Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is associated with the formation of renal cysts. We have devised a therapeutic approach, based on reversing the cyst phenotype from secretion to absorption by using VX-809, a modulator of the cystic fibrosis transmembrane regulator trafficking and processing. Our goal is to test VX-809 in RC/RC mice bearing the R3277C human mutation to demonstrate its therapeutic potential. We found that by 5 months of age, RC/RC mice had large cysts and impaired renal function, but when treated with VX-809 between the ages of 3 and 5 months, or 6 and 8 months, the cyst area was reduced in both groups, suggesting that VX-809 had shrunk previously existing cysts. After 2 months of treatment, the cyst size was lower than that of untreated animals of the same age. Our co-localization studies confirmed that cystic fibrosis transmembrane conductance regulator (CFTR) is found predominately at the apical membrane in the untreated animals of each age group, consistent with its role in Cl− secretion; after VX-809 treatment, the basolateral membrane co-localization of CFTR increased ~4-fold, accompanied by a decrease of ~2–3-fold in its apical co-localization, indicating that VX-809 alters the phenotype to favor fluid absorption. Sodium/hydrogen exchanger and epithelial sodium channel, found in normal kidneys at the apical membrane, were almost absent from the cysts. VX-809 restored both levels toward normal. HSP27 is highly expressed in RC/RC mice and lowered toward normal by VX-809. Our demonstration of cyst reduction, improved renal function, and generation of an absorptive phenotype all strongly support the therapeutic potential of VX-809 as a treatment for ADPKD. We show here in an animal model of slowly progressing cyst formation typical of human ADPKD that VX-809 reduces the growth of already established cysts. The magnitude of the effect in the RC/RC mouse model when compared to previous experiments using the same mouse model to evaluate tolvaptan indicates that CFTR modulators warrant further development as a treatment for ADPKD.
Keywords: absorption, CFTR, cysts, polycystic kidney disease, secretion, VX-809
1 ∣. INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is characterized by a slowly progressing enlargement of multiple renal cysts that leads to a decline in renal function and culminates in renal failure in 50% of all patients.1 Two proteins are associated with ADPKD, polycystins (PC)1 and PC2. Malfunction of either PC1 or PC2 leads to cyst formation,2 a hallmark of the disease.
1.1 ∣. The RC/RC mouse model
To develop a mouse model derived directly from a human mutation, Hopp and collaborators3 generated a mouse model bearing an R3277C mutation in PC1. This folding mutant produces a lower-than-normal quantity of mature PC1 protein, as assessed in kidney lysates of 1-month-old mice; this phenotype is consistent with a defect in the processing of the mature protein. The maturation of many folding mutants, such as the now-classic F508-del mutation, is increased when cells are grown at low temperature.4 A similar temperature sensitivity has been observed for the R3277C mutant,3 with higher amounts of mature protein noted when kidney collecting duct cells are grown at 33°C rather than 37°C; the authors of the study estimated that the R3277C mutant reduces the steady-state levels of the mature protein by ~60% when compared to wild-type levels, suggesting that this mouse model is hypomorphic in PC1 function.
As in humans, the pkd1RC/RC mice develop a slowly progressing disease over a year, as compared to pkd1RC/null mice, which show rapidly progressing disease.3 This rapid progression is consistent with the hypomorphic mice produced by Jiang and collaborators,5 which retain ~20% of wt PC1 function. Taken together, these studies point to a dosage effect on the severity of the disease, with the absence of PC1 protein being embryonic lethal, ~20% of the normal level overcoming the embryonic lethality, and 40% of the normal level causing slowly progressing disease.
The pkd1RC/RC mouse model has classical characteristics of ADPKD, including increased cAMP, which is known to fuel cyst expansion,6 and cAMP-dependent increased cellular proliferation, which is well known to occur in ADPKD.7
The pkdRC/RC mouse model displays an interesting progression in terms of the origin of the cysts: In young mice, 90% of the cysts are of proximal tubule origin and only 8% of collecting duct origin, whereas at 3 months, 76% of the cysts originate from the collecting ducts.3 A similar progression in cyst origin from proximal tubule to collecting duct occurs in human ADPKD.8
Because of its predominance of collecting duct cysts, the pkdRC/RC mouse model has been useful for the study of tolvaptan, a vasopressin receptor 2 (V2) antagonist.9 The inner medullary duct plays a role in water conservation (see Ref. [10]) through the action of arginine vasopressin (AVP). AVP binds to the V2 receptor, resulting in a transient increase in [iCa2+] that is detectable over 100 s and is not blocked by the removal of extracellular Ca2+, indicating that AVP stimulates Ca2+ release from the ER.11 AVP also increases cAMP, which in turn increases the trafficking of aquaporin 2 to the plasma membrane.12 Tolvaptan,9 which targets the vasopressin 2 receptor and lowers intracellular cAMP, is now approved to treat ADPKD. Hopp13 and collaborators have treated pkd1rc/rc mice with an AVP analog, desmopressin (dDAVP), and shown a dramatic increase in cyst burden and cAMP levels. They also treated the mice with tolvaptan for 1–6 months and noted a clear reduction in cyst size. This study clearly points out the deleterious effects of increased cAMP in contributing to cyst burden and the benefit of reducing cAMP as a treatment for ADPKD.
1.2 ∣. Role of CFTR in the genesis of ADPKD and as a target for therapies
One of the targets of increased cAMP in cystic kidneys is CFTR, which is well known to play key role in cAMP-driven fluid secretion in the airways and gastrointestinal tract.14,15 It is interesting that patients with cystic fibrosis do not have primary defects in renal function,16 indicating a minor role for CFTR in normal renal physiology. However, CFTR is indeed present in normal kidneys17 and is known to be involved in Cl− secretion into kidney cysts.14,15,18
Given its role in fluid secretion into the cysts, one might predict that inhibitors of CFTR could be used therapeutically in ADPKD to reduce fluid secretion.19-23 This is indeed the case: Verkman and colleagues have found an almost complete reduction of cyst growth in mouse models of ADPKD with two such inhibitors, CFTRinh-172 and Ph-GlyH-101. Also, CFTR inhibitor treatment of pkd−/− mice, another model of ADPKD, has been shown to slow kidney enlargement and cyst expansion and preserve renal function.22
We have demonstrated that cyst growth can be also reduced by VX-809, a corrector that actually restores CFTR function24 and was originally designed to rescue cystic fibrosis patients with the F508 CFTR mutation,25 a folding mutant common among patients with CF.26 We found that in a Pkd1-knockout mouse model, CFTR is predominately located in the endoplasmic reticulum and the apical plasma membrane of cysts, consistent with its role in cAMP-dependent fluid secretion. Surprisingly, treatment of cystic mice with VX-809 significantly increases CFTR’s co-localization with the basolateral membrane. We have proposed that a change in the balance of CFTR away from the luminal membrane and toward the basolateral membrane induced by VX-809 treatment creates an environment that is more favorable for fluid absorption thereby reducing cyst size.
