Abstract
Background
Diet is an important determinant of systemic pH and acid–base regulation. A frequent consumption of acid-inducing foods (including processed meats and cheese) combined with a low intake of base-inducing foods (such as fruits, legumes and vegetables) increases Dietary Acid Load (DAL), which has been associated with an increased risk for certain cancers. DAL also appears to be of paramount importance in cancer survivors, in whom it was associated with increased mortality and poor overall physical health. Literature on DAL in cancer survivors, however, is scarce and limited to a few studies.
Methods
Using cross-sectional data from the National Health and Nutrition Examination Surveys (NHANES), we sought to quantify DAL in U.S. cancer survivors and contrasted the results to the general population. DAL was estimated using established formulas (Potential Renal Acid Load (PRAL) and Net Endogenous Acid Production (NEAP)).
Results
Our study comprised 19,413 participants, of which 1444 were self-reported cancer survivors. Almost 63% of cancer survivors were female (weighted proportion) with a mean age of 61.75 (0.51) years. DAL scores were consistently higher in cancer survivors (as compared to the general population) after adjustment for confounders in multivariate regression models. These differences, however, were not statistically significant (p = 0.506 for NEAPF, 0.768 for PRALR and 0.468 for NEAPR, respectively). Notably, DAL scores were positive throughout (> 0 mEq/d) in cancer survivors, suggesting an acidifying diet. Specific examples include mean PRALR scores > 11 mEq/d in cancer survivors aged 55 years and mean NEAPF scores > 50 mEq/d in cancer survivors aged 40–60 years).
Conclusions
The acidifying diet in this sample of cancer survivors warrants caution and requires further investigation. Comparably high DAL scores have been associated with adverse health outcomes and an increased mortality in previous studies in breast cancer survivors. Thus, increased awareness as well as additional clinical trials in this field are urgently warranted.
Keywords: Dietary acid load, Acidosis, Cancer, Survivorship, Nutrition, Epidemiology, Nutrient intake, Prevention
Background
Diet affects the human acid-base status [1], and may influence systemic pH, metabolism and acid–base homeostasis [2, 3]. Dietary acid load (DAL) is determined by the balance of base-inducing foods (such as fruits and vegetables) and acid-inducing foods (including meats, eggs, and cheese) [4, 5]. Plant foods are abundant in metabolizable organic anions and generally decrease DAL, with green leafy vegetables (celery, broccoli, spinach), raisins and berries having a particularly alkalinizing effect [5, 6]. In contrast, the oxidation of sulfur-containing amino acids (methionine, cysteine and homocysteine) found in meat and meat products generates sulfate and thereby increases DAL [7, 8].
A high DAL has been positively associated with insulin resistance and type-2-diabetes [4, 9], chronic kidney disease [10], and cardiovascular disorders [11] in numerous epidemiological studies.
It has been argued that diet-dependent acid load could also predispose individuals to an increased cancer risk [12, 13], given that chronic acid–base imbalances can affect cellular and molecular activities that lead to tissue inflammation and cell transformation, which both may stimulate carcinogenesis or tumor progression [13–16]. Keramati et al. recently performed a systematic review and meta-analysis exploring potential association of a high DAL and cancer risk [17]. The authors emphasized that a high DAL may lead to a decreased adiponectin secretion (which plays a pivotal role in the development and progression of multiple malignancies [18]) and to an elevation of cortisol production and circulating IGF-1 levels [17]. The latter is a potent stimulant of several signaling pathways through binding to its cell surface receptor and induces cancer cell proliferation, survival, and migration [17, 19]. The authors also suggest that an acidogenic diet may increase carcinogenesis by elevation of insulin resistance [17].
In fact, DAL has been associated with an increased risk for certain types of cancer in various epidemiological studies [12, 20–26]. Positive associations of a high DAL have been reported with regard to prostate [20], breast [12, 22], lung [23], colorectal [24, 25], and pancreatic cancer [21]. In their meta-analysis, Keramati et al. pooled 10 studies and found that individuals with the highest DAL scores had a 66% increased risk of cancer (compared to those with the lowest DAL, p < 0.001) [17].
Although the total number of available studies in this particular field is still limited, there is now accumulating evidence that DAL could be an important modifiable risk factor to prevent from certain cancers.
Of note, the majority of studies focused on DAL as a risk factor in healthy individuals, while few studies addressed the role of DAL in cancer survivors [27–31]. Data from the Women’s Healthy Eating and Living (WHEL) study suggested that a higher DAL increased total mortality and breast cancer-specific mortality in a cohort of 2950 early stage breast cancer survivors [27]. An increased DAL was also significantly associated with increased plasma C-reactive protein (CRP) and hemoglobin A1c (HbA1c) levels, and reduced overall physical health in breast cancer survivors [28, 29]. In light of this mounting evidence, several experts called for adding DAL scores to dietary guidelines for breast cancer survivors [27]. However, the data supporting this call is based on a single cohort of breast cancer survivors from the WHEL study.
Additional trials and investigations using data from other cohorts are urgently warranted to gain a better (and more detailed) understanding of DAL in cancer survivors. We sought to address this gap in the literature and investigated DAL in U.S. cancer survivors from the National Health and Nutrition Examination Surveys (NHANES). The main aims of our study were twofold: a) to quantify DAL in U.S. cancer survivors, and b) to compare the results to the general U.S. population.
Methods
Study design and population
Our investigation is based on cross-sectional, population-based data from the NHANES [32]. NHANES is a major program of the National Center for Health Statistics (NCHS) designed to assess the health and nutritional status of non-institutionalized adults and children in the United States of America. The NHANES program began in the early 1960s and examined approximately 5000 people annually in 15 different counties across the country since 1999 [33]. The NHANES is an ongoing program which has two major components: an interview and an examination component. The interview includes demographic, socioeconomic, dietary, and other health-related questions [32]. The examination component consists of physiological and medical measurements, as well as laboratory tests administered by specialized medical personnel. Detailed information on both components may be obtained from the NHANES website [32].
NHANES data has been frequently used to investigate cancer-related and health-related questions [34–40]. For our present study, we used data from multiple NHANES cycles (2007/2008, 2009/2010, 2011/2012, and 2013/2014) to increase the potential sample size for analyses stratified by population subgroups. NHANES was approved by the National Center for Health Statistics research ethics review board [41]. Written informed consent was obtained from all participants.
Data collection
We used data from various NHANES modules, including demographic data, anthropometric data, dietary data and questionnaire data. Demographic data included age (in years), gender (female and male), race/ethnicity (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, Other Race - Including Multi-Racial), marital status (married or living with a partner, widowed/divorced/separated, never married), education level (less than 9th grade, 9-11th grade, high school graduate/ general education diploma or equivalent, some college or associate degree, college graduate or above) and annual household income (under $20,000 or over $20,000).
