Abstract
Objectives
The aim of this study was to assess the impact of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) on the quality of life (QoL) of people with ME/CFS and their relative or partner (family member).
Design
A patient-partner, multinational, subject-initiated, cross-sectional online survey.
Setting
International survey using ME/CFS charities, support groups and social media.
Participants
Participants were self-selected with recruitment via social media. Inclusion criteria were aged 18 years or over and reported diagnosis of ME/CFS by health professional. 1418 people with ME/CFS and their 1418 family members from 30 countries participated in the survey. Participants with ME/CFS had a mean age of 45.8 years (range 18–81) and were predominantly women (1214 (85.6%) of 1418). Family members had a mean age of 51.9 years (range 18–87) and were predominantly men (women: 504 (35.5%) of 1418). 991 (70%) family members were partners of the people with ME/CFS.
Interventions
EuroQoL-5 Dimension (EQ-5D-3L), completed by people with ME/CFS, and Family Reported Outcome Measure (FROM-16) questionnaire, completed by family members.
Results
The mean overall health status on a Visual Analogue Scale for people with ME/CFS was 33.8 (0=worst, 100=best). People with ME/CFS were most affected by ability to perform usual activities, pain, mobility, self-care and least impacted by anxiety. For family members, the overall mean FROM-16 score was 17.9 (0=no impact, 32=worst impact), demonstrating a major impact on QoL. Impact on QoL was significantly correlated between the person with ME/CFS and their family member (p<0.0001). Family members were most impacted emotionally by worry, frustration and sadness and personally by family activities, holidays, sex life and finances.
Conclusions
To the best of our knowledge, this is the largest study on the impact of the QoL of persons with ME/CFS and their family members. While open participation surveys are limited by selection bias, this research has revealed a significant worldwide burden of ME/CFS on the QoL of people with ME/CFS and their family members.
Keywords: neurology, social medicine, public health
Strengths and limitations of this study.
International study with patient and public involvement in the study design.
Use of validated quality of life questionnaires for persons with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and their family members.
Patients were only included in the data analysis if they reported a healthcare professional diagnosis of ME/CFS.
However, recruitment was biased towards English-speaking participants
Open participation can lead to sampling bias, limiting the generalisability of these findings.
Introduction
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a chronic, complex, debilitating disease, with existing literature demonstrating a negative impact on health-related quality of life (QoL),1 worse than for many other diseases.2 There is growing international acknowledgement of the impact of ME/CFS on caregivers,3 but there is only a small scale pilot study, using the Family Reported Outcome Measure (FROM-16) which showed that QoL of partners and other family members is greatly impaired, suggesting that ME/CFS impact goes far beyond the affected person.4 There is therefore very little information about the partner/family impact, a gap in ME/CFS knowledge which this study aims to address.
ME/CFS is characterised by multisystem symptoms exacerbated by mild exertion, pain, sleep disruption, orthostatic intolerance, cognitive dysfunction and severe and disabling fatigue not improved by rest.5 ME/CFS occurs globally with a prevalence of up to 0.89%6 though prevalence and impact are underestimated in many countries.7 Often triggered by a virus, the COVID-19 pandemic may increase ME/CFS prevalence8 and there needs to be improved international recognition of chronic post viral disease burden on QoL of sufferers and families.
This study’s aim was to measure the impact of ME/CFS on the QoL of those affected and expand knowledge by conducting a large-scale international study on the impact on QoL of their partners or family members. In addition we aimed to determine correlation of QoL data between the persons with ME/CFS and their family members.
Methods
This was a multinational, subject-initiated, cross-sectional survey to assess the impact of ME/CFS on the lives of patients and their partner or family member using the EuroQoL-5 Dimension (EQ-5D-3L)9 and FROM-1610 questionnaires.
REDCap (Research Electronic Data Capture), a secure web platform11 12 was used for the survey, which was distributed via ME/CFS organisations, websites and social media platforms.
Patient and public involvement
The study was co-designed by patients and clinical researchers. Patients with ME/CFS and their family members were involved at all stages of the study design and actively contributed to identifying the research questions and designing the research. Two of the authors, involved have ME/CFS: one is a clinician and the other a patient representative. Patient partners were directly involved in developing the ethics application and disseminating the surveys via patient charities and online. Burden of intervention and time required to participate in the survey was also assessed.
Questionnaires
EQ-5D-3L
This is a generic instrument measuring an individual’s health status.9 13 It has five dimensions (questions) on mobility, usual activities, self-care, pain and discomfort and anxiety and depression. Three dimensions have three possible responses: no problems, some problems and inability. The responses for the other two dimensions are: no problems, moderate problems and extreme problems. Each response is coded from 1 to 3 and combined as a series of five digits describing the ‘EQ-5D self-reported health state’ or ‘EQ-5D profile’.14 The EQ-5D-3L has 234 possible health states. EQ-5D profiles can be converted to a single number, the ‘EQ-5D value’, ‘1’ represents full health and ‘0’ dead.15 Values <0 indicate a health state worse than death. Overall health status is recorded on a Visual Analogue Scale (VAS), from 0 (worst imaginable health) to 100 (best imaginable health).
FROM-16
This questionnaire measures current QoL impact on a healthy person of having a partner or family member with a health condition.10 It can be completed by anyone over the age of 18 years, concerning the impact of the health condition of a patient of any age. There are 16 questions covering the domains ‘Emotional’ (6 questions) and ‘Personal and social life’ (10 questions). Each question is scored from 0 to 2 (0=not at all, 1=a little, 2=a lot), with a score range of 0–32, ‘0’ meaning no impact and ‘32’ meaning greatest possible impact.
