Abstract
Drug carriers to deliver macrolide antibiotics, such as azithromycin, show promise as antibacterial agents. Macrolide drug carriers have largely focused on improving the drug stability and pharmacokinetics, while reducing adverse reactions and improving antibacterial activity. Recently, macrolides have shown promise in treating inflammatory conditions by promoting a reparative effect and limiting detrimental pro-inflammatory responses, which shifts the immunologic setpoint from suppression to balance. While macrolide drug carriers have only recently been investigated for their ability to modulate immune responses, the previous strategies that deliver macrolides for antibacterial therapy provide a roadmap for repurposing the macrolide drug carriers for therapeutic interventions targeting inflammatory conditions. This review describes the antibacterial and immunomodulatory activity of macrolides, while assessing the past in vivo evaluation of drug carriers used to deliver macrolides with the intention of presenting a case for increased effort to translate macrolide drug carriers into the clinic.
Keywords: Antibiotic, azithromycin, drug delivery, immune modulation, macrolides
Graphical Abstract
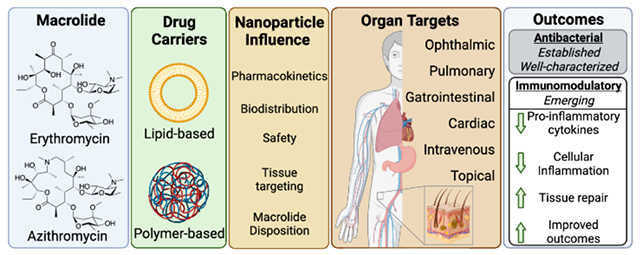
1. Introduction
Antibiotic macrolides are a class of polyketide natural products that inhibit bacterial protein biosynthesis resulting in bacteriostatic activity.1 Since the discovery of erythromycin in 1952,2 several macrolide derivatives have been synthesized and investigated for their antibacterial properties and more recently for their immunomodulatory properties.1 Collectively, macrolide antibiotics account for 28 million prescriptions in the US per year3 and efforts to improve the pharmacokinetics and bioavailability of macrolide antibiotics using drug delivery vehicles remain an active area of research. Given the breadth of studies investigating the delivery of macrolides using nano- and micro-carriers, a review of the in vivo drug delivery literature provides a foundation to utilize the same approaches to investigate immunomodulatory drug delivery with macrolides.
The immunomodulatory profiles of macrolides have been studied in animal models and human clinical trials leading to the discovery that they promote alternative macrophage polarization to a more regulatory phenotype resulting in tissue repair and reduced tissue damage.1 The exact mechanism of macrolide-based macrophage polarization is poorly understood, but azithromycin has been shown to inhibit NFκB and STAT1 signaling pathways in vitro.4 Efforts to determine the mechanism of action for their immunomodulatory properties are ongoing, and the utility of macrolides in treating patients with a variety of inflammatory conditions has been proposed and is currently being investigated. Importantly, macrolide antibiotics have been reported to induce arrhythmia, QT prolongation, and sudden cardiac death in subjects with preexisting heart conditions. The risk of cardiac complications has tempered enthusiasm for their clinical utility in sterile inflammatory indications,5 which poses a new opportunity to identify drug carriers that reduce the risk of cardiac toxicity, while harnessing the immunomodulatory activity of the macrolides to improve patient outcomes.6 As new macrolides are being developed, drug delivery vehicles that improve the pharmacokinetics and pharmacodynamics of the macrolides are necessary to enhance the therapeutic potential of these drugs. Additionally, formulations designed for antibacterial applications offer a promising approach to harness the immunomodulatory properties of the macrolides to reduce the burden of inflammatory disease in patients.
Given the wide-spread use of erythromycin, clarithromycin, azithromycin, and roxithromycin to treat bacterial infections and their implementation in drug carriers, this review focuses mainly on these four macrolide compounds. Additionally, we focus our attention on drug carriers that are designed to be taken up by cells as opposed to large (>10 μm) microspheres and tablets that improve the pharmacokinetics of the free drug by extended release. This is particularly important when considering the cardiotoxicity of macrolides and the potential to modulate immune responses of pro-inflammatory phagocytes. Additionally, several drug carriers have been developed and tested in vitro without subsequent in vivo evaluation, therefore we focus particular attention on macrolide drug carriers that have advanced to in vivo testing. The selection criteria utilized for this review provide a well-defined scientific scope that furnishes a clear and concise overview of the field to generate informed discussions on the utility of drug carriers using macrolides to treat a variety of inflammatory conditions.
This review is organized to first describe the therapeutic application of the individual macrolides as well as an overview of their immunomodulatory profiles in vitro and in vivo, followed by an in-depth analytical review of the drug carriers designed to deliver erythromycin, azithromycin, clarithromycin, and roxithromycin for in vivo use. Finally, we will offer our perspective of the field and a commentary of the potential utility of macrolide carriers to modulate pro-inflammatory immune responses.
2. The Macrolides
2.1. History, Development, and Utility
Macrolides are composed of a macrocyclic lactone ring of different sizes, with one or more deoxy-sugar or amino sugar residues attached. Macrolides have a high affinity for the 50s ribosome in bacteria, which is highly conserved across bacterial species, allowing macrolides a broad anti-microbial spectrum of activity. The compounds are categorized based on the size of the macrocyclic lactone ring having 12-, 14-, 15-, or 16-member structures (Figure 1). The first-generation macrolides were produced by and isolated from strains of Streptomyces,7 with erythromycin, a 14-membered molecule isolated from Streptomyces erythraeus, being the first to reach the market in 1951.2 Erythromycin has been used successfully since then to treat many types of clinical infections, but its use is limited due to its lack of stability at low pH, its relatively poor penetration into tissues, and gastrointestinal tolerability issues in patients. These drawbacks led to the development of second-generation macrolides, as well as inclusion in drug carriers to improve their therapeutic utility.
Figure 1.
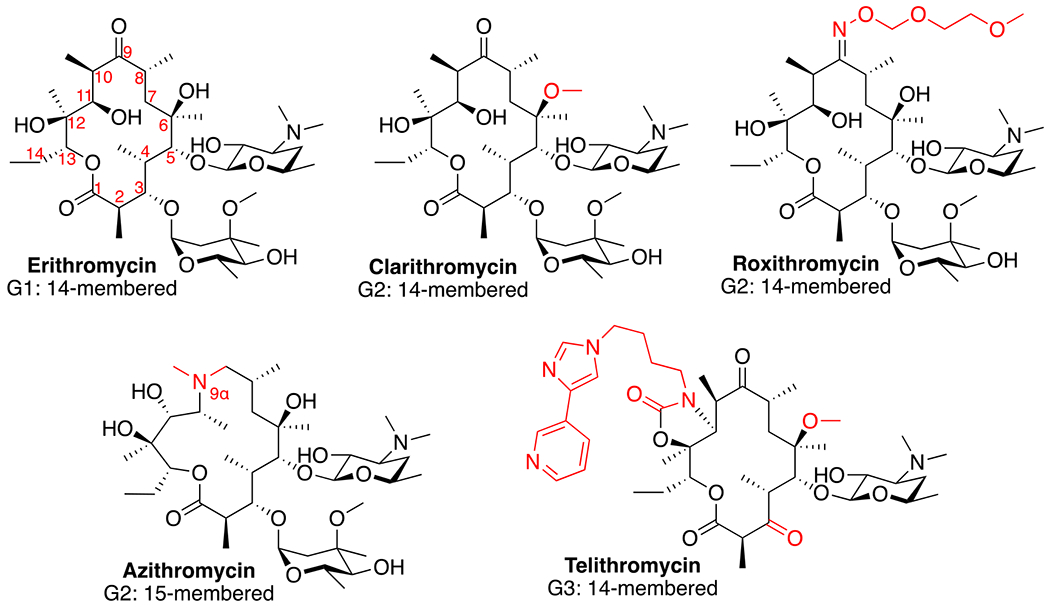
Structural comparison of macrolide derivative over three generations. Numbering of carbon atoms in the ring structure is shown for erythromycin and structural changes from erythromycin for each of the other derivatives are highlighted in red.
Second-generation agents began to emerge in the late 1980s and provided improved use profiles. While these drugs moderately improved upon the in vitro antimicrobial spectrum of erythromycin, the more impactful advances included the characteristics of their pharmacokinetic properties and enhanced tolerability in patients. Semi-synthetic derivatives were developed leading to the emergence of clarithromycin8 and azithromycin9, 10 which obtained approval and were used widely throughout the world. Additionally, a third macrolide roxithromycin was approved and has been used extensively in Europe.11 Despite these advances, the second-generation agents did little to decrease the emergence of macrolide resistance. The two most common resistance mechanisms associated with macrolides are the reduced binding affinity of the drugs due to modification of either the binding site or the compound, and the expression of bacterial efflux pumps that target macrolides.12, 13 The third generation macrolide agents, the ketolides, were developed to overcome resistance mechanisms due to multiple chemical modifications including replacing the cladinose with a ketone at position 3,14 which is the basis of this class led by the most successful ketolide, telithromycin (Figure 1). However, FDA surveillance data demonstrated several cases of acute liver failure and liver toxicity associated with telithromycin, which led to FDA warnings and restricted use in 2007.15 As a result, telithromycin was withdrawn from the market due to financial reasons, and currently there are no other ketolides in use.
Macrolides are used to treat many infection types including upper and lower respiratory tract infections,16–19 gastrointestinal infections,20, 21 skin/soft tissue infections,22 and sexually transmitted infections.23 When macrolides bind to the 50s bacterial ribosomal subunit, protein synthesis is inhibited by preventing transpeptidation and translocation reactions. Conversely, macrolides have an extremely low affinity for binding to eukaryotic ribosomes.24 Macrolide antibiotics are active against many Gram-positive bacterial pathogens as well as possessing limited activity against Gram-negative organisms.25 Macrolides are also effective against atypical bacterial species including the common community-acquired pneumonia pathogens.26–28 Additionally, the improved tissue penetration of second generation macrolides as a result of enhanced lipophilicity leads to more effective treatment of intracellular pathogens,29 and enhanced ability to accumulate within the lung tissues.30–32 The clinical use of these agents consistently results in high rates of positive treatment outcomes for these infections, and the macrolides remain a major component of the antimicrobial arsenal for the treatment of pneumonia and a variety of other infectious agents.
2.2. Pharmacokinetics and Pharmacodynamics
Most enhancements in clinical efficacy of the second-generation macrolides are due to improvements in their pharmacokinetic profiles. Compared to erythromycin, clarithromycin’s advantages include improved acid stability, a longer half-life, and more extensive biodistribution, which are also the goal of macrolide drug delivery vehicles. The methoxy group at position 6 of clarithromycin prevents formation of hemiketal and spiroketal degradation products of erythromycin in the presence of the low pH of the gut.33 The absence of these degradation products is thought to prevent some of the gastrointestinal intolerance associated with erythromycin.34 Clarithromycin is rapidly absorbed after oral administration and has a half-life of 3.5-5 hours following single dose administration, compared to 1.5-3 hours for erythromycin.35, 36 This allows for twice daily dosing of clarithromycin, which is a significant clinical advantage over erythromycin due to increases in patient adherence. Like the other second-generation agents,37–40 clarithromycin exhibits much higher tissue concentrations as compared to those found in the serum. After a 500 mg oral dose in human subjects, the concentration of clarithromycin was 17.5 mg/kg in the lungs compared to 2.5 mg/L in the serum after 4 hours. Studies in mice demonstrate that clarithromycin achieves high concentrations in the lungs, GI tract, kidney, and spleen.41 The combination of better intracellular penetration and improved intracellular acid stability is thought to improve its activity against intracellular pathogens.42
The agent with the most interesting and desirable pharmacokinetic profile is azithromycin. In addition to an enhancement of its Gram-negative activity, azithromycin is considerably more stable than erythromycin at low pH, has increased tissue penetration, and a longer elimination half-life. The oral bioavailability of azithromycin is approximately 37% and is rapidly distributed into tissues and intracellular compartments.30 This is reflected in its extremely low peak plasma concentration of 0.4 mg/L from a 500 mg oral dose. Accumulation in plasma after daily dosing of 500 mg for 5 days resulted in an increase in peak serum concentrations to only 0.62 mg/L. Azithromycin therefore has a very large volume of distribution of 23-31 L/kg, with high concentrations achieved in most tissues including the lungs, skin, sputum, and cervix, and body fluids as compared to the blood. Penetration into the cerebrospinal fluid is, however, poor. Additionally, it is advantageous that azithromycin has low protein binding characteristics. Binding of azithromycin to human plasma proteins is 50% at a concentration of 0.02 to 0.05 mg/L, but when increasing the azithromycin concentration to 1 mg/L, protein binding becomes saturated and exhibits an overall binding of only 7%.30 This is much lower than that of erythromycin and roxithromycin, and results in higher free azithromycin concentrations. Because azithromycin accumulates in granulocytes, specific phagocyte-driven delivery to inflamed tissues results in high drug concentrations in infected tissues.43–46 Because of its slow tissue release, azithromycin displays a terminal half-life of up to 68 hours in humans.30 The clearance rate of azithromycin is not altered significantly in elderly patients with mild renal impairment, nor in patients with mild to moderate hepatic impairment.47, 48 These properties make azithromycin less attractive as a candidate for drug delivery using nanoparticles. However, other considerations such as off target toxicity and harnessing the immunomodulatory properties of the drug positions azithromycin to benefit from inclusion within a nanoformulation.
2.3. Immune Modulation
2.3.1. Cell Signaling
The immunomodulatory properties of the macrolide antimicrobials, especially azithromycin, have been extensively evaluated, leading to their use for the treatment of conditions that have an inflammatory component to their pathology. Early studies demonstrated that erythromycin could suppress cytokine production.49, 50 While clarithromycin51 and roxithromycin52, 53 induce similar anti-inflammatory effects, azithromycin has been the macrolide most extensively studied. Mechanistic investigations demonstrate activity through the regulation of cellular processes involved in inflammation including NF-κB signaling,54–59 inflammasome activation,60, 61 inhibition of phospholipase-A2,62 and autophagy flux.63, 64
Azithromycin causes decreases in NF-κB nuclear translocation, an effect that is associated with the suppression of inflammatory gene expression and cytokine production in both in vitro and animal studies of various models of inflammation and infection.54, 55, 57, 58, 65–67 The impact of the drug on cytokine and chemokine expression leads to reduced immune cell migration, changes in epithelial cell barrier function, and decreased expression of adhesion molecules on endothelial cells of the vasculature.55 Despite impacting several immune mechanisms, instead of an overall immunosuppression, azithromycin modulates the inflammatory response, mainly in macrophages, to one characterized as regulatory. Therefore, azithromycin has the potential to be useful for a number of conditions in which inflammation contributes to the associated pathophysiology. Despite being extensively studied, the primary immunomodulatory mechanism of azithromycin, along with its binding target, is yet to be discovered. Detailed reviews of the immunomodulation induced by azithromycin have recently been published.1, 4
2.4.2. Impact on Immune Cells
The immunomodulatory evaluation of azithromycin has primarily focused on its impact on macrophages. Macrophages reside in almost all body tissues and respond to infection or damage, thereby initiating and coordinating inflammatory responses as well as regulating remodeling and repair mechanisms. Macrophages take on distinct functional phenotypes, including the classical, inflammatory phenotype (termed M1) activated by cytokines such as TNFα or IFNγ when stimulated by foreign antigens or molecules released during cellular damage,68 and the alternative phenotype (termed M2) when activated by the cytokines interleukin (IL)-4 or IL-13, which creates a cell that, in most settings, regulates inflammation and orchestrates remodeling and repair processes.69, 70 Azithromycin has been demonstrated to polarize macrophages to an M2 alternative-like phenotype in vitro in both a mouse cell line and in human monocytes stimulated to undergo M1 activation.58, 71 Azithromycin treatment decreases pro-inflammatory cytokine production and the M1 effector protein iNOS, while increasing the expression of M2 macrophage proteins including arginase, CCL18, and mannose receptor (Figure 2). Subsequently, M2 macrophage polarization was shown to be related to azithromycin’s impact on the interaction between the NF-κB and STAT-1 signaling pathways.56 These effects were confirmed in a study of P. aeruginosa pneumonia in mice, as azithromycin treatment induced regulatory macrophage function within the first few days after being infected with P. aeruginosa-laden agarose beads.72 Treatment led to a decrease in inflammation, a blunting of the influx of neutrophils into the lungs, reduced pulmonary fibrosis, and improved survival.73 These results correspond to the clinical utility of chronic azithromycin therapy in patients with cystic fibrosis, in whom P. aeruginosa drives inflammation and pulmonary functional decline.74–76
Figure 2.
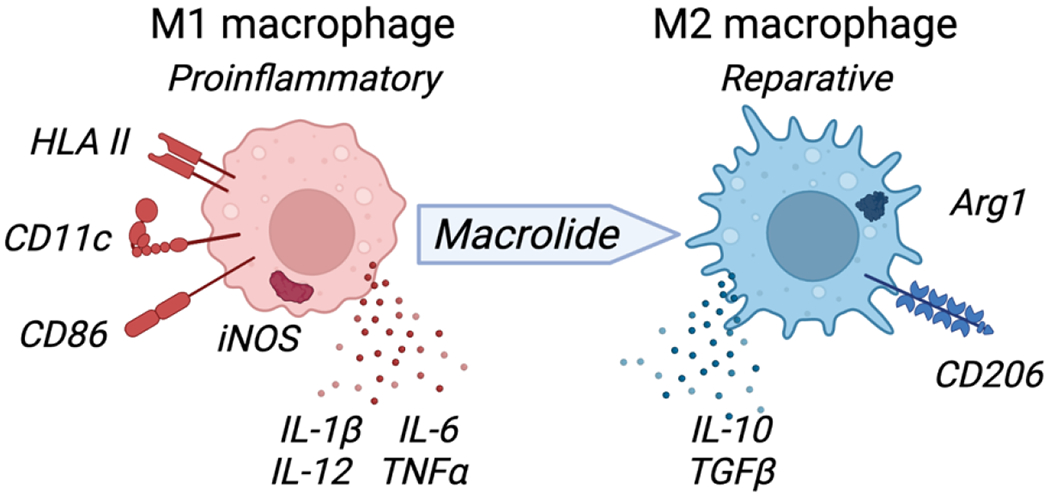
Macrolides promote macrophage polarization to a reparative M2-like phenotype. M1 macrophages treated with macrolides decrease expression of pro-inflammatory markers (HLA-II, CD11c, CD86, iNOS and inflammatory cytokines (IL1β, IL-12, IL-6, TNFα) and increase M2-like reparative phenotype markers including anti-inflammatory cytokines (IL10, TGFβ), Arginase (Arg1) and mannose receptor (CD206). Figure created with BioRender.com.
