Abstract
High-dose chemotherapy and autologous hematopoietic cell transplantation (HDT-AHCT) remains an effective therapy in lymphoma. Over the past several decades, HDT with BEAM (carmustine, etoposide, cytarabine, and melphalan) and CBV (cyclophosphamide, carmustine, and etoposide) have been the most frequently used preparatory regimens for AHCT in Hodgkin (HL) and non-Hodgkin lymphoma (NHL). This article reviews alternative combination conditioning regimens, as well as novel transplant strategies that have been developed, to reduce transplant-related toxicity while maintaining or improving efficacy. These data demonstrate that incorporation of maintenance therapy posttransplant might be the best way to improve outcomes.
Introduction
High-dose chemotherapy and autologous hematopoietic cell transplantation (HDT-AHCT) is an established therapeutic approach in lymphoma treatment, either as upfront therapy, or most commonly, in the relapsed or refractory (rel/ref) setting [1–5]. However, lymphoma recurrence continues to be the major cause for transplant failure. Efforts to develop more effective high-dose regimens include dose intensification of the regimens or integrating new agents into the combination regimens. In this article, we first review the traditional transplant approaches with established efficacy in lymphoma subtypes, followed by more novel conditioning regimens and transplant strategies to mitigate risk of relapse.
History of HDT-AHCT
The concept of a steep dose–response relationship for anticancer drugs dates back to the 1960s when Skipper and coworkers predicted a log cell kill model for antineoplastic drugs. In this model, the relationship between tumor cell kill and drug dose was exponential, with the number of cells killed by a given dose of drug being proportional to both the dose of the drug and the number of cells exposed to the drug [6, 7]. HDT exploits the steepness of the dose–response relationship between chemotherapeutic drugs and fractional cell kill [8, 9]. The steepness of the dose–response curve implies that a disproportionately high number of cancer cells are killed when drug doses are increased.
Initial use of HDT followed by autologous bone marrow cell infusion for lymphomas was reported in 1959 and 1960s [10–13]. In 1978, investigators at the National Cancer Institute reported successful treatment of resistant malignant lymphoma and Burkitt lymphoma with HDT and AHCT [14, 15]. HDT-AHCT as a successful treatment for patients with relapsed Hodgkin lymphoma (HL) was first reported in the 1980s [16–18]. Subsequent trials have demonstrated HDT-AHCT as the standard of care for management of rel/ref lymphomas [5, 19–22] or as consolidation of a first remission for mantle cell and T cell lymphoma [2, 4, 23–31]. The Center for International Blood and Marrow Transplant Research (CIBMTR) has reported a marked increase between 1994 and 1995 and between 2004 and 2005 in the number of HDT-AHCT performed in North America from 2573 to 3164 for non-Hodgkin lymphoma (NHL) and from 906 to 1302 for HL [32]. Interestingly, the proportion of patients aged ≥60 years who underwent AHCT during the same period rose from <7 to 35%, including an increase in those who are at least 70 years old from <1 to 5%. This increase may reflect changing demographics, patient selection, improved access, and safety of HDT-AHCT in this population, as well as better acceptance by the third-party payers because of its success.
HDT-AHCT in lymphoma subtypes
Hodgkin lymphoma
Durable remissions can be obtained using HDT-AHCT in HL patients whose disease has relapsed or was refractory to conventional therapy [33–36]. Two randomized trials have shown improvement in disease-free survival (DFS) with HDT-AHCT compared to conventional chemotherapy [19, 20]. Investigators in the British National Lymphoma Investigation trial prospectively randomized rel/ref HL patients to either chemotherapy or HDT-AHCT. The 3-year event-free survival (EFS) was 53% in transplanted patients, compared to 10% in the chemotherapy group (p = 0.025). The risk of disease progression was significantly lower (p = 0.005) in transplanted patients, although no significant differences in overall survival (OS) were observed. In the other randomized trial, conducted by the German Hodgkin Study Group and the European Society of Blood and Marrow Transplantation (EBMT), patients received two cycles of salvage chemotherapy with Dexa-BEAM (dexamethasone, carmustine, etoposide, cytarabine, melphalan). Responding patients then received either two additional cycles of Dexa-BEAM or high-dose BEAM-AHCT. The freedom from treatment failure at 3 years was 55% in subjects undergoing transplant, compared with 34% in patients who received Dexa-BEAM alone (p = 0.019). OS did not significantly differ between the two groups (p = 0.331). Both of these randomized clinical trials, however, were closed early because of poor accrual, mainly due to patient preferences, where they did not want to get randomized to chemotherapy-only arm. This issue has been a known challenge in executing and completing transplant vs no-transplant studies across many disease histologies.
Non-Hodgkin lymphoma
Diffuse large B cell lymphoma
HDT-AHCT has become the standard consolidative therapy in rel/ref aggressive NHL [5]. This practice is based on the PARMA trial, a randomized, multicenter trial that compared HDT-AHCT vs chemotherapy in 215 patients who had relapsed but chemotherapy-sensitive intermediate-to-high-grade NHL [5]. All patients had achieved a first remission (complete remission 1 (CR1)) with an anthracycline-containing chemotherapy regimen. At the time of relapse, patients received two cycles of DHAP (dexamethasone, high-dose cytarabine, and cisplatin). Subsequently, they were randomized to receive either four more cycles of DHAP or HDT-AHCT. After a median follow-up of 63 months, the response rate was 84% after HDT-AHCT vs 44% after conventional therapy. At 5 years, EFS and OS were 46% and 53% in the transplant group compared to 12% (p = 0.001) and 32% (p = 0.038) in the chemotherapy only group, respectively. These differences might have been even larger in favor of transplant, were it not for the fact that patients whose disease progressed on the conventional arm could cross over to the transplant arm. This study demonstrated survival benefit in patients affected with relapsed chemotherapy-sensitive NHL who underwent HDT-AHCT as compared to conventional chemotherapy and therefore established this approach as the standard treatment.
Follicular lymphoma (FL)
FL is frequently accompanied by bone marrow infiltration. Therefore, one of the concerns with HDT-AHCT in FL is the risk of reinfusing the lymphoma cells after HDT. Schouten and colleagues [3] designed a randomized trial to address two questions: (1) whether HDT-AHCT is more effective than standard treatment in improving survival in relapsed FL patients, and (2) whether ex vivo purging of the hematopoietic cell graft could positively impact progression-free survival (PFS) and OS. This randomized CUP trial (Chemotherapy vs Unpurged vs Purged arm) evaluated the role of HDT-AHCT vs conventional therapy in 140 patients who had rel/ref FL. The 4-year OS was 46% for the chemotherapy arm, 71% for the unpurged graft arm, and 77% for the purged graft transplant arm. There was a similar advantage for the transplant groups in 2-year PFS, which was 26, 58, and 55%, respectively (p = 0.0037). This trial showed that HDT-AHCT results in improved PFS and OS in patients with relapsed FL. It further showed that purging of the hematopoietic cell graft does not impact transplant outcomes.
In a study by the GELA/GOELAMS group, 175 patients with rel/ref FL underwent either conventional chemotherapy or HDT-AHCT. At a median follow-up of 31 months, the 3-year OS was significantly higher in patients who received HDT-AHCT at first relapse (92% vs 63%; p = 0.0003) [37]. Furthermore, Casulo and colleagues [38] evaluated outcomes of FL patients experiencing early therapy failure within 2 years of frontline chemoimmunotherapy in 2 cohorts of patients: (1) non-transplant patients, from the National LymphoCare (NLCS) database and (2) patients who underwent HDT-AHCT, from the CIBMTR. There was no difference in 5-year OS between the 2 groups (60% vs 67%, respectively; p = 0.16). However, patients receiving HDT-AHCT within 1 year of treatment failure had higher 5-year OS than those without transplant (73% vs 60%, p = 0.05). Results from the above studies support consideration of HDT-AHCT in patients with chemotherapy-sensitive relapsed FL, especially in those with early relapsed disease within 2 years of frontline therapy. Long-term outcomes of HDT-AHCT in FL demonstrating plateaus in PFS curves [39–41] suggest cure in a select group of patients with this therapy.
Mantle cell lymphoma (MCL)
Several studies have shown improved outcomes with HDT-AHCT in MCL patients in first remission [2, 23–28, 42–44]. In a randomized trial, the European MCL Network compared consolidation with high-dose cyclophosphamide plus total body irradiation (TBI) and AHCT to maintenance therapy with alpha-interferon in 122 patients, after subjects achieved first remission using a CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone)-like induction therapy. A longer PFS in the transplant arm of 39 vs 17 months (p = 0.01) was observed, even though the 3-year OS was not significantly superior [28]. In a subsequent pooled analysis of three studies presented as an abstract form only, the median OS seemed to be superior in the AHCT arm after extended follow-up (90 vs 54 months, p = 0.034) [45]. In a phase 2 study by the Nordic Lymphoma Group (MCL-2) in 160 patients with MCL who received induction treatment with augmented CHOP [cyclophosphamide 1200 mg/m2 (instead of 750 mg/m2), doxorubicin 75 mg/m2 (instead of 50 mg/m2), vincristine, and prednisone] alternating with high-dose cytarabine combined with rituximab, followed by HDT-AHCT in responders, the 6-year OS, EFS, and PFS were 70, 56, and 66% respectively. In the intent-to-treat analysis, the 10-year OS and EFS for all 160 patients was 58% and 43%, respectively [27]. In a phase 2 trial using sequential R (rituximab)-CHOP/R-DHAP followed by HDT-AHCT, Delarue et al. reported an overall response rate (ORR) of 95% with median EFS of 83 months and a 75% survival rate at 5 years [43]. In a recent analysis of the CIBMTR data, 159 patients received HDT-AHCT or allogeneic hematopoietic cell transplant (allo-HCT) for MCL in first or subsequent remissions [46]. Both transplant strategies resulted in similar OS. However, the study suggests that the optimal timing for transplant is early in the disease course defined as first partial or CR, with no more than two prior lines of therapy. Freedman and colleagues reported outcomes of 28 patients who underwent HDT-AHCT for MCL at the completion of induction (n = 8) or salvage (n = 20) therapy [47]. The 4-year DFS and OS for all 28 patients were estimated to be 31% and 62%, respectively. The 8 patients transplanted in CR1 experienced better DFS than the 20 patients transplanted after relapse (49 vs 21 months, p = 0.03). In a large registry study of AHCT in 191 MCL patients, the 2- and 5-year OS were 76% and 50% and PFS were 55% and 33%, respectively. Patients with chemosensitive disease but not in first CR were 2.99 times (95% confidence interval (CI): 1.66–5.38, P < 0.001) more likely to die than patients transplanted in CR1 [48]. The above studies demonstrate that the outcomes of HDT-AHCT in MCL beyond CR1 is less favorable and associated with higher relapse rates.