Examination data comprised body weight, height and body mass index (BMI), which were obtained from the body measures dataset and treated as a continuous variable.
Dietary data
Dietary data were obtained from the nutritional assessment component of the NHANES, which included a 24-hour dietary recall interview for all participants [42]. The main objective of this module was to obtain detailed dietary intake information from NHANES participants [43]. All dietary interviews were carried out in private rooms in the NHANES mobile examination centers and were conducted in person by specifically trained dietary interviewers fluent in both English and Spanish [42, 43]. The specifically equipped dietary interview room contained a standard set of measuring guides that were used to help the respondent report the volume and dimensions of the food items consumed. The dietary intake data were used to estimate the types (and amounts) of foods and beverages consumed during the 24-hour period prior to the interview (midnight to midnight). Based on these data, energy intake and nutrient intake were estimated.
The NHANES dietary interview component, called “What We Eat in America” is conducted as a partnership between the U.S. Department of Health and Human Services and the U.S. Department of Agriculture (USDA) [42]. All “What We Eat in America” data was collected using USDA’s dietary data collection instrument, called the Automated Multiple Pass Method [44]. The AMPM is a fully computerized recall method designed to provide accurate means of collecting intakes for large-scale national surveys [42, 44]. Additional information may be obtained from the NHANES website. The detailed dietary examination protocol and data collection methods are fully documented in the NHANES dietary interviewer’s procedure manuals [45].
Dietary data used for this study included daily energy intake (kcal/d), fiber intake (g/d), and intake of macronutrients (protein, fat and carbohydrate; all reported in g/d) and selected micronutrients necessary for the DAL estimations (calcium, magnesium, phosphorus and potassium; all reported in mg/d).
Cancer status
Cancer survivorship status was self-reported and based on the question “Have you ever been told by a doctor or other health professional that you had cancer or a malignancy of any kind?” from the medical conditions section. To stratify analyses by cancer sites, we made use of the question “What kind of cancer was it?”, which was included in the same module. In a first model, we combined all cancer types regardless of their site, except for skin cancers (non-melanoma and unspecified skin cancers) which were not included in this model. Afterwards, we performed sub-analyses stratified by specific cancer sites, including prostate cancer and breast cancer. Breast and prostate cancer were chosen in light of their aforementioned associations with DAL [20, 22, 27–30], and because of the modest case numbers (at least 60 cases for each cancer type) per cycle.
Dietary acid load estimations
We described the employed methods for the calculation of DAL in our previous publications in detail [5, 22]. In brief, we used the formulas developed by Remer & Manz and Frassetto et al. to calculate Net Endogenous Acid Production (NEAP) [2] and Potential Renal Acid Load (PRAL) from diet [6, 46, 47]. PRALR (in mEq/d) was calculated according to the modified Remer formula:
Net endogenous acid production was estimated based on Remer’s formula (NEAPR) and included average intestinal absorption rates of ingested protein and micronutrients (PRALR) and anthropometry-based estimates for organic acid excretion (OAest) [6]:
OAest (mEq/d) was calculated as follows:
Body surface area was calculated with the formula of Du Bois and Du Bois:
NEAPF was estimated based on the formula by Frassetto et al., which considers daily total protein intake and potassium intake.
The reader is referred to the work of Parmenter et al. for additional background information on all three employed formulas [48, 49]. Negative DAL scores (PRALR < 0 mEq/d) indicate an alkaline-forming potential, whereas positive scores (PRALR > 0 mEq/d) indicate an acid-forming potential.
Statistics
We used STATA 14 statistical software (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP) for our statistical analysis. Stata survey commands were used to account for the NHANES survey design characteristics and population weights. Based on the NHANES guidelines, we generated an 8-year weight (2007–2014) to obtain weighted percentages adjusted to the US adult population. We compared all aforementioned variables between self-identified cancer survivors and the general population (who denied a previous diagnosis of cancer). Participants were not matched for age, sex, ethnicity, cancer status or any other variable. For this study, only participants with a full dataset were considered in the final analysis.
Continuous variables were described with their mean and standard error in parenthesis (if normally-distributed) or median and interquartile range in parenthesis (when not-normally distributed). For categorical variables we reported the number of observations (n) as well as the weighted proportions in parenthesis. Reliability of estimated proportions was assessed based on the 2017 NCHS guidelines [50], which consider the Korn–Graubard confidence interval (CI), CI widths, sample size, and degrees of freedom to assess reliability of a proportion and to determine whether it can be presented [51]. For this step, we also made use of the user-written post-estimation command “kg_nchs” in Stata [51]. Unreliable proportions, that is proportions that did not met the NCHS standards were clearly marked.
Continuous and normally distributed variables were compared between cancer survivors and the general population using two-sample Student’s t-tests. We assessed potential associations between cancer survivorship status and categorical variables using STATA’s design-adjusted Rao-Scott test. Moreover, we conducted a series of linear regression analyses to examine the relationship between all 3 DAL scores (NEAPF, PRALR, NEAPR) and a selected set of independent variables. Predictor variables were chosen based on previous studies in the field and included age, gender, race/ethnicity, body mass index, total energy intake and cancer status [52–54].
Adjustment for total energy intake was necessary because cancer survivors were, on average, significantly older and had a lower total energy intake as compared to the general population. Only candidate predictors of interest and a bivariate relationship of significance (p < 0.25) with the response variables (DAL scores) were included in the multivariate logistic models. Multivariate linear regression models were constructed for all cancer sites combined, and for breast and prostate cancer, respectively. Margin plots were used to display marginal predicted values of NEAPF, PRALR and NEAPR stratified by cancer survivorship status at all possible increments of 5 units in age. Statistical significance was determined at α = 0.05 and all employed tests for statistical significance were two-sided.
Results
The total sample for this study comprised 19,413 participants. Our sample included 1444 self-reported cancer survivors. The remaining 17,696 participants denied a diagnosis of cancer in the past.
Cancer survivors had a mean age of 61.75 years and almost 63% (weighted proportion) were female (Table 1). More than 80% of cancer survivors were of Non-Hispanic White origin, and almost 2/3 were married or lived with a partner. Mean BMI was slightly higher in cancer survivors (29.20 kg/m2) as compared to the general population (28.85 kg/m2), however, this difference was not statistically significant (p = 0.210). Table 1 shows other demographic data characterizing our sample of cancer survivors.
Table 1.
