Abstract
Background
Machado-Joseph Disease (MJD), or Spinocerebellar Ataxia Type 3 (SCA3), is a genetic disorder that causes progressive muscle weakness, loss of motor control, ataxia and permanent physical disability. Sleep disturbances are associated with MJD but remain poorly understood.
Objective
To investigate frequency and characteristics of sleep disorders and their association with health-related quality of life and psychosocial wellbeing for Aboriginal Australians living with MJD.
Methods
A convenience sample of MJD participants n = 24 participated in a semi-attended, ambulatory diagnostic sleep study to capture polysomnography, actigraphy and sleep diary data. Self-report measures collected were the Pittsburgh Sleep Quality Index (PSQI), STOP-BANG Questionnaire for Obstructive Sleep Apnoea (OSA), International Restless Legs Syndrome Study Group rating scale (IRLS), Kessler-5 (K5) and EuroQoL-5 Dimension (EQ5D). Caregivers (n = 22) reported EQ-5D, K5 and bed partners’ sleep behaviour (Mayo Sleep Questionnaire-Informant). Environmental factors were measured.
Results
We observed Nocturia, Sleep Related Leg Cramps, OSA, REM Behaviour Disorder, and RLS, respectively in 100%, 71%, 47%, 43%, and 33% of participants with a significant positive correlation between Body mass index (BMI) and Apnoea hypopnea index (AHI). The majority of sleep was spent in non-rapid eye movement sleep (NREM)-N2 stage (77.8% (67.7, 81.6)). Overcrowding (92%) and overnight care needs (42%) interrupted sleep. MJD participants and caregivers reported high psychological distress (K5 median 12.5 IQR 7, 16.5 & 8 IQR 6, 12 respectively).
Conclusion
Poor sleep quality and sleep disturbances are prevalent among this cohort. Disease manifestations and environmental factors are driving factors. Larger sample sizes are required to predict risk factors and confirm observed associations.
Keywords: Machado-Joseph disease”, “Spinocerebellar Ataxia type 3”, “Ataxia”, “Sleep”, “Aboriginal”, “Indigenous”
Highlights
-
•
Aboriginal People living with MJD in Australia experience numerous sleep disorders.
-
•
Majority of sleep was spent in non-rapid eye movement sleep.
-
•
Overcrowding and overnight care needs interrupt sleep.
-
•
MJD participants and caregivers reported high psychological distress.
Abbreviations
- ATXN
Ataxin protein gene
- AHI
Apnoea hypopnea index
- AI
Arousal Index
- ASA
Australian Sleep Association
- BD
Bronwyn Daniels
- BMI
Body mass index
- BRPT
Board Registered Polysomnographic Technologists
- CAG
Cytosine, adenine and guanine
- CAQDS
Computer Assisted Qualitative Data Software
- CIs
Confidence Intervals
- DLG
Desireé LaGrappe
- DPH
Darwin Private Hospital
- DRSH
Darwin Respiratory and Sleep Health service
- ESS
Epworth Sleepiness Scale
- EQ5D
EuroQol- 5 Dimension
- GL
Gayangwa Lalara
- HREC
Human Research Ethics Committee
- IRLS
International Restless Legs Syndrome Scale
- IQRs
Interquartile ranges
- JGW
Julie Gungunbuy Wunungmurra
- K-5
Kessler- 5 scale
- LM
Libby Massey
- MJD
Machado-Joseph Disease
- MJDF
MJD Foundation (Australia)
- NATA
National Association of Testing Authorities
- NREM
Non-rapid eye movement
- NT
Northern Territory
- OSA
Obstructive sleep apnoea
- PSQI
Pittsburgh sleep quality index
- PLM
Periodic limb movement
- PSG
Polysomnography
- RBD
Rapid Eye Movement sleep behaviour disorder
- REM
Rapid eye movement
- RLS
Restless legs syndrome
- RPSGT
Registered polysomnographic technologist
- SCA3
Spinocerebellar Ataxia Type 3
- SDB
Sleep-disordered breathing
- SRLC
Sleep related leg cramps
- SQoL
Subjective quality of life
- TEHS
Top End Health Service
1. Introduction
Machado-Joseph Disease (MJD), or Spinocerebellar Ataxia Type 3 (SCA3), is an autosomal dominant, hereditary ataxia (Saute and Jardim, 2015). MJD is caused by an unstable cytosine, adenine and guanine (CAG) repeat DNA sequence. The resulting misfolded protein (Ataxin-3) leads to permanent destruction of neuronal cells predominantly in the cerebellum (Jacobi et al., 2015). Progressive muscle weakness, loss of voluntary motor control, ataxia and permanent physical disability are prominent features (Jacobi et al., 2015; Bettencourt et al., 2010). In common with other spinocerebellar ataxias, MJD presents with diffuse neurodegenerative involvement, resulting in both the classic motor and high frequency of non-motor manifestations (Bettencourt et al., 2010; Pedroso et al., 2013a). This is consistent with increasing recognition that the cerebellum rather than being a purely motor control structure also has non motor functions (Pedroso et al., 2013b; Grimaldi and Manto, 2012).
Non-motor manifestations also reduce quality of life, such as cramps, fatigue, cognitive affective disturbances and dystonia (Pedroso et al., 2013a). Sleep disturbances are frequently reported among people with MJD from the American continent (Pedroso et al., 2011a, 2011b, 2013a, 2013b; Silva et al., 2016), 5, 7-9; however, prevalence among other populations is sparsely explored. The cohort of this investigation are Indigenous Australians residing in remote communities who identify as Aboriginal. In Australia, the term Indigenous encompasses Aboriginal and Torres Strait Islander people. A general experience of poor health and low socioeconomic status compared to non-Indigenous Australians requires locally effective disease management approaches within the context (Vallesi et al., 2018).
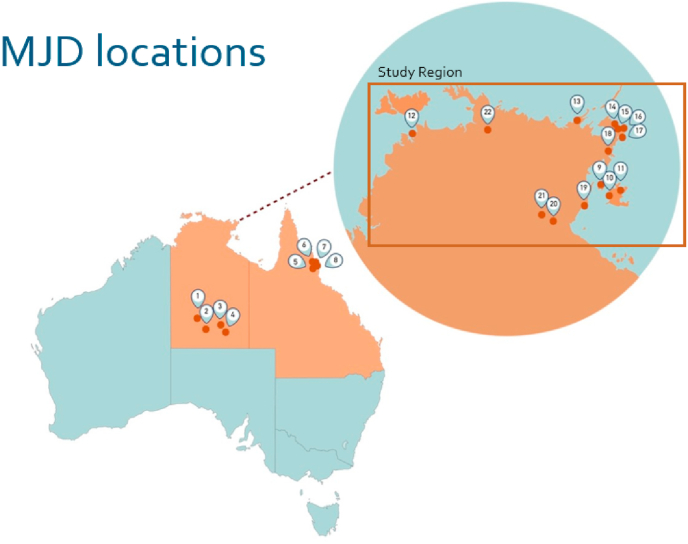
Aboriginal and Torres Strait Islander people comprise less than five per cent of the Australian population (Australian Institute of Health and Welfare, 2020a). The population demographics of the Northern Territory (NT) of Australia however, are unique: thirty percent (30%) identify as Aboriginal and/or Torres Strait Islander of which the majority (81%) live in remote or very remote communities (Australian Bureau of Statistics, 2020a; Australian Bureau of Statistics, 2020b; Australian Statistical Geography Standard Edition, 2020). Global MJD prevalence is highest in the remote East Arnhem region of the NT (Carr et al., 2019a) where the MJD Foundation (MJDF) has provided support to Aboriginal families living with the disease since 2008 (Fig. 1). This cohort reports consistent impaired sleep quality, with ensuing reduced quality of life. Their sleep experience is important to formally investigate due to the broad impacts on individuals, caregivers, families and communities, and prior reports of high sleep disorder prevalence among global MJD cohorts and other Aboriginal and Torres Strait Islander people in the region (Garg et al., 2020; Heraganahally et al., 2020a, 2020b, 2020c; Heraganahally and White, 2019; Kruavit et al., 2017; Mehra et al., 2020). Darwin Respiratory and Sleep Health service (DRSH) and the MJDF evaluated sleep disorder prevalence among a sample of the study population, as a critical step to provide appropriate care. The study assessed clinical parameters and caregivers’ experiences alongside associations with clinical sleep disturbance, polysomnography (PSG), actigraphy, health-related quality of life and psychosocial wellbeing.
