Abstract
The activity of telithromycin, a new ketolide, was evaluated in vitro and in vivo against Mycobacterium avium complex (MAC) strains. The MIC of telithromycin for several M. avium isolates obtained from the blood of AIDS patients ranged from 16 to >128 μg/ml (MIC at which 90% of isolates are inhibited, >128 μg/ml), and the compound did show activity in the macrophage system at concentrations greater than 8 or 16 μg/ml, but this was dependent on the MAC strain used. Telithromycin was then administered to mice infected with MAC strain 101 for 4 weeks at doses of 100, 200, or 400 mg/kg of body weight/day. Treatment with 100 and 200 mg/kg/day was bacteriostatic, but at 400 mg/kg/day telithromycin was bactericidal for MAC strains. The frequency of the emergence of resistance to telithromycin was low despite prolonged usage (12 weeks). This study demonstrates that telithromycin is active in vivo against MAC and warrants further evaluation.
Isolates of the Mycobacterium avium complex (MAC) are intracellular pathogens associated with both lung disease and disseminated infection in patients with AIDS (12, 14). Efficacious treatment for MAC infection is based on the use of new macrolides such as clarithromycin, azithromycin, and roxithromycin (3, 10, 22). However, additional drugs with anti-MAC activity are needed.
Telithromycin (HMR3647) is a ketolide, i.e., a semisynthetic derivative of erythromycin A, which differs from erythromycin A by substitution of a 3-keto group for l-cladinose. Previously, we reported that HMR3004 was active against MAC in vivo, although its effect was bacteriostatic (5). HMR3004 has a C11-C12 carbamate on which a quinoline group is attached through a propyl chain, but it is no longer in clinical development. Telithromycin, in contrast, has a carbamate group linked to an imidazolium and pyridinium nucleus at C11-C12 (1, 9). Telithromycin has been shown to have potent activity against gram-positive organisms (17), Toxoplasma gondii (2), and Legionella pneumophila (11). In addition, telithromycin was shown to accumulate in polymorphonuclear neutrophils and macrophages in a nonsaturable fashion (18).
Resistance to clarithromycin has been shown to develop after a few months of therapy (10), and clarithromycin-resistant strains are cross resistant to azithromycin, roxithromycin, and ketolides (19). Previous work raised the possibility that the use of macrolides that achieve high concentrations in tissue, such as azithromycin and telithromycin, might be associated with a lower frequency of reistance than macrolides with high concentrations in serum, such as clarithromycin.
We have examined the anti-MAC activity of telithromycin both in vitro and in vivo. Furthermore, we have assessed the development of resistance to telithromycin during the course of prolonged experimental MAC infection.
MATERIALS AND METHODS
Antimicrobial agents.
Telithromycin (formerly, HMR3647) was provided by Roussel Uclaf (Romanville, France).
The potency of the drug was confirmed by using standard American Type Culture Collection (ATCC) quality control strains Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 29213. Telithromycin was prepared for administration by gavage by suspending it in 0.2% Tween 80 plus 2.5% gum arabic (Sigma Chemical Co.), as described previously (5).
Mycobacteria.
The strains of MAC used in the present study (strains 100, 101, 102, 103, 104, 105, 107, 108, 109, 110, 111, 113, 116, 117, 128, 501, 502, 502, 504, 505, 506, 507, and 508) were isolated from the blood of human immunodeficiency virus-infected patients (each strain was isolated from a different AIDS patient) with disseminated MAC disease, and each isolate was identified as M. avium by using a commercially available DNA probe (Gen-Probe Inc., San Diego, Calif.). MAC strain 101 (serovar 1), MAC strain 109 (serovar 4), and MAC strain 100 (serovar 8) were used for all macrophage assays. MAC strain 101 was used in the animal studies. MAC 101 is a virulent strain in the beige mouse test system, and this strain causes reproducible levels of infection and mortality in beige mice (8). MAC organisms were cultured in Middlebrook 7H10 agar medium (Difco Laboratories, Detroit, Mich.) supplemented with oleic acid, albumin, dextrose, and catalase (OADC; Difco) for 10 days at 37°C. Only transparent colony types were used in the studies. For the macrophage and mouse studies, the colonies were harvested and suspended in Hanks' balanced salt solution to concentrations of 1 × 108 and 3 × 108 CFU/ml, respectively, by comparison with a McFarland no. 1 turbidity standard; samples were plated onto 7H10 agar to confirm the concentration of the inoculum.
