Abstract
Chemotherapy resistance is frequently observed in gastric cancer patients and is associated with poor prognosis; tryptophan (Trp) catabolism has been recognized as a key metabolic regulator of many types of cancer progression. Regulatory T cells (Tregs) and Trp metabolite kynurenine (Kyn) were analyzed using tumor tissues. Chemotherapy resistance induced by IL-10 or Treg was detected by flow cytometry assay. The activation of STAT3/BCL2 signaling pathways in gastric cells cocultured by Treg was illustrated by western blotting. Patients' Treg and human gastric cancer organoid model were established to examine the anticancer effects of STAT3 inhibitor. We found that a higher level of IL-10 secreted by Kyn-induced Tregs was responsible for the 5-fluorouracil-induced resistance of gastric cancer cell lines. STAT3 and BCL2 knockout significantly abrogated Treg supernatant- or IL-10-induced chemoresistance in SGC7901 and BGC823 cell lines. Furthermore, STAT3 inhibitor significantly reduced the organoid and clonogenicity of organoids cocultured with Treg. Our data suggested that tumor-derived Kyn may hyperactivate Tregs and induce chemoresistance through the IL-10/STAT3/BCL2 signaling pathway.
Keywords: gastric cancer, kynurenine, chemoresistance, Treg, STAT3
Introduction
Gastric cancer remains the third leading cause of cancer-related deaths worldwide, and to date, the treatment of gastric cancer patients mainly involves surgical resection and chemotherapy, including driamycin, 5-fluorouracil (5-Fu), and other targeted therapy drugs (Fitzmaurice et al., 2019; Wei et al., 2020). However, chemotherapy resistance is frequently observed and accounts for treatment failure as well as a poor survival rate in gastric cancer patients (Biagioni et al., 2019; Chen et al., 2020). Therefore, studies on the molecular mechanisms of chemoresistance in gastric cancer would help provide novel therapeutic targets and prognostic biomarkers for gastric cancer.
The tumor microenvironment is closely associated with chemoresistance in many cancers (Yeldag et al., 2018). Recent studies have shown that alterations in energy metabolism in the tumor microenvironment not only support aberrant proliferation of tumor cells but also participate in the regulation of chemotherapy resistance (Reina-Campos et al., 2017; Li et al., 2018b; Wang et al., 2018). Accumulating evidence indicates that tryptophan (Trp) catabolism is a well-established pathway for immune suppression in tumors (Triplett et al., 2018).
The majority of Trp catabolism-mediated immune suppression in tumors occurs through the kynurenine (Kyn) pathway (Opitz et al., 2020). A previous study has shown that the Kyn pathway promotes CD4+ T cell exhaustion in melanoma (Rad Pour et al., 2019), and another study has reported that the interaction of Kyn with aryl hydrocarbon receptor leads to the generation of regulatory T cells (Tregs) (Mezrich et al., 2010; Gutiérrez-Vázquez and Quintana, 2018). Our recent study demonstrated the role of the Kyn pathway in resistance to anti-PD-1 immunotherapy through inducing cytotoxic CD8+ T cell exhaustion in colorectal cancer (Wu and Zhu, 2021).
The expression of indoleamine 2,3-dioxygenase (IDO), which catalyses Trp into Kyn, has been found to be associated with poor response to neoadjuvant chemotherapy and prognosis, suggesting the role of Trp metabolism in the chemoresistance of tumor cells (Zhao et al., 2020). Recent studies have reported that the Kyn pathway is activated in the serum of gastric cancer patients, and these studies have demonstrated that the expression level of IDO1 is higher in four of seven gastric cancer cell lines and that it promotes cell migration through Kyn (Choi et al., 2016; Xiang et al., 2019). In this study, we sought to determine whether the activated Kyn pathway plays a role in chemotherapy for gastric cancer and the underlying molecular mechanisms. Our study provides new insight into developing novel therapeutic targets for clinical therapy in gastric cancer patients.
Materials and Methods
Patients and ethics
Patients diagnosed with stomach adenocarcinoma at the First Affiliated Hospital of Jinzhou Medical University were enrolled in this study. The study was approved by the ethics committee and informed consent was obtained from all participants (No. 202145). Detailed information is provided in Supplementary Data S1. Tumor samples were obtained from 20 patients with stomach adenocarcinoma who underwent chemotherapy at the First Affiliated Hospital of Jinzhou Medical University (Liaoning, China) between 2019 and 2021. Patients received chemotherapy regimens of cisplatin and 5-Fu. Therapeutic effects were evaluated according to the standard of RECIST (Response Evaluation Criteria in Solid Tumors). Complete response (CR) was defined as disappearance of all lesions in both primary tumor and lymph nodes; partial response (PR) was defined as at least a 30% reduction in the sum of the longest diameter of target lesions; progressive disease (PD) was defined as at least a 20% increase in the sum of the longest diameter of target lesions; and stable disease (SD) was defined as neither sufficient shrinkage to qualify as PR nor sufficient increase to qualify as PD. CR and PR were classified as chemosensitive, whereas SD and PD were classified as chemoresistant.
The tumor samples included 10 chemoresistant gastric cancer (GC) and 10 chemosensitive GC tissues. All samples were collected from patients with informed consent, and all related procedures were performed with the approval of the internal review and ethics boards of the indicated hospitals.
