Abstract
Since the late 1990s, tumor necrosis factor alpha (TNF-α) inhibitors (anti-TNFs) have revolutionized the therapy of immune-mediated inflammatory diseases (IMIDs) affecting the gut, joints, skin and eyes. Although the therapeutic armamentarium in IMIDs is being constantly expanded, anti-TNFs remain the cornerstone of their treatment. During the second decade of their application in clinical practice, a large body of additional knowledge has accumulated regarding various aspects of anti-TNF-α therapy, whereas new indications have been added. Recent experimental studies have shown that anti-TNFs exert their beneficial effects not only by restoring aberrant TNF-mediated immune mechanisms, but also by de-activating pathogenic fibroblast-like mesenchymal cells. Real-world data on millions of patients further confirmed the remarkable efficacy of anti-TNFs. It is now clear that anti-TNFs alter the physical course of inflammatory arthritis and inflammatory bowel disease, leading to inhibition of local and systemic bone loss and to a decline in the number of surgeries for disease-related complications, while anti-TNFs improve morbidity and mortality, acting beneficially also on cardiovascular comorbidities. On the other hand, no new safety signals emerged, whereas anti-TNF-α safety in pregnancy and amid the COVID-19 pandemic was confirmed. The use of biosimilars was associated with cost reductions making anti-TNFs more widely available. Moreover, the current implementation of the “treat-to-target” approach and treatment de-escalation strategies of IMIDs were based on anti-TNFs. An intensive search to discover biomarkers to optimize response to anti-TNF-α treatment is currently ongoing. Finally, selective targeting of TNF-α receptors, new forms of anti-TNFs and combinations with other agents, are being tested in clinical trials and will probably expand the spectrum of TNF-α inhibition as a therapeutic strategy for IMIDs.
Keywords: Anti-TNF, Autoimmune, Fibroblasts, Mortality, Biosimilar, Biomarkers, COVID-19
Introduction
The crucial role of tumor necrosis factor alpha (TNF-α) in the pathogenesis of several immune-mediated inflammatory diseases (IMIDs), primarily those affecting joints, skin, gut and eye, has been widely established. The first evidence of the role of TNF-α in inflammatory arthritis pathophysiology derived from the human TNF transgenic mouse model [1]. The favorable effect of blocking TNF-α was also demonstrated in these experiments for the first time. It is now clear that TNF-α constitutes a common effector module of the inflammatory cascade that occurs in several IMIDs [2]. Following impressive results of randomized clinical trials in the mid-1990s, and since their first approval in 1998, agents targeting TNF-α (anti-TNFs) have been proven effective for the treatment of various IMIDs. During the first decade of their use, concerns for anti-TNF-α safety, that may arise from the inhibition of prophylactic actions of TNF-α in infection and cancer, were alleviated. Millions of IMIDs patients have been safely treated, while clinical experience regarding optimization of their use accumulated [3].
In the past 10 years, anti-TNFs indications have expanded to include non-radiographic axial spondyloarthritis (AxSpA), paediatric enthesitis-related arthritis, paediatric ulcerative colitis (UC), hidradenitis suppurativa (including adolescents) and non-infectious uveitis (including children) (Table 1). Moreover, the therapeutic potential of anti-TNFs has been also demonstrated in several disorders, such as Behcet’s disease (BD), sarcoidosis, Takayasu arteritis, idiopathic inflammatory myopathies, synovitis-acne-pustulosis-hyperostosis-osteitis (SAPHO) syndrome, polyarteritis nodosa, adenosine deaminase 2 deficiency (DADA2) and pyoderma gangrenosum [4–11].
Table 1.
anti-TNF-α indications by chronological order of first approval (either by Food and Drug Administration-FDA or European Medicines Agency-EMA)
| Indication | Drug, year of first approval |
|---|---|
| Rheumatoid Arthritis |
Etanercept, 1998 Infliximab, 1999 Adalimumab, 2002certolizumab pegol, 2009 Golimumab, 2009 |
| Crohn’s Disease | Infliximab, 1998Adalimumab, 2007Certolizumab pegol, 2008 |
| Juvenile Idiopathic Arthritis |
Etanercept, 1999 Adalimumab, 2008 Golimumab, 2016 |
| Psoriatic Arthritis |
Etanercept, 2002 Infliximab, 2004 Adalimumab, 2005 Golimumab, 2009 Certolizumab pegol, 2013 |
| Axial Spondyloarthritis | Infliximab, 2003Eanercept, 2003Adalimumab, 2006Golimumab, 2009Certolizumab pegol, 2013 |
| Plaque Psoriasis |
Etanercept, 2004 Infliximab, 2005 Adalimumab, 2007 Certolizumab pegol, 2018 |
| Ulcerative Colitis |
Infliximab, 2005Adalimumab, 2012 Golimumab, 2013 |
| Paediatric Crohn’s Disease |
Infliximab, 2006 Adalimumab, 2012 |
| Paediatric Plaque Psoriasis | Etanercept, 2008Adalimumab, 2015 |
| Paediatric Ulcerative Colitis | Infliximab, 2011Adalimumab, 2020 |
| Non-radiographic Axial Spondyloarthritis | Adalimumab, 2012Certolizumab pegol, 2013Etanercept, 2014Glimumab, 2015 |
| Paediatric Enthesitis-Related Arthritis | Adalimumab, 2014 |
| Hidradenitis Suppurativa | Adalimumab, 2015 |
| Adolescent Hidradenitis Suppurativa | Adalimumab, 2016 |
| Non-infectious Uveitis | Adalimumab, 2016 |
| Paediatric non-infectious Uveitis | Adalimumab, 2017 |
Herein, we aimed to summarize new lessons learned during the second decade of anti-TNFs utilization in clinical practice. Besides a better understanding of the mechanisms of action of TNF-α inhibition that brought synovial fibroblast and gut myofibroblasts on stage, the long-term effects of anti-TNF-α treatment taught us that the natural history of chronic inflammatory diseases may be indeed altered [12–14]. Long-term effects of anti-TNFs on bone and gut pathophysiology, as well as their impact on systemic manifestations of chronic inflammation, such as atherosclerosis and cardiovascular (CV) events, have become evident and led to improved mortality rates [15].
Accumulating “real-world” data on additional millions of patients with various background conditions further established anti-TNFs’ excellent safety profile, which was extended to include pregnant women. Anti-TNFs safety regarding CV disease and cancer was further confirmed in patients with RA older than 50 years in comparison to newer drugs, such as Janus kinase (JAK) inhibitors [16]. The current COVID-19 pandemic revealed also unexpected beneficial effects of anti-TNF-α agents [17]; anti-TNF-α use protects against severe COVID-19 infection and hospitalization due to COVID-19 and is being examined for the treatment of COVID-19 [18–20]. Moreover, we have learned to better use TNF-α inhibition in order to apply treat-to-target strategy in IMIDs [21], including anti-TNF-α intensification, combination with immunomodulators, tapering, discontinuation, cycling, and/or switching to other biologics. While we have learned a lot on therapeutic drug monitoring (TDM), robust biomarkers of response remain to be established. The introduction of biosimilars had major socioeconomic impact, giving the opportunity to increased use of anti-TNF-α therapies worldwide. Finally, we briefly describe the future of anti-TNF-α therapy, including the next generation of anti-TNF-α drugs, as well as the possible combination of anti-TNFs with other biologic and targeted therapies.
For this narrative review, an exhaustive literature search was conducted by all authors independently, using PubMed via MEDLINE, Scopus and EMBASE databases. The included publication dates focused from 2010 to March 2022 in order to capture the advances during the second decade of anti-TNF-α use and COVID-19 pandemic, but earlier publications were also taken into consideration. The main terms used for database search were “TNF-α”, “TNF alpha”, “TNF receptor”, “anti-TNF-α”, “TNF inhibitor”, “COVID-19”, “safety”, but literature search was not only confined to these. This review was designed taking into account previously published considerations on narrative review writing [22].
Mechanisms of action of anti-TNFs in target organs: the central role of mesenchymal cells
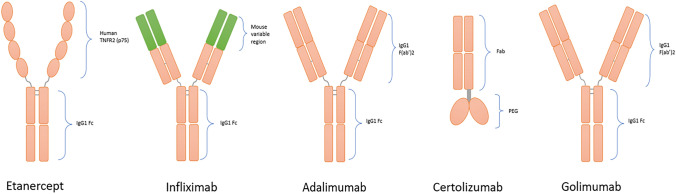
Early studies have established that anti-TNFs, namely the anti-TNF-α monoclonal antibodies (mAbs) infliximab, adalimumab, certolizumab and golimumab, and the soluble TNF-α receptor etanercept (Fig. 1), work by neutralizing the activity of soluble TNF-α and preventing its binding to TNFα receptor 1 (TNFR1) and TNFR2. TNFR1 is constitutively expressed on the membrane of almost all cell types and is associated with proinflammatory and cytotoxic responses [23]. On the other hand, TNFR2 is expressed on immune cells, mainly monocytes and T cells, as well as on activated mesenchymal cells, mediating cellular activation, proliferation, migration and other pathogenic functions on synovial fibroblasts [23, 24].
Fig. 1.
Molecular structure of approved anti-TNFs. The five approved anti-TNFs, presented in chronological order of first approval. Etanercept is a fusion protein of extracellular domain (p75) of human TNFR2 and Fc fragment of IgG1; infliximab is a mouse/human chimeric monoclonal IgG1 anti-TNF-α antibody; adalimumab is a humanized IgG1 monoclonal anti-TNF-α antibody; certolizumab is a Fc-free Fab region of a recombinant humanized IgG1 monoclonal anti-TNF-α antibody, conjugated to PEG; golimumab is a human IgG1 monoclonal anti-TNF-α antibody. Anti-TNF-a: tumor necrosis factor alpha inhibitor, PEG: polyethylene glycol, TNFR2: tumor necrosis factor receptor 2
Different TNF-α-mediated (auto)immune processes are involved in the initiation and perpetuation of IMIDs. The differential efficacy of anti-TNFs seen in clinical practice suggests that the mechanisms of therapeutic action are not distinct and may overlap. Briefly, anti-TNF-α treatment acts in the immune system as follows: (a) downregulating pro-inflammatory cytokines, chemokines, acute phase proteins and adhesion molecules expression, (b) increasing circulating regulatory T cells and (c) reducing the migration of inflammatory cells from blood to the inflamed tissue. Etanercept also neutralizes lymphotoxin, whereas anti-TNFs have cell-killing properties by directly binding to transmembrane TNF-α expressed in various cells. As Wu et al. recently showed, binding of anti-TNF-α to transmembrane TNF-α, affects intracellular signaling and, in addition to programmed cell death, may result either to suppression of cytokine production or cell growth arrest [25].
In addition to the effects on immune cells, anti-TNF-α mechanisms of action also include attenuation of vascular permeability and angiogenesis, as well as deactivation of epithelial, endothelial and mesenchymal cells. Recent advances in basic research have indeed shown that anti-TNF-α treatment works by interfering with TNF-α signaling in mesenchymal cells [26]. For example, mesenchymal-specific TNFR1 triggering is indispensable for arthritis development in acute and chronic TNF-dependent mouse models. While inhibitor kappa B kinase 2 (IKK2) in joint mesenchymal cells is necessary for cartilage destruction and bone erosion, in its absence synovitis still develops, as a result of local immunogenic synovial fibroblasts necroptosis. IKK2 deletion affects arthritic and anti-apoptotic gene expression leading to hypersensitization of synovial fibroblasts to TNF/receptor interacting serine/threonine kinase 1 (Ripk1)-mediated necroptosis via district mechanisms, depending on acute or chronic TNF-α signals. Moreover, Ripk3 is dispensable for TNF-mediated arthritis, yet it is required for synovitis in mice with mesenchymal-specific IKK2 deletion, clearly showing that TNFR1-IKK2-Ripk-mixed-lineage kinase domain-like (MLKL) signalling pathway orchestrates arthritogenic and death responses in synovial fibroblasts and that combinatorial inhibition of nuclear factor kappa beta (NF-κB) and MLKL/RIPKs may offer a therapeutic potential [26].
As has been demonstrated in TNF transgenic mice models, synovial fibroblasts and intestine myofibroblasts are activated early by TNF-TNFR1 signaling, produce several matrix degrading enzymes and are sufficient targets to induce TNF-driven inflammatory polyarthritis, Crohn's-like inflammatory bowel disease (IBD) and sacroiliitis [27]. Thus, mesenchymal cells are necessary targets of TNF-α in the development of spondyloarthritis-related disorders [27]. Importantly, mesenchymal cell activation by TNF-α has been shown to participate also in additional sites, such as in cardiac valvular cell activation and valve thickening [28]. This possibly explains several comorbidities (e.g. cardiac and lung pathologies) that may be associated with mesenchymal cell activation, interstitial inflammation and damage in TNF-α-driven rheumatic diseases.
