Abstract
Background
In individuals with cystic fibrosis there are no established targets for participation in physical activity, nor have any ideal strategies to promote participation in physical activity been identified
Objectives
To evaluate the effect of treatment to increase participation in physical activity in people with cystic fibrosis.
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register using the terms 'physiotherapy and exercise'.
Date of the most recent search: 05 December 2013.
Additionally, we conducted searches of the electronic databases MEDLINE, CINAHL (Ebscohost), PsycINFO (OvidSP) and the Physiotherapy Evidence Database (PEDro). We also searched for potentially relevant, completed but unpublished studies, on several clinical trials registers.
Date of the most recent searches: 10 September 2012.
Selection criteria
All randomised and quasi‐randomised controlled studies which investigated strategies designed to promote increased participation in daily physical activity for individuals with cystic fibrosis.
Data collection and analysis
Two authors independently selected studies for inclusion, assessed the risk of bias and extracted data. Any disagreements were resolved by discussion and consensus, or in arbitration with a third author.
Main results
Four studies (199 participants) met the inclusion criteria and were predominantly conducted in children with cystic fibrosis. Only one study had a combined cohort of adult and paediatric participants. The description of study methods was inadequate to assess the risk of bias, particularly with regard to blinding of assessors and selective reporting. One study was conducted in an inpatient setting with follow up in the outpatient setting; while the remaining three studies were conducted in individuals with stable respiratory disease in the outpatient setting. All included studies used exercise training to promote participation in physical activity, with the duration of the intervention period ranging from 18 days to three years. No improvement in physical activity participation was reported with any intervention period less than or equal to six months. Improvements in physical activity participation were only seen where follow up occurred beyond 12 months. There was no significant impact on quality of life from any of the intervention strategies.
Authors' conclusions
Although participation in physical activity is generally regarded as beneficial for people with cystic fibrosis, there is a lack of evidence regarding strategies to promote the uptake and the continued participation in physical activity for this population. This review provides very limited evidence that activity counselling and exercise advice, undertaken over at least six months, to engage in a home exercise programme may result in improved physical activity participation in people with cystic fibrosis. Further research is needed to determine the effect of strategies such as health coaching or telemedicine applications, in promoting the uptake and adherence to regular participation in physical activity. In addition, establishing the ideal duration of any interventions that promote physical activity, including exercise training programmes, will be important in addressing issues relating to participation in physical activity for people with cystic fibrosis.
Plain language summary
Strategies to promote participation in physical activity for people with cystic fibrosis
Physical activity refers to any movement of the body generated by the muscles and which burns energy. Physical activity includes exercise, but also includes activity as a part of work, chores or transport. Participation in regular physical activity is important for health and well‐being. For most people with cystic fibrosis, physical activity and exercise are routinely recommended, but participation in prescribed programmes is often poor. The best way to encourage people with cystic fibrosis to do more physical activity during their day is unclear. This review aimed to evaluate the strategies that encourage people with cystic fibrosis to participate in daily physical activity. There were four included studies with a total of 199 participants which investigated the effect of exercise training on participation in physical activity. These were mostly conducted in children. The study methods and results were not clearly reported, so it was difficult to tell if the results were influenced by the way in which participants were assessed, or the nature of the outcomes reported. The training programmes ranged from 18 days to three years. In two studies the exercise training programmes were supervised and in two studies they were unsupervised and home‐based. Due to differences in the study design and the outcomes measured, we could not combine data from different studies. None of the studies reported any improvement in participation in physical activity when the exercise training lasted less than six months. There was very limited evidence that using a home‐exercise programme, for at least six months after receiving activity counselling and exercise advice, improved participation in physical activity in people with cystic fibrosis. No training program showed significant effects on quality of life. It is unknown whether strategies such as health coaching or Internet‐based advice may help promote regular participation in physical activity in people with cystic fibrosis.
Background
For a glossary of terms please see the appendices (Appendix 1).
Description of the condition
Cystic fibrosis (CF) is a progressive, life‐limiting, multi‐system disease that predominantly affects children and young adults. The lungs of people with CF are affected by bronchiectasis progressing to respiratory failure (Koch 1993). Participation in regular physical activity and exercise is encouraged in CF (Yankaskas 2004) since exercise participation affords health benefits including enhanced clearance of pulmonary secretions (Bradley 2008; Dwyer 2011), improved blood glucose control and bone mineral accretion (Bradley 2008).
Description of the intervention
Physical activity refers to any bodily movement that results in an increase in energy expenditure above baseline resting energy expenditure (Casparen 1985). Physical activity and exercise are terms commonly used interchangeably; however, a subtle distinction exists between the two. Physical activity includes structured exercise and sport activities, as well as activities involved in play, work, transportation, chores and recreational pursuits (WHO 2010). Habitual physical activity implies the performance of physical activity within the context of regular daily life (Clanchy 2011). Physical activity participation can be quantified in a number of ways which can include recording the number of steps taken in a day, the amount of time spent in moderate intensity activity, daily energy expenditure or self‐reported activity participation using a diary or questionnaire. Participation in regular physical activity for the general population has a number of health benefits, including reducing the risk of cardiovascular disease, improving glycaemic control, and helping overcome obesity (Haskell 2007; Thompson 2003). In addition, physical activity participation can improve mental health and quality of life (WHO 2010).
Despite the benefits of regular physical activity participation in the general population, only one quarter of adults in the USA report participating in 30 minutes of activity of at least moderate intensity on five or more days of the week (Kahn 2002). Furthermore, the 2012 Australian Health Survey reported participation in moderate‐vigorous physical activity during the preceding week by only 30% of people older than 15 years of age (ABS 2012). Consequently, strategies to promote physical activity participation are of great importance. Physical activity promotion strategies can take a number of forms and may include mass‐media campaigns for health behaviour change, point‐of decision prompts to encourage increased activity or individually tailored interventions (CDC 2011).
Physical activity may also have specific health benefits for people with particular health disorders. In people with chronic illness, the ability to participate in regular physical activity can be hampered by factors such as the environment (natural and built), financial cost and emotional and psychological barriers (Rimmer 2004). These barriers contribute to people with chronic illness being less likely to report participation in moderate and vigorous physical activity compared to healthy populations (Marcus 2000). In some respiratory populations supervised exercise training have been used to change behaviour and result in increased participation in physical activity. Some examples of these are pulmonary rehabilitation, educational or motivational sessions (Conn 2008); walking programmes using pedometer feedback and Internet‐based monitoring (Moy 2012); and text‐messaging support (Nguyen 2009).
The effect of strategies to improve physical activity participation in people with CF has not previously been reviewed. For people with CF, healthcare professionals generally regard participation in physical activity as beneficial (Bradley 2008). The nature of treatments that can promote physical activity participation for people with CF may be quite variable, and could include such interventions as exercise training (supervised or unsupervised), reminders, counselling or education. Identifying the most effective strategies to promote increased participation in physical activity could therefore have long‐term health effects. As well as increases in physical activity, such health improvements may be reflected as increases in exercise capacity, respiratory function and quality of life, all of which are associated with improved prognostic outcomes in CF (Hebestreit 2006; Schneiderman‐Walker 2005; Troosters 2009).
How the intervention might work
Strategies likely to be successful in promoting increased physical activity participation are those which provide education regarding the health benefits of physical activity (Pate 1995), provide physical skills practice (Andersen 1998) and overcome perceived barriers by fostering enjoyment of activity and providing motivation (Pate 1995).
Why it is important to do this review
In an earlier Cochrane Review targeting previously sedentary healthy adults and excluding those who had pre‐existing medical conditions, it was reported that professional advice with continued support could encourage short‐term improvement in participation in physical activity, with more research required to identify the best long‐term strategies (Foster 2005). GIven that the population included in that review were healthy, it is unknown whether the interventions identified which target behaviour adaptation or involve strategies for delivering physical activity programmes, can be applied to people with CF.
Previous reviews in CF have focused on exercise participation and measures of exercise or aerobic capacity (Bradley 2008). This review will enable a comparison of the relative efficacy of strategies that promote participation in physical activity, and thus allow clinicians to identify treatment options suited to encouraging increased participation in physical activity for people with CF. In addition, this review may identify avenues for future research into novel strategies, that encourage increased participation in physical activity whilst maintaining the strict infection control procedures, which are mandatory to prevent cross infection in people with CF (Saiman 2004).
Objectives
To evaluate the effect of treatment to increase physical activity participation in people with CF.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised controlled studies.
Types of participants
People with CF aged over five years, with any degree of disease severity. Diagnosis of CF confirmed by clinical criteria and sweat testing or genotype analysis.
Types of interventions
We included all strategies which could be considered to promote increased participation in daily physical activity for individuals with CF (refer to examples below), including interventions in both inpatient and outpatient settings. Where the study intervention commenced in the inpatient setting, the study needed to include follow up in the discharge period in order to evaluate the effects of the intervention on physical activity in daily life.
Potential interventions include:
one‐off one‐to‐one counselling or advice;
self‐directed or unsupervised participation in a prescribed physical activity programme;
supervised physical activity session in the home;
supervised physical activity session in a facility;
on‐going face‐to‐face counselling or advice;
telephone support;
written material;
Internet‐based or tele‐health advice and motivation;
monitoring device for motivation, e.g. pedometer
Specific comparisons examined were:
one or more interventions to promote physical activity versus no intervention;
one or more interventions to promote physical activity versus a placebo intervention (e.g. attention control);
one intervention to promote physical activity compared to another intervention to promote physical activity.
We also included studies whose goal was to assess physiological outcomes as a response to a physical exercise intervention, but which included physical activity participation as part of that assessment.
Types of outcome measures
Primary outcomes
-
Participation in physical activity (change from baseline where possible, measured either subjectively, e.g. by an activity diary, or objectively using a monitoring device e.g. a pedometer)
intensity of physical activity (measured or estimated using objective measures e.g. activity monitoring, or self‐report of physical activity e.g. International Physical Activity Questionnaire (IPAQ)
time spent in physical activity (measured in minutes per week, sessions per week, etc)
energy expenditure (in calories or joules)
step count (using a monitoring device such as a pedometer)
Health‐related quality of life measured by generic or disease specific assessments, or both
Secondary outcomes
Exercise capacity (either maximal or submaximal where measured directly or by a standard field test)
-
Pulmonary function tests (change in per cent (%) predicted or absolute measures from baseline, or rate of decline)
forced expiratory volume in one second (FEV1)
forced vital capacity (FVC)
forced expiratory flows between 25% and 75% of expired volume (FEF25-75)
Adverse events (e.g. musculoskeletal injuries)
Body composition in terms of body mass index (BMI) and lean body mass
Bone mineral density (defined on dual energy X‐ray absorptiometry (DXA) scans)
Adherence to the intervention programme
Compliance with other CF treatments, e.g. airway clearance techniques and nebulised medication; any measure of compliance such as pill counts, self‐report diaries, electronic monitoring
Cost evaluation
Search methods for identification of studies
Electronic searches
We searched for relevant trials from the Group's Cystic Fibrosis Trials Register using the terms: physiotherapy AND exercise.
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of The Cochrane Library), quarterly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cochrane Cystic Fibrosis and Genetic Disorders Group Module.
Date of the search of the Group's Cystic Fibrosis Trials Register: 05 December 2013.
In addition, we searched MEDLINE, CINAHL (Ebscohost), PsycINFO (OvidSP) and the Physiotherapy Evidence Database (PEDro) for all years available (Appendix 2). We searched for potentially relevant, completed but unpublished studies, by searching the clinical trials registers listed below using the search terms 'physical activity' or 'physical fitness' or 'habitual activity' and 'cystic fibrosis' and 'promotion' or 'education' or 'uptake' or 'encourage' or 'increase' or 'start'.
WHO International Clinical Trials Register (incorporating clinicaltrials.gov);
European Clinical Trials Register; and
Current Controlled Trials register (incorporating the UK clinical trials register).
Date of the search of CINAHL, PsychINFO, PEDro and clinical trials registers: 10 September 2012.
Searching other resources
We reviewed the reference list of all included studies for any additional trials suitable for inclusion.
Data collection and analysis
Selection of studies
Two authors (NC and AH) independently assessed the titles and abstracts of trials identified by the searches. The same two authors assessed full‐text copies of potentially relevant trials for inclusion based upon the defined criteria. Each author compiled a list of studies that they believed met the inclusion criteria. The authors compared these lists and resolved any disagreements that occurred by discussion and consensus, with arbitration by the third author (JA) as necessary.