Although it is likely that azithromycin impacts the recruitment, activation, and function of other immune cell types through its impact on macrophages, the drug also has a direct effect on neutrophils and lymphocytes. Neutrophils often play a destructive role in airway diseases.77–79 Azithromycin accumulates in neutrophils and has been shown to modulate neutrophil extracellular trap (NET) release in vitro as well as decrease neutrophil oxidative burst in human clinical studies.80–82 In addition to impacting T cell subset disposition as demonstrated in a number of animal and human studies using either azithromycin or clarithromycin,83–86 the drug also suppresses T cell function through altering mTOR signaling.87 Despite these direct effects, a majority of the effects of macrolides on these cells are likely to be indirect.
Macrophage polarization with azithromycin is also effective in reducing inflammatory injury in a murine model of acute myocardial infarction (AMI).6, 88 Monocytes and neutrophils migrate to the heart post-AMI and contribute to damaging the myocardium.89, 90 Neutrophil and inflammatory macrophages are associated with defective tissue remodeling and eventually to the development of heart failure in the long-term.91, 92 Azithromycin has been shown to reduce the migration of neutrophils into the myocardium after an AMI is induced in mice. The drug caused a shift in the macrophage response to one dominated by M2-like macrophages, and the resulting effect was associated with decreased scar size and a reduction in mortality.6, 88 Similar evaluations have been characterized in neuroinflammatory conditions in animals including stroke, spinal cord injury, and retinal ischemia.93–96
2.4.3. Macrolide Safety
The macrolides are generally well-tolerated by patients, and among the adverse reactions evaluated in clinical studies, gastrointestinal side effects are reported with the highest incidence. As noted above, the second-generation agents improve upon these symptoms when compared to erythromycin. Macrolides cause significant incidence of headache, allergic rashes, and hepatotoxicity. Clinical study data that was pooled together comparing tolerability of azithromycin to erythromycin shows a decrease in gastrointestinal adverse events reported with azithromycin of 9.6% compared to 20.6% for erythromycin.97 Other rare effects of azithromycin include liver toxicity, interstitial nephritis, and hearing loss, although the vast majority of events are classified as mild or moderate.97–100 In addition to gastrointestinal effects, clarithromycin can cause headache, central nervous system effects, and hepatotoxicity.101 A study of high-dose clarithromycin in elderly patients was associated with nausea, vomiting, metallic taste, and central nervous system effects while a lower dose exposure significantly reduced the adverse reaction incidence.102 For roxithromycin, the majority of adverse events reported are gastrointestinal, and there are also several case reports of liver toxicity with the drug.38
As is the case with all anti-infectives, antimicrobial resistance results from use and exposure to macrolides. Clinical use of these agents not only leads to the development of macrolide resistance but to the resistance of other classes of anti-infectives as well. Macrolide resistance can result from the expression of bacterial efflux proteins, alteration of bacterial membrane permeability, modification of the bacterial ribosomal binding site of the macrolides, or through modification of the macrolide molecules themselves.12, 103 Significant rates of macrolide resistance have emerged for many clinically-relevant pathogens including S. pneumoniae, S. aureus, M. pneumoniae, H. pylori, and Neisseria gonorrhoeae.104–108 The use of chronic azithromycin therapy in patients with cystic fibrosis has been reported to lead to resistance emergence in pathogens including S. pneumoniae and S. aureus,109, 110 however a recent retrospective cohort study utilizing data from the Cystic Fibrosis Foundation Patient Registry found that there was little increased risk of infection with resistant pathogens in patients being treated with chronic azithromycin therapy.111 A recent study in Niger, Sub-Saharan Africa, demonstrated the impact of collateral damage by performing surveillance cultures where azithromycin was distributed twice yearly to preschool children. Macrolide resistance increased 7.5-fold as a result, and in addition resistance increased to other drug classes including the beta-lactams.112 Therefore, macrolides exposure can increase the risk of resistance development, which leads to treatment failures and the need for alternative agents. While the use of macrolides as immune modulators is attractive and will likely continue to drive the repurposing of these agents, chronic therapy may be limited by the potential to cause resistance.
Despite a large therapeutic window, a rare but serious off-target effect of the macrolides is cardiotoxicity that includes QT prolongation and arrhythmia.5 The macrolides inhibit the hERG1 potassium channel on cardiomyocytes causing prolongation of the action potential thereby prolonging the QT interval.113–115 Characterization of this effect in clinical studies led the U.S. Food and Drug Administration to issue a warning concerning the cardiotoxicity of azithromycin.116–120 The use of azithromycin may be associated with the increased cardiac risk or death, and although the data are conflicting, the use of azithromycin should be closely monitored in patients with pre-existing cardiac problems, arrhythmias, baseline QT prolongation, or electrolyte disturbances.117, 118, 121 This could impact the utility of the drug for the treatment of AMI, as patients are prone to arrhythmia immediately after a cardiac event. In the animal studies above evaluating the immunomodulatory properties of azithromycin, mice that were administered azithromycin intravenously immediately post-AMI suffered from a high incidence of cardiac failure and death.6
2.4.4. Clinical Studies of Immunomodulation
Azithromycin has been evaluated in several clinical studies, primarily in patients with chronic inflammatory lung diseases. Azithromycin therapy improves pulmonary function in patients suffering from panbronchiolitis,122, 123 slows the decline of pulmonary function in patients recovering from lung transplantation that develop bronchiolitis obliterans syndrome,81, 124 and decrease the frequency of exacerbations in patients with chronic obstructive pulmonary disease (COPD).125, 126 Efficacy is likely due to a reduction in inflammatory cytokine production and a blunting of neutrophil influx by the macrolides, although the antimicrobial effects of the agents also likely contribute. Long-term azithromycin therapy has also been studied in patients with asthma, although results have varied among the trials with some demonstrating advantages including reductions in acute exacerbations and improved quality of life, while others reported no clinical benefits.127–130 A recent meta-analysis that included results from 7 randomized controlled trials using chronic azithromycin therapy for asthma demonstrated no beneficial impacts on clinical outcomes.131 The bulk of azithromycin’s therapeutic utility in targeting inflammation has been evaluated in patients with cystic fibrosis (CF). These patients endure repetitive lung inflammation that stems from a thickening of the mucus in their lungs due to defective chloride channels in the airways.132 Short-term longitudinal azithromycin therapy has been evaluated in patients with CF in a series of clinical trials.74–76, 133, 134 Recently, the first long-term study in patients with CF showed that azithromycin reduced pulmonary functional decline over a 3 year period.135 These studies demonstrate that azithromycin is safe and effective in decreasing inflammation in the lungs of patients with CF, as well as reduces the frequency of pulmonary exacerbation and the need for antimicrobial therapy.75, 136, 137
Immunomodulatory investigations of the macrolides are active for many disease states and have progressed to phase 3 or 4 clinical trials for several conditions. For most, the effect is likely due to a combination of antimicrobial and immunomodulatory effects. Although the clinicaltrials.gov database is not all-inclusive, a search of the phase 3 and phase 4 registered studies provides a glimpse of the research currently being conducted evaluating the immunomodulatory properties of macrolides.138 We searched the database for registered trials for erythromycin, clarithromycin, roxithromycin, and azithromycin, eliminating trials with endpoints that do not involve utilization of the immunologic effects of the drugs and enumerated the remaining clinical trials in Table 1. Example studies are also provided in the table, which were included based on their ongoing status or available published data. In addition to the respiratory diseases discussed above, clinical trials using macrolides as immunomodulators included studies that are either active or completed for the treatment of Crohn’s disease, inflammation associated with viral infection, pulmonary sarcoidosis, rheumatoid arthritis, multiple myeloma, and dry eye disease.
Table 1.
Summary of Phase 3 and 4 clinical trials using macrolides as immunomodulatory therapeutics.
| Condition | Macrolide – Trialsa | Example Trials | Effect of macrolides – Key findings | |
|---|---|---|---|---|
| Asthma | Azithromycin Clarithromycin Erythromycin |
10 2 1 |
NCT01444469 | AZM – Under-enrolled; No statistical benefit139 |
| NCT00852579 | AZM – Lack of efficacy140 | |||
| NCT04669288 | AZM – Ongoing | |||
| NCT01272635 | AZM – Treatment reduced severity141 | |||
| NCT00318708 | CLR – No improved asthma control142 | |||
| COPD | Azithromycin | 6 | NCT02135354 | AZM – Reduced rate of hospital readmission143 |
| NCT04069312 | AZM – Ongoing | |||
| NCT04481555 | AZM – Ongoing | |||
| Bronchiectasis | Azithromycin Roxithromycin |
3 1 |
NCT02107274 | AZM – Decreased incidence of exacerbation144 |
| NCT04122040 | ROX – Completed – No results reported | |||
| Bronchiolitis obliterans | Azithromycin Clarithromycin Erythromycin |
2 1 1 |
NCT01307462 | AZM – May halt pulmonary decline145 |
| NCT01009619 | AZM – Reduced allograft dysfunction146 | |||
| Bronchopulmonary dysplasia | Azithromycin | 1 | NCT03485703 | AZM – Decreased cytokines, ventilation and death147 |
| COVID-19 | Azithromycin | 31 | NCT04321278 | AZM – Lack of efficacy (w/ hydroxychloroquine)148 |
| NCT04381962 | AZM – Lack of efficacy149 | |||
| NCT04334382 | AZM – Ongoing | |||
| NCT04370782 | AZM – Completed – No results reported | |||
| NCT04332107 | AZM – Lack of efficacy150 | |||
| NCT04673214 | AZM – Modest efficacy as combination therapyb | |||
| NCT04344379 | AZM – Completed – No results reported | |||
| Crohn’s Disease | Azithromycin Clarithromycin |
1 1 |
NCT01596894 | AZM – Improved induction of remission151 |
| NCT00269386 | CLR – Completed – No results reported | |||
| Cystic fibrosis | Azithromycin | 5 | NCT02677701 | AZM – Lack of efficacyb |
| NCT00431964 | AZM – Reduced neutrophils and inflammation136 | |||
| NCT00411736 | AZM – Completed – No results reported | |||
| NCT02054156 | AZM – Reduced pulmonary exacerbations152 | |||
| NCT01270074 | AZM – Completed – No results reported | |||
| Dry eye disease | Azithromycin | 5 | NCT01105624 | AZM – Improved contact lens comfort153 |
| NCT03162497 | AZM – Ongoing | |||
| NCT03953118 | AZM – Ongoing | |||
| Viral pneumonia | Azithromycin Clarithromycin |
4 1 |
NCT02426112 | AZM – Reduced respiratory exacerbation154 |
| NCT01779570 | AZM – Reduced systemic inflammation155 | |||
| NCT05026749 | AZM – Ongoing | |||
| Lung transplantation | Azithromycin | 2 | NCT01915082 | AZM – Anti-inflammatory, but no improved function156 |
| NCT01109160 | AZM – Completed – No results reported | |||
| Multiple myeloma | Clarithromycin | 3 | NCT02516696 | CLR – Ongoing |
| NCT04287660 | CLR – Ongoing | |||
| Cardiovascular disease | Clarithromycin | 1 | NCT00121550 | CLR – Increased cardiovascular mortality157 |
| Sepsis | Clarithromycin | 3 | NCT03345992 | CLR – Completed – No results reported |
| NCT01223690 | CLR – Completed – No results reported | |||
| NCT00297674 | CLR – Improved survival, decreased hospitalization158 | |||
| Rheumatoid arthritis | Roxithromycin | 1 | NCT00439062 | ROX – Improved signs and symptoms159 |
Number of relevant phase 3 and phase 4 clinical trials listed in clinicaltrials.gov
Data available from clinicaltrials.gov and not peer reviewed.
Macrolide therapy has been explored for the treatment of Crohn’s disease, a chronic relapsing inflammatory bowel disease characterized by inflammation and neutrophil influx into the intestinal epithelium, leading to ulceration. The role of intracellular bacteria and their interplay with specific aspects of the immune response remain unclear.160 The impact of azithromycin treatment on Crohn’s disease was evaluated with the addition of azithromycin to metronidazole therapy for 8 weeks. Its addition did not improve the response to therapy, but azithromycin did improve the rate of induction of remission from the disease.151 Pathology of Crohn’s is from a combination of infection and autoimmunity and therefore the drug could be impacting both. Clarithromycin was also studied in a randomized trial in patients with active Crohn’s disease, and when compared to placebo after 3 months of therapy, no differences were observed in response or remission rates.161
The impact of azithromycin on sarcoidosis is thought to be via antimicrobial and immunomodulatory activity. The pathology of sarcoidosis consists of abnormal function of several components of the immune system that leads to the formation of granulomas.162 Hyperpolarized T cell responses and an impaired regulatory T cell defect lead to excessive cytokine production.163 Macrophages are thought to contribute to this pathology through prolonged antigen exposure and inflammatory activation.164 Most current therapies for pulmonary sarcoidosis involve immunosuppression, although recently an anti-mycobacterial treatment regimen for sarcoidosis with concomitant levofloxacin, ethambutol, azithromycin, and rifamycin (CLEAR therapy) has been investigated in clinical trials due to the presence of mycobacterial proteins and DNA in sarcoid lesions.165 An early trial using CLEAR demonstrated improved respiratory parameters, functional capacity, and quality of life in the subset of subjects that completed 8 weeks of therapy.166 However, a randomized, placebo-controlled trial recently completed showed no benefit from CLEAR therapy versus placebo in 49 subjects with pulmonary sarcoidosis treated for 16 weeks.167 A related study of chronic cutaneous sarcoidosis did demonstrate a benefit of decreased granuloma burden and lesion severity compared to placebo.168 Finally, an open-label study of azithromycin alone for the treatment of sarcoidosis, based on the drugs impact on the mTOR pathway, is underway.
Clarithromycin is being studied in a number of trials for multiple myeloma, a plasma cell cancer in which myeloma cells are supported by the bone marrow microenvironment through which abnormal levels of cytokines and growth factors are produced and support tumor proliferation.169 Clarithromycin is being used in combination with a cocktail of other drugs including glucocorticoids, proteasome inhibitors, and other immunomodulators in an attempt to improve the dismal treatment outcomes of relapsed or refractory multiple myeloma.170, 171 Clarithromycin increases the exposure to glucocorticoids by inhibiting their metabolism through cytochrome P450 enzyme 3A4 which is thought to be involved in its benefit.172 Additionally, clarithromycin is thought to reduce tumor growth through the reduction of TNFα and IL-6, by altering plasma cell-bone marrow stromal interactions through immune mechanisms, and through impacting autophagy.173–175 Studies that are exploring synergistic mechanisms of clarithromycin with other agents are active for multiple myeloma at this time (Table 1).
Azithromycin has produced favorable clinical study results in its ability to reduce pulmonary inflammation associated with viral infection. In a study of patients with influenza, azithromycin added as an adjunct therapy to oseltamivir decreased plasma concentrations of several inflammatory cytokines and was associated with faster symptomatic resolution.155 Additionally, there are several studies that have been completed or are ongoing in pediatric patients infected with respiratory syncytial virus (RSV) treated with azithromycin that have produced mixed results.176–179 Most recently, azithromycin is being extensively studied for its immunomodulatory role in the treatment of severe acute respiratory syndrome (SARS)-coronavirus 2 (CoV-2) infection. At the time of this writing, there are 31 active or completed studies of azithromycin for the treatment of Coronavirus Disease 19 (COVID-19) in the clinicaltrials.gov database. Several immune effects of azithromycin are hypothesized to be beneficial for SARS-CoV-2 infection including a reduction in viral burden, a blunting of cytokine storm that is associated with severe COVID-19, and promotion of regulatory immune mechanisms (reviewed in 4). Likely due to the complex immune response that are in some ways helpful and in other ways harmful to the infected patient, study results have been mixed, and the role of azithromycin therapy for COVID-19 remains undetermined.
3. Macrolide Drug Delivery
Several drug carriers have been investigated to reduce the tendency of the macrolides to undergo hydrolysis and reduce the risk of adverse reactions, while enhancing the antibacterial activity at different anatomic locations. Most of the drug carriers reported in the literature are designed to enhance the retention of the antibiotic at the site of infection, and a limited number of macrolide formulations have been reported for their use as immunomodulatory agents. Macrolide delivery to specific tissues has thus far relied on routes of administration to achieve therapeutic drug concentrations, and the formulation parameters have successfully altered the in vivo drug disposition while also reducing adverse events including irritation at the site of injection and cardiac toxicity. Herein, we summarize the in vivo reports of macrolide formulations with special attention to both the drug carrier, target organ, and associated pharmacokinetic properties of the macrolides, when available. The following sections describe both lipid and polymer carriers and organized by the route of administration and/or target tissue.
Lipid-based drug delivery systems are highly tailorable platforms designed to improve the pharmacokinetics and bioavailability of therapeutic payloads to target tissues. Lipid-based drug delivery has been used to administer macrolides through topical, pulmonary, and intravenous routes to achieve therapeutic doses at target tissues. Liposomes are the most commonly studied formulations for macrolide delivery given their utility in other diseases.180 Liposomes are vesicular delivery vehicles composed of a lipid bilayer for incorporation of lipophilic drugs and an aqueous core for entrapment of water-soluble drugs. Other lipid-based drug carriers include ethosomes, niosomes, and micelles. Ethosomes are liposomal in nature, but have high ethanol content, which have proven useful for topical delivery.181 Niosomes are liposomal in architecture but are prepared from non-ionic surfactants and serve as an alternative to liposomal delivery systems.182 Micelles are monolayer vesicles capable of solubilizing hydrophobic drugs in a lipophilic core. Each formulation has been investigated for its ability to stably incorporate macrolides to impart antibacterial activity in vivo.