It is not unusual for CFTR to be present and functional at the basolateral membrane. In the sweat duct, CFTR participates in the absorption of Cl− out of the sweat.27 In these ducts, CFTR is located at both the apical and basolateral membranes.28
Bidaud-Meynard and collaborators29 have demonstrated that in airway cells, basolateral CFTR delivery occurs via both biosynthetic (~35%) and endocytic (~65%) pathways and that basolateral channels are retrieved via basolateral-to-apical transcytosis, enhancing CFTR’s apical expression by two-fold and suppressing its degradation. Given that CFTR has been observed in the basolateral membranes of both secretory and absorptive epithelia, the observation that VX-809 increases the localization of CFTR at the basolateral membrane indicates that VX-809 increases the stability of wt CFTR at the basolateral membrane.
1.3 ∣. CFTR correctors
Correctors have been identified to treat cystic fibrosis that act on F508-del CFTR either directly or indirectly.30 One of these correctors has reached the clinic, namely VX-809, which restores the trafficking of F508-del, the major mutant in CF, to the plasma membrane.25 How VX-809 rescues CFTR function is still unclear. Some researchers suggest that correctors act directly on CFTR however, we have shown that VX-809 alters a proteostatic network of proteins31 to rescue CFTR.4 For example, we have shown that VX-809 can reduce heat shock factor, HSP27, levels both in airway cells bearing the F508-del mutation and in cystic epithelial cells where PC1 has been ablated.4,32 We also found that HSP27 and VX-809 operate via a common pathway to alter the trafficking of CFTR.4
The goal of the present study is to advance the therapeutic potential of CFTR correctors in the RC/RC mouse model.
2 ∣. METHODS
2.1 ∣. Reagents
2.1.1 ∣. Antibodies for Western blotting
CFTR (217) (a kind gift of Dr J. Riordan, Department of Biochemistry and Biophysics and Cystic Fibrosis Center of North Carolina); vinculin (sc-73614), HSP27 (sc-13132), NHE3 (Novus, #NBP1-82574), ENaC (Invitrogen #PA1-920A). Horseradish peroxidase conjugated sheep anti mouse secondary (NA934V, 1:10,000) was purchased from Sigma Aldrich.
2.1.2 ∣. Antibodies for confocal studies
CFTR 596 antibody (Obtained from Cystic Fibrosis Center of North Carolina), NHE3 (Novus,#NBP1-82574), ENaC (Invitrogen #PA1-920A), Na+/K+ ATPase (Abcam #Ab7671); WGA-AlexaFluor 488 (Invitrogen #W11261) and secondary goat anti-mouse-Alexa Fluor 594 (Life Technologies #A11032), anti-mouse Alexa Fluor 488 (Life Technologies #A-11001), anti-rabbit IgG-Alexa Fluor 488 (Life Technologies #A-11008), and anti-rabbit IgG-AlexaFluor 594 (Life Technologies #A-11012) were used. Citrate buffer (DAKO#S1699) and Fluoro-gel (EMS#17985-10) were used for antigen retrieval and mounting the slides.
2.2 ∣. Mouse strain and treatment
All animal use complied with the Johns Hopkins Animal Care and Use Committee. Pkd1RC/RC mice on a C57BL/6 background were provided by Peter C. Harris (Mayo Clinic, Rochester, Minnesota). Animals were bred in a single cage with two females and one male. The resulting pubs were used as a cohort, with equal numbers of males and females in each cohort separated at one month of age. When animals in a cohort reached their experimental age as noted in the Figures, they were all analyzed a contemporaneously. Treated animals received VX-809 (30 mg/kg body weight) once every 48 h (i.e., every other day) from 3 to 5, 6 to 8 months of age. Age-matched and no cyst-induced Pkd1fl/fl; Pax8rtTA; TetO-cre mice on a C57BL/6 background (the same background as the RC/RC mice), provided by the Baltimore PKD Center, were used as controls. The latter animals were bred solely for use in this study.
2.3 ∣. Biochemical determinations
Blood was obtained by cardiac puncture from mice under 3% to 5% isoflurane/oxygen. Blood urea nitrogen (BUN), creatinine, aspartate aminotransferase (AST), alanine transaminase (ALT) and albumin (ALB) analyses were performed by spectrophotometric assay using a Vet Ace™ (Alfa Wassermann; West Caldwell, NJ) Automated Clinical Chemistry analyzer at Phenocore, the Phenotyping and Pathology Core laboratory of the Johns Hopkins University.
2.4 ∣. Magnetic resonance imaging acquisition
Mice were imaged with an advanced Bruker 9.4T horizontal-bore spectrometer (Bruker BioSpin, Billerica, MA, USA); the scanner had a 16.4 vertical wide-bore magnet and was equipped with an active radiofrequency (RF) decoupling feature. Prior to scanning, the mice were first anesthetized in 2% isoflurane in a chamber containing an oxygen and air mixture then transferred to the coil's animal holder. Anesthesia was maintained throughout the scan. A control system (Model 1030; SA Instruments Inc., Stony Brook, NY, USA) was used for respiratory monitoring and gating of the signal generated by a balloon sensor. Scout images were first obtained in three planes (axial, coronal, and sagittal) to locate the kidneys and determine the parameters of the volumetric scan. Complete coverage of the kidneys was obtained by using an axial TurboRARE T2-weighted acquisition reconstructed with 0.1-mm in-plane resolution and a 1-mm slice thickness (matrix size = 256 × 256 × Z, with Z chosen to be large enough to cover the full extent of the kidneys). MRI scans were obtained with the same magnification, field of view (FOV), and slice thickness (0.5 mm). The % of the area that was cystic was measured using ImageJ (provided by the NIH).
