Abstract
Introduction
The objective of the Chinese Neonatal Network (CHNN) is to provide a platform for collaborative research, outcomes evaluation and quality improvement for preterm infants with gestational age less than 32 weeks in China. The CHNN is the first national neonatal network and has the largest geographically representative cohort from neonatal intensive care units (NICUs) in China.
Methods and analysis
Individual-level data from participating NICUs will be collected using a unique database developed by the CHNN on an ongoing basis from January 2019. Data will be prospectively collected from all infants <32 weeks gestation or <1500 g birth weight at 58 participating NICUs. Infant outcomes and inter-institutional variations in outcomes will be examined and used to inform quality improvement measures aimed at improving outcomes. Information about NICU environmental and human resource factors and processes of neonatal care will also be collected and analysed for association with outcomes. Clinical studies, including randomised controlled trials will be conducted using the CHNN data platform.
Ethics and dissemination
This study was approved by the ethics review board of Children’s Hospital of Fudan University, which was recognised by all participating hospitals. Waiver of consent were granted at all sites. Only non-identifiable patient level data will be transmitted and only aggregate data will be reported in CHNN reports and publications.
Keywords: neonatology, protocols & guidelines, neonatal intensive & critical care, perinatology, qualitative research
Strengths and limitations of this study.
This is the largest prospective, geographically representative, hospital-based cohort study of very preterm infants in China.
Data definitions and data collection protocols were standardised.
Data were collected on both outcomes and clinical practices to facilitate risk analysis and quality improvement.
Limitations include lack of generalisability since participating hospitals were all tertiary hospitals.
Outcomes data were not available for infants who were discharged against medical advice, which may bias the results
Introduction
Preterm infants are of significant public health concern worldwide due to their high risk of mortality and morbidities.1–3 The incidence of preterm births is 7.3% in China and increasing steadily, with approximately 0.2 million infants born at less than 32 weeks gestational age (GA) born each year.4 5 In October 2015, China announced a national plan to end the one-child policy that had been implemented for more than 40 years, allowing some couples to have two children. A national descriptive before-and-after comparative study showed that the announcement of China’s universal two-child policy was associated with a rise in births in China, and with women giving birth being more likely to be multiparous and to be aged 35 and over,6 with an increased likelihood of preterm births.
The population of China represents almost one-fifth of the global population. In China, preterm infant survival in neonatal intensive care units (NICUs) has significantly improved in recent years.7 8 Neonatologists in China have made progress in NICU care, and introduced advances such as breast milk feeding,9 NICU infection control,10 family-integrated care of preterm infants11 and better treatment of bronchopulmonary dysplasia in very low birth weight infants.12 However, previous studies mostly involved small groups of NICUs during a defined research period, and were subject to selection bias. This has led to problems of generalisation in the context of the huge Chinese population with regional differences in population characteristics, medical expertise, economic development and health insurance coverage. It also presents difficulties for translation of clinical evidence into expert consensus practice guidelines,13 14 even though there is strong demand for them in China.
Previous authors have demonstrated that establishing a national neonatal network and database is an important step towards understanding the status of neonatal care and outcomes in a country and can provide an effective platform for collaborative care, quality improvement (QI), research and knowledge translation.15–19 Our goal is to establish a national Chinese Neonatal Network (CHNN) in the People’s Republic of China, with a standardised database for collaborative care, QI and research. This manuscript outlines the research protocol for CHNN.
Objectives
The specific objectives of CHNN are to: (1) Establish an ongoing prospectively collected standardised database of very preterm infants (VPI) with GA <32 weeks or <1500 g birth weight among NICUs in China, as a platform for surveillance, QI and research, (2) Assess and monitor major outcomes of VPI and their risk determinants, as well as inter-institutional variations in care practices and outcomes to identify clinical practices associated with good or poor outcomes, (3) Implement the Evidence-based Practice for Improving Quality (EPIQ) programme to improve quality of care and outcomes, (4) Conduct collaborative research, including epidemiological, clinical and health services studies and randomised controlled trials.
