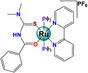
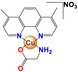
Abstract
Cancer is a heterogeneous and multifactorial disease that causes high mortality throughout the world; therefore, finding the most effective therapies is a major research challenge. Currently, most anticancer drugs present a limited number of well‐established targets, such as cell proliferation or death; however, it is important to consider that the worse progression of cancer toward pathological stages implies invasion and metastasis processes. Medicinal Inorganic Chemistry (MIC) is a young area that deals with the design, synthesis, characterization, preclinical evaluation, and mechanism of action of new inorganic compounds, called metallodrugs. The properties of metallic ions allow enriching of strategies for the design of new drugs, enabling the adjustment of physicochemical and stereochemical properties. Metallodrugs can adopt geometries, such as tetrahedral, octahedral, square planar, and square planar pyramid, which adjusts their arrangement and facilitates binding with a wide variety of targets. The redox properties of some metal ions can be modulated by the presence of the bound ligands to adjust their interaction, thereby opening a range of mechanisms of action. In this regard, the mechanisms of action that trigger the biological activity of metallodrugs have been generally identified by: (a) coordination of the metal to biomolecules (for instance, cisplatin binds to the N7 in DNA guanine, as Pt‐N via coordination of the inhibition of enzymes); (b) redox‐active; and (c) ROS production. For this reason, a series of metallodrugs can interact with several specific targets in the anti‐invasive processes of cancer and can prevent metastasis. The structural base of several metal compounds shows great anticancer potential by inhibiting the signaling pathways related to cancer progression. In this minireview, we present the advances in the field of antimetastatic effects of metallodrugs.
Keywords: cancer, invasion, metallodrugs, metastasis, transition metals
In this review, we examine the use of metallodrugs for cancer treatment, focussing on the advances in this type of therapy and the importance of these compounds, with emphasis on Pt, Ru, Au, Zn, and Cu as antimetastatic agents. In addition, we discuss the different mechanisms of action by which metallodrugs exert their therapeutic effects.

Abbreviations
- ADAMs
a disintegrin and metalloproteinases
- ATF3
activating transcription factor 3
- Cas
casiopeínas®
- CCN1
CCN1 family
- COX‐1
cyclooxygenase‐1
- CYR61
cysteine‐rich angiogenic inducer 61
- DNA
deoxyribonucleic acid
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- EMT
epithelial‐mesenchymal transition
- ER
endoplasmic reticulum
- FN1
fibronectin 1
- HPMC‐NP
nanoplatform formed of hyaluronic acid‐paclitaxel and marimastat/β‐casein complexes
- IL(s)
interleukins
- MAP
mitogen‐activated protein
- MAPK
mitogen‐activated protein kinases
- MIC
medicinal inorganic chemistry
- MMPs
matrix metalloproteinases
- MT1‐MMP
membrane type I‐matrix metalloproteinase
- NSAIDs
nonsteroidal anti‐inflammatory drugs
- PGE2
prostaglandins
- ROS
reactive oxygen species
- SMAD3
SMAD Family Member 3
- SOD
superoxide dismutase
- TGF‐β
transforming growth factor‐β
- TNF‐α
tumor necrosis factor α
- VEGF
vascular endothelial growth factor
- VEGFR‐2
vascular endothelial growth factor receptor 2
- WHO
World Health Organization
Cancer
Cancer is a group of diseases in which the constant proliferation of neoplastic cells results in tumor formation. These tumors can invade the surrounding tissues and spread to distant organs [1, 2]. There are more than 200 different types of cancer and according to the World Health Organization (WHO), cancer is a significant cause of morbidity and mortality worldwide, regardless of the level of human development. By the end of 2020, there were 19,3 million new cancer cases reported and around 10,0 million cancer‐related deaths [3]. In addition, as is mentioned by Hanahan and Weinberg in ‘The Hallmarks of Cancer’ and ‘Hallmarks of: The Next Generation of Cancer’, it is important, to recall that each cancer type has proven to be a complex, heterogeneous, and multifactorial disease, and knowledge of this provides guidelines for a better molecular understanding [2, 4], guiding the development of effective new cancer therapies. Most of the drugs against cancer are directed against a limited number of well‐established targets (proliferation or cellular death), which reflects the difficulty, time, and cost involved in identifying and validating new therapeutic targets [5]. Additionally, it is important to consider that cancer progression toward aggressive pathological stages or worse prognosis involves invasion and metastasis processes.
Invasion and metastasis
Invasion is defined as the movement of a cell to a site or tissue normally occupied by another cell type, generally crossing a basement membrane, whereas metastasis is the establishment of a tumor cell in an organ other than the one of origin, which is not physically connected [2, 6]. Both events allow neoplastic cells to disperse, complicating treatment and causing 90% of cancer deaths [7, 8, 9, 10]. During the dissemination process, neoplastic cells follow a stepwise process known as the metastatic cascade, consisting in local invasion of surrounding tissues, intravasation, survival in the circulatory system, arrest in distant organs, extravasation, and metastasis. Neoplastic cells undergo epithelial–mesenchymal transition (EMT) in order to invade and metastasize. The transforming growth factor‐β (TGF‐β) signaling pathway is crucial, particularly during intermediate stages, to induce EMT. The TGF‐β‐induced EMT has been associated with tumor metastasis, disease recurrence, and an increase in drug and radiation resistance [11, 12]. At a cellular level, EMT is characterized by the loss of cellular adhesion and increased cellular mobility and invasiveness, which results from the cadherin switch from E‐cadherin to N‐cadherin. This switch is responsible for epithelial to fibroblast phenotypic changes, apoptosis resistance, morphological changes, cytoskeleton reorganization, and extracellular matrix degradation [10, 13, 14]. The degradation of the extracellular matrix and basement membrane is essential for invasion and metastasis development. However, some extracellular matrix components, such as interstitial collagen, are resistant to proteolytic cleavage and are only susceptible to matrix metalloproteinases (MMPs) [9, 15], such as gelatinase‐A (MMP2), gelatinase‐B (MMP9), and collagenase‐3 (MMP13). These MMPs are considered indicators of tumor aggressiveness and an unfavorable cancer prognosis [10, 16].
Nowadays, the role of the tumor microenvironment in cancer progression is gaining significant attention. This complex interaction between the stroma and cancer cells results in a dynamic feedback loop with biochemical and biophysical signals that assist the metastatic transition of cancerous cells [12, 17]. In that context a noteworthy protein is cysteine‐rich angiogenic inducer 61 (CYR61) a member of the CCN1 family (CCN1), a matricellular protein that in humans is encoded by the CYR61 gene. CCN1 is an important extracellular matrix protein that participates in various tumorigenic processes, such as cellular adhesion, migration, apoptosis, and angiogenesis. CCN1 regulates the endothelial overexpression of N‐cadherin by activating the nuclear translocation and signaling of β‐catenin. In turn, N‐cadherin facilitates the interaction between cancer and endothelial cells and promotes infiltration of aggressive cells into the lymphatic and blood circulation for metastasis [17]. Another key component of tumor development and metastasis is angiogenesis, a complex process involving the highly regulated interaction between multiple signaling molecules. The vascular endothelial growth factor (VEGF) and its receptor 2 (VEGFR‐2) are important pro‐angiogenic signaling molecules that exert an important role in angiogenesis and metastasis. These molecules promote neovascularization through migration, proliferation, and mobilization of endothelial progenitor cells [18]. In Fig. 1, we summarize the general process of the mechanism of metastasis.
Fig. 1.

General process of metastasis. During the metastasis process, tumor cells follow a series of steps called the metastatic cascade, which consist of local invasion of nearby tissues, intravasation, survival in the circulation, arrest in distant organs, extravasation, and the establishment of metastasis. In order to carry out these processes, malignant cells lose their epithelial characteristics and acquire mesenchymal properties, this process is known as epithelial‐mesenchymal transition (EMT). At the cellular level, EMT is characterized by the loss of cell adhesion, increased mobility and invasiveness, and the secretion of extracellular matrix metalloproteases (MMPs); these changes are caused by the activation of signaling pathways such as TGF‐β or WNT / β‐catenin, increasing the expression of genes such as Snail1, Snail2, Twist, and Zeb‐1. Another key aspect for tumor development and metastasis is angiogenesis, a complex process that involves a highly regulated interaction of multiple signaling molecules. One of the most relevant pro‐angiogenic signaling molecules is vascular endothelial growth factor (VEGF) and its cognate receptor 2 (VEGFR‐2), because it promotes the formation of new blood vessels through cell migration, proliferation, and mobilization of endothelial progenitor cells; in addition, some interleukins play an important role in inflammation and the progression of metastasis, such as IL‐5, IL‐6, IL‐8, IL‐12, and IL‐17A. (Created with BioRender.com.).
Metastasis as a therapeutic target
The development of new therapeutic approaches specifically designed to prevent or treat metastasis must consider the genetic and phenotypic differences of metastatic cells. An alternative approach could be to target the neoplastic cell metastatic‐enabling mechanisms, that is, protease liberation, EMT, and angiogenesis.
An example of this approach is the nanoplatform formed of hyaluronic acid‐paclitaxel and marimastat/β‐casein complexes (HPMC‐NP). HPMC‐NP reduced the expression and activity of MMPs in 4T1 and tumor cells; it also inhibited tumor growth and suppressed the development of metastasis and angiogenesis in the 4T1 metastatic breast cancer model [19].
