Abstract
Wastewater-based surveillance (WBS) has been widely used as a public health tool to monitor the emergence and spread of SARS-CoV-2 infections in populations during the COVID-19 pandemic. Coincident with the global vaccination efforts, the world is also enduring new waves of SARS-CoV-2 variants. Reinfections and vaccine breakthroughs suggest an endemic future where SARS-CoV-2 continues to persist in the general population. In this treatise, we aim to explore the future roles of wastewater surveillance. Practically, WBS serves as a relatively affordable and non-invasive tool for mass surveillance of SARS-CoV-2 infection while minimizing privacy concerns, attributes that make it extremely suited for its long-term usage. In an endemic future, the utility of WBS will include 1) monitoring the trend of viral loads of targets in wastewater for quantitative estimate of changes in disease incidence; 2) sampling upstream for pinpointing infections in neighborhoods and at the building level; 3) integrating wastewater and clinical surveillance for cost-efficient population surveillance; and 4) genome sequencing wastewater samples to track circulating and emerging variants in the population. We further discuss the challenges and future developments of WBS to reduce inconsistencies in wastewater data worldwide, improve its epidemiological inference, and advance viral tracking and discovery as a preparation for the next viral pandemic.
Keywords: Wastewater surveillance, Endemic, SARS-CoV-2, Cost-effective, Epidemiology, COVID-19
Graphical abstract
1. Introduction
Wastewater-based surveillance (WBS), which was used for detecting the silent circulation of polio and non-polio enteroviruses (Manor et al., 2014; World Health Organization, 2003), has, with the advent of the COVID-19 pandemic, been adapted as a public health tool for monitoring the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in communities. The presence of SARS-CoV-2 RNA in wastewater was first demonstrated in the Netherlands, United States, Spain, and Australia in April 2020 (Ahmed et al., 2020; Medema et al., 2020; Randazzo et al., 2020; Wu et al., 2020). Since then, the WBS field has undergone considerable growth in the optimization of experimental protocols (Ahmed et al., 2022; Pérez-Cataluña et al., 2021), testing scales, and epidemiological modeling analyses (McMahan et al., 2021) as well as numerous observational case studies on a local or national scale (Shah et al., 2022). In April 2022, the World Health Organization (WHO) published interim guidelines for the environmental surveillance of SARS-CoV-2 as a complementary method to clinical diagnostics, thus further legitimizing WBS as an important public health tool (World Health Organization, 2022). With the emergence of SARS-CoV-2 variants of concern (VOCs), the applications of WBS have also expanded from merely indicating the presence of the virus to tracking the occurrence and prevalence of VOCs circulating in the community.
Vaccinations have been shown to be the most effective tool available to date to control the COVID-19 pandemic. Reinfections of naturally immunized or fully vaccinated individuals, however, have been frequently reported for the Delta and recently emerged Omicron variants (Brown, 2021; Cavanaugh, 2021; Griffin, 2021; Lafaie et al., 2020; Vitale et al., 2021; Wang et al., 2021; Wolfe et al., 2022; Yuan et al., 2020), suggesting that SARS-CoV-2 will most likely not be completely eradicated and the virus likely persisting in populations and becoming endemic like seasonal influenza or common human coronaviruses. On the other hand, the effectiveness of current COVID-19 vaccines against severe illness and hospitalization (Pilishvili et al., 2021; Thompson et al., 2020), together with progress in the ongoing global vaccination campaigns, the economic costs of lockdowns, as well as pandemic fatigue among the public (Azzolino and Cesari, 2022; Haktanir et al., 2021) has motivated policy changes towards endemicity.
As the classification of SARS-CoV-2 transitions from pandemic to endemic, the objectives and focus of WBS will necessarily change. Here, we explore the roles of wastewater surveillance in a COVID-19 endemic future, and discuss future developments that are important for the field as preparation for the next viral pandemic.
2. Why will we need wastewater surveillance in an endemic future?
While clinical testing remains the gold standard, albeit imperfect, for verifying SARS-CoV-2 infection in individuals, the costs associated with reagents, equipment, and personnel pose substantial economic burden for a long-term, population-level surveillance program. On the contrary, WBS, in part due to its ‘composite’ nature (samples are commonly taken downstream of defined populations including the inflows of wastewater treatment facilities), is comparatively cost-effective (Safford et al., 2022; Shrestha et al., 2021). Taking a sewershed with 10,000 people as an example, WBS only needs to test one sample for mass screening of SARS-CoV-2. Clinical testing, on the other hand, may require 10,000 individual tests and bears the associated costs for sample collection and investments in high-throughput infrastructure. While wastewater surveillance programs cost more capital expenditure on infrastructure setup at the start, the need for a long-term surveillance program for endemic SARS-CoV-2 increases the cost effectiveness of such investments. Pragmatically weighing costs and benefits, a well-designed wastewater surveillance program that delivers reliable results would do much to inform pandemic response. Thus, WBS is especially attractive for longitudinal mass surveillance of SARS-CoV-2 infections.
Second, wastewater sampling on the population level is non-invasive, since samples are collected from wastewater treatment plants and sewers in comparison to nasopharyngeal or anal swabs. The former also shields individuals from privacy concerns, though these are then relegated to the community level. While the majority of individuals have acquiesced, to a large degree, in the collection of their personal data including locations, personal mobility, and relationship, and health status during the pandemic (Bentotahewa et al., 2021), it would be challenging to maintain this over a long endemic phase, due to potential concerns over its impact on civil liberties (Gable et al., 2020). Moreover, sampling wastewater affords an opportunity to avoid the potential social stigma experienced by some COVID-19 patients and healthcare workers (Bhanot et al., 2021; Thompson et al., 2020).
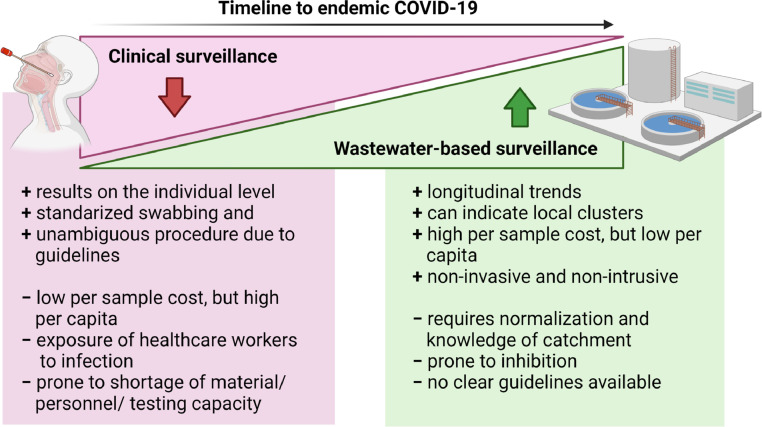
Further, the acceptance of COVID-19 as endemic will likely be accompanied by the roll back of routine diagnostics for communities from current emergency / pandemic response levels (Fig. 1 ). Subsequently, a reduction in the frequency of clinical PCR tests, in part due to increasing substitution by at-home rapid tests, and decreased societal willingness to get tested are to be expected. The antigen-based self-tests with reduced sensitivity, coupled with decreased testing behavior, could also impact the accuracy of government estimates of disease incidence. Thus, decentralization of individual testing and asymptomatic carriage and viral shedding despite vaccination (Riemersma et al., 2021) consolidate the relevance of WBS as an independent indicator of infection trends. These practical considerations suggest that WBS will be the most feasible and effective tool for monitoring SARS-CoV-2 infection in the population in an endemic future. However, caveats and challenges to the implementation of WBS, in the form of non-unified standards, uncertainty and variability of measurements remain to be overcome, and these will be discussed in the later section.