Beige mice were infected intravenously with 100 μl of the original suspension (3 × 107 MAC 101 bacteria per ml). Before infection of the macrophages, the suspension was vortex agitated for 2 min and passed through a 23-gauge needle five times to disperse clumps. Microscopic observation confirmed the dispersion of the inoculum.
In vitro susceptibility testing.
MICs were determined by a radiometric broth macrodilution method and the T100 method of data analysis (16). The inoculum for susceptibility testing was prepared by picking 5 to 10 colonies from a 7H11 agar plate and placing them into 7H9 broth; it was then tested directly or frozen at −70°C. The inoculum was adjusted to approximately 5 × 104 CFU/ml by comparison with a McFarland no. 1 turbidity standard. Isolates that clumped and that could not be easily dispersed were shaken with glass beads. Controls included the inoculum undiluted without added drug (no-drug control), the inoculum diluted 1:100 (99% control), and the inoculum diluted 1:1,000 (99.9% control). In addition, one vial was inoculated with a suspension of mycobacteria which were boiled for 5 min prior to inoculation in order to monitor the non-growth-related release of carbon dioxide in the BACTEC system. The period of observation and the end points were determined by daily monitoring of the control and test cultures, but a period of 7 days was sufficient for most isolates. MAC 101 was tested against amikacin to control for overall test performance.
Human macrophage studies.
The source of macrophages was the human monocyte cell line U937 cultured in RPMI 1640 medium (pH 7.2) (Gibco, Chicago, Ill.) supplemented with 5% fetal bovine serum (Sigma Chemical Co.) and 2 mM l-glutamine. The cells were grown to a density of 5 × 108 cells per ml and were then centrifuged, washed, and resuspended in supplemented RPMI 1640 medium. The concentration of cells was adjusted to 106 cells per ml, and 1 ml of the cell suspension was added to each well of a 24-well tissue culture plate (Costar, Cambridge, Mass.). Monolayers were treated with 0.5 mg of phorbol myristate acetate per ml for 24 h to stimulate maturation of the monocytes. The monolayers were monitored for the number of cells, and no difference in the extent of cell detachment was observed among the several treatment and control groups.
M. avium strains were cultured for 10 days in Middlebrook 7H10 agar (Difco Laboratories). On the day of the experiment, the bacteria were harvested, washed twice in Hank's balanced salt solution, and suspended in Hank's balanced salt solution; and a dispersed inoculum was prepared as described above. The turbidity of the suspension was adjusted so that it was equivalent to that of a McFarland no. 1 turbidity standard, and the suspension was diluted to a final concentration of approximately 5 × 107 CFU/ml. Each monolayer was infected with 100 μl of the final suspension, and the actual number of CFU per milliliter for the final suspension was determined by quantitative plate counts. Four hours after infection, the number of CFU of mycobacteria per well of the macrophage monolayer was determined by lysing the macrophages and performing quantitative plate counts to establish the initial inoculum (baseline), as reported previously (4). The infected monolayers were then treated with telithromycin (concentration range, 1 to 128 μg/ml). Drug and medium were replenished daily for 4 days. After the treatment period (4 days), the medium was removed and the monolayers were lysed as described previously (4). Briefly, ice-cold sterile water (0.5 ml) was added to each monolayer well, and the mixture was allowed to stand for 10 min at room temperature. Then, 0.5 ml of a second lysing solution (1.1 ml of Middlebrook 7H9 broth plus 0.4 ml of 0.25% sodium dodecyl sulfate [SDS] in phosphate buffer) was added to each well, and the mixture was allowed to stand for an additional 10 min. The wells were vigorously scraped with a rubber policeman, and the macrophage lysates were resuspended in 0.5 ml of 20% bovine serum in sterile water to neutralize the SDS. The suspension was vortex agitated for 2 min to ensure complete lysis of the macrophages. Finally, the lysate was sonicated for 5 s to disrupt clumps of bacilli. To control for the osmotic stability of the mycobacteria, a suspension of mycobacteria alone was plated for quantitation before and after being subjected to the lysis procedure as described above, and in each instance there was no change in the number of CFU per milliliter before or after the lysis treatment procedure.
