Abstract
Background:
Emerging evidence has highlighted the moderating effect of childhood maltreatment (CM) in shaping neurobiological abnormalities in major depressive disorder (MDD). However, whether neural mechanisms underlying stress sensitivity in MDD are affected by history of childhood maltreatment is unclear.
Methods:
213 medication-free female participants were recruited for a functional magnetic resonance imaging study assessing the effects of psychosocial stress on neural responses. The Montreal Imaging Stress Task was administrated to 44 female MDD patients with CM (MDD/CM), 32 female MDD patients without CM (MDD/noCM), 43 female healthy controls (HCs) with CM (HC/CM), and 94 female HCs without CM (HC/noCM). A CM (CM, noCM) × Diagnosis (MDD, HC) whole-brain voxel-wise analysis was run to assess putative group differences in neural stress responses.
Results:
A significant CM × Diagnosis interaction emerged in the medial prefrontal cortex (mPFC). Bonferroni-corrected simple effects analysis clarified that (1) the MDD/CM group had less mPFC deactivation than the HC/CM group, (2) the MDD/noCM group exhibited greater mPFC deactivation than the HC/noCM group, and (3) the MDD/CM group exhibited less mPFC deactivation relative to the MDD/noCM group. In addition, the mPFC-seed psychophysiological interaction analysis revealed that individuals in the CM groups had significantly greater stress-related mPFC-left superior frontal gyrus and mPFC-right posterior cerebellum connectivity relative to the noCM groups.
Conclusions:
Findings highlight distinct neural abnormalities in MDD depending on prior CM history, particularly potentiated stress-related mPFC recruitment among MDD individuals reporting CM. Moreover, CM history was generally associated with disruption in functional connectivity centered on the mPFC.
Keywords: functional neuroimaging, major depressive disorder, adverse childhood experience
1. Introduction
Major depressive disorder (MDD) is a highly heterogeneous psychiatric disorder. MDD patients with and without a history of childhood maltreatment (CM) exhibit distinct clinical course, including different onset time, severity, comorbidity, and treatment response (Teicher & Samson, 2013). Mounting neuroimaging evidence suggests that CM is associated with abnormalities in brain development within neural circuits critically implicated in threat detection (Hein et al., 2020; White et al., 2019), emotion regulation (Jenness et al., 2021; McLaughlin et al., 2015), reward anticipation (Dillon et al., 2009; Hein et al., 2020), and cognitive control (Bruce et al., 2013; Jankowski et al., 2017) (for review, see Teicher et al (2016)). In addition, initial findings indicate that individuals with MDD and a history of CM exhibited smaller hippocampal volume (Colle et al., 2017; Gerritsen et al., 2015; Yuan et al., 2020) and weaker functional connectivity within the prefrontal-limbic-thalamic-cerebellar circuit relative to individuals with MDD but no CM (Wang et al., 2014). Notably, several studies found that the abnormalities seen in MDD in terms of hippocampal atrophy (Opel et al., 2014) and reduced fractional anisotropy across various white matter tracts were abolished when regressing out the effects of CM (Meinert et al., 2019), suggesting some neural alterations underlying MDD could be the consequence of CM, rather than MDD. In this context, neural abnormalities in MDD may be further shaped by past CM.
Increased stress sensitivity has emerged as an important intermediate phenotype of MDD (Berghorst & Pizzagalli, 2010), therefore investigating the potential role of CM on stress sensitivity is of high significance. Prior neuroimaging findings revealed that MDD patients exhibited altered neural stress response in limbic-striatal-prefrontal regions (Admon et al., 2015; Holsen et al., 2011; Ming et al., 2017). Moreover, neuroimaging studies in healthy individuals reported that higher CM is associated with increased stress-induced activation in the amygdala (Grimm et al., 2014; Seo et al., 2019), hippocampus (Grimm et al., 2014; Seo et al., 2019), anterior cingulate cortex (Grimm et al., 2014), dorsal medial prefrontal cortex (van Harmelen et al., 2014), cerebellum (Seo et al., 2019), medial temporal lobe (Seo et al., 2019), insula (Zhong et al., 2020), precuneus (Zhong et al., 2020) and decreased stress-induced activation in the ventromedial and dorsolateral prefrontal cortex (Purcell et al., 2021), as well as increased stress-induced amygdala-hippocampus connectivity (Fan et al., 2015). Although these findings are not always consistent, they suggest that CM may impact neural stress responses in neural circuitries partly overlapping with regions consistently implicated in MDD (e.g., amygdala, hippocampus, prefrontal cortex). However, it is still unclear whether differences exist in stress circuitry between individuals with MDD with vs. without a history of CM. This important question can only be addressed by specifically comparing maltreated/non-maltreated MDD individuals to the corresponding maltreated/non-maltreated healthy individuals.
Toward this aim, we evaluated neural activation during a psychosocial stressor in maltreated and non-maltreated healthy controls (HC) and first-episode unmedicated MDD patients. The Montreal Imaging Stress Task (Dedovic et al., 2005), a reliable and widely-used psychosocial stressor, was administrated to induce psychosocial stress. Whole-brain analyses using a 2 × 2 factorial design were run, with the between-subject factors of CM and Diagnosis. In light of the overlapping stress-related neural circuits (i.e., limbic-prefrontal regions) implicated in MDD and CM, we hypothesized that the abnormal stress-related neural activation of MDD with past CM vs. HC with CM could differ from the abnormal stress-related neural activation of MDD without a history of CM vs. HC without CM, particularly in limbic-prefrontal regions.
2. Methods and Materials
2.1. Participants
Participants with a MDD diagnosis (N = 77) were recruited from the outpatient department of the Second Xiangya Hospital affiliated with Central South University. Healthy participants (N = 143) were recruited from two colleges and the community through advertisements and posters. All participants were unmedicated. Two psychiatrists conducted psychiatric evaluations using the Structured Clinical Interview for DSM-IV-TR Axis I disorders-Patient Edition. Patients meeting DSM-IV-TR Axis I disorders criteria for their first episode were recruited, with exclusion criteria for potential confounding effects of antidepressant medications, multiple episodes and comorbidities. Only females were included in light of abundant evidence of sex differences in stress responses at both the behavioral and neural levels (Goldfarb et al., 2019; Seo et al., 2017; Wang et al., 2007). See Supplemental Methods for detailed eligibility criteria. All participants were aware of the study’s purpose and provided informed written consent. The study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of the Second Xiangya Hospital of Central South University.
Six HC subjects and 1 patient with MDD were excluded because of excessive head motion (see Supplemental Methods for detailed exclusion criteria), leaving 76 female MDD patients and 137 female HCs available for analyses.