Fig. 1.
The shift from extensive (pandemic level) clinical testing towards a more sustainable, cost-efficient, and non-invasive population-level surveillance program conferred by wastewater-based surveillance, in the transition towards endemic COVID-19.
3. Future roles of wastewater surveillance
3.1. Trend monitoring - quantitative estimate of changes in disease incidence
As SARS-CoV-2 becomes endemic, municipal wastewater signals for the virus may perpetually stay positive, impairing the utility of qualitative information like the binary presence or absence of detectable viral shedding within a targeted community. Instead, quantitative monitoring of the longitudinal trends of viral concentrations in wastewater may yield more effective health information. Accurate trending would require a framework for regular wastewater sampling and virus quantification to establish a reference level of viral concentration corresponding to a certain infection incidence (such as 1 new case a day in 10,000 people) in each sewershed. Rising trends would then suggest the onset of emerging infections in a population and subsequently trigger (non-pharmaceutical) infection control and interventions, such as localized quarantine or mass swabbing orders. Falling trends, on the other hand, would indicate the adequacy of ongoing health management policies.
Differences in geography, climate (i.e., precipitation, and temperature), population size and vaccine coverage (including rationing of boosters), imply that the concentration of SARS-CoV-2 RNA that forms the reference line may be specific and dynamic for each sewershed and dependent on the sample logistics and processing methods. Moreover, the reference levels of SARS-CoV-2 in wastewater can be influenced by the difference in viral shedding rates amongst variants of concern and the proportion of vulnerable and immunized individuals in a population. Thus, developing a response framework to deal with the baseline levels of SARS-CoV-2 “variant de jour” and to detect the inflexion point in the temporal trends of viral concentrations would be essential for wastewater surveillance for population health in the endemic future.
3.2. Moving sampling upstream - qualitative assessment of positivity to track infections in smaller sewersheds
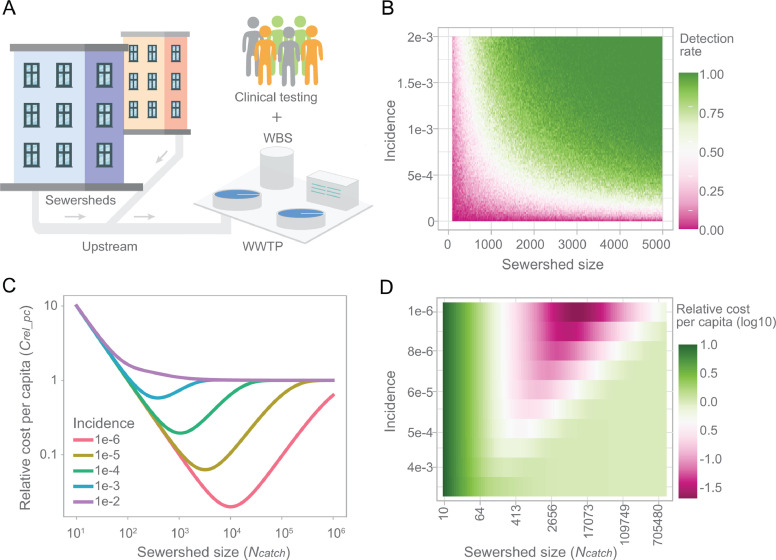
In a SARS-CoV-2 endemic future, we anticipate increased emphasis on wastewater sampling upstream of the wastewater treatment plant (Fig. 2 A). Depending on the extent of granularity desired for the targeted populations, wastewater samples would be collected at multiple levels like neighborhoods (Rios et al., 2021), schools (Gibas et al., 2021; Harris-Lovett et al., 2021; Karthikeyan et al., 2021), and housing estates (Wong et al., 2021), down to the individual building level (Gibas et al., 2021), at prisons, care homes, and food manufacturing sites for instance. Such flexibility enables precision in pinpointing locations where infections are taking place (Liu et al., 2022; Thompson et al., 2020; Yeager et al., 2021). Compared to large wastewater treatment plants, collecting samples from smaller sewersheds allows for the identification of neighborhood hotspots and enables the public health authorities to prioritize areas for more focused clinical testing, possibly ring fencing disease prior to the onset of larger outbreaks (Mota et al., 2021), and importantly, increases sensitivity for detecting changes in viral trends (Hewitt et al., 2022). However, it also poses challenges for sampling such as installation and maintenance in limited space as well as safety concerns, and passive sampling could be an alternative strategy because of its flexibility and ease of use (Habtewold et al., 2022; Schang et al., 2021).
Fig. 2.
Wastewater surveillance in endemic COVID-19. (A) Schematic diagram showing the integration of upstream wastewater sampling and clinical testing for endemic COVID-19. (B) An illustration of the relationship between the sewershed size, incidence, and positive detection rate for wastewater samples, based on a previous study (Wu et al., 2021). (C-D) Cost-effectiveness through integration of wastewater surveillance and clinical tests. Both incidence and sewershed size impact the total cost for mass surveillance of viral infections in the population. A minimum cost can be achieved through strategically customizing the sewershed size for wastewater surveillance based on the infection incidence. ; the range for v is (10−6, 10−2); and range for Ncatch is (10, 106).
We anticipate a modular system whereby qualitative assessment of positivity at catchments of different sizes could be used to identify disease incidence during the early outbreak of an emergent variant. The probability of SARS-CoV-2 detection in wastewater is impacted by infection incidence and population size in the sewershed, and the highest detection rate is mostly achieved from high incidence rate and large population size ((Wu et al., 2021), Fig. 2B). Such a qualitative relationship could help us to evaluate the detection efficiency and potentially to estimate new infections in the sub-catchments (Black et al., 2021; Claro et al., 2021).
3.3. Integrating WBS and clinical testing for cost-effective mass surveillance
It is expensive and challenging to maintain a clinical testing program with the current capacity for an endemic SARS-CoV-2. A long term COVID-19 surveillance program needs to be economically sustainable, of which wastewater surveillance and clinical testing should be integrated to achieve mass surveillance of infected individuals in the population in a cost-efficient way. This would be especially useful should containment and ring-fencing be necessary following the emergence of highly virulent variants. To better understand the economics of this integrated system, we developed an equation (Eq. (1)) to measure the total cost of conducting WBS in modular catchments, followed up by conducting clinical tests on individuals living in the WBS-positive catchments. In this equation, the population (with a size Npop) is divided into different sub-sewersheds (each with a population size of Ncatch) and the total cost for WBS (CtotWBS) is the number of sub-sewersheds multiplied by the cost of one wastewater sample (CWBS). The total cost of clinical testing (Ctotind) relies on the cost of an individual clinical test (Cind) and the probability of a given sewershed having positive cases (fp), which is determined by the incidence (v). The relative total per capita cost (Crel_pc) is measured relative to the cost of an individual clinical test (Eq. (2)).
| (1) |
where
| (2) |
Setting , we evaluated relative per capita cost over different sewershed sizes (Ncatch). We observe a regime of small incidence rates where there is a sewershed size that minimizes total cost by optimizing the tradeoff between WBS cost and individual cost (Fig. 2C). This optimal situation happens when many sewersheds can be eliminated as negative via WBS, so fewer individuals need to undergo clinical testing. We extend this analysis to a more densely sampled range of incidences and sewershed sizes and find that low incidence rates with sewershed sizes in the thousands minimize total cost, whereas higher incidence rates or larger catchment sizes lead to a relative total cost of 1 (Fig. 2D). The latter situation is equivalent to applying clinical testing to every individual in the population because all sewersheds are positive by WBS.