Study design
Multiple survey versions were piloted in November 2020, enabling refining wording for clarity, ensuring ease of use and to identify and resolve technical issues. Feedback confirmed that the questionnaires were easy to answer and most persons with ME/CFS completed the EQ-5D questionnaire within 5 min. The preferred order of questionnaires was identified, with the questionnaire for the person with ME/CFS presented first followed by the family member/partner questionnaire, with the option to return later. Following participant comments, a few minor changes were made, for instance to obviate any confusion resulting from having more than one family member with ME/CFS.
The participant eligibility criteria were being a person with ME/CFS aged 18 or over. Participating family members also had to be aged 18 years or over. Data were only analysed if the person with ME/CFS confirmed diagnosis with a healthcare professional (HCP).
Informed consent was obtained via a tick box question for the participant with ME/CFS. Participants completed basic demographic questions including if they had a diagnosis of ME/CFS from a HCP. To ascertain how many met ME/CFS criteria, participants were asked to select their symptoms from a tick box list adapted from the systemic exertion intolerance disease (SEID) US Institute of Medicine criteria for ME/CFS,16 a clinical diagnostic tool comprising five ME/CFS symptoms. The criteria include technical language, hence a plain English version was devised specifically for this study. ME/CFS is diagnosed if all the first three symptoms and at least one of the last two are present.
Participants answered the EQ-5D-3L and then chose either their partner or another family member to complete the survey second part. The family member/partner could participate in the study immediately, or was invited via email link by the person with ME/CFS. Similar to the person with ME/CFS, a link to the participant information was provided and consent was given via a tick box question. Family members/partners then completed basic demographic data questions and the FROM-16. The recruitment time window was the only limit to the number of participants.
Statistical analysis
Only data from participants with ME/CFS who reported a formal diagnosis by a HCP and their family members were included in the final analysis. Duplicate entries were identified by email address and matching demographics: only the second was analysed. Microsoft Excel, SPSS and GraphPad Prism V.9 were used for data handling and statistical analysis, involving descriptive statistics and non-parametric statistical tests including Spearman rank correlation coefficient.
Results
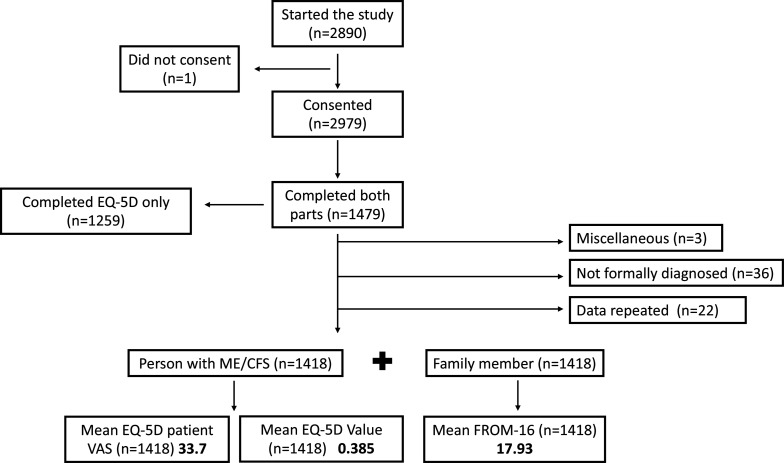
The survey was carried out from 1 December 2020 to 31 March 2021. It was started 2980 times. One participant withdrew consent; therefore, 2979 records were generated. The first part of the survey was completed by 2668 participants, including the EQ-5D-3L. The second part of the survey was completed by 1479 family members/partners. Only the 1479 records that were fully completed by both patient and family members/partners were analysed further. Twenty-five records were excluded either because they were duplicates (n=22) or for other reasons (n=3). From the remaining 1454 records a further 36 were excluded for not having a formal diagnosis of ME/CFS from a HCP. The final analysis included 1418 survey responses representing 2836 participants (persons with ME/CFS and their family member/partner) (figure 1).
Figure 1.
Participant numbers. Flow diagram demonstrating the basis for participant inclusion/exclusion from the analysis of the study. Following this protocol, 1418 patients with ME/CFS and their corresponding family members were identified for analysis. EQ-5D, EuroQoL 5 dimensions; FROM-16, Family Reported Outcome Measure; ME/CFS, myalgic encephalomyelitis/chronic fatigue syndrome; VAS, Visual Analogue Scale.
Demographic profile of participants
Table 1 shows the participant demographics. Persons with ME/CFS and their family members worldwide participated in the study however most responses came from the UK (58.8%) and other English-speaking countries, including the USA (11.2%), Canada (5%) and Australia (5.8%) (table 2). The average time since diagnosis of ME/CFS was 13.9 years, (median 11) with 15 patients diagnosed for 1 year and 8 patients for >50 years.
Table 1.
Participant demographic characteristics
| Person with ME/CFS | Family member | |
| Number | 1418 | 1418 |
| Time since diagnosis | 13.9 years | n/a |
| Mean age | 45.8 (18–81) | 51.9 (18–87) |
| Female | 1214 (85.6%) | 504 (35.5%) |
| Male | 196 (13.8%) | 902 (63.6%) |
| Other | 8 (<1%) | 12 (<1%) |
| Separate household | 149 (10.5%) | |
| Lives alone | 158 (11.1%) | |
| Relationship of person with ME/CFS to family member | ||
| Partner/spouse | 991 (69.9%) | |
| Parent | 76 (5.4%) | |
| Sibling | 288 (20.3%) | |
| Child | 28 (1.9%) | |
| Other | 35 (2.5%) | |
| >1 family member has ME/CFS | 160 (11%) | |
| Family member has ME/CFS | 49 (3%) | |
ME/CFS, myalgic encephalomyelitis/chronic fatigue syndrome.