Fig. 1.
Map showing communities across Australia where families affected by MJD reside and study region (Top End of the Northern Territory of Australia).
Source: MJD Foundation.
2. Methods
2.1. Setting and sample
Data were collected over 6 months in late 2017 to early 2018 from MJDF clients from three Aboriginal language groups in two remote communities and the capital city of the NT. MJD participants aged over 18 years had a molecular diagnosis or a minimum of three generations affected by MJD. All provided informed consent. Caregiver participants (i.e., sleep observers) were selected through referral sampling. Exclusion criteria included pregnancy, domestic and family violence or another acute crisis.
2.2. Study design
The multiple methods design comprised non-interventional, prospective assessments of frequency and characteristics of sleep disorders. Aboriginal co-researchers (BD, JWG, GL) were engaged from each language group to ensure language and cultural support and to maximise protocol adherence. They assisted in recruitment, delivery of instruments and face to face interactions. The quantitative arm utilised survey questionnaires to capture subjective measures of sleep quality, health-related quality of life and psychosocial wellbeing. To record physical activity and sleep patterns, objective evaluation was conducted by PSG and actigraphy (Lawrence and Muza, 2018; Collop et al., 2007). The qualitative arm, which will be reported on elsewhere, comprised unstructured interviews asking participants to describe their sleep and its impact on daily living. The design aimed to contextualise survey results and minimise response/social desirability bias related to cultural and linguistic differences (Amery, 2017). To minimise recall bias, medical histories were confirmed from medical records and chart abstractions. Well recognised social and environmental determinants of Aboriginal health, such as overcrowding, were measured to explore confounding (Australian Institute of Health and Welfare, 2020b). Overcrowding was defined as per the Australian Government's housing assistance measure using the Canadian National Occupancy Standard (when a minimum of 1 additional bedroom is required to adequately meet the housing needs of the occupants) (Australian Institute of Health and Welfare, 2021).
2.3. Polysomnography details
Each participant undertook a single night semi-attended sleep study. These were conducted in community to maximise adherence and engagement following high failure rates in previous unattended clinical studies of this cohort. 'In lab' conditions replicated in motels mimicked private sleep clinics. Studies for participants with severe-stage disease were conducted at their usual residence (e.g., supported accommodation or aged care facility). An upskilled registered nurse (DLG) performed PSGs with on call/in-person support from a qualified, registered polysomnographic technologist (RPSGT). In lieu of videorecording, MJD participants were discretely observed half hourly through an observation window to record position and aberrant behaviours, such as snoring or acting out dreams. MJD participants were also asked to complete a pre-PSG survey about typical sleep hygiene, patterns, latency, etc. and a post-PSG Morning Questionnaire (MornQ) to capture similar data for the night of the sleep study. Observations, survey administration and interviews were conducted by DLG and LM with support from GL, BD, and JGW.
PSGs were analysed and scored manually at DRSH by a qualified RPSGT utilising American Academy of Sleep Medicine recommendations (Jorgensen et al., 2020). Data extraction from PSG included information on sleep architecture and the presence and severity of sleep-disordered breathing (SDB). Obstructive Sleep Apnoea (OSA) was assessed using the apnoea hypopnea index (AHI) criteria. Participants were considered to have SDB/OSA if the total AHI was >5/hour. Oxygen saturation, arousal index (AI), periodic limb movement (PLM) and other abnormal behaviours, such as REM Behaviour Disorder (RBD), were analysed.
2.4. Actigraphy data
Actigraphy data was collected as an adjunct to PSG and sleep diaries to provide additional information about sleep latency. Actigraphy data were recorded on Actiwatch 2 (Philips Respironics, USA) monitors worn on the MJD participant's wrist for three consecutive nights with 30-s epoch length. Data were analysed using Actiware Software version 6.0.
2.5. Sleep diary data
For seven days, MJD participants were supported by data collectors (DLG and LM) and co-researchers (BD, JWG, GL) to document: time of food, alcohol, caffeine and any sedative medication intake; time to bed and awakening; time to sleep onset; duration of awakenings; and total sleep time. On the advice of the Aboriginal co-researchers, the number of days that sleep diary and actigraphy data were collected was reduced to seven days to increase adherence and decrease research burden.
2.6. Process
PSG was conducted on the initial night of data collection and actigraphy monitors were typically provided concurrently. Sleep diary data collection typically commenced on the same night to capture the previous 24 h in addition to the night of the PSG and continued for 5 nights for a total of seven days, allowing triangulation and verification of the data. Information about potential sources of sleep disturbance, such as substance use, nocturia and environmental conditions, was collected by researcher developed survey questions. Standardised survey questionnaires described below were also administered.
2.7. Standardised survey questionnaires
The Scale for assessment and rating of ataxia (SARA) was administered to MJD participants and scored by a single physiotherapist (PT) to omit bias from potential low interrater reliability. MJD participants were also supported to complete questionnaires measuring sleep apnoea (STOP-BANG)(Chung et al., 2016); sleep quality (Pittsburgh Sleep Quality Index (PSQI)) (Buysse et al., 1989), and restless leg syndrome (International Restless Legs Scale (IRLS)) (Group, 2003). The Mayo Sleep Questionnaire - Informant was administered to caregivers to establish sleep observer records of MJD participants’ sleep behaviours and presence of RBD (sn 100% sp 95%) (Boeve et al., 2013). All survey questionnaires were previously validated and showed high levels of reliability and validity across domains.
2.8. Quality of life & psychological parameter assessment
The EuroQol- 5 Dimension EQ5D to measure health-related quality of life and the modified Kessler- 5 (K5) to measure psychological distress were administered to all participants. Whilst not validated in this context, the EQ5D is commonly accepted as a reliable tool for use in Aboriginal Health research (Lavrencic et al., 2020). The K5 previously demonstrated validity and reliability among Indigenous Australians (McNamara et al., 2014).
2.9. Statistical consideration
Within this study, multiple statistical tests were utilised due to the diversity of data sources, scales and distributions. The equality of medians test (used to compare distribution of non-parametric data) identifies the median values for the overall cohort and compares the proportion of cases (i.e., MJD participants) and controls (i.e., caregivers) who are below the median value. The two-tailed proportions z-test was used to compare the equality of proportions between two populations for categorical parameters. Quantile regression estimates how a one unit change in an independent variable modifies a specified percentile of the dependant variable in the population and is used for non-parametrically distributed data. The median was selected as the independent variable in this study. Univariate linear regression was used for uniformly distributed data and estimates the effect of a change in an independent variable on the mean of the dependant variable. Logistic regression (used for continuous parameters) estimated the effect of a change in an independent variable on the change in a binary outcome.
