Abstract
The microbicidal activity of sodium lauryl sulfate (SLS) against human immunodeficiency virus type 1 (HIV-1) was studied in cultured cells. Pretreatment of HIV-1NL4-3 with SLS decreased, in a concentration-dependent manner, its infectivity when using 1G5 as target cells. In the absence of a viral pretreatment period or when 1G5 cells were pretreated with SLS, the surfactant-induced inactivation of viral infectivity was less pronounced, especially at concentrations between 375 and 550 μM. SLS had no effect on HIV-1 when the virus was adsorbed to 1G5 cells by a 2-h incubation period. SLS almost completely inhibited the fusion process by decreasing the attachment of HIV-1 to target cells. SLS also inhibited the infectivity of HIV-1-based luciferase reporter viruses pseudotyped with the amphotropic murine leukemia virus envelope (which enters cells in a CD4-, CCR5-, and CXCR4-independent manner), indicating that SLS may inactivate other envelope viruses. In contrast, no effect was seen with vesicular stomatitis virus envelope glycoprotein G (which enters cells through receptor-mediated endocytosis) pretreated with up to 700 μM SLS. SLS also decreased, in a dose-dependent manner, the HIV-1-dependent syncytium formation between 1G5 and J1.1 cells after a 24-h incubation. The reduction of luciferase activity was more pronounced when J1.1 cells (which express HIV-1 proteins on their surface) were pretreated with SLS rather than 1G5 cells. Taken together, our results suggest that SLS could represent a candidate of choice for use in vaginal microbicides to prevent the sexual transmission of HIV and possibly other pathogens causing sexually transmitted diseases.
The number of individuals infected with human immunodeficiency virus type 1 (HIV-1) is growing dramatically throughout the world. HIV-1 infection is now the sixth leading cause of death among persons aged 15 to 24 years in the United States (8). In 1996, AIDS was the leading cause of death among African American women aged 24 to 44 years (26). Recent statistics (as of the end of 2000) from the World Health Organization indicated that there are about 36.1 million people infected with HIV-1 worldwide. Of that number, 16.4 million (47.3%) are women living with HIV or AIDS. An estimated 5.3 million new cases of HIV-1 infection occurred during the year 2000 (i.e., approximately 15,000 infections each day). AIDS deaths totaled 3 million in 2000 for a cumulative death total of 21.8 million since the beginning of the epidemic. Globally, heterosexual transmission may account for as much as 85 to 90% of the new cases of HIV-1 infection.
As there is, until now, no vaccine against HIV-1, preventive measures are the only tools that can reduce the transmission of this retrovirus. The consistent and careful use of latex condoms represents an effective method to prevent the sexual transmission of HIV-1, but their use is not generalized. In Africa, the most intensive prevention programs were able to increase condom use only to approximately 70% of all sexual intercourse by female prostitutes (31). Moreover, a national U.S. survey made by the Centers for Disease Control and Prevention showed that condom use decreases markedly with age. Indeed, the percentage of condom use at last sexual intercourse for grade 12 students was only 50%, and this value decreased to 37 and 15% for individuals aged 25 to 29 and 45 to 49 years, respectively. Consequently, doubts arise about the possibilities of condom promotion in controling the AIDS epidemic. The situation may be similar for homosexual men from developed countries, where relapse to unsafe sexual practices, including lack of condom use for anal intercourse, has been documented in several studies (14, 36). More attention is now given to female-controlled methods for the prevention of HIV-1 infection since many women are unable to negotiate condom use with their sexual partners (19, 20, 26, 49, 52, 56). The development of safe and effective topical microbicides is actually a very high priority for the World Health Organization, the National Institutes of Health, and the Centers for Disease Control and Prevention in the field of sexually transmitted diseases (STDs) and HIV prevention.
Most currently available vaginal formulations use the spermicide nonoxynol-9, a nonionic surfactant, as a microbicide. In vitro, nonoxynol-9 inactivates enveloped viruses such as herpes simplex virus (HSV), HIV-1, and other microorganisms, including Chlamydia trachomatis and Neisseria gonorrhoeae (3, 5, 21, 27, 58). However, nonoxynol-9 does not inactivate nonenveloped papillomaviruses (24). The potential efficacy of nonoxynol-9 against HIV-1 has never been clearly established, and results of clinical trials are controversial (21, 30, 47, 58, 62). Recently, nonoxynol-9 was demonstrated to be ineffective since its use resulted in an increased rate of HIV-1 infection compared to the placebo group (55). The frequent use of nonoxynol-9 was associated with an increased incidence of vulvar ulcers and vulvitis, which could increase the risk of HIV-1 infection (30, 51, 62). Consequently, there is an urgent need to develop novel compounds for use in topical vaginal formulations to efficiently reduce sexually transmitted infections.
