This ancillary analysis of the Lifestyle Interventions and Independence For Elders Study evaluates whether a moderate-intensity exercise intervention can affect the estimated glomerular filtration rate per cystatin C change in older adults.
Key Points
Question
Can a moderate-intensity physical activity and exercise intervention slow the rate of decline of estimated glomerular filtration rate per cystatin C in sedentary older adults?
Findings
In this ancillary analysis of a randomized clinical trial of 1199 adults aged 70 to 89 years, those randomized to the physical activity and exercise intervention had statistically significantly lower decline in estimated glomerular filtration rate per cystatin C over 2 years compared with those in the health education arm.
Meaning
Clinicians should consider prescribing physical activity and moderate-intensity exercise for older adults to slow the rate of decline of kidney function.
Abstract
Importance
Observational evidence suggests that higher physical activity is associated with slower kidney function decline; however, to our knowledge, no large trial has evaluated whether activity and exercise can ameliorate kidney function decline in older adults.
Objective
To evaluate whether a moderate-intensity exercise intervention can affect the rate of estimated glomerular filtration rate per cystatin C (eGFRCysC) change in older adults.
Design, Setting, and Participants
This ancillary analysis of the Lifestyle Interventions and Independence For Elders randomized clinical trial enrolled 1199 community-dwelling, sedentary adults aged 70 to 89 years with mobility limitations and available blood specimens. The original trial was conducted across 8 academic centers in the US from February 2010 through December 2013. Data for this study were analyzed from March 29, 2021, to February 28, 2022.
Interventions
Structured, 2-year, partially supervised, moderate-intensity physical activity and exercise (strength, flexibility) intervention compared with a health education control intervention with 2-year follow-up. Physical activity was measured by step count and minutes of moderate-intensity activity using accelerometers.
Main Outcomes and Measures
The primary outcome was change in eGFRCysC. Rapid eGFRCysC decline was defined by the high tertile threshold of 6.7%/y.
Results
Among the 1199 participants in the analysis, the mean (SD) age was 78.9 (5.2) years, and 800 (66.7%) were women. At baseline, the 2 groups were well balanced by age, comorbidity, and baseline eGFRCysC. The physical activity and exercise intervention resulted in statistically significantly lower decline in eGFRCysC over 2 years compared with the health education arm (mean difference, 0.96 mL/min/1.73 m2; 95% CI, 0.02-1.91 mL/min/1.73 m2) and lower odds of rapid eGFRCysC decline (odds ratio, 0.79; 95% CI, 0.65-0.97).
Conclusions and Relevance
Results of this ancillary analysis of a randomized clinical trial showed that when compared with health education, a physical activity and exercise intervention slowed the rate of decline in eGFRCysC among community-dwelling sedentary older adults. Clinicians should consider targeted recommendation of physical activity and moderate-intensity exercise for older adults as a treatment to slow decline in eGFRCysC.
Trial Registration
ClinicalTrials.gov Identifier: NCT01072500
Introduction
Older adults have the highest burden of chronic kidney disease (CKD). More than 37% of adults 70 years and older in the US have CKD based on an estimated glomerular filtration rate (eGFR) of less than 60 mL/min/1.73 m2.1 Chronic kidney disease has strong and independent associations with multiple adverse outcomes, including cardiovascular events, physical decline, falls, fractures, cognitive decline, hospitalizations, and all-cause mortality.2,3,4,5,6,7,8 Although new therapies are being studied to slow the progression of kidney function decline in older adults, existing strategies are predominantly pharmacological treatments of hypertension and diabetes.9,10 However, these medications have a higher incidence of adverse events in older adults, which may be exacerbated by polypharmacy.11,12,13 Lifestyle interventions would be ideal options to slow CKD onset and progression in older adults, but heretofore, clinical trial evidence to support physical activity for preservation of kidney health has been lacking.
Multiple, well-powered observational studies have consistently identified a strong association between greater physical activity and slower declines in eGFR.14,15 The association of physical activity with slower kidney function decline spans the spectrum of CKD and across all age groups but has particularly strong associations in older adults.16,17,18,19,20,21,22,23,24 For example, in the Cardiovascular Health Study, participants in the highest quartile of physical activity had a 28% lower risk of rapid kidney function decline after adjusting for confounding factors.14 While these findings support the hypothesis that physical activity interventions could slow progression of CKD, the observational designs of these studies preclude causal inference. Thus, randomized trials are needed to test the effects of physical activity interventions on declining kidney function and have been described as a high priority for future research.25
The Lifestyle Interventions and Independence for Elders (LIFE) Study was designed to investigate the health effects of a moderate-intensity physical activity and exercise intervention compared with health education in sedentary older adults with mobility limitations.26 The intervention led to a statistically significant reduction in the trial’s primary end point, incident mobility disability, which developed in 30.1% of the physical activity group and 35.5% of the health education group (hazard ratio, 0.82; 95% CI, 0.69-0.98).27 The present ancillary study measured blood concentrations of cystatin C at baseline and during follow-up; the goal was to evaluate whether randomization to a structured physical activity and exercise intervention vs a control group of “successful aging” health education would change the rate of decline in eGFR per cystatin C (eGFRCysC) in this older population over 2 years. We chose cystatin C as the indicator of changes in kidney function because it is less influenced by physical activity and changes in health status than blood creatinine.28 We evaluated the effects of randomization to each study arm on changes of kidney function over 2 years and sought to evaluate potential heterogeneity by relevant subgroups; we also observed whether there was an association between the amount of measured physical activity and decline of eGFRCysC.
