Abstract
Extracellular vesicles (EVs) are cell-derived membrane structures exerting major effects in physiological as well as pathological processes by functioning as vehicles for the delivery of biomolecules to their target cells. An increasing number of effects previously attributed to cell-based therapies have been recognized to be actually mediated by EVs derived from the respective cells, suggesting the administration of purified EVs instead of living cells for cell-based therapies. In this review, we focus on the heterogeneity of EVs derived from mesenchymal stem/stromal cells (MSC) and summarize upstream process parameters that crucially affect the resulting therapeutic properties and biological functions. Hereby, we discuss the effects of the cell source, medium composition, 3D culture, bioreactor culture and hypoxia. Furthermore, aspects of the isolation and storage strategies influences EVs are described. Conclusively, optimization of upstream process parameters should focus on controlling MSC-derived EV heterogeneity for specific therapeutic applications.
Graphical Abstract
Keywords: Extracellular vesicles, Mesenchymal Stem Cells, Cell Culture Conditions, Scalability, Regenerative Medicine
Introduction
Intercellular communication has long been attributed to soluble factors and adhesion molecules, mediating cell-to-cell interactions. In the last decade, the biological significance of extracellular vesicles (EVs) has gained much recognition, especially their functions in intercellular communication in both, physiological and pathological settings [1].
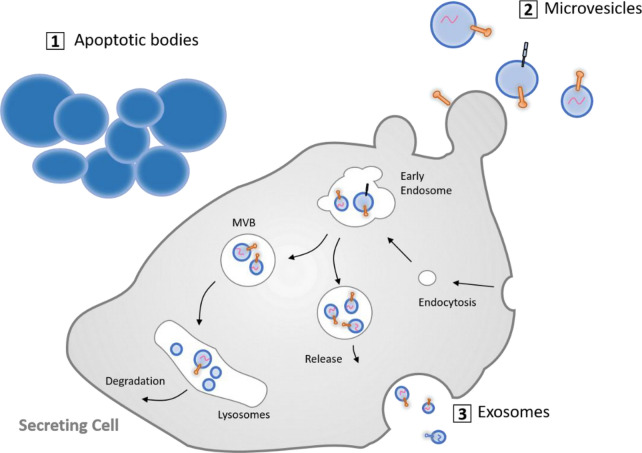
Multiple types of EVs have been described with different sites of cellular origin (reviewed in van der Pol et al. [2]) and with distinct molecular and biological properties. Three major EV subtypes (Fig. 1) have been classified based on their size and biogenesis, namely (i) exosomes (40–150 nm in diameter), (ii) microvesicles (100–1000 nm), and (iii) apoptotic bodies (> 1000 nm). Exosomes represent the most extensively studied EV species, and their secretion was originally described as a process that can complement and supplement lysosomal and proteasomal degradation for the removal of obsolete membrane and cytosolic materials [3]. They are formed by the intraluminal invagination of the membrane of the late endosome/multi-vesicular body (MVB) and subsequent fusion of MVBs with the plasma membrane (reviewed in Kreimer et al. [4] and van der Pol et al. [5]). Microvesicles derive from the plasma membrane and are continuously released from the cell membrane of apparently all cells under physiological conditions, although their release can be further triggered under pathological conditions [6]. Apoptotic bodies, finally, result from the disassembly of apoptotic cells into subcellular fragments. The formation of apoptotic bodies can promote efficient removal of cell debris by means of macrophages, and they were previously regarded as “sealed containers” for substances from dying cells, until the discovery that they are capable of delivering their cargo to healthy recipient cells, as well.
Fig. 1.
Subtypes of extracellular vesicles in eukaryotic cells. Cells can release three different types of EVs: (i) apoptotic bodies are generated during programmed cell death by membrane blebbing, (ii) microvesicles are shed by outward budding and fission of the plasma membrane, and (iii) exosomes are formed as intraluminal vesicles via inward budding of early endosomes, giving rise to multivesicular bodies (MVBs), which either fuse with lysosomes or with the plasma membrane, leading to the secretion of exosomes. Illustration
adapted from Gustafson et al. [7]
The function of EVs is closely linked to their cargo (Fig. 2), which can include functional mRNA, miRNA, lipids, and proteins. Transfer of this cargo to adjacent or distant recipient cells makes EVs important messengers in cell–cell communication. Beyond their cargo, EV surface molecules are of critical functional significance as they (i) establish connections with the surrounding milieu and with cells, (ii) determine EV mobility, (iii) mediate cellular uptake, (iv) affect immune recognition of EVs by the innate and adaptive immune systems, and (v) may represent effector molecules (such as FasL) [8, 9]. Moreover, these EV surface molecules enable the identification, affinity isolation, and molecular classification of EVs and their use as biomarkers [10].
Fig. 2.

Composition of extracellular vesicles (EVs). EVs are lipid-bound vesicles secreted by most cells into the extracellular space. They consist of lipids, nucleic acids, and proteins, which are specifically associated with the plasma membrane, cytosol, and those related to lipid metabolism of the parent cell. The cargo of EVs can be transferred to target cells and induce biological effects that alter cell behavior
Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles
Mesenchymal stem cells (MSCs) are multipotent, nonhematopoietic adult stem cells that are characterized by their capability to differentiate into mesenchymal lineages such as chondrocytes, osteoblasts and adipocytes as well as non-mesenchymal lineages including hepatocytes and neuronal cell types. MSCs have the ability of colony formation, self-renewal, and secretion of trophic factors such as cytokines and growth factors, which play major roles in physiological and pathological processes. For these reasons, MSCs have been extensively used for wound healing and immunomodulation by administration and migration to the damaged site, engraftment and subsequent differentiation into the desired tissue [11]. Numerous clinical trials have been conducted using MSC as therapeutic agents to treat diseases, such as multiple sclerosis, osteoarthritis, cardiovascular disease (CVD), Alzheimer’s disease, kidney disease, diabetes mellitus, knee cartilage injuries, organ transplantation, and graft-versus-host disease (GvHD). By August 2021, the National Institutes of Health clinical trial database www.clinicaltrials.gov contained over 1,100 registered clinical trials in the category of stem cell therapies. There is solid evidence that MSCs exert their effects mainly through strong paracrine action on the neighboring cells via the secretion of trophic bioactive factors, such as growth factors, cytokines and chemokines [12]. In addition to these soluble factors, it has become evident that MSC-derived EVs are part of the stem cell secretome and play a major role in mediating the effects of stem cells [13]. Moreover, cell-free therapies using EVs could circumvent disadvantages associated with MSC therapies, namely low survival rate of cells upon adminstration, morphological changes during therapy, and the possibility of dedifferentiation into undesired tissue cell types [14–16]. A search on clinicaltrials.gov revealed that by September 2021 84 trials of EVs from different sources were registered worldwide (Fig. 3). However, only 4 were related to MSC-derived EVs (search term: mesenchymal stem cell-derived extracellular vesicles) and 16 to MSC-derived exosomes (search term: mesenchymal stem cell-derived exosomes), indicating the novelty and potential of this source (Fig. 4).
Fig. 3.
Global clinical trials on extracellular vesicles for cell-free therapy. World map indicating the number of clinical trials registered globally to date (September 2021, search term: extracellular vesicles)
Fig. 4.
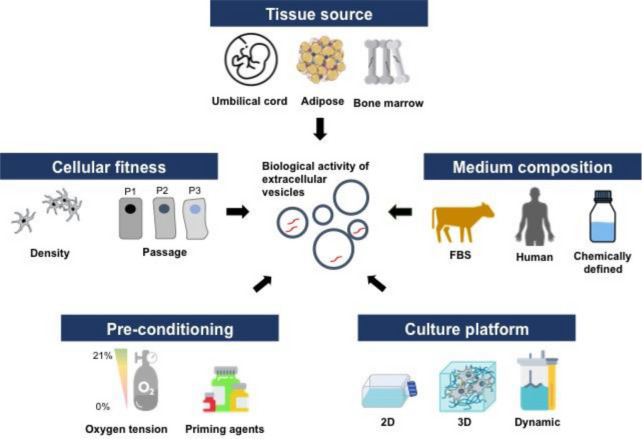
Key considerations for MSC-derived EV production. EVs are cellular products that are impacted by various culture conditions including tissue source, cell state (cellular passage, cell density during cultivation) as well as medium composition and culture platforms. Different culture conditions have been shown to influence the biological function of EVs. Therefore, careful considerations of these parameters are required upon manufacturing EVs for therapies
MSC-derived EVs have successfully been used to treat GvHD and are considered less immunogenic compared to their parent cells due to their lower content of major histocompatibility complex (MHC) molecules. These characteristics of MSC-derived EVs and their inability to form tumors make them strong candidates for cell-free therapy [17]. For example, MSC-derived EVs have been found to protect against myocardial ischemia (MI) [18], to reverse radiation toxicity [19], attenuate mitochondrial damage [20], and to enhance survival after acute kidney injury (AKI) through the transfer of MSC-EV specific miRNAs, such as hsa-let-7b and hsa-let-7 g miRNAs [21].