Table 2.
Countries of residence (participants with myalgic encephalomyelitis/chronic fatigue syndrome)
| Patient country | Number |
| UK | 834 |
| USA | 159 |
| Australia | 82 |
| Canada | 71 |
| Norway | 40 |
| Germany | 34 |
| Netherlands | 32 |
| Sweden | 31 |
| Ireland | 24 |
| New Zealand | 24 |
| Belgium | 14 |
| Italy | 10 |
| Spain | 10 |
| Japan | 9 |
| Denmark | 8 |
| France | 6 |
| South Africa | 6 |
| Finland | 5 |
| Switzerland | 5 |
| Austria | 3 |
| Portugal | 2 |
| China | 1 |
| Croatia | 1 |
| Czech Republic | 1 |
| Ghana | 1 |
| Iceland | 1 |
| Poland | 1 |
| Senegal | 1 |
| Trinidad and Tobago | 1 |
| Uruguay | 1 |
Reflecting the female preponderance for ME/CFS, far more women responded (85.6%) than men, eight did not answer this question. Only 11.1% (n=158) of participants with ME/CFS lived alone. Those that lived with others mainly shared with a life partner or family member, with only 14 people stating they lived with people outside that description. Most family members who participated lived with the person with ME/CFS, with only 149 living in a separate household and one unknown.
One hundred and sixty family members reported having more than one family member with ME/CFS and 49 family members were themselves ME/CFS sufferers. Two persons failed to answer this question.
All persons with ME/CFS completed five questions based on SEID criteria (table 3). Most respondents, already diagnosed by a HCP, also met these diagnostic criteria. However, 93 respondents lacked the symptoms for the SEID ME/CFS diagnosis criteria but stated they had a medical diagnosis, and therefore were included in the analysis. Eighty participants did not have one or more of the three required symptoms for diagnosis, including less able to do normal things (n=14), symptoms worse after physical, mental or emotional activity (n=12), sleep unrefreshing or disturbed (n=54). Twelve participants stated they did not have two of the three criteria, with one stating they experienced none of the five criteria. Of the 36 (2.5%) people without an ME/CFS medical diagnosis not included in the data analysis, most reported ME/CFS diagnosis criteria symptoms. Another chronic health condition was reported by 604 (42.6%) of the participants with ME/CFS.
Table 3.
Participants with myalgic encephalomyelitis/chronic fatigue syndrome and the systemic exertion intolerance disease criteria
| Symptom | Yes | No |
| Less able to do normal things | 1404 (99%) | 14 (1%) |
| Worse after physical, mental or emotional activity | 1406 (99%) | 12 (1%) |
| Sleep unrefreshing/disturbed | 1364 (96.2%) | 54 (3.8%) |
| Brain fog | 1382 (97.5%) | 36 (2.5%) |
| Worse symptoms/dizziness when upright | 1103 (77.8%) | 315 (22.2%) |
EQ-5D health profile of persons with ME/CFS
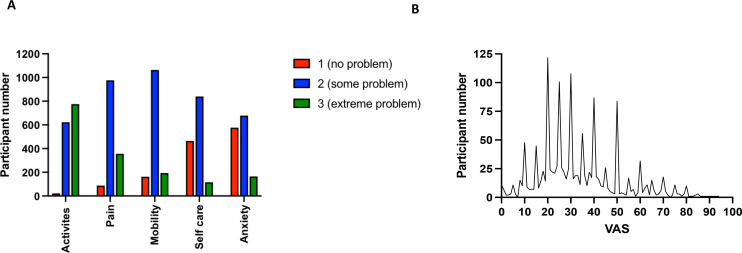
Figure 2 gives the EQ-5D results. Strikingly 98.5% (n=1397) of participants had problems performing their usual activities. Over half (n=775) were unable to perform their usual activities at all. Pain was the next most affected dimension with 93.9% (n=1331) experiencing some (n=976) and extreme (n=355) pain and discomfort. Mobility was affected in 88.6% (n=1256), with participants experiencing some problems (n=1063) with walking or confined to bed (n=193). In terms of self-care, 67.3% (n=954) had some problems or were unable to wash or dress themselves. Anxiety and depression was the least affected dimension, as 40.6% (n=576) participants reported they were not anxious or depressed at all, while 59.4% were either moderately (n=678) or extremely (n=164) anxious or depressed. The average EQ-5D VAS score of patients with ME/CFS was 33.7, (SD 17.5, median 47.5, range 0–94) (figure 2B).
Figure 2.
EQ-5D health profile. The EQ-5D health states of the person with ME/CFS. (A) Patients were asked about the following five dimensions, each representing a different aspect of health; usual activities, pain/discomfort, mobility, self-care and anxiety/depression. Each dimension has three levels (1=no problem, 2=some problem, 3=extreme problem), with the patient indicating their health state by identifying the level representative of their individual condition. (B) A graph showing the range of patient answers as they were asked to rate their health on a Visual Analogue Scale (VAS), with 0 representing worst imaginable health state and 100 best imaginable health state. The average VAS score of patients with ME/CFS was 33.7. EQ-5D, EuroQoL 5 dimensions; ME/CFS, myalgic encephalomyelitis/chronic fatigue syndrome.