Methods and analysis
Participating hospitals
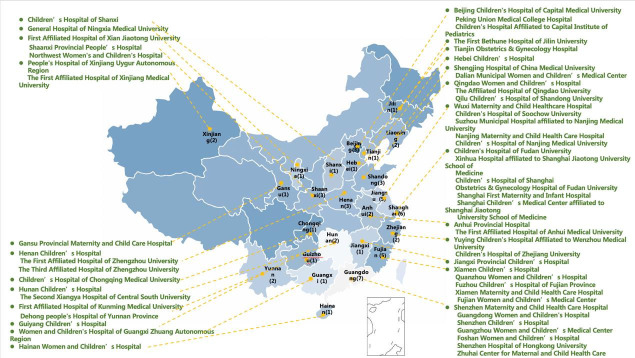
CHNN was founded in 2018 with 58 participating hospitals from 25 provinces throughout mainland China (figure 1). No hospitals in Tibet, Taiwan and Hong Kong were included. CHNN hospitals are tertiary referral hospitals with Grade A level III NICUs authorised by the Health Administration of China and have recognised expertise in caring for high-risk neonates. They were selected to be representative of different regions of the country but do not include all hospitals providing NICU care to VPI, and together they care for approximately 5% of all VPI in China. They include all government-designated neonatal centres of excellence in China, including 4 national children’s medical centres, 5 regional children’s medical centres and 30 provincial perinatal or children’s medical centres. The other hospitals comprise major referral centres in large cities across China. Forty-three hospitals were perinatal centres with birthing facilities, and 14 hospitals were freestanding children’s hospitals that admitted only outborn infants. The median number of NICU beds was 40 (IQR, 30–62), and the median number of intermediate-level and continuing care neonatal beds was 66 (IQR, 40–91). For perinatal centres, the median number of annual deliveries was 10 280 (IQR, 6273–15 423). The median number of full-time equivalent neonatologists was 19 (IQR, 12–27), and the median number of NICU nurses was 42 (IQR, 30–65).20
Figure 1.
Map of 58 participating hospitals in Chinese Neonatal Network (CHNN). There were 58 member hospitals from 25 provinces in China that are participating in the CHNN. The distribution of the 58 member hospitals is almost geographically throughout the mainland China.
Study population
This is a prospective, hospital-based cohort. Data will be collected on all infants <32+0 weeks GA or birth weight <1500 g admitted to participating NICUs. Stillborn, delivery room death and infants transferred to non-participating hospitals within 24 hours after birth will be excluded. Re-admissions and transfers between participating hospitals will be tracked as data from the same infants. Infants will be followed until NICU discharge/transfer or death.
Patient and public involvement
The study responds to patient concerns about quality of care and perceived variation in quality of care among different hospitals. Outcomes of QI initiatives will be published and made available to patients.
Achieving the four specific objectives of CHNN
Objective 1: Establish an ongoing prospectively collected standardised database.
Governance
The executive committee of CHNN comprises 13 senior neonatology leaders representing different regions of the country and include 2 co-chairpersons, 2 vice-presidents and 3 secretary generals. The committee is responsible for setting policies and research agendas, and oversees the activities and operations of the network and coordinating centre.
Coordinating centre and data collection
The CHNN Coordinating and Data Centre is located at the Children’s Hospital of Fudan University, Shanghai, China. The Data Centre is equipped with dedicated servers that are managed by the Information Technology Department of the Children’s Hospital of Fudan University in compliance with hospital, municipal and national standards for data security. A unique scalable customised database with built-in error checking based on MS Access was created in-house. A standard manual of operations and definitions was provided to all participating centres. Dedicated data abstractors under the supervision of the site principal investigator are responsible for data acquisition in each hospital and centralised training sessions were organised to teach data abstractors to carry out data collection and uploading to the CHNN database. Data abstractors enter data directly from patients charts into a dedicated computer at each hospital and electronically transmitted data to the CHNN Coordinating Centre. Only non-identifiable patient information will be transferred and only aggregate data results will be reported and published. Data quality is ensured at multiple levels. The data entry programme has built-in error checking. Additional data checks are performed quarterly by the coordinating centre for quality and completeness, and site-specific data quality reports will be fed back to each site and data records returned for corrections if needed. Data quality audit using data re-abstraction of randomly selected patient charts will be performed annually. Site investigators will be responsible for data quality control in each site. An annual report will be produced for the network.
Data variables
Data variables were selected by the CHNN executive committee (table 1). Definitions of variables were standardised and mapped to the The International Statistical Classification of Diseases, version 10 (ICD-10)21 and SNOMED22 dictionaries as appropriate. Mortality is defined as death due to any cause prior to discharge home. Bronchopulmonary dysplasia is defined as oxygen requirement at 36 weeks post-menstrual age.23 Severe neurological injury is defined as ≥ stage 3 intraventricular haemorrhage (IVH) with ventricular dilatation or parenchymal injury (including periventricular leucomalacia) with or without IVH, according to the Papile classification.24 Retinopathy of prematurity is defined as ≥ stage 3 according to the International Classification25 or need for laser surgery or intraocular injections of anti-vascular endothelial growth factor agents. Necrotising enterocolitis is defined as ≥ stage 2 according to Bell’s criteria.26 Nosocomial infection is defined as culture-proven sepsis (blood or cerebrospinal fluid positive for pathogenic organism) at >3 days or 72 hours postnatal age.27 Haemodynamically significant patent ductus arteriosus (PDA) is defined as PDA requiring pharmacological treatment or surgical ligation.28
Table 1.