Early biophysical studies with erythromycin and azithromycin identified parameters necessary to improve macrolide encapsulation efficiency within multilamellar vesicles, indicating that formulations containing phosphatidylglycerol provide optimal macrolide encapsulation.183 The optimization may be due to anionic charge of the lipids capable of interacting with the tertiary amine present in the desosamine carbohydrate of the macrolides. Nonetheless, these studies were followed by Straubinger and colleagues, who reported that up to 33 mol% azithromycin, with respect to phospholipid content, remained stably incorporated and exhibited antibacterial activity in vitro.184 Stable incorporation of therapeutics in the lipid bilayer is dependent on the partition coefficient (LogP) of the molecule being formulated. Hydrophilic drugs exhibit a LogP < 1.7 and are ideal for encapsulation in the liposomal aqueous core, while drugs with a LogP > 5.0 are lipophilic and ideal for incorporation in the lipid bilayer.185 The macrolides exhibit theoretical LogP values (Molinspiration Chemoinformatics, www.molinspiration.com) (erythromycin = 2.3, azithromycin = 2.7, clarithromycin = 2.9, roxithromycin = 2.8) that fall between the ideally hydrophobic and hydrophilic ranges leading to their potential integration in either the bilayer or aqueous interior. Successful strategies to improve the lipophilicity of macrolides have been achieved through ion pairing (Figure 3) with lipophilic additives including octadecanesulfonate186 and cholesteryl hemisuccinate.187 While not all ion pairing additives will improve the encapsulation efficiency of the macrolide, cholesteryl hemisuccinate is an ideal anion for interaction with the tertiary amine of the macrolides resulting in an increased encapsulation efficiency in the bilayer from 74% to 96%.187 Based on the stable incorporation of macrolides in lipid-based carriers through ion pairing or other means, the in vivo evaluation of the formulations for a variety of applications have been tested.
Figure 3.
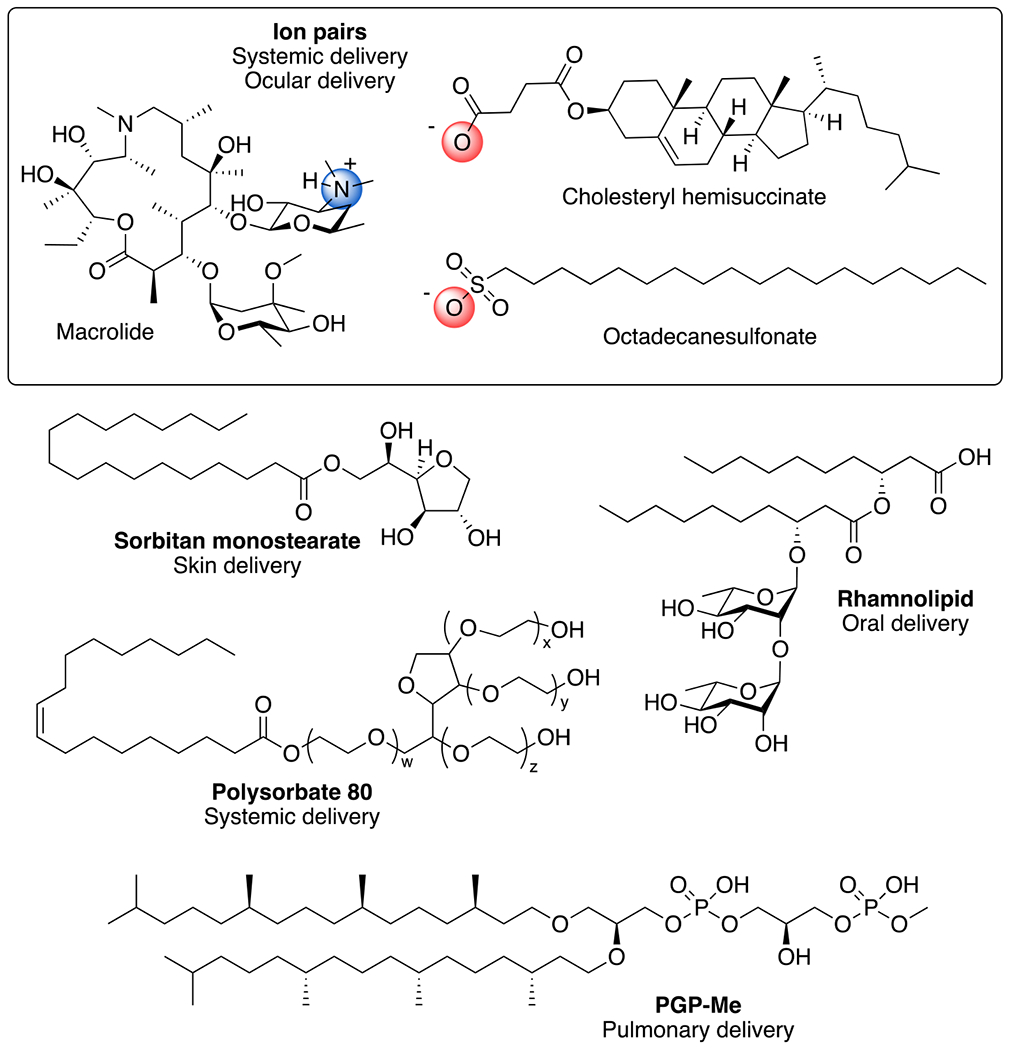
Examples of reagents used for lipid-based macrolide delivery. Ion pairs between the cation of the desosamine of azithromycin is shown with anions from cholesteryl hemisuccinate and octadecanesulfonate. Sorbitan monostearate used to prepare niosomes for skin delivery; rhamnolipid used for oral liposome delivery; polysorbate 80 used for systemic noisome delivery; PGP-Me for nanoarchaeosome pulmonary delivery.
Polymer-based delivery systems, like lipid-based systems, are designed to improve the solubility, bioavailability, and efficacy of the drug payload. Various polymers have been developed to deliver various therapeutic moieties,188, 189 yet few have been utilized for nanoparticle delivery of macrolides. Of the polymeric architectures designed for macrolide delivery, Azasite® is the most widely used, which is an FDA approved formulation consisting of polycarbophil, poloxamer 407 (Figure 4) for mucoadhesion and delayed drug release for the treatment of dry eye disease and ophthalmic infections.190 Additional studies using dendrimers and linear polymer architectures have been evaluated particularly for their in vitro immunomodulatory activity.
Figure 4.
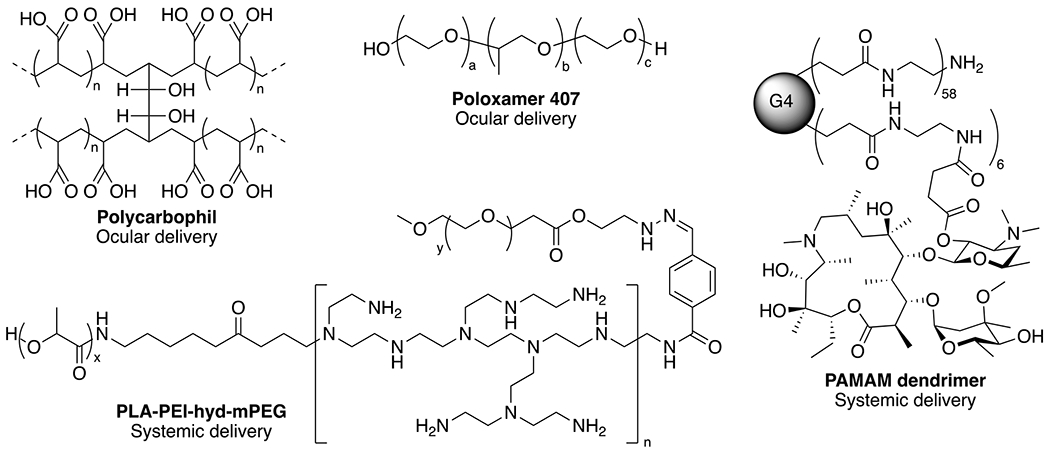
Examples of polymer architectures utilized for delivery of macrolides for antibacterial and immunomodulatory activity. Non-covalent polymer formulations to encapsulate macrolides include polycarbophil, poloxamer and PLE-PEI-hyd-mPEG, while PAMAM dendrimers are used for covalent conjugation of azithromycin.
Poly(amidoamine) (PAMAM) dendrimers are one such architecture that have been employed to improve the solubility, antibacterial and immunomodulatory properties of erythromycin.191, 192 While non-covalent mixtures of generation 2 and 3 PAMAM dendrimers were shown to increase erythromycin solubility more than 7-fold, there was no increase in antibacterial activity.192 Covalent attachment of erythromycin to the generation 4 dendrimer decreased the pro-inflammatory nitrite levels in macrophages, while preserving the antibacterial activity, with the goal of reducing infection and inflammation in periprosthetic inflammation.191 Similar covalent PAMAM constructs containing azithromycin were shown to prevent Chlamydia infections in vitro.193 Alternatively, chitosan modified by ionic interaction with oleic or linoleic acid provided a platform for clarithromycin encapsulation with tissue repair potential due to the wound healing effects of chitosan and fatty acids, while delivering antimicrobial agents.194 The potential utility of these formulations has yet to be realized in vivo, and their clinical relevance remains unclear particularly for PAMAM dendrimers, which have thus far failed to translate into the clinic as a drug carrier for other therapeutics. Nonetheless, continued investigation using in vivo models are necessary to demonstrate their potential as antimicrobial and immunomodulatory agents. Other lipid- and polymer-based constructs have been evaluated in both in vitro and in vivo models and the following sections highlight the utility of these systems for the delivery of macrolides through specific routes of administration, and describes the aspects of development and characterization of these formulations that are still lacking.
3.1. Transdermal Delivery
The skin serves as an ideal location for therapeutic activity due to the tissue access for topical applications. However, several challenges exist to ensure that the therapeutic payload can penetrate deep enough to act on infectious agents or immune cells, without passing through the tissue and bypassing the desired target. Investigation of the therapeutic potential of lipid-based formulations to administer macrolides topically resulted in several early studies with liposomes for delivery across the stratum corneum into the deeper skin strata. An early head-to-head study of erythromycin formulations in hairless mice indicated that liposomal emulsions, prepared by mixing liposomes with isopropyl myristate or mineral oil, achieve higher concentrations of drug in the deeper skin strata as compared to conventional emulsions and standard alcohol solutions of the drug.195 In this case, liposomes were composed of glyceryl dilaureate, cholesterol, and PEG-10-stearyl ether to form nonionic formulations, which alone achieved higher concentrations of drug in the living skin strata as compared to conventional emulsions and alcohol solutions, but liposomal emulsions offered the most significant uptake of erythromycin eight hours after administration.195 Another comparative study of liposomal formulations in ex vivo tissue indicated that azithromycin is retained in the deep skin layers when delivered in a deformable anionic liposome composed of 70% PC lipids, while cationic liposomes fail to migrate past the stratum corneum due to the rigidity of the formulation and the interaction with anionic keratinocytes on the skin surface.196 Some of these same formulations were also evaluated for treatment of cervicovaginal bacterial infections.197 Conventional liposomes, PEGylated liposomes and deformable PEGylated liposomes all exhibit compatibility with cervical cells and resulted in a 2-fold increase in tissue distribution in porcine vaginal tissue as compared to free drug, which penetrated through the tissue rather than being retained.197 While these data suggest that cationic formulations are not ideal for drug delivery to deep skin layers, their utility in other tissue may be more useful. In particular, their ability to disrupt endosomal compartments for delivery into the cytosol has been demonstrated and optimized formulations with properly titrated cationic lipids may be useful in intravenous delivery when targeting neutrophils and monocytes for immune modulation.
Similar to liposomal formulations, niosomes (prepared from sorbitan monostearate) (Figure 3) and ufosomes (prepared from sodium oleate) containing different amorphous roxithromycin dispersions resulted in enhanced penetration across the stratum corneum layer into the epidermal/dermal layers of the skin in an ex vivo model.198 While stratum corneum penetration and skin retention is critical for effective antibacterial activity, these studies utilized ex vivo skin sections and did not evaluate the in vivo antibacterial or immunomodulatory efficacy after administration. Furthermore, these formulations must be compared to other similar formulations including commercially available EMgel and Theramycin Z to properly assess their clinical potential, first in animal models of infection or inflammation and subsequently in human trials.
The development of additional technologies to deliver macrolides to the skin led to several additional studies evaluating the efficacy of macrolide delivery using experimental animal models of infection. One such technology is an ethosomal delivery system composed of phospholipids (2%), ethanol (30%), and water (68%), which was designed specifically to improve skin permeation.181 Ethosomal formulations containing erythromycin result in complete eradication of S. aureus infection in an experimental mouse model.199 Notably, the ethosomes were capable of penetrating the skin barrier to distribute throughout the tissue reaching the deep dermal strata where S. aureus are known to populate.199, 200 While promising in mouse models, ethosomal formulations containing macrolides have not advanced to the clinic.
Additional engineering control was designed in liposomal formulations to enhance active drug release in the target tissue. Jeong and colleagues developed a liposomal formulation with dual macrolide and photodynamic activity susceptible to lipase-driven drug release in the presence of bacteria.201 Liposomes were prepared using DPPC and cholesterol with erythromycin integrated in the lipid bilayer, which were then incubated with the polysaccharide, pullulan, conjugated to pheophorbide A to form a polysaccharide shell (Figure 5). In the presence of lipase from P. acnes, the pullulan is hydrolyzed and the liposomes are disrupted to release both erythromycin and pheophorbide A, a photodynamic therapy agent, for dual antibacterial therapy.201 While erythromycin-only liposomes reduced P. acnes in a nude mouse model of infection, only the dual therapy resulted in undetectable levels of infection with significant reduction in inflammatory cells in the skin.201 In such cases, where the macrolide has antibacterial activity, it is difficult to untangle the antibacterial effects and immunomodulatory effects that lead to reduced immune cell infiltration. Nonetheless, these data support further exploration of such formulations in inflammatory skin conditions such as psoriasis in which photodynamic therapy has shown promise as a treatment modality202 and coupled with azithromycin could offer a synergistic effect to reduce the burden of disease in patients.203, 204
Figure 5.
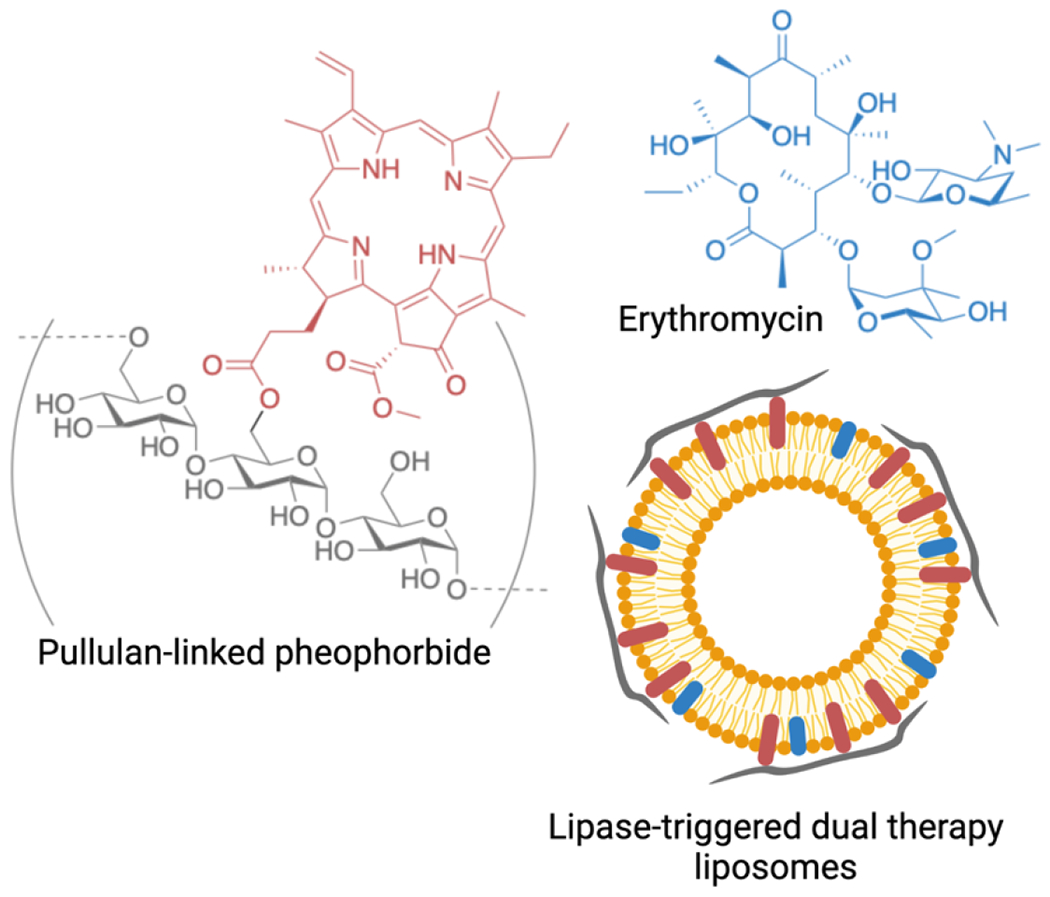
Lipase sensitive dual therapy liposomes prepared with erythromycin (denoted in blue) in the liposomal bilayer, and pullulan coating (denoted in grey) with hydrolysable pheophorbide (denoted in red) anchors in the bilayer. Figure prepared with Biorender.com.
Polymer nanoparticles prepared from poly(ε-caprolactone) to non-covalently encapsulate roxithromycin were developed for delivery into the hair follicle.205 The nanoparticles, which were under 400 nm in diameter, were further encapsulated in a pluronic lecithin organogel to enhance follicular targeting, however, the nanoparticles alone were able to reach deep follicles without the organogel in an ex vivo human scalp section.205 The same team then explored the utility of solid lipid nanoparticles prepared from glyceryl behenate and poloxamer to deliver roxithromycin in an ex vivo model and to the forearm and calf of a volunteer specifically to study the anti-inflammatory activity of the drug.206 The formulation resulted in minimal toxicity using the EpiDerm™ skin irritation test, and efficient and improved delivery to the hair bulb of ex vivo human scalp and chest skin sections were achieved, as compared to non-particulate formulations.206 The formulations were also shown to penetrate the skin of a volunteer, however the small cohort size (n=1), and inadequate control conditions (i.e. bathing, follicle density) preclude from drawing conclusions of this data. Nevertheless, these studies provide a promising platform for future investigation for the treatment of hair loss and acne through anti-inflammatory treatment with macrolides delivered to the hair follicles.206
Two clinical trials were conducted using liposomal formulations of azithromycin for cutaneous leishmaniasis, which is a chronic parasitic disease.207, 208 In both studies, liposomal formulations composed of phosphatidyl choline and cholesterol, were applied to skin lesions and compared to intralesional glucantime,207 or oral azithromycin alone.208 Although both studies include a relatively small number of patients and the patient cohorts are not adequately controlled, both studies indicated that liposomal azithromycin is safe and offered some pharmacologic benefit. Endpoints examined in these human studies included components of inflammation including ulceration, induration, and erythema—therefore, efficacy likely was due to a combination of antimicrobial and anti-inflammatory mechanisms. These data coupled with other mouse studies highlight the potential utility of formulations containing azithromycin to treat inflammatory skin conditions and may increase the efficacy of the drug over current commercially available topical and oral macrolide formulations.