Furthermore, EVs could as well provide a natural alternative to standard drug delivery systems as they possess low immunogenicity and cytotoxicity. Nanoparticle-based drug delivery systems based on polymeric micelles, liposomes and nano-sized polymer-drug conjugates serve to improve the pharmacokinetics and biodistribution of chemical and biological therapeutic agents [22]. Their application, however, is associated with concerns regarding their potential immunogenicity and cytotoxicity and their rapid clearance upon clinical administration [23, 24]. The protein and RNA in EVs are encapsulated by a lipid layer, providing a protective barrier, which increases the success rate of delivery to the target cells [25, 26]. Indeed, numerous studies indicate the efficiency of MSC-derived EVs as carriers of chemotherapeutics [27], as well as RNA-based- [28] and anti-inflammatory drugs [29]. Different uptake mechanisms have been proposed in the literature, including phagocytosis or fusion of EVs with the plasma membrane of recipient cells. In addition, cells might permit the selective uptake of EVs depending on their surface receptor repertoire [18].
The undeniable potential of MSC-derived EVs in regenerative medicine leads to new possibilities and growing interest of the scientific community [13]. However, despite the therapeutic promise and success of MSC-derived EVs, the use of these EVs in clinical settings will require the resolution of several critical issues, such as (i) large-scale production and isolation methods, (ii) methods for rapid and accurate quantification and characterization of EVs, (iii) precise characterization of the cargo, (iv) pharmacokinetics, targeting and transfer mechanisms of EVs to the target sites, and (v) safety profiles to determine the optimal clinical dosage and possible toxicities upon repeated administration [30–32]. Furthermore, there is increasing evidence showing that the properties and biological functions of EVs are influenced by different manufacturing parameters such as cell source, culture conditions as well as enrichment protocols and characterization strategies [32].
Hence, this review provides a summary on the effects of various parameters, particularly upstream process parameters, on therapeutically relevant properties and biological functions of MSC-derived EVs. Additionally, several downstream process parameters, such as isolation methods and storage strategies, will be discussed as these methods are crucial for the improvement of the purity and yield of MSC-EVs.
Influences of process parameters on the quality and heterogeneity of MSC-derived extracellular vesicles
MSC sources for EV production
The composition of EVs is largely determined by the cell source and by the physiological state of their parent cells [30, 33]. Indeed, studies have shown that the secretome of BM-MSC-EVs highly inhibit the accumulation of inflammatory and apoptotic cell and mediates the maturation, proliferation and activation of B cells by exerting differential mRNA expression of relevant genes [34]. Whereas, umbilical cord-derived MSC-EVs (ucMSC-EVs) suppress oxidative stress in cisplatin-induced AKI by activating ERK1/2 pathway, promote angiogenesis for fracture healing and improve proliferation and migration of skin cells for wound healing [35]. Shekari et al. [36] summarized in a recent review article that bone marrow MSC (43% of all publications included in the systematic review, used as MSC source for EV derivation) were the preferred source of EVs in different disease categories, except for studies that involved the skin, liver and the vasculature as well as reproductive systems. Other MSC sources listed were placenta-derived EVs (Plac-MSC-EVs), adipose tissue MSC-EVs (AD-MSC-EV), pluripotent stem cell-derived MSC (Pluri-MSC-EVs), and derived from other tissue-derived EVs (TD-MSC-EV). Pluri-MSC-EVs were prevalently used for treatments of the liver, inflammation, transplantation and musculoskeletal diseases, which could be related to their low immunogenicity compared to MSCs from other sources. Contrarily, AD-MSC-EVs were not widely used in cancer or pancreatic diseases, but rather for treatment of skin and inflammation and transplantation diseases. Interestingly, Plac-MSC-EVs were used for a diversity of aforementioned disease categories except for autoimmune conditions [36]. La Greca et al. [37] reported differences in the proteome profile of iPSC-derived, iPSC-MSC-derived (PD-MSC) and MSC-derived EVs. Apparently, iPSC-derived EVs share a greater number of proteins with their respective cells, as compared to PD-MSC-derived EVs. This suggests that upon differentiation from iPSCs to PD-MSCs, a change of the EV protein composition is mediated and therefore EVs from PD-MSCs acquire a more specific protein footprint and functionality related to the stem cell niche [37]. This indicates that MSCs from different sources, even from the same donor, indeed vary in their molecular composition as presented in Table 1. Consequently, these variations could therefore have influenced the functional differences as reported in the aforementioned studies. However, the authors of the respective studies did not discuss their choice of EV source for a particular disease model. Hence, further investigation needs to be performed to determine which MSC source for EV production is most suited for a particular disease. Besides the cell source, other parameters such as culture conditions, harvesting period, as well as enrichment methods impact the structural and functional EV heterogeneity [38, 39], which will be addressed in the following sections.
Table 1.
Specific surface markers identified in purified samples from different MSC sources
| Harvest [hours] |
EV marker | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CD9 | CD63 | CD81 | CD59 | Alix | TSG 101 |
Hsp 70/90 |
|||
| iPSC-MSC | 72 | + | + | + | + | − | − | − | Lai [14] |
| 24 | + | + | + | La Greca [37] | |||||
| 24 | + | + | + | + | + | Zhao [40] | |||
| Adipose tissue | 24 | − | − | + | − | + | − | − | Otero-Ortega [41] |
| 24 | + | + | + | + | Conolly [42] | ||||
| 48 | + | + | + | Zhu [43] | |||||
| 24 | + | + | + | + | + | + | Durcin [44] | ||
| 48 | + | + | Eirin [45] | ||||||
| Umbilical cord | 36 | + | − | + | Zhang [46] | ||||
| 24–48 | + | + | + | + | − | − | + | Kilpinen [47] | |
| 48 | + | + | + | − | − | − | − | Wang [48] | |
| 24 | + | + | Zou [49] | ||||||
| 48 | + | + | + | Zhang [50] | |||||
| Bone marrow | 24 | − | + | + | + | − | − | − | Kim [51] |
| 72 | + | + | + | + | − | + | − | Haraszti [52] | |
| 7 days | − | + | − | − | + | + | − | Barile [53] | |
| 24 | + | Angulski [54] | |||||||
| 48 | + | + | Shi [55] | ||||||
Upstream process parameters
The possibility to influence the EV phenotype by using different cell culture techniques might present a novel strategy for the production of “customized EVs” for cell-free therapy. However, uncertainties regarding certain characteristics, including the risk of teratoma formation, rapid clearance from blood after administration as well as their potential for hypertrophy, raise safety concerns and represent challenges for their translation into clinical application. Culture parameters including cultivation time, shear stress, oxygen supply, medium composition, as well as cell-material interactions have been shown to impact MSC characteristics, which subsequently affect the properties of released EVs [56].
Effects of exogenous serum-derived EVs
The composition of the culture medium appears to have an impact on EV production. Fetal bovine serum (FBS), human serum, or human platelet lysate (HPL) are crucial media supplements, but also constitute a major source of EVs and EV-like particles. Especially the use of FBS raises concerns as it may contain contaminating particles such as viral proteins, toxins and mycoplasma due to inconsistent manufacturing processes [57]. This, in turn, issues another challenge for the isolation of EVs, which will be further addressed in Sect. 3.3, as these particles are co-enriched in EV samples upon exposure to the cell culture [58]. In this context, HPL represents a superior serum alternative since the manufacturing processes are more controllable and provide consistent quality compared to FBS. Furthermore, as HPL allows for xeno-free culture of MSCs it facilitates the translation into clinical trials [59]. However, HPL also contains similar quantities of exogenous serum-derived EVs and other nanoparticles such as growth factors and protein aggregates. As of today, it is not evident that HPL-derived vesicles negatively impact the therapeutic functionalities of MSC-EV preparations obtained from HPL-containing culture medium. Hence, understanding whether exogenous serum EVs or EV-like particles support or counteract specific therapeutic effects of MSC-EVs is necessary. Nevertheless, as the composition of MSC-EV preparations is heterogeneous, the characterization and depiction of the distinct effects that are exerted specifically by MSC-EVs remain challenging. Despite this, about 83% of all registered studies have implemented serum-containing media [60].
Various protocols for the depletion of serum EVs or serum-free conditions have been proposed over the past few years to avoid this contamination with serum-derived EVs (Table 2). The use of EV-depleted FBS or human serum reduced cell growth-promoting activity in most cell types but enhanced growth upon supplementation with isolated FBS-derived EVs. In conclusion, exogenous serum EVs substantially influence the behavior of cultured cells [61]. In another study, human AD-MSC were cultured in EV-depleted medium and demonstrated similar proliferation rates and no significant differences in cell and EV morphology compared to the non-depleted serum medium [62]. Haraszti et al. reported an increase in the EV activity, in regards to siRNA transfer, despite the decrease of yield after serum deprivation of MSCs, which indicates that their biogenesis is differentially regulated under stress [63]. Hence, further investigations are needed to validate the impact of serum-free or EV-depleted medium towards the biological function of secreted EVs. Other medium-related parameters such as the presence of liposomes, calcium, and increased pH as well as the induction of oxidative stress have been reported to increase the EV production in different cell lines [64].
Table 2.