Continuous parameters were tested for normality with Shapiro Wilks distribution test. Non-parametric parameters were presented as medians (interquartile ranges (IQRs)), normally distributed parameters as means (95% confidence intervals (CIs)), and categorical parameters as numbers (%). Psychosocial subjective questionnaire results were compared between MJD participants and caregivers via equality of medians test. A two-tailed proportions z-test was utilised to determine if an equal proportion of MJD participants and caregivers were in the 'low to moderate' or 'high to very high' distress brackets. The effect of using different sleep quality reporting types (i.e., subjective MornQ questionnaire, Sleep Diary, or objective PSG or actigraphy) to measure total sleep time and sleep latency was compared to the use of the pre-PSG self-report via quantile regression, reporting beta coefficients and 95% confidence intervals. PSG and actigraphy parameters where data were present for both were tested against each other by equality of medians test. The effects of potential risk factors (sex, age, alcohol and other drug use, disease severity, comorbidities) on sleep disorder presence (RBD and OSA) were tested by univariate logistic regression for continuous variables reporting odds ratios (ORs) (95% CIs). The effects of the aforementioned risk factors on total AHI were tested by univariate linear regression reporting beta coefficients (95% CIs). All data were analysed in STATA IC 15 (StataCorp, Texas) by TH and alpha set to 0.05.
3. Ethics approval
The Human Research Ethics Committee of the NT, Top End Health Service and Menzies School of Health Research (Reference no: HREC 17–2939) granted approvals. The community engaged approach followed the National Health and Medical Research Council guidelines for ethical conduct in research with Aboriginal and Torres Strait Islander people and communities with approvals granted by local land councils and community-controlled organisations (Council and Australia Co, 2018).
4. Results
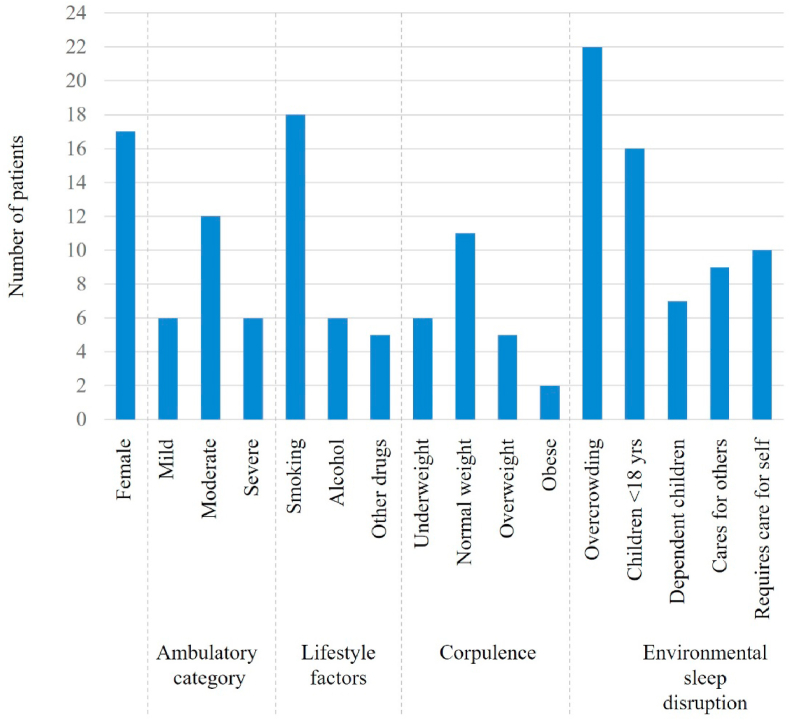
All eligible participants were approached, all agreed to participate (100%) and no participants withdrew. The total number of participants were n = 24 MJD participants and n = 22 caregivers (total n = 46). Two MJD participants did not have a regular adult caregiver. Most MJD participants were female (71%) with a mean age 45.6 years (95% CI 41.4, 49.7) (Table 1, Fig. 1), typically normal weight (46%) or underweight (25%), with a high prevalence of smoking history (75%). Three-quarters (75%) had some degree of independent ambulation. SARA (Schmitz- Hübsch et al., 2006) scores indicated moderate stage disease (median 14.5 IQR 7, 28.5). MJD participants reported a median 13.9 years (IQR 10.3, 17.4) living with MJD symptoms.
Table 1.
Demographic, clinical and lifestyle related factors of the study participants.
| Clinical Variables | Parameters | N = 24 |
|---|---|---|
| Age | Current age (years)a | 45.6 (41.4, 49.7) |
| Age of symptom onseta | 31.7 (27, 36.4) | |
| Length of time with MJD symptoms (years)a | 13.9 (10.3, 17.4) | |
| MJD severity | CAG (n = 11)b | 70 (69, 72) |
| SARAb | 14.5 (7, 28.5) | |
| Comorbidities | Number concurrent chronic diseasesb | 3 (2, 5) |
| Nocturia | 24 (100%) | |
| Nocturia – times per nightb | 1 (1, 2) |
Abbreviations: MJD, Machado-Joseph disease; CAG, cytosine-adenine-guanine; SARA, Scale for the assessment and rating of ataxia.
data expressed as mean (95% confidence interval).
data expressed as median (interquartile range).
MJD participants reported higher levels of psychological distress (K5 median 12.5 IQR 7, 16.5) than caregivers (8 IQR 6, 12); however, the difference was not significant (Table 2). A greater proportion were likewise classed as having high or very high psychological distress (K5 >11) (58%) in comparison to caregivers (36%). Subjective health-related quality of life was lower among MJD participants; however, there was significant variability among caregivers.
Table 2.
Psychosocial parameters for MJD participants and caregivers.
| Patient | Caregivers | p-value | Remote Indigenous Australians (previous reports) (Cunningham and Paradies, 2012) | |
|---|---|---|---|---|
| EQ-5D SQoLa | 67.5 (52.5, 80) | 77 (55, 90) | 0.675 | |
| K5 scorea | 12.5 (7, 16.5) | 8 (6, 12) | 0.164 | |
| Low distress (score 5–11) | 10 (42%) | 14 (64%) | 0.094 | 73% |
| High distress (score 12–25) | 14 (58%) | 8 (36%) | 0.094 | 27% |
Abbreviations: EQ-5D, EuroQol 5 dimension; SQoL, Subjective quality of life; K5, Kessler 5 scale.
Continuous parameters were tested via equality of medians test, categorical parameters tested via two-tailed proportions z-test.
Data expressed as median (interquartile range).
Self-reported and observer-reported sleep scales were available for the majority of participants (Table 3). Most reported low (50%) to moderate (29%) risk for OSA on STOP-Bang and moderate impairment by PSQI (median 10 (6.5, 14)), yet half (47%) of sleep observers reported possible presence of OSA for the MJD participants. All participants (100%) noted nocturia as a proximate reason for sleep disturbance or waking along with frequent (88%) mention of pain and cramping. Sleep observers also reported presence of RBD by MAYO- Informant in nearly half (43%) of MJD participants. RLS was reported in a minority by both self (29%) and sleep observer (33%) report.
Table 3.
Self-reported and observer reported sleep indices.
| Tool | Measure | Outcome |
|---|---|---|
| PSQI | Global Score | 10 (6.5, 14) |
| IRLS | Global Score | 0 (0, 11) |
| Nil | 17 (71%) | |
| Mild (1–10) | 5 (21%) | |
| Moderate (11–20) | 1 (4%) | |
| Severe (21–30) | 1 (4%) | |
| STOP-Bang | Global Score | 2 (2, 3) |
| Low risk (0–2) | 12 (50%) | |
| Moderate risk (3–5) | 7 (29%) | |
| High risk (>5) | 5 (21%) | |
| MAYO - Informant (n=21) | RBD | 9 (43%) |
| RLS | 7 (33%) | |
| Sleepwalk | 0 (0%) | |
| OSA | 10 (47%) | |
| SLRC | 15 (71%) |
Abbreviations: PSQI, Pittsburgh sleep quality index; IRLS, International restless legs scale; RBD, REM sleep behaviour disorder; RLS, Restless leg syndrome; OSA, Obstructive sleep apnoea; SLRC, Sleep related leg cramps; MAYO, Mayo Sleep Questionnaire-Informant.
