Abstract
Objective
To identify predictors of young Chinese patients with ischaemic stroke outcome and recurrence of stroke.
Design
Through considered the risk factors, neuroimaging findings, distribution, vascular stenosis, and the post-stroke treatment of young Chinese patients with ischemic stroke.
Setting
The First Hospital of Jilin University.
Participants
We enrolled 579 patients (median age 45 years, range 15–49, men 81.0%) treated for the first occurrence of ischaemic stroke between January 2014 and December 2017.
Main outcome measures
We assessed stroke outcome based on the modified Rankin Scale (mRS) scores and recurrence of cerebrovascular events at 12 months. Multivariate logistic regression was used to identify the independent predictors of unfavourable outcomes (mRS score ≥2) and recurrence.
Results
We observed stenosis in 295 patients (50.9%)—middle cerebral artery stenosis was the most common (18.1%). Of all 579 included patients, normal or mild, moderate and severe stenoses or occlusions were observed in 51.8%, 6.0% and 42.1% of patients, respectively. Unfavourable outcomes were observed in 91 patients (15.7%), including 10 patients (1.7%) who died. The rate of stroke recurrence was 7.9%. Independent predictors of unfavourable outcomes included a high National Institutes of Health Stroke Scale score (OR 1.151, 95% CI 1.094 to 1.210; p<0.001) and severe vascular stenosis or occlusion (OR 1.867, 95% CI 1.181 to 2.952; p=0.008). Predictors of recurrence included age of ≥45 years (OR 2.072, 95% CI 1.066 to 4.025; p=0.032) and atrial fibrillation (OR 15.207, 95% CI 4.273 to 54.120; p<0.001).
Conclusions
Our research shows that when developing prevention strategies for young people, measures that focus on mitigating risk factors should be considered. In addition, vascular screening of young populations is also of vital importance for stroke prevention and poor prognosis prediction.
Keywords: stroke, stroke medicine
Strengths and limitations of this study.
The long-term prognosis and recurrence of ischaemic stroke in young patients in Northeast China were analysed to provide a better reference for clinical situations.
The relationship between the vascular condition and prognosis and recurrence was analysed, and the degree of vascular stenosis was correlated with prognosis, indicating the importance of monitoring vascular stenosis in young people.
The large sample size of the study may provide useful information for improving the prognosis and quality of life of young people.
A limitation of the study is that it is a single-centre retrospective study.
Introduction
Ischaemic stroke is a significant cause of disability and mortality. Approximately 10% of ischaemic stroke cases occur among individuals under the age of 45 years.1 The reduced life expectancy in this population threatens to exacerbate the global burden of disease, as well as the consequent socioeconomic loads imposed on their families and society.2–5
The rates of mortality and burden vary greatly between countries.6 Although the majority of research and efforts related to the prevention of ischaemic stroke have been conducted in high-income countries, low-income and middle-income countries account for more than 85% of the incidents of stroke.7 Furthermore, while data on stroke onset in young patients are limited in general, it is especially lacking in low-income and middle-income countries.8 As stroke aetiologies and risk factors may reportedly vary by age, race and region,9–11 this disparity warrants attention. Recent studies describe the latest developments in the epidemiology, aetiology and treatment of ischaemic stroke in young people. However, studies on secondary prevention are still lacking, and global efforts to assess risk factors, aetiology and prognosis changes are needed.12 13
The findings obtained from research on ischaemic stroke in young patients are inconsistent. Studies have alternately reported male and female predominance.14–18 Furthermore, while several predictors of poor clinical outcomes and recurrence following stroke onset in young patients have been proposed, they remain unclear. A recent study showed that a nomogram composed of hypertension, diabetes, smoking status, stroke cause and years of education can predict the risk of stroke recurrence after ischaemic stroke in young adults.19 Some studies reported the baseline National Institutes of Health Stroke Scale (NIHSS) as the only predictor of outcome.20 21 One report suggested that age of >35 years, sex and cardiac disease were independent predictors of recurrent stroke, myocardial infarction or death from any cause,22 whereas no predictive value was found in another study.23 Furthermore, the literature lacks comparative data concerning how such predictors vary according to geography or race.
The investigation of the prognosis of young patients who had ischaemic stroke and the predictors of outcome and recurrence should be of paramount importance. The aim of this study was to identify predictors of young Chinese patients with ischaemic stroke outcome and recurrence of stroke; therefore, we investigated demographic data, risk factors, aetiologies, neuroimaging features, distribution, vascular stenosis and the poststroke treatment.
Materials and methods
We retrospectively analysed patients who had ischaemic stroke who were hospitalised in the department of neurology of the First Hospital of Jilin University between January 2014 and December 2017. All patient information came from the stroke database. The inclusion criteria were as follows: (1) age of onset between 15 years and 49 years, (2) discharge diagnosis of ischaemic stroke, (3) patients who had first-ever stroke and (4) undergoing imaging examination. None of the patients died in the hospital. The flowchart of the study is shown in figure 1. We defined ischaemic stroke as a sudden focal neurological deficit with an imaging-confirmed infarct or a neurological deficit that lasted for at least 24 hours with or without corresponding ischaemic lesions identified on brain imaging.
Figure 1.
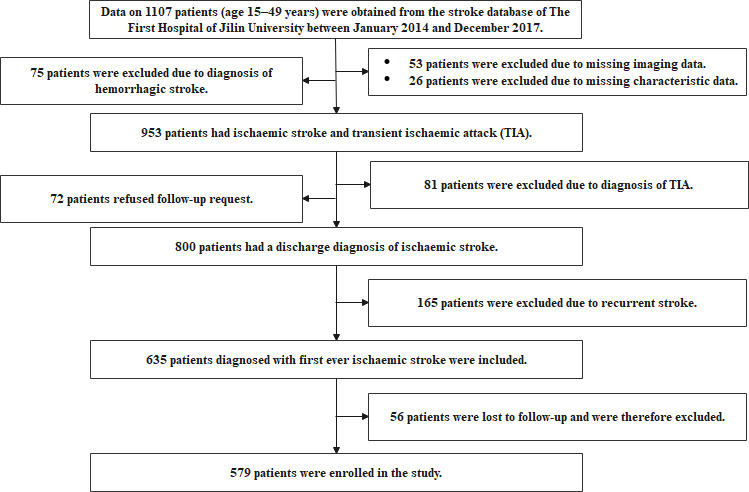
Flowchart of the study.