Methods for the depletion of EVs in serum additives for cell culture medium
| Method | References | |
|---|---|---|
| Ultracentrifugation | 120,000 g, 18 h, 4 °C, SW32 Ti rotor (Beckman Coulter, Brea, CA, USA) | [66] |
| Ultrafiltration | Amicon ultra-15 centrifugal filters (UFC910024, 100 K filters and benchtop Merk Millipore Ltd., Tullagreen, Carrigtwohill, Co. Cork, Ireland), 3,000 g, 55 min, 4 °C | [62] |
| Tangential flow filtration (TFF) | hollow fiber-modified polyethersulfone (mPES) membrane filter column (area 1,600 cm2, 500 kDa molecular weight cut off) operated on a KR2i TFF System (Repligen, USA) | [67] |
| Commercially available exosome-depleted serum or medium | MesenCult™-ACF Plus (STEMCELL Technologies, China); | [68] |
| Exo-FBS™ (System Biosciences, Mountain View, CA, USA); | [69] | |
| OxiumTMEXO (patent No. PCT/CL2019/100175); | [70] | |
| RoosterCollect EV Pro™ (RoosterBio Inc., Frederick, MD, USA) | [71] | |
| Fibrinogen and fibrin depletion | Hydrogel formation was facilitated for 4 h at room temperature (RT) followed by overnight incubation at 4 °C. The resulting coagulated medium was heated to 37 °C for 1 h to enable a complete fibrin clotting. Afterward, a collapse was induced by vigorous shaking followed by centrifugation at 3000 g for 10 min at RT. Finally, the clear medium supernatant was filtered through a 0.22 µm filter (Merck Millipore, Billerica, MA, USA) | [72] |
In conclusion, certain factors need to be considered upon using serum-free or serum-depleted culture medium: (i) a switch to these medium compositions could cause an alteration of extracellular RNAs, (ii) starvation leads to a stress response of the cells, which could change distinct cellular processes and increase/decrease of EV production, and (iii) some components of serum may persist in the culture after changing to serum-free conditions [65].
Effects of 3D and bioreactor culture
The production of EVs has most commonly been performed in 2D tissue culture polystyrene flasks. However, planar surfaces do not represent the native microenvironment of cells, which affects the cellular behavior and, consequently, the nature of the cellular secretome. Recent findings show the cultivation of MSCs in a three-dimensional (3D) microenvironment provides continuous production of MSC-derived EVs with similar properties to in vivo EVs and enhanced therapeutic potential for different disease models. Indeed, MSC-derived EVs from 3D hollow fiber bioreactor (HFB) cultivation were superior to 2D MSC-EVs as they significantly improved renal function, attenuated inflammatory factors, and suppressed T cell and macrophage infiltration in a murine model of cisplatin-induced acute kidney injury [73]. Another study reported an increase of immunomodulatory cytokines including TGF-b1 and TLR4/NF-kB negative regulator let-7b-5p in MSC-derived EVs from a microcarrier-based (2.5D) cultivation in a spinner flask [74]. These findings suggest that 3D culture systems could facilitate MSCs to release more potent EV populations, in terms of their functionality.
Furthermore, the limited surface area provided in 2D flasks generates over-confluent cell monolayers, if not properly controlled. Patel et al. [75] reported density- and passage-related differences in the bioactivity of MSC-derived EVs. In this study, MSCs of different passage numbers were cultured in cell culture-treated flasks at distinct seeding densities. Vesicle collection from conditioned medium was performed after 24 h. High cell seeding densities (104 cells/cm2) and passage number (> 5) resulted in reduced production per cell and diminished angiogenic bioactivity, while no significant differences were observed in regards to size (30–200 nm) and surface marker profiles. Increased MSC passage number was associated with alterations in genes involving cell cycle, protein ubiquitination, and apoptosis, all of which may result in decreased cellular activity [76]. It is thus likely that this diminished activity also impacts function, indicating that it is essential to maintain MSCs in a non‐senescent state to retain the therapeutic potential of MSC-derived EVs. As to the influence of cell density on EV release, the reduced release at higher seeding density could be due to metabolic effects. Additionally, it has been proposed that reduced cell–cell contacts at low seeding densities may also play a role in the observed increase in production, since EV release may be a compensatory intercellular communication mechanism. This is supported by the finding that the depletion of EVs from the culture microenvironment results in increased EV release, suggesting that continuous perfusion culture systems could increase the yield.
Another important factor is the harvesting period of EVs, which defines the period in which the cell is allowed to produce and release EVs into the culture medium. Common harvesting periods chosen by different groups range between 24 h and 7 days [77]. Lee et al. [78] reported an optimal harvesting period of 48 h for adipose tissue-derived MSC-derived EVs (adMSC-EVs), whereas Almeria and Weiss et al. [79] obtained the highest vesicle concentration after six days of adMSC culture, which included medium changes every other day. Overall, these studies highlight the need for careful consideration of the parameters of cell passage number and cell seeding density in the production of therapeutic EVs at laboratory scale as well as for the design of large‐scale manufacturing protocols.
The demand for high yields as a prerequisite for potential clinical applications of EVs requires novel culture strategies to scale up production and enhance the bioactivity of EVs. The use of dynamic, scalable culture systems has been promoted to meet this demand (Table 3). Furthermore, bioreactors enable continuous culture and monitoring of critical process parameters including O2 concentration and pH [80]. Currently, three main bioreactors are prominently used to produce high yields of MSC: (1) multilayer-stacked Cell-factories, (2) hollow fiber-based bioreactors, and (3) stirred-tank bioreactors [81]. These systems have garnered attention for EV production due to their successful expansion rate at large scales. While these systems have already been tested for MSC expansion, very few studies (less than 50 publications in PubMed using the search string “3D mesenchymal stem cell derived extracellular vesicles”) have yet been published regarding 3D MSC-derived EV production, warranting further investigations [65]. Cao et al. [73] reported that 2D and HFB-MSC-derived EVs did not differ significantly regarding their surface marker profiles, size, or morphology, however, an up to 19.4-fold increased yield was observed for HFB-MSC-derived EVs. Similarly, Yun et al. reported a 7.5-fold higher EV yield as well as enhanced therapeutic efficacy for HFB-MSC-EV as compared to 2D MSC-EVs [82]. Whereas, the cultivation of hUC-MSCs in a microcarrier-based culture was demonstrated to increase the yield of EVs up to 20-fold compared to 2D cultures [52]. Additional studies describe similar findings with culture systems such as a 3D-printed scaffold-perfusion bioreactor [83, 84], spheroid/aggregate/organoid culture [85], and 2.5D surfaces (e.g. microcarriers).
Table 3.
Bioreactor systems for MSC-EV production
| In vitro system | Origin of EVs | Yield | Harvest time | Medium supplement | Study |
|---|---|---|---|---|---|
| 10-layer Nunc™ EasyFill™ Cell Factory™ (2D) systems (Thermo Fisher Scientific, USA) | UC-MSC | 1.36 × 109 ± 3.49 × 108 up to 5.96 × 109 ± 7.11 × 108 particles/mL | 48 h over 6 days | OxiumTMEXO | [70] |
| Quantum (3D) bioreactor culture system (Terumo BCT, USA) | BM MSC-derived Evs | 1.04 × 1010 particles/mL | 48 h over 12 days | α MEM supplemented with 1% L-glutamine, 5% human platelet lysate, and 1% penicillin-strep- tomycin | [31] |
| Microcarrier-based (2.5D) culture in stirred tank bioreactor | UC-MSC | 27-fold | 48 h | serum-/xenofree StemPro medium (A1067501; Life Technologies, USA) | [52] |
| Microcarrier-based (2.5D) cultivation in spinner flask | hBM-MSC | 1 × 1011 particles/mL | 48 h over 7 days | 5% fetal bovine serum (FBS) | [74] |
| Hollow fiber (3D) bioreactor (Fibercell Systems, USA) | hBM-MSCs | 5.5 × 1010 particles/mL | 24 h over 25 days | RoosterCollect-EV ser-/xeno-free medium (RoosterBio Inc., cat.#M2001) | [71] |
| Microcarrier-based (2.5D) cultivation in Vertical-Wheel™ | AD-MSC | 3.1 ± 1.3 × 1011 | 48 h | DMEM low glucose, 5% v/v UltraGRO™-PURE, Antibiotic–Antimycotic 1x | [87] |
| BM-MSC | 2.8 ± 0.1 × 1011 | ||||
| UC-MSC | 4.1 ± 1.7 × 1011 EV particles |
Overall, the available publications demonstrate an increased potential of 3D and dynamic culture systems towards improved yield and bioactivity. Appropriate adjustments of related bioreactor parameters such as oxygen supply, hydrodynamic shear stress, metabolic byproducts and pH balance are required as they differ for each cell type. Furthermore, standardization of protocols is required to progress into translational studies [86].