Of the possible 234 EQ-5D-3L profiles, participants with ME/CFS expressed 94 unique profiles. Only three participants had a profile 11 111, indicating no problems in any dimension. Similarly, 12 participants had a profile 33 333 indicating extreme problems in all dimensions. Ten profiles accounted for 56.5% of EQ-5D-3L profiles (table 4). The profile 22 321 was the most frequent (n=128) indicating some problems with mobility and self-care, inability to perform usual activities, moderate pain/discomfort and no anxiety/depression. The profiles 22 222 and 22 322 were found in equal measure (n=117) the only difference is that 22 222 means moderate problems in all dimensions whereas 22 322 indicates moderate problems in all dimensions and inability to perform usual activities.
Table 4.
The 10 most frequent EQ-5D health states of participants with myalgic encephalomyelitis/chronic fatigue syndrome, sorted according to EQ-5D value severity
| EQ-5D state | EQ-5D value | Frequency | % frequency | |
| Least severe | 21 221 | 0.659 | 72 | 5.07 |
| 21 222 | 0.596 | 86 | 6.06 | |
| 22 221 | 0.566 | 70 | 4.93 | |
| 22 222 | 0.503 | 117 | 8.25 | |
| 21 321 | 0.394 | 55 | 3.87 | |
| 21 322 | 0.331 | 42 | 2.96 | |
| 22 321 | 0.301 | 128 | 9.02 | |
| 22 322 | 0.238 | 117 | 8.25 | |
| 22 331 | 0.214 | 43 | 3.03 | |
| Most severe | 22 332 | 0.151 | 77 | 5.43 |
EQ-5D, EuroQoL 5 Dimensions.
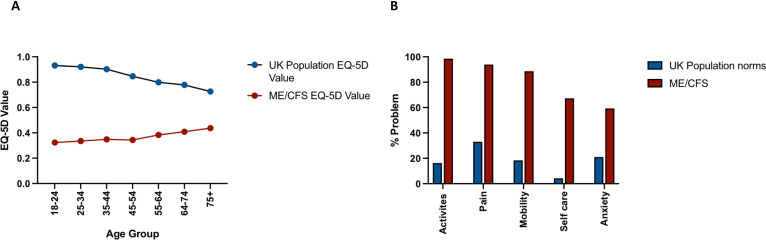
The EQ-5D-3L profile can be converted into a single summary number or EQ-5D value allowing for comparison with the general population. Our results demonstrate strikingly lower EQ-5D values in each age group for persons with ME/CFS compared with the general UK population.17 Similarly, persons with ME/CFS reported much higher percentages of ‘problems’ in each of the EQ-5D dimensions compared with the UK population norm (figure 3).
Figure 3.
EQ-5D value of ME/CFS versus population norm. The EQ-5D results of patients with ME/CFS compared with the UK population norm. (A) The average EQ-5D value of varying age groups for participants with ME/CFS of our study, compared with the UK population. (B) The percentage of participants with ME/CFS who reported a problem (level 2 or 3) for each of the EQ-5D dimensions as compared with the UK population norm. EQ-5D, EuroQoL 5 dimensions; ME/CFS, myalgic encephalomyelitis/chronic fatigue syndrome.
QoL of family members/partners of participants with ME/CFS
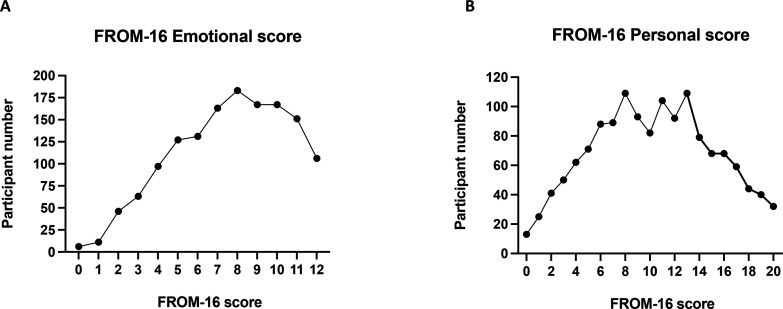
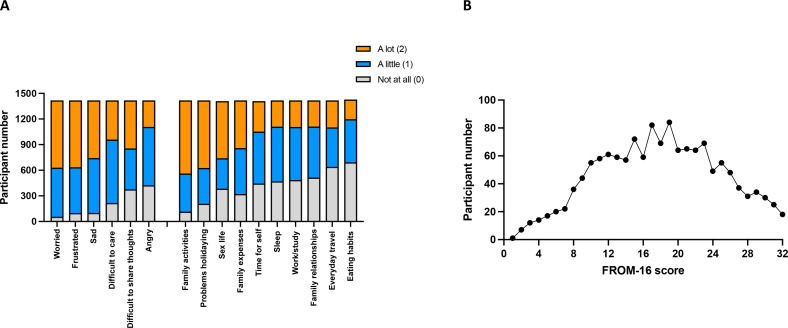
The FROM-16 examined the effects of a person’s ME/CFS on their family member’s emotions and personal/social life. Family members, on average, scored 7.62 (SD=2.81, median=8, max=12,) in the emotional domain and 10.31 (SD=4.9, median=10, max=20) in the personal and social life domain (figure 4). The average overall FROM-16 score (figure 5) was 17.93 out of a total of 32 (SD=6.95, median=18) demonstrating a major impact of ME/CFS on family members.