Cell culture and reagents
SGC7901 and BGC723 gastric cancer cell lines were purchased from the National Infrastructure of Cell Line Resource (Beijing, China). Cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cell lines were tested for mycoplasma routinely every 3 months using a mycoProbe mycoplasma detection kit (R&D Systems). Kyn and puromycin were purchased from MCE.
Generation of BCL2 or STAT3 knockout using CRISPR-Cas9
SGC7901 and BGC723 gastric cancer cell lines were transfected with sgRNA against BCL2 or STAT3 cloned in PX459 (Addgene). In brief, 2 × 105 SGC7901 and BGC723 gastric cancer cells were seeded in six-well plates for 24 h. SGC7901 and BGC723 cells were transfected with 5 μg of PX459 using Lipofectamine 8000 (Beyotime, China) in Gibco Opti-MEM reduced serum medium (Thermo Fisher Scientific). After 48 h, puromycin (2 μg/mL) was used for selection for 14 days, and cells were maintained in puromycin (0.5 μg/mL).
The following primers were used:
SGGFP: 5′-CACCGGGGCGAGGAGCTGTTCACCG-3′;
BCL2-SG1: 5′-CATTATAAGCTGTCGCAGAG-3′;
BCL2-SG2: 5′-TGGCGCACGCTGGGAGAACA-3′;
STAT3-SG1: 5′-AGCTACAGCAGCTTGACACA-3′; and
STAT3-SG2: 5′-ATCTTGACTCTCAATCCAAG-3′;
CCK-8 assay
In brief, 3000 cells were cultured into 96-well plates and incubated overnight at 37°C. The cells were then treated with indicated chemotherapeutic agents, other treatment for 48 h. Cell viability was assessed using the CCK-8 assay (Solarbio, China). After adding 100 μL CCK-8 solution, cells were then incubated for 2 h at 37°C. An automatic microplate reader was used for detection of the OD values at the wavelength of 450 nm.
Apoptosis assay
Cell apoptosis was evaluated with an APC Annexin V Apoptosis Detection Kit (BD). Apoptosis was measured by flow cytometry using an APC Annexin V Apoptosis Detection Kit (BioLegend).
Enzyme-linked immunosorbent assay
For tissue enzyme-linked immunosorbent assay (ELISA), tissues were collected from patients, 0.5 g of tumor tissue and 1.5 mL lysis buffer into homogenizer tube (preloaded with glass beads) on ice. After tissue homogenization, the protein suspension was centrifuged for 15 min at 12,000 g at 4°C. The supernatant is further used as the ELISA assay.
Tregs were seeded at 1 × 105 cells/mL in X-VIVO15 supplemented with 5% FBS for 72 h. The supernatant of Tregs were harvested for 15 min at 4700 g and cell culture supernatants were collected from Kyn-treated CD4 T cells for analysis of IL-10 concentration by ELISA using the Human IL-10 ELISA Kit (R&D Systems), Kyn from tumor tissue and serum was quantified using a KYN ELISA Kit (E4629; BioVision) following the manufacturer's protocol.
Western blot
SGC7901 and BGC723 gastric cells were lysed by RIPA buffer (Beyotime), and protein concentrations were measured by a BCA protein assay kit (Solarbio). Total protein was separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a nitrocellulose filter membrane (Beyotime). The membranes were blocked and incubated with BCL2 antibody (No. 15071 1:1000; CST), STAT3 antibody (No. 9139 1:1000; CST), and p-STAT3 antibody (No. 9145 1:1000; CST) followed by incubation with a secondary antibody (1:3000; Solarbio). The protein bands were detected with ECL detection reagent (Thermo Fisher Scientific), and the images were quantified using a Tanon 4600SF system (Tanon, China).
Flow cytometry
Gastric cancer tissues were cut into small pieces, digested with collagenase/DNase I, and filtered through 70 μm cell strainers to produce a single cell suspension.
First, live/dead fixable was used as a live/dead marker to gate out the dead cells. The samples were then surface-labeled with fluorochrome-conjugated antibodies against CD3 and CD4 for 30 min, and the transcription factor fixation/permeabilization buffer (Biolegend) was used for FOXP3 staining.
Samples were analyzed by flow cytometry (BD), and data were analyzed using FlowJo V10 software.
Treg cell culture
Blood samples were collected from healthy controls after informed consent was provided. All procedures were approved by the institutional review board of the First Affiliated Hospital of Jinzhou Medical University Hospital.
Peripheral blood mononuclear cells (PBMCs) were obtained by Ficoll density-gradient centrifugation. Total CD4+ T cells purified with naive CD4+ T cells were enriched by negative selection (EasySep™ naive CD4+ T Cell enrichment kits; STEMCELL, Canada). Isolated human-naive CD4+ T cells cultured in X-VIVO 15 serum-free medium (LONZA) were activated with anti-CD3 antibody (1 μg/mL; Biolegend) and anti-CD28 antibody (1 μg/mL; Biolegend) in a 96-well plate. For Treg culture, T cells were cultured with the addition of IL-2 (100 U/mL; PeproTech), TGF-β1 (PeproTech), and in the presence or absence of Kyn. After 6 days of differentiation, cells were harvested and assayed by flow cytometry.
