Abstract
Background
There are only limited data to guide the management of infectious risk for Pneumocystis jiroveci pneumonia (PCP) in patients with inflammatory bowel disease (IBD). We evaluated the frequency of admissions for PCP among patients with IBD, as well as the temporal trend in PCP admission rates and the contribution of non-IBD risk factors to the development of infection.
Methods
The National Inpatient Sample from 2016-2017 was queried for all admissions involving both PCP and either Crohn’s disease or ulcerative colitis. Inpatient outcomes associated with PCP and additive risk factors for development of PCP within the IBD patient population were assessed using multivariate regression. Linear regression was performed on data from 2002-2017 to measure infectious trends over time.
Results
There were an estimated 225 admissions involving PCP among patients with IBD from 2016-2017 nationwide, representing 0.035% of total admissions. IBD patients with PCP faced a 4.67-fold higher adjusted odds of inpatient mortality (95% confidence interval 1.72-12.66), while 49% of patients with IBD who developed PCP had an unrelated risk factor. The most common factors were HIV and congenital immunodeficiency, both of which were associated with PCP in adjusted regression. The infectious incidence of PCP increased by 141% from 2002 to 2017 (P=0.003).
Conclusions
National admissions data indicate that significant PCP is rare in IBD patients. Routine PCP prophylaxis is probably not necessary, although further study of high-risk subgroups of patients is required. The rising incidence of PCP indicates a need for continued surveillance.
Keywords: Crohn’s disease, ulcerative colitis, epidemiology
Introduction
Pneumocystis jiroveci pneumonia (PCP) is an opportunistic infection associated with impaired cell-mediated immunity. Initially an HIV-defining illness, it is increasingly associated with non-HIV immunocompromised populations [1]. Non-HIV patients have been found to have a more rapid and aggressive clinical course, with higher rates of respiratory failure and mortality [2]. There are multiple reports of PCP in patients with inflammatory bowel disease (IBD) [3-5], as in other rheumatologic and hematologic conditions treated with immunomodulatory therapies. Current data and recommendations guide the use of antimicrobial prophylaxis in many immunosuppressed populations, but standardization for the management of IBD is lacking. Different professional societies offer varying recommendations, or no recommendations at all, with respect to which combinations of therapies warrant prophylaxis against PCP [6,7]. As a result, clinical practice patterns vary widely by provider and institution, with one study reporting prophylaxis rates as low as 11%, with even lower use outside of academic medical centers [8]. Furthermore, much of this practice is based on assumptions from other diseases that may not apply to patients with IBD.
The lack of data on the risk of PCP in the IBD population limits the formulation of comprehensive national guidelines regarding the need for prophylaxis. To date, no study has looked at the scope of morbidity and mortality of PCP among patients with IBD on a national level. We therefore sought to explore the frequency of admissions for PCP among the IBD patient population using a National Inpatient Sample (NIS) dataset. Given the clinical experience and prior literature suggesting that such infections occur infrequently, we hypothesized there would be very low rates of hospitalization for PCP among patients with IBD. Furthermore, we hypothesized that the incidence of PCP admissions among patients with IBD has not significantly risen in the past 2 decades, given the gradual shift from steroids to more modern immunomodulator therapies with lower risk. We additionally sought to explore risk factors in patients with IBD for development of PCP to better define which, if any, cohort warrants prophylaxis.
Materials and methods
This study utilized data and research tools provided by the NIS from 2016 and 2017, produced by the Healthcare Cost and Utilization Project and sponsored by the Agency for Healthcare Research and Quality [9-11]. This database is an all-payer stratified sample of 20% of admissions in the United States, weighted to represent the entire country and drawn from 97% of the population. Information includes primary and secondary diagnoses, demographic and hospital characteristics, primary/secondary payer information, and illness severity measures [12]. Per institutional policy, Institutional Review Board approval was not required in view of the anonymized nature of the data.
