Summary
Viscosification of carbon dioxide by polymers can make large scale CO2 sequestration safe and efficient. We present solubility of branched hydrocarbon oligomers in CO2 and viscosification measurements at relevant subsurface conditions. Polymers of 1-decene (P1D) with about 20 repeating units are found to be effective in CO2 viscosification, increasing it by 6.5-fold at 1.8 wt% concentration at 308 K and 31 MPa. We reason that methyl groups and branching promote solubility and viscosification. Low molecular weight oligomers can have lower solubility in CO2 than higher molecular weight ones and the trend in solubility is non-monotonic at constant pressure and temperature. Analysis of solubility trend of P1D oligomers in CO2 advances our understanding of molecular structure and functionality and opens the path to engineering of oligomers effective in viscosification and widespread use of CO2.
Subject areas: Chemical engineering, Organic chemistry, Polymers
Graphical abstract

Highlights
-
•
Increasing P1D size has non-monotonic solubility trend in CO2 at moderate pressures
-
•
P1D solubility in CO2 has monotonic trend at higher pressures
-
•
Methyl groups enhance solubilization in CO2
-
•
An exponential trend observed for relative viscosity with polymer molecular size
Chemical engineering; Organic chemistry; Polymers
Introduction
The United States Geological Survey (USGS) in 2013 estimated the potential carbon dioxide storage capacity of the United States to be around 3000 metric gigatons. The US Energy Information Administration (EIA) estimates that the total CO2 emission from various sources in 2020 was about 5.1 billion metric tons in the USA and about 33.1 billion metric tons worldwide. Hydrocarbon energy production has made major advances to minimize release of pollutants and toxic substances into the atmosphere and oceans. Hydrocarbon fuels have also become clean-burning resources. The major issue is climate change from emission of substantial amounts of CO2 into the atmosphere. Numerous studies based on concentration of CO2 in the atmosphere and temperature have found a correlation between the two (Petit et al., 1999). In the past 400,000 years, there have been several cycles of global warming. The cycles correspond to the period of higher CO2 concentration (Firoozabadi, 2016). From around 1960, there has been a continuous increase in CO2 concentration in the atmosphere from a yearly average of around 318 to 412 ppm in 2018. Until about 1900, the concentration of CO2 had been close to or below 300 ppm. There may be delays in global warming from CO2 concentration, but the general belief in the scientific community is that global warming and other consequences may arrive somewhat later. There is no shortage of space in the subsurface to permanently sequester CO2 (Firoozabadi and Myint, 2010). Advanced technologies for carbon capture are being investigated with the focus on using metal-organic frameworks (MOF) compared to the conventional amine absorption methods (Britt et al., 2009; Liang et al., 2019). The captured CO2 can be used in the production of useful chemicals (Fiorani et al., 2015), including synthesis of cyclic organic carbonates (Arayachukiat et al., 2017; Al Maksoud et al., 2020; Natongchai et al., 2021). Use of CO2 may also help to drastically improve efficiency of oil recovery (CO2-IOR) and hydraulic fracturing (Feng et al., 2021). CO2 sequestration in saline aquifers, CO2-IOR, and CO2 fracturing depend on CO2 viscosification. CO2 has a liquid-like density but gas-like viscosity at subsurface conditions. Its low viscosity makes it inefficient for improved oil recovery (IOR) because of low sweep efficiency. CO2 may give rise to early breakthroughs from displacement of liquids in cores and from injection in large scale fields.
Viscosification of CO2 by low concentration of polymers has been investigated since the early 1980s (Heller et al., 1985; Enick et al., 2012). Numerous studies using polymers have been conducted to determine the increase in viscosity of CO2 (Hunter et al., 1993; Huang et al., 2000; Davani et al., 2012). There has not been much progress to find cost-effective and environmentally friendly materials for the intended applications. The polymers which seem effective without porous media become ineffective in contact with porous media (Zaberi et al., 2020). Owing to complexities in mixing and measurements, some of the results in the literature vary by a wide range between different authors. Fluorine-based polymers are commonly studied; they are found to be readily soluble in CO2 (Xu and Enick, 2001), viscosifying CO2 by two to three orders of magnitude at 4 to 5 wt%. Recently, close to a 4-fold viscosity increase by 1 wt% polyfluoroacrylate has been reported at temperature and pressure conditions of 297 K and 21 MPa (Zaberi et al., 2020). Fluoropolymers are expensive, potentially harmful for the environment, and adsorb strongly onto the rock formation (Trickett et al., 2010). Hydrocarbon polymers have also been examined by various authors. Poly-1-decene (P1D) with six repeating units and poly vinyl ethyl ether (PVEE) (Gu et al., 2013) show limited effectiveness. P1D with six repeating units has been found to be more effective in CO2 viscosification compared to other hydrocarbon polymers (Heller et al., 1985; Zhang et al., 2011; Al Hinai et al., 2018). Zhang et al. (2011) report large increase in CO2 viscosity by P1D with six repeating units. Their work has been disputed in the literature. Al-Hinai et al. (2018) use the same commercial P1D (molecular weight of around 910) at pressure and temperature conditions of 50 to 55 MPa and 358 to 377 K, respectively. They report CO2 viscosity to increase by a factor of 1.2 to 2.77 for P1D concentrations of 0.8 to 5 wt% at the aforementioned pressure and temperature ranges. Lemaire et al. (2021), in a recent review paper, investigate P1D with six repeating units along with other oligomers and heavy molecular weight polymers and report 0-10% increase in viscosity for 2 and 4 wt% of P1D in CO2 at 34 and 48 MPa, respectively, at temperature of 297 K. A main suggestion in the recent literature review of CO2 viscosification by Lemaire et al. (2021) is that CO2-soluble low molecular weight polymers and oligomers should be considered as modest thickeners, increasing relative viscosity by say less than a factor of two. The use of nanoparticles in combination with hydrocarbon polymers is being investigated to viscosify CO2 (Gandomkar and Sharif, 2020; Gallo et al., 2021). Gandomkar and Sharif (2020) added up to 500 ppm of graphene oxide to a very high molecular weight P1D (MW 640000) and measured a considerable increase in relative viscosity from 4.5 to 22.9 using 300 ppm of graphene oxide at 353 K, with a reduction in cloud point from 24 to 16 MPa. Gallo et al. (2021) found silicone resins to be efficient in thickening supercritical CO2 (relative viscosification of 4.2 at 328 K and 18 MPa, for 6.90 wt% nanoparticle concentration). Nanoparticles have also been mixed with CO2 to achieve better mobility control in EOR applications (Jafari et al., 2015; Dezfuli et al., 2020). In another recent review article, Pal et al. (2022) have reiterated the advances made in CO2 viscosification for applications in EOR and fracking and classified the CO2 thickeners into five main categories, including cosolvents, polymeric materials, and small molecule-associating compounds.