Sodium lauryl sulfate (SLS) is an anionic surfactant with protein denaturant potency. Ward and Ashley first demonstrated that SLS is a potent inactivator of nonenveloped rotavirus and poliovirus infectivities at quite low concentrations and under very mild conditions (57). More recently, SLS was shown to inhibit the infectivities of rabbit, bovine, and human papillomaviruses as well as that of the enveloped HSV-2 and HIV-1 in vitro (25, 29). Previous studies from our laboratory have also demonstrated that SLS inhibits the infectivities of different HSV strains in in vitro and in vivo models (44). Furthermore, a gel formulation containing 2.5% SLS was also effective in preventing infectious virus from colonizing mouse vaginal mucosa following intravaginal challenge with HSV-2 (48). This formulation was very well tolerated by the vaginal mucosa of rabbits after daily administration for 14 days. This suggests that SLS could be a potential candidate for use as a microbicide in vaginal formulations to prevent the sexual transmission of HIV-1, herpes, and other pathogens causing STDs. In the present study, we have investigated the mechanism involved in the inactivation of HIV-1 by SLS in cultured cells.
MATERIALS AND METHODS
Cell lines.
The human embryonic kidney cell line 293T expressing the simian virus 40T antigen was maintained in Dulbecco modified Eagle medium (DMEM; Gibco-BRL, Burlington, Ontario, Canada) supplemented with 10% fetal bovine serum (FBS; Hyclone Laboratories, Logan, Utah), l-glutamine (2 mM), penicillin G (100 U/ml) and streptomycin (100 mg/ml). 293T cells were kindly provided by W. C. Greene (The J. Gladstone Institutes, San Francisco, Calif.). Uninfected CD4+ T cells included Jurkat E6.1 (59) and the Jurkat E6.1-derived 1G5 cell line (provided by the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health), which harbors two copies of a stable transfected plasmid made of the luciferase reporter gene downstream of the HIV-1 long-terminal-repeat (LTR) region (2). We also used the Jurkat E6.1-derived J1.1 cell line, which is latently infected with HIV-1 (43). All cell lines were grown in RPMI 1640 (Gibco-BRL) supplemented with 10% heat-inactivated FBS, l-glutamine (2 mM), penicillin G (100 U/ml), and streptomycin (100 mg/ml). Cell cultures were maintained at 37°C in a 5% CO2 atmosphere.
Production of viral particles.
pNL4-3, a plasmidic preparation originating from an infectious molecular clone of a T-tropic strain of HIV-1, was provided by the AIDS Repository Program. The NL4-3 luciferase backbone (pNL4-3-Luc-E−R+), a vector that carries the gene for firefly luciferase inserted into the nef gene of the pNL4-3 provirus and contains a frameshift at the 5′ end of env (nucleotide 5950) that prevents expression of the envelope glycoproteins (9, 12), and the plasmid DNA encoding the amphotropic murine leukemia virus (A-MLV) full-length envelope proteins were generously provided by N. R. Landau (The Salk Institute for Biological Studies, La Jolla, Calif.). Upon transfection, pNL4-3 directs the production of replication and infection-competent virions (1). The pHCMV-G expressing the broad-host-range vesicular stomatitis virus envelope glycoprotein G (VSV-G) from the human cytomegalovirus promoter has been described previously (61). The production of HIV-1 recombinant luciferase-encoding progeny virions or HIV-1 virions pseudotyped with the envelope proteins from A-MLV or VSV-G envelopes was performed according to a previously described protocol (22). Briefly, 293T cells were transfected by the calcium phosphate protocol with 10 μg of pNL4-3 proviral DNA vector or cotransfected with 5 μg of both pNL4-3-luc-E−R+ and the plasmid DNA encoding the A-MLV or VSV-G envelopes for the pseudotyped virions. Supernatant of transfected cells was harvested 48 h posttransfection, and HIV-1 particles were quantified using a p24 enzymatic assay (Organon Teknika, Durham, N.C.).
Viral infection assay.
1G5 and Jurkat E6.1 cells (105) were infected with HIV-1NL4-3 and luciferase-encoding pseudotyped virions (10 ng of p24), respectively, in a final volume of 200 μl of complete culture medium containing SLS for 2 h at 37°C. Cells were then washed once with phosphate buffered saline (PBS; pH 7.4), resuspended in 200 μl of complete culture medium, transferred in a 96-well flat-bottomed tissue culture plate (Becton Dickinson, Lincoln Park, N.J.), and allowed to grow for 72 h at 37°C in a 5% CO2 atmosphere. In some experiments, HIV-1NL4-3 (10 ng of p24) or 1G5 cells (105) were pretreated with SLS (0 to 700 μM) for 1 h at 37°C in a final volume of 100 μl of complete culture medium and washed once with PBS prior to infection. In another set of experiments, virus (10 ng of p24) was adsorbed to cells (105) by a 2-h incubation period prior to the addition of SLS, which was then maintained in contact with cells for 72 h. Infection was monitored by measuring the luciferase activity as described previously (4). In brief, 100 μl of cell-free culture supernatant was withdrawn from each well, and 25 μl of 5× cell culture lysis buffer (125 mM Tris phosphate, 10 mM dithiothreitol, 5% Triton X-100, 50% glycerol [pH 7.8]) was added before incubation for 30 min at room temperature. Thereafter, an aliquot of this cell lysate (20 μl) was mixed with 100 μl of luciferase assay buffer [210 mM Tricine, 1.07 mM (MgCO3)4·Mg(OH)2·5H2O, 2.67 mM MgSO4, 0.1 mM EDTA, 270 μM coenzyme A, 470 μM luciferin (Biosynth International, Naperville, Ill.), 530 μM ATP, 33.3 mM dithiothreitol], and luciferase activity was evaluated using a microplate luminometer (Dynex Technologies, Chantilly, Va.).