Our primary outcome of interest was hospitalization for PCP, as defined as an ICD-10 code of B59.0 listed in any position among the hospitalization problems (including as a secondary problem). We subsequently compared the frequency of PCP admissions among adult patients with IBD to the general inpatient population. Admissions among patients with IBD were defined as those with either Crohn’s disease (ICD-10: K50.0-K50.9) or ulcerative colitis (ICD-10: K51.0-K51.9) included in any position on the hospitalization problem list for the admission. Patients classified as having “indeterminate colitis” by ICD-10 K52.3 were not included in order to maintain the specificity of our study population for patients with a confirmed diagnosis of IBD. Given the infrequency of PCP, patients with Crohn’s disease and ulcerative colitis were evaluated together in our primary analysis.
The demographic and hospital characteristics and inpatient outcomes of mortality, length of stay, and total charges for patients admitted for PCP were compared with the overall IBD inpatient population using a chi-square test (discrete variables) or bivariate linear regression (continuous variables). To adjust for probable differences in the underlying comorbidity burdens between these 2 groups, an Elixhauser Comorbidity Index was calculated for each patient on the basis of 29 medical comorbidities known to be associated with an increased risk of mortality, as outlined in Supplementary Table 1 (44.9KB, pdf) . This is a validated index, specific to the NIS, that has superior predictive ability compared to the Charlson comorbidity index [13-15]. We then conducted a survey-adjusted analysis on inpatient mortality and length-of-stay, using multivariate logistic and linear regression, respectively. All analyses were survey-adjusted according to the guidelines of the Healthcare Cost and Utilization Project. Missing data was <1% and therefore admissions missing a predictive factor were excluded from the analyses.
To assess the contribution of risk factors independent from underlying IBD in the development of PCP, the prevalence of well-established infectious risk factors was evaluated and compared between all IBD inpatients and those who developed PCP. A full list of the risk factors used is given in Supplementary Table 1 (44.9KB, pdf) . They include: HIV; lymphoma; leukemia; congenital immunodeficiencies; solid organ transplantation; post-transplant lymphoproliferative disorders; connective tissue disorders; and stem cell transplantation. The relative prevalence of chronic steroid use among those with PCP and those without was compared using chi-square testing; constraints of the NIS dataset prevented evaluation of other specific IBD therapies. Survey-adjusted multivariate logistic regression, controlled for age and comorbidity status, was performed to explore the relationship between the most significant known PCP risk factors—including HIV, congenital immunodeficiency, receipt of a solid organ transplant, and chronic steroids—and the development of infection.
Finally, in order to assess the change in hospitalization rates for PCP over time with the introduction of multiple new biologic medications, NIS datasets from 2002-2017 [16] were queried for all admissions involving PCP and either Crohn’s disease or ulcerative colitis. Linear regression was performed, evaluating for any trend in PCP rates among patients with IBD during this time period; for comparison, this analysis was repeated amongst the general inpatient population.
Results
In total, there were an estimated 641,265 all-cause admissions involving patients with IBD nationwide from 2016-2017. Of these admissions, only 235 involved management of PCP, representing 0.035% of the total admissions in this cohort during this time period.
Comparisons between the baseline characteristics of IBD patients admitted with PCP and those of the overall IBD population are provided in Table 1. In general, those admitted with PCP were older (58.9 vs. 53.3, P=0.007), more likely to be male (55.6% vs. 43.7%, P=0.117), and had higher comorbidity burdens than those admitted for other reasons (Elixhauser score 17.3 vs. 5.6, P<0.001), all of which is consistent with previously described PCP risk factors.
Table 1.
