Abstract
To establish whether a feline model can predict nucleoside analogue behavior in human semen, zidovudine (ZDV) and lamivudine (3TC) pharmacokinetic parameters (PKs) were determined in the blood and seminal plasma of healthy cats. Our results show considerable similarity in ZDV and 3TC PKs between cats and humans. As in humans, ZDV and 3TC tend to accumulate in feline seminal plasma. Area under the blood plasma concentration-time curve was predictive of seminal plasma excretion. The felid model offers a unique in vivo experimental alternative for investigating the pharmacokinetics of nucleoside analogues in the male genital tract.
Antiretroviral therapeutics, such as nucleoside and nonnucleoside reverse transcriptase inhibitors and protease inhibitors, have improved the quality of life and life expectancy for many people infected with human immunodeficiency virus type 1 (HIV-1) (34). Such compounds have also shown promise as prophylactic alternatives for lowering the risk of acquisition of new infections in susceptible populations. Most notably, administration of zidovudine (ZDV; 3′-azido-3′-deoxythymidine) or nevirapine to HIV-1-seropositive pregnant women significantly reduces transmission to newborns (6). ZDV treatment also decreases the risk of HIV-1 infection in occupationally exposed health care workers (2). There is growing interest in the efficacy of antiretroviral prophylaxis in the context of venereal exposure, the most common route of HIV-1 acquisition. Because ethical and practical constraints may limit direct study of virus transmission in sexually exposed human populations, the correlation of appropriate animal model data to human data offers a valuable avenue for evaluating antiretroviral therapeutic strategies that target virus in genital secretions.
Domestic cats infected with the felid lentivirus feline immunodeficiency virus (FIV) develop an acquired immune deficiency syndrome that parallels the syndrome in HIV-infected people (24). This small-animal model is a useful and cost-effective system for the study of lentivirus immunopathogenesis and antiviral interventions (4). FIV is susceptible to nucleoside analogues, such as ZDV, zalcitabine, didanosine, and lamivudine (3TC; β-d-2′,3′-dideoxy-3′-thiacytidine), in vitro at concentrations similar to those observed with HIV-1 (22, 23, 29, 31). The sensitivities of FIV and HIV-1 to the active triphosphate forms of ZDV and 3TC are also similar (21, 27). Furthermore, infected feline patients treated with ZDV show delayed onset of viremia, reduced plasma virus loads, and clinical improvement (9–12, 28). 3TC has been studied in vivo only recently in this species; infected cats receiving experimental bone marrow transplants and 3TC in combination with ZDV demonstrated some clinical benefit (36). Because FIV is shed in semen and can be transmitted by artificial insemination (15, 16), the feline system may represent a biologically relevant in vivo alternative for examining the effect of pharmacological agents on seminal virus transmission. In preparation for testing the effects of antiretroviral therapy in FIV-infected male cats, we conducted a preliminary analysis of the pharmacokinetics (PKs) of a nucleoside analogue combination commonly used in HIV-1-infected and -exposed individuals, ZDV plus 3TC, in the blood plasma and seminal plasma of healthy, uninfected cats.
Three specific-pathogen-free, male domestic cats (Harlan Sprague Dawley, Inc., Indianapolis, Ind.) were used in this study. Each cat was 19 months old and weighed approximately 4 kg. The animals were free of disease and were maintained in accordance with the American Association for Accreditation of Laboratory Animal Care standards and the “Guide for the Care and Use of Laboratory Animals” prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Resources, National Resources Council (20).