Data extraction and management
Two authors (NC and AH) independently extracted data using specially developed, standardised data extraction forms. One author (NC) entered the data into the Cochrane software Review Manager (RevMan 2011) and a second author (AH) reviewed it. They resolved any disagreements by discussion between review authors and, if necessary arbitration by the third author (JA).
We have reported data by type of intervention (supervised exercise training or unsupervised exercise training), and based on the time‐point at which follow‐up data were reported. We categorised these time‐points as:
less than or equal to one month (short‐term);
from one to six months; and
greater than six months.
Assessment of risk of bias in included studies
Two authors (NC and AH) independently rated the risk of bias of the reviewed studies using the standardised grading system described in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011). We assessed the following domains as having either a 'high', 'low' or 'unclear' risk of bias:
random sequence generation;
allocation concealment;
blinding (of participants, personnel and outcome assessors);
incomplete outcome data sets;
selective outcome reporting;
other sources of bias.
The authors resolved any disagreements by discussion to reach a consensus.
Measures of treatment effect
Where possible, the authors expressed the treatment effect for continuous outcomes in their original metrics using mean differences (MD) and 95% confidence intervals (CI). If trials had measured continuous outcomes using different scales, the authors planned to express the treatment effect as a standardised mean difference (SMD) with 95% CI. We report results for dichotomous data as risk ratios (RR) with 95% CIs.
If there had been more than one study with a specific intervention reporting outcomes at the same time‐point, we had intended to perform a meta‐analysis using the Cochrane statistical package RevMan, however, such data were not available (RevMan 2011).
Unit of analysis issues
Cross‐over studies were not included in the review. If future versions of this review include cross‐over studies, we will use the generic inverse variance method for the analysis of data.
Dealing with missing data
The review authors contacted the investigators of the studies included in this review for additional information where necessary.
In order to present MD and 95% CI data from the study by Hebestreit, the authors took the MD between groups from the results published in table 2 of the manuscript (Hebestreit 2010), and we calculated the 95% CI by multiplying the standard error (SE) by 1.96. We applied this method for all MD and 95% CI data presented pertaining to this paper.
Assessment of heterogeneity
The authors assessed clinical heterogeneity and intended to conduct meta‐analyses where they agreed that the trials were sufficiently clinically homogenous in terms of study population, interventions and outcomes. However, we were not able to combine any studies in a meta‐analysis; if we are able to do this in future updates, we will describe any heterogeneity between the included studies using the standard chi2 test and assess the impact of any heterogeneity on the meta‐analysis using the I2 statistic (Higgins 2011): I2 = [Q‐df/Q] x 100%, where Q is the chi2 statistic and df is its degrees of freedom (Higgins 2011). This describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance). We will use the following guide to interpret the I2 values:
0% to 40% might not be important;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity;
75% to 100% considerable heterogeneity.
Assessment of reporting biases
We sought to reduce reporting bias through the following measures:
we performed comprehensive searches to identify randomised controlled studies;
we attempted to seek out and include relevant unpublished studies;
we had planned to identify reporting biases using funnel plots to assess for small study effects (Higgins 2011), however this was precluded by the small number of included studies.
Data synthesis
If meta‐analyses are possible in future updates, we will use a fixed‐effect model, unless there is substantial heterogeneity (i.e. over 50%) (Higgins 2011), in which case we will use a random‐effects model. For outcomes where there were insufficient data, or a high degree of heterogeneity, we did not perform a meta‐analysis and instead presented a narrative synthesis.
Subgroup analysis and investigation of heterogeneity
We intended to undertake the following subgroup analyses:
children versus adults;
supervised versus unsupervised interventions.
However, the small number of included trials precluded these subgroup analyses. We will undertake these analyses in future updates of this review if sufficient numbers of studies are available.
Sensitivity analysis
If a sufficient number of studies are available in future updates, we will perform a sensitivity analysis by including only studies with an overall low risk of bias. If all studies are found to have a high risk of bias, we will perform a sensitivity analysis after the exclusion of studies that did not conceal allocation.
Results
Description of studies
Results of the search
Electronic searches retrieved a total of 567 citations. After the removal of duplicates and screening of records, 10 full‐text reports of studies and three abstracts were reviewed. An additional four potential references were identified from the reference lists of the full‐text studies. Of the total 14 full‐text studies reviewed, four studies met the criteria for inclusion and 10 were excluded. Of the three published abstracts, one study was excluded and two are awaiting classification and will be assessed for eligibility in a future update if further information becomes available (Alarie 2013; Marostica 2012). Please refer to the figures for the PRISMA diagram of study selection (Figure 1).
1.
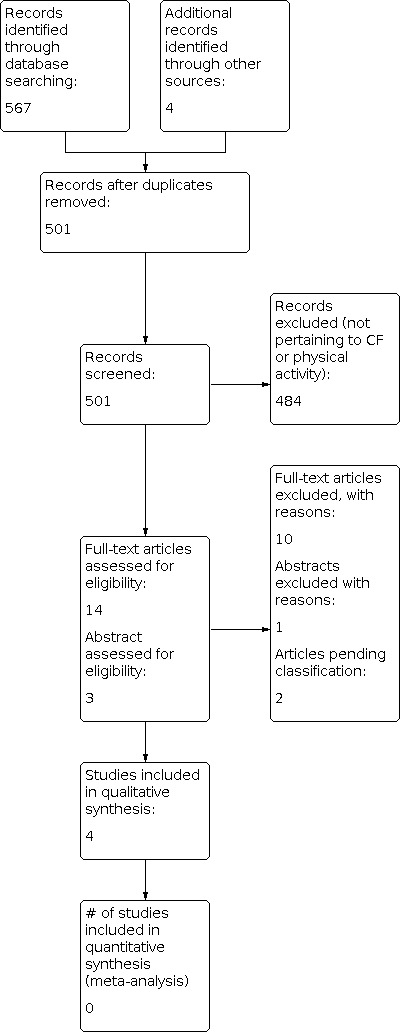
Study flow diagram.
Included studies
Trial Characteristics
All four included studies were randomised controlled studies. One study was conducted in the inpatient setting with follow up in the outpatient setting (Selvadurai 2002). The remaining three studies were conducted in people with CF with stable respiratory disease in the outpatient setting (Hebestreit 2010; Klijn 2004; Schneiderman‐Walker 2000).
The duration of the intervention period of the included studies ranged from 18 days (Selvadurai 2002) to three years (Schneiderman‐Walker 2000); with follow‐up periods of one month (Selvadurai 2002), three months (Klijn 2004), two years (Hebestreit 2010) and three years (Schneiderman‐Walker 2000).
Participants
A total of 199 individuals with CF participated in the four included studies. The number of participants recruited in each study ranged from 23 (Klijn 2004) to 72 (Schneiderman‐Walker 2000). Two studies reported the number of male versus female participants, 28 male versus 38 female (Selvadurai 2002), and 19 male versus 19 female (Hebestreit 2010). Two studies did not report the breakdown of participant gender (Klijn 2004; Schneiderman‐Walker 2000). The mean age of participants ranged from 13.2 years (Selvadurai 2002) to 19.5 years (Hebestreit 2010). Mean FEV1 % predicted ranged from 57% predicted (Selvadurai 2002) to 89% predicted (Schneiderman‐Walker 2000). Three studies were conducted solely in paediatric patients (Klijn 2004; Schneiderman‐Walker 2000; Selvadurai 2002), with one study being undertaken in a combined group of paediatric and adult patients (Hebestreit 2010).
All studies reported no significant differences in baseline characteristics between the intervention and control group in terms of measured parameters including pulmonary function, weight, height and body mass.
Interventions
All included studies used exercise interventions to promote physical activity. The specific interventions assessed in the studies were anaerobic training (Klijn 2004), predominantly aerobic training (Schneiderman‐Walker 2000) or a combination of aerobic training and resistance training (Hebestreit 2010; Selvadurai 2002). Two studies used supervised, prescribed exercise strategies (Klijn 2004; Selvadurai 2002), the remaining two studies provided activity counselling and exercise or activity prescription planning at baseline with telephone and clinic consultation throughout the intervention period (Hebestreit 2010; Schneiderman‐Walker 2000).
Outcomes
Participation in physical activity and exercise capacity were reported in all studies. Quality of life (QOL) was reported by three studies, all using different methods. Selvadurai used the 'Quality of Well‐being scale' in hospitalised children undertaking supervised training (Selvadurai 2002); Klijn used the 'CF Questionnaire (CFQ)' (Klijn 2004); and Hebestreit used the 'Revised German CF questionnaire' (Hebestreit 2010).The 'Revised German CF questionnaire' is a translation of the originally developed CFQ (Schmidt 2009). The CFQ assesses nine domains relating to health‐related QOL and three domains relating to symptoms, as well as subjective health perception (Henry 2003; Wenninger 2003). In stable patients a change in score of four points on the respiratory scale of the questionnaire corresponds to the minimum clinically important difference (MCID) (Quittner 2009).
All included studies assessed pulmonary function (FEV1 and FVC), while two studies also assessed FEF25-75 (Klijn 2004; Schneiderman‐Walker 2000). Adherence to the intervention and compliance with other CF‐related treatments were reported in two studies (Klijn 2004; Schneiderman‐Walker 2000). None of the included studies presented a cost analysis of the intervention. Only one study reported the occurrence of adverse events, however it was unclear if these events prevented participants from completing outcome assessments (Selvadurai 2002).
Excluded studies
On reviewing the full‐text papers, 10 potentially relevant studies were excluded. One additional study, available only in abstract form, was also excluded (Petrovic 2013).
Seven of the excluded studies were not randomised controlled studies (Bernard 2004; Gulmans 1999; Hind 2008; Orenstein 1981; Swisher 2010; Tunzin 1998; van Doorn 2010). One study was only conducted in the inpatient setting, with no outpatient follow up (Kuys 2011). Three studies only assessed physiological outcomes of exercise intervention and not physical activity participation (Moorcroft 2004; Petrovic 2013; Sosa 2012).
Risk of bias in included studies
Included studies were rated as low risk of bias for some aspects, and unclear for others. The authors' judgements of risk of bias for each study are outlined in their respective 'Risk of bias' table, and presented in the 'Risk of bias summary table' (Figure 2).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two studies were allocated low risk of bias for random sequence generation having identified the use of "random computer number generation" (Schneiderman‐Walker 2000) or "selection of pre‐folded papers" indicating group allocation (Hebestreit 2010). Two studies were rated as having an unclear risk of bias as the method of sequence generation was not described (Klijn 2004; Selvadurai 2002).
Three trials had a low risk of selection bias as allocation was concealed by using "concealed envelopes" (Klijn 2004; Selvadurai 2002) or "folded paper in an opaque bag" (Hebestreit 2010). For one study the method of concealment was not stated and it was therefore judged to have an unclear risk of bias (Schneiderman‐Walker 2000).
Blinding
It was not possible to blind the participants in any included study, nor were caregivers blind to group allocation in any of the included studies. In one study, assessors of pulmonary function were "unaware of each patient's group assignment" (Schneiderman‐Walker 2000). However, it is unclear whether assessors of other outcomes were also blind to group allocation. One study reported that "the primary researcher was blinded for the experimental condition", however, it was unclear if this researcher performed outcome assessments (Klijn 2004). In the two remaining studies there was insufficient information presented to determine if outcome assessors were blind to group allocation (Hebestreit 2010; Selvadurai 2002).
Overall, the risk of bias for blinding was unclear, as the extent to which unblinded assessment may have affected outcomes would vary depending upon the outcome; for example, assessor knowledge of participant group allocation could affect the reporting of QOL outcomes, or the amount of encouragement provided may influence the outcome of exercise tests. The influence of unblinded outcome assessors may be less likely to affect objective measures of physical activity such as activity monitoring.
Incomplete outcome data
Two studies were rated as having a low risk of bias for incomplete outcome data (Klijn 2004; Selvadurai 2002). One study was rated as having a high risk (Hebestreit 2010) and one as having an unclear risk of bias (Schneiderman‐Walker 2000). All studies did report dropouts, with the proportion of dropouts at the final data‐collection time‐point ranging from 0% (Selvadurai 2002) to 26% (Hebestreit 2010). Selvadurai reported that two participants were unable to complete all aspects of the intervention programme; however, it is presumed these participants were still able to complete outcome assessments as the paper identifies "no children withdrew from the study" (Selvadurai 2002). One study stated that an intention‐to‐treat analysis was performed; however, only the results of those participants who had at least two years of follow up were presented (Schneiderman‐Walker 2000). In this study seven participants dropped out prior to the two‐year follow‐up assessment; the authors stated that an intention‐to‐treat analysis revealed similar results to those reported. At two‐year follow up, there was a 26% dropout in the study by Hebestreit, with no statement of analysis by intention‐to‐treat (Hebestreit 2010). There were three dropouts reported in the Klijn study (n = 23) (Klijn 2004).