Cellular viability.
1G5 cells were incubated with RPMI 1640 + 10% FBS (control) or with different concentrations of SLS in complete culture medium for 72 h at 37°C. Cell viability and proliferation was then monitored with a tetrazolium salt [MTS; 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; Promega, Madison, Wis.], which in the presence of phenazine methosulfate is reduced by living cells to yield a formazan product that can be assayed colorimetrically (7). The effect of SLS on the viability and proliferation of Jurkat E6.1 cells (in the attachment-fusion and pseudotyped virus experiments) and of cocultures of J1.1 and 1G5 cells (in the syncytium-formation experiments) was also evaluated.
HIV-1 attachment and entry assays.
For the attachment assay, HIV-1NL4-3 (30 ng of p24) was pretreated with SLS (375 or 500 μM) in complete culture medium for 1 h at 37°C. Thereafter, Jurkat E6.1 cells (3 × 105) were washed once in ice-cold PBS and were infected with pretreated virus for 90 min on ice to allow virus attachment. Samples were washed three times with ice-cold PBS and dispensed in a 96-well plate. Quantification of bound viruses was determined using a commercial p24 enzymatic assay. The entry assay was performed according to a previously described protocol with some modifications (35, 42). Briefly, HIV-1NL4-3 (50 ng of p24) was pretreated with SLS (375 or 500 μM) in complete culture medium for 1 h at 37°C. Jurkat E6.1 cells (2.5 × 106) were washed once with room temperature PBS and were infected with pretreated virus for 2 h at 37°C. Afterwards, cells were washed twice with ice-cold PBS and resuspended in 1 ml of ice-cold FBS-free DMEM in the presence of 0.1 mg of pronase (Boehringer Mannheim, Laval, Quebec, Canada). Cells were then incubated for 5 min on ice and immediately washed twice with ice-cold DMEM containing 10% FBS and three times with ice-cold PBS to remove pronase. Cells were resuspended in 0.6% Triton X-100-containing RPMI medium, incubated for 10 min at room temperature under constant agitation, and stored at −85°C until assayed for p24 content.
Syncytium assay.
Syncytium formation was evaluated following a previously described luciferase quantitative assay based on the transfer of the Tat protein from the HIV-1 chronically infected J1.1 cells to the 1G5 cells, which harbor two copies of a stable transfected plasmid made of the luciferase reporter gene downstream of the HIV-1 LTR region (4). Briefly, 100 μl of J1.1 chronically infected cells (105 cells/ml) were mixed and incubated with 1G5 cells (105 cells/ml) for 24 h at 37°C in the presence of different concentrations of SLS. In some experiments, 1G5 or J1.1 cells were pretreated for 1 h at 37°C in the presence of SLS and then washed thoroughly before starting the coculture experiment. After a 24-h incubation period, cells were lysed and luciferase activity was determined as described above.
RESULTS
Inactivation of HIV-1NL4-3.
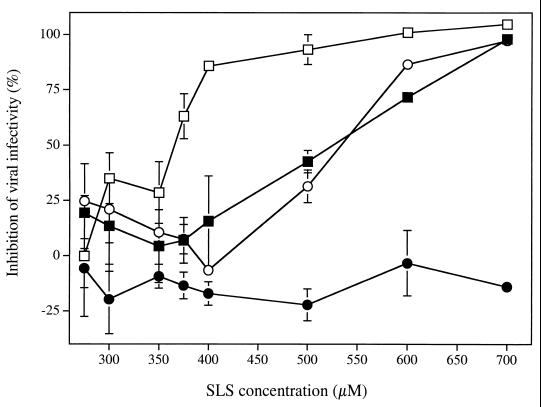
Figure 1 shows the effect of increasing SLS concentration on HIV-1NL4-3 infectivity to 1G5 cells under different experimental conditions. Pretreatment of the virus with SLS for 1 h at 37°C inhibited its infectivity to 1G5 cells in a concentration-dependent manner. The concentrations of SLS which inhibited 50 and 90% of virus infectivity were 365.0 ± 8.5 and 453.9 ± 30.0 μM, respectively. In the absence of a pretreatment period, the concentration of SLS which inhibited 50 and 90% of virus infectivity increased to 533.2 ± 10.7 and 606.1 ± 4.3 μM, respectively. Similarly, when 1G5 cells were pretreated with SLS, these values increased to 524.5 ± 15.2 and 663.0 ± 2.2 μM, respectively. In contrast, when SLS was added to cells infected with HIV-1NL4-3 (following a 2-h adsorption period), no significant inactivation of the virus could be observed, even at a concentration of 700 μM. The incubation of 1G5 cells with up to 700 μM SLS for 72 h did not affect their viability. The 50% cytotoxic concentration of SLS for this cell line was 829.1 μM.
