Abstract
Introduction and objectives
Tocilizumab is an interleukin-6 receptor-blocking agent proposed for the treatment of severe COVID-19; however, limited data are available on their efficacy. The aim of this study was to assess the effect of tocilizumab on the outcomes of patients with COVID-19 pneumonia by using propensity-score-matching (PSM) analysis.
Methods
A retrospective observational analysis of hospitalized COVID-19 adult patients admitted to the Vall d’Hebron Hospital was performed between March and April 2020. We used the logistic regression to analyze the effect of tocilizumab on mortality, as main outcome, and PSM analysis to further validate their effect. Secondary outcomes were length-of-stay (LOS) and intensive-care-unit (ICU) stay. Same outcomes were also assessed for early tocilizumab administration, within 72 h after admission. Patients were selected by matching their individual propensity for receiving therapy with tocilizumab, conditional on their demographic and clinical variables.
Results
A total of 544 COVID-19 patients were included, 197 (36.2%) were treated with tocilizumab of whom 147 were treated within the first 72 h after admission; and 347 were included in the control group. After PSM analyses, the results showed no association between tocilizumab use and overall mortality (OR = 1.03, 95%CI: 0.63–1.68). However, shorter ICU-stay in the tocilizumab group was found compared to the control group (Coefficient −4.27 95%CI: −6.63 to −1.92). Similar results were found in the early tocilizumab cohort.
Conclusions
The administration of tocilizumab in patients with moderate to severe COVID-19 did not reduce the risk of mortality in our cohort of patients, regardless of the time of administration.
Keywords: Tocilizumab, Propensity-score-matched, Mortality, COVID-19, Pneumonia, Observational study
Abstract
Introducción y objetivos
El tocilizumab es un agente bloqueador del receptor de la interleucina 6 propuesto para el tratamiento de la COVID-19 grave; sin embargo, se dispone de datos limitados sobre su eficacia. El objetivo de este estudio fue evaluar el efecto de tocilizumab en los resultados de los pacientes con neumonía por COVID-19 mediante un análisis de emparejamiento por propensity-score-matching (PSM, «puntuación de propensión»).
Métodos
Se realizó un análisis observacional retrospectivo de los pacientes adultos con COVID-19 ingresados en el Hospital Vall d’Hebron entre marzo y abril de 2020. Se utilizó la regresión logística para analizar el efecto de tocilizumab en la mortalidad, como resultado principal, y el análisis PSM para validar aún más su efecto. Los resultados secundarios fueron la duración de la estancia y la estancia en la unidad de cuidados intensivos (UCI). También se evaluaron los mismos resultados para la administración temprana de tocilizumab, dentro de las 72 h posteriores al ingreso. Los pacientes se seleccionaron mediante el emparejamiento de su propensión individual a recibir tratamiento con tocilizumab, condicionado a sus variables demográficas y clínicas.
Resultados
Se incluyeron 544 pacientes de COVID-19, 197 (36,2%) fueron tratados con tocilizumab, de los cuales 147 fueron tratados dentro de las primeras 72 h tras el ingreso; y 347 fueron incluidos en el grupo control. Tras los análisis PSM, los resultados no mostraron ninguna asociación entre el uso de tocilizumab y la mortalidad global (OR = 1,03; IC del 95%: 0,63-1,68). Sin embargo, se encontró una menor estancia en la UCI en el grupo de tocilizumab en comparación con el grupo de control (coeficiente −4,27; IC del 95%: −6,63 − −1,92). Se encontraron resultados similares en la cohorte de tocilizumab temprano.
Conclusiones
La administración de tocilizumab en pacientes con COVID-19 moderada a grave no redujo el riesgo de mortalidad en nuestra cohorte de pacientes, independientemente del momento de la administración.
Palabras clave: Tocilizumab, Propensity-score-matched, Mortalidad, COVID-19, Neumonía, Estudio observacional
Introduction
The coronavirus disease 2019 (Covid-19) pandemic was announced by the WHO Europe Regional Committee in March.1 Since then, millions of confirmed cases have been identified throughout the whole world (more than 1.9 million in Spain), causing substantial morbidity and mortality from the severe acute respiratory syndrome induced by the coronavirus-2 (SARS-CoV-2).