2.5 ∣. Immunoblotting
Tissue lysates were prepared from flash-frozen kidney samples stored in liquid nitrogen. Samples were centrifuged at 15,000× g for 10 min at 4°C to pellet insoluble material. Supernatants were mixed with 2X Laemmli sample buffer (BioRad#1610737), and protein samples were run on 4% to 15% SDS-PAGE gels (BioRad#4561084) before transfer to a polyvinylidene fluoride membrane (Bio-Rad, #1704157). The membranes were incubated with the indicated primary antibodies overnight and then washed with TBS-Tween 20 buffer. A horseradish peroxidase–conjugated secondary antibody was incubated for 1 h, and then Super Signal West Dura extended-duration ECL Prime (Thermo Fisher #78438) was used for detection with an Amersham Imager 600 (Cytiva). Band intensities were analyzed using IQTL 8.2 software. For experimental proteins, membranes were cut into strips corresponding to the molecular weight of the protein and treated with the corresponding experimental antibodies. For the loading control the cut portion corresponding to the molecular weight of vinculin was stripped with Restore western blot stripping buffer (Thermo scientific #21059), blocked and re-incubated with vinculin primary antibody. Each data point was ratioed to their individual vinculin values.
2.6 ∣. Immunostaining
Kidneys were obtained from pkd1RC/RC and control mice under isoflurane/oxygen anesthesia and fixed in 4% paraformaldehyde for 24 h and then paraffin-embedded and sectioned. Sections were deparaffinized with xylene and rehydrated with a descending ethanol series into phosphate-buffered saline solution, then either stained with hematoxylin and eosin or processed for immunofluorescent staining and confocal laser scanning microscopy. Whole-kidney images were obtained with a Nikon fluorescence dissection microscope equipped with an Olympus OM-D E-M5 Mark II digital camera (Nikon, Tokyo, Japan). The total kidney area and total cystic area were measured using ImageJ (provided by the NIH). Cystic index results are expressed as percentages. For immunofluorescent staining, deparaffinized sections were processed for heat-induced citrate buffer antigen retrieval, washed three times with PBS and blocked. Tissue was then permeabilized with PBS containing 1% BSA and 0.2% Triton X-100. After blocking, the sections were incubated with appropriate primary antibody overnight at 4°C and then reacted with labeled secondary antibody. Following antibody staining and washings, the sections were mounted on glass slides using Fluoro-gel and covered with coverslips. Images were captured using a Zeiss LSM 880 confocal microscope.
2.7 ∣. Calculation of Pearson′s coefficient
To calculate the coefficient,33 three animals for each condition were used for staining. Each dot indicates an individual cyst area from an image. 6–12 cyst areas for each condition were measured using the Zen 2012 software (Zeiss Corp.) by outlining the cyst cells using a cursor. Statistics were determined using the number of experimental measurements.
3 ∣. RESULTS
As mentioned above, in the RC/RC mouse model, ADPKD develops slowly, as it does in humans.3 To study this slow development further, we monitored cyst growth at two different times: 5, and 8 months of age. At each stage, we treated animals with VX-809 every other day beginning months prior to necropsy, i.e., at 3, and 6 months, respectively. The goal was to determine the extent of rescue of the phenotype and whether the age of the animal affected the outcome.
3.1 ∣. 5-month-old mice
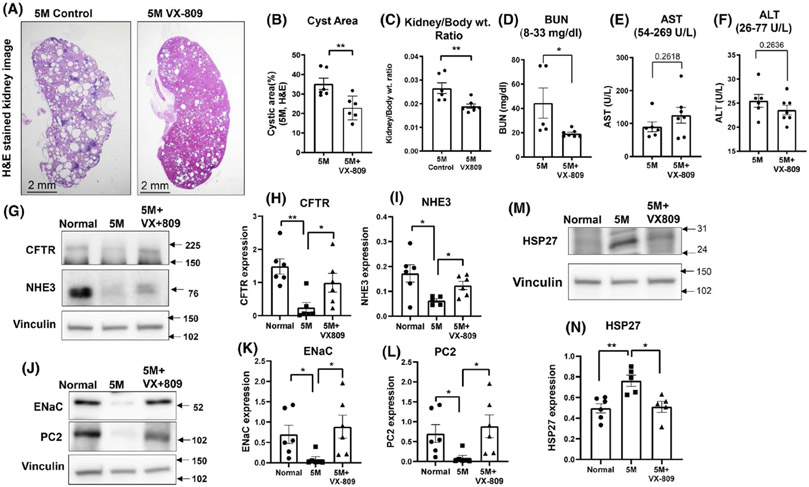
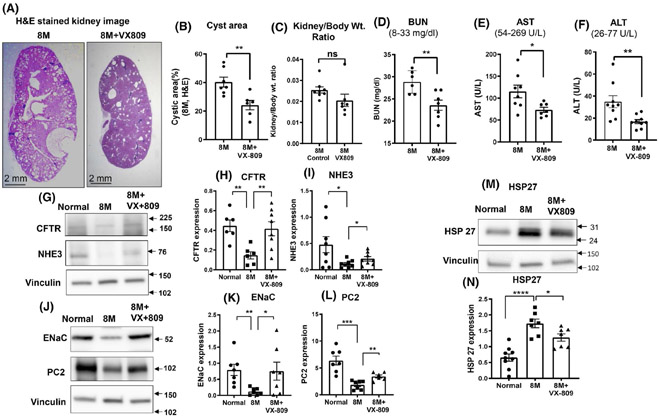
As is strikingly evident by 5 months of age (Figure 1), RC/RC mice develop a robust cystic phenotype, with cysts comprising a large fraction of the total kidney area. Important for our study is the observation that VX-809 treatment of the RC/RC mice for 2 months prior to necropsy (from 3 to 5 months of age) led to a significant reduction in the cyst area and kidney to body weight ratio. To take this one step further, at necropsy we analyzed blood samples from the mice and measured circulating levels of AST, ALT, and BUN. These parameters are indicators of liver and kidney function (normal values are displayed in each graph). The BUN levels were above normal in the cystic animals and were reduced by about half after treatment with VX-809. All the other measurements were within the normal range and were unaffected by VX-809. These data are consistent with an improvement in renal function as a result of VX-809 treatment.
FIGURE 1.