FIG. 1.
Effect of SLS on the infectivity of HIV-1NL4-3 to 1G5 cells under different experimental (or infection) conditions. (i) Cell-free virus (□) was pretreated with increasing SLS concentrations for 1 h at 37°C and washed with PBS. 1G5 cells were then infected with HIV-1NL4-3 (10 ng of p24/105 cells) for 2 h at 37°C, washed, and maintained in fresh culture medium in the absence of SLS (control luciferase activity was 33.1 ± 6.5 relative light units [RLU]). (ii) 1G5 cells (■) were pretreated with increasing SLS concentrations for 1 h at 37°C and washed with PBS. 1G5 cells were then infected with HIV-1NL4-3 (10 ng of p24/105 cells) for 2 h at 37°C, washed, and maintained in fresh culture medium in the absence of SLS (control luciferase activity was 23.6 ± 2.9 RLU). (iii) SLS and the virus were added simultaneously to cells, incubated for 2 h, washed, and maintained in fresh culture medium in the absence of SLS (○) (control luciferase activity was 36.8 ± 3.3 RLU). (iv) HIV-1NL4-3 was adsorbed to cells by a 2-h incubation period prior to the addition of SLS, which was maintained in contact with cells for 72 h (●) (control luciferase activity was 96.3 ± 10.5 RLU). Luciferase activity was measured 72 h after infection. Results are means (± standard deviations) of triplicates and are representative of three independent experiments.
Inhibition of HIV-1 attachment and entry.
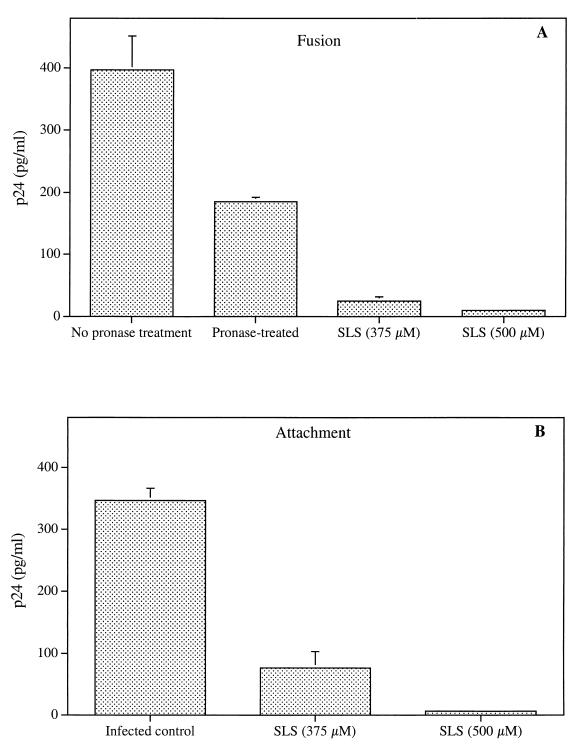
Figure 2A shows the effect of SLS on the process of viral entry into cells. Jurkat E6.1 cells were incubated with SLS-pretreated HIV-1NL4-3 for 2 h at 37°C. Cells were then treated with pronase to remove all bound viruses which had not fused with the cell membrane. Cells were washed thoroughly, and the amount of virus which had fused with and penetrated into the target cells was determined by using a p24 enzymatic assay. Jurkat E6.1 cells incubated with the virus in the absence of a pronase treatment gave a higher p24 value (i.e., bound and fused viruses) than pronase-treated cells (i.e., fused virus only). Interestingly, when the virus was pretreated with SLS followed by treatment of cells with pronase, an important decrease in p24 levels was observed compared to that of pronase-treated infected cells. Inhibitions of the fusion process to 86.5 and 94.6% of control values were observed following pretreatment with 375 and 500 μM SLS, respectively. In order to determine whether this effect resulted from an inhibition of the earliest steps of viral entry, we evaluated the effect of SLS on viral attachment to target cells. In contrast to the fusion process, which is energy-dependent and required a 37°C incubation period, HIV-1 binding can be achieved at 4°C. Bound viruses can then be quantitated directly by a simple p24 enzymatic assay from washed cells. Figure 2B shows that pretreatment of the virus with SLS inhibited its binding to Jurkat E6.1 cells, as demonstrated by the decrease of virus-associated p24 levels compared to that of control infected cells. Inhibitions of the attachment step by 77.9 and 98.1% of control values were observed following pretreatment with 375 and 500 μM SLS, respectively. Similar results were obtained when using 1G5 cells (data not shown).
FIG. 2.