Pre-conditioning with cytokines and hypoxia
MSC-derived EVs contain factors that promote tissue regeneration by immunomodulation [88] and enable targeted therapies via the introduction of genetic information, such as miRNAs [18]. MSCs have been investigated and applied in cell-based therapy for years due to their immunomodulatory, inflammatory, and regenerative capacity. To enhance their therapeutic efficacy, priming of MSCs with cytokines, pharmaceutical drugs, or further culture conditions was investigated [89]. The efficiency of MSCs in affecting immunomodulatory processes is known to be altered by their extracellular environment, which translates into the MSC secretome including EVs [89]. Similar to cellular priming, EVs can be pre-conditioned to exhibit increased efficacy upon certain biological functions. Interestingly, priming, by both cytokines and hypoxia, influenced on the yield, cargo, and surface markers of MSC-derived EVs, but did not significantly influence their size and morphology [90].
EV production seems to increase upon stimulation with different cytokine mixtures that drive a specific response or force the expression of certain genes by the producer cells [91]. Several studies investigated the effect of inflammatory stimulation, including pro-inflammatory treatment with IFN-γ, TNF- α, and IL-1 on the immunomodulatory efficacy and therapeutic applicability of primed MSC-derived EVs.
Moreover, there is convincing evidence for the effectiveness of hypoxia-preconditioned MSC-derived EVs in immune modulation. Oxygen concentration regulates hypoxia-inducible factor-1 (HIF-1)-mediated transcription of various genes, such as VEGF, fibroblast growth factor 2 (FGF-2), hepatocyte growth factor (HGF), and insulin-like growth factor 1 (IGF-1), which maintain the stem cell fate in terms of proliferation and differentiation [92]. Hypoxia (1–10% O2) [93] is common in various adult human tissues as depicted in Fig. 5. Contrary to the MSC niche, which has been reported to reside at physiological O2 concentrations of 2–9%, standard laboratory conditions involve an ambient (normoxic) O2 level of 20% [94]. Therefore, comparative studies on the impact of normoxic and hypoxic conditions towards MSC functionality have emerged, and many of these studies have reported markedly different patterns of gene regulation under hypoxic cultivation of MSCs [95]. Indeed, hypoxia-preconditioning was observed to alter properties of MSC-derived EVs and to effect enhanced secretion, compositional changes of bioactive molecules [96], improved immunomodulation [97], angiogenic potential [79], reduction of reactive oxygen species (ROS), intracellular adenosine triphosphate (ATP) recovery, as well as inhibition of apoptosis [98]. MSC-derived EVs produced under hypoxic conditions showed an increase in proteins associated with chemotaxis (e.g. CCL3, MCP2, MCP4 and CSF-1) and angiogenesis, and the expression of CD9 and CD81 was statistically higher in hypoxic-conditioned EVs (p < 0.05) [99]. Similarly, those effects could be replicated by HIF-1 overexpression in normoxic cultured MSCs [100]. Bian et al. observed that the generation of human BM-MSC-EVs under hypoxia (1% O2 for 72 h) resulted in an improved cardiac regeneration in a rat myocardial infarction model by increasing angiogenesis at the infarcted area [101], supporting the potential of hypoxic preconditioning for regenerative applications [102].
Fig. 5.

Physiological oxygen (O2) concentrations in different tissues. Illustration adapted [93]
Isolation and purification methods
EVs overlap in size and density with each other as well as with cellular components and organelles, including mitochondria [103]. On top of the diverse composition and function of EV subpopulations, such as exosomes or microvesicles, recent findings indicate that EV subpopulations released from different areas of the same cell (apical and basolateral EV) differ regarding their protein composition [56]. The distinction of populations and the designation of biological functions to individual populations—critical aspects for their potential therapeutic application—remains a challenge.
Protocols for sample preparation and MSC-EV enrichment influence not only the quantity but also the quality of EVs. Common isolation methods are based on physico-chemical properties of MSC-EVs, such as their density and size, or on the interaction with EV surface proteins (Table 4) [104, 105]. Ultracentrifugation at 100,000–200,000×g, has been used as the “golden standard” EV isolation method for many years, was reported to damage and disintegrate EVs due to the high g forces. Furthermore, sample viscosity, centrifugation time, as well as the rotor type (swing-out vs. fixed angle) affect EV isolation by centrifugation. Another disadvantage is that EV isolates could still be contaminated with proteins, which makes them unuseful for clinical application [104, 106]. Various studies have compared the different isolation techniques for MSC-EVs regarding criteria such as the resulting vesicle concentration and yield, size distribution, surface marker profiles, as well as the functional activity of the isolated MSC-EV populations [104, 107, 108]. Recent studies reported higher purity and functionality of MSC-EVs isolated by SEC rather than differential centrifugation (dUC). Nevertheless, the bottleneck includes high labor intensity and complete clearance of co-contamination with protein aggregates as well as lipoproteins is still not ensured [109, 110].
Table 4.
Common isolation protocols used for MSC-derived EVs
| Method | References | |
|---|---|---|
| Differential centrifugation (dUC) |
Prior to the ultracentrifugation (100,000–200,000 × g, 1-2 h, 4 °C) several low to intermediate-speed centrifugation steps are required to remove cells, cell debris, apoptotic bodies, and aggregates: 300–400 × g for 10 min sediment cells 1500–2000 × g for 15–20 min. at 4 °C remove cell debris 10,000 × g 15–30 min at 4 °C removal of other structures with a higher buoyant density that MSC-EVs |
[79] |
| Density gradient isolation | Hereby, a continuous density gradient is formed by layering different concentrations of iodixanol. The MSC-EV-rich conditioned medium (CM) is overlaid on top and subjected to high-speed centrifugation (100,000 × g, 18 h, 4 °C), resulting in gradient fractions containing EV-like vesicles of different concentrations. Subsequently, these fractions are further processed in another high-speed centrifugation step (100,000 × g, 1-2 h, 4 °C) to separate MSC-EVs from other proteins and nucleoproteins | [111] |
| Size-exclusion chromatography (SEC) | CM is concentrated using a 100 kDa molecular weight cut-off filter to reduce total volume prior to the loading onto the column. The most common stationary phase used for EV isolation using SEC is Sepharose CL-2B, which is extensively washed and then packed into a column or syringe. The CM is loaded on top and EV-rich fractions are collected immediately and pooled after elution and again concentrated for further analytical procedures | [109] |
| Precipitation/Phase separation | The majority of protocols use polyethylene glycol (PEG)-based volume exclusion which precipitates EVs to a pellet. Hereby, CM is centrifuged at intermediate speed (6,000–10,000 × g, 45 min, 4 °C), filtered (0.22 µm), added to PEG solution to a final concentration of 10% (or 75 mM), and incubated for 8–16 h at 4 °C. Subsequently, the suspension is centrifuged and the EV-rich pellet is washed a few times with 0.9% NaCl. Lastly, the suspension is ultracentrifuged (100,000 × g, 130 min, 4 °C) and the resulting pellet is dissolved in buffer | [112] |
Kamei et al. recently compared phosphatidyl serine (PS) affinity-based method (MagCapture Exosome, isolation Kit PS), polymer precipitation (ExoQuick, Total Exosome Isolation Reagent, and Exo-PREP), and size-exclusion chromatography (SEC) (qEV column) for the isolation of MSC-derived EVs and found that size, protein content, and yield varied depending on the method of isolation. In summary, results from that study show the highest purity obtained from PS affinity method compared to the other methods described. However, the outcome was connected with high EV loss and saturation of EV binding to the MagCapture beads. These observations demonstrate a disadvantage for clinical translation using the PS affinity method. On the other hand, SEC resulted in high protein concentration in fractions 7–9, which indicates a more effective collection of MSC-derived EVs [113]. Overall, the difficulty in isolating MSC-EVs in high yield or purity remains due to their small size and physicochemical heterogeneity. Hence, there is an urgent need to advance the technology to address this problem. Liangsupree et al. have recently summarized current and novel isolation techniques for EVs beyond ultracentrifugation and precipitation-based techniques [114]. The methods are categorized into (a) size-, (b) charge-, and (c) affinity-based techniques, which are listed in Table 5. Although most of these novel techniques have not been studied for MSC-EVs yet they represent promising approaches for the generation of highly purified MSC-EV isolates in the future.
Table 5.
List of promising modern isolation and separation techniques for MSC-EVs [114]
| Technique | Separation system | Advantages | Purity | Sample volume |
|---|---|---|---|---|
| Size-exclusion chromatography (SEC) | IZON® qEV column |
Removal of co-contaminants including HDLs, albumin Yield better functionality of EVs compared to UC Less compositional and structural alterations comparted to precipitation techniques |
+ + + | 100 µl—10 ml |
| Sepharose® CL-4B | + + + | 1 – 10 ml | ||
| Filtration-based | Centrifugal filter unit |
Defined MWCO ranging from 10 – 100 kDa Simple and easy handling Cost- and time-effective |
+ | Up to 10 ml |
| Tangential Flow Filtration (TFF) | Higher concentration of EVs | + | > 10 ml | |
| Hydrostatic filtration dialysis (HFD) |
No centrifugation step Low EV loss |
+ | > 10 ml | |
| Flow field-flow fractionation | asymmetrical flow field-flow fractionation (AsFlFFF or AF4) |
Cross-flow can be modified Optimization between runs possible to enhance separation efficiency More flexible compared to sec Gentle fractionation |
+ + | > 10 ml |
| Deterministic lateral displacement (DLD) pillar array | Enables separation of exosomes in the size range of 20 to 110 nm | + + | > 10 ml | |
| Charge-based | Anion-exchange chromatography (AIEC) |
Shorter isolation time (< 3 h for 1 L of cell culture supernatant) Yield intact evs |
+ + | Up to 1L |
| Electrophoresis and dielectrophoresis (DEP) | Subpopulations separated based on electrophoretic mobilities acquire information on properties of charged and non-charged EVs | + + | > 10 ml | |
| Affinity-based | Magnetic beads |
Highly selective and specific Isolate evs originating from different cell types |
+ + + | 100 µl–1 ml |
Storage and logistics
Next to isolation, storage can cause alterations in functionality. Generally, samples should be processed immediately after collection to preserve the stability and integrity of the membrane vesicles and to avoid aggregation of the EV preparations [115]. Approaches for EV preservation include (i) cryopreservation [116], (ii) freeze-drying [117], and (iii) spray-drying [118]. Studies on long-term storage of EVs have reported temperatures of − 20 °C as the upper limit under which EV from human embryonic kidney (HEK) 293 T cells, endothelial colony-forming cells (ECFCs,) and MSCs remain stable [119], whereas the optimal mode of storage was in the range of − 80 to − 70 °C [120]. As of today, however, no general standards regarding sample storage and processing of preparations have been defined [121].