However, this model has some limitations. The equation assumes that the incidence rate is equal across sewersheds, that sewersheds are the same size, and does not account for laboratory variability or PCR inhibition when conducting WBS. Additionally, we chose an arbitrary cost ratio where WBS for one catchment is 100-fold more expensive than an individual clinical test. While these assumptions will not necessarily be fulfilled in all neighborhoods, this simplified model is instructive to show that the size of the sewersheds is a strong determinant of the total cost of combined WBS and clinical surveillance. Thus, when officials are deciding how to implement modular WBS in their population, they should consider optimizing the sewershed sizes to be most cost-effective, given the cost ratio between WBS and individual clinical tests in their municipality.
3.4. Population-level variant tracking
As COVID-19 transitions into an endemic status, WBS will become an exceedingly important tool for monitoring the spread and emergence of SARS-CoV-2 variants. VOCs possess sets of mutations that confer increased transmissibility and/or altered antigenicity to circumvent human immune responses and have been responsible for generating sequential waves of COVID-19 infections and deaths. Given the threat of a potential ‘super variant’, regular wastewater-based variant surveillance for SARS-CoV-2 in defined populations is crucial for each sewershed. Wastewater variant surveillance has shown to provide estimates about the infection trend of clinically relevant mutations or variants (Nieuwenhuijse et al., 2020) and further, the potential to precede detection in clinical samples in targeted populations as revealed by recent studies detecting SARS-CoV-2 genomic variants in wastewater (Bar-Or et al., 2021; Carcereny et al., 2021; Jahn et al., 2021; Joshi et al., 2021). While identifying emerging variants de novo through WBS remains a challenge due to wastewater being complex mixtures of genotypes, it remains useful for a spatiotemporal tracking of SARS-CoV-2 mutations at the population level for a consistent detection of emerging variants (Amman et al., 2022).
We anticipate that technologies that have been developed for variant monitoring in wastewater will continue to play an important role for monitoring the evolution of SARS-CoV-2 in the endemic phase, when clinical testing and sequencing will probably decrease. WBS, coupled with genomic sequencing, would play a prominent role in maintaining a watchful eye on the evolution and emergence of SARS-CoV-2 variants circulating in the community as has previously been demonstrated (Crits-Christoph et al., 2021; Rothman et al., 2021; Smyth et al., 2022). Quantitative, VOC-specific PCR-based assays (Lee et al., 2022, 2021b, 2021a), RT-ddPCR (Gering et al., 2021; Heijnen et al., 2021) and nested RT-PCR (La Rosa et al., 2021) will continue to provide a cost-effective means for identifying and quantifying the distribution of VOCs in populations. Moreover, inferring transmission fitness of different VOCs from wastewater sequencing data will be another attractive but challenging application in endemic COVID-19 as discussed in the next section.
4. Challenges and future developments of wastewater surveillance
4.1. Inconsistencies in wastewater data
To date (February 2022), wastewater surveillance of SARS-CoV-2 has been carried out in 58 countries around the world (Naughton et al., 2021). However, the true utility of WBS for public health decision-making for COVID-19 remains yet to be realized due to sources of uncertainty which impact measurement quality and the interpretation of wastewater data (Li et al., 2021; Wade et al., 2022). This variability is present in almost every step from how the samples are collected and concentrated to the use of different reagents across laboratories and agencies (Ahmed et al., 2022; Wade et al., 2022). Wastewater composition could also lead to differences in PCR inhibition (Ahmed et al., 2022). Further, studies on spiked samples suggest that the recovery rate of viruses from wastewater could effectively be less than 10% and vary up to 7 orders of magnitude when tested across laboratories (Pecson et al., 2021). These sources of variability and limited recovery efficiency pose technical hurdles to relating viral load in wastewater to caseload in the community. Adopting US-EPA methods (US EPA, 2015) to assess the surface water quality into WHO recommendations (Deborah, 1996) and ISO norms (ISO 15216–2, 2019) could be a blueprint for such an endeavor to reduce uncertainties pertaining to wastewater viral measurements.
4.2. Uncertain epidemiological inference
While WBS may often be mentioned interchangeably or in combination with wastewater-based epidemiology, obtaining epidemiological estimates from wastewater data is challenging to achieve. Some of the attempts to correlate the concentration of SARS-CoV-2 in wastewater to the number of infected individuals have been proposed by (Wu et al., 2020; Wurtzer et al., 2020; Xiao et al., 2021), as well as others (Fernandez-Cassi et al., 2021; McMahan et al., 2021; Nourbakhsh et al., 2021; Saththasivam et al., 2021). The precision of epidemiological estimates extensively relies on the accuracy of quantification for viral RNA shed into wastewater throughout the course of SARS-CoV-2 infection, which is challenging to obtain especially during the pre-symptomatic phase. Using statistical methods, such as a beta or gamma distribution-based fitting, to infer the temporal viral shedding dynamics from clinical data has partially solved the problem (Ferretti et al., 2020; He et al., 2020; Wu et al., 2022), although more efforts are needed to improve model efficiencies in integrating the heterogeneous clinical datasets subjected to missing data points, methodological biases and inconsistent medical record metadata.
Furthermore, the lack of clinical viral shedding data from vaccinated (single or multiple doses) and unvaccinated individuals, patients with varied severity of symptoms, as well as across SARS-CoV-2 variants also hampers a wide range of WBE endeavours, including calculating the incidence of infection in sewersheds, estimating the effective reproduction number of SARS-CoV-2 in a population (Huisman et al., 2021) and inferring transmission fitness of VOCs from wastewater data (Caduff et al., 2021). On the other hand, viral concentrations in wastewater are impacted by the transient flow rates, water pH, viral degradation and detention in wastewater lines (Amoah et al., 2022; Khan et al., 2021; Simpson et al., 2021), further undermining WBS data applications.
All in all, these uncertainties call for the development of improved frameworks including optimized viral concentrating methods, accurately quantified viral degradation and recovery efficiency, and rigorous measurement of viral shedding dynamics in infected individuals across symptoms, age groups, and SARS-CoV-2 variants. These efforts will lead to better quantifying the relationship between the virus load in a sewershed and the number of infected individuals present for maximizing the utility of wastewater data for SARS-CoV-2 management.