Methods
Design
The LIFE Study was a phase 3, multicenter, randomized clinical trial of a moderate-intensity physical activity and exercise program vs a “successful aging” health education program that ran from February 2010 through December 2013 and involved 1635 sedentary older adults.26 Briefly, the study was conducted across 8 centers in the US (see Supplement 1 and eAppendix in Supplement 2 for details). Inclusion criteria targeted men and women aged 70 to 89 years with the following characteristics: (1) sedentary lifestyle, defined by reporting less than 20 min/d of regular physical activity and less than 125 min/wk of moderate physical activity; (2) at high risk of disability based on a score of less than 10 (but above 4) on the Short Physical Performance Battery; (3) ability to complete the 400-meter walk test without an assistive device; (4) absence of cognitive impairment, defined by score greater than 80 on the Modified Mini-Mental State Examination; and (5) willingness to consent to randomization. Exclusion criteria included unstable chronic disease and factors that would likely affect adherence to the intervention, or underlying conditions that might limit survival to study completion. Although patients undergoing dialysis were excluded, there were no specific exclusions related to nondialysis CKD. This study was approved by the institutional review board at the University of California, San Francisco, and all patients in the LIFE Study provided informed consent prior to participation.
Interventions
All participants underwent randomization to 1 of the 2 study arms, physical activity and exercise vs health education, using a block algorithm, stratified by field center and participant sex. The intervention arm underwent a combined activity and functional exercise intervention. The activity component was based on walking, with a target of 150 min/wk, and the functional exercise component included strength, flexibility, and balance training. Participants were expected to attend exercise sessions at their field center twice weekly and to conduct home-based activities 3 to 4 times weekly throughout the trial duration. Exercise sessions were individualized, and participants were expected to progress toward a goal of 30 minutes daily walking, 10 minutes of lower-extremity strength training, 10 minutes of balance training, and large-muscle flexibility exercises. Intensity started low and increased over the first weeks of the intervention. Participants were asked to walk at a self-perceived exertion of 13 (“somewhat hard”) on the original Borg Rating of Perceived Exertion scale and perform lower-extremity strength exercises at a self-perceived exertion of 15 to 16 (“hard”). The health education control arm of the trial involved weekly workshops over the first 26 weeks, followed by monthly workshops. These sessions addressed a variety of health topics relevant to older adults but did not specifically address physical activity. Further detail is included in eMethods in Supplement 2.
Measurements
Baseline assessments relevant to the present study included self-reported sociodemographic information (age, sex, self-reported race and ethnicity, and education), medical history (presence of diabetes, hypertension, and cardiovascular disease), and physical examination (body mass index and systolic blood pressure). For both arms of the trial, physical activity was assessed using accelerometers (wGT3X-BT [ActiGraph]) at baseline and at 6, 12, and 24 months of follow-up; on each occasion, participants wore the accelerometer for 7 days. Only data collected at baseline, 12 months, and 24 months were used for these analyses because they were contiguous with the kidney function assessments. Details of these measures are described elsewhere.29,30 The accelerometers reported the total step counts/d and also the minutes spent in moderately demanding tasks of daily living (>760 activity counts/min), expressed in minutes per week.31 All accelerometer data were adjusted for wear time.
During the trial, plasma specimens were collected at the baseline, year 1, and year 2 visits, and immediately stored at −80 °C. The specimens were shipped from the National Institute of Aging’s specimen bank to the Kidney Health Research Collaborative’s biomarker laboratory at the San Francisco VA Health Care System. Cystatin C was measured using the BN II nephelometer (Siemens). For each participant, all cystatin C measures were conducted concurrently to avoid assay drift. We used the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation to estimate GFR from cystatin C, age, and sex.32 To evaluate a clinically important threshold of eGFRCysC decline, we defined an outcome of rapid kidney function decline based on percentage loss from baseline; a priori, we chose the highest tertile of eGFRCysC percentage decline over 2 years as the binary threshold.
Statistical Analysis
Baseline characteristics were summarized by randomization groups using means (SDs) for continuous variables or counts and percentages for discrete variables. The t tests, Wilcoxon rank sum tests, and χ2 tests were used to compare normally distributed, non-normally distributed, and discrete characteristics, respectively, between participants with and without cystatin C measures and by randomization arm.
The association of the intervention on the primary outcome of change in kidney function (eGFRCysC) was analyzed using the repeated measures analysis of covariance with an unstructured covariance matrix. Covariates included baseline kidney function, sex and field center (stratified variables), intervention, clinic visit (year 1 and year 2), and intervention-by-visit interaction. Contrasts were used to estimate the average effects over the 2-year follow-up period. To determine whether there was heterogeneity in the effect of intervention across subgroups, we tested for potential interactions between the intervention arm and the following factors: age (median), sex, race and ethnicity (Black, White, other [including Asian, non-White Hispanic, and multiracial, which were grouped together owing to the small number of participants reporting within these categories]), hypertension, diabetes, cardiovascular disease (CVD), and baseline CKD (eGFRCysC <60 mL/min/1.73 m2). We also conducted analyses stratified by these subgroups. The association between intervention groups with the binary rapid kidney function decline outcome was examined using the marginal model with generalized estimating equations, the logit link function, binomial distribution, and the unstructured covariance matrix. Contrasts were also used to estimate the average effects over the 2 years.
We next evaluated associations between measured activity during follow-up with changes in kidney function, using both the continuous and binary eGFRCysC outcomes. The associations between total step count and activity time with changes in kidney function were determined using repeated measures analysis of covariance with the unstructured covariance matrix. The covariates in the basic model included the baseline kidney function, sex and field center (stratified variables), intervention, clinic visit, and intervention-by-visit interaction. Because these analyses were not comparing randomized groups, we also conducted a second model that additionally adjusted for age, body mass index, systolic blood pressure, race and ethnicity, education, diabetes, CVD, and hypertension. Total step count and activity time were each analyzed as standardized variables (per SD) and by quartiles. Contrasts were used to estimate the average effects over the 2-year follow-up period. The associations of measured activity with rapid kidney function decline were examined using the marginal model with generalized estimating equations, the logit link function, binomial distribution, and the unstructured covariance matrix, with sequential adjustment for the covariates listed above. We used B-splines within the linear mixed effects model to examine whether the association between achieved total steps and change in eGFRCysC is linear. For all analyses, SAS, version 9.4 (SAS Institute), and R, version 3.6.1 (R Foundation), were used, and a 2-sided P value less than .05 was considered to indicate statistical significance.