Conclusion
The use of MSC-derived EVs instead of stem cells confers several advantages, such as an improved safety profile, lower immunogenicity, as well as the ability to cross biological barriers. Furthermore, potential complications, including stem-cell-induced tumor formation, entrapment in the lung microvasculature, or immune rejection may be avoided by using MSC-derived EVs [1]. Despite promising results in preclinical trials, the use of MSC-derived EVs in clinical settings requires the resolution of several critical issues, including large-scale production, standardized isolation, quantification, and characterization procedures for MSC-derived EVs. Furthermore, an enhanced understanding of their targeting mechanisms and pharmacokinetics, as well as the determination of the optimal clinical dosage is still ongoing [122]. These aspects represent key elements for a successful EV-based therapy preventing risks, such as potential side effects on healthy cells, uncontrolled biodistribution and targeting, limited loading capacity, and insufficient clinical-grade production [123]. In this review, we summarized upstream process parameters that crucially affect the therapeutic properties and biologic functions of MSC-derived EVs. Critical upstream process parameters are (i) cell source, passage number, seeding density and confluence, (ii) medium composition, (iii) choice of 3D culture method and bioreactor type, and (iv) pre-conditioning of cells with cytokines or hypoxia. Additionally, critical downstream process parameters including isolation, purification, storage strategy as well as the characterization of MSC-derived EVs need to be considered for the manufacturing of clinical-grade EVs. The use of three-dimensional microenvironments, including bioreactors, for large-scale MSC-derived EV production is increasing nowadays and indicates a more efficient approach compared to traditional two-dimensional cell culture [73, 87, 124]. Combinations of different isolation methods, such as SEC and ultrafiltration-based method, currently garner great attention as it demonstrates to provide both high yield and high purity of selective MSC-derived EVs for the desired application [110]. Moreover, all these process parameters have to be aligned and optimized towards each particular target treatment resulting in unique processes despite not a universally valid solution. Overall, MSC-derived EVs indicate great benefits for biomedical applications, however, still significant challenges remain. Hence, continuous development and optimization of technologies are required to achieve higher efficiency and/or purity for the production and isolation of clinical-grade MSC-derived EVs.
Acknowledgements
No applicable.
Abbreviations
- EVs
Extracellular Vesicles
- MSC
Mesenchymal stem cell
- MVB
Multivesicular body
- RNA
Ribonucleic acid
- mRNA
Messenger ribonucleic acid
- FasL
Fas ligand
- CVD
Cardiovascular disease
- GvHD
Graft-versus-host disease
- MHC
Major histocompatibility complex
- MI
Myocardial ischaemia
- AKI
Acute kidney injury
- iPSC
Induced pluripotent stem cell
- PD-MSC
Induced pluripotent mesenchymal-derived stem cell
- Plac-MSC-EVs
Placenta-derived EVs
- AD-MSC-EVs
Adipose tissue-derived MSC-EVs
- TD-MSC-EVs
Tissue-derived EVs
- BM-MSC-EVs
Bone marrow-derived MSC-EVs
- ucMSC-EVs
Umbilical cord-derived MSC-EVs
- FBS
Fetal bovine serum
- HPL
Human platelet lysate
- siRNA
Small interfering RNA
- RT
Room temperature
- 3D
Three-dimensional
- HFB
Hollow fiber bioreactor
- TGF-b1
Transforming growth factor beta 1
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- miRNA
Micro RNA
- IFN-γ
Interferon gamma
- TNF-α
Tumor necrosis factor
- IL1
Interleukin-1
- HIF-1
Hypoxia-inducible factor-1
- VEGF
Vascular endothelial growth factor
- FGF-2
Fibroblast growth factor 2
- HGF
Hepatocyte growth factor
- IGF-1
Insulin-like growth factor 1
- ROS
Reactive oxygen species
- ATP
Adenosine triphosphate
- CCL3
Chemokine ligand 3
- MCP2
Monocyte chemoattractant protein 2
- MCP4
Monocyte chemoattractant protein 4
- CSF-1
Colony stimulating factor 1
- PBF
Phosphate buffered saline
- dUC
Differential ultracentrifugation
- SEC
Size-exclusion chromatography
- MWCO
Molecular weight cut-off
- HEK 293T
Human embryonic kidney 293T cells
- EFCFs
Endothelial colony forming cells
Author contributions
CA performed literature research, designed the figures and wrote the manuscript. SK performed literature research and revised the manuscript. VK, DE and CK designed the concept of the review and revised the manuscript. All authors read and approved the final manuscript.
Funding
No applicable.
Availability of data and materials
No applicable.
Declarations
Ethics approval and consent to participate
No applicable.
Consent for publication
No applicable.
Competing interests
No applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ciarra Almeria, Email: ciarra.almeria@boku.ac.at.
Sebastian Kreß, Email: sebastian.kress@boku.ac.at.
Viktoria Weber, Email: viktoria.weber@donau-uni.ac.at.
Dominik Egger, Email: dominik.egger@boku.ac.at.
Cornelia Kasper, Email: cornelia.kasper@boku.ac.at.
References
- 1.Gowen A, Shahjin F, Chand S, Odegaard KE, Yelamanchili SV. Mesenchymal stem cell-derived extracellular vesicles: challenges in clinical applications. Front Cell Dev Biol. 2020;8:149. doi: 10.3389/fcell.2020.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 3.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–9420. doi: 10.1016/S0021-9258(18)48095-7. [DOI] [PubMed] [Google Scholar]
- 4.Kreimer S, Belov AM, Ghiran I, Murthy SK, Frank DA, Ivanov AR. Mass-spectrometry-based molecular characterization of extracellular vesicles: Lipidomics and proteomics. J Proteome Res. 2015;14:2367–2384. doi: 10.1021/pr501279t. [DOI] [PubMed] [Google Scholar]
- 5.van der Pol E, Böing AN, Gool EL, Nieuwland R. Recent developments in the nomenclature, presence, isolation, detection and clinical impact of extracellular vesicles. J Thromb Haemost. 2016;14:48–56. doi: 10.1111/jth.13190. [DOI] [PubMed] [Google Scholar]
- 6.Borroto-Escuela DO, Agnati LF, Bechter K, Jansson A, Tarakanov AO, Fuxe K. The role of transmitter diffusion and flow versus extracellular vesicles in volume transmission in the brain neural-glial networks. Philos Trans R Soc B Biol Sci. 2015;370:1–14. doi: 10.1098/rstb.2014.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gustafson D, Veitch S, Fish JE. Extracellular vesicles as protagonists of diabetic cardiovascular pathology. Front Cardiovasc Med. 2017 doi: 10.3389/fcvm.2017.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buzás EI, Tóth E, Sódar BW, Szabó-Taylor K. Molecular interactions at the surface of extracellular vesicles. Semin Immunopathol. 2018;40:453–464. doi: 10.1007/s00281-018-0682-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q, Xu J. Functional proteins of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. 2019;10:1–11. doi: 10.1186/s13287-019-1484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shifrin DA, Beckler MD, Coffey RJ, Tyska MJ. Extracellular vesicles: Communication, coercion, and conditioning. Mol Biol Cell. 2013;24:1253–1259. doi: 10.1091/mbc.e12-08-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. Mesenchymal stem cells: A new trend for cell therapy. Acta Pharmacol Sin. 2013;34:747–754. doi: 10.1038/aps.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katare R, Stroemer P, Hicks C, Stevanato L, Patel S, Corteling R, Miljan E, Vishnubhatla I, Sinden J, Madeddu P. Clinical-grade human neural stem cells promote reparative neovascularization in mouse models of hindlimb ischemia. Arterioscler Thromb Vasc Biol. 2014;34:408–418. doi: 10.1161/ATVBAHA.113.302592. [DOI] [PubMed] [Google Scholar]
- 13.Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: Toward cell-free therapeutic applications. Mol Ther. 2015;23:812–823. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: A novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6:481–492. doi: 10.2217/rme.11.35. [DOI] [PubMed] [Google Scholar]
- 15.Camussi G, Deregibus MC, Cantaluppi V. Role of stem-cell-derived microvesicles in the paracrine action of stem cells. Biochem Soc Trans. 2013;23:283–287. doi: 10.1042/BST20120192. [DOI] [PubMed] [Google Scholar]
- 16.Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9:1–9. doi: 10.1186/s13287-018-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15:4142–4157. doi: 10.3390/ijms15034142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee Y, El Andaloussi S, Wood MJA. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012 doi: 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- 19.Wen S, Dooner M, Cheng Y, et al. Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia. 2016;30:2221–2231. doi: 10.1038/leu.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao M, Liu S, Wang C, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate mitochondrial damage and inflammation by stabilizing mitochondrial DNA. ACS Nano. 2021;15:1519–1538. doi: 10.1021/acsnano.0c08947. [DOI] [PubMed] [Google Scholar]
