Introduction: Definition, Classification, and Main Functions of Neuroglia
The human brain has a considerable complexity. In a rather limited volume, it contains a population of more than 200 billion neural cells, including neurones and neuroglia. Altogether, these neural cells form intricate networks connecting the various parts that make up this organ through trillions of chemical and electrical synapses. The concept of neuroglia was initially formalized by Rudolf Virchow who introduced it in the mid-1800s. According to Virchow, neuroglia was a “substance also which lies between the proper nervous parts, holds them together and gives the whole its form in a greater or lesser degree” (Virchow 1860). The neuroglia is present in both the peripheral nervous system (PNS) and the central nervous system (CNS) (Fig. 1). The PNS neuroglia arises from the neural crest, similarly to peripheral neurones, and is classified into Schwann cells (Kidd et al. 2013), satellite glial cells (Hanani and Verkhratsky 2021), olfactory ensheathing cells (Ruitenberg et al. 2006), and enteric glia (Grubisic et al. 2018). The neuroglia cells of the CNS are divided into macroglia cells (ectodermal, neuroepithelial origin) and microglia (mesodermal, myeloid origin) (Verkhratsky and Butt 2013). Macroglia is further classified into astroglia, oligodendroglia, and NG-2 glia, the latter also known as oligodendrocyte progenitor cells, or synantocytes, or polydendrocytes (Verkhratsky and Butt 2013). Each of these populations listed above can, in turn, be divided into further subtypes, making the complexity that these cells possess to parallel the multitude of functions they govern. The large number of subtypes of glial cells fueled for years the belief that in the human brain glial cells outnumber neurones by a factor of 10 up to 50 (Bear et al. 2007; Kandel et al. 2000). However, the views of numerical preponderance of glial cells in the brain and spinal cord with respect to the number of neurones have been proven erroneous, because none of the concepts that had been adopted as a demonstration of big glial numbers has been corroborated experimentally (Hilgetag and Barbas 2009; von Bartheld et al. 2016). It is generally agreed upon that the total number of neuronal and non-neuronal cells in the human brain is almost on par. Nonetheless, even if not in a linear manner, the evolution of the nervous system paralleled with trend of an increase in glia to neurone ratio (Verkhratsky et al. 2019), suggesting glial involvement in cerebral superior functions, although the largest numbers of glial cells are observed in the largest brains of whales and elephants.
Fig. 1.
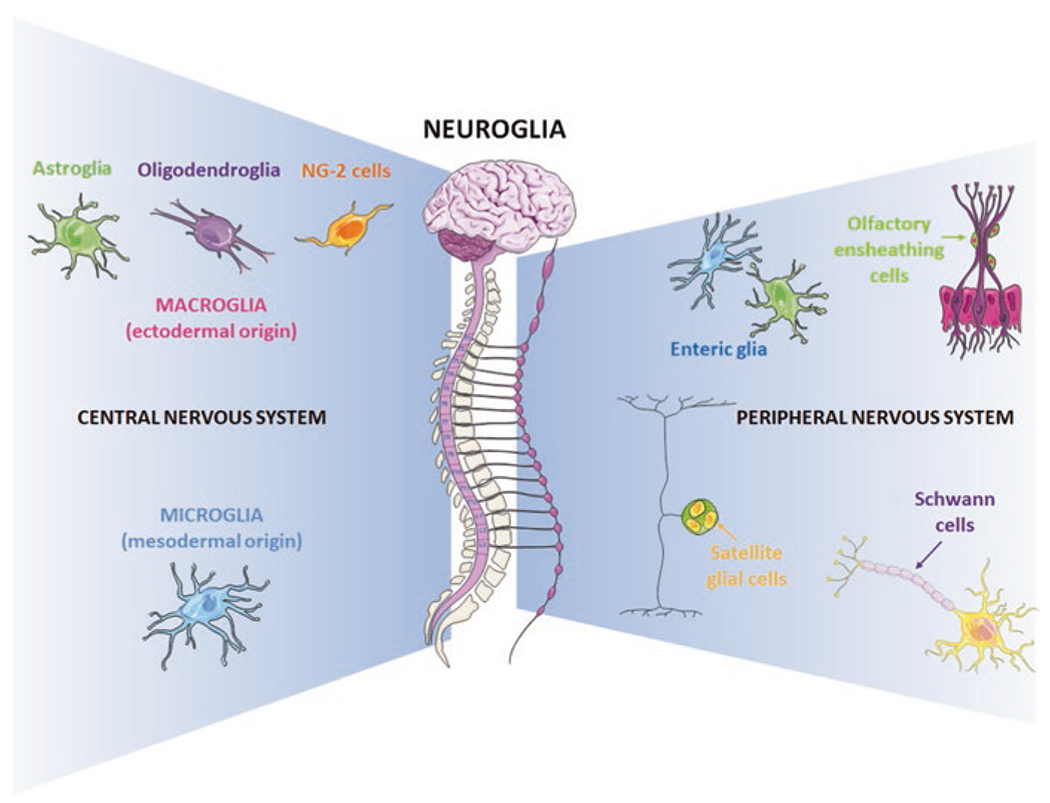
Neuroglia classification
The unifying fundamental function of all types of glial cells, regardless of their origin, structure, morphological appearance, and function, is the maintenance of the homeostasis of the nervous system (Verkhratsky and Butt 2013). This function is of crucial importance in the healthy brain, when neuroglia perform the normal housekeeping duties, as well as in pathology, when glial cells react to unusual stimuli and undergo morphofunctional modifications aimed at restoring brain homeostasis. Any deviation from this delicate equilibrium may have serious consequences in the correct development or functioning of the brain. The homeostatic support of neuroglia takes place at all levels of brain organization, thus allowing the brain to function properly.
Microglia
Microglia are the main type of immune cells that permanently reside in the CNS. Unlike all other brain parenchymal cells that have multiple neuroectodermal lineages, microglia originate from a mesodermal source. Microglia are of the myeloid origin, colonize the CNS very early in evolution, and are conserved across species (Ginhoux et al. 2010; Monier et al. 2007; Schlegelmilch et al. 2011; Swinnen et al. 2013; Verney et al. 2010). For a long time studies on microglia have been focused on their function as resident macrophages and their role in the immune response (Cartier et al. 2014; Prinz et al. 2011; Prinz and Priller 2014). Being the main immunocompetent cells of the nervous system, microglia fulfill fundamental defensive function by the virtue of their phagocytic capacity and ability to secrete numerous pro- and anti-inflammatory factors. Through phagocytosis, microglia can incorporate waste products, cellular debris, and pathogens (Nayak et al. 2014). Advances in the available technologies have enabled a better understanding of the microglial functions across different conditions (Tay et al. 2019). Microglia is fundamental for brain development, activity, and plasticity (Tay et al. 2017), including the creation and remodeling of synapses. Through the modulation of synapse number and synaptic activity, microglia can regulate the processes of learning, memory, and cognition (Weinhard et al. 2018). Microglia also regulate neurogenesis, neuronal density and connectivity, as well as neuronal survival and turnover (Shigemoto-Mogami et al. 2014). Most of these processes begun during the period of perinatal development and persist through to the late adolescence/adulthood (Sellner et al. 2016; Sierra et al. 2010; Ueno et al. 2013). Microglial functions are based on the scavenging of cellular debris as well as the intense exchange of communication between microglia and neurones, achieved through the production and release of numerous neurotrophic mediators (Tay et al. 2017). This former property explains the reason why microglial numerical preponderance occurs in areas containing debris or apoptotic neurones as well as in regions with high density of neural precursor cells where microglia can drive neuronal turnover during development (Ayata et al. 2018; Cunningham et al. 2013; Swinnen et al. 2013). Microglia can be considered as key contributors to normal brain functioning, mainly because these cells regularly scan the surrounding environment and adapt their morphology and functions to restore homeostasis. Therefore, dysfunctions of microglial cells could have deleterious consequences at any stage of human life. During the pre- and perinatal brain development, the modification of microglial functions could impair essential processes such as neural connectivity and synaptic plasticity (Kettenmann et al. 2013). In the same way during adult life, changes to microglial functions could cause a remodeling of the neuronal circuits with serious consequences on learning and memory (Weinhard et al. 2018). In conclusion, dysfunctional microglia play a fundamental role in the onset, evolution, and outcome of neurological diseases throughout life span (Scuderi and Verkhratsky 2020; Tay et al. 2018).