Furthermore, the TGF‐β signaling pathway is a potential therapeutical target due to its pleiotropic functions, which regulate cellular growth, differentiation, apoptosis, motility, invasion, and angiogenesis. Inhibiting this signaling pathway could improve survival in metastatic cancer patients; Galunisertib, one of the most promising drugs in clinical phase, is a small‐molecule selective inhibitor of the TGF‐β receptor type I (RI), a serine/threonine kinase, and thus inhibits the TGF‐β signaling pathway; this drug shows potent anti‐invasive activity [20]. Additionally, Galunisertib showed antiproliferative activity in ex vivo models, which suggests that inhibition of TGF‐β has antitumor effects, and that the tumor microenvironment mediates these effects [20, 21]. In the same way, angiogenesis has emerged as a therapeutic target due to the importance of blood vessels in cancer development and metastasis. To date, several of the drugs used to treat different types of cancer, such as Apatinib, Axitinib, Bevacizumab, Imatinib, and Ramucirumab, inhibit angiogenesis through VEGF and other angiogenic factors [18].
Overall, metastasis represents a complex challenge due to its systemic process. The use of more than one therapeutic agent is required for its effective inhibition. Therefore, combination or multiple‐target therapies are essential to stop metastasis development.
History of metallodrugs and their use in cancer
The use of metals for medicinal purposes has been practiced since the time of ancient civilizations, including Egyptian, Chinese, Greek, and Roman. Copper was used to sterilize wounds [22], gold to treat the skin of people affected by smallpox and skin ulcers [23], and silver to cure wounds and infections [24]. Nowadays, it is possible to find several commercial inorganic compounds for the treatment of various health problems. For example, aluminum hydroxide, aluminum carbonate, calcium carbonate, and sodium bicarbonate are used to treat stomach afflictions [25]; auranofin is prescribed to treat arthritis [26]; and bismuth subsalicylate used to treat gastrointestinal diseases [27].
Regarding cancer, treatment has three fundamental pillars: surgery, radiotherapy, and chemotherapy. The latter is the most common option, and could be a key approach to stop the metastatic process due to its systemic approach [6]. Cisplatin, an antineoplastic chemotherapy agent, is a ‘metalating’ compound with a square planar structure and a central platinum ion. The term ‘methylating’ is used for organic molecules and denotes the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group; however metalating in the case of metallodrugs is the union formed between a metal atom and biomolecules to form M‐biomolecules [28, 29]. Cisplatin was the first member of the platinum‐based metallodrug family. This family also includes carboplatin and oxaliplatin, used to treat testicular, ovarian, breast, cervical, gastric, colon, prostate, and small‐cell and non‐small‐cell lung cancer [30]. Arsenic trioxide, another metallodrug, is used to treat adult patients with acute promyelocytic leukemia [31]. For example, the superoxide dismutase (SOD) biomimetic activity of the manganese‐based M‐404003 and Ca4[Mn(DPDP)5] compounds, have been evaluated in clinical studies to determine their potential use as analgesics in postsurgery treatments [32]. Other examples of clinically relevant metallodrugs are photoactivatable metal anticancer drugs and drug candidates as mentioned by Anthony et al., in 2020, for example, Photosens® (Al III), TOOKAD® soluble (Pd II), approved in clinical phase III and TLD‐1433 (Ru II), which is in clinical phase II [33].
Platinum compounds
Throughout history, some positive health effects of inorganic compounds have been discovered serendipitously; one of the most referenced examples among inorganic chemists is the compound cis‐[PtCl2(NH3)2], known as cisplatin. With the initial aim of studying the influence of electric fields on the mitosis of Escherichia coli, Barnett Rosenberg et al., in 1960 [30], observed that bacteria stopped replicating in the presence of platinum electrodes and wrongly proposed that one of the compounds generated from the experimental materials and conditions, [(NH4)2][PtCl6], was responsible for the observed inhibition [34, 35]. Many experiments later they found that UV light caused a series of chemical reactions in the solution and then identified the [PtCl4(NH3)2] complex as the active chemical. Subsequently, knowing that neutral platinum compounds with a cis conformation are more active complexes, they altered the oxidation state of platinum to synthesize the cis‐[PtCl2(NH3)2] compound; this showed excellent biological activity, thus raising the prospect of studying the antitumor properties of this compound [36, 37].
Further studies focused on evaluating the effect of this compound on tumor cell lines. Eventually, the first inorganic antineoplastic compound for clinical use emerged [30]. Cisplatin activates both the intrinsic mitochondrial pathway and the extrinsic death receptor pathway of apoptosis (Fig. 2A). Cisplatin inspired numerous inorganic chemists to generate new metal‐based compounds. In the last few decades, the studies performed by inorganic chemists have allowed the development of new metal‐based drugs, thereby completing the basic and nonclinical research studies required to initiate human clinical studies.
Fig. 2.

(A) Mechanism of action of cisplatin. Modern studies have revealed that copper transporter protein CTR1 is responsible for cisplatin uptake. Cisplatin activates both the intrinsic mitochondrial pathway and the extrinsic death receptor pathway of apoptosis. In addition, ER stress may also be induced. Among the three pathways, the intrinsic pathway, involving the mitochondria, is the major one. The administration of cisplatin causes cellular stress and results in the alteration of the mitochondrial membrane, leading to the release of apoptogenic factors such as apoptosis‐inducing factor (AIF), endonuclease G and cytochrome C, from the mitochondria into the cytosol. After being released from the mitochondria, endonuclease G and AIF accumulate in the nucleus, leading to apoptosis in a caspase‐independent manner while the released cytochrome C binds to the adaptor protein Apaf‐1 and induces its conformational changes, activating caspase 9, which, in turn, leads to activation of several downstream caspases for caspase‐dependent apoptosis. The ER‐stress pathway is also involved in apoptosis during cisplatin administration. Caspase 12, which localizes at the cytosolic face of the ER and is activated by ER stress, is the key initiator caspase in the ER pathway and in the extrinsic pathway. Binding of the death receptors by ligands results in the recruitment and activation of caspase 8, which leads to the activation of downstream caspases to trigger apoptosis. Furthermore, cisplatin induces oxidative stress by triggering the formation of reactive oxygen species (ROS), such as hydroxyl radical and, superoxides, which depends on the concentration of cisplatin and time of exposure. ROS are thought to be responsible for peroxidation of lipids, depletion of sulfhydryl groups, and alterations in various signal transduction pathways, which can cause DNA damage and consequently apoptosis of cells. Although cisplatin can bind to various biomolecules, it is generally considered that DNA is the major biological target, forming adducts. Adapted from ‘Apoptosis Extrinsic and Intrinsic Pathways’, by BioRender.com (2020) (Retrieved from https://app.biorender.com/biorender‐templates). (B) Platin drugs in the general process of metastasis. Cisplatin induces the expression of the transcription regulation factor ATF3, which suppresses a variety of genes related to the rearrangement of the cytoskeleton, the extracellular matrix, filopodia, and cell adhesion, including TGFβ/SMAD3 signaling and the β‐catenin signaling pathway (and consequently compromises MET), and cell migration in vitro and cancer metastasis in vivo. Similarly, PIP platinum can increase cell adhesion and block cell migration/invasion by inhibiting the Wnt signaling pathway due to nuclear translocation of β‐catenin (which is necessary for the activation of Wnt signaling) and inducing the translocation of β‐catenin in the cell membrane, which favors cell adhesion through E‐Cadherin. (Created with BioRender.com.).
Currently, cisplatin is one of the chemotherapy agents used to treat cancer in situ; it is also used in combined therapies against metastatic cancer, with favorable results. For example, Franciosi et al. (2011) in a prospective study evaluated a cisplatin/etoposide therapy against brain metastases derived from breast carcinoma, non‐small‐cell lung carcinoma, and melanoma in patients previously subjected to radiotherapy. This study observed that the cisplatin/etoposide combined therapy is effective in patients with brain metastasis derived from breast cancer and non‐small‐cell lung cancer [38].
Furthermore, in a small nonclinical study, tocilizumab increased the cytotoxic effects of cisplatin in vitro and in vivo in a triple‐negative breast cancer model by inhibiting the EMT and increasing apoptosis. These results indicate that tocilizumab/cisplatin combined therapy can suppress the metastatic effect of highly aggressive breast cancer cells [39].
Recently, a study reported various findings regarding the role of cisplatin in the EMT. Cisplatin induces the expression of activating transcription factor 3 (ATF3), which suppresses various genes associated with cytoskeleton rearrangement, the extracellular matrix, filopodia, and cellular adhesion, including the TGFβ/SMAD3 signaling pathway. This inhibition suppresses the transcription of Fibronectin 1 (FN1), which regulates the EMT and cellular migration. In a neoadjuvant chemotherapy setting, cisplatin and paclitaxel block cancer metastasis by inhibiting the colonization of target organs with neoplastic cells and cancer growth. Based on these findings, a model has been proposed in which cisplatin activates ATF3, inhibiting the positive reciprocal regulation loop between FN1 and TGF‐β, thus blocking the EMT, and thereby compromising cellular migration in vitro and metastasis in vivo [40] (Fig. 2B). Similarly, it is important to mention that in a study by He et al. 2018, carboplatin has been shown to promote apoptosis and inhibit HN‐3 cell migration. [41]
Despite its effectiveness against a considerable number of tumors, cisplatin has serious side effects, such as nephrotoxicity, myelotoxicity, ototoxicity (tinnitus), peripheral neuropathy, and gastrointestinal problems, which affect the patient's quality of life [42].