2.2. Assessment of Childhood Maltreatment
The Childhood Trauma Questionnaire (CTQ) was used to assess childhood maltreatment (Bernstein et al., 1998; He et al., 2019). The CTQ consists of five subscales: emotional abuse, physical abuse, sexual abuse, emotional neglect, physical neglect; with five items per scale rated on a 5-point Likert scale. Individuals were classified as experiencing childhood maltreatment when they met the respective moderate-to-severe cut-off score on at least one subscale according to Bernstein et al (1998) (emotional abuse ≥ 12; physical abuse ≥10; sexual abuse ≥ 8; emotional neglect ≥ 15; physical neglect ≥10). Forty-three of the 137 HCs were classified as HCs with CM (“HC/CM”), whereas the remaining 94 reported no CM (“HC/noCM”); 44 of 76 MDD patients were classified as MDD with CM (“MDD/CM”), and the remaining individuals reported no CM (“MDD/noCM”).
2.3. Montreal Imaging Stress Task (MIST)
The MIST, which involves uncontrollability and social evaluative threat, was administrated to induce acute psychosocial stress (Dedovic et al., 2005). Briefly, the MIST was conducted using a block design with three 7-min imaging runs. Each run consisted of three conditions: a rest condition (30s) without task requirement; a control condition (90s) in which participants answered arithmetic questions without a time limit; and a stress condition (90s) in which subjects had to answer arithmetic questions with a time limit and a visible performance bar. Each condition was presented twice in each run. See Supplemental Methods and Fig.1 for details. The contrast of interest for fMRI analyses was the stress condition minus the control condition.
Fig. 1. Overview of experimental design.
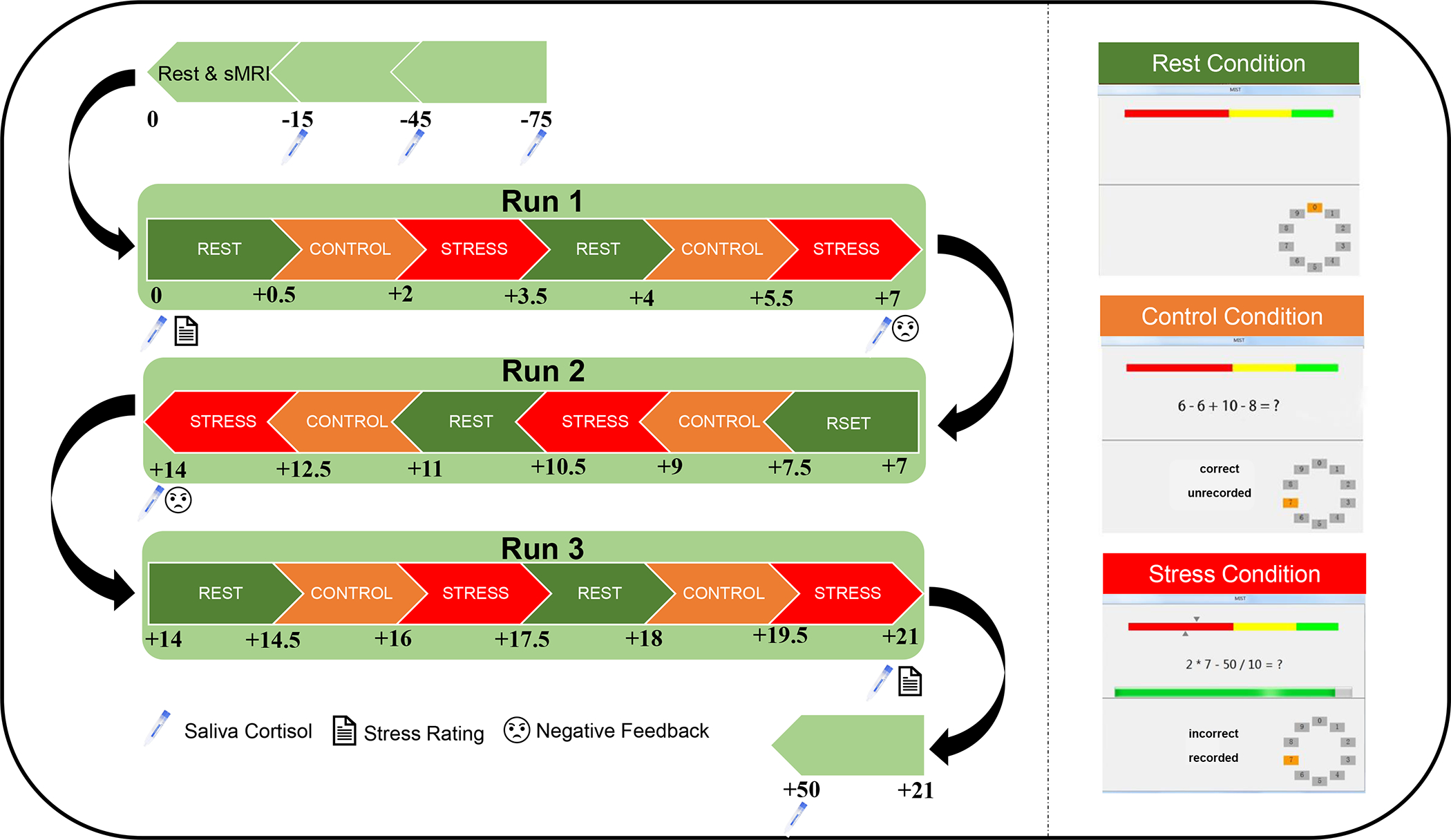
The MIST includes 3 runs, and each run lasts 7 minutes. Eight saliva samples were collected across the MIST and subjective stress levels were collected immediately before and after the MIST. Scripted negative feedback was given after the first and the second MIST runs. sMRI, structural magnetic resonance imaging.
2.4. Stress Response Measurement
Self-reported subjective stress ratings and cortisol concentrations (through saliva) were collected across the MIST to evaluate stress responses. Subjective stress responses were indexed by subtracting the pre-MIST stress rating from the post-MIST stress rating. To evaluate changes of cortisol concentration throughout the MIST, 8 saliva samples were collected with a Salivette (Sarstedt, Nümbrecht, Germany) in the scanner during the interval of scanning. Cortisol concentration was assessed using a human cortisol ELISA Kit (Bio-Swamp, Shanghai, China). Saliva samples were collected upon participants’ arrival (t = −75 min), after 30-minutes rest (t = −45 min), after entering the scanner (t = −15 min), after 15-minutes anatomical and resting-state scans (t = 0 min), after each MIST run (3 runs; t = +7/14/21 min), and after leaving the scanner (t = +50 min). Following established procedures, the area under the curve with respect to ground (AUCg; index of the overall cortisol output) and the area under the curve with respect to increase (AUCi; index of the cortisol changes) over the stress exposure [cort4 (t = 0 min) to cort8 (t = +50 min)] were calculated to measure the cortisol response (Pruessner et al., 2003). Both the AUCg and AUCi were calculated on the natural log-transformed cortisol concentrations.
2.5. fMRI Data Acquisition and Preprocessing
See Supplemental Methods.
2.6. Statistical Analysis
Psychological and Physiological data. Time (8 timepoints) × CM (CM, noCM) × Diagnosis (HC, MDD) repeated-measures ANCOVA analyses with age as a covariate were used to assess the main effect of time on cortisol concentration and subjective stress rating separately. In addition, Diagnosis × CM ANCOVA analyses were conducted to measure the group effects on subjective stress responses (post-stress minus pre-stress) and cortisol stress responses (AUCg, AUCi).
fMRI data.