Summary of patient and hospital characteristics for IBD admissions stratified by pneumocystis pneumonia status
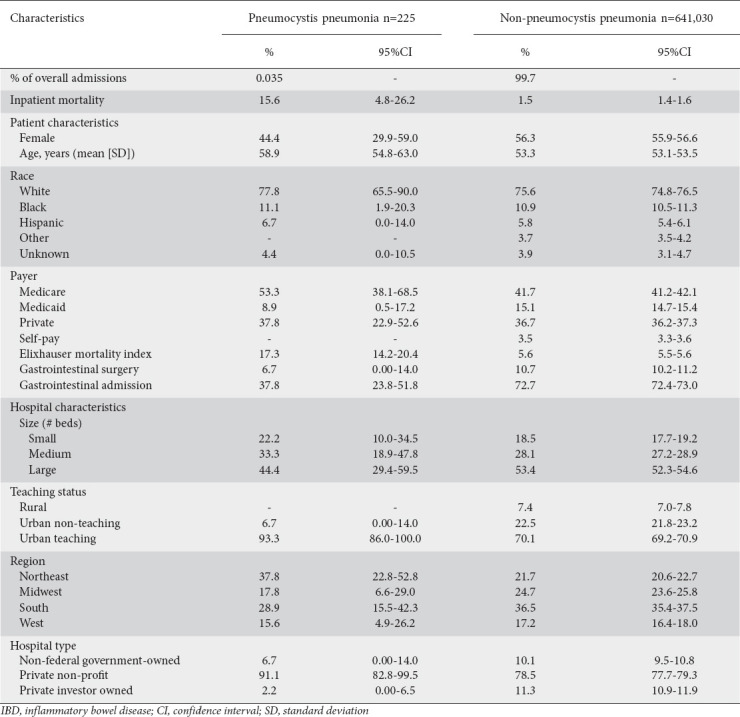
Patients with both IBD and PCP faced a higher unadjusted inpatient mortality rate (15.6% vs. 1.5%, P=0.015) compared to patients with IBD admitted for alternative reasons. This was confirmed on multivariate analysis of the IBD population, which demonstrated that PCP was associated with a 4.67-fold increase in the odds (95% confidence interval [CI] 1.72-12.66; P=0.003) of inpatient mortality, after controlling for comorbidity burden, hospital characteristics, patient age and inpatient surgery (Table 2). Among patients without IBD, those with PCP had 5.29 times higher odds of inpatient mortality (95%CI 4.76-5.85; P<0.001) compared to those admitted for alternative diagnoses.
Table 2.
Predictors of mortality and length of stay among IBD inpatients
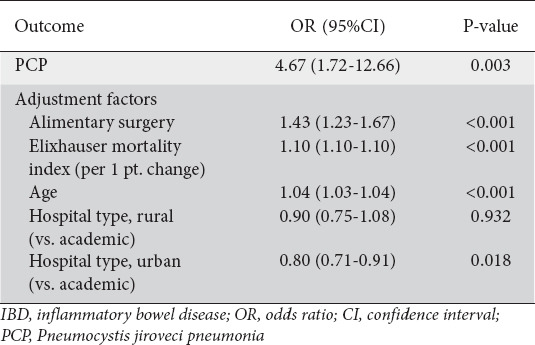
Length of stay among patients admitted with both IBD and PCP was a mean of 16.8 days, compared to 5.3 days among those IBD admissions unrelated to PCP (P<0.001). After adjustment in multivariate linear regression, the magnitude of this difference was only slightly reduced to 9.47 days (95%CI 4.97-13.96; P<0.001).