The final macrophage lysate suspension was serially diluted, and 0.1 ml was plated onto 7H10 agar. The plates were allowed to dry at room temperature for 15 min and incubated at 37°C in 5% CO2 for 2 weeks. Duplicate plates were prepared for each well, and the results were reported as the mean number of CFU per milliliter of macrophage lysate. Each assay was performed in triplicate, and each experiment was repeated six times.
Animal studies.
The potential therapeutic efficacy of telithromycin was determined by using the beige mouse test system as described previously (4, 6, 20). This system uses 6- to 7-week-old female C57BL/6 bg+ bg+ mice (Jackson Laboratories, Bar Harbor, Maine). Briefly, the mice were infected through the caudal vein with 3 × 107 MAC 101 bacteria, and after 7 days, treatment was initiated with telithromycin at a dosage of 100, 200, and 400 mg/kg of body weight per day for 4 weeks (chosen on the basis of simulated kinetics in humans). The drug was administered by daily gavage for 28 days. A control group of mice was infected but received sucrose syrups in place of the antibiotic. An additional group of mice was examined 7 days after infection in order to establish the level of infection before the initiation of therapy. Sixteen mice were used for each of the control and experimental groups. At the termination of therapy, the livers and spleens of the control and treated mice were aseptically removed, weighed, and then homogenized in 5 ml of 7H9 broth (Difco) with a tissue homogenizer. The tissue suspensions were serially diluted in 7H9 broth and plated onto 7H11 agar plates supplemented with OADC for quantitation of viable bacteria. The number of mycobacteria in the blood was determined by collecting 0.05 ml of blood at day 7 and day 28. The number of CFU per milliliter of blood was determined by inoculating the blood into 4 ml of BACTEC 12B medium (Johnston Laboratories, Sparks, Nev.) and by the T100 method of data analysis as described previously (16).
Emergence of resistance.
Detection of the emergence of resistance was performed as described previously (7). Briefly, the mice were infected with 4 × 107 CFU intravenously, and after 7 days, 10 mice per experiment were harvested to establish the baseline level of infection. Telithromycin was administered at 200 mg/kg/day by gavage over the entire period (up to 12 weeks). Control mice received the same vehicle without drug. Fifteen mice were harvested per experimental group at 0, 8, and 12 weeks after the initiation of therapy, and the spleens were removed and cultured in a quantitative manner. To decrease the possibility of carryover, mice were killed 48 h after they received the last dose of the drug.
Twelve to 20 mice were used per group or time point. The mice were killed, and the spleens were removed by aseptic dissection. The organs were weighed and homogenized in Middlebrook 7H9 broth. Aliquots of the suspensions were plated onto 7H11 agar with OADC and onto 7H11 agar with OADC and telithromycin (128 μg/ml). The frequency of resistance was determined by counting the number of CFU per gram of tissue growing on plates with and without antibiotic. The plates were incubated for 8 to 10 days at 37°C, and the number of CFU were counted. Both the total number of colonies and the number of resistant colonies per mouse were taken into consideration to calculate the frequency of resistance. MAC strains isolated from mice that developed microbiological evidence of telithromycin resistance were tested in vitro to determine the MIC of telithromycin with the BACTEC system, as described above. If more than one colony was isolated from a culture, a sweep of all colonies was taken for testing.
Resistance to telithromycin was defined as an MIC greater than 128 μg/ml, based on the similarity of pharmacokinetics between telithromycin and azithromycin (9, 11).
Statistical analysis.
The differences between results for the untreated control and the experimental groups in the macrophage experiments at identical time points were determined by the Mann-Whitney nonparametric test. The statistical significance of the differences between the number of organisms recovered from the spleens, livers, and blood was evaluated by one- or two-variable analysis of variance. Differences between the results for experimental groups and between the results for the experimental groups and the control groups were considered statistically significant if P values were <0.05.
RESULTS
MIC studies.
The MICs at which 50 and 90% of isolates were inhibited for 24 MAC strains (blood isolates) were 128 and 128 μg/ml, respectively. Seventeen of the individual strains tested were resistant to telithromycin at concentrations of 128 μg/ml or higher, and the range of MICs for the 23 strains tested varied from 32 to >128μg/ml.
Human macrophage studies.