For the first-level analysis, a general linear model including rest, control and stress conditions was conducted for each participant using Statistical Parametric Mapping (SPM12; The Wellcome Centre for Human Neuroimaging, London, UK). The first-level individual contrasts (stress minus control) were then submitted to group-level analyses. See Supplemental Fig.S1A for the uncorrected whole-brain t-map (stress vs. control). For the group-level analyses, a whole-brain Diagnosis × CM ANCOVA analysis with age as a covariate was conducted to probe possible group effects. All imaging results were corrected using cluster-level family-wise error rate (FWE) correction of p < 0.05 surpassing an initial p < 0.001 voxel threshold.
3. Results
3.1. Demographic and clinical characteristics
Clinical and demographic characteristics of four groups are summarized in Table 1. In the current sample, the MDD group was significantly older than the HC group (i.e., across both CM and noCM groups; F(1,209) = 26.01, p < 0.001, η2 = 0.111 ). With regard to years of education, a significant CM × Diagnosis interaction effect emerged (F(1,209) = 25.06, p = 0.008, η2 = 0.033), with both the MDD/CM group (pBonferroni = 0.030) and the HC/noCM group (pBonferroni = 0.003) having more years of education than the MDD/noCM group. In addition, there was a significant CM × Diagnosis interaction on CTQ score (F(1,209) = 4.12, p = 0.04, η2 = 0.019 ), with the MDD/CM group (vs. HC/CM; pBonferroni < 0.001) and the MDD/noCM group (vs. HC/noCM; pBonferroni = 0.045) exhibiting higher CTQ scores than their respectively HC group. See Table 1 for other demographic and clinical characteristics across groups.
Table 1.
Demographic and clinical characteristics
| Characteristics | HC/noCM (N = 94) | HC/CM (N = 43) | MDD/noCM (N = 32) | MDD/CM (N = 44) | Diagnosis | CM | Diagnosis × CM | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | F | P | F/t | P | F | P | |
|
| ||||||||||
| Age (Years) | 21.54 (4.24) | 20.93 (4.39) | 25.38 (8.30) | 25.91 (7.68) | 26.01 | < 0.001 | 0.001 | 0.979 | 0.41 | 0.521 |
| Education (Years) | 14.65 (1.58) | 14.12 (1.64) | 13.48 (2.29) | 14.43 (2.29) | 2.37 | 0.126 | 0.57 | 0.451 | 7.16 | 0.008 † |
| HAMD | - | - | 22.03 (4.69) | 22.82 (4.71) | - | - | −0.72 | 0.474 | - | - |
| BDI-II | 4.59 (4.34) | 6.72 (5.70) | 27.86 (10.23) | 30.96 (9.53) | 522.95 | < 0.001 | 6.34 | 0.013 | 0.22 | 0.640 |
| STAI-S | 36.26 (7.44) | 41.74 (7.95) | 57.53 (11.55) | 59.07 (10.08) | 217.39 | < 0.001 | 7.19 | 0.008 | 2.27 | 0.133 |
| CTQ Sum | 32.27 (4.00) | 44.75 (8.99) | 35.21 (5.02) | 51.98 (10.70) | 23.20 | < 0.001 | 192.24 | < 0.001 | 4.12 | 0.044 ‡ |
| Sexual abuse | 5.15 (0.49) | 5.81 (1.47) | 5.05 (0.18) | 6.20 (1.68) | 0.82 | 0.368 | 33.51 | < 0.001 | 2.51 | 0.115 |
| Physical abuse | 5.23 (0.69) | 6.28 (2.26) | 5.51 (0.95) | 7.95 (4.21) | 8.65 | 0.004 | 27.65 | < 0.001 | 4.43 | 0.037 § |
| Emotional abuse | 6.20 (1.48) | 8.35 (3.16) | 7.06 (2.06) | 11.27 (4.57) | 20.55 | < 0.001 | 58.30 | < 0.001 | 6.16 | 0.014 ¶ |
| Physical neglect | 6.77 (1.49) | 10.71 (2.58) | 6.70 (1.51) | 10.36 (2.73) | 0.46 | 0.501 | 156.79 | < 0.001 | 0.21 | 0.649 |
| Emotional neglect | 8.91 (2.37) | 13.60 (4.29) | 10.89 (2.57) | 16.18 (4.38) | 21.28 | < 0.001 | 102.10 | < 0.001 | 0.37 | 0.546 |
HAMD, Hamilton Depression Rating Scale; BDI-II, Beck Depressive Inventory-II; STAI, State and Trait Anxiety Inventory; CTQ, Childhood Trauma Questionnaire; HC, healthy controls; MDD, major depressive disorder; CM, childhood maltreatment. *p <0.05, **p < 0.01, ***p < 0.001.
MDD/CM > MDD/noCM *; HC/noCM > MDD/noCM **
MDD/noCM > HC/noCM *; MDD/CM > HC/CM ***; HC/CM > HC/noCM ***; MDD/CM > MDD/noCM***
MDD/CM > HC/CM **; HC/CM > HC/noCM *; MDD/CM > MDD/noCM ***
MDD/CM > HC/CM ***; HC/CM > HC/noCM ***; MDD/CM > HC/CM ***
3.2. Stress manipulation check
A Time (8 timepoints) × CM (CM, noCM) × Diagnosis (HC, MDD) repeated-measures ANCOVA analysis revealed a significant main effect of Time on subjective stress rating (F(1,200) = 63.99, p < 0.001, η2 = 0.242) and cortisol concentration (F(7,1120) = 8.93, p < 0.001, η2 = 0.053), with increased cortisol concentration (T50 vs. T0, pBonferroni < 0.001; Fig. 2A) and subjective stress level (post-MIST vs. pre-MIST, pBonferroni < 0.001; Fig. 2B) after the onset of the MIST, which indicated that psychosocial stress was successfully induced by the MIST. However, we did not observe significant Time × Diagnosis, Time × CM, or Time × Diagnosis × CM interaction effects with regard to both subjective stress ratings and cortisol concentration (ps > 0.05), suggesting the groups had similar affective and cortisol responses.
Fig. 2. Subjective and cortisol stress responses.