T cell lymphoma
There are no randomized prospective studies in T cell lymphoma comparing HDT-AHCT to conventional therapy in first line or relapsed setting. However, favorable outcomes have been reported by employing HDT-AHCT as consolidation in first remission [4, 29–31, 49–52]. The largest phase 2 study of upfront HDT-AHCT [4] included 160 patients with peripheral T cell lymphoma (PTCL) who received CHOEP (cyclophosphamide, doxorubicin, etoposide, vincristine, and prednisone) for 6 cycles and those in CR or partial remission underwent HDT-AHCT (n = 115). By intent-to-treat analysis, the 5-year OS and PFS were 51% and 44%, respectively. In another prospective phase 2 study by Corradini and coworkers [50], the estimated 12-year OS, DFS, and EFS were 34, 55 and 30%, respectively, in 46 of the 62 patients who received upfront HDT-AHCT. OS and EFS were significantly better in patients with ALK-positive anaplastic large-cell lymphoma (ALCL), as compared with the remaining PTCL. Analyzing separately the subgroup of PTCL unspecified, the 12-year OS and EFS projections were 37% and 25%, respectively. A prospective German study [52] evaluated outcomes of 111 patients who were planned to undergo upfront HDT-AHCT for T cell lymphoma. Seventy-five (68%) patients received transplantation. By intent-to-treat analysis, the estimated 5-year OS, DFS, and PFS rates were 44, 54 and 39%, respectively. Several retrospective studies in patients with T cell lymphoma have shown promising outcomes of HDT-AHCT when employed in first remission, less favorable outcomes in second remission, and disappointing results in refractory setting [53–56]. One of the largest retrospective study performed by CIBMTR reported outcomes of 241 patients with T cell lymphoma undergoing AHCT (n = 115) or allo-HCT (n = 126) [55]. The 3-year PFS and OS of AHCT recipients beyond CR1 were 42% and 53%, respectively. Among allo-HCT recipients who received transplantations beyond CR1, the 3-year PFS and OS were 31% and 50% respectively. Of note, this study suggests that HDT-AHCT at relapse may be a potential option for select patients, particularly in those with ALCL histology. In the absence of randomized controlled studies, available evidence from retrospective and non-randomized prospective studies suggest that HDT-AHCT offers greater effectiveness earlier in the disease course. In patients with rel/ref T cell lymphoma, while HDT-AHCT may be a potential option in select patients who achieve CR, allo-HCT remains a valuable treatment strategy for appropriately selected patients.
Chemotherapeutic agents commonly used in AHCT
Treatment regimens prior to AHCT are administered for tumor cytoreduction and to ideally eradicate disease. The cornerstones in HDT are alkylating agents such as melphalan, busulfan, thiotepa, cyclophosphamide, and bendamustine. Alkylators do not generally show cross-resistance and have steep concentration–response [57]. Their activity is dependent on the extent of DNA damage and repair. Thus combination of alkylating agents with drugs known to inhibit DNA damage repair is expected to result in synergistic effect. In HDT, alkylators are frequently combined with other agents from the same or other classes to improve efficacy and overcome drug resistance. Etoposide, a topoisomerase II inhibitor, causes DNA break and cell-cycle arrest. This mechanism of action may promote synergistic cytotoxicity with alkylators [58]. The suggested mechanism of the synergistic cell killing of nucleoside analogs such gemcitabine is that they inhibit DNA synthesis and repair, which results in DNA damage, making it more accessible to DNA alkylation [59, 60]. Carmustine (BCNU) is a nitrosourea commonly used in HDT. However, in conventional doses, BCNU is limited by delayed marrow toxicity, pulmonary fibrosis, and hepatic renal dysfunction. When BCNU is used in combinations that include cyclophosphamide, there is an increased risk of pneumonitis and veno-occlusive disease (VOD) [61, 62]. Based on the synergism for antitumor effect of chemotherapeutic agents and their none or low overlapping toxicities, several combination regimens for HDT-AHCT in lymphoma have been developed. However, there are no prospective randomized studies to compare different conditioning regimens for AHCT in lymphoma. The most widely used high-dose conditioning regimens in HL and NHL are those based on a carmustine backbone, BEAM (carmustine, etoposide, cytarabine and melphalan) and CBV (cyclophosphamide, carmustine, and etoposide). This is demonstrated by the large retrospective registry study of patients with NHL and HL (n = 4917) who underwent AHCT from 1995 to 2008 [63]. The most common preparatory regimens used were BEAM (n = 1730), CBV (n = 1853), BuCy (busulfan, cyclophosphamide) (n = 789), and TBI-containing regimens (n = 545). The 1-year incidence of idiopathic pulmonary syndrome was the highest in recipients of CBV (hazard ratio (HR) 1.9) and TBI (HR 2.0) compared to BEAM. While the 1-year transplant-related mortality (TRM) was 4–8% and similar between regimens, in patients with diffuse large B cell lymphoma (DLBCL) and HL, BEAM was associated with lower mortality. A retrospective analysis of the EBMT database [64] comparing BEAC (carmustine, etoposide, cytarabine, and cyclophosphamide) to a matched cohort of NHL patient conditioned with BEAM showed no difference in toxicity or outcome. The 2-year PFS and OS were 63% and 78% for BEAC and 63% and 77% for BEAM-conditioned patients [p = not significant (ns) for PFS and OS]. The 1-year cumulative incidence of non-relapse mortality (NRM) was 4% in the BEAC cohort and 3% in the BEAM group (p = ns).
Novel carmustine-free HDT-AHCT in lymphoma
In recent years, owing to restricted availability, high drug acquisition cost, and toxicity concerns, investigators around the world have substituted carmustine with other agents (Table 1). Two retrospective studies evaluated replacement of carmustine in the BEAM regimen using thiotepa and cyclophosphamide, i.e., the TECAM regimen (thiotepa, etoposide, cyclophosphamide, cytarabine, and melphalan) [65, 66]. In 212 NHL and HL patients who had undergone TECAM-AHCT from 2000 to 2013, Grisariu and co-workers [65] reported no idiopathic pneumonitis, but 6 patients died of treatment-related toxicity during the first 100 days. The 3-year OS among DLBCL and HL patients was 61% (95% CI, 0.490–0.722) and 82% (95% CI, 0.701–0.904), respectively. The 3-year PFS was 49% (95% CI, 0.36–0.60) for DLBCL patients and 50% (95% CI, 0.37–0.61) for HL patients. Joffe and colleagues [66] compared outcomes of TECAM to BEAM in 125 consecutive patients affected with B cell lymphomas who underwent AHCT between. TECAM (n = 65) and BEAM (n = 60) had comparable results [3-year PFS 49% vs 62%, p = 0.16; 3-year OS 64% vs 71%, p = 0.44; TRM 1.6% vs 5%, p = 0.35] without a difference in toxicity or time to engraftment. In the EBMT registry-based retrospective study, thiotepacontaining preparative regimens were compared to BEAM [67]. No significant differences were identified between thiotepa-based and BEAM regimen for any survival end points. In a more detailed analysis, where 47 TEAM-treated patients were compared with 75 matched BEAM recipients, there were no significant differences between the two groups for any survival end points [67]. In addition, the frequency of common infectious and non-infectious complications including secondary malignancies was comparable between TEAM and BEAM. The above studies indicate that thiotepa-based HDT can be an alternative regimen to traditional BEAM, with comparable efficacy and safety profile. These results justify further evaluation of this regimen in a prospective, multicenter study.
Table 1.
Carmustine-free BEAM and CBV-like regimens
| Reference | Conditioning regimen patients (n) | Histology | Median age (range) | Early TRM | PFS/OS | Comments |
|---|---|---|---|---|---|---|
|
| ||||||
| Grisariu et al. [65] | TECAM 212 2000–2013 Phase 2 | NHL HL (DLBCL, FL, MCL, TCL) | 46 (16–71) | 6 by d100 | 3-y PFS/OS DLBCL 49/61% HL 50/82% | 4 died of infection, 2 from MOF |
| Joffe et al. [66] | TECAM 65 (1999–2006) vs BEAM 60 (2007–2014) | NHL HL | 55 (16–75) vs 43 (16–68) | 1.6% vs 5% (p = ns) | 3-y PFS/OS 49/64% vs 62%/71% (p = ns) | Comparable efficacy and safety |
| Sellner et al. [67] | TEAM 110 vs BEAM 199 (2003–2011) | NHL HL | 44 (19–66) vs 44 (19–72) | 1% vs 2% | 30-mo PFS/OS 49/77 vs 62/77 (p = ns) | Comparable efficacy and toxicities |
| Visani et al. [68] | BeEAM 43 | NHL HL | 47(18–70) | 0 | 30-mo DFS/OS 65/90% | Mucositis, diarrhea, liver enzyme/bilirubin |
| Chantepie et al. [69] | BeEAM 474 | NHL HL | 56 (17–71) | 3.3% | ARF 132 (27.9%) (Gr>2; 12.3%) | |
| Saleh et al. [106] | BeEAM 34 vs BEAM 68 | NHL HL | 49 (21–68) vs 52 (21–71) | 0 for both | Comparable PFS OS not reached | Gr > 3 diarrhea and fever higher in BeEAM |
| Tsang et al. [107] | BeEAM 26 vs BEAM 52 (2009–2016) | HL | 34 (17–68) | 0 | 2-y PFS/OS 63%/91% vs 56%/87% | Grade 3–4 mucositis more common with Be-EAM 35% than BEAM 10% |
| Boisjoly et al. [108] | BEAM/BEAC 96 vs BeEAM 50 (2012–2016) | NHL HL | 55 (23–69) vs 57 (26–70) | 0 | OS comparable | Be-EAM more toxic (Pit recovery, ARF, TPN, infection, ICU), but cost-effective |
| Nathan et al. [109] | R-BeEAM 22 vs R-BEAM 36 (2011–2013) | B-NHL | 60 (19–75) | 1 sepsis death in BeEAM on d26 | Similar 2-y PFS and OS | Grade 3–1 mucositis more in Be-EAM 59.1% vs 5.6% |
| Ninos et al. [110] | BEAM 40 vs BeEAM 40 (2012–2017) | 54 | Comparable engraftment outcomes | |||
| Jaimovich et al. [70] | Benda-CV 54 | NHL HL | 47 (18–74) | 1.9% | 6-mo OS 93% | Gr 3–4 fever, cardiac, nausea, diarrhea, mucositis respiratory |
| Jain et al. [111] | BACE 17 (2012–2014) | NHL HL | 0 | Neutropenic sepsis 14 Klebsiella 8 No pulmonay toxicity | ||
| Chakrabartty et al. [112] | BACE 12 (2014–2016) | NHL HL | 0 | No Gr 3–4 non-heme toxicity | ||
| Kim et al. [71] | NEAM 69 | NHL | 42 (20–66) | 3% | EFS 17 mo 2-y OS 64% | Liver toxicity 10% Renal 3% Cardiac 3% |
| Sharma et al. [73] | TEAM 17 vs BEAM 34 (2001–2012) | NHL HL | 12 | 2-y PFS/OS 41/62 vs 44/61 (p = ns) | Similar efficacy and toxicities | |
| Kothari et al. [74] | TEAM 50 vs BEAM 50 | NHL HL | 52 (45–52) 51 (45–52) | 4% vs 2% (p = ns) | 3-y PFS/OS 69/86% vs 75/86% (p = ns) | Comparable efficacy and toxicities |
| Sakellari et al. [113] | BuEM 50 vs BEAM 87 | NHL HL | 0% vs 3.4% p = ns | PFS/OS 65/78 vs 63/76 (p = ns) | ||
| Berber et al. [114] | BuCyE 31 vs BEAM 11 (2010–2015) | NHL HL | 26–54 24–46 |
6.5% | Comparable efficacy and toxicities | |
| Zhang et al. [115] | BuCyE 294 (1999–2010) | NHL HL | 47 (17–69) 2–3.5% | 2-y OS 71% 5-y OS 63% | Hyperbili 38% No VOD No gr3–4 neurotoxicity |
|
| Kim et al. [116] | BuCyE 22 vs BEAM 43 | NHL | 46 (28–65) 46 9% (15–65) | EFS/OS 11/22 mo vs 16/30 mo p = ns | Comparable efficacy and toxicities | |
| Kim et al. [77] | BuCyE 64 | NHL | 43 (18–65) 3.1% | 3-y PFS/OS 70/72% | 4 VOD | |
| Wadehra et al. [75] | BuCyE 127 | HL | 33 (14–67) 5.5% | 5-y PFS/OS 48/51% | 4 died from hepatic toxicity, 3 from pneumonitis Second malignancy 9% 3 MDS/AML, 1 bladder ca |
|
| Hanel et al. [117] Copelan et al. [76] | BuCyE 53 (1990–2000) BuCyE 382 (1992–1998) | NHL HL NHL | 46 (18–64) 4% 16–72 10 (2.6%) in 1 year |
3-y PFS/OS 31%/43% 3-y PFS 46% | VOD 6%, Liver tox mucositis 11 VOD; 4 MDS/AML Cause of death: 4 respiratory failure 3 VOD 2 infection 1 bleeding |
|
TRM treatment-related mortality, PFS progression-free survival, DFS disease-free survival, EFS event-free survival, OS overall survival, d day, y years, mo months, NS not significant, NHL non-Hodgkin lymphoma, HL Hodgkin lymphoma, TCL T cell lymphoma, BEAM [carmustine, etoposide, cytarabine, melphalan], CBV [cyclophosphamide, carmustine, etoposide], R rituximab, TECAM [thiotepa, etoposide, cyclophosphamide, cytarabine, melphalan], BeEAM [bendamustine, etoposide, cytarabine, melphalan], Benda-CV [bendamustine, cyclophosphamide, etoposide], BACE [bendamustine, cytarabine, cyclophosphamide, etoposide], BEAC [carmustine, etoposide, cytarabine, cyclophosphamide], NEAM [mitoxantrone, etoposide, cytarabine, melphalan], LEAM [lomustine, etoposide, cytarabine, melphalan], BuCyE [busulphan, cyclophosphamide, etoposide], ARF acute renal failure, VOD veno-occlusive disease, AML acute myeloid leukemia, MDS myelodysplastic syndrome, MOF multiorgan failure
Viani and colleagues substituted bendamustine for carmustine in BEAM (BeEAM) [68] in 43 NHL patients undergoing AHCT. No grade III–IV nephrotoxicity, interstitial pneumonitis, idiopathic pneumonia, or cardiotoxicity were observed. No episode of hepatic VOD was reported. TRM at day 100 was 0%. In a French multicenter study of 474 lymphoma patients where BeEAM regimen was used, the observed grade 1–4 toxicities included mucositis (83.5%), gastroenteritis (53%), skin toxicity (34%), colitis (29%), liver toxicity (19%), pneumonitis (5%), and cardiac rhythm disorders (4%). Acute renal failure (ARF) was observed in 132 cases (27.9%). Organ toxicities and death were more frequent in patients who developed post-conditioning renal failure. In a multivariate analysis, pre-transplant chronic renal failure, bendamustine dose 160 mg/m2 and age were independent prognostic factors for ARF [69]. Multiple other studies have compared BEAM to BeEAM retrospectively, showing comparable engraftment rates and survivals, but with a slight increase in BeEAM-associated toxic effects (Table 1). Other conditioning regimens using bendamustine are BACE (bendamustine, cytarabine, cyclophosphamide, and etoposide) and Benda-CV (bendamustine, cyclophosphamide, and etoposide). Jaimovich et al. [70] conducted a multi-center, prospective phase 2 study evaluating the safety and efficacy of Benda-CV. Toxicity profile was similar to that usually observed in the AHCT setting.