4.3. Challenging variant tracking and viral discovery
Using wastewater-based surveillance to monitor the emergence of SARS-CoV-2 variants has the potential to become an exceedingly important and versatile tool in a COVID-19 endemic future. Accurate quantification of low-frequency variants by WBS remains a technological challenge due to the dilution and decay of SARS-CoV-2 in wastewater and the lack of normalization parameters to confidentially indicate the population equivalents contributing to a sewershed at any given time. To further complicate things, new variants usually start to occur in a small group of people, which necessitates the development of sensitive and reliable bioinformatics approaches to discover the emerging low-frequency variants in wastewater. Metagenomic shotgun sequencing of either untargeted (Crits-Christoph et al., 2021) or targeted amplicons (Bar-Or et al., 2021; Fontenele et al., 2021; Nemudryi et al., 2020; Pérez-Cataluña et al., 2022; Swift et al., 2021) enables the discovery of viral genetic mutations to infer variants that are circulating in the sewershed. Reliable lineage assignment from viral metagenomic dataset remains putative (Fontenele et al., 2021) though methodological improvements have been made (Karthikeyan et al., 2021). This is further hampered by the low concentration and impaired integrity of SARS-CoV-2 RNA in wastewater samples. Thus, optimizations in experimental protocol and bioinformatics analysis are necessary to promote wastewater surveillance to be a more feasible tool for timely proactive variant tracking.
The COVID-19 pandemic has brought to the fore the need to develop expansive yet cost-effective methods for the detection and discovery of emerging zoonotic agents. To this end, WBS coupled with next generation sequencing represents a highly promising tool. As wastewater is a complex matrix containing a myriad of microorganisms, the detection of the pathogens requires either amplification or metagenomics with an implemented viral nucleotide acid enrichment (Crits-Christoph et al., 2021; Rothman et al., 2021), both of which rely on, and are biased by, a priori knowledge of genomic information on those biological agents. Pathogen detection using molecular methods independent of prior knowledge need to be developed for early warning surveillance of novel viruses with risk to human health.
5. Conclusions
We have discussed the utility of and needed methodologies for WBS as COVID-19 and its epidemiology evolve into endemicity and identified the following key conclusions:
-
•
WBS continues to be a cost-effective and non-invasive tool for mass surveillance of SARS-CoV-2 infection in the long run.
-
•
Decentralization of individual testing and asymptomatic carriage despite vaccination underscore the importance of WBS of SARS-CoV-2 as an independent indicator of infection trends.
-
•
The objectives and potential of WBS in an endemic phase will shift from early case detection to providing situational assessment about the trend of viral infection in the population.
-
•
Cost-effective integration of wastewater and clinical surveillance could be achieved through optimizing sewershed size.
-
•
WBS remains essential for population level variant tracking to anticipate emerging variants of concern, although further improvements in methodologies are necessary for proactive variant tracking.
-
•
Wastewater research should optimize frameworks to improve recovery efficiency and reduce inconsistencies in wastewater data towards improving epidemiological inference.
-
•
Methods developed in the context of SARS-CoV-2 and its analyses could be of invaluable benefit for future wastewater monitoring work on discovering emerging zoonotic pathogens and for early recognition of future pandemics.
Declaration of Competing Interest
E.J.A. is an advisor to Biobot Analytics, Inc. and holds shares in the company.
Acknowledgements
This work was supported by the MIT Center for Microbiome Informatics and Therapeutics, funding from the Massachusetts Consortium on Pathogen Readiness (MassCPR) and China Evergrande Group (EJA), the National Research Foundation, Prime Minister's Office, Singapore, under its Campus for Research Excellence and Technological Enterprise (CREATE) program funding to the Singapore-MIT Alliance for Research and Technology (SMART) Antimicrobial Resistance Interdisciplinary Research Group (AMR IRG), the Intra-CREATE Thematic Grant (Cities) grant NRF2019-THE001–0003a to JT and EJA and funding from the Singapore Ministry of Education and National Research Foundation through an RCE award to Singapore centre for Environmental Life Sciences Engineering (SCELSE) to SW and JT. FW is supported by the Faculty Startup funding from the Center of Infectious Diseases at UTHealth, the UT system Rising STARs award, and the Texas Epidemic Public Health Institute (TEPHI), which is housed within and supported administratively by The University of Texas Health Science Center at Houston (UTHealth).
References
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Simpson S.L., Bertsch P.M., Bibby K., Bivins A., Blackall L.L., Bofill-Mas S., Bosch A., Brandão J., Choi P.M., Ciesielski M., Donner E., D'Souza N., Farnleitner A.H., Gerrity D., Gonzalez R., Griffith J.F., Gyawali P., Haas C.N., Hamilton K.A., Hapuarachchi H.C., Harwood V.J., Haque R., Jackson G., Khan S.J., Khan W., Kitajima M., Korajkic A., La Rosa G., Layton B.A., Lipp E., McLellan S.L., McMinn B., Medema G., Metcalfe S., Meijer W.G., Mueller J.F., Murphy H., Naughton C.C., Noble R.T., Payyappat S., Petterson S., Pitkänen T., Rajal V.B., Reyneke B., Roman F.A., Rose J.B., Rusiñol M., Sadowsky M.J., Sala-Comorera L., Setoh Y.X., Sherchan S.P., Sirikanchana K., Smith W., Steele J.A., Sabburg R., Symonds E.M., Thai P., Thomas K.V., Tynan J., Toze S., Thompson J., Whiteley A.S., Wong J.C.C., Sano D., Wuertz S., Xagoraraki I., Zhang Q., Zimmer-Faust A.G., Shanks O.C. Minimizing errors in RT-PCR detection and quantification of SARS-CoV-2 RNA for wastewater surveillance. Sci. Total Environ. 2022;805 doi: 10.1016/j.scitotenv.2021.149877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amman Fabian, Markt Rudolf, Endler Lukas, Hupfauf Sebastian, Agerer Benedikt, Schedl Anna, Richter Lukas, Zechmeister Melanie, Bicher Martin, Heiler Georg, Triska Petr, Thornton Matthew, Penz Thomas, Senekowitsch Martin, Laine Jan, Keszei Zsofia, Daleiden Beatrice, Steinlechner Martin, Neiderstätter Harald, Scheffknecht Christoph, Vogl Gunther, Weichlinger Günther, Wagner Andreas, Slipko Katarzyna, Masseron Amandine, Radu Elena, Allerberger Franz, Popper Niki, Bock Christoph, Schmid Daniela, Oberacher Herbert, Kreuzinger Norbert, Insam Heribert, Bergthaler Andreas. National-scale surveillance of emerging SARS-CoV-2 variants in wastewater. medRxiv. 2022 doi: 10.1101/2022.01.14.21267633. [DOI] [PubMed] [Google Scholar]
- Amoah I.D., Abunama T., Awolusi O.O., Pillay L., Pillay K., Kumari S., Bux F. Effect of selected wastewater characteristics on estimation of SARS-CoV-2 viral load in wastewater. Environ. Res. 2022;203 doi: 10.1016/j.envres.2021.111877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzolino D., Cesari M. Fatigue in the COVID-19 pandemic. The Lancet Healthy Longevity. 2022;3:e128–e129. doi: 10.1016/S2666-7568(22)00029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or I., Weil M., Indenbaum V., Bucris E., Bar-Ilan D., Elul M., Levi N., Aguvaev I., Cohen Z., Shirazi R., Erster O., Sela-Brown A., Sofer D., Mor O., Mendelson E., Zuckerman N.S. Detection of SARS-CoV-2 variants by genomic analysis of wastewater samples in Israel. Sci. Total Environ. 2021;789 doi: 10.1016/j.scitotenv.2021.148002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentotahewa V., Hewage C., Williams J. Frontiers in Big Data 4. 2021. Solutions to big data privacy and security challenges associated with COVID-19 surveillance systems. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanot D., Singh T., Verma S.K., Sharad S. Stigma and discrimination during COVID-19 pandemic. Front. Public Health. 2021;8 doi: 10.3389/fpubh.2020.577018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J., Aung P., Nolan M., Roney E., Poon R., Hennessy D., Crosbie N.D., Deere D., Jex A.R., John N., Baker L., Scales P.J., Usher S.P., McCarthy D.T., Schang C., Schmidt J., Myers S., Begue N., Kaucner C., Thorley B., Druce J., Monis P., Lau M., Sarkis S. Epidemiological evaluation of sewage surveillance as a tool to detect the presence of COVID-19 cases in a low case load setting. Sci. Total Environ. 2021;786 doi: 10.1016/j.scitotenv.2021.147469. [DOI] [Google Scholar]
