Abstract
Objective
To investigate longitudinal changes in the blood concentration of fibroblast growth factor 23 (FGF23) from midlife to late life and their major predictors in the general population.
Patients and Methods
In 14,444 participants of the Atherosclerosis Risk in Communities Study, we analyzed the association of 31,095 measurements of serum intact FGF23 with age using data from 3 visits (visit 2 [N=13,460; mean age, 57 years]; visit 3 [N=12,323; mean age, 60 years]; and visit 5 [N=6122; mean age, 76 years]) and a linear mixed-effects model. Among 5804 participants who had FGF23 measurements at both visits 3 and 5, we explored predictors of FGF23 change from midlife to late life using linear regression models. Prespecified risk factors were estimated glomerular filtration rate, body mass index, ever smoking, ever drinker, diabetes, hypertension, history of cardiovascular disease, total cholesterol, and high-density lipoprotein cholesterol.
Results
Median FGF23 concentrations were 41.9 pg/mL (interquartile interval [IQI], 33.9 to 51.8 pg/mL) at visit 2, 38.3 pg/mL (IQI, 30.6 to 48.3 pg/mL) at visit 3, and 55.0 pg/mL (IQI, 44.4 to 70.3 pg/mL) at visit 5. A linear mixed-effects model showed that the association of FGF23 with age was nonlinear, with a slight decline or no change in age 45-60 years and a monotonic increase in age greater than or equal to 65 years (FGF23, +10 to 15 pg/mL per 10 years of age). In a multivariable linear regression model, significantly greater increases in FGF23 were noted, with midlife estimated glomerular filtration rate less than 60 mL/min per 1.73 m2 vs more than or equal to 60 mL/min per 1.73 m2 (ΔFGF23, +4.4 pg/mL [95% CI, 0.9 to 8.0]), diabetes vs no diabetes (ΔFGF23, +6.2 pg/mL [95% CI, 4.1 to 8.3]), and hypertension vs no hypertension (ΔFGF23, +4.1 pg/mL [95% CI, 2.7 to 5.4]).
Conclusion
FGF23 did not show any major changes in midlife but increased linearly in late life. Reduced kidney function, diabetes, and hypertension were robustly associated with a greater increase in FGF23. Further investigations are needed to understand the potential mechanisms linking these conditions to an increase in FGF23 concentrations.
Abbreviations and Acronyms: ARIC, Atherosclerosis Risk in Communities; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; FGF23, fibroblast growth factor 23
Fibroblast growth factor 23 (FGF23) is an endocrine hormone that is essential for maintaining normal blood phosphorus concentrations by inducing phosphaturia and suppressing vitamin D production. The gene encoding FGF23 was first identified in patients with autosomal dominant hypophosphatemic rickets,1 who had missense mutations in FGF23 leading to excess production of FGF23. Since an assay for FGF23 measurement in human blood was first developed in 2003,2 a growing body of evidence suggests that elevated FGF23 levels are associated with adverse outcomes, including cardiovascular disease (CVD) and mortality.3, 4, 5
Reduced kidney function is the most common cause of elevated FGF23 concentrations,6,7 as a compensatory mechanism to maintain normal blood phosphorus levels in this clinical condition. As kidney function usually declines with aging, it is plausible that the level of FGF23 changes over the lifespan. However, data on the association of FGF23 with age are sparse. A few studies have assessed changes in FGF23 in patients with advanced chronic kidney disease8,9; however, no study has investigated longitudinal changes in FGF23 from midlife to late life and their predictors in the general population. Such knowledge will help us understand the pathophysiological involvement of FGF23 beyond its link to bone mineral disorders and kidney diseases.
We analyzed more than 30,000 measurements of FGF23 collected over 20 years and across 3 study visits in nearly 15,000 participants in the community-based Atherosclerosis Risk in Communities (ARIC) Study. The objectives of this analysis were to characterize the changes in FGF23 concentrations from midlife to late life and to identify their major predictors.