- 21.Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS ONE. 2012 doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamaly N, He JC, Ausiello DA, Farokhzad OC. Nanomedicines for renal disease: current status and future applications. Nat Rev Nephrol. 2016;12:738–753. doi: 10.1038/nrneph.2016.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu D, Yang F, Xiong F, Gu N. The smart drug delivery system and its clinical potential. Theranostics. 2016;6:1306–1323. doi: 10.7150/thno.14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinde E, Thammasiraphop K, Duong HTT, Yeow J, Karagoz B, Boyer C, Gooding JJ, Gaus K. Pair correlation microscopy reveals the role of nanoparticle shape in intracellular transport and site of drug release. Nat Nanotechnol. 2017;12:81–89. doi: 10.1038/nnano.2016.160. [DOI] [PubMed] [Google Scholar]
- 25.Hartjes TA, Mytnyk S, Jenster GW, van Steijn V, van Royen ME. Extracellular vesicle quantification and characterization: Common methods and emerging approaches. Bioengineering. 2019 doi: 10.3390/bioengineering6010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106:148–156. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Yuan ZQ, Kolluri KK, Gowers KHC, Janes SM. TRAIL delivery by MSC-derived extracellular vesicles is an effective anticancer therapy. J Extracell Vesicles. 2017 doi: 10.1080/20013078.2017.1265291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimbo K, Miyaki S, Ishitobi H, Kato Y, Kubo T, Shimose S, Ochi M. Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem Biophys Res Commun. 2014;445:381–387. doi: 10.1016/j.bbrc.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Pascucci L, Coccè V, Bonomi A, et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release. 2014;192:262–270. doi: 10.1016/j.jconrel.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 30.György B, Szabó TG, Pásztói M, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendt M, Kamerkar S, Sugimoto H, et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI insight. 2018 doi: 10.1172/jci.insight.99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El Andaloussi S, Mäger I, Breakefield XO, Wood MJA. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 33.Cai J, Wu J, Wang J, Li Y, Hu X, Luo S, Xiang D. Extracellular vesicles derived from different sources of mesenchymal stem cells: Therapeutic effects and translational potential. Cell Biosci. 2020;10:1–14. doi: 10.1186/s13578-019-0370-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khare D, Or R, Resnick I, Barkatz C, Almogi-Hazan O, Avni B. Mesenchymal stromal cell-derived exosomes affect mRNA expression and function of B-Lymphocytes. Front Immunol. 2018;9:3053. doi: 10.3389/fimmu.2018.03053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbaszadeh H, Ghorbani F, Derakhshani M, Movassaghpour A, Yousefi M. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles: a novel therapeutic paradigm. J Cell Physiol. 2020;235:706–717. doi: 10.1002/jcp.29004. [DOI] [PubMed] [Google Scholar]
- 36.Shekari F, Nazari A, Assar Kashani S, Hajizadeh-Saffar E, Lim R, Baharvand H. Pre-clinical investigation of mesenchymal stromal cell-derived extracellular vesicles: a systematic review. Cytotherapy. 2021;23:277–284. doi: 10.1016/j.jcyt.2020.12.009. [DOI] [PubMed] [Google Scholar]
- 37.La Greca A, Solari C, Furmento V, et al. Extracellular vesicles from pluripotent stem cell-derived mesenchymal stem cells acquire a stromal modulatory proteomic pattern during differentiation. Exp Mol Med. 2018;50:1–12. doi: 10.1038/s12276-018-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther. 2008;16:782–790. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raj DAA, Fiume I, Capasso G, Pocsfalvi G. A multiplex quantitative proteomics strategy for protein biomarker studies in urinary exosomes. Kidney Int. 2012;81:1263–1272. doi: 10.1038/ki.2012.25. [DOI] [PubMed] [Google Scholar]
- 40.Zhao Q, Hai B, Kelly J, Wu S, Liu F. Extracellular vesicle mimics made from iPS cell-derived mesenchymal stem cells improve the treatment of metastatic prostate cancer. Stem Cell Res Ther. 2021;12:29. doi: 10.1186/s13287-020-02097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otero-Ortega L, Laso-García F, Frutos D-D, M, Rodríguez-Frutos B, Pascual-Guerra J, Fuentes B, Díez-Tejedor E, Gutiérrez-Fernández M, White matter repair after extracellular vesicles administration in an experimental animal model of subcortical stroke. Sci Rep. 2017;7:1–11. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Connolly KD, Guschina IA, Yeung V, Clayton A, Draman MS, Von Ruhland C, Ludgate M, James PE, Rees DA. Characterisation of adipocyte-derived extracellular vesicles released pre-and post-adipogenesis. J Extracell Vesicles. 2015 doi: 10.3402/jev.v4.29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu YZ, Hu X, Zhang J, Wang ZH, Wu S, Yi YY. Extracellular vesicles derived from human adipose-derived stem cell prevent the formation of hypertrophic scar in a rabbit model. Ann Plast Surg. 2020;84:602–607. doi: 10.1097/SAP.0000000000002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Durcin M, Fleury A, Taillebois E, et al. Characterisation of adipocyte-derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. J Extracell Vesicles. 2017 doi: 10.1080/20013078.2017.1305677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eirin A, Zhu XY, Puranik AS, Woollard JR, Tang H, Dasari S, Lerman A, Van Wijnen AJ, Lerman LO. Comparative proteomic analysis of extracellular vesicles isolated from porcine adipose tissue-derived mesenchymal stem/stromal cells. Sci Rep. 2016;6:1–12. doi: 10.1038/srep36120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang B, Shi Y, Gong A, et al. HucMSC Exosome-Delivered 14-3-3ζ Orchestrates Self-Control of the Wnt Response via Modulation of YAP during cutaneous regeneration. Stem Cells. 2016;34:2485–2500. doi: 10.1002/stem.2432. [DOI] [PubMed] [Google Scholar]
- 47.Kilpinen L, Impola U, Sankkila L, et al. Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. J Extracell Vesicles. 2013;2:21927. doi: 10.3402/jev.v2i0.21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L, Abhange KK, Wen Y, Chen Y, Xue F, Wang G, Tong J, Zhu C, He X, Wan Y. Preparation of engineered extracellular vesicles derived from human umbilical cord mesenchymal stem cells with ultrasonication for skin rejuvenation. ACS Omega. 2019;4:22638–22645. doi: 10.1021/acsomega.9b03561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zou XY, Yu Y, Lin S, Zhong L, Sun J, Zhang G, Zhu Y. Comprehensive miRNA analysis of human umbilical cord-derived mesenchymal stromal cells and extracellular vesicles. Kidney Blood Press Res. 2018;43:152–161. doi: 10.1159/000487369. [DOI] [PubMed] [Google Scholar]
- 50.Zhang N, Zhu J, Ma Q, Zhao Y, Wang Y, Hu X, Chen J, Zhu W, Han Z, Yu H. Exosomes derived from human umbilical cord MSCs rejuvenate aged MSCs and enhance their functions for myocardial repair. Stem Cell Res Ther. 2020;11:273. doi: 10.1186/s13287-020-01782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim HS, Choi DY, Yun SJ, Choi SM, Kang JW, Jung JW, Hwang D, Kim KP, Kim DW. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res. 2012;11:839–849. doi: 10.1021/pr200682z. [DOI] [PubMed] [Google Scholar]
- 52.Haraszti RA, Miller R, Stoppato M, et al. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol Ther. 2018;26:2838–2847. doi: 10.1016/j.ymthe.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barile L, Cervio E, Lionetti V, et al. Cardioprotection by cardiac progenitor cell-secreted exosomes: role of pregnancy-associated plasma protein-A. Cardiovasc Res. 2018;114:992–1005. doi: 10.1093/cvr/cvy055. [DOI] [PubMed] [Google Scholar]
- 54.Angulski ABB, Capriglione LG, Batista M, Marcon BH, Senegaglia AC, Stimamiglio MA, Correa A. The protein content of extracellular vesicles derived from expanded human umbilical cord blood-derived CD133+ and human bone marrow-derived mesenchymal stem cells partially explains why both sources are advantageous for regenerative medicine. Stem Cell Rev Reports. 2017;13:244–257. doi: 10.1007/s12015-016-9715-z. [DOI] [PubMed] [Google Scholar]
- 55.Shi S, Zhang Q, Xia Y, You B, Shan Y, Bao L, Li L, You Y, Gu Z. Mesenchymal stem cell-derived exosomes facilitate nasopharyngeal carcinoma progression. Am J Cancer Res. 2016;6:459–472. [PMC free article] [PubMed] [Google Scholar]