VX-809 slows cyst growth and improves renal function. Representative images of H&E-stained kidney sections from a cystic 5-month-old RC/RC control mouse or mouse treated with VX-809 every other day for 60 days beginning at 3 months of age (A). Cyst area, measured with ImageJ (provided by National Institutes of Health), is expressed as a percentage. Columns represent the mean ± SE of the % cyst area (B). Statistical analysis was performed using an unpaired t-test. **p < .01. (C) Kidney to body weight ratio was measured using wet weight. Dots = number of animals. Liver and kidney function tests. Blood samples were taken from control, treated animals, and serum was analyzed for kidney and liver function. Values measured were blood urea nitrogen (BUN) (D); aspartate aminotransferase (AST) (E); alanine transaminase (ALT) (F); and. Analyses were performed by spectrophotometric measurement using a Vet Ace clinical chemistry automated random-access analyzer (Alfa Wasserman Diagnostic Technologies). Values are means ± SEM. *p < .05. Statistical analysis was performed using an unpaired t-test. Dots = number of animals. CFTR, NHE3, ENaC, and PC2 Expression. Representative images of Western blotting for CFTR, NHE3, ENaC, and PC2 expression in normal (WT) mice, untreated RC/RC mice, and RC/RC mice treated with VX-809 (30 mg/kg) every other day for 60 days beginning at 3 months of age. Animals were necropsied at 5 months. (G&J). Summary data for CFTR, NHE3, ENaC, and PC2 expression, respectively. (H&I, K&L). HSP27 expression (M). Summary data for Hsp27 (N). Columns represent averages ± standard errors. *p < .05. **p < .01. Data is ratioed to the loading control, vinculin. Statistical analysis was performed using an unpaired t-test. Dots = number of animals
We previously noted a change in key proteins involved in renal function (NHE3 and ENaC) and cyst formation (CFTR) in an inducible animal model of ADPKD.24 To determine whether these changes also occur in the RC/RC mice, we measured the protein levels of CFTR, NE1E3, and ENaC (Figure 1G-L) and found that all three were severely reduced in cystic kidneys when compared to normal values and were raised toward normal values by VX-809. We analyzed (Figure 1J,L) the binding partner of PC1, PC2,34 and found it to be reduced to nearly undetectable levels in RC/RC kidneys. Again, restoration occurred with VX-809 treatment. Finally, we assessed levels of Hsp27 which we had previously found upregulated in an inducible model of ADPKD.32 Again, we found that Hsp27 levels were elevated in the RC/RC mouse and reduced by treatment of VX-809 (Figure 1M,N).
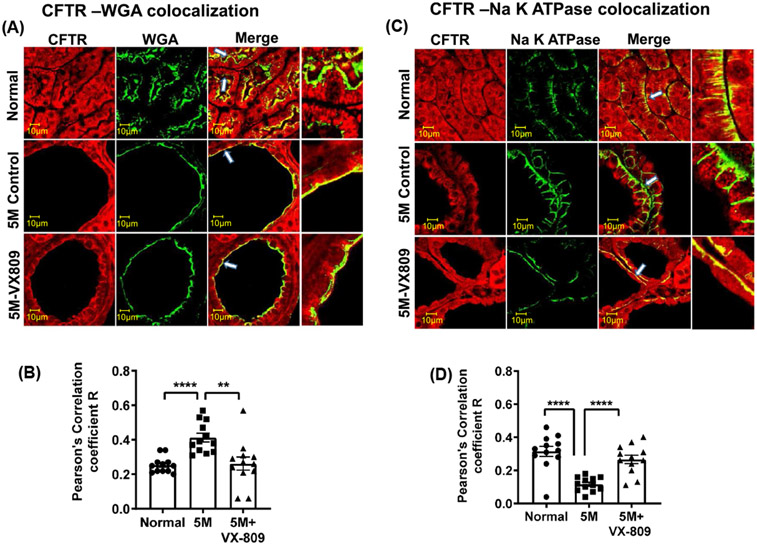
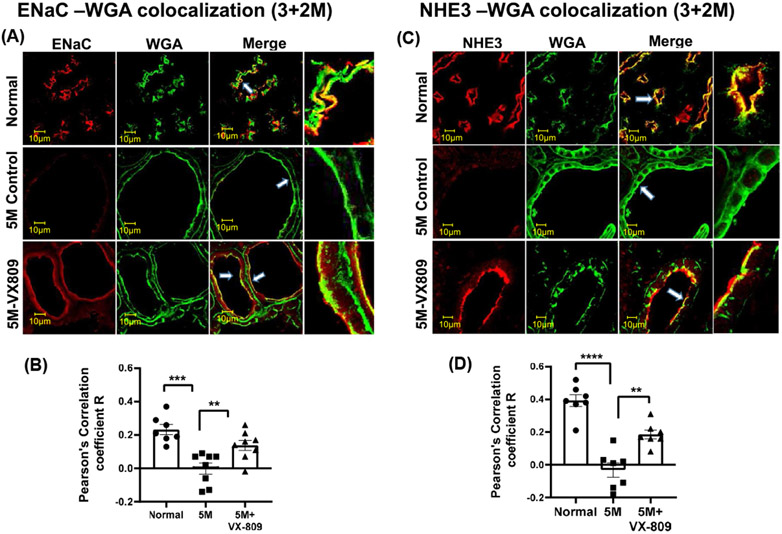
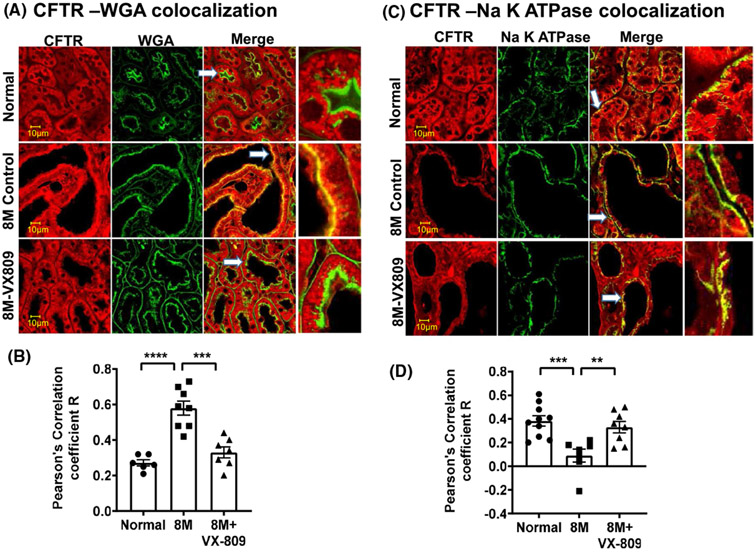
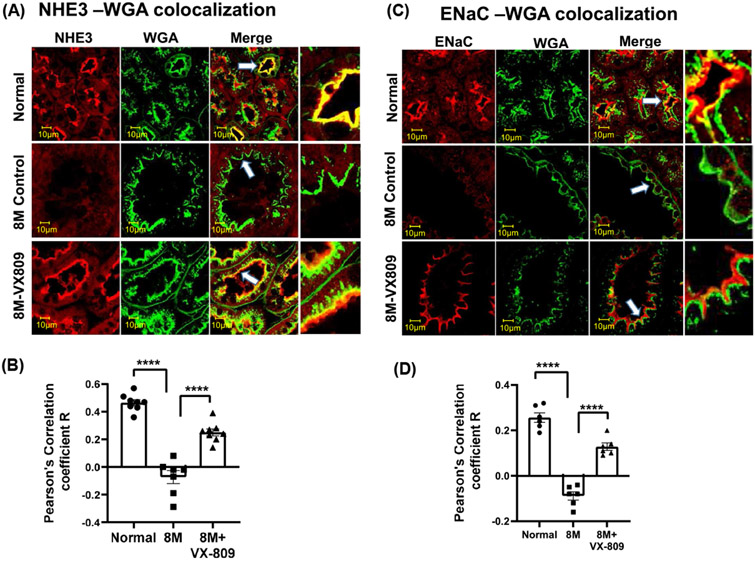
Next, we asked whether their localization in the kidney is also altered. As we have previously demonstrated in normal kidneys, CFTR co-localized with the apical membrane marker wheat germ agglutinin (WGA) and to an even greater extent with the basolateral marker, the Na+/K+ ATPase solute pump (Figure 2A-D). Consistent with its role in cyst formation,15 CFTR was present to a greater extent in the apical membrane of the cysts than in normal kidneys. CFTR was located in the basolateral membrane in normal kidneys but was virtually absent from the basolateral membrane in the RC/RC cystic kidneys. VX-809 treatment decreased the co-localization of CFTR with the apical membrane and increased the CFTR colocalization with the basolateral membrane marker. Consistent with their role in Na+ absorption,35 ENaC and NFIE3 were present in the apical membrane of normal kidneys but were mostly absent from the cystic RC/RC kidneys (Figure 3). Again, VX-809 treatment restored ENaC and NHE3 to their normal location in the apical membrane.