Results
Participant Outcomes
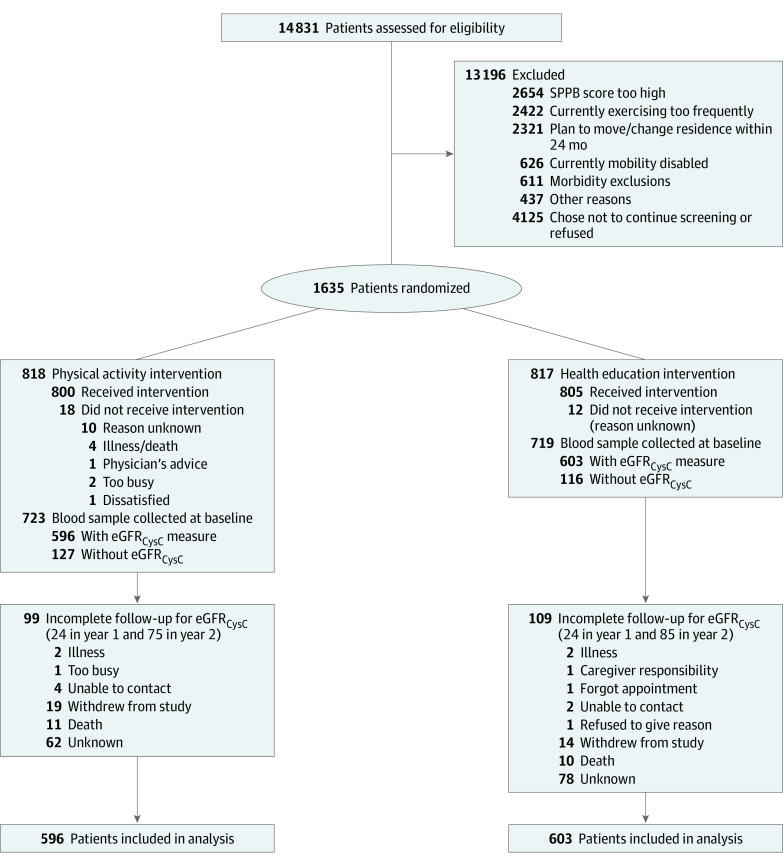
Among the 1635 participants in the LIFE Study, 1199 had available biospecimens for cystatin C measurement (Figure 1); these participants appeared similar to those without biospecimens apart from there being a higher prevalence of diabetes in participants who had available samples (eTable 1 in Supplement 2). The mean (SD) age of included participants was 78.9 (5.5) years, and 800 (66.7%) were women; 216 (18.0%) self-reported as Black, 904 (75.4%) as White, and 79 (6.6%) as other race and/or ethnicity, and the mean (SD) eGFRCysC at baseline was 54 (17) mL/min/1.73 m2 (Table 1). Key risk factors at baseline for rapidly declining kidney function were diabetes (n = 327 [27.3%]), hypertension (n = 855 [71.7%]), CVD (n = 354 [29.5%]), and an eGFRCysC less than 60 mL/min/1.73 m2 (n = 796 [66.4%]).
Figure 1. CONSORT Diagram.
eGFRCysC indicates estimated glomerular filtration rate by cystatin C; SPPB, Short Physical Performance Battery.
Table 1. Baseline Characteristics of Participants in the LIFE Study, Stratified by Randomization Arm.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Physical activity and exercise (n = 596) | Health education (n = 603) | Overall (n = 1199) | |
| Age, mean (SD), y | 78.5 (5.1) | 79.3 (5.3) | 78.9 (5.2) |
| Sex | |||
| Female | 390 (65.4) | 410 (68.0) | 800 (66.7) |
| Male | 206 (34.6) | 193 (32.0) | 399 (33.3) |
| Race and ethnicity | |||
| Black | 122 (20.5) | 94 (15.6) | 216 (18.0) |
| White | 437 (73.3) | 467 (77.4) | 904 (75.4) |
| Othera | 37 (6.2) | 42 (7.0) | 79 (6.6) |
| Education | |||
| ≤High school | 203 (34.2) | 197 (32.7) | 400 (33.4) |
| College | 255 (42.9) | 246 (40.9) | 501 (41.9) |
| Postgraduate | 136 (22.9) | 159 (26.4) | 295 (24.7) |
| BMI, mean (SD) | 30.3 (5.7) | 30.2 (6.1) | 30.2 (5.9) |
| Diabetes status | |||
| No diabetes | 285 (47.8) | 291 (48.3) | 576 (48.0) |
| Impaired fasting glucose | 157 (26.3) | 139 (23.1) | 296 (24.7) |
| Diabetes | 154 (25.8) | 173 (28.7) | 327 (27.3) |
| Cardiovascular disease | 166 (27.9) | 188 (31.2) | 354 (29.5) |
| Hypertension | 421 (71.0) | 434 (72.5) | 855 (71.7) |
| Systolic blood pressure, mean (SD), mmHg | 127.6 (18.5) | 127.3 (18.1) | 127.5 (18.3) |
| eGFRCysC, mean (SD), mL/min/1.73 m2 | 54.0 (17.3) | 53.4 (17.1) | 53.7 (17.2) |
| eGFRCysC <60 mL/min/1.73 m2 | 394 (66.1) | 402 (66.7) | 796 (66.4) |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; eGFRCysC, estimated glomerular filtration rate by cystatin C; LIFE, Lifestyle Interventions and Independence for Elders.
The Other category includes participants who self-reported as Asian, non-White Hispanic, and multiracial. These participants were grouped together owing to the small number reported within each category.
At baseline, the mean (SD) step counts for participants in the intervention and control arms were 2693 (1396) and 2729 (1576) steps, respectively. At years 1 and 2, those in the intervention arm had recorded step counts that were 20% and 15% higher than the control arm, respectively; recorded activity times were 22% higher at both year 1 and 2 (eFigure in Supplement 2).