Chinese Neonatal Network data variables
| Categories | Variables |
| Demographic | Birth weight gestation, sex, ethnicity, small for gestational age. |
| Obstetric/perinatal risks | Maternal age education, smoking, drugs in pregnancy, antenatal care, antenatal complications, hypertension preterm labour, preterm rupture of membranes, maternal diabetes, group B Strep chorioamnionitis, multiple gestation, antenatal steroids, delivery mode presentation, drug treatment, delivery complications, Apgar score, resuscitation. |
| NICU care | Admission illness severity (SNAP, TRIPS), fluids, nutrition, assisted respiration, antibiotics, drug therapy, intravenous lines, catheters procedures, surgery. |
| Outcomes |
|
| Resource use | Duration of hospitalisation, duration of ventilation, duration of oxygen therapy, use of central catheters, parenteral nutrition, surgery. |
BPD, bronchopulmonary dysplasia; NEC, necrotising enterocolitis; NI, nosocomial infection; NICU, neonatal intensive care unit; ROP, retinopathy of prematurity; SNAP, score for neonatal acute physiology; SNI, severe neurological injury; TRIPS, transport risk index of physiologic stability.
Objective 2: Assess and monitor major outcomes of very preterm infants and their risk determinants, and inter-institutional variations in care and outcomes.
Infant characteristics and outcomes will be summarised as counts and percentages for categorical variables and using the means and SD or the medians and IQRs for continuous variables. Subgroup analysis and inter-institutional comparisons will use the χ2 test for categorical variables and analysis of variance F-test or Mood’s median test for continuous variables. Multivariable logistic regression models with adjustment for confounders will be used to assess risk factors associated with outcomes.
To study inter-institutional variations in primary and secondary outcomes, the ratios and associated 95% CIs will be calculated, and ‘caterpillar plots’ will be used to graphically display differences between participating sites. The ‘indirect standardisation’ approach will be used to compute the standardised outcome ratios in order to adjust for multiple baseline characteristics. Each NICU can compare its own observed rate with the average CHNN rate to identify the relative level of its own unit. For each outcome, the expected number of events will be computed as the sum of predicted probabilities from a multivariable model (logistic regression or zero inflated negative binomial models based on data distribution) derived using data from all NICUs with adjustment for confounders. NICU standardised outcome ratios will be graphically displayed using ‘funnel’ plots with 95% prediction intervals for comparison between NICUs. Pair-wise comparisons between NICUs will be performed using multivariate regression models adjusted for confounders. The generalised estimating equation models will be used for adjusting analyses for infant cluster within the CHNN. Hierarchical random-effects regression models will be used to allow for variation at unit level. In addition, statistical significance will be evaluated by applying a Bonferroni correction to account for multiple pair-wise comparisons.
Associations of clinical practices and other factors with outcomes will be assessed under the general framework of individual patient-level data meta-analyses. Random-effects models with adjustment for confounding variables and important risk factors will be used to assess the association and residual variation due to unknown or unmeasured unit-specific factors. These analyses will identify treatment practices and healthcare services with significant effects on outcomes, which subsequently can be targeted for implementation or improvement. Analyses (two-sided tests) based on 10 000 yearly admissions evaluating the effects of treatments or practices (assuming 50% exposure) on outcomes (incidence 1% to 40%) will be able to detect relative risks of 1.6 to 1.1 with a statistical power of 80% and type I error rate of 5%. This power calculation is based on the anticipated data collection by CHNN during the first year. Safety and outcome improvements will be monitored within each unit using control charts and χ2 tests for differences from baseline in outcome rates. Multivariable logistic regression analyses will pool data from units within CHNN network to assess changes in outcomes over time with adjustment for potential confounders and important risk factors and accounting for clustering.
Objective 3: Implement EPIQ programme to improve quality of care and outcomes.