Selective reporting
Overall, included studies were judged as having unclear risk of reporting bias as there was insufficient information to determine the effect of the slight variations in reporting evidence.
Other potential sources of bias
Other potential sources of bias were identified in three studies (Hebestreit 2010; Klijn 2004; Selvadurai 2002). In the supervised anaerobic training study, the discussion suggests participants were instructed not to alter their physical activity participation outside of supervised training, however, the nature of this instruction was not outlined in the methods (Klijn 2004). In a study of activity counselling and exercise advice compared to control, logistic and financial support (for example gym fees) was offered if needed, to enable participation in the intervention programme (Hebestreit 2010). In one study, participation in physical activity as assessed using accelerometer was conducted in a subgroup of participants who had previously had their baseline levels of physical activity established as a part of another study (Selvadurai 2002). The inclusion criteria for this alternate study were not stated.
Effects of interventions
Four studies have been included in a narrative synthesis. There were no studies included in a quantitative synthesis, as a consequence of the variation in the type and duration of studies, the methods of measuring outcomes and measurement units, the time‐points at which outcomes were assessed, and the lack of reported data.
Supervised exercise training
Two studies, with a total of 86 participants reported the outcomes of supervised exercise training on participation in physical activity (Klijn 2004; Selvadurai 2002). In one study participants in the intervention group undertook anaerobic training, while control group participants received no intervention and continued with their normal daily activities (Klijn 2004). The second study of supervised exercise training was undertaken in the inpatient environment (Selvadurai 2002). Aerobic or resistance training (or a combination of both) was performed by children in the intervention groups, whilst control group participants received standard chest physiotherapy without any physical training (Selvadurai 2002).
Primary outcomes
1. Participation in physical activity
a. At less than or equal to one month
One study reported the effects of a supervised, inpatient programme of aerobic or resistance exercise on physical activity at one month following hospital discharge (Selvadurai 2002). There was no significant difference in physical activity participation between those who undertook aerobic training in the hospital and a control group who undertook no physical training sessions, MD 1.20 MJ/day (95% CI ‐0.47 to 2.87 MJ/day). Similarly, there was no significant difference in physical activity participation between those who undertook a resistance training program and the control group with no physical training at one month following discharge, MD 0.65 MJ/day (95% CI ‐0.86 to 2.16 MJ/day) (Analysis 1.1).
1.1. Analysis.

Comparison 1: Supervised exercise training compared to no intervention, Outcome 1: Participation in physical activity (MJ/day)
In one study comparing supervised anaerobic training and a control group who undertook no exercise training, the authors stated that there were no significant changes in physical activity participation for either group at the conclusion of the 12‐week training programme (Klijn 2004). No data for activity participation were reported.
b. At one month up to six months
For the study comparing supervised anaerobic training and a control group who undertook no exercise training, the authors stated that there were no significant changes in physical activity participation for either group at at three‐months follow up (Klijn 2004). No data for activity participation were reported.
c. At over six months
No studies of supervised exercise training reported outcomes for physical activity participation at over six months.
2. Change in QOL
a. At less than or equal to one month
In one study, the supervised, inpatient aerobic training group significantly improved quality of well‐being score at one month following discharge compared to a no physical training control group, MD 0.10 points (95% CI 0.03 to 0.17 points) (Selvadurai 2002) (Analysis 1.2). Inpatient resistance training produced no change in QOL score compared to a control group with no physical training at one month post discharge, MD 0.03 points (95% CI ‐0.04 to 0.10 points) (Analysis 1.2).
1.2. Analysis.

Comparison 1: Supervised exercise training compared to no intervention, Outcome 2: Change in quality of life
In the second study, at the completion of a 12‐week supervised anaerobic training programme, children in the training group had a mean increase in QOL score for the domain of physical functioning only (Klijn 2004). Physical functioning score changed by a mean 18.1 points in the anaerobic training group, compared to a 3.9 point change in the no intervention control group. A between‐group analysis was not presented.
b. Over one month and up to six months
No studies of supervised exercise training assessed QOL at one to six months.
c. At over six months
No studies of supervised exercise training assessed QOL at over six months.
Secondary outcomes
1. Change in exercise capacity
Two studies of supervised exercise training assessed exercise capacity. One used a cycle ergometry test (Klijn 2004), with the second study using a treadmill test (Selvadurai 2002).
a. At less than or equal to one month
In the Selvadurai study, supervised, inpatient aerobic training resulted in a significant improvement in change in VO2 peak measured on a treadmill compared to no physical training control at both discharge from hospital, MD 8.53 ml/kg/min (95% CI 4.85 to 12.21 ml/kg/min) and one month following discharge, MD 4.91 ml/kg/min (95% CI 1.13 to 8.69 ml/kg/min) (Selvadurai 2002) (Analysis 1.3). Supervised, inpatient resistance training resulted in no difference in change in VO2 peak relative to the no physical training control group at discharge from hospital, MD 1.95 ml/kg/min (95% CI ‐1.61 to 5.51 ml/kg/min), or at one month following discharge, MD ‐0.40 ml/kg/min (95% CI ‐4.03 to 3.23 ml/kg/min) (Selvadurai 2002) (Analysis 1.3).
1.3. Analysis.

Comparison 1: Supervised exercise training compared to no intervention, Outcome 3: Change in exercise capacity (ml/kg/min)
In the Klijn study, at completion of a 12‐week supervised anaerobic training programme, the intervention group had a significantly greater improvement in VO2 peak measured by cycle ergometry, when compared to a no physical training control group, MD 2.10 ml/kg/min (95% CI 0.12 to 4.08 ml/kg/min) (Klijn 2004) (Analysis 1.3).
b. Over one month up to six months
In one study, at three‐month follow up, a 12‐week supervised anaerobic training programme resulted in no significant difference in exercise capacity from baseline for the treatment group (Klijn 2004). No data were reported.
c. At over six months
No studies of supervised exercise training reported results for change in exercise capacity at over six months.
2. Pulmonary function tests
a. Change in FEV1 (% predicted)
Selvadurai reported data for change in FEV1 following supervised training at discharge from hospital (end intervention) and one month following discharge (Selvadurai 2002).
i. At less than or equal to one month
In the Selvadurai study there was no clear benefit seen when comparing supervised aerobic training during hospitalisation to a no training control group at discharge, MD 2.03% (95% CI ‐2.31 to 6.37%), and at one‐month follow up, MD 1.53% (95% CI ‐2.93 to 5.99%) (Selvadurai 2002) (Analysis 1.4). However, resistance training during hospitalisation resulted in a significantly greater improvement in FEV1 compared to the no training control group at both discharge from hospital, MD 5.58% (95% CI 1.34 to 9.82%), and one month post discharge, MD 5.08% (95% CI 0.66 to 9.50%) (Selvadurai 2002) (Analysis 1.4).
1.4. Analysis.

Comparison 1: Supervised exercise training compared to no intervention, Outcome 4: Change in FEV1 (% predicted)
In one study, there was no significant change in pulmonary function (including FEV1) at end of intervention for participants undertaking 12‐weeks supervised anaerobic training compared to no exercise training control group (Klijn 2004). No data were presented.
ii. Over one month up to six months
In one study, there was no significant change in pulmonary function (including FEV1) at three‐months follow up in participants who had completed either a 12‐week supervised anaerobic training programme or no exercise training control group (Klijn 2004). No data were presented.
iii. At over six months
No studies of supervised exercise training reported outcomes for change in FEV1 at over six months.
b. Change in FVC (% predicted)
i. At less than or equal to one month
There were no changes in FVC when supervised, inpatient aerobic training was compared to a no training control group at either discharge from hospital, MD 0.06% (95% CI ‐2.55 to 2.67%) or at one month post discharge, MD ‐0.11%, 95% CI (‐2.64 to 2.42%) (Selvadurai 2002) (Analysis 1.5). Similarly, supervised, inpatient resistance training compared to no training control resulted in no change in FVC at hospital discharge, MD 0.17%, 95% CI (‐2.31 to 2.65%) or at one month post discharge, MD 0.06% (95% CI ‐2.42 to 2.54%) (Selvadurai 2002) (Analysis 1.5).
1.5. Analysis.

Comparison 1: Supervised exercise training compared to no intervention, Outcome 5: Change in FVC (% predicted)
In one study there was no significant change in pulmonary function (including FVC) at end of intervention for participants who had undertaken 12‐weeks supervised anaerobic training compared to no exercise training control group (Klijn 2004). No data were presented.
ii. Over one month up to six months
In one study, there was no significant change in pulmonary function (including FVC) at three‐months follow up in participants who had completed either a 12‐week supervised anaerobic training programme or no exercise training control group (Klijn 2004). No data were presented.
iii. At over six months
No studies of supervised exercise training reported outcomes for change in FVC at over six months.
c. Change in FEF25-75 (%predicted)
No studies of supervised exercise training reported outcomes for change in FEF25-75.
3. Adverse events
No adverse events related to the study interventions were reported. One participant in the Selvadurai study (aerobic training group) suffered a sprained ankle which was reported as unrelated to the training programme (Selvadurai 2002).
4. Body composition
Both studies of supervised exercise training programmes reported outcomes for body composition (Klijn 2004; Selvadurai 2002).
a. At less than or equal to one month
Fat‐free mass in kilograms was reported in two studies (Klijn 2004; Selvadurai 2002). In the Selvadurai study, inpatient aerobic training had no effect on the change in fat‐free mass at discharge from hospital compared to a control group with no physical training, MD 0.01 kg (95% CI ‐0.19 to 0.21 kg) or at one month post discharge, MD 0.04 kg (95% CI ‐0.19 to 0.27 kg) (Selvadurai 2002) (Analysis 1.6).In the supervised, inpatient resistance training group the children had a significantly greater improvement in fat‐free mass at both discharge, MD 1.80 kg (95% CI 1.57 to 2.03 kg), and at one month after discharge from hospital, MD 1.71 kg (95% CI 1.46 to 1.96 kg), compared to a no physical training control group (Selvadurai 2002) (Analysis 1.6).
1.6. Analysis.

Comparison 1: Supervised exercise training compared to no intervention, Outcome 6: Change in fat‐free mass (kg)
In children undertaking a 12‐week supervised anaerobic training programme there was no difference in fat‐free mass compared to a no exercise control group at the end of the intervention, MD ‐0.40 kg (95% CI ‐1.14 to 0.34 kg) (Klijn 2004) (Analysis 1.6).
b. Over one month up to six months
In children undertaking a 12‐week supervised anaerobic training programme there was no difference in fat‐free mass compared to a control group with no exercise at three‐months follow up, MD 0.30 kg (95% CI ‐1.27 kg to 1.87 kg) (Klijn 2004) (Analysis 1.6).
c.At over six months
No studies of supervised exercise training reported outcomes for body composition at over six months.
5. Change in bone mineral density (defined on dual energy X‐ray absorptiometry (DXA) scans)
Bone mineral density was not measured or reported in any of the studies of supervised exercise training.
6. Adherence to intervention
One study of supervised exercise training reported on adherence to the intervention (Klijn 2004).
a. At less than or equal to one month
Attendance at a supervised, outpatient anaerobic training programme was rated as excellent with a mean attendance rate of 98% of sessions (SD 4%) (Klijn 2004).
b. Over one month up to six months
No studies of supervised exercise training reported outcomes for adherence to the intervention at one to six months.
c. At over six months
No studies of supervised exercise training reported outcomes for adherence to the intervention at over six months.
7. Compliance with other CF treatments
Compliance with other CF treatments was not measured or reported in any of the studies of supervised exercise training.
8.Cost evaluation
Cost evaluation was not assessed or reported in any of the studies of supervised exercise training.
Unsupervised exercise training
Two studies, including 110 participants reported the outcomes of unsupervised exercise training on physical activity participation (Hebestreit 2010; Schneiderman‐Walker 2000). In both studies, control group participants received no intervention and participated in their usual physical activity. Participants in the intervention groups received activity counselling and exercise or activity prescription planning at baseline (with telephone and clinic consultation throughout the intervention period) to undertake either predominantly aerobic training (Schneiderman‐Walker 2000) or a combination of aerobic and resistance training (Hebestreit 2010).