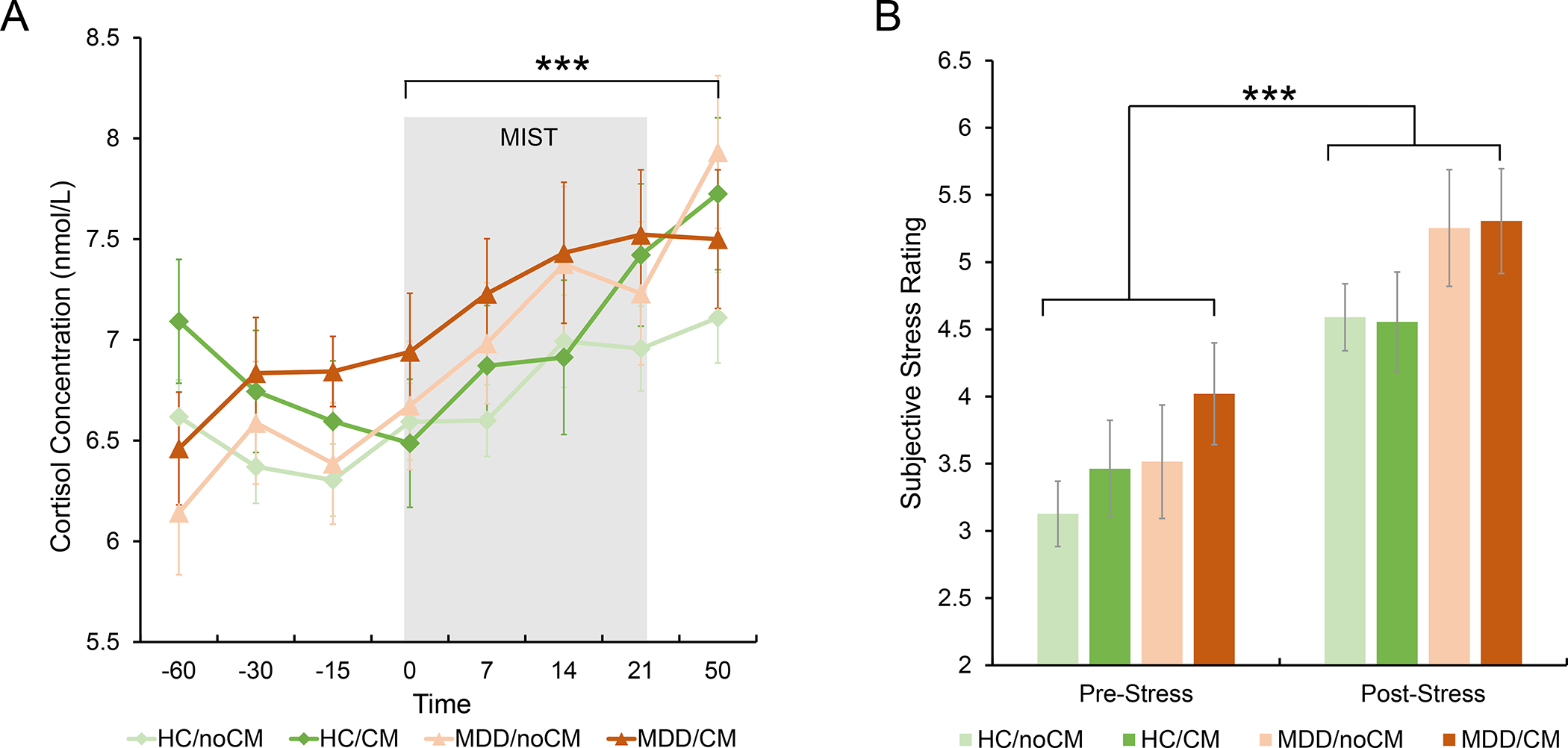
(A) Significant main effect of Time in cortisol concentration over the stress exposure. (B) Significant main effect of Time in subjective stress rating over the stress exposure. Estimated-mean are plotted, and error bar represents standard error (SE). HC, healthy controls; MDD, major depressive disorder; CM, childhood maltreatment; MIST, Montreal Imaging Stress Task. *** p Bonferroni< 0.001.
In addition, we investigated group effects on subjective stress level changes (post-stress minus pre-stress) and cortisol stress responses (AUCg and AUCi). No significant main effects of Diagnosis, CM or interaction effect of Diagnosis × CM emerged (ps > 0.05; Table S1).
3.3. Group effects on stress-related neural activation
A whole-brain CM × Diagnosis ANCOVA with age as a covariate revealed a significant CM × Diagnosis interaction effect in a cluster in the medial prefrontal cortex (mPFC, k = 143, x/y/z = −2/54/6, F(1,208) = 21.86, pFWE = 0.01; Fig. 3A; Table 2), which was the only cluster exhibiting an interaction effect surviving multiple comparison correction. Main effects of Diagnosis and CM did not survive FWE correction (pFWE > 0.05). The results were confirmed when not including age as a covariate (see Supplemental Results; Table S2).
Fig. 3. Significant CM × Diagnosis interaction in the medial prefrontal cortex.
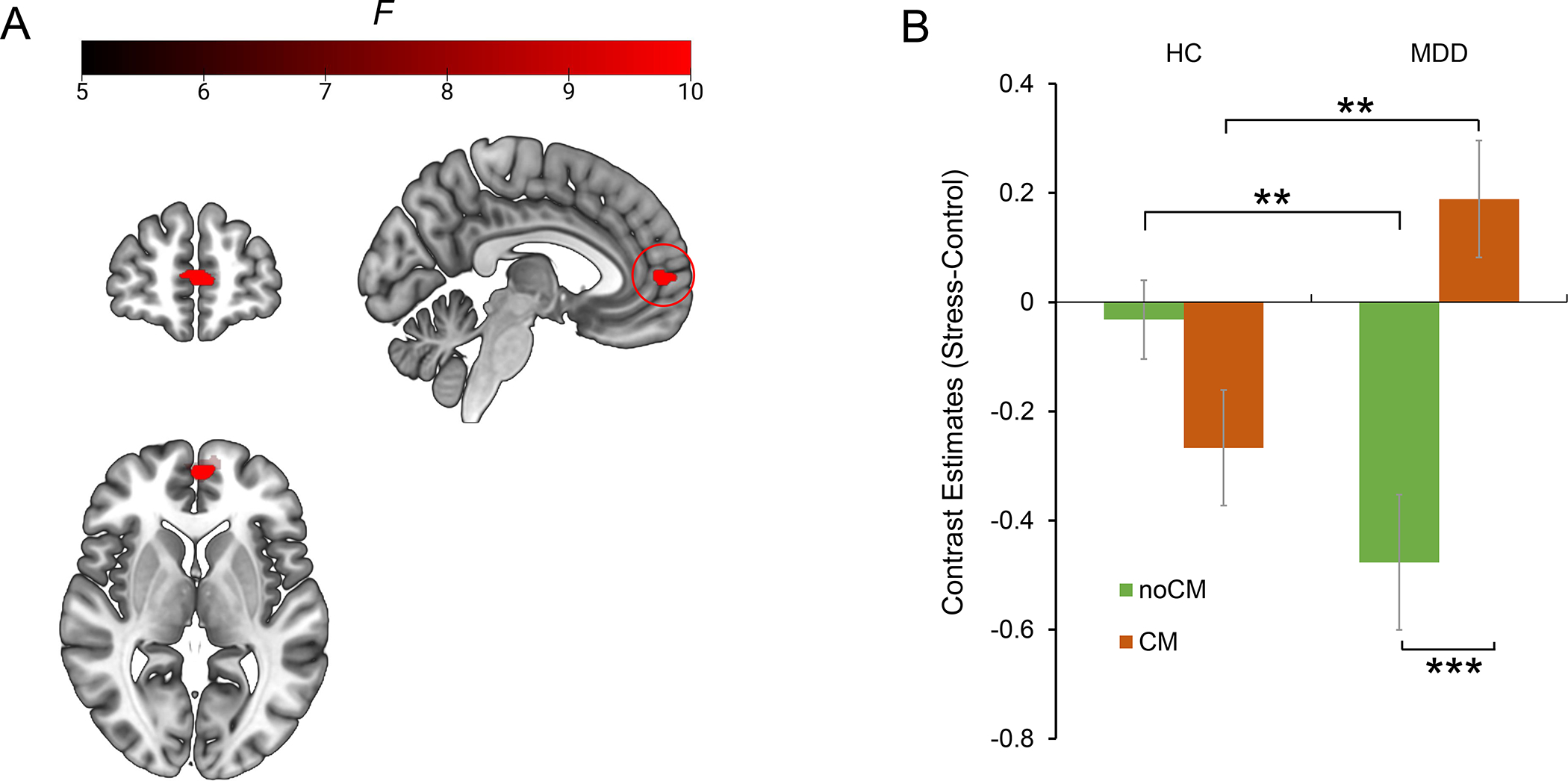
(A) location of mPFC exhibiting significant CM × Diagnosis interaction. (B) Bonferroni simple effects analysis of mPFC. Estimated-mean are plotted, and error bar represents SE. HC, healthy controls; MDD, major depressive disorder; CM, childhood maltreatment. ** p Bonferroni < 0.01, *** p Bonferroni < 0.001.