Oligodendrocyte and NG-2 Glia
Oligodendrocytes are cells of fundamental importance for the CNS because they form the myelin sheath necessary for a fast and efficient transmission of the nervous impulse. Oligodendrocytes originate from the oligodendrocyte precursor cells (OPCs) that arise from multipotent neural stem cells (NSCs), mainly localized in the ventricular zones of the brain from which they migrate to the developing CNS where they become active oligodendrocytes. This process starts shortly before birth and continues throughout life (Bergles and Richardson 2015) as a significant amount of OPCs persists in the adult brain. These OPCs have been also identified as NG-2 glia because they express CSPG4, the NG2 chondroitin sulfate proteoglycan (Almeida and Lyons 2017). The differentiation of NG-2 glia into oligodendrocytes is essential for myelin repair in the adult brain (Ortiz et al. 2019), and for ensheathing new neuronal connections with myelin in response to new experiences (McKenzie et al. 2014; Xiao et al. 2016). These observations suggest that neurotransmission drives the differentiation of NG-2 glia and are consistent with the evidence that NG-2 glia exhibit a wide range of ion channels and neurotransmitter receptors (Larson et al. 2016), and respond to synaptic transmission (Bergles et al. 2000). Despite these findings, further studies are required to decipher how neuronal activity drives NG-2 glia conversion in oligodendrocytes. Indeed, data acquired so far demonstrated that blocking, or stimulating, synaptic signaling has only weak effects on NG-2 glia, suggesting that neurotransmitters alone are not sufficient to start oligodendrogenesis (Butt et al. 2019).
Several factors modulate OPC migration, proliferation, differentiation, and myelination (Elbaz and Popko 2019). These factors include extrinsic as well as intrinsic transcription factors, epigenetic modulators, and signaling pathways (Elbaz and Popko 2019). Oligodendrocytes express many receptors belonging to different classes suggesting that these cells receive impulses from different signaling pathways indispensable for their development and functions, mainly the formation of myelin (Butt et al. 2019; Habermacher et al. 2019; Kiray et al. 2016; Patel and Klein 2011). For instance, it has been demonstrated that the Wnt signaling controls OPC expansion throughout life (Azim et al. 2017) and that estrogen favors oligodendrocyte differentiation and myelination by regulating cholesterol homeostasis (Voskuhl et al. 2019).
Myelin is mostly composed of lipids (about 70%, of which the primary component is cholesterol) and proteins (about 30%, of which the main components are the myelin basic protein and proteolipid protein) (Muller et al. 2013; Saher and Stumpf 2015). Although oligodendrocytes seem capable of de novo synthesis of cholesterol, it has been suggested that the lipid used to form the myelin sheath comes from astrocytes, as the blood-brain barrier (BBB) does not allow dietary cholesterol to enter the CNS (Kiray et al. 2016). Myelin also contains gap junctions formed by connexins, which are fundamental for ion homeostasis and axonal metabolism and integrity (Vejar et al. 2019). Besides the establishment of the optimal conditions for rapid electrical conduction, myelin is also required for axonal integrity (Alexandra et al. 2018). The underlying mechanisms are not fully clarified, but recent evidence indicates oligodendrocytes as essential for fulfilling axonal metabolic needs. They indeed provide glucose (Meyer et al. 2018) and lactate to axons (Funfschilling et al. 2012; Lee et al. 2012) depending on the axonal activity requirements (Micu et al. 2018; Saab et al. 2016).
Given the above, dysfunction or loss of oligodendroglia or of their ability to make the myelin sheath can cause devastating effects on CNS function and eventually lead to neuronal death. Moreover, the pleiotropism of factors involved in oligodendrocyte development and myelination helps to ensure that the disruption of any single factor does not result in their loss of function. On the other side, they represent multiple targets that could be involved in oligodendrocyte pathologies offering exciting new perspectives of research.
Astrocytes
Astroglia (to which astrocytes belong) are a class of highly heterogeneous in form and function neural cells of the ectodermal, neuroepithelial origin; these cells maintain homeostasis and defence of the CNS (Verkhratsky and Nedergaard 2018). Astrocytes reside in the white and gray matter of the brain and the spinal cord (Verkhratsky and Butt 2013). Numerous distinct morphological and functional subtypes of astrocytes have been identified, including (i) protoplasmic astrocytes of the gray matter; (ii) fibrous astrocytes of the white matter; (iii) velate astrocytes, localized in brain regions where neurones are small and densely packed (e.g., the olfactory bulb or the granular layer of the cerebellar cortex); (iv) radial glia, which are the pluripotent neural cell precursors that mostly disappear at birth; (v) radial astrocytes, which comprise the cerebellar Bergmann glia, the retinal Müller glia, radial glia-like neural stem cells of the neurogenic niches, and tanycytes; (vi) pituicytes, localized in the neurohypophysis; (vii) the iron-enriched astrocytes, named Gomori astrocytes, localized in the hypothalamus and the hippocampus; (viii) perivascular astrocytes, whose endfeet connect with blood vessels and are fundamental for the establishment of the glia limitans barriers; (ix) juxtavascular astrocytes somata of which are in close apposition with blood vessels; (x) ependymocytes, which are choroid plexus cells, lining the ventricles and producing the cerebrospinal fluid, and retinal pigment epithelial cells, which line up the retinal space; and specialized astrocytes observed only in the brain of higher primates (including humans) which include (xi) interlaminar astrocytes, (xii) polarized astrocytes, and (xiii) varicose projection astrocyte; functions of all these types are still unclear (Colombo 2018; Verkhratsky et al. 2018, 2019).