ZDV (5 mg/kg of body weight) and 3TC (5 mg/kg) (Glaxo Wellcome, Inc., Research Triangle Park, N.C.) were compounded together into single-dose capsules and administered orally every 12 h for 7 days. For blood plasma PK calculations, up to eight blood plasma samples (e.g., collected at approximately 0, 0.5, 1, 2, 4, 8, 11, and 12 h postdose) were obtained by jugular catheterization after dose 1 (day 1, first dose condition) and again after dose 13 (day 7, steady-state condition). In order to obtain first-dose and steady-state trough concentrations (C12) in seminal plasma, four ejaculates were collected during the 7-day study (e.g., two samples around dose 1 and two samples around dose 13) by an electroejaculation method standardized for this species (16). To avoid any confounding accumulation of drug and to obtain a clean baseline, cats were ejaculated approximately 1 h before dose 1 and dose 13 (C−1). Cats were ejaculated again 12 h after dose 1 and dose 13, but prior to doses 2 and 14, respectively. A fifth semen sample was collected from cat 1 at 1 h after dose 9 by artificial vagina as described previously (30). Blood plasma and seminal plasma specimens were separated from cellular constituents by centrifugation at 2,000 × g for 10 min and stored at −70°C until assayed.
Drug concentrations were determined by liquid chromatography and tandem mass spectroscopy as described by Pereira et al. (26). PK parameters were obtained by noncompartmental analyses using WinNonlin (version 3.0; Pharsight Corp., Mountain View, Calif.), and statistics were analyzed with SAS JMP 3.2.1 software (SAS Institute, Cary, N.C.) using nonparametric methods (with Bonferroni's correction for multiple comparisons; P < 0.01). Anemia has been reported as a side effect of nucleoside analogue administration in cats (primarily at doses higher than those used in this study) (7, 8, 10); therefore, packed cell volumes were determined at days 0 and 7.
PK data for each drug in feline blood plasma obtained during the first 12 h and steady-state 12-h intervals are summarized in Table 1. Blood plasma PK values from studies of healthy human volunteers are included for comparison (Combivir; Zidovudine and Lamivudine Product Information, 2000, Glaxo Wellcome, Inc., Research Triangle Park, N.C.; C. Cremieux, C. Katlama, H. Prevot, S. Leclerc, C. Gillotin, D. Demarles, P. Vial, and F. Raffi, Abstr. XIIIth Int. AIDS Conf., abstr. 4125, 2000; references 19 and 35). The cats remained healthy during the study and anemia was not observed. In blood plasma, the C12 values for ZDV and 3TC did not differ significantly between first dose and steady state in the cats (P > 0.25); the median value (range) for AZT C12 at first dose was 68.8 ng/ml (31.7 to 102.4 ng/ml) and 79.5 ng/ml (46.3 to 152.4 ng/ml) at steady state, and the C12 values for 3TC were 164.0 ng/ml (38.7 to 173.4 ng/ml) at first dose and 244.5 ng/ml (99.3 to 273.9 ng/ml) at steady state.
TABLE 1.
ZDV and 3TC PK parameters in feline blood plasma after first dose and steady statea
| Analysis group | Tmax (h) | Cmax (μg/ml) | AUC (μg · h/ml) | CLF (liter/h/kg) | t1/2 (h) | VF (liter/kg) |
|---|---|---|---|---|---|---|
| ZDV | ||||||
| Feline (5 mg/kg) first dose | 1.02 (0.05–1.98; 0.55) | 3.67 (2.67–4.66; 4.03) | 10.28 (9.16–11.40; 10.81) | 0.49 (0.43–0.55; 0.46) | 1.69 (1.43–1.94; 1.62) | 1.19 (1.00–1.38; 1.29) |
| Feline (5 mg/kg) steady state | 1.69 (1.28–2.10) | 3.65 (3.54–3.76) | 9.65 (9.13–10.17) | 0.56 (0.52–0.60) | 1.75 (1.60–1.90) | 1.39 (1.37–1.40) |
| Human (3–6 mg/kg) | 0.75 (0.25–1.50) | 1.15 (0.71–1.85) | 1.46 (1.11–1.91) | 2.94 (2.24–4.21) | 2.08 (1.91–2.27) | 1.60 (1.23–1.97) |
| 3TC | ||||||
| Feline (5 mg/kg) first dose | 1.17 (0.31–2.03; 1.02) | 2.86 (2.62–3.08; 2.83) | 8.35 (6.22–10.48; 8.31) | 0.62 (0.46–0.78; 0.60) | 2.39 (1.96–2.83; 2.59) | 2.10 (1.83–2.37; 2.17) |
| Feline (5 mg/kg) steady state | 1.69 (1.28–2.10) | 3.89 (3.27–4.51) | 10.36 (9.03–11.68) | 0.49 (0.46–0.62) | 2.12 (2.09–2.15) | 1.54 (1.27–1.80) |
| Human (1.5–3 mg/kg) | 1.24 (0.50–3.00) | 1.40 (1.17–1.68) | 5.53 (4.58–6.67) | 0.38 (0.29–0.47) | 9.79 (8.51–10.65) | 1.30 (1.07–1.53) |
First-dose values include mean (95% confidence interval; median). Steady-state values are calculated from two cats and include mean (range). Human data are as reported in Combivir, zidovudine and lamivudine product information, 2000 (Glaxo Wellcome, Inc.); Cremieux et al., XIIIth Int. AIDS Conf.; and references 19 and 35.