Patients and Methods
Study Population
The ARIC Study enrolled 15,792 participants aged 45-64 years from 1987 to 1989 (visit 1) from 4 US communities (Forsyth County, NC; Jackson, MS; suburbs of Minneapolis, MN; and Washington County, MD).10 Serum FGF23 concentrations were measured at 3 study visits that occurred in 1990-1992 (visit 2), 1993-1995 (visit 3), and 2011-2013 (visit 5). The present study included participants who self-identified as White or Black race and had at least one FGF23 measurement done. Data on FGF23 concentrations were available for approximately 95% of the participants at each visit: 13,460 of 14,161 participants at visit 2; 12,323 of 12,821 participants at visit 3; and 6122 of 6514 participants at visit 5. Accordingly, we analyzed 31,095 FGF23 measurements in 14,444 participants. All participants provided written informed consent. The study was approved by the institutional review board at each study site and conducted in accordance with the Declaration of Helsinki.
FGF23 Measurements and FGF23 Change From Midlife to Late Life
In the ARIC Study, FGF23 concentrations were measured in 2 ancillary studies. The first study measured FGF23 in 2011-2012 in stored samples collected from participants at visit 2.4 The second study measured FGF23 in 2018-2019 in stored samples collected from participants at visit 3 (1993-1995) and visit 5 (2011-2013). Both ancillary studies measured intact FGF23 in serum samples using the 2-site enzyme-linked immunosorbent assay kit (Kainos Laboratories, Inc) at the Advanced Research and Diagnostic Laboratory, University of Minnesota. The laboratory interassay coefficients of variation were 8.1% and 5.4% at mean concentrations of 23.7 and 87.2 pg/mL, respectively. To evaluate the drift over time, we conducted measurements in 2018 in a random sample of 99 serum samples collected at visit 2. There were no significant differences in FGF23 concentrations between the assays in the 2 ancillary studies (P value for difference, .358; mean FGF23 difference, −1.2 pg/mL with mean FGF23 of 45 pg/mL; interassay coefficient of variation, 13%).
Other Variables
Data on participant characteristics were obtained at each visit. Age, sex, race, smoking history (ever vs never smoker), and alcohol consumption (ever vs never drinker) were self-reported. Body mass index was calculated as body weight in kilograms divided by the square of height in meters. Diabetes was defined as a self-reported diagnosis of diabetes by a physician, fasting glucose more than or equal to 126 mg/dL, random glucose more than or equal to 200 mg/dL, or taking antidiabetic medications. Hypertension was defined as a systolic blood pressure of more than equal to 140 mmHg, diastolic blood pressure of more than or equal to 90 mmHg, or taking antihypertensive medication. Serum concentrations of total cholesterol and high-density lipoprotein cholesterol were measured using an enzymatic method. Estimated glomerular filtration rate (eGFR) was calculated using the 2012 Chronic Kidney Disease Epidemiology equation using serum creatinine and cystatin C.11 The history of CVD was defined as self-reported history of coronary heart disease, heart failure, or stroke at visit 1, or incident cases between visit 1 and the relevant study visits.4
Statistical Analyses
Participant characteristics were compared across the 3 study visits (ie, visits 2, 3, and 5). We used histograms to display the distributions of FGF23 concentrations at each visit. FGF23 concentrations at the 2.5th and 97.5th percentiles were reported as the lower and upper limits of the reference interval (central 95%).12 Because each individual had up to 3 FGF23 measurements, we used a linear mixed-effects model to assess the association of FGF23 concentrations with age, treating individuals as a random effect. We regressed FGF23 on age, which was modeled using a restricted cubic spline with knots at 50, 60, 70, and 80 years. To avoid erroneous influence by extreme values of FGF23, we winsorized values lower or higher than the 1st and 99th percentiles, respectively.13 The analysis was first performed for the entire population, and then was repeated on the basis of sex (men vs women) and race (White vs Black).
We examined factors associated with FGF23 change from midlife to late life, as calculated by FGF23 at visit 5 (2011-2013) subtracted by FGF23 at visit 3 (1993-1995) in 5804 participants. We used the difference in FGF23 between visits 3 and 5 for this analysis because FGF23 concentrations at visits 3 and 5 were simultaneously measured using the same lot of enzyme-linked immunosorbent assay kits, as described above, and overall we did not observe any major changes between visits 2 and 3 (Supplemental Figure 1, available online at http://www.mcpiqojournal.org).
