Abstract
Objective
To evaluate NKTR-358, a polyethylene glycol-interleukin-2 conjugate composition designed to selectively induce regulatory T cells (Tregs), in first-in-human studies.
Methods
Healthy volunteers and patients with systemic lupus erythematosus (SLE) received single- or multiple-dose (biweekly) NKTR-358 or placebo in two sequential, randomized, phase 1 studies (single ascending dose [SAD; NCT04133116] and multiple ascending dose [MAD; NCT03556007]). Primary objectives were safety and tolerability; secondary objectives included pharmacokinetics (PK) and immune effects of NKTR-358; exploratory objectives included effects on SLE disease activity.
Results
There were eight ascending dose cohorts in the SAD study (0.3–28.0 μg/kg: n = 76; placebo: n = 24) and four in the MAD study (3–24.0 μg/kg: n = 36; placebo: n = 12). Most adverse events (AEs) were grade 1–2 injection-site reactions, with no treatment‐related serious or severe AEs, or deaths. PK data showed dose proportionality and prolonged exposure (mean half-life: 7.4–12.9 days). Dose-dependent, selective, and sustained increases in percentages and absolute numbers of total CD4+ Tregs and CD25bright Tregs were observed, with no significant changes in conventional CD4+ and CD8+ T cells, and low-level increases in natural killer cells. At the highest doses tested, administration of NKTR-358 resulted in a 12–17-fold increase in CD25bright Tregs over baseline that was sustained for 20–30 days.
Conclusion
NKTR-358 was well tolerated, had a suitable PK profile for biweekly dosing, and led to marked and selective dose-dependent increases in CD25bright Tregs, with no significant changes in conventional T cells. These results provide strong support for further testing in SLE and other inflammatory diseases.
Keywords: Interleukin-2, IL-2 receptor, Systemic lupus erythematosus, Regulatory T cells, Autoimmune disease
Abbreviations
- AEs
adverse events
- AUC
area under the curve
- BMI
body mass index
- CLASI-A
cutaneous lupus erythematosus disease area and severity index–activity
- Cmax
maximum observed concentration
- IL-2
interleukin-2
- IL-5
interleukin-5
- MAD
multiple ascending dose
- MedDRA
Medical Dictionary for Regulatory Activities
- NCI-CTCAE
National Cancer Institute Common Terminology Criteria for Adverse Events
- NK
natural killer
- PD
pharmacodynamics
- PEG
polyethylene glycol
- PK
pharmacokinetics
- rhIL-2
recombinant human IL-2
- SAD
single ascending dose
- SD
standard deviation
- SEM
standard error of the mean
- SLE
systemic lupus erythematosus
- SLEDAI
SLE disease activity index
- Tcons
conventional T cell subsets
- TEAE
treatment-emergent adverse event
- Teffs
T effector cells
- Tregs
regulatory T cells
- TSDR
Treg-specific demethylation region
1. Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease with multi-organ involvement and diverse clinical manifestations. Current treatment options primarily include corticosteroids and immunosuppressants, which have been shown to provide partial clinical benefit but are associated with substantial toxicity and morbidity. Recent treatment advances have been limited, and the focus of most therapeutics in development has been on inhibition of specific immune pathways potentially involved in disease pathogenesis. There remains a high unmet need for effective and safer treatment options [1,2].
SLE is characterized by a loss of immune self-tolerance, the production of pathogenic autoantibodies, and the accrual of tissue damage [1,3]. Impaired interleukin-2 (IL-2) production and dysfunction of regulatory T cells (Tregs) have been identified as key immunologic defects leading to the breakdown of immune self-tolerance in SLE [4,5]. IL-2 is a cytokine that has an essential role in preserving immune homeostasis and maintaining a balance between Tregs and T effector cells (Teffs). At higher levels, IL-2 stimulates the proliferation, differentiation, and function of Teffs and natural killer (NK) cells, which has resulted in it being used therapeutically at high doses to promote antitumor immune responses [5,6]. Conversely, another important role for IL-2 is to expand and stimulate Tregs, which control immune system over-activation and prevent autoimmunity [5,7]. The contrasting effects of IL-2 on T cell subsets are due to differences in IL-2 receptor biology between these T cell subsets. Tregs are induced at low IL-2 concentrations due to constitutive expression of the high-affinity IL-2Rαβγ complex, whereas Teffs and NK cells mostly express the intermediate-affinity IL-2Rβγ chains that require higher IL-2 concentrations for activation [5,8,9]. In light of these differential effects, low-dose recombinant human IL-2 (rhIL-2) therapy has been evaluated for its ability to selectively induce Tregs and thereby treat autoimmune diseases, including SLE [4,[10], [11], [12], [13]]. Despite promising early results, low-dose rhIL-2 treatment is limited by a narrow therapeutic window for Treg selectivity and a short half-life, necessitating frequent administration [14].
NKTR-358 (also known as LY3471851) is a composition of polyethylene glycol (PEG) conjugates of rhIL-2 in which the rhIL-2 polypeptide (the same amino acid sequence as aldesleukin) has been stably covalently attached to PEG chains [15]. NKTR-358 has reduced binding affinity for IL-2Rβ compared with rhIL-2, resulting in a different binding selectivity for cells expressing IL-2Rβ versus IL-2Rα [15]. This design property renders Tregs with high-affinity (IL-2Rαβγ) receptors more sensitive than Teffs to activation by NKTR-358. In preclinical models, NKTR-358 demonstrated a markedly prolonged half-life compared with rhIL-2 and a sustained and selective proliferation and activation of Tregs with minimal effects on Teffs and other conventional (non-Treg) CD4+ and CD8+ T cell subsets (Tcons) [15]. Furthermore, repeated dosing of NKTR-358 in cynomolgus monkeys led to cyclical increases in Tregs with no loss of activity over the 6-month treatment period. NKTR-358 administration also suppressed lupus-like disease in a murine model of SLE [15].
Here, we report the results of two randomized, phase 1 studies – a single ascending dose (SAD) study in healthy volunteers and a multiple ascending dose (MAD) study in patients with SLE – each assessing the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of NKTR-358 versus placebo.
2. Material & methods
2.1. Study designs and treatment
The SAD study (NCT04133116) was a first-in-human, randomized, double-blind, placebo-controlled, phase 1 study in healthy volunteers conducted in a single center in the US. The study enrolled 100 participants in eight ascending dose cohorts. Each cohort included nine participants randomized to NKTR-358 and three to placebo. Participants received a single dose of NKTR-358 or placebo subcutaneously on Day 1 of each dosing cohort, with a starting dose of 0.3 μg/kg. A sentinel dosing approach was applied at each dose level whereby two participants were randomized to receive NKTR-358 or placebo and monitored for possible side effects for at least 7 days before the remainder of the cohort was randomized. Dose escalation was informed by safety and tolerability, cytokine levels, the expected NKTR-358 exposure based on PK simulations, as well as Treg and Tcon responses. Each cohort was followed for 50 days. A protocol amendment allowed four additional participants to be enrolled to receive open-label NKTR-358 at 20 μg/kg to evaluate additional PD parameters following safety review.
The MAD study (NCT03556007) was a randomized, double-blind, placebo-controlled, phase 1 study in patients with SLE conducted at 11 centers in the US. The study randomized 48 patients to four sequential ascending dose cohorts, with nine patients receiving NKTR-358 and three receiving placebo per cohort. Patients received three doses of NKTR-358 or placebo subcutaneously on a biweekly schedule, with doses given on Days 1, 15, and 29 at a starting dose of 3 μg/kg. Subsequent NKTR-358 dose levels were based on safety findings, as well as Treg, Tcon, and NK cell responses, cytokine levels, and available PK data. Patients were followed for 50 days after the last dose.