In this study of seronegative cats receiving ZDV in combination with 3TC, ZDV blood plasma PKs were similar to those reported in uninfected cats given ZDV alone (7, 10), suggesting that, as in people, 3TC coadministration did not substantially alter ZDV PK (19). 3TC PK data have not been reported previously for this species. Comparisons of time to maximum drug concentration (Tmax) values in Table 1 indicate that these nucleoside analogues are rapidly absorbed in cats and humans after oral administration, with maximal blood plasma concentrations (Cmax) attained within approximately 1.5 h. ZDV is eliminated primarily by the hepatic glucuronidation pathway, and the higher Cmax, higher area under the curve (AUC), and lower clearance (CLF) observed in cats as compared with humans may be related, in part, to deficiencies in glucuronidation in feline species (3). ZDV half-life (t1/2) and VF were somewhat lower in cats than in humans. Feline 3TC blood plasma Cmax and AUC were higher in cats than in humans. This may reflect the higher dose administered to the cats. The CLF values were similar between species, though the feline 3TC VF was greater than seen in humans, resulting in a shorter calculated plasma t1/2. Unlike ZDV, 3TC undergoes minimal metabolism and is eliminated via renal secretion (14).
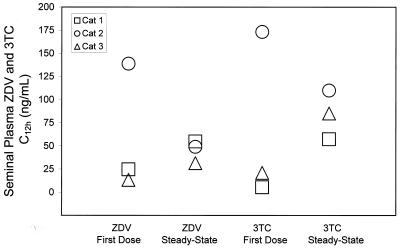
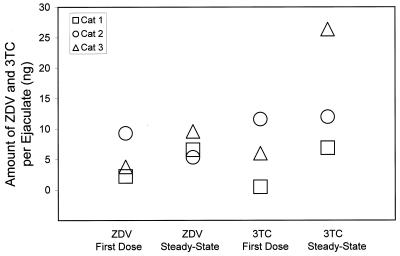
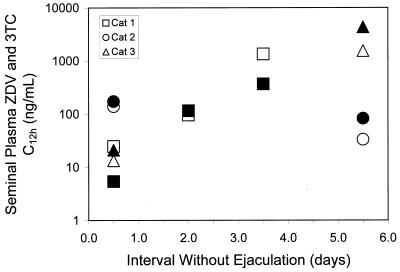
The C12 values for ZDV and 3TC in feline semen are shown in Fig. 1. The mean ± standard deviation for the seminal plasma concentration of ZDV was 58.9 ± 69.4 ng/ml after the first dose and 45.1 ± 12.2 ng/ml at steady state. For 3TC, seminal plasma drug concentrations were 66.5 ± 92.7 ng/ml after the first dose and 84.1 ± 26.3 ng/ml at steady state. The reason for the very high concentrations observed in cat 3 at first dose is uncertain. In general, variability in parent drug concentration was similar to what has been described in humans (25). The formation of semen is complex and the composition of ejaculated fluid is highly modified as it passes through secretory tissues. Correcting for ejaculate volume, the total amount of drug excreted in the feline ejaculates (seminal plasma drug concentration ejaculate volume) demonstrated a tendency toward accumulation at steady state (Fig. 2). Indeed, after an extended time without ejaculation (Fig. 3), concentrations were usually higher than those observed during the two 12-h collection periods, indicating that in cats, like humans (1, 13, 25), accumulation in this biological compartment extends beyond a single dosing interval. Systemic drug exposure over a dosing interval in the cats, as measured by AUC (Table 1) was positively correlated with seminal plasma excretion (Spearman's correlation coefficient, 0.67, P = 0.03), suggesting that approximately 40% of the variability in semen drug concentrations can be explained by systemic exposure to ZDV and 3TC.