Visual cells with mechanical mixing are generally used to measure solubility of various chemicals and polymers in CO2. The viscosity of CO2-polymer fluid is commonly measured by capillary tubes or by falling cylinder viscometers. A brief summary of different setups from the literature is presented in Table 1.
Table 1.
Methods used in solubility and viscosity measurements by various authors
| Study | Main component for solubility test | Temperature (K) | Pressure (MPa) | Mixing mechanism for solubility apparatus | Viscometer type |
|---|---|---|---|---|---|
| Heller et al. (1985) | Sapphire tube (entire sample volume visible) | 298–331 | 10–24 | Magnetic stirring bar | Falling cylinder |
| Bae and Irani (1993) | Ruska double windowed cell | 328 | 17 | Oscillating shaft, manual rocking | Capillary |
| Huang et al. (2000) | Variable volume, windowed cell | 295 | 6–48 | Rocking of cell | Falling cylinder |
| Xu and Enick (2001) | 298–373 | 45 | |||
| Kilic et al. (2019) | 295 | 10–15 | Impeller fixed at top of sample volume | ||
| Zhang et al. (2011) | See-through, windowed cell | 329 | 21 | Not mentioned | Capillary |
| Shi et al. (2015) | Fixed volume view chamber with two sapphire windows on opposite walls | 318–343 | 21 | Installed stirrer, maximum speed of 1,200 rpm | N/A |
| Sun et al. (2018) | Variable volume cell, windows made of quartz glass | 308 | 15–30 | Magnetic stirrer | Falling ball |
| Al-Hinai et al. (2018) | Windowed cell | 377 | 55 | No stirrer | Capillary |
| Chen et al. (2020) | Autoclave with adjustable volume (visibility) | 308 | 5–40 | Not mentioned | Falling ball |
Goicochea and Firoozabadi (2019a) have investigated molecular structures of CO2 with heptadecafluorodecyl acrylate (HFDA), P1D, and PVEE polymers via dissipative particle dynamics (DPD) simulations and then determine the increase in viscosity of CO2 by these polymers (Goicochea and Firoozabadi, 2019b). The branched structure of P1D with six repeating units is found to be more effective in solubilizing in CO2 compared to linear chains. For non-fluorinated polymers, the viscosity increase with concentrations is linear as opposed to power law viscosity increase with concentration for fluorinated polymer HFDA. The intermolecular π-stacking from the styrene functionality strongly increases the aggregation, leading to significant increase in viscosity. The effect of water on the solubility and viscosification of CO2 by the polymers is also examined. Water is observed to promote folding of PVEE molecules, thereby increasing the CO2 viscosity because of higher resistance to flow provided by the aggregation of folded molecules. Conversely, P1D and HFDA molecules are not affected by water from molecular simulations.
After the publication of the work of Goicochea and Firoozabadi in 2019 (2019a; 2019b), Zaberi et al. (2020) have studied the solubility and CO2 thickening effect of polyfluoroacrylate (PFA). At T = 297 K, the cloud point pressure is measured to range from 9.5 to 10 MPa for 1 to 8 wt% PFA concentration in CO2. The solubility of PFA is significantly reduced in the presence of light hydrocarbons in CO2, with cloud point pressure increasing to 21 MPa for a concentration of 0.5 wt% PFA in a mixture of 85 wt% CO2 and 15 wt% hydrocarbons consisting of n-hexane (C6) to eicosane (C20), at T = 297 K. Close to four-time increase in CO2 viscosity is obtained by 1 wt% PFA at T = 297 K and P = 21 MPa. Even with increased viscosity, PFA is rendered not applicable for CO2 mobility control because of high adsorption of the polymer onto rock surface. Increase in pressure drop by a factor of 30 is recorded during coreflooding in Berea sandstone rock using the CO2-PFA mixture.
Zhang et al. (2021) have synthesized polyether-based polymers by adding heptamethyltrisiloxane to improve solubility of the polymer in CO2 through reduced polymer-polymer interaction and improved chain flexibility. They add phenyl groups to the polymer to increase intermolecular π-π association and increase the CO2 viscosification of the polymer. At P = 20 MPa and T = 308 K, a 4.8-fold increase in CO2 viscosification is obtained using the polymer with 18.7 mole percent of phenyl group at a concentration of 1 wt% polymer in CO2. This ratio gives the optimal amount of phenyl group to achieve maximum thickening and further increase in phenyl group content is found to reduce the viscosity. The increase in intramolecular π stacking rather than intermolecular association leads to a decrease in effectiveness. Despite the fact that the molecules synthesized by Zhang et al. (2021) are very effective in viscosification, strong adsorption to the rock surface may make their performance similar to the results of Zaberi et al. (2020). Chen et al. (2020) report on CO2 thickening by poly ether carbonates and find CO2 viscosity to increase by a factor of 11 and 3.9 by addition of 0.95 wt% of phenyl-centered four-chain poly (ether-carbonate) and 0.72 wt% phenyl-centered tri-chain poly (ether-carbonate), respectively, at P = 30 MPa and T = 308 K. Ether group has one oxygen atom directly connected to an alkyl group. The presence of the heteroatoms is likely to have adverse adsorption effects on rock surface. In our work, we observe strong sandstone rock adsorption by polymers with phenol functionality.
There are six important characteristics of functional molecules for CO2 viscosification in relation to CO2 sequestration and some other important applications. These essential features relate to:1- solubility, 2- viscosification, 3-adsorption onto the rock surface, 4- solubility in the aqueous phase, 5- shear thinning and reversibility in performance, and 6- environmental considerations. Solubility and viscosification are the key elements of the process. In addition, low adsorption, low solubility in water and brine, reversibility in shear from change in pressure, and environmental friendliness are also required in the application of viscosified CO2. Based on the above six considerations, the published molecules may not be effective in CO2 sequestration. High adsorption is a serious issue in both fluorinated and oxygen-containing polymers. These considerations rule out the use of published molecules for the intended applications. The objective of this work is introduction of a molecule that meets the six requirements and can be used in increasing the efficiency and safety of CO2 sequestration.