Demographic, anthropometric and clinical characteristics – a comparison between cancer survivors and the general population
| General population n = 17,969 |
Cancer Survivors: n = 1444 |
p a | |
|---|---|---|---|
| Gender | < 0.001 | ||
| Male | n = 8783 (48.66) | n = 632 (37.21) c | |
| Female | n = 9186 (51.34) | n = 812 (62.79) c | |
| Ethnicity | < 0.001 | ||
| Mexican American | n = 2781 (8.75) | n = 106 (3.04) c | |
| Other Hispanic | n = 1800 (5.48) | n = 96 (2.80) c | |
| Non-Hispanic White | n = 7730 (66.94) | n = 911 (83.35) c | |
| Non-Hispanic Black | n = 3918 (11.76) | n = 266 (7.40) c | |
| Other Race - Including Multi-Racial | n = 1740 (7.06) | n = 65 (3.22) c | |
| Marital Status | < 0.001 | ||
| Married/living with a partner | n = 10,570 (62.97) | n = 870 (65.19) | |
| Widowed/Divorced/Separated | n = 3842 (17.28) | n = 477 (28.59) c | |
| Never married | n = 3557 (19.74) | n = 97 (6.21) c | |
| Annual household income | 0.586 | ||
| Under $20,000 | n = 4000 (15.05) | n = 354 (15.69) | |
| Over $20,000 | n = 13,969 (84.95) | n = 1090 (84.31) | |
| Education level | 0.144 | ||
| Less than 9th grade | n = 1799 (5.29) | n = 152 (5.04) | |
| 9-11th grade b | n = 2756 (11.82) | n = 216 (10.78) | |
| High school graduate/GED or equivalent | n = 4136 (22.68) | n = 314 (20.46) | |
| Some college or AA degree | n = 5252 (31.50) | n = 404 (31.69) | |
| College graduate or above | n = 4026 (28.71) | n = 358 (32.02) c | |
| Age (in years) | 45.36 (0.28) | 61.75 (0.51) | < 0.001 d |
| Body mass index (in kg/m2) | n = 28.85 (0.08) | n = 29.20 (0.26) | 0.210 |
Categorical variables are shown as n (weighted %), continuous variables as means (standard error)
a The p-value is based on a design-based Rao-Scott F-Test and tests for a potential association between cancer survivor status and the respective demographic, anthropometric or clinical characteristics (available for categorical variables only)
b includes 12th grade without diploma
c indicates significant differences in the proportions
d indicates significant differences in the means
Energy intake in cancer survivors was significantly lower as compared to the general population (1881.02 kcal/d vs 2201.78 kcal/d). Table 2 shows daily energy-adjusted nutrient intake (in g/1000 kcal or mg/1000 kcal, respectively) for both groups.
Table 2.
Nutrient intake – a comparison between cancer survivors and the general population
| General population n = 17,969 |
Cancer Survivors: n = 1444 |
p | |
|---|---|---|---|
| Energy intake (kcal/day) | 2201.78 (10.67) | 1881.02 (28.90) | < 0.001 |
| Protein intake (g/1000 kcal) | 39.19 (0.16) | 39.32 (0.49) | 0.796 |
| Carbohydrate intake (g/1000 kcal) | 121.87 (0.38) | 121.50 (1.05) | 0.712 |
| Fat (g/1000 kcal) | 37.23 (0.14) | 38.13 (0.33) | 0.016 |
| Magnesium intake (mg/1000 kcal) | 146.60 (0.81) | 153.88 (2.02) | < 0.001 |
| Potassium intake (mg/1000 kcal) | 1307.81 (6.69) | 1422.01 (17.81) | < 0.001 |
| Calcium intake (mg/1000 kcal) | 464.54 (2.44) | 493.46 (7.88) | < 0.001 |
| Phosphorus intake (mg/1000 kcal) | 656.18 (2.13) | 670.76 (7.52) | 0.051 |
| Fiber intake (g/1000 kcal) | 8.16 (0.07) | 8.75 (0.19) | 0.001 |
Data shown as mean and standard error in parenthesis. A p-value < 0.05 indicates significant differences between both groups
Energy adjusted intake of fat, fiber, potassium, magnesium, and calcium was significantly higher in cancer survivors. Energy-adjusted phosphorus intake also tended to be higher in this group (670.76 mg/1000 kcal vs 656.18 mg/1000 kcal), however, the difference was not statistically significant.