The heterogeneity of astroglia correlates with the multiplicity of functions that they perform. For instance, astroglia (i) control the levels of neurotransmitters, ions, reactive oxygen species, and metabolites (Deitmer and Rose 1996; Hertz et al. 1999; Kirischuk et al. 2007; Kofuji and Newman 2004); (ii) drive neurogenesis (Verkhratsky and Nedergaard 2018); (iii) regulate synapse formation, pruning, and elimination (Kettenmann et al. 2011, 2013; Pfrieger 2010); (iv) form and maintain the myelin sheath (Butt et al. 2019; Kuhn et al. 2019); and (v) control the BBB and the blood flow (Abbott et al. 2010; Attwell et al. 2010) (Fig. 2). The type and relative number of astrocytes vary among brain regions (Verkhratsky et al. 2019). Despite their great variety of morphology and functions, all astrocytes are best at performing their homeostatic function. To this end, astrocytes cooperate to form, through gap junctions, cellular networks called syncytia, comprised of apposing membranes of adjacent astrocytes pierced by hundreds of intercellular channels or connexons (Giaume et al. 2010). Gap junctions represent highly specialized areas for the transport of second messengers, ions, and bioactive molecules (Houades et al. 2008; Roux et al. 2011). Networks between astrocytes and oligodendrocytes have been identified in both the hippocampus and the neocortex (Butt and Ransom 1989; Griemsmann et al. 2015; Pastor et al. 1998) and named “panglial syncytia.”
Fig. 2.
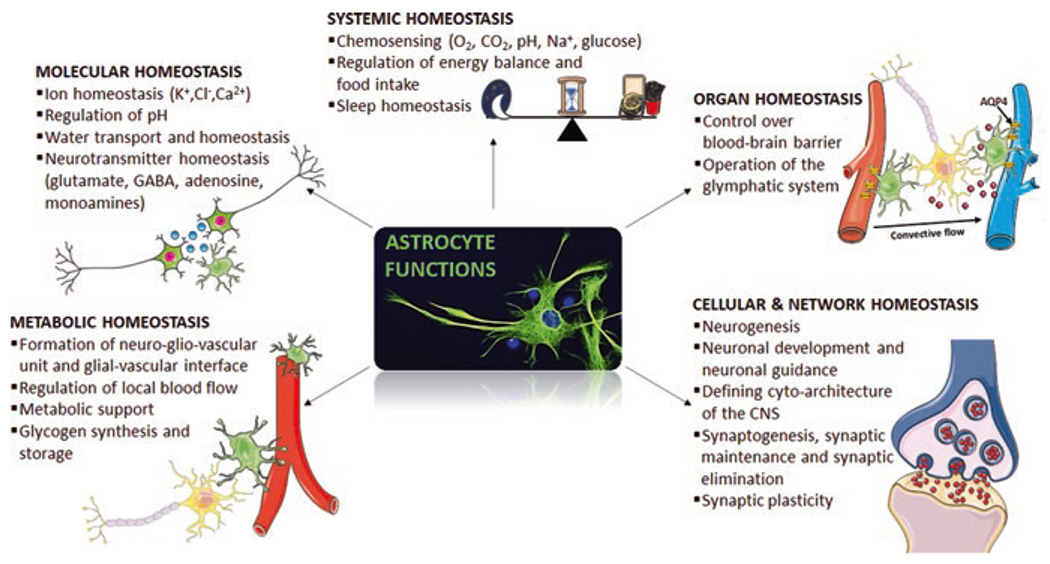
Astrocytes as homeostatic cells of the CNS
Astrocytic membrane carries a multitude of receptors for neurotransmitters and neurohormones, ion channels, and membrane transporter systems. Astrocytes integrate the signals from all other cells to operate their homeostatic function and foster neuronal activity. Channels for K+ (voltage-independent, voltage-gated and Ca2+-dependent K+ channels), Na+ (voltage-gated, specific type of Na+ channels regulated by extracellular Na+ concentration and epithelial Na+ channels), and Ca2+ (voltage-gated, Orai, and Ca2+ release channels), as well as for many other ions, have been registered (for a comprehensive review, refer to Verkhratsky and Nedergaard 2018). Also, astrocytes express receptors for almost all neuroactive agents (Kettenmann and Zorec 2013; Verkhratsky 2010), including adenosine receptors (Dare et al. 2007; Pilitsis and Kimelberg 1998), purinoreceptors (Franke et al. 2001; Fumagalli et al. 2003; Verkhratsky et al. 2009), GABA receptors (MacVicar et al. 1989; Nilsson et al. 1993), glycine receptors (Kirchhoff et al. 1996; Pastor et al. 1995), acetylcholine receptors (Graham et al. 2003; Sharma and Vijayaraghavan 2001), monoamines receptors (Hertz et al. 2010; Miyazaki et al. 2004; Shelton and McCarthy 2000), cannabinoid receptors (Navarrete and Araque 2008; Navarrete and Araque 2010), and both ionotropic and metabotropic glutamate receptors (Lalo et al. 2006; Sun et al. 2013; Verkhratsky and Butt 2013; Verkhratsky and Chvatal 2020). Lastly, numerous membrane transporter systems for different ions and neuroactive substances complete the complex astrocytic machinery required to exert their homeostatic function, such as the Na+-K+ ATPase (Hertz et al. 2015), Ca2+-ATPases (Verkhratsky and Nedergaard 2018), as well as plasmalemmal transporters for GABA (Ribak et al. 1996), glycine (Zafra et al. 1995), glutamate (Verkhratsky and Rose 2020), glutamine (Scalise et al. 2016), and monocarboxylates (Halestrap 2012). In this way, astrocytes control the CNS microenvironment by adjusting extracellular neurotransmitters, ions, and pH, regulating blood flow through the release of vasoactive molecules, and buffering reactive oxygen species (Parpura and Verkhratsky, 2012). It has been demonstrated the ability of a single astrocyte to be in contact with several neurones. In this way, they finely regulate synaptic transmission by tuning neurotransmitter levels in the synaptic cleft (Verkhratsky and Nedergaard 2018). Astrocytes are fundamental components of the BBB where their presence is essential for a protective function and the control of cerebral flow, thus regulating the communication between the CNS and the periphery (Verkhratsky and Parpura 2015). Astrocytes are also a part of the so-called gliocrine system, releasing around 200 molecules, mainly neurotrophic factors, and energy substrates, fundamental for the maintenance of CNS functions (Verkhratsky et al. 2016).
Given the above, all types of glial cells contribute to neuropathological developments. As astrocytes are a part of neural networks, interacting with neurones, with other glial cells, and with blood vessels, they are the key players in maintaining the structural and functional integrity of the brain tissue. The role of astrocytes in driving neuronal function and survival both in physiology and pathology has been widely documented (Verkhratsky and Nedergaard 2018). The hypothesis that astrocyte dysfunctions allow the creation of a disease-permissive context, which may favor neuronal deficits and death, has gained great attention in the recent years. Here, we provide a brief recap of the evidence accumulated so far on the active role of astrocytes in neuropsychiatric disorders, which are discussed in detail in the following chapters.
Astrogliopathology in Neuropsychiatric Disorders
Considering the above-mentioned multiple homeostatic and supportive functions that astrocytes perform, it becomes clear that any changes in the physiological performance of these cells are having a role in the etiology or progression of neuropsychiatric pathologies. Astrocyte impairments can be generic or disease-specific, and they often differ depending on the stage of the disease (Pekny et al. 2016). To complicate this scenario, human diseases are frequently modified both by age and by the presence of other comorbidities. Schematically, we can divide astrogliopathies into three main categories: (i) reactive astrogliosis; (ii) astroglial atrophy, characterized by degeneration and loss of function; and (iii) pathological remodeling of astrocytes (Verkhratsky et al. 2017a, 2019) (Fig. 3). It should be remembered that all three of these reactions are considered pathological, as well as that they can occur simultaneously or singly.
Fig. 3.