Table 2.
Group differences in neural stress responses to acute psychosocial stress.
| Brain Regions | BA | MNI coordinates |
F | Cluster size | p uncorr | pFWE | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
|
| ||||||||
| Whole-brain activity: CM × Diagnosis interaction | ||||||||
| mPFC | 10 | −2 | 54 | 6 | 21.86 | 143 | < 0.001 | 0.010 |
| −12 | 60 | 6 | 17.23 | |||||
| mPFC-seed connectivity: main effect of CM | ||||||||
| Posterior Cerebellum | 30 | −72 | −36 | 24.01 | 155 | < 0.001 | 0.001 | |
| 34 | −68 | −42 | 14.61 | |||||
| SFG | 8, 9 | −20 | 48 | 42 | 22.89 | 139 | < 0.001 | 0.003 |
| −20 | 50 | 34 | 16.04 | |||||
| −26 | 40 | 46 | 15.44 | |||||
CM, childhood maltreatment; mPFC, medial prefrontal cortex; SFG, superior frontal gyrus; FWE, family-wise error rate correction.
Bonferroni-corrected simple effects analyses clarified that the MDD/CM group exhibited less mPFC deactivation in comparison to the HC/CM group (pBonferroni = 0.003; Fig.3B), whereas the MDD/noCM group exhibited greater mPFC deactivation in comparison to the HC/noCM group (pBonferroni = 0.002; Fig.3B). Moreover, the MDD/CM group had less mPFC deactivation in comparison to the MDD/noCM group (pBonferroni < 0.001; Fig. 3B); finally, the HC/CM group showed a trend of greater mPFC deactivation relative to HC/noCM group (pBonferroni = 0.066; Fig. 3B). All these results remained significant after excluding two extreme outliers who had values outside the 1st quartile ± 3×interquartile range of the contrast values of mPFC activation (1, MDD/CM; 1, HC/noCM).
3.4. Group effects on stress-related mPFC-seed connectivity
A psychophysiological interaction (PPI) analysis was performed using the mPFC cluster as a seed to determine possible CM and MDD associations with stress-modulated functional connectivity. See Supplemental Methods for the detailed processes of PPI analysis. Fig. 4A shows the main effect of stress on mPFC-seed functional connectivity, which highlights a pattern of decreased connectivity with default mode network (DMN) regions and increased connectivity with dorsal prefrontal/parietal regions. A whole-brain CM × Diagnosis ANCOVA on PPI contrasts revealed a significant main effect of CM on connectivity between mPFC and left SFG (CM > noCM; k = 139, x/y/z = −20/48/42, F(1,208) = 22.89; pFWE = 0.003; Fig. 4B; Table 2) and right posterior cerebellum (CM > noCM; k = 155, x/y/z = 30/−72/−36; F(1,208) = 24.01, pFWE = 0.001; Fig. 4C; Table 2). No main effects of Diagnosis or CM × Diagnosis interaction effect emerged (pFWE >0.05). The results were confirmed when not including age as a covariate (see Supplemental Results; Table S2).
Fig. 4. mPFC-seed functional connectivity changes to acute psychosocial stress.
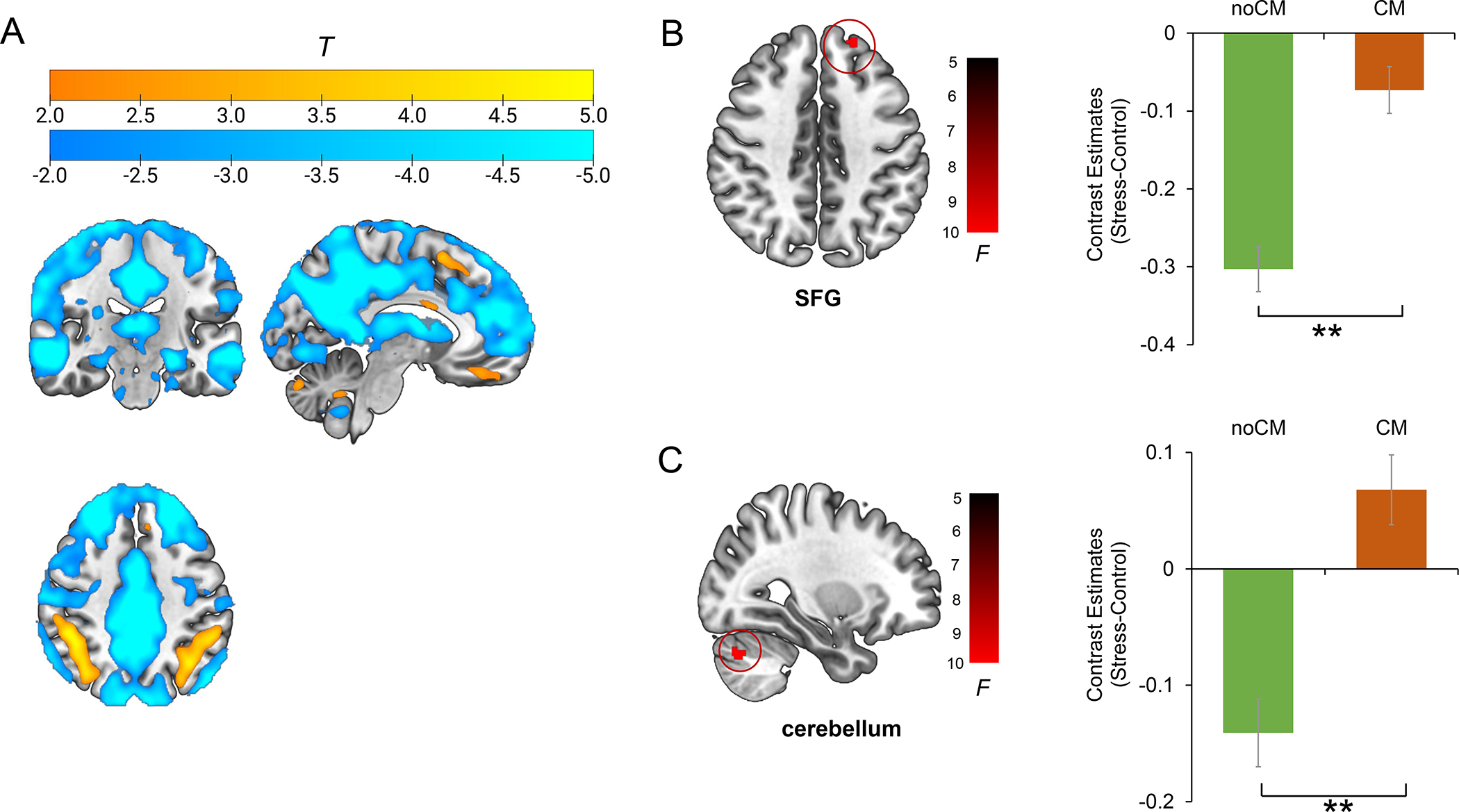
(A) main effect of stress on mPFC-seed functional connectivity. (B) Main effect of CM on mPFC-SFG connectivity (stress-control). (C) main effect of CM on mPFC-cerebellum connectivity (stress-control). Estimated-mean are plotted, and error bar represents SE. SFG, superior frontal gyrus; CM, childhood maltreatment. **p FWE < 0.01.