Figure 4.
Emotional and personal and social domain FROM-16 score. FROM-16 score range for the family members of participants with myalgic encephalomyelitis/chronic fatigue syndrome in (A) the emotional domain (max score 12) and (B) the personal/social domain (max score 20), with higher scores indicating greater impact on the family members quality of life. FROM-16, Family Reported Outcome Measure.
Figure 5.
Overall FROM-16 score. Total FROM-16 scores for the family members of participants with ME/CFS. (A) Family members were asked about different aspects of their lives. Each question had three responses (0=not at all, 1=a little, 2=a lot). Responses have been sorted from the most impact on family member lives to the least, in both the emotional and personal domains. (B) The FROM-16 score range of family members, with 0 representing no impact on family member quality of life and 32 the greatest impact of patients ME/CFS on family members quality of life. The average score in this study was 17.93 out of a possible 32. FROM-16, Family Reported Outcome Measure; ME/CFS, myalgic encephalomyelitis/chronic fatigue syndrome.
ME/CFS had a significant impact on family member’s emotions. Of the 1418 respondents, 96.1% (n=1362) felt worried due to their family member’s ME/CFS, making it the most affected emotion. Frustration and sadness with their family member’s ME/CFS were also highly prevalent with 93% (n=1319) experiencing frustration and 92.9% (n=1317) experiencing sadness. Caring for their family members was found difficult by 84.7% (n=1201), 73.4% (n=1041) found it difficult to talk to someone about their thoughts and 70% (n=994) of respondents were a little or a lot angry because of their family member’s ME/CFS.
In the personal and social domain, the greatest impact was in the area of family activities with 91.8% (n=1302) respondents reporting family activities affected. Similarly, 85.3% (n=1210) experienced problems with holidays. 72.9% (n=1034) stated their sex life was affected and 77.3% (n=1096) felt their finances were impacted in that their family expenses increased. Finding time for themselves was found hard by 68.6% (n=973) of respondents. Sleep, work or study and family relationships were almost equally affected with 66.9% (n=948) reporting a negative impact on their sleep, 65.7% (n=932) a negative impact on their work or study and 63.8% (n=904) found their family relationships with other family members were affected due to their family member’s ME/CFS. Everyday travel and eating habits of family members were the least affected of all the areas, with 54.8% (n=777) indicating a problem with everyday travel and 51.8% (n=735) reporting an effect on their eating habits.
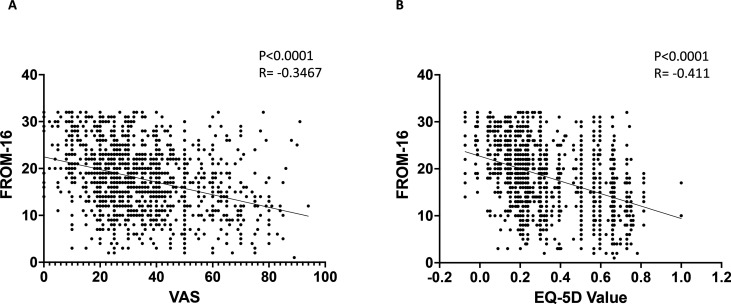
In order to determine the relationship between the person with ME/CFS and their family members QoL, we used Spearmans rank correlation as the data were not normally distributed. We found a significant negative correlation between the total FROM-16 score of family members and the patients VAS score (p<0.0001, R=−0.3467) (figure 6). Furthermore, a similar moderate but significant negative correlation was calculated using the total FROM-16 score and the EQ-5D value of patients (p<0.0001, R=−0.411,) (figure 6), supporting the fact that family member QoL is significantly impacted by a family member’s ME/CFS.
Figure 6.
Correlation of FROM-16 scores with VAS and EQ-5D values. Correlation of total FROM-16 scores with (A) VAS health state of patients and (B) the EQ-5D values of patients. (A) Scatter plot illustrating the relationship between total FROM-16 scores and patient EQ-5D VAS. (B) Scatter plot illustrating the relationship between total FROM-16 scores with the EQ-5D values of patients. The solid lines represent the linear fit of data. Figures shows the p value and R value as analysed by Spearman’s rank correlation test. EQ-5D, EuroQoL 5 dimensions; FROM-16, Family Reported Outcome Measure; ME/CFS, myalgic encephalomyelitis/chronic fatigue syndrome; VAS, Visual Analogue Scale.
The inherent biases in the method of recruitment to this study make it difficult to draw any meaningful comparison between FROM-16 scores from different countries or regions of the world. However, when examined, the mean FROM-16 score from UK was 17.79 (SD=6.99, median=18, n=834), Europe 18 (SD=6.99, median=18, n=228), North America 18.38 (SD=6.92, median=18.5, n=230) and Rest of World 17.96 (SD=6.68, median=18, n=126). The mean EQ-5D value from the different regions were also similar with the UK mean of 0.359 (SD=0.218, median=0.301), Europe mean 0.351 (SD=0.205, median=0.267), North America mean 0.341 (SD=0.201, median=0.264) and Rest of World mean EQ-5D value 0.389 (SD=0.217, median=0.264).