Study drug assignments in both studies occurred in a double-blinded fashion (unless otherwise stated) in accordance with a computer-generated randomization scheme prepared by the study sponsor, with participants, investigators, and study site personnel blinded to treatment assignment.
The studies were conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, and applicable local laws. Approvals were obtained from the appropriate institutional review boards. All participants provided written informed consent prior to study entry.
2.2. Study populations
The SAD study enrolled healthy adults aged 18–55 years. Participants were excluded if they had previous or concurrent immune-mediated disease, other relevant medical conditions, or were taking confounding medications. Additional eligibility criteria are provided in the Supplementary Materials and Methods.
In the MAD study, eligible patients had been diagnosed with SLE for at least 6 months, meeting at least four of the 11 American College of Rheumatology criteria for SLE (at least one of which must have been: positive antinuclear antibody test titer of ≥1:80 at screening; above normal anti-double-stranded DNA antibodies at screening; or above normal anti-Smith antibody at screening). Patients were required to have minimal-to-moderate SLE disease activity, and to be on a stable dose of concomitant medications for ≥8 weeks prior to study start. Patients with active lupus nephritis or central nervous system disease were excluded. If a patient was taking prednisone, the dose had to be ≤10 mg/day for a minimum of 8 weeks prior to screening and stable for a minimum of 2 weeks. Additional eligibility criteria are provided in the Supplementary Materials and Methods.
2.3. Outcomes
The primary objective was to evaluate the safety and tolerability of NKTR-358 administered as a single subcutaneous dose in healthy volunteers (SAD study) and as multiple doses in patients with SLE (MAD study). Secondary objectives were to characterize the PK of NKTR-358 following single and multiple doses, as well as the effects of NKTR-358 on the time course and extent of changes in the number and/or activity of circulating Tregs and Treg subpopulations, Tcon subsets, and major non-T cell subsets. Safety laboratories, blood cytokine levels, changes in gene expression, and anti-drug antibodies were also quantified. Disease activity was assessed as an exploratory endpoint in the MAD study, as measured by the SLE disease activity index (SLEDAI), cutaneous lupus erythematosus disease area and severity index–activity (CLASI-A), and joint counts.
2.4. Assessments
Safety and tolerability were assessed by monitoring adverse events (AEs), vital signs, physical examination, electrocardiography, and laboratory findings. AEs were collected throughout the study until completion (50 days after last dose) or at early termination visits. AEs were coded based on Medical Dictionary for Regulatory Activities (MedDRA) version 20.0 in the SAD study and version 21.0 in the MAD study and assessed for severity using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03.
Serum samples collected at pre-dose, during the study, and at the final study visit or early termination visit were used for the immunogenicity (anti-drug antibodies) testing.
Plasma PK sampling schedules, assays, and PK parameter calculation methods are summarized in the Supplementary Materials and Methods.
Immunophenotyping was performed by multicolor flow cytometry to quantify immune cell subsets using whole blood collected at pre-dose and throughout the study, until completion (50 days after the last dose) or the early termination visit. PD parameters included the absolute numbers and percentages of Tregs, Tcons, and NK cells, and percentage and expression level of functional markers of Treg activation (Ki67, ICOS, Helios, and CTLA4). Total Tregs were identified as CD4+FoxP3+CD25+ cells; CD25bright Tregs have been previously identified as a Treg subpopulation with high activity and suppressive capacity [16] and were identified as the CD4+FoxP3+CD25+ subpopulation with the highest CD25 expression (see Supplementary Fig. 1 and Supplementary Materials and Methods). CD4+ T cells were CD3+CD4+ Tcons; CD8+ T cells were CD3+CD8+ Tcons; and total NK cells were CD3–CD56+ cells, with NKbright (CD3–CD56+++CD16– cells), and NKdim (CD3–CD56+CD16+) subsets also identified in the MAD study (see Supplementary Materials and Methods).
The methylation status of the Treg-specific demethylation region (TSDR) of the FoxP3 gene was evaluated using the Epiontis ID quantitative polymerase chain reaction-based assay using whole blood collected at multiple time points from pre-dose through Day 50 post-dose [17].
2.5. Statistical analysis
All analyses were descriptive in nature and were performed using SAS® Version 9.4 or later. No formal sample size calculations were performed. All participants who received at least one dose of study drug (NKTR-358 or placebo) were included in the safety analysis. Those who also had adequate data were included in the PK and PD analyses.
The relationship between Tregs identified by flow cytometry and by epigenetic analysis was assessed using Spearman's correlation coefficient using GraphPad Prism software v8.4.2 (GraphPad; San Diego, CA).
3. Results
3.1. Disposition and baseline characteristics
In the SAD study, 100 participants were enrolled and randomized to receive a single dose of NKTR-358 in eight ascending dose cohorts ranging from 0.3 μg/kg to 28.0 μg/kg (n = 76) or placebo (n = 24; Supplementary Fig. 2A). Seven participants who received NKTR-358 and two who received placebo discontinued treatment either because they were lost to follow-up (n = 6) or withdrew from the study (n = 3). None of the participants discontinued due to an AE. All participants were included in the safety and PD analyses, and 70 of 76 participants who received NKTR-358 had adequate data points for the PK analysis. All participants received the full dose of study drug except one who was randomized to receive 13.5 μg/kg but only received 6.75 μg/kg due to syringe malfunction. Dose escalation was stopped based on a non-clinical no observed adverse effect exposure level ceiling that was not to be exceeded.
The MAD study enrolled 48 patients with SLE with minimal-to-moderate disease activity who were randomized to receive NKTR-358 in four ascending dose cohorts ranging from 3 μg/kg to 24 μg/kg (n = 36) or placebo (n = 12; Supplementary Fig. 2B). Three patients who received NKTR-358 discontinued treatment because they withdrew consent (n = 2) or because of an AE (n = 1). One patient who withdrew consent for treatment also withdrew from the study. All patients received at least one dose of NKTR-358 or placebo and were included in the safety and PD analyses, while one patient of the 36 who received NKTR-358 had no measurable drug concentration and was excluded from the PK analysis.
Within each study, no meaningful differences were observed between the NKTR-358 and placebo groups with respect to demographics and baseline characteristics (Table 1). Across studies, more participants were female in the MAD study (96%) versus the SAD study (40%), reflecting the SLE disease population.
Table 1.
Demographics and baseline disease characteristics.