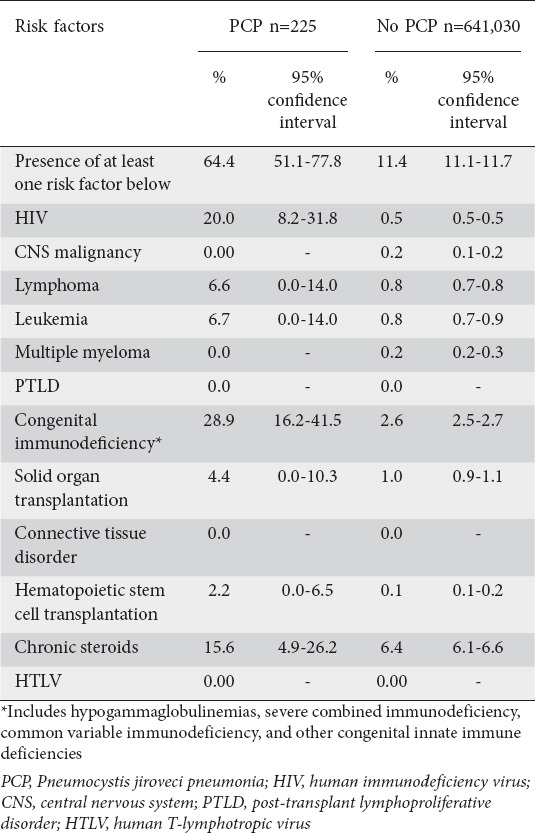
Notably, 48.9% (95%CI 34.9-62.9) of patients with IBD hospitalized for PCP were found to have an additional identifiable risk factor not directly related to their underlying IBD (Table 3). Most commonly, 20% of PCP patients had underlying HIV, compared to 0.5% of the non-PCP IBD cohort (P<0.001). Furthermore, almost one third had an underlying congenital immunodeficiency, including hypogammaglobulinemia, severe combined immunodeficiency, or common variable immunodeficiency (Table 3).
Table 3.
Summary of concurrent infectious risk factors present among inflammatory bowel disease admissions stratified by PCP status
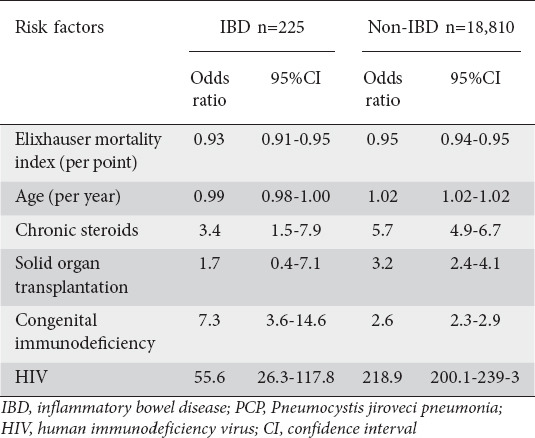
Patients with PCP and IBD were more often exposed to chronic steroids (15.6%, 95%CI 4.9-26.2) compared to non-PCP IBD patients (6.4%, 95%CI 6.1-6.6; P=0.10). When adjusted in multivariate regression, patients with IBD admitted for PCP had 3.4 times higher odds of chronic steroid use than the overall IBD inpatient population (95%CI 1.5-7.9; Table 4). Interestingly, chronic steroid use represented a less significant exposure among those with PCP than both positive HIV status (odds ratio [OR] 55.6, 95%CI 26.3-117.8; P<0.001) and congenital immunodeficiency (OR 7.3, 95%CI 3.6-14.6; P<0.001) (Table 4).
Table 4.
Risk factors for PCP infection among IBD and non-IBD admissions
Overall, there was a 141% increase in the rate of annual PCP admissions among the IBD population from 2002-2017, increasing from 58 cases in 2002 to 140 cases in 2017. In bivariate linear regression, the hospitalization rate for PCP among patients with IBD increased by a mean of 5.02 admissions per year (P=0.003) (Fig. 1). Comparatively, the national trend during that time period revealed a 47% decrease in annual PCP admissions from 2002 to 2017 among the overall inpatient population. This translated into an average decrease of 635.7 admissions per year during that time period (P<0.001) (Fig. 2).
Figure 1.
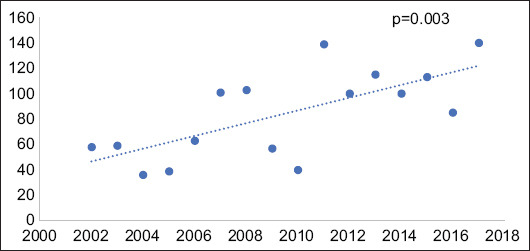
Volume of Pneumocystis jiroveci pneumonia (PCP) admissions among inflammatory bowel disease (IBD) patients from 2002-2017. Linear regression evaluating change in annual admission rates for PCP among patients with IBD, showing a statistically significant positive correlation with an average increase of 5.02 admissions annually
Figure 2.