Collectively, these studies indicate that lipid- and polymer-based carriers for macrolides are a reasonable strategy for the treatment of skin infection to penetrate the stratum corneum and deliver macrolides to skin strata that are commonly populated by bacteria and other infectious agents. Given these results and the cutaneous manifestations that arise due to autoimmune disorders and other inflammatory conditions, macrolide delivery is a promising therapeutic strategy for topical autoimmune therapy to modulate the inflammatory milieu associated with disease progression, without the immunosuppressive adverse effects of other therapeutic agents.
3.2. Intravenous Delivery
The most common route of administration for nanocarriers is through intravenous administration to enable direct delivery of formulations into the blood to target tissues of interest and avoid first-pass metabolism of orally delivered drugs. However, some macrolides administered intravenously cause severe pain at the injection site of patients,209, 210 while also posing a risk of cardiac arrhythmia.5 Due to these complications, lipid nanoparticles have been developed to improve the therapeutic index of these drugs by minimizing the off-target effects, while improving the drug targeting, pharmacokinetics, and biodistribution.
Lipid nanospheres prepared from a mixture of triglyceride, phospholipids and egg lecithin were used to encapsulate clarithromycin in ~200 nm particles to evaluate the formulation’s ability to reduce skin irritation.211 Irritation tests in rat paws and rabbit ear veins demonstrated a 2-fold reduction in rat paw licking times and reduced vascular engorgement and erythrocyte aggregation in clarithromycin nanoparticle dosed animals as compared to free clarithromycin.211 Similar formulations also exhibit robust antimicrobial activity in an in vitro growth assay with multidrug-resistant tuberculosis, but efficacy studies were never reported in vivo.212 The reduced irritation with lipid nanoparticle formulations were duplicated in three similar studies using liposomes prepared with phospholipids and ion pairs with cholesterol sulfate, cholesteryl hemisuccinate, or various fatty acids for ion pairing with clarithromycin, resulting in vesicles under 100 nm in diameter.213–215 In rat and rabbit irritation tests, these formulations reduced rat paw licking 5-fold, and the overall reduction in inflammation as assessed by pathology indicated that lipid carriers containing clarithromycin exhibit reduced irritation as compared to the free drug.213–215 Similar results were obtained with a tocopherol succinate emulsion of clarithromycin tested in rabbit ear veins.216 Collectively, these data support the use of formulations to improve drug tolerability, which is relevant to both antimicrobial and immunomodulatory activity, but do not address the need to target specific tissues for therapeutic intervention.
Cationic niosomes prepared from polysorbate 80 (Figure 3), cholesterol, and octadecylamine were investigated for their potential to deliver azithromycin orally or intravenously.217 While oral administration of the nano/microparticles was investigated, the absorption and bioavailability of azithromycin from the niosomes were measured instead of cellular uptake of the whole nanoparticle. However, intravenous administration of the large niosomes (~ 5.9 μm) exhibit increased uptake in the lung relative to other organs and in comparison to small niosomes (~0.95 μm) and free azithromycin.217 While these data only reflect azithromycin concentration, the altered lung accumulation likely has more to do with large particle size accumulating in the lung, rather than its drug release properties. Nonetheless, these formulations present opportunities to harness particle size to target the macrolides to the lungs, particularly in patients with reduced respiratory capacity due to infection or inflammation, who are unable to achieve therapeutic doses through inhalation.
Antibacterial efficacy of liposomal clarithromycin was evaluated in vivo using a murine model of methicillin resistant S. aureus (MRSA) infection.218 Liposomes were prepared with hydrogenated soy phosphatidyl choline (HSPC), cholesterol and distearoyl phosphatidylethanolamine (DSPE)-conjugated polyethylene glycol (PEG2000) in a 15:10:1 ratio with daptomycin encapsulated in the aqueous interior and clarithromycin incorporated in the bilayer.218 While clarithromycin or daptomycin formulations alone did not result in a survival benefit in mice inoculated with MRSA by tail vail injection, formulations containing both drugs did reduce the bacterial load in liver, spleen, lung, kidney, and blood.218 These data indicate that the antibacterial agents encapsulated in the formulations elicit either a systemic effect on bacteria, or the formulations are trafficking to sites of bacterial colonization and inflammation due to the migratory pathways of phagocytic immune cells. Similar antibacterial effects were found with clarithromycin liposomes modified with wheat germ agglutinin (WGA), which targets carbohydrates on bacterial epithelial cell membranes.219
Building upon the studies focused on reducing adverse reactions with the macrolides, we have shown that liposomal formulations containing azithromycin reduce cardiac toxicity and arrhythmia in a murine model of myocardial infarction.6 Notably, mice treated with free azithromycin within 30 minutes of left anterior descending (LAD) coronary artery ligation experience arrhythmia, reduced cardiac output, and a high rate of mortality as compared to those treated with an equivalent dose of azithromycin in a liposome.6 Mice treated with liposomal azithromycin exhibit a maintained heart rate and minimal mortality in the first three days after LAD ligation, in addition to long term benefits 30 days after ligation.6 Indeed, mice treated with liposomal azithromycin also exhibit reduced cardiac inflammatory markers as compared to untreated controls and experience reduced cardiac scar size due to the immunomodulatory effects of azithromycin.6 These data support the premise for investigating azithromycin as a treatment for acute myocardial infarction in patients, and development of a liposomal formulation for clinical use is critical given the inherent cardiotoxicity of the free drug in patients with cardiac complications.5 Continued development of safe and effective azithromycin formulations that reduce the cardiac toxicity will potentially mitigate the risk of arrhythmia and sudden cardiac death seen with the free drug, while reducing inflammation and improving patient cardiovascular outcomes.
A polymer-based micelle containing azithromycin was reported to target P. aeruginosa biofilms.220 The micelles are formed from triblock copolymers of poly(lactic acid)-polyethylenimine-polyethylene glycol (PLA-PEI-PEG) to form a hydrophilic interior for azithromycin encapsulation and a hydrazone linkage between PEI and PEG for pH-sensitive destabilization of the micelles. Upon intravenous delivery to mice infected subcutaneously with P. aeruginosa, polymer micelles containing azithromycin reduced bacterial burden by two orders of magnitude as compared to only one order of magnitude reduction with free azithromycin.220 The additional benefit of the nanoparticle may be due to the passive targeting of the formulation to the infected tissue, or the sustained release of azithromycin from the drug carrier leading to extended systemic exposure and clearance. The exact mechanism that led to the improved bacterial clearance was not determined. If passive targeting is the primary source of therapeutic benefit in this case, similar polymer constructs would be advantageous in the treatment of inflammatory diseases due to the trafficking of phagocytic immune cells to sites of injury.
Albumin microspheres prepared by the emulsion polymerization method to encapsulate clarithromycin resulting in 7-15 μm particles were used to evaluate their accumulation in lung and liver after intravenous administration in naïve mice.221 Notably, clarithromycin was not observed in the liver, but increased over time in the lung to peak at 6 hours. While the microspheres were designed to target the lungs, clarithromycin was not quantified in other organs and the percent injected dose was not reported.221 Similar constructs were prepared with azithromycin and evaluated in albino mice after tail vein injections.222 In this case, azithromycin particles were observed in the blood, liver, spleen and lungs. While free azithromycin equally distributed in each of the organs, the microparticles achieved 40-fold increase in the lungs relative to other tissue and a 9-fold increase in concentration and AUC in the lung relative to free-azithromycin.222 These studies were conducted in naïve mice without infection or injury and the influence of inflammation on the pharmacokinetics and biodistribution of these particles is unclear. The increased lung accumulation is notable and important to determine if a change in distribution is observed in the context of lung inflammation.
The murine chronic lung infection model of P. aeruginosa was used to evaluate the in vivo activity of PAMAM dendrimers conjugated with azithromycin (Figure 5) and electrostatically modified with a PEG-block-poly-L-lysine (PEG-b-PLys) diblock copolymer.223 The nanoparticles were designed to be administered intravenously to target pulmonary infection and disassemble in the acidic biofilm milieu. The pH sensitive nanoparticles were shown to accumulate in the lungs of mice, while those with similar architecture that lacked pH sensitivity failed to accumulate and be retained. While all the formulations were in the 100 nm range, it is unclear how pH sensitive degradation influenced accumulation and retention in the lungs. Nonetheless, the formulations targeted the pulmonary tissue and reduced the bacterial burden in mice leading to reduced alveolar fibrosis and recovery of lung structure.223 Mice treated with the pH sensitive particles also experienced lower pro-inflammatory cytokines (IL1β, IL6, MIP2, TNFα, G-CSF), as well as significantly reduced neutrophil and macrophage populations, which is a hallmark of azithromycin immunomodulatory activity.223 These data are in contrast to the albumin microspheres and the lipid-based delivery system in which 5.9 μm niosomes, but not 0.95 μm niosomes accumulated in the lungs after administration.217 Additional studies are necessary to ascertain why the nanoparticles are performing as they are. Nevertheless, these data highlight the potential to target the lungs with nanoparticles or microparticles after systemic administration.
Collectively, these data highlight the benefits of encapsulating macrolides in different carriers for systemic drug delivery and positions the field well for continued exploration of these formulations in the context of immune modulation. While lipid- and polymer-based drug delivery systems have been utilized for a variety of therapeutics, there is relatively scant literature on the pharmacokinetics and biodistribution of the formulations when macrolides are present. It is unlikely that macrolides would have a significant role in altering the liposomal characteristics to significantly affect their distribution, but the disposition of the drug released from the formulations also needs to be assessed for clinical translation. Given the propensity for phagocytic immune cells to traffic to inflamed tissues, the drug and carrier disposition must be assessed in both healthy and disease-bearing laboratory animals and patient cohorts to fully assess the therapeutic potential of these macrolide delivery vehicles.
3.3. Ophthalmic Delivery
Inflammatory conditions and infections of the eye have also been treated extensively with macrolides given the FDA approval of Azasite® eye drops, a polymer based formulation of azithromycin.224 Nonetheless, the clearance of active azithromycin from the surface of the eye with tears have led to studies focused on retaining the drug concentration in the corneal, conjunctival, retinal and choroidal layers where dry eye and other infections persist. Lipid based delivery systems have shown some promise in improving the benefit of macrolide delivery to the eye to treat infection225 and dry eye disease.187 Solid lipid nanoparticles formed with lipid, surfactant, and cosurfactant to stably incorporate clarithromycin are shown to enhance drug permeation through goat corneas, which the authors attribute to nanoparticle permeation rather than drug release and subsequent permeation.225 In rabbit eyes, the Cmax of azithromycin delivered in the nanoparticles was 150% higher than the free azithromycin solution with a 2.8-fold increase in area-under-the-curve (AUC),225 which positions this formulation as an ideal candidate for continued evaluation to treat ocular infections. Similarly, chitosan coated niosomes loaded with azithromycin achieved similar drug exposure in the cornea and conjunctiva after optimization for particle diameter, entrapment, zeta potential and adhesion force to the corneal mucosal membrane.226 Given that the pathology of these ophthalmic conditions simultaneously include both infection and inflammation, it is critical to evaluate parameters of both when using the formulations, as patients are likely to benefit from both antimicrobial and immunomodulatory mechanisms.
Liposomal azithromycin formulations were also used to treat dry eye disease, which is an inflammatory condition characterized by corneal damage and discomfort.187 In this case, azithromycin is used as an immunomodulatory drug to reduce the inflammation on the corneal surface. Formulations are prepared with azithromycin/cholesteryl hemisuccinate ion pairs (Figure 3) and administered to rat eyes after chemical induction of dry eye. Notably, treatment with liposomal azithromycin resulted in fully healed cornea and conjunctiva with reduced inflammatory infiltration observed by histology, which was not observed with the azithromycin solution alone and is likely due to the corneal permeation of azithromycin when incorporated in a liposomal formulation.187 The improved penetration of azithromycin in the eye when delivered in a liposome and the reduced inflammation after treatment support the continued investigation of liposomal formulations for topical delivery to treat inflammatory eye conditions.
Chitosan nanoparticles have been developed to deliver clarithromycin or azithromycin to the eye as a treatment for bacterial conjunctivitis and other infections.227, 228 Optimized chitosan nanoparticles prepared by ionotropic gelation result in particles less than 200 nm in diameter with positive zeta potential that are capable of increasing the drug retention in the cornea and permeation into the tissue of ex vivo goat eyes due to the mucoadhesive properties of the nanoparticles.227 A more complex architecture utilized poly-lactic acid (PLA) as a hydrophobic core for azithromycin and/or triamcinolone encapsulation and an outer hydrophilic shell of chitosan resulting in positively charged nanoparticles under 300 nm in diameter.228 An extensive biodistribution evaluation in mouse eyes was conducted after administration of the nanoparticles in eye drops as compared to PLA nanoparticles lacking chitosan. Both formulations resulted in significant uptake in the conjunctiva, sclera, choroid and retina with chitosan containing nanoparticles at highest intensity in the choroid.228 Chitosan nanoparticles were retained in the eye for more than 4 hours as compared to a peak at 30 minutes for chitosan-free nanoparticles.228 While the nanoparticles were not tested for efficacy in vivo, they remained active against E. coli and S. aureus in vitro and also exhibit anti-inflammatory activity with reduced TNFα and IL1β in BV-2 microglial cells, with synergistic effects when both azithromycin and triamcinolone are included in the nanoparticle.228 These data provide optimism for future clinical development and offer an avenue to explore the development of other drug carriers with synergistic therapeutic effects in ophthalmic inflammatory conditions.
3.4. Oral Delivery
Oral delivery to enhance the bioavailability, biodistribution and pharmacokinetics of small molecule therapeutics remains an active area of research in drug delivery. Few macrolide formulations have been developed to deliver macrolides to infected tissue in the GI tract,229, 230 while others have investigated the use of formulations to improve oral bioavailability of the drugs.231, 232 Although formulations with improved oral bioavailability are important to consider, those that reach their target organs without releasing their therapeutic payload are important to develop for repurposing as immune modulation agents and the focus of this discussion. Oral delivery to the gut mucosa requires several challenges to be overcome including significant pH changes in the stomach and intestine, as well as the capacity to penetrate the mucus to reach the mucosal surface.
To address these challenges for targeting infections in the stomach, Li et al prepared chitosan nanoparticles with a lipid shell containing rhamnolipids and DSPE-PEG2000.229 Rhamnolipids (Figure 3) have the potential to disrupt biofilm formation, while chitosan serves as an antimicrobial agent with PEG facilitating penetration through the mucus to the epithelial layer to deliver clarithromycin to H. pylori infected cells. Both in vitro and ex vivo models were used to demonstrate that PEG can minimize interactions of the nanoparticle with the mucus layer. Importantly, the authors highlight that PEGylated nanoparticles less than 200 nm in diameter with slightly negative surface charge are ideal for mucus penetration to target H. pylori biofilms.229 Although not used for inflammation, the data generated from these studies can be applied to inflammatory gut conditions in which nanoparticles must pass the mucus layer for macrolide-based immunomodulatory therapy to reduce the burden of disease in patients.
Synergistic drug activity was also explored in the treatment of gastric infection using oral administration of mucoadhesive nanoparticles.233 Glutaraldehyde crosslinked gliadin nanoparticles with or without Concanavalin A conjugated to the nanoparticle surface were prepared with amoxicillin, clarithromycin and omeprazole as a triple therapy to eradicate H. pylori.233 The particles measured ~500 nm in diameter and were administered orally to mice for five consecutive days after infection.233 Single doses were found to be retained in the stomach for over 3 h, and the multiple dosing scheme resulted in almost complete eradication of bacterial burden.233 However, stomach ulceration persisted in all treatment conditions.233 These studies were paralleled by the same group using chitosan-glutamic acid conjugates of 550 nm and 900nm, resulting in similar bacterial clearance rates and recovery in an H. pylori infection model.234
To enhance targeting of PLGA particles to H. pylori infection in mice, gastric epithelial cells were used to coat a clarithromycin-loaded polymer core and then administered orally to infected mice.235 Nanoparticles of about 100 nm in diameter resulted in a reduction of bacterial burden by over 3 orders of magnitude as compared to only 0.5 orders of magnitude with free clarithromycin. This antibacterial effect is encouraging, but it is unclear if this concept can be translated efficiently to target other tissues in the context of inflammation. In particular, Crohn’s disease, ulcerative colitis, and peptic ulcer disease are all inflammatory gastrointestinal conditions that may benefit from targeted macrolide immunomodulation therapy. Clinical trials with free azithromycin and clarithromycin have been conducted in patients with Crohn’s disease151 (Table 1), and the improved targetability with cell coated nanoparticles may result in improved clinical outcomes for these patients.
3.5. Pulmonary Delivery
Although large particles have been shown to target the lungs after intravenous administration, lipid based delivery systems have also been designed for direct pulmonary delivery of macrolides to treat infections and reduce biofilm formation in the lungs.236, 237 The potential to deliver macrolides in lipid nanoparticles relies on the stability of the formulations to be nebulized, while retaining their structural integrity. To address this, nanoarcheosomes prepared from a bacterial derived lipids known as diphytanylglycerol-phospho(glycerol methylphosphate) (PGP-Me) (Figure 3), were evaluated for their stability after nebulization.236 Due to the branched tails of phytanic acid used to prepare the archaeosomes, the formulations exhibit higher structural stability during nebulization and storage.236 Similarly, niosomes prepared from Span 60, cholesterol with clarithromycin exhibit optimal encapsulation efficiency and stability with minimal in vitro cytotoxicity.237 The niosomal formulation improves the pharmacokinetic parameters of the drug when administered to rats by inhalation, thus supporting the use of niosomes for phagocyte targeting. However, this study evaluates drug disposition in circulation after release from the formulation, rather than cell selective uptake and activity in the lung tissue. Continued evaluation of immune cell uptake is warranted as a strategy to treat lung pathologies. This delivery method could be applied to patients with cystic fibrosis or bronchiolitis obliterans syndrome, among other inflammatory conditions where macrolides have already been demonstrated to have efficacy. Increasing concentration in the lungs and limiting systemic distribution remains advantageous to reduce the dosages required, while reducing exposure to bacteria in other anatomic sites that can lead to the emergence of antimicrobial resistance.
4. Summary and Future Outlook
The need for immunomodulatory therapies capable of targeting specific tissues to balance the immune system, reduce inflammation, and promote beneficial outcomes are necessary to clinical care for a variety of diseases. The immunomodulatory effects of macrolides with the foundational work using drug carriers to deliver macrolides for antibacterial activity, provides a clear path toward repurposing the macrolides and drug carriers for immune modulation therapy, without the risks associated with systemic immune suppression. While the majority of drug carriers have been evaluated specifically for the antibacterial activity, a few formulations have shown immunomodulatory activity and the innovation landscape is available for repurposing drug carriers to deliver macrolides for immune modulation (Table 2). The business paradigm of invention, innovation, and imitation applied to cancer nanomedicines by Venditto and Szoka189 can also be applied here to macrolide drug delivery for immune modulation. The technologies and observations made in these areas position this field for new inventions and innovations to advance macrolide drug delivery into the clinic for immune modulation.