| SAD (healthy volunteers) |
MAD (patients with SLE) |
|||
|---|---|---|---|---|
| NKTR-358 (n = 76) | Placebo (n = 24) | NKTR-358 (n = 36) | Placebo (n = 12) | |
| Age, years, mean (SD) | 33.3 (9.6) | 34.0 (13.0) | 47.2 (12.5) | 47.8 (8.3) |
| Sex, n (%) | ||||
| Male | 48 (63) | 12 (50) | 2 (6) | 0 |
| Female | 28 (37) | 12 (50) | 34 (94) | 12 (100) |
| Race, n (%) | ||||
| White | 40 (53) | 12 (50) | 24 (67) | 8 (67) |
| Black or African American | 33 (43) | 11 (46) | 10 (28) | 2 (17) |
| Asian | 0 | 1 (4) | 1 (3) | 1 (8) |
| Multiple races | 3 (4) | 0 | 0 | 1 (8) |
| Not reported | 0 | 0 | 1 (3) | 0 |
| BMI, kg/m2, mean (SD) | 26.1 (3.7) | 26.6 (2.9) | 26.9 (3.0) | 26.7 (4.6) |
| Concomitant medication, n (%) | 26 (34) | 8 (33) | 36 (100) | 12 (100) |
| Medication of interest | ||||
| Hydroxychloroquine | 0 | 0 | 12 (33) | 4 (33) |
| Hydroxychloroquine sulfate | 0 | 0 | 12 (33) | 2 (17) |
| Prednisone | 0 | 0 | 11 (31) | 1 (8) |
| Azathioprine | 0 | 0 | 4 (11) | 0 |
| Methotrexate | 0 | 0 | 2 (6) | 0 |
| Mycophenolate | 0 | 0 | 1 (3) | 2 (17) |
| Duration of SLE, months, mean (SD) | NA | NA | 9.5 (8.9) | 14.3 (9.7) |
| SLEDAI-2K score, mean (SD, range) | NA | NA | 6.0 (2.8, 0–10) | 5.2 (2.2, 2–10) |
| CLASI-A activity score, mean (SD, range) | NA | NA | 4.1 (4.7, 0–22) | 2.7 (3.2, 0–9) |
| Joint counts, mean (SD, range) | ||||
| Swelling | NA | NA | 6.0 (6.9, 0–24) | 2.8 (4.5, 0–13) |
| Tenderness | NA | NA | 10.1 (9.6, 0–28) | 6.8 (3.9, 1–13) |
Abbreviations: BMI: body mass index; CLASI-A: cutaneous lupus erythematosus disease area and severity index–activity; MAD: multiple ascending dose; NA: not applicable; SAD: single ascending dose; SD: standard deviation; SLE: systemic lupus erythematosus; SLEDAI: SLE disease activity index.
3.2. Safety
Overall, 83% and 97% of participants who received single and multiple doses of NKTR-358 experienced a treatment-emergent AE (TEAE) versus 25% and 58% with placebo, respectively (Table 2). All TEAEs, except one in each study, were mild or moderate (grades 1–2) in severity. In participants receiving NKTR-358, TEAEs were primarily injection site reactions, most frequently injection site erythema. Injection site reactions usually began 1–2 days after injection. One participant who received a single dose of NKTR-358 at 28 μg/kg developed grade 1 pyrexia, decreased appetite, nausea, vomiting, diarrhea, tachycardia, and myalgia that were attributed to elevated cytokine levels (cytokine-release syndrome). Clinical manifestations started 2 days after administration of drug and resolved without treatment within 4 days. One patient in the MAD study (NKTR-358 at 24 μg/kg) had two treatment-related TEAEs of grade 1 flu-like symptoms following the second and third infusion, which resolved without treatment after 2 days. Although clinical manifestations were labeled as cytokine-release syndrome by the investigator, no clinically relevant hematology, chemistry, or elevated cytokine values were associated with either of the two episodes. Two serious TEAEs were reported following treatment with NKTR-358 (grade 4 attempted suicide at Day 45 in the SAD study and grade 3 migraine on Day 50 in the MAD study), neither of which were considered related to study drug. One patient had treatment (NKTR-358 at 24 μg/kg) discontinued due to moderate hypereosinophilia in the MAD study. Increased eosinophil levels resolved without treatment, and there were no associated clinical manifestations. No deaths were reported in either study.
Table 2.
Summary of treatment-emergent adverse events in the single ascending dose and multiple ascending dose studies.
| n (%) | SAD (healthy volunteers) |
MAD (patients with SLE) |
||
|---|---|---|---|---|
| NKTR-358 (n = 76) | Placebo (n = 24) | NKTR-358 (n = 36) | Placebo (n = 12) | |
| Any TEAEs | 63 (83) | 6 (25) | 35 (97) | 7 (58) |
| Grade 3–4 | 1 (1)a | 0 | 1 (3)b | 0 |
| Grade 5 | 0 | 0 | 0 | 0 |
| Any treatment-related TEAEs | 55 (72) | 2 (8) | 33 (92) | 1 (8) |
| Serious TEAEs | 1 (1)a | 0 | 1 (3)b | 0 |
| TEAEs leading to discontinuation | 0 | 0 | 1 (3)c | 0 |
| Injection site-related TEAEs | ||||
| Injection site erythema | 50 (66) | 0 | 28 (78) | 0 |
| Injection site pain | 34 (45) | 0 | 8 (22) | 0 |
| Injection site swelling | 17 (22) | 0 | 2 (6) | 0 |
| Injection site pruritus | 11 (14) | 0 | 22 (61) | 0 |
| Injection site induration | 5 (7) | 0 | 2 (6) | 0 |
| Injection site reaction | 0 | 0 | 14 (39) | 0 |
| Injection site warmth | 0 | 0 | 5 (14) | 0 |
| Injection site edema | 0 | 0 | 3 (8) | 0 |
| Most common TEAEs | ||||
| Headache | 9 (12) | 5 (21) | 1 (3) | 0 |
| Back pain | 4 (5) | 1 (4) | 0 | 1 (8) |
| Urinary tract infection | 1 (1) | 0 | 4 (11) | 2 (17) |
| Nasopharyngitis | 0 | 0 | 3 (8) | 0 |
| Eosinophilia | 0 | 0 | 3 (8) | 0 |
| Insomnia | 0 | 0 | 3 (8) | 0 |
| Fatigue | 0 | 0 | 2 (6) | 0 |
| Maculo-papular rash | 0 | 0 | 2 (6) | 0 |
| Sinusitis | 0 | 0 | 2 (6) | 0 |
Abbreviations: MAD: multiple ascending dose; SAD: single ascending dose; SLE: systemic lupus erythematosus; TEAE: treatment-emergent adverse event.
Grade 4 attempted suicide not considered related to study drug.
Grade 3 migraine not considered related to study drug.
Treatment-related grade 2 eosinophilia.
In the SAD study, no clinically meaningful changes from baseline were observed for clinical laboratory values, and no laboratory abnormality was reported as a TEAE. In the MAD study, the only observed laboratory abnormalities were shifts from normal baseline to high post-baseline eosinophil values across NKTR-358 dose cohorts; three patients experienced hypereosinophilia (eosinophil count >1.5 × 109 cells/L) at the 12 or 24 μg/kg doses. All events were mild or moderate in severity, and each of these events started after the second dose of NKTR-358 on Day 21 or 22, was not associated with clinical manifestations, and resolved after the last dose of study drug. No clinically significant vital sign or electrocardiogram abnormalities were observed in either study. No NKTR-358 anti-drug antibodies were detected at any dose or any time point in either study.
3.3. Pharmacokinetics
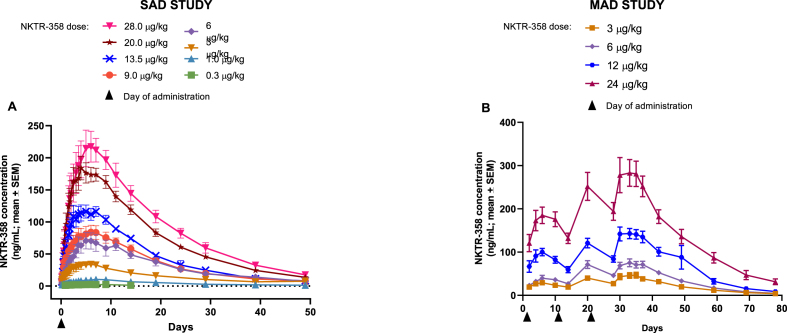
NKTR-358 showed approximately linear PK, with maximum observed concentration (Cmax) and area under the curve (AUC) increasing proportionally with dose (Fig. 1). NKTR-358 reached maximum plasma concentrations approximately 5–7 days post-dose, and subsequently declined with a mean half-life of 7.4–12.9 days (Supplementary Tables 1 and 2).
Fig. 1.