Among the 5804 participants who had FGF23 measured both at visits 3 and 5, we used linear regression models to estimate FGF23 change as a dependent variable modeled as a continuous variable, regressed on prespecified clinical factors at visit 3: high body mass index (≥30 kg/m2 vs <30 kg/m2), smoking history (ever vs never smoker), alcohol consumption (ever vs never drinker), diabetes (yes vs no), hypertension (yes vs no), history of CVD (yes vs no), low eGFR (<60 mL/min per 1.73 m2 vs ≥60 mL/min per 1.73 m2), high total cholesterol (≥200 mg/dL vs <200 mg/dL), and low high-density lipoprotein cholesterol (<40 mg/dL vs ≥40 mg/dL). We also modeled FGF23 change as a categorical variable by quartile, and deployed logistic regression models to estimate the odds ratios for the highest quartile of FGF23 change, compared with the remaining 3 quartiles combined. For both linear and logistic regression analyses, multivariable models included the independent variables of age (continuous), sex (men vs women), race (White vs Black), and clinical factors as listed above. All analyses were performed using Stata version 15. A 2-sided P value of <.05 was considered statistically significant.
Results
Distributions of FGF23 in Midlife and Late Life
Of the 14,444 participants who had at least one FGF23 measurement, 8016 (55.5%) were women and 10,767 (74.5%) were White. The mean age was 57 years at visit 2, 60 years at visit 3, and 76 years at visit 5 (Table 1). The prevalence of diabetes, hypertension, history of CVD, and low eGFR (<60 mL/min per 1.73 m2) was higher at visit 5 than at visit 2 or 3.
Table 1.
Characteristics of ARIC Participants With FGF23 Measurement at Visit 2 (1990-1992), Visit 3 (1993-1995), and Visit 5 (2011-2013)
| Characteristic | Visit 2, 1990-1992 (n=13,460) | Visit 3, 1993-1995 (n=12,323) | Visit 5, 2011-2013 (n=6,122) |
|---|---|---|---|
| FGF23 (pg/mL), median (IQI) | 42 (34 and 52) | 38 (31 and 48) | 55 (44 and 70) |
| Age (y), mean (SD) | 57 (5.7) | 60 (5.7) | 76 (5.3) |
| Women, n (%) | 7,548 (56) | 6,843 (56) | 3,601 (59) |
| White, n (%) | 10,053 (75) | 9,552 (78) | 4,745 (78) |
| Body mass index (kg/m2), mean (SD) | 28 (5.4) | 28 (5.5) | 29 (5.7) |
| Body mass index ≥30 kg/m2, n (%) | 3,923 (29) | 4,061 (33) | 2,052 (34) |
| Ever smoker, n (%) | 8,045 (60) | 7,228 (59) | 3,353 (55) |
| Ever drinker, n (%) | 10,394 (77) | 9,273 (75) | 4,541 (74) |
| Diabetes, n (%) | 1,560 (12) | 1,853 (15) | 1,975 (32) |
| Hypertension, n (%) | 4,828 (36) | 5,001 (41) | 4,500 (74) |
| History of CVD, n (%) | 967 (7.2) | 1,033 (8.4) | 1,124 (18) |
| HDL-C (mg/dL), mean (SD) | 50 (17) | 52 (18) | 52 (14) |
| HDL-C <40 mg/dL, n (%) | 3,927 (29) | 3,092 (25) | 1,104 (18) |
| Total cholesterol (mg/dL), mean (SD) | 210 (40) | 208 (38) | 182 (42) |
| Total cholesterol ≥200 mg/dL, n (%) | 7,805 (58) | 6,953 (56) | 1,861 (30) |
| eGFR (mL/min per 1.73 m2), mean (SD) | 95 (17) | 84 (16) | 65 (18) |
| eGFR <60 mL/min per 1.73 m2, n (%) | 382 (2.8) | 820 (6.7) | 2,326 (38) |
ARIC, Atherosclerosis Risk in Communities; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; FGF23, fibroblast growth factor 23; HDL-C, high-density lipoprotein cholesterol; IQI, interquartile interval.
Distributions of FGF23 concentrations at visit 2 (Figure 1A) and visit 3 (Figure 1B) were overall normal and largely similar (median FGF23, 41.9 pg/mL [95% reference interval, 21.1 and 83.3 pg/mL] at visit 2 and 38.3 pg/mL [95% reference interval, 18.0 and 80.4 pg/mL] at visit 3, respectively). The distribution of FGF23 at visit 5 was slightly skewed to the right and had a wider distribution compared with those at visit 2 or 3 (Figure 1C) (median FGF23, 55.0 pg/mL [95% reference interval, 28.1 and 128.3 pg/mL]).
