Abstract
Use of tobacco products during pregnancy is associated with increased risk for neurodevelopmental disorders in the offspring. Preclinical models of developmental nicotine exposure have offered valuable insights into the neurobiology of nicotine’s effects on the developing brain and demonstrated lasting effects of developmental nicotine exposure on brain structure, neurotransmitter signaling and behavior. These models have facilitated discovery of novel compounds as candidate treatments for attention deficit hyperactivity disorder, a neurodevelopmental disorder associated with prenatal nicotine exposure. Using these models the significance of heritability of behavioral phenotypes from the nicotine-exposed pregnant female or adult male to multiple generations of descendants has been demonstrated. Finally, research using the preclinical models has demonstrated synergistic interactions between developmental nicotine exposure and repetitive mild traumatic brain injury that contribute to “worse” outcomes from the injury in individuals with attention deficit hyperactivity disorder associated with developmental nicotine exposure.
Keywords: Nicotine, Mouse, Attention, Memory, Kappa opioid receptor, Traumatic brain injury, Transgenerational transmission
1. Introduction
Nicotine use continues to be a significant public health concern throughout the world, despite compelling evidence for its harmful health effects. In the late 20th century public health and legislative efforts led to a significant downturn in cigarette smoking in the United States (Centers for Disease, 1999, 2021). However, the 21st century saw the introduction of electronic nicotine delivery systems such as e-cigarettes, and an accompanying resurgence in nicotine use (Jaber et al., 2018; U.S. Surgeon General’s Report, 2016). As a result, nicotine use is once again becoming a public health concern.
Electronic cigarettes (e-cigarettes) are marketed as “safer” alternatives for traditional, combustible cigarettes. However, e-cigarettes contain nicotine, in some cases at levels higher than those in combustible cigarettes. In fact, all forms of tobacco products whether combustible cigarettes, e-cigarettes or chewing tobacco are marketed and used precisely because of their nicotine content. Nicotine is the neuro-active, addictive constituent in all of these products (Dwyer et al., 2008; Dwyer et al., 2009; Harvey et al., 2004; Rose, 2006; Slotkin, 2004). Therefore, the assumptions of e-cigarette “safety” may be misplaced (D’Angelo et al., 2019; Kandra et al., 2014; Ma et al., 2021; Saw et al., 2013; Tong et al., 2013; Whittington et al., 2018).
Independent of the harmful effects of nicotine, the delivery systems also can contribute to health effects of tobacco products, including neurobehavioral effects, because the chemical additives contained in the delivery vehicle can be harmful. For example, combustible cigarettes are notorious for the thousands of chemicals such as aldehydes, arsenic, creosols, cyanide and polycyclic hydrocarbons that they contain either as additives or as natural constituents of the tobacco leaf itself (Centers for Disease Control and Prevention et al., 2010). E-cigarettes contain a delivery vehicle called e-liquid or e-juice consisting of a cocktail of harmful chemicals including propylene glycol (Muthumalage et al., 2017; Rubinstein et al., 2018; Sassano et al., 2018). Thus, all tobacco products contain harmful chemicals in addition to nicotine, and as the technology for nicotine delivery has evolved over the centuries from tobacco leaves to electronic cigarettes, one characteristic has remained invariant: A cocktail of chemicals that can harm human health.
More men use tobacco products than women (Gowing et al., 2015; Makadia et al., 2017; U.S. Surgeon General’s Report, 2014). However, women show unique vulnerabilities to nicotine’s harmful effects. Women are more susceptible to nicotine dependence, withdrawal symptoms and smoking-related health effects such as cancer and coronary artery disease compared to men (Allen et al., 2014; Cross et al., 2017; O’Dell and Torres, 2014; Perkins, 2001). Nicotine use by women warrants additional considerations because nicotine use by pregnant and nursing women not only places their own health at risk but also the health of their children.
It is well known that use of tobacco products during pregnancy increases the risk for pulmonary, metabolic, immunological, and neurodevelopmental disorders in the children (McEvoy and Spindel, 2017; Zacharasiewicz, 2016). Clinical and preclinical studies offer compelling evidence that developmental nicotine exposure increases the risk for attention deficit hyperactivity disorder, conduct disorder, aggression, developmental delays, working memory deficits, as well as novelty-seeking, risk-taking and drug addiction in the offspring (Biederman et al., 2012; Faraone et al., 2018; Jacobsen et al., 2009; Martin et al., 2020; Pagani, 2014; Polli et al., 2020b; Wickstrom, 2007; Zhang et al., 2021c; Zhang et al., 2018; Zhu et al., 2017; Zhu et al., 2014a; Zhu et al., 2012).
There is yet another, perhaps more serious and less well appreciated consequence of nicotine use by pregnant women. It is the risk of transmission of the adverse effects from the pregnant mother who uses nicotine or the children who are exposed to nicotine in utero, to their descendants in multiple generations (Buck et al., 2019a; Buck et al., 2019b; Golding et al., 2017; Golding et al., 2020; Miller et al., 2014a; Miller et al., 2014b; Williams et al., 2019; Zhu et al., 2014a). Such “transgenerational transmission” likely increases the population at risk for neurodevelopmental disorders 2–3-fold above current estimates and produces public health and socioeconomic impacts that may last for at least 3 generations or 90 years.
Nicotine use by men also can influence the mental health of their offspring (McCarthy and Bhide, 2021), suggesting that nicotine use by either parent (i.e., father or mother) can produce adverse effects on offspring health. Nicotine use by the father around the time of conception of the child increases the child’s risk for cancer, disorders of the immune and metabolic systems as well as cognitive deficits (Biederman et al., 2020; Marczylo et al., 2012; Northstone et al., 2014; Soubry, 2018; Zhu et al., 2014b).
Turning to the consequences of nicotine use by pregnant women for their offspring, preclinical models of prenatal and early postnatal nicotine exposure have offered significant mechanistic and phenomenological insights into the neurobiology underlying nicotine’s effects on the developing brain as well as the link between developmental nicotine exposure and neurodevelopmental disorders. Preclinical models are valuable tools to extend and verify clinical data because cause-effect associations can be analyzed more rigorously in pre-clinical models than in human subjects. Preclinical models also permit a detailed analysis of the effects of dose, route and duration of nicotine exposure, as well as delineation of independent effects of each substance in a multiple exposure paradigm.
We review here nearly two decades of work from our laboratory using mouse models of developmental nicotine exposure, and the relevance of the findings to ADHD and related neurodevelopmental disorders. Specifically, we discuss the validity of the mouse models as preclinical models of ADHD and illustrate the use of the mouse models to facilitate drug discovery, investigation of heritability of behaviors arising from nicotine exposure and examination of potential synergistic interactions between ADHD and concussions. We also discuss studies showing that paternal nicotine exposure can produce significant adverse effects on offspring behaviors.
2. Mouse models of developmental nicotine exposure
Preclinical research on developmental nicotine exposure has relied principally on rodent models. Pioneering work by Slotkin, Levin, Pauly and colleagues established rodent models of developmental nicotine exposure as reliable and valuable preclinical models of human developmental nicotine exposure (England et al., 2017; Levin et al., 1993; Matta et al., 2007; Navarro et al., 1989; Navarro et al., 1988; Pauly and Slotkin, 2008; Pauly et al., 2004; Rosenthal and Slotkin, 1977; Roy et al., 2002; Slotkin, 1998, 2002; Slotkin et al., 1987a; Slotkin et al., 1987c; Trauth et al., 2000). These foundational studies showed that developmental nicotine exposure produces lasting changes in brain neuro-chemistry, receptor signaling mechanisms and behavior. The timing, route and dose of nicotine administration established by these investigators has served as a model for subsequent studies, including our own.
2.1. Developmental nicotine exposure paradigm
We used mouse models of prenatal and early postnatal nicotine exposures (Fig. 1). Female C57BL/6 or Swiss Webster mice were exposed to nicotine (100–200 μg/ml) in drinking water beginning 3-weeks prior to mating so that the mice could attain some tolerance to drinking nicotine-containing water by the time they were bred. The daily nicotine exposure continued throughout pregnancy and ceased at the time of parturition [prenatal-only exposure (Zhu et al., 2017; Zhu et al., 2012)] or continued until the offspring were weaned [prenatal and postnatal exposures; (Martin et al., 2020; Zhang et al., 2021c; Zhang et al., 2018)]. On average, the mice consumed 600–1200 μg (i.e., 20–40 mg/kg bodyweight) nicotine per day. The prenatal-only exposure represents human exposures during the first and second trimesters of pregnancy, whereas the pre- and postnatal exposures represent exposure throughout the 3 trimesters of the human pregnancy (Clancy, 2007; Clancy et al., 2001; Clancy et al., 2007; Semple et al., 2013).
Fig. 1.
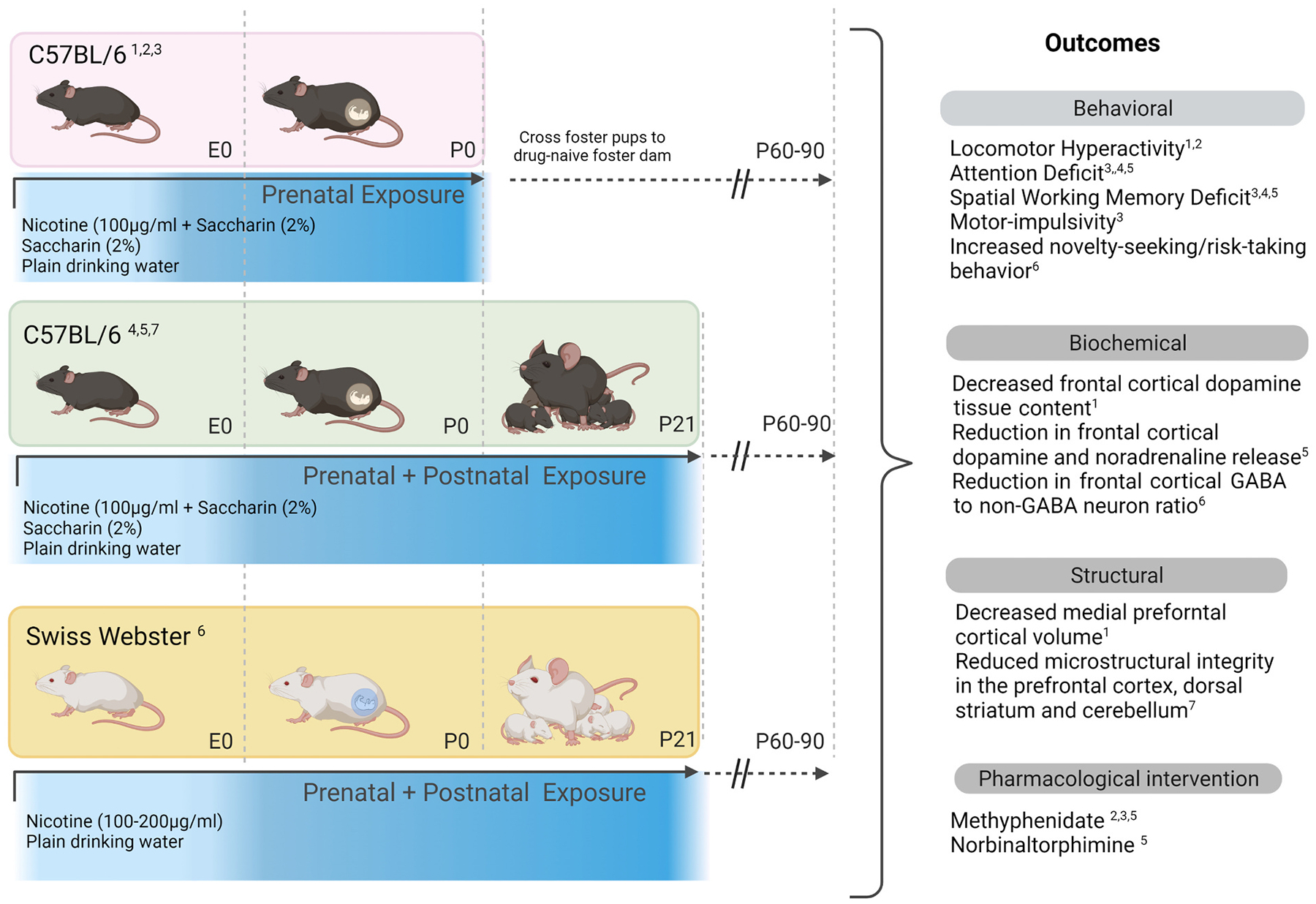
Mouse models of developmental nicotine exposure showing the experimental design and a summary of the outcomes. Three mouse models were used: A) Prenatal exposure in C57BL/6 strain, B) Prenatal + postnatal exposure in C57BL/6 strain, and C) Prenatal + postnatal exposure in Swiss Webster strain. Nicotine was supplied in drinking water (100–200 ng/ml) sweetened with 2% saccharin. Control groups included mice exposed to 2% saccharin in drinking water and plain drinking water. The prenatal period corresponded approximately to the first two trimesters of human pregnancy whereas the prenatal + postnatal periods to all three trimesters. The offspring from in the prenatal exposure paradigm (from all three exposure groups) were cross-fostered to drug naïve nursing dams within 1–2 days of birth. The offspring in the prenatal + postnatal exposure paradigm were raised by biological mothers. In all three paradigms, the offspring were subjected to behavioral, biochemical, structural and pharmacological assays around 60–90 days age. Citations: 1 Zhu et al., 2012; 2 Zhu et al., 2014a, 2014b; 3 Zhu et al., 2017; 4 Zhang et al., 2018; 5 Zhang et al., 2021c; 6 Martin et al., 2020; 7 Present data.
