Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by multisystem inflammation and the presence of circulating autoantibodies directed against self-antigens, with approximately 10% to 15% of cases presenting in childhood.1 The etiology is unknown; however, risk factors include female sex, genetic predisposition, and environmental factors that can induce both the onset of the disease and flares of existing disease.2 Diagnosing SLE is often challenging since it may present in a myriad of ways and may affect nearly any organ system. We present an unusual case of SLE in an adolescent male following a liver transplant with an unexpected full engraftment of the hematopoietic system. This patient did not have any known risk factors for SLE; therefore, we speculate that the unanticipated hematopoietic transplant resulted in the acquisition of genetic variants from the donor, that were associated with the development of SLE.
Case report
A 15-year-old boy developed a new rash on the face, neck, chest, and arms. Thirteen years earlier, he had undergone a cadaveric liver transplant for fulminant liver failure of unknown etiology. The donor was an ABO-compatible, deceased boy with a 0/6 human leukocyte antigen mismatch. Three months after the successful liver transplant, the patient developed chronic graft-versus-host disease (cGVHD) of his skin, eyes (photosensitivity), and gastrointestinal tract, which was treated with oral steroids and tacrolimus. The intestinal graft-versus-host disease symptoms improved with treatment, but the cGVHD of the skin persisted. Nine months after the transplant, amplification of variable number tandem repeats in flow-sorted peripheral blood cells showed the complete engraftment of the donor lymphoid and myeloid cell lineages, with 100% of CD3+, CD19+, CD56+, and CD33+ cells derived from the donor.
Five years after the transplant, while receiving tacrolimus (goal level of 3-5 ng/mL) and low-dose oral budesonide, the patient developed Evans syndrome with a Coombs-positive hemolytic anemia (hemoglobin 8.7 g/dL, reticulocytes 5.5%, spherocytes seen on peripheral smear) and thrombocytopenia (platelets 4000/μL). Over the next 4 years, he received 3 separate courses of rituximab and 12 total infusions of intravenous immunoglobulin, with eventual resolution. The treatment with tacrolimus and oral budesonide continued, with no further evidence of cGVHD or cytopenias. Seven months prior to developing the new skin rash, the tacrolimus was discontinued, and he remained on budesonide alone.
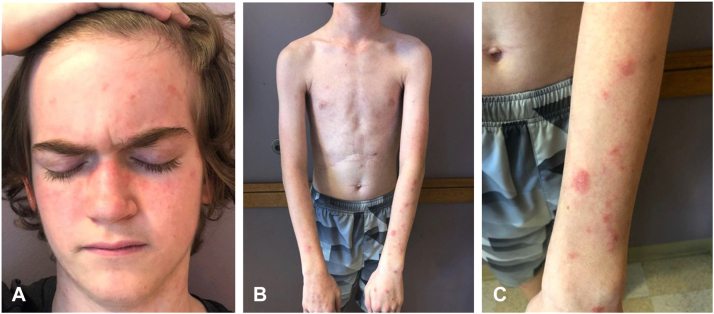
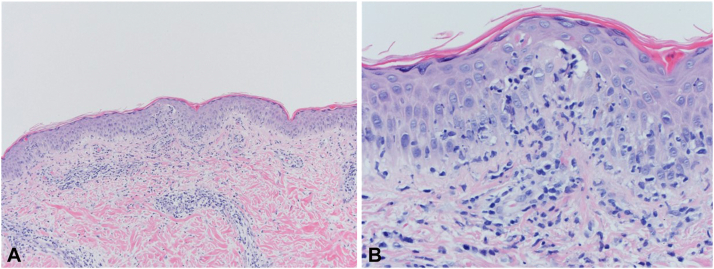
At presentation, he had a rash but was otherwise asymptomatic and systemically well. A physical examination was normal except for the skin, which revealed scaly, pink patches, and plaques on the neck, chest, and arms and in a malar distribution on the face (Fig 1). A skin biopsy revealed interface dermatitis suggestive of either cGVHD or connective tissue disease (Fig 2). A laboratory evaluation revealed elevated antinuclear antibodies (>1:2560, homogenous), elevated anti–double-stranded DNA antibodies (394 IU/mL), proteinuria, and serum hypocomplementemia (C3 55 mg/dL, C4 <6.0 mg/dL.) Other pertinent laboratory results are shown in Table I. A kidney biopsy demonstrated class IV lupus nephritis. The patient was classified as having SLE based on fulfilling the 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for SLE.3 At this time; he began treatment with hydroxychloroquine, prednisone, and mycophenolate. One year later, he remained systemically well, the rash had resolved, the proteinuria had decreased, and the serum anti–double-stranded DNA and complement levels were normal.
