Abstract
Peritoneal washings used for cytologic evaluation are collected at the outset of surgical exploration of women with gynecologic cancers to assist in determining extent of disease and follow-up therapy.
While there are similarities to ascites, these samples have differences that must be recognized in order to avoid false positive interpretations. Non-neoplastic mesothelial alterations including heterogeneous reactive changes, endosalpingiosis , endometriosis and tumor rupture are typically not seen in ascites samples but can be seen in peritoneal washings from women with malignancies that have not extended to the peritoneal cavity.
Awareness of these potential pitfalls and knowledge of the associated tumor type will facilitate accurate interpretation. When these caveats are recognized, peritoneal washing cytology results are a useful adjunct in helping to determine patient follow-up in women with gynecologic malignancies.
Keywords: Peritoneal washings, gynecologic neoplasms, endosalpingiosis, endometriosis, ovarian neoplasms, endometrial cancer, cervical cancer

INTRODUCTION
Involvement of serosa by cancer cells, even in the absence of effusion, correlates with poor prognosis. In this subset of cases without effusion, retrieval of a representative specimen may be achieved as washings, lavages, brushings, scrapings, and touch imprints for staging various cancers.[1]
Peritoneal washing cytology (PWC) was first described by Keettel and Elkins[2-4] in 1956 and has gained acceptance as a part of the surgical–pathologic evaluation of gynecologic malignancy. Creasman and Rutledge[5] found that PWC results correlated well with prognosis in ovarian, endometrial, and cervical malignancies. Many subsequent studies reinforced the value of peritoneal washing (PW).[6-11] Staging protocols for ovarian and endometrial carcinoma[12] include PW. More recently, concerns have been expressed that unrecognized benign patterns could result in cytologic overcalls.[13-16]
Because the association of positive PWC with worse prognosis has been controversial, the current FIGO staging system has de-emphasized PWC in treatment decisions, although it can still be performed and reported.[12,17,18]
Some centers have expanded the use of PWC to evaluation of some non-gynecologic cancers. Positive PWC has been associated with worse prognosis for gastric[19] and pancreatic cancers[20] in some studies.
SPECIMEN COLLECTION PROCEDURE
For staging purposes, the peritoneal cytologic sample must be obtained as soon as the peritoneal cavity is entered. Occasionally, tumor cells are inadvertently spilled into the peritoneal cavity during the course of the exploratory laparotomy and removal of the primary tumor. The significance of tumor cells identified in washings performed after iatrogenic contamination of the peritoneum is not known. • While artificial contamination of the peritoneum with tumor is disquieting, the prognostic value of PWC is predicated on the assumption that the results reflect the natural biology of the patient’s tumor. This dilemma can be avoided when the cytologic samples are collected as an initial step in the exploration.
If there is pre-existing spontaneous fluid in the pelvis, this may be collected and labeled as ‘peritoneal fluid’ or, if excessive, as ‘ascites.’ An 100 mL volume of saline, preferably a balanced salt solution such as peritoneal dialysis fluid, is instilled into the peritoneal cavity, agitated, aspirated, and sent for cytologic evaluation.
In order to save costs, Sharifi et al[21] recommended that peritoneal washings collected during laparotomy for disease presumed to be non-neoplastic be held and sent for cytology only if malignancy is subsequently diagnosed. However, abrading the mesothelial surfaces is traumatic, and delay in processing an unfixed PW specimen can result in compromised cellular detail. Presumably, a frozen section or other intraoperative pathologic consultation needed by the surgeon to plan the surgical can delay the processing. If this approach is contemplated, it would be wise to utilize balanced salt solution for the washings.
INTERPRETATION APPROACH
• Although washing the peritoneal cavity with saline or balanced salt solution may be expected to yield a cell sample similar to ascitic fluid, there are differences that must be appreciated in order to accurately evaluate these samples. Rather than the freely exfoliated cells seen in spontaneous ascitic fluid, many of the cells present in peritoneal washings have been mechanically stripped from the underlying connective tissue. In addition, cell types that are not commonly seen in ascites (probably because they are normally shed in very low numbers) may be stripped from the surfaces.[14-15] The differences between ascites and peritoneal washings are shown in Table 1. Awareness of these differences will aid in interpretation. It should be noted that, unlike spontaneous effusions,[22] a “second population” in peritoneal washings does not necessarily represent malignancy.
Table 1:
Comparison of cell patterns in peritoneal washings and ascites.
| Characteristic | Ascites | Washings |
|---|---|---|
| Collection | Spontaneous exfoliation | Mechanically abraded cells |
| Aggregation | Three-dimensional groups | Two-dimensional sheets |
| Cell shape | Rounded shapes | Flat mesothelial cells |
BENIGN CONDITIONS
Peritoneal washings are typically performed when there is clinical suspicion of malignancy. Particularly for ovarian lesions, the washing will be performed before a histologic diagnosis is known. It is important that benign conditions that can be confused with malignancy be understood and excluded [Table 2] prior to rendering a malignant interpretation in peritoneal washings. Indeed, the nonneoplastic changes found in peritoneal washings are the key differences between intraoperative washings and spontaneous ascites. This difference is particularly important in cases in which a primary malignant tumor is identified but no intraperitoneal extension has been observed histologically.
Table 2:
Changes associated with reactive and benign pathologies in peritoneal washings.
| Pathology | Cytologic finding | Pitfall |
|---|---|---|
| Inflammatory conditions | Mixture of mesothelial patterns in the same sample | Can be confused with cancer |
| Bulky benign tumors: • Leiomyomas • Ovarian fibrothecomas |
Mixture of mesothelial patterns in the same sample | Can be confused with cancer |
| Adhesions | Capillary tangles | |
| Detached ciliary tufts | Anucleated cytoplasmic fragments with cilia | |
| Surface reactions • Endosalpingiosis • Endometriosis • Collagen globules (Collagen balls) • Surface component of benign tumors |
Epithelial cell clusters with psammoma bodies Endometrial epithelial cells and stroma; hemosiderin-laden macrophages Small round bodies of collagenized stroma and flat mesothelial cells Epithelial cells forming cast-like group of ovarian surface clefts |
Pattern overlaps with serous tumors of low malignant potential and carcinoma Can be confused with cancer Overlaps with low-grade carcinomas |
| Ruptured benign epithelial cyst or cystic tumor | Sheets and groups of epithelial cells | Can be confused with carcinoma |
MESOTHELIAL CELL POLYMORPHISM
Because the mesothelial cells in peritoneal washings are typically stripped from the underlying connective tissue, these cells are commonly present as flat sheets [Figure 1] of varying cell numbers.[13-15,23,24] If large, these sheets are frequently folded or rolled. In general, these sheets are easily recognized as such and create little difficulty. Sometimes the sheets are squeezed together so that the nucleocytoplasmic ratio is artifactually distorted [Figure 2]. The cells typically are arranged in a uniform honeycomb pattern. Single cells with flattened, polygonal shapes can also be seen in some cases [Figure 3]. Nuclei are centrally placed with a roundoval, occasionally bilobed appearance [Figure 4]. The nuclei in normal mesothelial cells can vary considerably in a single sample [Figure 5]. The chromatin pattern is finely granular and a single micronucleolus is usually seen in the well-preserved mesothelial cells [Figure 6]. The cytoplasm is thin with a polygonal shape. The staining quality of the cytoplasm is variable, although usually faintly cyanophilic. Occasionally, multinucleated mesothelial cells are present as a part of these sheets, with six or more nuclei forming a ring within the center of a giant cytoplasm [Figure 7]. Degeneration of the mesothelial cells in peritoneal washing samples shows as paranuclear vacuoles that impinge on the nucleus [Figure 8]. This may give the appearance of jagged, angulated nuclear contours.
Figure 1:
Flat sheet of normal mesothelial cells in a peritoneal washing. This cell pattern reflects the manner of collecting the cells in a peritoneal washing and highlights the difference from spontaneously exfoliated cells in effusions. (Modified PAP stain, 20X.)
Figure 2:
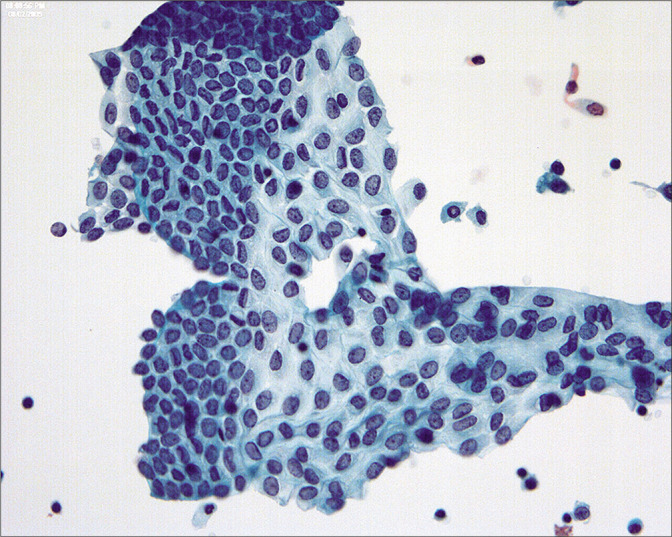
The mesothelial sheets are subject to distortion so that the nuclear-cytoplasmic ratio can show considerable variation in reactive mesothelial cells. (Modified PAP stain, 40X.)
Figure 3:
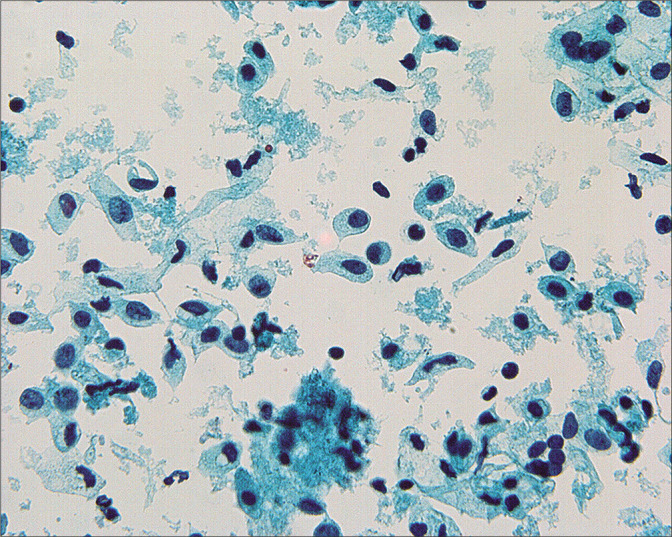
In some cases, the mesothelial cells are found as predominantly single cells. (Modified PAP stain, 60X.)
Figure 4:
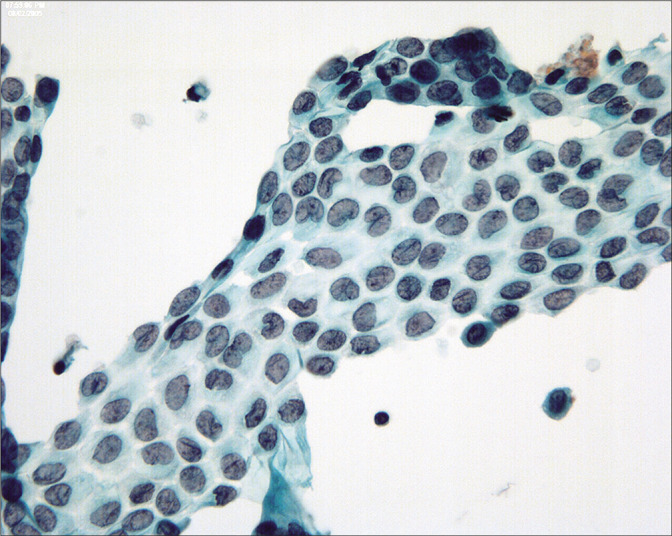
Although generally round-oval, mesothelial nuclei in these samples can sometimes appear bi-lobated and multi-lobated. Some nuclei also show longitudinal grooves. (Modified PAP stain, 60X.)