3.5. Correlation analysis
See Supplemental Methods and Results (Table S3) for the associations between neural activation/connectivity and cortisol response measures as well as with depressive symptoms.
3.6. Sensitivity analyses
Sensitivity analyses were conducted to test whether different types of CM (i.e., sexual abuse, physical abuse, emotional abuse, physical neglect, emotional neglect, abuse maltreatment, neglect maltreatment) differentially affected the main findings (i.e., mPFC deactivation, functional connectivity between mPFC-SFG; functional connectivity between mPFC-cerebellum). Generally, the results of these sensitivity analyses revealed that the MDD/CM group exhibited higher stress-related effects in comparison to MDD/noCM in terms of activation/connectivity regardless of which kind of criteria were used to identify maltreated individuals (Table S4). Finally, we considered the dimension model of adversity proposed by McLaughlin and colleagues (2019) which proposes that distinct dimensions (threat vs. deprivation) of adversity may have differential effects on neural development. When applying either abuse (threat) or neglect (deprivation) criteria, our findings of MDD/CM vs. MDD/noCM remained significant (see composite score section in Table S4). See Supplemental Methods for details of performing sensitivity analyses.
4. Discussion
The overarching goal of the current study was to test whether CM and MDD interacted in shaping neural patterns in response to a well-established acute psychosocial stressor. The fMRI findings revealed that the MDD/CM group and MDD/noCM group exhibited dissociable, stress-related mPFC responses when compared to HCs with a history of CM. Specifically, the MDD/CM group exhibited reduced stress-related mPFC deactivation relative to HC/CM group, whereas the MDD/noCM group exhibited greater stress-related mPFC deactivation in comparison to the HC/noCM group. In addition, participants reporting CM (irrespective of MDD diagnosis) exhibited greater stress-related mPFC-SFG connectivity and mPFC-cerebellum connectivity relative to participants without past CM. Collectively, these fMRI findings provide novel insights into the potential interaction between CM and MDD psychopathology.
Consistent with our hypothesis, the MDD/CM and MDD/noCM group exhibited distinct neural alterations during psychosocial stress processing in the mPFC. The mPFC is a critical region involved in the pathophysiology of MDD and has been linked to CM (Belleau et al., 2019; Cassiers et al., 2018; Hart & Rubia, 2012). Critically, the mPFC is regarded as an important region of the DMN which is implicated in self-referential processing (Gusnard et al., 2001). Thus, the observed increased deactivation of mPFC in MDD/noCM (vs. HC/noCM) and decreased deactivation in MDD/CM (vs. HC/CM) may implicate reduced engagement of the DMN amongst those with MDD and no CM and greater engagement of the DMN amongst those with MDD and with CM in response to stress. This suggests that both patterns of decreased- and increased- involvement of the DMN under stress may be maladaptive. The increased mPFC deactivation seen in the MDD/noCM group (vs. HC/noCM) could reveal less neural resources were allocated to DMN network with the aim to maintain task performance during psychosocial stress processing. The negative correlation between stress-related mPFC deactivation and depressive symptoms observed in MDD/noCM group further suggest that this neural pattern could be maladaptive. For the MDD/CM group, the decreased mPFC deactivation (vs. HC/CM) could reveal increased self-focused thinking under stress. Overall, the current findings suggest that the MDD/CM group may have a unique neurobiological profile during stress compared to the MDD group without a history of CM.
Of note, the mPFC has also been implicated in stress regulation (Herman et al., 2005). In line with this, in the current study, the mPFC was characterized by decreased connectivity with DMN regions and increased connectivity with dorsal prefrontal/parietal regions (stress vs. control; see Fig.3A). As an alternate interpretation, the greater deactivation of mPFC in the MDD/noCM group vs. the HC/noCM may implicate stress regulation dysfunction; whereas less mPFC deactivation observed in the MDD/CM group vs. the MDD/noCM group could provide evidence supporting the stress acceleration theory. This theory suggests that childhood maltreatment might facilitate the maturation of the stress/threat regulation circuit (i.e., mPFC and amygdala) in order to adapt to CM (Callaghan & Tottenham, 2016). However, this speculation still needs further verification with a more specific task design for investigating stress regulation.
In contrast to prior findings (Grimm et al., 2014; Seo et al., 2019; Zhong et al., 2020), we did not observe significant neural alterations in the HC/CM group, although the HC/CM vs. HC/noCM comparison yielded a trend toward significantly greater deactivation in mPFC. Our prior study – which included 48 HC/CM (24 male/24 female) and 48 HC-noCM (15 male/33 female) participants also included in the current analyses – found that the HC/CM group exhibited significantly greater activation in the dorsolateral prefrontal cortex, insula, precuneus, and greater deactivation in the ventral mPFC relative to the vs. HC/noCM group (Zhong et al., 2020). Both studies found that, relative to the HC/noCM group, the HC/CM group exhibited a tendency of greater deactivation in the mPFC, which highlights an important role of mPFC in terms of the interaction between stress and CM. However, the current study, which focused on female participants only, did not replicate other prior findings even though the sample partly overlapped (33/94 female HC/noCM; 24/43 female HC/CM). Possible explanations for this lack of replication are (1) the fact that the effect of CM on brain function might be sex-specific (Colich et al., 2017; Tiwari & Gonzalez, 2018; White et al., 2020) and (2) the improved fMRI preprocessing method used in the current study. Further investigations are warranted to address this question.
