This randomized clinical trial examines the change from baseline in drop seizure frequency, response rates, and improvement on the Clinical Global Impression scale among patients with Lennox-Gastaut syndrome.
Key Points
Question
Is adjunctive fenfluramine effective in patients with Lennox-Gastaut syndrome (LGS)?
Findings
In this randomized clinical trial of 263 patients with LGS, use of 0.7-mg/kg/d fenfluramine resulted in a greater reduction in drop seizures than with placebo, more patients achieving a 50% or greater reduction in drop seizure frequency, and greater reduction in generalized tonic-clonic seizure frequency. Treatment-emergent adverse events included decreased appetite, but no patient developed valvular heart disease or pulmonary hypertension.
Meaning
Findings from this trial suggest that fenfluramine may be a safe and effective treatment option for patients with LGS.
Abstract
Importance
New treatment options are needed for patients with Lennox-Gastaut syndrome (LGS), a profoundly impairing, treatment-resistant, developmental and epileptic encephalopathy.
Objective
To evaluate the efficacy and safety of fenfluramine in patients with LGS.
Design, Setting, and Participants
This multicenter, double-blind, placebo-controlled, parallel-group randomized clinical trial was conducted from November 27, 2017, to October 25, 2019, and had a 20-week trial duration. Patients were enrolled at 65 study sites in North America, Europe, and Australia. Included patients were aged 2 to 35 years with confirmed diagnosis of LGS and experienced 2 or more drop seizures per week during the 4-week baseline. Using a modified intent-to-treat method, data analysis was performed from November 27, 2017, to October 25, 2019. The database lock date was January 30, 2020, and the date of final report was September 11, 2021.
Interventions
Patients were randomized to receive either a 0.7-mg/kg/d or 0.2-mg/kg/d (maximum 26 mg/d) dose of fenfluramine or placebo. After titration (2-week period), patients were taking their randomized dose for 12 additional weeks.
Main Outcomes and Measures
Primary efficacy end point was percentage change from baseline in drop seizure frequency in patients who received 0.7 mg/kg/d of fenfluramine vs placebo.
Results
A total of 263 patients (median [range] age, 13 [2-35] years; 146 male patients [56%]) were randomized to the 0.7-mg/kg/d fenfluramine group (n = 87), 0.2-mg/kg/d fenfluramine group (n = 89), or placebo group (n = 87). The median percentage reduction in frequency of drop seizures was 26.5 percentage points in the 0.7-mg/kg/d fenfluramine group, 14.2 percentage points in the 0.2-mg/kg/d fenfluramine group, and 7.6 percentage points in the placebo group. The trial met its primary efficacy end point: patients in the 0.7-mg/kg/d fenfluramine group achieved a −19.9 percentage points (95% CI, −31.0 to −8.7 percentage points; P = .001) estimated median difference in drop seizures from baseline vs placebo. More patients in the 0.7-mg/kg/d fenfluramine group achieved a 50% or greater response (22 of 87 [25%]; P = .02) vs placebo (9 of 87 [10%]). Site investigators and caregivers gave a much improved or very much improved rating on the Clinical Global Impression of Improvement scale to more patients in the 0.7-mg/kg/d fenfluramine group than patients in the placebo group (21 [26%] vs 5 [6%]; P = .001). The seizure subtype that appeared most responsive to fenfluramine was generalized tonic-clonic seizure (120 of 263 [46%]), with a decrease in frequency of 45.7% in the 0.7-mg/kg/d fenfluramine group and 58.2% in the 0.2-mg/kg/d fenfluramine group compared with an increase of 3.7% in the placebo group. Most common treatment-emergent adverse events included decreased appetite (59 [22%]), somnolence (33 [13%]), and fatigue (33 [13%]). No cases of valvular heart disease or pulmonary arterial hypertension were observed.
Conclusions and Relevance
Results of this trial showed that, in patients with LGS, fenfluramine compared with placebo provided a significantly greater reduction in drop seizures and may be a particularly advantageous choice in patients who experience generalized tonic-clonic seizures.
Trial Registration
ClinicalTrials.gov Identifier: NCT03355209
Introduction
Lennox-Gastaut syndrome (LGS) is a developmental and epileptic encephalopathy characterized by multiple seizure types, diffuse slow spike-and-wave complexes on electroencephalograms (≤2.5 Hz), and cognitive impairment.1,2 Most patients do not respond to treatment and have lifelong disability.
Drop seizures are hallmark features of LGS, particularly tonic seizures, which occur in approximately 56% of cases.1,3 Most patients with LGS develop between 3 and 5 seizure types that wax and wane during disease progression.4,5 Convulsive seizures (eg, generalized tonic-clonic [GTC] seizures) are also commonly observed and usually occur in later stages of LGS but sometimes may precede core seizure types.6 In addition to being associated with bodily injury and hospitalizations,7,8 GTC seizures are a primary risk factor of sudden unexpected death in epilepsy (SUDEP). Patients with GTC seizures have an approximately 10-fold greater risk for SUDEP than patients with other seizure types.9
Seizures typically remain poorly controlled despite polypharmacy with multiple treatment options, including valproate, cannabidiol, felbamate, rufinamide, topiramate, lamotrigine, and clobazam. Recent randomized clinical trials (RCTs) have reported that patients with LGS had tried up to 18 to 28 medications (median: 6-8) before study enrollment to improve seizure frequency.10,11,12 In these RCTs, patients with LGS who were treated with cannabidiol (which is now approved for LGS) or soticlestat (which is in development) experienced an estimated −21.6% to −14.8% difference in drop seizure frequency compared with patients who received placebo.10,11,12
In 2020, fenfluramine was approved in the US, European Union, and UK for treatment of Dravet syndrome (DS). Results from 3 recent phase 3, placebo-controlled RCTs demonstrated that adjunctive fenfluramine compared with placebo significantly reduced monthly convulsive seizure frequency by 54% to 65% in patients with DS.13,14,15 In an investigator-initiated phase 2 study, 13 patients with LGS achieved a 53% median reduction in convulsive seizures (including GTC, tonic, atonic, and focal seizures with a motor component) after 20 weeks of fenfluramine treatment, with 62% of the patients achieving 50% or greater reductions and 33% of the patients achieving 75% or greater reductions in seizure frequency.16 In this phase 3 RCT, we aimed to evaluate the efficacy and safety of fenfluramine in patients with LGS.
Methods
This double-blind, placebo-controlled, parallel-group, phase 3 RCT was conducted from November 27, 2017, when the first patient was enrolled, to October 25, 2019, when the last patient completed the last visit. See the complete trial protocol in Supplement 1. Patients were enrolled at 65 study sites in North America, Europe, and Australia (eFigure 1 in Supplement 2). The trial complied with current International Conference on Harmonisation Good Clinical Practice Guidelines and was approved by the applicable regulatory authorities and independent ethics committee or institutional review board at each participating institution. All patients or their legal representatives provided written informed consent before enrollment and assent where applicable. We followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Patients were notified of the trial through advertising at the study sites, and information was provided by study site personnel. Lennox-Gastaut syndrome diagnosis for each patient was confirmed by the Epilepsy Study Consortium, an independent panel of experts.