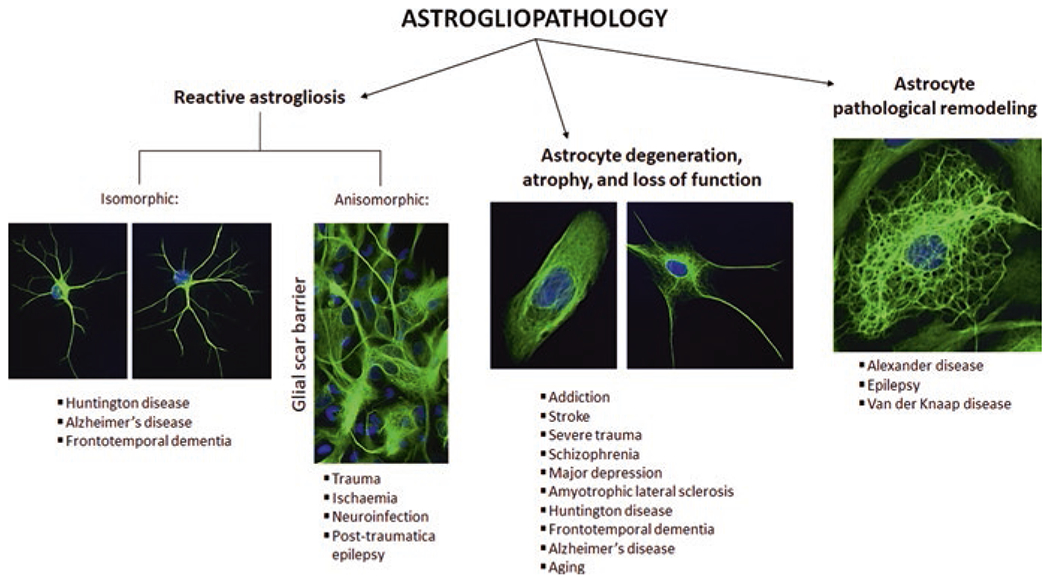
Astrocyte contribution to neuropsychiatric disorders
Reactive Astrogliosis in Neuropsychiatric Disorders
Reactive astrogliosis represents the most studied type of astrocytic response (Escartin et al. 2021), which has been considered for a long time the stereotypic and universal response to pathology. According to the severity, reactive astrogliosis can be classified as mild to moderate astrogliosis, diffuse severe astrogliosis, and severe astrogliosis with scar formation (Sofroniew 2009, 2014). According to cellular morphology, two types of reactive astrogliosis have been identified: the isomorphic one, which is reversible and characterized by the preservation of the territorial astroglial domains, and the anisomorphic astrogliosis, characterized by a lack of maintenance of the territorial domains, presence of cell migration, territorial overlap, and ultimately scar formation (Pekny et al. 2016). Histopathologically, reactive astrocytes display hypertrophic extensions due to the upregulation of vimentin and glial fibrillary acidic protein (GFAP), two cytoskeletal intermediate filaments/proteins (Hol and Pekny 2015; Sofroniew 2014). Broadly speaking, reactive astrocytes undergo numerous morphological and functional modifications, acquiring different phenotypes that are believed to be disease specific (Pekny et al. 2016; Escartin et al. 2021).
Reactive astrogliosis is an evolutionary-conserved defensive program aimed at isolating the damaged region, increasing neuroprotection, and starting the reparation of the damaged nervous tissue, as well as the BBB. Growing experimental evidence supports this notion, demonstrating that the suppression of reactive astrogliosis often increases the extent of the traumatic brain injury, exacerbates post-traumatic synaptic loss, and aggravates disease progression (Li et al. 2008; Okada et al. 2006; Pekny et al. 1999, 2014, 2016). Thus, astrocytic reactivity is broadly considered neuroprotective, albeit in some circumstances, especially if sustained for a too long time, it can become a maladaptive process, the consequences of which may override the initial benefits.
Reactive astrogliosis has been widely documented in numerous neurological diseases, including multiple sclerosis, Alzheimer’s disease, and autism spectrum disorders (Bronzuoli et al. 2018a, b; das Neves et al. 2020; Scuderi et al. 2018; Scuderi and Verkhratsky 2020; Tang et al. 2006; Zeidan-Chulia et al. 2014). Accumulating evidence indicates the presence of reactive astrocytes even in the course of some neuropsychiatric disorders. For instance, the astrocytic responses to chronic alcohol use may also lead to secondary activation of gliosis-like astrocyte responses (Miguel-Hidalgo 2009; Miguel-Hidalgo and Rajkowska 2003).
Astrocyte Degeneration, Atrophy, and Loss of Function in Neuropsychiatric Disorders
Astrodegeneration is characterized by morphological atrophy and functional asthenia of astrocytes. Thickness and extension of astrocyte branches appear reduced, while some of their homeostatic functions are compromised. This astrocytic response has been detected in several neurodegenerative and neuropsychiatric disorders (Heneka et al. 2010; Verkhratsky et al. 2014; Verkhratsky et al. 2017b). Schizophrenia, major depressive disorder, alcohol abuse disorder, and obsessive-compulsive disorders are all characterized by a reduction in the number or the packing density of astrocytes, accompanied by failure of their homeostatic function, especially in glutamate homeostasis (Aida et al. 2015; Czeh and Nagy 2018; Korbo 1999; Rajkowska et al. 2002; Rajkowska and Stockmeier 2013). Aberrant glutamate metabolism and transport, as well as the subsequent alteration in Ca2+ homeostasis, likely provoke an alteration in neurotransmission and excitotoxic neuronal death, both resulting in psychotic symptoms (Verkhratsky et al. 2014). Of note, Miguel-Hidalgo in his chapter published in this book offers evidence in the field of alcohol abuse disorder suggesting that the reduced number of astrocytes and the shrinkage of their processes may impair some of their critical functions. For instance, it has been shown that alcohol inhibits astrocyte proliferation, as well as DNA and protein synthesis, in cultured neonatal astrocytes (Davies and Cox 1991; Guerri and Renau-Piqueras 1997). Similar findings have also been achieved analyzing postmortem human brain tissue (Kane et al. 1996). In the chapter by Kruyer and Scofield, the emerging research highlighting the critical contribution of astrocytes to the encoding and expression of motivated behaviors relevant to drug addiction is extensively discussed. The chapter by Tanaka discusses the role of astrocytic control of the synaptic efficacy and its dysfunction in the pathophysiology of obsessive-compulsive and related disorders.
Astrocyte Pathological Remodeling in Cognitive Disorders
Astrocytes can undergo modifications in their intracellular cascade signaling or in their functional properties acquiring a pathological phenotype. This process is called pathological remodeling, and it has been implicated in the progression of several neurological diseases (Ferrer 2018; Pekny et al. 2016). Astrocytic pathological remodeling has been documented in diseases with severe damage to the developing white matter, mainly leukodystrophies. These are a group of hereditary diseases characterized by the accumulation of substances in the myelin that, therefore, gradually undergoes destruction. Alexander’s disease is a rare neurodegenerative disease of astrocytes that display sporadically mutated GFAP gene that causes early and severe leukomalacia (Messing et al. 2012). Pathological remodeling in astrocytes has also been observed in other pathologies, that is, mesial temporal lobe epilepsy and Van der Knaap disease (Bedner et al. 2015; Lanciotti et al. 2013; Verkhratsky et al. 2019).
