016/j.toxlet.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Chen Q., Zong Z., Gao X., Zhao Y., Wang J. Preparation and characterization of nanostarch-based green hard capsules reinforced by cellulose nanocrystals. Int. J. Biol. Macromol. 2021;167:1241–1247. doi: 10.1016/j.ijbiomac.2020.11.078. [DOI] [PubMed] [Google Scholar]
- Choi J.I., Chae S.J., Kim J.M., Choi J.C., Park S.J., Choi H.J., Bae H., Park H.J. Potential silver nanoparticles migration from commercially available polymeric baby products into food simulants. Food Addit. Contam. Part A Chem Anal Control Expo Risk Assess. 2018;35:996–1005. doi: 10.1080/19440049.2017.1411611. [DOI] [PubMed] [Google Scholar]
- Coles D., Frewer L.J. Nanotechnology applied to European food production – a review of ethical and regulatory issues. Trends Food Sci. Technol. 2013;34:32–43. doi: 10.1016/j.tifs.2013.08.006. [DOI] [Google Scholar]
- Committee, E. S Guidance on the risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain. EFSA J. 2011;9 doi: 10.2903/j.efsa.2011.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowcher D.P., Xu Y., Goodacre R. Portable, quantitative detection of Bacillus bacterial spores using surface-enhanced Raman scattering. Anal. Chem. 2013;85:3297–3302. doi: 10.1021/ac303657k. [DOI] [PubMed] [Google Scholar]
- Cushen M., Kerry J., Morris M., Cruz-Romero M., Cummins E. Migration and exposure assessment of silver from a PVC nanocomposite. Food Chem. 2013;139:389–397. doi: 10.1016/j.foodchem.2013.01.045. [DOI] [PubMed] [Google Scholar]
- Cushen M., Kerry J., Morris M., Cruz-Romero M., Cummins E. Evaluation and simulation of silver and copper nanoparticle migration from polyethylene nanocomposites to food and an associated exposure assessment. J. Agric. Food Chem. 2014;62:1403–1411. doi: 10.1021/jf404038y. [DOI] [PubMed] [Google Scholar]
- De Azeredo H.M. Antimicrobial nanostructures in food packaging. Trends Food Sci. Technol. 2013;30:56–69. [Google Scholar]
- Dekkers S., Krystek P., Peters R.J., Lankveld D.P., Bokkers B.G., Van Hoeven-Arentzen P.H., Bouwmeester H., Oomen A.G. Presence and risks of nanosilica in food products. Nanotoxicology. 2011;5:393–405. doi: 10.3109/17435390.2010.519836. [DOI] [PubMed] [Google Scholar]
- De Moura R.M., Mattoso L.H.C., Zucolotto V. Development of cellulose-based bactericidal nanocomposites containing silver nanoparticles and their use as active food packaging. J. Food Eng. 2012:520–524. doi: 10.1016/j.jfoodeng.2011.10.030. [DOI] [Google Scholar]
- Dimitrijevic M., Karabasil N., Boskovic M., Teodorovic V., Vasilev D., Djordjevic V., Kilibarda N., Cobanovic N. Safety aspects of nanotechnology applications in food packaging. Proc. Food Sci. 2015;5:57–60. doi: 10.1016/j.profoo.2015.09.015. [DOI] [Google Scholar]
- Divya K., Jisha M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2017;16:101–112. doi: 10.1007/s10311-017-0670-y. [DOI] [Google Scholar]
- Durán N., Marcato P.D. Nanobiotechnology perspectives. Role of nanotechnology in the food industry: a review. Int. J. Food Sci. Tech. 2013;48:1127–1134. doi: 10.1111/ijfs.12027. [DOI] [Google Scholar]
- Dwyer J.T., Fulgoni V.L., Clemens R.A., Schmidt D.B., Freedman M.R. Is "processed" a four-letter word? The role of processed foods in achieving dietary guidelines and nutrient recommendations. Adv. Nutr. 2012;3:536–548. doi: 10.3945/an.111.000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Information Council, E Processed food: what is the purpose of food processing? [Online] 2017. https://www.eufic.org/en/food-production/article/processed-food-qa Available.