- 56.Vagner T, Chin A, Mariscal J, Bannykh S, Engman DM, Di Vizio D. Protein composition reflects extracellular vesicle heterogeneity. Proteomics. 2019;19:e1800167. doi: 10.1002/pmic.201800167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lehrich BM, Liang Y, Fiandaca MS. Foetal bovine serum influence on in vitro extracellular vesicle analyses. J Extracell Vesicles. 2021 doi: 10.1002/jev2.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aswad H, Jalabert A, Rome S. Depleting extracellular vesicles from fetal bovine serum alters proliferation and differentiation of skeletal muscle cells in vitro. BMC Biotechnol. 2016;16:1–12. doi: 10.1186/s12896-016-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schallmoser K, Bartmann C, Rohde E, Reinisch A, Kashofer K, Stadelmeyer E, Drexler C, Lanzer G, Linkesch W, Strunk D. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 2007;47:1436–1446. doi: 10.1111/j.1537-2995.2007.01220.x. [DOI] [PubMed] [Google Scholar]
- 60.Gardiner C, Di Vizio D, Sahoo S, Ry CT, Witwer KW, Wauben M, Hill AF Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. 10.3402/jev.v5.32945 [DOI] [PMC free article] [PubMed]
- 61.Eitan E, Zhang S, Witwer KW, Mattson MP Extracellular vesicleÁdepleted fetal bovine and human sera have reduced capacity to support cell growth. 10.3402/jev.v4.26373 [DOI] [PMC free article] [PubMed]
- 62.Kornilov R, Puhka M, Mannerström B, Hiidenmaa H, Peltoniemi H, Siljander P, Seppänen-Kaijansinkko R, Kaur S. Efficient ultrafiltration-based protocol to deplete extracellular vesicles from fetal bovine serum. J Extracellular Vesicles. 2018 doi: 10.1080/20013078.2017.1422674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haraszti RA, Miller R, Dubuke ML, et al. Serum deprivation of mesenchymal stem cells improves exosome activity and alters lipid and protein composition. Science. 2019;16:230–241. doi: 10.1016/j.isci.2019.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Emam SE, Ando H, Lila ASA, Shimizu T, Ukawa M, Okuhira K, Ishima Y, Mahdy MA, Ghazy F. A novel strategy to increase the yield of exosomes (extracellular vesicles) for an expansion of basic research. Biol Pharm Bull. 2018;41:733–742. doi: 10.1248/bpb.b17-00919. [DOI] [PubMed] [Google Scholar]
- 65.Witwer KW, Van Balkom BWM, Bruno S, et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles. 2019 doi: 10.1080/20013078.2019.1609206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Antònia Forteza-Genestra M, Antich-Rosselló M, Calvo J, Gayà A, Monjo M, Ramis JM. Purity Determines the Effect of Extracellular Vesicles Derived from Mesenchymal Stromal. Cells. 2022 doi: 10.3390/cells9020422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolf M, Vari B, Blöchl C, et al. Extracellular vesicles from therapeutic grade allogeneic human placental stromal cells induce angiogenesis and modulate immunity. BioRxiv. 2019;23:1–40. [Google Scholar]
- 68.Zhang K, Yu L, Li F-R, et al. Topical application of exosomes derived from human umbilical cord mesenchymal stem cells in combination with sponge spicules for treatment of photoaging. Age. 2020 doi: 10.2147/IJN.S249751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guerreiro EM, Vestad B, Steffensen LA, Aass HCD, Saeed M, Øvstebø R, Costea DE, Galtung HK, Søland TM. Efficient extracellular vesicle isolation by combining cell media modifications, ultrafiltration, and size-exclusion chromatography. PLoS ONE. 2018;13:1–17. doi: 10.1371/journal.pone.0204276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Figueroa-Valdés AI, de la Fuente C, Hidalgo Y, María Vega-Letter A, Tapia-Limonchi R, Khoury M, Alcayaga-Miranda F. A chemically defined, xeno- and blood-free culture medium sustains increased production of small extracellular vesicles from mesenchymal stem cells. Frontier. 2021;9:619930. doi: 10.3389/fbioe.2021.619930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gobin J, Muradia G, Mehic J, et al. Hollow-fiber bioreactor production of extracellular vesicles from human bone marrow mesenchymal stromal cells yields nanovesicles that mirrors the immuno-modulatory antigenic signature of the producer cell. Stem Cell Res Ther. 2021;12:1–20. doi: 10.1186/s13287-021-02190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laner-Plamberger S, Lener T, Schmid D, et al. Mechanical fibrinogen-depletion supports heparin-free mesenchymal stem cell propagation in human platelet lysate. J Transl Med. 2015;13:1–10. doi: 10.1186/s12967-015-0717-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cao J, Wang B, Tang T, et al. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Res Ther. 2020;11:206. doi: 10.1186/s13287-020-01719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim H, Lee MJ, Bae EH, et al. Comprehensive molecular profiles of functionally effective MSC-derived extracellular vesicles in immunomodulation. Mol Ther. 2020;28:1628–1644. doi: 10.1016/j.ymthe.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patel DB, Gray KM, Santharam Y, Lamichhane TN, Stroka KM, Jay SM, Steven JC. Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell-derived extracellular vesicles. Bioeng Transl Med. 2017 doi: 10.1002/btm2.10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, Raposo G. The Tetraspanin CD63 Regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21:708–721. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Balkom BWM, Gremmels H, Giebel B, Lim SK. Proteomic signature of mesenchymal stromal cell-derived small extracellular vesicles. Proteomics. 2019 doi: 10.1002/pmic.201800163. [DOI] [PubMed] [Google Scholar]
- 78.Lee JY, Kim E, Choi SM, Kim DW, Kim KP, Lee I, Kim HS. Microvesicles from brain-extract-treated mesenchymal stem cells improve neurological functions in a rat model of ischemic stroke. Sci Rep. 2016 doi: 10.1038/srep33038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Almeria C, Weiss R, Roy M, Tripisciano C, Kasper C, Weber V, Egger D. Hypoxia conditioned mesenchymal stem cell-derived extracellular vesicles induce increased vascular tube formation in vitro. Front Bioeng Biotechnol. 2019 doi: 10.3389/fbioe.2019.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thippabhotla S, Zhong C, He M. 3D cell culture stimulates the secretion of in vivo like extracellular vesicles. Sci Rep. 2019;9:1–14. doi: 10.1038/s41598-019-49671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Robb KP, Fitzgerald JC, Barry F, Viswanathan S. Mesenchymal stromal cell therapy: progress in manufacturing and assessments of potency. Cytotherapy. 2019;21:289–306. doi: 10.1016/j.jcyt.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 82.Yan L, Wu X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Biol Toxicol. 2020;36:165–178. doi: 10.1007/s10565-019-09504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patel DB, Luthers CR, Lerman MJ, Fisher JP, Jay SM. Enhanced extracellular vesicle production and ethanol-mediated vascularization bioactivity via a 3D-printed scaffold-perfusion bioreactor system. Acta Biomater. 2019;95:236–244. doi: 10.1016/j.actbio.2018.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pizzicannella J, Diomede F, Gugliandolo A, Chiricosta L, Bramanti P, Merciaro I, Orsini T, Mazzon E, Trubiani O. 3D printing PLA/gingival stem cells/EVs upregulate miR-2861 and-210 during osteoangiogenesis commitment. Int J Mol Sci. 2019 doi: 10.3390/ijms20133256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abdollahi S. Extracellular vesicles from organoids and 3D culture systems. Biotechnol Bioeng. 2020 doi: 10.1002/bit.27606. [DOI] [PubMed] [Google Scholar]
- 86.Antoni D, Burckel H, Josset E, Noel G. Three-dimensional cell culture: a breakthrough in vivo. Int J Mol Sci. 2015;16:5517–5527. doi: 10.3390/ijms16035517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Almeida FM, Bernardes N, Oliveira FD, et al. Scalable Production of Human Mesenchymal Stromal Cell-Derived Extracellular Vesicles Under Serum-/Xeno-Free Conditions in a Microcarrier-Based Bioreactor Culture System. Int J Mol Sci. 2020 doi: 10.3389/fcell.2020.553444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mardpour S, Hamidieh AA, Taleahmad S, Sharifzad F, Taghikhani A, Baharvand H. Interaction between mesenchymal stromal cell-derived extracellular vesicles and immune cells by distinct protein content. J Cell Physiol. 2019;234:8249–8258. doi: 10.1002/jcp.27669. [DOI] [PubMed] [Google Scholar]
- 89.Noronha NDC, Mizukami A, Caliári-Oliveira C, Cominal JG, Rocha JLM, Covas DT, Swiech K, Malmegrim KCR. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res Ther. 2019;10:1–21. doi: 10.1186/s13287-018-1105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Andrews S, Maughon T, Marklein R, Stice S. Priming of MSCs with inflammation-relevant signals affects extracellular vesicle biogenesis, surface markers, and modulation of T cell subsets. BioRxiv. 2020;6:648. [Google Scholar]