Kim and colleagues [71] replaced carmustine in BEAM with mitoxantrone (NEAM) in 69 patients harboring chemosensitive, aggressive NHL. Median EFS was 17.9 months, with an estimated 2-year OS of 64.2%. Febrile neutropenia was seen in 61 patients (88.4%). Grade 3 or 4 hepatic toxicity developed in 7 patients (10.1%), grade 3 or 4 renal toxicity in 2 patients (2.9%), and grade 3 or 4 cardiac toxicity in 2 patients (2.9%). Two patients (2.9%) developed TRM [71].
A large multicenter retrospective study conducted by Olivieri and colleagues [72] compared safety and efficacy of BEAM and FEAM (fotemustine, etoposide, cytarabine, and melphalan). FEAM conditioning resulted in higher rates of gastrointestinal and infectious toxicities. Mortality from infection was higher in the FEAM group (HR 1.99; 95% CI:1.02–3.88, p = 0.04). This study does not support fotemustine substitution for carmustine due to concerns of higher toxicity.
Lomustine has been used instead of carmustine in the BEAM regimen [73, 74]. The largest comparison of the LEAM (lomustine, etoposide, cytarabine, and melphalan) vs BEAM showed no significant differences in NRM, OS, PFS, and in any of the toxicity parameters between the two cohorts. The most common grade 3–4 toxicities observed in both approaches were stomatitis (30%), diarrhea (50%), and nausea (16% for LEAM and 6% for BEAM). Grade 3–4 hepatic and renal toxicity was infrequent [74].
Busulfan has replaced carmustine in several lymphoma conditioning regimens. Wadehra and co-workers reported the outcomes of 127 HL patients who underwent BuCyE (busulfan, cyclophosphamide, and etoposide)-AHCT [75]. The regimen was well tolerated, with 5.5% TRM at 100 days. At a median follow-up of 6.7 years, the 5-year PFS was 48%, and the 5-year OS was 51%. Five patients died between 5.3 and 9.3 years of late complications, including secondary myelodysplasia or acute myeloid leukemia (MDS/AML) (2%), bladder cancer (1%), pulmonary toxicity (1%), and an overall 9% 8-year risk of second solid malignancy. In the largest study involving 382 NHL patients using BuCyE conditioning [76], mucositis was the most common toxicity. Severe hepatic VOD occurred in 11 patients (2.9%) and MDS/AML (1%) [76]. In another study, Kim and colleagues [77] evaluated the efficacy and toxicity of BuCyE in 64 patients with rel/ref NHL. Hepatic VOD was observed in 4 patients, and 2 (3.1%) died from treatment-related complications. At a median follow-up of 16.4 months, 15 patients (23.4%) had progressed, while 13 subjects (20.3%) had died of disease. The estimated 3-year OS and PFS overall for all patients were 72% and 70%, respectively. Other studies of BuCyE regimen are listed in Table 1 [77].
Over the past years, several other regimens have been developed (Table 2). Tarella and coworkers [78] reported a 7-year OS and 6.7-year failure-free survival projection of 77% and 69%, respectively, with melphalan and mitoxantrone (Mito/Mel). The toxicities included grade 3–4 mucositis, cardiotoxicity, sepsis, colitis, and deep vein thrombosis. There was one fatal event due to severe pancytopenia following abdominal radiation.
Table 2.
Other transplant conditioning approaches in lymphoma
| Reference | Conditioning patients (n) | Histology | Median age (range) | Early TRM | PFS/OS | Comments |
|---|---|---|---|---|---|---|
|
| ||||||
| Nieto et al. [80] | GemBuMel 133 2007–11 | HL 80 NHL 46 MM 7 |
41(18–65) | 2 | 2-y EFS/OS HL 54/72% BNHL 60/89% TNHL 70/70% MM 43/43% | Major toxicity was mucositis 2 died from infection |
| Nieto et al. [83] | GemBuMel 84 BuMel 39 BEAM 57 2005–10 | HL | 32 (17–65) | 0 | 3-y EFS/OS 57/82 33/52 39/59 | GemBuMel improved outcomes compared with BuMel and BEAM |
| Nieto et al. [82] | GemBuMel 80 vs BEAM 45 | HL | 31(13–65) vs 38 (17–65) | 0 | 2-y PFS/OS 65/89 vs 51/73 | More mucositis, dermatitis, transaminase, Hyperbili in GemBuMel |
| Nieto et al. [118] | GemBuMel 32 vs BEAM 84 | TCL | 47 (16–67) vs 59 (14–78) | 0 | DFS/OS 76/75% vs 40/60% | GemBuMel appear superior |
| Davidson et al. [84] | Gem/mel 42 | NHL HL | 52 (38–66) | 0 | 10-mo PFS 59%, OS 93% | Gr 3–1 non-hem events: mucositis (7), colitis (5), pneumonia (4), sepsis (2), diarrhea (1), esophagitis (1), emesis (1), transaminitis (1), pulmonary edema (1), stroke (1) |
| Tarella et al. [78] | Mito/Mel 113 | DLBCL | 48 (18–66) | 4.5% infect | 4-y EFS/OS 76/73% | Gr 3–4 mucositis, cardiac, sepsis, colitis, DVT |
| Villa etal. [119] | VP 16/MET. 50 | NHL | 50 (29–63) | 2% d100 | 3-y PFS/OS 42%/54% | |
| Ji et al. [120] | ChiCGB 43 2015–17 | Poor-risk NHL HL | 34 (22–62) | 0 | Median f/u 10 mo (3–26 mo) PFS 78% OS 89% |
Grade 3 neutropenic fever, mucositis, dermatitis |
| Chahoud et al. [90] | R-BEAM 73 vs RIT-BEAM 40 | DLBCL | 52 (19–69) | 5-y PFS/OS 62%/73% vs 65%m% | Addition of 90YIT does not confer a further survival benefit | |
| Bento et al. [89] | BEAM 1973 R-BEAM 207 RIT-BEAM 179 | FL | 56(19–77)55 (25— 72) 55 (25–70) | 3% in all | 63/82% 63/88% 62/86% (p = ns) | No difference in relapse, TRM, EFS, OS |
| Chow Victor et al. [91] | RIT-CyE 108 | NHL | 52 (32–60) | d30 0 d100 2.8% |
5-y PFS 50–60% OS 67–80% | 2nd cancer in 5.6% |
TRM treatment-related mortality, PFS progression-free survival, DFS disease-free survival, OS overall survival, EFS event-free survival, d day, y years, mo months, NS not significant, NHL non-Hodgkin lymphoma, HL Hodgkin lymphoma, TCL T cell lymphoma, BNHL B cell non-Hodgkin lymphoma, TNHL T cell non-Hodgkin lymphoma, MM multiple myeloma, BEAM [carmustine, etoposide, cytarabine, melphalan], CBV [cyclophosphamide, carmustine, etoposide], R rituximab, GemBuMel [gemcitabine, busulphan, melphalan], MITO mitoxantrone, ChiCGB [chiamide, cladribine, gemcitabine, busulfan], CyE [cyclophosphamide, etoposide], ARF acute renal failure, VOD veno-occlusive disease, RIT radioimmunotherapy, DVT deep venous thrombosis
Crump and colleagues [79] reported safety and efficacy of etoposide and melphalan (Eto/Mel) conditioning regimen in 73 patients with rel/ref HL. The most common toxicities were mucositis and diarrhea. However, cardiopulmonary toxicities and VOD were observed too. The 7 deaths related to AHCT were from infection (n = 3), interstitial pneumonitis (n = 3), and intracranial hemorrhage (n = 1). All cases of pneumonitis had received mantle and lung radiation immediately before AHCT.
Nieto and colleagues evaluated a combination of gemcitabine, busulfan and melphalan (GemBuMel) as conditioning regimen for AHCT in refractory NHL and HL [80–83]. Mucositis was the major toxicity. Two patients died from early posttransplant infections. Overall and complete response rates, respectively, were 87% and 62% (HL), 100% and 69% (B-NHL), 66% and 66% (T-NHL), and 71% and 57% (myeloma). At median follow-up of 24 months, the EFS and OS rates, respectively, were 54% and 72% (HL), 60% and 89% (B-NHL), 70% and 70% (T-NHL,) and 43% and 43% (myeloma). Furthermore, when this regimen was compared to BEAM conditioning, there were no transplant-related deaths in either cohort. Toxicities included mucositis, dermatitis, tranaminitis, and hyperbilirubinemia. At a median follow-up of 34.5 months, GemBuMel was associated with a better 2-year PFS (65% vs 51%; p = 0.008) and overall survival (89% vs 73%; p = 0.0003). GemMel regimen in NHL and HL [84] resulted in several grade 3–4 nonhematologic toxicities, including mucositis, infectious colitis, pneumonia, sepsis, non-infectious diarrhea, esophagitis, emesis, transaminitis, pulmonary edema, and stroke.
Taken together, the above studies show that the substitution of carmustine with other chemotherapy agents such as thiotepa, bendamustine, lomustine, mitoxantrone, and busulfan in HDT is safe, with comparable engraftment and survival outcomes. While the incidence of pulmonary complications in the carmustine-free regimens is lower, other non-hematologic complications such as renal and gastrointestinal toxicities with bendamustine, VOD with busulfan, and cardiologic and liver toxicities with mitoxantrone need to be taken into consideration when choosing an alternative regimen. Although GemBuMel seems to be a reasonable alternative conditioning regimen in refractory disease, one cannot discount investigator bias in choosing the approach.