Figure 5:
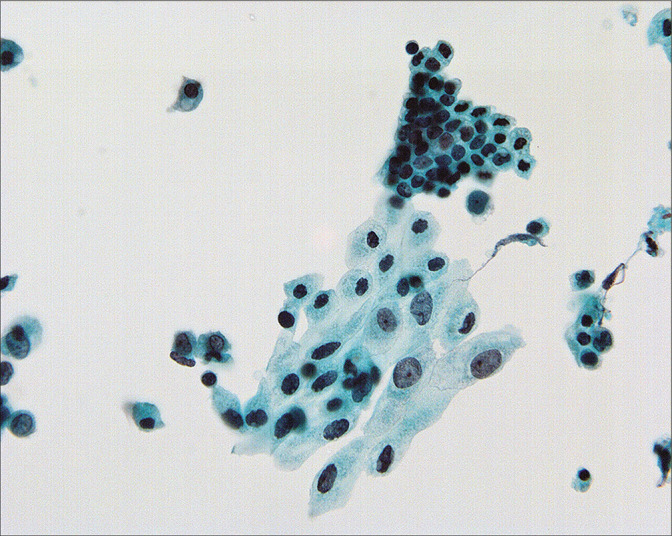
Nuclear size in mesothelial cells can vary considerably in peritoneal washings and it is important not to overcall in such cases. All of the cells in this field are reactive. (Modified PAP stain, 60X.)
Figure 6:
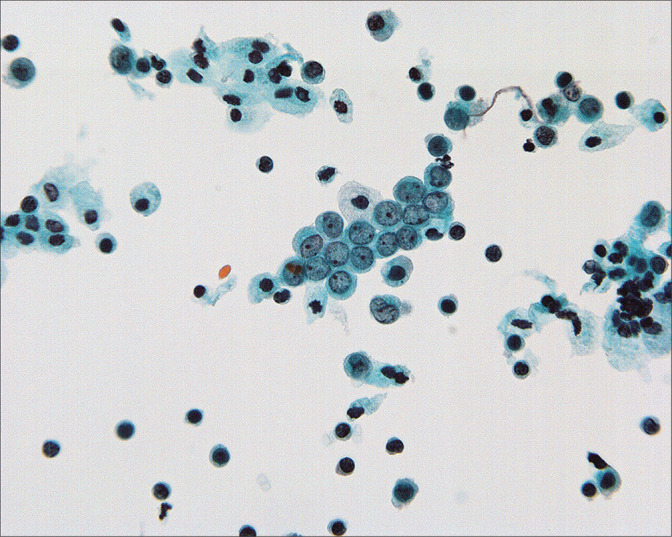
Normal mesothelial cells with well-preserved round-oval nuclei, finely granular chromatin, and single small nucleoli. (Modified PAP stain, 60X.)
Figure 7:
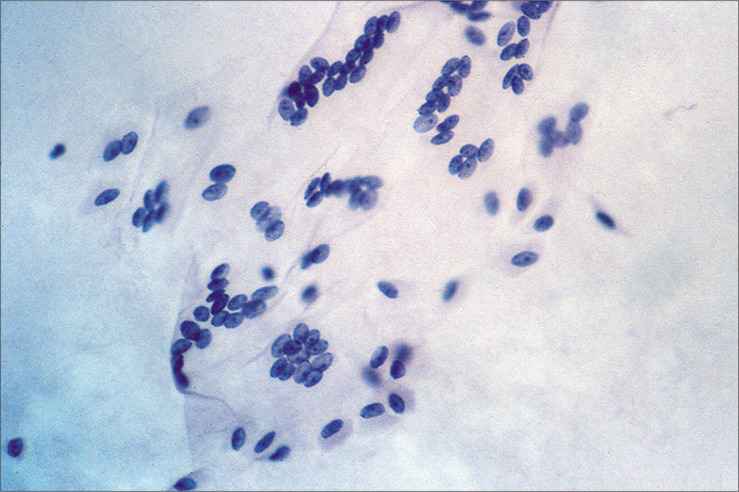
Multinucleated mesothelial cell sheets can sometimes be seen, generally against a background of previous laparotomy or other mesothelial injury. (Modified PAP stain, 20X.)
Figure 8:
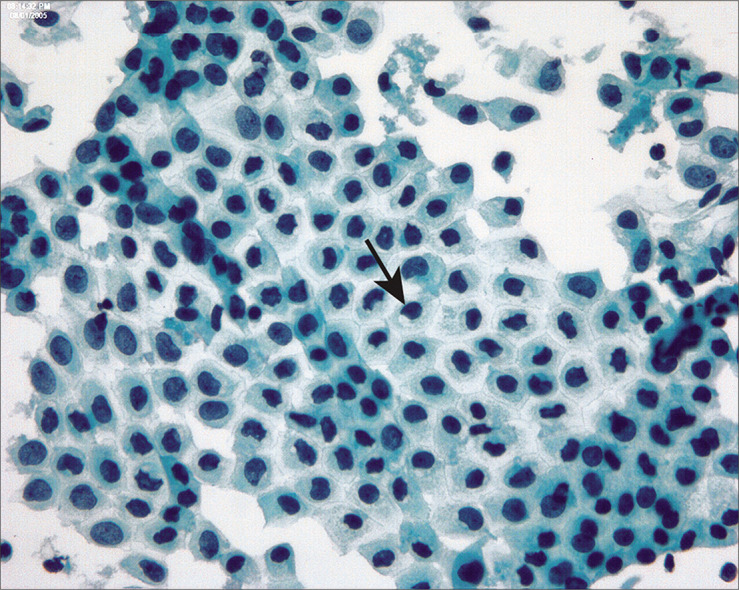
Paranuclear vacuoles develop in degenerating mesothelial cells that may alter nuclear contours (arrow). (Modified PAP stain, 60X.)
It is important to recognize that mesothelial cells in washings can have a variety of appearances in the same sample, particularly in cases in which there is an inflammatory component such as pelvic inflammatory disease,[13,14] preoperative rupture of an ovarian cyst,[14,15] or cystic neoplasm.[14,15] Inflammatory lesions of the gastrointestinal tract such as diverticulitis or appendicitis can have a similar effect. Healing of intraperitoneal inflammatory conditions is accompanied by adhesion formation in which a fibrinous exudate is replaced by mesothelial-lined fibrovascular connective tissue. Vascular tangles [Figure 9] composed of longitudinal capillaries wrapped in tangled fibrillar material can be seen in peritoneal washings as the cytologic residua of lysed adhesions.[14]
Figure 9:
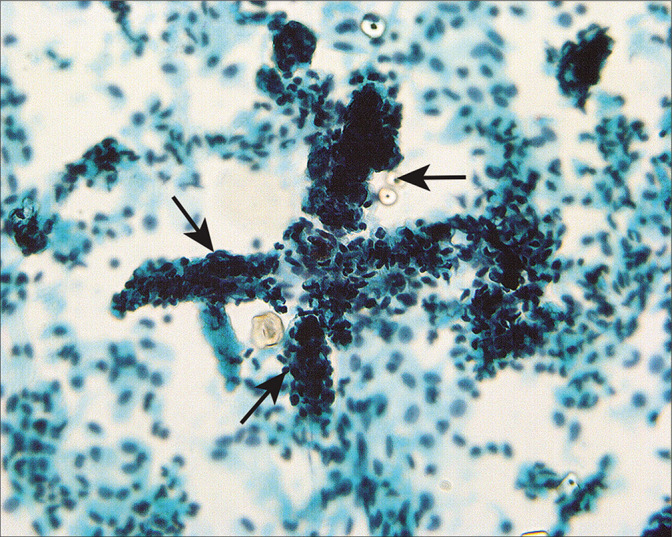
Structures composed of tangled fibrillary material (arrows) appear to represent capillaries and associated connective tissue material that reflect lysed adhesions. This pattern can be seen associated with healed inflammation or endometriosis. (Modified PAP stain, 60X.)
In some cases with large benign tumors, such as uterine leiomyomas or ovarian fibrothecomas [Figure 10], the mesothelial cells can be highly reactive and raise concerns for malignancy. This is particularly true in cases with Meigs’ syndrome[14] in which ovarian fibrothecomas are associated with reactive ascites. In such cases, there is a rather uniform distribution of reactive changes in the mesothelial cells, so that there is a spectrum of reactive changes in a single population rather than two discrete patterns.[22] Correlation with the surgical pathology specimen will generally help to clarify the nature of the process. Rarely, we have seen malignant cells from extragenital malignancies in peritoneal washings from women undergoing surgery for benign female genital tract disease.[14,15] In these cases, the malignant cells were unequivocal and did not merge into the spectrum of reactive mesothelial cells.
Figure 10:
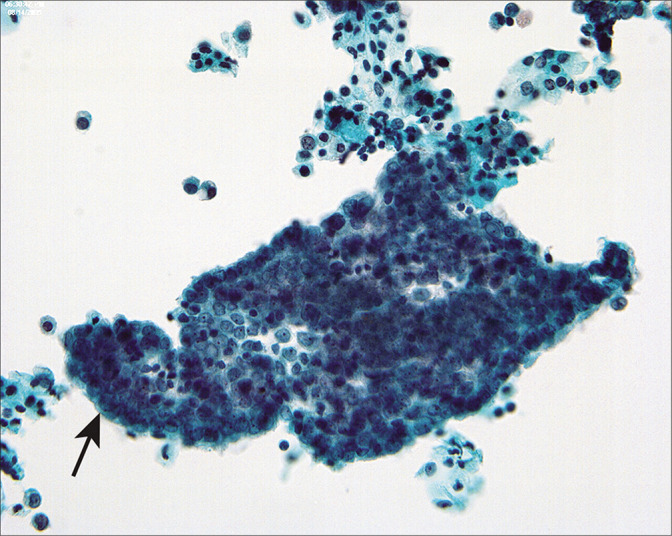
Mesothelial sheets associated with large ovarian fibromas and spontaneous peritoneal fluid can have a rolled-up, pseudopapillary appearance (arrow). (Modified PAP stain, 40X.)
Epithelial cells from ruptured cystic endometriosis, benign cystic ovarian tumors, and bowel mucosa have been described in peritoneal washings.[14] Although cytologically bland, these cells are clearly foreign to the peritoneal cavity and, therefore, can cause concern. Rupture of benign cystic tumors can also spill three-dimensional epithelial cell groups into the peritoneal washings.[14] Discussion with the surgeon or review of the operative note can clarify this issue in problematic cases.
MESOTHELIAL SURFACE REACTIONS
The peritoneal surface in women has been referred to as the ‘secondary müllerian system’ because of its embryologic relationship with the müllerian ducts and because of the müllerian-derived lesions that appear to develop de novo in the peritoneum.[26] It is important that anyone who examines peritoneal washings be familiar with these lesions so as not to interpret cells from these lesions as malignant.
Endosalpingiosis
Endosalpingiosis can be described as ectopic fallopian tube epithelium[27-30] involving pelvic structures and lymph nodes.