The search for less toxic treatments led to the development of new generation drugs. Carboplatin, a second‐generation drug, is used to treat ovarian, head and neck, breast, testicular, brain, gallbladder, cervical, and small‐cell lung cancer. Carboplatin is less ototoxic, nephrotoxic, and neurotoxic than cisplatin. However, the side effects associated with neurotoxicity significantly increase with age, which is why it is used to treat pediatric neuroblastoma. Additionally, hearing loss characteristic of cisplatin treatment is not observed in patients treated with carboplatin. Moreover, the main adverse effect that limits the maximum dose to be administered is thrombocytopenia [43, 44].
Oxaliplatin is less effective, as well as less cytotoxic, nephrotoxic, and ototoxic than cisplatin. Additionally, thrombocytopenia is less frequent than with carboplatin. Its most significant limitation is that it causes peripheral neuropathy, affecting the sense of touch. This is accompanied by numbness and tingling after exposure to the cold. This drug is mainly used to treat colon cancer, even after metastasis. Finally, it is important to emphasize that gastrointestinal effects are common when using this group of drugs [43, 44, 45].
Several chemotherapy agents inhibit cancer progression with serious side effects. Additionally, some types of tumors are resistant or refractory to cisplatin treatment. Therefore, the study of metal‐based compounds with antineoplastic effects has been promoted [42, 46]. Ideally, these compounds would avoid some of the most severe side effects of current chemotherapy while also seeking a possible cost reduction. Among these new metallodrugs, those composed of metals such as ruthenium, gold, zinc, and copper stand out. In this section, we will review some of the most promising metallodrug candidates.
Ruthenium compounds
Some of the ruthenium‐based molecular complexes have an electrostatic affinity for DNA and can reversely bind to the double helix [47]. For this reason, tumor cell cycle arrest at different checkpoints is expected, resulting in apoptosis. Consequently, these molecular complexes are considered potential candidates for synthesizing new antineoplastic molecules [48]. The NAMI‐A, KP1019, and KP1339 complexes are being evaluated in clinical trials [49]. Although the mechanisms of action of these compounds are still under study, several research groups have reported interesting findings. For example, their mechanism of action is not only through DNA binding; these compounds can also generate reactive oxygen species (ROS) and inhibit protein kinases. Furthermore, these complexes have shown some selectivity and the ability to overcome the resistance faced by platinum‐based therapeutic agents.
NAMI‐A is selective for metastatic cancer cells and acts through the TGF‐β pathway [50]. KP1019, also known as FFC14A, induces significant upregulation of 284 genes and downregulation of 76 genes, some of which are associated with cell cycle arrest through ROS, mitogen‐activated protein (MAP) kinases [51], and chromatin assembly. KP1339 is being evaluated in solid tumors, such as non‐small‐cell lung cancer and colorectal carcinoma, and gastrointestinal neuroendocrine tumors. Although the mechanism of action is unclear, reports indicate that oxidative stress‐related molecules are involved [52]. However, the interaction of these compounds in metastatic systems has not been sufficiently studied.
Gold compounds
In the last few decades, researchers have focused on the study of coated gold nanoparticles. Due to their size, optical properties, chemical stability, and biocompatibility, these nanoparticles are promising candidates for several biomedical applications, including cancer treatment [53]. Therefore, gold‐based compounds with antineoplastic potential have been recently synthesized.
Some interesting Au (I) phosphane antitumor compounds have been reported, such as [Au(d2pypp)2]Cl, [Au(PPh3)]Cl, [Au2(dppe)]Cl2, and [Au3(dpmp)]Cl3 [26]; in addition, a heterometallic compound [(η‐C5H5)2TiMe(μ‐mba)Au(PR3)] has been reported [54], with its mode of action identified as mitochondrial dysfunction or autophagy [54, 55, 56, 57].
Bis‐[4,5‐dichloro‐(N‐methyl‐N’(2‐hydroxy‐2‐phenyl)ethyl‐imidazole‐2‐ylidene)gold(I)][dichloro‐gold] (AuL7) is a gold‐based compound with potential antimetastatic activity in the breast cancer metastatic cell line MDA‐MB‐231. This compound inhibits tubulin polymerization and topoisomerase II; it also increases oxidative stress and caspases 3, 7 and 9, which causes cellular arrest at the G2‐M checkpoint, resulting in apoptosis [58].
Furthermore, the synthesis of hybrid metallic structures to increase therapeutic efficacy has been explored due to their unusual properties compared to their building units, with different physicochemical properties [53]. The study of these hybrid compounds has broadened their application scope and improved their overall yield. The results of these studies suggest that incorporating two different biologically active metals in the same molecule improves antitumor activity due to the specific interactions between the metals and their different biological targets (cooperative effect) or the improved physicochemical properties of the heterometallic compound (synergism) [59].
Zinc compounds
The study of new metallodrugs changed its focus to biological compounds formed by essential metals, due to the fact that there are biological mechanisms for their absorption and elimination, as well as a greater capacity for interaction with biochemical processes, particularly metals such as zinc and copper. For example, the active sites of most phosphatase enzymes have bivalent metallic ions and exhibit ease of ligand exchange, flexibility of the coordination environment, and physiological abundance (zinc is the second most abundant metal ion in biological systems).
Dasgupta et al. (2020) reported the cytotoxicity of zinc complexes as anticancer agents in different tumor cell lines, such as HCT116 (human colorectal carcinoma), HepG2 (human hepatocellular carcinoma), and A549 (non‐small‐cell lung carcinoma), with significant levels of DNA fragmentation [60].
Copper compounds
Copper (II) metallodrugs have emerged as an attractive chemotype against cancer due to their ability to generate ROS and reactive nitrogen species, resulting in oxidative damage and cellular death. Some of these copper complexes inhibit topoisomerases or can bind to DNA. Furthermore, these complexes can also affect cell cycle checkpoints and death effector proteins [61]. Casiopeínas® (Cas), a family of copper(II)‐containing compounds, have a diimine (N‐N) type bidentate ligand in their coordination sphere, that is, phenanthroline or bipyridine; and the second charged ligand is of N‐O type (α‐aminoacidate) or O‐O donor (acetylacetonate or salicylaldehyde). Casiopeínas are soluble in water, methanol, and dextrose solution intravenous. The antitumor activity of these compounds has been described in different in vitro and in vivo cancer models [62, 63]. As for the mechanism of action, Casiopeínas induces apoptosis by increasing endonuclease G, DNA fragmentation, and activating caspase 3 [64]. They also increase mitochondrial ROS and release of cytochrome C [65]. Recently, other research groups have synthesized new copper(II)‐containing coordination compounds with a similar structure to Casiopeínas. These compounds have shown cytotoxic and antiproliferative activities in different cancer cell lines, such as osteosarcoma (HOS), breast cancer (MCF7, MDA‐MB‐231), melanoma (G361, 518A2), colon cancer (HCT‐116), cervical cancer (HeLa), ovarian carcinoma (A2780, SKOV‐3), and cisplatin‐resistant ovarian carcinoma (A2780R) [66, 67, 68]. Phenanthroline, doxycycline, and some flavonoids are among their main ligands. In Table 1, we present a list of the most studied metallodrugs for cancer treatment.
Table 1.
Metallodrugs most studied for cancer treatment.
