Abstract
Tetracycline (TET) is a front-line antibiotic for the treatment of chlamydial infections in both humans and animals, and the emergence of TET-resistant (Tetr) Chlamydia is of significant clinical importance. Recently, several Tetr chlamydial strains have been isolated from swine (Sus scrofa) raised in production facilities in Nebraska. Here, the intracellular development of two Tetr strains, R19 and R27, is characterized through the use of tissue culture and immunofluorescence. The strains grow in concentrations of up to 4 μg of TET/ml, while a TET-sensitive (Tets) swine strain (S45) and a strain of the human serovar L2 (LGV-434) grow in up to 0.1 μg of TET/ml. Although inclusions form in the presence of TET, many contain large aberrant reticulate bodies (RBs) that do not differentiate into infectious elementary bodies. The percentage of inclusions containing typical developmental forms decreases with increasing TET concentrations, and at 3 μg of TET/ml 100% of inclusions contain aberrant RBs. However, upon removal of TET the aberrant RBs revert to typical RBs, and a productive developmental cycle ensues. In addition, inclusions were found that contained both C. suis R19 and Chlamydia trachomatis L2 after sequential infection, demonstrating that two biologically distinct chlamydial strains could both develop within a single inclusion.
The chlamydiae are important pathogens of humans and animals, causing a wide variety of significant diseases. In humans, Chlamydia trachomatis is the causative agent of trachoma, the leading cause of preventable blindness worldwide, as well as the cause of the most commonly reported sexually transmitted bacterial infection (10, 32). Chlamydia pneumoniae causes pneumonia and has recently been linked to atherosclerosis (20). Several other chlamydial species are important veterinary pathogens and cause diverse clinical syndromes in many animals. Chlamydiae have been isolated from swine (Sus scrofa), and these strains are associated with pneumonia, enteritis, conjunctivitis, pericarditis, perinatal mortality, and reproductive disorders. The majority of these swine strains are believed to be C. trachomatis-like organisms and have recently been reclassified as Chlamydia suis (12). Chlamydia pecorum and Chlamydia abortus (formerly “C. psittaci”) have also been identified in swine. The two resistant strains examined here are very similar to C. trachomatis and have only recently been reclassified as C. suis (7, 12, 28).
Chlamydiae are obligate intracellular pathogens that develop within a nonacidified vacuole termed an inclusion. Within the inclusion, chlamydiae undergo a unique biphasic developmental cycle that consists of two functionally and morphologically distinct developmental forms. Elementary bodies (EBs) are infectious and are involved in extracellular survival and transmission. Shortly after entry, EBs differentiate into noninfectious reticulate bodies (RBs), which are metabolically active and divide by binary fission. After several rounds of replication, RBs redifferentiate back into EBs, the cells lyse, and new infections can result.
As early as 1950, RBs were detected that existed in an altered, aberrant state (33). Since then, persistent chlamydial infections have been established in numerous cell culture systems using a variety of strains. In these infections, the chlamydiae deviate from the normal developmental cycle, remaining viable but persisting in a nonproductive state of growth. Aberrancy can be induced by exposure to antimicrobial agents such as penicillin, immunological agents such as gamma interferon, or through nutrient deprivation. These conditions generally delay maturation of the RB and block RB-to-EB transitions. It has been proposed that chlamydiae exploit this aberrant growth stage to persist in clinical infections and possibly exacerbate the disease process (6).
Uncomplicated acute chlamydial infections are generally cured with proper antibiotics, although the ability to effectively treat chronic or persistent infections is not well understood (11). Acute chlamydial infections are often asymptomatic and escape detection. This is thought to lead to severe complications, such as pelvic inflammatory disease, salpingitis and ectopic pregnancy in humans (17), and wasting syndrome and abortions in animals (8, 29). Many antibiotics are effective in treating chlamydial infections. Yet in both animals and humans, chlamydial infections are primarily treated with tetracycline (TET) or a TET derivative. The Centers for Disease Control and Prevention recommends treating individuals with either a 7-day course of doxycycline (a TET derivative) or a single dose of azithromycin (9). Livestock infections are most commonly treated with TET. In addition, livestock feed has been supplemented with TET for the past 50 years to ward off infections and promote growth (13). However, the introduction of antibiotics into animal feeds has encouraged the selection of resistant organisms (31).
The emergence of Tetr chlamydiae, in both human and animal populations, is of great concern. There are nine documented cases of human C. trachomatis isolates recovered in clinical settings that exhibited resistance to TET or a TET derivative. In 1990, Jones et al. (19) collected five isolates that were resistant to multiple antibiotics, including TET, doxycycline, erythromycin, sulfamethoxazole, and clindamycin. A second human Tetr C. trachomatis isolate was recovered in France in 1997 (22). This isolate was resistant to TET but sensitive to all other antibiotics tested, including azithromycin, erythromycin, ofloxacin, and pristinamycin. Recently, Somani et al. (30) reported the isolation of three urogenital isolates that were resistant to doxycycline, azithromycin, and ofloxacin. The mechanisms responsible for these resistant phenotypes are not known. All of the above Tetr chlamydiae were documented after treatment failure or suspected treatment failure with TET, and most isolates were shown to lose their resistance properties after several passages in TET-free media (19, 22).