With regard to mPFC-seed connectivity, we found that the noCM group exhibited decreased mPFC-SFG connectivity in the stress vs. control comparison, whereas the CM group exhibited relatively stable mPFC-SFG connectivity across both conditions. Although not frequently mentioned, the SFG (BA8, 9) is also reported as a part of the DMN (Meindl et al., 2010). Consistent with this literature, this region was deactivated in the stress vs. rest and control vs. rest comparison (see Supplemental Fig. S1). Along this line, the relatively stable mPFC-SFG connectivity (stress vs. control) observed in CM individuals may reveal maladaptive self-referential processing during psychosocial stress processing. In addition, the noCM group has decreased mPFC-cerebellum connectivity (stress vs. control), whereas the CM group has stable mPFC-cerebellum connectivity across conditions. The cerebellum activity and cerebellum-related connectivity are not frequently investigated in stress research. However, several studies have reported that acute stress may induce the activation of the cerebellum (Kogler et al., 2017; Seo et al., 2011, 2019). One recent review (Moreno-Rius, 2019) proposed that the traumatic/repeated stress may induce dysfunction of cerebellum-based predictive system, and thus promote overestimation of environment-associated negative outcomes and reduce appropriate actions, which may explain why the CM individuals exhibited altered mPFC-cerebellum coupling when experiencing acute psychosocial stress. However, these connectivity findings are relatively novel in terms of the neural mechanism underlying CM; accordingly, replications are warranted.
Some limitations of the current study should be mentioned. First, menstrual cycle information, which could affect neural stress responses (Goldstein et al., 2010), was not collected. Second, the classification of maltreatment was based on retrospective self-reported measurements, although it has been proposed by others that this limitation is not as severe as we may anticipate (Brewin et al., 1993). Third, various stress components induced by the MIST include uncontrollability and social evaluative threat (Dedovic et al., 2009), which may add the complexity of interpretation. Fourth, the first-episode, non-comorbid unmedicated female MDD sample is less representative of the community, which may limit the generalization of findings. Fourth, the unmatched age, years of education and CTQ score across groups is a limitation that should be mentioned. Finally, the onset time of childhood maltreatment was not collected. Because distinct brain regions have different maltreatment-sensitivity periods (Pechtel & Pizzagalli, 2011; Teicher et al., 2016), additional studies are needed to test whether the neural alterations observed in this study are affected by the onset time of CM.
In spite of these limitations, the current study represents the first exploration of the potential interaction between CM and MDD psychopathology in terms of neural stress reactivity and some novel findings emerged. The MDD/CM and MDD/noCM patients exhibited opposite neural stress responses in mPFC in relative to HCs, which provides evidence for distinct neurobiological abnormalities in MDD with vs. without CM. In addition, compared to those without CM history, individuals with a history of CM exhibited higher mPFC-SFG and mPFC-cerebellum connectivity independent of MDD diagnosis, revealing potential general neural consequences of CM on the development of stress circuitry in females.
Supplementary Material
Acknowledgment:
This project was supported by National Natural Science Foundation of China (82071532 to S. Yao) and Natural Science Foundation of Changsha (kq2007044 to C. Li). Ms. Dong was funded by China Scholarship Council (201906370240). The content is solely the responsibility of the authors. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interests:
Over the past 3 years, Dr. Pizzagalli has received consulting fees from Albright Stonebridge Group, BlackThorn Therapeutics, Boehringer Ingelheim, Compass Pathway, Concert Pharmaceuticals, Engrail Therapeutics, Neuroscience Software, Neurocrine Biosciences, Otsuka Pharmaceuticals, and Takeda Pharmaceuticals; one honorarium from Alkermes; honoraria from the Psychonomic Society for editorial work; and research funding from NIMH, Dana Foundation, Brain and Behavior Research Foundation, Millennium Pharmaceuticals. In addition, he has received stock options from BlackThorn Therapeutics and Compass Pathway. All other authors declare that they have no disclosures in association with this work.
References
- Admon R, Holsen LM, Aizley H, Remington A, Whitfield-Gabrieli S, Goldstein JM, & Pizzagalli DA (2015). Striatal hypersensitivity during stress in remitted individuals with recurrent depression. Biological Psychiatry, 78(1), 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghorst LH, & Pizzagalli DA (2010). Defining depression endophenotypes. Next Generation Antidepressants: Moving beyond Monoamines to Discover Novel Treatment Strategies for Mood Disorders, 70. [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, & Foote J (1998). Childhood trauma questionnaire. Assessment of Family Violence: A Handbook for Researchers and Practitioners. [Google Scholar]
- Brewin CR, Andrews B, & Gotlib IH (1993). Psychopathology and early experience: a reappraisal of retrospective reports. Psychological Bulletin, 113(1), 82. [DOI] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Graham AM, Moore WE, Peake SJ, & Mannering AM (2013). Patterns of brain activation in foster children and nonmaltreated children during an inhibitory control task. Development and Psychopathology, 25(4pt1), 931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, & Tottenham N (2016). The stress acceleration hypothesis: Effects of early-life adversity on emotion circuits and behavior. Current Opinion in Behavioral Sciences, 7, 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colle R, Segawa T, Chupin M, Dong MNTKT, Hardy P, Falissard B, Colliot O, Ducreux D, & Corruble E (2017). Early life adversity is associated with a smaller hippocampus in male but not female depressed in-patients: a case–control study. BMC Psychiatry, 17(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colich NL, Williams ES, Ho TC, King LS, Humphreys KL, Price AN, Ordaz SJ, & Gotlib IH (2017). The association between early life stress and prefrontal cortex activation during implicit emotion regulation is moderated by sex in early adolescence. Development and Psychopathology, 29(5), 1851–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, & Pruessner JC (2005). The Montreal Imaging Stress Task: Using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. Journal of Psychiatry and Neuroscience, 30(5), 319–325. [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, Rexroth M, Wolff E, Duchesne A, Scherling C, Beaudry T, Lue SD, Lord C, Engert V, & Pruessner JC (2009). Neural correlates of processing stressful information: An event-related fMRI study. Brain Research, 1293, 49–60. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, & Pizzagalli DA (2009). Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biological Psychiatry, 66(3), 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Pestke K, Feeser M, Aust S, Pruessner JC, Böker H, Bajbouj M, & Grimm S (2015). Amygdala–hippocampal connectivity changes during acute psychosocial stress: joint effect of early life stress and oxytocin. Neuropsychopharmacology, 40(12), 2736–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen L, van Velzen L, Schmaal L, van der Graaf Y, van der Wee N, van Tol M-J, Penninx B, & Geerlings M (2015). Childhood maltreatment modifies the relationship of depression with hippocampal volume. Psychological Medicine, 45(16), 3517–3526. [DOI] [PubMed] [Google Scholar]