For consistency with previous reports, in some studies we included the artificial sweetener saccharin as a vehicle to mask nicotine’s bitter taste in the drinking water (Zhang et al., 2021c; Zhu et al., 2014a; Zhu et al., 2012). We included two groups of controls in these studies: Mice that were exposed to saccharin alone and mice that were exposed to plain drinking water without additives. These controls facilitated analysis of the independent effects of saccharin. We found that prenatal and early postnatal exposures to saccharin did not produce significant effects on behavioral, neuroanatomical, or neurochemical features. In other studies (Martin et al., 2020) we excluded the use of saccharin as a sweetener upon discovering that the consumption of nicotine-containing drinking water by mice was not significantly affected by the addition of saccharin (Martin et al., 2020; McCarthy et al., 2018). We point out that in other studies, in which male mice were exposed to saccharin, behavioral changes were observed in the offspring derived from (i.e., sired by) the saccharin-exposed male mice (McCarthy et al., 2020). The behavioral changes produced by the paternal saccharin exposure are described under the heading “Transgenerational Transmission”.
Our nicotine exposure paradigm produced plasma cotinine levels (cotinine is a relatively stable metabolite of nicotine and its plasma content is used as a reliable indicator of nicotine exposure) of approximately 130 ng/ml (Martin et al., 2020), which is comparable to the plasma cotinine levels reported in other mouse models of developmental nicotine exposure (Pauly et al., 2004) and in cigarette smokers (Matta et al., 2007; Nagano et al., 2010).
We used the oral nicotine delivery method because it does not involve exposing the mice to the stress associated with daily systemic nicotine administrations (subcutaneous, intraperitoneal, or intravenous) or the stress of subcutaneous implantation of osmotic pumps for nicotine delivery. We recognize that nicotine delivery via the drinking water may not produce the “peaks and valleys” in maternal plasma nicotine content associated with inhalation of cigarette smoke (Benowitz et al., 1982), and in that sense it does not mimic cigarette smoking during pregnancy. However, peaks and valleys in fetal nicotine concentrations following maternal cigarette smoking are unlikely because of the differences in nicotine pharmacokinetics between adults and fetuses (Wickstrom, 2007). Another significant difference between cigarette smoking and nicotine delivery via drinking water is that nearly 70% of the orally delivered nicotine is catabolized in the liver. Thus, the route and dose of nicotine are among the most significant variables affecting the pharmacokinetics of nicotine and the outcome measures in preclinical models [Reviews in (Centner et al., 2020; Matta et al., 2007; Polli and Kohlmeier, 2020)].
Our prenatal-only nicotine exposure paradigm required us to cross-foster offspring from all prenatal exposure conditions (nicotine + saccharin, saccharin-only and plain drinking water) within 1–2 days of birth, to drug naïve foster dams [Fig. 1, (Zhu et al., 2017; Zhu et al., 2014a; Zhu et al., 2012)]. The cross-fostering was used to restrict nicotine exposure to the prenatal period. To account for potential independent effects of cross-fostering, we cross-fostered offspring from all three groups: nicotine + saccharin, saccharin only and plain drinking water. Cross-fostering could introduce at least two technical artifacts: 1) Effects of sudden withdrawal from nicotine upon cross fostering to a non-nicotine exposed nursing dam, and 2) effects of stress associated with cross-fostering (Muhammad and Kolb, 2011). Sudden nicotine withdrawal occurs in virtually every preclinical model of developmental nicotine exposure. It occurs soon after birth in the prenatal-only models and around the time of weaning in the pre- and postnatal models. A gradual step-up or step-down method of nicotine exposure can avoid abrupt nicotine withdrawal (Paz et al., 2007). However, we did not take such measures in our mouse models. The stress associated with cross-fostering was controlled in our studies by cross-fostering offspring from all prenatal exposure groups.
2.2. Breeding success, pregnancy, and litter metrics
Major adverse outcomes of cigarette smoking during pregnancy in humans include preterm delivery and low birth weight (Froggatt et al., 2020; Inoue et al., 2017; Ko et al., 2014). However, we did not observe statistically significant effects on the length of the pregnancy, the number of pups born, offspring sex ratio at birth, or offspring birth-weight. Breeding success (i.e., fertility) was not affected. In addition, the nicotine exposure did not affect offspring developmental milestones such as the time of eye opening or the time of appearance of fur in the offspring. Thus, our nicotine exposure paradigms did not replicate all the effects on pregnancy or pregnancy outcomes reported in humans. A higher dose of nicotine than the dose used here may be necessary to produce those effects. In fact, other studies using higher nicotine dose report effects on litter size as well as birth weight in rodent models (Newman et al., 1999; Santiago and Huffman, 2012; Slotkin et al., 1993).
2.3. Behavior
When the offspring in the nicotine exposed and control groups reached 2–3 months of age, we examined spontaneous locomotor activity (home cage activity monitoring for 24 h.), attention (object based attention), spatial working memory (Y-maze), motor impulsivity (cliff avoidance reflex) and novelty-seeking/risk-taking behavior (elevated plus maze). These behaviors were chosen for analysis to evaluate the possibility that the nicotine exposure contributed to behavioral changes consistent with ADHD. Attention deficit, hyperactivity and impulsivity are core symptoms of ADHD whereas working memory deficit and risk-taking behavior are co-morbidities (Antshel et al., 2010; Biederman, 2007; Biederman et al., 2004; Faraone et al., 2018; Fried et al., 2016; Silverstein et al., 2020).
We observed locomotor hyperactivity, attention deficit, spatial working memory deficit, motor impulsivity and novelty-seeking/risk-taking behavior in the offspring [Fig. 1 (Martin et al., 2020; Zhang et al., 2021c; Zhang et al., 2018; Zhu et al., 2017; Zhu et al., 2012)]. There were differences in the behavioral outcomes between prenatal-only and pre- and postnatal nicotine exposure models as well as between the C57BL/6 and SW strains. The locomotor hyperactivity and motor impulsivity were observed in the prenatal-only model but not in the pre-and postnatal exposure model. Attention deficit and spatial working memory deficit were observed in the C57BL/6 strain in the prenatal only as well as the pre- and postnatal exposure models. However, these deficits were not present in the pre- and postnatal exposure paradigm using the Swiss Webster strain. The novelty-seeking/risk-taking behavior was observed in the Swiss Webster in the pre- and postnatal exposures paradigm but not the C57BL/6 strain in either paradigm. Finally, many of the behavioral changes were observed in male but not female mice, consistent with the differences in the behavioral manifestations of ADHD between boys and girls (Biederman et al., 2002; Gaub and Carlson, 1997). Thus, the duration of the nicotine exposure, the strain of the mouse, and sex of the offspring influenced the behavioral outcomes.
Multiple reports in the literature suggest that differences in the timing of the nicotine exposure can introduce variability in the outcomes. For example, two studies examined the effects of nicotine exposure occurring at each one of six different windows of prenatal and postnatal development from conception to postnatal day 7 on cognitive and emotional behaviors (Alkam et al., 2013a; Alkam et al., 2013b). Nicotine exposure occurring from the 12th day of gestation until birth produced significant effects on these behaviors compared to exposures that began prior to or after the 12th day of gestation.
A plausible explanation for the differences in behavioral outcomes due to the differences in the timing of the exposure is that peak occurrence of the different developmental events (such as neurogenesis, gliogenesis, programmed cell death and synaptic reorganization) is at different times during the pre- and postnatal development. Therefore, a developmental event may be impacted particularly severely if the nicotine exposure overlapped with its peak. Another possibility is that with longer nicotine exposure (Martin et al., 2020; Zhang et al., 2021c; Zhang et al., 2018) the potential for adaptation to occur within the neural systems may be proportionately greater, and these adaptations may influence the behavioral outcomes (Zhang et al., 2018).
The differences in the novelty-seeking/risk-taking behavior between the SW and C57BL/6 strains of mice are supported by other reports of strain differences in the effects of nicotine [Review in (Zhang et al., 2018) (Marks et al., 1986a; Marks et al., 1986b)]. However, at the present time, detailed insights into the precise mechanisms that may contribute to the strain differences are not available.
Sex-dependent changes in behaviors such as hyperactivity, nicotine preference and pre-pulse inhibition have been reported in rodent models of developmental nicotine exposure (Klein et al., 2003; Pauly et al., 2004; Polli et al., 2020b; Popke et al., 1997; Romero and Chen, 2004; Shacka et al., 1997). Differences in hypothalamic-pituitary axis signaling, estrogen receptor signaling, neurotransmitter receptor signaling have been proposed as potential mechanisms for these sex differences (Andersen and Teicher, 2000; Pauly, 2008; Torres and O’Dell, 2016).
Thus, the findings from preclinical studies can be influenced significantly by technical and biological variables. These variables include timing of the nicotine exposure, use of vehicles such as sweeteners, type of nicotine exposure (aerosol, smoke or nicotine per se) the dose, frequency and route of nicotine administration, the strain and sex of the animal and the age at which the outcome measures are evaluated. Whereas the design of each study is justified by its specific goals and the specific hypotheses being tested, the influence of each variable should be considered while comparing outcomes from different studies and drawing conclusions about translational relevance.
2.4. Monoamine neurotransmitter content and synaptic release in the frontal cortex
We examined changes in monoamine neurotransmitter content and synaptic release in the frontal cortex in male C56BL/6 mice in our mouse models (Fig. 1). We focused on monoamines because of the association of these neurotransmitters with the symptoms as well as pharmacological treatment of ADHD (Berridge et al., 2006; Kuczenski et al., 1997; Kuczenski and Segal, 1997, 2005; Spencer et al., 2006; Spencer et al., 2013; Spencer et al., 2005; Volkow et al., 2012). We examined male mice because most of the behavioral changes were present in male but not female mice.
We found significant reductions in dopamine tissue content in the frontal cortex in our C57BL/6 prenatal nicotine exposure model (Zhu et al., 2014a; Zhu et al., 2012). In addition, in vivo microdialysis studies in the C57BL/6 pre- and postnatal nicotine exposure model showed significant reductions in dopamine and noradrenaline but not serotonin release in the frontal cortex (Zhang et al., 2021c). These findings are supported by another report that examined monoamine levels in the frontal cortex following pre- and postnatal nicotine exposure (Alkam et al., 2017).
The changes were not limited to dopamine and noradrenaline. In another study using transgenic SW mice in which the GFP reporter is under the control of the GAD67 promoter (McCarthy and Bhide, 2012; McCarthy et al., 2011; Tamamaki et al., 2003), we found that developmental nicotine exposure produced significant reductions in the GABA to non-GABA neuron ratio in the frontal cortex (Martin et al., 2020), suggesting a dampening of the frontal cortical inhibitory tone. Other studies have reported changes in glutamate and acetylcholine receptor signaling following developmental nicotine exposure (Aoyama et al., 2016; Baumann and Koch, 2017; Gavini et al., 2021; Parameshwaran et al., 2012; Polli et al., 2020a; Polli et al., 2020b; Slotkin et al., 2004; Vaglenova et al., 2008).
Nicotine’s effects on the developing brain are mediated via nicotinic acetylcholine receptors, which are expressed in the fetal brain by a variety of neurons including those that contain neurotransmitters such as dopamine or GABA (Navarro et al., 1989; Slotkin et al., 1987a; Slotkin et al., 1986; Slotkin et al., 1987b; Slotkin et al., 1987c). Therefore, it is not surprising that effects of developmental nicotine exposure are not limited to the cholinergic system but are manifested by dopamine, noradrenaline, GABA and glutamate neurotransmitter systems as well.