In a previous report (1), eight Tetr C. suis strains were isolated from swine that resided on farms in either Nebraska or Iowa. Resistance appeared stable, as it was retained after 10 to 15 passages in TET-free media. Six of these eight strains were also resistant to sulfadiazine. Here we further characterize two of the eight resistant C. suis swine strains, R19 and R27, initially identified by Andersen and Rogers (1). These strains were isolated from pigs on a Nebraska farm and exhibit resistance to both TET and sulfadiazine at concentrations up to 4 and 20 μg/ml, respectively. Although inclusions formed in the presence of TET, they often contained large aberrant RBs that retained the ability to differentiate into EBs upon removal of the antibiotic. These strains traffic the fluorescent lipid C6-NBD-Cer and can develop within a single inclusion with a strain of the human C. trachomatis serovar L2.
MATERIALS AND METHODS
Cell culture.
The human epithelial cell line HeLa 229 (CCL 2.1; American Type Culture Collection, Rockville, Md.) was cultured in minimal essential medium (MEM) supplemented with 10% (vol/vol) fetal bovine serum (FBS) and 10 μg of gentamicin/ml (all reagents were from Gibco, Bethesda, Md.). All cells were cultured at 37°C in 5% CO2.
Chlamydial infections.
HeLa cells were grown to 2 × 105 cells on sterile 12-mm-diameter glass coverslips in 24-well trays. EBs were diluted in Hanks' balanced salt solution (HBSS; Gibco), and cell monolayers were inoculated. Plates were centrifuged at 750 × g for 1 h at room temperature (RT). The inocula were removed and replaced with fresh medium with or without the indicated concentrations of TET (Sigma, St. Louis, Mo.), and the infection proceeded for 50 h. At 50 h postinfection (hpi), cells were either fixed for 10 min in 100% methanol and stored in phosphate-buffered saline (PBS) or were prepared for other experiments as described below.
Production of infectious chlamydiae in the presence of TET.
Experiments were designed to quantify the number of infectious EBs present in an infected monolayer of cells. At 50 hpi, infected cells were lysed with 0.5 ml of sterile water for 1 min on ice. HBSS (0.5 ml) was added and the lysate was mixed. Three hundred microliters of lysate was placed on monolayers of HeLa cells grown on sterile 12-mm-diameter glass coverslips at 2 × 105 cells per well, and infections were performed as described above. At 50 hpi, cells were fixed in 100% methanol for 10 min and stored in PBS. To quantify EB production over time infections were performed similarly, and at appropriate time points indicated in Fig. 2 cells were lysed and used to infect new monolayers of HeLa cells. Fifty hours postinfection cells were fixed for 10 min in 100% methanol and stored in PBS.
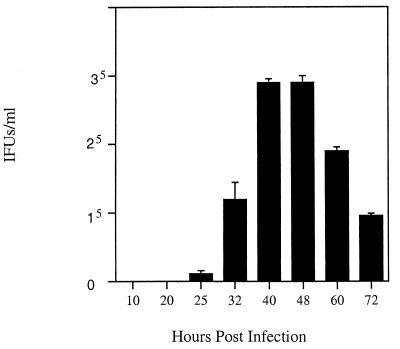
FIG. 2.
Temporal analysis of EB production by C. suis strain R19. HeLa cells were infected with C. suis R19, and EBs were harvested at the time points indicated. Cells were lysed, and the lysate was used to infect new monolayers of HeLa cells. At 50 hpi, cells were fixed and inclusion-forming units (IFUs) were enumerated using immunofluorescense with antibodies directed against HSP60. Values are averages of results from three wells, and standard deviations are represented with positive error bars.
Protein radiolabeling.
HeLa cells were grown to 1.2 × 106 cells per well in 6-well trays. C. trachomatis serovar L2 or C. suis strain R19 EBs were diluted in HBSS and added to the HeLa cell monolayers at a multiplicity of infection (MOI) of 2. Plates were centrifuged at 750 × g for 1 h at RT. The inocula were removed and replaced with fresh medium and supplemented with emitine (2 μg/ml) and with or without the appropriate concentration of TET. The infection was allowed to proceed for 24 h. At 24 hpi, 35S-labeled methionine and cysteine (1.25 Ci/mol; Amersham, Piscataway, N.J.) was diluted to 70 μCi/ml in methionine- and cysteine-free RPMI 1640 medium (ICN Biomedicals, Aurora, Ohio) supplemented with 0.5% (vol/vol) FBS and 10 μg of gentamicin/ml was added to each well. Cells were incubated for 5 h, after which cells were lysed in sample buffer (1% sodium dodecyl sulfate, 50 mM Tris-HCl [pH 6.8], 1% 2-mercaptoethanol, 10% glycerol), boiled for 5 min, and electrophoresed through a sodium dodecyl sulfate–12.5% acrylamide gel. After electrophoresis the gel was incubated in 10% acetic acid and 45% methanol for 30 min and then equilibrated in 1% glycerol. Gels were dried and placed onto X-ray film for 4 h at RT.