The baseline information collected from the patients included their (1) sociodemographic characteristics; (2) clinical features, including their level of consciousness assessed according to the Glasgow Coma Scale (GCS) and neurological deficits evaluated according to the NIHSS score at admission; (3) traditional risk factor variables (either self-reported history or diagnosed after stroke), including hypertension, hyperlipidaemia, diabetes, coronary artery disease, smoking (never smoked, ex-smoker or current smoker) and alcohol consumption within 3 months of the stroke event; (4) Oxfordshire Community Stroke Project clinical classification of stroke, including total anterior circulation syndrome, partial anterior circulation syndrome, lacunar syndrome and posterior circulation syndrome; and (5) pathological subtype according to the Trial of Org 10172 in Acute Stroke Treatment criteria, including large-artery atherosclerosis, small-vessel disease, cardioembolism (CE), other determined aetiology and undetermined aetiology. All patients were judged by two neurology specialists and divided into groups. In case of disagreement, the patient was not included in this study. All patients underwent a range of routine blood tests, electrocardiography and brain imaging on admission. We recorded the receipt of antiplatelet, antihypertensive or hypoglycaemic agents, as well as lipid-lowering or homocysteine-lowering therapies and traditional Chinese medicine following the stroke.
All patients underwent MRI with a 1.5 T scanner or CT to confirm and locate the cerebral infarction. Intracranial and extracranial artery stenosis in patients who had ischaemic stroke were assessed with carotid Doppler ultrasonography, transcranial Doppler, magnetic resonance angiography, computed tomographic angiography or digital subtraction angiography. The degree and distribution of stenosis were determined according to the neuroimaging results.
Using the modified Rankin Scale (mRS) score measured at the 12-month follow-up, we dichotomised outcomes into favourable (mRS score 0–1) and unfavourable (mRS score 2–6). The population characteristics of individuals with scores of 0–1, 2, 3–5 and 6 were described. Recurrent cerebral event was defined as a new, persisting (≥24 hour) neurological deficit occurring >24 hours after the index event that was non-attributable to other causes of neurological deterioration24; these data were collected during annual follow-up visits or structured telephone interviews.
All statistical analyses were performed using SPSS V.23.0. Two-sided probability values of p<0.05 were considered statistically significant. Pearson’s χ2 and Fisher’s exact tests were used to compare categorical variables across groups. Means and SD were provided for normally distributed variables and compared across groups using Student’s t-tests. Medians with IQRs were provided for non-normally distributed variables and compared across groups using non-parametric tests. Two non-parametric tests were used to analyse the differences between groups. The Mann-Whitney U test was used to compare two groups, and the Kruskal-Wallis test was used to compare a large number of groups. Logistic regression was used to determine factors associated with unfavourable outcomes and recurrence at 12 months of follow-up. Univariate analyses were followed by multivariate analyses adjusted for age (as a continuous variable), sex and initially significant factors. The multivariate analyses also considered plausible interactions between main effects. Data are presented with ORs and 95% CIs.
Results
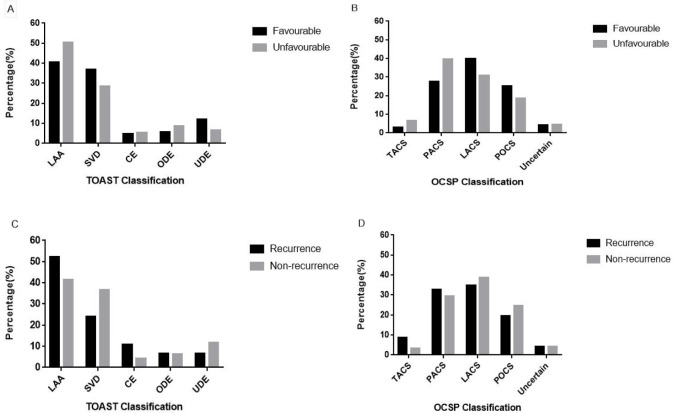
We enrolled 579 patients who had ischaemic stroke (median age 45 years, men 81.0%). The specific flowchart is shown in figure 1. The median initial GCS score was 15 (IQR 13–15), and the median NIHSS score on admission was 3 (IQR 1–6). The median body mass index was 24.77 (IQR 23.44–27.04). We observed at least one stroke risk factor in 552 patients (95.3%) and two or more in 476 (82.2%). We found the following traditional risk factor variables among the included patients: hypertension (n=328, 56.6%), hyperlipidaemia (n=317, 54.7%), diabetes (n=146, 25.2%), hyperhomocysteinaemia (n=157, 27.1%), coronary artery disease (n=30, 5.2%), atrial fibrillation (AF) (n=11, 1.9%), heart failure (n=5, 0.9%), valvular heart disease (n=6, 1.0%), cancer (n=3, 0.5%), alcohol consumption (n=307, 53.0%), current smoking (n=350, 60.4%), a family history of stroke (n=105, 18.1%) and migraine (n=20, 3.5%). The most frequent pathological classifications were large-artery atherosclerosis (42.1%) and small-vessel disease (35.6%); CE (4.8%) was relatively uncommon. The frequencies of partial anterior circulation syndrome, total anterior circulation syndrome, lacunar syndrome and posterior circulation syndrome were 29.5%, 3.6%, 38.5% and 24.2%, respectively; the strokes of 24 patients (4.1%) were of an uncertain clinical classification (figure 2). There were 91 (15.7%) patients with unfavourable clinical outcomes: 81 had mRS scores of 2–5, and 10 (1.7%) died prior to the 12-month follow-up. The distribution of all mRS scores is shown in figure 3. Forty-six patients (7.9%) experienced a recurrent cerebrovascular event. Table 1 compares the clinical data of the patients according to outcome and recurrence at 12 months post stroke. All baseline characteristics and clinical features, including the GCS (p<0.001) and NIHSS (p<0.001) scores on admission, differed significantly between patients with favourable and unfavourable outcomes. Age of ≥45 years (p=0.033) and the presence of AF (p=0.001) differed significantly between the recurrence and non-recurrence groups. Table 2 describes the population characteristics of individuals with a score of 0–1, 2, 3–5 and 6.
Figure 2.
TOASTand OCSP findings according to unfavourable outcomes and recurrence. (A) Comparison of TOAST classification between favourable and unfavourable outcomes. (B) Comparison of OCSP classification between favourable and unfavourable outcomes. (C) Comparison of TOAST classification between recurrence and non-recurrence. (D) Comparison of OCSP classification between recurrence and non-recurrence. CE, cardioembolism; LAA, large-artery atherosclerosis; LACS, lacunar syndrome; OCSP, Oxfordshire Community Stroke Project; PACS, partial anterior circulation syndrome; POCS, posterior circulation syndrome; SVD, small-vessel disease; TACS, total anterior circulation syndrome; TOAST, Trial of Org 10172 in Acute Stroke Treatment; UDE, undetermined aetiology.