Discussion
To the best of our knowledge this is the largest study on the impact on the QoL of persons with ME/CFS and their family members. Our study confirmed that ME/CFS has a considerable negative impact on QoL. The most common EQ-5D-3L profiles demonstrated that people with ME/CFS experience problems across all domains with similar severity: the problems are not confined or localised to one aspect. None of the 10 most frequent profiles in our survey reported a level 3 ‘a lot’ for anxiety. The average EQ VAS score in our study was 33.8 (SD=17.5, median=47.5). The higher the EQ VAS, the better the QoL. The mean EQ VAS for the representative UK population is 82.75. Our data demonstrate that the QoL of family members of persons with ME/CFS is more impaired than in other conditions.18 19 In our study, in the emotional domain of FROM-16, worry was the most frequently impacted item (96.1%, n=1362), frustration was experienced by 93% (n=1319) and sadness by 92.9% (n=1317).
The study strengths include the patient co-design, with patient involvement at the heart of the research team, wide international dissemination of the survey and the very large numbers of participants. There has been controversy over diagnostic criteria for ME/CFS. Participants with ME/CFS were only included in the data analysis if they reported a HCP diagnosis of ME/CFS. Of these participants, 93.4% also fulfilled the SEID criteria for ME/CFS diagnosis. The four required symptoms of the 2021 ME/CFS National Institute for Health and Care Excellence (NICE) guideline criteria20 are similar to the three required symptoms, and the first of the two additional symptoms, of the SEID diagnostic criteria. This diagnostic confirmation is a major study strength; however, a limitation of the study was that it was not possible to independently verify that a HCP diagnosis of ME/CFS had been made. Other limitations include open participation recruitment bias towards English speaking self-selected people active on social media. This may not be representative of the overall ME/CFS population. Those more severely affected may not have responded because of ME/CFS’s debilitating physical effects. Conversely, they may have been more motivated to take part. Online delivery precluded checking whether assistance was given completing forms or whether the family member or patient allowed others to see their responses. Lack of anonymity within the family may have influenced some responses. Data on ethnic background was not collected.
In contrast to the high level of QoL impact revealed in our study, the EQ-5D-3L profiles from a survey in England17 21 reported that 56.2% of the general public have an EQ-5D profile of 11 111, indicating no problems in any dimension. An EQ-5D profile can be converted into an EQ-5D value, with a value of 1 indicating the best possible health. The mean EQ-5D value for persons with ME/CFS in our study was 0.36 (SD=0.21). In comparison, the mean EQ-5D value for the UK representative sample is 0.86 (SD=0.23).22 Myers and Wilks23 in their ME/CFS study reported a mean EQ-5D value of 0.56 (SD=0.35), representing a QoL impact between the UK representative sample and our participants with ME/CFS. Hvidberg et al2 reported an EQ-5D mean value of 0.47 in Danish patients with ME/CFS, much lower than the representative Danish population mean of 0.85. Their study demonstrated that the EQ-5D value for ME/CFS was the lowest of 20 chronic conditions. Nacul et al,24 using the 36-Item Short Form Survey (SF-36) in a UK population also demonstrated that the QoL of people with ME/CFS was lower than 10 other chronic conditions. Our findings of greatly impaired QoL are consistent with these studies.
The EQ VAS score in our study was in contrast with a higher VAS score of 54.3 (SD=23.3) in the Myers study.23 Brenna et al25 conducted a survey of persons with ME/CFS in Italy, Latvia and the UK. Latvian respondents (n=74) reported the least impaired QoL (VAS mean=57.3, SD=16.3), Italian respondents (n=84) had a mean VAS score of 34.6 (SD=20.8) and the UK respondents (n=440) had a mean score of 31.5 (SD=19.8). A Swedish study by Jonsjö et al26 involving 106 patients with ME/CFS reported a mean EQ-5D value of 0.3 (SD=0.33) and a mean VAS score of 29.8 (SD=15.7).
Most previous studies on the impact on family members of persons with ME/CFS have focused on children with ME/CFS27–29 making comparisons difficult, however in a pilot study, Brittain et al4 compared the impact of ME/CFS on UK patients and on family members, using WHOQoL-BRef and FROM-16. That study demonstrated that poor QoL of the person with ME/CFS is associated with a high impact on the QoL of family members. There was no significant difference (p=0.07) between the mean family impact for the Brittain study (mean FROM-16 score=19.9, n=42) compared with our current international study (mean score=17.9, n=1418). Chantarasap et al18 assessed the impact on the QoL of family members of 248 patients diagnosed with various different cancers including haematological malignancies. The mean FROM-16 score was 11.75 (significantly lower than in our study p<0.0001) with the mean scores in the emotional domain=4.1, and personal and social life domain=7.1. The mean FROM-16 scores in our study indicate that family members of patients with ME/CFS have a much lower QoL. In a recent cross-sectional international study19 measuring the impact of COVID-19 on survivors and their partners or family members, the mean FROM-16 score at 15 (n=735) was also high, but significantly less impacted than in our study (p<0.0001). The mean symptom duration for post COVID-19 symptoms was 12.8 weeks, but it is clear that a subset of long COVID-19 patients matching ME/CFS diagnostic criteria is now emerging and a repeat study of those who remain symptomatic after a year would be interesting.
The median EQ-5D values and FROM-16 scores from the UK, Europe, North America and the Rest of the World are very similar, emphasising the uniform impact experienced by family members across the world. However, it is not possible to be certain of the generalisability of the data due to the recruitment selection bias.
ME/CFS needs to be acknowledged as a serious disease, causing significant impact on health and QoL, not only of the individual but also of their family. Education for healthcare practitioners must be updated to reflect this. It would be possible to screen for these impacts using EQ-5D or FROM-16 in routine clinics. The medical encounter can be vastly improved by acknowledging the impact on family members and providing practical advice and support to both people with ME/CFS and their family members.