Tocilizumab, a monoclonal humanized antibody which selectively targets the interleukin-6 (IL-6) receptor, is currently approved for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, and giant cells arteritis. Due to its capacity for blocking the IL-6 receptor, it has become one of the therapeutic options for the management of the cytokine release syndrome. Initially, it was successfully used in several small series of SARS-CoV-2 patients, showing some benefit in laboratory parameters as well as in clinical prognostic.2, 3, 4, 5, 6, 7
Recent published data from randomized control trials (RCTs) might suggest a potential role for tocilizumab in COVID-19; but they also revealed doubts about its efficacy regarding a reduction in mortality.8, 9, 10 However, the results from the largest observational studies associated the use of tocilizumab with lower risk of death among COVID-19 patients.11, 12 Given the controversy, a retrospective observational study was performed in order to analyze the clinical impact on mortality due to SARS-CoV-2 in our hospital, comparing outcomes of patients treated with tocilizumab with those not treated.
Methods
Patients
A retrospective observational analysis was performed of electronic medical records of hospitalized COVID-19 patients admitted to the Vall d’Hebron Barcelona Campus Hospital (VHBCH)¸ a reference public tertiary care hospital. Consecutive adults aged ≥18 years admitted with X-ray-confirmed pneumonia caused by laboratory-confirmed COVID-19 infection were suitable for the study. Data concerning medical history, demographic, comorbidities, laboratory findings and treatments during hospital stay, admissions and outcomes were extracted from electronic medical, pharmacy and nursing records. Patients with tocilizumab were identified from the pharmacy records of all patients to whom tocilizumab was dispensed. All patients discharged (deceased or alive) between March 2020 and April 2020 after hospital admissions in VHBCH for COVID-19 were included. Due to the high rate of hospitalization during this period, patients were either discharged or moved to other hospitals or nursing home facilities. Therefore, all the discharge status known, i.e., either during hospitalization in VHBCH or the subsequent hospitalization after being moved, were included. The observation period ended at final discharge. The research protocol was authorized by the Ethics Committee of the Hospital, without the need of informed consent from patients due to the retrospective nature of the study.
Patients hospitalized for <48 h or those with no need of oxygen therapy for >36 h were excluded. Patients who received another anti-IL-6 receptor antibody, such as sarilumab, were also excluded. To reduce indication bias, all patients included in the study should meet criteria for tocilizumab therapy in accordance with the Spanish Agency of Medicines and Medical Devices (AEMPS) during the study period for the treatment of COVID-19 infection.13 Thus, patients included in the tocilizumab group should meet these criteria before tocilizumab administration, meanwhile patients included in the control group (without tocilizumab therapy) should meet these criteria within the first 72 h after admission. The AEMPS tocilizumab administration criteria were: severe pneumonia caused by COVID-19, and presence of one of the following laboratory parameters: IL-6 >40 μL/L, D-dimer >1500 mcg/mL or if patient exhibited persistently rising D-dimer parameter. Patients with liver enzymes 5 times over the upper limit of normality or concomitant severe bacterial infection were not eligible for tocilizumab treatment. The final decision to use tocilizumab was at the discretion of the treating clinician.
All patients admitted in VHBCH with confirmed COVID-19 infection were treated with a standard pharmacological protocol, including antiviral drugs (lopinavir plus ritonavir twice a day), hydroxychloroquine 400 mg/day and antibiotic prophylaxis (azithromycin and ceftriaxone). Tocilizumab was initially administered at a dosage of 8 mg/kg (max 800 mg) by two consecutive administrations 12 h apart. Nevertheless, due to medication shortages, new indications for dose of tocilizumab were set by the AEMPS. Thus, patients during the study period received either 600 mg (400 mg for weight <75 kg) followed by a second dose of 400 mg 12 h apart, or a unique dose of 600 mg (400 mg for weight <75 kg). Every patient was fully informed about the off-label character of the treatment with tocilizumab in COVID-19. Signed or verbal consent was obtained from all patients before using tocilizumab. If the patient was incapable of executing permission, informed consent was given by the closest relative. Since corticoids given orally or parenterally showed benefit in COVID-19 patients, administration of systemic corticoids was also recorded, despite their limited and controversial use during the first wave of the pandemic.14
Outcomes
The primary endpoint for this analysis was all-cause mortality. Death was assessed either as occurring during hospitalization or during the stay at a nursing home. Secondary outcomes included in-hospital mortality, mortality of patients admitted to intensive care unit (ICU), and length of hospital stay (in-hospital LOS), LOS at any healthcare facilities, including nursing homes (total LOS) and intensive care unit (ICU) stay. LOS was calculated from day of admission to the day of discharge alive. Secondary analysis was performed with patients who received tocilizumab within the first 72 h after admission to analyze whether early administration of tocilizumab could affect mortality or LOS of patients with COVID-19 pneumonia.