Patients and Eligibility Criteria
Children and adults, aged 2 to 35 years, with Epilepsy Study Consortium–confirmed LGS diagnosis who were using stable antiseizure medication (ASM) regimens (≥1 and ≤4 concomitant ASMs) were eligible for enrollment if they met the following criteria: (1) onset of seizures at age 11 years or younger; (2) multiple seizure types, including tonic and tonic or atonic seizures; (3) stable 4-week seizure baseline with 2 or more drop seizures per week of GTC, secondary GTC (ie, focal to bilateral tonic-clonic seizures), tonic, atonic, or tonic or atonic seizure; (4) abnormal cognitive development; and (5) medical history showing electroencephalogram evidence of abnormal background activity with slow spike-and-wave pattern (<2.5 Hz).
Key exclusion criteria were degenerative neurological disease; history of hemiclonic seizures in the first year of life; only drop seizure clusters; previous or current exclusionary cardiovascular or cardiopulmonary abnormality that was detected on echocardiogram, electrocardiogram, or physical examination; and concomitant cannabidiol use (cannabidiol was not an approved medication anywhere in the world at the time of study enrollment).
Race and ethnicity data were self-reported by patients or their caregivers. The race and ethnicity categories were Asian, Black or African American, White, other (American Indian or Alaskan Native and Native Hawaiian or Other Pacific Islander), and unknown (not reported or missing).
Trial Procedures
After a 4-week baseline to establish baseline seizure frequency, patients were randomized 1:1:1 using an interactive web response system to receive fenfluramine (0.7- or 0.2-mg/kg/d dose, with a maximum of 26 mg/d) or placebo (Figure 1; eFigure 1 in Supplement 2). Treatment was administered double-blind. Because of the 26-mg dose limit, fenfluramine was supplied in 3 concentrations to prevent unblinding given the volume assigned by a unique randomization number from the interactive web response or interactive voice response systems.
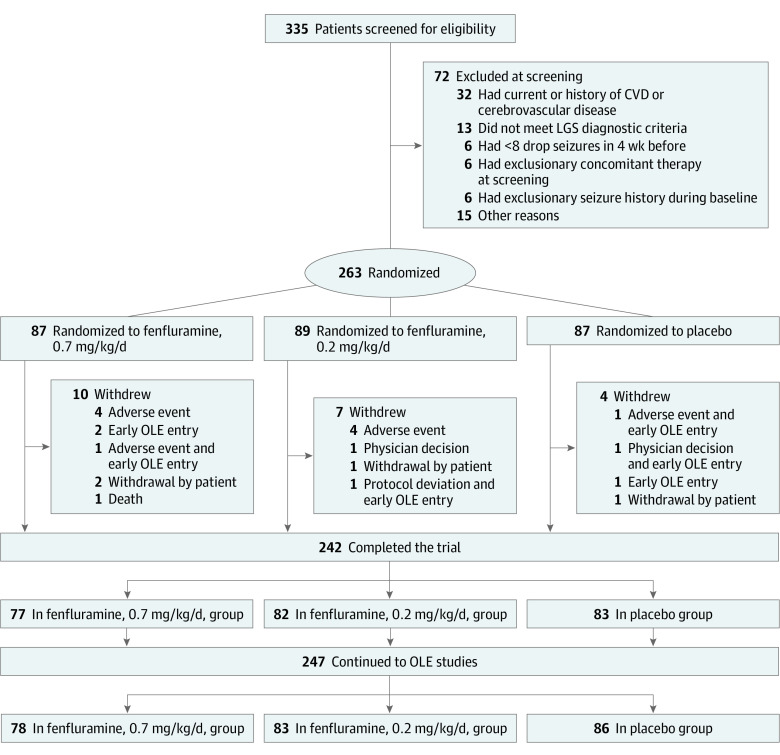
Figure 1. CONSORT Diagram.
Screening exclusion included patients who did not meet at least 1 entry or randomization criteria. A patient who did not meet 2 or more of these criteria may be counted in multiple categories. Seven patients discontinued the first part of the trial early and were allowed to enter the open-label extension (OLE) studies. Two patients who completed the trial did not continue to the OLE studies. CVD indicates cardiovascular disease; LGS, Lennox-Gastaut syndrome.
The placebo had the same appearance as the active drug. Randomization was stratified by patient weight (<37.5 kg or ≥37.5 kg). During the first 2 weeks (titration period), patients in the 0.7-mg/kg/d fenfluramine group were titrated to the target dose, whereas patients in the other 2 treatment groups started at their target dose (eTable 1 in Supplement 2). Patients remained at their assigned dose for 12 weeks (maintenance period). Changes in antiseizure medications, dietary therapy, and vagus nerve stimulation settings were not permitted during the trial.
At the end of the maintenance period, eligible patients could either continue to an open-label extension (OLE) study or discontinue treatment. Patients who discontinued treatment underwent a blinded downward taper of medication. Caregivers (eg, parents or legal guardians) recorded doses, any rescue medication, and number and type of seizures in electronic diaries.
Safety
Treatment-emergent adverse events (TEAEs) were recorded throughout the 14-week titration and maintenance period. Standardized color Doppler echocardiogram assessed cardiac valve function or structure and any evidence of pulmonary arterial hypertension at trial baseline (day −28 to day −15), maintenance period (day 40 to day 54), and end of trial (between day 90 and day 113). Patients who discontinued treatment underwent cardiac safety follow-up visits at 3 and 6 months after administration of the last dose or at country-specific intervals (in some cases, up to 24 months).
All valves were assessed to detect valvular regurgitation and morphological abnormalities. Potential signs or symptoms of valvular heart disease included any development of valve thickening; valve movement abnormalities and/or regurgitation (mitral, ≥moderate; aortic, ≥mild); left ventricular dysfunction; or left atrial enlargement. The rate of valvular regurgitation was graded as absent, trace, mild, moderate, or severe. Results were adjudicated if necessary by the Echo Core Lab and the International Cardiac Advisory Board. Echocardiogram criteria have been described previously.17 Briefly, pulmonary arterial hypertension was defined as pulmonary artery systolic pressure exceeding 35 mm Hg. Electrocardiograms were also evaluated for any abnormality at comparable times, with 1 additional assessment before randomization.
Efficacy End Points
The primary end point was the percentage change from baseline in Epilepsy Study Consortium–confirmed drop seizures (with a per-protocol definition including the following types: GTC, secondary GTC [focal to bilateral tonic-clonic], tonic, atonic, or tonic or atonic) in the 0.7-mg/kg/d fenfluramine group vs the placebo group. Key secondary end points by treatment group, unless specified, were the percentage change from baseline in frequency of drop seizures in the 0.2-mg/kg/d fenfluramine group, 50% or greater responder rate, and proportion of patients who achieved improvement (minimally, much, or very much improved) on the Clinical Global Impression-Improvement (CGI-I) scale. Additional secondary outcomes included CGI-I rated by caregivers, subgroup analyses by seizure type, change in frequency of all countable motor seizures (GTC, tonic, clonic, atonic, tonic or atonic, and clearly recognizable focal), and number of days free of drop seizures.