FIG. 1.
Trough concentrations (C12, in nanograms per milliliter) of ZDV and 3TC in seminal plasma from domestic cats after first dose (day 1) and at steady state (day 7).
FIG. 2.
The excretion (seminal plasma volume times trough drug concentration, in nanograms) of ZDV and 3TC in ejaculates from domestic cats after first dose and steady state.
FIG. 3.
Log graph of trough concentrations (C12, in nanograms per milliliter) of ZDV (open symbols) and 3TC (solid symbols) in feline seminal plasma with increasing time between semen collection. Semen was collected from all cats during day 1 and day 7; semen was also collected from cat 1 during day 5.
In a human study that analyzed serial paired semen and blood specimens obtained up to 500 days posttreatment, it was apparent that ZDV and 3TC can attain much higher concentrations in semen relative to blood (25). Median human seminal plasma to blood plasma (SP/BP) ratios for ZDV and 3TC were 5.9 (range, 1.0 to 13.5) and 9.1 (range, 2.3 to 16.1), respectively, supporting the sequestration or selective transport of nucleoside analogues into the seminal compartment, a characteristic that lends support for the administration of these drugs to prevent sexual spread of infection. In cats, median SP/BP ratios were also >1 in specimens collected more than 12 h after a previous ejaculation; the median SP/BP ratios were 3.01 (range, 2.55 to 220.30) for ZDV and 1.33 (range, 1.04 to 45.04) for 3TC.
Penetration of antiretroviral agents in the genital compartment is highly variable, not only between subjects but also between different compounds. Like ZDV and 3TC, stavudine, efavirenz, and nevirapine achieve seminal drug concentrations above the 50% inhibitory concentration for HIV-1. In contrast, the protease inhibitors saquinavir and ritonavir achieve low-to-undetectable concentrations (32, 33; J. Kim, K. Gotzkowksy, J. Eron, D. Manion, W. Fiske, M. Cohen, and A. Kashuba, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1171, 2000). Thus, drug exposure in genital secretions cannot be predicted solely on the basis of physicochemical properties, such as plasma protein binding, partition coefficients, and dissociation constants (17). To avoid suboptimal drug levels that could permit covert virus replication in the reproductive tract and dissemination of drug-resistant isolates (5), PK modeling for specific compounds or combination of compounds is essential. Relevant animal models suitable for assessing the potential pharmacological prevention of HIV-1 sexual transmission are limited. There are no rodent lentivirus systems that mirror the clinicopathologic effects of HIV-1. Simian immunodeficiency virus causes AIDS in rhesus macaques and closely approximates the virologic characteristics of HIV-1, but seminal plasma from this species frequently forms an insoluble coagulum precluding sample analysis. In cats, seminal plasma can be easily collected and this study confirms for the first time that nucleoside analogues can be quantified in this compartment in this small-animal species.
Like most animal models, there are limitations in using cats to address semen antiviral characteristics, and more detailed comparisons of the PK properties of these compounds and their triphosphorylated metabolites are warranted to fully validate this model. As noted earlier, felids have a low capacity for hepatic glucuronidation of drug metabolites (3). Anatomically, the feline and human genital tracts differ. Nearly 70% of human seminal plasma forms in the seminal vesicles, but cats, like other carnivores, lack this accessory organ. In addition, the ejaculate volume in cats is quite small (50 to 350 μl), limiting some types of analyses. Although FIV is susceptible to nucleoside analogues, it is not susceptible to currently prescribed HIV-1 protease inhibitors. This is a rapidly evolving area, however, and protease inhibitors active against both viruses are under development (18). Despite these differences, our results show considerable similarity in ZDV and 3TC PKs in humans and cats, suggesting that the feline system may be useful for experimental investigation of antiviral agents in the male genital tract.