- Evans S.J., Clift M.J., Singh N., De Oliveira Mallia J., Burgum M., Wills J.W., Wilkinson T.S., Jenkins G.J., Doak S.H. Critical review of the current and future challenges associated with advanced in vitro systems towards the study of nanoparticle (secondary) genotoxicity. Mutagenesis. 2017;32:233–241. doi: 10.1093/mutage/gew054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhoodi M., Mousavi S.M., Sotudeh-Gharebagh R., Emam-Djomeh Z., Oromiehie A. Migration of aluminum and silicon from PET/clay nanocomposite bottles into acidic food simulant. Packag. Technol. Sci. 2014;27:161–168. doi: 10.1002/pts.2017. [DOI] [Google Scholar]
- Faridi Esfanjani A., Assadpour E., Jafari S.M. Improving the bioavailability of phenolic compounds by loading them within lipid-based nanocarriers. Trends Food Sci. Technol. 2018;76:56–66. doi: 10.1016/j.tifs.2018.04.002. [DOI] [Google Scholar]
- Gasa-Falcon A., Acevedo-Fani A., Oms-Oliu G., Odriozola-Serrano I., Martín-Belloso O. Development, physical stability and bioaccessibility of β-carotene-enriched tertiary emulsions. J. Funct.Foods. 2020;64 doi: 10.1016/j.jff.2019.103615. [DOI] [Google Scholar]
- Gilbert J., Cheng C.J., Jones O.G. Vapor barrier properties and mechanical behaviors of composite hydroxypropyl methylcelluose/zein nanoparticle films. Food Biophys. 2017;13:25–36. doi: 10.1007/s11483-017-9508-1. [DOI] [Google Scholar]
- Griffiths P.R., De Haseth J.A. Fourier Transform infrared spectrometry. Anal. Bioanal. Chem. 2008;391:2379–2380. [Google Scholar]
- Grimsrud T.K., Andersen A. Evidence of carcinogenicity in humans of water-soluble nickel salts. J. Occup. Med. Toxicol. 2010;5(7) doi: 10.1186/1745-6673-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamad A.F., Han J.H., Kim B.C., Rather I.A. The intertwine of nanotechnology with the food industry. Saudi J. Biol. Sci. 2018;25:27–30. doi: 10.1016/j.sjbs.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Hwang H.M. Nanotechnology in food science: functionality, applicability, and safety assessment. J. Food Drug Anal. 2016;24:671–681. doi: 10.1016/j.jfda.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Deng H., Hwang H.M. The current application of nanotechnology in food and agriculture. J. Food Drug Anal. 2019;27:1–21. doi: 10.1016/j.jfda.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Chen S., Bing X., Gao C., Wang T., Yuan B. Nanosilver migrated into food-simulating solutions from commercially available food fresh containers. Packag. Technol. Sci. 2011;24:291–297. doi: 10.1002/pts.938. [DOI] [Google Scholar]
- Jain K.K. 2017. Nanomolecular Diagnostics. The Handbook of Nanomedicine. [Google Scholar]
- Jaiswal M., Dudhe R., Sharma P.K. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech. 2015;5:123–127. doi: 10.1007/s13205-014-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarzebski M., Fathordoobady F., Guo Y., Xu M., Singh A., Kitts D.D., Kowalczewski P.L., Jezowski P., Pratap Singh A. Pea protein for hempseed oil nanoemulsion stabilization. Molecules. 2019;24 doi: 10.3390/molecules24234288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeevahan J., Chandrasekaran M. Nanoedible films for food packaging: a review. J. Mater. Sci. 2019;54:12290–12318. doi: 10.1007/s10853-019-03742-y. [DOI] [Google Scholar]