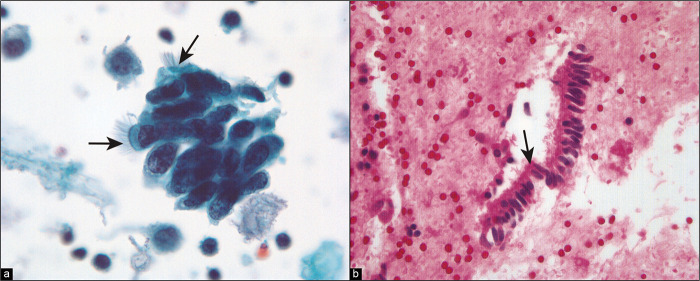
It is seen as cystic invaginations or papillary formations on the surfaces of the ovaries, paratubal tissues, and omentum as well as within pelvic and para-aortic lymph nodes. Microcalcifications and psammoma bodies are frequent [Figure 11]. Some authors attribute this entity to mesothelial metaplasia,[26] although others have suggested that it results from the implantation of inflamed epithelium from the fallopian tubes.[28] The major significance of endosalpingiosis is that it should be distinguished from metastatic carcinoma. [14,15,27,29,31-36]
Figure 11:
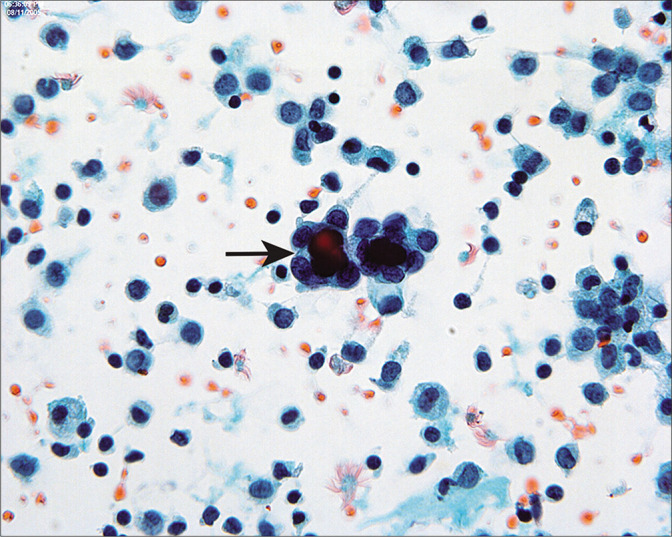
Psammoma bodies are surrounded by cuboidal epithelium (arrow). This pattern is typical of endosalpingiosis, although it can be seen with low-grade serous tumors. Typically in the latter instance, multiple abnormal cell groups will be present, with or without psammoma bodies. If only one or two groups are present (as seen in this case), they are unlikely to represent peritoneal extension of a serous carcinoma, although review of the histology is often helpful. (Modified PAP stain, 60X.)
Cytologically, endosalpingiosis has been described as small aggregates and papillary fragments of cells characterized by cylindrical cell shapes with oval nuclei set in scant cytoplasm.[13,14,31,32,34,35] In some cases, cilia and psammoma bodies can be seen.[14,20,27-36] The epithelial cells surrounding psammoma bodies often have a scalloped appearance [see Figure 11]. Nuclear features are usually bland, with oval to round nuclei, finely distributed chromatin and single round micronucleoli.[34-36] The necessity and occasional difficulty in distinguishing endosalpingiosis from serous neoplasia has been described in several reports.[14,15,29,32,34-38] This problem is compounded by the fact that endosalpingiosis and serous neoplasms (both benign and malignant) will coexist in some patients.[14,15,29,35-38] In endosalpingiosis, cell groups with the typical features are present only sparsely in the peritoneal washing. When there are many cell groups and/or large cell clusters that suggest endosalpingiosis, the possibility that they represent a serous neoplasm should be considered. Correlation with histology can be very helpful in these instances.
Endometriosis
Endometriosis is defined as ectopic functional endometrium composed of glands and stroma.[30] It is found on the mesothelial surfaces of the pelvis, especially ovaries, fallopian tubes, uterus, and posterior cul-de-sac (between the uterus and rectum). The pathogenesis remains unclear. Endometriosis is associated with peritoneal adhesions and frequently forms hemorrhagic cysts due to the cyclic bleeding associated with the menstrual cycle, with subsequent fibrosis. Occasionally, cystic ovarian endometriosis may be large enough to suggest an ovarian tumor clinically.[30]
Endometriotic epithelial cells and stroma have occasionally been reported in peritoneal washings.[14,39,40] In our experience, this is a rare event that usually correlates with rupture of an endometriotic cyst.[14] Epithelial cells from ruptured endometriosis can sometimes cause concern for malignancy[14,23] until correlated with histology. More typically, hemosiderin-laden macrophages are seen, but these must be considered a non-specific finding as they can be found in any situation in which intraperitoneal bleeding has occurred.
BENIGN OVARIAN TUMORS
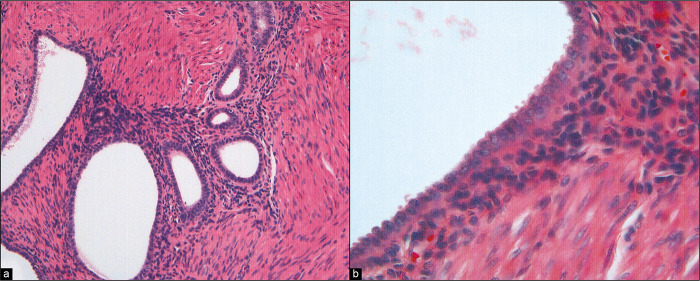
Rarely, three-dimensional cast-like groups [Figure 12] can be seen in association with benign epithelial-stromal tumors of the ovary.[14,15] These are characterized by a distinctive molded outline of the cell group with a smooth common epithelial border. Nuclei are bland and ordered to the periphery of the cell group. It has been suggested that these structures represent ovarian surface epithelial cells extracted as a unit from surface invaginations of fibromatous tumors.[15] Although the origin of these structures is unclear, their significance at this time is the recognition that they occur in benign lesions. Similar structures can sometimes be found in malignant cases. However, in such circumstances, the diagnosis should be made on the basis of other cells and groups that are diagnostic of malignancy. A number of studies[3-7,11,14,15,34,41] have reported abnormal cytology in patients with benign ovarian tumors. In most instances, these cells represent (1) a surface epithelial component of the tumor, (2) reactive germinal epithelium (mesothelium) of the ovary, or (3) contents of a cystic tumor that ruptured and dislodged tumor cells into the peritoneum.[14,15] In the last instance, interpretation and clinical follow-up should be determined by the histologic diagnosis of the ovarian tumor.
Figure 12:
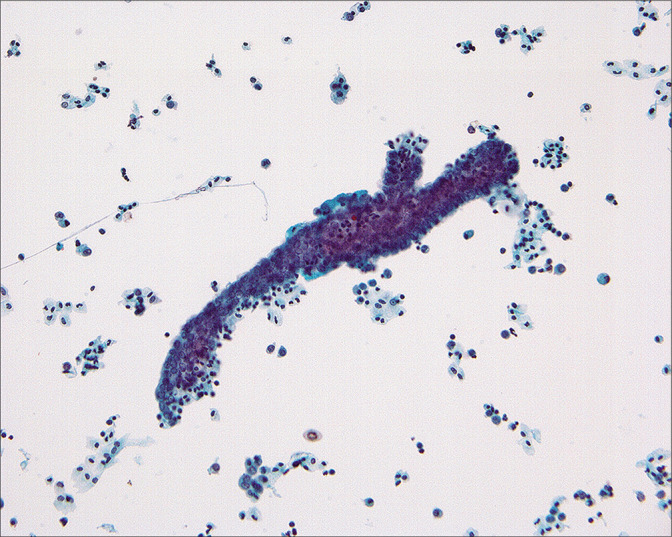
Cast-like groups that appear to have been removed as a unit from an irregular ovarian surface have been found in association with benign tumors, particularly adenofibromas. Although they can occasionally be found in the peritoneal washings of women with malignant tumors, the diagnosis of malignancy should only be made in cases that have other cells or groups with the typical features of malignancy. (Modified PAP stain, 20X.)
MISCELLANEOUS NON-NEOPLASTIC CHANGES IN PERITONEAL WASHINGS
Other cell types may sometimes be recognized in peritoneal washings. These include skeletal muscle fibers, presumably from the abdominal incision, and, rarely, adipose tissue, again presumably from the abdominal incision.[14,15]
Hemosiderin laden macrophages[6,9,14,15,39] can be seen in cases in which intraperitoneal bleeding has occurred. These include endometriosis, ruptured tubal pregnancy, prior surgical procedures, and malignancy. This is therefore a highly non-specific finding with little diagnostic significance.
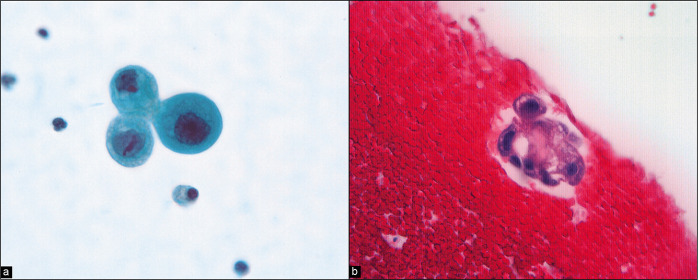
Collagen balls[42,43] [Figure 13] are microscopic nodules that appear to represent small protrusions from the surface of the ovary. In peritoneal washings, they are seen as roundoval nodules of collagenized stroma surrounded by flattened mesothelial cells. Their primary importance is to recognize them as non-neoplastic structures and not to confuse them with malignancy. It is recommended to designate these structures as collagen globules [Figure 13].[1]
Figure 13:
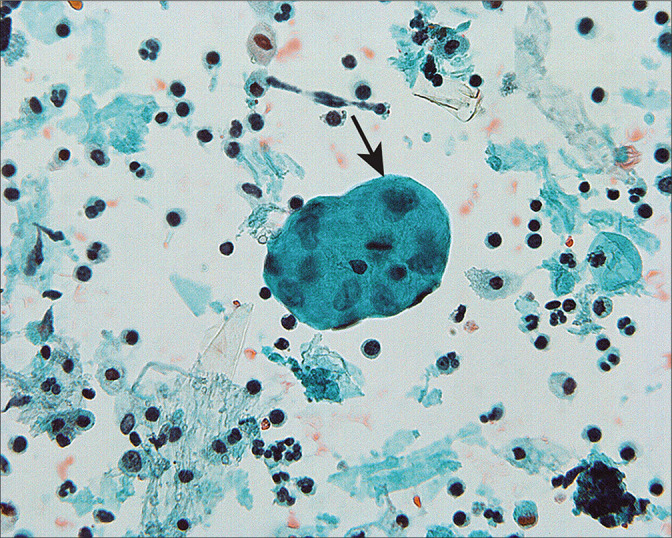
Collagen balls are microscopic nodules that appear to represent small, mesothelium-lined surface protrusions (arrow). (Modified PAP stain, 60X.)
Detached ciliary tufts [Figure 14] are a common finding[44] in PWC. They are seen as anucleated cell fragments composed of cilia and a small portion of attached cytoplasm. Sidawy et al[44] described them in women from 21 to 47 years of age, and speculated that they represent physiologic shedding of cilia from fallopian tube epithelium during the luteal phase of the menstrual cycle.