Acknowledgments
This study was supported by PHS grant K01 RR00109 from the National Center for Research Resources (H.L.J.), the UNC-CH Center for AIDS Research, NIH grant 9P30 AI50410 (H.L.J., A.D.M.K., and A.S.P.), and PHS NIH training grant NIO 7001 (A.S.P.).
We thank Lori Scappino, Deborah Anderson, Janet Dow, Debbie Gaffney, and Linda English at the college of Veterinary Medicine, North Carolina State University, and John Dunn at Glaxo Wellcome, Inc., Research Triangle Park, N.C., for technical assistance.
REFERENCES
- 1.Anderson P L, Noormohamed S E, Henry K, Brundage R C, Balfour H H, Jr, Fletcher C V. Semen and serum pharmacokinetics of zidovudine and zidovudine-glucuronide in men with HIV-1 infection. Pharmacotherapy. 2000;20:917–922. doi: 10.1592/phco.20.11.917.35263. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Case-control study of HIV seroconversion in health-care workers after percutaneous exposure to HIV-infected blood—France, United Kingdom, and United States, January 1988–August 1994. Morb Mortal Wkly Rep. 1995;44:929–933. [PubMed] [Google Scholar]
- 3.Court M H, Greenblatt D J. Molecular genetic basis for deficient acetaminophen glucuronidation by cats: UGT1A6 is a pseudogene, and evidence for reduced diversity of expressed hepatic UGT1A isoforms. Pharmacogenetics. 2000;10:355–369. doi: 10.1097/00008571-200006000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Elder J H, Dean G A, Hoover E A, Hoxie J A, Malim M H, Mathes L, Neil J C, North T W, Sparger E, Tompkins M B, Tompkins W A, Yamamoto J, Yuhki N, Pedersen N C, Miller R H. Lessons from the cat: feline immunodeficiency virus as a tool to develop intervention strategies against human immunodeficiency virus type 1. AIDS Res Hum Retrovir. 1998;14:797–801. doi: 10.1089/aid.1998.14.797. [DOI] [PubMed] [Google Scholar]
- 5.Eron J J, Vernazza P L, Johnston D M, Seillier-Moiseiwitsch F, Alcorn T M, Fiscus S A, Cohen M S. Resistance of HIV-1 to antiretroviral agents in blood and seminal plasma: implications for transmission. AIDS. 1998;12:F181–F189. doi: 10.1097/00002030-199815000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Guay L A, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, Sherman J, Bakaki P, Ducar C, Deseyve M, Emel L, Mirochnick M, Fowler M G, Mofenson L, Miotti P, Dransfield K, Bray D, Mmiro F, Jackson J B. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 7.Hart S, Nolte I. Long-term treatment of diseased, FIV-seropositive field cats with azidothymidine (AZT) J Vet Med. 1995;42:397–409. doi: 10.1111/j.1439-0442.1995.tb00392.x. [DOI] [PubMed] [Google Scholar]
- 8.Hartmann K, Donath A, Beer B, Egberink H F, Horzinek M C, Lutz H, Hoffmann-Fezer G, Thum I, Thefeld S. Use of two virustatica (AZT, PMEA) in the treatment of FIV and of FeLV seropositive cats with clinical symptoms. Vet Immunol Immunopathol. 1992;35:167–175. doi: 10.1016/0165-2427(92)90129-e. [DOI] [PubMed] [Google Scholar]
- 9.Hartmann K, Donath A, Kraft W. AZT in the treatment of feline immunodeficiency virus infection. Part 1. Feline Pract. 1995;23:16–21. [Google Scholar]
- 10.Hartmann K, Donath A, Kraft W. AZT in the treatment of feline immunodeficiency virus infection. Part 2. Feline Pract. 1995;23:13–20. [Google Scholar]
- 11.Hayes K A, Lafrado L J, Erickson J G, Michael Marr J, Mathes L. Prophylactic ZDV therapy prevents early viremia and lymphocyte decline but not primary infection in feline immunodeficiency virus-inoculated cats. J Acquir Immune Defic Syndr. 1993;6:127–134. [PubMed] [Google Scholar]