Along these lines, the mesenchymal hypothesis to explain the pathogenesis of RA is gaining momentum and several elegant studies analyzing human RA synovial tissue at the single-cell level have produced exciting results [29]. Such studies indicated that synovial lining fibroblasts (Thy1-) were found to be predominantly responsible for driving articular damage, whereas sub-lining layer fibroblasts (Thy1 +) are mainly pro-inflammatory [30]. More recent evidence revealed an endothelial-cell-instigated Notch-mediated pathway in perivascular sub-lining synovial fibroblasts, that establishes a positional gradient for sub-lining synovial fibroblasts differentiating to lining synovial fibroblasts [31]. So far, studies in humans have not provided robust evidence on how synovial fibroblasts transition from homeostasis to pathology. Preliminary single-cell RNA and ATAC-seq chromatin profiling of synovial fibroblasts from the TNF transgenic mouse model indicated that development of TNF-α-driven arthritis primes the emergence of distinct pathology-associated synovial fibroblasts subtypes and revealed key transcription factors such as Bach1 and Runx1 to drive arthritogenesis [32]. Taken together, these recent studies provided deeper mechanistic understanding of the dynamics of synovial fibroblast subpopulations during pathogenesis of arthritis, paving the way to the discovery of mesenchymal cell-targeted therapeutics beyond anti-TNFs.
Effects of long-term TNF-α inhibition in IMIDs
Long-term effects on bone pathophysiology in inflammatory arthritides
Along with interleukin-6 (IL-6), TNF-α stimulates synovial fibroblasts to produce receptor activator of NF-κB ligand (RANKL) promoting osteoclasts’ differentiation and activation [33]. Moreover, TNF-α leads to increased synovial levels of Dickkopf-related protein 1 (DKK-1), an inhibitor of osteoblastogenesis [34]. As a result, TNF-α orchestrates a process of local (expressed as bone erosions and juxta-articular osteoporosis) and systemic bone loss in patients with inflammatory arthritis [12, 35, 36]. Indeed, patients with rheumatoid arthritis (RA), psoriatic arthritis (PsA) and AxSpA exhibit increased rates of systemic osteoporosis and osteoporotic fractures. On the other hand, bone loss in PsA and AxSpA is accompanied by pathological bone formation, in which TNF-α contributes in a lesser degree than other cytokines, such as IL-17 and IL-23 [37].
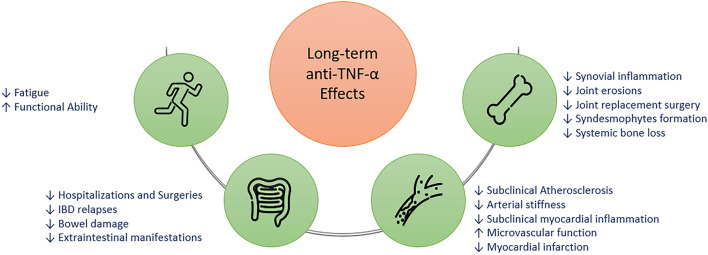
Blockade of TNF-α has been proven beneficial on bone metabolism in inflammatory arthritis (Fig. 2) [38]. Anti-TNF-α therapy controls synovial inflammation and leads to significant reduction of joint erosions in RA and PsA [12, 13]. Importantly, peripheral arthritis remains radiologically stable in AxSpA patients under infliximab, especially in the hip [39], resulting also in reduced hip replacement surgery rate [40, 41]. Through ameliorating inflammatory joint destruction, anti-TNF-α therapy led to 40% reduction of joint replacement surgery in older RA patients [42], while the benefit was stronger when treatment was introduced early in the disease course [43].
Fig. 2.
Long-term beneficial effects of anti-TNFs. Beneficial effects of anti-TNFs in bones, joints, intestine and cardiovascular system. IBD: Inflammatory bowel disease
Although initially thought that syndesmophytes formation was not hindered, but even accelerated, by anti-TNFs [44], newer long-term data have questioned this hypothesis [45]. The radiographic progression in axial disease is decelerated in patients that anti-TNF-α treatment sufficiently suppresses inflammation markers [46]. Systemic bone loss is also ameliorated by anti-TNFs in RA and AxSpA patients [12, 38, 47, 48]. Although an increase in bone mass and improvement in bone turnover markers is reported in anti-TNF-α -treated patients, less data are available for the possible impact on fracture occurrence [38, 48, 49].
Long-term effects on intestinal inflammation
The natural history of IBD is characterized by cumulative damage to the intestinal wall, which may lead to permanent injury and structural complications, such as strictures and fistulae in the case of Crohn’s disease (CD) and “lead pipe-like” dysfunctional colon in UC. Such abnormalities often necessitate surgical interventions and serve as objective indications of disease severity. There is now ample evidence that anti-TNF-α therapy has substantially decreased the incidence of complication-related hospitalizations by 50% and surgeries by 33–77% in patients with IBD, compared to placebo and the period before anti-TNF-α introduction [14, 50, 51]. In paediatric IBD, it was shown that adherence to recent guidelines with increased use of anti-TNFs resulted in significantly higher cumulative probability of a relapse-free and surgery-free course [52]. Taken together, those studies support an important role for TNF-α as a mediator of intestinal inflammation in IBD and emphasize the significance of its inhibition for the prevention of irreversible bowel damage, as well as the feasibility of this approach.
It should also be noted that, despite the expansion of available therapeutic options, anti-TNF-α agents remain the only agents that are indicated for the full spectrum of local and systemic manifestations in patients with IBD. Such indications include perianal fistulizing disease, acute severe UC and post-operative prevention of CD recurrence [53, 54]. More importantly, joint, skin and/or eye inflammation co-exists in at least one-third of patients with IBD and anti-TNFs represent the only biological therapy that is suitable for the treatment of such extra-intestinal manifestations [55].
Impact on atherosclerosis and cardiovascular events
It is known that IMIDs patients carry an increased risk for atherosclerotic CV events. Chronic systemic inflammation leads in acceleration of subclinical atherosclerosis [56]. In RA and AxSpA patients, low disease activity or remission induced by biologic disease modifying anti-rheumatic drugs (bDMARDs), mainly anti-TNFs, resulted in stabilization of subclinical atherosclerosis [57, 58], while a meta-analysis shows amelioration of atherosclerosis and arterial stiffness in anti-TNF-α-treated patients with inflammatory arthritis (RA, PsA, AxSpA) [59]. Anti-TNF-α therapy improves microvascular function in AxSpA patients [60] and subclinical myocardial inflammation in patients with inflammatory arthritis [61]. Additionally, myocardial infarction rates are reduced in RA patients under anti-TNF-α treatment, compared to those receiving conventional synthetic DMARDs (csMARDs) [62]. Last, anti-TNF’s beneficial effect seems to further extend to improvement of insulin resistance, functional ability and fatigue [63, 64].
Impact on mortality
The effect of anti-TNFs gets beyond the control of disease activity. In general, mortality of patients with IMIDs has decreased over the past two decades. Mortality rate of RA patients declined significantly only a few years after the introduction of anti-TNFs in clinical practice [65], while recent data suggest that patients with RA, PsA and AxSpA exhibit comparable mortality rate with the general population the past 5 years before the COVID-19 pandemic [15]. Regarding RA, lower number of deaths was observed in adalimumab-treated patients compared to the expected age- and sex-matched individuals from the general population [66]. The reduction in mortality of patients with IMIDs can be mainly attributed to diminished CV mortality, due to amelioration of subclinical atherosclerosis and atherosclerotic CV events [65].
Safety signals with chronic TNF-α inhibition
During the past decade, no new issues have arisen from long-term extension studies and real-life cohorts regarding anti-TNFs safety [66]. On the contrary, several points have been clarified. The hypothesis that anti-TNFs might make patients susceptible to lymphoma development has been rejected [67–71]. As shown from several meta-analyses, anti-TNF-α use is possibly not associated with an increased long-term malignancy risk in patients with inflammatory arthritis and IBD [71–74]. In addition, anti-TNFs exhibit a lower hazard ratio for cancer development compared to tofacitinib in RA patients aged over 50 years [16]. As for non-melanoma skin cancer (NMSC), some studies have shown that its incidence is increased in anti-TNF-α -treated patients [68, 75]. However, data from two large registries did not confirm these findings; in contrast, they showed that patients with RA, regardless of anti-TNF-α use, are in increased risk for NMSC development, compared to the general population [73, 76]. In RA patients, data for melanoma risk in anti-TNF-α -treated patients are conflicting, but the risk is possibly comparable to patients not exposed to anti-TNFs [68, 77]. Importantly, anti-TNF-α use in patients with malignancy history has not been associated with an increased risk for malignancy recurrence or new primary cancer development [78]. Moreover, the risk is not influenced by the time interval between initial cancer diagnosis and anti-TNF-α initiation [78]. There is currently no specific recommendation about when to start an anti-TNF-α in a patient with malignancy history. However, treatment with anti-TNFs should be avoided in patients with active malignancy.
As for active tuberculosis incidence, the introduction of screening methods prior to anti-TNF-α initiation led to a significant decline in (de novo or reactivation) tuberculosis cases in these patients [79]. Regarding other chronic infections, anti-TNF-α use is not related with an increased exacerbation rate of human immunodeficiency virus (HIV) infection [80, 81], while chronic hepatitis B and C reactivation rates remain low during anti-TNF-α treatment [82, 83]. In addition, skin psoriasis, an anti-TNF-α-related paradoxical adverse reaction [84], was proven to be induced also by other biologic agents used in IMIDs [85]. Moreover, although elevation of serum uric acid levels after 3 months of anti-TNF-α therapy have been observed, its clinical significance remains to be elucidated [86].
Based on the role of RANKL in the immune system, safety concerns had been raised for the combination of anti-TNFs with denosumab. Accumulating data demonstrated that the co-administration of these agents does not increase the risk for infection, compared to monotherapy with anti-TNF-α [87] and to those receiving zoledronic acid instead [88].
A growing body of evidence is now available about different anti-TNFs use during pregnancy and lactation. Anti-TNF-α use during pregnancy in women with IMIDs has not been associated with significantly increased rates of congenital malformations [89]. According to the IBD Parenthood Project, ideal scheduling of the final dose of an anti-TNF-α biologic prior to delivery is dictated by the half-life of the drug [90]. In “EULAR points to consider for use of anti-rheumatic drugs before pregnancy and during pregnancy and lactation” a slightly different perspective for women with inflammatory arthritis is suggested [91]. To be noted, as certolizumab pegol does not cross the placenta, it may be continued throughout pregnancy [91, 92]. Anti-TNF-α agents may be resumed 24 h after vaginal delivery and 48 h after cesarean delivery if there are no pregnancy-related or infectious contra-indications. Importantly, all anti-TNFs can be continued during lactation [91] and paternal exposure to anti-TNFs during conception period has not been associated with adverse pregnancy outcomes in their partners [93].
Last, reassuring “real-life” data about demyelinating disorders development after anti-TNF-α initiation have recently been seen the light [94]. It is still debatable if some sporadic cases are a coincidence or anti-TNF-α adverse events [95]; anti-TNFs’ use is either way contraindicated in patients with known concomitant demyelinating disorders.
Anti-TNFs in the COVID-19 era
The safety profile of anti-TNF-α emerged as an important issue for patients with IMIDs during the COVID-19 pandemic. Despite initial concerns due to fear of immunosuppression, only a small proportion of patients discontinued anti-TNF-α during the COVID-19 pandemic [96]. Experimental data suggest that anti-TNF-α-treated patients might be susceptible to be infected by SARS-CoV-2 [97]. Indeed, a threefold increased risk for COVID-19 infection was noted in patients with rheumatic diseases under anti-TNFs in a recent nationwide survey [18]. However, it seems that these patients do not carry an increased risk for serious COVID-19 disease [17, 18]. On the contrary, anti-TNF-α use was associated with reduced risk for hospitalization in SARS-CoV-2-infected patients with rheumatic diseases [98, 99], psoriasis [100] and IBD [101].
COVID-19 is an infection with a clear prothrombotic component [102–104]. TNF-α is elevated in patients with severe COVID-19, contributes in COVID-19 cytokine storm and acts as a major mediator in inflammation‐driven capillary leak syndrome that leads to lung injury [20, 104]. On this basis, the therapeutic potential of anti-TNF-α in COVID-19 infection emerged [20]. Ongoing trials examine the use of infliximab in serious COVID-19 infection, showing promising results, and etanercept has shown some effectiveness in case reports [105]. Interestingly, a trial with adalimumab was terminated early [106].
Importantly, anti-SARS-CoV-2 antibody development after vaccination are not hindered by anti-TNF-α use [107], as has been reported with rituximab, in patients with rheumatic diseases [108, 109]. Anti-SARS-CoV-2 antibody levels are comparable between PsA and AxSpA patients under anti-TNFs, anti-IL-17 and methotrexate [110, 111]. However, the sustainability of humoral response to COVID-19 vaccination in patients under anti-TNF-a agents is debatable, especially in IBD [112–114]. Thus, an earlier booster regimen might be crucial for these patients [112–114].
Optimization of anti-TNF-α use in IMIDs
Anti-TNFs as the basis for treat-to-target strategy
Implementation of bDMARDs in the clinical practice inevitably altered the therapeutic strategy in patients with IMIDs. Being the first bDMARD class applied, anti-TNFs made remission or low disease activity a feasible target. As a result, the “treat-to-target” strategy has been adopted in the management recommendations of several IMIDs [21]. This concept consists of the following: (a) baseline stratification of patients according to their individual risk for a complicated disease course; (b) pre-defined therapeutic targets that are predictive of a favorable long-term outcome of the disease; (c) modifications of the administered therapy until the target is met and, (d) periodical reassessment of the patient to ensure long-term maintenance of therapeutic target accomplishment. Physicians should maximize the possible control of disease activity to achieve remission or low disease activity as soon as possible. In patients with early CD, finetuning of anti-TNF-α therapy based on combinatorial clinical and biomarker endpoints resulted in better clinical and endoscopic outcomes than symptom-driven decisions alone [115]. A similar strategy has shown to improve disease outcomes compared to conventional follow-up in patients with RA [116–118] and PsA [119], while its value is still under debate in AxSpA [120]. For RA, physicians should also keep in mind that an anti-TNF-α without Fc portion of immunoglobulin (certolizumab pegol) might be more efficacious that other anti-TNF-α in patients with high rheumatoid factor levels, as was recently shown [121]. Moreover, in line with the “treat-to-target” strategy, patients with persistent monoarthritis, usually of the knee, might benefit from intra-articular anti-TNF-α infusion and etanercept is the agent with the better outcomes reported [122].
