Introduction
Atypical postradiation vascular proliferation (APRVP) is a diagnosis with heterogeneous clinical and histopathologic features. In this report, we discuss a case of a patient with metastatic peripheral nerve-sheath tumor, treated with surgical excision and radiation, and new-onset erythema of the right breast, who was found to have histologic findings consistent with APRVP outside the field of prior radiation exposure.
Case report
A 49-year-old woman with metastatic high-grade malignant peripheral nerve-sheath tumor of the left upper extremity, status post multiple surgical resections of the left axilla and radiation to the left axilla and left side of the chest wall 2 years previously (2 treatments to the left axilla with a cumulative dose of 99 gray [Gy], 1 treatment to the left side of the chest wall with a cumulative dose of 50 Gy), neurofibromatosis type 1, and chronic lymphedema of the right lower extremity, was admitted to the hospital for worsening shortness of breath and concern for cellulitis of the left side of the chest. Given clinical concern for pneumonia and cellulitis, the patient received cefepime and vancomycin in the emergency department. On admission, the patient was narrowed to cefepime and doxycycline.
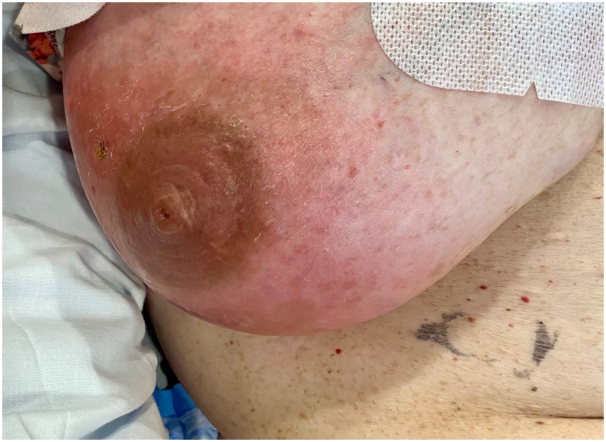
Five days later, the patient developed new-onset, asymptomatic erythema around the right areola. The erythema began in the distal, dependent tip of the right breast, and subsequently expanded outwards. Examination later that day demonstrated a well-defined, blanchable, nontender erythematous patch on the right breast with no fluctuance, warmth, or nodularity (Fig 1).
Fig 1.
Clinical image of a well-defined, blanchable, nontender erythematous patch on the right breast.
Dermatology was consulted to evaluate the etiology of the new-onset erythema of the right breast. Although there was initial concern for cellulitis spreading locally from the left side of the chest, this was deemed unlikely, considering the antimicrobial coverage and lack of tenderness, edema, and asymmetric warmth of the right breast. Given the patient’s lack of symptoms, the erythema was determined to be most likely lymphangitis or stasis. However, with the patient’s history of high-grade malignant peripheral nerve-sheath tumor, a 4-mm punch biopsy of the right breast erythematous patch was performed to rule out metastasis.
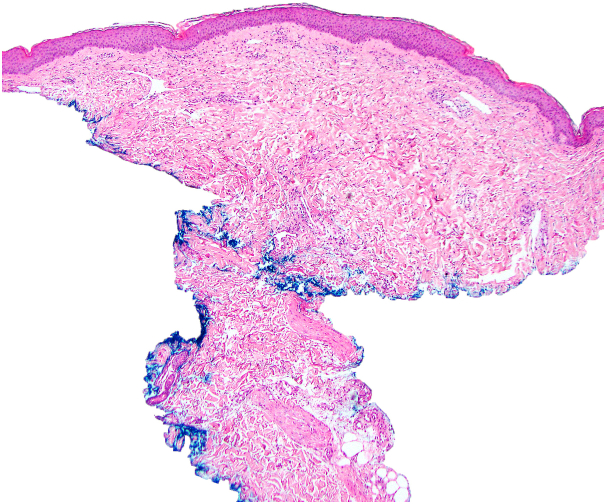
Histopathology of the right breast punch biopsy demonstrated vascular dilation with prominent endothelial cells and mixed inflammatory infiltrate, including scattered eosinophils and neutrophils (Fig 2). The increased number of ectatic and angulated vascular spaces with prominent endothelial cells was consistent with APRVP. Gram, methenamine silver, Nocardia, and periodic acid–Schiff stains were negative. Immunohistochemistry for MYC was performed but inconclusive given the limited amount of tissue remaining from the biopsy. An earlier hematoxylin-eosin–stained preparation was destained and used to repeat the immunohistochemistry for MYC; however, the attempt was not successful.