We ran multiple linear regression analyses to predict DAL scores (NEAPF, PRALR, NEAPR) from gender, age, race/ethnicity, total energy intake, BMI, and cancer survivor status (which indirectly defined nutrient intake as shown in Table 2). After adjusting for covariates, we found no significant associations between being a self-identified cancer survivor and all 3 DAL scores (NEAPF, PRALR, NEAPR). Figure 1 displays marginal predicted values of NEAPF, PRALR and NEAPR in cancers survivors at all possible increments of 5 units in age. DAL scores tended to be higher in cancer survivors, however, the differences were not statistically significant. DAL scores declined with higher age but remained positive throughout (e.g. PRALR > 0 mEq/d), indicating an acidifying potential. Whether cancer survivors modified their diet subsequent to their cancer diagnosis was not ascertainable from out data.
Fig. 1.
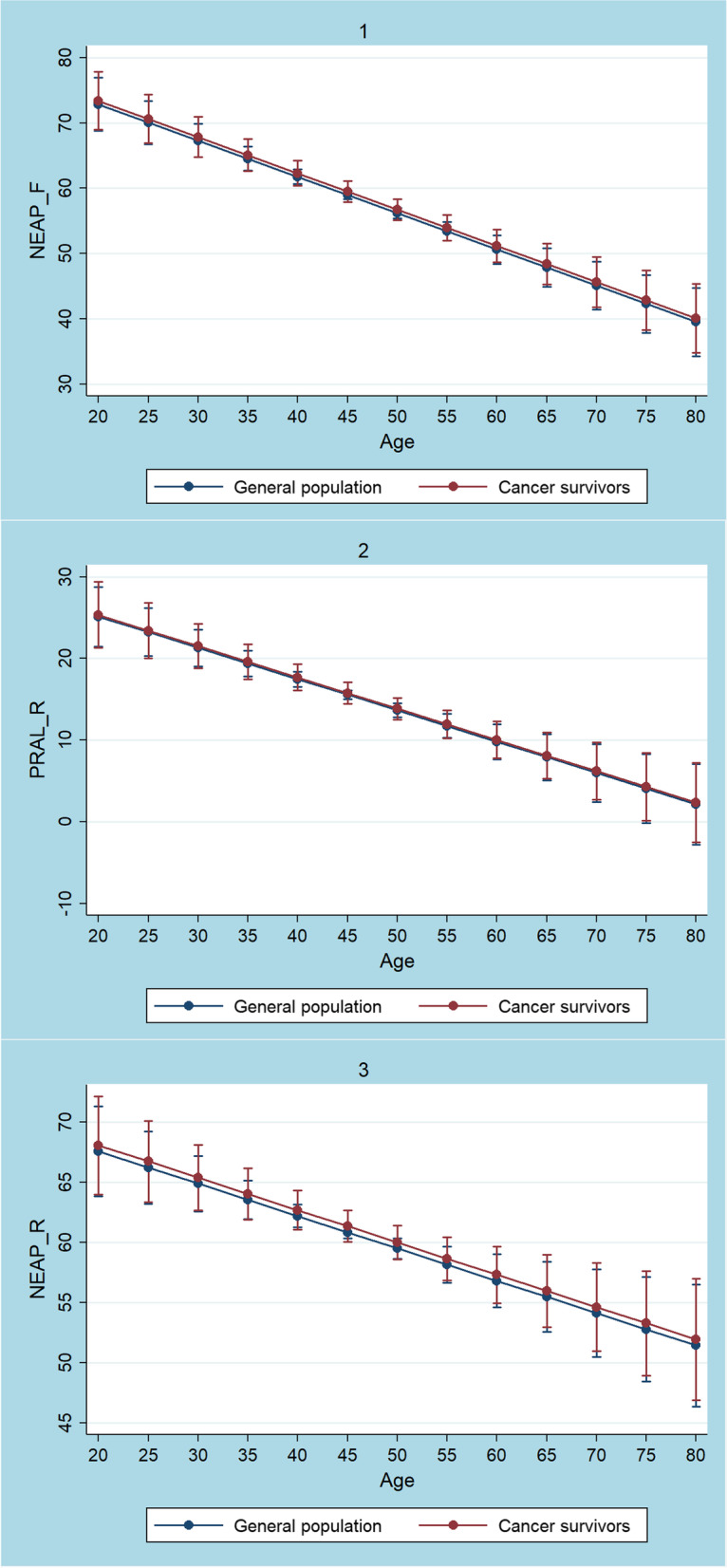
Plots of marginal predicted values for NEAPF (1), PRALR (2) and NEAPR (3) based on the employed regression models in cancer survivors and in the general population
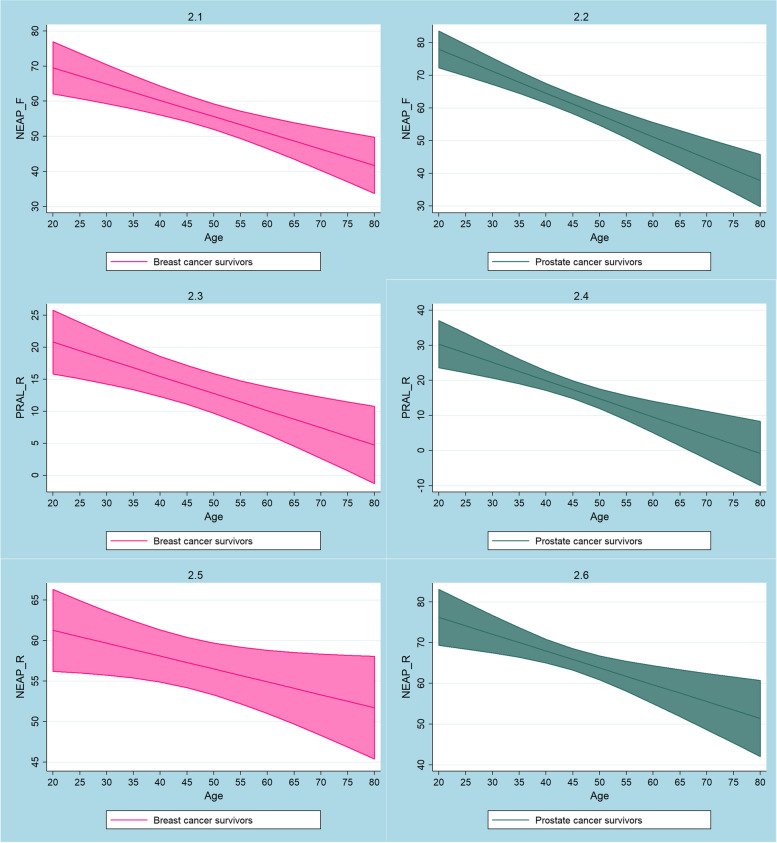
Marginal predicted values of NEAPF, PRALR and NEAPR in breast cancer and prostate cancer survivors are shown in Fig. 2. Again, all 3 DAL scores tended to decline with higher age but remained positive throughout. DAL scores were not significantly higher in breast- and prostate cancer survivors as compared to the general population after adjusting for covariates.
Fig. 2.
Plots of marginal predicted values (and confidence intervals) for NEAPF (2.1 and 2.2), PRALR (2.3 and 2.4) and NEAPR (2.5 and 2.6) based on the employed regression models in breast cancer survivors (pink) and prostate cancer survivors (emerald)
Table 3 shows the multivariate linear regression models to predict DAL scores (NEAPF, PRALR, and NEAPR) from gender, age, race, total energy intake, BMI, and cancer survivor status.
Table 3.
Multivariate linear regression models to predict DAL scores from gender, age, race, total energy intake, BMI, and cancer survivor status
| NEAPF | p-value | PRALR | p-value | NEAPR | p-value | |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | – | – | – | |||
| Female | −4.53 (−5.40 – (−3.66)) | < 0.001 | −3.69 (−4.55 – (−2.83)) | < 0.001 | −9.49 (−10.34 – (−8.64)) | < 0.001 |
| Ethnicity | ||||||
| Mexican American | – | – | – | |||
| Other Hispanic | 0.68 (−1.14–2.49) | 0.460 | −3.09 (−4.82 – (− 1.35)) | 0.001 | − 2.58 (− 4.32 – (−0.84)) | 0.004 |
| Non-Hispanic White | −2.14 (− 3.60 – (−0.67)) | 0.005 | −3.96 (− 5.39 – (− 2.54)) | < 0.001 | − 1.07 (− 2.54–0.39) | 0.148 |
| Non-Hispanic Black | 6.12 (4.66–7.58) | < 0.001 | − 0.78 (− 2.19–0.62) | 0.273 | 1.75 (0.31–3.20) | 0.018 |
| Other Race - Including Multi-Racial | −0.39 (− 2.51–1.73) | 0.714 | − 3.06 (− 5.11 – (− 1.01)) | 0.004 | − 2.24 (− 4.28 – (− 0.20)) | 0.032 |
| Cancer Survivor | ||||||
| No | – | – | – | |||
| Yes | 0.54 (−1.07–2.14) | 0.506 | 0.21 (− 1.19–1.60) | 0.768 | 0.50 (− 0.92–1.92) | 0.486 |
| Body mass index (in kg/m2) | 0.34 (0.26–0.42) | < 0.001 | 0.28 (0.22–0.35) | < 0.001 | 0.91 (0.84–0.97) | < 0.001 |
| Total energy intake (in kcal/d) | 0.0014 (0.001–0.0018) | < 0.001 | 0.0098 (0.0089–0.0105) | < 0.001 | 0.010 (0.092–0.0108) | < 0.001 |
| Age (in years) | −0.56 (− 0.71 – (− 0.40) | < 0.001 | −0.38 (− 0.52 – (− 0.24) | < 0.001 | −0.27 (− 0.41 – (− 0.12) | < 0.001 |
| Age squared (in years) | 0.0025 (0.001–0.004) | < 0.001 | 0.0019 (0.0005–0.0032) | 0.009 | 0.0003 (− 0.0011–0.0018) | 0.635 |
Coefficients are displayed with their 95% confidence intervals and p-values. The symbol “- “indicates the reference category
Apart from cancer survivor status, all entered variables added statistically significantly to the prediction.