- Kessler R. Engineered nanoparticles in consumer products: understanding a new ingredient. Environ. Health Perspect. 2011;119:a120–a125. doi: 10.1289/ehp.119-a120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil I., Yehye W.A., Etxeberria A.E., Alhadi A.A., Dezfooli S.M., Julkapli N.B.M., Basirun W.J., Seyfoddin A. Nanoantioxidants: recent trends in antioxidant delivery applications. Antioxidants. 2019;9:1–30. doi: 10.3390/antiox9010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N., Izumi H., Morimoto Y. Review of toxicity studies of carbon nanotubes. J. Occup. Health. 2017;59:394–407. doi: 10.1539/joh.17-0089-RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywoszynska K., Witkowska D., Swiatek-Kozlowska J., Szebesczyk A., Kozlowski H. General aspects of metal ions as signaling agents in health and disease. Biomolecules. 2020;10:1–29. doi: 10.3390/biom10101417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Sharma N., Maitra S.S. In vitro and in vivo toxicity assessment of nanoparticles. Int. Nano Lett. 2017;7:243–256. doi: 10.1007/s40089-017-0221-3. [DOI] [Google Scholar]
- Kumari M., Mukherjee A., Chandrasekaran N. Genotoxicity of silver nanoparticles in Allium cepa. Sci. Total Environ. 2009;407:5243–5246. doi: 10.1016/j.scitotenv.2009.06.024. [DOI] [PubMed] [Google Scholar]
- Lin Q.B., Li H., Zhong H.N., Zhao Q., Xiao D.H., Wang Z.W. Migration of Ti from nano-TiO(2)-polyethylene composite packaging into food simulants. Food Addit. Contam. Part A Chem Anal Control Expo Risk Assess. 2014;31:1284–1290. doi: 10.1080/19440049.2014.907505. [DOI] [PubMed] [Google Scholar]
- Lipasek R.A., Ortiz J.C., Taylor L.S., Mauer L.J. Effects of anticaking agents and storage conditions on the moisture sorption, caking, and flowability of deliquescent ingredients. Food Res. Int. 2012;45:369–380. doi: 10.1016/j.foodres.2011.10.037. [DOI] [Google Scholar]
- Liu R., Liu D., Liu Y., Song Y., Wu T., Zhang M. Using soy protein SiOx nanocomposite film coating to extend the shelf life of apple fruit. Int. J. Food Sci. Tech. 2017;52:2018–2030. doi: 10.1111/ijfs.13478. [DOI] [Google Scholar]
- Mallakpour S., Sadaty M.A. Thiamine hydrochloride (vitamin B1) as modifier agent for TiO2 nanoparticles and the optical, mechanical, and thermal properties of poly(vinyl chloride) composite films. RSC Adv. 2016;6:92596–92604. doi: 10.1039/c6ra18597e. [DOI] [Google Scholar]
- Marshall J.L. Springer; US: 1991. Scanning Electron Microscopy and Energy Dispersive X-ray (SEM/EDX) Characterization of Solder Solderability and Reliability; pp. 173–224. 1991. [DOI] [Google Scholar]
- Mcclements D.J. The future of food colloids: next-generation nanoparticle delivery systems. Curr. Opin. Colloid Interface Sci. 2017;28:7–14. doi: 10.1016/j.cocis.2016.12.002. [DOI] [Google Scholar]
- Mcclements D.J., Xiao H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. NPJ Sci. Food. 2017;1(6) doi: 10.1038/s41538-017-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmood T., Ahmed A., Ahmed Z. Food-Grade nanoemulsions for the effective delivery of beta-carotene. Langmuir. 2021;37:3086–3092. doi: 10.1021/acs.langmuir.0c03399. [DOI] [PubMed] [Google Scholar]
- Mendoza-Gómez V., Calderón-Santoyo M., Bautista-Rosales P.U., Ortiz-Basurto R.I., Jimenez-Sánchez D.E., Ragazzo-Sánchez J.A. Antibrowning agents added in polymeric coating applied to avocado fruit slices. Interciencia. 2017;42:812–817. [Google Scholar]
- Mohanta Y.K., Behera S.K. Biosynthesis, characterization and antimicrobial activity of silver nanoparticles by Streptomyces sp. SS2. Bioproc. Biosyst. Eng. 2014;37:2263–2269. doi: 10.1007/s00449-014-1205-6. [DOI] [PubMed] [Google Scholar]
- Mohanta T.K., Mohanta N., Mohanta Y.K., Parida P., Bae H. Genome-wide identification of Calcineurin B-Like (CBL) gene family of plants reveals novel conserved motifs and evolutionary aspects in calcium signaling events. BMC Plant Biol. 2015;15(189) doi: 10.1186/s12870-015-0543-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanta Y.K., Nayak D., Biswas K., Singdevsachan S.K., Abd Allah E.F., Hashem A., Alqarawi A.A., Yadav D., Mohanta T.K. Silver nanoparticles synthesized using wild mushroom show potential antimicrobial activities against food borne pathogens. Molecules. 2018;23 doi: 10.3390/molecules23030655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro C.A., Levy R.B., Claro R.M., De Castro I.R.R., Cannon G. A new classification of foods based on the extent and purpose of their processing. Cad. Saúde Pública. 2010;26:2039–2049. doi: 10.1590/s0102-311x2010001100005. Rio de Janeiro. [DOI] [PubMed] [Google Scholar]