Statistical analysis
This study was designed to investigate the effect of tocilizumab administration on the outcomes of these patients, using the propensity score–matching (PSM) method to eliminate the influence of other confounding factors. To minimize indication bias, patients to be included in each analysis were selected by matching their individual propensity for receiving therapy with tocilizumab (versus not receiving), conditional on their demographic and clinical variables. The following variables were fitted to a logistic regression model to derive their propensity score (PS): age, sex, chronic-obstructive-pulmonary-disease (COPD) or asthma, obesity, hypertension, diabetes, cancer or haematology malignancy, and a ratio of arterial oxygen saturation measured by pulse oximetry to fraction of inspired oxygen (SpO2/FiO2-R). The variable SpO2/FiO2-R was the worst SpO2/FiO2-R within the 24 h prior to tocilizumab administration. Meanwhile, the SpO2/FiO2-R in the control group was the worst SpO2/FiO2-R within the first 72 h after admission, regardless of fluctuations.
Overall survival was estimated by using multiple logistic regression model. PS driven analysis (inverse-probability-of-treatment weighting – IPTW) and PSM were performed to account for confounding by indication bias in the logistic regression model analysis. Multivariable logistic regression was carried out with tocilizumab use as a binary outcome to obtain the predicted probability of tocilizumab use, which was taken as PS. In IPTW, for the group with tocilizumab therapy, the weight was equal to 1/PS, while for the control group, the weight was equal to 1/(1-PS). Patients were matched on the basis of PS using nearest-neighbour matching without replacement, a calliper value was set as less than 10% of the standard deviation of PSs and a variable matching ratio of 1:2. Adequacy matching for no major imbalance of each baseline covariate was assessed by comparing distributions of PS, where a difference of less than 0.20 was considered an acceptable balance. Since some patients received several doses of tocilizumab, logistic regression was identically carried out with tocilizumab as lineal variable to assess the effect of multiple doses on the outcomes studied. Baseline characteristics and outcomes of patients who received tocilizumab and those who did not were compared using Students’ t test, Mann–Whitney test for continuous variables, and chi-square test or Fisher exact test for categorical variables. Statistical analyses were performed using Stata version 14 (Stata Corp., TX, USA) and statistical significance was set as p < 0.05.
Results
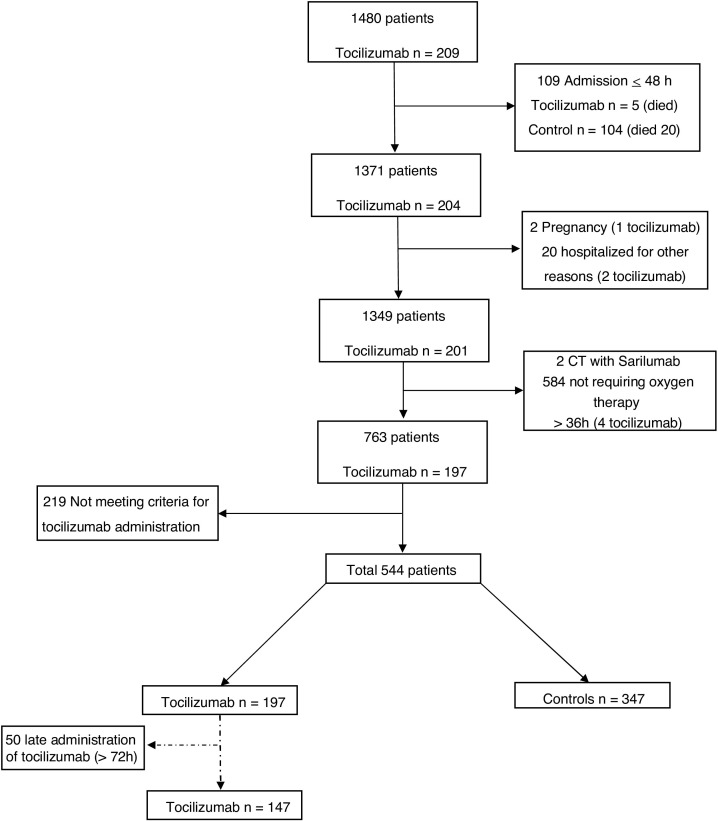
A total of 1480 COVID-19 patients were admitted to and discharged from VHBCH from March 2020 to April 2020, of whom 544 were included in this analysis, after exclusion of patients hospitalized for < 48 h (n = 109), for no need of oxygen therapy > 36 h (n = 584), being included in a clinical trial with sarilumab (n = 2) or other exclusion reasons (Fig. 1 ). Among the included individuals, 347 patients (73.8%) were included in the control group, 197 (36.2%) were treated with tocilizumab (148 patients received a single dose, 47 patients received 2 and 2 patients 3 doses of tocilizumab), of whom 147 were treated within the first 72 h after admission. Characteristics of COVID-positive patients included in the study are listed in Table 1 .