Fig 1.
Systemic lupus erythematosus. Scaly, pink patches and plaques on the malar distribution of the (A) face, (B) neck and chest, and (C) extensor arms.
Fig 2.
Systemic lupus erythematosus. Hematoxylin and eosin staining of skin biopsies showing (A) interface dermatitis and (B) necrotic keratinocytes.
Table I.
Patient’s relevant laboratory values
| Laboratory test | Result | Reference range |
|---|---|---|
| ANA titer | >1:2560, homogenous | <1:40 |
| Anti–double-stranded DNA antibody | 394 IU/mL | ≤10 IU/mL |
| C3 complement | 55 mg/dL (0.55 g/L) | 80-156 mg/dL (0.8-1.56 g/L) |
| C4 complement | <6.0 mg/dL (<0.06 g/L) | 12-43 mg/dL (0.12-0.43 g/L) |
| Anti-SSA antibody | <100 AU/mL | <100 AU/mL |
| Anti-SSB antibody | 110 AU/mL | <100 AU/mL |
| Anticardiolipin antibody | 15 GPL units | ≤14 GPL units |
| Anti-RNP antibody | <100 AU/mL | <100 AU/mL |
| Anti-Smith antibody | <100 AU/mL | <100 AU/mL |
| Lupus anticoagulant | Not detected | Not detected |
| Serum albumin | 3.9 g/dL (39 g/L) | 3.8–5.4 g/dL (38-54 g/L) |
| BUN | 20 mg/dL (7.14 mmol/L) | 5-20 mg/dL (1.7 - 7.14 mmol/L) |
| Creatinine | 0.54 mg/dL (41 mmol/L) | 0.5-1.06 mg/dL (38-80 mmol/L) |
| AST | 24 U/L (0.40 mkat/L) | 16-38 IU/L (0.26-0.63 mkat/L) |
| ALT | 15 U/L (0.25 mkat/L) | 10-35 IU/L (0.16-0.58 mkat/L) |
| Urine protein | ≥300 mg/dL (≥3.00 g/L) | 0 mg/dL (0 g/dL) |
| 24-hour urinary creatinine | 1291 mg/24 h (11.4 mmol/d) | 290-1870 mg/24 h (2563-16530 mmol/24 h) |
| 24-hour urinary protein | 3409 mg/24 h (3.409 g/d) | 0-71 mg/24 h (0.071 g/d) |
ALT, Alanine aminotransferase; ANA, antinuclear antibody; AST, aspartate aminotransferase; BUN, blood urea nitrogen; RNP, ribonucleoprotein; SSA, Sjögren's syndrome-related antigen A; SSB, Sjögren's syndrome-related antigen B.
Discussion
Graft-versus-host disease most commonly arises after hematopoietic stem cell transplants, with estimations of cGVHD occurring in 6% of pediatric patients.4 cGVHD arising after a pediatric solid organ transplant is rare. A recent review identified 70 total cases, with an incidence of 1.5% following liver transplants.5 cGVHD is most commonly seen in liver transplants as compared to other solid organ transplants, likely due to the large number of donor lymphocytes in the donor portal tract and liver parenchyma at the time of transplantation.6 Other autoimmune diseases, including cytopenias, inflammatory bowel disease, and alopecia areata, may occur following solid organ transplantation and have been reported in 4.5% of pediatric liver transplant recipients.7 The risk of autoimmunity after a pediatric hematopoietic stem cell transplant is higher, with up to 14% of children developing vitiligo, and prior cGVHD is a risk factor.8
There have been prior published reports of SLE following hematopoietic stem cell transplants occurring after autologous transplants but not solid organ or allogeneic hematopoietic stem cell transplants.9 The unusual inadvertent marrow engraftment, along with the liver transplant, likely transferred donor lymphoid cells with a genetic predisposition to the development of SLE. Our patient was an adolescent male and did not have any other risk factors for SLE, including no known family history of SLE or related diseases. The donor was also a male, did not have a known family history of SLE, and, therefore, most likely transferred lymphoid cells with a de novo genetic predisposition to the development of SLE. Neither the donor nor our patient had a clinical indication to undergo further analysis to confirm the presence of genetic variations that are known to be associated with SLE; therefore, we were unable to adequately test this hypothesis.