- Naghdi M., Metahni S., Ouarda Y., Brar S.K., Das R.K., Cledon M. Instrumental approach toward understanding nano-pollutants. Nanotech. Environ. Eng. 2017;2:1–17. doi: 10.1007/s41204-017-0015-x. [DOI] [Google Scholar]
- Nakagawa K. In: Nano- and Microencapsulation for Foods. KWAK H.-S., editor. John Wiley & Sons, Ltd; 2014. Nano- and microencapsulation of flavor in food systems. [Google Scholar]
- Naseer B., Srivastava G., Qadri O.S., Faridi S.A., Islam R.U., Younis K. Importance and health hazards of nanoparticles used in the food industry. Nanotechnol. Rev. 2018;7:623–641. doi: 10.1515/ntrev-2018-0076. [DOI] [Google Scholar]
- Nebu J., Sony G. 2017. Raman Spectroscopy. Spectroscopic Methods for Nanomaterials Characterization. [Google Scholar]
- Nejadmansouri M., Hosseini S.M.H., Niakosari M., Yousefi G.H., Golmakani M.T. Physicochemical properties and storage stability of ultrasound-mediated WPI-stabilized fish oil nanoemulsions. Food Hydrocolloids. 2016;61:801–811. doi: 10.1016/j.foodhyd.2016.07.011. [DOI] [Google Scholar]
- Nile S.H., Baskar V., Selvaraj D., Nile A., Xiao J., Kai G. Nanotechnologies in food science: applications, recent trends, and future perspectives. Nano-Micro Lett. 2020;12(45) doi: 10.1007/s40820-020-0383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou L., Song B., Liang H., Liu J., Feng X., Deng B., Sun T., Shao L. Toxicity of graphene-family nanoparticles: a general review of the origins and mechanisms. Part. Fibre Toxicol. 2016;13(57) doi: 10.1186/s12989-016-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes A.J., Asencio C.M., Llabot J.M., Allemandi D.A., Palma S.D. Nanoencapsulation in the food industry: manufacture, applications and characterization. J. Food Bioeng. Nanoprocessing. 2016;1(1):56–79. [Google Scholar]
- Pathakoti K., Manubolu M., Hwang H.M. Nanostructures: current uses and future applications in food science. J. Food Drug Anal. 2017;25:245–253. doi: 10.1016/j.jfda.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan N., Singh S., Ojha N., Shrivastava A., Barla A., Rai V., Bose S. Facets of nanotechnology as seen in food processing, packaging, and preservation industry. BioMed Res. Int. 2015 doi: 10.1155/2015/365672. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primozic M., Knez Z., Leitgeb M. (Bio)nanotechnology in food science-food packaging. Nanomaterials. 2021;11:2–29. doi: 10.3390/nano11020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Products, I. R. A. 2009.
- Qadri O.S., Younis K., Srivastava G., Srivastava A.K. Nanotechnology in packaging of fresh fruits and vegetables. Emerg. Postharvest Treat. Fruit Veg. 2018:147–166. [Google Scholar]
- Ranjan S., Dasgupta N., Chakraborty A.R., Melvin Samuel S., Ramalingam C., Shanker R., Kumar A. Nanoscience and nanotechnologies in food industries: opportunities and research trends. J. Nanoparticle Res. 2014;16(2464) doi: 10.1007/s11051-014-2464-5. [DOI] [Google Scholar]
- Reifarth M., Hoeppener S., Schubert U.S. Uptake and intracellular fate of engineered nanoparticles in mammalian cells: capabilities and limitations of transmission electron microscopy-polymer-based nanoparticles. Adv. Mater. 2018;30 doi: 10.1002/adma.201703704. [DOI] [PubMed] [Google Scholar]
- Roberto M.M., Christofoletti C.A. How to assess nanomaterial toxicity? Environ. Human Health Approch. 2019 doi: 10.5772/intechopen.88970. [DOI] [Google Scholar]
- Rodriguez-Saona L.E. 2011. Use of FTIR for Rapid Authentication and Detection of Adulteration of Food. [DOI] [PubMed] [Google Scholar]