- Brown C.M. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings — Barnstable County, Massachusetts, July 2021. MMWR Morb. Mortal. Wkly Rep. 2021;70 doi: 10.15585/mmwr.mm7031e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caduff, L., Dreifuss, D., Schindler, T., Devaux, A.J., Ganesanandamoorthy, P., Kull, A., Stachler, E., Fernandez-Cassi, X., Beerenwinkel, N., Kohn, T., Ort, C., Julian, T.R., 2021. Inferring Transmission Fitness Advantage of SARS-CoV-2 Variants of Concern in Wastewater Using Digital PCR. https://doi.org/10.1101/2021.08.22.21262024. [DOI] [PMC free article] [PubMed]
- Carcereny A., Martínez-Velázquez A., Bosch A., Allende A., Truchado P., Cascales J., Romalde J.L., Lois M., Polo D., Sánchez G., Pérez-Cataluña A., Díaz-Reolid A., Antón A., Gregori J., Garcia-Cehic D., Quer J., Palau M., Ruano C.G., Pintó R.M., Guix S. Monitoring emergence of the SARS-CoV-2 B1.1.7 Variant through the Spanish National SARS-CoV-2 wastewater surveillance system (VATar COVID-19) Environ. Sci. Technol. 2021;55:11756–11766. doi: 10.1021/acs.est.1c03589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh A.M. Reduced risk of reinfection with SARS-CoV-2 after COVID-19 vaccination — Kentucky, May–June 2021. MMWR Morb. Mortal. Wkly Rep. 2021;70 doi: 10.15585/mmwr.mm7032e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claro I.C.M., Cabral A.D., Augusto M.R., Duran A.F.A., Graciosa M.C.P., Fonseca F.L.A., Speranca M.A., Bueno R.de F. Long-term monitoring of SARS-COV-2 RNA in wastewater in Brazil: a more responsive and economical approach. Water Res. 2021;203 doi: 10.1016/j.watres.2021.117534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crits-Christoph A., Kantor R.S., Olm M.R., Whitney O.N., Al-Shayeb B., Lou Y.C., Flamholz A., Kennedy L.C., Greenwald H., Hinkle A., Hetzel J., Spitzer S., Koble J., Tan A., Hyde F., Schroth G., Kuersten S., Banfield J.F., Nelson K.L. Genome sequencing of sewage detects regionally prevalent SARS-CoV-2 variants. mBio. 2021;12 doi: 10.1128/mBio.02703-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deborah, D. (Ed.), 1996. Water quality assessments: a guide to the use of biota, sediments and water environmental monitoring, 2. ed. ed. E & FN Spon, London.
- Fernandez-Cassi X., Scheidegger A., Bänziger C., Cariti F., Tuñas Corzon A., Ganesanandamoorthy P., Lemaitre J.C., Ort C., Julian T.R., Kohn T. Wastewater monitoring outperforms case numbers as a tool to track COVID-19 incidence dynamics when test positivity rates are high. Water Res. 2021;200 doi: 10.1016/j.watres.2021.117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti L., Wymant C., Kendall M., Zhao L., Nurtay A., Abeler-Dörner L., Parker M., Bonsall D., Fraser C. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science. 2020;368 doi: 10.1126/science.abb6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenele R.S., Kraberger S., Hadfield J., Driver E.M., Bowes D., Holland L.A., Faleye T.O.C., Adhikari S., Kumar R., Inchausti R., Holmes W.K., Deitrick S., Brown P., Duty D., Smith T., Bhatnagar A., Yeager R.A., Holm R.H., von Reitzenstein N.H., Wheeler E., Dixon K., Constantine T., Wilson M.A., Lim E.S., Jiang X., Halden R.U., Scotch M., Varsani A. High-throughput sequencing of SARS-CoV-2 in wastewater provides insights into circulating variants. medRxiv. 2021 doi: 10.1101/2021.01.22.21250320. 2021.01.22.21250320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gable L., Ram N., Ram J.L. Legal and ethical implications of wastewater monitoring of SARS-CoV-2 for COVID-19 surveillance. J. Law Biosci. 2020;7 doi: 10.1093/jlb/lsaa039. lsaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gering, E., Colbert, J., Schmedes, S., Duncan, G., Lopez, J., Motes, J., Weiss, J., Azarian, T., Tekin, O., Blanton, J., 2021. ddPCR Reveals SARS-CoV-2 Variants in Florida Wastewater. https://doi.org/10.1101/2021.04.08.21255119.
- Gibas C., Lambirth K., Mittal N., Juel M.A.I., Barua V.B., Roppolo Brazell L., Hinton K., Lontai J., Stark N., Young I., Quach C., Russ M., Kauer J., Nicolosi B., Chen D., Akella S., Tang W., Schlueter J., Munir M. Implementing building-level SARS-CoV-2 wastewater surveillance on a university campus. Sci. Total Environ. 2021;782 doi: 10.1016/j.scitotenv.2021.146749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J.B. SARS-CoV-2 infections and hospitalizations among persons aged ≥16 Years, by vaccination status — Los Angeles County, California, May 1–July 25, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70 doi: 10.15585/mmwr.mm7034e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habtewold J., McCarthy D., McBean E., Law I., Goodridge L., Habash M., Murphy H.M. Passive sampling, a practical method for wastewater-based surveillance of SARS-CoV-2. Environ. Res. 2022;204 doi: 10.1016/j.envres.2021.112058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haktanir A., Can N., Seki T., Kurnaz M.F., Dilmaç B. Do we experience pandemic fatigue? current state, predictors, and prevention. Curr. Psychol. 2021:1–12. doi: 10.1007/s12144-021-02397-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Lovett S., Nelson K.L., Beamer P., Bischel H.N., Bivins A., Bruder A., Butler C., Camenisch T.D., De Long S.K., Karthikeyan S., Larsen D.A., Meierdiercks K., Mouser P.J., Pagsuyoin S., Prasek S.M., Radniecki T.S., Ram J.L., Roper D.K., Safford H., Sherchan S.P., Shuster W., Stalder T., Wheeler R.T., Korfmacher K.S. Wastewater surveillance for SARS-CoV-2 on college campuses: initial efforts, lessons learned, and research needs. Int. J. Environ. Res. Public Health. 2021;18:4455. doi: 10.3390/ijerph18094455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X., Lau Y.C., Wong J.Y., Guan Y., Tan X., Mo X., Chen Y., Liao B., Chen W., Hu F., Zhang Q., Zhong M., Wu Y., Zhao L., Zhang F., Cowling B.J., Li F., Leung G.M. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- Heijnen, L., Elsinga, G., Graaf, M. de, Molenkamp, R., Koopmans, M.P.G., Medema, G., 2021. Droplet Digital RT-PCR to detect SARS-CoV-2 variants of concern in wastewater. https://doi.org/10.1101/2021.03.25.21254324. [DOI] [PMC free article] [PubMed]
- Hewitt J., Trowsdale S., Armstrong B.A., Chapman J.R., Carter K.M., Croucher D.M., Trent C.R., Sim R.E., Gilpin B.J. Sensitivity of wastewater-based epidemiology for detection of SARS-CoV-2 RNA in a low prevalence setting. Water Res. 2022;211 doi: 10.1016/j.watres.2021.118032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman, J.S., Scire, J., Caduff, L., Fernandez-Cassi, X., Ganesanandamoorthy, P., Kull, A., Scheidegger, A., Stachler, E., Boehm, A.B., Hughes, B., Knudson, A., Topol, A., Wigginton, K.R., Wolfe, M.K., Kohn, T., Ort, C., Stadler, T., Julian, T.R., 2021. Wastewater-based estimation of the effective reproductive number of SARS-CoV-2. https://doi.org/10.1101/2021.04.29.21255961. [DOI] [PMC free article] [PubMed]
- ISO 15216-2, 2019. Microbiology of the food chain — Horizontal method for determination of hepatitis A virus and norovirus using real-time RT-PCR — Part 2: method for detection [WWW Document]. ISO. URL https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/07/42/74263.html (accessed 12.2.21).