Mean concentration–time profiles of NKTR-358 across dose cohorts after A) a single dose in the SAD study in healthy volunteers and B) biweekly multiple doses in the MAD study in patients with SLE.
Abbreviations: MAD: multiple ascending dose; SAD: single ascending dose; SEM: standard error of the mean.
3.4. Pharmacodynamics
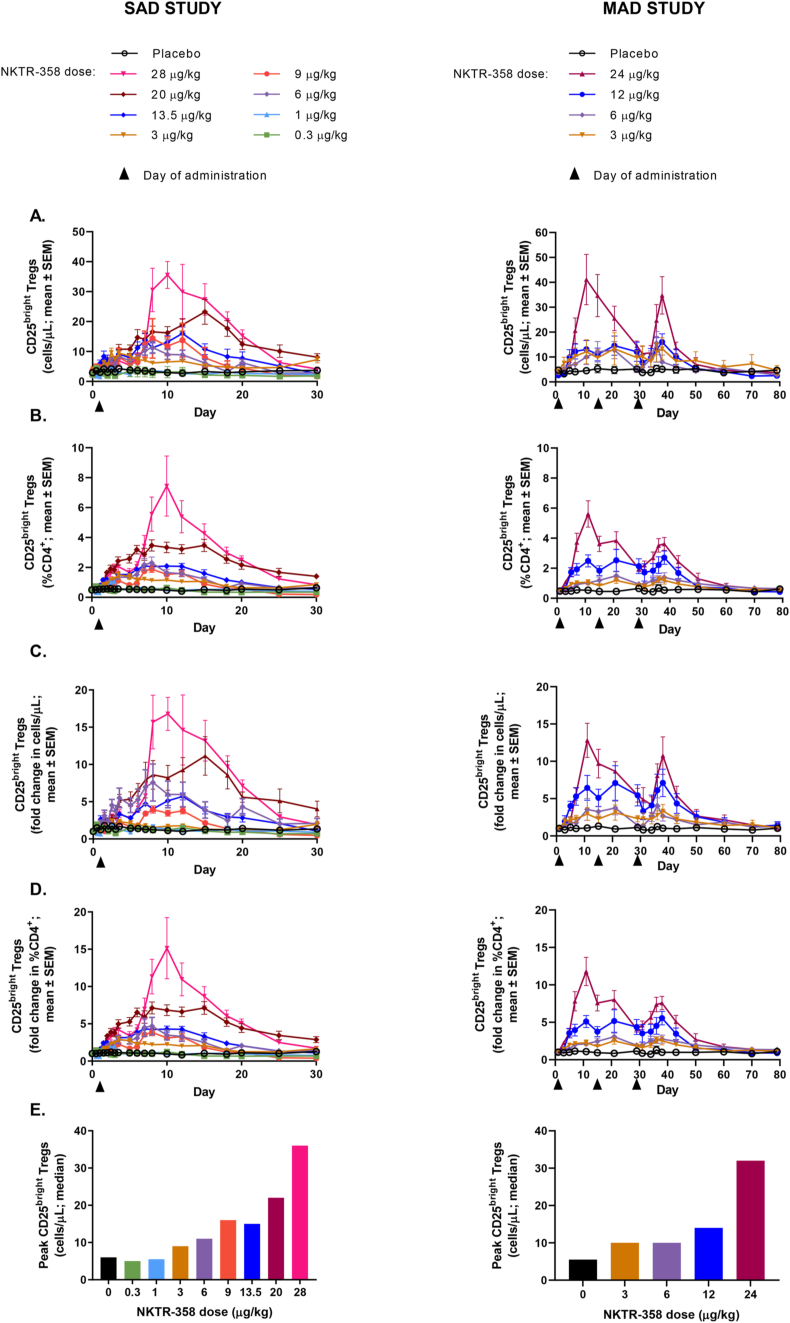
At doses ≥3 μg/kg, NKTR-358 led to a dose-dependent and sustained increase in the absolute numbers and percentages of total Tregs (CD4+FoxP3+CD25+ cells) (Supplementary Fig. 3A and B) and CD25bright Tregs (CD4+FoxP3+CD25bright cells) in both healthy volunteers and patients with SLE (Fig. 2A-D). While there was greater variability in baseline absolute numbers of Tregs in patients with SLE compared to healthy volunteers, the dose-dependent increase in the frequency of CD25bright Tregs as a percentage of CD4+ T cells was clearly observed in both patients with SLE and healthy participants. At the highest doses tested in both studies, there was a maximum 17-fold (SAD) and 12-fold (MAD) mean peak increase over baseline in the numbers of CD25bright Tregs, and levels peaked around Day 10 and remained above baseline for 20–30 days following administration of the last dose. In the SLE population, the magnitude of the response appeared modestly attenuated in several patients with repeat dosing at 24 μg/kg, but the mean CD25bright Treg population remained above baseline levels through approximately Day 60. Importantly, following the first dose of NKTR-358, a similar dose-dependent induction of CD25bright Tregs was seen in both healthy volunteers and patients with SLE (Fig. 2E). Disease activity and medications did not have significant impacts on the observed response, including the degree of Treg induction (data not shown).
Fig. 2.
Changes from baseline in the numbers and percentages of Tregs in peripheral blood after a single dose of NKTR-358 in the SAD study and after multiple doses of NKTR-358 in the MAD study. A) absolute number of CD25bright Tregs; B) percentage of CD25bright Tregs in total CD4+ T cells; C) fold-change in the absolute number of CD25bright Tregs; D) fold-change in percentage of CD25bright Tregs in total CD4+ T cells; E) peak level of post-baseline CD25bright Tregs after 1 dose of NKTR-358.
Abbreviations: MAD: multiple ascending dose: SAD, single ascending dose: SEM, standard error of the mean.
Using the methylation status of the FoxP3 gene as an additional method for identifying NKTR-358-induced Tregs, we observed a significant correlation between NKTR-358-induced Tregs identified by flow cytometry (CD4+FoxP3+CD25+ cells) and by epigenetic analysis (percentage demethylation of FoxP3 TSDR) after treatment with 28 μg/kg NKTR-358 (SAD) and 24 μg/kg NKTR-358 (MAD) (Supplementary Fig. 4A and B).
Several approaches were used to evaluate the activity of the NKTR-358-induced Tregs. NKTR-358 led to a dose-dependent increase in the percentage of CD25bright Tregs expressing Ki67 – a marker of proliferation and, therefore, of Treg activation – at doses ≥12 μg/kg in both studies (Supplementary Fig. 5A). A maximum of 6-fold (SAD) and 5-fold (MAD) mean peak increases over baseline in Ki67+CD25bright Tregs were observed. Similarly, a sustained induction of markers associated with suppressive Treg activity, including Helios and CTLA4, was observed in the higher dose groups, with similar magnitudes of increase in both studies (Supplementary Fig. 5B and 5C). The percentage of ICOS+ total Tregs, which also has an important role in Treg suppressive function, increased in response to NKTR-358 in the SAD study (Supplementary Fig. 5D), but the effect was more difficult to determine in the MAD study due to increased variability across patients (data not shown).