C. trachomatis L2 and C. suis R19 fusion experiments.
HeLa cells were grown to 2 × 105 cells on sterile 12-mm-diameter glass coverslips in 24-well trays. Cells were infected with C. suis strain R19 diluted in HBSS at an MOI of 1, and the plate was centrifuged at 750 × g for 1 h at RT. The inocula were removed and replaced with fresh MEM. After a 16-h culture period, cells were infected with L2 at an MOI of 2 in HBSS. The plate was placed on a rocker platform for 1 h at RT. The inocula were removed and replaced with fresh MEM. Cells were fixed 46 h after the initial R19 infection with 100% methanol and stored in PBS.
Immunofluorescence studies.
Infected and mock-infected cells fixed with methanol were incubated in 2% bovine serum albumin (BSA)–PBS at RT for 20 min on a rocker platform. This solution was aspirated from each well, primary antibody in 2% BSA–PBS was added, and the plate was rocked for 1 h at RT, followed by three washes with PBS. A secondary antibody conjugated to the appropriate fluorophore was added in 2% BSA–PBS and incubated in the dark for 1 h at RT. Cells were washed three times with PBS, and coverslips were inverted onto a drop of Vectashield mounting medium (Vector Laboratories, Burlingame, Calif.) on a microscope slide. Fluorescent and differential interference contrast (DIC) images were collected and examined on a Leica DMLB microscope equipped with appropriate fluorescence filters. Images were collected digitally using a Spot Camera (Diagnostic Instruments, Sterling Heights, Mich.) and processed with Adobe Photoshop 5.0 (Adobe Software; San Jose, Calif.).
Production of peptide antibody against R19 MOMP.
Rabbit antiserum against a peptide (CGAGKVEDKGSAGELC) in the strain R19 major outer membrane protein (MOMP) was produced by Genemed Synthesis, Inc. (San Francisco, Calif.). The peptide was linked to keyhole limpet hemocyanin and administered in complete Freund's adjuvant. Three subsequent booster injections were given with incomplete Freund's adjuvant, followed by enzyme-linked immunosorbent assay analysis to confirm the specificity and relative concentration of the antibody.
RESULTS
Chlamydial resistance patterns.
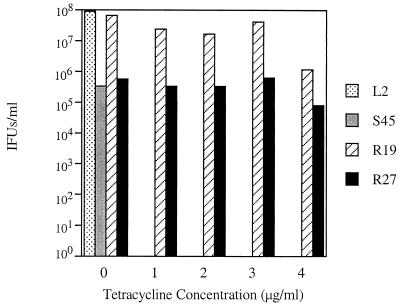
HeLa cells infected with chlamydial strains were cultured in the presence of various concentrations of TET for 50 h and examined for inclusion development by fluorescence microscopy. The C. trachomatis serovar L2 was highly sensitive to TET, as reported previously (18, 21). Inclusions, though rare, were observed in extremely low concentrations of TET (0.1 μg/ml and lower); alternatively, development was completely inhibited at concentrations of 0.25 μg/ml and higher (data not shown). These results were consistent with those for the Tets C. suis strain, S45. In contrast with these results, two C. suis strains, R19 and R27, exhibited approximately 40 times the resistance to TET that L2 showed. Both strains formed inclusions in TET concentrations up to and including 4 μg/ml and were completely inhibited at 5 μg/ml (Fig. 1). Although growth of the Tetr isolates was detected at the highest concentrations of TET (4 μg/ml), the formation of inclusions was reduced by 85 to 97%. Strains R19 and R27 were very similar in resistance properties and phenotypic characteristics, and all further studies were conducted using R19.
FIG. 1.
TET resistance patterns of several chlamydial strains. Infected cells were fixed at 50 hpi, and inclusion-forming units (IFUs) were enumerated using immunofluorescent microscopy and a monoclonal antibody against HSP60. The human C. trachomatis serovar, L2, was sensitive to a TET concentration of 0.25 μg/ml. Similar results were observed with the sensitive C. suis strain, S45. The two resistant C. suis strains, R19 and R27, formed inclusions at a TET concentration of 4 μg/ml, and no growth of R19 or R27 was observed at 5 μg of TET/ml. Values are averages of results from three wells.
Quantitative and qualitative analysis of chlamydial development in the presence of TET.