Statistical Analysis
A total sample size of 225 (75 patients per group) was estimated to have 90% power to detect a difference at α = .05, assuming an estimated mean decrease of 30% or greater in drop seizures for fenfluramine vs placebo and assuming 50% SD and 20% attrition.18
Safety analyses were performed using data from all randomized patients who received 1 dose or more of fenfluramine or placebo. The primary and secondary end point analyses used the modified intent-to-treat population (ie, all patients who received ≥1 dose of fenfluramine or placebo with ≥1 week of seizure diary data).
The primary efficacy analysis was a nonparametric rank analysis of covariance with treatment group and weight (<35 kg or ≥35 kg) as factors; rank baseline seizure frequency as a covariate; and the monthly frequency of drop seizures as assessed during the combined titration and maintenance period as the outcome variable. The Hodges-Lehmann method was used to estimate the median percentage reduction in drop seizure frequency over the titration and maintenance period. Statistical analyses for the primary and secondary end point analyses were controlled for multiplicity using a serial gatekeeping approach to maintain the type I error rate at α = .05 across analyses (eTable 2 in Supplement 2). End points were assessed for statistical significance only if all end points that were higher in the hierarchy were significant at the α = .05 level. Nominal P values were reported even if the gatekeeping test failed, although those from end points below a failure in the testing hierarchy did not indicate statistical significance.
The proportion of patients who achieved 50% or greater reduction in drop seizure frequency over the titration and maintenance period was analyzed using a logistic regression model, with the same factors and covariate as the primary analysis of covariance. The CGI-I ratings were assessed using the Cochran-Mantel-Haenszel test (fenfluramine vs placebo) to compare patients who were rated by caregivers or investigators as showing any improvement (score of ≤3 vs score of ≥4) or clinically meaningful improvement (score of ≤2 vs score of ≥3).
Adverse event frequencies were summarized in the safety population by treatment group using the preferred Medical Dictionary for Regulatory Activities. Results of the electrocardiogram and Doppler echocardiogram analyses were summarized by treatment group using descriptive statistics. Data analysis was performed from November 27, 2017, to October 25, 2019. The database lock date was January 30, 2020, and the date of final report was September 11, 2021. All statistical calculations and plots were performed with SAS, version 9.4 (SAS Institute Inc).
Results
A total of 335 patients were screened for eligibility, of whom 72 were excluded for various reasons, such as having a history of cardiovascular or cerebrovascular disease or not meeting echocardiogram or electrocardiogram requirements (Figure 1). The remaining 263 eligible patients were randomized to the 0.7-mg/kg/d fenfluramine group (n = 87), 0.2-mg/kg/d fenfluramine group (n = 89), and placebo group (n = 87). Among the randomized patients, 21 withdrew from the study early, with the most common reason being adverse events across all groups. A total of 242 patients completed the trial, and 247 entered the OLE study (Figure 1).
Patients had a median (range) age of 13 (2-35) years (Table 1) and consisted of 146 male (56%) and 117 female (44%) individuals. Most frequent investigator-designated primary causes of LGS were unknown (63 of 263 [24%]), structural (33 [13%]), and genetic (55 [21%]). Median (range) number of ASMs used previously was 7 (1-20). The most common ASMs were levetiracetam (177 [67%]), valproate (151 [57%]), topiramate (151 [57%]), clobazam (124 [47%]), and rufinamide (117 [44%]). At baseline, 233 of 263 patients (89%) were using 2 to 4 concomitant ASMs (median [range] number, 3 [1-5]). Of these ASMs, the 5 most common were valproate (147 [56%]), clobazam (119 [45%]), lamotrigine (88 [33%]), levetiracetam (60 [23%]), and rufinamide (53 [20%]). At baseline, the median (range) drop seizure frequency for all patients was 77 (2-2943) per 28 days. The median (range) frequency was higher in the 0.7-mg/kg/d and 0.2-mg/kg/d fenfluramine groups than in the placebo group (83 [7-1803] and 85 [4-2943] vs 53 [2-1761] events per 28 days, respectively).
Table 1. Patient Demographic and Baseline Characteristics.
| Characteristic | Patients, No. (%) | ||
|---|---|---|---|
| Placebo group | Fenfluramine group | ||
| 0.2 mg/kg/d | 0.7 mg/kg/d | ||
| No. of patients | 87 | 89 | 87 |
| Age, mean (SD), y | 14 (8) | 13 (8) | 13 (7) |
| Median (range), y | 13 (2-35) | 13 (3-35) | 13 (2-35) |
| Age group, y | |||
| 2-<6 | 9 (10) | 17 (19) | 12 (14) |
| 6-<12 | 23 (26) | 24 (27) | 25 (29) |
| 12-<18 | 29 (33) | 23 (26) | 25 (29) |
| 18-35 | 26 (30) | 25 (28) | 25 (29) |
| Sex | |||
| Male | 46 (53) | 46 (52) | 54 (62) |
| Female | 41 (47) | 43 (48) | 33 (38) |
| Race and ethnicitya | |||
| Asian | 2 (2) | 3 (3) | 4 (5) |
| Black or African American | 4 (5) | 5 (6) | 3 (3) |
| White | 71 (82) | 67 (75) | 70 (80) |
| Otherb | 0 | 1 (1) | 0 |
| Unknown, not reportedc | 10 (11) | 13 (15) | 10 (11) |
| Region | |||
| North America | 44 (51) | 45 (51) | 43 (49) |
| Europe | 41 (47) | 43 (48) | 38 (44) |
| Australia | 2 (2) | 1 (1) | 6 (7) |
| BMI, mean (SD) | 20 (5) | 20 (5) | 20 (5) |
| Median (range) | 18 (11-36) | 19 (20-47) | 19 (10-37) |
| Baseline weight, kg | |||
| <37.5 | 42 (48) | 42 (47) | 40 (46) |
| ≥37.5 | 45 (52) | 47 (53) | 47 (54) |
| Baseline median seizure frequency per 28 d, No. (range) | |||
| Drop seizures | 53 (2-1761) | 85 (4-2943) | 83 (7-1803) |
| All countable seizures | |||
| Motord | 68 (14-1761) | 106 (4-2943) | 111 (10-1897) |
| Motor and nonmotor | 120 (14-1761) | 138 (14-2967) | 152 (10-5472) |
| Previous ASM usee | |||
| Mean (SD) | 7 (4) | 7 (4) | 8 (4) |
| Median (range) | 6 (1-19) | 7 (1-18) | 7 (1-20) |
| Concomitant ASM use | |||
| Mean (SD) | 3 (1) | 3 (1) | 3 (1) |
| Median (range) | 3 (1-4) | 3 (1-5) | 3 (1-4) |
| Concomitant ASMs in ≥20% of subgroupf | |||
| Valproate (all forms) | 49 (56) | 52 (58) | 46 (53) |
| Clobazam | 38 (44) | 36 (40) | 45 (52) |
| Lamotrigine | 29 (33) | 30 (34) | 29 (33) |
| Levetiracetam | 20 (23) | 17 (19) | 23 (26) |
| Rufinamide | 18 (21) | 17 (19) | 18 (21) |
| Causeg | |||
| Unknown | 22 (25) | 24 (27) | 17 (20) |
| Structural | 13 (15) | 11 (12) | 9 (10) |
| Genetic | 15 (17) | 19 (21) | 21 (24) |
| Metabolic | 1 (1) | 3 (3) | 2 (2) |
| Infectious | 3 (3) | 1 (1) | 2 (2) |
| Immune | 0 | 0 | 1 (1) |
Abbreviations: ASM, antiseizure medication; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Race and ethnicity were self-reported by patients or their caregivers.