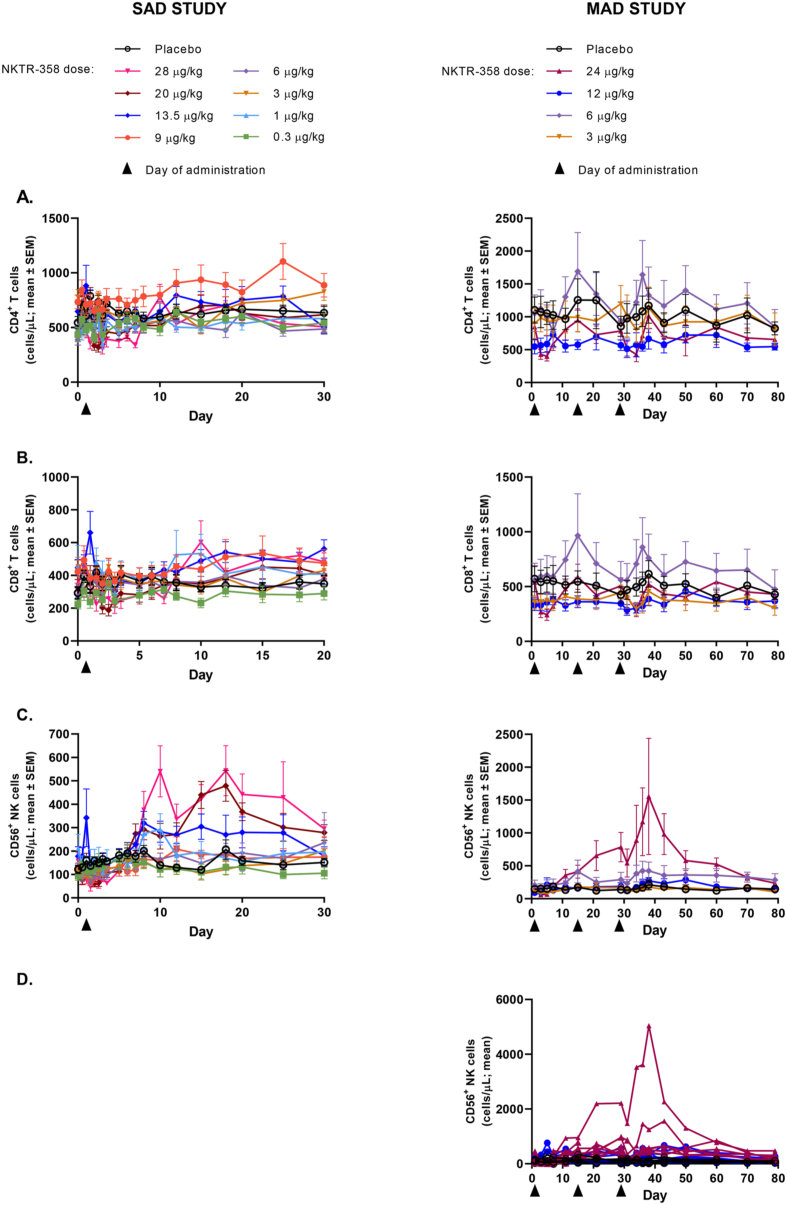
No meaningful changes from baseline in the number of Tcon cell populations (CD4+, CD8+) were observed at any of the dose levels tested (Fig. 3A and B). Due to the relatively small numbers of total Tregs as compared to the total population of CD4+ T cells, there was no difference when CD4+ Tcons were analyzed with CD4+FoxP3+CD25+ Tregs excluded from the CD4+ population or from the total CD4+ population. Low-level increases in total CD56+ NK cells were observed at the higher dose levels tested in both studies (Fig. 3C). In the MAD study, the observed increase in absolute number of NK cells was driven largely by two patients whose values were notably higher than the other patients in the cohort (Fig. 3D). The CD56bright population was more sensitive to NKTR-358 than the CD56dim population, showing greater induction following NKTR-358 administration, with the ratio of CD56bright to CD56dim NK cells increased 16-fold over pre-dose at 24 μg/kg (Supplementary Fig. 6).
Fig. 3.
Mean (SE) changes in absolute number of Tcon and NK cells in peripheral blood after a single dose of NKTR-358 in the SAD study and with multiple doses of NKTR-358 in the MAD study. A) CD4+ T cells; B) CD8+ T cells; C) total CD56+ NK cells; D) total CD56+ NK cells from individual SLE patients given the highest dose (24 μg/kg).
Abbreviations: MAD: multiple ascending dose; NK: natural killer; SAD: single ascending dose; SEM: standard error of the mean; Tcons: conventional T cells.
NKTR-358 administration in patients with SLE resulted in selective expansion of Tregs to levels similar to healthy volunteers in the SAD study, and maintenance of this selectivity after multiple administrations. At 24 μg/kg, there was a 12-fold increase from baseline in the mean peak CD25bright Tregs:Tcon ratio after the first administration and a 7-fold increase after the third administration (data available for six patients only; Supplementary Fig. 7).
3.5. SLE disease activity
In the MAD study in patients with SLE, exploratory analyses examined disease activity as measured by changes in SLEDAI, CLASI-A score, and joint counts. Importantly, the study was not designed to study effects on disease activity, and all analyses were limited by the small numbers of patients per cohort, minimal baseline disease activity in a proportion of patients, and the short duration of treatment (1 month). No NKTR-358 treatment-related changes in SLEDAI or joint scores were apparent. In an exploratory analysis of patients with a CLASI-A baseline score ≥4 (n = 22), a dose-dependent reduction in CLASI-A score was seen with NKTR-358 treatment (Supplementary Fig. 8). One patient (24 μg/kg) experienced a reduction in CLASI-A score from 22 at baseline to 5 by Day 43 (2 weeks after last dose), and 7 of 18 patients had a ≥4-point reduction in CLASI-A score from baseline to Day 43.
4. Discussion
The biological functions of IL-2 are pleiotropic, supporting expansion and activation of both Teffs and Tregs, and IL-2 thus has the capability of promoting or down-regulating immune responses in health and disease [5,18]. Considerable evidence has implicated deficiencies in IL-2 and in Treg numbers and function in the pathogenesis of different autoimmune diseases, including SLE [4,[10], [11], [12], [13]]. IL-2's regulation of the Teff/Treg balance, as well as its ability to prevent the effector antibody-mediated autoimmune response and to control inflammation by blocking differentiation of pro-inflammatory T helper 17 cells, has supported the use of low-dose IL-2 in the treatment of autoimmune diseases [5]. Consistent with the well-understood immunomodulatory roles of IL-2, preliminary evidence of clinical efficacy with low-dose IL-2 in SLE and other inflammatory disease indications has been reported [11,[19], [20], [21]]. However, rhIL-2 has a short half-life, necessitating frequent dosing, and there is a narrow therapeutic window of selective Treg induction by IL-2 before Teffs are also induced [22,23]. NKTR-358 was identified through an in vivo screening campaign to overcome the shortcomings of rhIL-2, first by altering binding to the IL-2 receptor to favor Treg versus Tcon stimulation, and second by extending the half-life [15]. Our studies not only established that NKTR-358 was well tolerated, with a favorable safety profile, but also demonstrated the desired PK profile. Importantly, selective Treg stimulation, without the induction of Tcons, was achieved in healthy volunteers, and these findings were confirmed in patients with SLE, including following multiple doses of NKTR-358. The selective, sustained Treg induction, combined with optimal PK and infrequent administration, suggest that NKTR-358 has the potential to be a safe and effective treatment option for SLE.