Figure 1.
Histograms of FGF23 concentrations at visit 2 (1990-1992), visit 3 (1993-1995), and visit 5 (2011-2013). A, FGF23 at visit 2 (n=13,460). B, FGF23 at visit 3 (n=12,323). C, FGF23 at visit 5 (n=6122).
Serum FGF23 Concentrations in Midlife and Late Life
Figure 2 shows the expected concentration of FGF23 regressed on age using all available measurements of FGF23 and a linear mixed-effects model. Overall, the FGF23 concentration declined slightly from the age of 45 to 55 years but was largely constant below the age of 60 years. Beyond the age of 60-65 years, the FGF23 concentration monotonically increased with age (FGF23, +10 to 15 pg/mL per 10 years of age). The associations were consistent between men and women as well as between White and Black participants, without evident differences in FGF23 concentrations by sex or race (Supplemental Figure 2, available online at http://www.mcpiqojournal.org).
Figure 2.
Predicted concentrations of FGF23 across the age spectrum from midlife to late life: Atherosclerosis Risk in Communities Study 1990-2013.
Major Predictors of Changes in FGF23 From Midlife to Late Life
Of the 5804 participants who had FGF23 measured both at visits 3 and 5, the median FGF23 change between visits 3 and 5 was +17.4 pg/mL (interquartile interval, +6.5 to +31.4 pg/mL) (median elapsed years, 17.8 years [interquartile interval, 17.1 to 17.4 years]) (Supplemental Figure 3, available online at http://www.mcpiqojournal.org), and an increase in FGF23 was seen in 86.1%.
In crude linear regression models, eGFR less than 60 mL/min per 1.73 m2 (vs ≥60 mL/min per 1.73 m2) was associated with a greater increase in FGF23 (ΔFGF23, +6.5 pg/mL [95% CI, 3.0 to 10.1]) (Table 2). Additionally, diabetes (vs no diabetes) was associated with a greater increase in FGF23 (+6.3 pg/mL [4.3 to 8.4 pg/mL]), with a similar magnitude as eGFR less than 60 mL/min per 1.73 m2. Other considerable predictors in these crude models were hypertension (vs no hypertension) (ΔFGF23, +4.6 pg/mL [95% CI, 3.3 to 5.8]) and a history of CVD (vs no history of CVD) (+3.9 pg/mL [95% CI, 1.5 to 6.2]) (Table 2).
Table 2.
Univariate and Multivariable Linear Regression Models for Factors Associated With FGF23 Change From Midlife to Late life: ARIC Study 1993-2013a
| Characteristic at visit 3 | No. | FGF23 (pg/mL) |
Linear regression analysis |
|||
|---|---|---|---|---|---|---|
| Visit 3 | Visit 5 | Mean change from visit 3 to visit 5 (95% CI) | Crude (95% CI) | Multivariableb (95% CI) | ||
| eGFR | ||||||
| ≥60 mL/min per 1.73 m2 | 5605 | 39.7 | 60.2 | 20.7 (20.1-21.3) | Reference (0) | Reference (0) |
| <60 mL/min per 1.73 m2 | 161 | 52.9 | 80.0 | 27.2 (21.9-32.4) | +6.5 (3.0 to 10.1) | +4.4 (0.9 to 8.0) |
| Diabetes | ||||||
| No | 5246 | 39.9 | 60.0 | 20.3 (19.7-20.9) | Reference (0) | Reference (0) |
| Yes | 520 | 41.8 | 68.1 | 26.6 (24.3-28.9) | +6.3 (4.3 to 8.4) | +6.2 (4.1 to 8.3) |