Envoi
We concisely examined the neuroglia, the origin of these cells, their classification, and some of their functions. We have deepened the aspects connected to the homeostatic function of astrocytes, and then we tersely reviewed the modification that astrocytes undergo in neuropsychiatric diseases. In the follow-up chapters collected for this book, we explore the role of astrocytes in the progression of neuropathological diseases, particularly in neuropsychiatric disorders.
Acknowledgments
We are grateful to Giorgia Menegoni for her help in preparing the figures.
BL’s work is supported by the National Natural Science Foundation of China (grant number 8187185), LiaoNing Revitalization Talents Program (grant number XLYC1807137), the Scientific Research Foundation for Returned Scholars of Education Ministry of China (grant number 20151098), LiaoNing Thousand Talents Program (grant number 202078), and “ChunHui” Program of Education Ministry of China (grant number 2020703). CS’s work is supported by a grant from the Italian Ministry of Education, University and Research (2015KP7T2Y_002) and a grant from Sapienza University of Rome (RM11916B7A8D0225). VP’s work is supported by a grant from the National Institute of General Medical Sciences of the National Institutes of Health (R01GM123971). VP is an Honorary Professor at University of Rijeka, Croatia.
Contributor Information
Caterina Scuderi, Department of Physiology and Pharmacology “Vittorio Erspamer”, SAPIENZA University of Rome, Rome, Italy.
Alexei Verkhratsky, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK; Achucarro Center for Neuroscience, IKERBASQUE, Basque Foundation for Science, Bilbao, Spain.
Vladimir Parpura, Department of Neurobiology, The University of Alabama at Birmingham, Birmingham, AL, USA.
Baoman Li, Department of Forensic Analytical Toxicology, School of Forensic Medicine, China Medical University, Shenyang, China.
References
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37:13–25 [DOI] [PubMed] [Google Scholar]
- Aida T, Yoshida J, Nomura M, Tanimura A, Iino Y, Soma M, Bai N, Ito Y, Cui W, Aizawa H, Yanagisawa M, Nagai T, Takata N, Tanaka KF, Takayanagi R, Kano M, Götz M, Hirase H, Tanaka K (2015) Astroglial glutamate transporter deficiency increases synaptic excitability and leads to pathological repetitive behaviors in mice. Neuropsychopharmacology 2015(40):1569–7159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandra IM, Constanze D, Klaus-Armin N (2018) An emerging role of dysfunctional axonoligodendrocyte coupling in neurodegenerative diseases. Dialogues Clin Neurosci 20:283–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida RG, Lyons DA (2017) On myelinated axon plasticity and neuronal circuit formation and function. J Neurosci 37:10023–10034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA (2010) Glial and neuronal control of brain blood flow. Nature 468:232–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayata P, Badimon A, Strasburger HJ et al. (2018) Epigenetic regulation of brain region-specific microglia clearance activity. Nat Neurosci 21:1049–1060. 10.1038/s41593-018-0192-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim K, Angonin D, Marcy G, Pieropan F, Rivera A, Donega V, Cantu C, Williams G, Berninger B, Butt AM, Raineteau O (2017) Pharmacogenomic identification of small molecules for lineage specific manipulation of subventricular zone germinal activity. PLoS Biol 15:e2000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Connors BW, Paradiso MA (2007) Exploring the brain. Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- Bedner P, Dupper A, Huttmann K, Muller J, Herde MK, Dublin P, Deshpande T, Schramm J, Haussler U, Haas CA, Henneberger C, Theis M, Steinhauser C (2015) Astrocyte uncoupling as a cause of human temporal lobe epilepsy. Brain 138:1208–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, Jahr CE (2000) Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405:187–191 [DOI] [PubMed] [Google Scholar]
- Bergles DE, Richardson WD (2015) Oligodendrocyte development and plasticity. Cold Spring Harb Perspect Biol 8(2):a020453. 10.1101/cshperspect.a020453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronzuoli MR, Facchinetti R, Ingrassia D, Sarvadio M, Schiavi S, Steardo L, Verkhratsky A, Trezza V, Scuderi C (2018a) Neuroglia in the autistic brain: evidence from a preclinical model. Mol Autism 9:66. 10.1186/s13229-018-0254-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronzuoli MR, Facchinetti R, Steardo L Jr, Romano A, Stecca C, Passarella S, Steardo L, Cassano T, Scuderi C (2018b) Palmitoylethanolamide dampens reactive astrogliosis and improves neuronal trophic support in a triple transgenic model of Alzheimer’s disease: in vitro and in vivo evidence. Oxid Med Cell Longev 16:4720532. 10.1155/2018/4720532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AM, Ransom BR (1989) Visualization of oligodendrocytes and astrocytes in the intact rat optic nerve by intracellular injection of lucifer yellow and horseradish peroxidase. Glia 2:470–475 [DOI] [PubMed] [Google Scholar]
- Butt AM, Papanikolaou M, Rivera A (2019) Physiology of oligodendroglia. In: Verkhratsky A, Ho M, Zorec R, Parpura V (eds) Neuroglia in neurodegenerative diseases. Advances in experimental medicine and biology, vol 1175. Springer, Singapore. 10.1007/978-981-13-9913-8_5 [DOI] [Google Scholar]
- Cartier N, Lewis C-A, Zhang R, Rossi FMV (2014) The role of microglia in human disease: therapeutic tool or target? Acta Neuropathol 128:363–380. 10.1007/s00401-014-1330-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo JA (2018) Interlaminar glia and other glial themes revisited: pending answers following three decades of glial research. Neuroglia 1:7–20 [Google Scholar]
- Cunningham CL, Martínez-Cerdeño V, Noctor SC (2013) Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci 33:4216–4233. 10.1523/JNEUROSCI.3441-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Nagy SA (2018) Clinical findings documenting cellular and molecular abnormalities of glia in depressive disorders. Front Mol Neurosci 11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dare E, Schulte G, Karovic O, Hammarberg C, Fredholm BB (2007) Modulation of glial cell functions by adenosine receptors. Physiol Behav 92:15–20 [DOI] [PubMed] [Google Scholar]
- das Neves SP, Sousa JC, Sousa N, Cerqueira JJ, Marques F (2020) Altered astrocytic function in experimental neuroinflammation and multiple sclerosis. Glia 69(6):1341–1368. 10.1002/glia.23940 [DOI] [PubMed] [Google Scholar]
- Davies DL, Cox WE (1991) Delayed growth and maturation of astrocytic cultures following exposure to ethanol: electron microscopic observations. Brain Res 547:53–61 [DOI] [PubMed] [Google Scholar]
- Deitmer JW, Rose CR (1996) pH regulation and proton signalling by glial cells. Prog Neurobiol 48:73–103 [DOI] [PubMed] [Google Scholar]