Over 2 years of follow-up, the mean (SD) decline in eGFRCysC was 1.42 (1.20) mL/min/1.73 m2 at year 1 and 2.99 (2.74) mL/min/1.73 m2 at year 2. Participants in the intervention arm had a nearly 1 mL/min/1.73 m2 slower rate of eGFRCysC decline on average (0.96 mL/min/1.73 m2; 95% CI, 0.02-1.91 mL/min/1.73 m2) during the 2-year follow-up period and lower odds of rapid eGFRCysC decline (odds ratio, 0.79; 95% CI, 0.65-0.97) (Table 2). When subgroups were stratified and tested for effect modification, an interaction that reached statistical significance was only observed when stratifying for eGFRCysC less than 60 mL/min/1.73 m2; participants in the higher eGFRCysC stratum appeared to derive greater benefit (eTable 2 in Supplement 2).
Table 2. Differences in eGFRCysC Decline and Odds of Rapid Kidney Function Decline Over 2 Years in the Physical Activity and Exercise Arm vs the Health Education Arm (n = 1199).
| Intervention effecta | No. (95% CI) | P value |
|---|---|---|
| Decline of eGFRCysC, mL/min/1.73 m2 | 0.96 (0.02-1.91)b | .05 |
| Odds of rapid decline | 0.79 (0.65-0.97)c | .03 |
Abbreviation: eGFRCysC, estimated glomerular filtration rate by cystatin C.
Adjusted for baseline eGFRCysC, sex, clinical sites, intervention, visit, and intervention-by-visit interaction.
Positive value indicates a lower rate of eGFRCysC decline.
Rapid decline defined by the high tertile threshold of 6.7%/y.
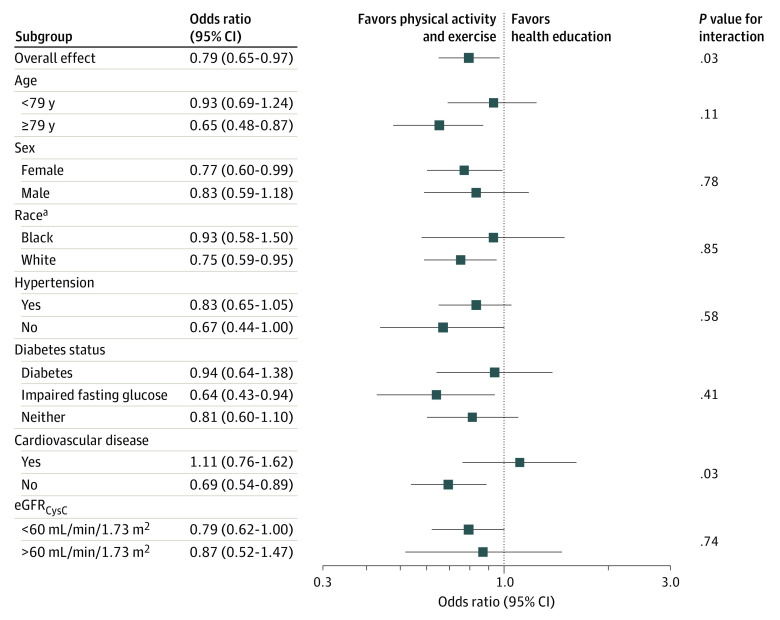
Overall, at year 2, 302 (29.1%) participants had rapidly declining kidney function, including 135 (25.9%) in the physical activity arm and 167 (32.2%) in the health education control group. Randomization to the physical activity arm statistically significantly lowered the odds of rapid kidney function decline by approximately 20% over the 2 years (Table 2). The intervention effect on rapid kidney decline appeared similar across nearly all subgroups evaluated; however, there was a statistically significant interaction by presence of CVD whereby the effect appeared stronger among those without CVD (Figure 2).
Figure 2. Effect of Randomization to Physical Activity and Exercise vs Health Education on Rapidly Declining Kidney Function, Overall and Stratified by Subgroups.
eGFRCysC indicates estimated glomerular filtration rate by cystatin C.
aThe Other category is excluded because there were too few participants to make a meaningful analysis by subgroup.
Observational Results
We next evaluated the association between achieved activity and eGFRCysC decline and rapid declining kidney function overall and within each trial arm. Overall, participants who were in the highest quartile of step count (≥3470 steps/d) had an approximately 2 mL/min/1.73 m2 slower decline in eGFRCysC and had about one-third reduction in odds of rapid kidney decline compared with participants with the lowest measured activity (≤1567 steps/d) (odds ratio, 0.62; 95% CI, 0.44-0.87; P = .005; Table 3). Participant time spent in moderate activity also had a linear association with slower declines in kidney function (Table 3).
Table 3. Difference in eGFRCysC Decline and Odds of Rapid Kidney Function Decline Over 2 Years in the Physical Activity and Exercise Arm vs the Health Education Arm by Quartile of Achieved Step Count and Minutes of Moderate-Intensity Activity (n = 1199)a.