Figure 14:
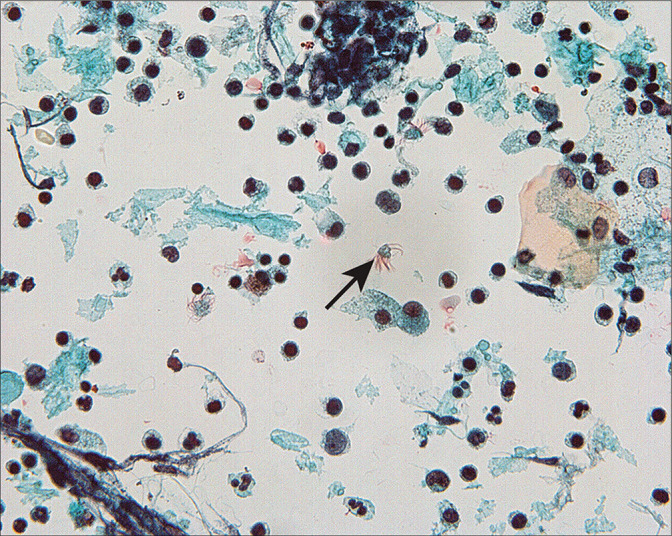
Detached ciliary tufts (arrow) are seen as anucleated cell fragments composed of cilia and a small fragment of residual cytoplasm. (Modified PAP stain, 60X.)
MALIGNANT DISEASE
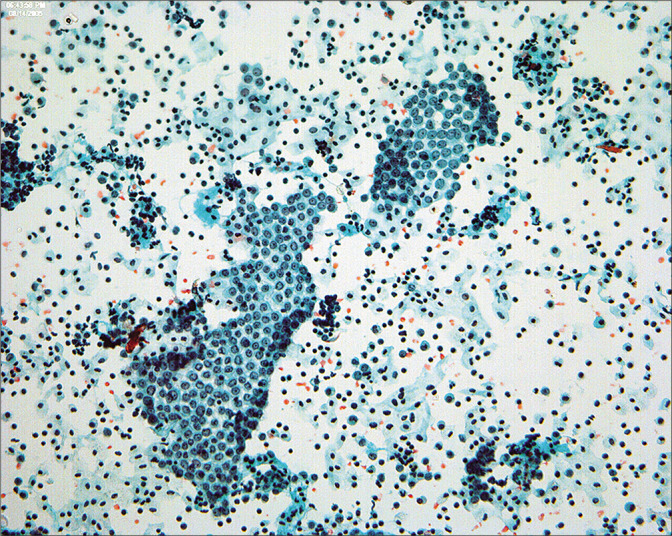
• Because most peritoneal washings are performed in patients with malignant tumors and the results have significant implications for prognosis and therapy. Interpretation of these samples requires not only familiarity with various pitfalls but also correlation with the patient’s primary lesion. The interpretation criteria for peritoneal washings are included in Table 3. Because of the large number of different tumor types that can be found in these samples, • it is important that the interpreter be familiar with the specific tumor morphology in the individual case with review of the histologic sections if possible prior to final sign-out.[13,15,16,23,24] Overall, the cytologic features of malignant tumors in peritoneal washings are little different from those in effusions, with the exception that large cell groups from intraperitoneal tumor can sometimes be dislodged into the washing [Figure 15]. Generally, these create minimal diagnostic difficulties.
Table 3:
| Classical criteria for malignancy, including: • Abnormal single cells and groups • Dysplastic nuclei • Nuclear enlargement • Irregular nuclear outline • Chromatin abnormalities • Nucleolar abnormalities |
| Abnormal cells outside the spectrum of reactive mesothelial cells and surface reactions |
| Abnormal cells compare well with those of the primary tumor. Comparative review with morphology in cell-block sections and surgical pathology of primary neoplasm is simple but very important practice |
Figure 15:
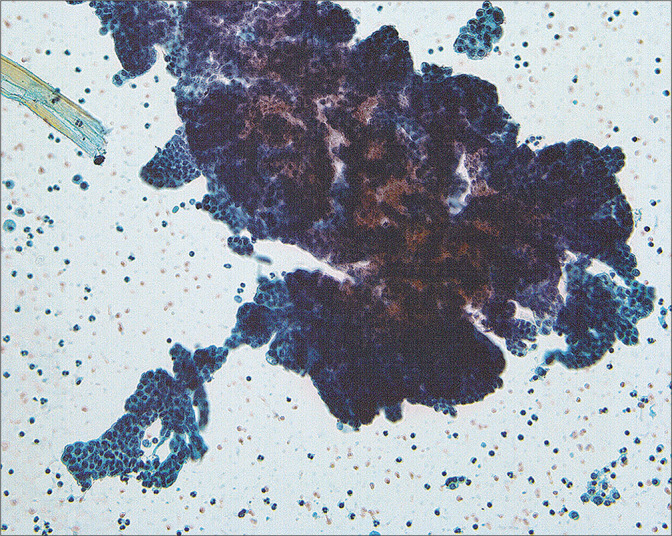
A large fragment of tumor cells can be dislodged from intraperitoneal malignancy in the course of taking the washings. This pattern is rare in spontaneous effusions. (Modified PAP stain, 20X.)
The most significant challenges arise in cases in which there is no clinical or histologic evidence of tumor outside the primary site. This scenario underlines the rationale for PWC and has been the subject of some controversy over the years. Mathew and Erozan[45] have reported that the PW diagnosis results in ‘upstaging’ 3.1% of patients with female genital tract malignancies. Mulvaney[46] found that peritoneal cytology upstaged 7–75% of female genital tract tumors, depending on the primary site. The more important issue is whether those women benefit by the upstaging and additional therapy that they frequently receive as a result. Fadare et al[47] found that, while 4.5% of endometrial cancer cases were upstaged because of PW results alone, there appeared to be little difference in their survival compared with controls.
In our series, after considerable efforts to identify problematic reactive and benign patterns,[14,15] and using the criteria outlined in Table 3, we found that the cases with positive cytology with an otherwise negative peritoneal cavity were uncommon (1.1%)[16] for the aggregate of female genital tract primaries. In addition, there was agreement in cytology and peritoneal histology in 92.1% patients who had thorough, oncologic exploratory surgeries and biopsy of suspicious peritoneal areas.[16] Women with positive PW had significantly worse survival than similar patients with negative cytology[16] for all female genital primary sites studied. These results reinforce the role of peritoneal cytology in female genital cancers. In addition, these observations also highlight the need for attention to the pitfalls that potentially may lead to overdiagnosis.
The role of ancillary studies in peritoneal washings remains to be established and should be approached with caution. • When informative, immunoperoxidase panels similar to those used in the interpretation of ascites fluid are applicable. However, immunoperoxidase stains to confirm the presence of malignant cells in peritoneal washings should be performed only with the understanding that the cells from the surface reactions such as endosalpingiosis and endometriosis can give positive results for epithelial and müllerian markers that could be erroneously interpreted as metastatic. In general, comparison of the cytologic pattern with that in the histology slides is sufficient to classify the cells in the PW. Cases that are most problematic are often those in which only a small number of worrisome cells are present. These cases would generally be difficult to categorize using immunohistochemistry because of sample limitations. Similarly, any use of molecular techniques also requires appropriate validation studies. Because of the high sensitivity of any test based on polymerase chain reaction techniques, the biologic and prognostic significance of a positive result must be verified before clinical use.
OVARIAN NEOPLASMS
48 to 60% of women with ovarian cancer have positive peritoneal cytology at laparotomy.[2,4,5,15,16,48] Although often accompanied by sheets of polymorphic mesothelial cells, the morphology of the malignant cells in the PWs is often similar to that seen in spontaneous effusions.[49] However, problematic patterns do occur. One such pattern is that of low-grade papillary serous lesions with psammoma bodies [Figure 16]. The differential diagnosis in these cases includes endosalpingiosis, surface components of a benign papillary serous cystadenoma or adenofibroma, serous tumors of low malignant potential (borderline or atypical proliferating), or low-grade serous adenocarcinoma [Table 4]. Endosalpingiosis and serous carcinoma represent the benign and malignant extremes of serous lesions. Endosalpingiosis is generally found as a few small cohesive cell clusters with psammoma bodies and bodies. Carcinomas typically show all of the features listed in Table 3, with some cytologic features overlapping with other lesions.
Figure 16:
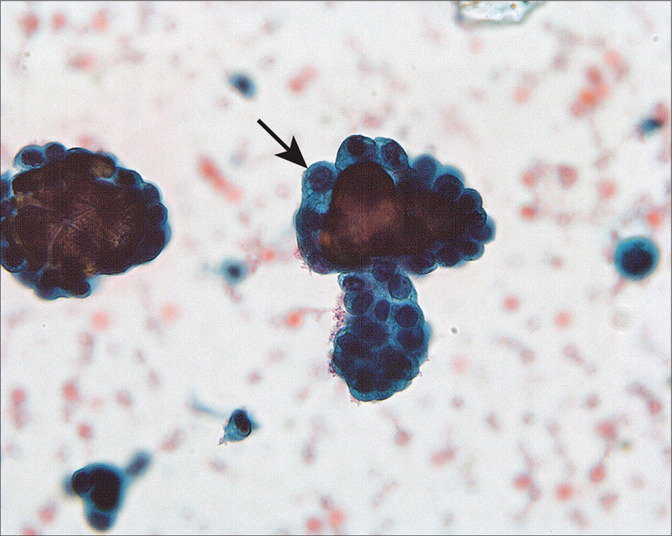
Groups of abnormal cells surrounding psammoma bodies (arrow) in a washing from a woman with an ovarian serous borderline tumor involving the peritoneal cavity. The large number of abnormal cells and groups suggested that the process was neoplastic rather than endosalpingiosis. The histologic comparison easily confirmed the neoplastic process. (Modified PAP stain, 60X.)
Table 4:
Comparison of cytologic criteria for distinguishing the spectrum of serous lesions in peritoneal washings.