- Jahn, K., Dreifuss, D., Topolsky, I., Kull, A., Ganesanandamoorthy, P., Fernandez-Cassi, X., Bänziger, C., Devaux, A.J., Stachler, E., Caduff, L., Cariti, F., Corzón, A.T., Fuhrmann, L., Chen, C., Jablonski, K.P., Nadeau, S., Feldkamp, M., Beisel, C., Aquino, C., Stadler, T., Ort, C., Kohn, T., Julian, T.R., Beerenwinkel, N., 2021. Detection and surveillance of SARS-CoV-2 genomic variants in wastewater. https://doi.org/10.1101/2021.01.08.21249379.
- Joshi, M., Kumar, M., Srivastava, V., Kumar, D., Rathore, D., Pandit, R., Joshi, C.G., 2021. First detection of SARS-CoV-2 Delta variant (B.1.617.2) in the wastewater of (Ahmedabad), India. https://doi.org/10.1101/2021.07.07.21260142.
- Karthikeyan S., Nguyen A., McDonald D., Zong Y., Ronquillo N., Ren J., Zou J., Farmer S., Humphrey G., Henderson D., Javidi T., Messer K., Anderson C., Schooley R., Martin N.K., Knight R. Rapid, large-scale wastewater surveillance and automated reporting system enable early detection of nearly 85% of COVID-19 cases on a university campus. mSystems. 2021;6 doi: 10.1128/mSystems.00793-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K., Tighe S.W., Badireddy A.R. Factors influencing recovery of SARS-CoV-2 RNA in raw sewage and wastewater sludge using polyethylene glycol-based concentration method. J Biomol Tech. 2021;32:172–179. doi: 10.7171/jbt.21-3203-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Mancini P., Bonanno Ferraro G., Veneri C., Iaconelli M., Lucentini L., Bonadonna L., Brusaferro S., Brandtner D., Fasanella A., Pace L., Parisi A., Galante D., Suffredini E. Rapid screening for SARS-CoV-2 variants of concern in clinical and environmental samples using nested RT-PCR assays targeting key mutations of the spike protein. Water Res. 2021;197 doi: 10.1016/j.watres.2021.117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafaie L., Célarier T., Goethals L., Pozzetto B., Grange S., Ojardias E., Annweiler C., Botelho-Nevers E. Recurrence or relapse of COVID-19 in older patients: a description of three cases. J. Am. Geriatr. Soc. 2020;68:2179–2183. doi: 10.1111/jgs.16728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, W.L., Gu, X., Armas, F., Chandra, F., Chen, H., Wu, F., Leifels, M., Xiao, A., Chua, F.J.D., Kwok, G.W., Jolly, S., Lim, C.Y., Thompson, J., Alm, E.J., 2021a. Quantitative SARS-CoV-2 tracking of variants Delta, Delta plus, Kappa and Beta in wastewater by allele-specific RT-qPCR. https://doi.org/10.1101/2021.08.03.21261298.
- Lee, W.L., Gu, X., Armas, F., Wu, F., Chandra, F., Chen, H., Xiao, A., Leifels, M., Chua, F.J.D., Kwok, G.W., Tay, J.Y., Lim, C.Y., Thompson, J., Alm, E.J., 2022. Quantitative detection of SARS-CoV-2 Omicron variant in wastewater through allele-specific RT-qPCR. https://doi.org/10.1101/2021.12.21.21268077.
- Lee W.L., Imakaev M., Armas F., McElroy K.A., Gu X., Duvallet C., Chandra F., Chen H., Leifels M., Mendola S., Floyd-O'Sullivan R., Powell M.M., Wilson S.T., Berge K.L.J., Lim C.Y.J., Wu F., Xiao A., Moniz K., Ghaeli N., Matus M., Thompson J., Alm E.J. Quantitative SARS-CoV-2 alpha variant B.1.1.7 tracking in wastewater by allele-specific RT-qPCR. Environ. Sci. Technol. Lett. 2021;8:675–682. doi: 10.1021/acs.estlett.1c00375. [DOI] [Google Scholar]
- Li X., Zhang S., Shi J., Luby S.P., Jiang G. Uncertainties in estimating SARS-CoV-2 prevalence by wastewater-based epidemiology. Chem. Eng. J. 2021;415 doi: 10.1016/j.cej.2021.129039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Ibaraki M., VanTassell J., Geith K., Cavallo M., Kann R., Guo L., Moe C.L. A sensitive, simple, and low-cost method for COVID-19 wastewater surveillance at an institutional level. Sci. Total Environ. 2022;807 doi: 10.1016/j.scitotenv.2021.151047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor Y., Shulman L.M., Kaliner E., Hindiyeh M., Ram D., Sofer D., Moran-Gilad J., Lev B., Grotto I., Gamzu R., Mendelson E. Intensified environmental surveillance supporting the response to wild poliovirus type 1 silent circulation in Israel, 2013. Euro Surveill. 2014;19:20708. doi: 10.2807/1560-7917.es2014.19.7.20708. [DOI] [PubMed] [Google Scholar]
- McMahan C.S., Self S., Rennert L., Kalbaugh C., Kriebel D., Graves D., Colby C., Deaver J.A., Popat S.C., Karanfil T., Freedman D.L. COVID-19 wastewater epidemiology: a model to estimate infected populations. The Lancet Planetary Health. 2021;5 doi: 10.1016/S2542-5196(21)00230-8. e874–e881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environ. Sci. Technol. Lett. 2020 doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Mota C.R., Bressani-Ribeiro T., Araújo J.C., Leal C.D., Leroy-Freitas D., Machado E.C., Espinosa M.F., Fernandes L., Leão T.L., Chamhum-Silva L., Azevedo L., Morandi T., Freitas G.T.O., Costa M.S., Carvalho B.O., Reis M.T.P., Melo M.C., Ayrimoraes S.R., Chernicharo C.A.L. Assessing spatial distribution of COVID-19 prevalence in Brazil using decentralised sewage monitoring. Water Res. 2021;202 doi: 10.1016/j.watres.2021.117388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton C.C., Roman F.A., Alvarado A.G.F., Tariqi A.Q., Deeming M.A., Bibby K., Bivins A., Rose J.B., Medema G., Ahmed W., Katsivelis P., Allan V., Sinclair R., Zhang Y., Kinyua M.N. Show us the data: global COVID-19 wastewater monitoring efforts, equity, and gaps. medRxiv. 2021 doi: 10.1101/2021.03.14.21253564. 2021.03.14.21253564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemudryi A., Nemudraia A., Wiegand T., Surya K., Buyukyoruk M., Cicha C., Vanderwood K.K., Wilkinson R., Wiedenheft B. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. Cell Reports Med. 2020;1 doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuijse D.F., Oude Munnink B.B., Phan M.V.T., Munk P., Venkatakrishnan S., Aarestrup F.M., Cotten M., Koopmans M.P.G. Setting a baseline for global urban virome surveillance in sewage. Sci. Rep. 2020;10:13748. doi: 10.1038/s41598-020-69869-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourbakhsh, S., Fazil, A., Li, M., Mangat, C.S., Peterson, S.W., Daigle, J., Langner, S., Shurgold, J., D'Aoust, P., Delatolla, R., Mercier, E., Pang, X., Lee, B.E., Stuart, R., Wijayasri, S., Champredon, D., 2021. A Wastewater-Based Epidemic Model for SARS-CoV-2 with Application to Three Canadian Cities. https://doi.org/10.1101/2021.07.19.21260773. [DOI] [PMC free article] [PubMed]