Organoid culture and growth
We harvested 10–20 mm gastric tumors from the patient, mechanically disrupted them with a scalpel and enzymatically digested them on a shaker (120 RPMs, 37°C, 1 h) in the presence of a collagenase solution, containing DMEM-F12 (Thermo Fisher Scientific), 2 mg/mL collagenase (Sigma, United Kingdom), 2 mg/mL trypsin (Thermo Fisher Scientific), 5% FBS (Thermo Fisher Scientific), 5 μg/mL insulin (Thermo Fisher Scientific), and 50 μg/mL gentamicin (Solarbio). Gastric cancer organoids were maintained at 37°C as 3D spheroid cultures in Matrigel. The organoids were cultured in human complete medium (advanced DMEM/Ham's F-12 supplemented with penicillin/streptomycin; Thermo Fisher Scientific) and GlutaMAX (Thermo Fisher Scientific). Minimal basal medium supplemented with 1 × B27 (Thermo Fisher Scientific) and 10 μM Y-27632 (Selleck) was used for Matrigel drops. Organoids were passaged by removing the medium and breaking the Matrigel with phosphate-buffered saline. Organoids were then trypsinized for 90 s at 37°C in a water bath with 1 × TrypLE (Thermo Fisher Scientific), and single cells were plated in drops of 10–15 μL. Pictures were taken every 24 h and analyzed. Relative cell growth was quantified. For organoid and Treg coculture, human Tregs from PBMC of gastric cancer patients were isolated by human CD4+CD25+ Treg Isolation kit (Cat. No. 130-091-041 or 130-091-301; Miltenyi Biotec, Germany) following the manufacturer's instructions.
Statistical and bioinformatics analysis
All statistical analyses were conducted using R software. For bioinformatics analysis, all statistical analyses and graphical work were performed in TIMER (Li et al., 2017). For survival analysis, The Cancer Genome Atlas (TCGA) RNA-Seq data from gastric cancer (Fragments Per Kilobase of exon model per Million mapped fragments [FPKM] value) were collected. Kaplan–Meier survival analysis between groups was performed using the ‘‘survival’’ R package. Pearson's test was used to describe the correlation between Kyn or IL-10 concentrations and the percentages of CD4+ T cells. Significant differences were considered when p < 0.05.
Results
Serum Kyn levels are increased and associated with Treg proportions in chemotherapy-resistant gastric cancer patients
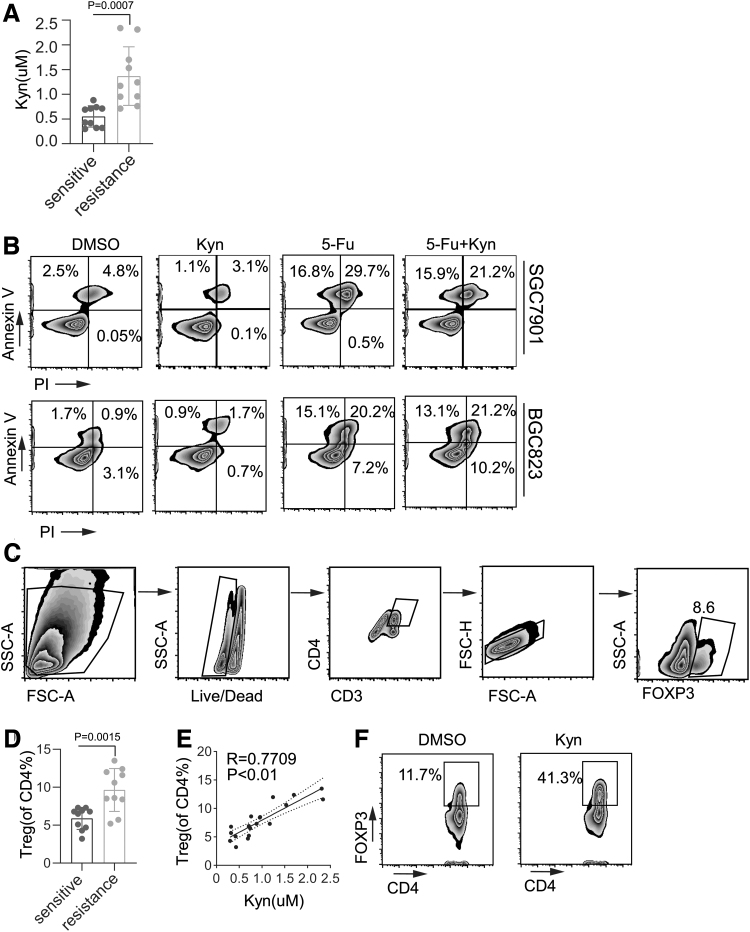
Kyn is a key metabolite for regulating T cell proliferation and survival and tumor progression. First, we compared Kyn expression between the chemotherapy-sensitive and chemotherapy-resistant groups and found higher expression of Kyn in the serum of chemoresistant gastric cancer patients (Fig. 1A). We speculate that Kyn may directly induce chemotherapy resistance in tumor. However, Kyn did not enhance the 5-Fu-induced cell death in the SGC7901 and BGC823 tumor cell lines in vitro (Fig. 1B). These results indicated that Kyn may promote chemotherapy resistance by influencing tumor immune microenvironment in gastric cancer patients.
FIG. 1.