Other included American Indian or Alaskan Native and Native Hawaiian or Other Pacific Islander.
Not reported or missing. Privacy laws in some regions and countries preclude disclosure of certain personal information.
Countable seizures included generalized tonic-clonic seizure, tonic seizure, clonic seizure, atonic seizure, tonic or atonic seizure, and clearly recognizable focal seizure.
Cannabidiol (72 [27%]); stiripentol (13 [5%]). Two patients (1%) had had a corpus callosotomy.
Additional concomitant therapies: vagus nerve stimulation (82 [31%]) and ketogenic diet (11 [4%]).
Causes were grouped into categories on the basis of investigator-designated primary International League Against Epilepsy classifications.
Drop Seizure Frequency
The median percentage reduction in drop seizure frequency was 26.5 percentage points in the 0.7-mg/kg/d fenfluramine group, 14.2 percentage points in the 0.2-mg/kg/d fenfluramine group, and 7.6 percentage points in the placebo group (Figure 2A). The study met its primary efficacy end point. Patients who received 0.7 mg/kg/d of fenfluramine achieved an estimated median difference from placebo of −19.9 percentage points (95% CI, −31.0 to −8.7 percentage points; P = .001) in drop seizure frequency compared with baseline level. In the 0.2-mg/kg/d fenfluramine group, the estimated median difference from placebo was −10.5 (95% CI, −25.0 to 4.0 percentage points; P = .09) in the titration and maintenance period (Figure 2A). Comparable results were observed at both doses in the maintenance period, with an estimated median difference of −20.3 (95% CI, −31.6 to −8.9 percentage points; P = .002) between the 0.7-mg/kg/d fenfluramine group and placebo group and 11.5 percentage points (95% CI, −26.3 to −3.3 percentage points; P = .08) between the 0.2-mg/kg/d fenfluramine group and the placebo group (Figure 2A).
Figure 2. Patient Response to Treatment From Prerandomization During the Combined Titration and Maintenance Period and/or Maintenance Only Period.
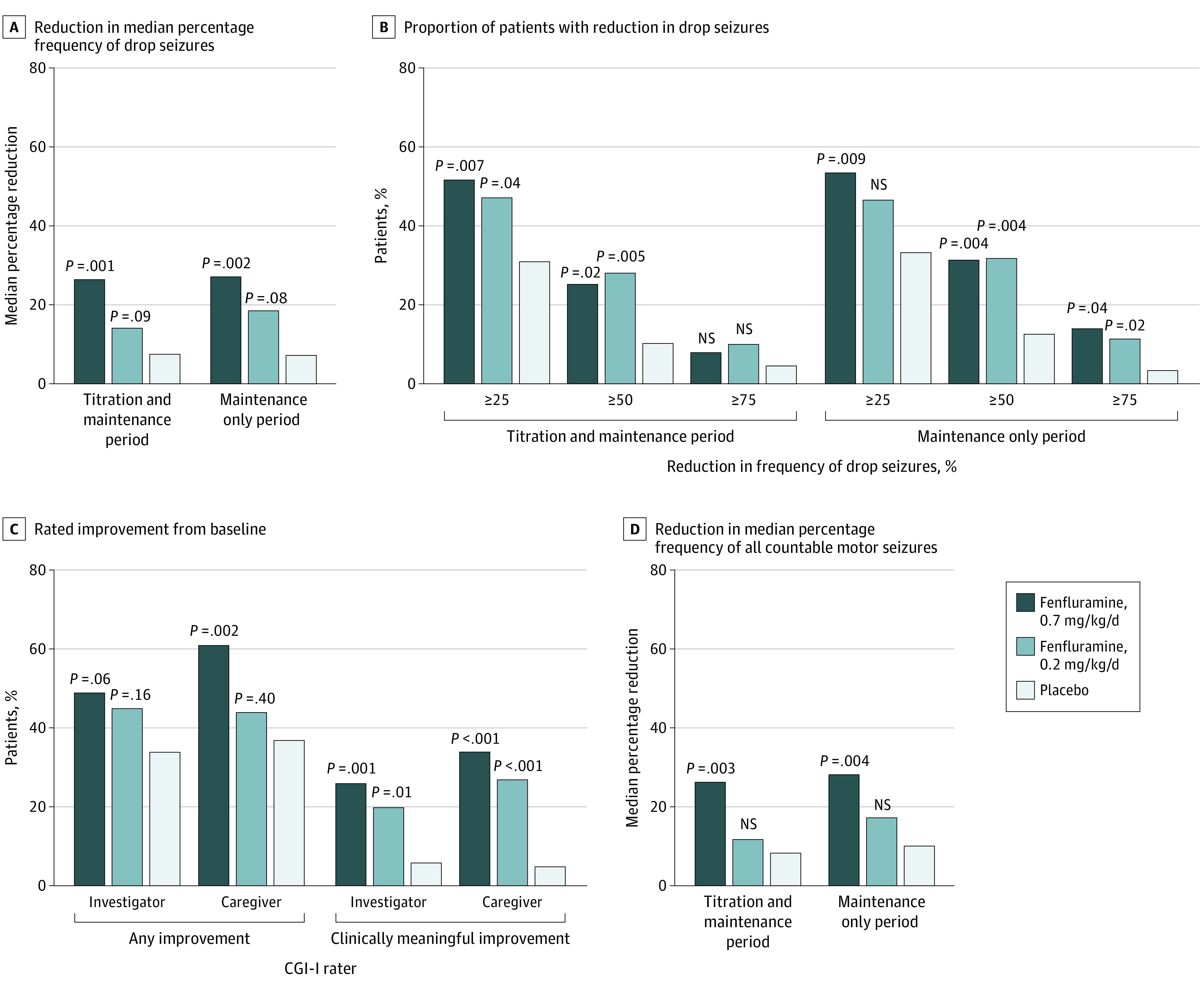
A, Estimated median difference from placebo was calculated using Hodges-Lehmann estimate. B, The 50%, 25%, and 75% or greater responder levels were included. C, P values were calculated using the Cochran-Mantel-Haenszel test. D, Countable motor seizures included generalized tonic-clonic (GTC) seizure, secondary GTC, tonic seizure, atonic seizure, tonic or atonic seizure, clonic seizure, hemiclonic seizure, and focal seizure. P values were statistically significant for the primary efficacy outcome and the 50% or greater responder rate for the 0.7-mg/kg/d fenfluramine group; all other P values were nominal. Distribution data are presented in eFigure 2 in the Supplement. CGI-I indicates Clinical Global Impression-Improvement scale; NS, not statistically significant.