EPIQ is a multifaceted system for QI that facilitates implementation of practice change.29 EPIQ is based on three features: (a) systematic review of evidence in the published literature, (b) quantitative and qualitative analysis of outcomes and practices to identify practices associated with good or poor outcomes for targeted change and (c) use of a collaborative network of clinicians, researchers and administrators to facilitate mutual learning and implement change. In Shanghai, Cao et al 30 demonstrated in the multicentre EPIQ-REIN study, that over a 1-year period, EPIQ reduced the rate of ventilator associated pneumonia from 48.84 per 1000 ventilator-days to 18.50 per 1000 ventilator-days (p<0.001), and rates of central line associated blood stream infection from 16.7 per 1000 central line days to 5.2 per 1000 central line days (p<0.01).
After the first year of data collection (baseline data), we will implement EPIQ in all CHNN NICUs and compare the results pre and post EPIQ implementation. The EPIQ programme has well established published protocols for training of personnel, coordination, literature review, risk analysis, identification of target outcomes, implementation and facilitation of practice changes, monitoring and surveillance, communication and safety assurance; and CHNN has well trained personnel, teams and organisation from the previous EPIQ-REIN experience.30 A national CHNN infrastructure for collaborative QI will be established. Hospitals will be organised into groups that target specific outcomes. Multidisciplinary groups will examine the literature, conduct systematic reviews and examine hospital specific data in China to develop practice guidelines and bundles of best practices for implementation in CHNN NICUs. Quarterly cycles of practice change will be introduced using PDSA (Plan-Do-Study-Act) cycles of rapid change, and outcomes will be monitored quarterly and results fed back to hospitals. An online teaching and communication web portal will be used to facilitate collaboration between sites, including site visits, discussions and planning. Safety will be monitored. Multivariable logistic regression analyses will pool data from units to assess changes in outcomes over time with adjustment for potential confounders and important practice related risk factors, which will be targeted for practice change.
Objective 4: Conduct collaborative research, including epidemiological, clinical and health services studies and randomised controlled trials.
The CHNN database platform will support additional research initiatives, including epidemiological, clinical and health services and randomised controlled trials. Research projects can be initiated by any CHNN member, approved by the CHNN Scientific Advisory Committee and coordinated through the CHNN Coordinating Centre, where data will also be collected and analysed. In future stages, limited data sets may be released to investigators using a secure electronic portal system.
Statistical analysis
All analyses of cohort data will be performed by a team of statisticians (including PhD and MSc statisticians) at the CHNN coordinating centre, under the leadership of the CHNN Secretary General supervising the centre. For specific research projects, relevant data may be released to the responsible research group for their analysis. Participating sites may perform analysis of their own individual site data.
Ethics and dissemination
Central ethics approval was obtained from the Ethics Committee of the Children’s Hospital of Fudan University (#CHFU 2018–296) and recognised by all participating sites. All participating sites have signed data transfer agreements with the CHNN Coordinating Centre and obtained ethics approval from their affiliated institutions or hospitals to allow sending de-identified data to the CHNN Coordinating Centre in compliance with national, provincial and local hospital regulations for ensuring patient privacy and confidentiality, and are consistent with international standards. All CHNN studies will not take individual patients, and waiver of consent were granted at all sites. The results of this registry will be disseminated by three methods: (1) An annual report will be published and provided to participating hospitals and regional health authorities. (2) Results will be reported at national and international scientific meetings. (3) Results will be published in domestic and international scientific journals.
Discussion
The CHNN collaboration will be the first national network to examine data from a large cohort of preterm infants that is representative of the different regions of China on an ongoing basis. This is important because China has a huge population, with many regional differences in social, economic and healthcare development. Unfortunately, the existing literature regarding preterm infants in China has been mostly limited to single-centre studies or multicentre studies with small numbers of participating sites.8 10 30 31 Findings from this national collaboration will provide a comprehensive picture of outcomes of very preterm infants in Chinese NICUs and establish a strong system for outcomes surveillance and monitoring trends in clinical practices and outcome variations. Data from CHNN will also provide robust estimates for development of clinical practice guidelines and healthcare recommendations that can be generalisable across the country.
QI is a key goal, especially for China where neonatal care is still developing and there is significant room for improvement. The adoption of EPIQ will provide a robust system for QI in Chinese NICUs. EPIQ was pioneered by the Canadian Neonatal Network, where Lee et al 29 first demonstrated 44% reduction in nosocomial infection and 15% reduction in bronchopulmonary dysplasia in a multicentre randomised controlled trial, and EPIQ is now practiced in all Canadian NICUs. In a comparative study of outcomes by the International Network for Evaluation of Outcomes, the implementation of EPIQ nationally was credited with rapidly improving neonatal outcomes in Canada in comparison with other countries.32 33 Subsequent studies in Canada and China provided confirmation of its efficacy.34–36 Since Chinese NICUs have experience using EPIQ, this will facilitate its implementation in Chinese NICUs and provide a proven method for improving quality of care and outcomes in China. However, we recognise that change can be difficult to implement, especially those that require making financial investments, adding staff or breaking cultural traditions. Notwithstanding possible early reluctance to follow these recommendations, the strength of evidence produced from the data, the pragmatic nature of the results and the examples provided by ‘early adopter’ NICUs will be the most persuasive elements of the CHNN collaboration.