Fig. 1.
Study flowchart.
Abbreviations: n, number; CT, Clinical Trial
1480 COVID-19 patients were admitted of whom 544 were included in the study, after exclusion of patients hospitalized for <48 h (n = 109), admitted for other medical reasons and incidentally found positive for COVID-19 pneumonia (n = 20), pregnancy (n = 2), for being included in a clinical trial with sarilumab (n = 2), for no need of oxygen therapy >36 h (n = 584), and not meeting criteria for tocilizumab administration (n = 219).
Table 1.
Characteristics of cohort patients at cohort entry.
| Demographics & comorbilities | Total (n = 544) | Tocilizumab |
Control (n = 347) | p Value* | ||
|---|---|---|---|---|---|---|
| All (n = 197) | Early (n = 147) | |||||
| Age (years; ± SD) | 62.1 ± 13.8 | 61.0 ± 13.5 | 60.0 ± 13.5 | 62.7 ± 13.9 | 0.18 | 0.05 |
| Male sex | 341 (62.7) | 132 (67.0) | 95 (64.6) | 209 (60.2) | 0.12 | 0.36 |
| Obesity | 197 (36.2) | 88 (44.7) | 66 (44.9) | 109 (31.4) | 0.002 | <0.01 |
| Asthma/COPD | 91 (16.7) | 38 (19.3) | 26 (17.7) | 53 (15.3) | 0.23 | 0.50 |
| Cancer/Haematology malignancy | 57 (10.5) | 19 (9.6) | 12 (8.2) | 38 (11.0) | 0.63 | 0.35 |
| Hypertension | 239 (43.9) | 84 (42.6) | 63 (42.9) | 155 (44.7) | 0.65 | 0.71 |
| Diabetes | 137 (25.2) | 43 (21.8) | 30 (20.4) | 94 (27.1) | 0.18 | 0.12 |
| Transplantation/VIH | 28 (5.2) | 17 (8.6) | 8 (5.4) | 11 (3.2) | 0.006 | 0.23 |
| Chronic renal disease | 42 (7.7) | 20 (10.2) | 11 (7.5) | 22 (6.3) | 0.11 | 0.64 |
| SpO2/FiO2 ratio (±SD) | 256.1 ± 100.2 | 209.6 ± 94.5 | 206.3 ± 94.5 | 282.5 ± 93.7 | <0.001 | <0.001 |
| Interleukin-6 μL/L (±SD) | 166.6 ± 644.1 (n = 509) | 151.2 ± 282.7 (n = 187) | 160.3 ± 258.0 (n = 145) | 175.5 ± 781.1 (n = 322) | 0.68 | 0.82 |
| D-dimer mcg/Ml (±SD) | 491.0 ± 1575.2 (n = 369) | 439.0 ± 508.4 (n = 138) | 436.8 ± 444.5 (n = 103) | 522.5 ± 1952.7 (n = 231) | 0.62 | 0.66 |
Data are presented as n (%) unless otherwise noted. SD: standard deviation
p Value obtained comparing Tocilizumab group (all/early) with Control group.
In unmatched analysis, patients treated with tocilizumab showed higher in-hospital and overall mortality (25.4 vs. 14.7%, risk ratio [RR] 1.73, 95% confidence interval [95% CI]: 1.22–2.45; and 25.9 vs. 15.6%, RR 1.48, 95% CI: 0.99–2.20, respectively, Table 2 ). In addition, differences in in-hospital LOS and total LOS were also found between tocilizumab and control group (13.2 vs. 10.0 days, p < 0.01; and 14.7 vs. 11.1 days, respectively, Table 2). A total of 91 patients were admitted to ICU during the study period. Overall mortality in these patients was 21.98%, no differences were found in mortality between the groups assessed (28.21% vs. 17.31%, RR 0.61 [95% CI: 0.28–1.33]). The ICU-stay was longer in non-tocilizumab patients compared to tocilizumab group (11.8 vs. 5.8 days, respectively; Table 2). The subgroup analysis of only patients who received tocilizumab within the first 72 h after admission revealed similar results (Table 2).
Table 2.