Effect of SLS on the fusion (A) and attachment (B) of HIV-1NL4-3 to Jurkat E6.1 cells. Panel A shows untreated and SLS-treated HIV-1NL4-3 viruses (30 ng of p24) incubated with Jurkat E6.1 cells (3 × 105) for 2 h at 37°C and then treated with pronase (0.1 mg/ml) for 5 min. Cells not treated with pronase correspond to bound and fused virus whereas cells treated with pronase correspond to fused virus only. Standard p24 quantification was subsequently performed after cell lysis. Panel B shows untreated and SLS-treated viruses (30 ng of p24) incubated with Jurkat E6.1 cells (3 × 105) for 90 min at 4°C. After extensive washing, cells were lysed and p24 quantification was performed. p24 levels measured in uninfected cells were subtracted from data as background values. Results are means (± standard deviations) of triplicates and are representative of three independent experiments.
Inactivation of pseudotyped viruses with VSV-G and AML-V env proteins.
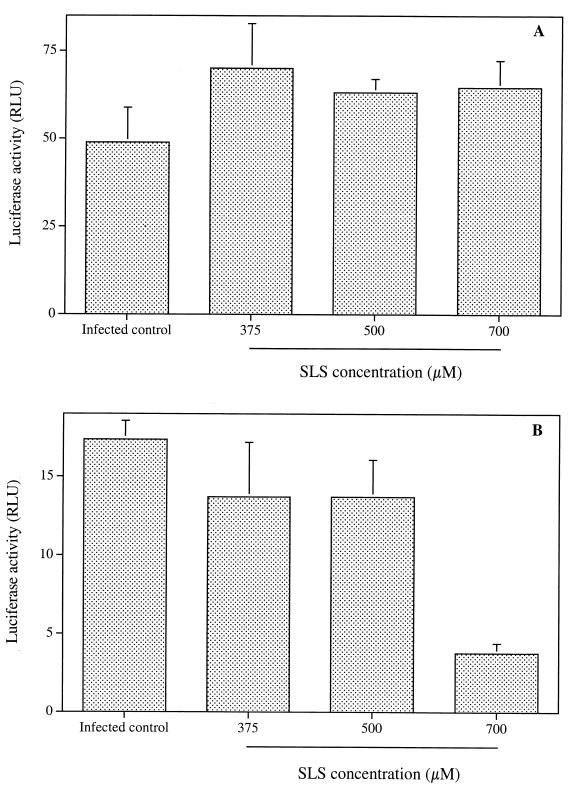
We evaluated the effect of SLS on luciferase reporter viruses bearing the VSV-G or AML-V envelope proteins. These pseudotyped viruses possess the genome and capsid shell of HIV-1 but bear either the AML-V or VSG-V envelope. These experiments allowed us to test whether SLS exerts its effect on HIV RNA, HIV-1 capsid proteins, or proteins present in the viral envelope. Figure 3A shows that SLS did not inhibit the infectivity of VSV-G pseudotyped virus, which enters cells through a receptor-mediated endocytosis, to Jurkat E6.1 cells, even at a concentration of 700 μM. In contrast, the infectivity of A-MLV pseudotyped virus, which enters cells by fusion via a CD4-, CCR5-, and CXCR4-independent mechanism, was severely reduced by SLS (Fig. 3B). For example, about 78% of AML-V infectivity was inhibited following pretreatment with 700 μM SLS. The incubation of Jurkat E6.1 cells with up to 700 μM SLS did not cause any loss of cell viability (data not shown).
FIG. 3.
Effect of SLS on the infectivity to Jurkat E6.1 cells. of HIV-1-based luciferase reporter viruses pseudotyped with VSV-G (A) and A-MLV (B) envelopes. Single-round infections were made with viruses pretreated with increasing SLS concentrations for 1 h at 37°C and then washed with PBS. Jurkat E6.1 cells were then infected with pretreated viruses (10 ng of p24) for 2 h at 37°C, washed, and maintained in fresh culture medium in the absence of SLS. Luciferase activity was measured at 72 h after infection. Luciferase activities for uninfected control cells were subtracted from data as background values. Results are means (± standard deviations) of triplicates and are representative of two independent experiments.
Inhibition of syncytium formation.