Anti-TNF-α cycling or switching to other biologics?
As said before, anti-TNFs are still considered the first-line bDMARDs used in several IMIDs, due to the established efficacy and safety profile. Nevertheless, a varying proportion of patients treated with a first anti-TNF-α agent will discontinue this therapy, mainly due to loss of response, rather than adverse effects [123, 124]. After failure of a first anti-TNF-α in a patient with RA, either a second anti-TNF-α or a drug with another mechanism of action can be chosen [21, 125]. However, in cases of primary failure, a switch to a non-anti-TNF-α bDMARD might be more efficacious than swapping to another anti-TNF-α [126], especially for seropositive RA patients (in favor of rituximab) and in those under bDMARD monotherapy (in favor of tocilizumab) [127]. Moreover, switching from monoclonal anti-TNFs (adalimumab or infliximab) to soluble anti-TNF-α receptor (etanercept) is associated with better clinical outcomes than the opposite [128]. Regarding AxSpA, recent studies imply that both a second anti-TNF-α and anti-IL-17 inhibitors are considerable options after failure of a first anti-TNF-α agent [129, 130]. For PsA, according to the last ACR guidelines, anti-TNF-α cycling is preferred over anti-IL-17 and anti-IL-12/23 inhibitor use after anti-TNF-α failure, except of the case of severe psoriasis or primary anti-TNF-α non-response [131]. More data are needed in psoriasis and IBD to guide the therapeutic strategy after anti-TNF-α failure [132, 133]. Eventually, the decision of second-line bDMARD treatment relies on the cause of anti-TNF-α non-response and should be individualized [134].
Therapeutic drug monitoring (TDM): Anti-drug antibodies & drug levels
In the era of “treat-to-target” approach and precision medicine, TDM, which consists of measuring drug levels and anti-drug antibodies (ADAs), is increasingly gaining attention [135]. TDM has been, almost exclusively, investigated for anti-TNF-α therapy, and applied in IBD to a much greater extent than in other IMIDs. A constantly expanding body of evidence indicates that TDM may be a valuable clinical tool for improving disease outcomes and can be applied in either reactive or pre-active fashion [136]. Reactive TDM calls for measuring trough levels and ADAs when a clinical endpoint is not met (“treat-to-target” approach). Pro-active TDM constitutes of dosage modifications until pre-determined serum drug levels are achieved. In case of treatment failure, either primary non-response or secondary failure to anti-TNFs, TDM may dictate the best therapeutic strategy, either by modifying the dosing scheme or switching out of anti-TNF-α therapy [135, 137].
Failure to respond to a biologic agent or loss of response after initial benefit are multifactorial phenomena. During induction, serum drug levels are affected by body mass index, gender, serum albumin, inflammatory burden, prior failure to bDMARDs, as well as tissue specific processes, such as loss in the stools, digestion by local proteases and recycling by neonatal Fc receptor. On the other hand, secondary loss of response may be mediated by either pharmacodynamic (mechanistic) or pharmacokinetic (immune or non-immune) factors.
Among the most recognized causes of secondary failure to anti-TNF-α therapy is the formation of ADAs [137]. This is influenced by several factors. Firstly, not all anti-TNFs have the same risk for ADA development; etanercept, compared to monoclonal anti-TNFs, exhibits low immunogenic potential [137]. Moreover, ADAs against mAbs (such as infliximab and adalimumab) have been associated with diminished drug effectiveness, whereas ADAs to etanercept are usually non-neutralizing [138]. Second concomitant therapy might alter this risk; methotrexate or thiopurine co-administration has been associated with decreased anti-TNF-α ADAs formation [139]. Additionally, the use of distinct antibiotics classes may also affect the risk of ADA formation in patients with IBD [140]. Importantly, ADAs against originator anti-TNF-α will cross-react with the relative biosimilar leading to treatment failure [137]. Although the precise clinical utility of TDM is still developing, patients who have higher serum drug levels always perform better in relation to a variety of disease outcomes, such as remission, drug survival and endoscopic scores [135].
Is anti-TNF tapering or discontinuation feasible?
In the bDMARDs era, adequate control of the disease activity has turned long-term remission in a realistic goal. As more patients manage to achieve sustained remission, a growing body of evidence suggests that anti-TNF-α treatment could be tapered or even discontinued in this group of patients (Table 2).
Table 2.
Data on disease outcomes after anti-TNF-α tapering or discontinuation. LDA: low disease activity
| Study | Outcome |
|---|---|
| Rheumatoid Arthritis | |
| Mangoni et al. [144] | 53% do not relapse after anti-TNF-α discontinuation |
| Tanaka et al. [147] |
LDA at 1-year follow-up: 91% in patients who continued adalimumab 62% in patients that discontinued adalimumab 79% in patients discontinued while in deep remission Adalimumab re-administration achieved LDA in 100% after 9 months |
| van Mulligen et al. [142] | Withdrawal of anti-TNF-α leads in numerically increased flare rate at 1 year, compared to cDMARD withdrawal |
| Curtis et al. [143] |
Withdrawal of etanercept leads in statistically lower remission rate at 1 year, compared to methotrexate withdrawal anti-TNF-α tapering or withdrawal feasible in 41% at 3-year follow-up |
| Sigaux et al. [146] | |
| Psoriatic Arthritis | |
| Ye et al. [151] |
Remission is maintained in 60–88.7% after tapering 48.3–83.3% of patients experience a flare within 22–29 months after anti-TNF-α discontinuation |
| Huynh et al. [152] | 55% do not relapse after anti-TNF-α discontinuation |
| Axial Spondylarthritis | |
| Navarro-Compan et al. [149] |
76–100% flare risk in a median 4 months after anti-TNF-α withdrawal 53–100% successful flare-free anti-TNF-α tapering |
| Landewe et al. [150] |
During 48 weeks, remission was maintained in: 83.7% in full dose certolizumab arm 79.0% in reduced dose certolizumab arm 20.2% in placebo (discontinuation) arm |
| Juvenile Idiopathic Arthritis | |
| Iglesias et al. [153] | 82% relapsed a mean 3 months after anti-TNF-α withdrawal |
| Cai et al. [154] | 87.1% of etanercept-treated patients maintained remission for 2 years after 50% dose reduction |
| Inflammatory Bowel Disease | |
| Gisbert et al. [155] |
58% of CD and 72% of UC patients remained in remission after 1 year In 80% of those who relapsed, remission was achieved after anti-TNF-α reinstitution |
| Kobayashi et al. [156] | 54% of UC patients was still in remission 1 year after infliximab discontinuation |
| Little et al. [157] | 50–93% remained in remission 1 year after anti-TNF-α tapering |
| Psoriasis | |
| Kim et al. [159] | In 69–83% of “relapsers” after drug discontinuation, remission was achieved after re-treatment with anti-TNF-α |
| Stinco et al. [160] |
50% did not relapse during the first 6 months after anti-TNF-α discontinuation Re-administration of anti-TNF-α resulted again in remission |
| Hansel et al. [161] | 60% maintained complete remission for at least 4 years after adalimumab discontinuation |
| Behcet’s Disease | |
| Sfikakis et al. [162] | Long-term remission in 40% after discontinuation |
In the latest EULAR and ACR guidelines for RA management, bDMARD tapering is considered a feasible practice in patients with sustained remission [21, 141]. This principle derives mainly from studies with anti-TNFs. Several studies after 2010 examined this issue. Tapering of anti-TNF-α or csDMARDs leads in similar flare rates after 9 months, but data from 12 months shows numerical less disease relapses in patients who continued anti-TNF-α treatment [142]. Indeed, in RA patients receiving etanercept and methotrexate combination, withdrawal of methotrexate led to less RA flares than etanercept withdrawal [143]. Thus, tapering of csDMARD might be a preferable option compared to anti-TNF-α discontinuation. Half patients with RA under anti-TNF-α can successfully discontinue treatment after achieving remission [144, 145], while this is less feasible for patients with established RA [145]. Long-term drug-free remission can be currently achieved in only a minority of RA patients and in about 30% a reduced anti-TNF-α dose can be maintained [146]. However, anti-TNF-α re-initiation restores remission in most cases [147]. It seems that early initiation of anti-TNF-α along with methotrexate and tight disease control provides the greatest potential of treatment holiday [148]. Moreover, sustaining deep remission was an independent predictor of better outcomes after adalimumab discontinuation [147].
Data in AxSpA patients support that anti-TNF-α withdrawal carries a high risk for disease flare (76–100%) in a median time of 4 months after drug discontinuation [149]. In contrast, flare-free tapering can be successful in about 53–100% of AxSpA patients receiving etanercept, adalimumab or infliximab [149]. In addition, a recent study demonstrated that AxSpA patients treated only for 12 months with certolizumab pegol showed comparable rates of flare-free remission when continuing on full-dose (200 mg per 2 weeks) or on reduced-dose (200 mg per 4 weeks) maintenance treatment thereafter [150]. Despite fewer data are available in PsA patients, short-term studies show a trend toward dose tapering and against treatment discontinuation in patients with well-controlled disease [151], although 55% of PsA patients might remain on remission after anti-TNF-α discontinuation [152]. Lower disease activity is a predictor of remission after tapering in PsA patients, while concomitant csDMARDs treatment does not have an impact on flare risk [151]. Importantly, drug re-institution effectively controls disease activity [151]. Anti-TNF-α discontinuation in children with juvenile idiopathic arthritis (JIA) led to high disease relapse rates (80–88%) [153], while gradual dose-lowering of etanercept in these patients is safe in maintaining remission [154].
Regarding IBD, 1 year after anti-TNF-α discontinuation 42% of CD patients and 28% of UC patients experience a disease flare [155]. In line with the aforementioned data, only 54% from UC patients who discontinued infliximab were in remission one year after drug withdrawal [156]. In another study, 0%–54% of IBD patients who tapered anti-TNF-α dose experienced a disease flare [157]. As in other diseases, remission was induced again in 80% of IBD patients that restarted the anti-TNF-α agent [155]. It should be noted, however, that in most IBD studies, patients who discontinued anti-TNFs maintained treatment with thiopurines [158]. On the other hand, only scarce data are available for psoriasis [159], but 50% of patients will still be in remission 6 months after anti-TNF-α withdrawal, especially those that had a complete response after drug initiation [160]. Adalimumab tapering is associated with 60% complete skin clearance within a follow-up of at least 4 years [161].
Discontinuation of anti-TNFs has been also reported in patients with BD. In a study from our group, about 40% of patients with severe BD in remission for at least 3 years might achieve long-term relapse-free remission of the disease after anti-TNF-α discontinuation [162]. Importantly, anti-TNF-α re-treatment was effective and safe in 82% of the patients that relapsed after treatment discontinuation [162].
Taken together, in patients with sustained remission available data strongly encourage dose reduction or increase of dosing intervals over drug discontinuation, as the latter carries a substantial risk of loss of remission. However, in those whom anti-TNF-α was suspended, drug re-administration is usually effective in restoring low disease activity.
Biomarkers of response
Given anti-TNF-α cost and their possible immunosuppressive effect, biomarkers capable of predicting patient’s response would guide physician’s therapeutic decisions for patients with IMIDs. In this line, many studies have examined several candidate molecules in a variety of clinical scenarios. Baseline higher serum calprotectin levels have been found in patients with RA that respond to etanercept or adalimumab [163]. Tao et al. showed that transcription signatures from peripheral blood mononuclear cells (PBMCs), monocytes and CD4 + T cells, of RA patients were different between adalimumab and etanercept responders and non-responders [164]. Another group applied multi-omics analyses in PBMCs from RA patients at the start of anti-TNF-α treatment and showed high expression of epiplakin 1 (EPPK1) and T-cell inhibitor chitinase-3-like 1 (CHI3L1) genes in those who responded [165]. Interestingly, a meta-analysis regarding patients with CD showed a discrepancy between proposed genomic and transcription biomarkers [166]. Treatment with anti-TNFs affects the levels of circulating microRNAs in RA patients [167]. Some studies have shown that serum levels of miR-22, miR-23, miR-27a, miR-223 and miR-886 before anti-TNF-α initiation could serve as predictors of patient’s response [167].
In IBD, search for predictive biomarkers is facilitated by the ability to obtain mucosal biopsies during endoscopy. This allows for the study of pre-treatment expression of local immunological and inflammatory mediators at the mRNA and protein levels and correlate them with subsequent response to treatment [168]. In one such study, non-response to anti-TNFs was associated with increased numbers of mucosal plasma cells and macrophages and elevated local expression of triggering receptor expressed on myeloid cells-1 (TREM-1), chemokine receptor type 2 (CCR2) and chemokine ligand 7 (CCL7) [169]. Another study with UC patients reported that the pretreatment mucosal expression of a combination of five genes predicted response to infliximab therapy [170]. In patients with colonic CD, another five-gene signature was found to discriminate between infliximab responders and non-responders [171]. Recently, the concept of molecular resistance via immunological pressure was proposed as an explanation for secondary loss of response to anti-TNFs. A significant upregulation of mucosal IL23p19, IL23R and IL17A expression was detected in patients who did not respond to anti-TNF-α therapy, in association with elevated number of mucosal T cells which expressed both TNFR2 and IL-23R and demonstrated high expression of inflammatory genes and resistance to apoptosis [172].