Figure 3.
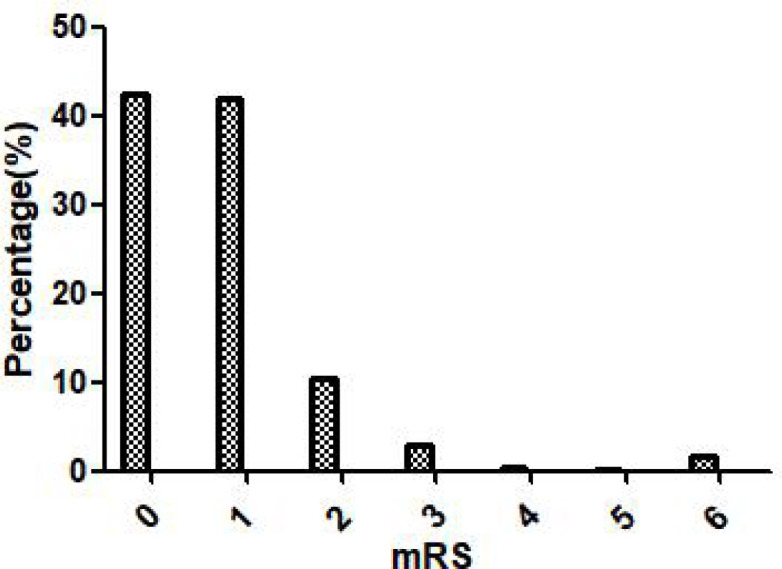
mRS score at the 12-month follow-up. mRS, modified Rankin Scale.
Table 1.
Comparison of the characteristic data about favourable/unfavourable outcomes and recurrence/non-recurrence
| Demographic data and risk factors | N (%) | N (%) | ||||
| mRS | P value | Recurrence | P value | |||
| Favourable | Unfavourable | Yes | No | |||
| Sex, male (%) | 395 (80.9) | 74 (81.3) | 0.933 | 33 (71.7) | 436 (81.8) | 0.095 |
| Age (years), median (IQR) | 45.0 (40.0–47.0) | 45.0 (41.0–47.0) | 0.451 | 46.0 (43.0–47.0) | 45.0 (40.0–47.0) | 0.190 |
| Age ≥45 years (%) | 251 (51.4) | 52 (57.1) | 0.317 | 31 (67.4) | 272 (51.0) | 0.033 |
| BMI (kg/m2), median (IQR) | 24.80 (23.50–26.87) | 24.73 (23.44–27.77) | 0.886 | 24.68 (23.55–26.81) | 24.77 (23.44–27.06) | 0.608 |
| Rural, n (%) | 231 (47.3) | 45 (49.5) | 0.711 | 23 (50.0) | 253 (47.5) | 0.741 |
| Referred to the hospital | 131 (26.8) | 24 (26.4) | 0.926 | 146 (26.6) | 9 (30.0) | 0.104 |
| GCS score, median (IQR) | 15 (13–15) | 13 (13–15) | <0.001 | 15 (13–15) | 15 (13–15) | 0.400 |
| Baseline NIHSS score, median (IQR) | 3 (1–6) | 6 (3–10) | <0.001 | 4 (1–9) | 3 (1–6) | 0.272 |
| Hypertension | 278 (57.0) | 50 (54.9) | 0.721 | 25 (54.3) | 303 (56.8) | 0.743 |
| Hyperlipidaemia | 266 (54.5) | 51 (56.0) | 0.787 | 24 (52.2) | 293 (55.0) | 0.715 |
| Diabetes | 117 (24.0) | 29 (31.9) | 0.111 | 14 (30.4) | 132 (24.8) | 0.396 |
| Hyperhomocysteinaemia | 136 (27.9) | 21 (23.1) | 0.345 | 11 (23.9) | 146 (27.4) | 0.611 |
| CHD | 26 (5.3) | 4 (4.4) | 1.000 | 2 (4.3) | 28 (5.3) | 1.000 |
| AF | 7 (1.4) | 4 (4.4) | 0.078 | 5 (10.9) | 6 (1.1) | 0.001 |
| Cancer | 1 (0.2) | 2 (2.2) | 0.066 | 0 (0) | 3 (0.6) | 1.000 |
| Heart failure | 4 (0.8) | 1 (1.1) | 0.576 | 1 (2.2) | 4 (0.8) | 0.340 |
| VHD | 5 (1.0) | 1 (1.1) | 1.000 | 2 (4.3) | 4 (0.8) | 0.075 |
| Migraine | 18 (3.7) | 2 (2.2) | 0.754 | 2 (4.3) | 18 (3.4) | 0.668 |
| Alcohol | 255 (52.3) | 52 (57.1) | 0.391 | 22 (47.8) | 285 (53.5) | 0.462 |
| Smoke | 0.276 | 0.285 | ||||
| Never | 169 (34.6) | 29 (31.9) | 20 (43.5) | 178 (33.4) | ||
| Ex-smoker | 23 (4.7) | 8 (8.8) | 1 (2.2) | 30 (5.6) | ||
| Current smoker | 296 (60.7) | 54 (59.3) | 25 (54.3) | 325 (61.0) | ||
| Family history of stroke | 88 (18.0) | 17 (18.7) | 0.883 | 9 (19.6) | 96 (18.0) | 0.793 |
AF, atrial fibrillation; BMI, body mass index; CHD, coronary heart disease; GCS, Glasgow Coma Scale; NIHSS, National Institutes of Health Stroke Scale; VHD, valvular heart disease.
Table 2.