As shown in Table 1, telithromycin had no inhibitory activity against intracellular MAC strains 100 and 101 at concentrations less than 16 μg/ml, while inhibitory activity against strain 109 was observed in the presence of 8 μg/ml after 4 days of treatment. Only the concentration of 128 μg/ml was bactericidal for MAC 100. No other bactericidal activity was observed in this system with the concentrations used.
TABLE 1.
Activity of telithromycin against intracellular MAC strains
| Drug dose (μg/ml) | No. of CFU/ml of macrophage lysate at day 4a
|
||
|---|---|---|---|
| MAC 100 | MAC 101 | MAC 109 | |
| None | (9.8 ± 0.1) × 105 | (8.2 ± 0.2) × 105 | (8.1 ± 0.2) × 105 |
| 1 | (9.6 ± 0.1) × 105 | (7.9 ± 0.1) × 105 | (7.6 ± 0.1) × 105 |
| 2 | (9.0 ± 0.1) × 105 | (7.6 ± 0.3) × 105 | (6.3 ± 0.2) × 105 |
| 4 | (7.9 ± 0.1) × 105 | (6.6 ± 0.4) × 105 | (5.5 ± 0.2) × 105 |
| 8 | (5.9 ± 0.1) × 105 | (5.6 ± 0.1) × 105 | (3.6 ± 0.1) × 105b |
| 16 | (4.8 ± 0.2) × 105b | (4.5 ± 0.2) × 105b | (2.5 ± 0.1) × 105b |
| 32 | (2.6 ± 0.3) × 105b | (3.5 ± 0.1) × 105b | (1.9 ± 0.2) × 105b |
| 64 | (1.6 ± 0.1) × 105b | (3.0 ± 0.1) × 105b | (1.6 ± 0.3) × 105b |
| 128 | (6.5 ± 0.1) × 104bc | (2.6 ± 0.4) × 105b | (1.2 ± 0.4) × 105b |
The numbers of intracellular bacteria at time zero (after infection but before treatment) were as follows: MAC 100, (8.5 ± 0.2) × 104; MAC 101, (7.9 ± 0.3) × 104; MAC 109, (7.4 ± 0.2) × 104.
P < 0.5 compared with the value for controls at 4 days (bacteriostatic activity).
P < 0.5 compared with the baseline level at time zero (bactericidal activity).
Therapeutic studies with mice.
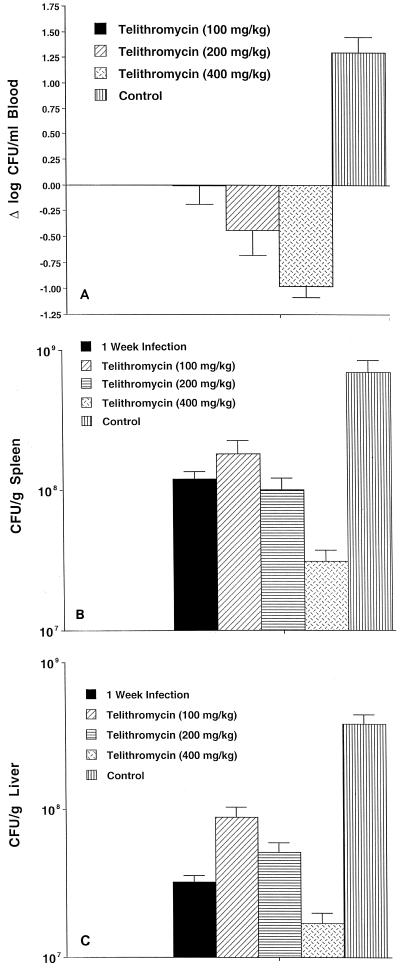
Telithromycin was administered orally at doses of 100, 200, and 400 mg/ml daily; these doses were based on the levels obtained in serum (Table 2). Telithromycin was not toxic, and its administration was not associated with increased rates of mortality. While 35% of the untreated control mice survived the experiment, 60% of the mice receiving 100 mg of telithromycin per ml, 75% of the mice receiving 200 mg of telithromycin per ml, and 85% of the mice receiving 400 mg of telithromycin per ml survived the period of the study. As shown in Fig. 1A to C, there was a dose-related effect according to the dose of telithromycin used. While concentrations of 100 and 200 mg/kg/day were bacteriostatic compared to the level of infection at day 7 (before treatment), the dose of 400 mg/kg showed bactericidal activity in both the livers and the spleens, and treatment at these doses resulted in a significant reduction (approximately 1 log) in the number of bacteria in the blood.