Fig 2.
Histologic findings of atypical postradiation vascular proliferation. Low-power view of punch biopsy with prominent ectatic dermal vasculature (hematoxylin-eosin stain; original magnification: ×40).
Given the histologic and immunohistochemical findings consistent with APRVP, no further diagnostic workup was pursued. The patient was treated with topical 0.05% betamethasone dipropionate as needed for symptomatic management, which led to decreased erythema on examination within 2 days. The patient subsequently expired from worsening hypoxia, likely due to aspiration pneumonia unrelated to the patient’s right breast erythema.
Discussion
First described in 1994 by Fineberg and Rosen1 and further categorized by Brenn and Fletcher2 in 2005, APRVP is a diagnosis with heterogeneous clinical and histologic features of unclear pathophysiologic basis.3 It is best described in patients with primary breast carcinoma treated with postoperative radiation therapy.3 Clinically, APRVPs are most often located on mammary skin, with commonly reported presentations including papules and plaques with erythema, telangiectasias, or induration, on average presenting 6 years after radiation.3,4 It has been associated with a history of lymphedema and recurrence of the primary tumor prior to APRVP onset, with the differential diagnosis typically including other benign vascular lesions and radiation-associated angiosarcoma (RAAS).3
Histologically, distinguishing APRVP from secondary angiosarcoma can be challenging, and the 2 pathologies may exist on a spectrum.5 Both lesions display abnormally dilated dermal vasculature with hyperchromatic and hobnailed cells.5 In RAAS, the vessels tend to dissect the collagen bundles and can involve the subcutis. The endothelial nuclei may be multilayered, and show atypia and mitoses.5 Although not needed, immunohistochemistry for MYC can be used to aid categorization, as MYC is usually amplified in RAAS but not in APRVPs.6
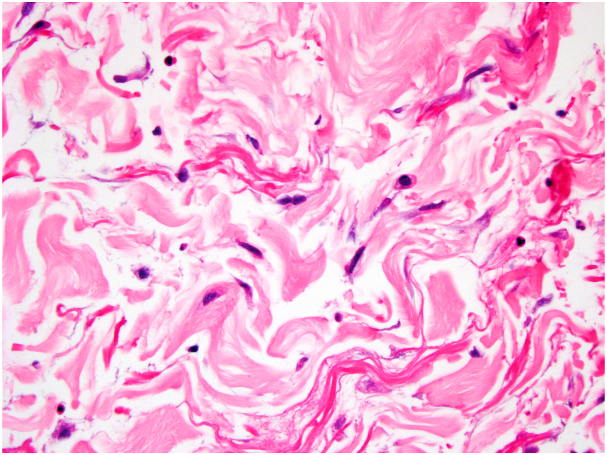
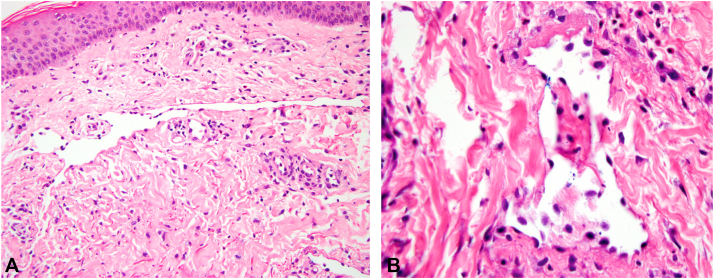
In our case, the patient had a history of chronic lymphedema and presented with erythema of the right breast. The patient's presentation is unusual, following a primary nerve-sheath tumor, rather than breast carcinoma, and with erythema appearing in an area outside the field of prior radiation exposure. However, the background stroma displayed hyperchromatic fibroblasts and collagenous changes typical of radiation effect (Fig 3). On hematoxylin-eosin staining, the lesion displayed the characteristic dermal stellate vessels with bland hobnailed endothelial cells (Fig 4). Negative immunohistochemistry for MYC would have further supported the diagnosis of APRVP.
Fig 3.
Stroma with hyperchromatic fibroblasts consistent with radiation effect (hematoxylin-eosin stain; original magnification: ×400).
Fig 4.