- Elbaz B, Popko B (2019) Molecular control of oligodendrocyte development. Trends Neurosci 42:263–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C, Galea E, Lakatos A, O’Callaghan JP, Petzold GC, Serrano-Pozo A, Steinhauser C, Volterra A, Carmignoto G, Agarwal A, Allen NJ, Araque A, Luis Barbeito L, Barzilai A, Bergles DE, Bonvento G, Butt AM, Chen W-T, Cohen-Salmon M, Cunningham C, Deneen B, De Strooper B, Díaz-Castro B, Farina C, Freeman M, Gallo V, Goldman JE, Goldman SA, Götz M, Gutiérrez A, Haydon PG, Heiland DH, Hol EM, Holt MG, Iino M, Kastanenka KV, Kettenmann H, Khakh BS, Koizumi S, Lee CJ, Liddelow SA, MacVicar BA, Magistretti P, Messing A, Mishra A, Molofsky AV, Murai K, Norris CM, Okada S, SHR O, Oliveira JF, Panatier A, Parpura V, Pekna M, Pekny M, Pellerin L, Perea G, Pérez-Nievas BG, Pfrieger FW, Poskanzer KE, Quintana FJ, Ransohoff RM, Riquelme-Perez M, Robel S, Rose CR, Rothstein J, Rouach N, Rowitch DH, Semyanov A, Sirko S, Sontheimer H, Swanson RA, Vitorica J, Wanner I-B, Wood LB, Wu J, Zheng B, Zimmer ER, Zorec R, Sofroniew MV, Verkhratsky A (2021) Working consensus on reactive astrocyte nomenclature, definitions and future directions. Nat Neurosci 24(3):312–325. 10.1038/s41593-020-00783-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I (2018) Astrogliopathy in tauopathies. Neuroglia 1:126–150 [Google Scholar]
- Franke H, Grosche J, Schadlich H, Krugel U, Allgaier C, Illes P (2001) P2X receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience 108:421–429 [DOI] [PubMed] [Google Scholar]
- Fumagalli M, Brambilla R, D’Ambrosi N, Volonte C, Matteoli M, Verderio C, Abbracchio MP (2003) Nucleotide-mediated calcium signaling in rat cortical astrocytes: role of P2X and P2Y receptors. Glia 43:218–230 [DOI] [PubMed] [Google Scholar]
- Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA (2012) Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485:517–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N (2010) Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci 11:87–99 [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M et al. (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330:841–845. 10.1126/science.1194637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AJ, Ray MA, Perry EK, Jaros E, Perry RH, Volsen SG, Bose S, Evans N, Lindstrom J, Court JA (2003) Differential nicotinic acetylcholine receptor subunit expression in the human hippocampus. J Chem Neuroanat 25:97–113 [DOI] [PubMed] [Google Scholar]
- Griemsmann S, Hoft SP, Bedner P, Zhang J, von Staden E, Beinhauer A, Degen J, Dublin P, Cope DW, Richter N, Crunelli V, Jabs R, Willecke K, Theis M, Seifert G, Kettenmann H, Steinhauser C (2015) Characterization of panglial gap junction networks in the thalamus, neocortex, and hippocampus reveals a unique population of glial cells. Cereb Cortex 25:3420–3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubisic V, Verkhratsky A, Zorec R, Parpura V (2018) Enteric glia regulate gut motility in health and disease. Brain Res Bull 136:109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerri C, Renau-Piqueras J (1997) Alcohol, astroglia, and brain development. Mol Neurobiol 15:65–81 [DOI] [PubMed] [Google Scholar]
- Habermacher C, Angulo MC, Benamer N (2019) Glutamate versus GABA in neuron-oligodendroglia communication. Glia 67(11):2092–2106. 10.1002/glia.23618 [DOI] [PubMed] [Google Scholar]
- Halestrap AP (2012) The monocarboxylate transporter family—structure and functional characterization. IUBMB Life 64:1–9 [DOI] [PubMed] [Google Scholar]
- Hanani M, Verkhratsky A (2021) Satellite glial cells and astrocytes, a comparative review. Neurochem Res:1–13. 10.1007/s11064-021-03255-8 [DOI] [PubMed] [Google Scholar]
- Heneka MT, Rodriguez JJ, Verkhratsky A (2010) Neuroglia in neurodegeneration. Brain Res Rev 63:189–211 [DOI] [PubMed] [Google Scholar]
- Hertz L, Dringen R, Schousboe A, Robinson SR (1999) Astrocytes: glutamate producers for neurons. J Neurosci Res 57:417–428 [PubMed] [Google Scholar]
- Hertz L, Lovatt D, Goldman SA, Nedergaard M (2010) Adrenoceptors in brain: cellular gene expression and effects on astrocytic metabolism and [Ca2+]i. Neurochem Int 57:411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Song D, Xu J, Peng L, Gibbs ME (2015) Role of the astrocytic Na+, K+-ATPase in K+ homeostasis in brain: K+ uptake, signaling pathways and substrate utilization. Neurochem Res 40:2505–2516 [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Barbas H (2009) Are there ten times more glia than neurons in the brain? Brain Struct Funct 213:365–366 [DOI] [PubMed] [Google Scholar]
- Hol EM, Pekny M (2015) Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol 32:121–130 [DOI] [PubMed] [Google Scholar]
- Houades V, Koulakoff A, Ezan P, Seif I, Giaume C (2008) Gap junction-mediated astrocytic networks in the mouse barrel cortex. J Neurosci 28:5207–5217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM (2000) Principles of neural science. McGrawhill, New York [Google Scholar]
- Kane CJ, Berry A, Boop FA, Davies DL (1996) Proliferation of astroglia from the adult human cerebrum is inhibited by ethanol in vitro. Brain Res 731:39–44 [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A (2011) Physiology of microglia. Physiol Rev 91:461–553 [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Kirchhoff F, Verkhratsky A (2013) Microglia: new roles for the synaptic stripper. Neuron 77:10–18 [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Zorec R (2013) Release of gliotransmitters and transmitter receptors in astrocytes. In: Kettenmann H, Ransom BR (eds) Neuroglia. Oxford University Press, New York, pp 197–211 [Google Scholar]
- Kidd GJ, Ohno N, Trapp BD (2013) Biology of Schwann cells. Handb Clin Neurol 115:55–79 [DOI] [PubMed] [Google Scholar]
- Kiray H, Lindsay SL, Hosseinzadeh S, Barnett SC (2016) The multifaceted role of astrocytes in regulating myelination. Exp Neurol 283:541–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff F, Mulhardt C, Pastor A, Becker CM, Kettenmann H (1996) Expression of glycine receptor subunits in glial cells of the rat spinal cord. J Neurochem 66:1383–1390 [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Kettenmann H, Verkhratsky A (2007) Membrane currents and cytoplasmic sodium transients generated by glutamate transport in Bergmann glial cells. Pflugers Arch 454:245–252 [DOI] [PubMed] [Google Scholar]
- Kofuji P, Newman EA (2004) Potassium buffering in the central nervous system. Neuroscience 129:1045–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbo L (1999) Glial cell loss in the hippocampus of alcoholics. Alcohol Clin Exp Res 23:164–168 [PubMed] [Google Scholar]
- Kuhn S, Gritti L, Crooks D, Dombrowski Y (2019) Oligodendrocytes in development, myelin generation and beyond. Cells 8(11):1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Kirchhoff F, North RA, Verkhratsky A (2006) NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J Neurosci 26:2673–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti A, Brignone MS, Bertini E, Petrucci TC, Aloisi F, Ambrosini E (2013) Astrocytes: emerging stars in leukodystrophy pathogenesis. Transl Neurosci 4(2):144–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson VA, Zhang Y, Bergles DE (2016) Electrophysiological properties of NG2 + cells: matching physiological studies with gene expression profiles. Brain Res 1638:138–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD (2012) Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487:443–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, Nodin C, Stahlberg A, Aprico K, Larsson K, Yabe T, Moons L, Fotheringham A, Davies I, Carmeliet P, Schwartz JP, Pekna M, Kubista M, Blomstrand F, Maragakis N, Nilsson M, Pekny M (2008) Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab 28:468–481 [DOI] [PubMed] [Google Scholar]