The flexibility of the CHNN data platform to accommodate additional studies like randomised controlled trials provides a unique opportunity for Chinese NICUs. China has a large population, which is conducive to conducting multicentre studies rapidly and efficiently. Once the CHNN data platform is fully functional, we anticipate that China will play a leading role in conducting large scale multicentre studies and clinical trials.
Limitations of CHNN are: (1) The data are from a select group of large tertiary NICUs with the highest level of neonatal care in China, and may not be generalisable to the whole population, (2) There may be biases inherent in large cohort databases of this nature, including reporting bias, selection bias and others, (3) Data on parent participation, pain management, skin-to-skin contact care and other important developmental aspects of modern neonatal care are not currently available but will be included in the future, (4) Data on resource use is limited.
In summary, the CHNN collaboration will serve as a strong national platform for collaborative research, outcomes evaluation and QI for VPI in China. The knowledge generated will have potential to benefit infants and families in China and internationally.
Supplementary Material
Acknowledgments
We would like to deeply thank Professor Jianxing Zhu, Professor Dezhi Mu and Professor Xing Feng for their dedicated support of the Chinese Neonatal Network. We would also like to thank Heather McDonald Kinkaid, PhD, from the Maternal-Infant Care Research Centre (MiCare) at Mount Sinai Hospital in Toronto, Canada, for editorial assistance; and Xiang Y. Ye, MSc, from the MiCare, for ongoing statistical supervision. MiCare is supported by the Canadian Institutes of Health Research and the Ontario Ministry of Health and Long-Term Care. We also thank all staffs from participating hospitals of the CHNN.
Footnotes
Collaborators: Group Information: Chairmen: Shoo K. Lee, MBBS, Mount Sinai Hospital, University of Toronto; Chao Chen, MD, Children’s Hospital of Fudan University. Vice-Chairmen: Lizhong Du, MD, Children's Hospital of Zhejiang University School of Medicine; Wenhao Zhou, Children’s Hospital of Fudan University. Site investigators of the Chinese Neonatal Network: Children's Hospital of Fudan University: Yun Cao, MD; The Third Affiliated Hospital of Zhengzhou University: Falin Xu, MD; Tianjin Obstetrics & Gynecology Hospital: Xiuying Tian, MD; Guangzhou Women and Children’s Medical Center: Huayan Zhang, MD; Children’s Hospital of Shanxi: Yong Ji, MD; Northwest Women's and Children's Hospital: Zhankui Li, MD; Gansu Provincial Maternity and Child Care Hospital: Bin Yi, MD; Shengjing Hospital of China Medical University: Xindong Xue, MD; Shenzhen Maternity and Child Health Care Hospital: Chuanzhong Yang, MD; Quanzhou Women and Children’s Hospital: Dongmei Chen, MD; Suzhou Municipal Hospital affiliated to Nanjing Medical University: Sannan Wang, MD; Guizhou Women and Children’s Hospital/Guiyang Children’s Hospital: Ling Liu, MD; Hunan Children’s Hospital: Xirong Gao, MD; The First Bethune Hospital of Jilin University: Hui Wu, MD; Fujian Maternity and Child Health Hospital,Affiliated Hospital of Fujian Medical University: Changyi Yang, MD; Nanjing Maternity and Child Health Care Hospital: Shuping Han, MD; Qingdao Women and Children’s Hospital: Ruobing Shan, MD; The Affiliated Hospital of Qingdao University: Hong Jiang, MD; Children’s Hospital of Shanghai: Gang Qiu, MD; Women and Children's Hospital of Guangxi Zhuang Autonomous Region: Xinnian Pan, MD; Children’s Hospital of Nanjing Medical University: Youyan Zhao, MD; Henan Children’s Hospital: Wenqing Kang, MD; The First Affiliated Hospital of Xinjiang Medical University: Mingxia Li, MD; Foshan Women and Children’s Hospital: Xuqiang Ye, MD; The First Affiliated Hospital of Anhui Medical University: Lili Wang, MD; Shanghai First Maternity and Infant Hospital: Jiangqin Liu MD; Yuying Children's Hospital Affiliated to Wenzhou Medical University: Zhenlang Lin, MD; Children’s Hospital of Chongqing Medical University: Yuan Shi, MD; The First Affiliated Hospital of Zhengzhou University: Xiuyong