Unanswered questions and future research
Not all people with ME/CFS have a family member or partner to complete the FROM-16. Several individuals wrote to the research team explaining their isolation, difficulty maintaining family relationships and/or lack of empathy of family members. Further research is needed to understand the wider impact of ME/CFS on families and on individuals.
FROM-16 score meaning descriptors have not yet been developed, therefore a logical arbitrary assumption has been made of the scale of severity as expressed by the FROM-16 scores. Our large data set may allow further work towards categorising family impact scores and increasing the international validity of FROM-16. A study of this scale provides direction for future qualitative and focus group research to identify why certain aspects of family QoL are impacted more than others and to identify and develop supportive interventions to make the greatest impact. FROM-16 could be used as an outcome measure to assess such novel interventions.
Conclusions
Despite the limitations of selection bias in open participation surveys, this research has revealed the significant worldwide burden of ME/CFS on the QoL of people with ME/CFS and on their family members’ QoL. Recognising this impact has the potential to lead to improvements in the standard of care and compassion we offer to our patients with ME/CFS and families.
Supplementary Material
Acknowledgments
We wish to thank the patients and family members/partners who contributed to this study. We would like to thank Action for M.E. and The M.E. Association who publicised the study in their patient magazines as well as online. We wish to thank patient support organisations approached, many of whom publicised the study, including Forward ME, ME Research UK, ReMEmber, Tymes Trust, BRAME, WAMES, MESiG, MEAction, IcanCME, SolveME, DecodeME, Edmesh, CureME. Deutsche Gesellschaft für ME/CFS Associazione Italiana, Emerge Australia, EUROMENE, European ME Alliance, ME Foreningen, Association Française du Syndrome de Fatigue Chronique, ME félag Íslands, RME, Irish ME Trust, Hope 4 ME & Fibro NI, Norges ME Forening, International Alliance for M.E., Solve CFS/ME, MECFS Foundation South Africa, ACAF, ME/CFS Friendship group in Gloucestershire, Irish ME/CFS Association, Leeds ME network, Lost Voices Stiftung, ME Trust, ME CFS phone support group. ME/cvs Vereniging, ME/CVS-Stichting Nederland, National CFIDS Foundation, New Jersey ME/CFS. Association, Oxfordshire Myalgic Encephalomyelitis Group for Action, Pandora. ReMEmberCFS, Steungroep ME en Arbeidsongeschiktheid, Sussex & Kent ME/CFS Society. The Grace Charity for M.E., Wisconsin Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Association, Fibroamérica, MECFS South Australia, ME CFS and Lyme Association of WA, Far North Coast MECFS Association, ME/CFS/FM Support Association QLD, ACT ME/Chronic Fatigue Syndrome, ME/CFS Australia, Japan ME Association. Društvo za fibromialgijo, ACSFCEM, ME/CFS Schweis, The Rocky Mountain CFS/ME & FM Association. We wish to acknowledge MEpedia which was used as a resource to identify national and international ME/CFS organisations.
Footnotes
Contributors: JV: conception of study, study design, data analysis, writing, reviewing and final approval of manuscript. NM: study design, data analysis, writing, reviewing and final approval of manuscript. RS: data analysis, writing, reviewing and final approval of manuscript. RE: study design, writing, reviewing and final approval of manuscript. AYF: study design, writing, reviewing and final approval of manuscript. The corresponding author JV acts as guarantor and attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: AYF is joint copyright owner of FROM-16, a family member is deputy chair of the NICE ME/CFS guideline committee. JV has been on an Advisory board for Amgen and received honorarium from L’Oreal and support for conference attendance from UCB pharma. NM is Chair of the CMRC education working group for ME/CFS Research Collaborative, member of Forward ME, director of Doctors with ME, witness for NICE education, member of ME education working groups ICANCME (Canada) and the Centre for Solutions (USA), a workshop participant in the James Lind Alliance ME/CFS Priority Setting Partnership and a supporter of Action for ME. NM has received consultancy fees from Learn about ME Project and Ono Pharmaceuticals as well as honorarium from GW4 ME/CFS Carers Project. RE is a member of the Patient Advisory Group to the CMRC, a member of the ME/CFS Friendship group in Gloucestershire, a workshop participant in the James Lind Alliance ME/CFS Priority Setting Partnership, a supporter of Action for ME and is both a patient with ME/CFS and a family member of a patient with ME/CFS.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. The authors agree to share data on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Ethical permission was granted by Cardiff University School of Medicine ethics committee (11 September 2020) (SMREC 20/86). Participants gave informed consent to participate in the study before taking part.
References
- 1.Eaton-Fitch N, Johnston SC, Zalewski P, et al. Health-Related quality of life in patients with myalgic Encephalomyelitis/Chronic fatigue syndrome: an Australian cross-sectional study. Qual Life Res 2020;29:1521–31. 10.1007/s11136-019-02411-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falk Hvidberg M, Brinth LS, Olesen AV, et al. The health-related quality of life for patients with myalgic encephalomyelitis / chronic fatigue syndrome (ME/CFS). PLoS One 2015;10:e0132421. 10.1371/journal.pone.0132421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Multidisciplinary Digital Publishing Institute . Experiences of living with severe chronic fatigue Syndrome/Myalgic encephalomyelitis. healthcare 2021.