| Hypertension | ||||||
| No | 3865 | 39.2 | 58.2 | 19.3 (18.7-20.0) | Reference (0) | Reference (0) |
| Yes | 1901 | 41.8 | 65.8 | 23.9 (22.8-25.0) | +4.6 (3.3 to 5.8) | +4.1 (2.7 to 5.4) |
| History of CVD | ||||||
| No | 5391 | 39.9 | 60.3 | 20.6 (20.0-21.2) | Reference (0) | Reference (0) |
| Yes | 375 | 42.4 | 67.1 | 24.5 (21.7-27.2) | +3.9 (1.5 to 6.2) | +2.2 (−0.2 to 4.5) |
| Ever smoker | ||||||
| No | 2649 | 40.1 | 60.7 | 20.9 (20.0-21.7) | Reference (0) | Reference (0) |
| Yes | 3117 | 40.0 | 60.7 | 20.8 (20.0-21.6) | −0.0 (−1.2 to 1.1) | +1.2 (−0.0 to 2.5) |
| Body mass index | ||||||
| <30 kg/m2 | 4018 | 38.7 | 59.2 | 20.6 (19.9-21.3) | Reference (0) | Reference (0) |
| ≥30 kg/m2 | 1748 | 43.2 | 64.1 | 21.3 (20.2-22.4) | +0.7 (−0.6 to 2.0) | −0.5 (−1.8 to 0.9) |
| HDL-C | ||||||
| ≥40 mg/dL | 4498 | 39.2 | 60.1 | 21.0 (20.4-21.7) | Reference (0) | Reference (0) |
| <40 mg/dL | 1268 | 42.9 | 62.7 | 20.3 (18.9-21.7) | −0.7 (−2.1 to 0.7) | −0.5 (−2.0 to 1.1) |
| Total cholesterol | ||||||
| <200 mg/dL | 2618 | 39.9 | 60.4 | 20.7 (19.8-21.6) | Reference (0) | Reference (0) |
| ≥200 mg/dL | 3148 | 40.1 | 61.0 | 21.0 (20.2-21.8) | +0.3 (−0.9 to 1.4) | −0.6 (−1.8 to 0.6) |
| Ever drinker | ||||||
| No | 1358 | 40.7 | 63.0 | 22.1 (20.9-23.4) | Reference (0) | Reference (0) |
| Yes | 4408 | 39.8 | 60.0 | 20.5 (19.8-21.1) | −1.6 (−3.0 to 0.3) | −1.1 (−2.6 to 0.4) |
ARIC, Atherosclerosis Risk in Communities; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; FGF23, fibroblast growth factor 23; HDL-C, high-density lipoprotein cholesterol.
Multivariable model was adjusted for age, sex, race, eGFR, diabetes, history of CVD, total cholesterol, ever smoker, body mass index, HDL-C, and ever drinker.
In a multivariable linear regression model, diabetes showed the highest degree of association (adjusted ΔFGF23, +6.2 pg/mL [95% CI, 4.1 to 8.3]). Other noteworthy predictors for a greater increase in FGF23 concentrations included low eGFR (+4.4 pg/mL [95% CI, 0.9 to 8.0]) and hypertension (+4.1 pg/mL [95% CI, 2.7 to 5.4]) (Table 2). The association was borderline significant for a history of CVD and ever smoking. We also confirmed that the coefficient for age was significant (adjusted ΔFGF23, +3.4 pg/mL [95% CI, 2.3 to 4.6] per +10 years).
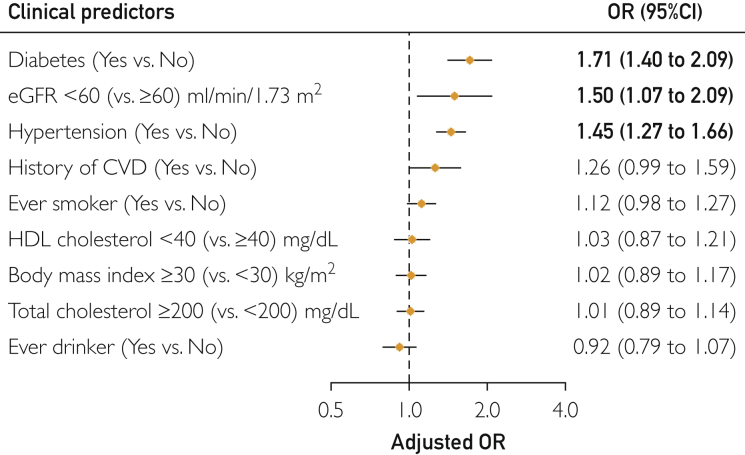
The associations in the case of low eGFR, diabetes, and hypertension were consistent and robust when FGF23 change was modeled as a categorical variable by quartile. In the multivariable logistic regression model, the adjusted odds ratios for the highest quartile of FGF23 change were 1.71 (95% CI, 1.40 to 2.09) for diabetes, 1.50 (95% CI, 1.07 to 2.09) for low eGFR, and 1.45 (95% CI, 1.27 to 1.66) for hypertension, compared with the remaining 3 quartiles combined (Figure 3). The associations were not significant for the other predictors.