Cheng, MD; The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China: Jiahua Pan, MD; Shaanxi Provincial People’s Hospital: Qin Zhang, MD; Children's Hospital of Soochow University: Xing Feng, MD; Wuxi Maternity and Child Healthcare Hospital: Qin Zhou, MD; People's Hospital of Xinjiang Uygur Autonomous Region: Long Li, MD; The Second Xiangya Hospital of Central South University: Pingyang Chen, MD; Qilu Children’s Hospital of Shandong University: Xiaoying Li, MD; Hainan Women and Children’s Hospital: Ling Yang, MD; Xiamen Children’s Hospital: Deyi Zhuang, MD; Xinhua Hospital affiliated to Shanghai Jiao Tong University School of Medicine: Yongjun Zhang, MD; Shanghai Children‘s Medical Center,Shanghai Jiao Tong University School of Medicine: Jianhua Sun, MD; Shenzhen Children’s Hospital: Jinxing Feng, MD; Children's Hospital Affiliated to Capital Institute of Pediatrics: Li Li, MD; Women and Children’s Hospital, School of Medicine, Xiamen university: Xinzhu Lin, MD; General Hospital of Ningxia Medical University: Yinping Qiu, MD; First Affiliated Hospital of Kunming Medical University: Kun Liang, MD; Hebei Provincial Children's Hospital: Li Ma, MD; Jiangxi Provincial Children’s Hospital: Liping Chen, MD; Fuzhou Children’s Hospital of Fujian Province: Liyan Zhang, MD; First Affiliated Hospital of Xian Jiao Tong University: Hongxia Song, MD; Dehong people's Hospital of Yunnan Province: Zhaoqing Yin, MD; Beijing Children's Hospital,Capital Medical University: Mingyan Hei, MD; Zhuhai Center for Maternal and Child Health Care: Huiwen Huang, MD; Guangdong Women and Children's Hospital: Jie Yang, MD; Dalian Municipal Women and Children’s Medical Center: Dong Li, MD; Peking Union Medical College Hospital: Guofang Ding, MD; Obstetrics & Gynecology Hospital of Fudan University: Jimei Wang, MD; Shenzhen Hospital of Hong Kong University: Qianshen Zhang, MD; Children's Hospital of Zhejiang University School of Medicine: Xiaolu Ma, MD. Advisor: Joseph Ting, MBBS; University of Alberta.
Contributors: Study concept and design: SKL, CC and MH. Drafting of the manuscript: MH and SKL. Critical revision of the manuscript for important intellectual content: MH, XL, YS, YC, JS, HW, SJ, XM, YW, HS, HZ, L-ZD, WZ, SKL and CC. Acquisition of data: MH, XL, YS, YC, JS, HW, SJ, XM and HS. Statistical analysis: YW. Interpretation of data: MH, XL, YS, YC, JS, HW, SJ, XM, HS, HZ, L-ZD, WZ, SKL and CC. Study supervision: MH, CC, SKL, WZ and L-ZD.
Funding: This work was jointly supported by Children’s Hospital of Fudan University, China, and the Maternal-Infant Care Research Centre at Mount Sinai Hospital, Toronto, Canada, which is supported by the Canadian Institutes of Health Research (CTP 87518) and the Ontario Ministry of Health and Long-Term Care. The funding agencies played no role in the study design; the collection, analysis and interpretation of data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographical or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
on behalf of the Chinese Neonatal Network (CHNN)*:
Shoo K. Lee, Chao Chen, Lizhong Du, Wenhao Zhou, Yun Cao, Falin Xu, Xiuying Tian, Huayan Zhang, Yong Ji, Zhankui Li, Bin Yi, Xindong Xue, Chuanzhong Yang, Dongmei Chen, Sannan Wang, Ling Liu, Xirong Gao, Hui Wu, Changyi Yang, Shuping Han, Ruobing Shan, Hong Jiang, Gang Qiu, Xinnian Pan, Youyan Zhao, Wenqing Kang, Mingxia Li, Xuqiang Ye, Lili Wang, Jiangqin Liu, Zhenlang Lin, Yuan Shi, Xiuyong Cheng, Jiahua Pan, Qin Zhang, Xing Feng, Qin Zhou, Long Li, Pingyang Chen, Xiaoying Li, Ling Yang, Deyi Zhuang, Yongjun Zhang, Jianhua Sun, Jinxing Feng, Li Li, Xinzhu Lin, Yinping Qiu, Kun Liang, Li Ma, Liping Chen, Liyan Zhang, Hongxia Song, Zhaoqing Yin, Mingyan Hei, Huiwen Huang, Jie Yang, Dong Li, Guofang Ding, Jimei Wang, Qianshen Zhang, Xiaolu Ma, and Joseph Ting
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. da Cunha Durães MI, Flor-De-Lima F, Rocha G. Morbidity and mortality of preterm infants less than 26 weeks of gestational age. Minerva Pediatr 2019;7120:12. 10.23736/S0026-4946.16.04609-0 [DOI] [PubMed] [Google Scholar]