- 91.Schorey JS, Bhatnagar S. Exosome function: From tumor immunology to pathogen biology. Traffic. 2008;9:871–881. doi: 10.1111/j.1600-0854.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saparov A, Ogay V, Nurgozhin T, Jumabay M, Chen WCW. Preconditioning of human mesenchymal stem cells to enhance their regulation of the immune response. Stem Cells Int. 2016 doi: 10.1155/2016/3924858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jagannathan L, Cuddapah S, Costa M. Oxidative stress under ambient and physiological oxygen tension in tissue culture. Curr Pharmacol Reports. 2016;2:64–72. doi: 10.1007/s40495-016-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haque N, Rahman MT, Abu Kasim NH, Alabsi AM. Hypoxic culture conditions as a solution for mesenchymal stem cell based regenerative therapy. Sci World J. 2013 doi: 10.1155/2013/632972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McGarry T, Biniecka M, Veale DJ, Fearon U. Hypoxia, oxidative stress and inflammation. Free Radic Biol Med. 2018;125:15–24. doi: 10.1016/j.freeradbiomed.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 96.Song S-W, Kim K-E, Choi J-W, et al. Proteomic analysis and identification of paracrine factors in mesenchymal stem cell-conditioned media under hypoxia. Cell Physiol Biochem. 2016;40:400–410. doi: 10.1159/000452555. [DOI] [PubMed] [Google Scholar]
- 97.Borges FT, Melo SA, Özdemir BC, Kato N, Revuelta I, Miller CA, Gattone VH, LeBleu VS, Kalluri R. TGF-β1-Containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol. 2013;24:385–392. doi: 10.1681/ASN.2012101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Collino F, Lopes JA, Corrêa S, Abdelhay E, Takiya CM, Wendt CHC, De Miranda KR, Vieyra A, Lindoso RS. Adipose-derived mesenchymal stromal cells under hypoxia: Changes in extracellular vesicles secretion and improvement of renal recovery after ischemic injury. Cell Physiol Biochem. 2019;52:1463–1483. doi: 10.33594/000000102. [DOI] [PubMed] [Google Scholar]
- 99.Hyland M, Mennan C, Wilson E, Clayton A, Kehoe O. Pro-inflammatory priming of umbilical cord mesenchymal stromal cells alters the protein cargo of their extracellular vesicles. Cells. 2020;9:726. doi: 10.3390/cells9030726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gonzalez-King H, Garciá NA, Ontoria-Oviedo I, Ciria M, Montero JA, Sepúlveda P. Hypoxia inducible factor-1a potentiates jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. Stem Cells. 2017;35:1747–1759. doi: 10.1002/stem.2618. [DOI] [PubMed] [Google Scholar]
- 101.Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med. 2014;92:387–397. doi: 10.1007/s00109-013-1110-5. [DOI] [PubMed] [Google Scholar]
- 102.Zhang HC, Bin LX, Huang S, et al. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev. 2012;21:3289–3297. doi: 10.1089/scd.2012.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Puhm F, Afonyushkin T, Resch U, et al. Mitochondria are a subset of extracellular vesicles released by activated monocytes and induce type I IFN and TNF responses in endothelial cells. Circ Res. 2019;125:43–52. doi: 10.1161/CIRCRESAHA.118.314601. [DOI] [PubMed] [Google Scholar]
- 104.Varderidou-Minasian S, Lorenowicz MJ. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: Challenges and opportunities. Theranostics. 2020;10:5979–5997. doi: 10.7150/thno.40122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Monguió-Tortajada M, Gálvez-Montón C, Bayes-Genis A, Roura S, Borràs FE. Extracellular vesicle isolation methods: rising impact of size-exclusion chromatography. Cell Mol Life Sci. 2019 doi: 10.1007/s00018-019-03071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Langevin SM, Kuhnell D, Orr-Asman MA, Biesiada J, Zhang X, Medvedovic M, Thomas HE. Balancing yield, purity and practicality: a modified differential ultracentrifugation protocol for efficient isolation of small extracellular vesicles from human serum. RNA Biol. 2019;16:5–12. doi: 10.1080/15476286.2018.1564465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Park KS, Bandeira E, Shelke GV, Lässer C, Lötvall J. Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. 2019;10:1–16. doi: 10.1186/s13287-018-1105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Börger V, Staubach S, Dittrich R, Stambouli O, Giebel B. Scaled isolation of mesenchymal stem/stromal cell-derived extracellular vesicles. Curr Protoc Stem Cell Biol. 2020;55:1–11. doi: 10.1002/cpsc.128. [DOI] [PubMed] [Google Scholar]
- 109.Mol EA, Goumans MJ, Doevendans PA, Sluijter JPG, Vader P. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomed Nanotechnol Biol Med. 2017;13:2061–2065. doi: 10.1016/j.nano.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 110.Monguió-Tortajada M, Roura S, Gálvez-Montón C, Pujal JM, Aran G, Sanjurjo L, Franquesa M, Sarrias MR, Bayes-Genis A, Borràs FE. Nanosized UCMSC-derived extracellular vesicles but not conditioned medium exclusively inhibit the inflammatory response of stimulated T cells: Implications for nanomedicine. Theranostics. 2017;7:270–284. doi: 10.7150/thno.16154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Collino F, Pomatto M, Bruno S, Lindoso RS, Tapparo M, Sicheng W, Quesenberry P, Camussi G. Exosome and microvesicle-enriched fractions isolated from mesenchymal stem cells by gradient separation showed different molecular signatures and functions on renal tubular epithelial cells. Stem Cell Rev Reports. 2017;13:226–243. doi: 10.1007/s12015-016-9713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sun Y, Liu G, Zhang K, Cao Q, Liu T, Li J. Mesenchymal stem cells-derived exosomes for drug delivery. Stem Cell Res Ther. 2021;12:561. doi: 10.1186/s13287-021-02629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kamei N, Nishimura H, Matsumoto A, Asano R, Muranaka K, Fujita M, Takeda M, Hashimoto H, Takeda-Morishita M. Comparative study of commercial protocols for high recovery of high-purity mesenchymal stem cell-derived extracellular vesicle isolation and their efficient labeling with fluorescent dyes. Nanomed Nanotechnol Biol Med. 2021;35:102396. doi: 10.1016/j.nano.2021.102396. [DOI] [PubMed] [Google Scholar]
- 114.Liangsupree T, Multia E, Riekkola ML. Modern isolation and separation techniques for extracellular vesicles. J Chromatogr A. 2021;1636:461773. doi: 10.1016/j.chroma.2020.461773. [DOI] [PubMed] [Google Scholar]
- 115.Jeyaram A, Jay SM. Preservation and storage stability of extracellular vesicles for therapeutic applications. AAPS J. 2018 doi: 10.1208/s12248-017-0160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kusuma GD, Barabadi M, Tan JL, Morton DAV, Frith JE, Lim R. To protect and to preserve: Novel preservation strategies for extracellular vesicles. Front Pharmacol. 2018 doi: 10.3389/fphar.2018.01199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hansen LJJ, Daoussi R, Vervaet C, Remon JP, De Beer TRM. Freeze-drying of live virus vaccines: A review. Vaccine. 2015;33:5507–5519. doi: 10.1016/j.vaccine.2015.08.085. [DOI] [PubMed] [Google Scholar]
- 118.Costantino HR, Firouzabadian L, Hogeland K, Wu C, Beganski C, Carrasquillo KG, Córdova M, Griebenow K, Zale SE, Tracy MA. Protein spray-freeze drying. effect of atomization conditions on particle size and stability. Pharm Res. 2000;17:1374–1383. doi: 10.1023/A:1007570030368. [DOI] [PubMed] [Google Scholar]
- 119.Sokolova V, Ludwig AK, Hornung S, Rotan O, Horn PA, Epple M, Giebel B. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surfaces B Biointerfaces. 2011;87:146–150. doi: 10.1016/j.colsurfb.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 120.Lee M, Ban JJ, Im W, Kim M. Influence of storage condition on exosome recovery. Biotechnol Bioprocess Eng. 2016;21:299–304. doi: 10.1007/s12257-015-0781-x. [DOI] [Google Scholar]
- 121.Li J, He X, Deng Y, Yang C. An update on isolation methods for proteomic studies of extracellular vesicles in biofluids. Molecules. 2019 doi: 10.3390/molecules24193516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yamamoto T, Kosaka N, Ochiya T. Latest advances in extracellular vesicles: from bench to bedside. Sci Technol Adv Mater. 2019;20:746–757. doi: 10.1080/14686996.2019.1629835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Meng W, He C, Hao Y, Wang L, Li L, Zhu G. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27:585–598. doi: 10.1080/10717544.2020.1748758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Burns AB, Doris C, Vehar K, Saxena V, Bardliving C, Shamlou PA, Phillips MI. Novel low shear 3D bioreactor for high purity mesenchymal stem cell production. PLoS ONE. 2021;16:e0252575. doi: 10.1371/journal.pone.0252575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No applicable.