Continuous parameters expressed as median (interquartile range) and categorical parameters as number (percentage).
Reported sleep latency varied according to when the questionnaire was administered, with the pre-PSG questionnaire reporting the greatest variance (median 15 min (5, 45)), and the morning/post PSG reporting the smallest (median 10 min, (7.5, 15)) (Table 4). Self-reported sleep time had the greatest variance in the pre-PSG questionnaire, yet comparable variance for the morning post-PSG and sleep diary. Self-reported measures (pre-PSG questionnaire, MornQ/post-PSG questionnaire and Sleep diary) were compared to objective measures (PSG and actigraphy) to determine level of agreeance (Table 4). Quantile regression was run utilising pre-PSG questionnaire as the reference value for sleep latency and total sleep time. Pre-PSG reported sleep latency tended to report lower values in comparison to sleep diary and MornQ/post-PSG reports; however, the difference was only significant in comparison to Actigraphy results with mean values 34.5 min (19.31, 49.69) higher. Total sleep time reports were similar. The pre-PSG report tended to overestimate total sleep time in comparison to each other reporting type. This was only significant in comparison to Actigraphy which reported total sleep time to be a mean 162.8 (247.71, 77.89) minutes shorter.
Table 4.
Comparison of self-reported measures (3) and objective measures (2).
| Self-report pre-PSG | Sleep diary | MornQ/post-PSG | PSG | Actigraphy | |
|---|---|---|---|---|---|
| Sleep latency (minutes) | 15 | 23.48 | 10 | 12.5 | 47.59 |
| (5, 45) | (11.09, 35.63) | (7.5, 15) | (4, 22) | (33.83, 65.25) | |
| Beta (95% CI) | Reference | 10.71 | −5 | −1 | 34.5 |
| (-4.15, 25.57) | (-20.19, 10.19) | (-16.57, 14.57) | (19.31, 49.69) | ||
| p-value | – | 0.156 | 0.515 | 0.899 | <0.001 |
| Total sleep time (minutes) | 475 | 433.9 | 390 | 384.9 | 312 |
| (358.8, 615) | (371.8, 522.6) | (300, 450) | (337.75, 430.75) | (244.8, 400.8) | |
| Beta (95% CI) | Reference | −37.16 | −80 | −71 | −162.8 |
| (-120.2, 45.89) | (-163.95, 3.95) | (-158.1, 16.1) | (-247.71, −77.89) | ||
| p-value | – | 0.377 | 0.062 | 0.109 | <0.001 |
Abbreviations: PSG, Polysomnography; CI, Confidence interval.
p-value resulting from quantile regression.
Data expressed as median (interquartile range).
Objective sleep data from either PSG or actigraphy was available for 22 MJD participants (Table 5). Two sleep studies failed. Significant differences were noted between PSG and actigraphy results. Actigraphy recordings reported significantly reduced sleep efficiency in comparison to PSG recordings (p < 0.001), predominantly due to lengthened sleep latency (47.59 min (33.83, 65.25) vs. 12.5 min (4, 22), p < 0.001) as time awake after sleep onset did not significantly differ (p = 0.757). The majority of sleep was spent in non-rapid eye movement sleep (NREM)-N2 stage (77.8% (67.7, 81.6)) with only a minor proportion in REM sleep (6% (0, 7.9)). Total arousal index (10.6 (7, 13.4)) was largely from spontaneous arousal (5.4 (2.7, 6.5)) as opposed to limb related or respiratory arousals. Median AHI was in the 'mild severity' (AHI 5–15) OSA range (10.8 (3.7, 14)) with most events occurring in the NREM stage of sleep (AHI 10.1 (3, 14.5)). Oxygen saturation remained relatively stable through awakenings and each sleep stage, however dipped occasionally to a minimum noted level (89% (85, 93)).
Table 5.
PSG and actigraphy parameters for MJD participants.
| PSG (n = 22) | Actigraphy (n = 22) | p-value | ||
|---|---|---|---|---|
| Sleep latency (minutes) | 12.5 (4, 22) | 47.59 (33.83, 65.25) | <0.001 | |
| REM latency (minutes) | 175 (68, 273) | – | – | |
| Time awake after sleep onset (minutes) | 55.5 (28.05, 71.5) | 51.37 (25.25, 84.5) | 0.757 | |
| Total sleep time (minutes) | 384.9 (337.75, 430.75) | 312 (244.8, 400.8) | 0.031 | |
| Sleep efficiency (percentage) | 86.55 (79.65, 91.05) | 69.31 (43.68, 73.42) | <0.001 | |
| Sleep stage breakdown (absolute time in minutes) | N1 | 48.5 (28, 64) | ||
| N2 | 286 (248.5, 339) | |||
| N3 | 24 (0, 26.5) | |||
| REM | 21.5 (0, 34.5) | |||
| Sleep stage breakdown (relative time as percentage) | N1 | 11.8 (7.1, 15.5) | ||
| N2 | 77.8 (67.7, 81.6) | |||
| N3 | 5.8 (0, 7.2) | |||
| REM | 6 (0, 7.9) | |||
| Arousal indexes | Total | 10.6 (7, 13.4) | ||
| Respiratory | 4 (1.3, 5.7) | |||
| Limb related | 0 (0, 0) | |||
| Spontaneous | 5.4 (2.7, 6.5) | |||
| AHI | Total | 10.8 (3.7, 14) | ||
| NREM | 10.1 (3, 14.5) | |||
| REM | 2.5 (0, 12.5) | |||
| Oxygen saturation | Awake | 97 (96, 98) | ||
| NREM | 96 (95, 97) | |||
| REM | 97 (94, 97) | |||
| Average | 96 (95, 97) | |||
| Minimum | 89 (85, 93) | |||
Abbreviations: PSG, Polysomnography; REM, Rapid eye movement; NREM, Non-rapid eye movement; N1, N2, N3, Non-rapid eye movement stage 1, 2, 3; AHI, Apnoea-hypopnea index.
p-value for difference between PSG and actigraphy via equality of medians test.
Data expressed as median (interquartile range).