Comparison of the characteristic data about mRS with a score of 0–1, 2, 3–5 and 6
| Demographic data and risk factors | N (%) | P value | |||
| mRS | |||||
| 0–1 | 2 | 3–5 | 6 | ||
| Sex, male (%) | 395 (80.9) | 52 (85.2) | 16 (80.0) | 6 (60.0) | 0.303 |
| Age (years), median (IQR) | 45.0 (40.0–47.0) | 45.0 (41.5–47.0) | 46 (39.5–47.5) | 45 (43.0–47.0) | 0.867 |
| Age ≥45 years (%) | 251 (51.4) | 34 (55.7) | 11 (55.0) | 7 (70.0) | 0.317 |
| BMI (kg/m2), median (IQR) | 24.80 (23.50–26.87) | 24.815 (23.66–27.78) | 25.24 (23.55–25.14) | 22.67 (21.26–24.16) | 0.087 |
| Rural, n (%) | 231 (47.3) | 29 (47.5) | 11 (55.0) | 5 (50.0) | 0.935 |
| Referred to the hospital | 131 (26.8) | 15 (24.6) | 6 (30.0) | 3 (30.0) | 0.930 |
| GCS score, median (IQR) | 15 (13–15) | 13 (13–15) | 15 (12–15) | 13.5 (13–15) | 0.004 |
| Baseline NIHSS score, median (IQR) | 3 (1–6) | 6 (3–10) | 8 (3–12) | 3 (1–6) | <0.001 |
| Hypertension | 278 (57.0) | 30 (49.2) | 13 (65.0) | 7 (70.0) | 0.460 |
| Hyperlipidaemia | 266 (54.5) | 35 (57.4) | 12 (60.0) | 4 (40.0) | 0.725 |
| Diabetes | 117 (24.0) | 19 (31.1) | 5 (25.0) | 5 (50.0) | 0.176 |
| Hyperhomocysteinaemia | 136 (27.9) | 14 (23.0) | 4 (20.0) | 3 (30.0) | 0.791 |
| CHD | 26 (5.3) | 2 (3.3) | 1 (5.0) | 1 (10.0) | 0.676 |
| AF | 7 (1.4) | 1 (1.6) | 1 (5.0) | 2 (20.0) | 0.011 |
| Cancer | 1 (0.2) | 0 (0) | 1 (5.0) | 1 (10.0) | 0.010 |
| Heart failure | 4 (0.8) | 0 (0) | 0 (0) | 1 (10.0) | 0.109 |
| VHD | 5 (1.0) | 0 (0) | 0 (0) | 1 (10.0) | 0.143 |
| Migraine | 18 (3.7) | 2 (3.3) | 0 (0) | 0 (0) | 1.000 |
| Alcohol | 255 (52.3) | 38 (62.3) | 10 (50.0) | 4 (40.0) | 0.391 |
| Smoke | 0.397 | ||||
| Never | 169 (34.6) | 20 (32.8) | 7 (35.0) | 2 (20.0) | |
| Ex-smoker | 23 (4.7) | 5 (8.2) | 1 (5.0) | 2 (20.0) | |
| Current smoker | 296 (60.7) | 36 (59.0) | 12 (60.0) | 6 (60.0) | |
| Family history of stroke | 88 (18.0) | 11 (18.0) | 3 (15.0) | 3 (30.0) | 0.752 |
AF, atrial fibrillation; BMI, body mass index; CHD, coronary heart disease; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; VHD, valvular heart disease.
MRI and CT revealed that 284 (49.1%) patients had new multiple brain infarctions followed by infarctions located in the pons (9.5%) and basal ganglia (9.5%). The distribution of ischaemic infarctions did not differ significantly between the groups with and without favourable outcomes or recurrence (p=0.145 and 0.624, respectively; table 2). Duplex sonography revealed that 364 patients had one or more plaques. We found that the stenosis observed in 295 patients (50.9%) was significantly associated with unfavourable outcomes at the 12-month follow-up (p=0.049, table 3). In these patients with stenosis, middle cerebral artery stenosis alone was the most common (18.1%). While non-significant, a diminished proportion of patients with no stenosis and an increased proportion of patients with middle cerebral artery stenosis were observed in the poor outcome and recurrence groups relative to the good outcome and non-recurrence groups (figure 4). The degree of stenosis was categorised according to severity: normal or mild, moderate, or severe stenosis or occlusion. Compared with individuals with a favourable outcome, individuals with an unfavourable outcome had severe stenosis or occlusion significantly (p=0.002) more often (57.1 vs 39.3%) and less often moderate (1.1% vs 7.0%) or normal/mild (41.8% vs 53.7%) stenosis (table 4).
Table 3.
Distribution of ischaemic infarctions according to favourable/unfavourable outcomes and recurrence/non-recurrence
| Distribution of ischaemic infarctions | mRS | P value | Recurrence | P value | ||
| Favourable | Unfavourable | 0.145 | Yes | No | 0.624 | |
| Without new cerebral infarction | 25 (5.1) | 3 (3.3) | 3 (6.5) | 25 (4.7) | ||
| Multiple brain infarctions | 229 (46.9) | 55 (60.4) | 27 (58.7) | 257 (48.2) | ||
| Frontal lobe | 8 (1.6) | 0 (0) | 0 (0) | 8 (1.5) | ||
| Parietal lobe | 6 (1.2) | 0 (0) | 0 (0) | 6 (1.1) | ||
| Occipital lobe | 3 (0.6) | 0 (0) | 0 (0) | 3 (0.6) | ||
| Temporal lobe | 2 (0.4) | 1 (1.1) | 0 (0) | 3 (0.6) | ||
| Medulla | 13 (2.7) | 5 (5.5) | 3 (6.5) | 15 (2.8) | ||
| Basal ganglia | 47 (9.6) | 8 (8.8) | 2 (4.3) | 53 (9.9) | ||
| Corona radiate | 41 (8.4) | 10 (11.0) | 2 (4.3) | 49 (9.2) | ||
| Cerebellum | 38 (7.8) | 2 (2.2) | 1 (2.2) | 39 (7.3) | ||
| Pons | 51 (10.5) | 4 (4.4) | 6 (13.0) | 49(92) | ||
| Thalamus | 22 (4.5) | 3 (3.3) | 2 (4.3) | 23 (4.3) | ||
| Mesencephalon | 3 (0.6) | 0 (0) | 0 (0) | 3 (0.6) | ||
mRS, modified Rankin Scale.
Figure 4.