As discussed earlier, there have been a number of publications on the performance of P1D with six repeating units on solubility in CO2 and viscosification. There are major differences among various authors in CO2 solubility and even wider differences in reported viscosity. Recent publications report a viscosity increase of only a few percent. One objective of this work is to present accurate measurements for the solubility and viscosification of P1D with six repeating units and then evaluate the potential for polymers with various number of 1-decene repeating units and provide a clear understanding of solubility in CO2 and viscosification. P1D with six repeating units has a molecular weight of about 910, but it has much higher solubility in CO2 compared with normal alkanes of half of its molecular weight. Our work covers a wide range of repeating units in P1D. The molecular weight of 1-decene and P1D molecules in our investigation vary from 140 to approximately 4200. Solubility of polymers and viscosification effects in CO2 are determined at pressures of 17, 24, and 31 MPa, and temperatures of 308 and 323 K. Throughout this work, the terms oligomer and polymer are used interchangeably, denoting polymers of low molecular weight with a maximum approximate molecular weight of 4200.
Results and discussion
The schematic of solubility and viscosity measurements are shown in Figures 1 and 2, respectively. Detailed description of the procedure is included in the STAR Methods section.
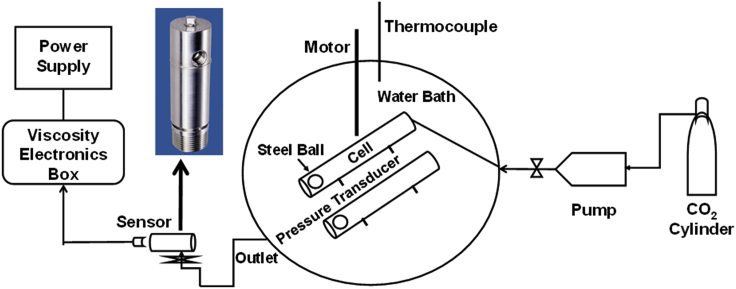
Figure 1.
Schematic of the Sapphire Rocking Cell Setup (insert image shows the cells with steel balls)
Figure 2.
Schematic of viscosity measurement by the moving piston viscometer
Solubility measurements
Figure 3 presents the essence of our measurements on solubility of 1-decene and P1D of various monomer units in CO2 at 308 K and three pressures of 17, 24, and 31 MPa. At 17 and 24 MPa, the highest solubility in CO2 is for P1D of 2300 MW: 1.5 wt% and 1.75 wt%, respectively. The non-monotonic trend in solubility is unusual and different from the monotonic trend in solubility of normal alkanes in CO2 as we will discuss shortly. At 31 MPa, the trend becomes monotonic but still one region (low MW P1D) shows slow decrease in CO2 solubility followed by a sharp decrease for heavy P1D molecules with 1-decene and P1D with 2 repeating units having highest solubility (2.1 wt%). There may be a probable shift in maximum with more data on P1D of repeating units between 3 and 6. The impurity in 1-decene and P1D molecules are not known. There may be some effect from impurity; however, the overall trend will not change. The non-monotonic solubility trend has not been reported previously for CO2 viscosification to the best of our knowledge.
Figure 3.
Solubility of 1-decene and poly-1-decene (P1D) molecules (different molecular weights) in supercritical CO2 at T = 308 K and P = 17, 24, and 31 MPa [Blue bars show the standard deviation of measured data]
The effect of increase in temperature on P1D solubility is examined at two temperatures: 308 and 323 K at constant pressure of 24 MPa (see Figure 4). There is a consistent trend of increase in solubility with increase in temperature. This is opposite to the solubility trend for n-alkanes (Shi et al., 2015) but in agreement with that of aromatics like toluene as reported in the literature (Ng and Robinson, 1978; Kim et al., 1986). Figures 3 and 4 reveal that the effect of pressure is greater than the effect of temperature in increasing the polymer solubility. The increase in solubility of P1D in CO2 with temperature is in agreement with literature data (Al Hinai et al., 2018).
Figure 4.
Solubility of 1-decene and poly-1-decene (P1D) molecules in supercritical CO2 at T = 308 K and 323 K and p = 24 MPa
The solubility measurements for a commercially available P1D with six repeating units (MW 910) from the literature are compared with our results in Figure 5. Our measurements agree with data of Heller et al. (1985) as well as data of Lemaire et al. (2021) at 296 K. Difference in measurements may be because of different techniques used in mixing; a summary of techniques is presented in Table 1.
Figure 5.
Solubility data of P1D (with 6 repeating units) from various sources
Solubility of alkanes and alkenes in CO2 with increasing molecular weight
For n-butane and 1-butene, the solubility is comparable in CO2 at T = 273 K and P = 0.3–3.4 MPa (Nagahama et al., 1974). CO2 is in liquid state under these conditions. Solubility of 1-hexene is lower than that of n-hexane in supercritical CO2 based on the work by Wagner and Wichterle (1987). They performed measurements at different temperatures and found 1-hexene solubility to increase with increase in temperature. The observation is supported by another study (Jennings and Teja, 1989). The increase in solubility with increase in temperature is in line with our measurements of 1-decene and P1D polymers. From our results, we see a significant lowering of solubility of 1-decene than that of n-decane at the same pressure and temperature conditions. The solubility data for n-decane, along with other heavier hydrocarbons, as extracted from literature studies, is presented in the next section. There is limited data in literature on solubility of heavier alkenes in supercritical CO2.
Effect of branching on hydrocarbon solubility in supercritical CO2
Molecular structure and shape and functionality may affect solubility in CO2. In this work, we find that the methyl group has a very strong effect on CO2 solubility. Figure 6 presents the solubility data for nC10, nC16, nC18 (Shi et al., 2015), nC20 (Schmitt and Reid, 1988), nC28, nC29, and nC32 (Chandler et al., 1996; Reverchon et al., 1993) alkanes as well as C30 branched alkane (squalane) (Sovova et al., 1997) and alkene (squalene) (Saure, 1996) at various pressures at temperature of 318 K. There is agreement for nC28 solubility from two separate studies. A decline in solubility is observed with increase in molecular weight of normal alkanes. The molecular weights of nC10 and nC32 are 142 and 450, respectively. When there is branching in alkane structure (addition of methyl group), there is a significant jump in solubility (see Figure 6). Squalane (C30H62) with eight methyl groups is around eight times more soluble in supercritical CO2 than nC32 with two methyl groups. Squalene has six double bonds and eight methyl groups. The two molecules (squalane and squalene) have comparable solubility in CO2. The implication is that the vast difference in CO2 solubility of squalene and nC32 of comparable molecular weight alkane is because of methyl functionality.