Mean outcomes differences before matching.
| All cohort outcomes | Total (n = 544) | Tocilizumab (n = 197) | Control (n = 347) | p Value |
|---|---|---|---|---|
| In hospital mortality | 101 (18.6) | 50 (25.4) | 51 (14.7) | 0.002 |
| Overall mortality | 105 (19.3) | 51 (25.9) | 54 (15.6) | 0.003 |
| Overall stay (days) | 12.3 ± 9.5 | 14.7 ± 9.4 | 11.1 ± 9.4 | 0.008 |
| In hospital stay (days) | 11.1 ± 6.4 | 13.2 ± 5.8 | 10.0 ± 6.6 | 0.001 |
| ICU stay (days) | 8.4 ± 6.3 | 5.8 ± 4.3 | 11.8 ± 7.0 | <0.001 |
| Mortality (ICU patients) | 20 (22.0) | 9 (17.3) | 11 (28.2) | 0.214 |
| Early administration outcomes | Total (n = 494) | Tocilizumab (n = 147) | Control (n = 347) | p Value |
|---|---|---|---|---|
| In hospital mortality | 83 (16.8) | 32 (21.8) | 51 (14.7) | 0.056 |
| Overall mortality | 87 (17.6) | 33 (22.5) | 54 (15.6) | 0.067 |
| Overall stay (days) | 11.7 ± 9.0 | 13.5 ± 12.0 | 11.0 ± 9.3 | 0.012 |
| In hospital stay (days) | 10.6 ± 6.2 | 12.2 ± 4.8 | 10.0 ± 6.6 | 0.002 |
| ICU stay (days) | 8.7 ± 6.6 | 5.7 ± 4.5 | 11.8 ± 7.0 | <0.001 |
| Mortality (ICU patients) | 18 (22.5) | 7 (17.1) | 11 (28.2) | 0.226 |
Data are presented as n (%) unless continuous variables (days) presented as mean ± SD.
The tocilizumab and control group were well matched after the PS, as described in supplementary figures (S1, S2) and Table 3 by comparing distributions of propensity score and standardized mean differences, respectively. After both PS analyses, the results showed no association between tocilizumab use and in-hospital and overall mortality (OR = 1.02, 95% CI: 0.62–1.68; and OR = 1.03, 95% CI: 0.63–1.68, after PSM analyses, respectively; Table 4 ). Similar results were found among the cohort with patients who received tocilizumab within the first 72 h (Table 4). Identically, after PS analysis, no difference in overall mortality was noted between tocilizumab and control group regarding the number of doses administered (OR = 1.00, 95% CI: 0.69–1.43).
Table 3.
Patient characteristics according to initial presentation, before and after matching.
| All cohort | |||||||
|---|---|---|---|---|---|---|---|
| Demographics & comorbilities | Before Matching |
After Matching |
|||||
| Tocilizumab (n = 197) | Control (n = 347) | Standardized difference (%) | Tocilizumab (n = 195) | Control (n = 347) | Standardized difference (%) | p Value | |
| Age (mean, years) | 61.0 | 62.7 | −12.2 | 61.0 | 62.7 | −12.0 | 0.23 |
| Male sex | 67.0 | 60.2 | 14.1 | 66.7 | 64.9 | 3.7 | 0.71 |
| Obesity | 44.7 | 31.4 | 27.5 | 44.1 | 44.6 | −1.1 | 0.92 |
| Asthma/COPD | 19.3 | 15.3 | 10.6 | 19.0 | 17.9 | 2.7 | 0.78 |
| Cancer/Haematology malignancy | 9.6 | 11.0 | −4.3 | 9.7 | 9.0 | 2.5 | 0.80 |
| Hypertension | 42.6 | 44.7 | −4.1 | 42.5 | 45.3 | −5.7 | 0.58 |
| Diabetes | 21.8 | 27.1 | −12.2 | 22.1 | 29.1 | 16.6 | 0.12 |
| Transplantation/VIH | 8.6 | 3.2 | 23.3 | 8.2 | 5.9 | 9.8 | 0.38 |
| Chronic renal disease | 10.2 | 6.3 | 13.9 | 9.2 | 10.5 | −4.7 | 0.42 |
| SpO2/FiO2 ratio (mean) | 209.6 | 282.5 | −77.4 | 210.4 | 202.4 | 8.5 | 0.67 |
| Early administration cohort | |||||||
|---|---|---|---|---|---|---|---|
| Demographics & comorbilities | Tocilizumab (n = 147) | Control (n = 347) | Standardized difference (%) | Tocilizumab (n = 146) | Control (n = 347) | Standardized difference (%) | p Value |
| Age (mean, years) | 60.0 | 62.7 | −19.4 | 60.1 | 60.8 | −4.7 | 0.69 |
| Male sex | 64.6 | 60.2 | 9.1 | 64.4 | 63.0 | 2.8 | 0.81 |
| Obesity | 44.9 | 31.4 | 28.0 | 44.5 | 37.3 | 14.9 | 0.21 |
| Asthma/COPD | 17.7 | 15.3 | 6.5 | 17.8 | 18.5 | −1.8 | 0.88 |
| Cancer/Haematology malignancy | 8.2 | 10.9 | −9.5 | 8.2 | 8.6 | −1.2 | 0.92 |
| Hypertension | 42.9 | 44.7 | −3.6 | 42.5 | 45.5 | −6.2 | 0.60 |
| Diabetes | 20.4 | 27.1 | −15.7 | 20.5 | 21.2 | −1.6 | 0.89 |
| Transplantation/VIH | 5.4 | 3.2 | 11.2 | 4.8 | 5.5 | −3.4 | 0.79 |
| Chronic renal disease | 7.5 | 6.3 | 4.5 | 6.8 | 6.2 | 2.7 | 0.81 |
| SpO2/FiO2 ratio (mean) | 206.3 | 282.5 | −81.4 | 206.5 | 198.4 | 8.6 | 0.48 |
Data are presented as % unless a unit is given (years and ratio).