Key Secondary Outcomes
A greater proportion of patients in the 0.7-mg/kg/d fenfluramine group (22 of 87 [25%]; P = .02) and the 0.2-mg/kg/d fenfluramine group (25 of 89 [28%]; P = .005) experienced a 50% or greater reduction in drop seizure frequency compared with patients in the placebo group (9 of 87 [10%]) (Figure 2B). More patients in the active treatment groups achieved a 25% or greater reduction in drop seizures compared with placebo (27 of 87 [31%]) during the titration and maintenance period. Similar results were seen in the maintenance period (Figure 2B). In the maintenance period, more patients in the 0.7-mg/kg/d fenfluramine group (27 of 87 [31%]; P = .004) and 0.2-mg/kg/d fenfluramine group (28 of 89 [32%]; P = .004) achieved a 50% or greater reduction in drop seizure frequency than placebo (11 of 87 [13%]). More patients in both active groups (12 of 87 [14%] in the 0.7-mg/kg/d fenfluramine group [P = .02]; 10 of 89 [11%] in the 0.2-mg/kg/d fenfluramine group [P = .04]) than in the placebo group (3 of 87 [3%]) achieved 75% or greater reduction in seizure frequency. At the 25% or greater responder level, 46 of 87 patients (54%) in the 0.7-mg/kg/d fenfluramine group (P = .009), 41 of 89 patients (47%) in the 0.2-mg/kg/d fenfluramine group (P = .10), and 29 of 87 (33%) in the placebo group achieved reduction in drop seizure frequency.
Near seizure freedom (defined as ≤1 observed drop seizure) was reported in 1 of 87 patients (1%) in the 0.7-mg/kg/d fenfluramine group, 2 of 89 patients (2%) in the 0.2-mg/kg/d fenfluramine group, and 1 of 87 patients (1%) in the placebo group. Seizure freedom was achieved by 1 of 89 patients (1%) in the 0.2-mg/kg/d fenfluramine group and 1 of 87 patients (1%) in the placebo group.
Site investigators rated patients as having clinically meaningful improvement on the CGI-I scale, giving a much improved or very much improved rating to more patients in the fenfluramine groups (21 of 80 patients [26%] in the 0.7-mg/kg/d fenfluramine group [P = .001]; 17 of 85 patients [20%] in the 0.2-mg/kg/d fenfluramine group [P = .01]) than in the placebo group (5 of 80 patients [6%]). Similarly, caregivers gave a clinically meaningful improvement rating to more patients in the fenfluramine groups (27 of 80 patients [34%] in the 0.7-mg/kg/d fenfluramine group [P < .001]; 23 of 85 patients [27%] in the 0.2-mg/kg/d fenfluramine group [P < .001]) than in the placebo group (4 of 81 patients [5%]) (Figure 2C). The percentages of patients who were rated by investigators as having any improvement on the CGI-I scale were 49% (39 of 80 patients) in the 0.7-mg/kg/d fenfluramine group (P = .06), 45% (38 of 85 patients) in the 0.2-mg/kg/d fenfluramine group (P = .16), and 34% (27 of 80 patients) in the placebo group (Figure 2C). Among caregivers, more patients in the fenfluramine groups were rated as having any improvement on the CGI-I scale (49 of 80 patients [61%] in the 0.7-mg/kg/d fenfluramine group [P = .002]; 37 of 85 patients [44%] in the 0.2-mg/kg/d fenfluramine group [P = .40]) than patients in the placebo group (30 of 81 [37%]).
Additional Secondary Outcomes
The percentage reduction in frequency of all countable motor seizures during the titration and maintenance period was 26.3% in the 0.7-mg/kg/d fenfluramine group, 11.8% in the 0.2-mg/kg/d fenfluramine group, and 8.4% in the placebo group. Comparable results were observed in the maintenance only period, with 28.3% in the 0.7-mg/kg/d fenfluramine group (P = .004), 17.3% in the 0.2-mg/kg/d fenfluramine group, and 10.2% in the placebo group (Figure 2D).
In seizure-type subgroup analyses, GTC seizures occurred at baseline in 46% of patients during the trial (120 of 263; n = 39-41 per group; median [range] baseline GTC frequency, 10-18 [0-198] per 28 days) and were more responsive to fenfluramine than other seizure types (Figure 3). The percentage reduction in GTC frequency during the titration and maintenance period was 45.7% (n = 38) in the 0.7-mg/kg/d fenfluramine group (P = .001) and 58.2% (n = 38) in the 0.2-mg/kg/d fenfluramine group (P < .001) compared with an increase of 3.7% (n = 38) in the placebo group. Results during the maintenance period were comparable, with 52.6% (n = 38) reduction in frequency of GTCs in the 0.7-mg/kg/d fenfluramine group (P = .001) and 61.7% (n = 38) reduction in frequency of GTCs in the 0.2-mg/kg/d fenfluramine group (P < .001), compared with an increase in frequency of GTCs of 2.6% (n = 38) in the placebo group.
Figure 3. Median Percentage Reduction at Baseline for the Combined Titration and Maintenance Period and Maintenance Only Period, by Seizure Type.
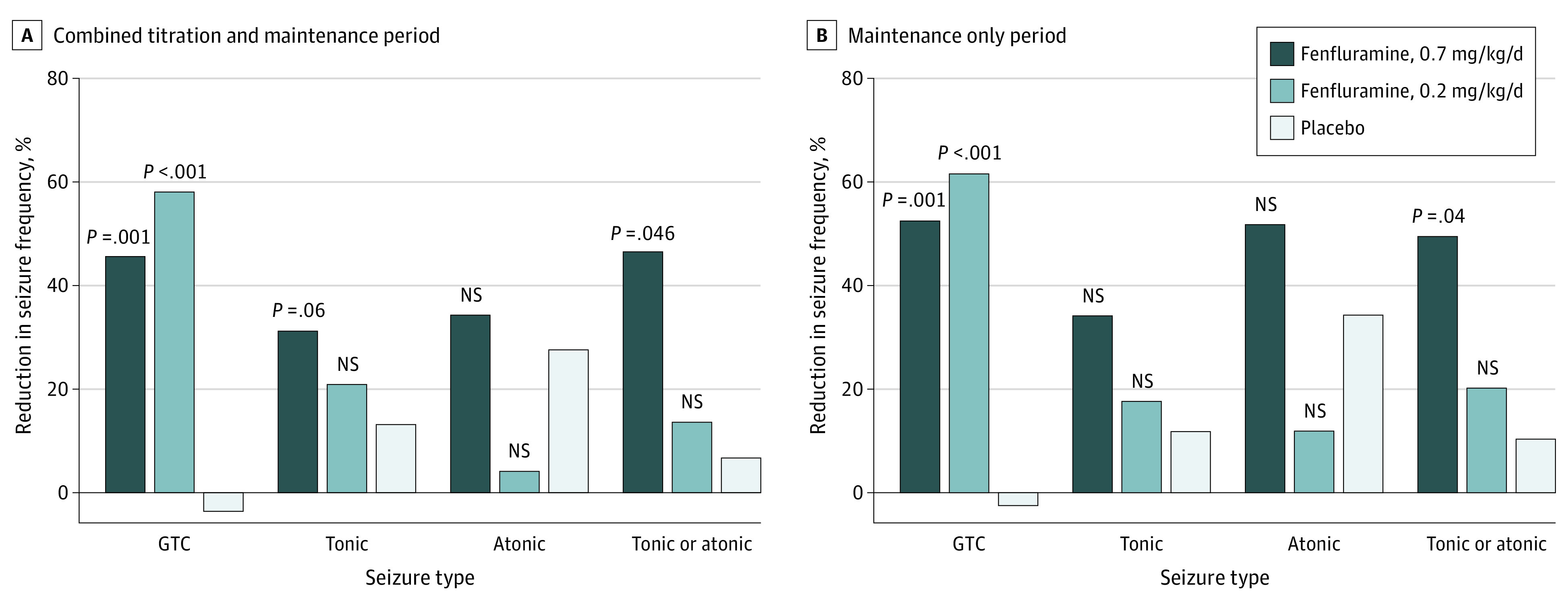
P values calculated using pairwise Wilcoxon rank sum test compared percentage changes from baseline between active treatment and placebo groups. All P values were nominal. Distribution data are presented in eFigure 2 in the Supplement. GTC indicates generalized tonic-clonic seizure; NS, not statistically significant.