| Criterion | Endosalpingiosis | Benign tumor | Low malignant potential | Micropapillary (low-grade serous carcinoma) | Carcinoma |
|---|---|---|---|---|---|
| Aggregation | Small 3-D papillary groups | Cast-like | Large 3-D groups | Large 3-D groups Finger-like | 3-D groups and single cells |
| Periphery | Cohesive | Cohesive | Cohesive | Cohesive | Frayed |
| Single cells | Absent | Absent | Rare | Variable | Characteristic |
| N/C ratio | Increased | Variable | Variable | Variable | Variable |
| Nuclear size | Normal | Normal | Normal-variable | Increased | Increased; pleomorphic |
| Nuclear shape | Round-oval | Round-oval | Variable | Variable | Pleomorphic |
| Chromatin | Uniform, finely granular | Uniform, finely granular | Variable | Variable | Clumped; variable |
| Nucleoli | Small, inconspicuous | Small | Small | Variable | Prominent |
| Psammoma bodies | Frequently present | Infrequent | Variable | Variable | Variable |
Typically, the borderline and malignant lesions have at least moderate cytologic atypia, and show both three-dimensional groups and single cells. Atypia may be present in more than 2–3 cell groups in the cytologic sample. In most such cases, the tumor will be found to involve the serosal surface of the ovary or peritoneal cavity by histology. Because of the degree of difficulty in reliably distinguishing well-differentiated serous carcinoma and borderline tumors on a cytologic basis,[23,24,50] we have reported these cases as ‘low-grade serous neoplasm’ [Figure 17] and rely upon the histologic appearance for precise categorization of the tumor. Similarly, Mulvaney et al[46] were not able to distinguish invasive and non-invasive serous implants in cytologic samples. Cheng et al[51] found that 33% of low malignant potential (LMP) cases had positive peritoneal cytology and reported a relationship of positive cytology with the presence of tumor on the peritoneal surface of the ovary. Sneige et al[52] found only moderate accuracy relating to the peritoneal washing result and the status of peritoneal biopsies at staging. Significantly, none of their patients with positive cytology and negative peritoneal biospies developed recurrence. Serous surface carcinomas (primary peritoneal carcinoma)[30] cannot be distinguished from primary ovarian serous carcinomas in peritoneal cytology [Figure 12].[49,53] In addition, it is advisable to review the cytology result in the context of the surgical pathology of the primary lesion prior to sign-out[16] because of the implications for determining the stage of disease and postoperative follow-up. Recent updates in FIGO staging protocols have modified the role of PWC in ovarian/tubal cancer staging. Positive PWC in Stage I ovarian neoplasms are assigned Stage 1C3, with follow-up individualized to the specific tumor type.[18]
Figure 17:
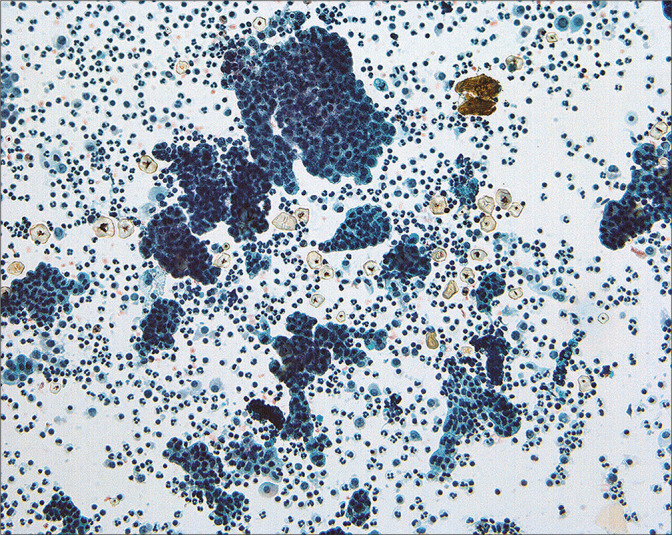
Groups of atypical cells interpreted as ‘low-grade serous tumor’ are present in this washing from a woman with intraperitoneal micropapillary serous tumor. While the cytologic pattern is that of a neoplasm, the precise categorization is difficult by cytology alone. Indeed, it is unclear whether this lesion is a well-differentiated carcinoma or a tumor of low malignant potential (borderline). (Modified PAP stain, 20X.)
While peritoneal washings have been widely used in the staging of ovarian neoplasms, Colgan et al [54] reported that peritoneal washings can detect occult carcinoma in women undergoing prophylactic oopherectomy, particularly those with BRCA mutations. However, Landon et al [55] performed a 10 year reappraisal of the clinical utility of peritoneal cytology in women undergoing prophylactic oopherectomy and noted that positive findings are uncommon and commented on the various pitfalls for the diagnosis of these specimens as reported here.
ENDOMETRIAL CARCINOMA
Peritoneal cytology in endometrial cancer cases has generated considerable controversy in the literature: 7.1–22.2% of cases of all stages had positive cytology in the various reports.[5,47,56-80] The most problematic is positive peritoneal cytology in stage 1 endometrial disease. Some confusion rests with the change from clinical to surgical–pathologic staging for endometrial cancers by FIGO in 1988. Some clinical stage 1 cases are found to have advanced surgical–pathologic stage cancers at exploration, so that is it important to clarify which staging system was used in the study. Follow-up studies[47,77,80] of women with positive cytology and endometrial carcinoma otherwise confined to the uterus have raised questions about the need for follow-up therapy for these women. Some studies[47,77] showed no significant difference in survival based on peritoneal cytology results, although others[80] did show differences.
Because of these mixed findings, PWC is no longer included in the FIGO staging system for endometrial carcinoma[12], although it may be performed. When performed, PWC results should be reported for clinical consideration.
There are two general categories of endometrial carcinoma that carry different risks for progressive disease:
Endometrioid type [see Figure 15], which is associated with hormonal abnormalities and has a typically indolent course
Serous type, which is generally unassociated with hormones and frequently has an aggressive course.
Whereas both tumor types show the depth of myometrial invasion as a key indicator of increasing risk, serous carcinoma is well known to be associated with intraperitoneal disease in the absence of demonstrable myometrial invasion.[30]
Endometrioid carcinoma of the endometrium involves the peritoneal cavity late in the disease and is infrequently associated with positive peritoneal cytology when the tumor is confined to the uterus.[16] It is important to consider and exclude various pitfalls in peritoneal washings [see Table 2] when evaluating these samples. Because well-differentiated endometrioid carcinomas of the endometrium typically have rather small nuclei [Figure 18], groups of reactive mesothelial cells in the washings of endometrial cancer cases can sometimes cause confusion, particularly in the background of serosal adhesions. Comparison with the histology can sometimes be difficult because of the rather bland cytologic appearance of the tumor cells. • In our experience, low-grade endometrioid tumors that are histologically confined to the uterus are rarely associated with positive peritoneal cytology in the absence of deep myometrial invasion.[15,16] Occasionally, positive peritoneal washings in endometrial cancers can be seen with deep myometrial invasion, presumably through lymphatic permeation.[64,69] • Some cases may be associated with transit of the tumor cells through patent fallopian tubes.[4,58,66,69] Progressive disease in cases with positive PWC in endometrioid carcinomas confined to the uterus (pathologic stage 1) are uncommon.[16,47] This raises concerns about the validity of the interpretation or possibility of iatrogenic contamination of the washings with tumor cells. Our experience suggests that this is relatively uncommon and is more likely to be associated with serous carcinomas of the endometrium.
Figure 18:
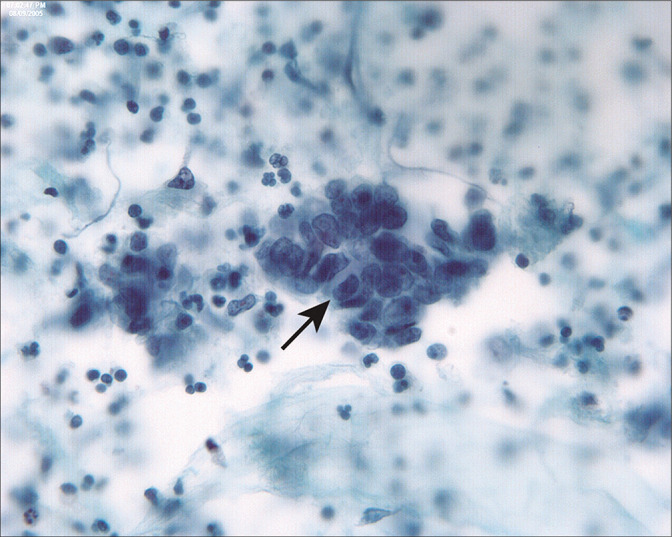
Endometrioid carcinoma of the endometrium typically involves the peritoneal cavity late in the disease, accompanied by deep myometrial invasion, vascular space invasion, and peritoneal tumor implants. In this case of low-grade endometrioid adenocarcinoma of the endometrium, tumor penetrated to the serosal surface and involved pelvic structures. In this three-dimensional group (arrow), the columnar cells are haphazardly arranged with dyspolaric nuclei. This latter feature helps to distinguish endometrioid carcinoma from tubal epithelium. (Modified PAP stain, 60X.)
Serous carcinomas of the endometrium in peritoneal fluids [Figure 19] are high-grade lesions, frequently with papillary clusters with, large, overtly malignant nuclei, and prominent nucleoli. In the absence of clinical history or histologic diagnosis of endometrial primary, these cases would be considered to be typical of high-grade epithelial cancers of the ovary. Some cases of serous carcinomas confined to an endometrial polyp[30] have been reported to be associated with disseminated carcinoma and peritoneal involvement in the absence of myometrial invasion. Unlike low grade endometrioid carcinomas, PWC for serous carcinomas can be positive when histologically confined to the uterus. This justifies the use of PWC in endometrial cancer patients despite the fact that it is no longer incorporated into the FIGO stage.
Figure 19:
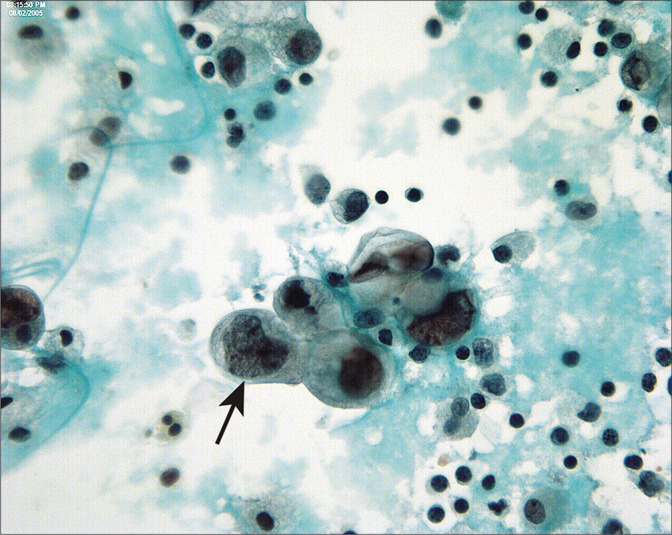
Serous carcinoma of endometrium in peritoneal washings are typically seen as three-dimensional groups and single cells with large nuclei and prominent nucleoli. Unlike endometrioid adenocarcinomas, peritoneal involvement is common and the abnormal cells are readily recognized as malignant (arrow). (Modified PAP stain, 60X.)
CERVICAL CARCINOMA
Cervical cancers grow below the peritoneal reflection, and thus are retroperitoneal until late in the disease. Peritoneal washings are not included in the staging for cervical cancer, because most of the studies have shown that positive cases are observed in association with other high-risk factors.[81-84] Peritoneal washings are positive in 7.0–21.0% of cervical cancer cases coming to surgery.[16,81-87] Our experience indicates that prognosis is very poor[16,83] when the washings are positive.
Squamous cell carcinomas are less likely to have positive peritoneal cytology than adenocarcinomas[88] for reasons that are not well understood. It is possible that squamous cancers induce a fibrogenic response that enmeshes the cancer cells and prevents them from desquamating.[88] When positive, the malignant cells in squamous cancers [Figure 20] can be found as cohesive three-dimensional groups or as isolated, highly atypical single cells with varying degrees of keratinization. • We have found that cohesive cells of nonkeratinizing squamous cell carcinoma can be mistaken for reactive mesothelial cells.[15] This is particularly problematic if the patient previously had radiation therapy to the pelvis.
Figure 20:
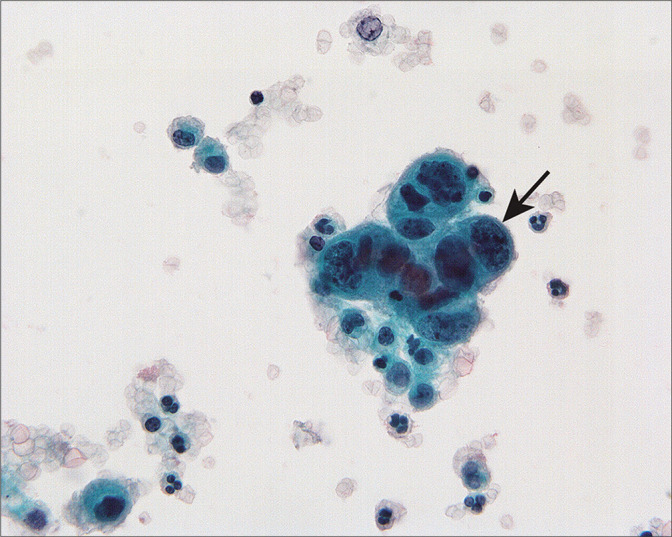
Cervical squamous carcinoma can be seen as three dimensional groups or single cells in peritoneal washings. This group of nonkeratinizing squamous carcinoma cells shows enlarged nuclei and increased nuclear-cytoplasmic ratio (arrow). Unless the histologic type is known, it is possible to overlook non-keratinizing squamous carcinoma as reactive mesothelial cells. (Modified PAP stain, 60X.)