Discussion
The present study sought to quantify DAL in U.S. cancer survivors. All examined acid load scores (NEAPF, PRALR, NEAPR) were higher in cancer survivors (compared to the general population) after adjustment for confounders. The differences, however, were not statistically significant. DAL scores were positive throughout (> 0 mEq/d), and suggested that cancer survivors in general consumed an acidifying diet that has been associated with adverse effects in the existing studies in cancer survivors [27–30].
Notably, it is important to highlight that we did not perform a case-control study, and that we did not match participants on any characteristics (which poses a bias-susceptible technical challenge because our data stem from a complex multistage, stratified, clustered and probability sampling design). Moreover, the employed NHANES data did not allow us to examine whether cancer survivors modified their diet after receiving a cancer diagnosis.
It is now widely accepted that a change in unhealthy lifestyle behaviors (e.g. smoking cessation, improving diet quality and increasing physical activity) in cancer survivors may help to reduce cancer treatment sequelae, and also reduces the risk for other common diseases such as cardiovascular disorders and obesity [55]. Several studies reported significant and long-term changes in dietary intake in cancer survivors [56–58].
A large Chinese trial found a substantial reduction in the consumption of red meat (p < 0.001), processed meat (p < 0.001), poultry (p < 0.001), and dairy products (p < 0.001) in cancer survivors at 18-months post-diagnosis [56]. A French study using data from the NutriNet-Santé cohort observed comparable trends, and reported a decrease in total energy intake (− 377.2 ± 243.5 kcal/d) and protein intake (− 17.4 ± 12.5 g/d) in cancer survivors [57]. The latter was also observed in a study from Malaysia examining dietary intake 2 years after a diagnosis of breast cancer [58]. An Australian study with more than 500 cancer survivors attending the Sydney Cancer Survivorship Center reported that the majority of survivors modified their diet after their cancer diagnosis [59].
We could not examine these associations due to the cross-sectional nature of our data and due to the lack of information on cancer-related comorbidities. As such, our data serve as a mere description of DAL scores in cancer survivors. The comparison to the general population (denying any prior cancer diagnosis) is therefore difficult, as well. We may only speculate why total energy intake was lower in cancer survivors and the same applies for the slightly higher energy-adjusted fat intake in the cancer survivor group (38.13 vs 37.23 g/1000 kcal, p = 0.016). It is conceivable that the lower total energy intake is related to the higher age of cancer survivors (61.75 vs 45.36 years). Yet, the cross-sectional nature of our data does not allow for causal attributions. Other scenarios (e.g. unintended therapy side-effects and sequelae including lack of appetite and nausea that lead to a reduced food intake) are also possible. Again, we cannot not prove or reject these hypotheses based on the type of our employed data.
As described in detail earlier, the major aim of this study was acid load quantification in the cancer survivor group. It is possible that cancer survivors in our cohort also modified their diet post diagnosis, but this remains subject to speculation. Although it is conceivable that this was the case (in light of the existing literature on dietary modifications in cancer survivors [56–59]), we may not prove it. Nonetheless, DAL scores were positive throughout in our cohort of cancer survivors, suggesting an acidifying diet (e.g. NEAPF ranging from 50 mEq/d to 60 mEq/d in cancer survivors aged 50 to 60) [60, 61]. This was the case in all 3 models examining cancer survivors in general (Fig. 1), and breast and prostate cancer survivors in particular (Fig. 2).
DAL scores in the range of 50 mEq/d to 60 mEq/d have been associated with adverse health effects and repercussions in analyses using data from the WHEL study in breast cancer survivors [27–30]. Comparable PRAL and NEAP scores have also been associated with an increased mortality in breast cancer survivors [27], and may contribute to reduced overall physical health [28]. Moreover, Wu et al. also reported associations between DAL and elevated plasma CRP and HbA1c levels in breast cancer survivors [29]. Both are important risk factors associated with cancer recurrence and comorbidities in in this particular group [62, 63]. A high DAL may contribute to systemic inflammation and hyperglycemia in cancer survivors [29], which, in turn, has been associated with a worse prognosis.
It is now widely accepted that a high DAL is associated with numerous health repercussions in both healthy and sick individuals [64, 65]. A high acid load decreases blood pH towards the lower end of the normal physiological range and induces low-grade mild metabolic acidosis that causes tissue damage and inflammation [4, 29, 64]. The latter is of particular concern in cancer patients, who have a reduced capacity to adjust their acid-base balance [66]. Local inflammation subsequent to an acidic microenvironment may initiate genomic instability on normal cells through the activation of cytokines, which may stimulate tumor invasion and metastases [15, 16, 67]. The combined evidence from basic research [13–16] and epidemiological investigations [29, 30, 32, 33] warrants consideration, and additional trials should investigate potential adverse effect of a high DAL in cancer survivors.
Our study provides evidence that a high diet-dependent acid load is common in cancer survivors in the NHANES and emphasizes the need for additional research in this area of current oncological interest. It is of utmost importance to highlight that DAL is an easily modifiable risk factor, as dietary interventions promoting more plant-based diets were shown to reduce acid load from diet [5, 68, 69]. The fact that our results still revealed a higher acid load in cancer survivors (who most likely modified and improved their diet after diagnosis) as compared to the general population reinforces this call.
Strengths and limitations
This study has several strengths and limitations that warrant further discussion. To the best of our knowledge, it is one of the first studies to quantify DAL in a large and nationally-representative cohort of cancer survivors (NHANES). As such, we may have built the foundation for additional research in the future in this field. Although we did not match participants (e.g. “cases and controls”) due to the specific nature of our data (complex multistage, stratified, clustered data), we used state-of-the-art multivariate linear regression techniques to adjust for confounders (such as age and total energy intake). An additional asset of our studies is the fact the we employed 3 different DAL scores (NEAPF, PRALR, NEAPR), and did not restrict our analysis to PRALR and NEAPF, which is often the case in epidemiological research. As such, our study also includes a marker based on anthropometric data (NEAPR).
Weaknesses include the missing cancer-specific parameters (e.g. duration since cancer diagnosis, the exact cancer stage, treatments received, hormonal status for breast cancer, etc.). This information would have been valuable to allow for a more detailed description of cancer cases. Discussing these parameters in the context of acid load scores would have certainly enriched our study but unfortunately they were not available in the NHANES. On the other hand, none of these cancer-specific parameters is directly related to the DAL calculations, which is based on nutrients and anthropometric data. Moreover, cancer status was self-reported (see methods) which could theoretically lead to bias. The lack of matching methods in the sense of a case-control study could also be interpreted as a weakness. Yet again, the major aim of this study was to quantify DAL in cancer survivors to gain a first overall impression, and to investigate whether elevated DAL scores were eventually a topic in cancer survivors or not. Our results suggest that additional trials in this field are urgently warranted, particularly with regard to specific cancers. Future studies should also examine additional associations between specific clinical outcomes and an elevated DAL.