Recent studies have also shown that response to anti-TNFs may be genetically determined. The most important associations have been reported for patients with IBD who bear a specific polymorphism in the gene encoding the Fc fragment of the low affinity IgG3a receptor, who showed increased ADA formation, enhanced infliximab elimination and elevated risk of relapse after infliximab discontinuation [173]. In addition, in patients with CD, ADA formation may be determined by the HLA class II gene variant HLA-DQA501 [174].
The clinical applications of such findings are obvious as much they may identify patients who will benefit from combination of anti-TNFs with immunomodulators or who should better receive non-anti-TNF-α therapies. It should be noted, however, that the majority of these reports remains to be validated in additional patient cohorts and in prospective manner, which explains why none has reached clinical practice yet.
Socioeconomic perspectives on anti-TNF-α therapy: the case for biosimilars
Biosimilar drugs are agents with highly similar chemical characteristics and biological activity to original bDMARDs. Due to the financial success of originator anti-TNFs, especially adalimumab, infliximab and etanercept, several industries have developed biosimilars of anti-TNFs during the past decade, according to strict requirements [175–177]. Several biosimilars have shown clinically similar effectiveness, safety and immunogenicity compared to originator drugs and have been approved for the treatment of IMIDs [177]. As expected, healthcare systems exhibit substantial cost savings from the use of biosimilars not only due to their reduced price, but also by the consequent fall in the originator anti-TNFs cost [175]. Moreover, the use of anti-TNF-α biosimilars is expected to broaden anti-TNF-α availability, as their lower cost will mitigate the inequity in drug accessibility that has been reported [177]. Advances in bDMARDs manufacturing, especially in molecular engineering, allow changes in protein formulation that might improve originator drug’s properties, introducing the term “biobetter” [175, 176]. An example of such innovative strategy is CT-P13, an infliximab biosimilar, which was the first infliximab to be effectively administered subcutaneously [175].
On the other hand, biosimilars introduction in clinical practice was accompanied by several issues. First, biosimilars’ availability differs between countries, especially due to different regulation policies [176]. Importantly, switching from a reference bDMARD to a biosimilar can lead in subjective increase of disease activity or pain in some patients [177], in contrast to the results from double-blind switch randomized clinical trials [178]. This can be mainly attributed to the nocebo effect, which is based on a pre-existing negative perception of the patient for the administered drug [178]. Patients with IMIDs are more prone to nocebo behavior and data from biosimilar studies indicate that discontinuation rate due to subjective complains is not negligible [179]. As nocebo behavior can lead in several consequences, such as polypharmacy and worse disease outcomes, physicians need to recognize patients with risk factors for nocebo behavior and cautiously communicate biosimilars efficacy and safety, in order to prevent nocebo phenomena [179]. This has become more important in the COVID-19 era, during which nocebo behavior may be intensified [180].
The future of anti-TNF-α therapy
The next generation of anti-TNF-α drugs
Although the efficacy of anti-TNFs has been established for several IMIDs, a proportion of patients do not respond adequately and this might be partially attributed to pharmacokinetics. Typically, anti-TNF-α are administered subcutaneously or intravenously. However, the doses needed to achieve sufficient drug concentrations locally can lead to increased immunosuppression and other adverse events. To overcome this issue, new forms of anti-TNFs are under development.
Korkmaz et al. delivered anti-TNF-α-enriched microneedles on psoriasiform skin lesions in mice resulting in reduced epidermal thickness and inflammation mediators’ levels [181]. Moreover, a bispecific antibody combining a single-chain variable fragment (scFv) specific for synovium and a scFv of adalimumab successfully delivered adalimumab to the inflamed synovium in a mouse model, ameliorating local inflammation and indicating a new targeted therapy potential for RA [182]. On the other hand, oral anti-TNF-α agents could be suitable for diseases confined to gastrointestinal tract, such as IBD [183, 184]. However, oral administration of antibodies is hindered mainly by the proteolytic enzymes and the acidic environment of the stomach; thus, alternative delivery methods have been investigated for anti-TNFs [183]. Rectal administration (infliximab), tablet coating (V565-adalimumab), anti-TNF-α loaded nanoparticles [185–187], avian polyclonal anti-TNF-α, antisense oligonucleotides in several formulations, intracolonic microRNA and per os- or rectal-administered small interfering RNA that silence TNF-α expression, have been studied with varying efficacy [183]. Lactic acid bacteria have been utilized as mucosal delivering system and anti-TNF-α scFv-producing Lactococcus lactis administration have been shown to improve histopathologic findings in colitis models [188].
Progranulin, an endogenous protein that binds both types of TNFR, and preligand-binding assembly domain (PLAD), a part of the TNFR extra-cellular domain, play a crucial role in the TNF-α signaling pathway. Therapeutic administration of either progranulin or soluble versions of TNFR1 or PLAD in mouse models led to beneficial results [189–191], opening the door to another type of non-antibody anti-TNF-α drugs [23, 192]. As said above, TNFR1 and TNFR2 mediate different signaling pathways [23]. Blockade of TNFR1 leads in activation and proliferation of Tregs and ameliorates progression of collagen-induced arthritis [193, 194]. In this line, a TNFR2 agonist was associated with Tregs expansion and suppression of cytotoxic CD8 + T cells, exhibiting an anti-inflammatory potential [195]. Thus, based also on preclinical data, specific targeting of TNFR1 or TNFR2 by antibodies, small molecules or aptamers may represent a safer and more effective treatment in IMIDs in the future [196–198]. For example, zafirlukast, a leukotriene inhibitor, downregulates TNFR1 pathway by PLAD-mediated TNFR1 dimerization [199].
Another approach of TNF-α pathway blockade that has been recently examined is the development of orally active small molecules (such as UCB-9260) that destabilize TNF-α trimer, impeding TNF-α-TNFR1 binding and signaling [200]. In addition, several natural products that exhibit a TNFα inhibitory effect are being identified using in silico methods [201]. Whether selective TNFR targeting or non-biologic anti-TNFs block TNF-α pathway in a more refined way than the current marketed biologic anti-TNFs, remains to be seen [23].
Combination of anti-TNFs with other targeted therapies
The current therapeutic unmet needs require the constant revisiting of established trends in IMIDs’ pharmacotherapy. Although combination therapies have the theoretical potential of a synergistic anti-inflammatory effect, two important issues should be noted. First, as immunosuppression is expected to be higher in such cases, safety issues should be addressed and avoided. Second, a combination therapy should not be relied on the mere co-administration of two DMARDs, but, instead, must be carefully designed to combine independent and additive, and not similar and overlapping, modes of action.
Combination therapies carry the advantage of synergic action of both drugs, usually allowing lower doses to be administered. In a recent experimental study, the tyrosine kinase inhibitor dasatinib was co-administered with sub-therapeutic doses (only 10% of the standard dose) of infliximab, adalimumab, golimumab or etanercept in the human TNF-dependent Tg197 arthritis mouse model [202]. The combination therapy led to amelioration of clinical and histopathological findings of arthritis, which was more profound with the combination of low-dose infliximab and dasatinib, to a degree similar to the therapeutic infliximab dose.
In patients with refractory IBD, co-administration of anti-TNFs with vedolizumab, ustekinumab or natalizumab has shown promising results in case reports and case series and was not accompanied by a substantial increase in the incidence of serious adverse events, such as serious infections [203]. Interestingly, dual inhibition of TNF-α and IL-17 in experimental spondyloarthritis model has been shown to ameliorate local inflammation, joint erosions and new bone formation in spine [204], while the effect of double blockade in bone erosion was more potent than neutralizing TNF-α or IL-17 alone [205]. In this line, a bi-specific fusion protein against TNF-α /IL-17A [206–208] is being under investigation in clinical trials in psoriasis.
Moreover, also recently, a novel antibody (ABBV-337) was developed; adalimumab was conjugated with a glucocorticoid receptor modulator, targeting both transmembrane TNFR and cytoplasmic glucocorticoid receptor through endocytosis. This strategy allows the anti-inflammatory effects of glucocorticoids, minimizing the adverse events of systemic glucocorticoids use. In a phase 2a proof-of-concept study in RA patients, ABBV-337 exhibited statistically significant better response than adalimumab alone at week 12 [209].
Conclusion
The introduction of anti-TNFs altered the disease course of several IMIDs by achieving remission or low disease activity in a substantial proportion of patients. These agents exhibit beneficial effect on the basic pathophysiological process, while their use led in a significant reduction in all-cause mortality, at least in rheumatic diseases. Anti-TNFs' known safety profile has been confirmed, proving safe during the COVID-19 pandemic and expanded their use within pregnancy and lactation. Moreover, their use changed the management of these diseases, adopting the “treat-to-target” strategy, treatment de-escalation strategies have been successfully applied and TDM has optimized anti-TNF-α use. The implementation of anti-TNF-α biosimilars made anti-TNF-α treatment more accessible and improved anti-TNFs’ cost-effectiveness. Several biomarkers are currently under investigation and are expected to utilize as useful prognostic tools in anti-TNF-α-treated patients. Last, development of new anti-TNF-α forms and sophisticated TNFR targeting might optimize drug bioavailability, enhance their local efficacy and minimize adverse effects, while combination with other agents might refine disease outcomes using lower drug doses. Although a proportion of patients with IMIDs do not respond to anti-TNFs and newer therapeutic targets are seeing the light toward this direction, anti-TNFs remain in the front stage of our therapeutic armamentarium.
Acknowledgements
GE wants to thank Melina Moschopoulou, Clinical Pharmacist, for her contribution in figures’ design.
Author contributions
GE: concept, design, literature search and drafted the work. GB: design, drafted the work. GDK: design, critically revised the work. GK: design, critically revised the work. PPS: concept, design, literature search, drafted and critically revised the work. All authors approved the final manuscript as submitted.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
Not applicable.
Declarations
No part of this review, including ideas and tables, is copied or published elsewhere.
Conflict of interest
GE declares no conflicts of interest for this review. GB has received advisor, lecturer and research grants from Abbvie, Adacyte Therapeutics, Aenorasis, Amgen, Cooper, Ferring, Genesis Pharma, Janssen, MSD, Mylan, Pfizer, Takeda, Vianex. GDK declares no conflicts of interest for this review. GK declares no conflicts of interest for this review. PPS has served as a consultant or on an advisory board and has received grant and research support from Abbvie, Actelion, Amgen, Boehringer Ingelheim, Enorasis, Faran, Pharmaserve-Lilly, Genesis Pharma, Gilead, Janssen, MSD, Novartis, Pfizer, Roche and UCB.
Research involving human participants and/or animals
Not applicable.