Table 4.
Adjusted clinical outcomes after PSM and IPTW.
| OR (95% CI) |
||
|---|---|---|
| PSM | IPTW | |
| Mortality outcomes | ||
| In hospital mortality | 1.02 (0.62–1.68) | 0.97 (0.59–1.57) |
| Overall mortality | 1.03 (0.63–1.68) | 0.95 (0.59–1.54) |
| Early administration | ||
| In hospital mortality | 0.86 (0.49–1.50) | 0.77 (0.44–1.33) |
| Overall mortality | 0.89 (0.51–1.53) | 0.76 (0.44–1.32) |
| Coefficient (B) [95% CI] |
||
|---|---|---|
| PSM | IPTW | |
| LOS outcomes | ||
| Overall stay | 1.46 [–0.54–3.46] | 1.28 [–0.56–3.15] |
| In hospital stay | 1.19 [–0.10–2.49] | 1.33 [–0.14–2.80] |
| ICU stay | –4.27 [–6.63 to –1.92] | –5.11 [–7.71 to –2.52] |
| Early administration | ||
| Overall stay | 0.14 [–1.93–2.22] | 0.03 [–1.82–1.88] |
| In hospital stay | –0.13 [–1.50–1.24] | 0.14 [–1.32–1.60] |
| ICU stay | –4.56 [–7.14 to –1.98] | –5.23 [–7.87 to –2.59] |
Data obtained using a multivariate logistic regression with tocilizumab as binary variable after PSM and IPTW analysis. Identically, linear variables, such as LOS, were analyzed using multivariable linear regression.
Abbreviations: OR: Odds Ratio. CI: Confidence Interval. PSM: propensity score matching. IPTW: inverse-probability-of-treatment weighting. LOS length of stay.
After matching, the results showed a tendency towards the association between tocilizumab use and longer hospital-LOS and total-LOS in the survivors (Table 4). Besides, shorter ICU-LOS in the tocilizumab group was found compared to the control group (Coefficient −5.11 95% CI: −7.71 to −2.52 in IPTW, Coefficient −4.27 95% CI: −6.63 to −1.92 in PSM). Identical to the results for the entire cohort, after IPTW and PSM, no association was found between early administration of tocilizumab and LOS (Table 4).
A total of 140 patients (25.7%) received systemic corticoids, mean dose and mean length of treatment were 417.95 mg of methylprednisolone/day and 4.28 days, respectively. Among patients receiving corticoids, 15 patients started corticoids within the first 2 days of admission, and 125 patients (89.3%) received corticoids within the last 5 days of admission. Use of corticoids was associated with a higher risk of mortality (44.3 vs. 10.6%, RR 3.32, 95% CI: 2.57–4.30). After matching, no difference in corticoids use were found between groups. Identically, no modification of tocilizumab effect was found after adding corticoids use on the outcomes assessed, such as overall mortality (OR 0.96; 95% CI: 0.57–1.61).
Discussion
In this real-life setting of our cohort study, patients with pneumonia caused by COVID-19 treated with tocilizumab had the same risk of death compared with those not treated with tocilizumab. The same results were found in the subgroup of patients with COVID-19 treated tocilizumab within the first 72 h after admission. In addition, no differences were found regarding the LOS of patients treated or not treated with tocilizumab, regardless of the time of administration. However, patients admitted to ICU and treated with tocilizumab showed lower stay in ICU compared with those not treated with tocilizumab.