Estimated median difference from placebo in GTC seizure frequency was −50.3 percentage points (95% CI, −76.7 to −23.8 percentage points; P = .001) in the 0.7-mg/kg/d fenfluramine group and −60.4 percentage points (95% CI, −84.9 to −36.0 percentage points; P < .001) in the 0.2-mg/kg/d fenfluramine group. Comparable results in the maintenance only period were observed, with −52.8 percentage points (95% CI, −80.3 to −25.3 percentage points; P = .001) in the 0.7- mg/kg/d fenfluramine group and −61.0 percentage points (95% CI, −85.5 to −36.5 percentage points; P < .001) in the 0.2-mg/kg/d fenfluramine group (eFigure 2 in Supplement 2). Percentage reduction in tonic or atonic seizure frequency was 46.7% (n = 13) in the 0.7-mg/kg/d fenfluramine group during the titration and maintenance period compared with 6.8% (n = 20) in the placebo group (P = .046) and was 49.6% during the maintenance only period compared with 10.5% (n = 20) in the placebo group (P = .04). Estimated median difference from placebo in tonic or atonic seizure frequency was −31.5 percentage points (95% CI, −61.1 to −2.0 percentage points; P = .046) in the 0.7-mg/kg/d fenfluramine group compared with −35.1 percentage points (95% CI, −67.1 to −3.1 percentage points; P = .04) in the maintenance only period (eFigure 2 in Supplement 2). Distribution data by seizure subtype are presented in eFigure 2 in Supplement 2.
Treatment-Emergent Adverse Events
Most patients (212 of 263 [81%]) experienced a TEAE (78 of 87 patients [90%] in the 0.7-mg/kg/d fenfluramine group; 69 of 89 [78%] in the 0.2-mg/kg/d fenfluramine group; 65 of 87 [75%] in the placebo group) (Table 2). More patients in the 0.7-mg/kg/d fenfluramine group (10 of 87 [11%]) compared with the 0.2-mg/kg/d fenfluramine group (4 of 89 [4%]) and the placebo group (4 of 87 [5%]) experienced 1 or more serious TEAE. In the 0.7-mg/kg/d fenfluramine group, 1 SUDEP was reported and was unrelated to fenfluramine use. The most common TEAEs included decreased appetite (59 of 263 [22%]), somnolence (33 of 263 [13%]), and fatigue (33 of 263 [13%]). More patients in the fenfluramine treatment groups than in the placebo group experienced decreased appetite (31 of 87 [36%] in the 0.7-mg/kg/d fenfluramine group; 18 of 89 [20%] in the 0.2-mg/kg/d fenfluramine group; 10 of 87 [11%] in the placebo group). Weight loss of 7% or more from baseline was reported in 7 of 87 patients (8%) in the 0.7-mg/kg/d fenfluramine group, 2 of 89 (2%) in the 0.2-mg/kg/d fenfluramine group, and 2 of 87 (2%) in the placebo group. The most frequent TEAEs leading to study withdrawal were seizure (3 patients in the 0.2-mg/kg/d fenfluramine group) and somnolence (3 patients in the 0.7-mg/kg/d fenfluramine group). Status epilepticus was observed in 3 of 87 patients (3%) in the 0.7-mg/kg/d fenfluramine group, 0 patients in the 0.2-mg/kg/d fenfluramine group, and 1 of 87 (1%) in the placebo group.
Table 2. Most Common (≥10%) Noncardiovascular Treatment–Emergent Adverse Events (TEAEs).
| Adverse event | Patients, No. (%) | |||
|---|---|---|---|---|
| Placebo group | Fenfluramine group | Overall | ||
| 0.2 mg/kg/d | 0.7 mg/kg/d | |||
| No. of patients | 87 | 89 | 87 | 263 |
| TEAE | ||||
| Any | 65 (75) | 69 (78) | 78 (90) | 212 (81) |
| Patients with ≥1 serious | 4 (5) | 4 (4) | 10 (11) | 18 (7) |
| TEAEs ≥10% | ||||
| Decreased appetite | 10 (11) | 18 (20) | 31 (36) | 59 (22) |
| Somnolence | 9 (10) | 9 (10) | 15 (17) | 33 (13) |
| Fatigue | 9 (10) | 8 (9) | 16 (18) | 33 (13) |
| Pyrexia | 10 (11) | 9 (10) | 7 (8) | 26 (10) |
| Diarrhea | 4 (5) | 10 (11) | 11 (13) | 25 (10) |
| Vomiting | 5 (6) | 12 (13) | 7 (8) | 24 (9) |
No cases of valvular heart disease or pulmonary arterial hypertension were observed at any time. One patient (0.7 mg/kg/d fenfluramine) had an end-of-study echocardiogram reading as mild aortic regurgitation without any changes in valve morphological structure and a subsequent diagnostic transesophageal echocardiogram revealed absent aortic regurgitation and a normal aortic valve.
Discussion
Lennox-Gastaut syndrome is a complex, highly treatment-resistant developmental and epileptic encephalopathy.1,3,19 Seizures for most patients with LGS do not respond to a large number of medications, including the multiple currently available ASMs approved for LGS. In this RCT, we demonstrated that 0.7 mg/kg/d of fenfluramine significantly reduced the frequency of drop seizures with efficacy that was comparable with that observed in recently reported clinical trials with similarly treatment-refractory LGS.10,11,12 Based on calculations from the responder analysis data obtained in this trial, the number needed to treat for a 50% responder level was 7 for the 0.7-mg/kg/d fenfluramine group and 6 for the 0.2-mg/kg/d fenfluramine group.
Current treatment strategies for LGS recommend polypharmacy with drugs of different classes and mechanisms of action.20 Fenfluramine has unique mechanisms of action among ASMs. Preclinical studies support dual pharmacological activity of enhancing specific serotonergic receptor activity and positively modulating σ-1 receptors, possibly reducing seizure activity by restoring the balance of GABA-mediated inhibition and glutamatergic excitation.21,22,23 Dual serotonergic or σ-1 receptor activity may also contribute to improvements in reported nonseizure outcomes, particularly improvements in executive function.24,25 These unique mechanisms of action may provide a novel treatment option for patients with LGS.