High-power view of dermal vessels in atypical postradiation vascular proliferation. A, Stellate abnormally dilated vessel. B, Vessel with hobnailing of endothelial nuclei (A and B, hematoxylin-eosin stain; original magnifications: A, ×200; B, ×400).
Although there have been previous case reports documenting benign vascular lesions after previous radiation therapy of the contralateral aspect of the breast, clinical and pathologic characterization of this presentation remains limited.7 Previous studies examining radiation exposure to the contralateral aspect of the breast after an average breast radiation therapy treatment of 45 to 65 Gy have demonstrated a potential mean exposure of 1.1 Gy.8 Even accounting for this patient’s higher total radiation dose, the expected scatter would be significantly lower than 50 to 60 Gy radiation doses associated with both APRVP and RAAS.1,2 As a result, in our case, there is an unclear relationship between previous radiation exposure and histologic findings diagnostic of APRVP.
Studies into the pathophysiology of RAAS have demonstrated a possible link between amplification of the MYC gene and the deregulated angiogenesis that leads to RAAS.9 Notably, fluorescence in situ hybridization and immunohistochemistry analyses have shown that APRVP lack expression of MYC.6 Few cases of APRVP have shown malignant transformation to RAAS, indicating, however, that there is likely another mechanism involved.9
Altogether, our case is an unusual clinical presentation that was determined to be a histologically confirmed diagnosis of APRVP outside the field of prior radiation therapy. This presentation, which was initially suspected to be a cellulitis, should be added to the differential diagnosis for pseudocellulitis in this patient population to prevent unnecessary antibiotics and hospitalization.10 Most critically, this case highlights a gap in the current understanding the pathophysiologic processes leading to APRVP.
Conflicts of interest
None disclosed.
Footnotes
Dr Davis and Author Yoon contributed equally to this article.
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Fineberg S., Rosen P.P. Cutaneous angiosarcoma and atypical vascular lesions of the skin and breast after radiation therapy for breast carcinoma. Am J Clin Pathol. 1994;102(6):757–763. doi: 10.1093/ajcp/102.6.757. [DOI] [PubMed] [Google Scholar]
- 2.Brenn T., Fletcher C.D. Radiation-associated cutaneous atypical vascular lesions and angiosarcoma: clinicopathologic analysis of 42 cases. Am J Surg Pathol. 2005;29(8):983–996. [PubMed] [Google Scholar]
- 3.Mattoch I.W., Robbins J.B., Kempson R.L., Kohler S. Post-radiotherapy vascular proliferations in mammary skin: a clinicopathologic study of 11 cases. J Am Acad Dermatol. 2007;57(1):126–133. doi: 10.1016/j.jaad.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 4.Zhong C.S., Raut C.P., Glynn R.J., Nambudiri V.E. Characteristics of atypical postradiation vascular proliferation: a retrospective review of 193 patients. J Am Acad Dermatol. 2020;83(5):1447–1450. doi: 10.1016/j.jaad.2019.10.036. [DOI] [PubMed] [Google Scholar]
- 5.Baker G.M., Schnitt S.J. Vascular lesions of the breast. Semin Diagn Pathol. 2017;34(5):410–419. doi: 10.1053/j.semdp.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Mentzel T., Schildhaus H.U., Palmedo G., Büttner R., Kutzner H. Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological, immunohistochemical and molecular analysis of 66 cases. Mod Pathol. 2012;25(1):75–85. doi: 10.1038/modpathol.2011.134. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H., Han M., Varma K., Dabbs D.J. Follow-up outcomes of benign vascular lesions of breast diagnosed on core needle biopsy: a study of 117 cases. Breast J. 2019;25(3):401–407. doi: 10.1111/tbj.13233. [DOI] [PubMed] [Google Scholar]
- 8.Stovall M., Smith S.A., Langholz B.M., et al. Dose to the contralateral breast from radiotherapy and risk of second primary breast cancer in the WECARE study. Int J Radiat Oncol Biol Phys. 2008;72(4):1021–1030. doi: 10.1016/j.ijrobp.2008.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo T., Zhang L., Chang N.E., Singer S., Maki R.G., Antonescu C.R. Consistent MYC and FLT4 gene amplification in radiation-induced angiosarcoma but not in other radiation-associated atypical vascular lesions. Genes Chromosomes Cancer. 2011;50(1):25–33. doi: 10.1002/gcc.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li D.G., Xia F.D., Khosravi H., et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154(5):537–543. doi: 10.1001/jamadermatol.2017.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]