The effects of tocilizumab against IL-6 receptor have been postulated to play a potential role in COVID-19.15, 16 Nevertheless, contrary to other observational studies that have been reported better results with tocilizumab compared to standard of care, our study did not find a benefit with tocilizumab in reducing mortality or LOS.6, 11, 12 The two largest observational studies, which to our knowledge are up to date at the time of writing, have exposed association between tocilizumab administration and lower mortality after multivariable adjustment.11, 12 Guaraldi et al. studied a cohort of COVID-19 patients similar to our cohort; assessing the effect of tocilizumab regardless of the time of administration.11 However, its end point was a composite outcome (invasive mechanical ventilation or death) and patients admitted to ICU were not included. In the STOP-COVID tocilizumab study of 3924 critically ill patients with COVID-19 admitted to ICUs, patients that were treated with tocilizumab within the first 2 days of ICU admission showed lower risk of death compared with those not treated with tocilizumab within the first 2 days of ICU admission.12 The observational studies above mentioned varied dosing (single or double), and subpopulations studied (moderate or critically ill patients). However, there was a lack of availability of inflammation biomarkers present in both studies. This point might be an important limitation due to the potential role of tocilizumab against the physiological inflammatory response to the virus. Therefore, all these differences in the observational studies might have been influenced in their study design, sample size and the results obtained. In contrast, all patients included in our cohort met the criteria for administration of tocilizumab, based on inflammation biomarkers; obtaining balanced groups.
Results in our study with early tocilizumab administration showed to be identical to those with the whole cohort, regardless of the time of administration. The RECOVERY study demonstrated that dexamethasone given for 10 days reduced 28-day mortality compared to usual care.14 Nevertheless, there was no benefit in patients who were randomized when they were not receiving oxygen (mortality 17% vs. 13%). These results may lead to think that immunomodulatory drugs, such as corticoids or tocilizumab, might have better outcomes depending on the time of their clinical conditions rather than depending on the time of admission. Notably, recent RCTs have failed to show reduction in mortality in COVID-19 patients treated with tocilizumab.10, 17, 18 Besides, preliminary results from two multicenter RCTs have been released, COVACTA and EMPACTA studies, showing both no decrease in mortality among COVID-19 patients treated with tocilizumab.8, 9 Our results are in line with those from the RCTs, showing no benefit in mortality in COVID-19 patients treated with tocilizumab, regardless of the time of administration, the number of doses of tocilizumab, or the subgroup of critically ill-patients admitted to ICU. However, our results regarding ICU patients might be also in line with other observational studies and preliminary results from RCTs. While our study showed shorter ICU stay in patients treated with tocilizumab, other observational studies and RCT found lower need of mechanical ventilation in patients treated with tocilizumab.9, 11, 12 In that sense, it might have a dual benefit, by reducing the need for intensive care, which is known to increase the risk of long-term complications, and also by limiting the burden on ICUs, leading to an increase in the availability of ICU beds. In addition, results from an institutional cohort showed lower mortality and less mechanical ventilation in patients treated with methylprednisolone and tocilizumab as rescue therapy.19 All these differences in results, study design and population treated could reflect possible presence of different sub-phenotypes with differential response to immunomodulatory therapies. Therefore, our results regarding ICU patients showed a possible benefit with tocilizumab in agreement with results from some observational studies and preliminary results from RCTs, might lead to assess the role tocilizumab in this subset of patients.9, 11, 12, 20
Nevertheless, there are several limitations to this study. Patients primarily received one or two doses of tocilizumab, depending on the time during the pandemic, availability of the drug and criteria for the use of tocilizumab from AEMPS, as they varied according to the situation during the pandemic. Other limitations of our study include the retrospective design, as it may have increased the risk selection bias. Notably, the crude mortality (before matching) was higher in the tocilizumab group, suggesting a possible selection bias towards more severe patients in the tocilizumab group (e.g. tocilizumab group showed worse SpO2/FiO2-R than control group before matching). While propensity score matching was used and resulted in well-matched arms, residual confounding by unmeasured variables cannot be excluded. Similarly, data collection did not include the duration of concomitantly administered medications, such as corticosteroids. However, the use of corticoids in our study should be considered during the pandemic on March and April as low and their use might be related to critical clinical situations. In fact, the use of corticoids in our cohort was associated with higher mortality; showing a possible trend to use them as a rescue therapy in worsening clinical situations. Finally, given that the study was a single centre cohort, a limited external validity is possible.
On the other hand, our study also has strengths. It was a large study that included patients from a real-life hospital setting. Our analysis used PSs and IPTW to create and analyze a comparison cohort similar to the one using tocilizumab, resulted in well-matched arms. Only patients who met these criteria, based on clinical and inflammatory biomarkers, for the use tocilizumab during the pandemic were included in the cohort. Besides, our analysis excluded patients who were still hospitalised before the beginning of the pandemic in Spain to avoid bias regarding baseline conditions in the cohort. Similarly, patients admitted <48 h were excluded to avoid immortal time bias. Death within the first 48 h of admission might show mortality in very critical situations, therefore patients might have received tocilizumab trying to offer a rescue treatment or, in contrast, just palliative care due to the poor clinical situation of the patient. Despite this exclusion might increase the risk of selection bias, excluding also mortality within the first 48 h, we think that better balanced groups were obtained excluding these patients. In addition, long follow-up period was possible for all the patients included. Since prolonged hospitalization and rehabilitation are common among patients hospitalized with severe COVID-19, mortality until final discharge was recorded, regardless of the last health institution that was in charge of the patient. Despite the inherent limitations of this study, this carefully retrospective study can provide insights into proper clinical management.