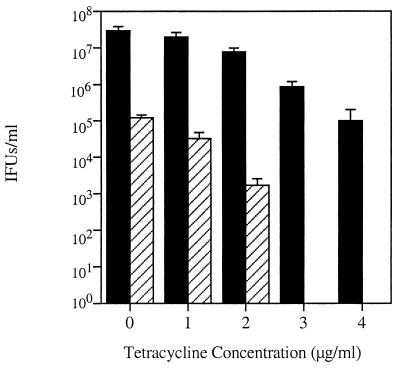
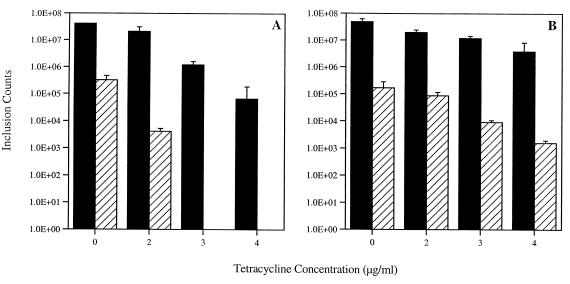
Experiments were performed to examine chlamydial growth and the production of EBs in infected monolayers under various concentrations of TET. Mature chlamydial inclusions are heavily laden with typical developmental forms, including both RBs and EBs. Under these conditions, if the inclusion is lysed then infectious EBs can be harvested that will productively infect new cells. An atypical inclusion, however, contains large, aberrant RBs that do not mature to infectious EBs. Thus, output experiments can be used to evaluate the production of infectious EBs under different culture conditions. In preliminary output experiments, maximal EB production was shown to occur between 40 and 50 hpi (Fig. 2). Experiments were then conducted that compared inclusion formation and the production of mature, infectious EBs in the presence of increasing concentrations of TET. Although the numbers of inclusions formed decreased with increasing concentrations of TET, inclusions developed at TET concentrations of up to 4 μg/ml (Fig. 3). The production of EBs is also inversely proportional to TET concentration, and at higher concentrations of TET (3 and 4 μg/ml) no EBs were present in the initial infection, as inclusions did not form in an output infection. These results indicate that 100% of the development was aberrant (Fig. 3).
FIG. 3.
Decreased growth of R19 and the effects of increasing TET concentrations. Infected cells were fixed at 50 hpi, and total numbers of inclusions were enumerated using immunofluorescence with antibodies against HSP60. These numbers, indicated with black bars, include both typical and aberrant inclusions. The hatched bars represent the number of inclusions formed after cells were lysed at 50 hpi, used to reinfect new cells, and cultured in the absence of TET. At 3 and 4 μg/ml, 100% of the RBs were aberrant and no EBs were present. Values are averages of results from three wells, and standard deviations are represented with positive error bars. IFUs, inclusion-forming units.
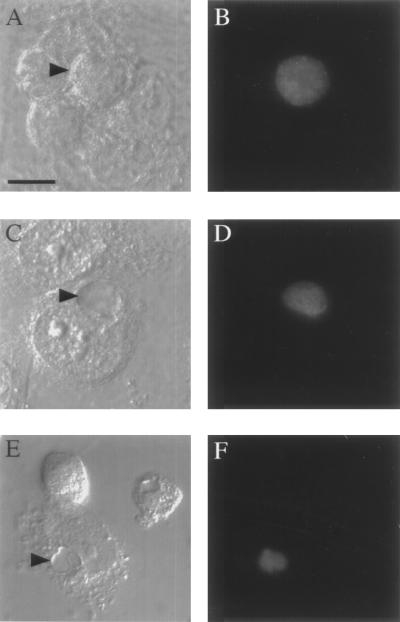
These results were confirmed by microscopic analysis. Monoclonal antibody directed against chlamydial heat shock protein (HSP60) was used as a probe for fluorescent microscopic analysis of methanol-fixed C. suis S45- and R19-infected HeLa cells. Strain S45 inclusions appeared similar to published L2 inclusions (Fig. 4A and B). The majority of R19 inclusions were typical when grown in the absence of TET (Fig. 4C and D), although approximately 1 to 5% of the inclusions contained aberrant RBs. As the concentration of TET was increased, an increasing percentage of inclusions appeared smaller and morphologically irregular and contained large aberrant RBs. Panels E and F of Fig. 4 show a single inclusion containing only three large aberrant RBs.
FIG. 4.
Inclusion morphology. (A and B) C. suis strain S45 formed typical inclusions when grown in the absence of TET. (C and D) C. suis strain R19 predominately formed typical inclusions when grown in the absence of TET. These inclusions are heavily laden with developmental forms. When grown in 2 μg of TET/ml, however, the majority of the inclusions contained only a few large aberrant RBs (E and F). Panels A, C, and E represent DIC micrographs; panels B, D, and F are fluorescent images labeled with antibodies against HSP60. Bar, 5 μm.