CASE STUDIES
Case 1
History
A 66-year-old woman underwent to hysterectomy and exploratory laparotomy because of an endometrial biopsy that showed endometrial carcinoma, endometrioid type. At surgery, multiple dense adhesions were found that made the surgery difficult. The peritoneal washings performed as part of the staging procedure showed a heterogenous population with flat mesothelial cell sheets and three-dimensional groups of cells with atypical nuclear features and occasional cilia [Figures 21, 22a, 22b].
Figure 21:
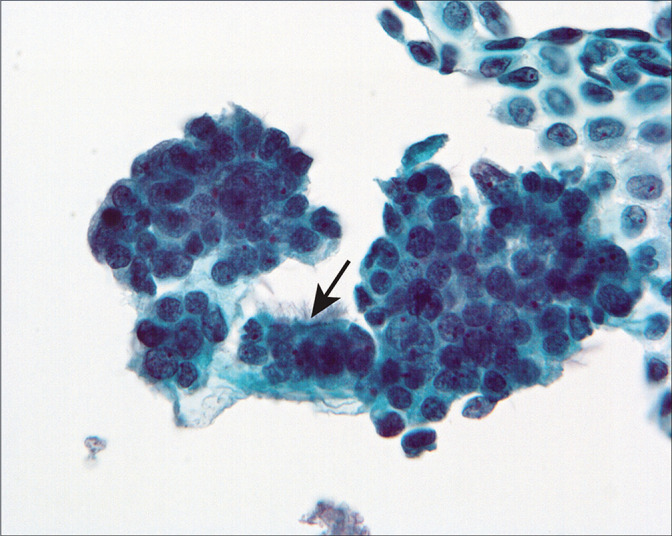
Three-dimensional cell groups are present, associated with a sheet of mesothelial cells. Some of the cells showed cilia (arrow). (Modified PAP stain, 40X.)
Figure 22:
(a) Higher-power view of abnormal cell cluster with cell crowding, increased nuclear size, clumped chromatin, prominent small nucleoli. Cilia (arrows) are prominent. (Modified PAP stain, 60X.). (b) Strip of columnar cells in the cell block, some of which show cilia (arrow). (HE, 60X.)
Diagnosis
Negative for carcinoma. Cell groups, not diagnostic for malignancy, consistent with endometriosis are present.
Discussion
Differential interpretations of these cell groups based on morphology include (1) metastatic endometrioid adenocarcinoma, (2) another simultaneous carcinoma such as endometrioid carcinoma of the ovary, (3) reactive fallopian tube epithelium, and (4) surface reaction such as endometriosis or endosalpingiosis.
Review of the differential diagnosis suggests that immunohistochemistry would not be helpful to distinguish benign versus malignant. • Most helpful was the review of the histology [Figure 23a,b], which demonstrated abundant, exuberant (but non-neoplastic) endometriosis involving the ovaries and multiple peritoneal sites. The process had features of endometriosis, including endometriotic stroma. Many epithelial cells in the glands showed prominent cilia. Although more common in endosalpingiosis, cilia may also be present in endometriosis. Thus, the overall impression was that of endometriosis in the peritoneal cavity. The hysterectomy [Figure 24] revealed FIGO G2 endometrioid adenocarcinoma that was confined to the endometrial cavity (stage 1), with vascular space invasion and greater than 50% myometrial invasion.
Figure 23:
(a) Histologic section of a peritoneal biopsy from this patient showing endometriosis, including endometrial stroma. (HE, 20X.). (b) Higher-power view of columnar epithelium lining an endometriotic cyst. (HE, 60X.)
Figure 24:
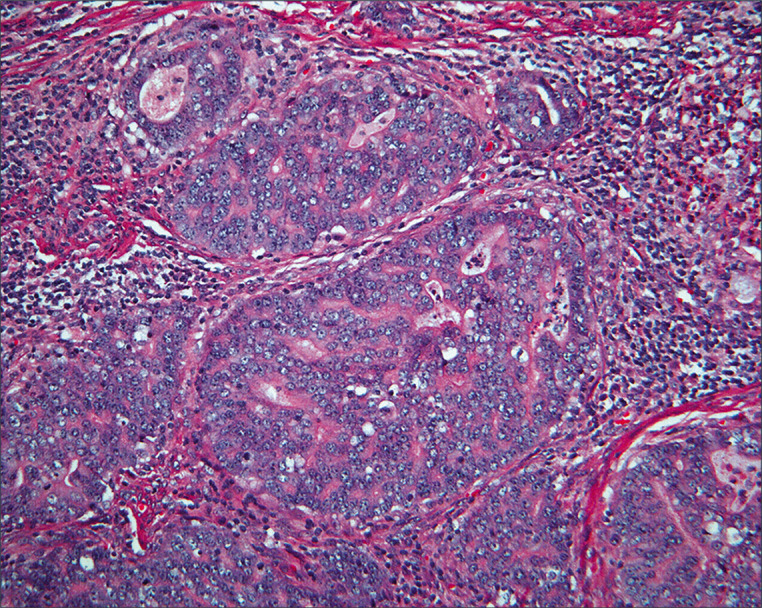
Histology of the endometrial carcinoma shows an endometrioid pattern with an open chromatin pattern and prominent nucleoli. (HE, 10X.)
Case 2
History
A 56-year-old woman underwent exploratory laparotomy for staging following a diagnosis of ovarian cancer by total hysterectomy with removal of both ovaries and fallopian tubes at another hospital. She had no other treatment for her tumor. The PW at re-exploration was rather sparsely cellular but showed isolated highly atypical single cells with rare small groups with occasional microcalcifications [Figures 25, 26]. A few sheets of mesothelial cells were also present in the specimen.
Figure 25:
(a) Higher-power view of isolated atypical cells with rounded shape, enlarged irregular nuclei, and prominent nucleoli. (Modified PAP stain, 60X.). (b) A similar cell group is present in the cell block. (HE, 60X.)
Figure 26:
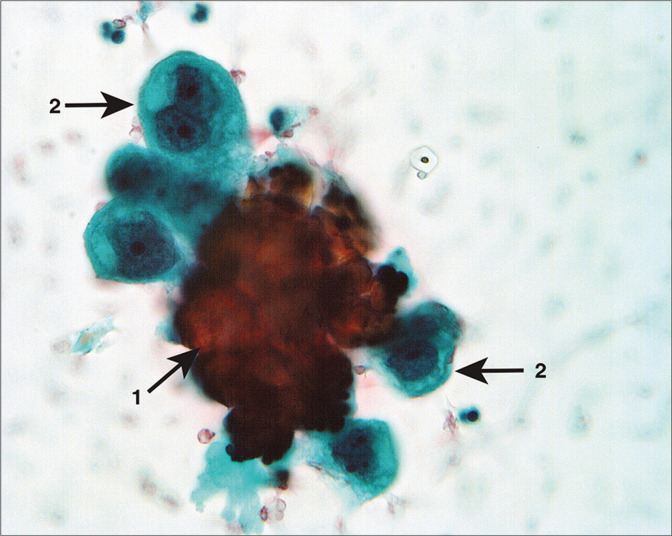
Atypical cell aggregate associated with microcalcification (arrow 1). The cells have a hobnail shape with enlarged nuclei and prominent large nucleoli (arrows 2). Despite the sparse number of cells in the sample, these cells were interpreted as neoplastic. (Modified PAP stain, 40X.)
Diagnosis
Neoplasm of uncertain biologic potential, consistent with low-grade serous tumor.
Discussion
Differential diagnosis based on PWC alone would be low-grade neoplasms, which include serous carcinoma and serous tumor of low-grade potential (borderline). Benign serous tumor or endosalpingiosis would be unlikely with this morphology. • Again, the key to the interpretation of this case was in the histology of the ovarian tumor removed at the outside hospital and the intraperitoneal biopsies removed following collection of the washing. Although the history was that of ovarian cancer, review of the woman’s tumor showed it to be a serous tumor of low-malignant potential with foci of micropapillary changes. This newly recognized entity is controversial in that some experts consider it to be a well-differentiated adenocarcinoma even in the absence of ovarian stromal invasion. However, its recognition is important because the follow-up is different from typical carcinoma in that, while tumor progression is more common than with usual tumors of low malignant potential, they respond poorly to chemotherapy, probably because of low mitotic activity. The peritoneal biopsies from the reexploration were positive for non-invasive serous implants [Figure 27]. The cytologic features of serous carcinoma, serous tumor of low malignant potential, and micropapillary serous tumors show considerable overlap in peritoneal washings. Similarly, the presence or absence of invasion in peritoneal implants of serous tumors cannot be definitively determined based upon the cytologic appearance alone. Correlation with the histology is the key to appropriate categorization.
Figure 27:
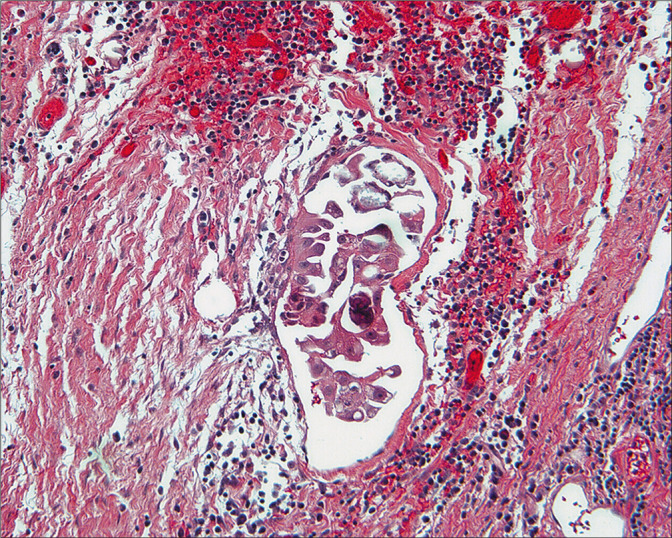
Histologic section of non-invasive implant of low-grade serous tumor. These cells compare well with the cytology. (Peritoneal biopsy at re-exploration; HE, 10X.)
Acknowledgment
The author thank Andrew Kumar, MD (Resident, Wayne State University School of Medicine, Detroit, MI, USA) and Janavi Kolpekwar for copy-editing support.
Footnotes
How to cite this article: Zuna RE. Diagnostic cytopathology of peritoneal washings. CytoJournal 2022;19:9.