| Model | Linear per SD | Quartile | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Continuous eGFRCysC change per achieved step count, mean (SD), mL/min/1.73 m2 | |||||
| Steps/d, range | 1487 | 139-1568 (n = 236) | 1568-2362 (n = 279) | 2362-3470 (n = 298) | 3470-13 598 (n = 313) |
| Basic adjusted | 0.87 (0.21) | 1 [Reference] | 0.87 (0.62) | 1.50 (0.66) | 2.86 (0.70) |
| P value | <.001 | NA | .16 | .002 | <.001 |
| Full adjusted | 0.65 (0.21) | 1 [Reference] | 0.43 (0.63) | 1.10 (0.66) | 2.01 (0.72) |
| P value | .002 | NA | .49 | .10 | .005 |
| Rapid kidney function decline per achieved step count, odds ratio (95% CI)b | |||||
| Steps/d, range | 1487 | 139-1568 (n = 236) | 1568-2362 (n = 279) | 2362-3470 (n = 298) | 3470-13 598 (n = 313) |
| Basic adjusted | 0.83 (0.74-0.92) | 1 [Reference] | 0.79 (0.59-1.06) | 0.71 (0.53-0.96) | 0.58 (0.42-0.79) |
| P value | <.001 | NA | .11 | .03 | <.001 |
| Full adjusted | 0.84 (0.75-0.94) | 1 [Reference] | 0.85 (0.63-1.14) | 0.74 (0.54-1.00) | 0.62 (0.44-0.87) |
| P value | .003 | NA | .27 | .05 | .005 |
| Continuous eGFRCysC change per achieved moderate-intensity activity in min, mean (SD), mL/min/1.73 m2c | |||||
| Activity counts/min, range | 24.5 | 0-10.2 (n = 289) | 10.2-20.2 (n = 289) | 20.2-36.8 (n = 269) | 36.8-179.1 (n = 214) |
| Basic adjusted | 0.73 (0.26) | 1 [Reference] | 1.63 (0.61) | 1.08 (0.64) | 2.39 (0.68) |
| P value | .005 | NA | .008 | .10 | <.001 |
| Full adjusted | 0.47 (0.27) | 1 [Reference] | 1.47 (0.61) | 0.77 (0.65) | 1.68 (0.70) |
| P value | .08 | NA | .02 | .23 | .02 |
| Rapid kidney function decline per achieved moderate-intensity activity in min, odds ratio (95% CIs) b , c | |||||
| Activity counts/min, range | 24.5 | 0-10.2 (n = 289) | 10.2-20.2 (n = 289) | 20.2-36.8 (n = 269) | 36.8-179.1 (n = 214) |
| Basic adjusted | 0.85 (0.74-0.97) | 1 [Reference] | 0.71 (0.54-0.94) | 0.73 (0.55-0.98) | 0.59 (0.43-0.80) |
| P value | .02 | NA | .02 | .03 | <.001 |
| Full adjusted | 0.87 (0.76-1.01) | 1 [Reference] | 0.72 (0.54-0.95) | 0.73 (0.54-0.99) | 0.63 (0.45-0.87) |
| P value | .07 | NA | .02 | .04 | .005 |
Abbreviation: eGFRCysC, estimated glomerular filtration rate by cystatin C.
Both basic-adjusted and full-adjusted models in the overall cohort additionally adjusted for intervention and intervention-by-visit interaction. The basic-adjusted model was adjusted for baseline eGFRCysC, sex, clinical sites, intervention, visit, and intervention-by-visit interaction. The full-adjusted model was adjusted for baseline eGFRCysC, sex, clinical sites, intervention, visit, intervention-by-visit interaction, age, body mass index, systolic blood pressure, race and ethnicity, education, diabetes status, cardiovascular disease, and hypertension.
Rapid kidney function decline was defined by the high tertile threshold of 6.7%/y.
More than 760 activity counts/min.
Discussion
In this ancillary analysis of the LIFE Study, we found that participants randomly assigned to the physical activity and exercise arm of the LIFE trial had a statistically significantly slower decline in eGFRCysC on average compared with participants assigned to the health education control arm. In addition, participants randomized to the physical activity and exercise group were less likely to experience rapid decline in kidney function. The magnitude of these effects from the combined physical activity and exercise intervention are consistent with the effect sizes reported by prior observational studies.14,33 Finally, while observational in nature, we observed a dose-dependent association of measured activity with slower declines in eGFRCysC and reduced likelihood of rapidly declining kidney function.
Despite a preponderance of observational data, to our knowledge, there have been relatively few clinical trials addressing the effects of physical activity or exercise interventions on changes in eGFRCysC. Several prior trials have supported the hypothesis that an exercise intervention could improve kidney health or adjust the course of kidney disease, but they had important differences from the LIFE Study. For example, prior trials were much smaller, with the largest including 180 participants; they recruited distinct target populations such as persons with CVD, diabetes, or kidney transplantation19,34,35,36; and they had much shorter interventions, averaging around a few months. Some prior trials found no effect from exercise; a 16-week intervention that combined aerobic and resistance exercise did not change eGFRCysC within its 150 participants with hypertension.37 Relative to these trials, the present study is distinguished by its focus on the older population, the large sample size, the longer follow-up, and the generalizability of the intervention. Prior exercise trials in CKD have often focused on moderate or vigorous activity interventions that may not be acceptable or feasible for many older adults38,39; in contrast, the LIFE intervention is both generalizable and scalable with its prioritization on walking and home-based exercise. In addition, the present study used cystatin C as the filtration marker for monitoring GFR because serum creatinine levels can be biased by changes in exercise-induced muscle activity.