Alternative and innovative transplant strategies in lymphoma
Radioimmunotherapy (RIT)-based conditioning regimen
Incorporation of RIT into a conditioning regimen for B cell NHL to increase transplant efficacy have been evaluated. Studies have shown this approach to be safe and associated with encouraging results [85, 86]. In a phase 1 study, Vose and colleagues [85] evaluated 23 patients with rel/ref B-NHL who underwent AHCT with BEAM combined with the radioimmunoconjugate iodine-131 tositumomab. The complete response rate after transplantation was 57%. Short- and long-term toxicities were similar to historic control patients treated with BEAM alone. With a median follow-up of 38 months, the OS and EFS were 55% and 39%, respectively. A phase 2 study conducted by Krishnan and co-workers [86] evaluated safety and efficacy of yttrium-90 ibritumomab tiuxetan combined with BEAM and AHCT in 41 B-NHL patients. At a median follow-up of 18.4 months, the estimated 2-year OS and PFS were 88.9% and 69.8%, respectively. Adverse events were similar to those seen historically with use of BEAM-AHCT alone and included grade 3–4 pulmonary toxicity in 10 patients. A randomized study of ibritumomab tiuxetan (RIT)-BEAM vs BEAM in rel/ref aggressive lymphoma showed that RIT-BEAM is safe and possibly more effective than BEAM alone [87]. The multicenter phase 3 trial, conducted by the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) where patients with chemosensitive, relapsed DLBCL were randomized to either rituximab (R)-BEAM (n = 113) or 131I-tositumomab (RIT)-BEAM (n = 111), showed no significant differences in 2-year PFS (48.6% vs 47.9%, p = 0.97) and 2-year OS (66% vs 61%, p = 0.38). TRM also was comparable (4.1% vs 4.9%, p = 0.97), although the RIT arm had a significantly higher mucositis score [88]. In FL, the effect of the addition of RIT or rituximab to BEAM was evaluated using data obtained from the EBMT registry. In that study, 3 cohorts of patients were compared: BEAM (n = 1973) [78], Y-Ibritumomab (RIT)- BEAM (n = 207), and R-BEAM (n = 179). The cumulative incidences of relapse at 2 years were 34, 34, and 32% for RIT-BEAM, R-BEAM, and BEAM, respectively. By multivariate analysis, there were no significant differences with RIT-BEAM or R-BEAM compared with BEAM for relapse, NRM, EFS, or OS [89]. Two other recent studies involving RIT-based conditioning are summarized in Table 2 [90, 91]. Of note, there is an ongoing phase 1 study evaluating escalating doses of 131-I monoclonal antibody BC8 (anti-CD45 antibody) followed by HDT-AHCT in rel/ref HL and NHL (ClinicalTrials.gov Identifier: NCT00860171). The published evidence, thus far, does not support routine addition of RIT to HDT-AHCT.
Tandem transplantations
Another approach to improve transplant outcomes in lymphoma have included the use of tandem transplants, i.e., two stem cell transplantations within a period of <6 months. Several retrospective and prospective studies suggest that tandem HDT-AHCT may improve the outcome of patients with high-risk rel/ref HL [92–96]. The prospective H96 trial conducted by the Lymphoma Study Association and Société Française de Greffe de Moell (LYSA/SFGM-TC) assessed the long-term results of this strategy. This multicenter phase 2 trial evaluated a risk-adapted strategy with single or tandem AHCT in 245 HL patients. Poor-risk patients (n = 150) received tandem AHCT, whereas intermediate-risk patients (n = 95) received a single AHCT. At a median follow-up of 10.3 years, 10-year freedom from second failure and OS rates were 64% (95% CI, 54–74%) and 70% (95% CI, 61–80%) for the intermediate-risk group and 41% (95% CI, 33–49%) and 47% (95% CI, 39 to 55%) for the poor-risk group, respectively. The 15-year cumulative incidences of second primary malignancies were higher in patients with tandem AHCT (24% vs 2%). In another phase 2 study performed by the US intergroup [96], 82 patients with rel/ref HL underwent tandem transplant. There were no TRM in the first year after AHCT. With a median follow-up of 6.2 years, the 5-year PFS and OS were 55% (95% CI: 44–64%), and 84% (95% CI: 74–90%), respectively. Deau and coworkers [97] evaluated the tolerance and efficacy of double AHCT or AHCT followed by allo-HCT in 120 rel/ref HL patients prospectively. Of those, 115 (96%) patients underwent a single AHCT, 44 (60%) had tandem AHCT, and 29 (40%) had AHCT followed by allo-HCT. The 2-year PFS rate for the whole population and for patients receiving tandem transplant was 56% (95% CI: 46–65%) and 71% (95% CI: 49–84%), respectively. Among tandem transplants, 20 deaths (17%) were observed, 10 of which were transplant related (6 allo-HCT and 4 AHCT). This study suggests that tandem HCT is effective in high-risk rel/ref HL patients, although TRM remains high. In the era of immunotherapies and targeted therapies, this strategy is less well defined. The role of tandem AHCT in NHL has not been established [98, 99].
Maintenance therapy after HDT-AHCT
Recurrent disease after HDT-AHCT remains the main cause of treatment failure in patients with rel/ref HL and NHL. The post-AHCT setting is characterized by a minimal disease state and a state of immune remodeling with gradual reconstitution of a full immune system. It represents the last effective intervention point for cure of rel/ref lymphoma. A variety of maintenance therapy approaches post-AHCT have been explored to decrease the risk of disease relapse, with varying success (Table 3).
Table 3.
Established and investigative maintenance strategies after autologous stem cell transplant
| Intervention | Study phase | Studies |
|---|---|---|
|
| ||
| Anti-CD20 monoclonal antibody: Rituximab | Phase III Phase III Phase III | DLBCL [100]: No impact on DFS or OS FL [39]: maintenance rituximab once every 2 monthsx4 doses is associated with a longer PFS (10-year PFS 54% vs 37%, p = 0.01), but no impact on OS MCL [102]: maintenance rituximab once every 2 monthsx3 years. 4-year EFS, PFS, and OS were 79, 83, and 89%, respectively, in the rituximab group, vs 61, 64, and 80% in the observation group (p = 0.001) |
| Anti-CD30 antibody drug conjugate: Brentuximab vedotin | Phase III | HL (high-risk rel/ref) [104]: maintenance BV every 3 weeks, for up to 16 cycles. Median PFS was 43 months for the BV group compared with 24 months for the placebo group |
| Anti-PD-1 monoclonal antibody: Pidilizumab | Phase II | DLBCL [121]: 3 doses of pidilizumab after AHCT: 16-month PFS and OS were 72 and 85%, respectively. Among patients who had measurable disease post-AHCT, ORR was 51% |
| Proteasome inhibitors: Bortezomib | Phase II Phase II | MCL [122]: bortezomib 1.3 mg/m2 weeklyx4, every 3 months and rituximab 375 mg/m2 weeklyx4 every 6 months for a total of 2 years. 2-year DFS and OS: 90.2% (95% Cl 66–97), and 94.7% (95% Cl 68–99), respectively MCL [44, 123]: After two doses of posttransplant rituximab, patients were randomized to bortezomib consolidation (1.3 mg/m2 on days 1,4, 8, and 11 for four cycles) or bortezomib maintenance (1.6 mg/m2 weekly 4 of 8 weeks for 18 months) on day 90 after transplant. The 2-year PFS rates were similar in both arms (84 and 89%, respectively). 5-year PFS rates compared to historical control (72.7% vs 51.5%, p = 0.0006) suggest a PFS benefit for post-autologous SCT bortezomib maintenance in MCL |
| Immune modulators: Lenalidomide | Phase I/II | DLBCL and HL [124, 125]: No dose-limiting toxicities in phase 1, and dose of 10 mg daily was determined appropriate for phase 2. At a median follow-up of 24 months, PFS and OS were 62% and 75%, respectively |
| Histone deacetylase inhibitor: Vorinostat | Phase I | HL and NHL [126]: starting day 60 post-AHCT for 21 consecutive days followed by a week off for up to 11 cycles. Of the 23 enrolled patients, 11 completed maintenance vorinostat treatment plan per protocol. Four patients were removed owing to progressive disease, and 7 were removed owing to toxicities. One patient developed lung cancer after 6 cycles. Given the toxicities observed, vorinostat at this schedule is not optimal for prolonged maintenance therapy |
AHCT autologous hematopoietic cell transplantation, NHL non-Hodgkin lymphoma, HL Hodgkin lymphoma, DLBCL diffuse large B cell lymphoma, FL follicular lymphoma, MCL mantle cell lymphoma, DFS disease-free survival, OS overall survival, EFS event-free survival, ORR overall response rate, BV brentuximab vedotin
Anti-CD20 monoclonal antibody
Rituximab, has been evaluated for maintenance treatment post-HDT-AHCT in B cell NHLs. In a prospective, phase 3 study, the Collaborative Trial in Relapsed Aggressive Lymphoma, 396 patients with rel/ref DLBCL were randomized to salvage chemotherapy with RICE (rituximab, Ifosfamide, carboplatin, and etoposide) or R-DHAP [100, 101]. Responding patients proceeded to HDT-AHCT and underwent a second randomization after transplant to either observation or rituximab maintenance for 1 year. There was no difference in response rates between the RICE and R-DHAP arms, 63.5% vs 62.8%, respectively, or in EFS, 26% vs 35%, respectively.Maintenance rituximab did not impact the EFS, PFS, or OS in DLBCL [100]. However, in a subset analysis based on sex that compared the rituximab and observation groups, the 3-year EFS was 43% (95% CI, 31–54%) in men and 69% (95% CI, 53–81%) in women (p = 0.1). This was attributed to higher rituximab clearance and hormone-related pharmacokinetic variations in males. Thus the impact of an increased dose of rituximab on survival requires further investigation. In FL, a randomized phase 3 study by EBMT evaluated the role of rituximab in vivo purging and maintenance therapy in patients with rituximab-naive, chemosensitive rel/ref FL. Patients were randomly assigned to in vivo purging with weekly rituximab 375 mg/m2 for 4 doses or observation prior to hematopoietic cell collection. After HDT-AHCT, patients underwent a second randomization to receive maintenance rituximab once every 2 months for a total of 4 doses. There was no difference in PFS between the purging group and observation group. Although rituximab maintenance was associated with a longer PFS (10-year PFS 54% vs 37%, p = 0.01), it did not impact OS [39]. Although While in DLBCL, maintenance rituximab after HDT-AHCT is not supported by published data, its use in FL is based on improvement in PFS. In MCL, a phase 3 trial examined induction chemoimmunotherapy followed by R-BEAM: 240 patients were randomized to either rituximab maintenance (once every 2 months for 3 years) or observation (120 patients per group). The rate of EFS, PFS, and OS at 4 years were 79, 83 and 89%, respectively, in the rituximab group, vs 61, 64 and 80% in the observation group (p = 0.001) [102]. This study established rituximab maintenance therapy after HDT-AHCT in MCL.
Anti-CD30 antibody–drug conjugate
Brentuximab vedotin (BV), an anti-CD30 antibody–drug conjugate is a microtubule-disrupting agent leading to cell-cycle arrest and apoptosis. In a phase 2 trial in patients with rel/ref HL after AHCT, the ORR was 75%, with 34% achieving a CR [103]. In HL, the phase 3 randomized ATHERA trial [104] randomized patients with rel/ref disease to maintenance BV or placebo every 3 weeks for up to 16 cycles. The most common toxicities were peripheral neuropathy and neutropenia. The median PFS was 43 months for the BV group compared with 24 months for the placebo group. Based on this study, BV was approved by the US Food and Drug Administration for early consolidation after HDT-AHCT in patients with high-risk rel/ref HL. Pro and coworkers demonstrated safety and efficacy of BV in 58 patients with rel/ref ALCL in a phase 2 study. Of the 38 patients who achieved CR, 16 received a consolidative AHCT with median PFS not reached [105].