- Goldfarb E. v., Seo D, & Sinha R (2019). Sex differences in neural stress responses and correlation with subjective stress and stress regulation. Neurobiology of Stress, 11, 100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, & Makris N (2010). Sex differences in stress response circuitry activation dependent on female hormonal cycle. Journal of Neuroscience, 30(2), 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Pestke K, Feeser M, Aust S, Weigand A, Wang J, Wingenfeld K, Pruessner JC, la Marca R, & Böker H (2014). Early life stress modulates oxytocin effects on limbic system during acute psychosocial stress. Social Cognitive and Affective Neuroscience, 9(11), 1828–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, & Raichle ME (2001). Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences, 98(7), 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein TC, Goetschius LG, McLoyd VC, Brooks-Gunn J, McLanahan SS, Mitchell C, Lopez-Duran NL, Hyde LW, & Monk CS (2020). Childhood violence exposure and social deprivation are linked to adolescent threat and reward neural function. Social Cognitive and Affective Neuroscience, 15(11), 1252–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Zhong X, Gao Y, Xiong G, & Yao S (2019). Psychometric properties of the Chinese version of the Childhood Trauma Questionnaire-Short Form (CTQ-SF) among undergraduates and depressive patients. Child Abuse & Neglect, 91, 102–108. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, & Figueiredo H (2005). Limbic system mechanisms of stress regulation: Hypothalamo-pituitary- adrenocortical axis. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 29(8), 1201–1213. [DOI] [PubMed] [Google Scholar]
- Holsen LM, Spaeth SB, Lee JH, Ogden LA, Klibanski A, Whitfield-Gabrieli S, & Goldstein JM (2011). Stress response circuitry hypoactivation related to hormonal dysfunction in women with major depression. Journal of Affective Disorders, 131(1–3), 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski KF, Bruce J, Beauchamp KG, Roos LE, Moore WE III, & Fisher PA (2017). Preliminary evidence of the impact of early childhood maltreatment and a preventive intervention on neural patterns of response inhibition in early adolescence. Developmental Science, 20(4), e12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness JL, Peverill M, Miller AB, Heleniak C, Robertson MM, Sambrook KA, Sheridan MA, & McLaughlin KA (2021). Alterations in neural circuits underlying emotion regulation following child maltreatment: a mechanism underlying trauma-related psychopathology. Psychological Medicine, 51(11), 1880–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogler L, Seidel EM, Metzler H, Thaler H, Boubela RN, Pruessner JC, Kryspin-Exner I, Gur RC, Windischberger C, Moser E, Habel U, & Derntl B (2017). Impact of self-esteem and sex on stress reactions. Scientific Reports, 7(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Peverill M, Gold AL, Alves S, & Sheridan MA (2015). Child maltreatment and neural systems underlying emotion regulation. Journal of the American Academy of Child & Adolescent Psychiatry, 54(9), 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Weissman D, & Bitrán D (2019). Childhood adversity and neural development: a systematic review. Annual Review of Developmental Psychology, 1, 277–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meindl T, Teipel S, Elmouden R, Mueller S, Koch W, Dietrich O, Coates U, Reiser M, & Glaser C (2010). Test–retest reproducibility of the default-mode network in healthy individuals. Human Brain Mapping, 31(2), 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinert S, Repple J, Nenadic I, Krug A, Jansen A, Grotegerd D, Förster K, Enneking V, Dohm K, & Schmitt S (2019). Reduced fractional anisotropy in depressed patients due to childhood maltreatment rather than diagnosis. Neuropsychopharmacology, 44(12), 2065–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming Q, Zhong X, Zhang X, Pu W, Dong D, Jiang Y, Gao Y, Wang X, Detre JA, & Yao S (2017). State-independent and dependent neural responses to psychosocial stress in current and remitted depression. American Journal of Psychiatry, 174(10), 971–979. [DOI] [PubMed] [Google Scholar]
- Moreno-Rius J (2019). The cerebellum under stress. Frontiers in Neuroendocrinology, 54, 100774. [DOI] [PubMed] [Google Scholar]
- Opel N, Redlich R, Zwanzger P, Grotegerd D, Arolt V, Heindel W, Konrad C, Kugel H, & Dannlowski U (2014). Hippocampal atrophy in major depression: a function of childhood maltreatment rather than diagnosis? Neuropsychopharmacology, 39(12), 2723–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, & Pizzagalli DA (2011). Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology, 214(1), 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, & Hellhammer DH (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28(7), 916–931. [DOI] [PubMed] [Google Scholar]
- Purcell JB, Goodman AM, Harnett NG, Davis ES, Wheelock MD, Mrug S, Elliott MN, Emery ST, Schuster MA, & Knight DC (2021). Stress-elicited neural activity in young adults varies with childhood sexual abuse. Cortex, 137, 108–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Ahluwalia A, Potenza MN, & Sinha R (2017). Gender differences in neural correlates of stress-induced anxiety. Journal of Neuroscience Research, 95(1–2), 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, & Sinha R (2011). Sex differences in neural responses to stress and alcohol context cues. Human Brain Mapping, 32(11), 1998–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Rabinowitz AG, Douglas RJ, & Sinha R (2019). Limbic response to stress linking life trauma and hypothalamus-pituitary-adrenal axis function. Psychoneuroendocrinology, 99, 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, & Samson JA (2013). Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. American Journal of Psychiatry, 170(10), 1114–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, & Ohashi K (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews Neuroscience, 17(10), 652–666. [DOI] [PubMed] [Google Scholar]
- Tiwari A, & Gonzalez A (2018). Biological alterations affecting risk of adult psychopathology following childhood trauma: a review of sex differences. Clinical Psychology Review, 66, 69–79. [DOI] [PubMed] [Google Scholar]
- van Harmelen A-L, Hauber K, Gunther Moor B, Spinhoven P, Boon AE, Crone EA, & Elzinga BM (2014). Childhood emotional maltreatment severity is associated with dorsal medial prefrontal cortex responsivity to social exclusion in young adults. PLoS One, 9(1), e85107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Korczykowski M, Rao H, Fan Y, Pluta J, Gur RC, McEwen BS, & Detre JA (2007). Gender difference in neural response to psychological stress. Social Cognitive and Affective Neuroscience, 2(3), 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Dai Z, Peng H, Tan L, Ding Y, He Z, Zhang Y, Xia M, Li Z, & Li W (2014). Overlapping and segregated resting-state functional connectivity in patients with major depressive disorder with and without childhood neglect. Human Brain Mapping, 35(4), 1154–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Voss JL, Chiang JJ, Wang L, McLaughlin KA, & Miller GE (2019). Exposure to violence and low family income are associated with heightened amygdala responsiveness to threat among adolescents. Developmental Cognitive Neuroscience, 40, 100709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JD, Arefin TM, Pugliese A, Lee CH, Gassen J, Zhang J, & Kaffman A (2020). Early life stress causes sex-specific changes in adult fronto-limbic connectivity that differentially drive learning. Elife, 9, e58301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Rubin-Falcone H, Lin X, Rizk MM, Miller JM, Sublette ME, Oquendo MA, Burke A, Ogden RT, & Mann JJ (2020). Smaller left hippocampal subfield CA1 volume is associated with reported childhood physical and/or sexual abuse in major depression: a pilot study. Journal of Affective Disorders, 272, 348–354. [DOI] [PubMed] [Google Scholar]
- Zhong X, Ming Q, Dong D, Sun X, Cheng C, Xiong G, Li C, Zhang X, & Yao S (2020). Childhood maltreatment experience influences neural response to psychosocial stress in adults: an fMRI study. Frontiers in Psychology, 10, 2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