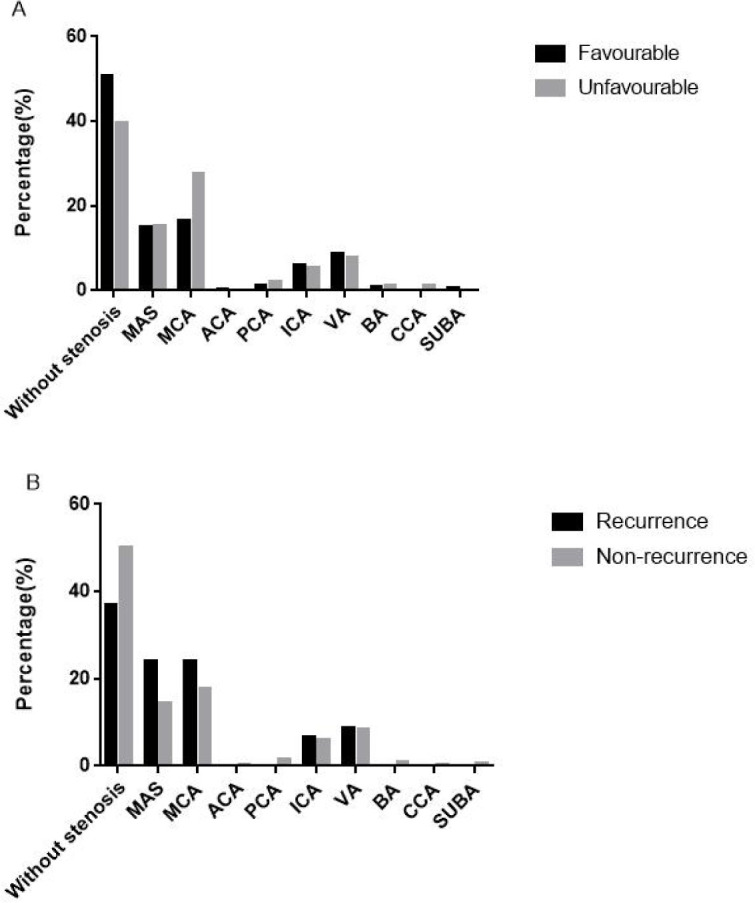
Distribution of artery stenosis according to unfavourable outcomes and recurrence. (A) Distribution of artery stenosis with favourable outcomes and unfavourable outcomes. (B) Distribution of artery stenosis with recurrence and non-recurrence. ACA, anterior cerebral artery; BA, basilar artery; CCA, common carotid artery; ICA, internal carotid artery; MAS, multiple artery stenosis; MCA, middle cerebral artery; PCA, posterior cerebral artery; SUBA, subclavian artery; VA, vertebral artery.
Table 4.
Comparison of different vascular imaging features
| mRS | Recurrence | |||||
| Favourable | Unfavourable | P value | Yes | No | P value | |
| Plaques | 311 (63.7) | 53 (58.2) | 0.320 | 28 (60.9) | 336 (63.0) | 0.770 |
| Stenosis | 240 (49.2) | 55 (60.4) | 0.049 | 29 (63.0) | 266 (49.9) | 0.087 |
| Distribution of stenosis | ||||||
| Intracranial/extracranial | 0.185 | 0.310 | ||||
| Normal | 248 (50.8) | 36 (39.6) | 17 (37.0) | 267 (50.1) | ||
| Intracranial | 180 (36.9) | 40 (44.0) | 22 (47.8) | 198 (37.1) | ||
| Extracranial | 35 (7.2) | 7 (7.7) | 3 (6.5) | 39 (7.3) | ||
| Both | 25 (5.1) | 8 (8.8) | 4 (8.7) | 29 (5.4) | ||
| Anterior/posterior | 0.264 | 0.398 | ||||
| Normal | 248 (50.8) | 36 (39.6) | 17 (37.0) | 267 (50.1) | ||
| Anterior | 153 (31.4) | 36 (39.6) | 19 (41.3) | 170 (31.9) | ||
| Posterior | 70 (14.3) | 15 (16.5) | 8 (17.4) | 77 (14.4) | ||
| Both | 17 (3.5) | 4 (4.4) | 2 (4.3) | 19 (3.6) | ||
| Degree of stenosis | 0.002 | 0.107 | ||||
| Normal or mild | 262 (53.7) | 38 (41.8) | 17 (37.0) | 283 (53.1) | ||
| Moderate | 34 (7.0) | 1 (1.1) | 4 (8.7) | 31 (5.8) | ||
| Severe or occlusion | 192 (39.3) | 52 (57.1) | 25 (54.3) | 219 (41.1) | ||
mRS, modified Rankin Scale.
Patients had the following poststroke therapies: antiplatelet (n=407, 70.3%) and traditional Chinese medicine (n=131, 22.6%). There were 328 patients with hypertension, and of them, 223 patients (70.0%) had antihypertensive therapy; 317 patients had hyperlipidaemia, of which 147 patients (46.4%) had lipid-lowering therapy; 146 of the patients had diabetes, of which 46 patients (31.5%) had hypoglycaemic therapy; 157 patients had hyperhomocysteinaemia, of which 91 patients (58.0%) underwent treatment. Homocysteine-lowering was a significant difference between patients with favourable and unfavourable outcomes (61.8% vs 33.3%, p=0.014). The proportion of other patients who received the relevant poststroke treatments did not differ significantly between the groups (table 5).
Table 5.
Therapy during poststroke
| Treatments | mRS | P value | Recurrence | P value | ||
| Therapy during poststroke | Favourable | Unfavourable | Yes | No | ||
| Antiplatelet | 345 (70.7) | 62 (68.1) | 0.623 | 35 (76.1) | 372 (69.8) | 0.370 |
| Antihypertensive | 193 (69.4) | 30 (60.0) | 0.188 | 15 (60.0) | 208 (68.6) | 0.373 |
| Hypoglycaemic agents | 37 (31.6) | 9 (31.0) | 0.951 | 6 (42.9) | 40 (30.3) | 0.336 |
| Lipid lowering | 122 (45.9) | 25 (49.0) | 0.679 | 11 (45.8) | 136 (46.4) | 0.956 |
| Traditional Chinese medicine | 107 (21.9) | 24 (26.4) | 0.352 | 6 (13.0) | 125 (23.5) | 0.105 |
| Homocysteine-lowering therapy | 84 (61.8) | 7 (33.3) | 0.014 | 5 (45.5) | 86 (58.9) | 0.384 |
mRS, modified Rankin Scale.
Multivariate logistic regression revealed a high NIHSS score on admission (OR 1.151, 95% CI 1.094 to 1.210; p<0.001) and severe stenosis or vascular occlusion (OR 1.867, 95% CI 1.181 to 2.952; p=0.008) to be independent predictors of unfavourable outcomes at 12 months. Age of ≥45 years (OR 2.072, 95% CI 1.066 to 4.025; p=0.032) and AF (OR 15.207, 95% CI 4.273 to 54.120; p<0.001) were found to be independent predictors of recurrence.