Aberrant RB reversion to EB upon TET removal.
Previous research has shown that aberrant chlamydiae can revert back to normal chlamydiae upon removal of the stressor (6). To test if this would also be the case upon removal of TET, infections were carried out for 24 h in the presence of TET and then the TET was removed and the infections were allowed to proceed for 26 more hours. At 50 hpi, parallel wells were either fixed for enumeration of inclusions or used for output experiments. In agreement with the data in Fig. 3, when C. suis strain R19 was cultured in the presence of TET for the entire course of infection, inclusions formed at all concentrations, although no EBs were produced at 3 or 4 μg of TET/ml (Fig. 5A). In contrast, when the drug was removed after 24 h and replaced with medium lacking TET, infectious EBs were produced in wells previously incubated in 3 or 4 μg of TET/ml (Fig. 5B).
FIG. 5.
Production of EBs from aberrant RBs upon removal of tetracycline. Infected cells were fixed at 50 hpi, and total numbers of inclusions were enumerated using immunofluorescence with antibodies against HSP60. These numbers, indicated with black bars, include both typical and aberrant inclusions. The hatched bars represent the number of inclusions formed after cells were lysed at 50 hpi, used to reinfect new cells, and cultured in the absence of TET. (A) EB production when R19 is cultured for 50 h in the indicated concentrations of TET. (B) EB production when R19 is cultured for the first 24 h in the indicated concentrations of TET and then cultured in no TET for the final 26 h. Values are averages of results from three wells, and standard deviations are represented with positive error bars.
Protein synthesis in the presence of TET.
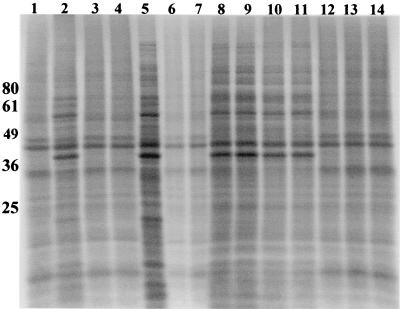
C. trachomatis L2- and C. suis S45- and R19-infected HeLa cells were labeled with 35S-labeled methionine and cysteine to visualize total protein production. While S45 and L2 protein production was completely inhibited in the presence of 1 μg of TET/ml, labeled chlamydial protein was detected during culture of R19 in the presence of TET concentrations of 1 and 2 μg/ml (Fig. 6). Incorporation of label by strain R19 during culture in TET concentrations above 2 μg/ml was not observed. This absence of labeling was likely due to decreased chlamydial numbers, as indicated by a much-reduced inclusion count at the higher TET concentrations (Fig. 1).
FIG. 6.
Overall protein production in response to TET. Overall protein production was examined through the use of radioactive methionine and cysteine. Lane 1, uninfected HeLa cell control; lane 2, L2-infected HeLa cells grown in the absence of TET; lane 3, L2 grown in the presence of 0.5 μg of TET/ml; lane 4, L2 grown in the presence of 1 μg of TET/ml; lane 5, S45-infected HeLa cells grown in the absence of TET; lane 6, S45 grown in the presence of 0.5 μg of TET/ml; lane 7, S45 grown in the presence of 1 μg of TET/ml; lane 8, R19-infected HeLa cells grown in the absence of TET; lanes 9 (0.5 μg/ml), 10 (1 μg/ml), 11 (2 μg/ml), 12 (3 μg/ml), 13 (4 μg/ml), and 14 (5 μg/ml) all represent R19 protein synthesis in the presence of TET. Molecular size markers are shown on the left in kilodaltons.
R19 and L2 development within a single inclusion.
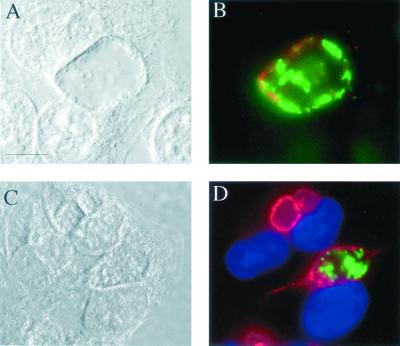
To test whether inclusions containing C. suis R19 and C. trachomatis L2, two biologically distinct chlamydial isolates, fuse to form one inclusion, HeLa cells were serially infected with both strains. Cells were first infected with strain R19, and 16 h later serovar L2 was added. The staggered infections eliminated the possibility that EBs from both species entered a single cell in the same phagocytic event. After being cultured, infected cells were visualized using monoclonal antibodies specific to the MOMP of each strain (Fig. 7A and B) or anti-R19 MOMP and anti-C. trachomatis IncA, an antibody that labels a protein localized to the C. trachomatis inclusion membrane (4) (Fig. 7C and D). In repeated experiments, single inclusions were found that contained both L2 and R19 EBs.
FIG. 7.