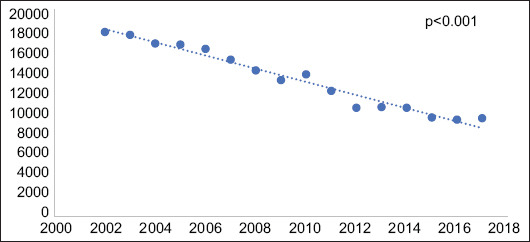
Volume of Pneumocystis jiroveci pneumonia (PCP) admissions nationwide from 2002-2017. Linear regression evaluating change in annual admission rates for PCP nationwide, showing a statistically significant negative correlation with an average decrease of 635.7 admissions annually
Discussion
Overall, national admissions data illustrate that PCP is associated with high mortality rates and longer lengths of stay among patients with IBD, as seen in other non-HIV patients. Incidence among IBD patients is rising, in contrast to the overall case burden, but it remains very rare, with only 225 admissions nationwide over a 2-year time span. Of all patients admitted with PCP and IBD, 49% had an additional risk factor contributing to their risk of developing the opportunistic infection. Given that there are currently an estimated 3 million individuals in the United States with IBD [17], these low case numbers suggest that serious PCP represents a very uncommon phenomenon among patients with IBD.
Our study is consistent with prior reports that found very low rates of PCP within cohorts of IBD patients; however, these studies were often confined to single centers or were retrospective case-control studies [18-21]. Long et al (2013) utilized a commercial dataset and found an greater but, overall, very low incidence of PCP among IBD patients (10.6/100,000) [18]. The relatively higher rates of infection reported in their study compared to ours may be partially explained by their inclusion of patients treated in the outpatient setting, while we only evaluated those who required inpatient-level care, and by differences due to over-coding. It is important to note that their analysis was regional and was confined to commercially insured patients, excluding higher risk subgroups such as the elderly and the uninsured. Similarly, Kojima et al (2020) reported a total of 9 cases in a population of over 4500 ulcerative colitis patients over a 12-year time period [20]; Cotter et al reported only 3 cases of PCP in over 16,000 years of IBD patient follow up [21]. Cumulatively, Lawrence et al found only 92 reported cases of PCP in the literature before 2017, though our study suggests this represents under-reporting of the total disease burden [3]. Okafor et al synthesized existing literature into a cost-effectiveness analysis that found no benefit to PCP prophylaxis in patients with IBD [8].
In spite of finding overall low infection rates in 2016-2017, we report a statistically significant increase in admissions for PCP among patients with IBD over the past 15 years, particularly notable when contextualized within the contemporary trend of decreasing PCP rates nationwide. Furthermore, although the prevalence of IBD within the United States increased by 50% during this time period [17], the corresponding rate of PCP admissions increased by 141%, suggesting that recent evolutions in IBD therapy could have contributed to this rise. Most notably, this period saw the introduction of biologic therapies [22], while there are several reported instances of PCP associated with anti-tumor necrosis factor (TNF) therapies, both within [4,5] and outside of IBD [23]. While this increase could theoretically have been offset by corresponding reductions in other immunomodulating therapies, prior studies have shown relative constancy in the use of other classes of IBD medications, including corticosteroids, as biologics became more prevalent [22]. Furthermore, it has been reported that nearly a fifth of patients started on steroids for IBD will still have a prolonged course [24], and up to 15% will be classified as having either steroid excess or dependence [25]. The use of steroids in conjunction with additional immunosuppressant agents has been empirically reported to increase patients’ risk of PCP [18,26-28], as well as having an additive lymphopenic effect [29]. Therefore, it seems possible that the rise of multidrug immunosuppressive therapy regimens, particularly those including corticosteroids, is contributing to an increasing risk of PCP among patients with IBD, as has been previously reported in smaller scale studies [18,28,30].