Informed consent
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Keffer J, Probert L, Cazlaris H, et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991;10:4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schett G, McInnes IB, Neurath MF. Reframing immune-mediated inflammatory diseases through signature cytokine hubs. N Engl J Med. 2021;385:628–639. doi: 10.1056/NEJMra1909094. [DOI] [PubMed] [Google Scholar]
- 3.Sfikakis PP. The first decade of biologic TNF antagonists in clinical practice: lessons learned, unresolved issues and future directions. Curr Dir Autoimmun. 2010;11:180–210. doi: 10.1159/000289205. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez-Cano D, Callejas-Rubio JL, Ruiz-Villaverde R, et al. Off-label uses of anti-TNF therapy in three frequent disorders: Behcet's disease, sarcoidosis, and noninfectious uveitis. Mediators Inflamm. 2013;2013:286857. doi: 10.1155/2013/286857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shuai ZQ, Zhang CX, Shuai ZW, et al (2021) Efficacy and safety of biological agents in the treatment of patients with Takayasu arteritis: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci 25: 250–62. 10.26355/eurrev_202101_24391. [DOI] [PubMed]
- 6.Ben Abdelghani K, Dran DG, Gottenberg JE, et al. Tumor necrosis factor-alpha blockers in SAPHO syndrome. J Rheumatol. 2010;37:1699–1704. doi: 10.3899/jrheum.091086. [DOI] [PubMed] [Google Scholar]
- 7.Ge Y, Li S, Chen F, et al. The effects of infliximab in treating idiopathic inflammatory myopathies: a review article. Dermatol Ther. 2021;34:e14976. doi: 10.1111/dth.14976. [DOI] [PubMed] [Google Scholar]
- 8.Melani AS, Bigliazzi C, Cimmino FA, et al. A comprehensive review of sarcoidosis treatment for pulmonologists. Pulm Ther. 2021 doi: 10.1007/s41030-021-00160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conticini E, Sota J, Falsetti P, et al. Biologic drugs in the treatment of polyarteritis nodosa and deficit of adenosine deaminase 2: a narrative review. Autoimmun Rev. 2021;20:102784. doi: 10.1016/j.autrev.2021.102784. [DOI] [PubMed] [Google Scholar]
- 10.Deuitch NT, Yang D, Lee PY, et al. TNF inhibition in vasculitis management in adenosine deaminase 2 deficiency (DADA2) J Allergy Clin Immunol. 2021 doi: 10.1016/j.jaci.2021.10.030. [DOI] [PubMed] [Google Scholar]
- 11.Maverakis E, Marzano AV, Le ST, et al. Pyoderma gangrenosum. Nat Rev Dis Primers. 2020;6:81. doi: 10.1038/s41572-020-0213-x. [DOI] [PubMed] [Google Scholar]
- 12.Manara M, Sinigaglia L. Bone and TNF in rheumatoid arthritis: clinical implications. RMD Open. 2015;1:e000065. doi: 10.1136/rmdopen-2015-000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Addimanda O, Possemato N, Caruso A, et al. The role of tumor necrosis factor-alpha blockers in psoriatic disease. therapeutic options in psoriatic arthritis. J Rheumatol Suppl. 2015;93:73–78. doi: 10.3899/jrheum.150642. [DOI] [PubMed] [Google Scholar]
- 14.Mao EJ, Hazlewood GS, Kaplan GG, et al. Systematic review with meta-analysis: comparative efficacy of immunosuppressants and biologics for reducing hospitalisation and surgery in Crohn's disease and ulcerative colitis. Aliment Pharmacol Ther. 2017;45:3–13. doi: 10.1111/apt.13847. [DOI] [PubMed] [Google Scholar]
- 15.Bournia VK, Fragoulis GE, Mitrou P, et al (2021) All-cause mortality in systemic rheumatic diseases under treatment compared with the general population, 2015–2019. RMD Open 7 10.1136/rmdopen-2021-001694. [DOI] [PMC free article] [PubMed]
- 16.Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316–326. doi: 10.1056/NEJMoa2109927. [DOI] [PubMed] [Google Scholar]
- 17.Salesi M, Shojaie B, Farajzadegan Z, et al (2021) TNF-alpha Blockers Showed Prophylactic Effects in Preventing COVID-19 in Patients with Rheumatoid Arthritis and Seronegative Spondyloarthropathies: A Case-Control Study. Rheumatol Ther: 1-16. 10.1007/s40744-021-00342-8 [DOI] [PubMed]
- 18.Gracia BDC, Saez L, Pallares L, et al. COVID GEAS: COVID-19 national survey in patients with systemic autoimmune diseases. Front Med (Lausanne) 2021;8:808608. doi: 10.3389/fmed.2021.808608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kokkotis G, Kitsou K, Xynogalas I, et al. Systematic review with meta-analysis: COVID-19 outcomes in patients receiving anti-TNF treatments. Aliment Pharmacol Ther. 2022;55:154–167. doi: 10.1111/apt.16717. [DOI] [PubMed] [Google Scholar]
- 20.Robinson PC, Richards D, Tanner HL, et al. Accumulating evidence suggests anti-TNF therapy needs to be given trial priority in COVID-19 treatment. Lancet Rheumatol. 2020;2:e653–e655. doi: 10.1016/S2665-9913(20)30309-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smolen JS, Landewe RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79:685–699. doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- 22.Gasparyan AY, Ayvazyan L, Blackmore H, et al. Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int. 2011;31:1409–1417. doi: 10.1007/s00296-011-1999-3. [DOI] [PubMed] [Google Scholar]
- 23.Sfikakis PP, Tsokos GC. Towards the next generation of anti-TNF drugs. Clin Immunol. 2011;141:231–235. doi: 10.1016/j.clim.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Sakkou M, Chouvardas P, Ntari L, et al (2018) Mesenchymal TNFR2 promotes the development of polyarthritis and comorbid heart valve stenosis. JCI Insight 3 10.1172/jci.insight.98864. [DOI] [PMC free article] [PubMed]
- 25.Wu B, Zhao TV, Jin K, et al. Mitochondrial aspartate regulates TNF biogenesis and autoimmune tissue inflammation. Nat Immunol. 2021;22:1551–1562. doi: 10.1038/s41590-021-01065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armaka M, Ospelt C, Pasparakis M, et al. The p55TNFR-IKK2-Ripk3 axis orchestrates arthritis by regulating death and inflammatory pathways in synovial fibroblasts. Nat Commun. 2018;9:618. doi: 10.1038/s41467-018-02935-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armaka M, Apostolaki M, Jacques P, et al. Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J Exp Med. 2008;205:331–337. doi: 10.1084/jem.20070906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ntari L, Sakkou M, Chouvardas P, et al. Comorbid TNF-mediated heart valve disease and chronic polyarthritis share common mesenchymal cell-mediated aetiopathogenesis. Ann Rheum Dis. 2018;77:926–934. doi: 10.1136/annrheumdis-2017-212597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davidson S, Coles M, Thomas T, et al. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat Rev Immunol. 2021;21:704–717. doi: 10.1038/s41577-021-00540-z. [DOI] [PubMed] [Google Scholar]
- 30.Croft AP, Campos J, Jansen K, et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature. 2019;570:246–251. doi: 10.1038/s41586-019-1263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei K, Korsunsky I, Marshall JL, et al. Notch signalling drives synovial fibroblast identity and arthritis pathology. Nature. 2020;582:259–264. doi: 10.1038/s41586-020-2222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armaka M, Konstantopoulos D, Tzaferis C, et al (2021) 10.1101/2021.08.27.457747.
- 33.Tunyogi-Csapo M, Kis-Toth K, Radacs M, et al. Cytokine-controlled RANKL and osteoprotegerin expression by human and mouse synovial fibroblasts: fibroblast-mediated pathologic bone resorption. Arthritis Rheum. 2008;58:2397–2408. doi: 10.1002/art.23653. [DOI] [PubMed] [Google Scholar]
- 34.Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 35.Osta B, Benedetti G, Miossec P. Classical and paradoxical effects of TNF-alpha on bone homeostasis. Front Immunol. 2014;5:48. doi: 10.3389/fimmu.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coury F, Peyruchaud O, Machuca-Gayet I. Osteoimmunology of bone loss in inflammatory rheumatic diseases. Front Immunol. 2019;10:679. doi: 10.3389/fimmu.2019.00679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gravallese EM, Schett G. Effects of the IL-23–IL-17 pathway on bone in spondyloarthritis. Nat Rev Rheumatol. 2018;14:631–640. doi: 10.1038/s41584-018-0091-8. [DOI] [PubMed] [Google Scholar]
- 38.Zerbini CAF, Clark P, Mendez-Sanchez L, et al. Biologic therapies and bone loss in rheumatoid arthritis. Osteoporos Int. 2017;28:429–446. doi: 10.1007/s00198-016-3769-2. [DOI] [PubMed] [Google Scholar]
- 39.Konsta M, Sfikakis PP, Bournia VK, et al. Absence of radiographic progression of hip arthritis during infliximab treatment for ankylosing spondylitis. Clin Rheumatol. 2013;32:1229–1232. doi: 10.1007/s10067-013-2263-x. [DOI] [PubMed] [Google Scholar]
- 40.Nystad TW, Furnes O, Havelin LI, et al. Hip replacement surgery in patients with ankylosing spondylitis. Ann Rheum Dis. 2014;73:1194–1197. doi: 10.1136/annrheumdis-2013-203963. [DOI] [PubMed] [Google Scholar]
- 41.Li L, Fu J, Xu C, et al. Hip Replacement in ankylosing spondylitis patients with advanced hip involvement: factors associated with bilateral total hip arthroplasty. Int J Gen Med. 2021;14:6857–6862. doi: 10.2147/IJGM.S336314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hawley S, Ali MS, Cordtz R, et al. Impact of TNF inhibitor therapy on joint replacement rates in rheumatoid arthritis: a matched cohort analysis of BSRBR-RA UK registry data. Rheumatology (Oxford) 2019;58:1168–1175. doi: 10.1093/rheumatology/key424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen YC, Chiu WC, Cheng TT, et al. Delayed anti-TNF therapy increases the risk of total knee replacement in patients with severe rheumatoid arthritis. BMC Musculoskelet Disord. 2017;18:326. doi: 10.1186/s12891-017-1685-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pedersen SJ, Chiowchanwisawakit P, Lambert RG, et al. Resolution of inflammation following treatment of ankylosing spondylitis is associated with new bone formation. J Rheumatol. 2011;38:1349–1354. doi: 10.3899/jrheum.100925. [DOI] [PubMed] [Google Scholar]
- 45.Baraliakos X, Haibel H, Listing J, et al. Continuous long-term anti-TNF therapy does not lead to an increase in the rate of new bone formation over 8 years in patients with ankylosing spondylitis. Ann Rheum Dis. 2014;73:710–715. doi: 10.1136/annrheumdis-2012-202698. [DOI] [PubMed] [Google Scholar]
- 46.Konsta M, Sakellariou GT, Rusman T, et al. Long-term effect of TNF inhibitors on radiographic progression in ankylosing spondylitis is associated with time-averaged CRP levels. Joint Bone Spine. 2021;88:105111. doi: 10.1016/j.jbspin.2020.105111. [DOI] [PubMed] [Google Scholar]
- 47.Gulyas K, Horvath A, Vegh E, et al. Effects of 1-year anti-TNF-alpha therapies on bone mineral density and bone biomarkers in rheumatoid arthritis and ankylosing spondylitis. Clin Rheumatol. 2020;39:167–175. doi: 10.1007/s10067-019-04771-3. [DOI] [PubMed] [Google Scholar]
- 48.Ashany D, Stein EM, Goto R, et al. The effect of TNF inhibition on bone density and fracture risk and of IL17 Inhibition on radiographic progression and bone density in patients with axial spondyloarthritis: a systematic literature review. Curr Rheumatol Rep. 2019;21:20. doi: 10.1007/s11926-019-0818-9. [DOI] [PubMed] [Google Scholar]
- 49.Maldonado-Perez MB, Castro-Laria L, Caunedo-Alvarez A, et al. Does the antitumor necrosis factor-alpha therapy decrease the vertebral fractures occurrence in inflammatory bowel disease? J Clin Densitom. 2019;22:195–202. doi: 10.1016/j.jocd.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 50.Guasch M, Canete F, Ordas I, et al. Changes in the requirement for early surgery in inflammatory bowel disease in the era of biological agents. J Gastroenterol Hepatol. 2020;35:2080–2087. doi: 10.1111/jgh.15084. [DOI] [PubMed] [Google Scholar]
- 51.Rungoe C, Langholz E, Andersson M, et al. Changes in medical treatment and surgery rates in inflammatory bowel disease: a nationwide cohort study 1979–2011. Gut. 2014;63:1607–1616. doi: 10.1136/gutjnl-2013-305607. [DOI] [PubMed] [Google Scholar]
- 52.D'Arcangelo G, Abi Nader E, Charbit-Henrion F, et al. Increased use of anti-tumor necrosis factor following the implementation of the ECCO-ESPGHAN guidelines and its impact on the outcome of pediatric crohn's disease: a retrospective single-center study. J Pediatr Gastroenterol Nutr. 2022;74:79–84. doi: 10.1097/MPG.0000000000003301. [DOI] [PubMed] [Google Scholar]
- 53.Raine T, Bonovas S, Burisch J, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis. 2021 doi: 10.1093/ecco-jcc/jjab178. [DOI] [PubMed] [Google Scholar]
- 54.Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in crohn's disease: medical treatment. J Crohns Colitis. 2020;14:4–22. doi: 10.1093/ecco-jcc/jjz180. [DOI] [PubMed] [Google Scholar]
- 55.Karmiris K, Avgerinos A, Tavernaraki A, et al. Prevalence and characteristics of extra-intestinal manifestations in a large cohort of greek patients with inflammatory bowel disease. J Crohns Colitis. 2016;10:429–436. doi: 10.1093/ecco-jcc/jjv232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arida A, Protogerou AD, Kitas GD, et al (2018) Systemic Inflammatory Response and Atherosclerosis: The Paradigm of Chronic Inflammatory Rheumatic Diseases. Int J Mol Sci 19 10.3390/ijms19071890. [DOI] [PMC free article] [PubMed]