C. trachomatis L2 and C. suis R19 growth within a single inclusion. HeLa cells sequentially infected with R19 (time, 0 h) and L2 (time, 16 h) were fixed with methanol and labeled with antibodies directed against L2 and R19. (A) DIC micrograph of an infected cell containing a single inclusion 50 h after infection with R19. (B) Fluorescent image of the cells shown in panel A labeled with antisera against L2 MOMP (red) and R19 MOMP (green). (C) DIC micrograph of infected cells fixed 32 h after infection with R19. (D) Fluorescent image of the cells shown in panel C labeled with antisera against L2 IncA (red) and R19 MOMP (green). The nuclei of cells shown in panel D are colored blue with 4′,6-diamino-2-phenylindole (Sigma). Bar, 5 μm.
DISCUSSION
TET and its derivatives are presently the drugs of choice for treating chlamydial infections because they are effective, relatively inexpensive, and have low toxicity (31). Azithromycin is also used in clinical settings but is more costly (24). For the last several decades, the appearance of antibiotic-resistant organisms has been increasing, and many of the traditional antibiotics used to fight pathogenic microorganisms are failing in clinical treatments. These data present an initial characterization of Tetr C. suis. There are presently nine reported Tetr C. trachomatis isolates and eight Tetr C. suis isolates. The strains examined here are resistant to both TET and sulfadiazine. The emergence of Tetr strains isolated from livestock raises the issue of antibiotic use in animal feeds (26). Chlortetracycline was the first feed additive, introduced in 1950, and has seen widespread use over the past 50 years (13). Sulfa drugs were also widely used as feed additives during the 1970s and early 1980s. With the emergence of several veterinary pathogens exhibiting resistance to these antibiotics and with the associated human health concerns with the consumption of milk and meats from antibiotic-feed animals, the use of antibiotics is now being reduced (2).
The swine strains characterized here, R19 and R27, are now classified as C. suis (7, 12). However, they are very similar to C. trachomatis (28) and share several characteristics. The inclusion morphology of S45 and R19 observed in the absence of TET is generally similar to that of L2 inclusions (14). Furthermore, this strain traffics a ceramide analog to the inclusion (data not shown), as previously demonstrated for other chlamydiae (15, 27). However, there are several traits unique to these isolates. During culture of these organisms in the absence of TET, large aberrant forms are observed in approximately 1 to 5% of the inclusion population. These forms are identical to those seen with the addition of TET. In addition, production of chlamydial proteins occurs in the presence of TET, as observed through radiolabeling and autoradiography (Fig. 6). Furthermore, C. suis R19 was not labeled by antibodies directed against C. trachomatis inclusion membrane proteins, including IncA (4), IncC, and D223 (3) (data not shown).
Several C. trachomatis isolates have been documented that exhibit resistance to TET or one of its derivatives (19, 22, 30). Jones et al. (19) collected five isolates resistant to TET as well as to doxycycline, erythromycin, sulfamethoxazole, and clindamycin. They reported that only a small population exhibited resistance. This resistant population was not stable, as some isolates lost resistance and others died upon passage in cell culture. Similarly, Somani et al. (30) reported three isolates exhibiting multiple drug resistance. These isolates were resistant to doxycycline, azithromycin, and ofloxacin. Only a small population of organisms were reported to exhibit this type of resistance. The final isolate, reported by Lefevre and Lepargneur (22), was resistant to TET and doxycycline and was sensitive to azithromycin, erythromycin, ofloxacin, and pristinamycin. Approximately 1% of the population was resistant, and the isolate was lost after several passages in tissue culture. Conversely, C. suis strain R19 exhibited stable resistance to TET and sulfadiazine. It retains resistance after 10 to 15 passages in TET-free media (1). In agreement with the other reports, the Tetr R19 strain displayed at least a 100-fold decrease in inclusion numbers when exposed to high TET concentrations (≥3 μg/ml).
Inclusions containing the Tetr swine strains were shown to contain aberrant RBs that were enlarged and that failed to differentiate into EBs when grown in the presence of TET. These aberrant RBs retain the ability to differentiate into EBs upon the removal of TET (Fig. 5). Chlamydiae have been previously shown to adapt to periods of stress by existing in a persistent, nonculturable state. These stressors include nutrient deficiency, treatment with β-lactams or other antimicrobial agents, and treatment with cytokines such as gamma interferon (6). Persisting aberrant forms may mediate chronic infection and may be associated with exacerbated disease (5, 20, 25). The micrographs shown in Fig. 4E and F and the culture data shown in Fig. 5 demonstrate that the aberrant forms induced during the culture of strain R19 at higher concentrations of TET parallel chlamydial development observed in other stressful culture conditions.