| Metal | Compound Name/Structure | Cancer types and cell lines | IC50 range | Action mechanisms | Migration and invasion | Metastasis | Refs. |
|---|---|---|---|---|---|---|---|
| Platin |
[Pt(C6H6O4)(NH3)2] (Carboplatin) |
Breast cancer (MCF7 and MDA‐MB‐231) |
DNA damage Apoptosis Generates increased ROS |
Decreases fibronectin‐1 (FN1) critical for activation of the TGFβ / SMAD3 signaling pathway, Decreases the expression FN1, vimentin, and inhibits β‐catenin Inhibits migration potential and invapod formation even in cells stimulated by TGF‐β |
Antitumor and antimetastatic activity in murine breast cancer model (4T1) It is conventionally used in combination with other drugs (Etoposide, Tocilizumab, etc.) to treat metastatic cancer |
[39, 40, 41] | |
|
[Pt(C35H40N3O4)(C18H15P)]ClO4 (PIP‐platin) |
Cervical carcinoma (HeLa), hepatocellular carcinoma (HepG2), breast cancer (MCF7), lung adenocarcinoma epithelial (A549), cisplatin‐resistant (A549/DDP). |
8–13 µm 30.50 µm |
Viability DNA damage Apoptosis Generates increased ROS |
Inhibits cell migration (Scratch assay) and invasion (Transwell assay) Inhibits the WNT signaling pathway and b‐catenin translocation |
Antitumor activity in murine breast cancer (4T1) | [75] | |
| Ruthenium |
Na[RuCl4(C2H6OS)(C3H4N2)] (NAMI) (C3H5N2)[RuCl4(C2H6OS)(C3H4N2)] (NAMI‐A) Na[RuCl4(C7H6N2)2] (KP1339) (C3H5N2)[ RuCl4(C7H6N2)2] (1019) |
Cervical carcinoma (HeLa) Colon (HCT116 and SW480) |
Generation of reactive oxygen species (ROS) in HCT116 and SW480 cells |
Transcriptome | Antitumor and antimetastatic activity in a murine model of ovarian cancer | [29, 71] | |
|
[Ru(C18H15P)2(C10H12N2OS)(C10H8N2)]PF6 (Ru(PPh3)2(O‐S)(bpy)PF6 |
Breast cancer (MCF7, MCF‐10A, and MDA‐MB‐231) |
8.81‐14.82 µm |
Viability Apoptosis (Caspase 3 and DNA damage) |
Reduces the expression of β1‐integrin, EGFR, p38 MAPK, and the activity of MPP2 | [81] | ||
|
[Ru3(O)(C2H3O2)5(C12H7N2)(C5H5N)2]PF6 [Ru3(O)(OAc)5(phen‐)(py)2]PF6 |
Murine melanoma (B16F10) | 25 µm | Interaction with the DNA double helix. | [79] | |||
| Ruthenium |
[Ru(C20H24N3)( C12H8N2)(CH3CN)2](PF6)2 [Ru(N‐C)(phen)(CH3CN)2](PF6)2 Ru8 |
Lung cancer (A549, A549 / cisR) Ovarian cancer (A2780) Liver cancer (Huh‐7) Murine melanoma (B16‐F10) |
6.9‐25 µm |
Viability Cell cycle arrest Apoptosis (caspase 9 and caspase 3) |
Decreases expression of MPP9 and EGFR‐p Inhibits angiogenesis |
Decreases angiogenesis in chicken embryos. Antitumor and antimetastatic activity in a murine model Ovarian cancer |
[71] |
|
[Ru(C15H13O3)( C25H22P2)2]PF6 Ru(Lap)(dppm)2]PF6 |
Breast cancer MDA‐MB‐231 |
2.7 µm |
Apoptosis Affects mitochondrial membrane potential |
Inhibits cell migration and chemotaxis (Scratch assay Boyden‐Chamber Assay) | [80] | ||
|
[Ru4(C10H14)4(C24H18N4O2)Cl6] Ru1
[Ru4(C10H14)4(C14H10N4O2)Cl6] Ru2 |
Cancer de pulmón (A549, A549cisR) Mama (MCF‐7) colon (LoVo) Hígado (HuH‐7) |
1.39– 17.24 | Apoptosis and cell cycle detection | Inhibits cell migration and invasion (Scratch assay and Transwell assay) | Less toxicity in contrast to cisplatin | [69] | |
| Ruthenium |
[Ru(CH3CO2)(C28H28P2)(C10H8N2)]PF6 [Ru(OAc)(dppb)(bipy)]PF6 |
Breast cancer (MCF7, MCF‐10A and MDA‐MB‐231) |
31.6–200 µm |
Affects cell morphology and cytoskeleton structure Apoptosis (Caspase 9, Caspase 3 and BCL‐2) Interaction with DNA |
Inhibits cell migration, invasion, and chemotaxis (Scratch assay, Boyden‐Chamber assay and Transwell assay) Reduces MPP2 activity |
[70] | |
| Gold |
[Au(C38H42N2)2][AuCl2] AuL6
[Au(C22H26N2][AuCl2] AuL7 |
Breast cancer (MDA‐MB‐231) |
2.10 µm |
Increase in reactive oxygen species Cell cycle arrest in G2 / M Activation of caspases 3/7 and 9 |
Inhibits tubulin polymerization and topoisomerase II activity | [58] | |
|
[Cl2(C10H14)Ru(C25H22P2)Au(C21H24N2)]ClO4 RANCE‐1 |
Human clear‐cell renal cell carcinoma (Caki‐1) | 8.7 µm |
Viability Apoptosis Cell cycle arrest |
Inhibits cell migration and invasion (Scratch assay and Transwell assay) Inhibition of VEGF secretion |
[66] | ||
| Gold |
[Au(C6H15P)(C14H19O9S)] Auranofin
[(C5H5)2(CH3)Ti(C7H4O2S)Au(C18H15P)] Titanocref
[(C5H5)2(CH3)Ti(C7H4O2S)Au(C6H15P)] Titanofin |
Clear cell renal cell carcinoma (Caki‐1) | 0.097– 2.8 µm |
Viability Apoptosis Cell cycle arrest |
Inhibits cell migration, chemotaxis and invasion (Scratch assay and Transwell assay) Inhibits angiogenesis: in human umbilical vein endothelial cells (HUVEC) in an ECM‐like matrix based on the length of the uninterrupted tubes (TL) and the number of branch points or nodes in the tubes (TN) Inhibition of VEGF Decreases the expression of pro‐inflammatory and prometastatic cytosines (TNF‐α, and interleukins) also decreases the expression of MMPs |
[78] | |
| Zinc |
[Zn(C12H6N2O2)(C15H14NO2)2] [Zn(phendione)(MFN)2]
[Zn(C12H6N2O2)(C14H14O3)2(H2O)2] [Zn(phendione)(NPR)2(H2O)2] |
Breast cancer (MDA‐MB‐231) |
≈ 1 µm | Caspase‐mediated apoptosis (Caspase 3, 8, and 9) |
Inhibits cell migration (Scratch assay) Decreases Vimentin and β‐Integrin Inhibits EMT Anti‐inflammatory Selective inhibition of COX‐1 and Prostaglandins |
[82] | |
| Zinc |
[Zn(C15H9O7)2] Q‐ZnCPX |
Urinary bladder transitional cell carcinoma (BFTC‐905) |
≈ 75 µm | Viability and proliferation |
Inhibits cell migration, chemotaxis and invasion (Scratch assay and Transwell assay) Decreases MMP14 and AKT‐P PI3K / AKT / mTOR pathway |
[83] | |
|
[Zn(C19H14N3O)2] [Zn(bimnap)2]
[Zn(C12H10N5)₂] [Zn(BnI)₂] |
Liver cancer (HepG2), skin cancer (SK‐MEL‐1), colon cancer (HT018) cervical cancer (Hela) and breast cancer (MDA‐MB‐231) |
6.66– 15.8 µm |
Viability Apoptosis |
Inhibits chemotaxis and cell invasion (Transwell assay) | [73] | ||
| 19–26.7 µm |
Viability Apoptosis |
Inhibits chemotaxis and cell invasion (Transwell assay) | [72] | ||||
| Copper |
[Cu(C2H4NO2)(C14H12N2)]NO3 Casiopeína II‐gly |
Ovarian cancer (CH1) Murine leukemia (L1210) Cervical cancer (HeLa, SiHa and CasKi) Breast cancer (MCF‐7) Colon (HCT15) Glioblastoma (C6, U87MG) Murine melanoma (B16) Lung cancer (A549, H157 and SKLU) and Neuroblastoma (CHP‐212, SK‐N‐SH) |
1 µm– 10 µm |
Inhibits viability and proliferation Apoptosis (Caspase‐3, Endonuclease G, Cytochrome C) Increased ROS in mitochondria |
Reduces the expression of proteins such as β‐catenin, GSK‐3β, Dvl affecting the Wnt signaling pathway Decreases the expression of genes related to migration such as TGFβ‐R1, AURKA, SNAI2, BMP4, BMP6, and N‐Cadherin |
[42, 87, 88, 89] | |
|
[Cu(C5H7O2)(C12H12N2)]NO3 Casiopeína III‐ia |
10 – 60 µm | Inhibits cell migration | [86] | ||||
|
[Cu(C5H7O2)(C14H12N2)]NO3 Casiopeína III‐La |
Cervical cancer (HeLa, SiHa) Colon (HCT15) Brain Tumor (glioma U373) Breast cancer (MCF‐7) |
1.7–4.3 µm |
Exerts an antiproliferative effect, promoting apoptotic cell death and inactivating the invasive process (wound healing and Transwell invasion assays) by generating ROS, inactivating GSK3β, activating JNK and ERK, and promoting the nuclear accumulation of β‐catenin. Decrease the levels of MMP9 and MMP2 and increases E‐cadherin expression |
||||
|
[Cu(C7H5O2)2] [Cu(trp)2] |
Breast cancer (MCF7 and MDA‐MB‐231) |
4–5.2 µm |
Viability Apoptosis |
Decrease MMP2 and MPP9 Inhibits cell migration and 3D cell culture invasion (Scratch assay) |
[74] | ||
|
[Cu(C12H10N5)₂] [Cu(BnI)₂] |
Liver cancer (HepG2), skin cancer (SK‐MEL‐1), colon cancer (HT018) cervical cancer (Hela) and breast cancer (MDA‐MB‐231) | 3.5–17.8 µm |
Viability Apoptosis |
Inhibits chemotaxis and cell invasion (Transwell assay) | [72] | ||
| Copper |
[Cu(C10H10NO5SBr)(C12H8N2)] [Cu(O‐N‐O)(phen)] |
cervical cancer (Hela and C33A) | 3.5–17.8 µm |
Viability/ Apoptosis (BCL‐2, Bax and Caspase 3) |
Inhibits cell migration, chemotaxis, and invasion (Scratch assay and Transwell assay) Inhibits cell migration and 3D cell culture invasion Reduces the expression of VEGFR‐2, FAK, AKT, AKT‐p |
In vivo antitumor activity (mice) and antiangiogenic (chicken embryos) | [93] |
|
[Cu2(C16H17N2O2Cl)2Cl2] [Cu2(BPClNOL)2Cl2] |
Neuroblastoma (H4) | N/A | Cell cycle arrest |
Inhibits chemotaxis and cell invasion (Transwell assay) Reduces invasion in 3D cultures, reduces gene expression: snail vimentin and increases E‐cadherin expression |
[85] | ||
|
[Cu(C39H39N3OP)Cl2]Br [Cu(CPT8)Cl2]Br |
Cervical cancer (HeLa), ovarian cancer (SKOV‐3), kidney cancer (HK‐2), and melanoma (B16F10) | 6.5– 21.57 µm | Viability/Cell cycle arrest |
3D cell culture invasion Reduces the expression of MMP2 and VEGFR1 |
[67] | ||
| Copper |
[Cu(C22H22N2O8)(C12H8N2)(H2O)2]NO3 [Cu(doxycycline)(phen)(H2O)2]NO3 |
Cervical cancer (HeLa) and breast cancer (MCF‐7) | 0.5– 0.87 µm | Viability |
Inhibits cell migration (Transwell assay) Reduces the expression of MMP2 |
[66] | |
|
[Cu(R1R2C9H3O4)2] |
Melanoma (518A2), colon cancer (HCT‐116), cervical cancer (KB‐V1 / Vbl), breast cancer (MCF‐7) and skin fibroblasts (HF). | 6.7– 50 µm | Viability/Cell cycle arrest |
Inhibits cell migration (Scratch assay) Reduces the expression of MMP2 and MMP7 β‐actin |
[68] |
Metallodrugs against migration and metastatic processes
Several studies have reported the inhibition of cancer migration and metastasis processes using metallodrugs. For example, ruthenium complexes and arene ligands have been successfully applied in different cancer cell lines, such as lung carcinoma (A549), MCF‐7, colon adenocarcinoma (LoVo), and hepatocarcinoma (HuH‐7), decreasing viability and proliferation, and inducing apoptosis. Furthermore, these complexes also decrease cellular migration and invasion [69]. Another ruthenium‐based compound, [Ru(OAc)(dppb)(bipy)]PF6, induces apoptosis (through activation of caspases −9 and −3, DNA interaction, and Bcl‐2 subexpression) and affects cellular morphology and cytoskeleton structure; it also decreases migration, chemotaxis, invasion, and MPP2 activity [70]. Additionally, Wang et al. (2020) reported that compound Ru8 efficiently inhibits the metastatic process against tumor cell invasion and migration, but also has potent antiangiogenic effects in vitro and in vivo models. In a metastatic A2780 tumor xenograft‐bearing mouse model, Ru8 administration outperformed NAMI‐A compound and cisplatin in its antitumor potential and metastasis inhibition capacity (Table 1) [71].