- 57.Arida A, Protogerou AD, Konstantonis G, et al. Atherosclerosis is not accelerated in rheumatoid arthritis of low activity or remission, regardless of antirheumatic treatment modalities. Rheumatology (Oxford) 2017;56:934–939. doi: 10.1093/rheumatology/kew506. [DOI] [PubMed] [Google Scholar]
- 58.Arida A, Protogerou AD, Konstantonis G, et al. Subclinical atherosclerosis is not accelerated in patients with ankylosing spondylitis with low disease activity: new data and metaanalysis of published studies. J Rheumatol. 2015;42:2098–2105. doi: 10.3899/jrheum.150316. [DOI] [PubMed] [Google Scholar]
- 59.Tam LS, Kitas GD, Gonzalez-Gay MA. Can suppression of inflammation by anti-TNF prevent progression of subclinical atherosclerosis in inflammatory arthritis? Rheumatology (Oxford) 2014;53:1108–1119. doi: 10.1093/rheumatology/ket454. [DOI] [PubMed] [Google Scholar]
- 60.Batko B, Maga P, Urbanski K, et al. Microvascular dysfunction in ankylosing spondylitis is associated with disease activity and is improved by anti-TNF treatment. Sci Rep. 2018;8:13205. doi: 10.1038/s41598-018-31550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ntusi NAB, Francis JM, Sever E, et al. Anti-TNF modulation reduces myocardial inflammation and improves cardiovascular function in systemic rheumatic diseases. Int J Cardiol. 2018;270:253–259. doi: 10.1016/j.ijcard.2018.06.099. [DOI] [PubMed] [Google Scholar]
- 62.Low AS, Symmons DP, Lunt M, et al. Relationship between exposure to tumour necrosis factor inhibitor therapy and incidence and severity of myocardial infarction in patients with rheumatoid arthritis. Ann Rheum Dis. 2017;76:654–660. doi: 10.1136/annrheumdis-2016-209784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van den Oever IAM, Baniaamam M, Simsek S, et al. The effect of anti-TNF treatment on body composition and insulin resistance in patients with rheumatoid arthritis. Rheumatol Int. 2021;41:319–328. doi: 10.1007/s00296-020-04666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Veldhuijzen van Zanten J, Sandoo A, Metsios GS, et al. Comparison of the effects of exercise and anti-TNF treatment on cardiovascular health in rheumatoid arthritis: results from two controlled trials. Rheumatol Int. 2019;39:219–225. doi: 10.1007/s00296-018-4183-1. [DOI] [PubMed] [Google Scholar]
- 65.Abhishek A, Nakafero G, Kuo CF, et al. Rheumatoid arthritis and excess mortality: down but not out. A primary care cohort study using data from Clinical Practice Research Datalink. Rheumatology (Oxford) 2018;57:977–981. doi: 10.1093/rheumatology/key013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burmester GR, Gordon KB, Rosenbaum JT, et al. Long-term safety of adalimumab in 29,967 adult patients from global clinical trials across multiple indications: an updated analysis. Adv Ther. 2020;37:364–380. doi: 10.1007/s12325-019-01145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mariette X, Matucci-Cerinic M, Pavelka K, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70:1895–1904. doi: 10.1136/ard.2010.149419. [DOI] [PubMed] [Google Scholar]
- 68.Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886–2895. doi: 10.1002/art.22864. [DOI] [PubMed] [Google Scholar]
- 69.Askling J, Baecklund E, Granath F, et al. Anti-tumour necrosis factor therapy in rheumatoid arthritis and risk of malignant lymphomas: relative risks and time trends in the Swedish Biologics Register. Ann Rheum Dis. 2009;68:648–653. doi: 10.1136/ard.2007.085852. [DOI] [PubMed] [Google Scholar]
- 70.Mercer LK, Galloway JB, Lunt M, et al. Risk of lymphoma in patients exposed to antitumour necrosis factor therapy: results from the British society for rheumatology biologics register for rheumatoid arthritis. Ann Rheum Dis. 2017;76:497–503. doi: 10.1136/annrheumdis-2016-209389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hellgren K, Dreyer L, Arkema EV, et al. Cancer risk in patients with spondyloarthritis treated with TNF inhibitors: a collaborative study from the ARTIS and DANBIO registers. Ann Rheum Dis. 2017;76:105–111. doi: 10.1136/annrheumdis-2016-209270. [DOI] [PubMed] [Google Scholar]
- 72.Thompson AE, Rieder SW, Pope JE. Tumor necrosis factor therapy and the risk of serious infection and malignancy in patients with early rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheum. 2011;63:1479–1485. doi: 10.1002/art.30310. [DOI] [PubMed] [Google Scholar]
- 73.Dreyer L, Mellemkjaer L, Andersen AR, et al. Incidences of overall and site specific cancers in TNFalpha inhibitor treated patients with rheumatoid arthritis and other arthritides - a follow-up study from the DANBIO Registry. Ann Rheum Dis. 2013;72:79–82. doi: 10.1136/annrheumdis-2012-201969. [DOI] [PubMed] [Google Scholar]
- 74.Nyboe Andersen N, Pasternak B, Basit S, et al. Association between tumor necrosis factor-alpha antagonists and risk of cancer in patients with inflammatory bowel disease. JAMA. 2014;311:2406–2413. doi: 10.1001/jama.2014.5613. [DOI] [PubMed] [Google Scholar]
- 75.Amari W, Zeringue AL, McDonald JR, et al. Risk of non-melanoma skin cancer in a national cohort of veterans with rheumatoid arthritis. Rheumatology (Oxford) 2011;50:1431–1439. doi: 10.1093/rheumatology/ker113. [DOI] [PubMed] [Google Scholar]
- 76.Mercer LK, Green AC, Galloway JB, et al. The influence of anti-TNF therapy upon incidence of keratinocyte skin cancer in patients with rheumatoid arthritis: longitudinal results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2012;71:869–874. doi: 10.1136/annrheumdis-2011-200622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mercer LK, Askling J, Raaschou P, et al. Risk of invasive melanoma in patients with rheumatoid arthritis treated with biologics: results from a collaborative project of 11 European biologic registers. Ann Rheum Dis. 2017;76:386–391. doi: 10.1136/annrheumdis-2016-209285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Waljee AK, Higgins PDR, Jensen CB, et al. Anti-tumour necrosis factor-α therapy and recurrent or new primary cancers in patients with inflammatory bowel disease, rheumatoid arthritis, or psoriasis and previous cancer in Denmark: a nationwide, population-based cohort study. The Lancet Gastroenterology & Hepatology. 2020;5:276–284. doi: 10.1016/s2468-1253(19)30362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Evangelatos G, Koulouri V, Iliopoulos A, et al (2020) Tuberculosis and targeted synthetic or biologic DMARDs, beyond tumor necrosis factor inhibitors. Ther Adv Musculoskelet Dis 12: 1759720X20930116. 10.1177/1759720X20930116. [DOI] [PMC free article] [PubMed]
- 80.Liang SJ, Zheng QY, Yang YL, et al. Use of etanercept to treat rheumatoid arthritis in an HIV-positive patient: a case-based review. Rheumatol Int. 2017;37:1207–1212. doi: 10.1007/s00296-017-3690-9. [DOI] [PubMed] [Google Scholar]
- 81.Narcisi A, Bernardini N, Orsini D, et al. Long-term safety and efficacy of adalimumab in psoriasis: a multicentric study focused on infections (connecting study) Postepy Dermatol Alergol. 2020;37:428–434. doi: 10.5114/ada.2020.96910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fidan S, Capkin E, Arica DA, et al. Risk of hepatitis B reactivation in patients receiving anti-tumor necrosis factor-alpha therapy. Int J Rheum Dis. 2021;24:254–259. doi: 10.1111/1756-185X.14034. [DOI] [PubMed] [Google Scholar]
- 83.Chen YM, Chen HH, Chen YH, et al. A comparison of safety profiles of tumour necrosis factor alpha inhibitors and rituximab therapy in patients with rheumatoid arthritis and chronic hepatitis C. Ann Rheum Dis. 2015;74:626–627. doi: 10.1136/annrheumdis-2014-206711. [DOI] [PubMed] [Google Scholar]
- 84.Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513–2518. doi: 10.1002/art.21233. [DOI] [PubMed] [Google Scholar]
- 85.Karamanakos A, Vergou T, Panopoulos S, et al. Psoriasis as an adverse reaction to biologic agents beyond anti-TNF-alpha therapy. Eur J Dermatol. 2021;31:307–317. doi: 10.1684/ejd.2021.4056. [DOI] [PubMed] [Google Scholar]
- 86.Hasikova L, Pavlikova M, Hulejova H, et al. Serum uric acid increases in patients with systemic autoimmune rheumatic diseases after 3 months of treatment with TNF inhibitors. Rheumatol Int. 2019;39:1749–1757. doi: 10.1007/s00296-019-04394-6. [DOI] [PubMed] [Google Scholar]
- 87.Zabihiyeganeh M, Mirzaei AM, Jahed SA (2018) AB1010 Risk of infection in patients with rheumatoid arthritis taking denosumab concurrent with biologic therapy. 1624.2-. 10.1136/annrheumdis-2018-eular.1317.
- 88.Curtis JR, Xie F, Yun H, et al. Risk of hospitalized infection among rheumatoid arthritis patients concurrently treated with a biologic agent and denosumab. Arthritis Rheumatol. 2015;67:1456–1464. doi: 10.1002/art.39075. [DOI] [PubMed] [Google Scholar]
- 89.Broms G, Granath F, Ekbom A, et al. Low risk of birth defects for infants whose mothers are treated with anti-tumor necrosis factor agents during pregnancy. Clin Gastroenterol Hepatol. 2016;14(234–41):e1–5. doi: 10.1016/j.cgh.2015.08.039. [DOI] [PubMed] [Google Scholar]
- 90.Mahadevan U, Robinson C, Bernasko N, et al. Inflammatory bowel disease in pregnancy clinical care pathway: a report from the american gastroenterological association IBD parenthood project working group. Am J Obstet Gynecol. 2019;220:308–323. doi: 10.1016/j.ajog.2019.02.027. [DOI] [PubMed] [Google Scholar]
- 91.Gotestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795–810. doi: 10.1136/annrheumdis-2015-208840. [DOI] [PubMed] [Google Scholar]
- 92.Mahadevan U, Wolf DC, Dubinsky M, et al (2013) Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 11: 286–92; quiz e24. 10.1016/j.cgh.2012.11.011. [DOI] [PMC free article] [PubMed]
- 93.Uyaroglu OA, Seyhoglu E, Erden A, et al. Pregnancy outcomes in partners of male ankylosing spondylitis patients treated with anti-tumour necrosis factor-alpha biologics: real-life results from a single-centre cross-sectional study. Rheumatol Int. 2020;40:1501–1507. doi: 10.1007/s00296-020-04518-3. [DOI] [PubMed] [Google Scholar]
- 94.Taylor TRP, Galloway J, Davies R, et al (2021) Demyelinating Events Following Initiation of Anti-TNFalpha Therapy in the British Society for Rheumatology Biologics Registry in Rheumatoid Arthritis. Neurol Neuroimmunol Neuroinflamm 8 10.1212/NXI.0000000000000992. [DOI] [PMC free article] [PubMed]
- 95.Kemanetzoglou E, Andreadou E. CNS demyelination with TNF-alpha blockers. Curr Neurol Neurosci Rep. 2017;17:36. doi: 10.1007/s11910-017-0742-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fragoulis GE, Evangelatos G, Arida A, et al. Treatment adherence of patients with systemic rheumatic diseases in COVID-19 pandemic. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217935. [DOI] [PubMed] [Google Scholar]
- 97.Keewan E, Beg S, Naser SA. Anti-TNF-alpha agents modulate SARS-CoV-2 receptors and increase the risk of infection through notch-1 signaling. Front Immunol. 2021;12:641295. doi: 10.3389/fimmu.2021.641295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Q, Liu J, Shao R, et al. Risk and clinical outcomes of COVID-19 in patients with rheumatic diseases compared with the general population: a systematic review and meta-analysis. Rheumatol Int. 2021;41:851–861. doi: 10.1007/s00296-021-04803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kridin K, Schonmann Y, Damiani G, et al. Tumor necrosis factor inhibitors are associated with a decreased risk of COVID-19-associated hospitalization in patients with psoriasis-A population-based cohort study. Dermatol Ther. 2021;34:e15003. doi: 10.1111/dth.15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bamias G, Kokkotis G, Christidou A, et al. The natural history of COVID-19 in patients with inflammatory bowel disease: a nationwide study by the Hellenic Society for the study of IBD. Eur J Gastroenterol Hepatol. 2021 doi: 10.1097/MEG.0000000000002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ahmed S, Zimba O, Gasparyan AY. Thrombosis in Coronavirus disease 2019 (COVID-19) through the prism of Virchow's triad. Clin Rheumatol. 2020;39:2529–2543. doi: 10.1007/s10067-020-05275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Icenogle T. COVID-19: infection or autoimmunity. Front Immunol. 2020;11:2055. doi: 10.3389/fimmu.2020.02055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fara A, Mitrev Z, Rosalia RA, et al. Cytokine storm and COVID-19: a chronicle of pro-inflammatory cytokines. Open Biol. 2020;10:200160. doi: 10.1098/rsob.200160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guo Y, Hu K, Li Y, et al. Targeting TNF-alpha for COVID-19: Recent Advanced and Controversies. Front Public Health. 2022;10:833967. doi: 10.3389/fpubh.2022.833967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fakharian A, Barati S, Mirenayat M, et al. Evaluation of adalimumab effects in managing severe cases of COVID-19: a randomized controlled trial. Int Immunopharmacol. 2021;99:107961. doi: 10.1016/j.intimp.2021.107961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jena A, Mishra S, Deepak P, et al. Response to SARS-CoV-2 vaccination in immune mediated inflammatory diseases: systematic review and meta-analysis. Autoimmun Rev. 2022;21:102927. doi: 10.1016/j.autrev.2021.102927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boyarsky BJ, Ruddy JA, Connolly CM, et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Evangelatos G, Kouna K, Fragoulis GE, et al (2021) Low levels of anti-SARS-CoV-2 antibodies after vaccination in rituximab- treated patients: Comment on article of Simon et al. Arthritis Rheumatol 10.1002/art.42011. [DOI] [PMC free article] [PubMed]