Table 2.
Summary of in vivo studies with lipid-based and protein-based drug carriers for antibacterial and immunomodulatory activity.
| Antibacterial activity | Immune modulation | |||
|---|---|---|---|---|
| Lipid-based | Polymer-based | Lipid-based | Polymer-based | |
| Topical | Liposome 195–197, 201, 207, 208 | Micelle220 | Lipid Nanoparticle206 | |
| Niosome198 | ||||
| Ethosome199, 200 | ||||
|
| ||||
| Pulmonary | Liposome218, 236 | Protein-based221, 222 | Dendrimer223 | |
| Niosome217 | Dendrimer223 | |||
|
| ||||
| Cardiac | Liposome6 | |||
|
| ||||
| Ophthalmic | Lipid Nanopartice225 | Polysaccharide227, 228 | Liposome187 | Polysaccharide228 |
| Niosome226 | ||||
|
| ||||
| Oral / Gastrointestinal | Liposome229 | Protein-based233 | ||
| Polysaccharide234 | ||||
| Poly Amino Acid235 | ||||
First, new macrolide derivatives continue to be investigated with the goal of uncoupling antibiotic properties from the immunomodulatory activity to avoid antibiotic resistance when used to treat inflammation. Additionally, these efforts must also focus on avoiding the known cardiotoxicity associated with macrolides, while ensuring that other toxicity and tolerability metrics are met for appropriate clinical utility. The advances thus far have led to several generations of macrolide derivatives that continue to address the shortcomings identified with previous generations. However, the exact mechanism of macrolide driven immune modulation is poorly understood and efforts to determine the eukaryotic target protein/s of macrolides are imperative to advance this field.
Second, the foundational evidence of macrolide formulations with lipid-based and polymer-based nanoparticles to improve tolerability and reduce toxicity offers a clear path for continued effort in this area. While only a small number of recent studies have demonstrated the utility of macrolide drug delivery to reduce inflammation, promote repair and improve outcomes, these data provide a clear path toward clinical translation. Many of the macrolide drug carriers have evaluated their formulations using in vitro and ex vivo systems without advancing into in vivo models. The few that have moved into an in vivo model have focused on reducing infection without performing the critical pharmacokinetic and biodistribution studies, which are necessary as the field moves toward modulating tissue specific inflammatory responses.
Finally, by combining efforts between macrolide development and established drug carriers to address fundamental questions associated with mechanism of action and drug disposition, new discoveries, inventions, and innovations will be made to advance macrolide drug carriers into the clinic. The field is currently poised for significant advances in the next 10 years, and this will be due to the efforts in all areas of the field of macrolide drug delivery.
Highlights.
Macrolide antibiotics exhibit both antibacterial and immunomodulatory activity.
Numerous clinical trials with macrolide antibiotics highlight the therapeutic immunomodulatory potential of these drugs.
Several drug carriers have been evaluated in vivo for their antibiotic properties.
Limited studies with macrolide drug carriers for immune modulation highlight the potential for repurposing drug carriers for macrolide immune modulation.
5. Acknowledgements
VJV would like to thank Prof. Szoka for his guidance while a postdoctoral fellow to navigate the field of liposome drug delivery, and more importantly to appreciate the importance of simple design parameters to make significant impacts in the areas of biotechnology development and translational drug delivery. VJV is supported by grants from the National Institutes of Health (R01HL152081, P20GM130456, R01NS116068).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: Drs. Venditto and Feola are inventors on a pending patent related to liposomal azithromycin.
6 References
- 1.Kricker JA; Page CP; Gardarsson FR; Baldursson O; Gudjonsson T; Parnham MJ, Nonantimicrobial Actions of Macrolides: Overview and Perspectives for Future Development. Pharmacol Rev 2021, 73 (4), 233–262. [DOI] [PubMed] [Google Scholar]
- 2.McGuire J; Bunch RL; Anderson RC; Boaz HE; Flynn EH; Powell HM; Smith JW, Ilotycin, a new antibiotic. Antibiot Chemother (Northfield) 1952, 2 (6), 281–283. [PubMed] [Google Scholar]
- 3.Center for Disease Control and Prevention Outpatient antibiotic prescriptions - United States; 2020. [Google Scholar]
- 4.Venditto VJ; Haydar D; Abdel-Latif A; Gensel JC; Anstead MI; Pitts MG; Creameans J; Kopper TJ; Peng C; Feola DJ, Immunomodulatory Effects of Azithromycin Revisited: Potential Applications to COVID-19. Front Immunol 2021, 12, 574425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giudicessi JR; Ackerman MJ, Azithromycin and risk of sudden cardiac death: Guilty as charged or falsely accused? Clev Clin J Med 2013, 80 (9), 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Darraji A; Donahue RR; Tripathi H; Peng H; Levitan BM; Chelvarajan L; Haydar D; Gao E; Henson D; Gensel JC; Feola DJ; Venditto VJ; Abdel-Latif A, Liposomal delivery of azithromycin enhances its immunotherapeutic efficacy and reduces toxicity in myocardial infarction. Sci Rep 2020, 10 (1), 16596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brockmann H; W H, Pikromycin, ein bitter schmeckendes antibioticum aus actinomyeceten. Chem Ber 1951, 84, 284–288. [Google Scholar]
- 8.Omura S; Morimoto S; Nagate T; Adachi T; Kohno Y, [Research and development of clarithromycin]. Yakugaku Zasshi 1992, 112 (9), 593–614. [DOI] [PubMed] [Google Scholar]
- 9.Girard AE; Girard D; English AR; Gootz TD; Cimochowski CR; Faiella JA; Haskell SL; Retsema JA; Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob Agents Chemother 1987, 31 (12), 1948–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Retsema J; Girard A; Schelkly W; Manousos M; Anderson M; Bright G; Borovoy R; Brennan L; Mason R, Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against gram-negative organisms. Antimicrob Agents Chemother 1987, 31 (12), 1939–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chantot JF; Bryskier A; Gasc JC, Antibacterial activity of roxithromycin: a laboratory evaluation. J Antibiot (Tokyo) 1986, 39 (5), 660–668. [DOI] [PubMed] [Google Scholar]
- 12.Wilson DN, Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat Rev Microbiol 2014, 12 (1), 35–48. [DOI] [PubMed] [Google Scholar]
- 13.Dinos GP, The macrolide antibiotic renaissance. Br J Pharmacol 2017, 174 (18), 2967–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryskier A, Ketolides-telithromycin, an example of a new class of antibacterial agents. Clin Microbiol Infect 2000, 6 (12), 661–669. [DOI] [PubMed] [Google Scholar]
- 15.Clay KD; Hanson JS; Pope SD; Rissmiller RW; Purdum PP 3rd; Banks PM, Brief communication: severe hepatotoxicity of telithromycin: three case reports and literature review. Ann Intern Med 2006, 144 (6), 415–420. [DOI] [PubMed] [Google Scholar]
- 16.Shulman ST; Bisno AL; Clegg HW; Gerber MA; Kaplan EL; Lee G; Martin JM; Van Beneden C, Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis 2012, 55 (10), 1279–1282. [DOI] [PubMed] [Google Scholar]
- 17.Lieberthal AS; Carroll AE; Chonmaitree T; Ganiats TG; Hoberman A; Jackson MA; Joffe MD; Miller DT; Rosenfeld RM; Sevilla XD; Schwartz RH; Thomas PA; Tunkel DE, The diagnosis and management of acute otitis media. Pediatrics 2013, 131 (3), e964–999. [DOI] [PubMed] [Google Scholar]
- 18.Daley CL; Iaccarino JM; Lange C; Cambau E; Wallace RJ; Andrejak C; Bottger EC; Brozek J; Griffith DE; Guglielmetti L; Huitt GA; Knight SL; Leitman P; Marras TK; Olivier KN; Santin M; Stout JE; Tortoli E; van Ingen J; Wagner D; Winthrop KL, Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clin Infect Dis 2020, 71 (4), 905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metlay JP; Waterer GW; Long AC; Anzueto A; Brozek J; Crothers K; Cooley LA; Dean NC; Fine MJ; Flanders SA; Griffin MR; Metersky ML; Musher DM; Restrepo MI; Whitney CG, Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019, 200 (7), e45–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung WK; Graham DY, Clarithromycin for Helicobacter pylori infection. Expert Opin Pharmacother 2000, 1 (3), 507–514. [DOI] [PubMed] [Google Scholar]
- 21.Yang JC; Lu CW; Lin CJ, Treatment of Helicobacter pylori infection: current status and future concepts. World J Gastroenterol 2014, 20 (18), 5283–5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens DL; Bisno AL; Chambers HF; Dellinger EP; Goldstein EJ; Gorbach SL; Hirschmann JV; Kaplan SL; Montoya JG; Wade JC, Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis 2014, 59 (2), 147–159. [DOI] [PubMed] [Google Scholar]
- 23.Workowski KA; Bachmann LH; Chan PA; Johnston CM; Muzny CA; Park I; Reno H; Zenilman JM; Bolan GA, Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm Rep 2021, 70 (4), 1–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bottger EC; Springer B; Prammananan T; Kidan Y; Sander P, Structural basis for selectivity and toxicity of ribosomal antibiotics. EMBO Rep 2001, 2 (4), 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirst HA; Sides GD, New directions for macrolide antibiotics: structural modifications and in vitro activity. Antimicrob Agents Chemother 1989, 33 (9), 1413–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bebear CM; Renaudin H; Aydin MD; Chantot JF; Bebear C, In-vitro activity of ketolides against mycoplasmas. J Antimicrob Chemother 1997, 39 (5), 669–670. [DOI] [PubMed] [Google Scholar]
- 27.Doucet-Populaire F; Capobianco JO; Zakula D; Jarlier V; Goldman RC, Molecular basis of clarithromycin activity against Mycobacterium avium and Mycobacterium smegmatis. J Antimicrob Chemother 1998, 41 (2), 179–187. [DOI] [PubMed] [Google Scholar]
- 28.Morozumi M; Iwata S; Hasegawa K; Chiba N; Takayanagi R; Matsubara K; Nakayama E; Sunakawa K; Ubukata K; Acute Respiratory Diseases Study, G., Increased macrolide resistance of Mycoplasma pneumoniae in pediatric patients with community-acquired pneumonia. Antimicrob Agents Chemother 2008, 52 (1), 348–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarez-Elcoro S; Yao JD, Antimicrobial Macrolides in Clinical Practice. In Macrolide Antibiotics: Chemistr, Biology, and Practice, Second ed.; Omura S, Ed. Elsevier: Amsterdam, 2003; pp 363–402. [Google Scholar]
- 30.Foulds G; Shepard RM; Johnson RB, The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother 1990, 25 Suppl A, 73–82. [DOI] [PubMed] [Google Scholar]
- 31.Retsema JA; Girard AE; Girard D; Milisen WB, Relationship of high tissue concentrations of azithromycin to bactericidal activity and efficacy in vivo. J Antimicrob Chemother 1990, 25 Suppl A, 83–89. [DOI] [PubMed] [Google Scholar]
- 32.Hardy DJ; Guay DR; Jones RN, Clarithromycin, a unique macrolide. A pharmacokinetic, microbiological, and clinical overview. Diagn Microbiol Infect Dis 1992, 15 (1), 39–53. [DOI] [PubMed] [Google Scholar]
- 33.Kurath P; Jones PH; Egan RS; Perun TJ, Acid degradation of erythromycin A and erythromycin B. Experientia 1971, 27 (4), 362. [DOI] [PubMed] [Google Scholar]
- 34.Omura S; Tsuzuki K; Sunazuka T; Marui S; Toyoda H; Inatomi N; Itoh Z, Macrolides with gastrointestinal motor stimulating activity. J Med Chem 1987, 30 (11), 1941–1943. [DOI] [PubMed] [Google Scholar]
- 35.Ferrero JL; Bopp BA; Marsh KC; Quigley SC; Johnson MJ; Anderson DJ; Lamm JE; Tolman KG; Sanders SW; Cavanaugh JH; et al. , Metabolism and disposition of clarithromycin in man. Drug Metab Dispos 1990, 18 (4), 441–446. [PubMed] [Google Scholar]
- 36.Nilsen OG, Comparative pharmacokinetics of macrolides. J Antimicrob Chemother 1987, 20 Suppl B, 81–88. [DOI] [PubMed] [Google Scholar]
- 37.Lassman HB; Puri SK; Ho I; Sabo R; Mezzino MJ, Pharmacokinetics of roxithromycin (RU 965). J Clin Pharmacol 1988, 28 (2), 141–152. [DOI] [PubMed] [Google Scholar]
- 38.Markham A; Faulds D, Roxithromycin. An update of its antimicrobial activity, pharmacokinetic properties and therapeutic use. Drugs 1994, 48 (2), 297–326. [DOI] [PubMed] [Google Scholar]
- 39.Wise R; Kirkpatrick B; Ashby J; Andrews JM, Pharmacokinetics and tissue penetration of roxithromycin after multiple dosing. Antimicrob Agents Chemother 1987, 31 (7), 1051–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrews JM; Ashby JP; Wise R, Factors affecting the in-vitro activity of roxithromycin. J Antimicrob Chemother 1987, 20 Suppl B, 31–37. [DOI] [PubMed] [Google Scholar]
- 41.Kohno Y; Yoshida H; Suwa T; Suga T, Uptake of clarithromycin by rat lung cells. J Antimicrob Chemother 1990, 26 (4), 503–513. [DOI] [PubMed] [Google Scholar]
- 42.Anderson R; Joone G; van Rensburg CE, An in-vitro evaluation of the cellular uptake and infraphagocytic bioactivity of clarithromycin (A-56268, TE-031), a new macrolide antimicrobial agent. J Antimicrob Chemother 1988, 22 (6), 923–933. [DOI] [PubMed] [Google Scholar]
- 43.Hand WL; Hand DL, Characteristics and mechanisms of azithromycin accumulation and efflux in human polymorphonuclear leukocytes. Int J Antimicrob Agents 2001, 18 (5), 419–425. [DOI] [PubMed] [Google Scholar]
- 44.McDonald PJ; Pruul H, Phagocyte uptake and transport of azithromycin. Eur J Clin Microbiol Infect Dis 1991, 10 (10), 828–33. [DOI] [PubMed] [Google Scholar]
- 45.Yanagihara K; Izumikawa K; Higa F; Tateyama M; Tokimatsu I; Hiramatsu K; Fujita J; Kadota J; Kohno S, Efficacy of azithromycin in the treatment of community-acquired pneumonia, including patients with macrolide-resistant Streptococcus pneumoniae infection. Intern Med 2009, 48 (7), 527–535. [DOI] [PubMed] [Google Scholar]
- 46.Ballow CH; Amsden GW; Highet VS; Forrest A, Pharmacokinetics of Oral Azithromycin in Serum, Urine, Polymorphonuclear Leucocytes and Inflammatory vs Non-Inflammatory Skin Blisters in Healthy Volunteers. Clin Drug Investig 1998, 15 (2), 159–167. [DOI] [PubMed] [Google Scholar]
- 47.Mazzei T; Surrenti C; Novelli A; Crispo A; Fallani S; Carla V; Surrenti E; Periti P, Pharmacokinetics of azithromycin in patients with impaired hepatic function. J Antimicrob Chemother 1993, 31 Suppl E, 57–63. [DOI] [PubMed] [Google Scholar]
- 48.Periti P; Mazzei T; Mini E; Novelli A, Clinical pharmacokinetic properties of the macrolide antibiotics. Effects of age and various pathophysiological states (Part I). Clin Pharmacokinet 1989, 16 (4), 193–214. [DOI] [PubMed] [Google Scholar]
- 49.Kudoh S; Azuma A; Yamamoto M; Izumi T; Ando M, Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am J Respir Crit Care Med 1998, 157 (6 Pt 1), 1829–1832. [DOI] [PubMed] [Google Scholar]
- 50.Oda H; Kadota J; Kohno S; Hara K, Erythromycin inhibits neutrophil chemotaxis in bronchoalveoli of diffuse panbronchiolitis. Chest 1994, 106 (4), 1116–1123. [DOI] [PubMed] [Google Scholar]
- 51.Kikuchi T; Hagiwara K; Honda Y; Gomi K; Kobayashi T; Takahashi H; Tokue Y; Watanabe A; Nukiwa T, Clarithromycin suppresses lipopolysaccharide-induced interleukin-8 production by human monocytes through AP-1 and NF-kappa B transcription factors. J Antimicrob Chemother 2002, 49 (5), 745–755. [DOI] [PubMed] [Google Scholar]
- 52.Abdelghaffar H; Vazifeh D; Labro MT, Comparison of various macrolides on stimulation of human neutrophil degranulation in vitro. J Antimicrob Chemother 1996, 38 (1), 81–93. [DOI] [PubMed] [Google Scholar]
- 53.Kamoi H; Kurihara N; Fujiwara H; Hirata K; Takeda T, The macrolide antibacterial roxithromycin reduces bronchial hyperresponsiveness and superoxide anion production by polymorphonuclear leukocytes in patients with asthma. J Asthma 1995, 32 (3), 191–197. [DOI] [PubMed] [Google Scholar]
- 54.Stellari FF; Sala A; Donofrio G; Ruscitti F; Caruso P; Topini TM; Francis KP; Li X; Carnini C; Civelli M; Villetti G, Azithromycin inhibits nuclear factor-κB activation during lung inflammation: an in vivo imaging study. Pharmacol Res Perspect 2014, 2 (5), e00058–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanoh S; Rubin BK, Mechanisms of Action and Clinical Application of Macrolides as Immunomodulatory Medications. Clin Microbiol Rev 2010, 23 (3), 590–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haydar D; Cory TJ; Birket SE; Murphy BS; Pennypacker KR; Sinai AP; Feola DJ, Azithromycin Polarizes Macrophages to an M2 Phenotype via Inhibition of the STAT1 and NF-κB Signaling Pathways. J Immunol 2019, ji1801228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cigana C; Assael BM; Melotti P, Azithromycin selectively reduces tumor necrosis factor alpha levels in cystic fibrosis airway epithelial cells. Antimicrob Agents Chemother 2007, 51 (3), 975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vrancic M; Banjanac M; Nujic K; Bosnar M; Murati T; Munic V; Stupin Polancec D; Belamaric D; Parnham MJ; Erakovic Haber V, Azithromycin distinctively modulates classical activation of human monocytes in vitro. Br J Pharmacol 2012, 165 (5), 1348–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li D-Q; Zhou N; Zhang L; Ma P; Pflugfelder SC, Suppressive effects of azithromycin on zymosan-induced production of proinflammatory mediators by human corneal epithelial cells. Investig Ophthalmol Vis Sci 2010, 51 (11), 5623–5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fendermon EA; Coon TA; Bednash JS; Weathington NM; McDyer JF; Mallampalli RK, Azithromycin decreases NAFP3 mRNA stability in monocytes to limit inflammasome-dependent inflammation. Respir Res 2017, 18 (1), 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gualdoni GA; Lingscheid T; Schmetterer KG; Hennig A; Steinberger P; Zlabinger GJ, Azithromycin inhibits IL-1 secretion and non-canonical inflammasome activation. Sci Rep 2015, 5, 12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Banjanac M; Munic Kos V; Nujic K; Vrancic M; Belamaric D; Crnkovic S; Hlevnjak M; Erakovic Haber V, Anti-inflammatory mechanism of action of azithromycin in LPS-stimulated J774A.1 cells. Pharmacol Res 2012, 66 (4), 357–362. [DOI] [PubMed] [Google Scholar]