Turning to zinc complexes, one with ‘bimnap’ type ligands derived from 1‐methyl‐2‐aminobenzimidazole and 2‐hydroxynaphthaldehyde, and another with a new ligand called BnI derived from benzimidazole and the zinc(II)complex, [Zn(BnI)₂], [72] have anticancer activity in five different human tumor cell lines: HepG2 (liver), SK‐MEL‐1 (skin), HT018 (colon), HeLa, and MDA‐MB‐231, decreasing viability and inducing apoptosis; these compounds also decrease chemotaxis‐induced migration [72, 73].
Among the copper‐containing complexes, [Cu(trp)2] decreases the viability of MCF‐7 and MDA‐MB‐231, induces apoptosis and decreases migration and invasion of 3D cell cultures, and decreases the formation of breast spheres and the expression of MMP2 and MPP9 [74]. Similarly, other copper‐containing metallodrugs also decrease the expression of these metalloproteinases and MMP9. The main ligands of these compounds are phenanthroline, doxycycline, and flavonoids. Some of these compounds exerts anti‐invasion activity in models in vitro [66, 67, 68]. [Cu(BnI)₂], [72] which has the same Bnl ligands, also has anticancer activity in different cancer cell lines, inducing apoptosis and decreasing migration capacity.
The evidence is very diverse and shows that metallodrugs can decrease the ability of tumor cells to migrate or invade. However, the mechanisms of action and cellular effects of these compounds require further analysis. Some metallodrugs have been studied in greater depth, increasing understanding of their participation against invasion and metastasis processes.
Proposed mechanisms of action
It is essential to continue studying novel platinum complexes to overcome drug resistance and improve pharmacological activity. An important example is PIP‐platinum, which inhibits the proliferation of several tumor cell lines, including a cisplatin‐resistant cell line of adenocarcinomic human (A549/DDP). This complex triggers mitochondrial dysfunction, cytochrome c release, and increases ROS, thus inducing apoptosis. Additionally, PIP‐platinum increases cellular adhesion and decreases cellular migration/invasion in three in vitro models by inhibiting the WNT signaling pathway that blocks nuclear translocation of β‐catenin, which promotes E‐cadherin‐mediated cellular adhesion (Fig. 2B). Of note, PIP‐platinum also showed antitumor activity in a breast cancer murine model (4T1) [75].
Another strategy employed by Pt drugs, namely, immunogenic cell death induced by photoactive Pt complexes, might be important for antimetastatic activity [76].
Moreover, it is important to mention that some complexes of other metals overcome the resistance to Pt, since they have different target sites or the resulting DNA damage is different; as a result, the potential of heterometallic compounds as cancer therapy is currently being explored. [59]. Elie et al. (2019) [77] compared the activity of two bimetallic compounds and one monometallic gold‐based compound in a kidney metastatic carcinoma cell line (Caki‐1). This study reported that three evaluated compounds: auranofin, titanofin, and titanocref (gold‐ and titanocene‐based metallic compounds), induce apoptosis; titanofin and titanocref are more efficient at arresting the cell cycle at G2/M, while titanocref is a more efficient inhibitor of cellular migration. Furthermore, the three compounds can inhibit angiogenesis. Although the signaling pathways by which these compounds act are not clear, markers associated with the processes of migration, invasion, and angiogenesis were detected, such as prometastatic IL(s), MMP(s), TNF‐α, and VEGF [77].
Another hybrid compound with potential antineoplastic activity belongs to the ruthenium‐gold family. This complex incorporates heterocyclic Au‐N ligands derived from [Cl2(p‐cymene)Ru(μ‐dppm)Au(NHC)]ClO4, known as RANCE‐1. Compared to auranofin, this compound reduces cellular migration and invasion more efficiently. This effect is accompanied by the modification of the levels of proteins related to oxidative stress and metastasis; this includes reduction of the mitochondrial protein TrxR, the angiogenesis mediator factor VEGF, the secretion of metalloproteinases (MMP‐1, MMP‐3, MMP‐7, MMP‐8, MMP‐9, MMP‐10, MMP‐12, and MMP‐13), ADAM proteolytic enzymes (ADAM8 and ADAM9), and proteases from the cathepsin family (B, D, L, S, and Z/X/P). Furthermore, this complex decreases the levels of inflammation and metastasis‐related interleukins (IL‐5, IL‐6, IL‐8, IL‐12, and IL‐17A). However, its mechanism of action is still unknown [77, 78].
Regarding ruthenium‐based compounds, those with PF6 as a counter ion are of great interest for the development of selective antineoplastic therapies in metastatic stages. The PF6 compounds with promising results are [Ru3(μ3‐O)(μ‐OAc)5(μ‐η1(C),η2(N,N)‐phen)(py)2]PF6, and [Ru(Lapachol)(bis(diphenylphosphine)methane)2]PF6. [Ru3(μ3‐O)(μ‐OAc)5(μ‐η1(C),η2(N,N)‐phen)(py)2]PF6 shows selective cytotoxicity in the murine melanoma cell line B16F10 compared to the fibroblast L929 cell lines at a concentration of IC50 = 25 µm. Although the mechanism of action remains unknown, it has been shown that this compound interacts with DNA through electrostatic attraction [79]. [Ru(Lapachol)(bis(diphenylphosphine)methane)2]PF6 induces mitochondrial apoptosis at a concentration of IC50 = 0.13 µm in the metastatic breast cancer cell line MDA‐MB‐231. Although no tissue selectivity was observed when compared with the MCF‐10A breast epithelial nontumor cell line, it shows greater tolerance to the effect of the compound at an IC50 of 2.7 µm [80]. However, Becceneri et al. (2020) reported that the Ru(PPh3)2(O‐S)(bpy)PF6 complex is cytotoxic and selective to triple‐negative breast cancer cells in 3D cultures, because has the ability to reduce the expression of β1‐integrin, EGFR, and p38 MAPK and decrease the activity of MPP2 [81].
Recently, Deb et al. (2020) [82] reported the synthesis, characterization, and biological evaluation of a zinc(II)‐naproxen and a zinc(II)‐mefenamic acid complex of 1,10‐phenanthroline‐5,6‐dione [82]. Both complexes exhibit antiproliferative activity in the human breast cancer cell line MDA‐MB‐231. The zinc(II)–naproxen complex induces apoptosis through activation of caspases −3, −8, and −9. Additionally, the anti‐inflammatory properties of nonsteroidal anti‐inflammatory drugs (NSAIDs) are also conserved in metallic complexes since both complexes inhibit the cyclooxygenase pathway (selective inhibition of COX‐1 at low concentration), decreasing the synthesis of prostaglandins (PGE2), which shows anti‐inflammatory activity. In vitro cell migration and EMT‐related genes (VIMENTIN and β1‐INTEGRIN) also decrease. Mechanistic studies indicate that ternary complexes are more active than cisplatin and can overcome cisplatin resistance in MDA‐MB‐231 cells [82].