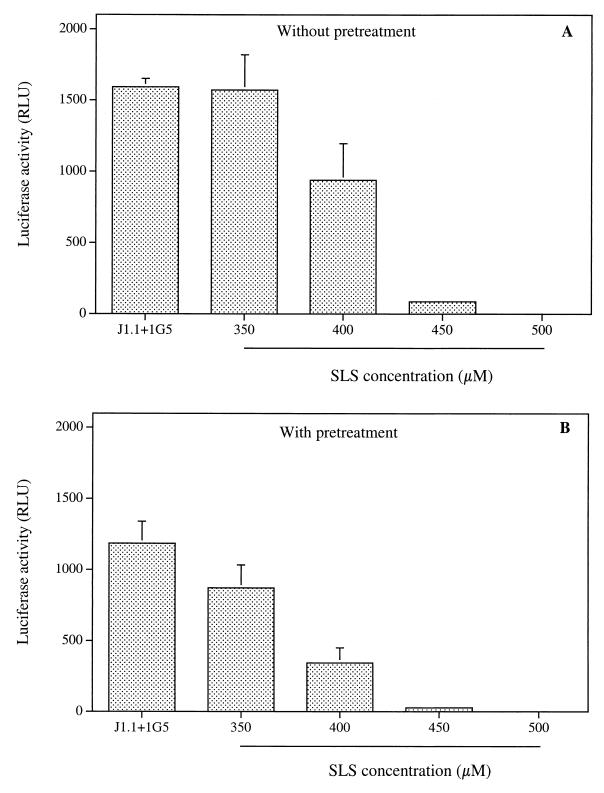
Because several compounds known to inhibit viral entry into cells have also been reported to be potent inhibitors of syncytium formation, we have evaluated the effect of SLS on this process using a previously described methodology (4). The principle of the assay is based on the use of two cell lines, which upon fusion allow the free diffusion of the viral Tat protein from the chronically infected J1.1 cells to 1G5 cells, which stably harbor an HIV-1 LTR-driven luciferase construct. Syncytium formation is quantitated in terms of the relative light units correlating with transcription of the luciferase reporter gene. Figure 4A shows the luciferase activity determined upon addition of J1.1 to 1G5 cells. Interestingly, the addition of SLS simultaneously to cocultured cells (without pretreatment) reduced in a concentration-dependent manner the syncytium-dependent luciferase activity induction. A complete inhibition of syncytium formation was obtained when the coculture experiment was done in the presence of 500 μM SLS. Pretreatment of J1.1 cells with SLS for 1 h at 37°C prior to the coculture with 1G5 cells induced a more pronounced decrease of the syncytium formation compared to that seen in the absence of a pretreatment period (Fig. 4B). A less pronounced decrease in HIV-1 LTR-driven gene activity was observed when 1G5 cells were pretreated with SLS for 1 h at 37°C prior to coculture with the chronically infected J1.1 cells (data not shown). The viability of cocultures of 1G5 and J1.1 cells was not affected following incubation with up to 500 μM SLS (data not shown).
FIG. 4.
Effect of SLS on the syncytium formation between J1.1 and 1G5 cells. Panel A shows 1G5 and J1.1 cells (105 cells) mixed in the absence (positive control) or in the presence of increasing SLS concentrations and incubated for 24 h at 37°C. Panel B shows J1.1 cells pretreated with increasing SLS concentrations, washed, and then incubated with 1G5 cells for 24 h at 37°C. Cells were lysed and assayed for luciferase activity. Luciferase activities for 1G5 cells alone were subtracted from data as background values. Results are means (± standard deviations) of triplicates and are representative of two independent experiments.
DISCUSSION
Heterosexual intercourse is now the most frequent cause of HIV-1 transmission worldwide (45). Since many women are unable to negotiate condom use with their sexual partners, more attention is now given to female-controlled methods for the prevention of HIV-1 infection (16). There is thus a great interest in the development of novel compounds, for use in vaginal microbicides, to reduce the sexual transmission of HIV-1 as well as other pathogens causing STDs. Previous studies have shown that SLS inhibits in vitro the infectivity of different HSV strains, HIV-1, and human papillomavirus (25, 44). SLS could thus represent a potential candidate for use as a vaginal microbicide to prevent the sexual transmission of HIV-1. In the present study, we have examined the mechanism by which SLS inactivates HIV-1 in cultured cells.
Results showed that SLS decreased, in a concentration-dependent manner, the infectivity of the T-tropic HIV-1NL4-3 to 1G5 cells, a T-cell line derived from Jurkat E6.1 cells. A complete inhibition of the infectivity was obtained following pretreatment of cell-free virus with 600 μM of SLS. Krebs et al. have also demonstrated that SLS inhibits the infectivity of the T-tropic HIV-1IIIB to HeLa-CD4-LTR-β-gal (HCLB) cells, as well as that of the macrophage-tropic HIV-1BaL and the dual-tropic HIV-189.6 to the P4-R5 indicator cell line (29). Both HeLa-derived cell lines stably express the cell surface protein CD4 (HCLB) or CD4 in conjunction with the chemokine receptor CCR5 (P4-R5) and also express the β-galactosidase under the control of the HIV-1 long terminal repeat, which can be transactivated during HIV infection. The inactivating potency of SLS was greater for the T-tropic HIV-1IIIB and for the dual-tropic HIV-189.6 (867 μM) than for the macrophage-tropic HIV-1BaL (1.73 mM). Wu et al. have shown that proteins exhibit differential sensitivity to denaturation by SLS (60). Thus, strain-specific differences in the amino acid sequences of viral glycoproteins may modify the conformation of the protein, making it more or less sensitive to denaturation by SLS. A characteristic of HIV-1 propagation in susceptible cells is the incorporation of several host-encoded proteins during the budding process of viral particles from infected cells. These include major histocompatibility complex class II determinants, β2-microglobulin, CD43, CD44, CD55, CD59, CD63, CD71, and adhesion receptors such as ICAM-1 and LFA-1. Several studies have suggested a contribution of virally acquired host cell proteins in virus infectivity (reviewed in reference 54). Therefore, differences in the inactivating potency of SLS may also vary between strains with the same tropism. The inhibitory potency of SLS against HIV-1NL4-3 infectivity was directly related to the duration of the viral pretreatment period, especially at SLS concentrations between 375 and 550 μM. The inactivating potency of SLS was also more efficient when the virus was pretreated rather than when cells were pretreated in this range of concentrations.