HTML of this article is available FREE at: https://dx.doi.org/10.25259/CMAS_02_07_2021
ABBREVIATIONS (IN ALPHABETIC ORDER)
3-D - Three dimensional
FIGO - International Federation of Gynecology and Obstetrics
N/C ratio - Nucleocytoplasmic ratio
LMP - Low malignant potential
PW - Peritoneal washing
PWC - Peritoneal washing cytology
References
- 1.Shidham VB, Layfield LJ. Introduction to the second edition of diagnostic cytopathology of serous fluids' as cytojournal monograph (CMAS) in open access. CytoJournal. 2021;18:30. doi: 10.25259/CMAS_02_01_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keettel WC, Elkins HB. Experience with radioactive colloidal gold in the treatment of ovarian carcinoma. Am J Obstet Gynecol. 1956;71(3):553–568. doi: 10.1016/0002-9378(56)90482-3. [DOI] [PubMed] [Google Scholar]
- 3.Keettel WC, Pixley E. Diagnostic value of peritoneal washings. Clin Obstet Gynecol. 1958;1(3):592–606. doi: 10.1097/00003081-195809000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Keettel WC, Pixley E, Buchsbaum HJ. Experience with peritoneal cytology in the management of gynecologic malignancies. Am J Obstet Gynecol. 1974;120(2):174–182. doi: 10.1016/0002-9378(74)90359-7. [DOI] [PubMed] [Google Scholar]
- 5.Creasman WT, Rutledge F. The prognostic value of peritoneal cytology in gynecological malignant disease. Am J Obstet Gynecol. 1971;110(6):773–781. doi: 10.1016/0002-9378(71)90571-0. [DOI] [PubMed] [Google Scholar]
- 6.Morton DG, Moore JG, Chang N. The clinical value of peritoneal lavage for cytologic examination. Am J Obstet Gynecol. 1961;31(6):1115–1125. doi: 10.1016/S0002-9378(15)33341-X. [DOI] [PubMed] [Google Scholar]
- 7.Marcus CC. Cytology of the pelvic peritoneal cavity in benign and malignant disease. Obstet Gynecol. 1962;20(12):701–712. [PubMed] [Google Scholar]
- 8.Graham JB, Graham RM. Cul-de-sac puncture in the diagnosis of early ovarian carcinoma. J Obstet Gynaecol Br Commonw. 1967;74(3):371–378. doi: 10.1111/j.1471-0528.1967.tb03961.x. [DOI] [PubMed] [Google Scholar]
- 9.DeBrux J, Depre-Froment J, Mintz M. Cytology of the peritoneal fluids sampled by coelioscopy or by cul-de-sac puncture: its value in gynecology. Acta Cytol. 1968;12(5):395–403. [PubMed] [Google Scholar]
- 10.Woodruff JD, Telinde RW. The histology and histogenesis of ovarian neoplasia. Cancer. 1976;38(Suppl 1):411–413. doi: 10.1002/1097-0142(197607)38:1<411::AID-CNCR2820380164>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Cefis F, Forni A, Carinelli S, Cangini L. Significance of ascitic fluid and peritoneal washing cytology in ovarian tumor diagnosis. Tumori. 1978;64(1):77–88. doi: 10.1177/030089167806400109. [DOI] [PubMed] [Google Scholar]
- 12.Kehoe S, Bhatla N. FIGO Cancer Report 2021. Int J Gynecol Obstet. 2021;155(S1):45–60. doi: 10.1002/ijgo.13866. [DOI] [PubMed] [Google Scholar]
- 13.Ziselman EM, Harkavy SE, Hogan M, West W, Atkinson B. Peritoneal washing cytology: uses and diagnostic criteria in gynecologic neoplasms. Acta Cytol. 1984;28(2):105–110. [PubMed] [Google Scholar]
- 14.Zuna RE, Mitchell ML. Cytologic finding in peritoneal washings associated with benign gynecologic disease. Acta Cytol. 1988;32(2):139–147. [PubMed] [Google Scholar]
- 15.Zuna RE, Mitchell ML, Mulick KA, Weijchert WM. Cytologic-histologic correlation of peritoneal washing cytology in gynecologic disease. Acta Cytol. 1989;33(3):327–336. [PubMed] [Google Scholar]
- 16.Zuna RE, Behrens A. Peritoneal washing cytology in gynecologic cancers: long-term follow-up of 355 patients. J Natl Cancer Inst. 1996;88(14):980–987. doi: 10.1093/jnci/88.14.980. [DOI] [PubMed] [Google Scholar]
- 17.Kasius JC, Pijnenborg JM, Lindemann K, Forsse D, van Zwol J, Kristensen GB, et al. Risk stratification of endometrial cancer patients: FIGO stage, biomarkers and molecular classification. Cancers (Basel) 2021;13:5848. doi: 10.3390/cancers13225848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prat J, FIGO Committee on Gynecologic Oncology FIGO's staging classification for cancer of the ovary, fallopian tube, and peritoneum: Abridged republication. J Gynecol Oncol. 2015;26:87–9. doi: 10.3802/jgo.2015.26.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakata T, Takahata T, Kimura T, Yasuhara I, Kojima T, Akazai Y, et al. A single-institution retrospective analysis of gastric carcinoma with positive peritoneal lavage cytology and without serosal invasion: A case series. Ann Med Surg (Lond) 2019;39:10–5. doi: 10.1016/j.amsu.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirabayashi K, Imoto A, Yamada M, Hadano A, Kato N, Miyajima Y, et al. Positive intraoperative peritoneal lavage cytology is a negative prognostic factor in pancreatic ductal adenocarcinoma: A retrospective single-center study. Front Oncol. 2015;5:182. doi: 10.3389/fonc.2015.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scharifi S, Ducatman BS, Wang HH, Fraser JL. Peritoneal washing cytology is unnecessary in gynecologic surgery for benign diseases. Cancer Cytopathol. 1999;87:259–62. doi: 10.1002/(SICI)1097-0142(19991025)87:5<259::AID-CNCR4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 22.Shidham VB, Layfield LJ. Approach to diagnostic cytopathology of serous effusions. CytoJournal. 2021;18:32. doi: 10.25259/CMAS_02_03_2021.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravinsky E. Cytology of peritoneal washings in gynecologic patients: diagnostic criteria and pitfalls. Acta Cytol. 1986;30(1):8–16. [PubMed] [Google Scholar]
- 24.Shield P. Peritoneal washing cytology. Cytopathology. 2004;15(3):131–141. doi: 10.1111/j.1365-2303.2004.00140.x. [DOI] [PubMed] [Google Scholar]
- 25.Shidham VB. The panorama of different faces of mesothelial cells. CytoJournal. 2021;18:31. doi: 10.25259/CMAS_02_02_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laughlan SC. The secondary müllerian system. Obstet Gynecol Surv. 1972;27:133–46. doi: 10.1097/00006254-197203000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Burmeister RE, Fechner RE, Franklin RR. Endosalpingiosis of the peritoneum. Obstet Gynecol. 1969;34(3):310–318. [PubMed] [Google Scholar]
- 28.Zinsser RR, Wheeler JE. Endosalpingiosis in the omentum: a study of autopsy and surgical material. Am J Surg Pathol. 1982;6(2):109–117. doi: 10.1097/00000478-198203000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Coffin CM, Adcock LL, Dehner LP. The second-look operation for ovarian neoplasms: a study of 85 cases emphasizing cytologic and histologic problems. Int J Gynecol Pathol. 1985;4(2):97–109. doi: 10.1097/00004347-198506000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Kurman RJ (ed) Blaustein's Pathology of the Female Genital Tract, 7th ed. Springer; 2019 doi: 10.1007/978-3-319-46334-6. [DOI] [Google Scholar]
- 31.Kern WH. Benign papillary structures with psammoma bodies in culdocentesis fluid. Acta Cytol. 1969;13(3):178–180. [PubMed] [Google Scholar]
- 32.Carlson CJ, Samuelson J, Dehner LP. Cytologic diagnosis of florid peritoneal endosalpingiosis: a case report. Acta Cytol. 1986;30(5):494–460. [PubMed] [Google Scholar]
- 33.Young OH, Belinson JL, Papillo JL Korson R. Psammona bodies in peritoneal washings. Acta Cytol. 1982;26(2):233–236. [PubMed] [Google Scholar]
- 34.Covell JL, Carry JB, Feldman PS. Peritoneal washings in ovarian tumors: potential sources of error in cytologic diagnosis. Acta Cytol. 1985;29(3):310–316. [PubMed] [Google Scholar]
- 35.Sneige N, Fernandez T, Copeland LJ, Katz RL. Müllerian inclusions in peritoneal washings: potential source of error in cytologic diagnosis. Acta Cytol. 1986;30(3):271–276. [PubMed] [Google Scholar]
- 36.Sidawy MK, Silverberg SG. Endosalpingiosis in female peritoneal washings: a diagnostic pitfall. Int J Gynecol Pathol. 1987;6(4):340–346. doi: 10.1097/00004347-198712000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Sneige N, Fanning CV. Peritoneal washing cytology in women: diagnostic pitfalls and clues for correct diagnosis. Diagn Cytopathol. 1992;8(6):632–642. doi: 10.1002/dc.2840080621. [DOI] [PubMed] [Google Scholar]
- 38.Schuldenfrei R, Janovaki NA. Disseminated endosalpingiosis associated with bilateral papillary serous cystadenocarcinoma of the ovaries. Am J Obstet Gynecol. 1962;84(8):282–289. doi: 10.1016/0002-9378(62)90136-9. [DOI] [PubMed] [Google Scholar]
- 39.Gaulier A, Jouret-Mourin A, Marsan C. Peritoneal endometriosis. Report of a case with cytologic. cytochemical and histopathologic study. Acta. 1983 Cytol;27(4):446–449. [PubMed] [Google Scholar]
- 40.Portuondo JA, Herron C, Echanojauregui AD, Reigo AG. Peritoneal flushing and biopsy in laparoscopically diagnosed endometriosis. Fertil Steril. 1982;38(5):538–541. doi: 10.1016/S0015-0282(16)46631-8. [DOI] [PubMed] [Google Scholar]
- 41.McGowan L, Bunnag B, Arias LB. Peritoneal fluid cytology associated with benign neoplastic ovarian tumors in woman. Am J Obstet Gynecol. 1972;113(6):961–966. doi: 10.1016/0002-9378(72)90665-5. [DOI] [PubMed] [Google Scholar]
- 42.Selvaggi SM. Diagnostic pitfalls of peritoneal washing cytology and the role of cell blocks in their diagnosis. Diagn Cytopathol. 2003;28(6):335–341. doi: 10.1002/dc.10290. [DOI] [PubMed] [Google Scholar]
- 43.Wojcik E, Naylor B. 'Collagen balls' in peritoneal washings: prevalence, morphology, origin and significance. Acta Cytol. 1992;36(4):466–470. [PubMed] [Google Scholar]
- 44.Sidawy MK, Charndra P, Oertel YC. Detached ciliary tufts in female peritoneal washings. A common finding. Acta Cytol. 1987;31(6):841–844. doi: 10.1097/00004347-198712000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Mathew S, Erozan Y. Significance of peritoneal washings in gynecologic oncology. The experience with 901 intraoperative washings at an academic medical center. Arch Pathol Lab Med. 1997;121(6):604–606. [PubMed] [Google Scholar]
- 46.Mulvaney N. Cytohistologic correlation in malignant peritoneal washings: analysis of 75 malignant fluids. Acta Cytol. 1996;40(6):1231–1239. doi: 10.1159/000333986. [DOI] [PubMed] [Google Scholar]