Sequential infection of C. suis R19 and C. trachomatis serovar L2 demonstrated that inclusions formed by these strains will fuse, and thus the intracellular developmental forms can occupy the same intracellular vacuole (Fig. 7). This creates an environment where the two strains can interact. Although stable gene transfer has not been documented in chlamydiae, the growing RB within an inclusion provides a likely environment where such exchange could occur. The existence of chlamydial phage and plasmids allows opportunities for genetic exchange that parallel common processes in other systems (16, 23).
Future research in this area will focus on the identification of candidate genes associated with the resistance phenotype. This identification will allow an expanded understanding of the origin of the resistance and allow rapid screening of both veterinary and human populations for the presence of resistant chlamydial strains.
ACKNOWLEDGMENTS
We thank Karin Everett and Wendy Hambly of the U.S. Department of Agriculture for providing R19 and R27 MOMP sequences. We also thank John Bannantine of the U.S. Department of Agriculture for providing the monoclonal antibody against L2 IncA.
This work was supported by the U.S. Department of Agriculture through Cooperative Agreement 58-3625-8–121. J. Lenart is supported by the Oregon State University Eckelman Fellowship program.
Footnotes
Oregon Agricultural Experimental Station Technical Paper 11775.
REFERENCES
- 1.Andersen A A, Rogers D G. Resistance to tetracycline and sulfadiazine in swine C. trachomatis isolates. In: Stephens R S, et al., editors. Chlamydial Infections. Proceedings of the Ninth International Symposium on Human Chlamydial Infection. 1998. pp. 313–316. [Google Scholar]
- 2.Anonymous. Government advisory committee calls for a reduction in antibiotic use. Vet Rec. 1999;145:266–267. [PubMed] [Google Scholar]
- 3.Bannantine J P, Griffiths R S, Viratyosin W, Brown W J, Rockey D D. A secondary structure motif predictive of protein localization to the chlamydial inclusion membrane. Cell Microbiol. 2000;2:35–47. doi: 10.1046/j.1462-5822.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- 4.Bannantine J P, Stamm W E, Suchland R J, Rockey D D. Chlamydia trachomatis IncA is localized to the inclusion membrane and is recognized by antisera from infected humans and primates. Infect Immun. 1998;66:6017–6021. doi: 10.1128/iai.66.12.6017-6021.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barth W F, Segal K. Reactive arthritis (Reiter's syndrome) Am Fam Physician. 1999;60:499–503. [PubMed] [Google Scholar]
- 6.Beatty W L, Morrison R P, Byrne G I. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol Rev. 1994;58:686–699. doi: 10.1128/mr.58.4.686-699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bush R M, Everett K D. Molecular evolution of the Chlamydiaceae. Int J Syst Evol Microbiol. 2001;51:203–220. doi: 10.1099/00207713-51-1-203. [DOI] [PubMed] [Google Scholar]
- 8.Carrasco L, Segales J, Bautista M J, Gomez-Villamandos J C, Rosell C, Ruiz-Villamor E, Sierra M A. Intestinal chlamydial infection concurrent with postweaning multisystemic wasting syndrome in pigs. Vet Rec. 2000;146:21–23. doi: 10.1136/vr.146.1.21. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Guidelines for treatment of sexually transmitted diseases. Morb Mortal Wkly Rep. 1998;47:1–111. [PubMed] [Google Scholar]
- 10.Dawson C, Schachter J. Can blinding trachoma be eliminated worldwide? Arch Ophthalmol. 1999;117:974. doi: 10.1001/archopht.117.7.974. [DOI] [PubMed] [Google Scholar]
- 11.Dean D, Suchland R J, Stamm W E. Evidence for long-term cervical persistence of Chlamydia trachomatis by omp1 genotyping. J Infect Dis. 2000;182:909–916. doi: 10.1086/315778. [DOI] [PubMed] [Google Scholar]
- 12.Everett K D. Chlamydia and Chlamydiales: more than meets the eye. Vet Microbiol. 2000;75:109–126. doi: 10.1016/s0378-1135(00)00213-3. [DOI] [PubMed] [Google Scholar]
- 13.Gustafson R H. Use of antibiotics in livestock and human health concerns. J Dairy Sci. 1991;74:1428–1432. doi: 10.3168/jds.S0022-0302(91)78299-4. [DOI] [PubMed] [Google Scholar]
- 14.Hackstadt T, Fischer E R, Scidmore M A, Rockey D D, Heinzen R A. Origins and functions of the chlamydial inclusion. Trends Microbiol. 1997;5:288–293. doi: 10.1016/S0966-842X(97)01061-5. [DOI] [PubMed] [Google Scholar]