Conclusions
The present study investigated DAL in U.S. cancer survivors from the NHANES and revealed a higher (yet non-significant) diet-dependent acid load in this cohort. DAL scores > 0 mEq/d suggested an acidifying diet in cancer survivors. This warrants attention, as comparable acid load scores have been associated with adverse health outcomes in previous studies in breast cancer survivors.
Acknowledgements
In memory of Stefan Skaper.
Abbreviations
- AMPM
Automated Multiple Pass Method
- BMI
Body Mass Index
- CI
Confidence Interval
- CRP
C Reactive Protein
- DAL
Dietary Acid Load
- HbA1c
Hemoglobin A1c
- NCHS
National Center for Health Statistics
- NEAP
Net Endogenous Acid Production
- NHANES
National Health and Nutrition Examination Survey
- PRAL
Potential Renal Acid Load
- USDA
U.S. Department of Agriculture
- WHEL
Women’s Healthy Eating and Living
Authors’ contributions
MAS and ALR contributed to the analysis and interpretation of data. MAS and ALR contributed to the preparation of the manuscript. MAS is the guarantor of this study, designed the idea, and validated data. Writing original draft: MAS. Writing—review and editing: MAS and ALR. Illustration: MAS. All authors reviewed and approved the final manuscript. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors received no financial support / funding.
Availability of data and materials
Data is publicly available online (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx). The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The present study is negligible risk research that involves existing collections of non-identifiable data about human beings. It is a de-identified secondary analysis of freely available data. Research was performed in accordance with the Declaration of Helsinki and approved by the NCHS Research Ethics Review Board (https://www.cdc.gov/nchs/nhanes/irba98.htm). NHANES was approved by the National Centre for Health Statistics research ethics review board and informed consent was obtained for all participants.
This work been approved by all co-authors.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Banerjee T, Crews DC, Wesson DE, Tilea A, Saran R, Rios Burrows N, et al. Dietary acid load and chronic kidney disease among adults in the United States. BMC Nephrol. 2014;15(1):137. doi: 10.1186/1471-2369-15-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahleova H, McCann J, Alwarith J, Rembert E, Tura A, Holubkov R, et al. A plant-based diet in overweight adults in a 16-week randomized clinical trial: the role of dietary acid load. Clin Nutr ESPEN. 2021;44:150–158. doi: 10.1016/j.clnesp.2021.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Vormann J, Remer T. Dietary, metabolic, physiologic, and disease-related aspects of acid-base balance: foreword to the contributions of the second international acid-base symposium. J Nutr. 2008;138(2):413S–414S. doi: 10.1093/jn/138.2.413S. [DOI] [PubMed] [Google Scholar]
- 4.Williams RS, Kozan P, Samocha-Bonet D. The role of dietary acid load and mild metabolic acidosis in insulin resistance in humans. Biochimie. 2016;124:171–177. doi: 10.1016/j.biochi.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Müller A, Zimmermann-Klemd AM, Lederer A-K, Hannibal L, Kowarschik S, Huber R, et al. A vegan diet is associated with a significant reduction in dietary acid load: post hoc analysis of a randomized controlled trial in healthy individuals. Int J Environ Res Public Health. 2021;18(19):9998. doi: 10.3390/ijerph18199998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc. 1995;95:791–797. doi: 10.1016/S0002-8223(95)00219-7. [DOI] [PubMed] [Google Scholar]
- 7.Adeva MM, Souto G. Diet-induced metabolic acidosis. Clin Nutr. 2011;30(4):416–421. doi: 10.1016/j.clnu.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Storz MA, Ronco AL. Reduced dietary acid load in U.S. vegetarian adults: Results from the National Health and Nutrition Examination Survey. Food Science & Nutrition. [cited 2022 Apr 12];n/a(n/a). Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/fsn3.2825 [DOI] [PMC free article] [PubMed]
- 9.Lee KW, Shin D. Positive association between dietary acid load and future insulin resistance risk: findings from the Korean genome and epidemiology study. Nutr J. 2020;19(1):137. doi: 10.1186/s12937-020-00653-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scialla JJ, Appel LJ, Astor BC, Miller ER, Beddhu S, Woodward M, et al. Estimated net endogenous acid production and serum bicarbonate in African Americans with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(7):1526–1532. doi: 10.2215/CJN.00150111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hejazi E, Emamat H, Sharafkhah M, Saidpour A, Poustchi H, Sepanlou S, et al. Dietary acid load and mortality from all causes, CVD and cancer: results from the Golestan cohort study. Br J Nutr. 2021:1–7. [DOI] [PubMed]
- 12.Park Y-MM, Steck SE, Fung TT, Merchant AT, Elizabeth Hodgson M, Keller JA, et al. Higher diet-dependent acid load is associated with risk of breast cancer: findings from the sister study. Int J Cancer. 2019;144(8):1834–1843. doi: 10.1002/ijc.31889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robey IF. Examining the relationship between diet-induced acidosis and cancer. Nutr Metab. 2012;9(1):72. doi: 10.1186/1743-7075-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillies RJ, Verduzco D, Gatenby RA. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat Rev Cancer. 2012;12(7):487–493. doi: 10.1038/nrc3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillies RJ, Pilot C, Marunaka Y, Fais S. Targeting acidity in cancer and diabetes. Biochim Biophys Acta Rev Cancer. 2019;1871(2):273–280. doi: 10.1016/j.bbcan.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moellering RE, Black KC, Krishnamurty C, Baggett BK, Stafford P, Rain M, et al. Acid treatment of melanoma cells selects for invasive phenotypes. Clin Exp Metastasis. 2008;25(4):411–425. doi: 10.1007/s10585-008-9145-7. [DOI] [PubMed] [Google Scholar]
- 17.Keramati M, Kheirouri S, Musazadeh V, Alizadeh M. Association of high dietary acid load with the risk of cancer: a systematic review and meta-analysis of observational studies. Front Nutr. 2022;9 [cited 2022 Apr 12]. Available from: https://www.frontiersin.org/article/10.3389/fnut.2022.816797. [DOI] [PMC free article] [PubMed]