Serum Kyn levels are increased and associated with Treg proportions in chemotherapy resistant gastric cancer patients. (A) ELISA analysis of the secretion of Kyn in tumor cells from chemotherapy-sensitive and chemotherapy-resistant tissues from gastric cancer patients. (B) SGC7901 or BGC823 cells were treated with DMSO, Kyn (100 μM), 5-Fu, or a combination of 5-Fu and Kyn for 48 h. Cell apoptosis was analyzed by flow cytometry. (C) Gating strategy for Treg analysis by flow cytometry. (D) Flow cytometry analysis of the percentage of Tregs in tumor cells from chemotherapy-sensitive and chemotherapy-resistant tumor tissues. (E) Correlation study between Tregs and Kyn in tumor cells from chemotherapy-sensitive and chemotherapy-resistant tissues from gastric cancer patients. (F) CD4+ T cells was purified in vitro. DMSO and Kyn (100 μM) were added on day 0, and CD4+ T cells were gated for Treg analyses on day 3. The percentage of Treg induction was determined by flow cytometry. Data are presented as the mean ± SD of three independent experiments. ELISA, enzyme-linked immunosorbent assay; 5-Fu, 5-fluorouracil; Kyn, kynurenine; SD, standard deviation; Tregs, regulatory T cells.
Of interest, we found that the percentage of Tregs was significantly higher in the peripheral blood of the resistance group than in the peripheral blood of the sensitive group (Fig. 1C, D), and the percentage of peripheral Tregs in the resistance group was positively correlated with the serum Kyn level (Fig. 1E). We further purified CD4+ T cells and induced Tregs in the presence of Kyn in vitro, and as expected, Kyn facilitated Treg induction in vitro (Fig. 1F). Therefore, these findings suggested that Kyn may induce increased Treg numbers in the tumor microenvironment, thus promoting chemotherapy resistance in gastric cancer.
Treg-derived IL-10 promotes chemoresistance in gastric cancer patients
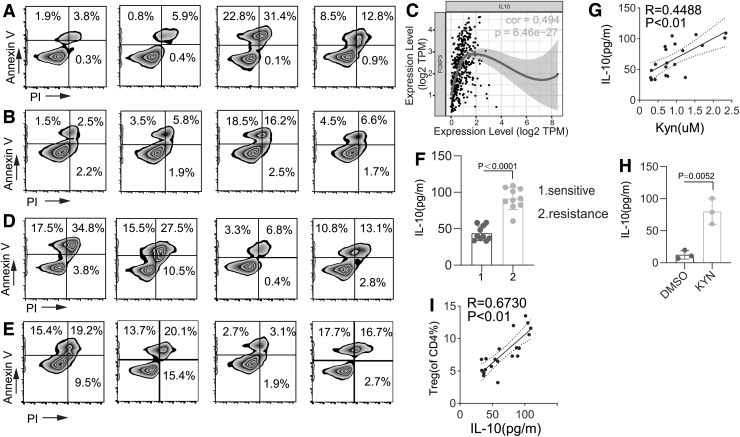
Next, we sought to explore the underlying molecular mechanisms of how Kyn/Treg promotes chemoresistance in gastric cancer patients. SGC7901 and BGC823 cell lines were cultured for 24 h in medium or culture supernatants from Treg cells followed by 5-Fu treatment. The above Treg supernatants were capable of reducing 5-Fu-induced gastric cancer cell apoptosis (Fig. 2A, B), suggesting Treg itself releases a certain factor(s) to induce chemotherapy resistance. Treg cells secrete inhibitory cytokines, including IL-10 and TGF-β, in a contact-independent manner. A previous study has shown that Tregs in gastric cancer mucosa express high levels of IL-10 but low levels of TGF-β (Stewart et al., 2013; Kindlund et al., 2017).
FIG. 2.
Treg-derived IL-10 promotes chemoresistance in gastric cancer patients. SGC7901 (A) and BGC823 (B) cell lines were cultured overnight in NC medium or supernatants from Treg cells and then treated with DMSO or 5-Fu. Cell apoptosis was analyzed by flow cytometry. (C) The relationship between IL-10 and FOXP3 was analyzed using TCGA dataset. SGC7901 (D) and BGC823 (E) cell lines were cultured overnight in NC medium or supernatants from Treg cells in the presence of IgG or anti-IL-10 mAb. (F) ELISA analysis of the secretion of IL-10 in tumor cells from chemotherapy-sensitive and chemotherapy-resistant tumor tissues. (G) Correlation study between IL-10 and Kyn in tumor cells from chemotherapy-sensitive and chemotherapy-resistant tissues from gastric cancer patients. (H) Correlation study between IL-10 and Tregs in tumor cells from chemotherapy-sensitive and chemotherapy-resistant tissues. (I) ELISA analysis of the secretion of IL-10 by CD4 cells in the presence of DMSO or Kyn. Data are presented as mean ± SD of three independent experiments. TCGA, The Cancer Genome Atlas.
In this study, we found a positive correlation of IL-10 and FOXP3 expression in TCGA gastric cancer tumor database (Fig. 2C). To explore whether Treg-derived IL-10 participates in chemoresistance in gastric cancer, we used an IL-10 mAb to neutralize IL-10 in Treg-derived culture supernatant. Both SGC7901 and BGC823 cell lines treated with Treg supernatant in the presence of anti-IL-10 mAb significantly abrogated Treg-induced chemoresistance in gastric cancer cells (Fig. 2D, E).
Moreover, serum IL-10 levels were significantly higher in the chemoresistance group and associated with the percentage of peripheral Tregs as well as serum Kyn levels (Fig. 2F–H). Induced Tregs in the presence of Kyn showed higher levels of IL-10 in the culture supernatant in vitro (Fig. 2I). The above results indicated that gastric cancer cell-derived Kyn induces Treg IL-10 secretion to promote chemotherapy resistance.