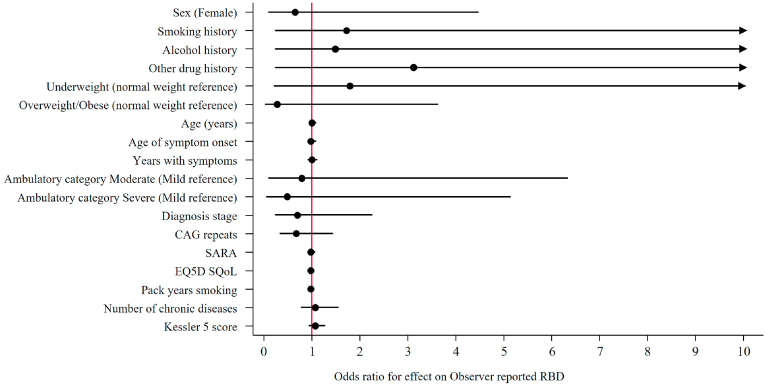
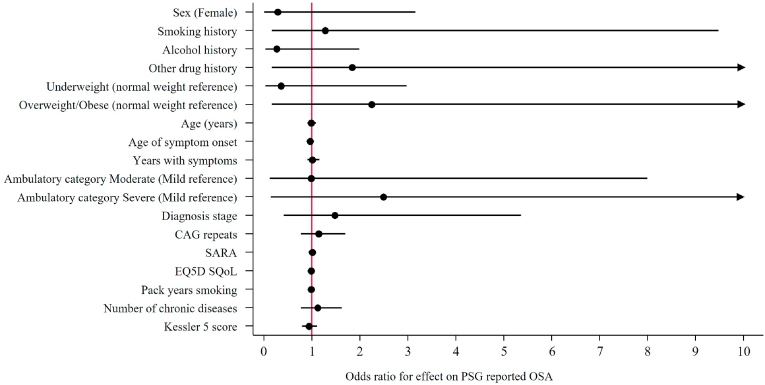
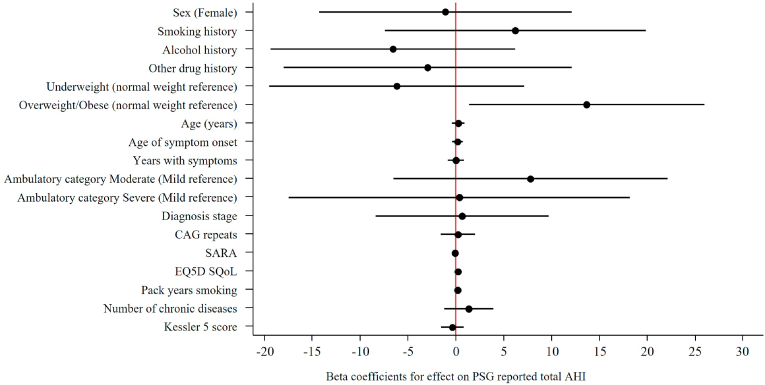
Logistic regression found no significant effect for independent parameters tested on the risk or odds ratios for RBD nor for PSG reported OSA (Fig. 2 & Fig. 3). Linear regression with total AHI as the outcome identified a significant positive correlation between BMI and AHI, particularly overweight/obese and AHI (Fig. 4). Each unit increase of BMI increased total AHI by 1.76 (95% CI 0.82, 2.70, p = 0.001), whereas the difference between overweight or obese MJD participants compared to normal weight MJD participants was 13.66 (95% CI 1.34, 25.99, p = 0.032). Although a significant effect of BMI on total AHI was noted, no significant effect was noted on the odds of OSA with increasing BMI (OR 1.08, 95% CI 0.90, 1.29, p = 0.431), nor the risk of OSA for MJD participants in the overweight/obese category (OR 2.25, 95% CI 0.19, 27.37, p = 0.525). No significant effect of MJD severity of disease (defined as CAG length, SARA, age of disease onset, years length of disease) on OSA nor RBD was found (see Fig. 5).
Fig. 2.
Clinical variables associated with MJD among study participants.
Fig. 3.
Univariate logistic regressions result of independent parameters effects on MAYO – Observer RBD outcome reporting Odds ratios (95% CIs). Notes: Other drug history includes Volatile substance abuse, cannabis and kava. Abbreviations, CI, Confidence interval; CAG, cytosine-adenine-guanine; SARA, Scale for the assessment and rating of ataxia; EQ-5D, EuroQol 5 dimension; SQoL, Subjective quality of life.
Fig. 4.
Univariate logistic regressions of independent parameters effects on PSG reported OSA reporting Odds ratios. Notes: Other drug history includes volatile substance abuse, cannabis and kava. Abbreviations, CI, Confidence interval; CAG, cytosine-adenine-guanine; SARA, Scale for the assessment and rating of ataxia; EQ-5D, EuroQol 5 dimension; SQoL, Subjective quality of life.
Fig. 5.
Univariate linear regressions of independent parameters effects on Total AHI reporting beta coefficients outcome. Notes: Other drug history includes volatile substance abuse, cannabis and kava. Abbreviations; AHI, Apnoea-hyopnea index CI, Confidence interval; CAG, cytosine-adenine-guanine; SARA, Scale for the assessment and rating of ataxia; EQ-5D, EuroQol 5 dimension; SQoL, Subjective quality of life.
5. Discussion
To the best of our knowledge this is the first study to comprehensively document sleep disturbances among Aboriginal Australians living with MJD.
Assessment of sleep quality and the range of sleep disorders experienced by Aboriginal Australians in this region is recently emerging. Evidence indicates a high prevalence of OSA associated with all-cause mortality, consistent with the regionally high rates of chronic health conditions (Heraganahally et al., 2020b, 2021). Relatedly, sleep quality and duration have been shown to affect obesity and cardiometric risk factors among the Indigenous Australian population without MJD (Deacon-Crouch et al., 2020; Yiallourou et al., 2021). Still, data showing the rates of other sleep disorders is sparse. Significantly, there is no current evidence of the impact of environmental factors such as overcrowding on sleep quality for Aboriginal Australians in this region, nor published evidence of rates of RLS, PLMS, RBD or SRLC.
The impact of these other sleep issues within the communities where people with MJD live is speculative. The sleep disorders identified in this MJD cohort are consistent with those found in other MJD populations; however, there may be other influential factors.
In our study, MJD participants tended to be within a healthy weight range, have an average age of disease onset of 32 years and have high levels of psychological distress. Several sleep related issues, such as OSA, RLS, PLMs, RBD and SRLC are prevalent and potentially lead to poor quality of sleep and life. No association between MJD severity of disease was found. The degree to which this lack of a predictive finding was related to sample size is unknown.
Previous clinical investigations of sleep in a subset of the older generation of this cohort (n = 7) found evidence of RBD in 3 of 7 (43%) individuals, REM without atonia in 5 of 7 (71%) and PLMS in 3 of 7 (43%) (Adelaide Institute for Sleep Health MJDF, 2008). In our study, we observed RBD, RLS, SRLC and OSA in 43%, 33%, 71% and 47% respectively.
While conditions such as RLS have been reported in spinocerebellar ataxia types 1, 2 and 3 (Pedroso et al., 2011a, Pedroso et al., 2013a), many of the sleep complaints from MJD participants (and their bed partners) in this cohort include reports of complex motor movements and vocalisations, indicative of RBD. Studies elsewhere report more frequent occurrences of RBD for MJD participants than controls (40% v 6%, p < 0.001) (Grimaldi and Manto, 2012). Likewise, this study observed the aforementioned sleep related disturbances, although with lower prevalence.
OSA is prevalent (42%) among people with MJD (Santos et al., 2018). In the current study, 47% of MJD participants had OSA. However, contrary to our previous studies with Aboriginal Australians in the general NT population, fewer were noted to have very severe OSA (Heraganahally et al., 2020b; Mehra et al., 2020; Yiallourou et al., 2021; Benn et al., 2021). Instead, participants of this study demonstrated a milder form of OSA with an AHI <15/hour. This may be due to our previous studies including participants with high pre-test probability of OSA and higher BMI, a main risk factor for severe OSA (Heraganahally et al., 2020b; Mehra et al., 2020; Jehan et al., 2017). In contrast, the current study participants were within normal weight ranges or under-weight (71%), which may account for the disparity.
The underlying mechanisms of RBD and other sleep disturbances in MJD is unclear. One mechanism postulated is dysfunction of brainstem structures that modulate muscle activity during REM sleep, either by ataxin-3 deposition or cell degeneration (Koeppen, 2018).