TABLE 2.
Telithromycin concentration in the blood of micea
| Time (h) | Concn in blood (μg/ml) for the following dose:
|
||
|---|---|---|---|
| 100 mg/kg | 200 mg/kg | 400 mg/kg | |
| 0.5 | 3.90 | 6.64 | 9.51 |
| 1 | 3.95 | 8.12 | 8.78 |
| 2 | 4.91 | 11.47 | 11.07 |
| 4 | 3.97 | 9.18 | 9.41 |
| 8 | 2.16 | 7.36 | 8.86 |
Three uninfected mice were given telithromycin orally for each time point and dose. Blood was obtained via the tail vein. Drug levels in tissue have been published previously (9).
FIG. 1.
Effect of telithromycin treatment on the number of viable MAC bacteria in blood (A), spleen (B), and liver (C) following 4 weeks of treatment. Sixteen mice were used per experimental group. Data represent the mean ± standard error.
Emergence of resistance.
Treatment with telithromycin resulted in a low frequency of resistance. Table 3 shows the number of mice from which telithromycin-resistant MAC strains were isolated over time and the frequency of resistance to the drug among these isolates. While the frequency of the emergence of strains resistant to telithromycin was statistically significantly greater compared with the frequency of emergence of resistant MAC strains isolated from untreated mice at both weeks 8 and 12, the frequency was nonetheless low. The telithromycin MICs for the resistant strains were 512 μg/ml or greater.
TABLE 3.
Incidence of telithromycin-resistant MAC and frequency of telithromycin resistance in splenic homogenates
| Treatment | No. of mice with resistant bacteria/total no. of mice tested
|
Frequency of resistancea
|
|||
|---|---|---|---|---|---|
| 8 wk | 12 wk | 0 wk | 8 wk | 12 wk | |
| Telithromycin | 12/20 | 6/16 | 0 | 9.0 × 10−7 | 7.4 × 10−6 |
| Control | 0/12 | 8/12 | 0 | 0 | 6.2 × 10−9 |
| P value | >0.05 | 0.01 | 0.01 | ||
Based on the number of bacteria per gram of tissue by plating spleen homogenate onto a 7H10 agar plate containing 128 μg of telithromycin per ml and compared with the number of bacteria per gram of tissue on plates without antibiotic. The telithromycin MICs for the resistant strains were 512 μg/ml or higher.
DISCUSSION
Telithromycin showed weak activity against MAC in vitro, with MICs of 16 or greater, but it was significantly active against MAC infection in vivo. The activity in vivo was dose dependent. Telithromycin was bacteriostatic at doses of 100 and 200 mg/kg/day, but it was bactericidal at a dose of 400 mg/kg/day. Telithromycin was significantly more active on a weight basis than HMR3004 for the treatment of MAC infection in a similar mouse system (5).
Other studies found that telithromycin is active for the treatment of infections caused by another intracellular pathogen, L. pneumophila, in guinea pigs (11) and T. gondii in murine models of infection (2).
The pharmacokinetics of telithromycin are compatible with those for a compound that achieves a very high concentration within cells and a low concentration in serum (18). In fact, our in vitro results suggest that a very high concentration within the cell is needed in order to inhibit the growth of or kill MAC. However, the findings obtained with the macrophage system appear to indicate that one needs a high concentration of telithromycin outside the cell to achieve the needed high concentration within the cell. A plausible explanation for this finding is that telithromycin penetrates macrophages very slowly and that the 4 days of the macrophage assay were not long enough to allow the drug to concentrate within the cells. It is possible that the macrophage system, with only 4 days of incubation, has a limited ability to identify active compounds when the concentration necessary to inhibit the growth or kill the intracellular pathogen is high.
A recent study has demonstrated that telithromycin accumulates up to 300 times within phagocytic cells and that it is poorly released from uninfected cells (21). In polymorphonuclear neutrophils, more than 75% of the molecule was recovered in the azurophil granule fraction. Since MAC inhibits phagosome fusion with the lysosome, this property may well explain why telithromycin administered at low concentrations for 4 days did not inhibit MAC strains in macrophages. In contrast, we observed that telithromycin was effective for the treatment of MAC infection. Thus, telithromycin is the second ketolide with anti-MAC activity in mice (5), although the anti-MAC activity was inferior to the activities of azithromycin and clarithromycin reported previously (13, 15).