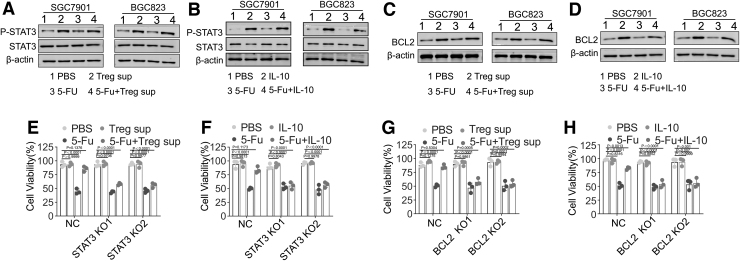
Tregs promote chemoresistance through the IL-10/STAT3/BCL2 signaling pathway
IL-10/STAT3/BCL2 is a well-known signaling pathway that is associated with drug resistance (Gritsko et al., 2006; Yang et al., 2015). Immunoblotting assays showed that SGC7901 and BGC823 cell lines cultured with either Treg supernatant or IL-10 significantly upregulated p-STAT3 and BCL2 expression (Fig. 3A–D). Positive correlations of STAT3 or BCL2 with FOXP3 expression were also detected in TCGA gastric cancer tumor database (Supplementary Fig. S1A, B). To further evaluate whether Treg-derived IL-10 promotes chemoresistance through the STAT3/BCL2 signaling pathway, we successfully knocked out STAT3 or BCL2 expression in SGC7901 and BGC823 cell lines, which was validated by an immunoblot assay (Supplementary Fig. S1C). STAT3 and BCL2 knockout significantly abrogated Treg supernatant- or IL-10-induced chemoresistance in SGC7901 and BGC823 cell lines (Fig. 3E–H).
FIG. 3.
Tregs promote chemoresistance through the IL-10/STAT3/BCL2 signaling pathway. (A) Western blotting assay of p-STAT3 and STAT3 expression in SGC7901 and BGC823 cells treated with PBS (IgG), 5-Fu (50 μg/mL), Treg supernatant, or a combination of 5-Fu and Treg supernatant. (B) Western blotting assay of p-STAT3 and STAT3 expression in SGC7901 and BGC823 cells treated with PBS (IgG), 5-Fu, IL-10, or a combination of 5-Fu and IL-10. (C) Western blotting assay of BCL2 expression in SGC7901 and BGC823 cells treated with PBS (IgG), 5-Fu, Treg supernatant, or a combination of 5-Fu and Treg supernatant. (D) Western blotting assay of BCL2 expression in SGC7901 and BGC823 cells treated with PBS (IgG), 5-Fu, IL-10, or a combination of 5-Fu and IL-10. (E) Proliferation of SGC7901-NC, SGC7901-STAT3 KO1, and SGC7901-STAT3 KO2 cells treated with PBS, 5-Fu, Treg supernatant, or a combination of 5-Fu and Treg supernatant. (F) Proliferation of BGC823-NC, BGC823-STAT3 KO1, and BGC823-STAT3 KO2 cells treated with PBS, 5-Fu, Treg supernatant, or a combination of 5-Fu and Treg supernatant. (G) Proliferation of SGC7901-NC, SGC7901-BCL2 KO1, and SGC7901-BCL2 KO2 cells treated with PBS, 5-Fu, Treg supernatant, or a combination of 5-Fu and Treg supernatant. (H) Proliferation of BGC823-NC, BGC823-BCL2 KO1, and BGC823-BCL2 KO2 cells treated with PBS, 5-Fu, Treg supernatant, or a combination of 5-Fu. Data are presented as the means ± SD of three independent experiments. PBS, phosphate-buffered saline. For better viewing of the data, please see the online version.
These data suggested that Kyn induces Tregs to produce IL-10 and promote chemoresistance through the IL-10/STAT3/BCL2 signaling pathway in gastric cancer cells.
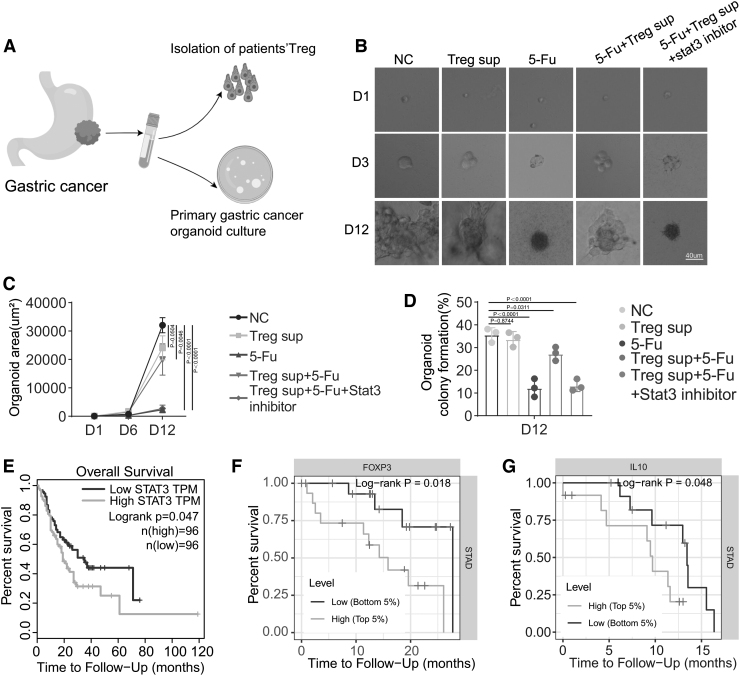
To evaluate the Treg-induced drug resistance in vivo, we finally established a human gastric cancer organoid model and cultured it with supernatant from blood sample-derived Tregs. The organoid area and organoid colony formation were analyzed to evaluate the effect of Tregs on organoid growth (Fig. 4A). Cultured with supernatant from Tregs reversed 5-Fu-inhibited organoid growth, confirming the role of Tregs in promoting chemoresistance in gastric cancer. However, this effect was abrogated by the STAT3 inhibitor, which significantly reduced the organoid area and clonogenicity of organoids (Fig. 4B–D). Finally, using TCGA tumor database, we found that patients with gastric cancer expressing high levels of STAT3, FOXP3, and IL-10 exhibited worse overall survival than patients expressing low levels of these molecules (Fig. 4E–G).