Figure 6.
Solubility of: (A) normal and branched alkanes and alkene, and (B) magnified data in supercritical CO2 at 318 K. [nC20 data at 320 K, squalene data at 313 K]
Solubility of: (A) n-alkanes (C10, C16, C18, and C28), branched alkane (C30: squalane), and branched alkene (C30: squalene), and (B) magnified solubility data of n-alkanes (C28, C29, and C32), branched alkane (C30: squalane) in supercritical CO2. nC20 data is at 320 K and C30 squalene data at 313 K, all the other data are at 318 K.
We are not aware of a discussion on the solubility of hydrocarbons of different molecular structures in CO2 in the literature. In 1-decene and in normal decane, both with a molecular weight of about 140, there is huge difference in CO2 solubility (Figures 3 and 6). The solubility of nC20 is about 3 folds higher than P1D with two repeating units; besides, both have a molecular weight of around 280. The trend in CO2 solubility completely reverses in P1D with six repeating units of about 910 molecular weight of g/mole, which is 10 times higher than nC32 that has a molecular weight of around 450 g/mole. The main difference between normal alkanes and P1D molecules is the number of methyl functionalities and branching. The single double-bond in P1D is not likely to contribute to the solubility in CO2. The number of methyl functionalities may have a significant effect on CO2 solubility. 1-decene has one methyl group, whereas nC10 has two methyl groups. P1D with six repeating units has 7 methyl groups; however, nC32 has only two methyl groups. It may be the combined contribution of molecular size (i.e., molecular weight), orientation, branching, and the number of methyl groups that determine CO2 solubility. In normal alkanes, the number of methyl groups is two irrespective of size. A decreasing solubility trend with size is observed. In P1D, as the molecular size increases, the number of methyl groups also increases. We observe a non-monotonic trend with size at 17 MPa and 24 MPa. In methyl groups, there are three hydrogen atoms. Methylene groups have two hydrogen atoms. The solubilization of P1D in CO2 may be assisted by hydrogen bonding between oxygen in CO2 and hydrogen in P1D (Goicochea and Firoozabadi, 2019b). Apart from the number of methyl groups, the molecular orientation, and position of the side chains, among other features, may affect solubility efficiency of these hydrocarbons in supercritical CO2. In our molecular simulations, we find that the number of CO2 molecules around a methyl group is higher than in the methylene group in normal alkanes. The number of CO2 molecules around a methyl group in P1D is consistently higher than in methyl groups in normal alkanes.
Functional groups such as fluorine increase solubility (Besnard et al., 2009); fluorine is environmentally undesirable and expensive. Fluorinated molecules promote adsorption to rock surfaces which rules them out for many applications. One main conclusion of our work is that P1D polymers without additional functional groups may be the best choice in achieving the desired solubility. The purpose of our work is to assess the contribution of structural features of P1D to solubility in CO2 and eventual viscosification without additional functional groups.
Based on Figure 3 and the aforementioned analysis on the effectiveness of methyl groups in enhancing solubility, we next present the viscosification data. The effect of water on relative viscosity is also examined to ensure the viability of polymer-viscosified CO2 to maintain its rheology in aquifers.
Viscosity measurements
The soluble concentration of polymers in CO2 is examined for viscosification at 308 K and pressures of 17, 24, and 31 MPa. Increase in molecular weight of a polymer is expected to result in higher viscosification of CO2, provided the molecule is soluble in CO2. Our measured viscosity and relative viscosity for P1D polymers (with respect to the viscosity of base CO2 at the same conditions) are portrayed in Figure 7. We observe an increasing trend of relative viscosity, which is approximately exponential. This trend for hydrocarbons without introduction of heteroatoms such as O or N is being reported for the first time and is the most important observation in our work and perhaps in CO2 viscosification. The trend is opposite to that of solubility presented in Figure 3.
Figure 7.
Relative viscosity of 1-decene and poly-1-decene (P1D) molecules in supercritical CO2 (compared to base case viscosity of CO2) at temperature of 308 K and pressures of 17, 24, and 31 MPa. Blue bars show the standard deviation of duplicate measurements
Viscosity data for P1D molecules of approximately 4000 and 4200 molecular weight in g/mol are not shown in Figure 7 because of differences in soluble concentrations but included in Table 2. P1D of molecular weight of 4000 gives a relative viscosity of 3.2 at 0.5 wt% concentration at 31 MPa. Because of higher molecular weight, even a significantly lower polymer concentration gives a moderate relative viscosity increase.
Table 2.
Relative Viscosity of 1-decene and poly-1-decene (P1D) molecules in supercritical CO2 (compared to base case viscosity of CO2) at temperatures of 308 and 323 K and pressures of 17, 24, and 31 MPa
| No. | Source | Polymer mol wt (g/mol) | Relative viscosity at 308 K |
||
|---|---|---|---|---|---|
| 17 MPa, 1 wt % | 24 MPa, 1.5 wt% | 31 MPa, 1.8 wt% | |||
| 1 | Sa | 140 | 1.0 | 1.3 | 1.5 |
| 2 | Lb | 280 | 1.1 | 1.3 | 1.6 |
| 3 | Sa | 910 | 1.5 | 2.3 | 2.5 |
| 4 | Lb | 2300 | 2.7 | 4.5 | 5.3 |
| 5 | Lb | 2900 | 4.1 | 5.9 | 6.5 |
| 6 | Lb | 4000 | 1.6 (0.2 wt%) | 2.8 (0.4 wt%) | 3.2 (0.5 wt%) |
| 7 | Br | 4200 | 1.6 (0.15 wt%) | 2.0 (0.25 wt%) | 3.0 (0.4 wt%) |
Sa- Sigma Aldrich, Lb- Lubrizol, Br- Brookfield.
The effect of increase in temperature on viscosification of 1-decene and P1D molecules is presented in Figure 8 from 308 to 323 K at pressure of 24 MPa. There is less than 10% reduction in relative viscosity with increase in temperature by 15 K. Temperature does not have a significant effect on the viscosity in the conditions examined. One reason for the mild effect of temperature on viscosity may be because of the fact that P1D molecules do not associate in CO2. Viscosifier molecules with oxygen functionality associate, and therefore as temperature increases, the viscosity may decrease significantly. The association characteristics have been shown by molecular simulations (Goicochea and Firoozabadi, 2019a).
Figure 8.