Primary outcomes
1. Participation in physical activity
a. At less than or equal to one month
No studies of unsupervised exercise training reported physical activity outcomes at less than or equal to one month.
b. Over one month up to six months
Activity counselling and exercise advice resulted in no significant difference in participation in vigorous physical activity as measured by accelerometry, when compared to a control group with no intervention at three‐ to six‐months follow up, MD 1.05 hours/week (95% CI ‐0.65 to 2.75 hours/week) (Hebestreit 2010). In order to present the MD and 95% CI data from the study by Hebestreit, the MD between groups was taken from the results published in table 2 of the manuscript (Hebestreit 2010), and the 95% CI was calculated by multiplying the SE by 1.96.
c. At over six months
At 12‐months follow up, activity counselling and exercise advice resulted in no significant difference in participation in vigorous physical activity as measured by accelerometry, when compared to a control group with no intervention, MD 2.08 hours/week (95% CI ‐1.84 to 6 hours/week) (Hebestreit 2010). However, at 18‐ to 24‐months follow up, participants who received activity counselling and exercise advice spent significantly more time in vigorous activity than participants in the control group, MD 1.63 hours/week (95% CI 0.02 to 3.24 hours/week); P = 0.047. Time spent in vigorous activity was determined via accelerometry over seven days.
In contrast, in the second study, physical activity participation was assessed using daily activity diaries in children with CF (Schneiderman‐Walker 2000). Activity participation was reported as significantly greater in the home exercise programme group compared to the no intervention control group at the end of one year (P = 0.06), two years (P = 0.006) and three years (P = 0.01) (Schneiderman‐Walker 2000). No data for activity participation were reported.
2. Change in quality of life
a. At less than or equal to one month
No studies of unsupervised exercise training assessed or reported on QOL at less than or equal to one month.
b. Over one month up to six months
Hebestreit reported that the only change in QOL was recorded in the subjective health perception category (Hebestreit 2010). Subjective health perception favoured the activity counselling and exercise advice group compared to the no intervention control group at three to six months, MD 9.91 points (95% CI 0.89 to 18.93 points) (P = 0.031).
c. At over six months
At 12‐month follow up the activity counselling and exercise advice group demonstrated no difference in score for subjective health perception from baseline when compared to a no intervention control group, MD ‐2.31 points (95% CI ‐15.46 to 10.84 points) (Hebestreit 2010). However, at 18‐ to 24‐months follow up, subjective health perception was better for the activity counselling and exercise advice group compared to the control group, MD 9.89 points (95% CI 0.64 to 19.14 points) (P = 0.036) (Hebestreit 2010).
Secondary outcomes
1. Change in exercise capacity
Both studies of unsupervised exercise training assessed exercise capacity using a cycle ergometry test (Hebestreit 2010; Schneiderman‐Walker 2000).
a. At less than or equal to one month
No studies of unsupervised exercise training assessed or reported on change in exercise capacity at less than or equal to one month.
b.Over one month up to six months
Six months activity counselling and exercise advice produced significant differences in change in VO2 peak at three to six months when compared to a control group with no intervention, MD 2.04 ml/kg/min (95% CI 0.08 to 4.0 ml/kg/min) (P = 0.041) (Hebestreit 2010).
c. At over six months
In one study, a home exercise programme resulted in no significant difference in the annual rate of decline of VO2 peak, over a three‐year period, compared to a control group with no intervention, MD 0.05 ml/kg/min (95% CI ‐1.10 to 1.20 ml/kg/min) (Schneiderman‐Walker 2000) (Analysis 2.1). Similarly, after 12 months there was no difference in VO2 peak between those who received six months activity counselling and exercise advice and a control group with no training, MD 0.70 ml/kg/min (95% CI ‐1.61 to 3.01 ml/kg/min) (Hebestreit 2010). However, at 18‐ to 24‐months follow up, six months activity counselling and exercise advice resulted in a significant difference in change in VO2 peak when compared to a no intervention control group, MD 3.73 ml/kg/min (95% CI 1.32 to 6.14 ml/kg/min) (P = 0.002) (Hebestreit 2010).
2.1. Analysis.

Comparison 2: Unsupervised exercise training compared to no intervention, Outcome 1: Annual rate of change in exercise capacity (ml/kg/min) (follow up > 6 months)
2. Pulmonary function tests
a. Change in FEV1 (% predicted)
Change in FEV1 was reported in both studies of unsupervised exercise training.
In one study, a trend toward a greater annual rate of decline in FEV1 was reported in the control group with no intervention when compared to a three‐year home exercise programme group, MD 2.01% (95% CI ‐0.06 to 4.08%) (Schneiderman‐Walker 2000) (Analysis 2.2). In the second study, activity counselling and exercise advice was found to have had no effect on FEV1 at any time‐point (Hebestreit 2010). The raw data supplied by the authors have generated the following results: at three‐months follow up MD ‐0.75% (95% CI ‐6.10 to 4.60%); at six‐months follow up, MD 2.42 (95% CI ‐5.42 to 10.26%); at 12‐months follow up, MD ‐0.36% (95% CI ‐8.02 to 7.30%); at 18‐months follow up, MD 5.66% (95% CI ‐2.88 to 14.20%); and at 24‐months follow up, MD ‐1.72% (95% CI ‐10.62 to 7.18%) (Analysis 2.3).
2.2. Analysis.

Comparison 2: Unsupervised exercise training compared to no intervention, Outcome 2: Annual rate of decline FEV1 (% predicted) (follow up > 6 months)
2.3. Analysis.

Comparison 2: Unsupervised exercise training compared to no intervention, Outcome 3: Change in FEV1 (% predicted)
b. Change in FVC (%predicted)
i. At less than or equal to one month
No studies of unsupervised exercise training assessed or reported on change in FVC at less than or equal to one month.
ii. Over one month up to six months
In one study, at three‐ to six‐months follow up there was no difference in change in the FVC for activity counselling and exercise advice compared to no intervention control, MD 0.5% (95% CI ‐4.3 to 5.3%) (P = 0.837) (Hebestreit 2010).
iii. At over six months
A significantly greater annual rate of decline in FVC was seen in the control group of the Schneiderman‐Walker study when compared to a three‐year home exercise programme group, MD 2.17% (95% CI 0.47 to 3.87%) (Schneiderman‐Walker 2000) (Analysis 2.4).
2.4. Analysis.

Comparison 2: Unsupervised exercise training compared to no intervention, Outcome 4: Annual rate of decline FVC (% predicted) (follow up > 6 months)
In the Hebestreit study, for activity counselling and exercise advice there was no difference in change in FVC when compared to a no intervention control group at 12 months, MD 2.71 % (95% CI ‐4.36 to 9.78 %) (Hebestreit 2010), however at 18 to 24 months the activity counselling and exercise advice group had significantly greater improvement in FVC compared to the no intervention control group, MD 6.06 % (95% CI 0.43 to 11.69 %) (P = 0.036).
c. Change in FEF25-75 (%predicted)
Outcomes for FEF25-75 were only reportedat over six months in a single study of unsupervised exercise training (Schneiderman‐Walker 2000).
i. At less than or equal to one month
No studies of unsupervised exercise training assessed or reported on change in FEF25-75 at less than or equal to one month.
ii. Over one month up to six months
No studies of unsupervised exercise training assessed or reported on change in FEF25-75 at one to six months.
iii. At over six months
In one study, a greater annual rate of decline in FEF25-75 was seen in the control group when compared to a home exercise programme group, but the difference was not significant, MD 0.80% (95% CI ‐2.20 to 3.80%) (Schneiderman‐Walker 2000) (Analysis 2.5).
2.5. Analysis.

Comparison 2: Unsupervised exercise training compared to no intervention, Outcome 5: Annual rate of decline FEF25-75 (% predicted) (follow up > 6 months)
3. Adverse outcomes
No adverse outcomes were reported in any studies of unsupervised exercise training.
4. Body composition
One study of unsupervised exercise training assessed and reported outcomes for body composition (Hebestreit 2010). Raw data supplied by the study authors was used to assess this outcome.
a. At less than or equal to one month
No studies of unsupervised exercise training assessed or reported on body composition at less than or equal to one month.
b. Over one month up to six months
Hebestreit reported significant improvements in lean body mass (kg) favouring participants who received activity counselling and exercise advice compared to the control group with no intervention at three‐month and six‐month follow up (Hebestreit 2010): three months, MD 1.76 kg (95% CI 0.97 to 2.55 kg); six months, MD 2.16 kg (95% CI 0.55 to 3.77 kg) (Analysis 2.6).
2.6. Analysis.

Comparison 2: Unsupervised exercise training compared to no intervention, Outcome 6: Change in lean body mass (kg)
However, for skin‐fold measurement (mm, across four sites) the results for three and six months were not statistically significant: at three‐months follow up, MD ‐0.47 mm (95% CI ‐4.42 to 3.48 mm); at six‐months follow up, MD ‐2.54 mm (95% CI ‐6.70 to 1.62 mm) (Analysis 2.7).
2.7. Analysis.

Comparison 2: Unsupervised exercise training compared to no intervention, Outcome 7: Change in skin fold measure (mm)
c. At over six months
Hebestreit found no significant difference in lean body mass (kg): at 12 months, MD 1.88 kg (95% CI ‐0.13 to 3.89 kg); at 18 months, MD 1.77 kg (95% CI ‐0.80 to 4.34 kg); and at 24‐months follow up, MD 1.28 kg (95% CI ‐1.43 to 5.07 kg) (Analysis 2.6).
Hebestreit found a significant difference in skin fold measurements (mm, across four sites) between participants who received activity counselling and exercise advice compared to no intervention control at 12‐months follow up, MD ‐5.54 mm (95% CI ‐10.86 to ‐0.22 mm) (Analysis 2.7). However, the results at the remaining time‐points were not significant: at 18‐months follow up, MD ‐5.03 mm (95% CI ‐10.74 to 0.68 mm); and at 24‐months follow up, MD ‐6.94 mm (95% CI ‐14.02 to 0.14 mm) (Analysis 2.7).
5. Change in bone mineral density (defined on DXA scans)
Bone mineral density was not measured or reported in any of the studies of unsupervised exercise training.
6. Adherence to intervention
Adherence to the intervention programme was described in two studies of unsupervised exercise training (Hebestreit 2010; Schneiderman‐Walker 2000).
a. At less than or equal to one month
No studies of unsupervised exercise training assessed or reported on adherence to the intervention at less than or equal to one month.
b. At over one month up to six months
No studies of unsupervised exercise training assessed or reported on adherence to the intervention at one to six months.
c. At over six months
A grading system of 0 to 2 (0 indicating poor compliance; 1 indicating partial compliance; 2 indicating full compliance) was used by exercise physiologists overseeing the three‐year home exercise programme (Schneiderman‐Walker 2000). Mean (SD) scores for exercise compliance in the intervention group did not change across the three years of the study (year 1: 1.51 (0.55); year 2: 1.51 (0.60); year 3: 1.49 (0.62)).
Hebestreit identified under‐reporting, or insufficient compliance with physical activity participation following activity counselling and exercise advice (Hebestreit 2010). This presents a possible explanation for increases in physical activity participation not reaching the prescribed target of three hours per week. Participants in the activity counselling and exercise advice group had a self‐reported increase in vigorous physical activity of 2.16 hours per week.
7. Compliance with other CF treatments
a. At less than or equal to one month
No studies of unsupervised exercise training assessed or reported on compliance with other CF treatments at less than or equal to one month.
b. Over one month up to six months
No studies of unsupervised exercise training assessed or reported on compliance with other CF treatments at one to six months.
c. At over six months
Compliance with other CF treatments was reported in one study (Schneiderman‐Walker 2000). Compliance with prescribed physiotherapy regimen was graded 0 to 2 (0 indicating poor compliance; 1 indicating partial compliance; 2 indicating full compliance) and scored by the exercise physiologists administering the intervention. Information regarding physiotherapy compliance was provided subjectively by the patient, and their parent when necessary, in order for a score to be given. Mean scores for compliance with physiotherapy routines were lower than compliance scores for exercise participation, but were not significantly different between the exercise group (year 1 physiotherapy compliance mean score: 0.69) and the control group (year 1 physiotherapy compliance mean score: 0.95). Measures of variability were not reported for physiotherapy compliance scores.
8. Cost evaluation
Cost evaluation was not assessed or reported in any of the studies of unsupervised exercise training.