Discussion
Reviewing the clinical data of young adults with stroke in the northeast of China, the present study found that severe neurological deficits and occlusion or severe vascular stenosis at presentation predicted unfavourable outcomes, while the age of ≥45 years and AF predicted recurrent stroke.
In our study, the most frequent pathological classification was large-artery atherosclerosis (42.1%), which is similar to the results of previous studies15 24 but different from the results of European and American studies.25 26 A recent Japanese multicentre prospective study showed that the most common causes of ischaemic stroke in young people were small-vessel disease and intracranial arterial dissection.27 These differences in stroke aetiology might be caused by differences in the distribution of risk factors or ethnic groups. Further, our study was retrospective and covered a long period of time; therefore, there were differences in diagnostic tests. Moreover, some patients may not have completed their transesophageal echocardiogram examination, which may also have caused the low percentage of CE.12 Li et al pointed out that the cause of stroke may be related to the higher prevalence of atherosclerosis in northern China.24
Consistent with previous studies,24 27 we found that men were more commonly affected by ischaemic stroke. The greater incidence of ischaemic stroke in men versus women in the present study could be explained by the following: (1) traditional risk factors—men have a significantly higher frequency of hyperlipidaemia than women (58.2% vs 40.0%, p=0.001); (2) differences in lifestyle—smoking and drinking are significantly more prevalent in men than in women (69.3% vs 22.7%, p<0.001; 62.9% vs 10.9%, p<0.001); and (3) men have a significantly higher frequency of hyperhomocysteinaemia than women (31.1% vs 10.0%, p<0.001). In our study, the median age of the patients was 45 years, which is higher than that in a Colombian study14 but similar to those in studies from Saudi Arabia and Finland.28 29 The inconsistent median age of the studies could be explained by the following: (1) differences in ethnicity and geographical location and (2) differences in cut-off age in studies about stroke in young patients,27 30 with lower age limits of 15–18 years and upper age limits of 45–65 years.12 Clinical outcomes were favourable in 84.3% of patients and unfavourable in 15.7%; 1.7% of the patients died within a year of the initial stroke event. A Korean study showed that 81.9% of patients have a favourable outcome, which is similar to our study results.30 The proportion of patients with poor outcomes was slightly lower than the proportion of 19.0% in the USA26 and 20.0% in Western Norway16 and significantly lower than in Israel (84.3%).31 The cumulative mortality at 12 months was lower than the rate of 4.5% in France and 4.7% in Finland.32 33 These results may be associated with a relatively higher incidence (75.1%) of mild stroke (NIHSS score ≤6). Previous studies have shown that, compared with the elderly, young patients who had ischaemic stroke have lower NIHSS scores at the time of their hospital admission. This is similar to the findings in a study involving a northern Chinese population,24 which suggested that young patients were more active in seeking medical attention; thus, they had low NIHSS scores at the time of their hospital admission. Stroke recurrence at 12 months was 7.9%—higher than the previously reported rate of 3.0%; a study in East China reported a recurrence rate of 3.5%.1 34
Although several previous studies have considered the risk factors for and aetiologies and clinical outcomes of ischaemic stroke in young patients, few studies have considered the relationship between infarction, vascular stenosis and prognosis.35 In addition, the factors associated with stroke recurrence in young ischaemic stroke have not been rigorously assessed.24 The presently performed multivariate logistic regression revealed a high NIHSS score and severe stenosis or occlusion to be independent predictors for unfavourable outcomes at 12 months. A study performed in Western Norway found that short-term outcomes were better among patients with infarctions located in the posterior than the anterior circulation.16 A Swiss study of 203 young adults with stroke found that a history of diabetes mellitus, the initial stroke severity and total anterior circulation syndrome were associated with adverse clinical outcomes, while age, sex, stroke risk factors other than diabetes mellitus and stroke aetiology had no predictive value for the clinical outcome at 3 months.1 A study performed in Italy concerning the long-term prognosis of cerebral ischaemia among young adults found that male sex, age of >35 years, stroke at entry and cardiac disease were predictors of poor outcomes.22 Additionally, older women were found to have worse functional outcomes after stroke than their male counterparts. This might be due to their age, a worse health status prior to the occurrence of stroke, the tendency to sustain more severe stroke and the limited applicability of small-purpose designed studies.36–38 In a Korean study, higher NIHSS scores at admission, higher initial blood glucose levels and systemic lupus erythematosus were associated with unfavourable outcomes at 3 months.30 A study in East China showed that NIHSS scores, pneumonia and the female sex were independent predictors of poor outcomes after 12 months.34 Whether age or sex predicts the clinical outcomes of young patients following stroke has remained controversial on account of the majority of previous studies having employed small simple sizes.1 16 22 Our multivariate logistic regression found that neither age nor sex was statistically a significant predictor of outcome. In addition, previous research shows that multivariate associations between baseline characteristics and poor outcomes at 3 months were similar to those at 12 months.39 This finding may be due to the majority of patients (95.0%) achieving neurological recovery within 11 weeks of the stroke event.40
Our study identified the age of ≥45 years and AF as independent predictors of stroke recurrence. The finding that 4.9% of a large sample of patients developed recurrent stroke in a Japanese study implicated hypertension and AF in early ischaemic stroke recurrence.41 A Chinese study on stroke recurrence associated a first-year recurrence rate of 11.2% with AF and smoking.42 Similar to the present investigation, a study performed in northern China showed that large-vessel atherosclerosis and small-vessel occlusion were the most common causes of stroke.24 By contrast, the study also implicated the NIHSS score on admission in the risk of recurrence. A recent study reported that age and diabetes mellitus were long-term risk factors for stroke recurrence.43 In our study, age of ≥45 years was an independent predictor of recurrence. We speculatively attribute this finding to the increased incidence of traditional risk factors, such as hypertension, in this age group. Other studies have found associations between stroke caused by CE and an increased risk of recurrence.44 45 We found that CE accounted for 10.9% of cases of recurrence and only 4.3% of cases without recurrence.
Limitations
First, this was a single-centre retrospective study. Only patients from the First Hospital of Jilin University were selected. The sample size was not sufficiently large. All young patients who had ischaemic stroke in the northeast were not included, which may have led to biased results. Second, this study depended on previously collected patient reports; hence, it was limited by incomplete data. In addition, as a single-centre study, the applicability and validity of our results should be confirmed by larger multicentre studies.