In an effort to further improve transplant outcomes in lymphoma and to reduce relapse rate, ongoing clinical trials are investigating a multitude of novel targeted agents for post-AHCT maintenance. These ongoing studies are summarized in Table 4.
Table 4.
Ongoing investigative maintenance strategies after autologous stem cell transplant
| Intervention | Study phase | Disease | Identifier | Clinical trial |
|---|---|---|---|---|
|
| ||||
| Lenalidomide | Pilot Study | HL | NCT01207921 | Lenalidomide maintenance therapy post-AHCT |
| Lenalidomide | Phase 1/2 | NHL | NCT01035463 | Lenalidomide maintenance after high-dose BEAM with or without rituximab |
| Lenalidomide | Phase 1/2 | DLBCL | NCT01241734 | Lenalidomide with RICE with lenalidomide maintenance post- AHCT |
| Lenalidomide | Phase 1/2 | HL and NHL | NCT01575860 | Maintenance lenalidomide in lymphoma after AHCT |
| Lenalidomide | Phase 3 | MCL | NCT02354313 | Lenalidomide vs observation after AHCT |
| Bortezomib | Phase 2 | MCL | NCT00310037 | Bortezomib after combination chemotherapy, rituximab, and AHCT |
| Bortezomib | Phase 2 | MCL | NCT01267812 | Weekly maintenance bortezomib and rituximab in MCL Post- AHCT |
| Bortezomib+vorinostat | Phase 2 | HL and NHL | NCT00992446 | Bortezomib+vorinostat as maintenance after AHCT |
| Ixazomib+Rituximab | Phase 1/2 | MCL | NCT02632396 | Ixazomib and rituximab after AHCT |
| Everolimus+Rituximab | Phase 2 | HL and NHL | NCT01665768 | Maintenance rituximab with mTOR inhibition after AHCT |
| Ibrutinib | Phase 3 | MCL | NCT02858258 | AHCT after a rituximab/ibrutinib/Ara-c induction and ibrutinib maintenance |
| Ibrutinib | Phase 3 | DLBCL (ABC subtype) | NCT02443077 | Ibrutinib during and following AHCT vs placebo |
| Romidepsin | Phase 2 | T-NHL | NCT01908777 | Maintenance therapy with romidepsin for T cell NHL after AHCT |
| Nivolumab | Phase 2 | HL | NCT03436862 | Nivolumab as maintenance therapy after AHCT |
| Pembrolizumab | Phase 2 | HL, DLBCL, T-NHL | NCT02362997 | Pembrolizumab after AHCT |
AHCT autologous hematopoietic cell transplantation, NHL non-Hodgkin lymphoma, HL Hodgkin lymphoma, T-NHL T cell non-Hodgkin lymphoma, MCL mantle cell lymphoma, DLBCL diffuse large B cell lymphoma, ABC activated B cell
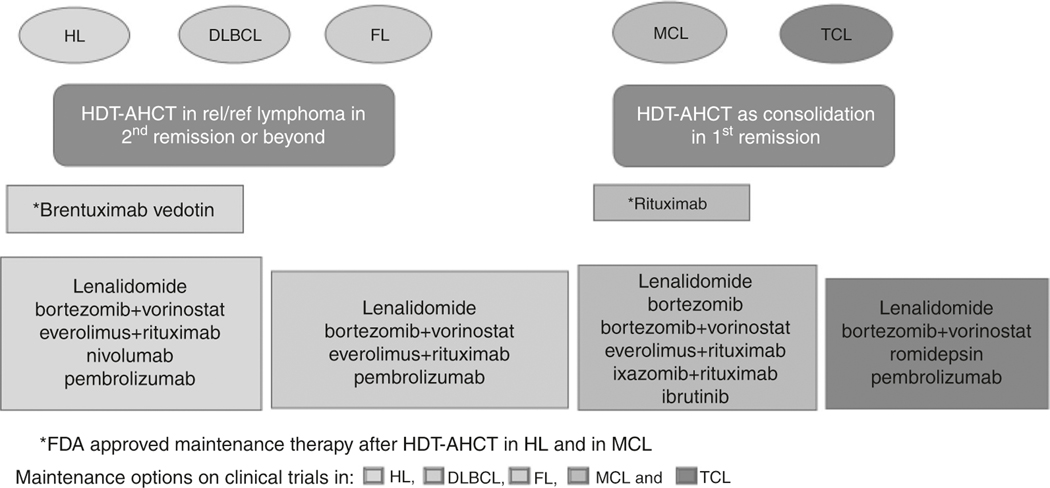
Transplant timing along with established and investigative maintenance agents in lymphoma are summarized in Fig. 1.
Fig. 1.
Schema for transplant and maintenance strategies in lymphoma. Transplant timing and maintenance options in lymphoma
HL: hodgkin lymphoma, DLBCL: diffuse large B-cell lymphoma, FL: follicular lymphoma,MCL: mantle cell lymphoma, TCL: T-cell lymphoma HDT-AHCT: high-dose therapy and autologous stem cell transplantation
In summary, in the era of personalized and targeted treatments in lymphoma, HDT-AHCT still plays an important role in disease control. Lymphoma recurrence, however, continues to be the major cause for treatment failure. Patients whose disease recurs after HDT-AHCT generally are considered incurable, with the exception of a small proportion who may be cured with an allo-HCT or with novel targeted agents. Various strategies have been investigated to improve outcomes of HDT-AHCT and reduce relapse, including advancements in supportive care, intensification of the conditioning regimen, incorporation of novel agents into the combination regimens, and innovative maintenance strategies after HDT-AHCT. The use of maintenance therapies appears to hold the greatest promise for leading future direction. While awaiting mature data on the currently accruing clinical trials, it is important to recognize the associated potential toxicities, logistic concerns, and potentially higher financial cost when selecting patients who may benefit from further treatment post-HDT-AHCT.
Acknowledgements
This research is supported in part by grants from the National Institute of Health, National Cancer Institute (P01 CA23766 and P30 CA008748). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Compliance with ethical standards
Conflict of interest HML has been a consultant and promotional speaker for Seattle Genetics. The other authors declare that they have no conflict of interest.
References
- 1.Philip T, Armitage JO, Spitzer G, Chauvin F, Jagannath S, Cahn JY, et al. High-dose therapy and autologous bone marrow transplantation after failure of conventional chemotherapy in adults with intermediate-grade or high-grade non-Hodgkin’s lymphoma. N Eng J Med. 1987;316:1493–8. [DOI] [PubMed] [Google Scholar]
- 2.Geisler CH, Kolstad A, Laurell A, Andersen NS, Pedersen LB, Jerkeman M, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multi-center study by the Nordic Lymphoma Group. Blood. 2008;112:2687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schouten HC, Qian W, Kvaloy S, Porcellini A, Hagberg H, Johnson HE, et al. High-dose therapy improves progression-free survival and survival in relapsed follicular non-Hodgkin’s lymphoma: results from the randomized European CUP trial. J Clin Oncol. 2003;21:3918–27. [DOI] [PubMed] [Google Scholar]
- 4.d’Amore F, Relander T, Lauritzsen GF, Jantunen E, Hagberg H, Anderson H, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30:3093–9. 10.1200/JCO.2011.40.2719. [DOI] [PubMed] [Google Scholar]
- 5.Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Eng J Med. 1995;333:1540–5. [DOI] [PubMed] [Google Scholar]
- 6.Skipper HE, Schabel FM Jr., Wilcox WS Experimental evaluation of potential anticancer agents. XIII. On the criteria and kinetics associated with “curability” of experimental leukemia. Cancer Chemother Rep. 1964;35:1–111. [PubMed] [Google Scholar]
- 7.Skipper HE. Perspectives in cancer chemotherapy: therapeutic design. Cancer Res. 1964;24:1295–302. [PubMed] [Google Scholar]
- 8.Skipper HE, Schabel FM Jr., Mellett LB, Montgomery JA, Wilkoff LJ, Lloyd HH, et al. Implications of biochemical, cytokinetic, pharmacologic, and toxicologic relationships in the design of optimal therapeutic schedules. Cancer Chemother Rep. 1970;54:431–50. [PubMed] [Google Scholar]
- 9.Skipper HE, Perry S. Kinetics of normal and leukemic leukocyte populations and relevance to chemotherapy. Cancer Res. 1970;30:1883–97. [PubMed] [Google Scholar]
- 10.Mc FW, Granville NB, Dameshek W. Autologous bone marrow infusion as an adjunct in therapy of malignant disease. Blood. 1959;14:503–21. [PubMed] [Google Scholar]
- 11.Clifford P, Clift RA, Duff JK. Nitrogen-mustard therapy combined with autologous marrow infusion. Lancet. 1961;1:687–90. [DOI] [PubMed] [Google Scholar]
- 12.Pegg DE. A quantitative study of bone marrow grafting: implications for human bone marrow infusion. Br J Cancer. 1962;16:400–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurnick NB. Autologous and isologous bone marrow storage and infusion in the treatment of myelo-suppresson. Transfusion. 1962;2:178–87. [DOI] [PubMed] [Google Scholar]
- 14.Appelbaum FR, Herzig GP, Ziegler JL, Graw RG, Levine AS, Deisseroth AB. Successful engraftment of cryopreserved autologous bone marrow in patients with malignant lymphoma. Blood. 1978;52:85–95. [PubMed] [Google Scholar]
- 15.Appelbaum FR, Deisseroth AB, Graw RG Jr., Herzig GP, Levine AS, Magrath IT, et al. Prolonged complete remission following high dose chemotherapy of Burkitt’s lymphoma in relapse. Cancer. 1978;41:1059–63. [DOI] [PubMed] [Google Scholar]
- 16.Phillips GL, Wolff SN, Herzig RH, Lazarus HM, Fay JW, Lin HS, et al. Treatment of progressive Hodgkin’s disease with intensive chemoradiotherapy and autologous bone marrow transplantation. Blood. 1989;73:2086–92. [PubMed] [Google Scholar]
- 17.Carella AM, Santini G, Santoro A, Coser P, Frassoni F, Martinengo M, et al. Massive chemotherapy with non-frozen autologous bone marrow transplantation in 13 cases of refractory Hodgkin’s disease. Eur J Cancer Clin Oncol. 1985;21:607–13. [DOI] [PubMed] [Google Scholar]
- 18.Jagannath S, Dicke KA, Armitage JO, Cabanillas FF, Horwitz LJ, Vellekoop L, et al. High-dose cyclophosphamide, carmustine, and etoposide and autologous bone marrow transplantation for relapsed Hodgkin’s disease. Ann Intern Med. 1986;104:163–8. [DOI] [PubMed] [Google Scholar]
- 19.Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341:1051–4. [DOI] [PubMed] [Google Scholar]
- 20.Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359:2065–71. [DOI] [PubMed] [Google Scholar]
- 21.Buadi FK, Micallef IN, Ansell SM, Porrata LF, Dispenzieri A, Elliot MA, et al. Autologous hematopoietic stem cell transplantation for older patients with relapsed non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2006;37:1017–22. 10.1038/sj.bmt.1705371. [DOI] [PubMed] [Google Scholar]
- 22.Lazarus HM, Carreras J, Boudreau C, Loberiza FR Jr., Armitage JO, Bolwell BJ, et al. Influence of age and histology on outcome in adult non-Hodgkin lymphoma patients undergoing autologous hematopoietic cell transplantation (HCT): a report from the Center For International Blood & Marrow Transplant Research (CIBMTR). Biol Blood Marrow Transplant. 2008;14:1323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khouri IF, Romaguera J, Kantarjian H, Palmer JL, Pugh WC, Korbling M, et al. Hyper-CVAD and high-dose methotrexate/cytarabine followed by stem-cell transplantation: an active regimen for aggressive mantle-cell lymphoma. J Clin Oncol. 1998;16:3803–9. [DOI] [PubMed] [Google Scholar]
- 24.Thieblemont C, Antal D, Lacotte-Thierry L, Delwail V, Espinouse D, Michallet AS, et al. Chemotherapy with rituximab followed by high-dose therapy and autologous stem cell transplantation in patients with mantle cell lymphoma. Cancer. 2005;104:1434–41. 10.1002/cncr.21313. [DOI] [PubMed] [Google Scholar]
- 25.Ritchie DS, Seymour JF, Grigg AP, Roberts AW, Hoyt R, Thompson S, et al. The hyper-CVAD-rituximab chemotherapy programme followed by high-dose busulfan, melphalan and autologous stem cell transplantation produces excellent event-free survival in patients with previously untreated mantle cell lymphoma. Ann Hematol. 2007;86:101–5. 10.1007/s00277-006-0193-2. [DOI] [PubMed] [Google Scholar]
- 26.LaCasce AS, Vandergrift JL, Rodriguez MA, Abel GA, Crosby AL, Czuczman MS, et al. Comparative outcome of initial therapy for younger patients with mantle cell lymphoma: an analysis from the NCCN NHL Database. Blood. 2012;119:2093–9. 10.1182/blood-2011-07-369629. [DOI] [PubMed] [Google Scholar]