This trial found a reduction in GTC seizures after fenfluramine use in patients who had GTC seizures at baseline. The magnitude of response was similar to the reduction in monthly convulsive seizure frequency that was observed in patients with DS, as reported in 2 RCTs13,14,15 and long-term OLE studies,26,27 for up to 3 years.
Generalized tonic-clonic seizures are commonly observed in patients with LGS, especially later in disease progression as the patient ages.1,3 Two studies with large populations of patients (n = 73-252) reported a range of 40% to 54.7% of patients with LGS presenting with GTC seizures at baseline.28,29 Moreover, GTC seizures often result in bodily injury and hospitalizations7,8 and represent the primary risk factor for SUDEP.30 A recent study demonstrated a substantial reduction in SUDEP for patients with DS using fenfluramine compared with published reports.31 Along with its favorable safety profile, fenfluramine has a high level of efficacy in GTC seizures, positioning fenfluramine as an ideal therapeutic option for patients with LGS who present with GTC seizures as part of their phenotype.
In the current patient population with LGS, fenfluramine had a safety profile that was comparable with that reported in RCTs in patients with DS, for whom the most common TEAEs were also decreased appetite, pyrexia, diarrhea, and fatigue.14,15 Weight loss occurred at similar rates in this trial, as observed in phase 3 studies of pediatric patients with DS.14,15 Fenfluramine (up to 2 years) had a minimal effect on growth of patients with DS compared with a reference population of historical control patients using standard ASM regimens.27,32 None of the patients in the present RCT withdrew because of weight loss or decreased appetite, which corroborates previous reports of fenfluramine for patients with DS.14,15,27 Doppler echocardiogram examinations did not identify pathological functional changes in cardiac valves or signs of pulmonary arterial hypertension in any patient during the trial. These findings support the cardiovascular safety of fenfluramine and are consistent with results in monitoring studies of patients with DS.17 No valvular heart disease or pulmonary arterial hypertension has been reported in patients with DS after a median fenfluramine treatment duration of 256 days17; these results have been more recently confirmed with up to 36 months of fenfluramine treatment,33 and long-term safety studies are ongoing in patients with LGS.
Limitations
A limitation of this study is its short duration. Long-term safety and efficacy evaluations in OLE studies are being conducted.34
Conclusions
This phase 3 RCT in patients with highly treatment-refractory LGS met its primary end point, demonstrating a reduction in the drop seizure frequency in patients who used 0.7 mg/kg/d fenfluramine. Fenfluramine was also highly effective in reducing GTC seizures, suggesting that fenfluramine may be a particularly advantageous choice in patients with this seizure subtype. Moreover, fenfluramine was generally well tolerated, with safety profiles that were comparable with those reported in previous RCTs and OLE studies for short- and long-term treatment of DS, including no observations of valvular heart disease or pulmonary arterial hypertension.
Trial Protocol
eFigure 1. Study Design
eFigure 2. Forest Plots: Distribution of Median Percentage Difference From Placebo in Seizure Frequency
eTable 1. Titration and Tapering Algorithms
eTable 2. Sequential Gatekeeping Procedure to Maintain Type 1 Error Rate at α = 0.05 for Pairwise Comparisons Between Active Treatment and Placebo
Data Sharing Statement
References
- 1.Cross JH, Auvin S, Falip M, Striano P, Arzimanoglou A. Expert opinion on the management of Lennox-Gastaut syndrome: treatment algorithms and practical considerations. Front Neurol. 2017;8:505. doi: 10.3389/fneur.2017.00505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gastraut H, Roger J, Soulayrol R, et al. Childhood epileptic encephalopathy with diffuse slow spike-waves (otherwise known as “petit mal variant”) or Lennox syndrome. Epilepsia. 1966;7(2):139-179. doi: 10.1111/j.1528-1167.1966.tb06263.x [DOI] [PubMed] [Google Scholar]
- 3.Arzimanoglou A, French J, Blume WT, et al. Lennox-Gastaut syndrome: a consensus approach on diagnosis, assessment, management, and trial methodology. Lancet Neurol. 2009;8(1):82-93. doi: 10.1016/S1474-4422(08)70292-8 [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, Paliwal VK, Agarwal V, Neyaz Z, Lal H, Goel G. Relationship of Lennox-Gastaut syndrome with perinatal event: a cross-sectional study. J Pediatr Neurosci. 2015;10(2):98-102. doi: 10.4103/1817-1745.159184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piña-Garza JE, Chung S, Montouris GD, Radtke RA, Resnick T, Wechsler RT. Challenges in identifying Lennox-Gastaut syndrome in adults: a case series illustrating its changing nature. Epilepsy Behav Case Rep. 2016;5:38-43. doi: 10.1016/j.ebcr.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arzimanoglou A. Dravet syndrome: from electroclinical characteristics to molecular biology. Epilepsia. 2009;50(suppl 8):3-9. doi: 10.1111/j.1528-1167.2009.02228.x [DOI] [PubMed] [Google Scholar]
- 7.Strzelczyk A, Schubert-Bast S, Simon A, Wyatt G, Holland R, Rosenow F. Epidemiology, healthcare resource use, and mortality in patients with probable Lennox-Gastaut syndrome: a population-based study on German health insurance data. Epilepsy Behav. 2021;115:107647. doi: 10.1016/j.yebeh.2020.107647 [DOI] [PubMed] [Google Scholar]
- 8.Arzimanoglou A, Resnick T. All children who experience epileptic falls do not necessarily have Lennox-Gastaut syndrome... but many do. Epileptic Disord. 2011;13(suppl 1):S3-S13. doi: 10.1684/epd.2011.0422 [DOI] [PubMed] [Google Scholar]
- 9.Sveinsson O, Andersson T, Mattsson P, Carlsson S, Tomson T. Clinical risk factors in SUDEP: a nationwide population-based case-control study. Neurology. 2020;94(4):e419-e429. doi: 10.1212/WNL.0000000000008741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devinsky O, Patel AD, Cross JH, et al. ; GWPCARE3 Study Group . Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378(20):1888-1897. doi: 10.1056/NEJMoa1714631 [DOI] [PubMed] [Google Scholar]
- 11.Hahn CD, Jiang Y, Villanueva V, et al. Efficacy, safety and tolerability of soticlestat (TAK-935/OV935) as adjunctive therapy in pediatric patients with Dravet Syndrome or Lennox-Gastaut Syndrome (ELEKTRA). Poster presented at: American Epilepsy Society Annual Meeting; December 4-8, 2020; virtual meeting. [Google Scholar]