With regard to the important issue of the emergence of resistance, treatment with telithromycin was apparently associated with a lower frequency of emergence of resistance than treatment with clarithromycin (7) and a frequency of emergence of resistance comparable to that obtained with azithromycin (7). Even though we did not compare the frequency of emergence of resistance to the three compounds side by side, we have evaluated the frequency of emergence of resistance to both clarithromycin and azithromycin for many years, always with consistent results. Although limited, our observations thus far suggest that compounds that achieve very high concentrations within cells may be associated with a lower frequency of emergence of resistance among MAC strains than compounds that achieve high concentrations in serum.
Clinical resistance to macrolides is primarily due to a single mutation in the 23s rRNA gene (19). Thus far, all macrolide-resistant strains that we have tested are also resistant to ketolides (data not shown). Therefore, ketolides cannot be used as alternative therapies when macrolides fail.
In summary, we described the anti-MAC activity of telithromycin, an antibiotic that, despite its poor activity in vitro, showed significant efficacy in the treatment of MAC infection in mice, likely due to its pharmacokinetic properties.
ACKNOWLEDGMENTS
We thank Karen Allen for the careful preparation of the manuscript.
This work was supported by contract NO 1-AI-25140 from the National Institutes of Health.
REFERENCES
- 1.Agouridas C, Bonnefoy A, Chantot J F. Antibacterial activity of RU 64004 (HMR 3004), a novel ketolide derivative active against respiratory pathogens. Antimicrob Agents Chemother. 1997;41:2149–2158. doi: 10.1128/aac.41.10.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araujo F G, Khan A A, Slifer T L, Bryskier A, Remington J S. The ketolide antibiotics HMR 3647 and HMR 3004 are active against Toxoplasma gondii in vitro and in murine models of infection. Antimicrob Agents Chemother. 1997;41:2137–2140. doi: 10.1128/aac.41.10.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson C A, Williams P L, Cohn D L, Becker S, Hojczyk P, Nevin T, Korvick J A, Heifets L, Child C C, Lederman M M, Reichman R C, Powderly W G, Notario G F, Wynne B A, Hafner R. Clarithromycin or rifabutin alone or in combination for primary prophylaxis of Mycobacterium avium complex disease in patients with AIDS: a randomized, double-blind, placebo-controlled trial. The AIDS Clinical Trials Group 196/Terry Beirn Community Programs for Clinical Research on AIDS 009 Protocol Team. J Infect Dis. 2000;181:1289–1297. doi: 10.1086/315380. [DOI] [PubMed] [Google Scholar]
- 4.Bermudez L E, Inderlied C B, Kolonoski P, Wu M, Barbara-Burnham L, Young L S. Activities of Bay Y 3118, levofloxacin, and ofloxacin alone or in combination with ethambutol against Mycobacterium avium complex in vitro, in human macrophages, and in beige mice. Antimicrob Agents Chemother. 1996;40:546–551. doi: 10.1128/aac.40.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bermudez L E, Inderlied C B, Kolonoski P, Wu M, Young L S. Activity of HMR3004 against Mycobacterium avium complex in vitro, in human macrophages and in beige mice. Clin Microbiol Infect. 1998;4:325–331. doi: 10.1128/aac.40.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bermudez L E, Kolonoski P, Young L S. Roxithromycin alone and in combination with either ethambutol or levofloxacin for disseminated Mycobacterium avium infections in beige mice. Antimicrob Agents Chemother. 1996;40:1033–1035. doi: 10.1128/aac.40.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bermudez L E, Petrofsky M, Kolonoski P, Young L S. Emergence of Mycobacterium avium populations resistant to macrolides during experimental chemotherapy. Antimicrob Agents Chemother. 1998;42:180–183. doi: 10.1128/aac.42.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertram M A, Inderlied C B, Yadegar S, Kolanoski P, Yamada J K, Young L S. Confirmation of the beige mouse model for study of disseminated infection with Mycobacterium avium complex. J Infect Dis. 1986;154:194–195. doi: 10.1093/infdis/154.1.194. [DOI] [PubMed] [Google Scholar]