- 12.Hayes K A, Phipps A J, Francke S, Mathes L E. Antiviral therapy reduces viral burden but does not prevent thymic involution in young cats infected with feline immunodeficiency virus. Antimicrob Agents Chemother. 2000;44:2399–2405. doi: 10.1128/aac.44.9.2399-2405.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henry K, Chinnock B J, Quinn R P, Fletcher C V, de Miranda P, Balfour H H., Jr Concurrent zidovudine concentrations in semen and serum determined by radioimmunoassay in patients with AIDS or AIDS related complex. JAMA. 1988;259:3023–3026. [PubMed] [Google Scholar]
- 14.Johnson M A, Moore K H, Yuen G J, Bye A, Pakes G E. Clinical pharmacokinetics of lamivudine. Clin Pharmacokinet. 1999;36:41–66. doi: 10.2165/00003088-199936010-00004. [DOI] [PubMed] [Google Scholar]
- 15.Jordan H L, Howard J G, Sellon R K, Wildt D E, Tompkins W A, Kennedy-Stoskopf S. Transmission of feline immunodeficiency virus in domestic cats via artificial insemination. J Virol. 1996;70:8224–8228. doi: 10.1128/jvi.70.11.8224-8228.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan H L, Howard J, Tompkins W A, Kennedy-Stoskopf S. Detection of feline immunodeficiency virus in semen from seropositive domestic cats (Felis catus) J Virol. 1995;69:7328–7333. doi: 10.1128/jvi.69.11.7328-7333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashuba A D M, Dyer J R, Kramer L M, Raasch R H, Eron J J, Cohen M S. Antiretroviral drug concentrations in semen: implications for sexual transmission of HIV-1. Antimicrob Agents Chemother. 1999;43:1817–1826. doi: 10.1128/aac.43.8.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee T, Le V, Lim D, Lin Y, Morris G M, Wong A L, Olson A J, Elder J H, Wong C. Development of a new type of protease inhibitors, efficacious against FIV and HIV variants. J Am Chem Soc. 1999;121:1145–1155. [Google Scholar]
- 19.Moore K H, Shaw S, Laurent A L, Lloyd P, Duncan B, Morris D M, O'Mara M J, Pakes G E. Lamivudine/zidovudine as a combined formulation tablet: bioequivalence compared with lamivudine and zidovudine administered concurrently and the effect of food on absorption. J Clin Pharmacol. 1999;39:593–605. doi: 10.1177/00912709922008209. [DOI] [PubMed] [Google Scholar]
- 20.National Research Council. Guide for the care and use of laboratory animals. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- 21.North T W, Cronn R C, Remington K M, Tandberg R T. Direct comparisons of inhibitor sensitivities of reverse transcriptases from feline and human immunodeficiency viruses. Antimicrob Agents Chemother. 1990;34:1505–1507. doi: 10.1128/aac.34.8.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.North T W, LaCasse R A. Testing anti-HIV drugs in the FIV model. Nat Med. 1995;1:410–411. doi: 10.1038/nm0595-410. [DOI] [PubMed] [Google Scholar]
- 23.North T W, North G L T, Pedersen N C. Feline immunodeficiency virus, a model for reverse transcriptase-targeted chemotherapy for acquired immune deficiency syndrome. Antimicrob Agents Chemother. 1989;33:915–919. doi: 10.1128/aac.33.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedersen N C, Ho E W, Brown M L, Yamamoto J K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987;235:790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- 25.Pereira A S, Kashuba A D, Fiscus S A, Hall J E, Tidwell R R, Trolani L, Dunn J A, Eron J J, Jr, Cohen M S. Nucleoside analogues achieve high concentrations in seminal plasma: relationship between drug concentration and virus burden. J Infect Dis. 1999;180:2039–2043. doi: 10.1086/315149. [DOI] [PubMed] [Google Scholar]