- 110.Benucci M, Damiani A, Infantino M, et al. Vaccination for SARS-CoV-2 in patients with psoriatic arthritis: Can therapy affect the immunological response? Front Med (Lausanne) 2022;9:811829. doi: 10.3389/fmed.2022.811829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Smetanova J, Strizova Z, Sediva A, et al. Humoral and cellular immune responses to mRNA COVID-19 vaccines in patients with axial spondyloarthritis treated with adalimumab or secukinumab. Lancet Rheumatol. 2022;4:e163–e166. doi: 10.1016/S2665-9913(21)00393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Geisen UM, Sumbul M, Tran F, et al (2021) Humoral protection to SARS-CoV2 declines faster in patients on TNF alpha blocking therapies. RMD Open 7 10.1136/rmdopen-2021-002008. [DOI] [PMC free article] [PubMed]
- 113.Vollenberg R, Tepasse PR, Kuhn JE, et al (2022) Humoral Immune Response in IBD Patients Three and Six Months after Vaccination with the SARS-CoV-2 mRNA Vaccines mRNA-1273 and BNT162b2. Biomedicines 10 10.3390/biomedicines10010171. [DOI] [PMC free article] [PubMed]
- 114.Jena A, James D, Singh AK, et al. Effectiveness and durability of COVID-19 vaccination in 9447 patients with IBD: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022 doi: 10.1016/j.cgh.2022.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn's disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017;390:2779–2789. doi: 10.1016/S0140-6736(17)32641-7. [DOI] [PubMed] [Google Scholar]
- 116.Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. The Lancet. 2004;364:263–269. doi: 10.1016/s0140-6736(04)16676-2. [DOI] [PubMed] [Google Scholar]
- 117.Verstappen SM, Jacobs JW, van der Veen MJ, et al. Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Computer assisted management in early rheumatoid arthritis (CAMERA, an open-label strategy trial) Ann Rheum Dis. 2007;66:1443–1449. doi: 10.1136/ard.2007.071092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.van Vollenhoven R. Treat-to-target in rheumatoid arthritis—Are we there yet? Nat Rev Rheumatol. 2019;15:180–186. doi: 10.1038/s41584-019-0170-5. [DOI] [PubMed] [Google Scholar]
- 119.Coates LC, Moverley AR, McParland L, et al. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open-label, randomised controlled trial. The Lancet. 2015;386:2489–2498. doi: 10.1016/s0140-6736(15)00347-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Molto A, López-Medina C, Van den Bosch FE, et al. Efficacy of a tight-control and treat-to-target strategy in axial spondyloarthritis: results of the open-label, pragmatic, cluster-randomised TICOSPA trial. Ann Rheum Dis. 2021;80:1436–1444. doi: 10.1136/annrheumdis-2020-219585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nakayama Y, Watanabe R, Murakami K, et al. Differential efficacy of TNF inhibitors with or without the immunoglobulin fragment crystallizable (Fc) portion in rheumatoid arthritis: the ANSWER cohort study. Rheumatol Int. 2022 doi: 10.1007/s00296-021-05086-w. [DOI] [PubMed] [Google Scholar]
- 122.Bello S, Bonali C, Serafino L, et al. Intra-articular therapy with tumor necrosis factor-alpha antagonists: an update. Reumatismo. 2014;65:257–263. doi: 10.4081/reumatismo.2013.721. [DOI] [PubMed] [Google Scholar]
- 123.Bolge SC, Goren A, Tandon N. Reasons for discontinuation of subcutaneous biologic therapy in the treatment of rheumatoid arthritis: a patient perspective. Patient Prefer Adherence. 2015;9:121–131. doi: 10.2147/PPA.S70834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Garcia-Lagunar MH, Gutierrez-Civicos MR, Garcia-Simon MS, et al. Reasons for discontinuation and adverse effects of TNFalpha inhibitors in a cohort of patients with rheumatoid arthritis and ankylosing spondylitis. Ann Pharmacother. 2017;51:388–393. doi: 10.1177/1060028016682330. [DOI] [PubMed] [Google Scholar]
- 125.Nam JL, Takase-Minegishi K, Ramiro S, et al. Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2016 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2017;76:1113–1136. doi: 10.1136/annrheumdis-2016-210713. [DOI] [PubMed] [Google Scholar]
- 126.Gottenberg JE, Brocq O, Perdriger A, et al. Non-TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first Anti-TNF drug: a randomized clinical trial. JAMA. 2016;316:1172–1180. doi: 10.1001/jama.2016.13512. [DOI] [PubMed] [Google Scholar]
- 127.Todoerti M, Favalli EG, Iannone F, et al (2018) Switch or swap strategy in rheumatoid arthritis patients failing TNF inhibitors? Results of a modified Italian Expert Consensus. Rheumatology (Oxford) 57: vii42-vii53. 10.1093/rheumatology/key195. [DOI] [PubMed]
- 128.Kobayakawa T, Kojima T, Takahashi N, et al. Drug retention rates of second biologic agents after switching from tumor necrosis factor inhibitors for rheumatoid arthritis in Japanese patients on low-dose methotrexate or without methotrexate. Mod Rheumatol. 2015;25:251–256. doi: 10.3109/14397595.2014.953668. [DOI] [PubMed] [Google Scholar]
- 129.Micheroli R, Tellenbach C, Scherer A, et al. Effectiveness of secukinumab versus an alternative TNF inhibitor in patients with axial spondyloarthritis previously exposed to TNF inhibitors in the swiss clinical quality management cohort. Ann Rheum Dis. 2020;79:1203–1209. doi: 10.1136/annrheumdis-2019-215934. [DOI] [PubMed] [Google Scholar]
- 130.Dougados M, Wei JC, Landewe R, et al. Efficacy and safety of ixekizumab through 52 weeks in two phase 3, randomised, controlled clinical trials in patients with active radiographic axial spondyloarthritis (COAST-V and COAST-W) Ann Rheum Dis. 2020;79:176–185. doi: 10.1136/annrheumdis-2019-216118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Singh JA, Guyatt G, Ogdie A, et al. Special article: 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis. Arthritis Rheumatol. 2019;71:5–32. doi: 10.1002/art.40726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gisbert JP, Chaparro M (2021) Primary Failure to an Anti-TNF Agent in Inflammatory Bowel Disease: Switch (to a Second Anti-TNF Agent) or Swap (for Another Mechanism of Action)? J Clin Med 10 10.3390/jcm10225318. [DOI] [PMC free article] [PubMed]
- 133.Kerdel F, Zaiac M. An evolution in switching therapy for psoriasis patients who fail to meet treatment goals. Dermatol Ther. 2015;28:390–403. doi: 10.1111/dth.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cantini F, Niccoli L, Nannini C, et al. Second-line biologic therapy optimization in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Semin Arthritis Rheum. 2017;47:183–192. doi: 10.1016/j.semarthrit.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 135.Wu JF. Therapeutic drug monitoring of biologics for patients with inflammatory bowel diseases: How, When, and for Whom? Gut Liver. 2021 doi: 10.5009/gnl210262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Syversen SW, Jorgensen KK, Goll GL, et al. Effect of therapeutic drug monitoring vs standard therapy during maintenance infliximab therapy on disease control in patients with immune-mediated inflammatory diseases: a randomized clinical trial. JAMA. 2021;326:2375–2384. doi: 10.1001/jama.2021.21316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707–718. doi: 10.1038/nrrheum.2017.187. [DOI] [PubMed] [Google Scholar]
- 138.Owczarczyk-Saczonek A, Owczarek W, Osmola-Mankowska A, et al. Secondary failure of TNF-alpha inhibitors in clinical practice. Dermatol Ther. 2019;32:e12760. doi: 10.1111/dth.12760. [DOI] [PubMed] [Google Scholar]
- 139.Bitoun S, Nocturne G, Ly B, et al. Methotrexate and BAFF interaction prevents immunization against TNF inhibitors. Ann Rheum Dis. 2018;77:1463–1470. doi: 10.1136/annrheumdis-2018-213403. [DOI] [PubMed] [Google Scholar]
- 140.Gorelik Y, Freilich S, Gerassy-Vainberg S, et al. Antibiotic use differentially affects the risk of anti-drug antibody formation during anti-TNFalpha therapy in inflammatory bowel disease patients: a report from the epi-IIRN. Gut. 2021 doi: 10.1136/gutjnl-2021-325185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fraenkel L, Bathon JM, England BR, et al. 2021 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2021;73:1108–1123. doi: 10.1002/art.41752. [DOI] [PubMed] [Google Scholar]
- 142.van Mulligen E, de Jong PHP, Kuijper TM, et al. Gradual tapering TNF inhibitors versus conventional synthetic DMARDs after achieving controlled disease in patients with rheumatoid arthritis: first-year results of the randomised controlled TARA study. Ann Rheum Dis. 2019;78:746–753. doi: 10.1136/annrheumdis-2018-214970. [DOI] [PubMed] [Google Scholar]
- 143.Curtis JR, Emery P, Karis E, et al. Etanercept or methotrexate withdrawal in rheumatoid arthritis patients in sustained remission. Arthritis Rheumatol. 2021;73:759–768. doi: 10.1002/art.41589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mangoni AA, Al Okaily F, Almoallim H, et al. Relapse rates after elective discontinuation of anti-TNF therapy in rheumatoid arthritis: a meta-analysis and review of literature. BMC Rheumatol. 2019;3:10. doi: 10.1186/s41927-019-0058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tanaka Y (2016) Stopping tumour necrosis factor-targeted biological DMARDs in rheumatoid arthritis. Rheumatology (Oxford) 55: ii15-ii22. 10.1093/rheumatology/kew352. [DOI] [PubMed]
- 146.Sigaux J, Bailly F, Hajage D, et al. Sustainability of TNF-blocker tapering in rheumatoid arthritis over 3 years: long-term follow-up of the STRASS (Spacing of TNF-blocker injections in Rheumatoid ArthritiS Study) randomised controlled trial. RMD Open. 2017;3:e000474. doi: 10.1136/rmdopen-2017-000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tanaka Y, Hirata S, Kubo S, et al. Discontinuation of adalimumab after achieving remission in patients with established rheumatoid arthritis: 1-year outcome of the HONOR study. Ann Rheum Dis. 2015;74:389–395. doi: 10.1136/annrheumdis-2013-204016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tanaka Y, Hirata S, Saleem B, et al. Discontinuation of biologics in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2013;31:S22–S27. [PubMed] [Google Scholar]
- 149.Navarro-Compan V, Plasencia-Rodriguez C, de Miguel E, et al. Anti-TNF discontinuation and tapering strategies in patients with axial spondyloarthritis: a systematic literature review. Rheumatology (Oxford) 2016;55:1188–1194. doi: 10.1093/rheumatology/kew033. [DOI] [PubMed] [Google Scholar]
- 150.Landewe RB, van der Heijde D, Dougados M, et al. Maintenance of clinical remission in early axial spondyloarthritis following certolizumab pegol dose reduction. Ann Rheum Dis. 2020;79:920–928. doi: 10.1136/annrheumdis-2019-216839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ye W, Tucker LJ, Coates LC. Tapering and discontinuation of biologics in patients with psoriatic arthritis with low disease activity. Drugs. 2018;78:1705–1715. doi: 10.1007/s40265-018-0994-3. [DOI] [PubMed] [Google Scholar]
- 152.Huynh DH, Boyd TA, Etzel CJ, et al. Persistence of low disease activity after tumour necrosis factor inhibitor (TNFi) discontinuation in patients with psoriatic arthritis. RMD Open. 2017;3:e000395. doi: 10.1136/rmdopen-2016-000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Iglesias E, Torrente-Segarra V, Bou R, et al. Non-systemic juvenile idiopathic arthritis outcome after reaching clinical remission with anti-TNF-alpha therapy: a clinical practice observational study of patients who discontinued treatment. Rheumatol Int. 2014;34:1053–1057. doi: 10.1007/s00296-013-2884-z. [DOI] [PubMed] [Google Scholar]
- 154.Cai Y, Liu X, Zhang W, et al. Clinical trial of etanercept tapering in juvenile idiopathic arthritis during remission. Rheumatol Int. 2013;33:2277–2282. doi: 10.1007/s00296-012-2642-7. [DOI] [PubMed] [Google Scholar]
- 155.Gisbert JP, Marin AC, Chaparro M. The risk of relapse after anti-tnf discontinuation in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2016;111:632–647. doi: 10.1038/ajg.2016.54. [DOI] [PubMed] [Google Scholar]
- 156.Kobayashi T, Motoya S, Nakamura S, et al. Discontinuation of infliximab in patients with ulcerative colitis in remission (HAYABUSA): a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol. 2021;6:429–437. doi: 10.1016/S2468-1253(21)00062-5. [DOI] [PubMed] [Google Scholar]
- 157.Little DHW, Tabatabavakili S, Shaffer SR, et al (2020) Effectiveness of Dose De-escalation of Biologic Therapy in Inflammatory Bowel Disease: A Systematic Review. Am J Gastroenterol 115: 1768–74. 10.14309/ajg.0000000000000783. [DOI] [PubMed]
- 158.Torres J, Boyapati RK, Kennedy NA, et al. Systematic review of effects of withdrawal of immunomodulators or biologic agents from patients with inflammatory bowel disease. Gastroenterology. 2015;149:1716–1730. doi: 10.1053/j.gastro.2015.08.055. [DOI] [PubMed] [Google Scholar]