- Sahu S.C., Hayes A.W. Toxicity of nanomaterials found in human environment. Toxicol. Res. Appl. 2017;1:1–13. doi: 10.1177/2397847317726352. [DOI] [Google Scholar]
- Salameh A.K., Taylor L.S. Deliquescence-induced caking in binary powder blends. Pharmaceut. Dev. Technol. 2006;11:453–464. doi: 10.1080/10837450600939057. [DOI] [PubMed] [Google Scholar]
- Sallak N., Motallebi Moghanjoughi A., Ataee M., Anvar A., Golestan L. Antimicrobial biodegradable film based on corn starch/Satureja khuzestanicaessential oil/Ag-TiO2nanocomposites. Nanotechnology. 2021;32 doi: 10.1088/1361-6528/ac0a15. [DOI] [PubMed] [Google Scholar]
- Samal D. Use of nanotechnology in food industry: a review. Int. J. Environ. Agric. Biotech. 2017;2:2270–2278. doi: 10.22161/ijeab/2.4.90. [DOI] [Google Scholar]
- Sánchez-González L., Pastor C., Vargas M., Chiralt A., González-Martínez C., Cháfer M. Effect of hydroxypropylmethylcellulose and chitosan coatings with and without bergamot essential oil on quality and safety of cold-stored grapes. Postharvest Biol. Technol. 2011;60:57–63. doi: 10.1016/j.postharvbio.2010.11.004. [DOI] [Google Scholar]
- Shafique M., Luo X. Nanotechnology in transportation vehicles: an overview of its applications, environmental, health and safety concerns. Materials. 2019;12(2493) doi: 10.3390/ma12152493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M.A., Bhat M.A., Davim J.P. Nanotechnology Applications for Improvements in Energy Efficiency and Environmental Management. IGI Global; 2015. [Google Scholar]
- Shang Y., Hasan M.K., Ahammed G.J., Li M., Yin H., Zhou J. Applications of nanotechnology in plant growth and crop protection: a review. Molecules. 2019;24(2558) doi: 10.3390/molecules24142558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V.K., Siskova K.M., Zboril R., Gardea-Torresdey J.L. Organic-coated silver nanoparticles in biological and environmental conditions: fate, stability and toxicity. Adv. Colloid Interface Sci. 2014;204:15–34. doi: 10.1016/j.cis.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Sharma S., Barkauskaite S., Jaiswal A.K., Jaiswal S. Essential oils as additives in active food packaging. Food Chem. 2021;343(128403) doi: 10.1016/j.foodchem.2020.128403. [DOI] [PubMed] [Google Scholar]
- Singh N., Nelson B.C., Scanlan L.D., Coskun E., Jaruga P., Doak S.H. Exposure to engineered nanomaterials: impact on DNA repair pathways. Int. J. Mol. Sci. 2017;18(1515) doi: 10.3390/ijms18071515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T., Shukla S., Kumar P., Wahla V., Bajpai V.K. Application of nanotechnology in food science: perception and overview. Front. Microbiol. 2017;8(1501) doi: 10.3389/fmicb.2017.01501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhan A., Muthukumarappan K., Wei L., Van Den Top T., Zhou R. Development of an activated carbon-based nanocomposite film with antibacterial property for smart food packaging. Mater. Today Commun. 2020;23 doi: 10.1016/j.mtcomm.2020.101124. [DOI] [Google Scholar]
- Sodano V., Gorgitano M.T., Quaglietta M., Verneau F. Regulating food nanotechnologies in the European Union: open issues and political challenges. Trends Food Sci. Technol. 2016;54:216–226. doi: 10.1016/j.tifs.2016.05.022. [DOI] [Google Scholar]
- Störmer A., Bott J., Kemmer D., Franz R. Critical review of the migration potential of nanoparticles in food contact plastics. Trends Food Sci. Technol. 2017;63:39–50. doi: 10.1016/j.tifs.2017.01.011. [DOI] [Google Scholar]
- Tai K., Liu F., He X., Ma P., Mao L., Gao Y., Yuan F. The effect of sterol derivatives on properties of soybean and egg yolk lecithin liposomes: stability, structure and membrane characteristics. Food Res. Int. 2018;109:24–34. doi: 10.1016/j.foodres.2018.04.014. [DOI] [PubMed] [Google Scholar]