2.5. Structural changes in the brain
We performed a pilot diffusion tensor imaging study in C57BL/6 male mice from the developmental nicotine + saccharin, saccharin alone or plain drinking water exposure groups (Zhang et al., 2021c; Zhang et al., 2018). We used an ex-vivo scanning protocol that was consistent with our prior work (Caffall et al., 2021). Only 3 brains from each developmental exposure group were imaged. Regions of interest (ROI) were identified by performing whole-brain voxel-wise independent-samples t-tests between the nicotine + saccharin, saccharin-only and plain drinking water using Analysis of Functional Neuroimages (AFNI) software. A voxel-wise threshold of p < 0.10 was used to identify ROIs for further analyses. Although preliminary and although based on a small sample, data from this analysis supported the hypothesis that the medial prefrontal cortex and dorsal striatum showed reduced microstructural integrity (i.e., reduced fractional anisotropy) in the developmentally nicotine exposed group (Fig. 2). These are the same two brain regions that were implicated by our behavioral and neurotransmitter data in this mouse model (Martin et al., 2020; Zhang et al., 2021c; Zhang et al., 2018; Zhu et al., 2017; Zhu et al., 2012). In an earlier study we had showed that the volume of the medial prefrontal cortex was significantly reduced in the prenatal nicotine exposure mouse model (Zhu et al., 2012). The imaging analysis also highlighted the ansiform lobule (Crus 1) of the cerebellum as a third region with reduced microstructural integrity in the developmentally nicotine exposed group (Fig. 2). This part of the lateral cerebellum or cerebro-cerebellum is implicated in ADHD and other cognitive disorders (Makris et al., 2015; Stoodley et al., 2010, 2012).
Fig. 2.

Summary of findings from the pilot neuroimaging study. Region of interest (ROI) analysis of fractional anisotropy (FA) in the diffusion tensor imaging study. Left column shows ROI, right column shows FA (mean ± SEM) by group. (A) medial prefrontal cortex; (B) dorsal striatum (C), ansiform lobule of the cerebellum. There is a significant decrease (p < 0.1) in FA values in the medial prefrontal cortex (A), dorso-lateral striatum (B) and the ansiform lobule of the cerebellum (C) in the pre- and postnatal nicotine exposure (PNE) group compared to the plain drinking water (WATER) or saccharin-only (SAC) exposure groups.
Changes identified by diffusion tensor imaging are reported to reflect histological changes in the gray matter regions in neurological disorders and during normal development (Kim et al., 2019; Mori and Zhang, 2006). Therefore, developmental nicotine exposure may produce changes in neuronal, glial, and synaptic numbers and organization. Neuroimaging of network function in ADHD has identified structural and functional deficits associated with the medial prefrontal cortex, dorsal striatum, and the cerebellum (Makris et al., 2009; Makris et al., 2015; Monuteaux et al., 2008; Seidman et al., 2006; Shaw et al., 2006; Shaw et al., 2012; Valera et al., 2010). Thus, our neuroimaging data represent a first step toward identification and characterization of network-wide structural and functional changes in the brain following developmental nicotine exposure and relating the changes to those reported in ADHD and other neurodevelopmental disorders.
2.6. Summary of findings
Our findings offer insights into the effects of developmental nicotine exposure on behavioral, neurochemical, and neuroanatomical parameters. These data facilitate an integrated, system-wide view of nicotine’s effects on the developing brain. A major theme that emerges is that developmental nicotine exposure may target neural networks involving the frontal cortex, striatum and the cerebellum and dopamine, noradrenaline, and GABA neurotransmission in the frontal cortex. Frontal cortex plays a critical role in the regulation of attention, working memory, impulsivity, and risk-taking behaviors (Arnsten et al., 1994; Arnsten and Jin, 2014; Arnsten and Pliszka, 2011; Robbins and Arnsten, 2009). Our data suggest that changes in frontal cortical network function, likely via changes in monoamine and GABA neurotransmission may be a key functional outcome of the effects of developmental nicotine exposure. The unique trajectory of the structural and functional development of the frontal cortical regions (Kolk and Rakic, 2021; Mills et al., 2014; Shaw et al., 2006; Shaw et al., 2012; Tsujimoto, 2008), which results in a characteristic maturational “delay” compared to the other brain regions may render these regions particularly vulnerable to the effects of nicotine, and other risk factors for neurodevelopmental disorders.
3. Developmental nicotine exposure mouse models as preclinical models of ADHD
Translational value of preclinical models of neuro-psychiatric conditions such as ADHD can be significant, because these models can offer novel insights into the neurobiological mechanisms of the disorder and facilitate discovery of novel therapeutics. Here we discuss how we have used our mouse models as preclinical models of ADHD as a first step toward these objectives.
3.1. Construct, face and predictive validities
We developed our mouse models based on evidence from the clinical literature that maternal cigarette smoking during pregnancy increased the risk for ADHD in the offspring (Altink et al., 2009; Biederman et al., 2012; Mick et al., 2002; Milberger et al., 1996; Milberger et al., 1998; Schmitz et al., 2006; Torabi et al., 2021). We had shown that the clinical presentations of ADHD due to maternal cigarette smoking during pregnancy are the same as the presentations of ADHD due to other causes (Biederman et al., 2012). Our findings (described in the previous sections) show that the mouse models replicate behavioral and neurochemical phenotypes that are consistent with ADHD. Therefore, our mouse models carry significant construct validity and face validity. Other reports in the literature support the notion that rodent models of developmental nicotine exposure are valid models of ADHD (Bryden et al., 2016; Buck et al., 2019b; Polli et al., 2020b; Russell, 2011; Yochum et al., 2014).
Next, we examined whether the mouse models carried predictive validity. In these studies, we examined the effects of methylphenidate, a classic stimulant drug with decades-long record of safety and efficacy in the treatment of ADHD in children and adults. In our prenatal nicotine exposure mouse model, a single administration of methylphenidate [0.75 mg/kg; intraperitoneal, which is equivalent to therapeutic dose administered to ADHD patients (Balcioglu et al., 2009)] reduced hyperactivity (Zhu et al., 2014a; Zhu et al., 2012), motor impulsivity, and improved attention as well as spatial working memory (Zhu et al., 2017). Methylphenidate improved attention and working memory in our pre- and postnatal nicotine exposure mouse model as well (Zhang et al., 2021c). In another study using in vivo microdialysis in awake, behaving mice in our pre- and postnatal nicotine exposure mouse model a single methylphenidate administration significantly increased dopamine and noradrenaline release in the frontal cortex (Zhang et al., 2021c). The increase in monoamine release (compared to the baseline level) was statistically significant between 30 min and 4 h. following the methylphenidate administration (Zhang et al., 2021c). The methylphenidate-induced improvement in attention and working memory was evident at 0.5 h. but not at 2.5 h., suggesting that the behavioral improvement occurred during the period of increased monoamine release (Zhang et al., 2021c). The behavioral responses to methylphenidate are consistent with the therapeutic effects of this drug in ADHD suggesting that our mouse models carry significant predictive validity as preclinical models of ADHD.
3.2. Evaluation of efficacy of candidate therapeutic compounds using the mouse model
One of the advantages of a well-characterized mouse model of a human disorder is the opportunity it offers to test the efficacy of novel therapeutic compounds. Since our developmental nicotine exposure mouse model fulfils the criteria for construct, face, and predictive validity, we used this model to test a candidate non-stimulant compound as a potential therapeutic for ADHD.
Discovery and development of non-stimulant drugs for the treatment of ADHD remains a high priority for the field. Stimulant drugs such as methylphenidate and amphetamines are mainstays of ADHD treatment because of their proven efficacy and safety. However, stimulants carry significant abuse potential (Spencer et al., 2018; Wilens et al., 2008). In fact, prescription stimulants are among the most frequently misused medications (Butler et al., 2021; Vosburg et al., 2021; Vosburg et al., 2020). As a result, there are significant concerns about the safety of stimulants among patients, prescribers and families of patients, and these concerns represent significant barriers to successful treatment of ADHD. Thus, there is an urgent need for a non-stimulant compound with the efficacy of stimulants but without the abuse potential. Here we show that the developmental nicotine exposure mouse model can be used successfully for evaluation of potential, novel non-stimulant candidates for the treatment of ADHD,
The non-stimulant compound that was examined was norbinaltorphimine, a selective kappa opioid receptor (KOR) antagonist. KORs are widely distributed throughout the central nervous system and are activated by the endogenous ligand dynorphin (DePaoli et al., 1994; Liu-Chen, 2004). KOR signaling plays a role in stress response, affective disorders and pain sensation. KOR antagonists show significant “beneficial” effects in animal models of depression, anxiety, and drug addiction (Bruchas et al., 2010; Carlezon and Krystal, 2016; Carlezon et al., 1998; Chavkin and Koob, 2016; Knoll and Carlezon, 2010; Van’t Veer et al., 2013). KORs are expressed by midbrain/brainstem monoamine neurons, and KORs serve as negative feedback regulators of dopamine and noradrenaline release at the synapse in the frontal cortex (Fuentealba et al., 2006; Margolis et al., 2003; Margolis et al., 2006). Therefore, KOR antagonists have the potential to increase frontal cortical dopamine and noradrenaline release by inhibiting the KOR-mediated negative feedback for neurotransmitter release.
We found that norbinaltorphimine dose-dependently increased dopamine and noradrenaline release in the frontal cortex of mice (Zhang et al., 2021c). In our pre- and postnatal nicotine exposure mouse model (C57BL/6 mice), a single administration of norbinaltorphimine (20 mg/kg; intraperitoneal) produced significant increases in the release of both the neurotransmitters beginning at 2.5 h. and lasting until 6.0 h. (Zhang et al., 2021c). As discussed previously, the mice in the pre- and postnatal nicotine exposure group have significant deficits in object based attention and working memory. The norbinaltorphimine administration produced significant improvements in both these behaviors at 2.5 h and 5.5 h., but not at 24 h. following the single administration. Thus, the increase in frontal cortical monoamine neurotransmitter release and the behavioral improvement showed temporal overlap following the norbinaltorphimine administration.
In a head-to-head comparison, the effects of norbinaltorphimine and methylphenidate on frontal cortical monoamine neurotransmitter release, attention and working memory were comparable in direction as well as magnitude. However, the effects of norbinaltorphimine lasted much longer than those of methylphenidate. Norbinaltorphimine’s CNS actions are known to be longer lasting compared to the action of other KOR antagonists. One of the intracellular mechanisms of norbinaltorphimine’s actions is phosphorylation of c-Jun N-terminal kinase (Bruchas et al., 2007; Chavkin et al., 2019; Melief et al., 2010; Melief et al., 2011; Munro et al., 2012). We found that norbinaltorphimine produced significant increases in phosphorylated c-Jun N-terminal kinase in the frontal cortex in our mouse model, and that the phosphorylation lasted for 24 h., coinciding with the neurotransmitter and behavioral changes (Zhang et al., 2021c). Thus, the developmental nicotine exposure mouse model proved to be a valuable tool for examining the efficacy of a KOR antagonist as a potential non-stimulant treatment for ADHD.
4. Transgenerational transmission
Heritability of environment-induced phenotypes from one generation to the next is gaining acceptability as a phenomenon with significant scientific and public health relevance. A growing list of environmental factors including stress, hormones and chemicals such as nicotine, cocaine, alcohol, pesticides are implicated in producing heritable effects (Crews et al., 2012; McCarthy and Bhide, 2021; McCarthy et al., 2020; McCarthy et al., 2018; Skinner, 2011a, 2011b; Skinner et al., 2011; Vassoler et al., 2014; Vassoler et al., 2013; Wimmer et al., 2019).
We examined whether the locomotor hyperactivity in the prenatally nicotine exposed mice was heritable from one generation to the next. We bred the prenatally nicotine exposed mice (F1 generation; the nicotine exposed dams are the F0 generation) with drug naïve partners to produce the next generation of offspring (F2 generation; Fig. 3). We found that locomotor hyperactivity, which was present in male and female mice in the F1 generation (Zhu et al., 2014a; Zhu et al., 2012) was also present in the F2 generation, demonstrating intergenerational transmission of hyperactivity. The transmission from F1 to F2 generation is intergenerational (rather than transgenerational) because the germline of the F1 generation was directly exposed to nicotine in utero as well as in the pre-weaning period. For the transmission to be transgenerational, the founder in the preceding generation should not be exposed to the environmental factor at any time.
Fig. 3.

Experimental design for the transgenerational transmission study. Female C57BL/6 mice (F0 generation) were exposed to nicotine in drinking water beginning 3 weeks before breeding with a drug naïve sire. The nicotine exposure of the females continued throughout pregnancy. On the day of birth, the offspring (F1 generation) were cross-fostered to drug naïve nursing dams. Both the male and female F1 mice were hyperactive. They were crossed with drug naïve partners to produce the F2 generation. F2 male and female mice from an F1 female founder but not F1 male founder were hyperactive. F2 female mice produced by F1 female founder were crossed with drug naïve males to produce the F3 genaration. The F2 male mice produced by an F1 female were not used for further breeding. Similarly, F2 male or female mice produced by an F1 male founder were not bred further. An identical breeding plan was used to generate F1, F2 and F3 mice from the saccharin-only exposed F0 females (not shown).