Relative viscosity of 1.5 wt% concentration of 1-decene and poly-1-decene (P1D) molecules in supercritical CO2 (to base case viscosity of CO2) at pressure of 24 MPa, and temperatures of 308 K, and 323 K
Effect of side chain length
We measure the viscosity of 2.5 wt% squalane dissolved in supercritical CO2 at pressure and temperature of 24 MPa and 308 K. There is only a 10–20% increase in viscosity. On evaluating the data with Figure 8, a comparable molecular weight of P1D (obtained through interpolation) provides a relative viscosity close to 1.5 at a lower concentration (1.5 wt% in CO2). One may interpret the difference as being in the chain length. P1D has considerably longer side chains compared to squalane. According to Graessley (1977), the solution viscosity of branched polymers may exceed that of linear polymers of comparable molecular weight at the same concentration of polymer in solvent, depending on branched structure and length of the branches.
We further compare the viscosification effect of P1D (molecular weight 2,300 g/mol) with PIB (molecular weight 2,250 g/mol) of considerably higher number of methyl groups than P1D but shorter branch lengths. Comparable molecular weight of the two polymers provides similar viscosification. P1D results in a relative viscosity of 3.6, whereas PIB, with more than four times the number of methyl groups than P1D, gives a relative viscosity of 3.5 at 1 wt% polymer concentration at T = 308 K and P = 24 MPa.
Effect of water on CO2 viscosification
We have examined the effect of water dissolved in CO2 in relation to viscosification at 308 K and 17 MPa. We add 0.15 wt% water to CO2, which is below the solubility limit. Addition of water to the polymer mixture in CO2 is not found to affect the viscosity significantly for P1D (MW 2900 g/mol). Presence of water lowers the relative viscosity from 4.1 to 3.9 for 1 wt% of polymer measured at 308 K and 17 MPa.
Conclusions
In this work we have found polymers of 1-decene with an unusual solubility characteristic. At supercritical pressure conditions of 17 MPa and 24 MPa, the solubility in CO2 is not monotonic. At 31 MPa, also a supercritical condition, there is a slow reduction in CO2 solubility with molecular weight followed by a sharp drop. Viscosification has the expected trend. For the same concentration, there is increase in viscosity from increase in molecular size. The trend is exponential. The P1D oligomers do not adsorb onto the rock and have very low solubility in the aqueous phase. They have all the desirable features to make CO2 sequestration safe. For most CO2 sequestration conditions, a five-to-six-fold increase in CO2 viscosity will allow significant sweep improvement and make CO2 storage safe.
In the past, only P1D with six repeating units, which has a moderate enhanced viscosifying effect, has been studied. There is a large difference in solubility and especially viscosification reports in the literature. In this study, we provide definitive measurements for both solubility and viscosity. We also report on a P1D molecule with about twenty repeating units which can provide a six-fold increase in CO2 viscosity at 31 MPa and 308 K. At higher pressure applications, P1D of high molecular weight at low concentration is an excellent candidate to provide much higher viscosification of CO2 for a broad range of applications. Our findings relate to the effect of methyl functionality and branching in relation to solubility and branching in viscosification.
The essence of our findings is summarized below:
-
1.
Polymers of P1D have a non-monotonic trend in solubility with increase in molecular size at moderate pressures, which turns to a monotonic trend at higher pressures.
-
2.
Presence of methyl groups enhances solubilization in CO2.
-
3.
An exponential clear-cut trend is observed for relative viscosity, with P1D (MW 2900 g/mol) providing the highest relative viscosity of 6.5, at 1.8 wt% concentration, 308 K and 31 MPa.
The sweep efficiency improvement of viscosified CO2 by P1D of approximately 2,900 g/mol molecular weight is significant (related data and results are being prepared for submission). The effectiveness is because of negligible adsorption onto the rock surface and very low solubility in brine.
Limitations of the study
Based on the solubility and viscosity measurements from this work, extensive coreflooding tests have been successfully conducted to gauge the polymer efficiency for sequestration and IOR applications. The findings have been submitted for publication. Ongoing studies are focused on synthesizing and testing polymers with higher relative viscosification at lower polymer concentrations. Systematic measurements conducted up to temperature of 90°C indicate a mild effect of temperature on relative viscosity of the polymer-CO2 mixture. This observation points toward an efficient polymer performance at subsurface conditions. Continuing studies in this direction are being carried out to consolidate our findings at higher temperature conditions. Molecular simulations are being conducted to gain molecular insight to methyl functionality on solubility.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| 1-decene | Sigma-Aldrich | Product#30650 |
| Poly-1-decene (Approx MW 280) | Lubrizol | N/A |
| Poly-1-decene (Approx MW 910) | Sigma-Aldrich | Product#462349 |
| Poly-1-decene (Approx MW 2300) | Lubrizol | N/A |
| Poly-1-decene (Approx MW 2900) | Lubrizol | N/A |
| Poly-1-decene (Approx MW 4000) | Lubrizol | N/A |
| Poly-1-decene (Approx MW 4200) | Brookfield | Part#B1060 |
| Polyisobutylene | Brookfield | Part#B10200 |
Resource availability
Lead contact
Further information and queries will be directed to the lead contact, Abbas Firoozabadi (abbas.firoozabadi@rice.edu).
Materials availability
This study did not generate new unique reagents.
Method details
P1D oligomers of varying molecular weights are sourced from Lubrizol, Sigma Aldrich, and Brookfield. 1-decene and P1D molecules are listed in Table 1. Viscosification of P1D in CO2 is compared with that of squalane (branched alkane C30 from Sigma Aldrich). Poly isobutylene (PIB) of approximate molecular weight 2,250, from Brookfield has been also investigated for viscosification of CO2. We have compared solubility and viscosification effects for equivalent molecular weight P1D and PIB, which differ in the length of branching, and number of methyl groups in the structures.
Table A. List of 1-decene, poly-1-decene, and polyisobutylene in our study
| No. | Sample | Source | Approximate molecular weight (g/mol) |
|---|---|---|---|
| 1 | 1-decene | Sigma Aldrich | 140 |
| 2 | Poly-1-decene | Lubrizol | 280 |
| 3 | Poly-1-decene | Sigma Aldrich | 910 |
| 4 | Poly-1-decene | Lubrizol | 2300 |
| 5 | Poly-1-decene | Lubrizol | 2900 |
| 6 | Poly-1-decene | Lubrizol | 4000 |
| 7 | Poly-1-decene | Brookfield | 4200 |
| 8 | Polyisobutylene | Brookfield | 2250 |
All the polymers listed above have a low solubility in deionized (DI) water, approximately about 0.05 wt%. pH measurements are conducted using 0.5 wt% concentration of polymers in DI water to measure pH of aqueous phase, without and with exposure to CO2. No change in pH is observed with addition of polymers to DI water compared to base case of DI water. Exposure to CO2 causes a pH reduction from 6.4 to 5.6; but no significant difference in pH is observed by adding polymer. The pH measurements are conducted at atmospheric conditions.