- 4.Brittain E, Muirhead N, Finlay AY, et al. Myalgic Encephalomyelitis/Chronic fatigue syndrome (ME/CFS): major impact on lives of both patients and family members. Medicina 2021;57:43. 10.3390/medicina57010043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toogood PL, Clauw DJ, Phadke S, et al. Myalgic Encephalomyelitis/Chronic fatigue syndrome (ME/CFS): where will the drugs come from? Pharmacol Res 2021;165:105465. 10.1016/j.phrs.2021.105465 [DOI] [PubMed] [Google Scholar]
- 6.Lim E-J, Ahn Y-C, Jang E-S, et al. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J Transl Med 2020;18:1–15. 10.1186/s12967-020-02269-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Njoku MGC, Jason LA, Torres-Harding SR. The prevalence of chronic fatigue syndrome in Nigeria. J Health Psychol 2007;12:461–74. 10.1177/1359105307076233 [DOI] [PubMed] [Google Scholar]
- 8.Friedman KJ, Murovska M, Pheby DFH, et al. Our evolving understanding of ME/CFS. Medicina 2021;57:200. 10.3390/medicina57030200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol group. Ann Med 2001;33:337–43. 10.3109/07853890109002087 [DOI] [PubMed] [Google Scholar]
- 10.Golics CJ, Basra MKA, Finlay AY, et al. The development and validation of the Family Reported Outcome Measure (FROM-16)© to assess the impact of disease on the partner or family member. Qual Life Res 2014;23:317–26. 10.1007/s11136-013-0457-y [DOI] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Minor BL, et al. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.EuroQol Group . EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 14.Devlin N, Parkin D, Janssen B. Analysis of EQ-5D profiles. methods for analysing and reporting EQ-5D data. Cham: Springer International Publishing, 2020: 23–49. [PubMed] [Google Scholar]
- 15.Devlin N, Parkin D, Janssen B. Analysis of EQ-5D values. methods for analysing and reporting EQ-5D data. Cham: Springer International Publishing, 2020: 61–86. [PubMed] [Google Scholar]
- 16.CDC . SEID us Institute of medicine criteria for ME/CFS, 2015. Available: https://www.cdc.gov/me-cfs/healthcare-providers/diagnosis/iom-2015-diagnostic-criteria.html [Accessed 07 Jun 2021].
- 17.Szende A, Janssen B, Cabases J. Self-Reported population health: an international perspective based on EQ-5D, 2014. [PubMed] [Google Scholar]
- 18.Chantarasap P, Johns NP, Pairojkul S, et al. Validation of the Thai version of the family reported outcome measure (FROM-16)© to assess the impact of disease on the partner or family members of patients with cancer. Health Qual Life Outcomes 2019;17:32. 10.1186/s12955-019-1091-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah R, Ali FM, Nixon SJ, et al. Measuring the impact of COVID-19 on the quality of life of the survivors, partners and family members: a cross-sectional international online survey. BMJ Open 2021;11:e047680. 10.1136/bmjopen-2020-047680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NICE . Myalgic encephalomyelitis (or encephalopathy)/chronic fatigue syndrome: diagnosis and management [Internet], 2021. Available: https://www.nice.org.uk/guidance/ng206 [Accessed 10 Mar 2022].
- 21.Feng Y, Devlin N, Herdman M. Assessing the health of the general population in England: how do the three- and five-level versions of EQ-5D compare? Health Qual Life Outcomes 2015;13:171–71. 10.1186/s12955-015-0356-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kind P, Hardman G, Macran S. Uk population norms for EQ-5D, York Centr for health economics. University of York, 1999. [Google Scholar]
- 23.Myers C, Wilks D. Comparison of Euroqol EQ-5D and SF-36 in patients with chronic fatigue syndrome. Qual Life Res 1999;8:9–16. 10.1023/A:1026459027453 [DOI] [PubMed] [Google Scholar]
- 24.Nacul LC, Lacerda EM, Campion P, et al. The functional status and well being of people with myalgic Encephalomyelitis/Chronic fatigue syndrome and their carers. BMC Public Health 2011;11:402. 10.1186/1471-2458-11-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenna E, Araja D, Pheby DFH. Comparative survey of people with ME/CFS in Italy, Latvia, and the UK: a report on behalf of the socioeconomics Working group of the European ME/CFS research network (EUROMENE). Medicina 2021;57:300. 10.3390/medicina57030300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonsjö MA, Wicksell RK, Holmström L, et al. Identifying symptom subgroups in patients with ME/CFS – relationships to functioning and quality of life. Fatigue: Biomedicine, Health & Behavior 2017;5:33–42. 10.1080/21641846.2017.1287546 [DOI] [Google Scholar]
- 27.Velleman S, Collin SM, Beasant L, et al. Psychological wellbeing and quality-of-life among siblings of paediatric CFS/ME patients: a mixed-methods study. Clin Child Psychol Psychiatry 2016;21:618–33. 10.1177/1359104515602373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Missen A, Hollingworth W, Eaton N, et al. The financial and psychological impacts on mothers of children with chronic fatigue syndrome (CFS/ME). Child Care Health Dev 2012;38:505–12. 10.1111/j.1365-2214.2011.01298.x [DOI] [PubMed] [Google Scholar]
- 29.Rangel L, Garralda ME, Jeffs J, et al. Family health and characteristics in chronic fatigue syndrome, juvenile rheumatoid arthritis, and emotional disorders of childhood. J Am Acad Child Adolesc Psychiatry 2005;44:150–8. 10.1097/00004583-200502000-00007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. The authors agree to share data on reasonable request.