Conclusions
This study has revealed predictors of long-term unfavourable outcomes and the recurrence of stroke in young adults. The NIHSS score on admission and severe stenosis or occlusion were associated with unfavourable long-term outcomes, and AF and age of ≥45 years with an increased risk of recurrence. Our data may provide useful information for refining the treatment of young patients who had ischaemic stroke and thus improve their prognoses and quality of life. Measures that are focused on mitigating risk factors should be considered when planning preventive strategies for young adults. In addition, vascular screening of young populations is also of vital importance for stroke prevention and poor prognosis prediction.
Supplementary Material
Acknowledgments
We are very grateful to members of the public who generously gave their time to feedback on the outline of this study, and we also thank the patient and the patient’s family for their cooperation and contributions to this study.
Footnotes
Contributors: YX and LW conceived and designed the manuscript. YL, LL and YC collected the patient information. YD and CL analysed the data. JG and LW wrote the paper and revised the manuscript. LW is the study guarantor. All authors approved the final version for publication.
Funding: This article was supported by the Natural Science Foundation of China (grant number 81971620) and the the Natural Science Foundation of Jilin Province.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and conforms with the World Medical Association's Declaration of Helsinki, and was approved by the ethics committee of the First Hospital of Jilin University (approval number 2018-397). As this was a retrospective study and the study results would not affect patient care, the ethics committee waived the need for informed consent.
References
- 1.Nedeltchev K, der Maur TA, Georgiadis D, et al. Ischaemic stroke in young adults: predictors of outcome and recurrence. J Neurol Neurosurg Psychiatry 2005;76:191–5. 10.1136/jnnp.2004.040543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giang KW, Björck L, Nielsen S, et al. Twenty-Year trends in long-term mortality risk in 17,149 survivors of ischemic stroke less than 55 years of age. Stroke 2013;44:3338–43. 10.1161/STROKEAHA.113.002936 [DOI] [PubMed] [Google Scholar]
- 3.Naess H, Waje-Andreassen U, Thomassen L, et al. Health-Related quality of life among young adults with ischemic stroke on long-term follow-up. Stroke 2006;37:1232–6. 10.1161/01.STR.0000217652.42273.02 [DOI] [PubMed] [Google Scholar]
- 4.Huang Y, Liao X, Song Z, et al. Evaluation of the influence of etiological factors on the economic burden of ischemic stroke in younger patients in China using the trial of ORG 10172 in acute stroke treatment (TOAST) classification. Med Sci Monit 2019;25:637–42. 10.12659/MSM.913977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naess H, Nyland H. Poststroke fatigue and depression are related to mortality in young adults: a cohort study. BMJ Open 2013;3. 10.1136/bmjopen-2012-002404. [Epub ahead of print: 01 Mar 2013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston SC, Mendis S, Mathers CD. Global variation in stroke burden and mortality: estimates from monitoring, surveillance, and modelling. Lancet Neurol 2009;8:345–54. 10.1016/S1474-4422(09)70023-7 [DOI] [PubMed] [Google Scholar]
- 7.Lopez AD, Mathers CD, Ezzati M, et al. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006;367:1747–57. 10.1016/S0140-6736(06)68770-9 [DOI] [PubMed] [Google Scholar]
- 8.Singhal AB, Biller J, Elkind MS, et al. Recognition and management of stroke in young adults and adolescents. Neurology 2013;81:1089–97. 10.1212/WNL.0b013e3182a4a451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pezzini A, Grassi M, Lodigiani C, et al. Predictors of long-term recurrent vascular events after ischemic stroke at young age: the Italian project on stroke in young adults. Circulation 2014;129:1668–76. 10.1161/CIRCULATIONAHA.113.005663 [DOI] [PubMed] [Google Scholar]
- 10.Berry JD, Dyer A, Cai X, et al. Lifetime risks of cardiovascular disease. N Engl J Med 2012;366:321–9. 10.1056/NEJMoa1012848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mogensen UB, Olsen TS, Andersen KK, et al. Cause-Specific mortality after stroke: relation to age, sex, stroke severity, and risk factors in a 10-year follow-up study. J Stroke Cerebrovasc Dis 2013;22:e59–65. 10.1016/j.jstrokecerebrovasdis.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 12.Ekker MS, Boot EM, Singhal AB, et al. Epidemiology, aetiology, and management of ischaemic stroke in young adults. Lancet Neurol 2018;17:790–801. 10.1016/S1474-4422(18)30233-3 [DOI] [PubMed] [Google Scholar]
- 13.George MG. Risk factors for ischemic stroke in younger adults: a focused update. Stroke 2020;51:729–35. 10.1161/STROKEAHA.119.024156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguilera-Pena MP, Cardenas-Cruz AF, Baracaldo I, et al. Ischemic stroke in young adults in Bogota, Colombia: a cross-sectional study. Neurol Sci 2021;42:639–45. 10.1007/s10072-020-04584-2 [DOI] [PubMed] [Google Scholar]
- 15.Tang M, Yao M, Zhu Y, et al. Sex differences of ischemic stroke in young adults-A single-center Chinese cohort study. J Stroke Cerebrovasc Dis 2020;29:105087. 10.1016/j.jstrokecerebrovasdis.2020.105087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naess H, Nyland HI, Thomassen L, et al. Incidence and short-term outcome of cerebral infarction in young adults in Western Norway. Stroke 2002;33:2105–8. 10.1161/01.STR.0000023888.43488.10 [DOI] [PubMed] [Google Scholar]
- 17.Rasura M, Spalloni A, Ferrari M, et al. A case series of young stroke in Rome. Eur J Neurol 2006;13:146–52. 10.1111/j.1468-1331.2006.01159.x [DOI] [PubMed] [Google Scholar]
- 18.Barinagarrementeria F, Figueroa T, Huebe J. Cerebral infarctionin people under 40 years I. etiologic analysis of 300 cases prospectively evaluated. Cerebrovasc Dis 1996;6:75–9. [Google Scholar]