Conclusions
The administration of tocilizumab in patients with moderate to severe COVID-19 did not reduce the risk of mortality, nor the LOS, in our cohort of patients regardless of the time of administration of tocilizumab. Further RCTs are needed to assess the potential role of tocilizumab in subset populations such as ICU patients.
Conflict of interest
The authors declare that they have no conflict of interest regarding the publication of this work.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.medcle.2021.03.036
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.WHO announces COVID-19 outbreak a pandemic. [Internet] Available from: https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic [accessed 30.12.20].
- 2.Antwi-Amoabeng D., Kanji Z., Ford B., Beutler B.D., Riddle M.S., Siddiqui F. Clinical outcomes in COVID-19 patients treated with tocilizumab: An individual patient data systematic review. J Med Virol. 2020;92:2516–2522. doi: 10.1002/jmv.26038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campochiaro C., Della-Torre E., Cavalli G., De Luca G., Ripa M., Boffini N., et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quartuccio L., Sonaglia A., Pecori D., Peghin M., Fabris M., Tascini C., et al. Higher levels of IL-6 early after tocilizumab distinguish survivors from nonsurvivors in COVID-19 pneumonia: a possible indication for deeper targeting of IL-6. J Med Virol. 2020;92:2852–2856. doi: 10.1002/jmv.26149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morena V., Milazzo L., Oreni L., Bestetti G., Fossali T., Bassoli C., et al. Off-label use of tocilizumab for the treatment of SARS-CoV-2 pneumonia in Milan, Italy. Eur J Intern Med. 2020;76:36–42. doi: 10.1016/j.ejim.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rojas-Marte G., Khalid M., Mukhtar O., Hashmi A.T., Waheed M.A., Ehrlich S., et al. Outcomes in patients with severe COVID-19 disease treated with tocilizumab: a case-controlled study. QJM. 2020;113:546–550. doi: 10.1093/qjmed/hcaa206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jordan S.C., Zakowski P., Tran H.P., Smith E.A., Gaultier C., Marks G., et al. Compassionate use of tocilizumab for treatment of SARS-CoV-2 pneumonia. Clin Infect Dis. 2020;71:3168–3173. doi: 10.1093/cid/ciaa812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Press release COVACTA study. Available from: https://www.roche.com./investors/updates/invupdate-2020-07-29.htm [accessed 30.12.20].
- 9.Salama C., Han J., Yau L., Reiss W.G., Kramer B., Neidhart J.D., et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermine O., Mariette X., Tharaux P.L., Resche-Rigon M., Porcher R., Ravaud P., CORIMUNO-19 Collaborative Group Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M., et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. Erratum in: Lancet Rheumatol. 2020 Oct;2(10):e591. doi: 10.1016/S2665-9913(20)30244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta S., Wang W., Hayek S.S., Chan L., Mathews K.S., Melamed M.L., et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181:41–51. doi: 10.1001/jamainternmed.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tratamientos disponibles para el manejo de la infección respiratoria por SARS-CoV-2. Available from: https://www.aemps.gob.es/laAEMPS/docs/medicamentos-disponibles-SARS-CoV-2-19-3-2020.pdf?x42633 [accessed 30.12.20]. Spanish.
- 14.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., et al. Dexamethasone in hospitalized patients with Covid-19 – preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu B., Xu X., Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. 2020;18:164. doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crisafulli S., Isgrò V., La Corte L., Atzeni F., Trifirò G. Potential role of anti-interleukin (IL)-6 drugs in the treatment of COVID-19: rationale, clinical evidence and risks. BioDrugs. 2020;34:415–422. doi: 10.1007/s40259-020-00430-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvarani C., Dolci G., Massari M., Merlo D.F., Cavuto S., Savoldi L., et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S., et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramiro S., Mostard R.L.M., Magro-Checa C., van Dongen C.M.P., Dormans T., Buijs J., et al. Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study. Ann Rheum Dis. 2020;79:1143–1151. doi: 10.1136/annrheumdis-2020-218479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.REMAP-CAP Investigators. Gordon A.C., Mouncey P.R., Al-Beidh F., Rowan K.M., Nichol A.D., et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021 doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.