- Thuong N.T., Dung T.A., Yusof N.H., Kawahara S. Controlling the size of silica nanoparticles in filler nanomatrix structure of natural rubber. Polymer. 2020;195 doi: 10.1016/j.polymer.2020.122444. [DOI] [Google Scholar]
- Toker R.D., Kayaman-Apohan N., Kahraman M.V. UV-curable nano-silver containing polyurethane based organic–inorganic hybrid coatings. Prog. Org. Coating. 2013;76:1243–1250. doi: 10.1016/j.porgcoat.2013.03.023. [DOI] [Google Scholar]
- Tosi M.M., Ramos A.P., Esposto B.S., Jafari S.M. Dynamic light scattering (DLS) of nanoencapsulated food ingredients. Char. Nanoencapsulated Food Ingredients. 2020 [Google Scholar]
- Tran V., Yiannaka A., Giannakas K. Market potential and economic impacts of food nanotechnology innovations. J. Know. Econ. 2017;10:776–811. doi: 10.1007/s13132-017-0494-9. [DOI] [Google Scholar]
- Uboldi C., Giudetti G., Broggi F., Gilliland D., Ponti J., Rossi F. Amorphous silica nanoparticles do not induce cytotoxicity, cell transformation or genotoxicity in Balb/3T3 mouse fibroblasts. Mutat. Res. 2012;745:11–20. doi: 10.1016/j.mrgentox.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Venkateshaiah A., Padil V.V.T., Nagalakshmaiah M., Waclawek S., Cernik M., Varma R.S. Microscopic techniques for the analysis of micro and nanostructures of biopolymers and their derivatives. Polymers. 2020;12(512) doi: 10.3390/polym12030512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner C. FOOD PACKAGING & HEALTH; 2013. Nanomaterials.https://www.foodpackagingforum.org/food-packaging-health/nanomaterials [Google Scholar]
- Weaver C.M., Dwyer J., Fulgoni V.L., King J.C., Leveille G.A., Macdonald R.S., Ordovas J., Schnakenberg D. Processed foods: contributions to nutrition. Am. J. Clin. Nutr. 2014;99:1525–1542. doi: 10.3945/ajcn.114.089284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y., Xu Z., Liu Y., Corke H., Sui Z. Investigation of food microstructure and texture using atomic force microscopy: a review. Compr. Rev. Food Sci. Food Saf. 2020;19:2357–2379. doi: 10.1111/1541-4337.12605. [DOI] [PubMed] [Google Scholar]
- Winkler H.C., Kornprobst J., Wick P., Von Moos, M L., Trantakis I., Schraner E.M., Bathke B., Hochrein H., Suter M., Naegeli H. MyD88-dependent pro-interleukin-1beta induction in dendritic cells exposed to food-grade synthetic amorphous silica. Part. Fibre Toxicol. 2017;14(21) doi: 10.1186/s12989-017-0202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Yin J.J., Wamer W.G., Zeng M., Lo Y.M. Reactive oxygen species-related activities of nano-iron metal and nano-iron oxides. J. Food Drug Anal. 2014;22:86–94. doi: 10.1016/j.jfda.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S.K. Realizing the potential of nanotechnology for agriculture and food technology. J. Tissue Sci. Eng. 2017;8 doi: 10.4172/2157-7552.1000195. [DOI] [Google Scholar]
- Yang C., Zhu B., Wang J., Qin Y. Structural changes and nano-TiO2 migration of poly(lactic acid)-based food packaging film contacting with ethanol as food simulant. Int. J. Biol. Macromol. 2019;139:85–93. doi: 10.1016/j.ijbiomac.2019.07.151. [DOI] [PubMed] [Google Scholar]
- Yin P., Lin Q., Duan Y. Applications of Raman spectroscopy in two-dimensional materials. J. Innov. Opt. Health Sci. 2020;13 doi: 10.1142/s1793545820300104. [DOI] [Google Scholar]
- Youssef A.M., El-Sayed S.M. Bionanocomposites materials for food packaging applications: concepts and future outlook. Carbohydr. Polym. 2018;193:19–27. doi: 10.1016/j.carbpol.2018.03.088. [DOI] [PubMed] [Google Scholar]
- Yu H., Park J.-Y., Kwon C.W., Hong S.-C., Park K.-M., Chang P.-S. An overview of nanotechnology in food science: preparative methods, practical applications, and safety. J. Chem. 2018:1–10. doi: 10.1155/2018/5427978. 2018. [DOI] [Google Scholar]
- Zhang Z. Chapter 1. Raman spectroscopic sensing in food safety and quality analysis. Sens. Tech. Food Saf. Qual. Control. 2017 [Google Scholar]