- 63.Renna M; Schaffner C; Brown K; Shang S; Tamayo MH; Hegyi K; Grimsey NJ; Cusens D; Coulter S; Cooper J; Bowden AR; Newton SM; Kampmann B; Helm J; Jones A; Haworth CS; Basaraba RJ; DeGroote MA; Ordway DJ; Rubinsztein DC; Floto RA, Azithromycin blocks autophagy and may predispose cystic fibrosis patients to mycobacterial infection. J Clin Invest 2011, 121 (9), 3554–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moriya S; Che XF; Komatsu S; Abe A; Kawaguchi T; Gotoh A; Inazu M; Tomoda A; Miyazawa K, Macrolide antibiotics block autophagy flux and sensitize to bortezomib via endoplasmic reticulum stress-mediated CHOP induction in myeloma cells. Int J Oncol 2013, 42 (5), 1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheung PS; Si EC; Hosseini K, Anti-inflammatory activity of azithromycin as measured by its NF-kappaB, inhibitory activity. Ocul Immunol Inflamm 2010, 18 (1), 32–37. [DOI] [PubMed] [Google Scholar]
- 66.Matsumura Y; Mitani A; Suga T; Kamiya Y; Kikuchi T; Tanaka S; Aino M; Noguchi T, Azithromycin may inhibit interleukin-8 through suppression of Rac1 and a nuclear factor-kappa B pathway in KB cells stimulated with lipopolysaccharide. JPeriodontol 2011, 82 (11), 1623–1631. [DOI] [PubMed] [Google Scholar]
- 67.Iwamoto S; Kumamoto T; Azuma E; Hirayama M; Ito M; Amano K; Ido M; Komada Y, The effect of azithromycin on the maturation and function of murine bone marrow-derived dendritic cells. Clin Exp Immunol 2011, 166 (3), 385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Locati M; Curtale G; Mantovani A, Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu Rev Pathol 2020, 15, 123–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mills CD; Kincaid K; Alt JM; Heilman MJ; Hill AM, M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 2000, 164 (12), 6166–73. [DOI] [PubMed] [Google Scholar]
- 70.Murray PJ; Wynn TA, Protective and pathogenic functions of macrophage subsets. Nature reviews. Immunology 2011, 11 (11), 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murphy BS; Bush HM; Sundareshan V; Davis C; Hagadone J; Cory TJ; Hoy H; Hayes D Jr.; Anstead MI; Feola DJ, Characterization of macrophage activation states in patients with cystic fibrosis. J Cyst Fibros 2010, 9 (5), 314–322. [DOI] [PubMed] [Google Scholar]
- 72.van Heeckeren AM; Schluchter MD, Murine models of chronic Pseudomonas aeruginosa lung infection. Lab Anim 2002, 36 (3), 291–312. [DOI] [PubMed] [Google Scholar]
- 73.Feola DJ; Garvy BA; Cory TJ; Birket SE; Hoy H; Hayes D; Murphy BS, Azithromycin Alters Macrophage Phenotype and Pulmonary Compartmentalization during Lung Infection with Pseudomonas. Antimicrob Agents Chemother 2010, 54 (6), 2437–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Equi A; Balfour-Lynn IM; Bush A; Rosenthal M, Long term azithromycin in children with cystic fibrosis: a randomised, placebo-controlled crossover trial. Lancet 2002, 360 (9338), 978–84. [DOI] [PubMed] [Google Scholar]
- 75.Saiman L; Marshall BC; Mayer-Hamblett N; Burns JL; Quittner AL; Cibene DA; Coquillette S; Fieberg AY; Accurso FJ; Campbell PW 3rd; Macrolide Study G, Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. J Am Med Assoc 2003, 290 (13), 1749–56. [DOI] [PubMed] [Google Scholar]
- 76.Wolter J; Seeney S; Bell S; Bowler S; Masel P; McCormack J, Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 2002, 57 (3), 212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nichols DP; Chmiel JF, Inflammation and its genesis in cystic fibrosis. Pediatr Pulmonol 2015, 50 Suppl 40, S39–56. [DOI] [PubMed] [Google Scholar]
- 78.Gao H; Ying S; Dai Y, Pathological Roles of Neutrophil-Mediated Inflammation in Asthma and Its Potential for Therapy as a Target. J Immunol Res 2017, 2017, 3743048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoenderdos K; Condliffe A, The neutrophil in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2013, 48 (5), 531–539. [DOI] [PubMed] [Google Scholar]
- 80.Bystrzycka W; Manda-Handzlik A; Sieczkowska S; Moskalik A; Demkow U; Ciepiela O, Azithromycin and Chloramphenicol Diminish Neutrophil Extracellular Traps (NETs) Release. Int J Mol Sci 2017, 18 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Verleden GM; Vanaudenaerde BM; Dupont LJ; Van Raemdonck DE, Azithromycin reduces airway neutrophilia and interleukin-8 in patients with bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 2006, 174 (5), 566–570. [DOI] [PubMed] [Google Scholar]
- 82.Culic O; Erakovic V; Cepelak I; Barisic K; Brajsa K; Ferencic Z; Galovic R; Glojnaric I; Manojlovic Z; Munic V; Novak-Mircetic R; Pavicic-Beljak V; Sucic M; Veljaca M; Zanic-Grubisic T; Parnham MJ, Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects. Eur J Pharmacol 2002, 450 (3), 277–289. [DOI] [PubMed] [Google Scholar]
- 83.Pukhalsky AL; Shmarina GV; Kapranov NI; Kokarovtseva SN; Pukhalskaya D; Kashirskaja NJ, Anti-inflammatory and immunomodulating effects of clarithromycin in patients with cystic fibrosis lung disease. Mediators Inflamm 2004, 13 (2), 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Williams AC; Galley HF; Watt AM; Webster NR, Differential effects of three antibiotics on T helper cell cytokine expression. J Antimicrob Chemother 2005, 56 (3), 502–506. [DOI] [PubMed] [Google Scholar]
- 85.Hodge S; Hodge G; Holmes M; Jersmann H; Reynolds PN, Increased CD8 T-cell granzyme B in COPD is suppressed by treatment with low-dose azithromycin. Respirology 2015, 20 (1), 95–100. [DOI] [PubMed] [Google Scholar]
- 86.Verleden SE; Vos R; Vandermeulen E; Ruttens D; Vaneylen A; Dupont LJ; Verbeken EK; Verleden GM; Van Raemdonck DE; Vanaudenaerde BM, Involvement of interleukin-17 during lymphocytic bronchiolitis in lung transplant patients. J Heart Lung Transplant 2013, 32 (4), 447–453. [DOI] [PubMed] [Google Scholar]
- 87.Ratzinger F; Haslacher H; Poeppl W; Hoermann G; Kovarik JJ; Jutz S; Steinberger P; Burgmann H; Pickl WF; Schmetterer KG, Azithromycin suppresses CD4(+) T-cell activation by direct modulation of mTOR activity. Sci Rep 2014, 4, 7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Al-Darraji A; Haydar D; Chelvarajan L; Tripathi H; Levitan B; Gao E; Venditto VJ; Gensel JC; Feola DJ; Abdel-Latif A, Azithromycin therapy reduces cardiac inflammation and mitigates adverse cardiac remodeling after myocardial infarction: Potential therapeutic targets in ischemic heart disease. PLoS ONE 2018, 13 (7), e0200474–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nahrendorf M; Swirski FK; Aikawa E; Stangenberg L; Wurdinger T; Figueiredo J-L; Libby P; Weissleder R; Pittet MJ, The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 2007, 204 (12), 3037–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dutta P; Courties G; Wei Y; Leuschner F; Gorbatov R; Robbins CS; Iwamoto Y; Thompson B; Carlson AL; Heidt T; Majmudar MD; Lasitschka F; Etzrodt M; Waterman P; Waring MT; Chicoine AT; van der Laan AM; Niessen HWM; Piek JJ; Rubin BB; Butany J; Stone JR; Katus HA; Murphy SA; Morrow DA; Sabatine MS; Vinegoni C; Moskowitz MA; Pittet MJ; Libby P; Lin CP; Swirski FK; Weissleder R; Nahrendorf M, Myocardial infarction accelerates atherosclerosis. Nature 2012, 487 (7407), 325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Panizzi P; Swirski FK; Figueiredo JL; Waterman P; Sosnovik DE; Aikawa E; Libby P; Pittet M; Weissleder R; Nahrendorf M, Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol 2010, 55 (15), 1629–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Epelman S; Liu PP; Mann DL, Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nature 2015, 15 (2), 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang B; Bailey WM; Kopper TJ; Orr MB; Feola DJ; Gensel JC, Azithromycin drives alternative macrophage activation and improves recovery and tissue sparing in contusion spinal cord injury. J Neuroinflam 2015, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gensel JC; Kopper TJ; Zhang B; Orr MB; Bailey WM, Predictive screening of M1 and M2 macrophages reveals the immunomodulatory effectiveness of post spinal cord injury azithromycin treatment. Sci Rep 2017, 7 (1), 3956–3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Amantea D; Certo M; Petrelli F; Tassorelli C; Micieli G; Corasaniti MT; Puccetti P; Fallarino F; Bagetta G, Azithromycin protects mice against ischemic stroke injury by promoting macrophage transition towards M2 phenotype. Exp Neurol 2016, 275 Pt 1, 116–125. [DOI] [PubMed] [Google Scholar]
- 96.Varano GP; Parisi V; Adornetto A; Cavaliere F; Amantea D; Nucci C; Corasaniti MT; Morrone LA; Bagetta G; Russo R, Post-ischemic treatment with azithromycin protects ganglion cells against retinal ischemia/reperfusion injury in the rat. Mol Vis 2017, 23, 911–921. [PMC free article] [PubMed] [Google Scholar]
- 97.Hopkins S, Clinical toleration and safety of azithromycin. Am J Med 1991, 91 (3A), 40S–45S. [DOI] [PubMed] [Google Scholar]
- 98.Mansoor GA; Panner BJ; Ornt DB, Azithromycin-induced acute interstitial nephritis. Ann Intern Med 1993, 119 (7 Pt 1), 636–637. [DOI] [PubMed] [Google Scholar]
- 99.Alsowaida YS; Almulhim AS; Oh M; Erstad B; Abraham I, Sensorineural hearing loss with macrolide antibiotics exposure: a meta-analysis of the association. Int J Pharm Pract 2021, 29 (1), 21–28. [DOI] [PubMed] [Google Scholar]
- 100.Lockwood AM; Cole S; Rabinovich M, Azithromycin-induced liver injury. Am J Health Syst Pharm 2010, 67 (10), 810–814. [DOI] [PubMed] [Google Scholar]
- 101.Brown BA; Wallace RJ Jr.; Griffith DE; Girard W, Clarithromycin-induced hepatotoxicity. Clin Infect Dis 1995, 20 (4), 1073–1074. [DOI] [PubMed] [Google Scholar]
- 102.Wallace RJ Jr.; Brown BA; Griffith DE, Drug intolerance to high-dose clarithromycin among elderly patients. Diagn Microbiol Infect Dis 1993, 16 (3), 215–221. [DOI] [PubMed] [Google Scholar]
- 103.Fyfe C; Grossman TH; Kerstein K; Sutcliffe J, Resistance to Macrolide Antibiotics in Public Health Pathogens. Cold Spring Harb Perspect Med 2016, 6 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Felmingham D; Canton R; Jenkins SG, Regional trends in beta-lactam, macrolide, fluoroquinolone and telithromycin resistance among Streptococcus pneumoniae isolates 2001-2004. J Infect 2007, 55 (2), 111–118. [DOI] [PubMed] [Google Scholar]
- 105.Shahsavan S; Emaneini M; Noorazar Khoshgnab B; Khoramian B; Asadollahi P; Aligholi M; Jabalameli F; Eslampour MA; Taherikalani M, A high prevalence of mupirocin and macrolide resistance determinant among Staphylococcus aureus strains isolated from burnt patients. Burns 2012, 38 (3), 378–382. [DOI] [PubMed] [Google Scholar]
- 106.Kawai Y; Miyashita N; Kubo M; Akaike H; Kato A; Nishizawa Y; Saito A; Kondo E; Teranishi H; Wakabayashi T; Ogita S; Tanaka T; Kawasaki K; Nakano T; Terada K; Ouchi K, Nationwide surveillance of macrolide-resistant Mycoplasma pneumoniae infection in pediatric patients. Antimicrob Agents Chemother 2013, 57 (8), 4046–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thung I; Aramin H; Vavinskaya V; Gupta S; Park JY; Crowe SE; Valasek MA, Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther 2016, 43 (4), 514–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.St Cyr S; Barbee L; Workowski KA; Bachmann LH; Pham C; Schlanger K; Torrone E; Weinstock H; Kersh EN; Thorpe P, Update to CDC’s Treatment Guidelines for Gonococcal Infection, 2020. MMWR Morb Mortal Wkly Rep 2020, 69 (50), 1911–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thornton CS; Grinwis ME; Sibley CD; Parkins MD; Rabin HR; Surette MG, Antibiotic susceptibility and molecular mechanisms of macrolide resistance in streptococci isolated from adult cystic fibrosis patients. J Med Microbiol 2015, 64 (11), 1375–1386. [DOI] [PubMed] [Google Scholar]
- 110.Tkadlec J; Varekova E; Pantucek R; Doskar J; Ruzickova V; Botka T; Fila L; Melter O, Characterization of Staphylococcus aureus Strains Isolated from Czech Cystic Fibrosis Patients: High Rate of Ribosomal Mutation Conferring Resistance to MLS(B) Antibiotics as a Result of Long-Term and Low-Dose Azithromycin Treatment. Microb Drug Resist 2015, 21 (4), 416–423. [DOI] [PubMed] [Google Scholar]
- 111.Cogen JD; Onchiri F; Emerson J; Gibson RL; Hoffman LR; Nichols DP; Rosenfeld M, Chronic Azithromycin Use in Cystic Fibrosis and Risk of Treatment-Emergent Respiratory Pathogens. Ann Am Thorac Soc 2018, 15 (6), 702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Doan T; Worden L; Hinterwirth A; Arzika AM; Maliki R; Abdou A; Zhong L; Chen C; Cook C; Lebas E; O’Brien KS; Oldenburg CE; Chow ED; Porco TC; Lipsitch M; Keenan JD; Lietman TM, Macrolide and Nonmacrolide Resistance with Mass Azithromycin Distribution. N Engl J Med 2020, 383 (20), 1941–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mishra A; Friedman HS; Sinha AK, The effects of erythromycin on the electrocardiogram. Chest 1999, 115 (4), 983–986. [DOI] [PubMed] [Google Scholar]
- 114.Stanat SJ; Carlton CG; Crumb WJ Jr.; Agrawal KC; Clarkson CW, Characterization of the inhibitory effects of erythromycin and clarithromycin on the HERG potassium channel. Mol Cell Biochem 2003, 254 (1-2), 1–7. [DOI] [PubMed] [Google Scholar]
- 115.Thomsen MB; Beekman JD; Attevelt NJ; Takahara A; Sugiyama A; Chiba K; Vos MA, No proarrhythmic properties of the antibiotics Moxifloxacin or Azithromycin in anaesthetized dogs with chronic-AV block. Br J Pharmacol 2006, 149 (8), 1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Carlier MB; Garcia-Luque I; Montenez JP; Tulkens PM; Piret J, Accumulation, release and subcellular localization of azithromycin in phagocytic and non-phagocytic cells in culture. Int J Tissue React 1994, 16 (5-6), 211–220. [PubMed] [Google Scholar]
- 117.Russo V; Puzio G; Siniscalchi N, Azithromycin-induced QT prolongation in elderly patient. Acta Biomed 2006, 77 (1), 30–32. [PubMed] [Google Scholar]
- 118.Kezerashvili A; Khattak H; Barsky A; Nazari R; Fisher JD, Azithromycin as a cause of QT-interval prolongation and torsade de pointes in the absence of other known precipitating factors. J Interv Card Electrophysiol 2007, 18 (3), 243–246. [DOI] [PubMed] [Google Scholar]
- 119.Matsunaga N; Oki Y; Prigollini A, A case of QT-interval prolongation precipitated by azithromycin. N Z Med J 2003, 116 (1185), U666. [PubMed] [Google Scholar]
- 120.Samarendra P; Kumari S; Evans SJ; Sacchi TJ; Navarro V, QT prolongation associated with azithromycin/amiodarone combination. Pacing Clin Electrophysiol 2001, 24 (10), 1572–4. [DOI] [PubMed] [Google Scholar]
- 121.Huang BH; Wu CH; Hsia CP; Yin Chen C, Azithromycin-induced torsade de pointes. Pacing Clin Electrophysiol 2007, 30 (12), 1579–1582. [DOI] [PubMed] [Google Scholar]
- 122.Hui D; Yan F; Chen RH, The effects of azithromycin on patients with diffuse panbronchiolitis: a retrospective study of 29 cases. J Thorac Dis 2013, 5 (5), 613–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li H; Zhou Y; Fan F; Zhang Y; Li X; Yu H; Zhao L; Yi X; He G; Fujita J; Jiang D, Effect of azithromycin on patients with diffuse panbronchiolitis: retrospective study of 51 cases. Intern Med 2011, 50 (16), 1663–1669. [DOI] [PubMed] [Google Scholar]
- 124.Shitrit D; Bendayan D; Gidon S; Saute M; Bakal I; Kramer MR, Long-term azithromycin use for treatment of bronchiolitis obliterans syndrome in lung transplant recipients. J Heart Lung Transplant 2005, 24 (9), 1440–1443. [DOI] [PubMed] [Google Scholar]
- 125.Albert RK; Connett J; Bailey WC; Casaburi R; Cooper JA Jr.; Criner GJ; Curtis JL; Dransfield MT; Han MK; Lazarus SC; Make B; Marchetti N; Martinez FJ; Madinger NE; McEvoy C; Niewoehner DE; Porsasz J; Price CS; Reilly J; Scanlon PD; Sciurba FC; Scharf SM; Washko GR; Woodruff PG; Anthonisen NR; Network CCR, Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011, 365 (8), 689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pomares X; Monton C; Espasa M; Casabon J; Monso E; Gallego M, Long-term azithromycin therapy in patients with severe COPD and repeated exacerbations. Int J Chron Obstruct Pulmon Dis 2011, 6, 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Johnston SL, IFN Deficiency in Asthma Attacks. Is Restoring Toll-like Receptor-7 Expression a New Treatment Approach in Severe Asthma? Am J Respir Crit Care Med 2016, 194 (1), 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gibson PG; Yang IA; Upham JW; Reynolds PN; Hodge S; James AL; Jenkins C; Peters MJ; Marks GB; Baraket M; Powell H; Taylor SL; Leong LEX; Rogers GB; Simpson JL, Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet 2017, 390 (10095), 659–668. [DOI] [PubMed] [Google Scholar]
- 129.Hahn DL; Grasmick M; Hetzel S; Yale S; Group AS, Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med 2012, 25 (4), 442–459. [DOI] [PubMed] [Google Scholar]
- 130.Brusselle GG; Vanderstichele C; Jordens P; Deman R; Slabbynck H; Ringoet V; Verleden G; Demedts IK; Verhamme K; Delporte A; Demeyere B; Claeys G; Boelens J; Padalko E; Verschakelen J; Van Maele G; Deschepper E; Joos GF, Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax 2013, 68 (4), 322–329. [DOI] [PubMed] [Google Scholar]
- 131.Tian BP; Xuan N; Wang Y; Zhang G; Cui W, The efficacy and safety of azithromycin in asthma: A systematic review. J Cell Mol Med 2019, 23 (3), 1638–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gentzsch M; Mall MA, Ion Channel Modulators in Cystic Fibrosis. Chest 2018, 154 (2), 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Saiman L; Anstead M; Mayer-Hamblett N; Lands LC; Kloster M; Hocevar-Trnka J; Goss CH; Rose LM; Burns JL; Marshall BC; Ratjen F; Group AZAS, Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 2010, 303 (17), 1707–1715. [DOI] [PubMed] [Google Scholar]
- 134.Clement A; Tamalet A; Leroux E; Ravilly S; Fauroux B; Jais JP, Long term effects of azithromycin in patients with cystic fibrosis: A double blind, placebo controlled trial. Thorax 2006, 61 (10), 895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nichols DP; Odem-Davis K; Cogen JD; Goss CH; Ren CL; Skalland M; Somayaji R; Heltshe SL, Pulmonary Outcomes Associated with Long-Term Azithromycin Therapy in Cystic Fibrosis. Am J Respir Crit Care Med 2020, 201 (4), 430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ratjen F; Saiman L; Mayer-Hamblett N; Lands LC; Kloster M; Thompson V; Emmett P; Marshall B; Accurso F; Sagel S; Anstead M, Effect of azithromycin on systemic markers of inflammation in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa. Chest 2012, 142 (5), 1259–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Southern KW; Barker PM; Solis-Moya A; Patel L, Macrolide antibiotics for cystic fibrosis. Cochrane Database Syst Rev 2012, 11, CD002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.US National Library of Medicine, ClinicalTrials.gov. Accessed Dec 21, 2021.