FIG. 4.
Tregs promote chemoresistance through the IL-10/STAT3/BCL2 signaling pathway. (A) Workflow of organoid cultures from gastric cancer tissues from patients. (B, C) Microphotographs of gastric cancer organoids in the indicated groups on days 1, 3, and 9. The area of the organoid area was calculated. (D) Colony formation analysis of gastric cancer organoids in the indicated groups on day 9. (E) The relationship between patient overall survival and STAT3 expression from TCGA dataset was analyzed. (F) The relationship between patient overall survival and FOXP3 expression from TCGA dataset was analyzed. (G) The relationship between patient overall survival and IL-10 expression from TCGA dataset was analyzed. Data are presented as means ± SD of three independent experiments.
Discussion
The development of clinical strategies to overcome chemoresistance is a central goal in gastric cancer research. In this study, we found an elevated Kyn level in chemotherapy-resistant gastric cancer patients compared with chemotherapy-sensitive gastric cancer patients. Further mechanistic study showed that Kyn promoted Treg induction and chemotherapy resistance through the IL-10/STAT3/BCL2 signaling pathway.
Trp catabolism has been implicated in the resistance of anti-PD1 and anti-CTLA-4 immunotherapy. Holmgaard et al. (2013) found that in a murine B16 model, the antitumor effect of anti-CTLA-4 therapy is significantly increased in IDO−/− mice. Botticelli et al. (2018, 2020) found that higher IDO activity, expressed as the Kyn/Trp ratio, predicts resistance to anti-PD-1 treatment. In a recent study, Nguyen et al. (2020) showed that colorectal cancer patients who failed cisplatin chemotherapy possess significantly higher Kyn/Trp ratios than the pretreatment baseline, indicating that Trp catabolism is involved in resistance to cisplatin chemotherapy. Our present study revealed a higher level of serum Kyn in chemotherapy-resistant gastric cancer patients, which significantly increased proportions of Tregs that promote chemotherapy resistance in gastric cancer patients.
The IDO/Kyn pathway has long been recognized as a critical regulator of immune system activity. IFN activates IDO1, whereas corticosteroids activate Trp-2,3-dioxygenase (TDO) (Spranger et al., 2013; Badawy, 2018). Corticosteroids have been used clinically to prevent and treat cancer-related adverse reactions, mainly for pretreatment before administration of certain chemotherapy agents, antiemetic treatment for radiotherapy, and chemotherapy-related vomiting, and anti-inflammatory treatment for inflammatory injuries (Santomasso et al., 2021). Corticosteroids may enhance differentiation of Treg through TDO/KYN to promote chemotherapy resistance, which is worthy of in-depth research in the future.
In this study, all our patients received corticosteroids treatment before chemotherapy, which led to us not knowing whether the tumor immune microenvironment will change in patients who did not receive glucocorticoid treatment. Li. et al. (2020) showed that TDO2 promotes the Epithelial-Mesenchymal Transition of hepatocellular carcinoma by activating Kyn-AhR pathway, thereby participating in the metastasis and invasion of HCC. Corticosteroid's treatment may cause liver metastasis, which may change tumor immune microenvironment compared with primary tumor. It is worth future investigation.
Previous studies have shown that Kyn plays a critical role in Treg induction (de Araújo et al., 2017; Nguyen et al., 2020). The Kyn/AhR signaling pathway epigenetically regulates Foxp3 expression and induces the generation and function of Tregs (Singh et al., 2011; Campesato et al., 2020; Qiu et al., 2020). Disruption of the Treg/Th17 balance has been implicated in the pathogenesis of gastric cancer (Li et al., 2013; Wang et al., 2017).
A recent study has found that TIM3+/LAG3+ Tregs contribute to KRAS-related chemoresistance and correlate with CD8 T cell exhaustion and ILC2 augmentation (Domvri et al., 2021). This study showed that coculture with Treg supernatant and an anti-IL-10 mAb significantly promoted 5-Fu-induced chemoresistance in gastric cancer cell lines, indicating that Treg-derived IL-10 promotes 5-Fu-induced gastric cancer cell line chemoresistance. Our study revealed a contact-independent manner of Tregs in inducing chemoresistance in cancer patients.
Several studies have demonstrated the role of STAT3 in regulating cancer chemoresistance in many cancers (Li et al., 2018a; Wang et al., 2018). The BCL2 gene is a well-characterized antiapoptotic gene, and the STAT3/BCL2 signaling pathway is also implicated in the drug resistance of B-non-Hodgkin's lymphoma and breast cancer (Alas and Bonavida, 2001; Yang et al., 2015). Analysis of TCGA tumor database showed that high levels of STAT3 exhibited worse overall survival in gastric cancer patients, and STAT3 or BCL2 knockout reversed Treg supernatant-induced gastric cancer chemoresistance. Together, these findings suggested the importance of the IL-10/STAT3/BCL2 signaling pathway in chemotherapy resistance in gastric cancer.