Figure 3.
Multivariable logistic regression models for factors associated with FGF23 change from midlife to late life: ARIC Study 1993-2013. The model was adjusted for age, sex, race, estimated glomerular filtration rate (eGFR), diabetes, history of cardiovascular disease (CVD), total cholesterol, ever smoker, body mass index, high-density lipoprotein (HDL) cholesterol, and ever drinker. Circles indicate point estimates. Horizontal lines indicate 95% confidence intervals. Odds ratios in bold indicate statistical significance. OR = odds ratio.
Discussion
In this study, we examined the changes in FGF23 from midlife to late life and their predictors in the general population. We found that FGF23 concentrations were mostly unchanged or slightly decreased with age in midlife but linearly increased with age in late life. This association was consistent between men and women, as well as between White and Black participants. When we assessed the predictors of FGF23 change from midlife to late life, we confirmed that reduced kidney function was an independent predictor for a greater FGF23 increase. Additionally, we found that diabetes and hypertension were significantly and robustly associated with a greater increase in FGF23 from midlife to late life.
FGF23 distributions (eg, range and shape of histograms) were not homogeneous between midlife and late life. Using the intact FGF23, which is a direct measure of the biologically active form, rather than C-terminal FGF23,8,14 we found that the central 95% reference intervals are approximately 20 and 80 pg/mL in midlife and 30 and 130 pg/mL in late life. Such knowledge may help guide future FGF23 studies by offering a reference range of the intact FGF23 in the general population.12 A wider FGF23 distribution at visit 5 relative to visits 2 and 3 may suggest diverse health conditions across individuals in late life compared with midlife, a phenomenon shown in other biomarkers.15
The association of FGF23 with age was nonlinear (ie, slightly negative sloping or no change in midlife, and positive sloping in late life), and these findings were generally consistent between sex and racial groups. The underlying reasons for a slightly negative slope in midlife are not fully clear and may be within the range of measurement variability. Consistent with our finding, a previous cross-sectional analysis of 1130 healthy male volunteers in Austria has shown that the association of FGF23 with age was slightly inverse in age less than 60 years and evidently positive in age approximately 65 years or older.14 Future studies that examine changes in FGF23 regulatory pathways during midlife and late life may provide insights into the underlying reasons for this nonlinear association.
An independent association between reduced kidney function and a greater increase in FGF23 is consistent with the level of FGF23 increasing with reduced kidney function in response to the reduced capacity of urinary phosphorus excretion.6,7 Although several factors such as diabetes, hypertension, and older age often coexist with reduced kidney function, the association of reduced kidney function with FGF23 change remained strong after accounting for these factors. Our finding further supports the relevance of kidney diseases in FGF23 increase.
Diabetes showed a strong and robust association for FGF23 change from midlife to late life. In diabetes, tubular epithelial cells in the kidney are hypertrophied in response to glycosuria,16, 17, 18 and the function of sodium/phosphate cotransporters is upregulated.19 The upregulated sodium/phosphate cotransporters increase phosphate influx,20 which may trigger the production of FGF23. Indeed, sodium/phosphate cotransporters are a prime target of FGF23.21 A previous study showed that the administration of insulin increased the level of FGF23 in patients with diabetes.22 However, only 3.6% of participants in our study were using insulin at visit 3, so we did not explore this question in our sample. Future studies are needed to examine the pathophysiological link between diabetes and FGF23 increase.
Hypertension was another condition robustly associated with a greater FGF23 increase. The activation of the renin-angiotensin-aldosterone system in hypertension may play some role because wild-type rodents treated with angiotensin II showed an increased expression of FGF23 in bone with elevated circulating FGF23 levels.23 Additionally, in a study of transgenic mice, the activated calcineurin-nuclear factor of activated T-cell signaling, which is a pathway that is involved in the development of vascular disease (eg, hypertension) and atherosclerosis,24 resulted in increased circulating FGF23 levels.25 Of note, the calcineurin-nuclear factor of activated T-cell pathway is also known to be activated by FGF23,26,27 suggesting their bidirectional interaction.