To examine if the effects were transmitted transgenerationally, we bred the F2 female mice (F2 males were not bred because they did not show hyperactivity) with drug naïve partners to produce the F3 generation (Fig. 3). We found significant hyperactivity in the F3 generation as well (Zhu et al., 2014a). Thus, hyperactivity produced by developmental nicotine exposure shows transgenerational transmission, via the maternal line of descent.
Around the time of publication of our study and since then, several preclinical (Buck et al., 2019a; Buck et al., 2019b) and clinical (Golding et al., 2017; Golding et al., 2020; Miller et al., 2014a; Miller et al., 2014b; Williams et al., 2019) studies have reported transgenerational transmission of multiple phenotypes produced by the developmental nicotine exposure.
Heritable consequences of nicotine use by fathers has received significant attention in recent years and some of the behavioral phenotypes transmitted from the nicotine-exposed father to his offspring are consistent with the symptoms associated with ADHD and other neurodevelopmental disorders [Review in (McCarthy and Bhide, 2021)]. For example, locomotor hyperactivity was observed in the first generation offspring derived from nicotine-exposed male mice (Dai et al., 2017; Hawkey et al., 2019; McCarthy et al., 2018; Zhang et al., 2020), suggesting that hyperactivity may be a heritable phenotype common to developmental (i.e., maternal) and paternal nicotine exposure. However, hyperactivity was not observed in the second generation derived from the nicotine-exposed males. Paternal nicotine exposure also decreased nicotine self-administration and increased contextual and cued fear responses in the first generation of descendants (Goldberg and Gould, 2019; Goldberg et al., 2019). Significant reversal learning deficits were observed in both the first and second generations derived from nicotine-exposed males suggesting that cognitive inflexibility, a symptom shared by developmental disorders such as autism spectrum disorder, schizophrenia and ADHD (Dajani and Uddin, 2015; Izquierdo et al., 2016; Klanker et al., 2013; Ozsivadjian et al., 2021) may be a heritable phenotype following paternal nicotine exposure (McCarthy et al., 2018).
Although prenatal and early postnatal (i.e., developmental or maternal) exposure to the artificial sweetener saccharin did not produce locomotor hyperactivity in the descendants in the mouse model (Zhu et al., 2014a), exposure of male mice to saccharin produced locomotor hyperactivity and motor impulsivity in the first generation of descendants (McCarthy et al., 2020). Co-exposure of male mice to nicotine and saccharin, which occurs when humans consume certain forms of smokeless tobacco (Miao et al., 2016) produced hyperactivity and working memory deficits in the first generation (McCarthy et al., 2020).
Thus, intergenerational and transgenerational transmission of multiple behavioral phenotypes was observed following paternal exposure to nicotine, saccharin or co-exposure to nicotine and saccharin.
4.1. Molecular mechanisms of transgenerational transmission
The transgenerational inheritance of nicotine-induced phenotypes whether following developmental (i.e., maternal) or paternal exposure does not follow the classic Mendelian pattern of heritability across generations, pointing toward epigenetic modification of germ cells as a plausible mechanism of heritability rather than genetic mutations in the germline (McCarthy et al., 2018). However, the ability of cigarette smoke and nicotine to produce germ line mutations is well-known (Beal et al., 2017; Marchetti et al., 2011; Yauk et al., 2007).
Epigenetic modification occurs by chemical modification of the DNA or histones as well as by the action of non-coding RNAs such as miRNAs (Brunner et al., 2014; Carrell, 2012; Hamatani, 2012). The effects of nicotine on epigenetic modification of germ cells have been studied in the spermatozoa more widely than in the oocyte, perhaps due to the ease of access to the male germ cells compared to the female germ cells. Studies in human subjects and preclinical models show that exposure to cigarette smoke, e-cigarette aerosol or direct exposure to nicotine (in preclinical models) produces significant changes in DNA methylation and miRNA expression in the spermatozoa (Altintas et al., 2021; Beal et al., 2017; Bline et al., 2020; Dai et al., 2017; Jenkins et al., 2017; Liu et al., 2022; Marczylo et al., 2012; McCarthy and Bhide, 2021; McCarthy et al., 2020; McCarthy et al., 2018; Murphy et al., 2020; Soubry, 2018; Zhang et al., 2020) supporting the possibility that epigenetic changes in the germ cells can mediate transgenerational transmission of nicotine-induced phenotypes. However, multiple epigenetic reprogramming events involving erasure and re-acquisition of epigenetic marks occur throughout embryonic and postnatal development. Therefore, how exactly epigenetic changes in the germ cells of one generation can produce changes in somatic cells and behaviors in multiple generations of descendants remains an area of intensive research (Spadafora, 2020).
5. Synergistic interaction between ADHD and repetitive mild traumatic brain injury
Recent evidence suggests a potentially concerning association between ADHD and concussions [also known as repetitive mild traumatic brain injury (mTBI)]. Individuals with untreated ADHD are more likely to engage in activities that carry a higher risk of concussions (Alosco et al., 2014; Biederman et al., 2015; Chasle et al., 2016; Cook et al., 2020; Iverson et al., 2016; Nelson et al., 2016). Moreover, concussions in individuals with untreated ADHD result in significant deficits in visual and verbal memory, and visual motor processing speed that are not observed in individuals without ADHD who suffer concussions (Cook et al., 2017; Kaye et al., 2019; Nowak et al., 2020). Student athletes with ADHD report increased incidence of symptoms such as fatigue and poor concentration following concussions (Biederman et al., 2015). Paradoxically, participation in sports and other organized physical activities is often recommended as part of ADHD management programs.
Since our mouse models of developmental nicotine exposure carry validity as preclinical models of ADHD, we examined whether we could gain further insights into potential link between ADHD and repetitive mTBI using our mouse model. We subjected C57BL/6 male mice that were exposed to nicotine during the pre- and postnatal periods (as well as mice in the control groups) to repetitive mTBI or sham procedure. In one study we used isoflurane anesthesia during the repetitive mTBI or sham procedure (Zhang et al., 2021b) whereas in another we used unanesthetized mice (Zhang et al., 2021a). Anesthetic agents can exert “neuroprotective” effects on the injured brain (Bailes et al., 2014; Petraglia et al., 2014a; Petraglia et al., 2014b). Moreover, using unanesthetized mice mimics “real-life” concussions in humans more closely. Collectively, our studies showed that the combination of developmental nicotine exposure and repetitive mTBI produced transient depression-like behavior and novelty-seeking/risk-taking behavior. These behaviors were not produced by either factor alone, but emerged only when the two factors were combined, demonstrating synergistic interactions between developmental nicotine exposure and repetitive mTBI.
In the non-nicotine exposed control group of mice, the repetitive mTBI produced transient deficits in object based attention, consistent with findings from human studies showing poor attention following concussion (Ling et al., 2015). In the developmentally nicotine exposed mice, deficits in object based attention were present at baseline (i.e., prior to repetitive mTBI), and those deficits persisted following the repetitive mTBI. It is possible that the repetitive mTBI had an “additive” effect on attention deficits in the developmentally nicotine exposed group that were not detectable by our method.
We could not evaluate the contribution of anesthesia to the behavioral outcomes fully because of the differences in the mTBI protocols (5 versus 7 mTBI episodes) between the two studies. However, depression-like behavior in the nicotine exposed mice and attention deficit in the non-nicotine exposed mice following the repetitive mTBI were observed in anesthetized as well as unanesthetized mice. Novelty-seeking/risk-taking behavior was analyzed only in the unanesthetized mice.
Our findings are consistent with findings from human studies that concussions produce “worse” outcomes in individuals with untreated ADHD, once again illustrating the translational value of the mouse models. We recognize that ADHD is a complex disorder to which multiple etiological and risk factors contribute. Therefore, it is possible that our findings apply to ADHD associated with developmental nicotine exposure only rather than to ADHD associated with other etiological factors.
6. Synopsis and prospective
The nicotine exposure mouse models offer a valuable experimental framework for examination of the effects of nicotine on the developing brain. Our research has identified neural networks involving the frontal cortex, striatum and the cerebellum and signaling via the neurotransmitters dopamine, noradrenaline and GABA in the frontal cortex as modulators of behavioral consequences of developmental nicotine exposure. The mouse models have facilitated identification of the selective kappa opioid receptor antagonist norbinaltorphimine as a candidate non-stimulant treatment for ADHD, analysis of transgenerational transmission of the effects of developmental nicotine exposure, and synergistic interactions between ADHD associated with developmental nicotine exposure and concussions. With the emergence of e-cigarette use during pregnancy as a major public health concern preclinical research is beginning to incorporate rodent models of e-cigarette or e-liquid exposures. These models have shown that developmental exposure to e-cigarette aerosol produces behavioral changes that overlap with the behavioral changes produced by developmental exposure to nicotine (Church et al., 2020; Lauterstein et al., 2016; Nguyen et al., 2018; Ponzoni et al., 2015; Smith et al., 2015). The public health implications of e-cigarette use during pregnancy are enormous. These implications, especially the potential for transgenerational transmission of the effects of developmental e-cigarette or e-liquid exposure, are beginning to be recognized. Research using preclinical models offers our best chance to build a sound scientific understanding of the consequences of nicotine use during pregnancy and to facilitate formulation of rational public education and public policy efforts.
Acknowledgements
We gratefully acknowledge funding support from the Florida Department of Health, The Jim and Betty Anne Rodgers Chair Fund and a research grant to the Florida State University Research Foundation by Avekshan, LLC.
Declaration of competing interest
Deirdre McCarthy has financial interest in Avekshan, LLC, which is disclosed to and is managed by the Florida State University Research Foundation. She is an inventor of following intellectual property through Florida State University: US Patent (#10,245,271 B2) and a pending US patent application 16/369,748.
Lin Zhang, Bradley Wilkes and David Vaillancourt: No conflict of interest to declare.
Joseph Biederman is currently receiving research support from the following sources: AACAP, Feinstein Institute for Medical Research, Genentech, Headspace Inc., NIDA, Pfizer Pharmaceuticals, Roche TCRC Inc., Sunovion Pharmaceuticals Inc., Takeda/Shire Pharmaceuticals Inc., Tris, and NIH. He receives honoraria from the Medlearning Inc. and MGH Psychiatry Academy for tuition-funded CME courses. Through MGH corporate licensing, Dr. Biederman has a US Patent (#14/027,676) for a non-stimulant treatment for ADHD, a US Patent (#10,245,271 B2) on a treatment of impaired cognitive flexibility, and a US patent (11,045,465 B2) on a method to prevent stimulant abuse. Dr. Biederman and his program have received royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Biomarin, Bracket Global, Cogstate, Ingenix, Medavent Prophase, Shire, Sunovion, and Theravance; these royalties were paid to the Department of Psychiatry at MGH. In 2020: Dr. Biederman received an honorarium for a scientific presentation from Tris, and research support from the Food & Drug Administration. In 2019, Dr. Biederman was a consultant for Akili, Avekshan, Jazz Pharma, and Shire/Takeda. He received research support from Lundbeck AS and Neurocentria Inc. Through MGH CTNI, he participated in a scientific advisory board for Supernus. In 2018, Dr. Biederman was a consultant for Akili and Shire.
Pradeep Bhide has financial interest in Avekshan, LLC, which is disclosed to and is managed by the Florida State University Research Foundation. He is an inventor of following intellectual property through Florida State University: US patents 14/027,676, 10,245,271 B2, 11,045,465 B2 and a pending application 16/369,748.