NKTR-358 administered subcutaneously was well tolerated at the doses tested, with a similar safety profile for both single and repeat administrations. No treatment-related grade ≥3 or serious TEAEs were observed, and TEAEs were primarily limited to mild or moderate injection site reactions. A safety profile dominated by local reaction at the injection site is consistent with other clinical trials with low-dose rhIL-2 [[11], [12], [13]], IL-2 muteins [24], and other cytokines. While increased eosinophil counts were seen in some patients at the highest dose levels, it is important to emphasize that no participants had associated clinical manifestations suggestive of a hypereosinophilic disorder, and eosinophil counts were cyclical, related to dosing, and resolved after treatment discontinuation. It is well documented that IL-2 can induce eosinophilia via IL-5 produced by proliferating type 2 innate lymphoid cells [5,25], and induction of IL-5 was observed in some participants in our studies (data not shown). Overall, there was no evidence of a clinical syndrome characteristic of severe cytokine-release syndrome in either study, nor was there an observed increased risk of AEs or laboratory abnormalities that are typically associated with high-dose rhIL-2 (aldesleukin), such as capillary leak syndrome, disseminated infections, autoimmune diseases, cardiopulmonary events, and hematologic toxicities [5,26]. Importantly, in contrast to other molecules designed to improve the half-life and Treg selectivity of IL-2 through mutation or fusion approaches, NKTR-358 utilizes the rhIL-2 aldesleukin sequence, which has minimal potential for the development of anti-drug antibodies. Indeed, no NKTR-358 anti-drug antibodies were detected in either the SAD or MAD studies.
The primary effect of NTKR-358 in our studies was a marked and selective, dose-dependent expansion of Tregs. Treg stimulation was sustained, with elevated Treg levels detectable for over 3 weeks after dosing, and restimulation was maintained through multiple administrations of NKTR-358. We focused on the CD25bright Tregs, the subpopulation which has been shown to have the highest suppressive capacity and to be inversely correlated with SLE disease activity [4,5]. Effects on total Tregs were also observed, but the magnitude of expansion was less than that observed for CD25bright Tregs. The functional activity of the NKTR-358-expanded Tregs in these phase 1 studies was further supported by an increase in expression of the proliferation marker Ki67, as well as an increase in expression of Helios and CTLA4, markers associated with increased Treg suppressive activity [27,28].
Importantly, we showed that the NKTR-358-dependent induction of Tregs was not associated with any meaningful changes in CD4+ and CD8+ Tcons across dose levels, and only low-level increases in total NK cells were observed in some patients at the highest doses tested. When NK cell subsets were examined in response to NKTR-358, the CD56bright population showed a larger increase than the CD56dim population. This result was not unexpected, as the CD56bright NK cells are considered more immature than the CD56dim NK cells and have been shown to constitutively express the high-affinity IL-2 receptor. This NK subpopulation is also more highly associated with production of immunoregulatory cytokines rather than high cytotoxic activity [29,30].
Despite a greater understanding of the mechanisms underlying SLE, corticosteroids and immunosuppressive agents are still considered mainstays of therapy for this disease [31,32]. Unfortunately, corticosteroids are associated with a myriad of complications that contribute to the long-term morbidity and poor outcome in some SLE patients [31,32], and immunosuppressive drugs further contribute to the long-term risk of infection [[32], [33], [34]]. Newer therapeutics recently approved and in development for the treatment of SLE [33,34] primarily target immune pathways, such as cytokines and key cellular populations, with inhibitory agents that may add to the immunosuppressive risk [32]. In contrast, low-dose IL-2, NKTR-358, and other IL-2-like molecules have the ability to restore immune homeostasis in SLE patients by increasing deficient IL-2 levels and by selectively stimulating Tregs. The resulting correction of the Treg/effector T cell imbalance may provide the opportunity to control disease activity without increasing immunosuppression, and also allow for a decrease in the use of steroids and immunosuppressive agents [5,21,35].
Considerable evidence indicates that both T cells and B cells are dysregulated in SLE [[36], [37], [38]]. T cell-dependent stimulation of B cells contributes to excess activation and production of autoantibodies which are a key hallmark of SLE [39,40]. Deposition of immune-complexes and excessive effector T cell activity in tissues can further lead to increased chronic inflammation and long-term organ damage [39,41]. In addition to selective stimulation of Tregs and suppression of effector T cell inflammatory responses, IL-2 agents have the ability to alter the balance and function of T follicular helper cells and T follicular regulatory cells, and more directly reduce autoantibody formation and immune complex deposition [4,5,[10], [11], [12], [13],[19], [20], [21]]. There also is evidence that Tregs have a potential role in regulating tissue damage and regeneration, through involvement of the amphiregulin-EGFR signaling axis and other pathways [41,42].
In conclusion, NKTR-358 was well tolerated in healthy volunteers and patients with minimal-to-moderate SLE. NKTR-358 elicited substantial dose-dependent increases in circulating CD25bright Tregs, with no significant changes in Tcons and low-level increases in NK cells. These immune effects extend previous preclinical studies showing the prolonged and Treg-selective action of NKTR-358. Due to the inherent limitations in phase 1 studies, including a relatively short treatment duration and a small number of patients, there was a limited ability to assess clinical disease activity in the NKTR-358 MAD study. Still, the results from this study showing dose-dependent reductions in CLASI-A scores in the subset of patients with higher baseline activity are encouraging. Together, the findings from these studies provide strong support for continued testing of NKTR-358 as a new therapeutic in SLE and other inflammatory diseases. A randomized, double-blind, placebo-controlled phase 2 study (ISLAND-SLE; NCT04433585) has been initiated to further evaluate NKTR-358 in patients with SLE.
Author statement
Christie Fanton: Conceptualization, Formal analysis; Funding acquisition, Investigation, Methodology, Resources, Writing – review & editing, Supervision, Visualization, Writing – original draft. Richard Furie: Conceptualization, Investigation, Writing – review & editing, Supervision. Vishala Chindalore: Investigation, Writing – review & editing, Supervision. Robert Levin: Investigation, Writing – review & editing, Supervision. Isam Diab: Investigation, Writing – review & editing, Supervision. Neha Dixit: Formal analysis; Methodology, Writing – review & editing, Visualization. Cat Haglund: Writing – review & editing, Visualization. Jacqueline Gibbons: Formal analysis, Writing – review & editing, Writing – original draft. Nathan Hanan: Formal analysis; Writing – review & editing. Daniel Dickerson: Formal analysis; Investigation, Writing – review & editing, Supervision. Jonathan Zalevsky: Conceptualization, Formal analysis; Funding acquisition, Investigation, Methodology, Resources, Writing – review & editing, Supervision, Visualization, Writing – original draft. Brian L. Kotzin: Conceptualization, Formal analysis; Funding acquisition, Investigation, Methodology, Resources, Writing – review & editing, Supervision, Visualization, Writing – original draft.
Funding
Funding support was provided by Nektar Therapeutics.