- 159.Kim B, Maverakis E, Raychaudhuri SP. Is it possible to discontinue tumor necrosis factor antagonists after psoriasis remission? Ann Dermatol. 2019;31:495–501. doi: 10.5021/ad.2019.31.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Stinco G, Balato N, Buligan C, et al (2019) A multicenter retrospective case-control study on Suspension of TNF-inhibitors and Outcomes in Psoriatic patients (STOP study). G Ital Dermatol Venereol 154: 392–9. 10.23736/S0392-0488.18.06156-4. [DOI] [PubMed]
- 161.Hansel K, Bianchi L, Lanza F, et al. Adalimumab dose tapering in psoriasis: predictive factors for maintenance of complete clearance. Acta Derm Venereol. 2017;97:346–350. doi: 10.2340/00015555-2571. [DOI] [PubMed] [Google Scholar]
- 162.Sfikakis PP, Arida A, Panopoulos S, et al. Brief report: drug-free long-term remission in severe behcet's disease following withdrawal of successful anti-tumor necrosis factor treatment. Arthritis Rheumatol. 2017;69:2380–2385. doi: 10.1002/art.40235. [DOI] [PubMed] [Google Scholar]
- 163.Tweehuysen L, den Broeder N, van Herwaarden N, et al. Predictive value of serum calprotectin (S100A8/A9) for clinical response after starting or tapering anti-TNF treatment in patients with rheumatoid arthritis. RMD Open. 2018;4:e000654. doi: 10.1136/rmdopen-2018-000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tao W, Concepcion AN, Vianen M, et al. Multiomics and machine learning accurately predict clinical response to adalimumab and etanercept therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2021;73:212–222. doi: 10.1002/art.41516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yoosuf N, Maciejewski M, Ziemek D, et al. Early prediction of clinical response to anti-TNF treatment using multi-omics and machine learning in rheumatoid arthritis. Rheumatology (Oxford) 2021 doi: 10.1093/rheumatology/keab521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gole B, Potocnik U (2019) Pre-Treatment biomarkers of anti-tumour necrosis factor therapy response in crohn's disease-a systematic review and gene ontology analysis. Cells 8 10.3390/cells8060515. [DOI] [PMC free article] [PubMed]
- 167.Evangelatos G, Fragoulis GE, Koulouri V, et al. MicroRNAs in rheumatoid arthritis: from pathogenesis to clinical impact. Autoimmun Rev. 2019;18:102391. doi: 10.1016/j.autrev.2019.102391. [DOI] [PubMed] [Google Scholar]
- 168.Atreya R, Neurath MF. Mechanisms of molecular resistance and predictors of response to biological therapy in inflammatory bowel disease. Lancet Gastroenterol Hepatol. 2018;3:790–802. doi: 10.1016/S2468-1253(18)30265-6. [DOI] [PubMed] [Google Scholar]
- 169.Gaujoux R, Starosvetsky E, Maimon N, et al. Cell-centred meta-analysis reveals baseline predictors of anti-TNFalpha non-response in biopsy and blood of patients with IBD. Gut. 2019;68:604–614. doi: 10.1136/gutjnl-2017-315494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Arijs I, Li K, Toedter G, et al. Mucosal gene signatures to predict response to infliximab in patients with ulcerative colitis. Gut. 2009;58:1612–1619. doi: 10.1136/gut.2009.178665. [DOI] [PubMed] [Google Scholar]
- 171.Arijs I, Quintens R, Van Lommel L, et al. Predictive value of epithelial gene expression profiles for response to infliximab in Crohn's disease. Inflamm Bowel Dis. 2010;16:2090–2098. doi: 10.1002/ibd.21301. [DOI] [PubMed] [Google Scholar]
- 172.Schmitt H, Billmeier U, Dieterich W, et al. Expansion of IL-23 receptor bearing TNFR2+ T cells is associated with molecular resistance to anti-TNF therapy in Crohn's disease. Gut. 2019;68:814–828. doi: 10.1136/gutjnl-2017-315671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Romero-Cara P, Torres-Moreno D, Pedregosa J, et al. A FCGR3A polymorphism predicts anti-drug antibodies in chronic inflammatory bowel disease patients treated with anti-TNF. Int J Med Sci. 2018;15:10–15. doi: 10.7150/ijms.22812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sazonovs A, Kennedy NA, Moutsianas L, et al. HLA-DQA1*05 carriage associated with development of anti-drug antibodies to infliximab and adalimumab in patients with crohn's disease. Gastroenterology. 2020;158:189–199. doi: 10.1053/j.gastro.2019.09.041. [DOI] [PubMed] [Google Scholar]
- 175.Kim H, Alten R, Cummings F, et al. Innovative approaches to biologic development on the trail of CT-P13: biosimilars, value-added medicines, and biobetters. MAbs. 2021;13:1868078. doi: 10.1080/19420862.2020.1868078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Burchiel SW, Aspbury R, Munday J. The search for biosimilars and biobetters. Drug Discov Today. 2019;24:1087–1091. doi: 10.1016/j.drudis.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 177.Smolen JS, Goncalves J, Quinn M, et al. Era of biosimilars in rheumatology: reshaping the healthcare environment. RMD Open. 2019;5:e000900. doi: 10.1136/rmdopen-2019-000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kravvariti E, Kitas GD, Mitsikostas DD, et al. Nocebos in rheumatology: emerging concepts and their implications for clinical practice. Nat Rev Rheumatol. 2018;14:727–740. doi: 10.1038/s41584-018-0110-9. [DOI] [PubMed] [Google Scholar]
- 179.Kravvariti E, Kitas GD, Sfikakis PP (2019) The role of the Nocebo effect in the use of biosimilars in routine rheumatology clinical practice. Mediterr J Rheumatol 30: 63–8. 10.31138/mjr.30.1.63. [DOI] [PMC free article] [PubMed]
- 180.Fragoulis GE, Evangelatos G, Arida A, et al (2020) Nocebo-Prone Behaviour in Patients with Autoimmune Rheumatic Diseases during the COVID-19 Pandemic. Mediterr J Rheumatol 31: 288–94. 10.31138/mjr.31.3.288. [DOI] [PMC free article] [PubMed]
- 181.Korkmaz E, Friedrich EE, Ramadan MH, et al. Therapeutic intradermal delivery of tumor necrosis factor-alpha antibodies using tip-loaded dissolvable microneedle arrays. Acta Biomater. 2015;24:96–105. doi: 10.1016/j.actbio.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ferrari M, Onuoha SC, Fossati-Jimack L, et al. Novel bispecific antibody for synovial-specific target delivery of anti-tnf therapy in rheumatoid arthritis. Front Immunol. 2021;12:640070. doi: 10.3389/fimmu.2021.640070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gareb B, Otten AT, Frijlink HW, et al (2020) Review: Local Tumor Necrosis Factor-alpha Inhibition in Inflammatory Bowel Disease. Pharmaceutics 12 10.3390/pharmaceutics12060539. [DOI] [PMC free article] [PubMed]
- 184.Griffiths OR, Landon J, Coxon RE, et al. Inflammatory bowel disease and targeted oral anti-TNFalpha therapy. Adv Protein Chem Struct Biol. 2020;119:157–198. doi: 10.1016/bs.apcsb.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 185.Li X, Yu M, Zhu Z, et al. Oral delivery of infliximab using nano-in-microparticles for the treatment of inflammatory bowel disease. Carbohydr Polym. 2021;273:118556. doi: 10.1016/j.carbpol.2021.118556. [DOI] [PubMed] [Google Scholar]
- 186.Wang X, Yan J, Wang L, et al. Oral delivery of anti-TNF antibody shielded by natural polyphenol-mediated supramolecular assembly for inflammatory bowel disease therapy. Theranostics. 2020;10:10808–10822. doi: 10.7150/thno.47601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Eder P, Zielinska A, Karczewski J, et al. How could nanobiotechnology improve treatment outcomes of anti-TNF-alpha therapy in inflammatory bowel disease? Current knowledge, future directions. J Nanobiotechnology. 2021;19:346. doi: 10.1186/s12951-021-01090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chiabai MJ, Almeida JF, de Azevedo MGD, et al. Mucosal delivery of Lactococcus lactis carrying an anti-TNF scFv expression vector ameliorates experimental colitis in mice. BMC Biotechnol. 2019;19:38. doi: 10.1186/s12896-019-0518-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tang W, Lu Y, Tian QY, et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;332:478–484. doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Deng GM, Zheng L, Chan FK, et al. Amelioration of inflammatory arthritis by targeting the pre-ligand assembly domain of tumor necrosis factor receptors. Nat Med. 2005;11:1066–1072. doi: 10.1038/nm1304. [DOI] [PubMed] [Google Scholar]
- 191.Deng GM, Liu L, Tsokos GC. Targeted tumor necrosis factor receptor I preligand assembly domain improves skin lesions in MRL/lpr mice. Arthritis Rheum. 2010;62:2424–2431. doi: 10.1002/art.27534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Williams A, Wang ECY, Thurner L, et al. Review: novel insights into tumor necrosis factor receptor, death receptor 3, and progranulin pathways in arthritis and bone remodeling. Arthritis & Rheumatology. 2016;68:2845–2856. doi: 10.1002/art.39816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.McCann FE, Perocheau DP, Ruspi G, et al. Selective tumor necrosis factor receptor I blockade is antiinflammatory and reveals immunoregulatory role of tumor necrosis factor receptor II in collagen-induced arthritis. Arthritis Rheumatol. 2014;66:2728–2738. doi: 10.1002/art.38755. [DOI] [PubMed] [Google Scholar]
- 194.Shibata H, Yoshioka Y, Abe Y, et al. The treatment of established murine collagen-induced arthritis with a TNFR1-selective antagonistic mutant TNF. Biomaterials. 2009;30:6638–6647. doi: 10.1016/j.biomaterials.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 195.Okubo Y, Mera T, Wang L, et al. Homogeneous expansion of human T-regulatory cells via tumor necrosis factor receptor 2. Sci Rep. 2013;3:3153. doi: 10.1038/srep03153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li P, Zheng Y, Chen X. Drugs for autoimmune inflammatory diseases: from small molecule compounds to anti-TNF biologics. Front Pharmacol. 2017;8:460. doi: 10.3389/fphar.2017.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang N, Wang Z, Zhao Y. Selective inhibition of tumor necrosis factor receptor-1 (TNFR1) for the treatment of autoimmune diseases. Cytokine Growth Factor Rev. 2020;55:80–85. doi: 10.1016/j.cytogfr.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 198.Fischer R, Kontermann RE, Pfizenmaier K. Selective targeting of TNF receptors as a novel therapeutic approach. Front Cell Dev Biol. 2020;8:401. doi: 10.3389/fcell.2020.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Weinelt N, Karathanasis C, Smith S, et al. Quantitative single-molecule imaging of TNFR1 reveals zafirlukast as antagonist of TNFR1 clustering and TNFalpha-induced NF-kB signaling. J Leukoc Biol. 2021;109:363–371. doi: 10.1002/JLB.2AB0420-572RR. [DOI] [PubMed] [Google Scholar]
- 200.O'Connell J, Porter J, Kroeplien B, et al. Small molecules that inhibit TNF signalling by stabilising an asymmetric form of the trimer. Nat Commun. 2019;10:5795. doi: 10.1038/s41467-019-13616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Papadopoulou D, Drakopoulos A, Lagarias P, et al (2021) In Silico Identification and Evaluation of Natural Products as Potential Tumor Necrosis Factor Function Inhibitors Using Advanced Enalos Asclepios KNIME Nodes. Int J Mol Sci 22 10.3390/ijms221910220. [DOI] [PMC free article] [PubMed]
- 202.Ntari L, Nikolaou C, Kranidioti K, et al. Combination of subtherapeutic anti-TNF dose with dasatinib restores clinical and molecular arthritogenic profiles better than standard anti-TNF treatment. J Transl Med. 2021;19:165. doi: 10.1186/s12967-021-02764-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gold SL, Steinlauf AF. Efficacy and safety of dual biologic therapy in patients with inflammatory bowel disease: a review of the literature. Gastroenterol Hepatol (N Y) 2021;17:406–414. [PMC free article] [PubMed] [Google Scholar]
- 204.Hammoura I, Fiechter RH, Bryant SH, et al (2022) Dual Blockade of TNF and IL-17A Inhibits Inflammation and Structural Damage in a Rat Model of Spondyloarthritis. Int J Mol Sci 23 10.3390/ijms23020859. [DOI] [PMC free article] [PubMed]
- 205.Koenders MI, Marijnissen RJ, Devesa I, et al. Tumor necrosis factor-interleukin-17 interplay induces S100A8, interleukin-1beta, and matrix metalloproteinases, and drives irreversible cartilage destruction in murine arthritis: rationale for combination treatment during arthritis. Arthritis Rheum. 2011;63:2329–2339. doi: 10.1002/art.30418. [DOI] [PubMed] [Google Scholar]
- 206.Fischer JA, Hueber AJ, Wilson S, et al. Combined inhibition of tumor necrosis factor alpha and interleukin-17 as a therapeutic opportunity in rheumatoid arthritis: development and characterization of a novel bispecific antibody. Arthritis Rheumatol. 2015;67:51–62. doi: 10.1002/art.38896. [DOI] [PubMed] [Google Scholar]
- 207.Nosenko MA, Atretkhany KN, Mokhonov VV, et al. VHH-based bispecific antibodies targeting cytokine production. Front Immunol. 2017;8:1073. doi: 10.3389/fimmu.2017.01073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zheng S, Shen F, Jones B, et al. Characterization of concurrent target suppression by JNJ-61178104, a bispecific antibody against human tumor necrosis factor and interleukin-17A. MAbs. 2020;12:1770018. doi: 10.1080/19420862.2020.1770018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Stoffel B, McPherson M, Hernandez A, et al (2021) Pos0365 Anti-Tnf Glucocorticoid Receptor Modulator Antibody Drug Conjugate for the Treatment of Autoimmune Diseases. Annals of the Rheumatic Diseases 80: 412.2–3. 10.1136/annrheumdis-2021-eular.2213.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.