FIGURE 2.
Co-localization of CFTR with WGA or Na+/K+ ATPase: Images in a normal (wt) mouse, untreated RC/RC mouse, and RC/RC mouse treated with VX-809 (30 mg/kg). Staining for CFTR (red) and WGA or Na+/K+ ATPase (green), and the merged image. Yellow denotes co-localization (A,C). Summary of Pearson's correlation coefficient R (B,D). Samples were incubated with primary antibody (CFTR 596 and 488 conjugated WGA or CFTR 596 and Na+/K+ ATPase). Anti-mouse-Alexa Fluor 594 secondary or Anti-Rabbit-Alexa Fluor 488 secondary antibodies were used. Scale bar is 10 μm. Dots = number of experimental measurements from three animals, (cortical and medullary regions)
FIGURE 3.
Co-localization of ENaC or NHE3 with WGA: Images of a normal (wt) mouse, untreated RC/RC mouse, and RC/RC mouse treated with VX-809 (30 mg/kg) every other day for 60 days beginning at 3 months of age. Mice were necropsied at 5 months. Staining indicates ENaC or NHE3 (red), WGA (green), and the merged image. Yellow denotes co-localization (A&C). Summary of Pearson's correlation coefficient R (B,D). Samples were incubated with primary antibody against ENaC or NHE3 and WGA. Anti-rabbit-Alexa Fluor 594 and Anti-mouse-Alexa Fluor 488 secondary antibodies were used. Dots = number of experimental measurements from 3 animals. (cortical for NHE3 and medullary regions for ENaC)
3.2 ∣. 8-month-old mice
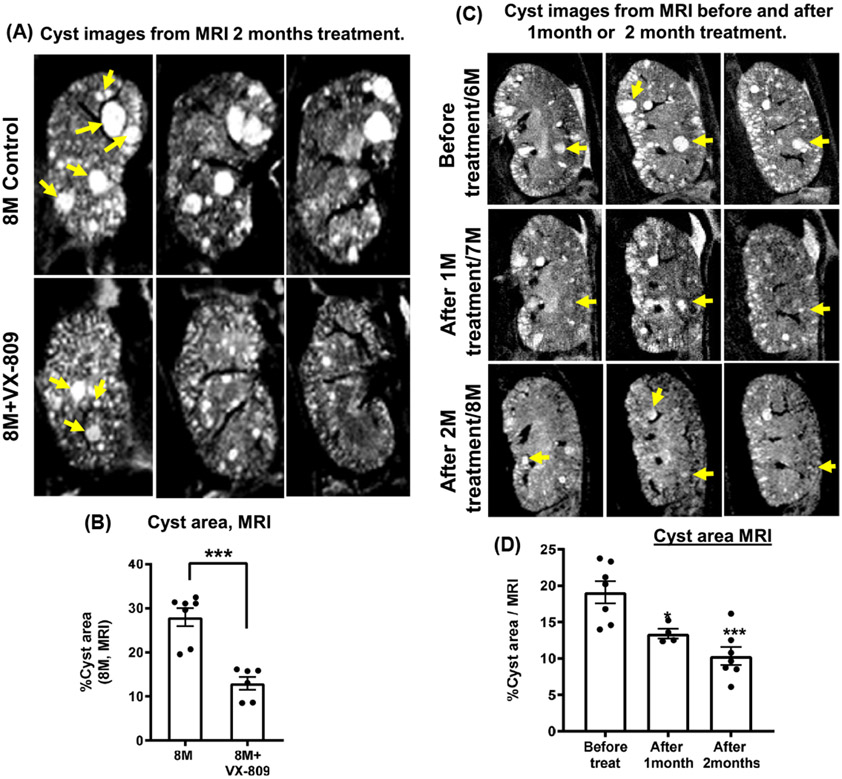
To study a later stage of cyst growth, we obtained magnetic resonance images (MRI) images of live mice (Figure 4A,B). Our data show that at 8 months of age, the cysts comprised ~30% of the area of the kidney in the RC/RC mice. What is important is that again, VX-809 treatment for 2 months (month 6 to 8) had shrunk the cysts by 8 months of age. Figure 4C,D shows one animal where MRI images were obtained at 6 months of age just prior to treatment with VX-809, at 7 months of age, 1 month after treatment and at 8 months of age, 2 months after treatment. Please note the reduction in cyst area both 1 and 2 months after treatment.
FIGURE 4.