- Pecson B.M., Darby E., Haas C.N., Amha Y.M., Bartolo M., Danielson R., Dearborn Y., Giovanni G.D., Ferguson C., Fevig S., Gaddis E., Gray D., Lukasik G., Mull B., Olivas L., Olivieri A., Qu Y., Consortium S.-C.-2 I. Reproducibility and sensitivity of 36 methods to quantify the SARS-CoV-2 genetic signal in raw wastewater: findings from an interlaboratory methods evaluation in the U.S. Environ. Sci.: Water Res. Technol. 2021;7:504–520. doi: 10.1039/D0EW00946F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Cataluña A., Chiner-Oms Á., Cuevas-Ferrando E., Díaz-Reolid A., Falcó I., Randazzo W., Girón-Guzmán I., Allende A., Bracho M.A., Comas I., Sánchez G. Spatial and temporal distribution of SARS-CoV-2 diversity circulating in wastewater. Water Res. 2022;211 doi: 10.1016/j.watres.2021.118007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Cataluña A., Cuevas-Ferrando E., Randazzo W., Falcó I., Allende A., Sánchez G. Comparing analytical methods to detect SARS-CoV-2 in wastewater. Sci. Total Environ. 2021;758 doi: 10.1016/j.scitotenv.2020.143870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilishvili T., Gierke R., Fleming-Dutra K.E., Farrar J.L., Mohr N.M., Talan D.A., Krishnadasan A., Harland K.K., Smithline H.A., Hou P.C., Lee L.C., Lim S.C., Moran G.J., Krebs E., Steele M.T., Beiser D.G., Faine B., Haran J.P., Nandi U., Schrading W.A., Chinnock B., Henning D.J., Lovecchio F., Lee J., Barter D., Brackney M., Fridkin S.K., Marceaux-Galli K., Lim S., Phipps E.C., Dumyati G., Pierce R., Markus T.M., Anderson D.J., Debes A.K., Lin M.Y., Mayer J., Kwon J.H., Safdar N., Fischer M., Singleton R., Chea N., Magill S.S., Verani J.R., Schrag S.J. Effectiveness of mRNA Covid-19 Vaccine among U.S. Health Care Personnel. N. Engl. J. Med. 2021;0 doi: 10.1056/NEJMoa2106599. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemersma, K.K., Grogan, B.E., Kita-Yarbro, A., Halfmann, P.J., Segaloff, H.E., Kocharian, A., Florek, K.R., Westergaard, R., Bateman, A., Jeppson, G.E., Kawaoka, Y., O'Connor, D.H., Friedrich, T.C., Grande, K.M., 2021. Shedding of infectious SARS-CoV-2 Despite Vaccination. https://doi.org/10.1101/2021.07.31.21261387.
- Rios G., Lacoux C., Leclercq V., Diamant A., Lebrigand K., Lazuka A., Soyeux E., Lacroix S., Fassy J., Couesnon A., Thiery R., Mari B., Pradier C., Waldmann R., Barbry P. Monitoring SARS-CoV-2 variants alterations in Nice neighborhoods by wastewater nanopore sequencing. The Lancet Regional Health – Europe. 2021:10. doi: 10.1016/j.lanepe.2021.100202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J.A., Loveless T.B., Kapcia J., Adams E.D., Steele J.A., Zimmer-Faust A.G., Langlois K., Wanless D., Griffith M., Mao L., Chokry J., Griffith J.F., Whiteson K.L. RNA viromics of Southern California wastewater and detection of SARS-CoV-2 single-nucleotide variants. Appl. Environ. Microbiol. 2021;87 doi: 10.1128/AEM.01448-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safford H.R., Shapiro K., Bischel H.N. Wastewater analysis can be a powerful public health tool—If it's done sensibly. Proc. Natl. Acad. Sci. 2022;119 doi: 10.1073/pnas.2119600119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saththasivam J., El-Malah S.S., Gomez T.A., Jabbar K.A., Remanan R., Krishnankutty A.K., Ogunbiyi O., Rasool K., Ashhab S., Rashkeev S., Bensaad M., Ahmed A.A., Mohamoud Y.A., Malek J.A., Abu Raddad L.J., Jeremijenko A., Abu Halaweh H.A., Lawler J., Mahmoud K.A. COVID-19 (SARS-CoV-2) outbreak monitoring using wastewater-based epidemiology in Qatar. Sci. Total Environ. 2021;774 doi: 10.1016/j.scitotenv.2021.145608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schang C., Crosbie N.D., Nolan M., Poon R., Wang M., Jex A., John N., Baker L., Scales P., Schmidt J., Thorley B.R., Hill K., Zamyadi A., Tseng C.-.W., Henry R., Kolotelo P., Langeveld J., Schilperoort R., Shi B., Einsiedel S., Thomas M., Black J., Wilson S., McCarthy D.T. Passive sampling of SARS-CoV-2 for wastewater surveillance. Environ. Sci. Technol. 2021;55:10432–10441. doi: 10.1021/acs.est.1c01530. [DOI] [PubMed] [Google Scholar]
- Shah S., Gwee S.X.W., Ng J.Q.X., Lau N., Koh J., Pang J. Wastewater surveillance to infer COVID-19 transmission: a systematic review. Sci. Total Environ. 2022;804 doi: 10.1016/j.scitotenv.2021.150060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S., Yoshinaga E., Chapagain S.K., Mohan G., Gasparatos A., Fukushi K. Wastewater-based epidemiology for cost-effective mass surveillance of COVID-19 in low- and middle-income countries: challenges and opportunities. Water. 2021;13:2897. doi: 10.3390/w13202897. [DOI] [Google Scholar]
- Simpson A., Topol A., White B.J., Wolfe M.K., Wigginton K.R., Boehm A.B. Effect of storage conditions on SARS-CoV-2 RNA quantification in wastewater solids. PeerJ. 2021;9:e11933. doi: 10.7717/peerj.11933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D.S., Trujillo M., Gregory D.A., Cheung K., Gao A., Graham M., Guan Y., Guldenpfennig C., Hoxie I., Kannoly S., Kubota N., Lyddon T.D., Markman M., Rushford C., San K.M., Sompanya G., Spagnolo F., Suarez R., Teixeiro E., Daniels M., Johnson M.C., Dennehy J.J. Tracking cryptic SARS-CoV-2 lineages detected in NYC wastewater. Nat. Commun. 2022;13:635. doi: 10.1038/s41467-022-28246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift C.L., Isanovic M., Correa Velez K.E., Norman R.S. Community-level SARS-CoV-2 sequence diversity revealed by wastewater sampling. Sci. Total Environ. 2021;801 doi: 10.1016/j.scitotenv.2021.149691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.R., Nancharaiah Y.V., Gu X., Lee W.L., Rajal V.B., Haines M.B., Girones R., Ng L.C., Alm E.J., Wuertz S. Making waves: wastewater surveillance of SARS-CoV-2 for population-based health management. Water Res. 2020;184 doi: 10.1016/j.watres.2020.116181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA, O., 2015. Approved CWA Microbiological Test Methods [WWW Document]. URL https://www.epa.gov/cwa-methods/approved-cwa-microbiological-test-methods (accessed 12.2.21).