References
- Alkam T, Kim HC, Hiramatsu M, Mamiya T, Aoyama Y, Nitta A, Yamada K, Nabeshima T, 2013a. Evaluation of emotional behaviors in young offspring of C57BL/6J mice after gestational and/or perinatal exposure to nicotine in six different time-windows. Behav. Brain Res 239, 80–89. [DOI] [PubMed] [Google Scholar]
- Alkam T, Kim HC, Mamiya T, Yamada K, Hiramatsu M, Nabeshima T, 2013b. Evaluation of cognitive behaviors in young offspring of C57BL/6J mice after gestational nicotine exposure during different time-windows. Psychopharmacology 230 (3), 451–463. [DOI] [PubMed] [Google Scholar]
- Alkam T, Mamiya T, Kimura N, Yoshida A, Kihara D, Tsunoda Y, Aoyama Y, Hiramatsu M, Kim HC, Nabeshima T, 2017. Prenatal nicotine exposure decreases the release of dopamine in the medial frontal cortex and induces atomoxetine-responsive neurobehavioral deficits in mice. Psychopharmacology (Berl) 234 (12), 1853–1869. [DOI] [PubMed] [Google Scholar]
- Allen AM, Oncken C, Hatsukami D, 2014. Women and smoking: the effect of gender on the epidemiology, health effects, and cessation of smoking. Curr. Addict. Rep 1 (1), 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Fedor AF, Gunstad J, 2014. Attention deficit hyperactivity disorder as a risk factor for concussions in NCAA division-I athletes. Brain Inj. 28 (4), 472–474. [DOI] [PubMed] [Google Scholar]
- Altink ME, Slaats-Willemse DI, Rommelse NN, Buschgens CJ, Fliers EA, Arias-Vasquez A, Xu X, Franke B, Sergeant JA, Faraone SV, Buitelaar JK, 2009. Effects of maternal and paternal smoking on attentional control in children with and without ADHD. Eur. Child Adolesc. Psychiatry 18 (8), 465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altintas A, Liu J, Fabre O, Chuang TD, Wang Y, Sakurai R, Chehabi GN, Barres R, Rehan VK, 2021. Perinatal exposure to nicotine alters spermatozoal DNA methylation near genes controlling nicotine action. FASEB J. 35 (7), e21702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH, 2000. Sex differences in dopamine receptors and their relevance to ADHD. Neurosci. Biobehav. Rev 24 (1), 137–141. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Faraone SV, Maglione K, Doyle AE, Fried R, Seidman LJ, Biederman J, 2010. Executive functioning in high-IQ adults with ADHD. Psychol. Med 40 (11), 1909–1918. [DOI] [PubMed] [Google Scholar]
- Aoyama Y, Toriumi K, Mouri A, Hattori T, Ueda E, Shimato A, Sakakibara N, Soh Y, Mamiya T, Nagai T, Kim HC, Hiramatsu M, Nabeshima T, Yamada K, 2016. Prenatal nicotine exposure impairs the proliferation of neuronal progenitors, leading to fewer glutamatergic neurons in the medial prefrontal cortex. Neuropsychopharmacology 41 (2), 578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Jin LE, 2014. Molecular influences on working memory circuits in dorsolateral prefrontal cortex. Prog. Mol. Biol. Transl. Sci 122, 211–231. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Pliszka SR, 2011. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol. Biochem. Behav 99 (2), 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS, 1994. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology 116 (2), 143–151. [DOI] [PubMed] [Google Scholar]
- Bailes JE, Dashnaw ML, Petraglia AL, Turner RC, 2014. Cumulative effects of repetitive mild traumatic brain injury. Prog. Neurol. Surg 28, 50–62. [DOI] [PubMed] [Google Scholar]
- Balcioglu A, Ren JQ, McCarthy D, Spencer TJ, Biederman J, Bhide PG, 2009. Plasma and brain concentrations of oral therapeutic doses of methylphenidate and their impact on brain monoamine content in mice. Neuropharmacology 57 (7–8), 687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann VJ, Koch U, 2017. Perinatal nicotine exposure impairs the maturation of glutamatergic inputs in the auditory brainstem. J. Physiol 595 (11), 3573–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MA, Yauk CL, Marchetti F, 2017. From sperm to offspring: assessing the heritable genetic consequences of paternal smoking and potential public health impacts. Mutat. Res 773, 26–50. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Kuyt F, Jacob III P, 1982. Circadian blood nicotine concentrations during cigarette smoking. Clin. Pharmacol. Ther 32 (6), 758–764. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC, 2006. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol. Psychiatry 60 (10), 1111–1120. [DOI] [PubMed] [Google Scholar]
- Biederman J, 2007. Advances in the neurobiology of ADHD. CNS Spectr. 12 (4 Suppl. 6), 6–7. [DOI] [PubMed] [Google Scholar]
- Biederman J, Mick E, Faraone SV, Braaten E, Doyle A, Spencer T, Wilens TE, Frazier E, Johnson MA, 2002. Influence of gender on attention deficit hyperactivity disorder in children referred to a psychiatric clinic. Am. J. Psychiatry 159 (1), 36–42. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Doyle AE, Seidman LJ, Wilens TE, Ferrero F, Morgan CL, Faraone SV, 2004. Impact of executive function deficits and attention-deficit/hyperactivity disorder (ADHD) on academic outcomes in children. J. Consult. Clin. Psychol 72 (5), 757–766. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Bhide PG, Woodworth KY, Faraone S, 2012. Does exposure to maternal smoking during pregnancy affect the clinical features of ADHD? Results from a controlled study. World J. Biol. Psychiatry 13 (1), 60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Feinberg L, Chan J, Adeyemo BO, Woodworth KY, Panis W, McGrath N, Bhatnagar S, Spencer TJ, Uchida M, Kenworthy T, Grossman R, Zafonte R, Faraone SV, 2015. Mild traumatic brain injury and attention-deficit hyperactivity disorder in young student athletes. J. Nerv. Ment. Dis 203 (11), 813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Fitzgerald M, Spencer TJ, Bhide PG, McCarthy DM, Woodworth KY, Saunders A, Faraone SV, 2020. Is paternal smoking at conception a risk for ADHD? A controlled study in youth with and without ADHD. J. Atten. Disord 24 (11), 1493–1496. [DOI] [PubMed] [Google Scholar]
- Bline AP, Dearfield KL, DeMarini DM, Marchetti F, Yauk CL, Escher J, Workshop P, 2020. Heritable hazards of smoking: applying the “clean sheet” framework to further science and policy. Environ. Mol. Mutagen 61 (9), 910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Yang T, Schreiber S, Defino M, Kwan SC, Li S, Chavkin C, 2007. Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. J. Biol. Chem 282 (41), 29803–29811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C, 2010. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 1314, 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner AM, Nanni P, Mansuy IM, 2014. Epigenetic marking of sperm by post-translational modification of histones and protamines. Epigenetics Chromatin 7 (1), 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryden DW, Burton AC, Barnett BR, Cohen VJ, Hearn TN, Jones EA, Kariyil RJ, Kunin A, Kwak SI, Lee J, Lubinski BL, Rao GK, Zhan A, Roesch MR, 2016. Prenatal nicotine exposure impairs executive control signals in medial prefrontal cortex. Neuropsychopharmacology 41 (3), 716–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck JM, O’Neill HC, Stitzel JA, 2019a. Developmental nicotine exposure elicits multigenerational disequilibria in proBDNF proteolysis and glucocorticoid signaling in the frontal cortices, striata, and hippocampi of adolescent mice. Biochem. Pharmacol 168, 438–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck JM, Sanders KN, Wageman CR, Knopik VS, Stitzel JA, O’Neill HC, 2019b. Developmental nicotine exposure precipitates multigenerational maternal transmission of nicotine preference and ADHD-like behavioral, rhythmometric, neuropharmacological, and epigenetic anomalies in adolescent mice. Neuropharmacology 149, 66–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SF, Faraone SV, Rostain AL, Newcorn JH, Antshel KM, Robbins RS, Green JL, 2021. Non-medical use of prescription stimulants among college students: non-oral routes of administration, risk factors, motivations, and pathways. Front. Psychiatry 12, 667118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffall ZF, Wilkes BJ, Hernandez-Martinez R, Rittiner JE, Fox JT, Wan KK, Shipman MK, Titus SA, Zhang YQ, Patnaik S, Hall MD, Boxer MB, Shen M, Li Z, Vaillancourt DE, Calakos N, 2021. The HIV protease inhibitor, ritonavir, corrects diverse brain phenotypes across development in mouse model of DYT-TOR1A dystonia. Sci. Transl. Med 13 (607). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA Jr., Krystal AD, 2016. Kappa-opioid antagonists for psychiatric disorders: from bench to clinical trials. Depress. Anxiety 33 (10), 895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA Jr., Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ, 1998. Regulation of cocaine reward by CREB. Science 282 (5397), 2272–2275. [DOI] [PubMed] [Google Scholar]
- Carrell DT, 2012. Epigenetics of the male gamete. Fertil. Steril 97 (2), 267–274. [DOI] [PubMed] [Google Scholar]
- Centers for Disease C, 1999. Achievements in public health, 1900–1999: tobacco use – United States, 1900–1999. MMWR Morb. Mortal. Wkly Rep 48 (43), 986–993.10577492 [Google Scholar]
- Centers for Disease, C., 2021. Smoking and Tobacco Use (Accessed 10/25/2021 2021).
- Centers for Disease Control and Prevention, Office on Smoking & Health, 2010. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the surgeon general. Chemistry and Toxicology of Cigarette Smoke and Biomarkers of Exposure and Harm. Centers for Disease Control and Prevention (US), Atlanta, GA. https://www.ncbi.nlm.nih.gov/books/NBK53014/. [PubMed] [Google Scholar]
- Centner AM, Bhide PG, Salazar G, 2020. Nicotine in senescence and atherosclerosis. Cells 9 (4), 1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasle V, Riffaud L, Longuet R, Martineau-Curt M, Collet Y, Le Fournier L, Pladys P, 2016. Mild head injury and attention deficit hyperactivity disorder in children. Childs Nerv. Syst 32 (12), 2357–2361. [DOI] [PubMed] [Google Scholar]
- Chavkin C, Koob GF, 2016. Dynorphin, dysphoria, and dependence: the stress of addiction. Neuropsychopharmacology 41 (1), 373–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, Cohen JH, Land BB, 2019. Repeated administration of norbinaltorphimine produces cumulative kappa opioid receptor inactivation. Front. Pharmacol 10, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JS, Chace-Donahue F, Blum JL, Ratner JR, Zelikoff JT, Schwartzer JJ, 2020. Neuroinflammatory and behavioral outcomes measured in adult offspring of mice exposed prenatally to E-cigarette aerosols. Environ. Health Perspect 128 (4), 47006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, 2007. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics 5, 79–94. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL, 2001. Translating developmental time across mammalian species. Neuroscience 105, 7–17. [DOI] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ, 2007. Extrapolating brain development from experimental species to humans. Neurotoxicology 28, 931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook NE, Huang DS, Silverberg ND, Brooks BL, Maxwell B, Zafonte R, Berkner PD, Iverson GL, 2017. Baseline cognitive test performance and concussion-like symptoms among adolescent athletes with ADHD: examining differences based on medication use. Clin. Neuropsychol 31 (8), 1341–1352. [DOI] [PubMed] [Google Scholar]
- Cook NE, Iaccarino MA, Karr JE, Iverson GL, 2020. Attention-deficit/hyperactivity disorder and outcome after concussion: a systematic review. J. Dev. Behav. Pediatr 41 (7), 571–582. [DOI] [PubMed] [Google Scholar]
- Crews D, Gillette R, Scarpino SV, Manikkam M, Savenkova MI, Skinner MK, 2012. Epigenetic transgenerational inheritance of altered stress responses. Proc. Natl. Acad. Sci. U. S. A 109 (23), 9143–9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross SJ, Linker KE, Leslie FM, 2017. Sex-dependent effects of nicotine on the developing brain. J. Neurosci. Res 95 (1–2), 422–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Wang Z, Xu W, Zhang M, Zhu Z, Zhao X, Zhang D, Nie D, Wang L, Qiao Z, 2017. Paternal nicotine exposure defines different behavior in subsequent generation via hyper-methylation of mmu-miR-15b. Sci. Rep 7 (1), 7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajani DR, Uddin LQ, 2015. Demystifying cognitive flexibility: implications for clinical and developmental neuroscience. Trends Neurosci. 38 (9), 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo DV, Bauman BL, Broussard CS, Tong VT, Ko JY, Kapaya M, Harrison L, Ahluwalia IB, 2019. Prevalence and maternal characteristics associated with receipt of prenatal care provider counseling about medications safe to take during pregnancy. Prev. Med 126, 105743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaoli AM, Hurley KM, Yasada K, Reisine T, Bell G, 1994. Distribution of kappa opioid receptor mRNA in adult mouse brain: an in situ hybridization histochemistry study. Mol. Cell. Neurosci 5 (4), 327–335. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, Broide RS, Leslie FM, 2008. Nicotine and brain development. Birth Defects Res. C. Embryo Today 84, 30–44. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM, 2009. The dynamic effects of nicotine on the developing brain. Pharmacol. Ther 122 (2), 125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England LJ, Aagaard K, Bloch M, Conway K, Cosgrove K, Grana R, Gould TJ, Hatsukami D, Jensen F, Kandel D, Lanphear B, Leslie F, Pauly JR, Neiderhiser J, Rubinstein M, Slotkin TA, Spindel E, Stroud L, Wakschlag L, 2017. Developmental toxicity of nicotine: a transdisciplinary synthesis and implications for emerging tobacco products. Neurosci. Biobehav. Rev 72, 176–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone S, Bhide PG, Biederman J, 2018. Neurobiology of attention deficit hyperactivity disorder. In: Charney D, Nestler EJ, Sklar P, Buxbaum JD (Eds.), Neurobiology of Mental Illness. Oxford University Press, New York, NY, pp. 865–878. [Google Scholar]