Our findings should be interpreted considering several limitations. First, the analytic sample for FGF23 change required participants to have FGF23 concentrations both at visits 3 and 5. We confirmed that participant characteristics were largely similar between those who did and did not attend visit 5, which took place on a median of 18 years later (Supplemental Table, available online at http://www.mcpiqojournal.org), but we cannot rule out the possible survival bias. Second, FGF23 measurements were performed using blood samples collected at study visits, and therefore, the timing of the visits did not necessarily reflect the biological reasoning. Nonetheless, the measurement timing at visits 3 and 5 occurred during midlife and late life, respectively. Third, our study population consisted of middle-aged and older adults residing in the United States. Extrapolation of our findings to other populations should be done cautiously. Finally, residual confounding is possible because of the observational nature of this study.
Conclusion
FGF23 concentrations did not show major changes in midlife but increased linearly in late life. We confirmed that reduced kidney function was a major predictor of greater FGF23 change from midlife to late life and also identified diabetes and hypertension as additional predictors. Further investigations are warranted to understand the potential mechanisms linking diabetes and hypertension with an increase in FGF23 concentrations.
Acknowledgments
We thank the staff and participants of the Atherosclerosis Risk in Communities Study for their important contributions.
Footnotes
Grant Support: The Atherosclerosis Risk in Communities Study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, and Department of Health and Human Services, under contract nos. HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, and HHSN268201700004I. Dr. Selvin was supported by NIH/NHLBI grant K24 HL152440, and visit 2 FGF23 measurements by R01-HL103706. This specific study was supported by research funding from Kyowa Kirin (Principal Investigator: Kunihiro Matsushita, MD, PhD).
Potential Competing Interests: Dr Matsushita received a personal fee from Kyowa Kirin outside of the submitted work. Author Karger serves as a consultant to Roche Diagnostics, and has received research support from Siemens Healthcare Diagnostics, both unrelated to this work. The other authors report no competing interests.
Supplemental material can be found online at http://www.mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.ADHR Consortium Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26(3):345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 2.Jonsson K.B., Zahradnik R., Larsson T., et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348(17):1656–1663. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 3.Ishigami J., Taliercio J.T., Feldman H.I., et al. Fibroblast growth factor 23 and risk of hospitalization with infection in chronic kidney disease: the chronic renal insufficiency cohort (CRIC) study. J Am Soc Nephrol. 2020;31(8):1836–1846. doi: 10.1681/ASN.2019101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lutsey P.L., Alonso A., Selvin E., et al. Fibroblast growth factor-23 and incident coronary heart disease, heart failure, and cardiovascular mortality: the Atherosclerosis Risk in Communities study. J Am Heart Assoc. 2014;3(3) doi: 10.1161/JAHA.114.000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishigami J., Grams M.E., Michos E.D., Lutsey P.L., Matsushita K. 25-Hydroxyvitamin D, fibroblast growth factor 23, and risk of acute kidney injury over 20 years of follow-up. Kidney Int Rep. 2021;6(5):1299–1308. doi: 10.1016/j.ekir.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isakova T., Wahl P., Vargas G.S., et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishigami J., Jaar B.G., Rebholz C.M., et al. Biomarkers of mineral and bone metabolism and 20-year risk of hospitalization with infection: the Atherosclerosis Risk in Communities study. J Clin Endocrinol Metab. 2017;102(12):4648–4657. doi: 10.1210/jc.2017-01868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isakova T., Cai X., Lee J., et al. Longitudinal FGF23 trajectories and mortality in patients with CKD. J Am Soc Nephrol. 2018;29(2):579–590. doi: 10.1681/ASN.2017070772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouma-de Krijger A., Bots M.L., Vervloet M.G., et al. Time-averaged level of fibroblast growth factor-23 and clinical events in chronic kidney disease. Nephrol Dial Transplant. 2014;29(1):88–97. doi: 10.1093/ndt/gft456. [DOI] [PubMed] [Google Scholar]