- MacVicar BA, Tse FW, Crichton SA, Kettenmann H (1989) GABA-activated Cl− channels in astrocytes of hippocampal slices. J Neurosci 9:3577–3583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, Richardson WD (2014) Motor skill learning requires active central myelination. Science 346:318–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing A, Brenner M, Feany MB, Nedergaard M, Goldman JE (2012) Alexander disease. J Neurosci 32:5017–5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer N, Richter N, Fan Z, Siemonsmeier G, Pivneva T, Jordan P, Steinhauser C, Semtner M, Nolte C, Kettenmann H (2018) Oligodendrocytes in the mouse corpus callosum maintain axonal function by delivery of glucose. Cell Rep 22:2383–2394 [DOI] [PubMed] [Google Scholar]
- Micu I, Plemel JR, Caprariello AV, Nave KA, Stys PK (2018) Axo-myelinic neurotransmission: a novel mode of cell signalling in the central nervous system. Nat Rev Neurosci 19:49–58 [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ (2009) The role of glial cells in drug abuse. Curr Drug Abuse Rev 2:76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Rajkowska G (2003) Comparison of prefrontal cell pathology between depression and alcohol dependence. J Psychiatr Res 37:411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki I, Asanuma M, Diaz-Corrales FJ, Miyoshi K, Ogawa N (2004) Direct evidence for expression of dopamine receptors in astrocytes from basal ganglia. Brain Res 1029:120–123 [DOI] [PubMed] [Google Scholar]
- Monier A, Adle-Biassette H, Delezoide A-L et al. (2007) Entry and distribution of microglial cells in human embryonic and fetal cerebral cortex. J Neuropathol Exp Neurol 66:372–382. 10.1097/nen.0b013e3180517b46 [DOI] [PubMed] [Google Scholar]
- Muller C, Bauer NM, Schafer I, White R (2013) Making myelin basic protein -from mRNA transport to localized translation. Front Cell Neurosci 7:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M, Araque A (2008) Endocannabinoids mediate neuron-astrocyte communication. Neuron 57:883–893 [DOI] [PubMed] [Google Scholar]
- Navarrete M, Araque A (2010) Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 68:113–126 [DOI] [PubMed] [Google Scholar]
- Nayak D, Roth TL, McGavern DB (2014) Microglia development and function. Annu Rev Immunol 32:367–402. 10.1146/annurev-immunol-032713-120240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Eriksson PS, Ronnback L, Hansson E (1993) GABA induces Ca2+ transients in astrocytes. Neuroscience 54:605–614 [DOI] [PubMed] [Google Scholar]
- Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H (2006) Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med 12:829–834 [DOI] [PubMed] [Google Scholar]
- Ortiz FC, Habermacher C, Graciarena M, Houry PY, Nishiyama A, Oumesmar BN, Angulo MC (2019) Neuronal activity in vivo enhances functional myelin repair. JCI Insight 5(9):e123434. 10.1172/jci.insight.123434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Verkhratsky A (2012) Neuroglia at the crossroads of homoeostasis, metabolism and signalling: evolution of the concept. ASN Neuro 4:201–205. 10.1042/AN20120019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor A, Chvatal A, Sykova E, Kettenmann H (1995) Glycine- and GABA-activated currents in identified glial cells of the developing rat spinal cord slice. Eur J Neurosci 7:1188–1198 [DOI] [PubMed] [Google Scholar]
- Pastor A, Kremer M, Moller T, Kettenmann H, Dermietzel R (1998) Dye coupling between spinal cord oligodendrocytes: differences in coupling efficiency between gray and white matter. Glia 24:108–120 [PubMed] [Google Scholar]
- Patel JR, Klein RS (2011) Mediators of oligodendrocyte differentiation during remyelination. FEBS Lett 585:3730–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Johansson CB, Eliasson C, Stakeberg J, Wallen A, Perlmann T, Lendahl U, Betsholtz C, Berthold CH, Frisen J (1999) Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J Cell Biol 145:503–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Wilhelmsson U, Pekna M (2014) The dual role of astrocyte activation and reactive gliosis. Neurosci Lett 565:30–38 [DOI] [PubMed] [Google Scholar]
- Pekny M, Pekna M, Messing A, Steinhauser C, Lee JM, Parpura V, Hol EM, Sofroniew MV, Verkhratsky A (2016) Astrocytes: a central element in neurological diseases. Acta Neuropathol 131:323–345 [DOI] [PubMed] [Google Scholar]
- Pfrieger FW (2010) Role of glial cells in the formation and maintenance of synapses. Brain Res Rev 63:39–46 [DOI] [PubMed] [Google Scholar]
- Pilitsis JG, Kimelberg HK (1998) Adenosine receptor mediated stimulation of intracellular calcium in acutely isolated astrocytes. Brain Res 798:294–303 [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J, Sisodia SS, Ransohoff RM (2011) Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci 14:1227–1235. 10.1038/nn.2923 [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J (2014) Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci 15:300–312. 10.1038/nrn3722 [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Makkos Z, Meltzer H, Overholser J, Stockmeier C (2002) Layer-specific reductions in GFAP-reactive astroglia in the dorsolateral prefrontal cortex in schizophrenia. Schizophr Res 57:127–138 [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Stockmeier CA (2013) Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets 14:1225–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribak CE, Tong WM, Brecha NC (1996) GABA plasma membrane transporters, GAT-1 and GAT-3, display different distributions in the rat hippocampus. J Comp Neurol 367:595–606 [DOI] [PubMed] [Google Scholar]
- Roux L, Benchenane K, Rothstein JD, Bonvento G, Giaume C (2011) Plasticity of astroglial networks in olfactory glomeruli. Proc Natl Acad Sci U S A 108:18442–18446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruitenberg MJ, Vukovic J, Sarich J, Busfield SJ, Plant GW (2006) Olfactory ensheathing cells: characteristics, genetic engineering, and therapeutic potential. J Neurotrauma 23:468–478 [DOI] [PubMed] [Google Scholar]
- Saab AS, Tzvetavona ID, Trevisiol A, Baltan S, Dibaj P, Kusch K, Mobius W, Goetze B, Jahn HM, Huang W, Steffens H, Schomburg ED, Perez-Samartin A, Perez-Cerda F, Bakhtiari D, Matute C, Lowel S, Griesinger C, Hirrlinger J, Kirchhoff F, Nave KA (2016) Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron 91:119–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saher G, Stumpf SK (2015) Cholesterol in myelin biogenesis and hypomyelinating disorders. Biochim Biophys Acta 1851:1083–1094 [DOI] [PubMed] [Google Scholar]
- Scalise M, Pochini L, Galluccio M, Indiveri C (2016) Glutamine transport. From energy supply to sensing and beyond. Biochim Biophys Acta 1857(8):1147–1157 [DOI] [PubMed] [Google Scholar]
- Schlegelmilch T, Henke K, Peri F (2011) Microglia in the developing brain: from immunity to behaviour. Curr Opin Neurobiol 21:5–10. 10.1016/j.conb.2010.08.004 [DOI] [PubMed] [Google Scholar]