VX-809 slows cyst growth. Sequential MRI images of the control kidney from a mouse and from a second mouse treated with VX-809 for 2 months beginning at 6 months of age (A). Graphs summarize the % cyst area (B). Sequence images of the same mouse before treatment, and 1 and 2 months after treatment (C). Graphs summarize the % cyst area (D). Arrows indicate that the cyst number or size in kidneys was reduced when compared to the untreated control group. Scans were captured using a Bruker 9.4T horizontal-bore magnetic resonance imaging (MRI) system. MRI scans were obtained under the same magnification, field of view (FOV), and slice thickness (0.5 mm). % area was measured using ImageJ (provided by the NIH). ***p < .001. Statistical analysis was performed using an unpaired t-test. Dots = number of animals
Figure 5A shows a section of the kidney of a treated and of an untreated RC/RC mouse at 8 months of age. Clearly the untreated animal has a cyst area that was consistent with the area measured in the MRI images. Following treatment, there was a significant reduction in the cyst area (Figure 5 A) but no change in the kidney/body weight ratio. One can see that the BUN levels (Figure 5D) were at the higher end of the normal range in the cystic animals and were reduced by treatment with VX-809. Both AST and ALT (Figure 5E,F) were also reduced by VX-809, which may indicate an improvement in liver function following VX-809 treatment. Taken together, these data are consistent with an improvement in renal and/or liver function as a result of treatment with VX-809.
FIGURE 5.
VX-809 slows cyst growth and improves renal function. Images of H&E-stained kidney sections from RC/RC mice at 8 months of age: a normal mouse, and an RC/RC cystic mouse treated with VX-809 for 2 months beginning at 6 months of age (A). See Figure 1 for details. Columns represent mean areas ± SE of % cyst area (B). Statistical analysis was performed using an unpaired t-test. n = 6, *p < .05. (C) Kidney to body weight ratio was measured using wet weight. Dots = number of animals. Liver and kidney function tests. Blood samples were taken from control and treated animals. Serum was analyzed for kidney and liver function. Values measured were BUN (D); AST (E); ALT (F). Values are means ± SEM. *p < .05, ***p < .001. Statistical analysis was performed using an unpaired t-test. Dots = number of animals. CFTR, NHE3, ENaC, and PC2 Expression. Representative images of Western blotting for CFTR, NHE3, ENaC, and PC2 expression in normal (WT) mice, untreated RC/RC mice, and RC/RC mice treated with VX-809 (30 mg/kg) every other day for 60 days beginning at 3 months of age. Animals were necropsied at 5 months. (G&J). Summary data for CFTR, NHE3, ENaC, and PC2 expression, respectively. (H&I, K&L). HSP27 expression (M). Summary data for Hsp27 (N). Columns represent averages ± standard errors. *p < .05. **p < .01. Data is ratioed to the loading control, vinculin. Statistical analysis was performed using an unpaired t-test.Dots = number of animals
We found that VX-809 produced a change in key proteins involved in renal function (ENaC and NHE3) and cyst formation (CFTR). To determine whether these changes also occur in older RC/RC mice, we again measured the protein levels of CFTR, ENaC, and NHE3 (Figure 5G-I) and found that all three were severely reduced in the cystic kidneys when compared to normal and elevated back toward normal values by VX-809. Consistent with the results in younger mice, we found severely reduced levels of PC2 (Figure 5K,L). Again, restoration occurred with VX-809 treatment. Finally, we again detected increased levels of HSP27 in the RC/RC mice that were reduced by VX-809 (Figure 5M,N).
Figure 6 shows confocal images of an 8-month-old normal mouse kidney and an RC/RC mouse kidney untreated or treated for 2 months with VX-809. There was strong co-localization of CFTR with the apical membrane marker (WGA) but not with the basolateral marker in the cystic mice, as compared to the normal animals. ENaC and NHE3 were present in the apical membranes in the normal kidneys but were mostly absent from these membranes in the cystic kidneys (Figure 7). Again, VX-809 restored the localization of CFTR (basolateral) as well as ENaC and NHE3 (both apical) toward that in normal mice.
FIGURE 6.
Co-localization of CFTR and WGA or Na+/K+ ATPase: Images in a normal (wt) mouse, untreated RC/RC mouse, and RC/RC mouse treated with VX-809 (30 mg/kg) every other day for 60 days beginning at 6 months of age. Staining indicates CFTR (red) and WGA or Na+/K+ ATPase (green), and the merged image. Yellow denotes co-localization (A,C). Summary of Pearson's correlation coefficient R (B,D). Samples were incubated with primary antibody (CFTR 596 and 488 conjugated WGA or CFTR 596 and Na+/K+ ATPase). Anti-mouse-Alexa Fluor 594 or Anti-Rabbit-Alexa Fluor 488 secondary antibodies were used. Scale bar is 10 μm. Dots = number of experimental measurements from three animals (cortical and medullary regions)
FIGURE 7.
Co-localization of ENaC or NHE3 with WGA: Images of a normal (wt) mouse, untreated RC/RC mouse, and RC/RC mouse treated with VX-809 (30 mg/kg) every other day for 60 days beginning at 6 months of age. Animals were necropsied at 8 months of age. Staining indicates ENaC or NHE3 (red), WGA (green), and the merged image. Yellow denotes co-localization (A,C). Summary of Pearson's correlation coefficient R (B,D). Samples were incubated with primary antibody against ENaC or NHE3 and WGA. Anti-rabbit-Alexa Fluor 594 and Anti-mouse-Alexa Fluor 488 secondary antibodies were used. Dots = number of experimental measurements from 3 animals (cortical for NHE3 and medullary regions for ENaC)
4 ∣. DISCUSSION
We have shown here that in human mutation model of ADPKD, the RC/RC mouse, VX-809 can reduce cyst growth and improve liver and kidney function. Several important takeaways are evident from this study. First, despite the differences in age of the mice examined and the degree of progression of their disease, in all of the treated mice, VX-809 consistently reduced cyst size. Second, the cystic kidneys retained a consistent phenotype as the animals aged, namely: CFTR was localized to the apical membrane, and ENaC and NHE3 were not. The protein levels of all three proteins were reduced in the RC/RC mice, pointing to a change in both the steady-state levels of the proteins and their location within the cystic cells. We show thatVX-809 restores CFTR, NHE3 and ENaC to their proper locations. How might VX-809 alter all three proteins? As we mention VX-809 alters a proteostatic network of proteins including Hsp274 that are known to affect the processing and trafficking36,37 pointing to a possible mechanism of how VX-809 restores CFTR, ENaC and NHE3 toward their properly functioning location.
4.1 ∣. Drug development using animal models
The RC/RC mouse exhibits a slowly progressing phenotype not unlike the human disease.3 The RC/RC mouse model also develops liver disease which becomes prominent in older animals and depends upon the genetic background.38 We observed improvement in the levels of the transaminases, AST and ALT only in the 8-month-old animals which is consistent with progression of liver disease in older animals. Interestingly, treatment of CF patients with CFTR modulators leads to increased transaminase levels39 which was not observed in our mouse studies either in younger or older animals.