Discussion
Summary of main results
This systematic review aimed to examine the effect of interventions designed to promote physical activity participation in people with CF. A search of the literature identified 15 potentially relevant studies, of which only four met the inclusion criteria. All four included studies, providing data on 199 participants, assessed the effect of an exercise programme on participation in physical activity. None of the included studies were primarily designed to increase participation in physical activity, but rather assessed the effect of the exercise training intervention on outcomes including participation in physical activity.
The effects of the interventions were varied, and most evident with longer‐term follow up. Inpatient, supervised aerobic training and resistance training did not result in significant changes in participation in physical activity at one month post‐discharge (Selvadurai 2002), nor did supervised anaerobic training at three‐month follow up (Klijn 2004). Six months of unsupervised exercise training following activity counselling and exercise advice had no effect on vigorous physical activity participation compared to a control group with no intervention when assessed at three to six months and 12‐months follow up (Hebestreit 2010). There was, however, a significant improvement in activity participation at 18‐ to 24‐months follow up, MD 1.63 hours per week (95% CI 0.02 to 3.24 hours per week) (Hebestreit 2010). Physical activity participation was also significantly greater in a home exercise programme group compared to a control group with no intervention at the end of each year over three years of follow up (year one (P = 0.06), year two (P = 0.006) and year three (P = 0.01)) (Schneiderman‐Walker 2000). These results indicate that exercise training strategies of longer duration (greater than six months), and those that require self‐directed behaviours, may have more impact on physical activity participation than short‐term, supervised interventions. Furthermore, that duration of follow up may be an important factor in assessing interventions to promote physical activity with improvements in physical activity participation only being evident when outcomes were measured at or after 12 months.
Type and duration of exercise training interventions had inconsistent effects on exercise capacity and physical activity participation. Inpatient, supervised, aerobic training had a positive short‐term influence on exercise capacity at one‐month follow up post‐discharge when compared to a control group with no physical training, but no influence on physical activity participation (Selvadurai 2002). Likewise, 12 weeks supervised anaerobic training improved exercise capacity compared to no training at the completion of the intervention period without concomitant changes in physical activity participation (Klijn 2004). Differences in exercise capacity following 12 weeks supervised anaerobic training were no longer evident at three month follow up. Whether changes in exercise capacity resulting from supervised inpatient aerobic training, or improvements in physical activity participation would become evident at longer‐term follow up is unknown.
The studies in this review suggest that changes in physical activity participation do not always occur simultaneously with changes in exercise capacity. Six months of activity counselling and exercise advice produced significant differences in change in VO2 peak between the intervention group and a no intervention control group at three‐ to six‐months and 18‐ to 24‐months follow up (Hebestreit 2010), however, improvement in physical activity participation was only seen concurrently at 18 to 24 months post intervention. Meanwhile a three‐year home exercise programme found no difference in annual rate of decline in VO2peak when compared to a no intervention control group, but did result in significant improvements in diary reports of activity participation when compared to the no intervention control group (Schneiderman‐Walker 2000). The inconsistent nature of the relationship between changes in exercise capacity and changes in physical activity participation, suggests that inferences regarding physical activity participation can rarely be determined based on exercise capacity alone, and vice versa. The only intervention with a significant effect on both exercise capacity and physical activity participation, and only at a single time‐point, was six months of activity counselling and exercise advice compared to a no intervention control when assessed at 18 to 24 months. It is unclear why this effect was not evident at other time‐points, or for other interventions. There is insufficient evidence to draw conclusions on the relationship between exercise capacity and physical activity participation, and how any such relationship may influence the type, duration and follow‐up period of interventions to promote physical activity participation.
Included studies reported limited effects of the interventions on QOL outcomes. Included studies reported effects on QOL that were unclear (Klijn 2004), inconsistent across time points (Hebestreit 2010) or too small to be clinically meaningful (Selvadurai 2002). Only one study reported an improvement in a single domain of QOL with a concurrent improvement in physical activity participation (Hebestreit 2010). This effect was only seen at a single assessment time‐point, and was not found in any other studies where the effect of an exercise training programme may have resulted in an improvement in QOL or physical activity participation, but not both at the same time. In contrast, exercise training undertaken as pulmonary rehabilitation in people with chronic obstructive pulmonary disease (COPD) achieves both large and clinically important changes in QOL (Lacasse 2006). This may suggest it is more difficult to achieve changes in QOL in a complex, multi‐system disease like CF with a singular mode of intervention (i.e. exercise training), than in other respiratory diseases. Additionally, it will be important to elucidate which elements of an activity counselling and exercise advice programme lends itself to simultaneously improve QOL and physical activity participation to see if this effect can be replicated at other time‐points, or when combined with other interventions.
Overall completeness and applicability of evidence
The included studies had intervention periods of variable length, ranging from 18 days (Selvadurai 2002) to three years (Schneiderman‐Walker 2000). The follow‐up periods ranged from short term (one month) to long term (three years). Predominantly, the included studies were conducted solely with paediatric participants. Only one study had a combined cohort of adult and paediatric participants (Hebestreit 2010). The included studies in this review comprised a relatively young (maximum mean age 19.5 years) CF population, with only mild to moderate CF lung disease. Consequently, whether the results of this review are applicable to older people with CF, and those with more severe lung disease, can not be stated with certainty. The included studies were conducted in both hospitalised patients receiving intravenous antibiotics, as well as in outpatients. The effect of respiratory exacerbations, or fluctuating health status over longer follow‐up periods, e.g. one to three years, on the reported results of physical activity participation is not clear.
The intervention strategies to promote participation in physical activity were all exercise training programmes. Only one study specified the exact nature of the exercises which constituted the training programme (available as an online supplement (Klijn 2004). Basic details of activity counselling and exercise advice were described (Hebestreit 2010) as was the development of a home exercise programme (Schneiderman‐Walker 2000). However, strategies known to effect change in physical activity behaviour, such as motivational interviewing, were not described (Brodie 2005). It is unclear whether the inclusion of such strategies would have produced greater improvements in physical activity participation when coupled with the chosen intervention.
No studies were identified which investigated strategies other than exercise training programmes for their impact on participation in physical activity. It should be considered that other strategies, such as psychological interventions, print material or telemedicine applications, may also have an effect on physical activity participation in this population. More evidence is needed in this important area.
Quality of the evidence
There are limited RCTs addressing physical activity participation in people with CF. The quality of the four included studies in this review was graded according to the standardised risk of bias tool as described in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011). As a result of limitations to reporting, it was difficult to accurately determine study quality. Given that all of the included studies involved exercise training interventions, double blinding was impracticable. Blinded assessors were identified for only one outcome in one study (Schneiderman‐Walker 2000). For the remaining outcome measures in this study, and for the remaining three studies, it is unclear what impact this may have had on outcome assessment. The inability to pool data for quantitative analysis of the primary outcomes underscores the limited evidence for exercise training programmes to promote participation in physical activity in people with CF.
Potential biases in the review process
Any type of physical activity measure was included in this review. This may pose a source of bias when comparing the results across studies, as some methods of physical activity assessment are more objective than others. For example, subjective physical activity assessment using questionnaires or activity diaries can have reduced accuracy given the need to rely upon participant (or caregiver) recall of events to provide information (Bradley 2010). Whilst objective measures of participation in physical activity, such as accelerometers or video observation, provide detailed information on participant movement; they are expensive and require technical expertise to operate, and may pose questions regarding inter‐device variability (Bradley 2010). Futhermore, data obtained from objective measurement devices will be influenced by the type of monitor chosen, where on the body the monitor is worn, and the number of days over which activity data are collected (Trost 2005).
Agreements and disagreements with other studies or reviews
A current Cochrane Review indicates there are positive effects on health‐related outcomes such as blood glucose control, bone mineral accretion and potentially clearance of pulmonary secretions associated with physical training for individuals with CF (Bradley 2008), however, the impact of this training on physical activity participation was not considered. A Cochrane Review of strategies to promote physical activity participation in adults without pre‐existing medical conditions identifies that counselling and support by a professional can encourage increased participation in the short to medium term (Foster 2005). Meanwhile, a meta‐analysis of interventions to promote physical activity in adults with chronic illness identified supervised exercise to be no more effective than education or motivational sessions, and that the greatest intervention effect was seen in education interventions which solely targeted physical activity (Conn 2008). This current review is the first to evaluate the impact of clinical interventions on participation in physical activity for people with CF.
Authors' conclusions
Implications for practice.
From this review, it is difficult to draw strong conclusions regarding the most effective strategies for promoting participation in physical activity in people with CF. Only a small number of randomised controlled studies have investigated interventions to promote physical activity in this group. All included studies investigated exercise training with no evaluation of the strategies that have been found to be effective in other populations (Eakin 2007; van Sluijs 2007; Vandelanotte 2007). The outcomes reported were variable and we were not able to calculate any pooled effects. In addition, the included studies were undertaken in relatively young people with CF, without severe disease. This review provides very limited evidence that activity counselling and exercise advice to undertake a home exercise programme, conducted over at least six months, may result in improved participation in physical activity by people with CF, when assessed in the longer term. The length of follow up required to see benefit in terms of participation in physical activity may have implications with regards to participant motivation and adherence.
Implications for research.
This review has only identified studies using exercise training to promote participation in physical activity in people with CF. Further research is needed to assess the effect of other strategies, which may include the use of print material, health coaching or telemedicine applications, on promoting the uptake and adherence to regular physical activity participation in this population. The use of the Internet to promote physical activity in a variety of adult populations, including those who are healthy, diabetic or overweight and those with physical disability, was found to have a small effect size (mean 0.44) with better outcomes achieved when there were at least five communications (e.g. email) with participants and when the intervention was of shorter duration (up to three months) (Vandelanotte 2007). In healthy adults, and those with chronic conditions, telephone interventions were effective in increasing physical activity participation, particularly when the intervention period ranged from six to 12 months, and included at least 12 telephone calls (Eakin 2007). In children and adolescents there is strong evidence that school‐based interventions, which include family or community involvement, effectively promote physical activity participation (van Sluijs 2007). Future research might focus on identifying whether similar or alternative strategies are effective in promoting participation in physical activity for different groups within the CF population, such as males compared to females, children compared to adults, and severe CF lung disease compared to mild or moderate disease.
What's new
| Date | Event | Description |
|---|---|---|
| 3 May 2022 | Amended | This review will no longer be updated since the remit of this review has been included in the expanded review 'Physical activity and exercise training in cystic fibrosis' which will be published in June 2022. |
History
Protocol first published: Issue 12, 2011 Review first published: Issue 12, 2013
Acknowledgements
The authors would like to acknowledge the authors of included studies who responded to requests for additional information, and the valued assistance of Tracey Remmington.