- 10.The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. the ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 11.Inker L.A., Schmid C.H., Tighiouart H., et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solberg H.E. International Federation of Clinical Chemistry (IFCC), Scientific Committee, Clinical Section, Expert Panel on Theory of Reference Values, and International Committee for Standardization in Haematology (ICSH), Standing Committee on Reference Values. Approved Recommendation (1986) on the theory of reference values. Part 1. The concept of reference values. J Clin Chem Clin Biochem. 1987;25(5):337–342. [PubMed] [Google Scholar]
- 13.Hastings C., Mosteller F., Tukey J.W., Winsor C.P. Low moments for small samples: a comparative study of order statistics. Ann Math Stat. 1947;18(3):413–426. 414. [Google Scholar]
- 14.Schoppet M., Hofbauer L.C., Brinskelle-Schmal N., et al. Serum level of the phosphaturic factor FGF23 is associated with abdominal aortic calcification in men: the STRAMBO study. J Clin Endocrinol Metab. 2012;97(4):E575–E583. doi: 10.1210/jc.2011-2836. [DOI] [PubMed] [Google Scholar]
- 15.Sebastiani P., Thyagarajan B., Sun F., et al. Age and sex distributions of age-related biomarker values in healthy older adults from the long life family study. J Am Geriatr Soc. 2016;64(11):e189–e194. doi: 10.1111/jgs.14522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf G., Ziyadeh F.N. Molecular mechanisms of diabetic renal hypertrophy. Kidney Int. 1999;56(2):393–405. doi: 10.1046/j.1523-1755.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- 17.Habib S.L. Alterations in tubular epithelial cells in diabetic nephropathy. J Nephrol. 2013;26(5):865–869. doi: 10.5301/jn.5000287. [DOI] [PubMed] [Google Scholar]
- 18.Morcos M., Sayed A.A., Bierhaus A., et al. Activation of tubular epithelial cells in diabetic nephropathy. Diabetes. 2002;51(12):3532–3544. doi: 10.2337/diabetes.51.12.3532. [DOI] [PubMed] [Google Scholar]
- 19.Li H., Ren P., Onwochei M., Ruch R.J., Xie Z. Regulation of rat Na+/Pi cotransporter-1 gene expression: the roles of glucose and insulin. Am J Physiol. 1996;271(6 Pt 1):E1021–E1028. doi: 10.1152/ajpendo.1996.271.6.E1021. [DOI] [PubMed] [Google Scholar]
- 20.Vinke J.S.J., Heerspink H.J.L., de Borst M.H. Effects of sodium glucose cotransporter 2 inhibitors on mineral metabolism in type 2 diabetes mellitus. Curr Opin Nephrol Hypertens. 2019;28(4):321–327. doi: 10.1097/MNH.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gattineni J., Alphonse P., Zhang Q., Mathews N., Bates C.M., Baum M. Regulation of renal phosphate transport by FGF23 is mediated by FGFR1 and FGFR4. Am J Physiol Renal Physiol. 2014;306(3):F351–F358. doi: 10.1152/ajprenal.00232.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winther K., Nybo M., Vind B., Pedersen S.M., Højlund K., Rasmussen L.M. Acute hyperinsulinemia is followed by increased serum concentrations of fibroblast growth factor 23 in type 2 diabetes patients. Scand J Clin Lab Invest. 2012;72(2):108–113. doi: 10.3109/00365513.2011.640407. [DOI] [PubMed] [Google Scholar]
- 23.Pi M., Ye R., Han X., et al. Cardiovascular interactions between fibroblast growth factor-23 and angiotensin II. Sci Rep. 2018;8(1):12398. doi: 10.1038/s41598-018-30098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crabtree G.R., Olson E.N. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109(suppl):S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 25.Matsui I., Oka T., Kusunoki Y., et al. Cardiac hypertrophy elevates serum levels of fibroblast growth factor 23. Kidney Int. 2018;94(1):60–71. doi: 10.1016/j.kint.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Faul C., Amaral A.P., Oskouei B., et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mhatre K.N., Wakula P., Klein O., et al. Crosstalk between FGF23- and angiotensin II-mediated Ca2+ signaling in pathological cardiac hypertrophy. Cell Mol Life Sci. 2018;75(23):4403–4416. doi: 10.1007/s00018-018-2885-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.