- 2. Huff K, Rose RS, Engle WA. Late preterm infants: morbidities, mortality, and management recommendations. Pediatr Clin North Am 2019;66:387. 10.1016/j.pcl.2018.12.008 [DOI] [PubMed] [Google Scholar]
- 3. Richter LL, Ting J, Muraca GM, et al. Temporal trends in neonatal mortality and morbidity following spontaneous and clinician-initiated preterm birth in Washington state, USA: a population-based study. BMJ Open 2019;9:e023004. 10.1136/bmjopen-2018-023004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Bureau of statistics population. Population. China statistic yeat book 2019. http://www.stats.gov.cn/tjsj/ndsj/2019/indexch.htm [Google Scholar]
- 5. Chen C, Zhang JW, Xia HW, et al. Preterm birth in China between 2015 and 2016. Am J Public Health 2019;109:1597–604. 10.2105/AJPH.2019.305287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li H-T, Xue M, Hellerstein S, et al. Association of China's universal two child policy with changes in births and birth related health factors: national, descriptive comparative study. BMJ 2019;366:l4680. 10.1136/bmj.l4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jiang S, Yan W, Li S, et al. Mortality and Morbidity in Infants <34 Weeks' Gestation in 25 NICUs in China: A Prospective Cohort Study. Front Pediatr 2020;8:33. 10.3389/fped.2020.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. SJ L, Yan SY, Zhou Y. Ventilator-Associated pneumonia among premature infants. Chin J Pediatr 2017;55:182–7. [DOI] [PubMed] [Google Scholar]
- 9. Sun H, Han S, Cheng R, et al. Testing the feasibility and safety of feeding preterm infants fresh mother's own milk in the NICU: a pilot study. Sci Rep 2019;9:941–9. 10.1038/s41598-018-37111-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang S, Yang Z, Shan R, et al. Neonatal outcomes following culture-negative late-onset sepsis among preterm infants. Pediatr Infect Dis J 2020;39:232–8. 10.1097/INF.0000000000002558 [DOI] [PubMed] [Google Scholar]
- 11. Hei M, Gao X, Gao X, et al. Is family integrated care in neonatal intensive care units feasible and good for preterm infants in China: study protocol for a cluster randomized controlled trial. Trials 2016;17:22. 10.1186/s13063-015-1152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiangsu Multicenter Study Collaborative Group for Breastmilk Feeding in Neonatal Intensive Care Units. Clinical characteristics and risk factors of very low birth weight infants with bronchopulmonary dysplasia: multicenter retrospective analysis [in Chinese]. Chin J Pediatr 2019;57:33–9. [DOI] [PubMed] [Google Scholar]
- 13. The Subspecialty Group of Neonatology,, the Society of Pediatrics,, Chinese Medical Association . the Editorial Board, Chinese Journal of Pediatrics. Expert consensus on clinical management of premature infants with Bronchopulmonary dysplasia [Chinese]. Chin J Pediatr 2020;58:358–65. [DOI] [PubMed] [Google Scholar]
- 14. Experts Working Group on Consensus on the Use of Human Milk Fortifier in Preterm Infants; Editorial Board Committee of Chinese Journal of Neonatology. Consensus o the use of human milk fortifier for preterm infants [in Chinese]. Chin J Neonatol 2019;34:321–8. [Google Scholar]
- 15. Rochow N, Landau-Crangle E, Lee S, et al. Quality indicators but not admission volumes of neonatal intensive care units are effective in reducing mortality rates of preterm infants. PLoS One 2016;11:e0161030. 10.1371/journal.pone.0161030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee SK, McMillan DD, Ohlsson A, et al. Variations in practice and outcomes in the Canadian NICU network: 1996-1997. Pediatrics 2000;106:1070–9. 10.1542/peds.106.5.1070 [DOI] [PubMed] [Google Scholar]
- 17., Fellman V, Hellström-Westas L, et al. , EXPRESS Group . One-Year survival of extremely preterm infants after active perinatal care in Sweden. JAMA 2009;301:2225–33. 10.1001/jama.2009.771 [DOI] [PubMed] [Google Scholar]
- 18. Bader D, Kugelman A, Boyko V, et al. Risk factors and estimation tool for death among extremely premature infants: a national study. Pediatrics 2010;125:696–703. 10.1542/peds.2009-1607 [DOI] [PubMed] [Google Scholar]