HIV-1 usually binds to target cells through interactions between the viral envelope gp120 complex and the cell surface CD4 molecule (13, 18, 28, 34). Thereafter, a high affinity domain of gp120 resulting from conformational changes allows an interaction with either CXCR4 (T-cell tropic isolates) or CCR5 (macrophage-tropic isolates) coreceptors, leading to a destabilization of gp120 trimers, exposure of the viral gp41 fusion peptide, and the fusion of viral and cellular membranes (6, 15, 40). Our studies showed that SLS decreased the fusion process by inhibiting the attachment of HIV-1NL4-3 to Jurkat E6.1 cells.
SLS at concentrations up to 700 μM did not inactivate HIV-1NL4-3 when the virus was adsorbed to 1G5 cells for a 2-h incubation period. SLS is an anionic surfactant with protein denaturant potency. Proteins are known to exhibit different susceptibilities to SLS denaturation. Consequently, it is possible that during pretreatment of the virus with SLS, the surfactant can alter the viral proteins, preventing the initial attachment to the cell surface without having any effect on virus stably attached to cells (i.e., following a 2-h adsorption period). These results suggest that SLS exerts its effect mainly on the attachment of the virus to target cells and not necessarily at the level of entry. However, these data do not imply that SLS did not exert any effect on the virus beyond the attachment step. Indeed, Krebs et al. have reported that SLS inactivated HIV-1IIIB associated with SupT1 cells that were infected 5 days prior to SLS treatment (29). However, higher concentrations were required to achieve an effect similar to that seen on cell-free virus. In addition, the inhibition remained incomplete (approximately 20% of control values) even following treatment of cell-associated virus with 1.75 mM SLS.
SLS, at concentrations similar to those inhibiting HIV-1NL4-3 infectivity, did not inactivate pseudotyped virus with a VSV-G envelope (which enters cells through receptor-mediated endocytosis), suggesting that no effect was exerted on the HIV-1 genome and capsid proteins. However, it is not excluded that SLS could inactivate this pseudotyped virus at higher concentrations because of the different sensitivity of proteins to denaturation by SLS. In contrast, SLS inhibited the infectivity of virons pseudotyped with A-MLV envelope (which enters cells through direct fusion with cell membrane), indicating that SLS may also affect enveloped viruses other than HIV-1. In this respect, we have already shown that SLS inactivated different HSV strains (44). The concentration of SLS required to inactivate the pseudotyped virus with A-MLV envelope was higher than that needed to inactivate HIV-1NL4-3 and was probably related to the denaturating potency of SLS.
The formation of giant multinucleated cells (termed syncytia) resulting from multiple cell-to-cell fusion events and leading to cell death is a well-characterized cytopathic effect associated with HIV-1 infection (11, 32, 33). As for HIV-1 viral entry, the formation of syncytia depends mainly on the interaction of CD4 and CXCR4 coreceptors of uninfected cells with gp120 on HIV-1 infected cells (13, 37, 50). Several compounds, such as soluble CD4, specific antibodies, complestatin, terpestacin, lysophosphatidylcholine, and lipophosphoglycan, act as inhibitors of viral entry into cells but also of syncytium formation (10, 17, 23, 38, 39, 41, 46, 53). SLS also completely prevented HIV-1-dependent syncytium formation between 1G5 and J1.1 (a cell line chronically infected with HIV-1) cells after a 24-h incubation period. The decrease of HIV-1 LTR-driven gene activity was more pronounced when J1.1 cells were pretreated with SLS, suggesting that this surfactant could exert an effect on HIV-1 envelope proteins present on their surface. At a concentration of 300 μM, SLS had no effect on syncytium formation whereas it decreased to about 50% the infectivity of HIV-1NL4-3 to 1G5 cells. SLS thus acts differently on the cell-to-cell fusion process and on virus-to-cell infectivity, probably because the denaturing potency of SLS on target proteins is different.
The syudy of the influence of SLS on the viability and proliferation of each cell line performed in parallel with all efficacy experiments showed that all SLS concentrations used were subcytotoxic and subcytostatic. Therefore, the observations reported here are mainly due to an effect exerted on the virus rather than on cells or on cell-cell interactions.