This animal model was used to evaluate tolvaptan, the drug currently approved to treat ADPKD. Although the time course of the experimental approach was different between our current study and that of Hopp and collaborators,13 it is useful to compare the therapeutic potential of VX-809 with that of tolvaptan in the same animal model. Cyst growth in ADPKD is fueled by cAMP.6 In the normal kidney, vasopressin binds to its receptor and increases cAMP to regulate water transport across the collecting duct.35 However, in ADPKD patients, vasopressin stimulation of cAMP increases cyst growth.6 Thus, tolvaptan was designed to circumvent the effect of vasopressin on cAMP by blocking vasopressin receptor 2.40
Hopp and collaborators13 treated the RC/RC mice with tolvaptan from age 1 to 6 months and saw an ~20% reduction in cyst volume when compared with the untreated controls. This reduction was accompanied by an approximately 13% reduction in cAMP. This is a relatively small effect of tolvaptan alone, but the results were amplified dramatically when tolvaptan was used in combination with pasireotide, a somatostatin ligand41; somatostatin receptor activation is known to reduce cAMP levels via an inhibitory G-protein.42 We have observed here a 40%–50% decrease in cyst area in response to 2 months of VX-809 treatment at all the ages of mice studied here. These data demonstrate that VX-809 treatment may indeed be a better option than tolvaptan, but more studies need to be performed, particularly in humans, to confirm this claim.
4.2 ∣. Role of CFTR
CFTR is known to participate in Cl− secretion in several organs in the body to create a thin layer of mucosal fluid that provides a hydrating function,43 but it also plays a role in salt absorption in the sweat duct.44 Although the experiments shown here do not address this question directly, VX-809, by favoring CFTR at the basolateral membrane, creates an absorptive phenotype, reducing fluid. This mechanism, which in essence would turn the physiology of the cyst against itself, would reduce the cyst size by removing fluid from the lumen. the results show that VX-809 can reduce the cyst size in RC/RC mice treated at 6 through 8 months of age by ~50% when compared to untreated animals of the same age. We have provided evidence for the movement of CFTR to the basolateral membrane in a previous manuscript.24 However, this study focuses on a hypomorphic variant which still retains function, thus other mechanisms may be involved in VX-809 reducing cysts in this model.
The absorption of fluid from the cysts would require a Na+ absorptive pathway to operate in parallel with CFTR-based pathways. Indeed, we have shown previously that the Na+/H+ exchanger (NHE3) and the epithelial Na+ channel (ENaC) are not prominent in the apical membrane of cystic epithelial cells, as expected for a secretory epithelium; however, following the application of VX-809, both are restored to their proper functional location in the apical membrane.24 Thus, VX-809 changes the secretory phenotype of the cystic epithelium toward one more favorable for absorption.
ACKNOWLEDGEMENTS
The authors thank Deborah McClellan, PhD for editing the manuscript. We thank the Mayo Clinic Robert M. and Billie Kelley Pirnie Translational PDK Center for providing the mouse model. We thank William B. Guggino, PhD for scientific disucssions and help with the manuscript.
Funding information
The work was funded by NIH Grant # DK125272 to LC
Abbreviations:
- ADPKD
autosomal dominant polycystic kidney disease
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AVP
arginine vasopressin
- BUN
blood urea nitrogen
- CFTR
cystic fibrosis transmembrane conductance regulator
- ENaC
epithelial sodium channel
- HSP
heat shock protein
- Na+/K+ ATPase
sodium/potassium adenosine triphosphatase
- NHE3
sodium/hydrogen exchanger
- PC
polycystin
- PKD
polycystic kidney disease
- RC/RC mouse
mouse model containing the R3277C human mutation
Footnotes
DISCLOSURES
LC has license and consulting agreements with RA Capital. These agreements did not influence the experiments conducted in this manuscript.
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study will be made available by the corresponding author upon reasonable request. All data generated or analyzed during this study are included in this published article. No data sets were generated.
REFERENCES
- 1.Calvet JP, Grantham JJ. The genetics and physiology of polycystic kidney disease. Semin Nephrol. 2001;21:107–123. [DOI] [PubMed] [Google Scholar]
- 2.Ong AC, Harris PC. A polycystin-centric view of cyst formation and disease: the polycystins revisited. Kidney Int. 2015;88:699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hopp K, Ward CJ, Hommerding CJ, et al. Functional polycys tin-1 dosage governs autosomal dominant polycystic kidney disease severity. J Clin Invest. 2012;122:4257–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopes-Pacheco M, Boinot C, Sabirzhanova I, Morales MM, Guggino WB, Cebotaru L. Combination of correctors rescue deltaF508-CFTR by reducing its association with Hsp40 and Hsp27. J Biol Chem. 2015;290:25636–25645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang ST, Chiou YY, Wang E, et al. Defining a link with autosomal-dominant polycystic kidney disease in mice with congenitally low expression of Pkd1. Am J Pathol. 2006;168:205–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grantham JJ, Mangoo-Karim R, Uchic ME, et al. Net fluid secretion by mammalian renal epithelial cells: stimulation by cAMP in polarized cultures derived from established renal cells and from normal and polycystic kidneys. Trans Assoc Am Physicians. 1989;102:158–162. [PubMed] [Google Scholar]
- 7.Yamaguchi T, Pelling JC, Ramaswamy NT, et al. cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracellular signal-regulated kinase pathway. Kidney Int. 2000;57:1460–1471. [DOI] [PubMed] [Google Scholar]
- 8.Torres VE, Harris PC. Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol. 2006;2:40–55; quiz 55. [DOI] [PubMed] [Google Scholar]
- 9.Higashihara E, Torres VE, Chapman AB, et al. Tolvaptan in autosomal dominant polycystic kidney disease: three years′ experience. Clin J Am Soc Nephrol. 2011;6:2499–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda Y, Han JS, Gibson CC, Knepper MA. Vasopressin and oxytocin receptors coupled to Ca2+ mobilization in rat inner medullary collecting duct. Am J Physiol. 1993;265:F15–F25. [DOI] [PubMed] [Google Scholar]