Appendices
Appendix 1. Glossary
| Physical activity | Any bodily movement produced by the skeletal muscles that results in energy expenditure. |
| Exercise | A component of physical activity. Exercise is planned and structured. |
| Intervention | A treatment or therapy, in this instance aiming to increase participation in physical activity. |
| Pulmonary | In relation to, or affecting, the lungs. |
| Secretions | A substance produced by and discharged from a part of the body e.g. pulmonary secretions ‐ a substance produced by the lungs. |
| Prognostic | Relating to prognosis; predicting the likely outcome of a disease. |
| Suppurative | The formation and/or discharge of pus e.g. suppurative lung disease ‐ a disease process producing pus in the lungs. |
Appendix 2. Search Strategy: CINAHL (EbscoHost)
| 1. randomized controlled trials. mh 2. clinical trials. mh 3. placebos. mh 4. humans NOT animals 5. 1 or 2 or 3 6. 4 and 5 7. Cystic Fibrosis. mh 8. CF. tx 9. mucoviscidosis. ti, ab 10. 7 or 8 or 9 11. 6 and 10 12. physical activity. mh 13. physical fitness. mh 14. habitual N3 activity. ti, ab 15. exercise. mh 16. exertion. mh 17. sport. ti, ab 18. 12 or 13 or 14 or 15 or 16 or 17 19. 11 and 18 20. promot*. tx 21. uptake. tx 22. encourage. tx 23. increase. tx 24. start. tx 25. educat*. tx 26. program*. tx 27. 20 or 21 or 22 or 23 or 24 or 25 or 26 28. 19 and 27 .ab. denotes a word in the abstract; .mh. denotes a CINAHL exact subject heading .ti. denotes a word in the title. .tx. Performs a keyword search of all the database's searchable fields |
Appendix 3. Search Strategy: MEDLINE (Ovid) & PsycINFO (Ovid)
| 1. randomized controlled trial. pt 2. controlled clinical trial. pt 3. randomized. ab 4. placebo. ab 5. randomly. ab 6. trial. ab 7. groups. ab 8. 1 or 2 or 3 or 4 or 5 or 6 or 7 9. cystic fibrosis or CF 10. fibrocystic adj5 disease adj5 pancreas 11. mucoviscidos$ 12. (cystic$ adj10 fibros$) 13. 9 or 10 or 11 or 12 14. 8 and 13 15. physical activity 16. physical fitness 17. habitual adj3 activity 18. exercise 19. exertion 20. sport 21. 15 or 16 or 17 or 18 or 19 or 20 22. 14 and 21 23. promot$ 24. uptake 25. increase 26. start 27. educat$ 28. program$ 29. 23 or 24 or 25 or 26 or 27 or 28 30. 22 and 29 |
Appendix 4. Search Strategy: PEDro
| Abstract and Title: Cystic Fibrosis Problem: Reduced exercise tolerance Sub discipline: Cardiothoracics Abstract and Title: Cystic Fibrosis Therapy: Fitness training Sub discipline: Cardiothoracics Abstract and Title: Cystic Fibrosis Therapy: Behaviour modification Sub discipline: Cardiothoracics |
Data and analyses
Comparison 1. Supervised exercise training compared to no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Participation in physical activity (MJ/day) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 Predominantly aerobic training (short‐term follow up ≦ 1 month) | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐0.47, 2.87] |
| 1.1.2 Predominantly resistance training (short‐term follow up ≦ 1 month) | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 0.65 [‐0.86, 2.16] |
| 1.2 Change in quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Predominantly aerobic training (short‐term follow up ≦ 1 month) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [0.03, 0.17] |
| 1.2.2 Predominantly resistance training (short‐term follow up ≦ 1 month) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.04, 0.10] |
| 1.3 Change in exercise capacity (ml/kg/min) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 Predominantly aerobic training (short term; end of intervention) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 8.53 [4.85, 12.21] |
| 1.3.2 Predominantly aerobic training (short‐term follow up ≦ 1 month) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 4.91 [1.13, 8.69] |
| 1.3.3 Predominantly anaerobic training (short‐term follow up; end of intervention) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [0.12, 4.08] |
| 1.3.4 Predominantly resistance training (short‐term follow up; end of intervention) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 1.95 [‐1.61, 5.51] |
| 1.3.5 Predominantly resistance training (short‐term follow up ≦ 1 month) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐4.03, 3.23] |
| 1.4 Change in FEV1 (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.4.1 Predominantly aerobic training (short‐term follow up; end of intervention) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 2.03 [‐2.31, 6.37] |
| 1.4.2 Predominatly aerobic training (short‐term follow up ≦ 1 month) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 1.53 [‐2.93, 5.99] |
| 1.4.3 Predominantly resistance training (short‐term follow up; end of intervention) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 5.58 [1.34, 9.82] |
| 1.4.4 Predominantly resistance training (short‐term follow up ≦ 1 month) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 5.08 [0.66, 9.50] |
| 1.5 Change in FVC (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 Predominantly aerobic training (short‐term follow up; end of intervention) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐2.55, 2.67] |
| 1.5.2 Predominantly aerobic training (short‐term follow up ≦ 1 month) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐2.64, 2.42] |
| 1.5.3 Predominantly resistance training (short‐term follow up; end of intervention) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐2.31, 2.65] |
| 1.5.4 Predominantly resistance training (short‐term follow up ≦ 1 month) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐2.42, 2.54] |
| 1.6 Change in fat‐free mass (kg) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.6.1 Predominantly aerobic training (short‐term follow up; end of intervention) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.19, 0.21] |
| 1.6.2 Predominantly aerobic training (short‐term follow up ≦ 1 month) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.19, 0.27] |
| 1.6.3 Anaerobic training (short‐term follow up; end of intervention) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.14, 0.34] |
| 1.6.4 Anaerobic training (follow up between 1 and 6 months) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐1.27, 1.87] |
| 1.6.5 Predominantly resistance training (short‐term follow up; end of intervention) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [1.57, 2.03] |
| 1.6.6 Predominantly resistance training (short‐term follow up ≦ 1 month) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 1.71 [1.46, 1.96] |
Comparison 2. Unsupervised exercise training compared to no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Annual rate of change in exercise capacity (ml/kg/min) (follow up > 6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2 Annual rate of decline FEV1 (% predicted) (follow up > 6 months) | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | 2.01 [‐0.06, 4.08] |
| 2.3 Change in FEV1 (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.3.1 At 3‐month follow up | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐6.10, 4.60] |
| 2.3.2 At 6‐month follow up | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 2.42 [‐5.42, 10.26] |
| 2.3.3 At 12‐months follow up | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐8.02, 7.30] |
| 2.3.4 At 18‐months follow up | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 5.66 [‐2.88, 14.20] |
| 2.3.5 At 24‐months follow up | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐1.72 [‐10.62, 7.18] |
| 2.4 Annual rate of decline FVC (% predicted) (follow up > 6 months) | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | 2.17 [0.47, 3.87] |
| 2.5 Annual rate of decline FEF25-75 (% predicted) (follow up > 6 months) | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐2.20, 3.80] |
| 2.6 Change in lean body mass (kg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.6.1 At 3‐months follow up | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 1.76 [0.97, 2.55] |
| 2.6.2 At 6‐months follow up | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 2.16 [0.55, 3.77] |
| 2.6.3 At 12‐months follow up | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 1.88 [‐0.13, 3.89] |
| 2.6.4 At 18‐months follow up | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 1.77 [‐0.80, 4.34] |
| 2.6.5 At 24‐months follow up | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 1.82 [‐1.43, 5.07] |
| 2.7 Change in skin fold measure (mm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.7.1 At 3‐months follow up | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐4.42, 3.48] |
| 2.7.2 At 6‐months follow up | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐2.54 [‐6.70, 1.62] |
| 2.7.3 At 12‐months follow up | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐5.54 [‐10.86, ‐0.22] |
| 2.7.4 At 18‐months follow up | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐5.03 [‐10.74, 0.68] |
| 2.7.5 At 24‐months follow up | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐6.94 [‐14.02, 0.14] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hebestreit 2010.
| Study characteristics | ||
| Methods | Randomised controlled study (60% allocation to intervention group); 1‐year intervention period, 2‐year follow up. Multicentre (3 German CF centres). | |
| Participants | 38 paediatric and adult CF patients. Intervention group n = 23, Control group n = 15. Participants recruited in outpatient setting from 1 of 3 German CF centres (Hannover, Wurzburg, Frankfurt). Mean (SD) age: Intervention group 19.5 (6.4) years; Control group 19.4 (5.3) years. No significant difference between groups at baseline in terms of spirometry (FEV1 % predicted, Intervention group 69.1 (17.2); Control group 75.6 (21.9)), participant gender or age. |
|
| Interventions | Intervention Group
Participants in the intervention group agreed to increase physical activity by a minimum 3 hours/week for the first 6 months of the study. Participants received in‐person counselling with an exercise specialist at baseline, 3 months and 6 months. An individualised exercise plan was devised for each participant in the intervention group based on exercise test outcome and clinical data, as well as patient preference for activity. Participants in the intervention group received fortnightly phone consults for the first 6 months of the study to check on activity behaviour and offer additional help if required. Control Group Participants in the control group were instructed to keep their physical activity level constant for the 1st 12 months of the study. During the 2nd year of the study, control participants were able to change their physical activity behaviour if they wished. |
|
| Outcomes | Outcomes assessed at 3, 6, 12, 18 and 24 months. Vigorous physical activity: hours/week ‐ self reported. Physical activity participation: hours/week ‐ Actigraph accelerometer count measured over 7 days (completed at 6‐month time‐point only). QOL: CFQ14+ ‐ German CF Questionnaire. Exercise capacity (VO2 peak): ml/kg/min ‐ highest VO2 achieved over 30 seconds during a continuous incremental cycle test. Work max: w/kg ‐ power maintained during 1‐minute of last stage completed of incremental exercise test. Muscle power: peak power (PP) and mean power (MP) determined via Wingate test. Lean body mass: kg. Skin fold thickness: mm ‐ sum of 4 measurements. Body fat %. Pulmonary function: FEV1 % predicted; FVC % predicted; RV/TLC % predicted. |
|
| Notes | Results presented for baseline, 3 ‐ 6 months, 12 months and 18 ‐ 24 months follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Recuited participants drew a folded paper ticket indicating their group randomisation. |
| Allocation concealment (selection bias) | Low risk | Participant randomisation occurred by the drawing of a folded paper ticket from an opaque bag. The ticket was destroyed following removal. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel aware of group allocation, the nature of the intervention would make this type of blinding difficult. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unknown if outcome assessments performed by blinded personnel. Activity participation via accelerometry and skin‐fold measures likely subjected to minimal assessment bias. Assessment of QOL and exercise capacity could be open to bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Fewer than 15% of assessed participants were excluded and withdrawals were described and time point indicated. At 24‐month follow‐up the dropout rate was 26%. Intention‐to‐treat analysis was not performed. |
| Selective reporting (reporting bias) | Unclear risk | Outcome measures reported as outlined. Data only presented for the QOL category where there was significant change. Only numerical data presented for spirometry parameter where a change was seen. Results of activity intensity (HR recording) was not presented due to compliance being too poor to gain valid data (communication from author). |
| Other bias | Unclear risk | Participants in the intervention group were offered logistic and financial support, if needed, to foster the realisation of the activity programme. |
Klijn 2004.
| Study characteristics | ||
| Methods | Randomised controlled study. | |
| Participants | 23 children with CF with a stable clinical condition (no requirement for oral or IV antibiotics in the 3 months preceding study testing; FEV1 >30%; absence of musculoskeletal disorders). | |
| Interventions | Intervention Group Intervention group participants received individual anaerobic training, supervised by a physiotherapist. 2 sessions per week for 12 weeks. Control Group Contol group participants were asked not to change their normal daily activities. |
|
| Outcomes | Body composition: fat‐free mass; body weight (kg); BMI. Pulmonary function tests (% predicted): FEV1; FVC; FEF25-75; RV/TLC. Peripheral muscle strength (isometric muscle force): presented as the total maximal muscle force (i.e. summed maximal force in all 4 muscle groups assessed). Anaerobic performance: Wingate anaerobic test (WanT) (mean power and peak power presented). Aerobic performance: standard, incremental progressive exercise test (bike) (VO2 peak ‐ ml/kg/min). Lactate: via blood sample drawn 3 minutes after peak aerobic exercise (mmol/L). Daily physical activity: Habitual Activity Estimation Scale (HAES). Total percentage of time active presented. QOL: CF questionnaire (CFQ). |
|
| Notes | 3 children withdrew ‐ results of remaining 20 participants presented. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Low risk | Use of concealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and physiotherapists conducting exercise training sessions aware of group allocation, the nature of the intervention would make this type of blinding difficult. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The paper notes that the primary researcher was blinded to group allocation, however, it is unclear whether this researcher undertook the outcome assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 dropouts are reported (1 from the treatment group, 2 from the control group). There was no intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | Access to the protocol was unavailable. Results are presented for only one domain on the QOL assessment. |
| Other bias | Unclear risk | The discussion section of the paper states "participants were asked not to change their activity level during the study period", however the means by which this was instructed and the proposed effect are not stated in the methods of the paper. |
Schneiderman‐Walker 2000.