Data availability statement
Data from this study may be shared with qualified researchers, upon request.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: CF, RF, VC, RL, ID, ND, CH, JG, NH, JZ and BK report financial support was provided by Nektar Therapeutics. CF reports a relationship with Nektar Therapeutics that includes: employment and equity or stocks. RF reports a relationship with Nektar Therapeutics that includes: consulting or advisory. RF reports a relationship with Genentech/Roche that incudes personal fees and grants. RF reports a relationship with AstraZeneca that includes: consulting or advisory. RF reports a relationship with Mallinckrodt Pharmaceuticals that includes: consulting or advisory. VC reports a relationship with AbbVie Inc that includes: funding grants. VC reports a relationship with Amgen Inc that includes: funding grants. VC reports a relationship with Boehringer Ingelheim Corp USA that includes: funding grants. VC reports a relationship with Boston Pharmaceuticals that includes: funding grants. VC reports a relationship with Eli Lilly and Company that includes: funding grants. VC reports a relationship with EMD Serono Inc that includes: funding grants. VC reports a relationship with Genentech that includes: funding grants. VC reports a relationship with GlaxoSmithKline USA that includes: funding grants. VC reports a relationship with Merck & Co Inc that includes: funding grants. VC reports a relationship with Nektar Therapeutics that includes: funding grants. VC reports a relationship with Novartis that includes: funding grants. VC reports a relationship with Pfizer that includes: funding grants. VC reports a relationship with Roche that includes: funding grants. VC reports that Pinnacle Research Group/Anniston Medical Clinic also received funding from Astellas Pharma Global Development Inc. RL reports a relationship with Gilead Sciences Inc that includes: consulting or advisory. RL reports a relationship with Exagen Inc that includes: consulting or advisory. RL reports a relationship with Myriad Genetics Inc that includes: consulting or advisory. RL reports a relationship with Sanofi that includes: paid expert testimony and speaking and lecture fees. RL reports a relationship with Regeneron Pharmaceuticals Inc that includes: paid expert testimony and speaking and lecture fees. RL reports a relationship with Bristol Myers Squibb Co that includes: paid expert testimony and speaking and lecture fees. RL reports a relationship with AbbVie that includes: paid expert testimony and speaking and lecture fees. ND reports a relationship with Nektar Therapeutics that includes: employment and equity or stocks. CH reports a relationship with Nektar Therapeutics that includes: employment and equity or stocks. JG reports a relationship with Nektar Therapeutics that includes: employment and equity or stocks. NH reports a relationship with GlaxoSmithKline that includes: employment. DD reports a relationship with PRA Health Sciences that includes: employment. DD reports a relationship with ICON plc that includes: employment. JZ reports a relationship with Nektar Therapeutics that includes: employment and equity or stocks. BK reports a relationship with Nektar Therapeutics that includes: employment and equity or stocks. ID has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The investigators would like to thank the patients, their families, and clinical teams for their participation in this study. This study was sponsored by Nektar Therapeutics. Editorial assistance was provided by BOLDSCIENCE Inc. and funded by Nektar Therapeutics.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtauto.2022.100152.
Appendix ASupplementary data
The following is the Supplementary data to this article:
References
- 1.Kaul A., Gordon C., Crow M.K., Touma Z., Urowitz M.B., van Vollenhoven R., Ruiz-Irastorza G., Hughes G. Systemic lupus erythematosus. Nat. Rev. Dis. Prim. 2016;2:16039. doi: 10.1038/nrdp.2016.39. [DOI] [PubMed] [Google Scholar]
- 2.Bakshi J., Segura B.T., Wincup C., Rahman A. Unmet needs in the pathogenesis and treatment of systemic lupus erythematosus. Clin. Rev. Allergy Immunol. 2018;55:352–367. doi: 10.1007/s12016-017-8640-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi J., Kim S.T., Craft J. The pathogenesis of systemic lupus erythematosus – an update. Curr. Opin. Immunol. 2012;24:651–657. doi: 10.1016/j.coi.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Spee-Mayer C., Siegert E., Abdirama D., Rose A., Klaus A., Alexander T., Enghard P., Sawitzki B., Hiepe F., Radbruch A., Burmester G.-R., Riemekasten G., Humrich J.Y. Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2016;75:1407–1415. doi: 10.1136/annrheumdis-2015-207776. [DOI] [PubMed] [Google Scholar]
- 5.Klatzmann D., Abbas A.K. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat. Rev. Immunol. 2015;15:283–294. doi: 10.1038/nri3823. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg S.A., Yang J.C., Topalian S.L., Schwartzentruber D.J., Weber J.S., Parkinson D.R., Seipp C.A., Einhorn J.H., White D.E. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA. 1994;271:907–913. [PubMed] [Google Scholar]
- 7.Malek T.R., Yu A., Vincek V., Scibelli P., Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 8.Yu A., Zhu L., Altman N.H., Malek T.R. A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity. 2009;30:204–217. doi: 10.1016/j.immuni.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Z., Zhang X., Su L., Xu L., Zheng Y., Sun J. Fine tuning subsets of CD4+ T cells by low-dosage of IL-2 and a new therapeutic strategy for autoimmune diseases. Int. Immunopharmacol. 2018;56:269–276. doi: 10.1016/j.intimp.2018.01.042. [DOI] [PubMed] [Google Scholar]
- 10.He J., Zhang X., Wei Y., Sun X., Chen Y., Deng J., Jin Y., Gan Y., Hu X., Jia R., Xu C., Hou Z., Leong Y.A., Zhu L., Feng J., An Y., Jia Y., Li C., Liu X., Ye H., Ren L., Li R., Yao H., Li Y., Chen S., Zhang X., Su Y., Guo J., Shen N., Morand E.F., Yu D., Li Z. Low-dose interleukin-2 treatment selectively modulates CD4 + T cell subsets in patients with systemic lupus erythematosus. Nat. Med. 2016;22:991–993. doi: 10.1038/nm.4148. [DOI] [PubMed] [Google Scholar]
- 11.Rosenzwajg M., Lorenzon R., Cacoub P., Pham H.P., Pitoiset F., El Soufi K., RIbet C., Bernard C., Aractingi S., Banneville B., Beaugerie L., Berenbaum F., Champey J., Chazouilleres O., Corpechot C., Fautrel B., Mekinian A., Regnier E., Saadoun D., Salem J.-E., Sellam J., Seksik P., Daguenel-Nguyen A., Doppler V., Mariau J., Vicaut E., Klatzmann D. Immunological and clinical effects of low-dose interleukin-2 across 11 autoimmune diseases in a single, open clinical trial. Ann. Rheum. Dis. 2019;78:209–217. doi: 10.1136/annrheumdis-2018-214229. [DOI] [PubMed] [Google Scholar]
- 12.He J., Zhang R., Shao M., Zhao X., Miao M., Chen J., Liu J., Zhang X., Zhang X., Jin Y., Wang Y., Zhang S., Zhu L., Jacob A., Jia R., You X., Li X., Li C., Zhou Y., Yang Y., Ye H., Liu Y., Su Y., Shen N., Alexander J., Guo J., Ambrus J., Lin X., Yu D., Sun X., Li Z. Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: a randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 2020;79:141–149. doi: 10.1136/annrheumdis-2019-215396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humrich J.Y., von Spee-Mayer C., Siegert E., Bertolo M., Rose A., Abdirama D., Enghard P., Stuhlmüller B., Sawitzki B., Huscher D., Hiepe F., Alexander T., Feist E., Radbruch A., Burmester G.-R., Riemekasten G. Low-dose interleukin-2 therapy in refractory systemic lupus erythematosus: an investigator-initiated, single-centre phase 1 and 2a clinical trial. Lancet Rheumatol. 2019;1:e44–e54. doi: 10.1016/S2665-9913(19)30018-9. [DOI] [PubMed] [Google Scholar]
- 14.Xu L., Song X., Su L., Zheng Y., Li R., Sun J. New therapeutic strategies based on IL-2 to modulate Treg cells for autoimmune diseases. Int. Immunopharmacol. 2019;72:322–329. doi: 10.1016/j.intimp.2019.03.064. [DOI] [PubMed] [Google Scholar]