- 139.Johnston SL; Szigeti M; Cross M; Brightling C; Chaudhuri R; Harrison T; Mansur A; Robison L; Sattar Z; Jackson D; Mallia P; Wong E; Corrigan C; Higgins B; Ind P; Singh D; Thomson NC; Ashby D; Chauhan A; Team AT, Azithromycin for Acute Exacerbations of Asthma : The AZALEA Randomized Clinical Trial. JAMA Intern Med 2016, 176 (11), 1630–1637. [DOI] [PubMed] [Google Scholar]
- 140.Cameron EJ; Chaudhuri R; Mair F; McSharry C; Greenlaw N; Weir CJ; Jolly L; Donnelly I; Gallacher K; Morrison D; Spears M; Evans TJ; Anderson K; Thomson NC, Randomised controlled trial of azithromycin in smokers with asthma. Eur Respir J 2013, 42 (5), 1412–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bacharier LB; Guilbert TW; Mauger DT; Boehmer S; Beigelman A; Fitzpatrick AM; Jackson DJ; Baxi SN; Benson M; Burnham CD; Cabana M; Castro M; Chmiel JF; Covar R; Daines M; Gaffin JM; Gentile DA; Holguin F; Israel E; Kelly HW; Lazarus SC; Lemanske RF Jr.; Ly N; Meade K; Morgan W; Moy J; Olin T; Peters SP; Phipatanakul W; Pongracic JA; Raissy HH; Ross K; Sheehan WJ; Sorkness C; Szefler SJ; Teague WG; Thyne S; Martinez FD, Early Administration of Azithromycin and Prevention of Severe Lower Respiratory Tract Illnesses in Preschool Children With a History of Such Illnesses: A Randomized Clinical Trial. J Am Med Assoc 2015, 314 (19), 2034–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sutherland ER; King TS; Icitovic N; Ameredes BT; Bleecker E; Boushey HA; Calhoun WJ; Castro M; Cherniack RM; Chinchilli VM; Craig TJ; Denlinger L; DiMango EA; Fahy JV; Israel E; Jaijour N; Kraft M; Lazarus SC; Lemanske RF Jr.; Peters SP; Ramsdell J; Sorkness CA; Szefler SJ; Walter MJ; Wasserman SI; Wechsler ME; Chu HW; Martin RJ; National Heart L; Blood Institute’s Asthma Clinical Research, N., A trial of clarithromycin for the treatment of suboptimally controlled asthma. J Allergy Clin Immunol 2010, 126 (4), 747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vermeersch K; Belmans A; Bogaerts K; Gyselinck I; Cardinaels N; Gabrovska M; Aumann J; Demedts IK; Corhay JL; Marchand E; Slabbynck H; Haenebalcke C; Vermeersch S; Verleden GM; Troosters T; Ninane V; Brusselle GG; Janssens W; investigators B. t., Treatment failure and hospital readmissions in severe COPD exacerbations treated with azithromycin versus placebo - a post-hoc analysis of the BACE randomized controlled trial. Respir Res 2019, 20 (1), 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lourdesamy AA; Muthukumaru U, Efficacy of azithromycin in the treatment of bronchiectasis. Respiraology 2014, 19 (8), 1178–1182. [DOI] [PubMed] [Google Scholar]
- 145.Williams KM; Cheng GS; Pusic I; Jagasia M; Burns L; Ho VT; Pidala J; Palmer J; Johnston L; Mayer S; Chien JW; Jacobsohn DA; Pavletic SZ; Martin PJ; Storer BE; Inamoto Y; Chai X; Flowers MED; Lee SJ, Fluticasone, Azithromycin, and Montelukast Treatment for New-Onset Bronchiolitis Obliterans Syndrome after Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant 2016, 22 (4), 710–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ruttens D; Verleden SE; Vandermeulen E; Bellon H; Vanaudenaerde BM; Somers J; Schoonis A; Schaevers V; Van Raemdonck DE; Neyrinck A; Dupont LJ; Yserbyt J; Verleden GM; Vos R, Prophylactic Azithromycin Therapy After Lung Transplantation: Post hoc Analysis of a Randomized Controlled Trial. Am J Transplant 2016, 16 (1), 254–261. [DOI] [PubMed] [Google Scholar]
- 147.Nunes C; Procianoy R; Corso A; Silviera R, Use of azithromycin for the prevention of lung injury in mechanically ventilated preterm neonates: A randomized controlled trial. Neonatology 2020, 117 (4), 522–528. [DOI] [PubMed] [Google Scholar]
- 148.Furtado RHM; Berwanger O; Fonseca HA; Correa TD; Ferraz LR; Lapa MG; Zampieri FG; Veiga VC; Azevedo LCP; Rosa RG; Lopes RD; Avezum A; Manoel ALO; Piza FMT; Martins PA; Lisboa TC; Pereira AJ; Olivato GB; Dantas VCS; Milan EP; Gebara OCE; Amazonas RB; Oliveira MB; Soares RVP; Moia DDF; Piano LPA; Castilho K; Momesso R; Schettino GPP; Rizzo LV; Neto AS; Machado FR; Cavalcanti AB; Investigators CC-BI, Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. Lancet 2020, 396 (10256), 959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hinks TSC; Cureton L; Knight R; Wang A; Cane JL; Barber VS; Black J; Dutton SJ; Melhorn J; Jabeen M; Moss P; Garlapati R; Baron T; Johnson G; Cantle F; Clarke D; Elkhodair S; Underwood J; Lasserson D; Pavord ID; Morgan S; Richards D, Azithromycin versus standard care in patients with mild-to-moderate COVID-19 (ATOMIC2): an open-label, randomised trial. Lancet Respir Med 2021, 9 (10), 1130–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Oldenburg CE; Pinsky BA; Brogdon J; Chen C; Ruder K; Zhong L; Nyatigo F; Cook CA; Hinterwirth A; Lebas E; Redd T; Porco TC; Lietman TM; Arnold BF; Doan T, Effect of Oral Azithromycin vs Placebo on COVID-19 Symptoms in Outpatients With SARS-CoV-2 Infection: A Randomized Clinical Trial. J Am Med Assoc 2021, 326 (6), 490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Levine A; Kori M; Kierkus J; Sigall Boneh R; Sladek M; Escher JC; Wine E; Yerushalmi B; Amil Dias J; Shaoul R; Veereman Wauters G; Boaz M; Abitbol G; Bousvaros A; Turner D, Azithromycin and metronidazole versus metronidazole-based therapy for the induction of remission in mild to moderate paediatric Crohn’s disease : a randomised controlled trial. Gut 2019, 68 (2), 239–247. [DOI] [PubMed] [Google Scholar]
- 152.Mayer-Hamblett N; Retsch-Bogart G; Kloster M; Accurso F; Rosenfeld M; Albers G; Black P; Brown P; Cairns A; Davis SD; Graff GR; Kerby GS; Orenstein D; Buckingham R; Ramsey BW; Group OS, Azithromycin for Early Pseudomonas Infection in Cystic Fibrosis. The OPTIMIZE Randomized Trial. Am J Respir Crit Care Med 2018, 198 (9), 1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nichols JJ; Bickle KM; Zink RC; Schiewe MD; Haque RM; Nichols KK, Safety and efficacy of topical azithromycin ophthalmic solution 1.0% in the treatment of contact lens-related dry eye. Eye Contact Lens 2012, 38 (2), 73–79. [DOI] [PubMed] [Google Scholar]
- 154.Ferrand RA; McHugh G; Rehman AM; Mujuru H; Simms V; Majonga ED; Nicol MP; Flaegstad T; Gutteberg TJ; Gonzalez-Martinez C; Corbett EL; Rowland-Jones SL; Kranzer K; Weiss HA; Odland JO; Group BT, Effect of Once-Weekly Azithromycin vs Placebo in Children With HIV-Associated Chronic Lung Disease: The BREATHE Randomized Clinical Trial. JAMA Netw Open 2020, 3 (12), e2028484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lee N; Wong CK; Chan MCW; Yeung ESL; Tam WWS; Tsang OTY; Choi KW; Chan PKS; Kwok A; Lui GCY; Leung WS; Yung IMH; Wong RYK; Cheung CSK; Hui DSC, Anti-inflammatory effects of adjunctive macrolide treatment in adults hospitalized with influenza: A randomized controlled trial. Antiviral Res 2017, 144, 48–56. [DOI] [PubMed] [Google Scholar]
- 156.Van Herck A; Frick AE; Schaevers V; Vranckx A; Verbeken EK; Vanaudenaerde BM; Sacreas A; Heigl T; Neyrinck AP; Van Raemdonck D; Dupont LJ; Yserbyt J; Verleden SE; Verleden GM; Vos R, Azithromycin and early allograft function after lung transplantation: A randomized, controlled trial. J Heart Lung Transplant 2019, 38 (3), 252–259. [DOI] [PubMed] [Google Scholar]
- 157.Jespersen CM; Als-Nielsen B; Damgaard M; Hansen JF; Hansen S; Helo OH; Hildebrandt P; Hilden J; Jensen GB; Kastrup J; Kolmos HJ; Kjoller E; Lind I; Nielsen H; Petersen L; Gluud C; Group CT, Randomised placebo controlled multicenter trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. Brit Med J 2006, 332 (7532), 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tsaganos T; Raftogiannis M; Pratikaki M; Christodoulou S; Kotanidou A; Papadomichelakis E; Armaganidis A; Routsi C; Giamarellos-Bourboulis EJ, Clarithromycin Leads to Long-Term Survival and Cost Benefit in Ventilator-Associated Pneumonia and Sepsis. Antimicrob Agents Chemother 2016, 60 (6), 3640–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ogrendik M; Karagoz N, Treatment of rheumatoid arthritis with roxithromycin: a randomized trial. Postgrad Med 2011, 123 (5), 220–227. [DOI] [PubMed] [Google Scholar]
- 160.Podolsky DK, Inflammatory bowel disease. N Engl J Med 2002, 347 (6), 417–429. [DOI] [PubMed] [Google Scholar]
- 161.Leiper K; Martin K; Ellis A; Watson AJ; Morris AI; Rhodes JM, Clinical trial: randomized study of clarithromycin versus placebo in active Crohn’s disease. Aliment Pharmacol Ther 2008, 27 (12), 1233–1239. [DOI] [PubMed] [Google Scholar]
- 162.Moller DR; Rybicki BA; Hamzeh NY; Montgomery CG; Chen ES; Drake W; Fontenot AP, Genetic, Immunologic, and Environmental Basis of Sarcoidosis. Ann Am Thorac Soc 2017, 14 (Supplement_6), S429–S436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Drent M; van den Berg R; Haenen GR; van den Berg H; Wouters EF; Bast A, NF-kappaB activation in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2001, 18 (1), 50–56. [PubMed] [Google Scholar]
- 164.Standiford TJ, Macrophage Polarization in Sarcoidosis: An Unexpected Accomplice? Am J Respir Cell Mol Biol 2019, 60 (1), 9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mitchell DN, Mycobacteria and sarcoidosis. Lancet 1996, 348 (9030), 768–769. [DOI] [PubMed] [Google Scholar]
- 166.Drake WP; Richmond BW; Oswald-Richter K; Yu C; Isom JM; Worrell JA; Shipley GR, Effects of broad-spectrum antimycobacterial therapy on chronic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2013, 30 (3), 201–211. [PMC free article] [PubMed] [Google Scholar]
- 167.Drake WP; Culver DA; Baughman RP; Judson MA; Crouser ED; James WE; Ayers GD; Ding T; Abel K; Green A; Kerrigan A; Sesay A; Bernard GR, Phase II Investigation of the Efficacy of Antimycobacterial Therapy in Chronic Pulmonary Sarcoidosis. Chest 2021, 159 (5), 1902–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Drake WP; Oswald-Richter K; Richmond BW; Isom J; Burke VE; Algood H; Braun N; Taylor T; Pandit KV; Aboud C; Yu C; Kaminski N; Boyd AS; King LE, Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. JAMA Dermatol 2013, 149 (9), 1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cho SF; Xing L; Anderson KC; Tai YT, Promising Antigens for the New Frontier of Targeted Immunotherapy in Multiple Myeloma. Cancers (Basel) 2021, 13 (23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Richardson PG; Oriol A; Beksac M; Liberati AM; Galli M; Schjesvold F; Lindsay J; Weisel K; White D; Facon T; San Miguel J; Sunami K; O’Gorman P; Sonneveld P; Robak P; Semochkin S; Schey S; Yu X; Doerr T; Bensmaine A; Biyukov T; Peluso T; Zaki M; Anderson K; Dimopoulos M; investigators O. t., Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): a randomised, open-label, phase 3 trial. Lancet Oncol 2019, 20 (6), 781–794. [DOI] [PubMed] [Google Scholar]
- 171.Stewart AK; Rajkumar SV; Dimopoulos MA; Masszi T; Spicka I; Oriol A; Hajek R; Rosinol L; Siegel DS; Mihaylov GG; Goranova-Marinova V; Rajnics P; Suvorov A; Niesvizky R; Jakubowiak AJ; San-Miguel JF; Ludwig H; Wang M; Maisnar V; Minarik J; Bensinger WI; Mateos MV; Ben-Yehuda D; Kukreti V; Zojwalla N; Tonda ME; Yang X; Xing B; Moreau P; Palumbo A; Investigators A, Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med 2015, 372 (2), 142–152. [DOI] [PubMed] [Google Scholar]
- 172.Spahn JD; Fost DA; Covar R; Martin RJ; Brown EE; Szefler SJ; Leung DY, Clarithromycin potentiates glucocorticoid responsiveness in patients with asthma: results of a pilot study. Ann Allergy Asthma Immunol 2001, 87 (6), 501–505. [DOI] [PubMed] [Google Scholar]
- 173.Matsuoka N; Eguchi K; Kawakami A; Tsuboi M; Kawabe Y; Aoyagi T; Nagataki S, Inhibitory effect of clarithromycin on costimulatory molecule expression and cytokine production by synovial fibroblast-like cells. Clin Exp Immunol 1996, 104 (3), 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nakamura M; Kikukawa Y; Takeya M; Mitsuya H; Hata H, Clarithromycin attenuates autophagy in myeloma cells. Int J Oncol 2010, 37 (4), 815–820. [PubMed] [Google Scholar]
- 175.Mark TM; Forsberg PA; Rossi AC; Pearse RN; Pekle KA; Perry A; Boyer A; Tegnestam L; Jayabalan D; Coleman M; Niesvizky R, Phase 2 study of clarithromycin, pomalidomide, and dexamethasone in relapsed or refractory multiple myeloma. Blood Adv 2019, 3 (4), 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Beigelman A; Isaacson-Schmid M; Sajol G; Baty J; Rodriguez OM; Leege E; Lyons K; Schweiger TL; Zheng J; Schechtman KB; Castro M; Bacharier LB, Randomized trial to evaluate azithromycin’s effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol 2015, 135 (5), 1171–1178 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Srinivasan M; Bacharier LB; Goss CW; Zhou Y; Boomer J; Bram S; Burgdorf D; Burnham CA; Casper T; Castro M; Coverstone A; Haslam M; Kanchongkittiphon W; Kuklinski C; Lian Q; Schechtman K; Storch GA; True K; Wallace MA; Yin-DeClue H; Ahrens E; Wang J; Beigelman A, The azithromycin to prevent wheezing following severe RSV bronchiolitis-II clinical trial: Rationale, study design, methods, and characteristics of study population. Contemp Clin Trials Commun 2021, 22, 100798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kneyber MC; van Woensel JB; Uijtendaal E; Uiterwaal CS; Kimpen JL; Dutch Antibiotics in, R. S. V. T. R. G., Azithromycin does not improve disease course in hospitalized infants with respiratory syncytial virus (RSV) lower respiratory tract disease: a randomized equivalence trial. Pediatr Pulmonol 2008, 43 (2), 142–149. [DOI] [PubMed] [Google Scholar]
- 179.Pinto LA; Pitrez PM; Luisi F; de Mello PP; Gerhardt M; Ferlini R; Barbosa DC; Daros I; Jones MH; Stein RT; Marostica PJ, Azithromycin therapy in hospitalized infants with acute bronchiolitis is not associated with better clinical outcomes: a randomized, double-blinded, and placebo-controlled clinical trial. J Pediatr 2012, 161 (6), 1104–1108. [DOI] [PubMed] [Google Scholar]
- 180.Nardo D; Henson D; Springer J; Venditto VJ, Modulating the immune reponse with liposomal delivery. In Nanomaterials for Clinical Applications: Case Studies in Nanomedicines, Pippa N; Demetzos C, Eds. Elsevier: Amsterdam, Netherlands, 2020; pp 159–211. [Google Scholar]