- 11.Star RA, Nonoguchi H, Balaban R, Knepper MA. Calcium and cyclic adenosine monophosphate as second messengers for vasopressin in the rat inner medullary collecting duct. J Clin Invest. 1988;81:1879–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou CL, Yip KP, Michea L, et al. Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct. Roles of ryanodine-sensitive Ca2+ stores and calmodulin. J Biol Chem. 2000;275:36839–36846. [DOI] [PubMed] [Google Scholar]
- 13.Hopp K, Hommerding CJ, Wang X, Ye H, Harris PC, Torres VE. Tolvaptan plus pasireotide shows enhanced efficacy in a PKD1 model. J Am Soc Nephrol. 2015;26:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan LP, Wallace DP, Grantham JJ. Epithelial transport in polycystic kidney disease. Physiol Rev. 1998;78:1165–1191. [DOI] [PubMed] [Google Scholar]
- 15.Hanaoka K, Devuyst O, Schwiebert EM, Wilson PD, Guggino WB. A role for CFTR in human autosomal dominant polycystic kidney disease. Am J Physiol. 1996;270:C389–C399. [DOI] [PubMed] [Google Scholar]
- 16.Cohen-Cymberknoh M, Shoseyov D, Kerem E. Managing cystic fibrosis: strategies that increase life expectancy and improve quality of life. Am J Respir Crit Care Med. 2011;183:1463–1471. [DOI] [PubMed] [Google Scholar]
- 17.Morales MM, Nascimento DS, Capella MA, Lopes AG, Guggino WB. Arginine vasopressin regulates CFTR and ClC-2 mRNA expression in rat kidney cortex and medulla. Pflugers Arch. 2001;443:202–211. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda M, Fong P, Cheng J, et al. A regulatory role of polycystin-1 on cystic fibrosis transmembrane conductance regulator plasma membrane expression. Cell Physiol Biochem. 2006;18:9–20. [DOI] [PubMed] [Google Scholar]
- 19.Hanaoka K, Guggino WB. cAMP regulates cell proliferation and cyst formation in autosomal polycystic kidney disease cells. J Am Soc Nephrol. 2000;11:1179–1187. [DOI] [PubMed] [Google Scholar]
- 20.Tradtrantip L, Sonawane ND, Namkung W, Verkman AS. Nanomolar potency pyrimido-pyrrolo-quinoxalinedione CFTR inhibitor reduces cyst size in a polycystic kidney disease model. J Med Chem. 2009;52:6447–6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verkman AS, Lukacs GL, Galietta LJ. CFTR chloride channel drug discovery–inhibitors as antidiarrheals and activators for therapy of cystic fibrosis. Curr Pharm Des. 2006;12:2235–2247. [DOI] [PubMed] [Google Scholar]
- 22.Yang B, Sonawane ND, Zhao D, Somlo S, Verkman AS. Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2008;19:1300–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Sheppard DN. Therapeutic potential of cystic fibrosis transmembrane conductance regulator (CFTR) inhibitors in polycystic kidney disease. Biodrugs. 2009;23:203–216. [DOI] [PubMed] [Google Scholar]
- 24.Yanda MK, Cha B, Cebotaru CV, Cebotaru L. Pharmacological reversal of renal cysts from secretion to absorption suggests a potential therapeutic strategy for managing autosomal dominant polycystic kidney disease. J Biol Chem. 2019;294:17090–17104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Goor F, Hadida S, Grootenhuis PD, et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci U S A. 2011;108:18843–18848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drumm M. What happens to DeltaF508 in vivo? J Clin Invest. 1999;103:1369–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quinton PM, Reddy MM. Cl- conductance and acid secretion in the human sweat duct. Ann N Y Acad Sci. 1989;574:438–446. [DOI] [PubMed] [Google Scholar]
- 28.Reddy MM, Quinton PM. Localization of Cl- conductance in normal and Cl- impermeability in cystic fibrosis sweat duct epithelium. Am J Physiol. 1989;257:C727–C735. [DOI] [PubMed] [Google Scholar]
- 29.Bidaud-Meynard A, Bossard F, Schnur A, et al. Transcytosis maintains CFTR apical polarity in the face of constitutive and mutation-induced basolateral missorting. J bioRxiv. 2018: 411322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedemonte N, Lukacs GL, Du K, et al. Small-molecule correctors of defective DeltaF508-CFTR cellular processing identified by high-throughput screening. J Clin Invest. 2005;115:2564–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. [DOI] [PubMed] [Google Scholar]
- 32.Yanda MK, Liu Q, Cebotaru L. A potential strategy for reducing cysts in autosomal dominant polycystic kidney disease with a CFTR corrector. J Biol Chem. 2018;293:11513–11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costes SV, Daelemans D, Cho EH, et al. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J. 2004;86:3993–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanaoka K, Qian F, Boletta A, et al. Co-assembly of polycystin-1 and −2 produces unique cation-permeable currents. Nature. 2000;408:990–994. [DOI] [PubMed] [Google Scholar]
- 35.Koeppen BM, Stanton BA. Renal Physiology E-Book: Mosby Physiology Monograph Series. Elsevier Health Sciences; 2012. [Google Scholar]
- 36.Skach WR. Pharmacological chaperoning: two “hits” are better than one. Biochem J. 2007;406:e1–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skach WR. CFTR: new members join the fold. Cell. 2006;127:673–675. [DOI] [PubMed] [Google Scholar]
- 38.Arroyo J, Escobar-Zarate D, Wells HH, et al. The genetic background significantly impacts the severity of kidney cystic disease in the Pkd1RC/RC mouse model of autosomal dominant polycystic kidney disease. Kidney Int. 2021;99:1392–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Staufer K. Current treatment options for cystic fibrosis-related liver disease. Int J Mol Sci. 2020;21:8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Devuyst O, Torres VE. Osmoregulation, vasopressin, and cAMP signaling in autosomal dominant polycystic kidney disease. Curr Opin Nephrol Hypertens. 2013;22:459–470. [DOI] [PubMed] [Google Scholar]
- 41.Schmid HA. Pasireotide (SOM230): development, mechanism of action and potential applications. Mol Cell Endocrinol. 2008;286:69–74. [DOI] [PubMed] [Google Scholar]
- 42.Friedlander G, Amiel C. Somatostatin and α2-adrenergic agonists selectively inhibit vasopressin-induced cyclic AMP accumulation in MDCK cells. FEBS Lett. 1986;198:38–42. [DOI] [PubMed] [Google Scholar]
- 43.Tarran R, Grubb BR, Gatzy JT, Davis CW, Boucher RC. The relative roles of passive surface forces and active ion transport in the modulation of airway surface liquid volume and composition. J Gen Physiol. 2001;118:223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quinton PM, Reddy MM. Regulation of absorption in the human sweat duct. Adv Exp Med Biol. 1991;290:159–170; discussion 170-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study will be made available by the corresponding author upon reasonable request. All data generated or analyzed during this study are included in this published article. No data sets were generated.