- Fried R, Chan J, Feinberg L, Pope A, Woodworth KY, Faraone SV, Biederman J, 2016. Clinical correlates of working memory deficits in youth with and without ADHD: a controlled study. J. Clin. Exp. Neuropsychol 38 (5), 487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froggatt S, Reissland N, Covey J, 2020. The effects of prenatal cigarette and e-cigarette exposure on infant neurobehaviour: a comparison to a control group. EClinicalMedicine 28, 100602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba JA, Gysling K, Magendzo K, Andres ME, 2006. Repeated administration of the selective kappa-opioid receptor agonist U-69593 increases stimulated dopamine extracellular levels in the rat nucleus accumbens. J. Neurosci. Res 84 (2), 450–459. [DOI] [PubMed] [Google Scholar]
- Gaub M, Carlson CL, 1997. Gender differences in ADHD: a meta-analysis and critical review. J. Am. Acad. Child Adolesc. Psychiatry 36 (8), 1036–1045. [DOI] [PubMed] [Google Scholar]
- Gavini K, Yang E, Parameshwaran K, 2021. Developmental nicotine exposure impairs memory and reduces acetylcholine levels in the hippocampus of mice. Brain Res. Bull 176, 1–7. [DOI] [PubMed] [Google Scholar]
- Goldberg LR, Gould TJ, 2019. Multigenerational and transgenerational effects of paternal exposure to drugs of abuse on behavioral and neural function. Eur. J. Neurosci 50 (3), 2453–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg LR, Zeid D, Kutlu MG, Cole RD, Lallai V, Sebastian A, Albert I, Fowler CD, Parikh V, Gould TJ, 2019. Paternal nicotine enhances fear memory, reduces nicotine administration, and alters hippocampal genetic and neural function in offspring. Addict. Biol 26 (1), e12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding J, Ellis G, Gregory S, Birmingham K, Iles-Caven Y, Rai D, Pembrey M, 2017. Grand-maternal smoking in pregnancy and grandchild’s autistic traits and diagnosed autism. Sci. Rep 7, 46179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding J, van den Berg G, Northstone K, Suderman M, Ellis G, Iles-Caven Y, Gregory S, Pembrey M, 2020. Grandchild’s IQ is associated with grandparental environments prior to the birth of the parents. Wellcome Open Res. 5, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing LR, Ali RL, Allsop S, Marsden J, Turf EE, West R, Witton J, 2015. Global statistics on addictive behaviours: 2014 status report. Addiction 110 (6), 904–919. [DOI] [PubMed] [Google Scholar]
- Hamatani T, 2012. Human spermatozoal RNAs. Fertil. Steril 97 (2), 275–281. [DOI] [PubMed] [Google Scholar]
- Harvey DM, Yasar S, Heishman SJ, Panlilio LV, Henningfield JE, Goldberg SR, 2004. Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology 175 (2), 134–142. [DOI] [PubMed] [Google Scholar]
- Hawkey AB, White H, Pippen E, Greengrove E, Rezvani AH, Murphy SK, Levin ED, 2019. Paternal nicotine exposure in rats produces long-lasting neurobehavioral effects in the offspring. Neurotoxicol. Teratol 74, 106808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, Naruse H, Yorifuji T, Kato T, Murakoshi T, Doi H, Subramanian SV, 2017. Impact of maternal and paternal smoking on birth outcomes. J. Public Health (Oxf.) 39 (3), 1–10. [DOI] [PubMed] [Google Scholar]
- Iverson GL, Atkins JE, Zafonte R, Berkner PD, 2016. Concussion history in adolescent athletes with attention-deficit hyperactivity disorder. J. Neurotrauma 33 (23), 2077–2080. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Brigman JL, Radke AK, Rudebeck PH, Holmes A, 2016. The neural basis of reversal learning: an updated perspective. Neuroscience 345, 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber RM, Mirbolouk M, DeFilippis AP, Maziak W, Keith R, Payne T, Stokes A, Benjamin E, Bhatnagar A, Blankstein R, Saxena A, Blaha MJ, Nasir K, 2018. Electronic cigarette use prevalence, associated factors, and pattern by cigarette smoking status in the United States from NHANES (National Health and nutrition examination Survey) 2013–2014. J. Am. Heart Assoc 7 (14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Picciotto MR, Heath CJ, Mencl WE, Gelernter J, 2009. Allelic variation of calsyntenin 2 (CLSTN2) modulates the impact of developmental tobacco smoke exposure on mnemonic processing in adolescents. Biol. Psychiatry 65 (8), 671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins TG, James ER, Alonso DF, Hoidal JR, Murphy PJ, Hotaling JM, Cairns BR, Carrell DT, Aston KI, 2017. Cigarette smoking significantly alters sperm DNA methylation patterns. Andrology 5 (6), 1089–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandra KL, Ranney LM, Lee JG, Goldstein AO, 2014. Physicians’ attitudes and use of e-cigarettes as cessation devices, North Carolina, 2013. PLoS One 9 (7), e103462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye S, Sundman MH, Hall EE, Williams E, Patel K, Ketcham CJ, 2019. Baseline neurocognitive performance and symptoms in those with attention deficit hyperactivity disorders and history of concussion with previous loss of consciousness. Front. Neurol 10, 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Shon SH, Joo SW, Yoon W, Lee JH, Hur JW, Lee J, 2019. Gray matter microstructural abnormalities and working memory deficits in individuals with schizophrenia. Psychiatry Investig. 16 (3), 234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klanker M, Feenstra M, Denys D, 2013. Dopaminergic control of cognitive flexibility in humans and animals. Front. Neurosci 7, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein LC, Stine MM, Pfaff DW, Vandenbergh DJ, 2003. Maternal nicotine exposure increases nicotine preference in periadolescent male but not female C57B1/6J mice. Nicotine Tob. Res 5 (1), 117–124. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA Jr., 2010. Dynorphin, stress, and depression. Brain Res. 1314, 56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko TJ, Tsai LY, Chu LC, Yeh SJ, Leung C, Chen CY, Chou HC, Tsao PN, Chen PC, Hsieh WS, 2014. Parental smoking during pregnancy and its association with low birth weight, small for gestational age, and preterm birth offspring: a birth cohort study. Pediatr. Neonatol 55 (1), 20–27. [DOI] [PubMed] [Google Scholar]
- Kolk SM, Rakic P, 2021. Development of prefrontal cortex. Neuropsychopharmacology 47 (1), 41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, 1997. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J. Neurochem 68 (5), 2032–2037. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, 2005. Stimulant actions in rodents: implications for attention-deficit/hyperactivity disorder treatment and potential substance abuse. Biol. Psychiatry 57 (11), 1391–1396. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Melega WP, Cho AK, Segal DS, 1997. Extracellular dopamine and amphetamine after systemic amphetamine administration: comparison to the behavioral response. J. Pharmacol. Exp. Ther 282 (2), 591–596. [PubMed] [Google Scholar]
- Lauterstein DE, Tijerina PB, Corbett K, Akgol Oksuz B, Shen SS, Gordon T, Klein CB, Zelikoff JT, 2016. Frontal cortex transcriptome analysis of mice exposed to electronic cigarettes during early life stages. Int. J. Environ. Res. Public Health 13 (4), 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Briggs SJ, Christopher NC, Rose JE, 1993. Prenatal nicotine exposure and cognitive performance in rats. Neurotoxicol. Teratol 15 (4), 251–260. [DOI] [PubMed] [Google Scholar]
- Ling H, Hardy J, Zetterberg H, 2015. Neurological consequences of traumatic brain injuries in sports. Mol. Cell. Neurosci 66 (Pt B), 114–122. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chen S, Pang D, Zhou J, Xu X, Yang S, Huang Z, Yu B, 2022. Effects of paternal exposure to cigarette smoke on sperm DNA methylation and long-term metabolic syndrome in offspring. Epigenetics Chromatin 15 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Chen LY, 2004. Agonist-induced regulation and trafficking of kappa opioid receptors. Life Sci. 75 (5), 511–536. [DOI] [PubMed] [Google Scholar]
- Ma C, Xi B, Li Z, Wu H, Zhao M, Liang Y, Bovet P, 2021. Prevalence and trends in tobacco use among adolescents aged 13–15 years in 143 countries, 1999–2018: findings from the global youth tobacco surveys. Lancet Child. Adolesc. Health 5 (4), 245–255. [DOI] [PubMed] [Google Scholar]
- Makadia LD, Roper PJ, Andrews JO, Tingen MS, 2017. Tobacco use and smoke exposure in children: new trends, harm, and strategies to improve health outcomes. Curr Allergy Asthma Rep 17 (8), 55. [DOI] [PubMed] [Google Scholar]
- Makris N, Biederman J, Monuteaux MC, Seidman LJ, 2009. Towards conceptualizing a neural systems-based anatomy of attention-deficit/hyperactivity disorder. Dev. Neurosci 31 (1–2), 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Liang L, Biederman J, Valera EM, Brown AB, Petty C, Spencer TJ, Faraone SV, Seidman LJ, 2015. Toward defining the neural substrates of ADHD: a controlled structural MRI study in medication-naive adults. J. Atten. Disord 19 (11), 944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti F, Rowan-Carroll A, Williams A, Polyzos A, Berndt-Weis ML, Yauk CL, 2011. Sidestream tobacco smoke is a male germ cell mutagen. Proc. Natl. Acad. Sci. U. S. A 108 (31), 12811–12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczylo EL, Amoako AA, Konje JC, Gant TW, Marczylo TH, 2012. Smoking induces differential miRNA expression in human spermatozoa: a potential transgenerational epigenetic concern? Epigenetics 7 (5), 432–439. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL, 2003. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J. Neurosci 23 (31), 9981–9986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL, 2006. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A 103 (8), 2938–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Romm E, Gaffney DK, Collins AC, 1986a. Nicotine-induced tolerance and receptor changes in four mouse strains. J. Pharmacol. Exp. Ther 237 (3), 809–819. [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC, 1986b. Dose-response analysis of nicotine tolerance and receptor changes in two inbred mouse strains. J. Pharmacol. Exp. Ther 239 (2), 358–364. [PubMed] [Google Scholar]
- Martin MM, McCarthy DM, Schatschneider C, Trupiano MX, Jones SK, Kalluri A, Bhide PG, 2020. Effects of developmental nicotine exposure on frontal cortical GABA-to-non-GABA neuron ratio and novelty-seeking behavior. Cereb. Cortex 30, 1830–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM, 2007. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology 190 (3), 269–319. [DOI] [PubMed] [Google Scholar]
- McCarthy DM, Bhide PG, 2012. Prenatal cocaine exposure decreases parvalbumin-immunoreactive neurons and GABA-to-projection neuron ratio in the medial prefrontal cortex. Dev. Neurosci 34 (2–3), 174–183. [DOI] [PubMed] [Google Scholar]