- 26.Pereira A S, Kenney K B, Cohen M S, Hall J E, Eron J J, Tidwell R R, Dunn J A. Simultaneous determination of lamivudine and zidovudine concentrations in human seminal plasma using high-performance liquid chromatography and tandem mass spectrometry. J Chromatogr Biomed Sci Appl. 2000;742:173–183. doi: 10.1016/s0378-4347(00)00162-6. [DOI] [PubMed] [Google Scholar]
- 27.Smith R A, Remington K M, Lloyd R M, Jr, Schinazi R F, North T W. A novel Met-to-Thr mutation in the YMDD motif of reverse transcriptase from feline immunodeficiency virus confers resistance to oxathiolane nucleosides. J Virol. 1997;71:2357–2362. doi: 10.1128/jvi.71.3.2357-2362.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smyth N R, Bennett M, Gaskell R M, McCracken C M, Hart C A, Howe J L. Effect of 3′azido-2′,3′-deoxythymidine (AZT) on experimental feline immunodeficiency virus infection in domestic cats. Res Vet Sci. 1994;57:220–224. doi: 10.1016/0034-5288(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 29.Smyth N R, McCracken C, Gaskell R M, Cameron J M, Coates J A, Gaskell C J, Hart C A, Bennett M. Susceptibility in cell culture of feline immunodeficiency virus to eighteen antiviral agents. J Antimicrob Chemother. 1994;34:589–594. doi: 10.1093/jac/34.4.589. [DOI] [PubMed] [Google Scholar]
- 30.Soyka N J, Jennings L L, Hamner C E. Artificial insemination in the cat (Felis catus) Lab Anim Care. 1970;20:198–204. [PubMed] [Google Scholar]
- 31.Tanabe-Tochikura A, Tochikura T S, Blakeslee J R, Jr, Olsen R G, Mathes L E. Anti-human immunodeficiency virus (HIV) agents are also potent selective inhibitors of feline immunodeficiency virus (FIV)-induced cytopathic effect: development of a new method for screening of anti-FIV substances in vitro. Antivir Res. 1992;19:161–172. doi: 10.1016/0166-3542(92)90075-g. [DOI] [PubMed] [Google Scholar]
- 32.Taylor S, Back D J, Workman J, Drake S M, White D J, Choudhury B, Cane P A, Beards G M, Halifax K, Pillay D. Poor penetration of the male genital tract by HIV-1 protease inhibitors. AIDS. 1999;13:859–860. doi: 10.1097/00002030-199905070-00017. [DOI] [PubMed] [Google Scholar]
- 33.Taylor S, van Heeswijk R P, Hoetelmans R M, Workman J, Drake S M, White D J, Pillay D. Concentrations of nevirapine, lamivudine and stavudine in semen of HIV-1-infected men. AIDS. 2000;14:1979–1984. doi: 10.1097/00002030-200009080-00014. [DOI] [PubMed] [Google Scholar]
- 34.Vella S, Palmisano L. Antiretroviral therapy: state of the HAART. Antivir Res. 2000;45:1–7. doi: 10.1016/s0166-3542(99)00068-6. [DOI] [PubMed] [Google Scholar]
- 35.Wang L H, Chittick G E, McDowell J A. Single-dose pharmacokinetics and safety of abacavir (1592U89), zidovudine, and lamivudine administered alone and in combination in adults with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1999;43:1708–1715. doi: 10.1128/aac.43.7.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto J K, Pu R, Arai M, Pollock D, Irausquin R, Bova F J, Fox L E, Homer B L, Gengozian N. Feline bone marrow transplantation: its use in FIV-infected cats. Vet Immunol Immunopathol. 1998;65:323–351. doi: 10.1016/s0165-2427(98)00165-2. [DOI] [PubMed] [Google Scholar]