- 19. Gale C, Santhakumaran S, Nagarajan S, et al. Impact of managed clinical networks on NHS specialist neonatal services in England: population based study. BMJ 2012;344:e2105. 10.1136/bmj.e2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cao Y, Jiang S, Sun J, et al. Assessment of neonatal intensive care unit practices, morbidity, and mortality among very preterm infants in China. JAMA Netw Open 2021;4:e2118904. 10.1001/jamanetworkopen.2021.18904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization . International statistical classification of diseases and related health problems 10th revision (ICD-10), 2010. Available: http://apps.who.int/classifications/icd10/browse/2010/enwebsite
- 22. International Health Terminology Standards Development Organisation . Snomed CT, 2012. Available: http://www.ihtsdo.org/snomed-ct/
- 23. Shennan AT, Dunn MS, Ohlsson A, et al. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988;82:527–32. 10.1542/peds.82.4.527 [DOI] [PubMed] [Google Scholar]
- 24. Papile LA, Burstein J, Burstein R, et al. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 GM. J Pediatr 1978;92:529–34. 10.1016/S0022-3476(78)80282-0 [DOI] [PubMed] [Google Scholar]
- 25. International Committee for the Classification of Retinopathy of Prematurity . The International classification of retinopathy of prematurity revisited. Arch Ophthalmol 2005;123:991–9. 10.1001/archopht.123.7.991 [DOI] [PubMed] [Google Scholar]
- 26. Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. therapeutic decisions based upon clinical staging. Ann Surg 1978;187:1–7. 10.1097/00000658-197801000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Freeman J, Epstein MF, Smith NE, et al. Extra hospital stay and antibiotic usage with nosocomial coagulase-negative staphylococcal bacteremia in two neonatal intensive care unit populations. Am J Dis Child 1990;144:324–9. 10.1001/archpedi.1990.02150270074029 [DOI] [PubMed] [Google Scholar]
- 28. Jasani B, Weisz DE, McNamara PJ. Evidence-Based use of acetaminophen for hemodynamically significant ductus arteriosus in preterm infants. Semin Perinatol 2018;42:243–52. 10.1053/j.semperi.2018.05.007 [DOI] [PubMed] [Google Scholar]
- 29. Lee SK, Aziz K, Singhal N, et al. Improving the quality of care for infants: a cluster randomized controlled trial. CMAJ 2009;181:469–76. 10.1503/cmaj.081727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cao Y, Jiang S, Zhou Q. Introducing evidence-based practice improvement in Chinese neonatal intensive care units. Transl Pediatr 2019;8:257–61. 10.21037/tp.2019.07.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. REIN-EPIQ Study Group . Outcomes of very low birth weight infants at discharge: a multicentered cross-sectional study of 25 tertiary neonatal intensive care units in China [Chinese]. Chin J Peirnat Med 2018;21:294–400. [Google Scholar]
- 32. Shah PS, Lee SK, Lui K. And on behalf of the International network for evaluating outcomes of neonates (iNeo). The International network for evaluating outcomes of very low birth weight, very preterm neonates (iNeo): a protocol for collaborative comparisons of international health services for quality improvement in neonatal care. BMC Pediatrics 2014;14:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lui K, Lee SK, Kusuda S. Trends in neonatal outcomes for very preterm and very low birth weight neonates in 11 countries. J Pediatr 2019;215:e14:32–40. [DOI] [PubMed] [Google Scholar]
- 34. Lee SK, Aziz K, Singhal N, et al. The evidence-based practice for improving quality method has greater impact on improvement of outcomes than dissemination of practice change guidelines and quality improvement training in neonatal intensive care units. Paediatr Child Health 2015;20:e1–9. [PMC free article] [PubMed] [Google Scholar]
- 35. Lee SK, Shah PS, Singhal N, et al. Association of a quality improvement program with neonatal outcomes in extremely preterm infants: a prospective cohort study. CMAJ 2014;186:E485–94. 10.1503/cmaj.140399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cronin CMG, Baker GR, Lee SK, et al. Reflections on knowledge translation in Canadian NICUs using the EPIQ method. Healthc Q 2011;14 Spec No 3:8–16. 10.12927/hcq.2011.22539 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.