- McCarthy DM, Bhide PG, 2021. Heritable consequences of paternal nicotine exposure: from phenomena to mechanisms. Biol. Reprod 105 (3), 632–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DM, Zhang X, Darnell SB, Sangrey GR, Yanagawa Y, Sadri-Vakili G, Bhide PG, 2011. Cocaine alters BDNF expression and neuronal migration in the embryonic mouse forebrain. J. Neurosci 31 (38), 13400–13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DM, Morgan TJ Jr., Lowe SE, Williamson MJ, Spencer TJ, Biederman J, Bhide PG, 2018. Nicotine exposure of male mice produces behavioral impairment in multiple generations of descendants. PLoS Biol. 16 (10), e2006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DM, Lowe SE, Morgan TJ, Cannon EN, Biederman J, Spencer TJ, Bhide PG, 2020. Transgenerational transmission of behavioral phenotypes produced by exposure of male mice to saccharin and nicotine. Sci. Rep 10 (1), 11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy CT, Spindel ER, 2017. Pulmonary effects of maternal smoking on the fetus and child: effects on lung development, respiratory morbidities, and life long lung health. Paediatr. Respir. Rev 21, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Bruchas MR, Chavkin C, 2010. Ligand-directed c-Jun N-terminal kinase activation disrupts opioid receptor signaling. Proc. Natl. Acad. Sci. U. S. A 107 (25), 11608–11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Carroll FI, Beguin C, Carlezon WA Jr., Cohen BM, Grimwood S, Mitch CH, Rorick-Kehn L, Chavkin C, 2011. Duration of action of a broad range of selective kappa-opioid receptor antagonists is positively correlated with c-Jun N-terminal kinase-1 activation. Mol. Pharmacol 80 (5), 920–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao S, Beach ES, Sommer TJ, Zimmerman JB, Jordt SE, 2016. High-intensity sweeteners in alternative tobacco products. Nicotine Tob. Res 18 (11), 2169–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick E, Biederman J, Faraone SV, Sayer J, Kleinman S, 2002. Case-control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy. J. Am. Acad. Child Adolesc. Psychiatry 41 (4), 378–385. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Chen L, Jones J, 1996. Is maternal smoking during pregnancy a risk factor for attention deficit hyperactivity disorder in children? Am. J. Psychiatry 153 (9), 1138–1142. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Jones J, 1998. Further evidence of an association between maternal smoking during pregnancy and attention deficit hyperactivity disorder: findings from a high-risk sample of siblings. J. Clin. Child Psychol 27 (3), 352–358. [DOI] [PubMed] [Google Scholar]
- Miller LL, Henderson J, Northstone K, Pembrey M, Golding J, 2014a. Do grandmaternal smoking patterns influence the etiology of childhood asthma? Chest 145 (6), 1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LL, Pembrey M, Davey Smith G, Northstone K, Golding J, 2014b. Is the growth of the fetus of a non-smoking mother influenced by the smoking of either grandmother while pregnant? PLoS One 9 (2), e86781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Goddings AL, Clasen LS, Giedd JN, Blakemore SJ, 2014. The developmental mismatch in structural brain maturation during adolescence. Dev. Neurosci 36 (3–4), 147–160. [DOI] [PubMed] [Google Scholar]
- Monuteaux MC, Seidman LJ, Faraone SV, Makris N, Spencer T, Valera E, Brown A, Bush G, Doyle AE, Hughes S, Helliesen M, Mick E, Biederman J, 2008. A preliminary study of dopamine D4 receptor genotype and structural brain alterations in adults with ADHD. Am. J. Med. Genet. B Neuropsychiatr. Genet 147B (8), 1436–1441. [DOI] [PubMed] [Google Scholar]
- Mori S, Zhang J, 2006. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51 (5), 527–539. [DOI] [PubMed] [Google Scholar]
- Muhammad A, Kolb B, 2011. Maternal separation altered behavior and neuronal spine density without influencing amphetamine sensitization. Behav. Brain Res 223 (1), 7–16. [DOI] [PubMed] [Google Scholar]
- Munro TA, Berry LM, Van’t Veer A, Beguin C, Carroll FI, Zhao Z, Carlezon WA Jr., Cohen BM, 2012. Long-acting kappa opioid antagonists nor-BNI, GNTI and JDTic: pharmacokinetics in mice and lipophilicity. BMC Pharmacol. 12, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PJ, Guo J, Jenkins TG, James ER, Hoidal JR, Huecksteadt T, Broberg DS, Hotaling JM, Alonso DF, Carrell DT, Cairns BR, Aston KI, 2020. NRF2 loss recapitulates heritable impacts of paternal cigarette smoke exposure. PLoS Genet. 16 (6), e1008756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthumalage T, Prinz M, Ansah KO, Gerloff J, Sundar IK, Rahman I, 2017. Inflammatory and oxidative responses induced by exposure to commonly used e-cigarette flavoring chemicals and flavored e-liquids without nicotine. Front. Physiol 8, 1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano T, Shimizu M, Kiyotani K, Kamataki T, Takano R, Murayama N, Shono F, Yamazaki H, 2010. Biomonitoring of urinary cotinine concentrations associated with plasma levels of nicotine metabolites after daily cigarette smoking in a male japanese population. Int. J. Environ. Res. Public Health 7 (7), 2953–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro HA, Seidler FJ, Whitmore WL, Slotkin TA, 1988. Prenatal exposure to nicotine via maternal infusions: effects on development of catecholamine systems. J. Pharmacol. Exp. Ther 244 (3), 940–944. [PubMed] [Google Scholar]
- Navarro HA, Seidler FJ, Eylers JP, Baker FE, Dobbins SS, Lappi SE, Slotkin TA, 1989. Effects of prenatal nicotine exposure on development of central and peripheral cholinergic neurotransmitter systems. Evidence for cholinergic trophic influences in developing brain. J. Pharmacol. Exp. Ther 251 (3), 894–900. [PubMed] [Google Scholar]
- Nelson LD, Guskiewicz KM, Marshall SW, Hammeke T, Barr W, Randolph C, McCrea MA, 2016. Multiple self-reported concussions are more prevalent in athletes with ADHD and learning disability. Clin. J. Sport Med 26 (2), 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MB, Shytle RD, Sanberg PR, 1999. Locomotor behavioral effects of prenatal and postnatal nicotine exposure in rat offspring. Behav. Pharmacol 10 (6–7), 699–706. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Li GE, Chen H, Cranfield CG, McGrath KC, Gorrie CA, 2018. Maternal E-cigarette exposure results in cognitive and epigenetic alterations in offspring in a mouse model. Chem. Res. Toxicol 31 (7), 601–611. [DOI] [PubMed] [Google Scholar]
- Northstone K, Golding J, Davey Smith G, Miller LL, Pembrey M, 2014. Prepubertal start of father’s smoking and increased body fat in his sons: further characterisation of paternal transgenerational responses. Eur. J. Hum. Genet 22 (12), 1382–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak MK, Ejima K, Quinn PD, Bazarian JJ, Mickleborough TD, Harezlak J, Newman SD, Kawata K, 2020. ADHD may associate with reduced tolerance to acute subconcussive head impacts: a pilot case-control intervention study. J. Atten. Disord 26 (1), 125–139, 1087054720969977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Torres OV, 2014. A mechanistic hypothesis of the factors that enhance vulnerability to nicotine use in females. Neuropharmacology 76 (Pt B), 566–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsivadjian A, Hollocks MJ, Magiati I, Happe F, Baird G, Absoud M, 2021. Is cognitive inflexibility a missing link? The role of cognitive inflexibility, alexithymia and intolerance of uncertainty in externalising and internalising behaviours in young people with autism spectrum disorder. J. Child Psychol. Psychiatry 62 (6), 715–724. [DOI] [PubMed] [Google Scholar]
- Pagani LS, 2014. Environmental tobacco smoke exposure and brain development: the case of attention deficit/hyperactivity disorder. Neurosci. Biobehav. Rev 44, 195–205. [DOI] [PubMed] [Google Scholar]
- Parameshwaran K, Buabeid MA, Karuppagounder SS, Uthayathas S, Thiruchelvam K, Shonesy B, Dityatev A, Escobar MC, Dhanasekaran M, Suppiramaniam V, 2012. Developmental nicotine exposure induced alterations in behavior and glutamate receptor function in hippocampus. Cell. Mol. Life Sci 69 (5), 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly JR, 2008. Gender differences in tobacco smoking dynamics and the neuropharmacological actions of nicotine. Front. Biosci 13, 505–516. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Slotkin TA, 2008. Maternal tobacco smoking, nicotine replacement and neurobehavioural development. Acta Paediatr. 97 (10), 1331–1337. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Sparks JA, Hauser KF, Pauly TH, 2004. In utero nicotine exposure causes persistent, gender-dependant changes in locomotor activity and sensitivity to nicotine in C57Bl/6 mice. Int. J. Dev. Neurosci 22 (5–6), 329–337. [DOI] [PubMed] [Google Scholar]
- Paz R, Barsness B, Martenson T, Tanner D, Allan AM, 2007. Behavioral teratogenicity induced by nonforced maternal nicotine consumption. Neuropsychopharmacology 32 (3), 693–699. [DOI] [PubMed] [Google Scholar]
- Perkins KA, 2001. Smoking cessation in women. Special considerations. CNS Drugs 15 (5), 391–411. [DOI] [PubMed] [Google Scholar]
- Petraglia AL, Dashnaw ML, Turner RC, Bailes JE, 2014a. Models of mild traumatic brain injury: translation of physiological and anatomic injury. Neurosurgery 75 (Suppl. 4), S34–S49. [DOI] [PubMed] [Google Scholar]
- Petraglia AL, Plog BA, Dayawansa S, Dashnaw ML, Czerniecka K, Walker CT, Chen M, Hyrien O, Iliff JJ, Deane R, Huang JH, Nedergaard M, 2014b. The pathophysiology underlying repetitive mild traumatic brain injury in a novel mouse model of chronic traumatic encephalopathy. Surg. Neurol. Int 5, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polli FS, Kohlmeier KA, 2020. Prenatal nicotine exposure in rodents: why are there so many variations in behavioral Outcomes? Nicotine Tob. Res 22 (10), 1694–1710. [DOI] [PubMed] [Google Scholar]
- Polli FS, Ipsen TH, Caballero-Puntiverio M, Osterbog TB, Aznar S, Andreasen JT, Kohlmeier KA, 2020a. Cellular and molecular changes in hippocampal glutamate signaling and alterations in learning, attention, and impulsivity following prenatal nicotine exposure. Mol. Neurobiol 57 (4), 2002–2020. [DOI] [PubMed] [Google Scholar]
- Polli FS, Scharff MB, Ipsen TH, Aznar S, Kohlmeier KA, Andreasen JT, 2020b. Prenatal nicotine exposure in mice induces sex-dependent anxiety-like behavior, cognitive deficits, hyperactivity, and changes in the expression of glutamate receptor associated-genes in the prefrontal cortex. Pharmacol. Biochem. Behav 195, 172951. [DOI] [PubMed] [Google Scholar]
- Ponzoni L, Moretti M, Sala M, Fasoli F, Mucchietto V, Lucini V, Cannazza G, Gallesi G, Castellana CN, Clementi F, Zoli M, Gotti C, Braida D, 2015. Different physiological and behavioural effects of e-cigarette vapour and cigarette smoke in mice. Eur. Neuropsychopharmacol 25 (10), 1775–1786. [DOI] [PubMed] [Google Scholar]
- Popke EJ, Tizabi Y, Rahman MA, Nespor SM, Grunberg NE, 1997. Prenatal exposure to nicotine: effects on prepulse inhibition and central nicotinic receptors. Pharmacol. Biochem. Behav 58 (4), 843–849. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF, 2009. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu. Rev. Neurosci 32, 267–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero RD, Chen WJ, 2004. Gender-related response in open-field activity following developmental nicotine exposure in rats. Pharmacol. Biochem. Behav 78 (4), 675–681. [DOI] [PubMed] [Google Scholar]
- Rose JE, 2006. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology 184 (3–4), 274–285. [DOI] [PubMed] [Google Scholar]
- Rosenthal RN, Slotkin TA, 1977. Development of nicotinic responses in the rat adrenal medulla and long-term effects of neonatal nicotine administration. Br. J. Pharmacol 60 (1), 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy TS, Seidler FJ, Slotkin TA, 2002. Prenatal nicotine exposure evokes alterations of cell structure in hippocampus and somatosensory cortex. J. Pharmacol. Exp. Ther 300 (1), 124–133. [DOI] [PubMed] [Google Scholar]
- Rubinstein ML, Delucchi K, Benowitz NL, Ramo DE, 2018. Adolescent exposure to toxic volatile organic chemicals from E-cigarettes. Pediatrics 141 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell VA, 2011. Overview of animal models of attention deficit hyperactivity disorder (ADHD). Curr. Protoc. Neurosci 9 (Unit9), 35. Chapter. [DOI] [PubMed] [Google Scholar]
- Santiago SE, Huffman KJ, 2012. Postnatal effects of prenatal nicotine exposure on body weight, brain size and cortical connectivity in mice. Neurosci. Res 73 (4), 282–291. [DOI] [PubMed] [Google Scholar]
- Sassano MF, Davis ES, Keating JE, Zorn BT, Kochar TK, Wolfgang MC, Glish GL, Tarran R, 2018. Evaluation of e-liquid toxicity using an open-source high-throughput screening assay. PLoS Biol. 16 (3), e2003904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saw A, Kim J, Lim J, Powell C, Tong EK, 2013. Smoking cessation counseling for asian immigrants with serious mental illness: using RE-AIM to understand challenges and lessons learned in primary care-behavioral health integration. Health Promot. Pract 14 (5 Suppl), 70S–79S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz M, Denardin D, Laufer Silva T, Pianca T, Hutz MH, Faraone S, Rohde LA, 2006. Smoking during pregnancy and attention-deficit/hyperactivity disorder, predominantly inattentive type: a case-control study. J. Am. Acad. Child Adolesc. Psychiatry 45 (11), 1338–1345. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, Kennedy DN, Caviness VS, Bush G, Aleardi M, Faraone SV, Biederman J, 2006. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol. Psychiatry 60 (10), 1071–1080. [DOI] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ, 2013. Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol 106–107, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shacka JJ, Fennell OB, Robinson SE, 1997. Prenatal nicotine sex-dependently alters agonist-induced locomotion and stereotypy. Neurotoxicol. Teratol 19 (6), 467–476. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, Giedd J, Castellanos FX, Rapoport J, 2006. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 63 (5), 540–549. [DOI] [PubMed] [Google Scholar]