Quercetin is a polyphenolic flavonoid with demonstrated anticancer effect. The quercetin‐zinc (Q‐ZnCPX) complex slightly reduces the viability of bladder cancer cells (BFTC‐905) at high concentrations. However, at low concentrations, Q‐ZnCPX drastically reduces cellular movement and the expression of invasion‐related proteins. The effect on cellular migration and invasion of Q‐ZnCPX was determined in three in vitro models. These processes were significantly decreased compared to the control, with concentrations ≥ 12,5 μM through the regulation of p‐AKT and MT1‐MMP [83]. AKT was described as an essential protein of the Akt / PI3K / PTEN signaling pathway; it is a serine/threonine protein kinase that, once activated through phosphorylation (p‐AKT), plays an important role in cancer progression [84] and metastasis. Meanwhile, MT1‐MMP (membrane type I‐matrix metalloproteinase) is one of the proteinases involved in cell migration. Among the copper metallodrugs, those containing the 2‐[(3‐chloro‐2‐hydroxy‐propyl)‐pyridin‐2‐ylmethyl‐amino]‐methyl‐phenol (L) ligand were reported to induce cell cycle arrest and decrease chemotaxis‐induced migration in neuroblastoma cells (H4). Additionally, this compound inhibits migration in 3D cultures, reduces the expression of EMT‐related genes, such as SNAIL and VIMENTIN, and increases E‐CADHERIN expression, enhancing cell adhesion [85]. Similarly, Cas III‐ia, besides showing a dose‐dependent antiproliferative activity in different tumor cell lines, can also decrease migration in the neuroblastoma cell line SK‐N‐SH [86]. Furthermore, the expression of genes induced by Cas II‐gly treatment were studied in HeLa cells, demonstrating that this compound can decrease the expression of migration‐related genes, such as: TGFβR1, AURKA, SNAI2, BMP4, BMP6, and N‐CADHERIN [42, 86, 87, 88]. Cas II‐gly was also shown to act on MALAT1, targeting miR‐17‐5p to inhibit FZD2 expression by inactivating the Wnt signaling pathway, thus inhibiting cell proliferation and promoting apoptosis in HeLa and CaSki cells [89]. This is important, since invasion and metastasis require the EMT. Particularly, the TGF‐β and Wnt signaling pathways are crucial to induce the EMT. Moreover, previous studies have reported that regardless of the mechanism of inhibition of the TGF‐β pathway and the EMT, motility, invasion capacity, and migration are decreased in different cancer cell lines [21, 90, 91]. It is important to highlight that Castillo‐Rodríguez et al. (2021) [92] reported that Casiopeína III‐La, in addition to its antiproliferative and pro‐apoptotic effects, has the ability to decrease the invasive capacity of glioma cells, through the induction of ROS and regulation of the Wnt/β‐catenin pathway [92]. Other interesting compounds include: CPT8, since it affects viability and cell cycle arrest in different cancer cell lines and reduces migration and invasion capacity in different in vitro models. Interestingly, this compound also decreases the expression of VEGF receptors [67, 93]. In a murine model, CTP8 showed antitumor activity and antiangiogenesis activity in a chicken embryo model [93]. In Fig. 3, we present a summary of some of the most promising metallodrugs for targeting metastasis.
Fig. 3.

Metallodrugs in the general process of the invasion and metastasis. In order to undergo invasion and metastasis, malignant cells require different processes, which allow them to acquire invasive properties (mobility, degradation of the extracellular matrix, etc.); these changes are caused by the activation of signaling pathways such as TGF‐β or Wnt/β‐catenin, increasing the expression of genes such as Snail1, Snail2, Twist, and Zeb‐1. It also needs to mold its microenvironment by means of signaling molecules such as VEGF or EGF (angiogenesis) and interleukins that have an important role in inflammation and the progression of metastasis (such as IL‐5, IL‐6, IL‐8, IL‐12, and IL‐17A). To date, different metallodrugs have been described that can intervene or inhibit these processes. (Created with BioRender.com.).
Conclusion
Since the emergence of the field of inorganic medicinal chemistry, various new drugs with a metal base or metallodrugs have been developed to combat various diseases such as cancer, specifically in those high‐risk tumors that develop invasive and metastatic properties.
In this review article, we described the mechanism of action of various platinum, ruthenium, gold, zinc, and copper‐based metal drugs. These mechanisms include effects on cell viability and apoptosis, cell cycle arrest, alteration of the cytoskeleton, inhibition of angiogenesis, and DNA damage. The current data from metallodrugs support the idea they may function as chemotherapeutic agents, because of their potential to inhibit signaling pathways for multiple aspects of cancer progression including tumor growth, angiogenesis, and metastasis.
It must be remembered that to understand how the pathways described above are altered by metallodrugs, it has been necessary to develop exhaustive analyses of cell biology to establish which cell organelles are capable of acting as biological targets. Thus, for example, we know that the permeability of the transition pore for mitochondria can be altered, which favors the generation of ROS and the entry of proteins such as Bax or the exit of Bcl‐2 and cytochrome C, which results in apoptosis. The formation of autophagosomes is also promoted to protect damaged cellular material or cause its degradation when they bind to lysosomes. Finally, in the cell nucleus, DNA is altered, as occurs with cisplatin, which fosters unions between the chains of the double helix, directly inhibiting DNA synthesis.
The use of various state‐of‐the‐art techniques, such as advanced synchrotron cryotechniques of light have made it possible to visualize the 3D cytoarchitecture in the entire cell with minimal disturbance, showing for example that iridium is 100 times more powerful than cisplatin.
Finally, metallomics allows the identification of genes that could be considered as pharmacological targets by virtue of a drug that acts on it. This has allowed the development of platforms such as drug target, which integrates information from at least 15 pharmacological databases that include information on drugs, drug targets, type of drug–target interaction, data sources, and other characteristics.
Undoubtedly, there is still a need for more research on current metallodrugs or even the development of new metal drugs.
Conflict of interest
The authors declare no conflict of interest.
Author contribution
MMG‐B: performed references research in data base formal analysis, investigation, writing—original draft, and visualization. CM performed formal analysis, review, and editing, LR‐A performed conceptualization, supervision, and writing—review and editing project administration.
Data accessibility
Data accessibility as open access.
Acknowledgements
This work was supported by CONACyT Project 314819; CONACyT grant 589762. This work is submitted in partial fulfillment of the requirements for the PhD degree (in Biochemical Sciences) of MMG‐B at the Universidad Nacional Autónoma de México. We thank Viridiana C. Hernández‐Cruz., for searching for references and proofreading and Adrian Espinoza‐Guillén for the structures in Table 1 and helpful discussions.
References
- 1. Cell Editorial Team . Cancer: the road ahead. Cell. 2017;168:545–6. [DOI] [PubMed] [Google Scholar]
- 2. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Cancer J Clin. 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 4. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. [DOI] [PubMed] [Google Scholar]
- 5. Hainaut P, Plymoth A. Targeting the hallmarks of cancer: towards a rational approach to next‐generation cancer therapy. Curr Opin Oncol. 2013;25:50–1. [DOI] [PubMed] [Google Scholar]
- 6. Ganesh K, Massagué J. Targeting metastatic cancer. Nat Med. 2021;27:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aceto N, Toner M, Maheswaran S, Haber DA. En Route to Metastasis: circulating tumor cell clusters and epithelial‐to‐mesenchymal transition. Trends can. 2015;1:44–52. [DOI] [PubMed] [Google Scholar]
- 8. Chiang AC, Massague J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Trans Targeted Therapy. 2020;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wirtz D, Konstantopoulos K, Searson PC. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer. 2011;11:512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Melzer C, Hass R, von der Ohe J, Lehnert H, Ungefroren H. The role of TGF‐β and its crosstalk with RAC1/RAC1b signaling in breast and pancreas carcinoma. Cell Commun Signal. 2017;15:19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Padua D, Massagué J. Roles of TGFβ in metastasis. Cell Res. 2008;19:89. [DOI] [PubMed] [Google Scholar]
- 13. Kalluri R, Weinberg RA. The basics of epithelial‐mesenchymal transition. J Clin Investig. 2009;119:1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang WG, Sanders AJ, Katoh M, Ungefroren H, Gieseler F, Prince M, et al. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin Cancer Biol. 2015;35(Suppl):S244–75. [DOI] [PubMed] [Google Scholar]