Early studies indicated RBD and RLS pathophysiology may be associated with dopamine deficiency, therefore suggesting dopaminergic treatment (Pedroso et al., 2013b; Grimaldi and Manto, 2012) However, there is some evidence extrastriatial and non-presynaptic dopamine pathways as well as non-motor features such as psychiatric manifestations play a greater part in RBD in people with MJD than dopaminergic system dysfunction (Silva et al., 2016; Pedroso et al., 2011b). This is not thought to rule out the ‘dopaminergic hypothesis’ in MJD. Further studies are needed to investigate beyond presynaptic terminal using.
Regardless of the cause, the effect on people living with MJD and their caregivers is detrimental and well documented (Carr et al., 2019b). In addition to risk of self-injury during muscle activation, sleep disturbances, which fragment sleep and reduce sleep efficiency, can lead to daytime sleepiness, fatigue, irritability and depression (Yuan et al., 2019) among other effects. We observed incontinence with nocturia in 100% of participants corresponding to urodynamic studies showing autonomic dysfunction among people with MJD (Yeh et al., 2005; Sakakibara et al., 2004).
It is highly probable that the variability between PSG and actigraphy results in our study indicates the influence of environmental factors on sleep for Aboriginal Australians living with MJD. The significant findings of decreased sleep latency and increased sleep efficiency and sleep time while overnight in motel accommodation were striking compared to home actigraphy results. Overcrowding affected 22/24 (92%) participants and roughly one third care for dependants or require their own care overnight. On average, there were 8 people per household (range 1–28) with 2 people per room (i.e., people with MJD who co-sleep disturb others’ sleep). This leads to excess noise and interruptions during sleep, including from visitors. Consistent reports of overcrowded and inappropriate housing structures for Aboriginal Australians emphasise these findings (Buergelt et al., 2017). Hunger due to food insecurity, another known issue associated with overcrowding for Aboriginal families, could influence sleep but was not explored (Vallesi et al., 2018).
5.1. Findings in context
The wider context is important when evaluating the sleep experience of this cohort. The impact of MJD extends beyond sleep and affects whole families. Each child of a person who carries the mutated gene has a 50% chance of developing disease. The mutation is typically expanded when passed to the next generation. Known as an 'anticipation effect,' this phenomenon results in more severe symptoms manifesting 8–10 years earlier in each subsequent generation(Sena et al., 2021). Multiple generations may be affected concurrently, profoundly impacting caregivers, who may also be affected or at risk of MJD (Carr et al., 2019b). The experience of caregivers may be reflected in the K5 scores, which showed psychological distress more than double previous reports among remote Aboriginal and Torres Strait Islander Australians (27%) (Cunningham and Paradies, 2012). Disease manifestations are compounded by geographic remoteness, lack of access to adequate health care services, and historical disadvantage (Carr et al., 2019b). As there is no known cure for MJD, treatment is limited to supportive care and symptom management (Carr et al., 2021). In tandem with generalised muscle weakness and psychological distress, sleep disturbances are debilitating for people with MJD, impairing the ability to engage with therapy or medical appointments and thus compromising quality of life. Consideration of participants’ living situations and preferences was integral to data collection integrity. The capacity to utilise trusted relationships, first language support and qualitative methods enabled assurance about the trustworthiness and credibility of results obtained from tools not previously validated among the study population. The use of validated sleep tools with appropriate support may therefore be a useful and valid clinical measure in remote settings where PSG is not feasible.
5.2. Limitations of the study
Resource limitations restricted feasibility to include a comparison group and eligible participants from other geographical regions. As MJD is a rare disease, the sample size is small. Several individuals passed away or were in crisis during recruitment, limiting study numbers. Notwithstanding, the sample represents >50% of the entire Aboriginal population diagnosed with MJD in Australia (recognising underdiagnosis occurs due to limited specialist services). Remoteness of the settings meant fully attended studies were not feasible, which eventuated in n = 2 (8%) failed sleep studies. Actigraphy was not sensitive to wheelchair bound participants with severe disease, thus their data was excluded. Participants were noted to have high levels of distress, presumed related to MJD, hardship and/or trauma; however, mood disorders were not independently screened beyond where a psychiatric disorder was confirmed from medical record review.
6. Conclusion
Poor sleep quality and sleep disturbances are prevalent among Aboriginal Australians living with MJD, confirmed by PSG and sleep quality standardised tools. Results suggest poor sleep affects people living with MJD regardless of disease stage and is associated with psychosocial distress, impacting quality of life for people with MJD and their caregivers. Sleep quality should be addressed during routine medical consultations and a sleep study offered to determine treatment options, in addition to a complete continence assessment and where indicated, management plan for incontinence and nocturia. Given the high prevalence of pain, discomfort and cramps at bedtime, timing of pain medication should be considered. Treatment options should be holistic and consider non-pharmaceutical interventions, such as exercise, social and emotional wellbeing supports, like facilitated social inclusion activities or counselling, and advocacy for improved housing conditions. Future studies with larger sample sizes are needed to confirm associations and explore the feasibility and acceptability of treatment options. This formative information would assist the design of a culturally-informed community trial to determine whether improvements to sleep quality can alleviate MJD symptoms and enhance quality of life. This study demonstrates where PSG is not feasible, the use of sleep quality standardised tools, in addition to a clinical assessment, is an acceptable alternative to diagnose sleep disorders when appropriate cultural and linguistic support is applied.
Funding
This work was supported by the MJD Foundation, Alyangula, NT, Australia and in-kind support from Darwin Respiratory and Sleep health, Darwin Private Hospital, Darwin, NT, Australia.
CRediT authorship contribution statement
Desireé LaGrappe: Conceptualization, and design, Data curation, interpretation of the data, Writing – original draft, of the paper, Writing – review & editing, Formal analysis, project management and administration. Libby Massey: Conceptualization, and design, interpretation of the data, writing – drafting of the paper, Writing – review & editing, Formal analysis, project management and administration. Anuk Kruavit: Conceptualization, and design, Formal analysis, writing – drafting of the paper. Timothy Howarth: Interpretation of the data, writing – drafting of the paper, Writing – review & editing, Formal analysis. Gayangwa Lalara: Conceptualization, and design - cultural consultation, recruitment. Bronwyn Daniels: Conceptualization, and design - cultural consultation, recruitment, Writing – review & editing. Julie Gungunbuy Wunungmurra: Conceptualization, and design - cultural consultation, recruitment, Writing – review & editing. Kimberley Flavell: Conceptualization, and design, Data curation, review and editing. Ruth Barker: Conceptualization, and design, interpretation of the data, Formal analysis, Writing – review & editing. Howard Flavell: Conceptualization, and design, Writing – review & editing. Subash S. Heraganahally: Conceptualization, and design, interpretation of the data, writing – drafting of the paper, Writing – review & editing, Formal analysis, revising it critically for intellectual content and final approval of the version to be published. Responsible for the overall content as guarantor and to be accountable.
Declaration of competing interest
DLG, LM, GL, BD, JGW were, or are employed by the MJD Foundation.
Acknowledgment:
The researchers acknowledge and thank all the individuals, families and communities involved who graciously participated for the benefit of their families and the international ataxia community. We extend our sincere gratitude to the staff of DRSH and the MJDF, notably the Aboriginal co-researchers, for their contributions towards this study. We would also like to thank Katarina Kamenar who volunteered her time as a graduate student to assist with data cleaning. This research was made possible by partnerships from the Northern Territory Department of Health and the Anindilyakwa Land Council, a major donor to the MJD Foundation's research program.
Contributor Information
Desireé LaGrappe, Email: D.LaGrappe@latrobe.edu.au.
Libby Massey, Email: libby.massey@mjd.org.au.
Anuk Kruavit, Email: aiy_krua@yahoo.com.
Timothy Howarth, Email: timothypaul.howarth@cdu.edu.au.