- Shaw P, Malek M, Watson B, Sharp W, Evans A, Greenstein D, 2012. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biol. Psychiatry 72 (3), 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein MJ, Faraone SV, Leon TL, Biederman J, Spencer TJ, Adler LA, 2020. The relationship between executive function deficits and DSM-5-defined ADHD symptoms. J. Atten. Disord 24 (1), 41–51. [DOI] [PubMed] [Google Scholar]
- Skinner MK, 2011a. Environmental epigenomics and disease susceptibility. EMBO Rep. 12 (7), 620–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK, 2011b. Role of epigenetics in developmental biology and transgenerational inheritance. Birth Defects Res. C Embryo Today 93 (1), 51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK, Manikkam M, Guerrero-Bosagna C, 2011. Epigenetic transgenerational actions of endocrine disruptors. Reprod. Toxicol 31 (3), 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, 1998. Fetal nicotine or cocaine exposure: which one is worse? J. Pharmacol. Exp. Ther 285, 931–945. [PubMed] [Google Scholar]
- Slotkin TA, 2002. Nicotine and the adolescent brain: insights from an animal model. Neurotoxicol. Teratol 24 (3), 369–384. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, 2004. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol. Appl. Pharmacol 198 (2), 132–151. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Greer N, Faust J, Cho H, Seidler FJ, 1986. Effects of maternal nicotine injections on brain development in the rat: ornithine decarboxylase activity, nucleic acids and proteins in discrete brain regions. Brain Res. Bull 17 (1), 41–50. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Cho H, Whitmore WL, 1987a. Effects of prenatal nicotine exposure on neuronal development: selective actions on central and peripheral catecholaminergic pathways. Brain Res. Bull 18 (5), 601–611. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Orband-Miller L, Queen KL, 1987b. Development of [3H]nicotine binding sites in brain regions of rats exposed to nicotine prenatally via maternal injections or infusions. J. Pharmacol. Exp. Ther 242 (1), 232–237. [PubMed] [Google Scholar]
- Slotkin TA, Orband-Miller L, Queen KL, Whitmore WL, Seidler FJ, 1987c. Effects of prenatal nicotine exposure on biochemical development of rat brain regions: maternal drug infusions via osmotic minipumps. J. Pharmacol. Exp. Ther 240 (2), 602–611. [PubMed] [Google Scholar]
- Slotkin TA, Lappi SE, Seidler FJ, 1993. Impact of fetal nicotine exposure on development of rat brain regions: critical sensitive periods or effects of withdrawal? Brain Res. Bull 31 (3–4), 319–328. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Southard MC, Adam SJ, Cousins MM, Seidler FJ, 2004. Alpha7 nicotinic acetylcholine receptors targeted by cholinergic developmental neurotoxicants: nicotine and chlorpyrifos. Brain Res. Bull 64 (3), 227–235. [DOI] [PubMed] [Google Scholar]
- Smith D, Aherrera A, Lopez A, Neptune E, Winickoff JP, Klein JD, Chen G, Lazarus P, Collaco JM, McGrath-Morrow SA, 2015. Adult behavior in male mice exposed to E-cigarette nicotine vapors during late prenatal and early postnatal life. PLoS One 10 (9), e0137953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubry A, 2018. POHaD: why we should study future fathers. Environ. Epigenet. 4 (2), dvy007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadafora C, 2020. Transgenerational epigenetic reprogramming of early embryos: a mechanistic model. Environ. Epigenet 6 (1), dvaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Madras BK, Faraone SV, Dougherty DD, Bonab AA, Fischman AJ, 2005. In vivo neuroreceptor imaging in attention-deficit/hyperactivity disorder: a focus on the dopamine transporter. Biol. Psychiatry 57 (11), 1293–1300. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Ciccone PE, Madras BK, Dougherty DD, Bonab AA, Livni E, Parasrampuria DA, Fischman AJ, 2006. PET study examining pharmacokinetics, detection and likeability, and dopamine transporter receptor occupancy of short- and long-acting oral methylphenidate. Am. J. Psychiatry 163 (3), 387–395. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Faraone SV, Madras BK, Bonab AA, Dougherty DD, Batchelder H, Clarke A, Fischman AJ, 2013. Functional genomics of attention-deficit/hyperactivity disorder (ADHD) risk alleles on dopamine transporter binding in ADHD and healthy control subjects. Biol. Psychiatry 74 (2), 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TJ, Bhide P, Zhu J, Faraone SV, Fitzgerald M, Yule AM, Uchida M, Spencer AE, Hall AM, Koster AJ, Feinberg L, Kassabian S, Storch B, Biederman J, 2018. The mixed opioid receptor antagonist naltrexone mitigates stimulant-induced euphoria: a double-blind, placebo-controlled trial of naltrexone. J. Clin. Psychiatry 79 (2), 17m11609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD, 2010. An fMRI study of intra-individual functional topography in the human cerebellum. Behav. Neurol 23 (1–2), 65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD, 2012. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage 59 (2), 1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T, 2003. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J. Comp. Neurol 467 (1), 60–79. [DOI] [PubMed] [Google Scholar]
- Tong VT, Dietz PM, Farr SL, D’Angelo DV, England LJ, 2013. Estimates of smoking before and during pregnancy, and smoking cessation during pregnancy: comparing two population-based data sources. Public Health Rep. 128 (3), 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torabi R, Jenkins S, Harker A, Whishaw IQ, Gibb R, Luczak A, 2021. A neural network reveals motoric effects of maternal preconception exposure to nicotine on rat pup behavior: a new approach for movement disorders diagnosis. Front. Neurosci 15, 686767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, O’Dell LE, 2016. Stress is a principal factor that promotes tobacco use in females. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 65, 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA, 2000. An animal model of adolescent nicotine exposure: effects on gene expression and macromolecular constituents in rat brain regions. Brain Res. 867 (1–2), 29–39. [DOI] [PubMed] [Google Scholar]
- Tsujimoto S, 2008. The prefrontal cortex: functional neural development during early childhood. Neuroscientist 14 (4), 345–358. [DOI] [PubMed] [Google Scholar]
- U.S. Surgeon General’s Report, U., 2014. The Health Consequences of Smoking —50 Years of Progress: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Washington, DC. [Google Scholar]
- U.S. Surgeon General’s Report, U., 2016. E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Washington, D.C. [Google Scholar]
- Vaglenova J, Parameshwaran K, Suppiramaniam V, Breese CR, Pandiella N, Birru S, 2008. Long-lasting teratogenic effects of nicotine on cognition: gender specificity and role of AMPA receptor function. Neurobiol. Learn. Mem 90 (3), 527–536. [DOI] [PubMed] [Google Scholar]
- Valera EM, Spencer RM, Zeffiro TA, Makris N, Spencer TJ, Faraone SV, Biederman J, Seidman LJ, 2010. Neural substrates of impaired sensorimotor timing in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry 68 (4), 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Veer A, Bechtholt AJ, Onvani S, Potter D, Wang Y, Liu-Chen LY, Schutz G, Chartoff EH, Rudolph U, Cohen BM, Carlezon WA Jr., 2013. Ablation of kappa-opioid receptors from brain dopamine neurons has anxiolytic-like effects and enhances cocaine-induced plasticity. Neuropsychopharmacology 38 (8), 1585–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC, 2013. Epigenetic inheritance of a cocaine-resistance phenotype. Nat. Neurosci 16 (1), 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Byrnes EM, Pierce RC, 2014. The impact of exposure to addictive drugs on future generations: physiological and behavioral effects. Neuropharmacology 76 (Part B(0)), 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Kollins SH, Wigal TL, Newcorn JH, Telang FW, Fowler JS, Logan J, Wong CT, Swanson JM, 2012. Methylphenidate-elicited dopamine increases in ventral striatum are associated with long-term symptom improvement in adults with attention deficit hyperactivity disorder. J. Neurosci 32 (3), 841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosburg SK, Robbins RS, Antshel KM, Faraone SV, Green JL, 2020. Characterizing pathways of non-oral prescription stimulant non-medical use among adults recruited from reddit. Front. Psych 11, 631792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosburg SK, Faraone SV, Newcorn JH, Rostain AL, Findling RL, Butler SF, Govoni TD, Green JL, 2021. Prescription stimulant nonmedical use among adolescents evaluated for substance use disorder treatment (CHAT). J. Atten. Disord 25 (13), 1859–1870. [DOI] [PubMed] [Google Scholar]
- Whittington JR, Simmons PM, Eltahawy EA, Magann EF, 2018. Bladder stone in pregnancy: a case report and review of the literature. Am. J. Case Rep 19, 1546–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom R, 2007. Effects of nicotine during pregnancy: human and experimental evidence. Curr. Neuropharmacol 5 (3), 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, Utzinger L, Fusillo S, 2008. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J. Am. Acad. Child Adolesc. Psychiatry 47 (1), 21–31. [DOI] [PubMed] [Google Scholar]
- Williams C, Suderman M, Guggenheim JA, Ellis G, Gregory S, Iles-Caven Y, Northstone K, Golding J, Pembrey M, 2019. Grandmothers’ smoking in pregnancy is associated with a reduced prevalence of early-onset myopia. Sci. Rep 9 (1), 15413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer ME, Vassoler FM, White SL, Schmidt HD, Sidoli S, Han Y, Garcia BA, Pierce RC, 2019. Impaired cocaine-induced behavioral plasticity in the male offspring of cocaine-experienced sires. Eur. J. Neurosci 49 (9), 1115–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauk CL, Berndt ML, Williams A, Rowan-Carroll A, Douglas GR, Stampfli MR, 2007. Mainstream tobacco smoke causes paternal germ-line DNA mutation. Cancer Res. 67 (11), 5103–5106. [DOI] [PubMed] [Google Scholar]
- Yochum C, Doherty-Lyon S, Hoffman C, Hossain MM, Zelikoff JT, Richardson JR, 2014. Prenatal cigarette smoke exposure causes hyperactivity and aggressive behavior: role of altered catecholamines and BDNF. Exp. Neurol 254, 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharasiewicz A, 2016. Maternal smoking in pregnancy and its influence on childhood asthma. ERJ Open Res. 2 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Spencer TJ, Biederman J, Bhide PG, 2018. Attention and working memory deficits in a perinatal nicotine exposure mouse model. PLoS One 13 (5), e0198064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zhang D, Dai J, Cao Y, Xu W, He G, Wang Z, Wang L, Li R, Qiao Z, 2020. Paternal nicotine exposure induces hyperactivity in next-generation via down-regulating the expression of DAT. Toxicology 431, 152367. [DOI] [PubMed] [Google Scholar]
- Zhang L, Levenson CW, Salazar VC, Biederman J, Zafonte R, Bhide PG, 2021a. Repetitive mild traumatic brain injury in an awake, unanesthetized mouse model of perinatal nicotine exposure produces transient novelty-seeking and depression-like behaviors. J. Neurotrauma 10.1089/neu.2021.0268. In Press. [DOI] [PubMed] [Google Scholar]
- Zhang L, Levenson CW, Salazar VC, McCarthy DM, Biederman J, Zafonte R, Bhide PG, 2021b. Repetitive mild traumatic brain injury in a perinatal nicotine exposure mouse model of attention deficit hyperactivity disorder. Dev. Neurosci 43 (1), 63–72. [DOI] [PubMed] [Google Scholar]
- Zhang L, McCarthy DM, Eskow Jaunarajs KL, Biederman J, Spencer TJ, Bhide PG, 2021c. Frontal cortical monoamine release, attention, and working memory in a perinatal nicotine exposure mouse model following kappa opioid receptor antagonism. Cereb. Cortex 31 (1), 483–496. [DOI] [PubMed] [Google Scholar]
- Zhu J, Zhang X, Xu Y, Spencer TJ, Biederman J, Bhide PG, 2012. Prenatal nicotine exposure mouse model showing hyperactivity, reduced cingulate cortex volume, reduced dopamine turnover, and responsiveness to oral methylphenidate treatment. J. Neurosci 32 (27), 9410–9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Lee KP, Spencer TJ, Biederman J, Bhide PG, 2014. Transgenerational transmission of hyperactivity in a mouse model of ADHD. J. Neurosci 34 (8), 2768–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JL, Olsen J, Liew Z, Li J, Niclasen J, Obel C, 2014. Parental smoking during pregnancy and ADHD in children: the danish national birth cohort. Pediatrics 134 (2), e382–e388. [DOI] [PubMed] [Google Scholar]
- Zhu J, Fan F, McCarthy DM, Zhang L, Cannon EN, Spencer TJ, Biederman J, Bhide PG, 2017. A prenatal nicotine exposure mouse model of methylphenidate responsive ADHD-associated cognitive phenotypes. Int. J. Dev. Neurosci 58, 26–34. [DOI] [PubMed] [Google Scholar]