- 12.Thiele EA, Marsh ED, French JA, et al. ; GWPCARE4 Study Group . Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a andomized, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085-1096. doi: 10.1016/S0140-6736(18)30136-3 [DOI] [PubMed] [Google Scholar]
- 13.Sullivan J, Lagae L, Cross JH, et al. Fenfluramine (FINTEPLA) in Dravet syndrome: results of a third randomized, placebo-controlled clinical trial (Study 3). Poster presented at: American Epilepsy Society Annual Meeting; December 4-8, 2020; virtual meeting. [Google Scholar]
- 14.Nabbout R, Mistry A, Zuberi S, et al. ; FaiRE, DS Study Group . Fenfluramine for treatment-resistant seizures in patients with Dravet syndrome receiving stiripentol-inclusive regimens: a randomized clinical trial. JAMA Neurol. 2020;77(3):300-308. doi: 10.1001/jamaneurol.2019.4113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lagae L, Sullivan J, Knupp K, et al. ; FaiRE DS Study Group . Fenfluramine hydrochloride for the treatment of seizures in Dravet syndrome: a andomized, double-blind, placebo-controlled trial. Lancet. 2019;394(10216):2243-2254. doi: 10.1016/S0140-6736(19)32500-0 [DOI] [PubMed] [Google Scholar]
- 16.Lagae L, Schoonjans AS, Gammaitoni AR, Galer BS, Ceulemans B. A pilot, open-label study of the effectiveness and tolerability of low-dose ZX008 (fenfluramine HCl) in Lennox-Gastaut syndrome. Epilepsia. 2018;59(10):1881-1888. doi: 10.1111/epi.14540 [DOI] [PubMed] [Google Scholar]
- 17.Lai WW, Galer BS, Wong PC, et al. Cardiovascular safety of fenfluramine in the treatment of Dravet syndrome: analysis of an ongoing long-term open-label safety extension study. Epilepsia. 2020;61(11):2386-2395. doi: 10.1111/epi.16638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng YT, Conry JA, Drummond R, Stolle J, Weinberg MA; OV-1012 Study Investigators . Randomized, phase III study results of clobazam in Lennox-Gastaut syndrome. Neurology. 2011;77(15):1473-1481. doi: 10.1212/WNL.0b013e318232de76 [DOI] [PubMed] [Google Scholar]
- 19.Asadi-Pooya AA. Lennox-Gastaut syndrome: a comprehensive review. Neurol Sci. 2018;39(3):403-414. doi: 10.1007/s10072-017-3188-y [DOI] [PubMed] [Google Scholar]
- 20.Amrutkar C, Riel-Romero RM. Lennox Gastaut Syndrome: StatPearls. StatPearls Publishing LLC; 2021. [PubMed] [Google Scholar]
- 21.Martin P, de Witte PAM, Maurice T, Gammaitoni A, Farfel G, Galer B. Fenfluramine acts as a positive modulator of sigma-1 receptors. Epilepsy Behav. 2020;105:106989. doi: 10.1016/j.yebeh.2020.106989 [DOI] [PubMed] [Google Scholar]
- 22.Sourbron J, Smolders I, de Witte P, Lagae L. Pharmacological analysis of the anti-epileptic mechanisms of fenfluramine in scn1a mutant zebrafish. Front Pharmacol. 2017;8:191. doi: 10.3389/fphar.2017.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin P, Reeder T, Sourbron J, de Witte PAM, Gammaitoni AR, Galer BS. An emerging role for sigma-1 receptors in the treatment of developmental and epileptic encephalopathies. Int J Mol Sci. 2021;22(16):8416. doi: 10.3390/ijms22168416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bishop KI, Isquith PK, Gioia GA, et al. Improved everyday executive functioning following profound reduction in seizure frequency with fenfluramine: analysis from a phase 3 long-term extension study in children/young adults with Dravet syndrome. Epilepsy Behav. 2021;121(pt A):108024. doi: 10.1016/j.yebeh.2021.108024 [DOI] [PubMed] [Google Scholar]
- 25.Bishop KI, Isquith PK, Gioia GA, et al. FINTEPLA (fenfluramine) treatment improves everyday executive functioning in patients with Lennox-Gastaut syndrome: analysis from a phase 3 clinical trial. Presented at: American Academy of Neurology Annual Meeting; April 17-22, 2021; virtual meeting. [Google Scholar]
- 26.Scheffer IE, Devinsky O, Perry MS, et al. Efficacy and tolerability of adjunctive FINTEPLA (fenfluramine HCl) in an open-label extension study of Dravet syndrome patients treated for up to 3 years. Poster presented at: American Epilepsy Society Annual Meeting; December 4-8, 2020; virtual meeting. [Google Scholar]
- 27.Sullivan J, Scheffer IE, Lagae L, et al. Fenfluramine HCl (Fintepla®) provides long-term clinically meaningful reduction in seizure frequency: analysis of an ongoing open-label extension study. Epilepsia. 2020;61(11):2396-2404. doi: 10.1111/epi.16722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felbamate Study Group in Lennox-Gastaut Syndrome . Efficacy of felbamate in childhood epileptic encephalopathy (Lennox-Gastaut syndrome). N Engl J Med. 1993;328(1):29-33. doi: 10.1056/NEJM199301073280105 [DOI] [PubMed] [Google Scholar]
- 29.Asadi-Pooya AA, Bazrafshan M, Farazdaghi M. Cluster analysis of a large dataset of patients with Lennox-Gastaut syndrome. Seizure. 2021;92:36-39. doi: 10.1016/j.seizure.2021.08.005 [DOI] [PubMed] [Google Scholar]
- 30.Sveinsson O, Andersson T, Carlsson S, Tomson T. The incidence of SUDEP: a nationwide population-based cohort study. Neurology. 2017;89(2):170-177. doi: 10.1212/WNL.0000000000004094 [DOI] [PubMed] [Google Scholar]
- 31.Cross JH, Galer BS, Gil-Nagel A, et al. Impact of fenfluramine on the expected SUDEP mortality rates in patients with Dravet syndrome. Seizure. 2021;93:154-159. doi: 10.1016/j.seizure.2021.10.024 [DOI] [PubMed] [Google Scholar]
- 32.Gil-Nagel A, Sullivan J, Ceulemans B, et al. Treatment with fenfluramine in patients with Dravet syndrome has no long-term effects on weight and growth. Epilepsy Behav. 2021;122:108212. doi: 10.1016/j.yebeh.2021.108212 [DOI] [PubMed] [Google Scholar]
- 33.Agarwal A, Farfel G, Gammaitoni AR, Wong PC, Pinto FJ, Galer BS. Serial echocardiographic assessment of patients with Dravet syndrome treated with fenfluramine (Fintepla®) for up to 3 years: no incidence of valvular heart disease or pulmonary artery hypertension. Poster presented at: American Epilepsy Society Annual Meeting; December 3-7, 2021; Chicago, IL. [Google Scholar]
- 34.Knupp KG, Scheffer IE, Ceulemans B, et al. FINTEPLA (fenfluramine) provides clinically meaningful reduction in frequency of seizures resulting in a drop in patients with Lennox-Gastaut syndrome for up to 1 year: interim analysis of an open-label extension study. Poster presented at: Annual Child Neurology Society Meeting; September 29-October 2, 2021; Boston, MA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Study Design
eFigure 2. Forest Plots: Distribution of Median Percentage Difference From Placebo in Seizure Frequency
eTable 1. Titration and Tapering Algorithms
eTable 2. Sequential Gatekeeping Procedure to Maintain Type 1 Error Rate at α = 0.05 for Pairwise Comparisons Between Active Treatment and Placebo
Data Sharing Statement