In spite of this increase in PCP admission rates, the rate of PCP in 2016-1017 does not support an absolute infectious risk of 3.5% [1], recommended for a favorable risk-benefit profile of PCP prophylaxis in non-HIV populations, in any meaningful segment of patients with IBD. We might also expect that this trend will be affected by the recent rise of newer biologics such as vedolizumab [31], ustekinumab, and tofacitinib, which are less frequently associated with PCP than are older anti-TNF therapies [32].
The primary limitation of our study pertains to the data constraints imposed by a national dataset that does not provide more granular clinical information on individual patients. We cannot say definitively the extent to which the low infection rates observed are driven by existing use of prophylaxis, though existing data suggest this remains an uncommon practice [8]. Similarly, we cannot explore the specific immunosuppressive therapies that patients were taking or the duration of their therapy, which would be helpful in tailoring more nuanced prophylaxis recommendations. In addition, we cannot comment on the validity or utility of proposed laboratory monitoring for PCP risk stratification among IBD patients, including T-lymphocyte counts [21], CD4 counts [21], and serum albumin levels [30]. Furthermore, the use of administrative coding poses the risk of diagnostic misclassification, although the use of ICD coding in IBD has been previously validated in a variety of contexts [33-36]. To further minimize this risk, only diagnostic codes specific for IBD were included, resulting in the potential exclusion of patients with undetermined IBD from our study. However, given that undetermined IBD exists on a spectrum between ulcerative colitis and Crohn’s disease, without different guidelines for clinical management and immunosuppressive therapy, we anticipate a similar relationship with PCP within this cohort. Overall, we believe these limitations are largely offset by the comprehensive nature of the surveillance data and a focus on clinically more severe infections warranting admissions.
In conclusion, national admissions data indicate that PCP is increasing in frequency among patients with IBD. We postulate that this at least partially due to the rise of multidrug regimens in IBD therapy. However, PCP represents an overall very rare complication of IBD therapy. Almost one half of patients with PCP have at least one additional risk factor unrelated to their underlying IBD. These data do not support the routine use of PCP prophylaxis in the majority of patients with IBD; however, further study is needed to determine whether there are small subsets of IBD patients with more profound immune compromise who might benefit. Continued population-level surveillance of PCP in patients with IBD is indicated to ensure that the observed rise does not change this calculus.
Summary Box.
What is already known:
Patients with inflammatory bowel disease (IBD) are increasingly exposed to multiple classes of medications reported to increase the risk of Pneumocystis jiroveci pneumonia (PCP)
The national incidence of infection among patients with IBD is unknown
What the new findings are:
Admission for PCP is rare among patients with IBD and 49% of cases are associated with independent risk factors
Annual admission rates are rising, in contrast to national trends
Routine PCP prophylaxis is likely not necessary in the IBD patient population
Biography
Massachusetts General Hospital and Harvard Medical School; Brigham and Women’s Hospital and Harvard Medical School; Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA
Footnotes
Conference Presentation: This work was presented at American College of Gastroenterology’s Annual Meeting (Virtual; October, 2020) as a poster presentation
Data Transparency: Data, analytical methods and study materials will not be made available to other researchers