- 15.Dixit N., Fanton C., Langowski J.L., Kirksey Y., Kirk P., Chang T., Cetz J., Dixit V., Kim G., Kuo P., Maiti M., Tang Y., VanderVeen L.A., Zhang P., Lee M., Ritz J., Kamihara Y., Ji C., Rubas W., Sweeney T.D., Doberstein S.K., Zalevsky J. NKTR-358: a novel regulatory T-cell stimulator that selectively stimulates expansion and suppressive function of regulatory T cells for the treatment of autoimmune and inflammatory diseases. J. Transl. Autoimmun. 2021;4:100103. doi: 10.1016/j.jtauto.2021.100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z., Li D., Tsun A., Li B. FOXP3 + regulatory T cells and their functional regulation. Cell. Mol. Immunol. 2015;12:558–565. doi: 10.1038/cmi.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wieczorek G., Asemissen A., Model F., Turbachova I., Floess S., Liebenberg V., Baron U., Stauch D., Kotsch K., Pratschke J., Hamann A., Loddenkemper C., Stein H., Volk H.D., Hoffmüller U., Grützkau A., Mustea A., Huehn J., Scheibenbogen C., Olek S. Quantitative DNA methylation analysis of FOXP3 as a new method for counting regulatory T cells in peripheral blood and solid tissue. Cancer Res. 2009;69:599–608. doi: 10.1158/0008-5472.CAN-08-2361. [DOI] [PubMed] [Google Scholar]
- 18.Abbas A.K., Trotta E., Simeonov D.R., Marson A., Bluestone J.A. Revisiting IL-2: biology and therapeutic prospects. Sci. Immunol. 2018;3 doi: 10.1126/sciimmunol.aat1482. [DOI] [PubMed] [Google Scholar]
- 19.Saadoun D., Rosenzwajg M., Joly F., Six A., Carrat F., Thibault V., Sene D., Cacoub P., Klatzmann D. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N. Engl. J. Med. 2011;365:2067–2077. doi: 10.1056/NEJMoa1105143. [DOI] [PubMed] [Google Scholar]
- 20.Koreth J., Matsuoka K., Kim H.T., McDonough S.M., Bindra B., Alyea E.P., Armand P., Cutler C., Ho V.T., Treister N.S., Bienfang D.C., Prasad S., Tzachanis D., Joyce R.M., Avigan D.E., Antin J.H., Ritz J., Soiffer R.J. Interleukin-2 and regulatory T cells in graft-versus-host disease. N. Engl. J. Med. 2011;365:2055–2066. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koreth J., Kim H.T., Jones K.T., Lange P.B., Reynolds C.G., Chammas M.J., Dusenbury K., Whangbo J., Nikiforow S., Alyea E.P., Armand P., Cutler C.S., Ho V.T., Chen Y.-B., Avigan D., Blazar B.R., Antin J.H., Ritz J., Soiffer R.J. Efficacy, durability, and response predictors of low-dose interleukin-2 therapy for chronic graft-versus-host disease. Blood. 2016;128:130–137. doi: 10.1182/blood-2016-02-702852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lotze M.T., Matory Y.L., Ettinghausen S.E., Rayner A.A., Sharrow S.O., Seipp C.A., Custer M.C., Rosenberg S.A. In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J. Immunol. 1985;135:2865–2875. [PubMed] [Google Scholar]
- 23.Bayer A.L., Pugliese A., Malek T.R. The IL-2/IL-2R system: from basic science to therapeutic applications to enhance immune regulation. Immunol. Res. 2013;57:197–209. doi: 10.1007/s12026-013-8452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visweswaraiah J., Sampson E., Petaipimol P., Monsef A., Kis-Toth K., Otipoby K.L., Sundy J., Higginson-Scott N., Viney J. Generation of PT101, a highly selective IL-2 mutein for treatment of autoimmune diseases. Ann. Rheum. Dis. 2021;80:13. doi: 10.1136/annrheumdis-2021-eular.2097. [DOI] [Google Scholar]
- 25.Van Gool F., Molofsky A.B., Morar M.M., Rosenzwajg M., Liang H.-E., Klatzmann D., Locksley R.M., Bluestone J.A. Interleukin-5–producing group 2 innate lymphoid cells control eosinophilia induced by interleukin-2 therapy. Blood. 2014;124:3572–3576. doi: 10.1182/blood-2014-07-587493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dutcher J.P., Schwartzentruber D.J., Kaufman H.L., Agarwala S.S., Tarhini A.A., Lowder J.N., Atkins M.B. High dose interleukin-2 (Aldesleukin) – expert consensus on best management practices-2014. J. Immunother. Cancer. 2014;2:26. doi: 10.1186/s40425-014-0026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakaguchi S., Miyara M., Costantino C.M., Hafler D.A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 28.Thornton A.M., Lu J., Korty P.E., Kim Y.C., Martens C., Sun P.D., Shevach E.M. Helios+ and Helios- Treg subpopulations are phenotypically and functionally distinct and express dissimilar TCR repertoires. Eur. J. Immunol. 2019;49:398–412. doi: 10.1002/eji.201847935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper M.A., Fehniger T.A., Turner S.C., Chen K.S., Ghaheri B.A., Ghayur T., Carson W.E., Caligiuri M.A. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 30.Ito S., Bollard C.M., Carlsten M., Melenhorst J.J., Biancotto A., Wang E., Chen J., Kotliarov Y., Cheung F., Xie Z., Marincola F., Tanimoto K., Battiwalla M., Olnes M.J., Perl S., Schum P., Hughes T.E., Keyvanfar K., Hensel N., Muranski P., Young N.S., Barrett A.J. Ultra-low dose interleukin-2 promotes immune-modulating function of regulatory T cells and natural killer cells in healthy volunteers. Mol. Ther. 2014;22:1388–1395. doi: 10.1038/mt.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruiz-Irastorza G., Bertsias G. Treating systemic lupus erythematosus in the 21st century: new drugs and new perspectives on old drugs. Rheumatology. 2020;59:v69–v81. doi: 10.1093/rheumatology/keaa403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yasuda S. Emerging targets for the treatment of lupus erythematosus: there is no royal road to treating lupus. Mod. Rheumatol. 2019;29:60–69. doi: 10.1080/14397595.2018.1493909. [DOI] [PubMed] [Google Scholar]
- 33.Vukelic M., Li Y., Kyttaris V.C. Novel treatments in lupus. Front. Immunol. 2018;9:2658. doi: 10.3389/fimmu.2018.02658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liossis S.N., Staveri C. What's new in the treatment of systemic lupus erythematosus. Front. Med. 2021;8:655100. doi: 10.3389/fmed.2021.655100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humrich J.Y., Riemekasten G. Restoring regulation - IL-2 therapy in systemic lupus erythematosus. Expet Rev. Clin. Immunol. 2016;12:1153–1160. doi: 10.1080/1744666X.2016.1199957. [DOI] [PubMed] [Google Scholar]
- 36.Rahman A., Isenberg D.A. Systemic lupus erythematosus. N. Engl. J. Med. 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 37.Dörner T., Jacobi A.M., Lee J., Lipsky P.E. Abnormalities of B cell subsets in patients with systemic lupus erythematosus. J. Immunol. Methods. 2011;363:187–197. doi: 10.1016/j.jim.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Xia X., Yang J., Wang S. Follicular regulatory T cells in systemic lupus erythematosus. J. Immunol. Res. 2021;2021:9943743. doi: 10.1155/2021/9943743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suárez-Fueyo A., Bradley S.J., Tsokos G.C. T cells in systemic lupus erythematosus. Curr. Opin. Immunol. 2016;43:32–38. doi: 10.1016/j.coi.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jucaud V., Ravindranath M.H., Terasaki P.I., Morales-Buenrostro L.E., Hiepe F., Rose T., Biesen R. Serum antibodies to human leucocyte antigen (HLA)-E, HLA-F and HLA-G in patients with systemic lupus erythematosus (SLE) during disease flares: clinical relevance of HLA-F autoantibodies. Clin. Exp. Immunol. 2016;183:326–340. doi: 10.1111/cei.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizui M., Tsokos G.C. Targeting regulatory T cells to treat patients with systemic lupus erythematosus. Front. Immunol. 2018;9:786. doi: 10.3389/fimmu.2018.00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boothby I.C., Cohen J.N., Rosenblum M.D. Regulatory T cells in skin injury: at the crossroads of tolerance and tissue repair. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.aaz9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this study may be shared with qualified researchers, upon request.