The two limitations of this article are as follows: first, our study is focused on gastric cancer; whether tumoral cell-derived Kyns active Tregs to enhance chemoresistance in other cancer types warrants future investigation. Second, although STAT3 inhibitor did improve the chemosensitivity of tumor cells, the dose and schedule may need to be further optimized to improve efficacy and/or reduce possible toxicity in mouse model or clinical trials.
Conclusion
In summary, we elucidated the effect of Kyn on chemoresistance through Treg activation and the IL-10/STAT3/BCL2 signaling pathway in gastric cancer patients. Our study provides a new potentially clinical strategy targeting Trp catabolism or the STAT3 signaling pathway to treat gastric cancer.
Supplementary Material
Authors' Contributions
Z.W. conceived, designed the study and draft the article. D.W. performed the experiments and conducted the data analysis.
Disclosure Statement
No competing financial interests exist.
Funding Information
This work is supported by Fund for Liaoning Science and Technology Foundation (No. 2019-ZD-0813).
Supplementary Material
References
- Alas, S., and Bonavida, B. (2001). Rituximab inactivates signal transducer and activation of transcription 3 (STAT3) activity in B-non-Hodgkin's lymphoma through inhibition of the interleukin 10 autocrine/paracrine loop and results in down-regulation of Bcl-2 and sensitization to cytotoxic drugs. Cancer Res 61, 5137–5144. [PubMed] [Google Scholar]
- Badawy, A.A. (2018). Targeting tryptophan availability to tumors: the answer to immune escape? Immunol Cell Biol 96, 1026–1034. [DOI] [PubMed] [Google Scholar]
- Biagioni, A., Skalamera, I., Peri, S., Schiavone, N., Cianchi, F., Giommoni, E., et al. (2019). Update on gastric cancer treatments and gene therapies. Cancer Metastasis Rev 38, 537–548. [DOI] [PubMed] [Google Scholar]
- Botticelli, A., Cerbelli, B., Lionetto, L., Zizzari, I., Salati, M., Pisano, A., et al. (2018). Can IDO activity predict primary resistance to anti-PD-1 treatment in NSCLC? J Transl Med 16, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botticelli, A., Mezi, S., Pomati, G., Cerbelli, B., Cerbelli, E., Roberto, M., et al. (2020). Tryptophan catabolism as immune mechanism of primary resistance to anti-PD-1. Front Immunol 11, 1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campesato, L.F., Budhu, S., Tchaicha, J., Weng, C.H., Gigoux, M., Cohen, I.J., et al. (2020). Blockade of the AHR restricts a Treg-macrophage suppressive axis induced by L-Kynurenine. Nat Commun 11, 4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z., Li, Y., Tan, B., Zhao, Q., Fan, L., Li, F., et al. (2020). Progress and current status of molecule-targeted therapy and drug resistance in gastric cancer. Drugs Today (Barcelona, Spain: 1998) 56, 469–482. [DOI] [PubMed] [Google Scholar]
- Choi, J.M., Park, W.S., Song, K.Y., Lee, H.J., and Jung, B.H. (2016). Development of simultaneous analysis of tryptophan metabolites in serum and gastric juice—an investigation towards establishing a biomarker test for gastric cancer diagnosis. Biomed Chromatogr 30, 1963–1974. [DOI] [PubMed] [Google Scholar]
- de Araújo, E.F., Feriotti, C., Galdino, N.A.L., Preite, N.W., Calich, V.L.G., and Loures, F.V. (2017). The IDO-AhR axis controls Th17/Treg immunity in a pulmonary model of fungal infection. Front Immunol 8, 880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domvri, K., Petanidis, S., Zarogoulidis, P., Anestakis, D., Tsavlis, D., Bai, C., et al. (2021). Treg-dependent immunosuppression triggers effector T cell dysfunction via the STING/ILC2 axis. Clin Immunol (Orlando, Fla) 222, 108620. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice, C., Abate, D., Abbasi, N., Abbastabar, H., Abd-Allah, F., Abdel-Rahman, O., et al. (2019). Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol 5, 1749–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritsko, T., Williams, A., Turkson, J., Kaneko, S., Bowman, T., Huang, M., et al. (2006). Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res 12, 11–19. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Vázquez, C., and Quintana, F.J. (2018). Regulation of the immune response by the aryl hydrocarbon receptor. Immunity 48, 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgaard, R.B., Zamarin, D., Munn, D.H., Wolchok, J.D., and Allison, J.P. (2013). Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med 210, 1389–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindlund, B., Sjöling, Å., Yakkala, C., Adamsson, J., Janzon, A., Hansson, L.E., et al. (2017). CD4(+) regulatory T cells in gastric cancer mucosa are proliferating and express high levels of IL-10 but little TGF-β. Gastric Cancer 20, 116–125. [DOI] [PubMed] [Google Scholar]
- Li, H., Chen, L., Li, J.J., Zhou, Q., Huang, A., Liu, W.W., et al. (2018a). miR-519a enhances chemosensitivity and promotes autophagy in glioblastoma by targeting STAT3/Bcl2 signaling pathway. J Hematol Oncol 11, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L., Wang, T., Li, S., Chen, Z., Wu, J., Cao, W., et al. (2020). TDO2 promotes the EMT of hepatocellular carcinoma through Kyn-AhR pathway. Front Oncol 10, 562823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q., Li, Q., Chen, J., Liu, Y., Zhao, X., Tan, B., et al. (2013). Prevalence of Th17 and Treg cells in gastric cancer patients and its correlation with clinical parameters. Oncol Rep 30, 1215–1222. [DOI] [PubMed] [Google Scholar]