Conflict of Interest: None
References
- 1.Green H, Paul M, Vidal L, Leibovici L. Prophylaxis of pneumocystis pneumonia in immunocompromised non-HIV-infected patients:systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc. 2007;82:1052–1059. doi: 10.4065/82.9.1052. [DOI] [PubMed] [Google Scholar]
- 2.Liu CJ, Lee TF, Ruan SY, Yu CJ, Chien JY, Hsueh PR. Clinical characteristics, treatment outcomes, and prognostic factors of pneumocystis pneumonia in non-HIV-infected patients. Infect Drug Resist. 2019;12:1457–1467. doi: 10.2147/IDR.S199761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawrence SJ, Sadarangani M, Jacobson K. Pneumocystis jirovecii pneumonia in pediatric inflammatory bowel disease:A case report and literature review. Front Pediatr. 2017;5:161. doi: 10.3389/fped.2017.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwama T, Sakatani A, Fujiya M, et al. Increased dosage of infliximab is a potential cause of Pneumocystis carinii pneumonia. Gut Pathog. 2016;8:2. doi: 10.1186/s13099-016-0086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omer O, Cohen P, Neong SF, Smith GV. Pneumocystis pneumonia complicating immunosuppressive therapy in Crohns disease:A preventable problem? Frontline Gastroenterol. 2016;7:222–226. doi: 10.1136/flgastro-2014-100458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahier JF, Magro F, Abreu C, et al. European Crohn's and Colitis Organisation (ECCO) Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443–468. doi: 10.1016/j.crohns.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Farraye FA, Melmed GY, Lichtenstein GR, Kane SV. ACG Clinical guideline:preventive care in inflammatory bowel disease. Am J Gastroenterol. 2017;112:241–258. doi: 10.1038/ajg.2016.537. [DOI] [PubMed] [Google Scholar]
- 8.Okafor PN, Wasan SK, Farraye FA. Pneumocystis jiroveci pneumonia in patients with inflammatory bowel disease:a survey of prophylaxis patterns among gastroenterology providers. Inflamm Bowel Dis. 2013;19:812–817. doi: 10.1097/MIB.0b013e31828029f4. [DOI] [PubMed] [Google Scholar]
- 9.Feuerstein JD, Alvarez D, Curran T, et al. Surgery for ulcerative colitis in geriatric patients is safe without increased risks compared to younger patients who undergo surgery for ulcerative colitis no title. Gastroenterology. 2017;152:S782. doi: 10.1097/MEG.0000000000001529. [DOI] [PubMed] [Google Scholar]
- 10.Montgomery CL, Rolfson DB, Bagshaw SM. Frailty and the association between long-term recovery after intensive care unit admission. Crit Care Clin. 2018;34:527–547. doi: 10.1016/j.ccc.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Opstelten JL, Vaartjes I, Bots ML, Oldenburg B. Mortality after first hospital admission for inflammatory bowel disease:a nationwide registry linkage study. Inflamm Bowel Dis. 2019;25:1692–1699. doi: 10.1093/ibd/izz055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agency for Healthcare Research and Quality. Overview of the National (Nationwide) Inpatient Sample (NIS) 2020. [Accessed 24 March 2022]. Available from: https://www.hcup-us.ahrq.gov/nisoverview.jsp .
- 13.Chu YT, Ng YY, Wu SC. Comparison of different comorbidity measures for use with administrative data in predicting short- and long-term mortality. BMC Health Serv Res. 2010;10:140. doi: 10.1186/1472-6963-10-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quan H, Sundararajan V, Halfon P, Fong A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 15.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Romero-Ortuno R, Forsyth DR, Wilson KJ, et al. The association of geriatric syndromes with hospital outcomes. J Hosp Med. 2017;12:83–89. doi: 10.12788/jhm.2685. [DOI] [PubMed] [Google Scholar]
- 17.Center for Disease Control and Prevention. Inflammatory Bowel Disease (IBD) in the United States. November 9th, 2021. [Accessed 14 April 2022]. Available from: https://www.cdc.gov/ibd/data-statistics.htm .