- 181.Touitou E; Dayan N; Bergelson L; Godin B; Eliaz M, Ethosomes - novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release 2000, 65 (3), 403–418. [DOI] [PubMed] [Google Scholar]
- 182.Bartelds R; Nematollahi MH; Pols T; Stuart MCA; Pardakhty A; Asadikaram G; Poolman B, Niosomes, an alternative for liposomal delivery. PLoS One 2018, 13 (4), e0194179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Stuhne-Sekalec L; Stanacev NZ; Djokic S, Liposomes as carriers of macrolides: preferential association of erythromycin A and azithromycin with liposomes of phosphatidylglycerol containing unsaturated fatty acid(s). J Microencapsul 1991, 8 (2), 171–83. [DOI] [PubMed] [Google Scholar]
- 184.Oh YK; Nix DE; Straubinger RM, Formulation and efficacy of liposome-encapsulated antibiotics for therapy of intracellular Mycobacterium avium infection. Antimicrob Agent Chemother 1995, 39 (9), 2104–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gregoriadis G; Perrie Y, Liposomes. In Encylcopedia of Life Sciences, John Wiley & Sons, Ltd.: Chichester, 2010. [Google Scholar]
- 186.Matschiner S; Neubert R; Wohlrab W, Optimization of topical erythromycin formulations by ion pairing. Skin Pharmacol 1995, 8 (6), 319–325. [DOI] [PubMed] [Google Scholar]
- 187.Ren T; Lin X; Zhang Q; You D; Liu X; Tao X; Gou J; Zhang Y; Yin T; He H; Tang X, Encapsulation of Azithromycin Ion Pair in Liposome for Enhancing Ocular Delivery and Therapeutic Efficacy on Dry Eye. Mol Pharmaceut 2018, 15 (11), 4862–4871. [DOI] [PubMed] [Google Scholar]
- 188.Venditto VJ; Simanek EE, Cancer therapies utilizing the camptothecins: a review of the in vivo literature. Mol Pharmaceut 2010, 7 (2), 307–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Venditto VJ; Szoka FCJ, Cancer nanomedicines: so many papers and so few drugs! Adv Drug Deliv Rev 2013, 65 (1), 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Opitz DL; Harthan JS, Review of Azithromycin Ophthalmic 1% Solution (AzaSite((R))) for the Treatment of Ocular Infections. Ophthalmol Eye Dis 2012, 4, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bosnjakovic A; Mishra MK; Ren W; Kurtoglu YE; Shi T; Fan D; Kannan RM, Poly(amidoamine) dendrimer-erythromycin conjugates for drug delivery to macrophages involved in periprosthetic inflammation. Nanomed 2011, 7 (3), 284–294. [DOI] [PubMed] [Google Scholar]
- 192.Winnicka K; Wroblewska M; Wieczorek P; Sacha PT; Tryniszewska EA, The effect of PAMAM dendrimers on the antibacterial activity of antibiotics with different water solubility. Molecules 2013, 18 (7), 8607–8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mishra MK; Kotta K; Hali M; Wykes S; Gerard HC; Hudson AP; Whittum-Hudson JA; Kannan RM, PAMAM dendrimer-azithromycin conjugate nanodevices for the treatment of Chlamydia trachomatis infections. Nanomed 2011, 7 (6), 935–944. [DOI] [PubMed] [Google Scholar]
- 194.Bonferoni MC; Sandri G; Dellera E; Rossi S; Ferrari F; Mori M; Caramella C, Ionic polymeric micelles based on chitosan and fatty acids and intended for wound healing. Comparison of linoleic and oleic acid. Eur J Pharm Biopharm 2014, 87 (1), 101–106. [DOI] [PubMed] [Google Scholar]
- 195.Jayaraman SC; Ramachandran C; Weiner N, Topical delivery of erythromycin from various formulations: an in vivo hairless mouse study. J Pharm Sci 1996, 85 (10), 1082–1084. [DOI] [PubMed] [Google Scholar]
- 196.Rukavina Z; Segvic Klaric M; Filipovic-Grcic J; Lovric J; Vanic Z, Azithromycin-loaded liposomes for enhanced topical treatment of methicillin-resistant Staphyloccocus aureus (MRSA) infections. Int J Pharm 2018, 553 (1-2), 109–119. [DOI] [PubMed] [Google Scholar]
- 197.Vanic Z; Rukavina Z; Manner S; Fallarero A; Uzelac L; Kralj M; Amidzic Klaric D; Bogdanov A; Raffai T; Virok DP; Filipovic-Grcic J; Skalko-Basnet N, Azithromycin-liposomes as a novel approach for localized therapy of cervicovaginal bacterial infections. Int J Nanomed 2019, 14, 5957–5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Csongradi C; du Plessis J; Aucamp ME; Gerber M, Topical delivery of roxithromycin solid-state forms entrapped in vesicles. Eur J Pharm Biopharm 2017, 114, 96–107. [DOI] [PubMed] [Google Scholar]
- 199.Godin B; Touitou E, Erythromycin ethosomal systems: physicochemical characterization and enhanced antibacterial activity. Curr Drug Deliv 2005, 2 (3), 269–75. [DOI] [PubMed] [Google Scholar]
- 200.Godin B; Touitou E; Rubinstein E; Athamna A; Athamna M, A new approach for treatment of deep skin infections by an ethosomal antibiotic preparation: an in vivo study. J Antimicrob Chemother 2005, 55 (6), 989–994. [DOI] [PubMed] [Google Scholar]
- 201.Jeong S; Lee J; Im BN; Park H; Na K, Combined photodynamic and antibiotic therapy for skin disorder via lipase-sensitive liposomes with enhanced antimicrobial performance. Biomaterials 2017, 141, 243–250. [DOI] [PubMed] [Google Scholar]
- 202.Tandon YK; Yang MF; Baron ED, Role of photodynamic therapy in psoriasis: a brief review. Photodermatol Photoimmunol Photomed 2008, 24 (5), 222–230. [DOI] [PubMed] [Google Scholar]
- 203.Huang SW; Chen YJ; Wang ST; Ho LW; Kao JK; Narita M; Takahashi M; Wu CY; Cheng HY; Shieh JJ, Azithromycin impairs TLR7 signaling in dendritic cells and improves the severity of imiquimod-induced psoriasis-like skin inflammation in mice. J Dermatol Sci 2016, 84 (1), 59–70. [DOI] [PubMed] [Google Scholar]
- 204.Saxena VN; Dogra J, Long-term oral azithromycin in chronic plaque psoriasis: a controlled trial. Eur J Dermatol 2010, 20 (3), 329–333. [DOI] [PubMed] [Google Scholar]
- 205.Glowka E; Wosicka-Frackowiak H; Hyla K; Stefanowska J; Jastrzebska K; Klapiszewski L; Jesionowski T; Cal K, Polymeric nanoparticles-embedded organogel for roxithromycin delivery to hair follicles. Eur J Pharm Biopharm 2014, 88 (1), 75–84. [DOI] [PubMed] [Google Scholar]
- 206.Wosicka-Frackowiak H; Cal K; Stefanowska J; Glowka E; Nowacka M; Struck-Lewicka W; Govedarica B; Pasikowska M; Debowska R; Jesionowski T; Srcic S; Markuszewski MJ, Roxithromycin-loaded lipid nanoparticles for follicular targeting. Int J Pharm 2015, 495 (2), 807–815. [DOI] [PubMed] [Google Scholar]
- 207.Rajabi O; Layegh P; Hashemzadeh S; Khoddami M, Topical liposomal azithromycin in the treatment of acute cutaneous leishmaniasis. Dermatol Ther 2016, 29 (5), 358–363. [DOI] [PubMed] [Google Scholar]
- 208.Abtahi-Naeini B; Hadian S; Sokhanvari F; Hariri A; Varshosaz J; Shahmoradi Z; Feizi A; Khorvash F; Hakamifard A, Effect of Adjunctive Topical Liposomal Azithromycin on Systemic Azithromycin on Old World Cutaneous Leishmaniasis: A Pilot Clinical Study. Iran J Pharm Res 2021, 20 (2), 383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.de Celis G; Gea E; Roig J; Latorre X; Martínez-Benazet J; Gómez J, Comparative tolerability of intravenous erythromycin and clarithromycin in hospitalized patients with community-aquired pneumonia. Clin Drug Invest 2002, 22, 393–398. [Google Scholar]
- 210.Zimmermann T; Laufen H; Riedel K-D; Treadway G; Wildfeuer A, Comparative tolerability of intravenous azithromycin, clarithromycin and erythromycin in healthy volunteers. Clin Drug Invest 2001, 21. [Google Scholar]
- 211.LingHao Q; Wei L, Formulation and evaluation of less-painful clarithromycin lipid microspheres. Arch Pharm Res 2007, 30 (10), 1336–1343. [DOI] [PubMed] [Google Scholar]
- 212.Luo L; Chen Q; Gong H; Liu L; Zhou L; He H; Zhang Y; Yin T; Tang X, Capacity of cholesteryl hemisuccinate in ion pair/phospholipid complex to improve drug-loading, stability and antibacterial activity of clarithromycin intravenous lipid microsphere. Colloids Surf B Biointerfaces 2018, 172, 262–271. [DOI] [PubMed] [Google Scholar]
- 213.Liu X; Sun W; Zhang B; Tian B; Tang X; Qi N; He H; Li H; Jin X, Clarithromycin-loaded liposomes offering high drug loading and less irritation. Int J Pharm 2013, 443 (1-2), 318–327. [DOI] [PubMed] [Google Scholar]
- 214.Ma Y; Li H; Guan S, Characteristics and evaluation of an injectable clarithromycin lipid-based complex in vitro and in vivo. Drug Deliv 2013, 20 (8), 349–355. [DOI] [PubMed] [Google Scholar]
- 215.Geng S; Liu X; Xu H; Cai C; Zhang Y; Yao Q; Xu H; Gou J; Yin T; Xiao W; Tang X, Clarithromycin ion pair in a liposomal membrane to improve its stability and reduce its irritation caused by intravenous administration. Expert Opin Drug Deliv 2016, 13 (3), 337–348. [DOI] [PubMed] [Google Scholar]
- 216.Li J; Nie S; Yang X; Wang C; Cui S; Pan W, Optimization of tocol emulsions for the intravenous delivery of clarithromycin. Int J Pharm 2008, 356 (1-2), 282–290. [DOI] [PubMed] [Google Scholar]
- 217.Zhong M; Feng Y; Liao H; Hu X; Wan S; Zhu B; Zhang M; Xiong H; Zhou Y; Zhang J, Azithromycin cationic non-lecithoid nano/microparticles improve bioavailability and targeting efficiency. Pharm Res 2014, 31 (10), 2857–2867. [DOI] [PubMed] [Google Scholar]
- 218.Li Y; Su T; Zhang Y; Huang X; Li J; Li C, Liposomal co-delivery of daptomycin and clarithromycin at an optimized ratio for treatment of methicillin-resistant Staphylococcus aureus infection. Drug Deliv 2015, 22 (5), 627–637. [DOI] [PubMed] [Google Scholar]
- 219.Meng Y; Hou X; Lei J; Chen M; Cong S; Zhang Y; Ding W; Li G; Li X, Multifunctional Liposomes Enhancing Target and Antibacterial Immunity for Antimicrobial and Anti-Biofilm Against Methicillin-Resistant Staphylococcus aureus. Pharm Res 2016, 33 (3), 763–775. [DOI] [PubMed] [Google Scholar]
- 220.Chen X; Guo R; Wang C; Li K; Jiang X; He H; Hong W, On-demand pH-sensitive surface charge-switchable polymeric micelles for targeting Pseudomonas aeruginosa biofilms development. J Nanobiotechnol 2021, 19 (1), 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ozkan Y; Dikmen N; Isimer A, Clarithromycin targeting to lung: optimization of the size, morphology and release characteristics of albumin microspheres. Acta Pol Pharm 2000, 57 (5), 375–380. [PubMed] [Google Scholar]
- 222.Ramaiah B; Nagaraja SH; Kapanigowda UG; Boggarapu PR; Subramanian R, High azithromycin concentration in lungs by way of bovine serum albumin microspheres as targeted drug delivery: lung targeting efficiency in albino mice. Daru 2016, 24 (1), 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gao Y; Wang J; Chai M; Li X; Deng Y; Jin Q; Ji J, Size and Charge Adaptive Clustered Nanoparticles Targeting the Biofilm Microenvironment for Chronic Lung Infection Management. ACS Nano 2020, 14 (5), 5686–5699. [DOI] [PubMed] [Google Scholar]
- 224.Friedlaender MH; Protzko E, Clinical development of 1% azithromycin in DuraSite, a topical azalide anti-infective for ocular surface therapy. Clin Ophthalmol 2007, 1 (1), 3–10. [PMC free article] [PubMed] [Google Scholar]
- 225.Nair AB; Shah J; Al-Dhubiab BE; Jacob S; Patel SS; Venugopala KN; Morsy MA; Gupta S; Attimarad M; Sreeharsha N; Shinu P, Clarithromycin Solid Lipid Nanoparticles for Topical Ocular Therapy: Optimization, Evaluation and In Vivo Studies. Pharmaceutics 2021, 13 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Eid HM; Naguib IA; Alsantali RI; Alsalahat I; Hegazy AM, Novel Chitosan-Coated Niosomal Formulation for Improved Management of Bacterial Conjunctivitis: A Highly Permeable and Efficient Ocular Nanocarrier for Azithromycin. J Pharm Sci 2021, 110 (8), 3027–3036. [DOI] [PubMed] [Google Scholar]
- 227.Bin-Jumah M; Gilani SJ; Jahangir MA; Zafar A; Alshehri S; Yasir M; Kala C; Taleuzzaman M; Imam SS, Clarithromycin-Loaded Ocular Chitosan Nanoparticle: Formulation, Optimization, Characterization, Ocular Irritation, and Antimicrobial Activity. Int J Nanomedicine 2020, 15, 7861–7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mahaling B; Baruah N; Ahamad N; Maisha N; Lavik E; Katti DS, A non-invasive nanoparticle-based sustained dual-drug delivery system as an eyedrop for endophthalmitis. Int J Pharm 2021, 606, 120900. [DOI] [PubMed] [Google Scholar]
- 229.Li P; Chen X; Shen Y; Li H; Zou Y; Yuan G; Hu P; Hu H, Mucus penetration enhanced lipid polymer nanoparticles improve the eradication rate of Helicobacter pylori biofilm. J Control Release 2019, 300, 52–63. [DOI] [PubMed] [Google Scholar]
- 230.Tran LTC; Gueutin C; Frebourg G; Burucoa C; Faivre V, Erythromycin encapsulation in nanoemulsion-based delivery systems for treatment of Helicobacter pylori infection: Protection and synergy. Biochem Biophys Res Commun 2017, 493 (1), 146–151. [DOI] [PubMed] [Google Scholar]
- 231.Ullah S; Shah MR; Shoaib M; Imran M; Elhissi AM; Ahmad F; Ali I; Shah SW, Development of a biocompatible creatinine-based niosomal delivery system for enhanced oral bioavailability of clarithromycin. Drug Deliv 2016, 23 (9), 3480–3491. [DOI] [PubMed] [Google Scholar]
- 232.Ullah S; Shah MR; Shoaib M; Imran M; Shah SW; Ali I; Ahmed F, Creatinine-based non-phospholipid vesicular carrier for improved oral bioavailability of Azithromycin. Drug Dev Ind Pharm 2017, 43 (6), 1011–1022. [DOI] [PubMed] [Google Scholar]
- 233.Ramteke S; Ganesh N; Bhattacharya S; Jain NK, Triple therapy-based targeted nanoparticles for the treatment of Helicobacter pylori. J Drug Target 2008, 16 (9), 694–705. [DOI] [PubMed] [Google Scholar]
- 234.Ramteke S; Ganesh N; Bhattacharya S; Jain NK, Amoxicillin, clarithromycin, and omeprazole based targeted nanoparticles for the treatment of H. pylori. J Drug Target 2009, 17 (3), 225–234. [DOI] [PubMed] [Google Scholar]
- 235.Angsantikul P; Thamphiwatana S; Zhang Q; Spiekermann K; Zhuang J; Fang RH; Gao W; Obonyo M; Zhang L, Coating nanoparticles with gastric epithelial cell membrane for targeted antibiotic delivery against Helicobacter pylori infection. Adv Ther (Weinh) 2018, 1 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Altube MJ; Martinez MMB; Malheiros B; Maffia PC; Barbosa LRS; Morilla MJ; Romero EL, Fast Biofilm Penetration and Anti-PAO1 Activity of Nebulized Azithromycin in Nanoarchaeosomes. Mol Pharm 2020, 17 (1), 70–83. [DOI] [PubMed] [Google Scholar]
- 237.Shilakari Asthana G; Sharma PK; Asthana A, In Vitro and In Vivo Evaluation of Niosomal Formulation for Controlled Delivery of Clarithromycin. Scientifica (Cairo) 2016, 2016, 6492953. [DOI] [PMC free article] [PubMed] [Google Scholar]