Of particular clinical importance, the benefits of exercise on kidney function were detectable even with relatively modest increases in physical activity. Despite the relative improvements in their step counts, the majority of participants in the physical activity and exercise group would still have been classified as sedentary throughout the follow-up period, and only about 1 in 12 achieved step counts (>5000 steps/d) that would classify them as active based on guideline recommendations for physical activity in older adults.40 Indeed, current guidelines assume a baseline activity of about 5000 steps/d (mostly of light intensity) and recommend an additional 150 min/wk of moderately intense physical activity in addition to 2 sessions per week of muscle-strengthening exercises.40,41,42,43 Similarly, the cutoff for moderately intense activity by accelerometer used in LIFE (>760 activity counts/min) is equivalent to the energy expended from household tasks such as vacuuming or trimming the lawn, which in other populations would constitute low intensity tasks by perceived exertion.31
Of note, the original LIFE Study did show an increased number of hospitalizations in the intervention arm.27 Although this difference did not rise to the level of statistical significance, it remains prudent to take into account individual limitations in physical function or ability prior to prescription of activity or exercise. Indeed, current recommendations for older or more frail individuals caution that activity and exercise should be increased slowly and individually prescribed according to each individual’s limits.43 However, the present results show that extreme levels of activity are not necessary to slow rates of decline of eGFRCysC. The findings in LIFE demonstrated that these targets are eminently achievable in older adults, and the present findings are thus clinically relevant for selecting the dose of exercise to prescribe for sedentary older adults who are at risk for the onset or progression of kidney disease. Overall, the effect of the intervention was substantial despite achieving only moderate separation in step counts between the 2 groups. The difference in eGFRCysC of approximately 1 mL/min/1.73 m2 over the 2 years may seem clinically small. However, robust data from an analysis of more than 60 000 patients in 47 randomized clinical trials demonstrated that a treatment effect of 1 mL/min/1.73 m2 over 2 years (0.5 mL/min/1.73 m2/y) overwhelmingly predicts a benefit effect on clinical CKD end points such as end-stage kidney disease.44
Beyond the effect of randomization, the observational analyses found that moderate-intensity physical activity and exercise have a beneficial association on eGFRCysC that appears linear. Even small increases in steps were associated on average with a slowing of eGFRCysC decline, and the beneficial effects appeared to increase incrementally with participant effort. Any type of intervention that motivates older adults to walk should therefore provide some benefit on kidney function. Similar observational findings were shown previously in LIFE within the intervention arm whereby higher measured exercise was associated with incremental improvements in physical function.29 Motivational barriers represent a key obstacle to physical activity and exercise among patients with CKD across the spectrum of age,38,45,46,47 and older adults report unique barriers to exercise. These data allow clinicians to add another evidence-based rationale for exercise for any older adult. By reporting the prominent improvements that physical activity can have on the trajectory of kidney disease, clinicians can empower patients toward lifestyle change and advocacy; this is especially important for kidney prognosis because many patients diagnosed with CKD feel helpless and unable to change their disease course.48,49,50 Prescription of activity and exercise therefore represents a potent tool for the clinician taking care of these older adults.
There are many other potential benefits of physical activity and exercise for older adults beyond CKD. Prior research has also shown the benefits of exercise on several kidney disease risk factors, including hypertension, CVD, and insulin resistance/diabetes.51 However, we currently do not understand the mechanisms by which exercise could have direct benefits on kidney function. Further research is required to explore the effect of a combined physical activity and exercise intervention on structural damage to the kidney.
Limitations
There are several limitations to be acknowledged. This is an ancillary study to an existing trial, and the original study was not designed to test the effect of physical activity and exercise on kidney function. Because the LIFE Study focused on sedentary older adults, we cannot be certain that exercise would reduce kidney function decline in other populations. The study was underrepresented in participants who were not White and had few participants with advanced CKD. There was limited power to assess for interactions between the intervention and certain subgroups on the kidney function outcomes. Owing to the timing of specimen collection, these analyses were limited to a 2-year follow-up, and there were no end-stage kidney disease events; we do not know whether the beneficial effects of physical activity would increase or diminish during longer follow-up. In addition, only 75% of the original LIFE cohort had available specimens to analyze for longitudinal eGFRCysC, although we found no considerable differences between participants with and without available specimens. The strong associations of achieved activity and exercise on eGFRCysC changes were observational and thus are subject to residual confounding despite adjustment for important covariates. There may also have been informative dropout, such as time lost to hospitalization. In addition, the trial only included 2 measures of achieved activity that could be used to evaluate a dose-response relationship between components of the intervention and changes in eGFRCysC: the step count and a measure of time in moderate-intensity activity. This limits the ability to distinguish which component was more beneficial for kidney function, the physical activity component or the muscle-strengthening exercises. The step count and time in moderate-intensity activity were highly correlated, suggesting that a majority of moderate-intensity activity was spent walking, but we cannot be certain that walking alone would achieve the desired effect on kidney function.
Conclusions