- 15.Hackstadt T, Scidmore M A, Rockey D D. Lipid metabolism in Chlamydia trachomatis-infected cells: directed trafficking of Golgi-derived sphingolipids to the chlamydial inclusion. Proc Natl Acad Sci USA. 1995;92:4877–4881. doi: 10.1073/pnas.92.11.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatt C, Ward M E, Clarke I N. Analysis of the entire nucleotide sequence of the cryptic plasmid of Chlamydia trachomatis serovar L1. Evidence for involvement in DNA replication. Nucleic Acids Res. 1988;16:4053–4067. doi: 10.1093/nar/16.9.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hillis S D, Owens L M, Marchbanks P A, Amsterdam L F, MacKenzie W R. Recurrent chlamydial infections increase the risks of hospitalization for ectopic pregnancy and pelvic inflammatory disease. Am J Obstet Gynecol. 1997;176:103–107. doi: 10.1016/s0002-9378(97)80020-8. [DOI] [PubMed] [Google Scholar]
- 18.Hsu A H, Knelsen B, Kasatiya S. In vitro tetracycline susceptibility of Chlamydia trachomatis in clinical specimens. Antonie Leeuwenhoek. 1987;53:191–196. doi: 10.1007/BF00393847. [DOI] [PubMed] [Google Scholar]
- 19.Jones R B, Van der Pol B, Martin D H, Shepard M K. Partial characterization of Chlamydia trachomatis isolates resistant to multiple antibiotics. J Infect Dis. 1990;162:1309–1315. doi: 10.1093/infdis/162.6.1309. [DOI] [PubMed] [Google Scholar]
- 20.Kuo C, Campbell L A. Is infection with Chlamydia pneumoniae a causative agent in atherosclerosis? Mol Med Today. 1998;4:426–430. doi: 10.1016/s1357-4310(98)01351-3. [DOI] [PubMed] [Google Scholar]
- 21.Lefevre J C, Bauriaud R. Comparative in vitro activities of pristinamycin and other antimicrobial agents against genital pathogens. Antimicrob Agents Chemother. 1989;33:2152–2154. doi: 10.1128/aac.33.12.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefevre J C, Lepargneur J P. Comparative in vitro susceptibility of a tetracycline-resistant Chlamydia trachomatis strain isolated in Toulouse (France) Sex Transm Dis. 1998;25:350–352. doi: 10.1097/00007435-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Liu B L, Everson J S, Fane B, Giannikopoulou P, Vretou E, Lambden P R, Clarke I N. Molecular characterization of a bacteriophage (Chp2) from Chlamydia psittaci. J Virol. 2000;74:3464–3469. doi: 10.1128/jvi.74.8.3464-3469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin D H, Mroczkowski T F, Dalu Z A, McCarty J, Jones R B, Hopkins S J, Johnson R B. A controlled trial of a single dose of azithromycin for the treatment of chlamydial urethritis and cervicitis. The Azithromycin for Chlamydial Infections Study Group. N Engl J Med. 1992;327:921–925. doi: 10.1056/NEJM199209243271304. [DOI] [PubMed] [Google Scholar]
- 25.Morrison R P. Persistent Chlamydia trachomatis infection: in vitro phenomenon or in vivo trigger of reactive arthritis? J Rheumatol. 1998;25:610–612. [PubMed] [Google Scholar]
- 26.Novick R P. The development and spread of antibiotic-resistant bacteria as a consequence of feeding antibiotics to livestock. Ann N Y Acad Sci. 1981;368:23–59. doi: 10.1111/j.1749-6632.1981.tb15430.x. [DOI] [PubMed] [Google Scholar]
- 27.Rockey D D, Fischer E R, Hackstadt T. Temporal analysis of the developing Chlamydia psittaci inclusion by use of fluorescence and electron microscopy. Infect Immun. 1996;64:4269–4278. doi: 10.1128/iai.64.10.4269-4278.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers D G, Andersen A A. Conjunctivitis caused by a swine Chlamydia trachomatis-like organism in gnotobiotic pigs. J Vet Diagn Investig. 1999;11:341–344. doi: 10.1177/104063879901100408. [DOI] [PubMed] [Google Scholar]
- 29.Shewen P E. Chlamydial infection in animals: a review. Can Vet J. 1980;21:2–11. [PMC free article] [PubMed] [Google Scholar]
- 30.Somani J, Bhullar V B, Workowski K A, Farshy C E, Black C M. Multiple drug-resistant Chlamydia trachomatis associated with clinical treatment failure. J Infect Dis. 2000;181:1421–1427. doi: 10.1086/315372. [DOI] [PubMed] [Google Scholar]
- 31.Speer B S, Shoemaker N B, Salyers A A. Bacterial resistance to tetracycline: mechanisms, transfer, and clinical significance. Clin Microbiol Rev. 1992;5:387–399. doi: 10.1128/cmr.5.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stamm W E. Chlamydia trachomatis infections: progress and problems. J Infect Dis. 1999;179(Suppl. 2):S380–S383. doi: 10.1086/513844. [DOI] [PubMed] [Google Scholar]
- 33.Weiss E. The effect of antibiotics on agents of the psittacosis-lymphogranuloma group. J Infect Dis. 1950;87:249–263. doi: 10.1093/infdis/87.3.249. [DOI] [PubMed] [Google Scholar]