- 47.Fadare O, Mariappan MR, Hileeto D, et al. Upstaging based solely on positive peritoneal washing does not affect outcome in endometrial cancer. Mod Pathol. 2005;18(5):673–680. doi: 10.1038/modpathol.3800342. [DOI] [PubMed] [Google Scholar]
- 48.Yoshimura S, Scully RE, Taft PD, Herrington JB. Peritoneal fluid cytology in patients with ovarian cancer. Gynecol Oncol. 1984;17(2):161–167. doi: 10.1016/0090-8258(84)90072-6. [DOI] [PubMed] [Google Scholar]
- 49.Shidham VB. Metastatic carcinoma in effusions. CytoJournal. 2022;19:4. doi: 10.25259/CMAS_02_09_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson TL, Kumar NC, Hopkins M, Hughes JD. Cytologic features of ovarian tumors of low malignant potential in peritoneal fluids. Acta Cytol. 1988;32(4):513–518. [PubMed] [Google Scholar]
- 51.Cheng L, Wolf NG, Rose PG, Rodriguez M, Abdul-Karim FW. Peritoneal washing cytology of ovarian tumors of low malignant potential: Correlation with surface ovarian involvement and peritoneal implants. Acta Cytol. 1998;42:1091–4. doi: 10.1159/000332094. [DOI] [PubMed] [Google Scholar]
- 52.Sneige N, Thomison JB, Malpica A, Gong Y, Ensor J, Silva EG. Peritoneal washing cytologic analysis of ovarian serous tumors of low malignant potential to detect peritoneal implants and predict clinical outcome. Cancer Cytopathol. 2012;120:238–44. doi: 10.1002/cncy.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tauchi PS, Caraway N, Truong LD, Kaplan AL, Ramzy I. Serous surface carcinoma of the peritoneum: useful role of cytology in differential diagnosis and followup. Acta Cytol. 1996;40(3):429–436. doi: 10.1159/000333894. [DOI] [PubMed] [Google Scholar]
- 54.Colgan TJ, Boerner SL, Murphy J, Cole DE, Narod S, Rosen B. Peritoneal lavage cytology: An assessment of its value during prophylactic oophorectomy. Gynecol Oncol. 2002;85:397–403. doi: 10.1006/gyno.2002.6638. [DOI] [PubMed] [Google Scholar]
- 55.Landon G, Stewart J, Deavers M, Lu K, Sneige N. Peritoneal washing cytology in patients with BRCA1 or BRCA2 mutations undergoing risk-reducing salpingooophorectomies: A 10-year experience and reappraisal of its clinical utility. Gynecol Oncol. 2012;125:683–6. doi: 10.1016/j.ygyno.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szpak CA, Creasman WT, Vollmer RT, Johnston WW. Prognostic value of cytologic examination of peritoneal washing in patients with endometrial carcinoma. Acta Cytol. 1981;25(6):640–646. doi: 10.1097/00006254-198210000-00017. [DOI] [PubMed] [Google Scholar]
- 57.Creasman WT, Disaia PJ, Blessing J, et al. Prognostic significance of peritoneal cytology in patients with endometrial cancer and preliminary data concerning therapy with intraperitoneal radiopharmaceuticals. Am J Obstet Gynecol. 1981;141(8):921–929. doi: 10.1016/S0002-9378(16)32684-9. [DOI] [PubMed] [Google Scholar]
- 58.Yazigi R, Piver MS, Blumenson L. Malignant peritoneal cytology as prognostic indicator in stage I endometrial cancer. Obstet Gynecol. 1983;62(3):359–362. doi: 10.1097/00006250-198309000-00019. [DOI] [PubMed] [Google Scholar]
- 59.Ide P. Prognostic value of peritoneal fluid cytology in patients with endometrial cancer stage I. Eur J Obstet Gynec Reprod Biol. 1984;18(5-6):343–349. doi: 10.1016/0028-2243(84)90056-X. [DOI] [PubMed] [Google Scholar]
- 60.Boronow RC, Morrow CP, Creasman WT, et al. Surgical staging of endometrial cancer: clinicopathologic findings of a prospective study. Obstet Gynecol. 1984;63(6):825–832. [PubMed] [Google Scholar]
- 61.Soper JT, Creasman WT, Clarke-Pearson DL, et al. Intraperitoneal chromic phosphate P32 suspension therapy of malignant peritoneal cytology in endometrial carcinoma. Am J Obstet Gynecol. 1985;153(2):191–196. doi: 10.1016/0002-9378(85)90112-7. [DOI] [PubMed] [Google Scholar]
- 62.Kennedy AW, Peterson RN, Becker SN, Nunez C, Webster KD. Experience with pelvic washings with stage I and II endometrial carcinoma. Gynecol Oncol. 1987;28(1):50–60. doi: 10.1016/S0090-8258(87)80008-2. [DOI] [PubMed] [Google Scholar]
- 63.Mazurka JL, Krepart GV, Loticki RJ. Prognostic significance of positive peritoneal cytology in endometrial carcinoma. Am J Obstet Gynecol. 1988;158(2):303–306. doi: 10.1016/0002-9378(88)90143-3. [DOI] [PubMed] [Google Scholar]
- 64.Imachi M, Tsukamoto Matsuyama T, Nakano H. Peritoneal cytology in patients with endometrial carcinoma. Gynecol Oncol. 1988;30(1):76–86. doi: 10.1016/0090-8258(88)90049-2. [DOI] [PubMed] [Google Scholar]
- 65.Konski A, Poulter C, Keys H, et al. Absence of prognostic significance, peritoneal dissemination and treatment advantage in endometrial cancer patients with positive peritoneal cytology. Int J Radiat Oncol Biol Phys. 1988;14(1):49–55. doi: 10.1016/0360-3016(88)90050-8. [DOI] [PubMed] [Google Scholar]
- 66.Heath R, Rosenman J, Varia M, Walton L. Peritoneal fluid cytology in endometrial cancer: its significance and the role of chromic phosphate (P32) therapy. Int J Radiat Oncol Biol Phys. 1988;15(4):815–822. doi: 10.1016/0360-3016(88)90112-5. [DOI] [PubMed] [Google Scholar]
- 67.Harouny VR, Sutton GP, Clark SA, et al. The importance of peritoneal cytology in endometrial carcinoma. Obstet Gynecol. 1988;72(3 Pt 1):394–398. [PubMed] [Google Scholar]
- 68.Brewington KC, Hughes RR, Coleman S. Peritoneal cytology as a prognostic indicator in endometrial carcinoma. J Reprod Med. 1989;34(10):824–826. [PubMed] [Google Scholar]
- 69.McLellan R, Dillon MB, Currie JL, Rosenshein NB. Peritoneal cytology in endometrial cancer: a review. Obstet Gynecol Surv. 1989;44(10):711–719. doi: 10.1097/00006254-198910000-00002. [DOI] [PubMed] [Google Scholar]
- 70.Hirai Y, Fujimoto I, Yamauchi K, et al. Peritoneal fluid cytology and prognosis in patients with endometrial carcinoma. Obstet Gynecol. 1989;73(3 Pt 1):335–338. doi: 10.1016/0020-7292(89)90237-3. [DOI] [PubMed] [Google Scholar]
- 71.Gu M, Shi W, Barakat RR, Thaler HT, Saigo PE. Peritoneal washings in endometrial carcinoma. A study of 298 patients with histopathologic correlation. Acta Cytol. 2000;44(5):783–789. doi: 10.1159/000328562. [DOI] [PubMed] [Google Scholar]
- 72.Lurain JR, Rumsey NK, Schink JC, Wallemark CB, Chmiel JS. Prognostic significance of positive peritoneal cytology in clinical stage I adenocarcinoma of the endometrium. Obstet Gynecol. 1989;74(2):175–179. [PubMed] [Google Scholar]
- 73.Turner DA, Gershenson DM, Atkinson N, Sneige N, Wharton AT. The prognostic significance of peritoneal cytology for Stage 1 endometrial cancer. Obstet Gynecol. 1989;74(5):775–780. [PubMed] [Google Scholar]
- 74.Grimshaw RN, Tupper WC, Fraser RC, Tompkins MG, Jeffrey JF. Prognostic value of peritoneal cytology in endometrial carcinoma. Gynecol Oncol. 1990;36(1):97–100. doi: 10.1016/0090-8258(90)90116-3. [DOI] [PubMed] [Google Scholar]
- 75.Morrow CP, Bundy BN, Kurman RJ, et al. Relationship between surgical-pathologic risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991;40(1):55–65. doi: 10.1016/0090-8258(91)90086-K. [DOI] [PubMed] [Google Scholar]
- 76.Wolfson AH, Sightler SE, Markoe AM, et al. The prognostic significance of surgical staging for carcinoma of the endometrium. Gynecol Oncol. 1992;45(2):142–146. doi: 10.1016/0090-8258(92)90276-O. [DOI] [PubMed] [Google Scholar]
- 77.Kadar N, Homesley HD, Maletano JH. Positive peritoneal cytology is an adverse factor in endometrial carcinoma only if there is other evidence of extrauterine disease. Gynecol Oncol. 1992;47(2):145–149. doi: 10.1016/0090-8258(92)90123-Z. [DOI] [PubMed] [Google Scholar]
- 78.Grigsby PW, Perez CA, Kuten A, et al. Clinical stage I endometrial cancer: prognostic factors for local control and distant metastasis and implication of the new FIGO surgical staging system. Int J Radiat Oncol Biol. 1992;22(5):905–911. doi: 10.1016/0360-3016(92)90786-H. [DOI] [PubMed] [Google Scholar]
- 79.Kennedy AW, Webster KD, Nunez C, Bauer LJ. Pelvic washings for cytologic analysis in endometrial carcinoma. J Reprod Med. 1993;38(1):637–642. [PubMed] [Google Scholar]
- 80.Obermair A, Geramou M, Tripcony L, et al. Peritoneal cytology: impact on disease-free survival in clinical stage I endometrioid adenocarcinoma of the uterus. Cancer Lett. 2001;164(1):105–110. doi: 10.1016/S0304-3835(00)00722-9. [DOI] [PubMed] [Google Scholar]
- 81.Abu-Ghazaleh S, Johnston WJ, Creasman WT. The significance of peritoneal cytology in patients with carcinoma of the cervix. Gynecol Oncol. 1984;17(2):139–48. doi: 10.1016/0090-8258(84)90069-6. [DOI] [PubMed] [Google Scholar]
- 82.Roberts WS, Bryon CSP, Cavenagh D, Roberts VC, Lyman GH. Peritoneal cytology and invasive carcinoma of the cervix. Gynecol Oncol. 1986;24(3):331–336. doi: 10.1016/0090-8258(86)90310-0. [DOI] [PubMed] [Google Scholar]
- 83.Zuna RE, Hansen K, Mann W. Peritoneal washing cytology in cervical carcinoma: analysis of 108 patients. Acta Cytol. 1990;39(5):645–651. [PubMed] [Google Scholar]
- 84.Morris PC, Haugen J, Anderson B, Buller R. The significance of peritoneal cytology in stage IB cervical cancer. Obstet Gynecol. 1992;80(2):196–198. [PubMed] [Google Scholar]
- 85.Kilgore LC, Orr JW, Hatch KD, Shingleton HM, Roberson J. Peritoneal cytology in patients with squamous cell carcinoma of the cervix. Gynecol Oncol. 1984;19(1):24–29. doi: 10.1016/0090-8258(84)90153-7. [DOI] [PubMed] [Google Scholar]
- 86.Imachi M, Tsukamoto N, Matsuyama T, Nakano H. Peritoneal cytology in patients with carcinoma of the uterine cervix. Gynecol Oncol. 1987;26(2):202–207. doi: 10.1016/0090-8258(87)90274-5. [DOI] [PubMed] [Google Scholar]
- 87.Ito K, Noda K. Peritoneal cytology in patients with uterine cervical carcinoma. Gynecol Oncol. 1992;47(1):75–79. doi: 10.1016/0090-8258(92)90080-3. [DOI] [PubMed] [Google Scholar]
- 88.Drescher CW, Hopkins MP, Roberts JA. Comparison of the pattern of metastatic spread of squamous cell cancer and adenocarcinoma of the uterine cervix. Gynecol Oncol. 1989;33(3):340–343. doi: 10.1016/0090-8258(89)90524-6. [DOI] [PubMed] [Google Scholar]