Results of this ancillary analysis of the LIFE Study demonstrate that when compared with a health education intervention, a physical activity and exercise intervention slowed the rate of decline in eGFRCysC and reduced the likelihood of rapidly declining eGFRCysC among community-dwelling sedentary older adults. Physical activity and exercise interventions should be considered as a treatment to slow decline of eGFRCysC in older adults.
Trial Protocol
eTable 1. Comparison of participants with and without cystatin C measures
eTable 2. Effect of randomization to the physical activity and exercise intervention group versus the health education control group of the LIFE trial on longitudinal changes in eGFRCysC over 2 years, stratified by key subgroups
eFigure
eMethods
eAppendix. Research Investigators for the LIFE Study
Nonauthor Collaborators. LIFE Investigators
Data Sharing Statement
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038-2047. doi: 10.1001/jama.298.17.2038 [DOI] [PubMed] [Google Scholar]
- 2.Rocco MV, Gassman JJ, Wang SR, Kaplan RM. Cross-sectional study of quality of life and symptoms in chronic renal disease patients: the Modification of Diet in Renal Disease Study. Am J Kidney Dis. 1997;29(6):888-896. doi: 10.1016/S0272-6386(97)90463-7 [DOI] [PubMed] [Google Scholar]
- 3.Fried LF, Biggs ML, Shlipak MG, et al. Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol. 2007;18(1):282-286. doi: 10.1681/ASN.2006050546 [DOI] [PubMed] [Google Scholar]
- 4.Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2005;16(7):2127-2133. doi: 10.1681/ASN.2005010005 [DOI] [PubMed] [Google Scholar]
- 5.Molsted S, Prescott L, Heaf J, Eidemak I. Assessment and clinical aspects of health-related quality of life in dialysis patients and patients with chronic kidney disease. Nephron Clin Pract. 2007;106(1):c24-c33. doi: 10.1159/000101481 [DOI] [PubMed] [Google Scholar]
- 6.Odden MC, Whooley MA, Shlipak MG. Depression, stress, and quality of life in persons with chronic kidney disease: the Heart and Soul Study. Nephron Clin Pract. 2006;103(1):c1-c7. doi: 10.1159/000090112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hailpern SM, Melamed ML, Cohen HW, Hostetter TH. Moderate chronic kidney disease and cognitive function in adults 20 to 59 years of age: Third National Health and Nutrition Examination Survey (NHANES III). J Am Soc Nephrol. 2007;18(7):2205-2213. doi: 10.1681/ASN.2006101165 [DOI] [PubMed] [Google Scholar]
- 8.Kittiskulnam P, Sheshadri A, Johansen KL. Consequences of CKD on functioning. Semin Nephrol. 2016;36(4):305-318. doi: 10.1016/j.semnephrol.2016.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rayego-Mateos S, Valdivielso JM. New therapeutic targets in chronic kidney disease progression and renal fibrosis. Expert Opin Ther Targets. 2020;24(7):655-670. doi: 10.1080/14728222.2020.1762173 [DOI] [PubMed] [Google Scholar]
- 10.Ruiz-Ortega M, Rayego-Mateos S, Lamas S, Ortiz A, Rodrigues-Diez RR. Targeting the progression of chronic kidney disease. Nat Rev Nephrol. 2020;16(5):269-288. doi: 10.1038/s41581-019-0248-y [DOI] [PubMed] [Google Scholar]
- 11.Mason NA. Polypharmacy and medication-related complications in the chronic kidney disease patient. Curr Opin Nephrol Hypertens. 2011;20(5):492-497. doi: 10.1097/MNH.0b013e328349c261 [DOI] [PubMed] [Google Scholar]
- 12.Roux-Marson C, Baranski JB, Fafin C, et al. Medication burden and inappropriate prescription risk among elderly with advanced chronic kidney disease. BMC Geriatr. 2020;20(1):87. doi: 10.1186/s12877-020-1485-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Formica M, Politano P, Marazzi F, et al. Acute kidney injury and chronic kidney disease in the elderly and polypharmacy. Blood Purif. 2018;46(4):332-336. doi: 10.1159/000492149 [DOI] [PubMed] [Google Scholar]
- 14.Robinson-Cohen C, Katz R, Mozaffarian D, et al. Physical activity and rapid decline in kidney function among older adults. Arch Intern Med. 2009;169(22):2116-2123. doi: 10.1001/archinternmed.2009.438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen IR, Wang SM, Liang CC, et al. Association of walking with survival and RRT among patients with CKD stages 3-5. Clin J Am Soc Nephrol. 2014;9(7):1183-1189. doi: 10.2215/CJN.09810913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo C, Tam T, Bo Y, Chang LY, Lao XQ, Thomas GN. Habitual physical activity, renal function and chronic kidney disease: a cohort study of nearly 200 000 adults. Br J Sports Med. 2020;54(20):1225-1230. doi: 10.1136/bjsports-2019-100989 [DOI] [PubMed] [Google Scholar]
- 17.Bellizzi V, Cupisti A, Capitanini A, Calella P, D’Alessandro C. Physical activity and renal transplantation. Kidney Blood Press Res. 2014;39(2-3):212-219. doi: 10.1159/000355799 [DOI] [PubMed] [Google Scholar]
- 18.Park S, Lee S, Kim Y, et al. Causal effects of physical activity or sedentary behaviors on kidney function: an integrated population-scale observational analysis and Mendelian randomization study. Nephrol Dial Transplant. 2021;gfab153. doi: 10.1093/ndt/gfab153 [DOI] [PubMed] [Google Scholar]
- 19.Lima PS, Campos ASD, Corrêa CS, et al. Effects of chronic physical activity on glomerular filtration rate, creatinine, and the markers of anemia of kidney transplantation patients. Transplant Proc. 2018;50(3):746-749. doi: 10.1016/j.transproceed.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 20.Gordon EJ, Prohaska TR, Gallant MP, et al. Longitudinal analysis of physical activity, fluid intake, and graft function among kidney transplant recipients. Transpl Int. 2009;22(10):990-998. doi: 10.1111/j.1432-2277.2009.00917.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martens RJH, van der Berg JD, Stehouwer CDA, et al. Amount and pattern of physical activity and sedentary behavior are associated with kidney function and kidney damage: the Maastricht Study. PLoS One. 2018;13(4):e0195306. doi: 10.1371/journal.pone.0195306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawkins MS, Sevick MA, Richardson CR, Fried LF, Arena VC, Kriska AM. Association between physical activity and kidney function: National Health and Nutrition Examination Survey. Med Sci Sports Exerc. 2011;43(8):1457-1464. doi: 10.1249/MSS.0b013e31820c0130 [DOI] [PubMed] [Google Scholar]
- 23.Parsons TJ, Sartini C, Ash S, et al. Objectively measured physical activity and kidney function in older men; a cross-sectional population-based study. Age Ageing. 2017;46(6):1010-1014. doi: 10.1093/ageing/afx091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosaki K, Tanahashi K, Matsui M, et al. Sedentary behaviour, physical activity, and renal function in older adults: isotemporal substitution modelling. BMC Nephrol. 2020;21(1):211. doi: 10.1186/s12882-020-01869-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group . KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021;99(3S):S1-S87. [DOI] [PubMed] [Google Scholar]