There have been significant differences in solubility and viscosity measurements reported by various authors as mentioned above. We have verified our measurements using different methods. In the following, we will first describe the equipment used in solubility and viscosity determination.
Solubility measurements
Solubility measurements are performed using a sapphire rocking cell setup from PSL Systemtechnik (RCS 2). A steel ball is placed inside each cell. The polymers are introduced into the cell after purge with CO2. Pressurized CO2 is then fed into the two cells, which are periodically rocked through −45° to 45° via an in-built motor system, at 4-second intervals. During rocking, the cells are also turned axially, ensuring that the steel balls are in contact with the polymer spread on the walls of the cells. Temperature of the system and pressure in the two cells are continuously recorded by data acquisition. For the experiments at 17 and 24 MPa, Teledyne ISCO 500 D syringe pumps are used to pressurize the cells with CO2, while a Vindum Engineering 6K piston displacement pump is used for the 31 MPa experiments.
Initially, the pressure is maintained between 6 and 7 MPa as temperature is increased from 297 to 308 K (297–323 K, for experiments at higher temperature), at a rate of 0.122 K/min. After stabilizing at the final desired temperature, the cells are further pressurized with CO2 to the final pressure. The temperature and pressure conditions are maintained, along with periodic rocking of the cells, for a duration of five hours. The solubility of the polymers at the final stabilized pressure and temperature are visually observed.
Careful visual measurements are conducted to determine the concentration of polymer solubility in CO2 at desired pressure and temperature. We first use two different concentrations of the polymer (one at a low value and the other at significantly higher value, likely above the solubility limit) in the two sapphire cells. If single phase is observed with no visible polymer remaining in the cell, that concentration is determined to be fully soluble. Once an approximate range is established (lower concentration limit - soluble, upper concentration limit- insoluble), we perform additional runs by increasing lower limit concentration and decreasing upper limit, to determine the solubility limit for the polymer at the given pressure and temperature. The difference between concentrations in the two cells is less than 5% in all the tests with low molecular weight polymers. It is less than 10% for the heavier polymers (molecular weight greater than 4000) due to low solubility. The method takes many iterations but provides a high degree of confidence in all measurements. The method also embeds repeatability.
Viscosity measurements
A moving piston viscometer is used for viscosity measurements. The viscometer unit is connected in line with the rocking cell setup. The Visco Pro 2,100 viscometer (from Cambridge Viscosity Inc) consists of a sensor in which a piston is moved from one end of the sensor to the other with electromagnetic coils. The sensor also includes a temperature probe. This system is highly accurate for our experiments as viscosity measurements can be performed directly after the solubility measurements, thereby requiring less fluid; the time is also saved. Less than 4 mL of fluid is required in the sensor to determine the viscosity. The sensor is initially purged with CO2, and then connected to the outlet of the solubility setup. By opening the outlet valves for the two sapphire cells, the mixture of CO2 and polymer flows into the viscometer. During flow of fluid into the viscometer, we stabilize flow by CO2 injection pump at one end of the system to stabilize the pressure at the desired value. Temperature control in the lines connecting the viscometer and rocking cell setup is through heating bands. Real-time readings of viscosity and temperature of the fluid in the sensor are recorded.
A second measurement setup is used for viscosity measurements of selected polymers at 24 MPa for comparison and all the measurements at 31 MPa. The setup is a custom-built accumulator with volume of 500 mL, maximum pressure of 48 MPa, and maximum temperature of 393 K. The accumulator consists of a magnetic drive mixer equipped with four turbine impellers, which facilitate rigorous mixing of the polymer with CO2. A speed of 1,500 rpm is employed for mixing. We allow several hours for complete mixing and stabilization based on periodic viscosity measurements. Once the mixture is prepared, the viscometer is connected to the accumulator. Very close viscosity results are obtained from the two independent setups, which confirm the measurement accuracy. The accumulator has also a piston; this setup is used for core flooding experiments, to investigate the efficiency of viscosified CO2 in sequestration in saline aquifers. Pressure drop across the rock is in agreement with the CO2 viscosification factor. The core flooding results are being prepared for submission.
Acknowledgement
The first part of the project for measurements was supported by ExxonMobil. The continuation of the project (viscosity measurements at 24 MPa, solubility and viscosity measurements at 31 MPa, and testing of the additional polymers supplied) was supported by Equinor. The support is much appreciated. We also acknowledge the initial solubility measurements for the screening of polymers performed by Sarah Chavez and her literature review of solubility measurements.
Author contributions
Conceptualization, T.K. and A.F.; Experimental work, T.K.; Writing – Review & Editing, T.K. and A.F.; Supervision, A.F.; Funding Acquisition, A.F.
Declaration of interests
The authors declare no competing interests.
Published: May 20, 2022
Data and code availability
-
•
All data generated in this study is included in the manuscript.
-
•
This study did not generate custom code.
-
•
Any additional information required to analyze the data reported in this paper is available from the lead contact upon request.