The development of autoimmune cytopenias in our patient may have been the initial manifestation of SLE given the known association between Evans syndrome and the subsequent development of SLE.10 The evolution of additional manifestations of SLE could have certainly been delayed by treatment with immunosuppressive and immunomodulating agents, eventually becoming apparent when this therapy was discontinued. Had the patient not received this therapy, we speculate that the development of the rash, nephritis, and autoantibodies associated with SLE would have occurred earlier. This case highlights the need for awareness of the potential for secondary autoimmunity, including SLE, in cases of solid organ transplantation, particularly when complicated by cGVHD and unexpected bone marrow engraftment.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Harry O., Yasin S., Brunner H. Childhood-onset systemic lupus erythematosus: a review and update. J Pediatr. 2018;196:22–30.e2. doi: 10.1016/j.jpeds.2018.01.045. [DOI] [PubMed] [Google Scholar]
- 2.Pons-Estel G.J., Ugarte-Gil M.F., Alarcón G.S. Epidemiology of systemic lupus erythematosus. Expert Rev Clin Immunol. 2017;13(8):799–814. doi: 10.1080/1744666X.2017.1327352. [DOI] [PubMed] [Google Scholar]
- 3.Aringer M., Costenbader K., Daikh D., et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78(9):1151–1159. doi: 10.1136/annrheumdis-2018-214819. [DOI] [PubMed] [Google Scholar]
- 4.Rocha V., Wagner J.E., Jr., Sobocinski K.A., et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and international bone marrow transplant registry working committee on alternative donor and stem cell sources. N Engl J Med. 2000;342(25):1846–1854. doi: 10.1056/NEJM200006223422501. [DOI] [PubMed] [Google Scholar]
- 5.Green T., Hind J. Graft-versus-host disease in paediatric solid organ transplantation: a review of the literature. Pediatr Transplant. 2016;20(5):607–618. doi: 10.1111/petr.12721. [DOI] [PubMed] [Google Scholar]
- 6.Smith D.M., Agura E., Netto G., et al. Liver transplant-associated graft-versus-host disease. Transplantation. 2003;75(1):118–126. doi: 10.1097/00007890-200301150-00022. [DOI] [PubMed] [Google Scholar]
- 7.Marcus N., Amir A.Z., Grunebaum E., et al. De novo allergy and immune-mediated disorders following solid-organ transplantation-prevalence, natural history, and risk factors. J Pediatr. 2018;196:154–160. doi: 10.1016/j.jpeds.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 8.Huang J.T., Song J.S., Hawryluk E.B., et al. Nonmalignant late cutaneous changes after allogeneic hematopoietic stem cell transplant in children. J Am Acad Dermatol. 2018;79(2):230–237. doi: 10.1016/j.jaad.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 9.Daikeler T., Labopin M., Di Gioia M., et al. Secondary autoimmune diseases occurring after HSCT for an autoimmune disease: a retrospective study of the EBMT Autoimmune Disease Working Party. Blood. 2011;118(6):1693–1698. doi: 10.1182/blood-2011-02-336156. [DOI] [PubMed] [Google Scholar]
- 10.Aladjidi N., Fernandes H., Leblanc T., et al. Evans syndrome in children: long-term outcome in a prospective French national observational cohort. Front Pediatr. 2015;3:79. doi: 10.3389/fped.2015.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]