- Li, S.S., Ma, J., and Wong, A.S.T. (2018b). Chemoresistance in ovarian cancer: exploiting cancer stem cell metabolism. J Gynecol Oncol 29, e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, T., Fan, J., Wang, B., Traugh, N., Chen, Q., Liu, J.S., et al. (2017). TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res 77, e108–e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezrich, J.D., Fechner, J.H., Zhang, X., Johnson, B.P., Burlingham, W.J., and Bradfield, C.A. (2010). An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol (Baltimore, Md: 1950) 185, 3190–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, D.J.M., Theodoropoulos, G., Li, Y.Y., Wu, C., Sha, W., Feun, L.G., et al. (2020). Targeting the kynurenine pathway for the treatment of cisplatin-resistant lung cancer. Mol Cancer Res 18, 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz, C.A., Somarribas Patterson, L.F., Mohapatra, S.R., Dewi, D.L., Sadik, A., Platten, M., et al. (2020). The therapeutic potential of targeting tryptophan catabolism in cancer. Br J Cancer 122, 30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, H., Zhang, J., Guo, Q., Zhang, Y., and Zhong, X. (2020). Prunella vulgaris L. attenuates experimental autoimmune thyroiditis by inducing indoleamine 2,3-dioxygenase 1 expression and regulatory T cell expansion. Biomed Pharmacother 2, 110288. [DOI] [PubMed] [Google Scholar]
- Rad Pour, S., Morikawa, H., Kiani, N.A., Yang, M., Azimi, A., Shafi, G., et al. (2019). Exhaustion of CD4+ T-cells mediated by the Kynurenine Pathway in Melanoma. Sci Rep 9, 12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reina-Campos, M., Moscat, J., and Diaz-Meco, M. (2017). Metabolism shapes the tumor microenvironment. Curr Opin Cell Biol 48, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santomasso, B.D., Nastoupil, L.J., Adkins, S., Lacchetti, C., Schneider, B.J., Anadkat, M., et al. (2021). Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO Guideline. J Clin Oncol 39, 3978–3992. [DOI] [PubMed] [Google Scholar]
- Singh, N.P., Singh, U.P., Singh, B., Price, R.L., Nagarkatti, M., and Nagarkatti, P.S. (2011). Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS One 6, e23522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger, S., Spaapen, R.M., Zha, Y., Williams, J., Meng, Y., Ha, T.T., et al. (2013). Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 5, 200ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, C.A., Metheny, H., Iida, N., Smith, L., Hanson, M., Steinhagen, F., et al. (2013). Interferon-dependent IL-10 production by Tregs limits tumor Th17 inflammation. J Clin Investig 123, 4859–4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett, T.A., Garrison, K.C., Marshall, N., Donkor, M., Blazeck, J., Lamb, C., et al. (2018). Reversal of indoleamine 2,3-dioxygenase-mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme. Nat Biotechnol 36, 758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M., Chen, B., Sun, X.X., Zhao, X.D., Zhao, Y.Y., Sun, L., et al. (2017). Gastric cancer tissue-derived mesenchymal stem cells impact peripheral blood mononuclear cells via disruption of Treg/Th17 balance to promote gastric cancer progression. Exp Cell Res 361, 19–29. [DOI] [PubMed] [Google Scholar]
- Wang, T., Fahrmann, J.F., Lee, H., Li, Y.J., Tripathi, S.C., Yue, C., et al. (2018). JAK/STAT3-regulated fatty acid β-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab 27, 136–150.e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, L., Sun, J., Zhang, N., Zheng, Y., Wang, X., Lv, L., et al. (2020). Noncoding RNAs in gastric cancer: implications for drug resistance. Mol Cancer 19, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, D., and Zhu, Y. (2021). Role of kynurenine in promoting the generation of exhausted CD8(+) T cells in colorectal cancer. Am J Transl Res 13, 1535–1547. [PMC free article] [PubMed] [Google Scholar]
- Xiang, Z., Li, J., Song, S., Wang, J., Cai, W., Hu, W., et al. (2019). A positive feedback between IDO1 metabolite and COL12A1 via MAPK pathway to promote gastric cancer metastasis. J Exp Clin Cancer Res 38, 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, C., He, L., He, P., Liu, Y., Wang, W., He, Y., et al. (2015). Increased drug resistance in breast cancer by tumor-associated macrophages through IL-10/STAT3/bcl-2 signaling pathway. Med Oncol (Northwood, London, England) 32, 352. [DOI] [PubMed] [Google Scholar]
- Yeldag, G., Rice, A., and Del Río Hernández, A. (2018). Chemoresistance and the self-maintaining tumor microenvironment. Cancers 10. [Epub ahead of print]; DOI: 10.3390/cancers10120471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y., Wei, L., Liu, J., and Li, F. (2020). Chemoresistance was correlated with elevated expression and activity of indoleamine 2,3-dioxygenase in breast cancer. Cancer Chemother Pharmacol 85, 77–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.