References
- Al Hinai N.M., Saeedi A., Wood C.D., Myers M., Valdez R., Sooud A.K., Sari A. Experimental evaluations of polymeric solubility and thickeners for supercritical CO2 at high temperatures for enhanced oil recovery. Energy Fuels. 2018;32:1600–1611. doi: 10.1021/acs.energyfuels.7b03733. [DOI] [Google Scholar]
- Al Maksoud W., Saidi A., Samantaray M.K., Abou-Hamad E., Poater A., Ould-Chikh S., Guo X., Guan E., Ma T., Gates B.C., Basset J.M. Docking of tetra-methyl zirconium to the surface of silica: a well-defined pre-catalyst for conversion of CO2 to cyclic carbonates. Chem. Commun. 2020;56:3528–3531. doi: 10.1039/C9CC07383C. [DOI] [PubMed] [Google Scholar]
- Arayachukiat S., Kongtes C., Barthel A., Vummaleti S.V.C., Poater A., Wannakao S., Cavallo L., D’Elia V. Ascorbic acid as a bifunctional hydrogen bond donor for the synthesis of cyclic carbonates from CO2 under ambient conditions. ACS Sustain. Chem. Eng. 2017;5:6392–6397. doi: 10.1021/acssuschemeng.7b01650. [DOI] [Google Scholar]
- Bae J.H., Irani C.A. A laboratory investigation of viscosified CO2 process. SPE Adv. Technol. Ser. 1993;1:166–171. doi: 10.2118/20467-PA. [DOI] [Google Scholar]
- Besnard M., Cabaço M.I., Danten Y. Transient complex formation in CO2− hexafluorobenzene mixtures: a combined Raman and ab initio investigation. J. Phys. Chem. A. 2009;113:184–192. doi: 10.1021/jp8068267. [DOI] [PubMed] [Google Scholar]
- Britt D., Furukawa H., Wang B., Glover T.G., Yaghi O.M. Highly efficient separation of carbon dioxide by a metal-organic framework replete with open metal sites. Proc. Natl. Acad. Sci. 2009;106:20637–20640. doi: 10.1073/pnas.0909718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler K., Pouillot F.L.L., Eckert C.A. Phase equilibria of alkanes in natural gas systems. 3. Alkanes in carbon dioxide. J. Chem. Eng. Data. 1996;41:6–10. doi: 10.1021/je950138a. [DOI] [Google Scholar]
- Chen R., Zheng J., Ma Z., Zhang X., Fan H., Bittencourt C. Evaluation of CO2-philicity and thickening capability of multichain poly (ether-carbonate) with assistance of molecular simulations. J. Appl. Polym. Sci. 2020;138 doi: 10.1002/app.49700. [DOI] [Google Scholar]
- Davani E., Falcone G., Teodoriu C., McCain W.D. Rolling ball viscometer calibration with gas over whole interest range of pressure and temperature improves accuracy of gas viscosity measurement. Ind. Eng. Chem. Res. 2012;51:15276–15281. doi: 10.1021/ie301751y. [DOI] [Google Scholar]
- Dezfuli M.G., Jafari A., Gharibshahi R. Optimum volume fraction of nanoparticles for enhancing oil recovery by nanosilica/supercritical CO2 flooding in porous medium. J. Pet. Sci. Eng. 2020;185:106599. doi: 10.1016/j.petrol.2019.106599. [DOI] [Google Scholar]
- Enick R.M., Olsen D.K., Ammer J.R., Schuller W. SPE Improved Oil Recovery Symposium. Society of Petroleum Engineers; 2012. Mobility and conformance control for CO2 EOR via thickeners, Foams, and gels--A literature review of 40 Years of research and pilot tests. [DOI] [Google Scholar]
- Feng Y., Haugen K., Firoozabadi A. Phase-field simulation of hydraulic fracturing by CO2, water and nitrogen in 2D and comparison with laboratory data. J. Geophys. Res. Solid Earth. 2021;126 doi: 10.1029/2021JB022509. [DOI] [Google Scholar]
- Fiorani G., Guo W., Kleij A.W. Sustainable conversion of carbon dioxide: the advent of organocatalysis. Green. Chem. 2015;17:1375–1389. doi: 10.1039/C4GC01959H. [DOI] [Google Scholar]
- Firoozabadi A. McGraw Hill Education; 2016. Thermodynamics and Applications of Hydrocarbon Energy Production. [Google Scholar]
- Firoozabadi A., Myint P.C. Prospects for subsurface CO2 sequestration. AIChE J. 2010;56:1398–1405. doi: 10.1002/aic.12287. [DOI] [Google Scholar]
- Gallo G., Erdmann E., Cavasotto C.N. Evaluation of silicone fluids and resins as CO2 thickeners for enhanced oil recovery using a computational and experimental approach. ACS Omega. 2021;6:24803–24813. doi: 10.1021/acsomega.1c03660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandomkar A., Sharif M. Nano composites performance as direct thickeners for gas based enhanced oil recovery, a new approach. J. Pet. Sci. Eng. 2020;194:107491. doi: 10.1016/j.petrol.2020.107491. [DOI] [Google Scholar]
- Gama Goicochea A., Firoozabadi A. Atomistic and Mesoscopic simulations of the structure of CO2 with fluorinated and nonfluorinated copolymers. The J. Phys. Chem. C. 2019;123:17010–17018. doi: 10.1021/acs.jpcc.9b04293. [DOI] [Google Scholar]
- Goicochea A.G., Firoozabadi A. CO2 viscosification by functional molecules from Mesoscale simulations. J. Phys. Chem. C. 2019;123:29461–29467. doi: 10.1021/acs.jpcc.9b08589. [DOI] [Google Scholar]
- Graessley W.W. Effect of long branches on the flow properties of polymers. Acc. Chem. Res. 1977;10:332–339. doi: 10.1021/ar50117a004. [DOI] [Google Scholar]
- Gu Y., Zhang S., She Y. Effects of polymers as direct CO2 thickeners on the mutual interactions between a light crude oil and CO2. J. Polym. Res. 2013;20:61. doi: 10.1007/s10965-012-0061-9. [DOI] [Google Scholar]
- Heller J.P., Dandge D.K., Card R.J., Donaruma L.G. Direct thickeners for mobility control of CO2 floods. Soc. Pet. Eng. J. 1985;25:679–686. doi: 10.2118/11789-PA. [DOI] [Google Scholar]
- Huang Z., Shi C., Xu J., Kilic S., Enick R.M., Beckman E.J. Enhancement of the viscosity of carbon dioxide using styrene/fluoroacrylate copolymers. Macromolecules. 2000;33:5437–5442. doi: 10.1021/ma992043+. [DOI] [Google Scholar]
- Hunter I.N., Marsh G., Matthews G.P., Smith E.B. Argon+ carbon dioxide gaseous mixture viscosities and anisotropic pair potential energy functions. Int. J. Thermophys. 1993;14:819–833. doi: 10.1007/BF00502110. [DOI] [Google Scholar]