- 27.Geisler CH, Kolstad A, Laurell A, Jerkeman M, Raty R, Andersen NS, et al. Nordic MCL2 trial update: six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem-cell support: still very long survival but late relapses do occur. Br J Haematol. 2012;158:355–62. 10.1111/j.1365-2141.2012.09174.x. [DOI] [PubMed] [Google Scholar]
- 28.Dreyling M, Lenz G, Hoster E, Van Hoof A, Gisselbrecht C, Schmits R, et al. Early consolidation by myeloablative radio-chemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105:2677–84. 10.1182/blood-2004-10-3883. [DOI] [PubMed] [Google Scholar]
- 29.Feyler S, Prince HM, Pearce R, Towlson K, Nivison-Smith I, Schey S, et al. The role of high-dose therapy and stem cell rescue in the management of T-cell malignant lymphomas: a BSBMT and ABMTRR study. Bone Marrow Transplant. 2007;40:443–50. 10.1038/sj.bmt.1705752. [DOI] [PubMed] [Google Scholar]
- 30.Jantunen E, Wiklund T, Juvonen E, Putkonen M, Lehtinen T, Kuittinen O, et al. Autologous stem cell transplantation in adult patients with peripheral T-cell lymphoma: a nation-wide survey. Bone Marrow Transplant. 2004;33:405–10. 10.1038/sj.bmt.1704367. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez J, Conde E, Gutierrez A, Arranz R, Leon A, Marin J, et al. The results of consolidation with autologous stem-cell transplantation in patients with peripheral T-cell lymphoma (PTCL) in first complete remission: the Spanish Lymphoma and Autologous Transplantation Group experience. Ann Oncol. 2007;18:652–7. [DOI] [PubMed] [Google Scholar]
- 32.McCarthy PL Jr., Hahn T, Hassebroek A, Bredeson C, Gajewski J, Hale G, et al. Trends in use of and survival after autologous hematopoietic cell transplantation in North America, 1995–2005: significant improvement in survival for lymphoma and myeloma during a period of increasing recipient age. Biol Blood Marrow Transplant. 2013;19:1116–23. 10.1016/j.bbmt.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sureda A, Arranz R, Iriondo A, Carreras E, Lahuerta JJ, Garcia-Conde J, et al. Autologous stem-cell transplantation for Hodgkin’s disease: results and prognostic factors in 494 patients from the Grupo Espanol de Linfomas/Transplante Autologo de Medula Osea Spanish Cooperative Group. J Clin Oncol. 2001;19:1395–404. 10.1200/JCO.2001.19.5.1395. [DOI] [PubMed] [Google Scholar]
- 34.Lazarus HM, Loberiza FR Jr., Zhang MJ, Armitage JO, Ballen KK, Bashey A, et al. Autotransplants for Hodgkin’s disease in first relapse or second remission: a report from the Autologous Blood and Marrow Transplant Registry (ABMTR). Bone Marrow Transplant. 2001;27:387–96. 10.1038/sj.bmt.1702796. [DOI] [PubMed] [Google Scholar]
- 35.Federico M, Bellei M, Brice P, Brugiatelli M, Nagler A, Gisselbrecht C, et al. High-dose therapy and autologous stem-cell transplantation versus conventional therapy for patients with advanced Hodgkin’s lymphoma responding to front-line therapy. J Clin Oncol. 2003;21:2320–5. 10.1200/JCO.2003.11.103. [DOI] [PubMed] [Google Scholar]
- 36.Moskowitz CH, Nimer SD, Zelenetz AD, Trippett T, Hedrick EE, Filippa DA, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97:616–23. [DOI] [PubMed] [Google Scholar]
- 37.Le Gouill S, De Guibert S, Planche L, Brice P, Dupuis J, Cartron G, et al. Impact of the use of autologous stem cell transplantation at first relapse both in naive and previously rituximab exposed follicular lymphoma patients treated in the GELA/GOELAMS FL2000 study. Haematologica. 2011;96:1128–35. 10.3324/haematol.2010.030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casulo C, Friedberg JW, Ahn KW, Flowers C, DiGilio A, Smith SM, et al. Autologous transplantation in follicular lymphoma with early therapy failure: a national lymphocare study and center for international blood and marrow transplant research analysis. Biol Blood Marrow Transplant. 2017. 10.1016/j.bbmt.2017.12.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pettengell R, Schmitz N, Gisselbrecht C, Smith G, Patton WN, Metzner B, et al. Rituximab purging and/or maintenance in patients undergoing autologous transplantation for relapsed follicular lymphoma: a prospective randomized trial from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2013;31:1624–30. 10.1200/JCO.2012.47.1862. [DOI] [PubMed] [Google Scholar]
- 40.Montoto S, Canals C, Rohatiner AZ, Taghipour G, Sureda A, Schmitz N, et al. Long-term follow-up of high-dose treatment with autologous haematopoietic progenitor cell support in 693 patients with follicular lymphoma: an EBMT registry study. Leukemia. 2007;21:2324–31. [DOI] [PubMed] [Google Scholar]
- 41.Kornacker M, Stumm J, Pott C, Dietrich S, Sussmilch S, Hensel M, et al. Characteristics of relapse after autologous stem-cell transplantation for follicular lymphoma: a long-term follow-up. Ann Oncol. 2009;20:722–8. [DOI] [PubMed] [Google Scholar]
- 42.Geisler CH, Kolstad A, Laurell A, Andersen NS, Pedersen LB, Jerkeman M, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multi-center study by the Nordic Lymphoma Group. Blood. 2008;112:2687–93. 10.1182/blood-2008-03-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delarue R, Haioun C, Ribrag V, Brice P, Delmer A, Tilly H, et al. CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: a phase 2 study from the Groupe d’Etude des Lymphomes de l’Adulte. Blood. 2013;121:48–53. 10.1182/blood-2011-09-370320. [DOI] [PubMed] [Google Scholar]
- 44.Damon LE, Johnson JL, Niedzwiecki D, Cheson BD, Hurd DD, Bartlett NL, et al. Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. J Clin Oncol. 2009;27:6101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoster EMB, Forstpointner R, Pfreundschuh M, Trümper L, Hallek M, Wörmann B, et al. Autologous stem cell transplantation and addition of rituximab independently prolong response duration in advanced stage mantle cell lymphoma. Blood. 2009;114:880. [Google Scholar]
- 46.Fenske TS, Zhang MJ, Carreras J, Ayala E, Burns LJ, Cashen A, et al. Autologous or reduced-intensity conditioning allogeneic hematopoietic cell transplantation for chemotherapy-sensitive mantle-cell lymphoma: analysis of transplantation timing and modality. J Clin Oncol. 2014;32:273–81. 10.1200/JCO.2013.49.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freedman AS, Neuberg D, Gribben JG, Mauch P, Soiffer RJ, Fisher DC, et al. High-dose chemoradiotherapy and anti-B-cell monoclonal antibody-purged autologous bone marrow transplantation in mantle-cell lymphoma: no evidence for long-term remission. J Clin Oncol. 1998;16:13–8. [DOI] [PubMed] [Google Scholar]
- 48.Vandenberghe E, Ruiz de Elvira C, Loberiza FR, Conde E, Lopez-Guillermo A, Gisselbrecht C, et al. Outcome of autologous transplantation for mantle cell lymphoma: a study by the European Blood and Bone Marrow Transplant and Autologous Blood and Marrow Transplant Registries. Br J Haematol. 2003;120:793–800. [DOI] [PubMed] [Google Scholar]
- 49.Reimer P, Rudiger T, Geissinger E, Weissinger F, Nerl C, Schmitz N, et al. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol. 2009;27:106–13. [DOI] [PubMed] [Google Scholar]
- 50.Corradini P, Tarella C, Zallio F, Dodero A, Zanni M, Valagussa P, et al. Long-term follow-up of patients with peripheral T-cell lymphomas treated up-front with high-dose chemotherapy followed by autologous stem cell transplantation. Leukemia. 2006;20:1533–8. 10.1038/sj.leu.2404306. [DOI] [PubMed] [Google Scholar]
- 51.Mercadal S, Briones J, Xicoy B, Pedro C, Escoda L, Estany C, et al. Intensive chemotherapy (high-dose CHOP/ESHAP regimen) followed by autologous stem-cell transplantation in previously untreated patients with peripheral T-cell lymphoma. Ann Oncol. 2008;19:958–63. 10.1093/annonc/mdn022. [DOI] [PubMed] [Google Scholar]
- 52.Wilhelm M, Smetak M, Reimer P, Geissinger E, Ruediger T, Metzner B, et al. First-line therapy of peripheral T-cell lymphoma: extension and long-term follow-up of a study investigating the role of autologous stem cell transplantation. Blood Cancer J. 2016;6:e452. 10.1038/bcj.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodriguez J, Caballero MD, Gutierrez A, Marin J, Lahuerta JJ, Sureda A, et al. High-dose chemotherapy and autologous stem cell transplantation in peripheral T-cell lymphoma: the GEL-TAMO experience. Ann Oncol. 2003;14:1768–75 [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez J, Caballero MD, Gutierrez A, Gandarillas M, Sierra J, Lopez-Guillermo A, et al. High dose chemotherapy and autologous stem cell transplantation in patients with peripheral T-cell lymphoma not achieving complete response after induction chemotherapy. The GEL-TAMO experience. Haematologica. 2003;88:1372–7. [PubMed] [Google Scholar]
- 55.Smith SM, Burns LJ, van Besien K, Lerademacher J, He W, Fenske TS, et al. Hematopoietic cell transplantation for systemic mature T-cell non-Hodgkin lymphoma. J Clin Oncol. 2013;31:3100–9. 10.1200/JCO.2012.46.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mehta N, Maragulia JC, Moskowitz A, Hamlin PA, Lunning MA, Moskowitz CH, et al. A retrospective analysis of peripheral T-cell lymphoma treated with the intention to transplant in the first remission. Clin Lymphoma Myeloma Leuk. 2013;13:664–70. 10.1016/j.clml.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Frei E 3rd, Holden SA, Gonin R, Waxman DJ, Teicher BA. Antitumor alkylating agents: in vitro cross-resistance and collateral sensitivity studies. Cancer Chemother Pharmacol. 1993;33:113–22. [DOI] [PubMed] [Google Scholar]
- 58.Chao NJ, Stein AS, Long GD, Negrin RS, Amylon MD, Wong RM, et al. Busulfan/etoposide--initial experience with a new preparatory regimen for autologous bone marrow transplantation in patients with acute nonlymphoblastic leukemia. Blood. 1993;81:319–23. [PubMed] [Google Scholar]
- 59.Plunkett W, Huang P, Searcy CE, Gandhi V. Gemcitabine: preclinical pharmacology and mechanisms of action. Semin Oncol. 1996;23(5 Suppl 10):3–15. [PubMed] [Google Scholar]
- 60.Valdez BC, Nieto Y, Murray D, Li Y, Wang G, Champlin RE, et al. Epigenetic modifiers enhance the synergistic cytotoxicity of combined nucleoside analog-DNA alkylating agents in lymphoma cell lines. Exp Hematol. 2012;40:800–10. 10.1016/j.exphem.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jones RB, Matthes S, Shpall EJ, Fisher JH, Stemmer SM, Dufton C, et al. Acute lung injury following treatment with high-dose cyclophosphamide, cisplatin, and carmustine: pharmacodynamic evaluation of carmustine. J Natl Cancer Inst. 1993;85:640–7. [DOI] [PubMed] [Google Scholar]