| Study characteristics | ||
| Methods | Randomised controlled study. 3‐year follow‐up period. | |
| Participants | 65 children with CF recruited in outpatient setting. Intervention group n = 30, Control group n = 35. Unkown proportion male to female participants. Participants recruited from the CF clinic at the Hospital for Sick Children in Toronto, Canada. Age range: 7 ‐ 19 years. Intervention Group: FEV1 % pred mean (SD) 89.2 (19.5); FVC % pred 92.6 (15.7); FEF25-75 %pred 76.3 (31.2). Lean body mass 38.2 (13.1) kg. Schwachman score (/100) 89.2 (9.1). VO2max 40.6 (7.6) ml/kg/min. Control Group: FEV1 % pred mean (SD) 87.9 (17.8); FVC % pred 90.1 (12.9); FEF25-75 % pred 72.9 (29.2). Lean body mass 34.1 (11.1) kg. Schwachman score (/100) 87.7 (9.5). VO2max 40.7 (7.9) ml/kg/min. |
|
| Interventions | Intervention Group
Participants in the intervention group were directed to participate in their preferred aerobic activities for 20 minutes on at least 3 occasions in a week (incorporating a warm‐up and cool‐down period). Participants received 1, in‐person counselling session with an exercise physiologist to educate them as to activity requirements for the study, and monitoring techniques. Monitoring techniques were reviewed during clinic (each 12 ‐ 16 weeks) and telephone consultations (each 4 ‐ 6 weeks). Control Group Control group participants were requested to maintain their usual levels of physical activity. Both intervention and control group participants recorded their physical activity in a diary each day during the study period ‐ recording date, activity type, duration and estimate of intensity. |
|
| Outcomes | Outcomes assessed at yearly intervals after recruitment to the study. Participation in physical activity: activity diary Exercise capacity (VO2max):ml/kg/min ‐ measured by cycle ergometry test using Godfrey protocol. Wmax % predicted Maximum HR: beats/min MaxVE: L/min VEmax/MVV: %/min Pulmonary function: FVC % predicted; FEV1 % predicted; FEF25-75 % predicted; PEFR; MVV Score of exercise compliance: range 0 ‐ 2 Questionnaire on feasibility of implementing regular exercise programme. CXR score: Brasfield score (/25) Disease score: Schwachman score (/100) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated to the home exercise programme group or control group using "computerised random number assignment" which was performed by a statistician. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel aware of group allocation, the nature of the intervention would make this type of blinding difficult. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Lung function outcome measure performed by blinded assessor. Unclear whether exercise capacity outcome performed by blinded assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts accounted for (n = 7). Pulmonary function results available for all participants ‐ indicating similar results with intention to treat analysis as those presented. Only results of participants who completed 2‐years follow up presented (n = 65). |
| Selective reporting (reporting bias) | Unclear risk | Physical, exercise and pulmonary function parameters presented as annual rates of change. All outcome measures reported. |
| Other bias | Low risk | None identified. |
Selvadurai 2002.
| Study characteristics | ||
| Methods | Randomised controlled study. Inpatient study with follow up in the outpatient period. | |
| Participants | 66 children with CF admitted to the Royal Alexandra Hospital for Children for treatment of a pulmonary exacerbation. Aged 8 ‐ 16 years. Aerobic training group: n = 22 (9 male); mean (SD) age 13.2 years (2.0); body mass 37.9 kg (7.4); FEV1 56.8 % predicted (17.9); VO2 33.8 ml/kg/min (17.0); Strength 155 Nm (19); QOL 0.62 (0.28). Resistance training group: n = 22 (10 male); mean (SD) age 13.1 years (2.1); body mass 38.1 kg (8.2); FEV1 58.0 % predicted (16.8); VO2 34.2 ml/kg/min (17.8); strength 156 Nm (21); QOL 0.60 (0.26). Control group: n = 22 (9 male); mean age (SD): 13.2 years (2.0); body mass: 38.5 kg (8.0); FEV1: 57.4 %predicted (17.3); VO2: 34.0 ml/kg/min (17.7); strength: 155 Nm (20); QOL: 0.62 (0.29). |
|
| Interventions | Participants randomised to one of 3 groups: Aerobic training group (n = 22); Resistance training group (n = 22); Control group (n = 22).
Aerobic training group: participants completed supervised aerobic activities for 5 sessions, each of 30‐minutes duration, for a week. Activities included treadmill running or stationary cycling at 70% of peak HR. If required, supplemental oxygen to maintain oxygen saturation of at least 90% administered. If dyspnoea scored reached 7 (modified 0 ‐ 10 point Borg score) session ceased prior to 30‐minutes. Resistance training group: participants undertook upper and lower limb resistance exercises using a non‐isokinetic resistance machine with built‐in graded incremental resistance dial. Load of 70% of maximal subjective resistance established at commencement of each session. 5 sets of 10 repetitions of each exercise completed. Supervised sessions 5 times per week were undertaken. Control Group: participants undertook standard chest physiotherapy but did not attend exercise training sessions. |
|
| Outcomes | Outcomes assessed at admission (within 36 hours) and discharge from hospital, and 1‐month post discharge from hospital. Weight: kg Height: cm Fat free mass: kg ‐ using skin fold thickness from four sites. Pulmonary function: FEV1 % predicted; FVC % predicted Exercise capacity: VO2 ‐ ml/kg/min; VCO2; V'E; RQ Lower limb strength (Nm): non‐dominant hamstring and quadriceps femoris strength measured using an isokinetic Cybex dynamometer. QOL: Quality of Well Being Scale* (administered on admission and 1‐month post discharge only). Physical activity participation: MJ/day ‐ activity diary and accelerometer count over 1‐week at 1‐month post discharge only. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes used to conceal participant allocation order. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel aware of group allocation, the nature of the intervention would make this type of blinding difficult. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. Awareness of group allocation by outcome assessors could have impacted upon exercise capacity testing, QOL measures and lung function testing. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 66 children randomised ‐ 2 participants missed some aspect of training sessions. The paper states "none of the children were withdrawn from the study" however the number of participants assessed at hospital discharge and one‐month post discharge is not specifically stated. It is assumed they continued on to assessment based on the statement of no withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Physical activity data completed by a subset of participants whom had previously undertaken equivalent assessment. All other outcome measures reported. |
| Other bias | Unclear risk | Subset of participants who completed physical activity assessment had previously had their baseline levels of physical activity established as a part of another study. The inclusion criteria for this alternate study were not stated. |
CF: cystic fibrosis CXR: chest X‐ray FEF25-75: forced expiratory flow from 25% to 75% of vital capacity FEV1: forced expiratory volume at one second FVC: forced vital capacity HR: heart rate kg: kilograms Max HR: maximum heart rate (beats per minute) MaxVE: maximum ventilation MJ: mega‐joules MVV: maximum voluntary ventilation Nm: Newton metres PEFR; peak expiratory flow rate QOL: quality of life RQ: respiratory quotients RV: residual volume SD: standard deviation TLC: total lung capacity VCO2:carbon dioxide production V'E: minute ventilation VO2: oxygen update (expressed in millilitres per kilogram of body weight per minute) VO2max: maximum oxygen update Wmax:maximum work capacity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bernard 2004 | Not a randomised controlled study. |
| Gulmans 1999 | Not a randomised controlled study. |
| Hind 2008 | Not a randomised controlled study. |
| Kuys 2011 | Focus on physiological outcomes of exercise intervention, not physical activity participation. Inpatient study, no follow up in the outpatient period. |
| Moorcroft 2004 | Focus on physiological outcomes of exercise intervention, not physical activity participation. |
| Orenstein 1981 | Not a randomised controlled study. |
| Petrovic 2013 | No assessment of physical activity participation |
| Sosa 2012 | Focus on physiological outcomes of exercise interventions, no physical activity participation. |
| Swisher 2010 | Not a randomised controlled study. |
| Tunzin 1998 | Not a randomised controlled study. |
| van Doorn 2010 | Not a randomised controlled study. |
Characteristics of studies awaiting classification [ordered by study ID]
Alarie 2013.
| Methods | Randomised controlled study |
| Participants | Children and adults with CF aged 6‐47 years |
| Interventions | High frequency chest wall oscillation versus PEP mask therapy |
| Outcomes | Physical activity participation measured by Habitual Activity Estimation Scale (HAES) |
| Notes |
Marostica 2012.
| Methods | Randomised controlled study. |
| Participants | Children and adolescents with CF aged 7 ‐ 20 years. |
| Interventions | Intervention (n = 17) (involved handing out a manual with guidelines for aerobic physical exercises and reinforcing recommendations in contacts by phone every 2 weeks) versus control (n = 17). |
| Outcomes | Spirometry, ergospirometry, nutritional parameters, quality of life domains (measured by the CFQ). |
| Notes |
CF: cystic fibrosis CFQ: Cystic Fibrosis Questionnaire
Differences between protocol and review
Studies where the primary focus was not activity promotion, but rather the assessment of physiological outcomes as a response to a physical exercise intervention were not originally intended to be included in the review. Such studies were included where they incorporated assessment of physical activity participation.
Contributions of authors
NSC conducted the search for trials and selected included trials; assessed trial quality; extracted, entered and analysed data; interpreted results and wrote the review. AEH selected included trials; assessed trial quality; checked extracted and entered data; edited the draft review. JAA was consulted on trials for inclusion and edited the draft review. All authors conceived the review.
Sources of support
Internal sources
No sources of support provided
External sources
-
National Health and Medical Research Council (NHMRC), Australia
PhD Scholarship
-
Cystic Fibrosis Australia Research Trust, Australia
PhD Stipend
Declarations of interest
Narelle Cox has received a PhD stipend from the National Health and Medical Research Council (NH&MRC), Australia. This review forms a part of those PhD studies. The NH&MRC will not interfere with the independence of the authors in regard to the conduct of the review and will not delay or prevent publication of the review.
Edited (no change to conclusions)
References
References to studies included in this review
Hebestreit 2010 {published and unpublished data}
- Hebestreit H, Kieser S, Junge S, Ballmann M, Hebestreit A, Schindler C, et al.Long-term effects of a partially supervised conditioning programme in cystic fibrosis. European Respiratory Journal 2010;35(3):578-83. [DOI] [PubMed] [Google Scholar]
Klijn 2004 {published data only (unpublished sought but not used)}
- Klijn PH, Oudshoorn A, Ent CK, Net J, Kimpen JL, Helders PJ.Effects of anaerobic training in children with cystic fibrosis: a randomized controlled study. Chest 2004;125(4):1299-305. 5500100000002558 [DOI] [PubMed] [Google Scholar]
Schneiderman‐Walker 2000 {published data only (unpublished sought but not used)}
- Reisman JJ, Schneiderman-Walker J, Corey M, Wilkes D, Pedder L, Levison H, et al.The role of an organized exercise program in cystic fibrosis - a three year study [abstract]. Pediatric Pulmonology 1995;Suppl 12:261. 5500100000001555 [Google Scholar]
- Schneiderman-Walker J, Pollock SL, Corey M, Wilkes DD, Canny GJ, Pedder L, et al.A randomized controlled trial of a 3-year home exercise program in cystic fibrosis. Journal of Pediatrics 2000;136(3):304-10. [DOI] [PubMed] [Google Scholar]
Selvadurai 2002 {published data only (unpublished sought but not used)}
- Selvadurai HC, Blimkie CJ, Meyers N, Mellis CM, Cooper PJ, Van Asperen PP.Randomized controlled study of in-hospital exercise training programs in children with cystic fibrosis. Pediatric Pulmonology 2002;33(3):194-200. [DOI] [PubMed] [Google Scholar]
- Selvadurai HC, Van Asperen PP, Cooper PJ, Mellis CM, Blimkie CJ.A randomised controlled study of in-hospital exercise training programs in children with cystic fibrosis (CF) [abstract]. Pediatric Pulmonology 1999;Suppl 19:287-8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bernard 2004 {published data only}
- Bernard RS and Cohen LL.Increasing adherence to cystic fibrosis treatment: A systematic review of behavioral techniques. Pediatric Pulmonology 2004;37(1):8-16. [DOI] [PubMed] [Google Scholar]
Gulmans 1999 {published data only}
- Gulmans VAM, Meer K, Brackel HJL, Faber JAJ, Berger R, Helders PJM.Outpatient exercise training in children with cystic fibrosis: Physiological effects, perceived competence and acceptability. Pediatric Pulmonology 1999;28:39-46. [DOI] [PubMed] [Google Scholar]
Hind 2008 {published data only}
- Hind K, Truscott JG, Conway SP.Exercise during childhood and adolescence: a prophylaxis against cystic fibrosis-related low bone mineral density? Exercise for bone health in children with cystic fibrosis. Journal of Cystic Fibrosis 2008;7(4):270-6. [DOI] [PubMed] [Google Scholar]
Kuys 2011 {published data only}
- Hall K, Peasey M, Wood M, Cobb R, Bell SC, Kuys S.The effects of Nintendo Wii® exercise training in adults with cystic fibrosis [abstract]. Journal of Cystic Fibrosis 2010;9(Suppl 1):S71, Abstract no: 275. [Google Scholar]
- Kuys SS, Hall K, Peasey M, Wood M, Cobb R, Bell SC.Gaming console exercise and cycle or treadmill exercise provide similar cardiovascular demand in adults with cystic fibrosis: a randomised crossover trial. Journal of Physiotherapy 2011;57(1):35-40. [DOI] [PubMed] [Google Scholar]
Moorcroft 2004 {published data only}
- Dodd ME, Moorcroft AJ, Webb AK.Improved fitness and decreased symptoms of muscle fatigue for upper and lower body following an individualised unsupervised home exercise training programme in adults with cystic fibrosis [abstract]. Pediatric Pulmonology 1998;Suppl 17:347. [Google Scholar]
- Moorcroft AJ, Dodd ME, Morris J, Webb AK.Individualised unsupervised exercise training in adults with cystic fibrosis: a 1 year randomised controlled trial. Thorax 2004;59(12):1074-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorcroft AJ, Dodd ME, Webb AK.Individualised home exercise training in cystic fibrosis - a one-year trial [abstract]. In: Proceedings of the 13th International Cystic Fibrosis Congress. 2000:153.