- 18.Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev. 2012;33(4):547–594. doi: 10.1210/er.2011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hua H, Kong Q, Yin J, Zhang J, Jiang Y. Insulin-like growth factor receptor signaling in tumorigenesis and drug resistance: a challenge for cancer therapy. J Hematol Oncol. 2020;13(1):64. doi: 10.1186/s13045-020-00904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ronco AL, Storz MA, Martínez-López W, Calderón JM, Golomar W. High dietary acid load is associated with prostate cancer risk: an epidemiological study. WCRJ. 2021;8:e2119. doi: 10.32113/wcrj_202111_2119. [DOI] [Google Scholar]
- 21.Shi L-W, Wu Y-L, Hu J-J, Yang P-F, Sun W-P, Gao J, et al. Dietary acid load and the risk of pancreatic cancer: a prospective cohort study. Cancer Epidemiol Biomark Prev. 2021;30(5):1009–1019. doi: 10.1158/1055-9965.EPI-20-1293. [DOI] [PubMed] [Google Scholar]
- 22.Ronco A, Calderón J, Mendoza B. Dietary acid load and breast cancer risk: a case-control study in Uruguay. Eur J Cancer. 2020;138:S122. doi: 10.1016/S0959-8049(20)30866-2. [DOI] [Google Scholar]
- 23.Ronco AL, Martínez-López W, Calderón JM, Golomar W. Dietary acid load and lung cancer risk: a case-control study in men. Cancer Treat Res Commun. 2021;28:100382. doi: 10.1016/j.ctarc.2021.100382. [DOI] [PubMed] [Google Scholar]
- 24.Jafari Nasab S, Rafiee P, Bahrami A, Rezaeimanesh N, Rashidkhani B, Sohrab G, et al. Diet-dependent acid load and the risk of colorectal cancer and adenoma: a case-control study. Public Health Nutr. 2021;24(14):4474–4481. doi: 10.1017/S1368980020003420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ronco AL, Martínez-López W, Calderón JM, Mendoza BA. Dietary acid load and colorectal cancer risk: a case-control study. World Cancer Res J. 2020;7:e1750. [Google Scholar]
- 26.Milajerdi A, Shayanfar M, Benisi-Kohansal S, Mohammad-Shirazi M, Sharifi G, Tabibi H, et al. A case-control study on dietary acid load in relation to glioma. Nutr Cancer. 2021;29:1–8. doi: 10.1080/01635581.2021.1957134. [DOI] [PubMed] [Google Scholar]
- 27.Wu T, Hsu F-C, Pierce JP. Increased acid-producing diet and past smoking intensity are associated with worse prognoses among breast cancer survivors: a prospective cohort study. J Clin Med. 2020;9(6):1817. doi: 10.3390/jcm9061817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tessou KD, Lemus H, Hsu F-C, Pierce J, Hong S, Brown L, et al. Independent and joint impacts of acid-producing diets and depression on physical health among breast cancer survivors. Nutrients. 2021;13(7):2422. doi: 10.3390/nu13072422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu T, Seaver P, Lemus H, Hollenbach K, Wang E, Pierce JP. Associations between dietary acid load and biomarkers of inflammation and hyperglycemia in breast cancer survivors. Nutrients. 2019;11(8):E1913. doi: 10.3390/nu11081913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chronister BNC, Wu T, Santella RM, Neugut AI, Wolff MS, Chen J, et al. Dietary acid load, serum polychlorinated biphenyl levels, and mortality following breast cancer in the Long Island breast cancer study project. Int J Environ Res Public Health. 2022;19(1):374. doi: 10.3390/ijerph19010374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu T, Hsu F-C, Pierce JP. Acid-producing diet and depressive symptoms among breast cancer survivors: a longitudinal study. Cancers (Basel) 2020;12(11):E3183. doi: 10.3390/cancers12113183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.NHANES - About the National Health and Nutrition Examination Survey. 2020 [cited 2022 Feb 11]. Available from: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm
- 33.NHANES - Participants - About. 2021 [cited 2022 Feb 11]. Available from: https://www.cdc.gov/nchs/nhanes/participant/participant-about.htm
- 34.Albanes D, Blair A, Taylor PR. Physical activity and risk of cancer in the NHANES I population. Am J Public Health. 1989;79(6):744–750. doi: 10.2105/AJPH.79.6.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lynch BM, Dunstan DW, Healy GN, Winkler E, Eakin E, Owen N. Objectively measured physical activity and sedentary time of breast cancer survivors, and associations with adiposity: findings from NHANES (2003–2006) Cancer Causes Control. 2010;21(2):283–288. doi: 10.1007/s10552-009-9460-6. [DOI] [PubMed] [Google Scholar]
- 36.Marinac CR, Natarajan L, Sears DD, Gallo LC, Hartman SJ, Arredondo E, et al. Prolonged nightly fasting and breast cancer risk: findings from NHANES (2009–2010) Cancer Epidemiol Biomark Prev. 2015;24(5):783–789. doi: 10.1158/1055-9965.EPI-14-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deshmukh AA, Shirvani SM, Likhacheva A, Chhatwal J, Chiao EY, Sonawane K. The association between dietary quality and overall and cancer-specific mortality among cancer survivors, NHANES III. JNCI Cancer Spectr. 2018;2(2) [cited 2022 Feb 11]. Available from: https://academic.oup.com/jncics/article/2/2/pky022/5026131. [DOI] [PMC free article] [PubMed]
- 38.Su LJ, Arab L. Nutritional status of folate and colon cancer risk: evidence from NHANES I epidemiologic follow-up study. Ann Epidemiol. 2001;11(1):65–72. doi: 10.1016/S1047-2797(00)00188-5. [DOI] [PubMed] [Google Scholar]
- 39.Petrova D, Catena A, Rodríguez-Barranco M, Redondo-Sánchez D, Bayo-Lozano E, Garcia-Retamero R, et al. Physical comorbidities and depression in recent and long-term adult cancer survivors: NHANES 2007–2018. Cancers. 2021;13(13):3368. doi: 10.3390/cancers13133368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karavasiloglou N, Pestoni G, Faeh D, Rohrmann S. Post-diagnostic diet quality and mortality in females with self-reported history of breast or gynecological cancers: results from the third National Health and Nutrition Examination Survey (NHANES III) Nutrients. 2019;11(11):2558. doi: 10.3390/nu11112558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.NHANES - NCHS Research Ethics Review Board Approval. 2021 [cited 2022 Feb 11]. Available from: https://www.cdc.gov/nchs/nhanes/irba98.htm
- 42.NHANES 2007–2008: Dietary Interview - Individual Foods, First Day Data Documentation, Codebook, and Frequencies. [cited 2022 Apr 13]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2007-2008/DR1IFF_E.htm
- 43.NHANES - Measuring Guides. 2019 [cited 2022 Apr 13]. Available from: https://www.cdc.gov/nchs/nhanes/measuring_guides_dri/measuringguides.htm
- 44.AMPM—USDA Automated Multiple-Pass Method: USDA ARS. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/ampm-usda-automatedmultiple-pass-method/ (Accessed on 31 Jan 2022).