Kimberley Flavell, Email: kimberley.flavell@nt.gov.au.
Ruth Barker, Email: ruth.barker@jcu.edu.au.
Howard Flavell, Email: howard.flavell@nt.gov.au.
Subash S. Heraganahally, Email: hssubhashcmc@hotmail.com.
Data availability
The data that has been used is confidential.
References
- Adelaide Institute for Sleep Health MJDF . northern territory; australia: 2008. Sleep Disorders Among MJD Patients from Groote Eylandt. [Google Scholar]
- Amery R. Recognising the communication gap in Indigenous health care. Med. J. Aust. 2017;207(1):13–15. doi: 10.5694/mja17.00042. [DOI] [PubMed] [Google Scholar]
- Australian Bureau of Statistics . ABS; 2020. National State and Territory Population.https://www.abs.gov.au/statistics/people/aboriginal-and-torres-strait-islander-peoples/estimates-aboriginal-and-torres-strait-islander-australians/jun-2016 [Internet] [Google Scholar]
- Australian Bureau of Statistics . ABS; 2020. Estimates of Aboriginal and Torres Strait Islander Australians.https://www.abs.gov.au/statistics/people/aboriginal-and-torres-strait-islander-peoples/estimates-and-projections-aboriginal-and-torres-strait-islander-australians/latest-release [Internet] [cited March 2022]. Available from: [Google Scholar]
- Australian Institute of Health and Welfare . Australian Institute of Health and Welfare; 2020. Profile of Indigenous Australians.https://www.aihw.gov.au/reports/australias-health/profile-of-indigenous-australians [Internet] [Google Scholar]
- Australian Institute of Health and Welfare . Australian Institute of Health and Welfare; 2020. Social Determinants and Indigenous Health.https://www.aihw.gov.au/reports/australias-health/social-determinants-and-indigenous-health [Internet] [Google Scholar]
- Australian Institute of Health and Welfare . Australian Institute of Health and Welfare; 2021. Housing Assistance in Australia.https://www.aihw.gov.au/reports/housing-assistance/housing-assistance-in-australia [Internet] [Google Scholar]
- Australian Statistical Geography Standard Edition 3. 2020. https://www.abs.gov.au/statistics/standards/australian-statistical-geography-standard-asgs-edition-3/latest-release [Internet] [cited 2021-2026]. Available from. [Google Scholar]
- Benn E., Wirth H., Short T., Howarth T., Heraganahally S.S. The Top End sleepiness scale (TESS): a new tool to assess subjective daytime sleepiness among Indigenous Australian adults. Nat. Sci. Sleep. 2021;13:315. doi: 10.2147/NSS.S298409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettencourt C., Santos C., Montiel R., Costa MdC., Cruz-Morales P., Santos L.R., et al. Increased transcript diversity: novel splicing variants of Machado–Joseph Disease gene (ATXN3) Neurogenetics. 2010;11(2):193–202. doi: 10.1007/s10048-009-0216-y. [DOI] [PubMed] [Google Scholar]
- Boeve B.F., Molano J.R., Ferman T.J., Lin S.-C., Bieniek K., Tippmann-Peikert M., et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in a community-based sample. J. Clin. Sleep Med. 2013;9(5):475–480. doi: 10.5664/jcsm.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buergelt P.T., Maypilama E.L., McPhee J., Dhurrkay G., Nirrpuranydji S., Mänydjurrpuy S., et al. Housing and overcrowding in remote indigenous communities: impacts and solutions from a holistic perspective. Energy Proc. 2017;121:270–277. [Google Scholar]
- Buysse D.J., Reynolds C.F., III, Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr. Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carr J.J., Lalara J., Lalara G., Smith M., Quaill J., Clough A.R., et al. What is the best way to keep walking and moving around for individuals with Machado-Joseph disease? A scoping review through the lens of Aboriginal families with Machado-Joseph disease in the Top End of Australia. BMJ Open. 2019;9(9) doi: 10.1136/bmjopen-2019-032092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr J.J., Lalara J., Lalara G., O'Hare G., Massey L., Kenny N., et al. ‘Staying strong on the inside and outside’ to keep walking and moving around: perspectives from aboriginal people with machado joseph disease and their families from the groote eylandt archipelago, Australia. PLoS One. 2019;14(3) doi: 10.1371/journal.pone.0212953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr J.J., Lalara J., Lalara G., Lalara G., Daniels B., Clough A.R., et al. Staying strong toolbox: Co-design of a physical activity and lifestyle program for aboriginal families with machado-joseph disease in the top end of Australia. PLoS One. 2021;16(2) doi: 10.1371/journal.pone.0244311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung F., Abdullah H.R., Liao P. STOP-Bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631–638. doi: 10.1378/chest.15-0903. [DOI] [PubMed] [Google Scholar]
- Collop N.A., Anderson W.M., Boehlecke B., Claman D., Goldberg R., Gottlieb D.J., et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J. Clin. Sleep Med. 2007;3(7):737–747. [PMC free article] [PubMed] [Google Scholar]
- Council NHaMR. In: Australia: Canberra. Australia Co, editor. National Health and Medical Research Council; 2018. Ethical Conduct in research with aboriginal and torres strait islander peoples and communities: guidelines for researchers and stakeholders. [Google Scholar]
- Cunningham J., Paradies Y.C. Socio-demographic factors and psychological distress in Indigenous and non-Indigenous Australian adults aged 18-64 years: analysis of national survey data. BMC Publ. Health. 2012;12(1):1–15. doi: 10.1186/1471-2458-12-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon-Crouch M., Begg S., Skinner T. Is sleep duration associated with overweight/obesity in Indigenous Australian adults? BMC Publ. Health. 2020;20(1):1–13. doi: 10.1186/s12889-020-09287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg H., Er X.Y., Howarth T., Heraganahally S.S. Positional sleep apnea among regional and remote Australian population and simulated positional treatment effects. Nat. Sci. Sleep. 2020;12:1123. doi: 10.2147/NSS.S286403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi G., Manto M. Topography of cerebellar deficits in humans. Cerebellum. 2012;11(2):336–351. doi: 10.1007/s12311-011-0247-4. [DOI] [PubMed] [Google Scholar]
- Group I.R.L.S.S. Validation of the international restless legs syndrome study group rating scale for restless legs syndrome. Sleep Med. 2003;4(2):121–132. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- Heraganahally S.S., White S. A cost-effective novel innovative box (C-Box) to prevent cockroach infestation of continuous positive airway pressure equipment: a unique problem in Northern Tropical Australia. Am. J. Trop. Med. Hyg. 2019;101(4):937. doi: 10.4269/ajtmh.19-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heraganahally S.S., Kerslake C., Issac S., Mingi J.J., Thomas I., Jayaram L., et al. Outcome of public hospital-funded continuous positive airway therapy device for patients with obstructive sleep apnoea: an Australian Perspective Study. Sleep. Vigilance. 2020;4(2):195–204. [Google Scholar]