- 62.Jones RB, Shpall EJ, Ross M, Coniglio D, Affronti ML, Peters WP. High-dose carboplatin, cyclophosphamide, and BCNU with autologous bone marrow support: excessive hepatic toxicity. Cancer Chemother Pharmacol. 1990;26:155–6. [DOI] [PubMed] [Google Scholar]
- 63.Chen YB, Lane AA, Logan B, Zhu X, Akpek G, Aljurf M, et al. Impact of conditioning regimen on outcomes for patients with lymphoma undergoing high-dose therapy with autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21:1046–53. 10.1016/j.bbmt.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robinson SP, Boumendil A, Finel H, Dreger P, Sureda A, Hermine O, et al. High-dose therapy with BEAC conditioning compared to BEAM conditioning prior to autologous stem cell transplantation for non-Hodgkin lymphoma: no differences in toxicity or outcome. A matched-control study of the EBMT-Lymphoma Working Party. Bone Marrow Transplant. 2018. 10.1038/s41409-018-0196-3. [DOI] [PubMed] [Google Scholar]
- 65.Grisariu S, Shapira MY, Or R, Avni B. Thiotepa, etoposide, cyclophosphamide, cytarabine, and melphalan (TECAM) conditioning regimen for autologous stem cell transplantation in lymphoma. Clin Lymphoma, Myeloma & Leuk. 2018;18:272–9. 10.1016/j.clml.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 66.Joffe E, Rosenberg D, Rozovski U, Perry C, Kirgner I, Trestman S, et al. Replacing carmustine by thiotepa and cyclophosphamide for autologous stem cell transplantation in Hodgkin’s and non-Hodgkin’s B-cell lymphoma. Bone Marrow Transplant. 2018;53:29–33. 10.1038/bmt.2017.205. [DOI] [PubMed] [Google Scholar]
- 67.Sellner L, Boumendil A, Finel H, Choquet S, de Rosa G, Falzetti F, et al. Thiotepa-based high-dose therapy for autologous stem cell transplantation in lymphoma: a retrospective study from the EBMT. Bone Marrow Transplant. 2016;51:212–8. 10.1038/bmt.2015.273. [DOI] [PubMed] [Google Scholar]
- 68.Visani G, Malerba L, Stefani PM, Capria S, Galieni P, Gaudio F, et al. BeEAM (bendamustine, etoposide, cytarabine, melphalan) before autologous stem cell transplantation is safe and effective for resistant/relapsed lymphoma patients. Blood. 2011;118:3419–25. 10.1182/blood-2011-04-351924. [DOI] [PubMed] [Google Scholar]
- 69.Chantepie SP, Garciaz S, Tchernonog E, Peyrade F, Larcher MV, Diouf M, et al. Bendamustine-based conditioning prior to autologous stem cell transplantation (ASCT): results of a French multicenter study of 474 patients from LYmphoma Study Association (LYSA) centers. Am J Hematol. 2018. 10.1002/ajh.25077. [DOI] [PubMed] [Google Scholar]
- 70.Jaimovich G, Ostriz MBR, Castro M, Riera L, Banchieri A, Foncuberta MC. et al. Autologous stem cell transplantation (ASCT) with benda-CV (bendamustine, cyclophosphamide, etoposide) in non-Hodgkin (non-HO) and Hodgkin (HL) lymphoma patients (pts). Biol Blood Marrow Transplant. 2017;23: S84 [Google Scholar]
- 71.Kim JW, Lee HJ, Yi HG, Kim BS, Bang SM, Kim JS, et al. Mitoxantrone, etoposide, cytarabine, and melphalan (NEAM) followed by autologous stem cell transplantation for patients with chemosensitive aggressive non-Hodgkin lymphoma. Am J Hematol. 2012;87:479–83. 10.1002/ajh.23150. [DOI] [PubMed] [Google Scholar]
- 72.Olivieri JMF, Pelosini M, Fama A, Rattotti S, Giannoccaro M, Carli G, et al. A comparison of the conditioning regimens BEAM and FEAM for autologous hematopoietic stem cell transplantation in lymphoma: an observational study on patients from Fondazione Italiana Linfomi (Fil). Biol Blood Marrow Transplant. 2018. 10.1016/j.bbmt.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 73.Sharma A, Kayal S, Iqbal S, Malik PS, Raina V. Comparison of BEAM vs. LEAM regimen in autologous transplant for lymphoma at AIIMS. +. 2013;2:489 10.1186/2193-1801-2-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kothari J, Foley M, Peggs KS, Mackenzie S, Thomson K, Morris E, et al. A retrospective comparison of toxicity and initial efficacy of two autologous stem cell transplant conditioning regimens for relapsed lymphoma: LEAM and BEAM. Bone Marrow Transplant. 2016;51:1397–9. 10.1038/bmt.2016.134. [DOI] [PubMed] [Google Scholar]
- 75.Wadehra N, Farag S, Bolwell B, Elder P, Penza S, Kalaycio M, et al. Long-term outcome of Hodgkin disease patients following high-dose busulfan, etoposide, cyclophosphamide, and autologous stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:1343–9. 10.1016/j.bbmt.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 76.Copelan EA, Penza SL, Pohlman B, Avalos BR, Goormastic M, Andresen SW, et al. Autotransplantation following busulfan, etoposide and cyclophosphamide in patients with non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2000;25:1243–8. 10.1038/sj.bmt.1702433. [DOI] [PubMed] [Google Scholar]
- 77.Kim JG, Sohn SK, Chae YS, Yang DH, Lee JJ, Kim HJ, et al. Multicenter study of intravenous busulfan, cyclophosphamide, and etoposide (i.v. Bu/Cy/E) as conditioning regimen for autologous stem cell transplantation in patients with non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2007;40:919–24. 10.1038/sj.bmt.1705841. [DOI] [PubMed] [Google Scholar]
- 78.Tarella C, Zallio F, Caracciolo D, Cuttica A, Corradini P, Gavarotti P, et al. High-dose mitoxantrone + melphalan (MITO/L-PAM) as conditioning regimen supported by peripheral blood progenitor cell (PBPC) autograft in 113 lymphoma patients: high tolerability with reversible cardiotoxicity. Leukemia. 2001;15:256–63. [DOI] [PubMed] [Google Scholar]
- 79.Crump M, Smith AM, Brandwein J, Couture F, Sherret H, Sutton DM, et al. High-dose etoposide and melphalan, and autologous bone marrow transplantation for patients with advanced Hodgkin’s disease: importance of disease status at transplant. J Clin Oncol. 1993;11:704–11. 10.1200/JCO.1993.11.4.704. [DOI] [PubMed] [Google Scholar]
- 80.Nieto Y, Thall P, Valdez B, Andersson B, Popat U, Anderlini P, et al. High-dose infusional gemcitabine combined with busulfan and melphalan with autologous stem-cell transplantation in patients with refractory lymphoid malignancies. Biol Blood Marrow Transplant. 2012;18:1677–86. 10.1016/j.bbmt.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nieto Y, Valdez BC, Thall PF, Ahmed S, Jones RB, Hosing C, et al. Vorinostat combined with high-dose gemcitabine, busulfan, and melphalan with autologous stem cell transplantation in patients with refractory lymphomas. Biol Blood Marrow Transplant. 2015;21:1914–20. 10.1016/j.bbmt.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nieto Y, Thall PF, Ma J, Valdez BC, Ahmed S, Anderlini P, et al. Phase II trial of high-dose gemcitabine/busulfan/melphalan with autologous stem cell transplantation for primary refractory or poor-risk relapsed hodgkin lymphoma. Biol Blood Marrow Transplant. 2018. 10.1016/j.bbmt.2018.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nieto Y, Popat U, Anderlini P, Valdez B, Andersson B, Liu P, et al. Autologous stem cell transplantation for refractory or poor-risk relapsed Hodgkin’s lymphoma: effect of the specific high-dose chemotherapy regimen on outcome. Biol Blood Marrow Transplant. 2013;19:410–7. 10.1016/j.bbmt.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davidson MSD, Duggan P, Daly A, Shafey M. Phase I/II study of infusional gemcitabine and high-dose melphalan conditioning prior to autologous stem cell transplantation for patients with relapsed/refractory lymphoma: interim safety analysis. Blood. 2017;130:4534. [Google Scholar]
- 85.Vose JM, Bierman PJ, Enke C, Hankins J, Bociek G, Lynch JC, et al. Phase I trial of iodine-131 tositumomab with high-dose chemotherapy and autologous stem-cell transplantation for relapsed non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:461–7. 10.1200/JCO.2005.05.117. [DOI] [PubMed] [Google Scholar]
- 86.Krishnan A, Nademanee A, Fung HC, Raubitschek AA, Molina A, Yamauchi D. et al. Phase II trial of a transplantation regimen of yttrium-90 ibritumomab tiuxetan and high-dose chemotherapy in patients with non-Hodgkin’s lymphoma. J Clin Oncol.2008;26:90–5. Doi: 10.1200/JCO.2007.11.9248. [DOI] [PubMed] [Google Scholar]
- 87.Shimoni A, Avivi I, Rowe JM, Yeshurun M, Levi I, Or R, et al. A randomized study comparing yttrium-90 ibritumomab tiuxetan (Zevalin) and high-dose BEAM chemotherapy versus BEAM alone as the conditioning regimen before autologous stem cell transplantation in patients with aggressive lymphoma. Cancer. 2012;118:4706–14. 10.1002/cncr.27418. [DOI] [PubMed] [Google Scholar]
- 88.Vose JM, Carter S, Burns LJ, Ayala E, Press OW, Moskowitz CH, et al. Phase III randomized study of rituximab/carmustine, etoposide, cytarabine, and melphalan (BEAM) compared with iodine-131 tositumomab/BEAM with autologous hematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: results from the BMT CTN 0401 trial. J Clin Oncol. 2013;31:1662–8. 10.1200/JCO.2012.45.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bento L, Boumendil A, Finel H, Le Gouill S, Amorim S, Monjanel H, et al. Radioimmunotherapy-augmented BEAM chemotherapy vs BEAM alone as the high-dose regimen for autologous stem cell transplantation (ASCT) in relapsed follicular lymphoma (FL): a retrospective study of the EBMT Lymphoma Working Party. Bone Marrow Transplant. 2017;52:1120–5. 10.1038/bmt.2017.88. [DOI] [PubMed] [Google Scholar]
- 90.Chahoud J, Sui D, Erwin WD, Gulbis AM, Korbling M, Zhang M, et al. Updated results of rituximab pre- and post-BEAM with or without (90)yttrium ibritumomab tiuxetan during autologous transplant for diffuse large B-cell lymphoma. Clin Cancer Res. 2018. 10.1158/1078-0432.CCR-17-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Victor A, Chow JR, Shields A, Fisher DR, Appelbaum FR, Cassaday RD, et al. A phase II trial evaluating the efficacy of radioiodinated tositumomab (Anti-CD20) antibody, etoposide and cyclophosphamide followed by autologous transplantation, for high-risk relapsed or refractory non-Hodgkin’s lymphoma.Blood. 2017;130:2018. [DOI] [PubMed] [Google Scholar]
- 92.Brice P, Divine M, Simon D, Coiffier B, Leblond V, Simon M, et al. Feasibility of tandem autologous stem-cell transplantation (ASCT) in induction failure or very unfavorable (UF) relapse from Hodgkin’s disease (HD). SFGM/GELA Study Group. Ann Oncol. 1999;10:1485–8. [DOI] [PubMed] [Google Scholar]