Orenstein 1981 {published data only}
- Orenstein DM, Franklin BA, Doershuk CF, Hellerstein HK, Germann KJ, Horowitz JG, et al.Exercise conditioning and cardiopulmonary fitness in cystic fibrosis. The effects of a three-month supervised running program. Chest 1981;80(4):392-8. [DOI] [PubMed] [Google Scholar]
Petrovic 2013 {published data only}
- Petrovic M, Kaluza I, Pohl W.Effects of individualised aerobic exercise training in adults with cystic fibrosis: A 4 year controlled trial [abstract]. Journal of Cystic Fibrosis 2013;12 Suppl 1:S28, Abstract no: WS14.4. 5500100000011658 [Google Scholar]
Sosa 2012 {published data only}
- Sosa ES, Groeneveld IR, Gonzalez-Saiz L, Lopez-Mojares LM, Villa-Asensi JR, Barrio Gonzalez MI, et al.Intrahospital weight and aerobic training in children with cystic fibrosis: A randomized controlled trial. Medicine and science in sports and exercise 2012;44(1):2-11. [DOI] [PubMed] [Google Scholar]
Swisher 2010 {published data only}
- Swisher AK, Moffett K.The effect of coaching on physical activity and quality of life in children and adolescents with cystic fibrosis: a quality improvement pilot study. http://ijahsp.nova.edu/articles/Vol8Num2/pdf/swisher.pdf (The Internet Journal of Allied Helath Sciences and Practice) (accessed 16 November 2012).
Tunzin 1998 {published data only}
- Tuzin BJ, Mulvihill MM, Kilbourn KM, Bertran DA, Buono M, Hovell MF, et al.Increasing physical activity of children with cystic fibrosis: a home-based family intervention. Pediatric Exercise Science 1998;10(1):57-68. [Google Scholar]
van Doorn 2010 {published data only}
- Doorn N.Exercise programs for children with cystic fibrosis: a systematic review of randomized controlled trials. Disability and Rehabilitation 2010;32(1):41-9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Alarie 2013 {published data only}
- Alarie N, Agnew LL, McIlwaine MP, Ratjen F, Davidson GF, Milner R, et al.Evaluation of physical activity using the habitual activity estimation scale (HAES) questionnaire in a multicenter study [abstract]. Journal of Cystic Fibrosis 2013;12 Suppl 1:S28, Abstract no: WS14.1. 5500100000011657 [Google Scholar]
Marostica 2012 {published data only}
- Marostica PJ, Hommerding P, Makarewicz G, Baptista R, Donadio MV.Efficacy of an educational intervention of physical activity for children and adolescents with cystic fibrosis [abstract]. Journal of Cystic Fibrosis 2012;11 Suppl 1:S36. [DOI] [PubMed] [Google Scholar]
Additional references
ABS 2012
- Australian Bureau of Statistics.Australian Health Survey: First Results 2011-2012. http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/4364.0.55.001main+features12011-12 (accessed 28 November 2013).
Andersen 1998
- Andersen RE, Crespo CJ, Bartlett SJ, Cheskin LJ, Pratt M.Relationship of physical activity and television watching with body weight and level of fatness among children. JAMA 1998;279(12):938-42. [DOI] [PubMed] [Google Scholar]
Bradley 2008
- Bradley JM, Moran F.Physical training for cystic fibrosis. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD002768. [DOI: 10.1002/14651858.CD002768.pub2] [DOI] [PubMed] [Google Scholar]
Bradley 2010
- Bradley JM, Kent L, Elborn JS, O'Neill B.Motion sensors for monitoring physical activity in cystic fibrosis: what is the next step? Physical Therapy Reviews 2010;15(3):197-203. [Google Scholar]
Brodie 2005
- Brodie DA, Inoue A.Motivational interviewing to promote physical activity for people with chronic heart failure. Journal of Advanced Nursing 2005;50(5):518-27. [DOI] [PubMed] [Google Scholar]
Casparen 1985
- Casparen CJ, Powell KE, Christenson GM.Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Reports 1985;100(2):126-31. [PMC free article] [PubMed] [Google Scholar]
CDC 2011
- US Department of Health and Human Services Centers for Disease Control and Prevention.Strategies to Prevent Obesity and Other Chronic Diseases: The CDC Guide to Strategies to Increase Physical Activity in the Community. http://www.cdc.gov/obesity/downloads/PA_2011_WEB.pdf (accessed 28 November 2013).
Clanchy 2011
- Clanchy KM, Tweedy SM, Boyd R.Measurement of habitual physical activity performance in adolescents with cerebral palsy: a systematic review. Developmental Medicine and Child Neurology 2011;53(6):499-505. [DOI] [PubMed] [Google Scholar]
Conn 2008
- Conn VS, Hafdahl AR, Brown SA, Brown LM.Meta-analysis of patient education intervention to increase physical activity among chronically ill adults. Patient Education and Counselling 2008;70:157-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dwyer 2011
- Dwyer TJ, Alison JA, McKeough ZJ, Daviskas E, Bye PTP.Effects of exercise on respiratory flow and sputum properties in patients with cystic fibrosis. Chest 2011;139(4):870-7. [DOI] [PubMed] [Google Scholar]
Eakin 2007
- Eakin EG, Lawler SP, Vandelanotte C, Owen N.Telephone Interventions for Physical Activity and Dietary Behavior Change: A systematic review. American Journal of Preventative Medicine 2007;32(5):419-34. [DOI] [PubMed] [Google Scholar]
Foster 2005
- Foster C, Hillsdon M, Thorogood M.Interventions for promoting physical activity. Cochrane Database of Systematic Reviews 2005, Issue 1. Art. No: CD003180. [DOI: 10.1002/14651858.CD003180.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haskell 2007
- Haskell WL, Lee I-M, Pate RR, Powel KE, Blair SN, Franklin BA, et al.Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine & Science in Sports & Exercise 2007;39(8):1423-34. [DOI] [PubMed] [Google Scholar]
Hebestreit 2006
- Hebestreit H, Kieser S, Rüdiger S, Schenk T, Junge S, Hebestreit A, et al.Physical activity is independently related to aerobic capacity in cystic fibrosis. European Respiratory Journal 2006;28(4):734-9. [DOI] [PubMed] [Google Scholar]
Henry 2003
- Henry B, Aussage P, Grosskopf C, Goehers JM.Development of the Cystic Fibrosis Questionnaire (CFQ) for Assessing Quality of Life in Pediatric and Adult Patients. Quality of Life Research 2003;12(1):63-76. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Kahn 2002
- Kahn EB, Ramsey LT, Brownson RC, Heath GW, Howze EH, Powell KE, et al.The effectiveness of interventions to increase physical activity: A systematic review. American Journal of Preventive Medicine 2002;22(4 Suppl):73-107. [DOI] [PubMed] [Google Scholar]
Koch 1993
- Koch C, Høiby N.Pathogenesis of cystic fibrosis. Lancet 1993;341(8852):1065-9. [DOI] [PubMed] [Google Scholar]
Lacasse 2006
- Lacasse Y, Goldstein R, Lasserson TJ, Martin S.Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No: CD003793. [DOI: 10.1002/14651858.CD003793.pub2] [DOI] [PubMed] [Google Scholar]
Marcus 2000
- Marcus BH, Forsyth LH, Stone EJ, Dubbert PM, McKenzie TL, Dunn AL, et al.Physical activity behavior change: issues in adoption and maintenance. Health Psychology 2000;19(1 Suppl):32-41. [DOI] [PubMed] [Google Scholar]
Moy 2012
- Moy ML, Weston NA, Wilson EJ, Hess ML, Richardson CR.A pilot study of an Internet walking program and pedometer in COPD. Respiratory Medicine 2012;106(9):1342-50. [DOI] [PubMed] [Google Scholar]
Nguyen 2009
- Nguyen HQ, Gill DP, Wolpin S, Steele BG, Benditt JO.Pilot study of a cell phone-based exercise persistence intervention post-rehabilitation for COPD. International Journal of Chronic Obstructive Pulmonary Disease 2009;4:301-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pate 1995
- Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, et al.Physical Activity and Public Health. JAMA 1995;273(5):402-7. [DOI] [PubMed] [Google Scholar]
Quittner 2009
- Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB.Determination of the Minimal Clinically Important Difference Scores for the Cystic Fibrosis Questionnaire-Revised Respiratory Symptom Scale in Two Populations of Patients With Cystic Fibrosis and Chronic Pseudomonas aeruginosa Airway Infection. Chest 2009;135(6):1610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- Review Manager (RevMan).Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rimmer 2004
- Rimmer JH, Riley B, Wang E, Rauworth A, Jurkowski J.Physical activity participation among persons with disabilities: Barriers and facilitators. American Journal of Preventive Medicine 2004;26(5):419-25. [DOI] [PubMed] [Google Scholar]
Saiman 2004
- Saiman L, Seigel J.Infection control in cystic fibrosis. Clinical Microbiology Reviews 2004;17(1):57-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmidt 2009
- Schmidt A, Wenninger K, Niemann N, Wahn U, Staab D.Health-related quality of life in children with cystic fibrosis: validation of the German CFQ-R. Health and Quality of Life Outcomes 2009;7:97-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schneiderman‐Walker 2005
- Schneiderman-Walker J, Wilkes DL, Strug L, Lands LC, Pollock SL, Selvadurai HC, et al.Sex differences in habitual physical activity and lung function decline in children with cystic fibrosis. Journal of Pediatrics 2005;147(3):321-6. [DOI] [PubMed] [Google Scholar]
Thompson 2003
- Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, Fletcher GF, Gordon NF, Pate RR, Rodriguez BL, Yancey AK, Wenger NK.Exercise and Physical Activity in the Prevention and Treatment of Atherosclerotic Cardiovascular Disease: A Statement From the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Arteriosclerosis, Thrombosis, and Vascular Biology 2003;23(8):e42-e49. [Google Scholar]
Troosters 2009
- Troosters T, Langer D, Vrijsen B, Segers J, Wouters K, Janssens W, et al.Skeletal muscle weakness, exercise tolerance and physical activity in adults with cystic fibrosis. European Respiratory Journal 2009;33(1):99-106. [DOI] [PubMed] [Google Scholar]
Trost 2005
- Trost SG, McIver KL, Pate RR.Conducting Accelerometer-Based ActivityAssessments in Field-Based Research. Medicine and Science in Sports and Exercise 2005;37(11 Suppl):S531-S543. [DOI] [PubMed] [Google Scholar]
Vandelanotte 2007
- Vandelanotte C, Spathonis KM, Eakin EG, Owen N.Website-Delivered Physical Activity Interventions. American Journal of Prevenative Medicine 2007;33(1):54-64. [DOI] [PubMed] [Google Scholar]
van Sluijs 2007
- Sluijs EMF, McMinn AM, Griffin SJ.Effectiveness of interventions to promote physical activity in children and adolescents: systematic review of controlled trials. BMJ 2007;335(7622):703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wenninger 2003
- Wenninger K, Aussage P, Wahn U, Staab D, German CFQ study group.The revised German Cystic Fibrosis Questionnaire: validation of a disease-specific health-related quality of life instrument. Quality of Life Research 2003;12(1):77-85. [DOI] [PubMed] [Google Scholar]
WHO 2010
- WHO.Global recommendations for physical activity and health. Geneva: WHO Library Cataloguing-in-Publication Data, 2010. [ISBN: 978 924159 9979] [Google Scholar]
Yankaskas 2004
- Yankaskas JR, Marshall BC, Sufian B, Simon RH, Rodman D.Cystic fibrosis adult care. Consensus conference report. Chest 2004;125(Suppl 1):1-39. [DOI] [PubMed] [Google Scholar]