- 16. Merdad A, Karim S, Schulten H‐J, Dallol A, Buhmeida A, Al‐Thubaity F, et al. Expression of Matrix Metalloproteinases (MMPs) in Primary Human Breast Cancer: MMP‐9 as a Potential Biomarker for Cancer Invasion and Metastasis. Anticancer Res. 2014;34:1355–66. [PubMed] [Google Scholar]
- 17. Emon B, Bauer J, Jain Y, Jung B, Saif T. Biophysics of Tumor Microenvironment and Cancer Metastasis ‐ A Mini Review. Comput Struct Biotechnol J. 2018;16:279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yehya AHS, Asif M, Petersen SH, Subramaniam AV, Kono K, Majid A, et al. Angiogenesis: Managing the Culprits behind Tumorigenesis and Metastasis. Medicina (Kaunas). 2018;54:8–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lv Y, Zhao X, Zhu L, Li S, Xiao Q, He W, et al. Targeting intracellular MMPs efficiently inhibits tumor metastasis and angiogenesis. Theranostics. 2018;8:2830–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Serova M, Tijeras‐Raballand A, Dos Santos C, Albuquerque M, Paradis V, Neuzillet C, et al. Effects of TGF‐beta signalling inhibition with galunisertib (LY2157299) in hepatocellular carcinoma models and in ex vivo whole tumor tissue samples from patients. Oncotarget. 2015;6:21614–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Neuzillet C, Tijeras‐Raballand A, Cohen R, Cros J, Faivre S, Raymond E, et al. Targeting the TGFβ pathway for cancer therapy. Pharmacol Ther. 2015;147:22–31. [DOI] [PubMed] [Google Scholar]
- 22. Borkow G, Gabbay J. Copper, an ancient remedy returning to fight microbial, fungal and viral infections. Curr Chem Biol. 2009;3:272–8. [Google Scholar]
- 23. Huaizhi Z, Yuantao N. China’s ancient gold drugs. Gold Bulletin. 2001;34:24–9. [Google Scholar]
- 24. Spear M. Silver: an age‐old treatment modality in modern times. Plastic Surg Nurs. 2010;30:90–3. [DOI] [PubMed] [Google Scholar]
- 25. Maton PN, Burton ME. Antacids revisited. Drugs. 1999;57:855–70. [DOI] [PubMed] [Google Scholar]
- 26. Roder C, Thomson MJ. Auranofin: repurposing an old drug for a golden new age. Drugs in R&D. 2015;15:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Keogan DM, Griffith DM. Current and potential applications of bismuth‐based drugs. Molecules. 2014;19:15258–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suryadi J, Bierbach U. DNA metalating‐intercalating hybrid agents for the treatment of chemoresistant cancers. Chemistry. 2012;18:12926–34. [DOI] [PubMed] [Google Scholar]
- 29. Alessio E, Messori L. NAMI‐A and KP1019/1339, two iconic ruthenium anticancer drug candidates face‐to‐face: a case story in medicinal inorganic chemistry. Molecules. 2019;24:1420–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shah N, Dizon DS. New‐generation platinum agents for solid tumors. Future Oncol. 2009;5:33–42. [DOI] [PubMed] [Google Scholar]
- 31. Mathews V, Chendamarai E, George B, Viswabandya A, Srivastava A. Treatment of acute promyelocytic leukemia with single‐agent arsenic trioxide. Mediterranean J Hematol Infect Dis. 2011;3:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bonetta R. Potential Therapeutic Applications of MnSODs and SOD‐Mimetics, Chemistry–A . European Journal. 2018;24:5032–41. [DOI] [PubMed] [Google Scholar]
- 33. Anthony EJ, Bolitho EM, Bridgewater HE, Carter OWL, Donnelly JM, Imberti C, et al. Metallodrugs are unique: opportunities and challenges of discovery and development. Chem Sci. 2020;11:12888–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosenberg B, Van Camp L, Grimley EB, Thomson AJ. The inhibition of growth or cell division in Escherichia coli by different ionic species of platinum(IV) complexes. J Biol Chem. 1967;242:1347–52. [PubMed] [Google Scholar]
- 35. Rosenberg B, Vancamp L, Krigas T. Inhibition of cell division in escherichia coli by electrolysis products from a platinum electrode. Nature. 1965;205:698–9. [DOI] [PubMed] [Google Scholar]
- 36. Rosenberg B. Platinum complexes for the treatment of cancer. Interdisc Sci Rev. 1978;3:134–47. [Google Scholar]
- 37. Rosenberg B, VanCamp L, Trosko JE, Mansour VH. Platinum compounds: a new class of potent antitumour agents. Nature. 1969;222:385–6. [DOI] [PubMed] [Google Scholar]
- 38. Franciosi V, Cocconi G, Michiara M, Di Costanzo F, Fosser V, Tonato M, et al. Front‐line chemotherapy with cisplatin and etoposide for patients with brain metastases from breast carcinoma, nonsmall cell lung carcinoma, or malignant melanoma: a prospective study. Cancer. 1999;85:1599–605. [PubMed] [Google Scholar]
- 39. Alraouji NN, Al‐Mohanna FH, Ghebeh H, Arafah M, Almeer R, Al‐Tweigeri T, et al. Tocilizumab potentiates cisplatin cytotoxicity and targets cancer stem cells in triple‐negative breast cancer. Mol Carcinog. 2020;59:1041–51. [DOI] [PubMed] [Google Scholar]
- 40. Wang H, Guo S, Kim SJ, Shao F, Ho JWK, Wong KU, et al. Cisplatin prevents breast cancer metastasis through blocking early EMT and retards cancer growth together with paclitaxel. Theranostics. 2021;11:2442–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. He PJ, Ge RF, Mao WJ, Chung PS, Ahn JC, Wu HT. Oxidative stress induced by carboplatin promotes apoptosis and inhibits migration of HN‐3 cells. Oncol Lett. 2018;16:7131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Espinal‐Enríquez J, Hernández‐Lemus E, Mejía C, Ruiz‐Azuara L. Network analysis shows novel molecular mechanisms of action for copper‐based chemotherapy. Front Physiol. 2016;6:406–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 2009;8:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hartmann JT, Lipp HP. Toxicity of platinum compounds. Expert Opin Pharmacother. 2003;4:889–901. [DOI] [PubMed] [Google Scholar]
- 45. Chaney SG, Campbell SL, Bassett E, Wu Y. Recognition and processing of cisplatin‐and oxaliplatin‐DNA adducts. Critical Rev Oncol. 2005;53:3–11. [DOI] [PubMed] [Google Scholar]
- 46. Lainé A‐L, Passirani C. Novel metal‐based anticancer drugs: a new challenge in drug delivery. Curr Opin Pharmacol. 2012;12:420–6. [DOI] [PubMed] [Google Scholar]
- 47. Gill MR, Thomas JA. Ruthenium (II) polypyridyl complexes and DNA—from structural probes to cellular imaging and therapeutics. Chem Soc Rev. 2012;41:3179–92. [DOI] [PubMed] [Google Scholar]
- 48. Lee SY, Kim CY, Nam T‐G. Ruthenium complexes as anticancer agents: a brief history and perspectives. Drug Design Dev Ther. 2020;14:5375–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thota S, Rodrigues DA, Crans DC, Barreiro EJ. Ru(II) Compounds: next‐generation anticancer metallotherapeutics? J Med Chem. 2018;61:5805–21. [DOI] [PubMed] [Google Scholar]
- 50. Brescacin L, Masi A, Sava G, Bergamo A. Effects of the ruthenium‐based drug NAMI‐A on the roles played by TGF‐β1 in the metastatic process. J Biol Inorg Chem. 2015;20:1163–73. [DOI] [PubMed] [Google Scholar]
- 51. Golla U, Swagatika S, Chauhan S, Tomar RS. A systematic assessment of chemical, genetic, and epigenetic factors influencing the activity of anticancer drug KP1019 (FFC14A). Oncotarget. 2017;8:98426–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Flocke LS, Trondl R, Jakupec MA, Keppler BK. Molecular mode of action of NKP‐1339–a clinically investigated ruthenium‐based drug–involves ER‐ and ROS‐related effects in colon carcinoma cell lines. Invest New Drugs. 2016;34:261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ding Y, Sun Z, Tong Z, Zhang S, Min J, Xu Q, et al. Tumor microenvironment‐responsive multifunctional peptide coated ultrasmall gold nanoparticles and their application in cancer radiotherapy. Theranostics. 2020;10:2296–4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fernández‐Gallardo J, Elie BT, Sadhukha T, Prabha S, Sanaú M, Rotenberg SA, et al. Heterometallic titanium–gold complexes inhibit renal cancer cells in vitro and in vivo . Chem Sci. 2015;6:5269–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Humphreys AS, Filipovska A, Berners‐Price SJ, Koutsantonis GA, Skelton BW, White AH. Gold(i) chloride adducts of 1,3‐bis(di‐2‐pyridylphosphino)propane: synthesis, structural studies and antitumour activity. Dalton Trans. 2007;4943–50. [DOI] [PubMed] [Google Scholar]
- 56. Rackham O, Nichols SJ, Leedman PJ, Berners‐Price SJ, Filipovska A. A gold(I) phosphine complex selectively induces apoptosis in breast cancer cells: implications for anticancer therapeutics targeted to mitochondria. Biochem Pharmacol. 2007;74:992–1002. [DOI] [PubMed] [Google Scholar]
- 57. Tian S, Siu F‐M, Kui SCF, Lok C‐N, Che C‐M. Anticancer gold(i)–phosphine complexes as potent autophagy‐inducing agents. Chem Commun. 2011;47:9318–20. [DOI] [PubMed] [Google Scholar]
- 58. Iacopetta D, Rosano C, Sirignano M, Mariconda A, Ceramella J, Ponassi M, et al. Is the way to fight cancer paved with gold? Pharmaceuticals. 2020;13:1–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pelletier F, Comte V, Massard A, Wenzel M, Toulot S, Richard P, et al. Development of bimetallic titanocene− ruthenium− arene complexes as anticancer agents: relationships between structural and biological properties. J Med Chem. 2010;53:6923–33. [DOI] [PubMed] [Google Scholar]