- 93.Castagna L, Magagnoli M, Balzarotti M, Sarina B, Siracusano L, Nozza A, et al. Tandem high-dose chemotherapy and autologous stem cell transplantation in refractory/relapsed Hodgkin’s lymphoma: a monocenter prospective study. Am J Hematol. 2007;82:122–7. 10.1002/ajh.20790. [DOI] [PubMed] [Google Scholar]
- 94.Morschhauser F, Brice P, Ferme C, Divine M, Salles G, Bouabdallah R, et al. Risk-adapted salvage treatment with single or tandem autologous stem-cell transplantation for first relapse/refractory Hodgkin’s lymphoma: results of the prospective multicenter H96 trial by the GELA/SFGM study group. J Clin Oncol. 2008;26:5980–7. 10.1200/JCO.2007.15.5887. [DOI] [PubMed] [Google Scholar]
- 95.Fung HC, Stiff P, Schriber J, Toor A, Smith E, Rodriguez T, et al. Tandem autologous stem cell transplantation for patients with primary refractory or poor risk recurrent Hodgkin lymphoma. Biol Blood Marrow Transplant. 2007;13:594–600. 10.1016/j.bbmt.2007.01.072. [DOI] [PubMed] [Google Scholar]
- 96.Smith EP, Li H, Friedberg JW, Constine LS, Rimsza LM, Cook JR, et al. Tandem autologous hematopoietic cell transplantation for patients with primary progressive or recurrent Hodgkin lymphoma: a SWOG and Blood and Marrow Transplant Clinical Trials Network Phase II Trial (SWOG S0410/BMT CTN 0703).Biol Blood Marrow Transplant. 2018;24:700–7. 10.1016/j.bbmt.2017.12.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deau B, Amorim S, Perrot A, Quittet P, Cornillon J, Chaoui D, et al. Tandem haematopoietic stem cell transplantation for high risk relapsed/refractory Hodgkin lymphoma: a LYSA study. Br J Haematol. 2018;181:341–9. 10.1111/bjh.15184. [DOI] [PubMed] [Google Scholar]
- 98.Hohloch K, Sahlmann CO, Lakhani VJ, Wulf G, Glass B, Hasenkamp J, et al. Tandem high-dose therapy in relapsed and refractory B-cell lymphoma: results of a prospective phase II trial of myeloablative chemotherapy, followed by escalated radio-immunotherapy with (131)I-anti-CD20 antibody and stem cell rescue. Ann Hematol. 2011;90:1307–15. 10.1007/s00277-011-1199-y. [DOI] [PubMed] [Google Scholar]
- 99.Monjanel H, Deconinck E, Perrodeau E, Gastinne T, Delwail V, Moreau A, et al. Long-term follow-up of tandem high-dose therapy with autologous stem cell support for adults with high-risk age-adjusted international prognostic index aggressive non-Hodgkin Lymphomas: a GOELAMS pilot study. Biol Blood Marrow Transplant. 2011;17:935–40. 10.1016/j.bbmt.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 100.Gisselbrecht C, Schmitz N, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Rituximab maintenance therapy after autologous stem-cell transplantation in patients with relapsed CD20(+) diffuse large B-cell lymphoma: final analysis of the collaborative trial in relapsed aggressive lymphoma. J Clin Oncol. 2012;30:4462–9. 10.1200/JCO.2012.41.9416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010. 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Le Gouill S, Thieblemont C, Oberic L, Moreau A, Bouabdallah K, Dartigeas C. et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Eng J Med. 2017;377:1250–60. 10.1056/NEJMoa1701769. [DOI] [PubMed] [Google Scholar]
- 103.Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30:2183–9. 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moskowitz CH, Nademanee A, Masszi T, Agura E, Holowiecki J, Abidi MH, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385:1853–62. 10.1016/S0140-6736(15)60165-9. [DOI] [PubMed] [Google Scholar]
- 105.Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, et al. Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood. 2017;130:2709–17. 10.1182/blood-2017-05-780049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saleh K, Danu A, Koscielny S, Legoupil C, Pilorge S, CastillaLlorente C, et al. A retrospective, matched paired analysis comparing bendamustine containing BeEAM versus BEAM conditioning regimen: results from a single center experience. Leuk Lymphoma. 2017. 10.1080/10428194.2017.1403019. [DOI] [PubMed] [Google Scholar]
- 107.Tsang EVD, Loscocco F, Gerrie AS, Visani G, Power M, Guiducci B, et al. Comparative effectiveness of high-dose bendamustine-EAM versus BEAM in patients with relapsed/refractory classical hodgkin lymphoma undergoing autologous stem cell transplantation. Blood. 2017;130:3291. [Google Scholar]
- 108.Boisjoly JA BP, Cohen S, Ahmad I, Bambace N, Bernard L, Delisle JS, et al. Impact of carmustine (BCNU) substitution for bendamustine in the conditioning regimen prior to autologous hematopoietic cell transplantation for the treatment of lymphoma. Blood. 2017;130:2022. [Google Scholar]
- 109.Nathan SBS, McWilliams D, Schultz K, Geswein L, Macie-jewski J, Venugopal P, et al. Rituxan-benda-eam (R-be-EAM) and rituxan-BEAM (R-BEAM) have similar efficacy in patients with in B cell non-Hodgkin lymphoma (B-NHL) patients (Pts) undergoing autologous stem cell transplantation (AutoSCT). Biol Blood Marrow Transplant. 2017;23:S139. [Google Scholar]
- 110.Ninos CJJ, Mably M, Reed M, Hutson P, Williams Z, Callander NS, et al. Outcomes of BeEAM (bendamustine, etoposide, cytarabine, melphalan) compared to BEAM (carmustine, etoposide, cytarabine, melphalan) for high dose chemotherapy followed by autologous stem cell transplant. Biol Blood Marrow Transplant. 2018;24:S134. [Google Scholar]
- 111.Jain NCA, Sood N, Arabandi A, Sengupta K, Chakrabartty J. Bendamustine based conditioning regime (BACE-bendamustine, cytarabine, cyclophosphamide and etoposide) for patients with lymphoma undergoing autologous stem cell transplant. Biol Blood Marrow Transplant. 2016;22:S121–2. [Google Scholar]
- 112.Chakrabartty JJN, Sarada P, Sengupta K, Sood N, Arabandi A, Chakravarti A, et al. Bendamustine-based conditioning regime (BACE-bendamustine, cytarabine, cyclophosphamide and etoposide) for patients with lymphoma undergoing autologous stem cell transplant. Biol Blood Marrow Transplant. 2017;23:S133. [Google Scholar]
- 113.Sakellari I, Mallouri D, Batsis I, Apostolou C, Konstantinou V, Abela EM, et al. Carmustine, etoposide, cytarabine and melphalan versus a newly designed intravenous busulfan-based Busulfex, etoposide and melphalan conditioning regimen for autologous hematopoietic cell transplant: a retrospective matched-pair analysis in advanced Hodgkin and non-Hodgkin lymphomas. Leuk Lymphoma. 2015;56:3071–81. 10.3109/10428194.2015.1028054. [DOI] [PubMed] [Google Scholar]
- 114.Berber I, Erkurt MA, Nizam I, Koroglu M, Kaya E, Kuku I, et al. Can BuCyE conditioning regimen be an alternative treatment to BEAM at autologous transplantation in malignant lymphoma patients?: a single center experience. Int J Clin Exp Med. 2015;8:16308–14. [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang H, Graiser M, Hutcherson DA, Dada MO, McMillan S, Ali Z, et al. Pharmacokinetic-directed high-dose busulfan combined with cyclophosphamide and etoposide results in predictable drug levels and durable long-term survival in lymphoma patients undergoing autologous stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1287–94. 10.1016/j.bbmt.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 116.Kim JE, Lee DH, Yoo C, Kim S, Kim SW, Lee JS, et al. BEAM or BuCyE high-dose chemotherapy followed by autologous stem cell transplantation in non-Hodgkin’s lymphoma patients: a single center comparative analysis of efficacy and toxicity. Leuk Res. 2011;35:183–7. 10.1016/j.leukres.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 117.Hanel M, Kroger N, Sonnenberg S, Bornhauser M, Kruger W, Kroschinsky F, et al. Busulfan, cyclophosphamide, and etoposide as high-dose conditioning regimen in patients with malignant lymphoma. Ann Hematol. 2002;81:96–102. 10.1007/s00277-001-0413-8. [DOI] [PubMed] [Google Scholar]
- 118.Nieto YBR, Hosing C, Jones RB, Valdez BC, Kingham A, Ahmed S, et al. Comparison of high-dose gemcitabine, busulfan and melphalan (GEM/BU/MEL) and BEAM in concurrent patient cohorts with mature T-cell non-Hodgkin’s lymphoma (T-NHL) receiving an autologous stem cell transplantation (ASCT). Biol Blood Marrow Transplant. 2016;22:S227–8. [Google Scholar]
- 119.Villa D, Crump M, Keating A, Panzarella T, Feng B, Kuruvilla J. Outcome of patients with transformed indolent non-Hodgkin lymphoma referred for autologous stem-cell transplantation. Ann Oncol. 2013;24:1603–9. 10.1093/annonc/mdt029. [DOI] [PubMed] [Google Scholar]
- 120.Ji JLT, Kuang P, Liu Z, Dong T, Liu J. Chidamide, a HDAC inhibitor, combined with cladribine, high dose gemcitabine and busulfan with autologous stem cell transplantation in patients with relapsed/refractory and poor-risk lymphoma. Blood. 2017;130:3288. [Google Scholar]
- 121.Armand P, Nagler A, Weller EA, Devine SM, Avigan DE, Chen YB, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol. 2013;31:4199–206. 10.1200/JCO.2012.48.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen RW, Palmer JM, Tomassetti S, Popplewell LL, Alluin J, Chomchan P, et al. Multi-center phase II trial of bortezomib and rituximab maintenance combination therapy in patients with mantle cell lymphoma after consolidative autologous stem cell transplantation. J Hematol Oncol. 2018;11:87 10.1186/s13045-018-0631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kaplan LD, Jung S-H, Stock W, Bartlett NL, Pitcher B, Byrd JC. et al. Bortezomib maintenance (BM) versus consolidation (BC) following aggressive immunochemotherapy and autologous stem cell transplant (ASCT) for untreated mantle cell lymphoma (MCL): CALGB (Alliance) 50403. Blood. 2015;126:337. [Google Scholar]
- 124.Feldman T, Mato AR, Chow KF, Protomastro EA, Yannotti KM, Bhattacharyya P, et al. Addition of lenalidomide to rituximab, ifosfamide, carboplatin, etoposide (RICER) in first-relapse/primary refractory diffuse large B-cell lymphoma. Br J Haematol. 2014;166:77–83. 10.1111/bjh.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jakub Svoboda LES, Daniel JLandsburg, Sunita DwivedyNasta, Anthony RMato, Wei-Ting Hwang, Sarah Jordan Nagle, et al. Lenalidomide maintenance after autologous stem cell transplant in patients with high-risk relapsed/refractory lymphomas is feasible and compares favorably to historical controls: results of a phase I/II trial. Blood. 2016;128:4639. [Google Scholar]
- 126.Hofmeister CC, Williams N, Geyer S, Hade EM, Bowers MA, Earl CT, et al. A phase 1 study of vorinostat maintenance after autologous transplant in high-risk lymphoma. Leuk Lymphoma. 2015;56:1043–9. 10.3109/10428194.2014.963073. [DOI] [PMC free article] [PubMed] [Google Scholar]