- Heraganahally S.S., Kruavit A., Oguoma V.M., Gokula C., Mehra S., Judge D., et al. Sleep apnoea among Australian Aboriginal and non-Aboriginal patients in the Northern Territory of Australia—a comparative study. Sleep. 2020;43(3):zsz248. doi: 10.1093/sleep/zsz248. [DOI] [PubMed] [Google Scholar]
- Heraganahally S.S., Zaw K.K., Tip S., Jing X., Mingi J.J., Howarth T., et al. Obstructive sleep apnoea and adherence to continuous positive airway therapy among Australian women. Intern. Med. J. 2020 doi: 10.1111/imj.15076. [DOI] [PubMed] [Google Scholar]
- Heraganahally S.S., Howarth T.P., Wirth H., Short T., Benn E. Validity of the new “top end sleepiness scale”(TESS) against the STOP‐bang tool in predicting obstructive sleep apnoea among indigenous Australian adults. Intern. Med. J. 2021 doi: 10.1111/imj.15633. [DOI] [PubMed] [Google Scholar]
- Jacobi H., du Montcel S.T., Bauer P., Giunti P., Cook A., Labrum R., et al. Long-term disease progression in spinocerebellar ataxia types 1, 2, 3, and 6: a longitudinal cohort study. Lancet Neurol. 2015;14(11):1101–1108. doi: 10.1016/S1474-4422(15)00202-1. [DOI] [PubMed] [Google Scholar]
- Jehan S., Zizi F., Pandi-Perumal S.R., Wall S., Auguste E., Myers A.K., et al. Obstructive sleep apnea and obesity: implications for public health. Sleep Med. Disorder.: Int. J. 2017;1(4) [PMC free article] [PubMed] [Google Scholar]
- Jorgensen G., Downey C., Goldin J., Melehan K., Rochford P., Ruehland W. An australasian commentary on the AASM manual for the scoring of sleep and associated events. Sleep Biol. Rhythm. 2020;18(3):163–185. [Google Scholar]
- Koeppen A.H. The neuropathology of spinocerebellar ataxia type 3/Machado-Joseph disease. Polylutamin. Disorder. 2018:233–241. doi: 10.1007/978-3-319-71779-1_11. [DOI] [PubMed] [Google Scholar]
- Kruavit A., Fox M., Pearson R., Heraganahally S. Chronic respiratory disease in the regional and remote population of the Northern Territory Top End: a perspective from the specialist respiratory outreach service. Aust. J. Rural Health. 2017;25(5):275–284. doi: 10.1111/ajr.12349. [DOI] [PubMed] [Google Scholar]
- Lavrencic L.M., Mack H.A., Daylight G., Wall S., Anderson M., Hoskins S., et al. Staying in touch with the community: understanding self-reported health and research priorities in older Aboriginal Australians. Int. Psychogeriatr. 2020;32(11):1303–1315. doi: 10.1017/S1041610219001753. [DOI] [PubMed] [Google Scholar]
- Lawrence G., Muza R. Assessing the sleeping habits of patients in a sleep disorder centre: a review of sleep diary accuracy. J. Thorac. Dis. 2018;10(Suppl. 1):S177. doi: 10.21037/jtd.2017.12.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara B.J., Banks E., Gubhaju L., Williamson A., Joshy G., Raphael B., et al. Measuring psychological distress in older aboriginal and Torres Strait islanders Australians: a comparison of the K‐10 and K‐5. Aust. N. Z. J. Publ. Health. 2014;38(6):567–573. doi: 10.1111/1753-6405.12271. [DOI] [PubMed] [Google Scholar]
- Mehra S., Ghimire R.H., Mingi J.J., Hatch M., Garg H., Adams R., et al. Gender differences in the clinical and polysomnographic characteristics among Australian aboriginal patients with obstructive sleep apnea. Nat. Sci. Sleep. 2020;12:593. doi: 10.2147/NSS.S258330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroso J.L., Bezerra M.L.E., Braga-Neto P., Pinheiro D.S., Minett T., Do Prado G.F., et al. Is neuropathy involved with restless legs syndrome in Machado-Joseph disease? Eur. Neurol. 2011;66(4):200–203. doi: 10.1159/000331008. [DOI] [PubMed] [Google Scholar]
- Pedroso J.L., Braga-Neto P., Felício A.C., Dutra L.A., Santos W.A., do Prado G.F., et al. Sleep disorders in machado–joseph disease: frequency, discriminative thresholds, predictive values, and correlation with ataxia-related motor and non-motor features. Cerebellum. 2011;10(2):291–295. doi: 10.1007/s12311-011-0252-7. [DOI] [PubMed] [Google Scholar]
- Pedroso J.L., Franca M.C., Jr., Braga‐Neto P., D'Abreu A., Saraiva‐Pereira M.L., Saute J.A., et al. Nonmotor and extracerebellar features in Machado‐Joseph disease: a review. Mov. Disord. 2013;28(9):1200–1208. doi: 10.1002/mds.25513. [DOI] [PubMed] [Google Scholar]
- Pedroso J.L., Braga-Neto P., Felício A.C., Minett T., Yamaguchi E., do Prado L.B.F., et al. Sleep disorders in Machado–Joseph disease: a dopamine transporter imaging study. J. Neurol. Sci. 2013;324(1–2):90–93. doi: 10.1016/j.jns.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Sakakibara R., Uchiyama T., Arai K., Yamanishi T., Hattori T. Lower urinary tract dysfunction in Machado–Joseph disease: a study of 11 clinical-urodynamic observations. J. Neurol. Sci. 2004;218(1–2):67–72. doi: 10.1016/j.jns.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Santos F.A.F., de Carvalho L.B.C., do Prado L.F., do Prado G.F., Barsottini O.G., Pedroso J.L. Sleep apnea in Machado-Joseph disease: a clinical and polysomnographic evaluation. Sleep Med. 2018;48:23–26. doi: 10.1016/j.sleep.2018.04.002. [DOI] [PubMed] [Google Scholar]
- Saute J.A.M., Jardim L.B. Machado Joseph disease: clinical and genetic aspects, and current treatment. Expert. Opinion. Orphan. Drug. 2015;3(5):517–535. [Google Scholar]
- Schmitz-Hübsch T., Du Montcel S.T., Baliko L., Berciano J., Boesch S., Depondt C., et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66(11):1717–1720. doi: 10.1212/01.wnl.0000219042.60538.92. [DOI] [PubMed] [Google Scholar]
- Sena L.S., dos Santos Pinheiro J., Saraiva‐Pereira M.L., Jardim L.B. Selective forces acting on spinocerebellar ataxia type 3/Machado–Joseph disease recurrency: a systematic review and meta‐analysis. Clin. Genet. 2021;99(3):347–358. doi: 10.1111/cge.13888. [DOI] [PubMed] [Google Scholar]
- Silva G.M.F., Pedroso J.L., Dos Santos D.F., Braga‐Neto P., Do Prado L.B.F., De Carvalho L.B.C., et al. NREM‐related parasomnias in Machado–Joseph disease: clinical and polysomnographic evaluation. J. Sleep. Res. 2016;25(1):11–15. doi: 10.1111/jsr.12330. [DOI] [PubMed] [Google Scholar]
- Vallesi S., Wood L., Dimer L., Zada M. In their own voice”—incorporating underlying social determinants into Aboriginal health promotion programs. Int. J. Environ. Res. Publ. Health. 2018;15(7):1514. doi: 10.3390/ijerph15071514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh T.-H., Lu C.-S., Chou Y.-H.W., Chong C.-C., Wu T., Han N.-H., et al. Autonomic dysfunction in Machado-Joseph disease. Arch. Neurol. 2005;62(4):630–636. doi: 10.1001/archneur.62.4.630. [DOI] [PubMed] [Google Scholar]
- Yiallourou S.R., Maguire G.P., Carrington M.J. Sleep quantity and quality and cardiometabolic risk factors in Indigenous Australians. J. Sleep. Res. 2021;30(2) doi: 10.1111/jsr.13067. [DOI] [PubMed] [Google Scholar]
- Yuan X., Ou R., Hou Y., Chen X., Cao B., Hu X., et al. Extra-cerebellar signs and non-motor features in Chinese patients with spinocerebellar ataxia type 3. Front. Neurol. 2019;10:110. doi: 10.3389/fneur.2019.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.