- Scuderi C, Bronzuoli MR, Facchinetti R, Pace L, Ferraro L, Broad KD, Serviddio G, Bellanti F, Palombelli G, Carpinelli G, Canese R, Gaetani S, Steardo L Jr, Steardo L, Cassano T (2018) Ultramicronized palmitoylethanolamide rescues learning and memory impairments in a triple transgenic mouse model of Alzheimer’s disease by exerting anti-inflammatory and neuroprotective effects. Transl Psychiatry 8(1):32. 10.1038/s41398-017-0076-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuderi C, Verkhratsky A (2020) The role of neuroglia in autism spectrum disorders. Prog Mol Biol Transl Sci 173:301–330. 10.1016/bs.pmbts.2020.04.011 [DOI] [PubMed] [Google Scholar]
- Sellner S, Paricio-Montesinos R, Spieß A et al. (2016) Microglial CX3CR128 promotes adult neurogenesis by inhibiting Sirt 1/p65 signaling independent of CX3CL1. Acta Neuropathol Commun 4:102. 10.1186/s40478-016-0374-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Vijayaraghavan S (2001) Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proc Natl Acad Sci U S A 98:4148–4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton MK, McCarthy KD (2000) Hippocampal astrocytes exhibit Ca2+-elevating muscarinic cholinergic and histaminergic receptors in situ. J Neurochem 74:555–563 [DOI] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y, Hoshikawa K, Goldman JE et al. (2014) Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J Neurosci 34:2231–2243. 10.1523/JNEUROSCI.1619-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJP et al. (2010) Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7:483–495. 10.1016/j.stem.2010.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32:638–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV (2014) Astrogliosis. Cold Spring Harb Perspect Biol 7:a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W, Lovatt D, Han X, Smith Y, Nedergaard M (2013) Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science 339:197–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen N, Smolders S, Avila A et al. (2013) Complex invasion pattern of the cerebral cortex by microglial cells during development of the mouse embryo. Glia 61:150–163. 10.1002/glia.22421 [DOI] [PubMed] [Google Scholar]
- Tang G, Xu Z, Goldman JE (2006) Synergistic effects of the SAPK/JNK and the proteasome pathway on glial fibrillary acidic protein (GFAP) accumulation in Alexander disease. J Biol Chem 281:38634–38643 [DOI] [PubMed] [Google Scholar]
- Tay TL, Savage JC, Hui CW et al. (2017) Microglia across the lifespan: from origin to function in brain development, plasticity and cognition. J Physiol (Lond) 595:1929–1945. 10.1113/JP272134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay TL, Béchade C, D’Andrea I et al. (2018) Microglia gone rogue: impacts on psychiatric disorders across the lifespan. Front Mol Neurosci 10:421. 10.3389/fnmol.2017.00421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay TL, Carrier M, Tremblay MÈ (2019) Physiology of microglia. In: Verkhratsky A, Ho M, Zorec R, Parpura V (eds) Neuroglia in neurodegenerative diseases. Advances in experimental medicine and biology, vol 1175. Springer, Singapore. 10.1007/978-981-13-9913-8_6 [DOI] [PubMed] [Google Scholar]
- Ueno M, Fujita Y, Tanaka T et al. (2013) Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci 16:543–551. 10.1038/nn.3358 [DOI] [PubMed] [Google Scholar]
- Vejar S, Oyarzun JE, Retamal MA, Ortiz FC, Orellana JA (2019) Connexin and pannexin-based channels in oligodendrocytes: implications in brain health and disease. Front Cell Neurosci 13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A (2010) Physiology of neuronal-glial networking. Neurochem Int 57:332–343 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Butt AM (2013) Glial physiology and pathophysiology. Wiley-Blackwell, Chichester, p 560 [Google Scholar]
- Verkhratsky A, Chvatal A (2020) NMDA receptors in astrocytes. Neurochem Res 45:122–133 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Krishtal OA, Burnstock G (2009) Purinoceptors on neuroglia. Mol Neurobiol 39:190–208 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Rodriguez JJ, Steardo L (2014) Astrogliopathology: a central element of neuropsychiatric diseases? Neuroscientist 20:576–588 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Parpura V (2015) Physiology of astroglia: channels, receptors, transporters, ion signaling and gliotransmission. Morgan & Claypool Publishers, p 172 [Google Scholar]
- Verkhratsky A, Matteoli M, Parpura V, Mothet JP, Zorec R (2016) Astrocytes as secretory cells of the central nervous system: idiosyncrasies of vesicular secretion. EMBO J 35(3):239–257. 10.15252/embj.201592705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Zorec R, Parpura V (2017a) Stratification of astrocytes in healthy and diseased brain. Brain Pathol 27:629–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Zorec R, Rodriguez JJ, Parpura V (2017b) Neuroglia: functional paralysis and reactivity in Alzheimer’s disease and other neurodegenerative pathologies. Adv Neurobiol 15:427–449 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Nedergaard M (2018) Physiology of astroglia. Physiol Rev 98:239–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Rose CR (2020) Na+-dependent transporters: the backbone of astroglial homeostatic function. Cell Calcium 85:102136. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Oberheim Bush NA, Nedergaard M, Butt AM (2018) The special case of human astrocytes. Neuroglia 1:21–29 [Google Scholar]
- Verkhratsky A, Ho MS, Zorec R, Parpura V (2019) The concept of neuroglia. In: Verkhratsky A, Ho M, Zorec R, Parpura V (eds) Neuroglia in neurodegenerative diseases. Advances in experimental medicine and biology, vol 1175. Springer, Singapore: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verney C, Monier A, Fallet-Bianco C, Gressens P (2010) Early microglial colonization of the human forebrain and possible involvement in periventricular white-matter injury of preterm infants. J Anat 217:436–448. 10.1111/j.1469-7580.2010.01245.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virchow R (1860) Cellular pathology. Robert M de Witt, New York [Google Scholar]
- von Bartheld CS, Bahney J, Herculano-Houzel S (2016) The search for true numbers of neurons and glial cells in the human brain: a review of 150 years of cell counting. J Comp Neurol 524:3865–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskuhl RR, Itoh N, Tassoni A, Matsukawa MA, Ren E, Tse V, Jang E, Suen TT, Itoh Y (2019) Gene expression in oligodendrocytes during remyelination reveals cholesterol homeostasis as a therapeutic target in multiple sclerosis. Proc Natl Acad Sci U S A 116:10130–10139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhard L, d’Errico P, Tay TL (2018) Headmasters: microglial regulation of learning and memory in health and disease. Molecular 5:63–89. 10.3934/molsci.2018.1.63 [DOI] [Google Scholar]
- Xiao L, Ohayon D, McKenzie IA, Sinclair-Wilson A, Wright JL, Fudge AD, Emery B, Li H, Richardson WD (2016) Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat Neurosci 19:1210–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra F, Aragon C, Olivares L, Danbolt NC, Gimenez C, Storm-Mathisen J (1995) Glycine transporters are differentially expressed among CNS cells. J Neurosci 15:3952–3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan-Chulia F, Salmina AB, Malinovskaya NA, Noda M, Verkhratsky A, Moreira JC (2014) The glial perspective of autism spectrum disorders. Neurosci Biobehav Rev 38:160–172 [DOI] [PubMed] [Google Scholar]