- 9.Bryskier A, Agouridas C, Chantot J F. Ketolides: new semi-synthetic 14-membered ring macrolides. In: Zinner S H, Young L S, Acar J, Neu H C, editors. Expanding indications of the new macrolides, azalides and streptogramins. New York, N.Y: Marcel Dekker, Inc; 1997. pp. 47–62. [Google Scholar]
- 10.Chaisson R E, Benson C A, Dube M P, Heifets L B, Korvick J A, Elkin S, Smith T, Craft J C, Sattler F R. Clarithromycin therapy for bacteremic Mycobacterium avium complex disease. A randomized, double-blind, dose-ranging study in patients with AIDS. AIDS Clinical Trials Group Protocol 157 Study Team. Ann Intern Med. 1994;121:905–911. doi: 10.7326/0003-4819-121-12-199412150-00001. [DOI] [PubMed] [Google Scholar]
- 11.Edelstein P H, Edelstein M A. In vitro activity of the ketolide HMR 3647 (RU 6647) for Legionella spp., its pharmacokinetics in guinea pigs, and use of the drug to treat guinea pigs with Legionella pneumophila pneumonia. Antimicrob Agents Chemother. 1999;43:90–95. doi: 10.1128/aac.43.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falkinham J O., III Epidemiology of infection by nontuberculous mycobacteria. Clin Microbiol Rev. 1996;9:177–215. doi: 10.1128/cmr.9.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandes P B, Hardy D J, McDaniel D, Hanson C W, Swanson R N. In vitro and in vivo activities of clarithromycin against Mycobacterium avium. Antimicrob Agents Chemother. 1989;33:1531–1534. doi: 10.1128/aac.33.9.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inderlied C B, Kemper C A, Bermudez L E. The Mycobacterium avium complex. Clin Microbiol Rev. 1993;6:266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inderlied C B, Kolonoski P T, Wu M, Young L S. In vitro and in vivo activity of azithromycin (CP 62, 993) against the Mycobacterium avium complex. J Infect Dis. 1989;159:994–997. doi: 10.1093/infdis/159.5.994. [DOI] [PubMed] [Google Scholar]
- 16.Inderlied C B, Young L S, Yamada J K. Determination of in vitro susceptibility of Mycobacterium avium complex isolates to antimycobacterial agents by various methods. Antimicrob Agents Chemother. 1987;31:1697–1702. doi: 10.1128/aac.31.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jamjian C, Biedenbach D J, Jones R N. In vitro evaluation of a novel ketolide antimicrobial agent, RU-64004. Antimicrob Agents Chemother. 1997;41:454–459. doi: 10.1128/aac.41.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miossec-Bartoli C, Pilatre L, Peyron P, N'Diaye E N, Collart-Dutilleul V, Maridonneau-Parini I, Diu-Hercend A. The new ketolide HMR3647 accumulates in the azurophil granules of human polymorphonuclear cells. Antimicrob Agents Chemother. 1999;43:2457–2462. doi: 10.1128/aac.43.10.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nash K A, Inderlied C B. Genetic basis of macrolide resistance in Mycobacterium avium isolated from patients with disseminated disease. Antimicrob Agents Chemother. 1995;39:2625–2630. doi: 10.1128/aac.39.12.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullam P M, Gordin F M, Wynne B A. Efficacy of rifabutin in the treatment of disseminated infection due to Mycobacterium avium complex. The Rifabutin Treatment Group. Clin Infect Dis. 1994;19:84–86. doi: 10.1093/clinids/19.1.84. [DOI] [PubMed] [Google Scholar]
- 21.Vazifeh D, Abdelghaffar H, Labro M T. Cellular accumulation of the new ketolide RU 64004 by human neutrophils: comparison with that of azithromycin and roxithromycin. Antimicrob Agents Chemother. 1997;41:2099–2107. doi: 10.1128/aac.41.10.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young L S, Wiviott L, Wu M, Kolonoski P, Bolan R, Inderlied C B. Azithromycin for treatment of Mycobacterium avium-intracellulare complex infection in patients with AIDS. Lancet. 1991;338:1107–1109. doi: 10.1016/0140-6736(91)91965-w. [DOI] [PubMed] [Google Scholar]