- Vitale J., Mumoli N., Clerici P., De Paschale M., Evangelista I., Cei M., Mazzone A. Assessment of SARS-CoV-2 reinfection 1 year after primary infection in a population in Lombardy, Italy. JAMA Intern Med. 2021 doi: 10.1001/jamainternmed.2021.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade M.J., Lo Jacomo A., Armenise E., Brown M.R., Bunce J.T., Cameron G.J., Fang Z., Farkas K., Gilpin D.F., Graham D.W., Grimsley J.M.S., Hart A., Hoffmann T., Jackson K.J., Jones D.L., Lilley C.J., McGrath J.W., McKinley J.M., McSparron C., Nejad B.F., Morvan M., Quintela-Baluja M., Roberts A.M.I., Singer A.C., Souque C., Speight V.L., Sweetapple C., Walker D., Watts G., Weightman A., Kasprzyk-Hordern B. Understanding and managing uncertainty and variability for wastewater monitoring beyond the pandemic: lessons learned from the United Kingdom national COVID-19 surveillance programmes. J. Hazard. Mater. 2022;424 doi: 10.1016/j.jhazmat.2021.127456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Kaperak C., Sato T., Sakuraba A. COVID-19 reinfection: a rapid systematic review of case reports and case series. J. Investig. Med. 2021;69:1253–1255. doi: 10.1136/jim-2021-001853. [DOI] [PubMed] [Google Scholar]
- Wolfe, M., Hughes, B., Duong, D., Chan-Herur, V., Wigginton, K.R., White, B.J., Boehm, A.B., 2022. Detection of SARS-CoV-2 variant Mu, Beta, Gamma, Lambda, Delta, Alpha, and Omicron in wastewater settled solids using mutation-specific assays is associated with regional detection of variants in clinical samples. https://doi.org/10.1101/2022.01.17.22269439. [DOI] [PMC free article] [PubMed]
- Wong J.C.C., Tan J., Lim Y.X., Arivalan S., Hapuarachchi H.C., Mailepessov D., Griffiths J., Jayarajah P., Setoh Y.X., Tien W.P., Low S.L., Koo C., Yenamandra S.P., Kong M., Lee V.J.M., Ng L.C. Non-intrusive wastewater surveillance for monitoring of a residential building for COVID-19 cases. Sci. Total Environ. 2021;786 doi: 10.1016/j.scitotenv.2021.147419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2022. Environmental surveillance for SARS-COV-2 to complement public health surveillance – Interim Guidance. URL https://www.who.int/publications-detail-redirect/WHO-HEP-ECH-WSH-2022.1.
- World Health Organization, 2003. Guidelines for environmental surveillance of poliovirus circulation (No. WHO/V&B/03.03).
- Wu F., Xiao A., Zhang J., Gu X., Lee W.L., Kauffman K., Hanage W., Matus M., Ghaeli N., Endo N., Duvallet C., Moniz K., Erickson T., Chai P., Thompson J., Alm E. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. mSystems. 2020;5 doi: 10.1128/mSystems.00614-20. 2020.04.05.20051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Xiao A., Zhang J., Moniz K., Endo N., Armas F., Bonneau R., Brown M.A., Bushman M., Chai P.R., Duvallet C., Erickson T.B., Foppe K., Ghaeli N., Gu X., Hanage W.P., Huang K.H., Lee W.L., Matus M., McElroy K.A., Nagler J., Rhode S.F., Santillana M., Tucker J.A., Wuertz S., Zhao S., Thompson J., Alm E.J. SARS-CoV-2 RNA concentrations in wastewater foreshadow dynamics and clinical presentation of new COVID-19 cases. Sci. Total Environ. 2022;805 doi: 10.1016/j.scitotenv.2021.150121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Xiao A., Zhang J., Moniz K., Endo N., Armas F., Bushman M., Chai P.R., Duvallet C., Erickson T.B., Foppe K., Ghaeli N., Gu X., Hanage W.P., Huang K.H., Lee W.L., McElroy K.A., Rhode S.F., Matus M., Wuertz S., Thompson J., Alm E.J. Wastewater surveillance of SARS-CoV-2 across 40 U.S. states from February to June 2020. Water Res. 2021;202 doi: 10.1016/j.watres.2021.117400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J.M., Maday Y., Teyssou R., Richard E., Almayrac J.L., Moulin L. Evaluation of lockdown effect on SARS-CoV-2 dynamics through viral genome quantification in waste water, Greater Paris, France, 5 March to 23 April 2020. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.50.2000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao A., Wu F., Bushman M., Zhang J., Imakaev M., Chai P.R., Duvallet C., Endo N., Erickson T.B., Armas F., Arnold B., Chen H., Chandra F., Ghaeli N., Gu X., Hanage W.P., Lee W.L., Matus M., McElroy K.A., Moniz K., Rhode S.F., Thompson J., Alm E.J. Metrics to relate COVID-19 wastewater data to clinical testing dynamics. medRxiv. 2021 doi: 10.1101/2021.06.10.21258580. 2021.06.10.21258580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager R., Holm R.H., Saurabh K., Fuqua J.L., Talley D., Bhatnagar A., Smith T. Wastewater sample site selection to estimate geographically resolved community prevalence of COVID-19: a sampling protocol perspective. GeoHealth. 2021;5 doi: 10.1029/2021GH000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B., Liu H.-.Q., Yang Z.-.R., Chen Y.-.X., Liu Z.-.Y., Zhang K., Wang C., Li W.-.X., An Y.-.W., Wang J.-.C., Song S. Recurrence of positive SARS-CoV-2 viral RNA in recovered COVID-19 patients during medical isolation observation. Sci. Rep. 2020;10:11887. doi: 10.1038/s41598-020-68782-w. [DOI] [PMC free article] [PubMed] [Google Scholar]