- 26.Fielding RA, Rejeski WJ, Blair S, et al. ; LIFE Research Group . The Lifestyle Interventions and Independence for Elders Study: design and methods. J Gerontol A Biol Sci Med Sci. 2011;66(11):1226-1237. doi: 10.1093/gerona/glr123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pahor M, Guralnik JM, Ambrosius WT, et al. ; LIFE Study Investigators . Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311(23):2387-2396. doi: 10.1001/jama.2014.5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shlipak MG, Matsushita K, Ärnlöv J, et al. ; CKD Prognosis Consortium . Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi: 10.1056/NEJMoa1214234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fielding RA, Guralnik JM, King AC, et al. ; LIFE Study Group . Dose of physical activity, physical functioning and disability risk in mobility-limited older adults: results from the LIFE study randomized trial. PLoS One. 2017;12(8):e0182155. doi: 10.1371/journal.pone.0182155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rejeski WJ, Marsh AP, Brubaker PH, et al. ; LIFE Study Investigators . Analysis and interpretation of accelerometry data in older adults: the LIFE study. J Gerontol A Biol Sci Med Sci. 2016;71(4):521-528. doi: 10.1093/gerona/glv204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthew CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc. 2005;37(11)(suppl):S512-S522. doi: 10.1249/01.mss.0000185659.11982.3d [DOI] [PubMed] [Google Scholar]
- 32.Inker LA, Schmid CH, Tighiouart H, et al. ; CKD-EPI Investigators . Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20-29. doi: 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson-Cohen C, Littman AJ, Duncan GE, et al. Physical activity and change in estimated GFR among persons with CKD. J Am Soc Nephrol. 2014;25(2):399-406. doi: 10.1681/ASN.2013040392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nylen ES, Gandhi SM, Kheirbek R, Kokkinos P. Enhanced fitness and renal function in type 2 diabetes. Diabet Med. 2015;32(10):1342-1345. doi: 10.1111/dme.12789 [DOI] [PubMed] [Google Scholar]
- 35.Takaya Y, Kumasaka R, Arakawa T, et al. Impact of cardiac rehabilitation on renal function in patients with and without chronic kidney disease after acute myocardial infarction. Circ J. 2014;78(2):377-384. doi: 10.1253/circj.CJ-13-0779 [DOI] [PubMed] [Google Scholar]
- 36.Toyama K, Sugiyama S, Oka H, Sumida H, Ogawa H. Exercise therapy correlates with improving renal function through modifying lipid metabolism in patients with cardiovascular disease and chronic kidney disease. J Cardiol. 2010;56(2):142-146. doi: 10.1016/j.jjcc.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 37.Barcellos FC, Del Vecchio FB, Reges A, et al. Exercise in patients with hypertension and chronic kidney disease: a randomized controlled trial. J Hum Hypertens. 2018;32(6):397-407. doi: 10.1038/s41371-018-0055-0 [DOI] [PubMed] [Google Scholar]
- 38.Sheshadri A, Johansen KL. Prehabilitation for the frail patient approaching ESRD. Semin Nephrol. 2017;37(2):159-172. doi: 10.1016/j.semnephrol.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilkinson TJ, McAdams-DeMarco M, Bennett PN, Wilund K; Global Renal Exercise Network . Advances in exercise therapy in predialysis chronic kidney disease, hemodialysis, peritoneal dialysis, and kidney transplantation. Curr Opin Nephrol Hypertens. 2020;29(5):471-479. doi: 10.1097/MNH.0000000000000627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tudor-Locke C, Bassett DR Jr. How many steps/day are enough? preliminary pedometer indices for public health. Sports Med. 2004;34(1):1-8. doi: 10.2165/00007256-200434010-00001 [DOI] [PubMed] [Google Scholar]
- 41.Nelson ME, Rejeski WJ, Blair SN, et al. ; American College of Sports Medicine; American Heart Association . Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1094-1105. doi: 10.1161/CIRCULATIONAHA.107.185650 [DOI] [PubMed] [Google Scholar]
- 42.Lee PG, Jackson EA, Richardson CR. Exercise prescriptions in older adults. Am Fam Physician. 2017;95(7):425-432. [PubMed] [Google Scholar]
- 43.Physical Activity Guidelines for Americans, 2nd edition. US Department of Health and Human Services . 2018. Accessed April 5, 2022. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
- 44.Inker LA, Heerspink HJL, Tighiouart H, et al. GFR slope as a surrogate end point for kidney disease progression in clinical trials: a meta-analysis of treatment effects of randomized controlled trials. J Am Soc Nephrol. 2019;30(9):1735-1745. doi: 10.1681/ASN.2019010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jagannathan R, Ziolkowski SL, Weber MB, et al. Physical activity promotion for patients transitioning to dialysis using the “Exercise is Medicine” framework: a multi-center randomized pragmatic trial (EIM-CKD trial) protocol. BMC Nephrol. 2018;19(1):230. doi: 10.1186/s12882-018-1032-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clarke AL, Young HM, Hull KL, Hudson N, Burton JO, Smith AC. Motivations and barriers to exercise in chronic kidney disease: a qualitative study. Nephrol Dial Transplant. 2015;30(11):1885-1892. doi: 10.1093/ndt/gfv208 [DOI] [PubMed] [Google Scholar]
- 47.Kendrick J, Ritchie M, Andrews E. Exercise in individuals with CKD: a focus group study exploring patient attitudes, motivations, and barriers to exercise. Kidney Med. 2019;1(3):131-138. doi: 10.1016/j.xkme.2019.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collein I, Sitorus R, Yetti K, Hastono SP. Facilitators and barriers to self-management of patients chronic kidney disease. Enferm Clin. 2021;31(suppl 2):S37-S40. doi: 10.1016/j.enfcli.2020.10.014 [DOI] [Google Scholar]
- 49.Costantini L, Beanlands H, McCay E, Cattran D, Hladunewich M, Francis D. The self-management experience of people with mild to moderate chronic kidney disease. Nephrol Nurs J. 2008;35(2):147-155. [PubMed] [Google Scholar]
- 50.van Dipten C, de Grauw WJC, Wetzels JFM, Assendelft WJJ, Scherpbier-de Haan ND, Dees MK. What patients with mild-to-moderate kidney disease know, think, and feel about their disease: an in-depth interview study. J Am Board Fam Med. 2018;31(4):570-577. doi: 10.3122/jabfm.2018.04.170459 [DOI] [PubMed] [Google Scholar]
- 51.Shlipak MG, Katz R, Kestenbaum B, Fried LF, Siscovick D, Sarnak MJ. Clinical and subclinical cardiovascular disease and kidney function decline in the elderly. Atherosclerosis. 2009;204(1):298-303. doi: 10.1016/j.atherosclerosis.2008.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Comparison of participants with and without cystatin C measures
eTable 2. Effect of randomization to the physical activity and exercise intervention group versus the health education control group of the LIFE trial on longitudinal changes in eGFRCysC over 2 years, stratified by key subgroups
eFigure
eMethods
eAppendix. Research Investigators for the LIFE Study
Nonauthor Collaborators. LIFE Investigators
Data Sharing Statement