- 60. Dasgupta S, Karim S, Banerjee S, Saha M, Das Saha K, Das D. Designing of novel zinc(ii) Schiff base complexes having acyl hydrazone linkage: study of phosphatase and anti‐cancer activities. Dalton Trans. 2020;49:1232–40. [DOI] [PubMed] [Google Scholar]
- 61. Molinaro C, Martoriati A, Pelinski L, Cailliau K. Copper complexes as anticancer agents targeting topoisomerases I and II. Cancers. 2020;12:2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chavez‐Gonzalez A, Centeno‐Llanos S, Moreno‐Lorenzana D, Sandoval‐Esquivel MA, Aviles‐Vazquez S, Bravo‐Gomez ME, et al. Casiopeina III‐Ea, a copper‐containing small molecule, inhibits the in vitro growth of primitive hematopoietic cells from chronic myeloid leukemia. Leuk Res. 2017;52:8–19. [DOI] [PubMed] [Google Scholar]
- 63. Bravo‐Gomez ME, Garcia‐Ramos JC, Gracia‐Mora I, Ruiz‐Azuara L. Antiproliferative activity and QSAR study of copper(II) mixed chelate [Cu(N‐N)(acetylacetonato)]NO3 and [Cu(N‐N)(glycinato)]NO3 complexes, (Casiopeinas). J Inorg Biochem. 2009;103:299–309. [DOI] [PubMed] [Google Scholar]
- 64. Trejo‐Solís C, Palencia G, Zúñiga S, Rodríguez‐Ropon A, Osorio‐Rico L, Torres Luvia S, et al. Cas IIgly Induces Apoptosis in Glioma C6 cells in vitro and in vivo through caspase‐dependent and caspase‐independent mechanisms. Neoplasia. 2005;7:563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kachadourian R, Brechbuhl HM, Ruiz‐Azuara L, Gracia‐Mora I, Day BJ. Casiopeina IIgly‐induced oxidative stress and mitochondrial dysfunction in human lung cancer A549 and H157 cells. Toxicology. 2010;268:176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Abosede OO, Vyas NA, Singh SB, Kumbhar AS, Kate A, Kumbhar AA, et al. Copper (II) mixed‐ligand polypyridyl complexes with doxycycline–structures and biological evaluation. Dalton Trans. 2016;45:3003–12. [DOI] [PubMed] [Google Scholar]
- 67. Shi X, Chen Z, Wang Y, Guo Z, Wang XJDT. Hypotoxic copper complexes with potent anti‐metastatic and anti‐angiogenic activities against cancer cells. Dalton Trans. 2018;47:5049–54. [DOI] [PubMed] [Google Scholar]
- 68. Spoerlein C, Mahal K, Schmidt H, Schobert RJJ. Effects of chrysin, apigenin, genistein and their homoleptic copper(II) complexes on the growth and metastatic potential of cancer cells. J Inorganic Biochem. 2013;127:107–15. [DOI] [PubMed] [Google Scholar]
- 69. Subarkhan MKM, Ren L, Xie B, Chen C, Wang Y, Wang H. Novel tetranuclear ruthenium (II) arene complexes showing potent cytotoxic and antimetastatic activity as well as low toxicity in vivo . Eur J Med Chem. 2019;179:246–56. [DOI] [PubMed] [Google Scholar]
- 70. Popolin CP, Reis JP, Becceneri AB, Graminha AE, Almeida MA, Corrêa RS, et al. Cytotoxicity and anti‐tumor effects of new ruthenium complexes on triple negative breast cancer cells. PLoS One. 2017;12:1932–6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang Y, Jin J, Shu L, Li T, Lu S, Subarkhan MKM, et al. New Organometallic Ruthenium(II) Compounds Synergistically Show Cytotoxic, Antimetastatic and Antiangiogenic Activities for the Treatment of Metastatic Cancer. Chemistry. 2020;26:15170–82. [DOI] [PubMed] [Google Scholar]
- 72. AlAjmi MF, Hussain A, Rehman M, Khan AA, Shaikh PA, Khan RAJI. Design, Synthesis, and Biological Evaluation of Benzimidazole‐Derived Biocompatible Copper(II) and Zinc(II) Complexes as Anticancer Chemotherapeutics. Intern J Mol Sci. 2018;19:1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hussain A, AlAjmi MF, Rehman M, Khan AA, Shaikh PA, Khan RAJM. Evaluation of transition metal complexes of benzimidazole‐derived scaffold as promising anticancer chemotherapeutics. Molecules. 2018;23:1232–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Balsa LM, Ruiz MC, Maria S, de la Parra L, Baran EJ, León IE. Anticancer and antimetastatic activity of copper(II)‐tropolone complex against human breast cancer cells, breast multicellular spheroids and mammospheres. J Inorg Biochem. 2020;204:110975. [DOI] [PubMed] [Google Scholar]
- 75. Li J, He X, Zou Y, Chen D, Yang L, Rao J, et al. Mitochondria‐targeted platinum(ii) complexes: dual inhibitory activities on tumor cell proliferation and migration/invasion via intracellular trafficking of β‐catenin. Metallomics. 2017;9:726–33. [DOI] [PubMed] [Google Scholar]
- 76. Novohradsky V, Pracharova J, Kasparkova J, Imberti C, Bridgewater HE, Sadler PJ, et al. Induction of immunogenic cell death in cancer cells by a photoactivated platinum(iv) prodrug. Inorganic Chem Front. 2020;7:4150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Elie BT, Pechenyy Y, Uddin F, Contel M. A heterometallic ruthenium‐gold complex displays antiproliferative, antimigratory, and antiangiogenic properties and inhibits metastasis and angiogenesis‐associated proteases in renal cancer. J Biol Inorg Chem. 2018;23:399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Elie BT, Fernández‐Gallardo J, Curado N, Cornejo MA, Ramos JW, Contel M. Bimetallic titanocene‐gold phosphane complexes inhibit invasion, metastasis, and angiogenesis‐associated signaling molecules in renal cancer. Eur J Med Chem. 2019;161:310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. da Silva CF, Ramos LCB, Rohrabaugh TN, Vandevord JM, da Silva RS, Turro C, et al. Exploring the structure of a ruthenium acetate cluster for biological purposes. Inorg Chem Commun. 2020;114:1387–7003. [Google Scholar]
- 80. Oliveira KM, Honorato J, Demidoff FC, Schultz MS, Netto CD, Cominetti MR, et al. Lapachol in the Design of a New Ruthenium(II)‐diphosphine complex as a promising anticancer metallodrug. J Inorg Biochem. 2021;214:111289. [DOI] [PubMed] [Google Scholar]
- 81. Becceneri AB, Fuzer AM, Plutin AM, Batista AA, Lelièvre SA, Cominetti MR. Three‐dimensional cell culture models for metallodrug testing: induction of apoptosis and phenotypic reversion of breast cancer cells by the trans‐[Ru (PPh 3) 2 (N, N‐dimethyl‐N‐thiophenylthioureato‐k 2 O, S)(bipy)] PF 6 complex. Inorganic Chem Front. 2020;7:2909–19. [Google Scholar]
- 82. Deb J, Lakshman TR, Ghosh I, Jana SS, Paine TK. Mechanistic studies of in vitro anti‐proliferative and anti‐inflammatory activities of the Zn(ii)–NSAID complexes of 1,10‐phenanthroline‐5,6‐dione in MDA‐MB‐231 cells. Dalton Trans. 2020;49:11375–84. [DOI] [PubMed] [Google Scholar]
- 83. Lee YH, Tuyet PT. Synthesis and biological evaluation of quercetin‐zinc (II) complex for anti‐cancer and anti‐metastasis of human bladder cancer cells. In Vitro Cell Dev Biol Anim. 2019;55:395–404. [DOI] [PubMed] [Google Scholar]
- 84. Cao F, Zhang C, Han W, Gao XJ, Ma J, Hu YW, et al. p‐Akt as a potential poor prognostic factor for gastric cancer: a systematic review and meta‐analysis. Oncotarget. 2017;8:59878–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Guerreiro JF, Gomes MAG, Pagliari F, Jansen J, Marafioti MG, Nistico C, et al. Iron and copper complexes with antioxidant activity as inhibitors of the metastatic potential of glioma cells. RSC Adv. 2020;10:12699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Velázquez‐Avalos LK, Vitela‐Ramos ML, Ruiz‐Azuara L, Mejía C. Migración, invasividad y ciclo celular en NB por Casiopeínas®. Nthe. 2019;31:50–9. [Google Scholar]
- 87. Anda‐Jáuregui G, Espinal‐Enríquez J, Hur J, Alcalá‐Corona SA, Ruiz‐Azuara L, Hernández‐Lemus E. Identification of Casiopeina II‐gly secondary targets through a systems pharmacology approach. Comput Biol Chem. 2019;78:127–32. [DOI] [PubMed] [Google Scholar]
- 88. Valencia‐Cruz AI, Uribe‐Figueroa LI, Galindo‐Murillo R, Baca‐Lopez K, Gutierrez AG, Vazquez‐Aguirre A, et al. Whole genome gene expression analysis reveals casiopeina‐induced apoptosis pathways. PLoS One. 2013;8:1932–6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Xu Y, Zhang Q, Lin F, Zhu L, Huang F, Zhao L, et al. Casiopeina II‐gly acts on lncRNA MALAT1 by miR‐17‐5p to inhibit FZD2 expression via the Wnt signaling pathway during the treatment of cervical carcinoma. Oncol Rep. 2019;42:1365–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Halder SK, Beauchamp RD, Datta PK. A specific inhibitor of TGF‐beta receptor kinase, SB‐431542, as a potent antitumor agent for human cancers. Neoplasia. 2005;7:509–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Leonel C, Borin TF, de Carvalho Ferreira L, Moschetta MG, Bajgelman MC, Viloria‐Petit AM, et al. Inhibition of Epithelial‐mesenchymal transition and metastasis by combined TGFbeta knockdown and metformin treatment in a canine mammary cancer xenograft model. J Mammary Gland Biol Neoplasia. 2017;22:27–41. [DOI] [PubMed] [Google Scholar]
- 92. Castillo‐Rodriguez RA, Palencia G, Anaya‐Rubio I, Perez JCG, Jimenez‐Farfan D, Escamilla‐Ramirez A, et al. Anti‐proliferative, pro‐apoptotic and anti‐invasive effect of the copper coordination compound Cas III‐La through the induction of reactive oxygen species and regulation of Wnt/beta‐catenin pathway in glioma. J Cancer. 2021;12:5693–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Qin X‐Y, Wang Y‐N, Liu H‐F, Luo Z‐H, Zhang P‐L, Li‐Fang H, et al. Anti‐cancer activities of metal‐based complexes by regulating the VEGF/VEGFR2 signaling pathway and apoptosis‐related factors Bcl‐2, Bax, and caspase‐9 to inhibit angiogenesis and induce apoptosis. Metallomics. 2020;12:92–103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data accessibility as open access.