- Jafari S., Khezrnejad A., Shahrokhi O., Ghazanfari M.H., Vossoughi M. Experimental investigation of heavy oil recovery by continuous/WAG injection of CO2 saturated with silica nanoparticles. Int. J. Oil Gas Coal Technol. 2015;9:169–179. doi: 10.1504/IJOGCT.2015.067494. [DOI] [Google Scholar]
- Jennings D.W., Teja A.S. Vapor-liquid equilibria in the carbon dioxide-1-hexene and carbon dioxide-1-hexyne systems. J. Chem. Eng. Data. 1989;34:305–309. doi: 10.1021/je00057a014. [DOI] [Google Scholar]
- Kilic S., Enick R.M., Beckman E.J. Fluoroacrylate-aromatic acrylate copolymers for viscosity enhancement of carbon dioxide. J. Supercrit. Fluids. 2019;146:38–46. doi: 10.1016/j.supflu.2019.01.001. [DOI] [Google Scholar]
- Kim C.H., Vimalchand P., Donohue M.D. Vapor-liquid equilibria for binary mixtures of carbon dioxide with benzene, toluene and p-xylene. Fluid Ph. Equilibria. 1986;31:299–311. doi: 10.1016/0378-3812(86)87014-5. [DOI] [Google Scholar]
- Lemaire P.C., Alenzi A., Lee J.J., Beckman E.J., Enick R.M. Thickening CO2 with direct thickeners, CO2-in-Oil emulsions, or nanoparticle dispersions: literature review and experimental validation. Energy Fuels. 2021;35:8510–8540. doi: 10.1021/acs.energyfuels.1c00314. [DOI] [Google Scholar]
- Liang W., Bhatt P.M., Shkurenko A., Adil K., Mouchaham G., Aggarwal H., Mallick A., Jamal A., Belmabkhout Y., Eddaoudi M. A tailor-made interpenetrated MOF with exceptional carbon-capture performance from flue gas. Chem. 2019;5:950–963. doi: 10.1016/j.chempr.2019.02.007. [DOI] [Google Scholar]
- Nagahama K., Konishi H., Hoshino D., Hirata M. Binary vapor-liquid equilibria of carbon dioxide-light hydrocarbons at low temperature. J. Chem. Eng. Japan. 1974;7:323–328. doi: 10.1252/jcej.7.323. [DOI] [Google Scholar]
- Natongchai W., Luque-Urrutia J.A., Phungpanya C., Solà M., D'Elia V., Poater A., Zipse H. Cycloaddition of CO2 to epoxides by highly nucleophilic 4-aminopyridines: establishing a relationship between carbon basicity and catalytic performance by experimental and DFT investigations. Org. Chem. Front. 2021;8:613–627. doi: 10.1039/D0QO01327G. [DOI] [Google Scholar]
- Ng H.J., Robinson D.B. Equilibrium-phase properties of the toluene-carbon dioxide system. J. Chem. Eng. Data. 1978;23:325–327. doi: 10.1021/je60079a020. [DOI] [Google Scholar]
- Pal N., Zhang X., Ali M., Mandal A., Hoteit H. Carbon dioxide thickening: a review of technological aspects, advances and challenges for oilfield application. Fuel. 2022 doi: 10.1016/j.fuel.2021.122947. [DOI] [Google Scholar]
- Petit J.R., Jouzel J., Raynaud D., Barkov N.I., Barnola J.M., Basile I., Bender M., Chappellaz J., Davis M., Delaygue G., et al. Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica. Nature. 1999;399:429–436. doi: 10.1038/20859. [DOI] [Google Scholar]
- Reverchon E., Russo P., Stassi A. Solubilities of solid octacosane and triacontane in supercritical carbon dioxide. J. Chem. Eng. Data. 1993;38:458–460. doi: 10.1021/je00011a034. [DOI] [Google Scholar]
- Saure C. 1996. Untersuchungen zur Anreicherung von Squalen und Tocopherolen mittels Gegenstromextraktion mit überkritischem Kohlendioxid. na. [Google Scholar]
- Schmitt W.J., Reid R.C. The solubility of paraffinic hydrocarbons and their derivatives in supercritical carbon dioxide. Chem Eng. Commun. 1988;64:155–176. doi: 10.1080/00986448808940234. [DOI] [Google Scholar]
- Shi Q., Jing L., Qiao W. Solubility of n-alkanes in supercritical CO2 at diverse temperature and pressure. J. CO2 Utilization. 2015;9:29–38. doi: 10.1016/j.jcou.2014.12.002. [DOI] [Google Scholar]
- Sovova H., Jez J., Khachaturyan M. Solubility of squalane, dinonyl phthalate and glycerol in supercritical CO2. Fluid Ph. Equilibria. 1997;137:185–191. doi: 10.1016/S0378-3812(97)00102-7. [DOI] [Google Scholar]
- Sun W., Sun B., Li Y., Huang X., Fan H., Zhao X., Sun H., Sun W. Thickening supercritical CO2 with π-stacked Co-polymers: molecular insights into the role of intermolecular interaction. Polymers. 2018;10:268. doi: 10.3390/polym10030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trickett K., Xing D., Enick R., Eastoe J., Hollamby M.J., Mutch K.J., Rogers S.E., Heenan R.K., Steytler D.C. Rod-like micelles thicken CO2. Langmuir. 2010;26:83–88. doi: 10.1021/la902128g. [DOI] [PubMed] [Google Scholar]
- Wagner Z., Wichterle I. High-pressure vapour—liquid equilibrium in systems containing carbon dioxide, 1-hexene, and n-hexane. Fluid Ph. Equilibria. 1987;33:109–123. doi: 10.1016/0378-3812(87)87006-1. [DOI] [Google Scholar]
- Xu J., Enick R.M. SPE Annual Technical Conference and Exhibition. Society of Petroleum Engineers; PA: 2001. Thickening carbon dioxide with the fluoroacrylate-styrene copolymer. SPE 71497. [DOI] [Google Scholar]
- Zaberi H.A., Lee J.J., Enick R.M., Beckman E.J., Cummings S.D., Dailey C., Vasilache M. An experimental feasibility study on the use of CO2-soluble polyfluoroacrylates for CO2 mobility and conformance control applications. J. Pet. Sci. Eng. 2020;184:106556. doi: 10.1016/j.petrol.2019.106556. [DOI] [Google Scholar]
- Zhang S., She Y., Gu Y. Evaluation of polymers as direct thickeners for CO2 enhanced oil recovery. J. Chem. Eng. Data. 2011;56:1069–1079. doi: 10.1021/je1010449. [DOI] [Google Scholar]
- Zhang Y., Zhu Z., Tang J. Investigation on modified polyether as an efficient CO2 thickener. New J. Chem. 2021;45:651–656. doi: 10.1039/D0NJ02442B. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
-
•
All data generated in this study is included in the manuscript.
-
•
This study did not generate custom code.
-
•
Any additional information required to analyze the data reported in this paper is available from the lead contact upon request.