- 18.Long MD, Farraye FA, Okafor PN, Martin C, Sandler RS, Kappelman MD. Increased risk of Pneumocystis jiroveci pneumonia among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1018–1024. doi: 10.1097/MIB.0b013e3182802a9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato T, Koshiba R, Kojima K, et al. P114 Risk factors and clinical characteristics for Pneumocystis jirovecii pneumonia in Japanese patients with ulcerative colitis. J Crohns Colitis. 2019;13(Suppl 1):S144–S145. [Google Scholar]
- 20.Kojima K, Sato T, Uchino M, et al. Clinical characteristics and risk factors for Pneumocystis jirovecii pneumonia during immunosuppressive treatment in patients with ulcerative colitis:a retrospective study. J Gastrointestin Liver Dis. 2020;29:167–173. doi: 10.15403/jgld-854. [DOI] [PubMed] [Google Scholar]
- 21.Cotter TG, Gathaiya N, Catania J, et al. Low risk of pneumonia from Pneumocystis jirovecii infection in patients with inflammatory bowel disease receiving immune suppression. Clin Gastroenterol Hepatol. 2017;15:850–856. doi: 10.1016/j.cgh.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu H, MacIsaac D, Wong JJ, et al. Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Aliment Pharmacol Ther. 2018;47:364–370. doi: 10.1111/apt.14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaur N, Mahl TC. Pneumocystis jiroveci (carinii) pneumonia after infliximab therapy:a review of 84 cases. Dig Dis Sci. 2007;52:1481–1484. doi: 10.1007/s10620-006-9250-x. [DOI] [PubMed] [Google Scholar]
- 24.Waljee AK, Wiitala WL, Govani S, et al. Corticosteroid use and complications in a US inflammatory bowel disease cohort. PLoS One. 2016;11:e0158017. doi: 10.1371/journal.pone.0158017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selinger CP, Parkes GC, Bassi A, et al. Assessment of steroid use as a key performance indicator in inflammatory bowel disease-analysis of data from 2385 UK patients. Aliment Pharmacol Ther. 2019;50:1009–1018. doi: 10.1111/apt.15497. [DOI] [PubMed] [Google Scholar]
- 26.Toruner M, Loftus EV, Jr, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929–936. doi: 10.1053/j.gastro.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Okafor PN, Nunes DP, Farraye FA. Pneumocystis jiroveci pneumonia in inflammatory bowel disease:when should prophylaxis be considered? Inflamm Bowel Dis. 2013;19:1764–1771. doi: 10.1097/MIB.0b013e318281f562. [DOI] [PubMed] [Google Scholar]
- 28.Mill J, Lawrance IC. Preventing infective complications in inflammatory bowel disease. World J Gastroenterol. 2014;20:9691–9698. doi: 10.3748/wjg.v20.i29.9691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glück T, Kiefmann B, Grohmann M, Falk W, Straub RH, Schölmerich J. Immune status and risk for infection in patients receiving chronic immunosuppressive therapy. J Rheumatol. 2005;32:1473–1480. [PubMed] [Google Scholar]
- 30.Yoshida A, Kamata N, Yamada A, et al. Risk factors for mortality in Pneumocystis jirovecii pneumonia in patients with inflammatory bowel disease. Inflamm Intest Dis. 2019;3:167–172. doi: 10.1159/000495035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bye WA, Jairath V, Travis SPL. Systematic review:the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3–15. doi: 10.1111/apt.14075. [DOI] [PubMed] [Google Scholar]
- 32.Vallabhaneni S, Chiller TM. Fungal infections and new biologic therapies. Curr Rheumatol Rep. 2016;18:29. doi: 10.1007/s11926-016-0572-1. [DOI] [PubMed] [Google Scholar]
- 33.Shrestha S, Olén O, Eriksson C, et al. The use of ICD codes to identify IBD subtypes and phenotypes of the Montreal classification in the Swedish National Patient Register. Scand J Gastroenterol. 2020;55:430–435. doi: 10.1080/00365521.2020.1740778. [DOI] [PubMed] [Google Scholar]
- 34.Stepaniuk P, Bernstein CN, Nugent Z, Singh H. Characterization of inflammatory bowel disease in elderly hospitalized patients in a large central Canadian health region. Can J Gastroenterol Hepatol. 2015;29:274–278. doi: 10.1155/2015/724359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz J, Stein DJ, Lipcsey M, Li B, Feuerstein JD. High rates of mortality in geriatric patients admitted for inflammatory bowel disease management. J Clin Gastroenterol. 2022;56:e20–e26. doi: 10.1097/MCG.0000000000001458. [DOI] [PubMed] [Google Scholar]
- 36.Lipcsey M, Stein DJ, DeVore ZG, Feuerstein JD. Rising rate of obesity in patients admitted for Crohn's disease increases costs but not mortality. J Clin Gastroenterol. 2021;55:716–720. doi: 10.1097/MCG.0000000000001421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