- 19.Yuan K, Chen J, Xu P, et al. A nomogram for predicting stroke recurrence among young adults. Stroke 2020;51:1865–7. 10.1161/STROKEAHA.120.029740 [DOI] [PubMed] [Google Scholar]
- 20.Neau JP, Ingrand P, Mouille-Brachet C, et al. Functional recovery and social outcome after cerebral infarction in young adults. Cerebrovasc Dis 1998;8:296–302. 10.1159/000015869 [DOI] [PubMed] [Google Scholar]
- 21.Ferro JM, Crespo M. Prognosis after transient ischemic attack and ischemic stroke in young adults. Stroke 1994;25:1611–6. 10.1161/01.STR.25.8.1611 [DOI] [PubMed] [Google Scholar]
- 22.Marini C, Totaro R, Carolei A. Long-Term prognosis of cerebral ischemia in young adults. National Research Council Study Group on stroke in the young. Stroke 1999;30:2320–5. 10.1161/01.str.30.11.2320 [DOI] [PubMed] [Google Scholar]
- 23.Kong KH, Chan KF, Tan ES. Functional outcome in young strokes. Ann Acad Med Singap 1995;24:172–6. [PubMed] [Google Scholar]
- 24.Li F, Yang L, Yang R, et al. Ischemic stroke in young adults of northern China: characteristics and risk factors for recurrence. Eur Neurol 2017;77:115–22. 10.1159/000455093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Putaala J, Metso AJ, Metso TM, et al. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki young stroke Registry. Stroke 2009;40:1195–203. 10.1161/STROKEAHA.108.529883 [DOI] [PubMed] [Google Scholar]
- 26.Ji R, Schwamm LH, Pervez MA, et al. Ischemic stroke and transient ischemic attack in young adults: risk factors, diagnostic yield, neuroimaging, and thrombolysis. JAMA Neurol 2013;70:51–7. 10.1001/jamaneurol.2013.575 [DOI] [PubMed] [Google Scholar]
- 27.Kono Y, Terasawa Y, Sakai K, et al. Risk factors, etiology, and outcome of ischemic stroke in young adults: a Japanese multicenter prospective study. J Neurol Sci 2020;417:117068. 10.1016/j.jns.2020.117068 [DOI] [PubMed] [Google Scholar]
- 28.Eltemamy MA, Tamayo A, Altarsha E, et al. Cerebrovascular risk profiles in a Saudi Arabian cohort of young stroke patients. Front Neurol 2021;12:736818. 10.3389/fneur.2021.736818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Dongen MME, Aarnio K, Martinez-Majander N, et al. Use of antihypertensive medication after ischemic stroke in young adults and its association with long-term outcome. Ann Med 2019;51:68–77. 10.1080/07853890.2018.1564358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon HS, Kim YS, Lee JM, et al. Causes, risk factors, and clinical outcomes of stroke in Korean young adults: systemic lupus erythematosus is associated with unfavorable outcomes. J Clin Neurol 2020;16:605–11. 10.3988/jcn.2020.16.4.605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rozenthul-Sorokin N, Ronen R, Tamir A, et al. Stroke in the young in Israel. incidence and outcomes. Stroke 1996;27:838–41. 10.1161/01.str.27.5.838 [DOI] [PubMed] [Google Scholar]
- 32.Leys D, Bandu L, Hénon H, et al. Clinical outcome in 287 consecutive young adults (15 to 45 years) with ischemic stroke. Neurology 2002;59:26–33. 10.1212/WNL.59.1.26 [DOI] [PubMed] [Google Scholar]
- 33.Putaala J, Curtze S, Hiltunen S, et al. Causes of death and predictors of 5-year mortality in young adults after first-ever ischemic stroke: the Helsinki young stroke Registry. Stroke 2009;40:2698–703. 10.1161/STROKEAHA.109.554998 [DOI] [PubMed] [Google Scholar]
- 34.Geng C, Lin Y, Tang Q, et al. Sex differences in clinical characteristics and 1-year outcomes of young ischemic stroke patients in East China. Ther Clin Risk Manag 2019;15:33–8. 10.2147/TCRM.S182232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mustanoja S, Putaala J, Haapaniemi E, et al. Multiple brain infarcts in young adults: clues for etiologic diagnosis and prognostic impact. Eur J Neurol 2013;20:216–22. 10.1111/j.1468-1331.2012.03872.x [DOI] [PubMed] [Google Scholar]
- 36.Gall SL, Tran PL, Martin K, et al. Sex differences in long-term outcomes after stroke: functional outcomes, handicap, and quality of life. Stroke 2012;43:1982–7. 10.1161/STROKEAHA.111.632547 [DOI] [PubMed] [Google Scholar]
- 37.Di Carlo A, Lamassa M, Baldereschi M, et al. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke 2003;34:1114–9. 10.1161/01.STR.0000068410.07397.D7 [DOI] [PubMed] [Google Scholar]
- 38.Wade DT. Stroke: rehabilitation and long-term care. Lancet 1992;339:791–3. 10.1016/0140-6736(92)91906-O [DOI] [PubMed] [Google Scholar]
- 39.Wei JW, Heeley EL, Wang J-G, et al. Comparison of recovery patterns and prognostic indicators for ischemic and hemorrhagic stroke in China: the ChinaQUEST (quality evaluation of stroke care and treatment) registry study. Stroke 2010;41:1877–83. 10.1161/STROKEAHA.110.586909 [DOI] [PubMed] [Google Scholar]
- 40.Jørgensen HS, Nakayama H, Raaschou HO, et al. Outcome and time course of recovery in stroke. Part II: time course of recovery. The Copenhagen stroke study. Arch Phys Med Rehabil 1995;76:406–12. 10.1016/S0003-9993(95)80568-0 [DOI] [PubMed] [Google Scholar]
- 41.Toyoda K, Okada Y, Kobayashi S. Early recurrence of ischemic stroke in Japanese patients: the Japan standard stroke Registry study. Cerebrovasc Dis 2007;24:289–95. 10.1159/000105682 [DOI] [PubMed] [Google Scholar]
- 42.Xu G, Liu X, Wu W, et al. Recurrence after ischemic stroke in Chinese patients: impact of uncontrolled modifiable risk factors. Cerebrovasc Dis 2007;23:117–20. 10.1159/000097047 [DOI] [PubMed] [Google Scholar]
- 43.Pennlert J, Eriksson M, Carlberg B, et al. Long-Term risk and predictors of recurrent stroke beyond the acute phase. Stroke 2014;45:1839–41. 10.1161/STROKEAHA.114.005060 [DOI] [PubMed] [Google Scholar]
- 44.Rutten-Jacobs LCA, Maaijwee NAM, Arntz RM, et al. Long-Term risk of recurrent vascular events after young stroke: the future study. Ann Neurol 2013;74:592–601. 10.1002/ana.23953 [DOI] [PubMed] [Google Scholar]
- 45.Toni D, Di Angelantonio E, Di Mascio MT, et al. Types of stroke recurrence in patients with ischemic stroke: a substudy from the profess trial. Int J Stroke 2014;9:873–8. 10.1111/ijs.12150 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.