- 45.Centers for Disease Control and Prevention, National Center for Health Statistics . National Health and Nutrition Examination Survey MEC in-person dietary interviewers procedure manual. Hyattsville: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 46.Frassetto LA, Todd KM, Morris RC, Sebastian A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr. 1998;68:576–583. doi: 10.1093/ajcn/68.3.576. [DOI] [PubMed] [Google Scholar]
- 47.Remer T, Dimitriou T, Manz F. Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. Am J Clin Nutr. 2003;77(5):1255–1260. doi: 10.1093/ajcn/77.5.1255. [DOI] [PubMed] [Google Scholar]
- 48.Parmenter BH, Slater GJ, Frassetto LA. Accuracy and precision of estimation equations to predict net endogenous acid excretion using the Australian food database. Nutr Diet. 2017;74(3):308–312. doi: 10.1111/1747-0080.12324. [DOI] [PubMed] [Google Scholar]
- 49.Parmenter BH, Dymock M, Banerjee T, Sebastian A, Slater GJ, Frassetto LA. Performance of predictive equations and biochemical measures quantifying net endogenous acid production and the potential renal acid load. Kidney Int Rep. 2020;5(10):1738–1745. doi: 10.1016/j.ekir.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parker JD, Talih M, Malec DJ, Beresovsky V, Carroll M, Gonzalez JF, et al. National Center for Health Statistics data presentation standards for proportions. Vital Health Stat 2. 2017;(175):1–22. [PubMed]
- 51.Ward BW. kg_nchs: a command for Korn–Graubard confidence intervals and National Center for Health Statistics’ data presentation standards for proportions. Stata J. 2019;19(3):510–522. doi: 10.1177/1536867X19874221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alam I, Alam I, Paracha PI, Pawelec G. Higher estimates of daily dietary net endogenous acid production (NEAP) in the elderly as compared to the young in a healthy, free-living elderly population of Pakistan. CIA. 2012;7:565–573. doi: 10.2147/CIA.S37158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lemann J. Relationship between urinary calcium and net acid excretion as determined by dietary protein and potassium: a review. Nephron. 1999;81(Suppl 1):18–25. doi: 10.1159/000046294. [DOI] [PubMed] [Google Scholar]
- 54.Gannon RHT, Millward DJ, Brown JE, Macdonald HM, Lovell DP, Frassetto LA, et al. Estimates of daily net endogenous acid production in the elderly UK population: analysis of the National Diet and Nutrition Survey (NDNS) of British adults aged 65 years and over. Br J Nutr. 2008;100(3):615–623. doi: 10.1017/S0007114508901240. [DOI] [PubMed] [Google Scholar]
- 55.Pinto BM, Eakin E, Maruyama NC. Health behavior changes after a cancer diagnosis: what do we know and where do we go from here? Ann Behav Med. 2000;22(1):38. doi: 10.1007/BF02895166. [DOI] [PubMed] [Google Scholar]
- 56.Lei Y-Y, Ho SC, Cheng A, Kwok C, Cheung KL, He Y-Q, et al. Dietary changes in the first 3 years after breast cancer diagnosis: a prospective Chinese breast cancer cohort study. Cancer Manag Res. 2018;10:4073–4084. doi: 10.2147/CMAR.S168562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fassier P, Zelek L, Lécuyer L, Bachmann P, Touillaud M, Druesne-Pecollo N, et al. Modifications in dietary and alcohol intakes between before and after cancer diagnosis: results from the prospective population-based NutriNet-Santé cohort. Int J Cancer. 2017;141(3):457–470. doi: 10.1002/ijc.30704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaharudin SH, Sulaiman S, Shahril MR, Emran NA, Akmal SN. Dietary changes among breast cancer patients in Malaysia. Cancer Nurs. 2013;36(2):131–138. doi: 10.1097/NCC.0b013e31824062d1. [DOI] [PubMed] [Google Scholar]
- 59.Tan SY, Wong HY, Vardy JL. Do cancer survivors change their diet after cancer diagnosis? Support Care Cancer. 2021;29(11):6921–6927. doi: 10.1007/s00520-021-06276-9. [DOI] [PubMed] [Google Scholar]
- 60.Remer T, Berkemeyer S, Rylander R, Vormann J. Muscularity and adiposity in addition to net acid excretion as predictors of 24-h urinary pH in young adults and elderly. Eur J Clin Nutr. 2007;61(5):605–609. doi: 10.1038/sj.ejcn.1602560. [DOI] [PubMed] [Google Scholar]
- 61.Lemann JrJ. Relationship between urinary calcium and net acid excretion as determined by dietary protein and potassium: a review. NEF. 1999;81(Suppl. 1):18–25. doi: 10.1159/000046294. [DOI] [PubMed] [Google Scholar]
- 62.McAndrew NP, Bottalico L, Mesaros C, Blair IA, Tsao PY, Rosado JM, et al. Effects of systemic inflammation on relapse in early breast cancer. Npj Breast Cancer. 2021;7(1):1–10. doi: 10.1038/s41523-020-00212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ayoub NM, Jaradat SK, Alhusban A, Tahaineh L. <p>glycosylated hemoglobin A1c is associated with anthropometric measurements and tumor characteristics in breast cancer patients. IJWH. 2020;12:139–149. doi: 10.2147/IJWH.S234408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Osuna-Padilla IA, Leal-Escobar G, Garza-García CA, Rodríguez-Castellanos FE. Dietary acid load: mechanisms and evidence of its health repercussions. Nefrologia (Engl Ed) 2019;39(4):343–354. doi: 10.1016/j.nefroe.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 65.Abshirini M, Bagheri F, Mahaki B, Siassi F, Koohdani F, Safabakhsh M, et al. The dietary acid load is higher in subjects with prediabetes who are at greater risk of diabetes: a case–control study. Diabetol Metab Syndr. 2019;11(1):52. doi: 10.1186/s13098-019-0447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sia P, Plumb TJ, Fillaus JA. Type B lactic acidosis associated with multiple myeloma. Am J Kidney Dis. 2013;62(3):633–637. doi: 10.1053/j.ajkd.2013.03.036. [DOI] [PubMed] [Google Scholar]
- 67.Estrella V, Chen T, Lloyd M, Wojtkowiak J, Cornnell HH, Ibrahim-Hashim A, et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013;73(5):1524–1535. doi: 10.1158/0008-5472.CAN-12-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cosgrove K, Johnston CS. Examining the impact of adherence to a vegan diet on acid-base balance in healthy adults. Plant Foods Hum Nutr. 2017;72(3):308–313. doi: 10.1007/s11130-017-0620-7. [DOI] [PubMed] [Google Scholar]
- 69.Knurick JR, Johnston CS, Wherry SJ, Aguayo I. Comparison of correlates of bone mineral density in individuals adhering to lacto-ovo, vegan, or omnivore diets: a cross-sectional investigation. Nutrients. 2015;7(5):3416–3426. doi: 10.3390/nu7053416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is publicly available online (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx). The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.