The mechanism by which SLS inactivates HSV-1 in vitro is quite different from that described above for HIV-1 (44). Indeed, SLS inhibited completely the infectivity of different herpesviruses to Vero cells at a concentration 10-fold lower (50 μM) than that required to inactivate HIV-1. SLS did not prevent the attachment of HSV-1 to Vero cells. Following pretreatment of viral particles with 50 μM SLS, viruses were able to enter into cells and to produce capsid shells devoid of a DNA core in the nuclei. The amount of the glycoprotein D gene produced in these cells remained unchanged compared to control infected cells suggesting that SLS could interfere with the maturation of the virus. Pretreatment of HSV-1 with 100 μM SLS highly damaged the virus, and only a few abnormal capsids were detected in cell nuclei. The mechanism by which SLS inactivates enveloped and nonenveloped viruses probably involves the denaturation of proteins. These proteins may play different roles in the viral replicative cycle, such as adhesion receptors, proteins involved in the encapsidation of herpes viral DNA (44). Therefore, SLS could represent a convenient tool to study the role of its target proteins in the pathogenesis of viral infection in cultured cells and animal models.
Taken together, these data and those of previous studies show that in vitro SLS demonstrates interesting inactivating potencies against HIV-1, HSV strains, and human papillomavirus (25, 29, 44). In a previous study, a gel formulation containing 2.5% SLS was shown to be effective in preventing infectious virus from colonizing mouse vaginal mucosa following intravaginal challenge with HSV-2 (48). In conclusion, SLS could represent a potential candidate for use in vaginal microbicides to prevent the sexual transmission of enveloped and nonenveloped viruses and possibly other pathogens causing STDs.
ACKNOWLEDGMENTS
We thank Nicolas Genois for constructive comments and helpful discussions.
M.J.T. is supported by an investigator (HIV/AIDS) award from the Canadian Institutes of Health Research. This work was supported by a grant from the American Foundation for AIDS Research (AMFAR) and the Canadian Foundation for AIDS Research (CANFAR).
J.B.-S. and J.P. are both first coauthors.
REFERENCES
- 1.Adachi A, Koenig S, Gendelman H E, Daugherty D, Gattoni-Celli S, Fauci A S, Martin M A. Productive, persistent infection of human colorectal cell lines with human immunodeficiency virus. J Virol. 1986;61:209–213. doi: 10.1128/jvi.61.1.209-213.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilar-Cordova E, Chinen J, Donehower L, Lewis D E, Belmont J W. A sensitive reporter cell line for HIV-1 tat activity, HIV-1 inhibitors, and T cell activation effects. AIDS Res Hum Retrovir. 1994;10:295–301. doi: 10.1089/aid.1994.10.295. [DOI] [PubMed] [Google Scholar]
- 3.Alexander N J. Future contraceptives. Sci Am. 1995;273:136–141. [PubMed] [Google Scholar]
- 4.Barbeau B, Fortin J F, Genois N, Tremblay M J. Modulation of human immunodeficiency virus type 1-induced syncytium formation by the conformational state of LFA-1 determined by a new luciferase-based syncytium quantitative assay. J Virol. 1998;72:7125–7136. doi: 10.1128/jvi.72.9.7125-7136.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourinbaiar A S, Lee-Huang S. Comparative in vitro study of contraceptive agents with anti-HIV activity: gramicidin, nonoxynol-9 and gossypol. Contraception. 1994;49:131–137. doi: 10.1016/0010-7824(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 6.Broder C C, Collman R G. Chemokine receptors and HIV. J Leukoc Biol. 1997;62:20–29. doi: 10.1002/jlb.62.1.20. [DOI] [PubMed] [Google Scholar]
- 7.Buttke T M, McCubrey J A, Owen T C. Use of an aqueous soluble tetrazolium/formazan assay to measure viability and proliferation of lymphocyte-dependent cell lines. J Immunol Methods. 1993;157:233–240. doi: 10.1016/0022-1759(93)90092-l. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Trends in sexual risk behaviors among high school students—United States, 1991–1997. Morb Mortal Wkly Rep. 1998;47:749–752. [PubMed] [Google Scholar]
- 9.Chen B K, Saksela K, Andino R, Baltimore D. Distinct modes of human immunodeficiency virus type 1 proviral latency revealed by superinfection of nonproductively infected cell lines with recombinant luciferase-encoding viruses. J Virol. 1994;68:654–660. doi: 10.1128/jvi.68.2.654-660.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clapham P R, Weber J N, Whitby D, McIntosh K, Dalgleish A G, Maddon P J, Deen K C, Sweet R W, Weiss R A. Soluble CD4 blocks the infectivity of diverse strains of HIV and SIV for T cells and monocytes but not for brain and muscle cells. Nature. 1989;337:368–370. doi: 10.1038/337368a0. [DOI] [PubMed] [Google Scholar]
- 11.Cohen J. HIV ‘cofactor’ comes in for more heavy fire. Science. 1993;262:1971. doi: 10.1126/science.7903478. [DOI] [PubMed] [Google Scholar]
- 12.Connor R I, Chen B K, Choe S, Landau N R. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 13.Dalgleish A G, Beverley P C L, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 14.De Wit J B F, Van Griensven G J P, Kok G, Sandfort T G M. Why do homosexual men relapse into unsafe sex? Predictors of resumption of unprotected anogenital intercourse with casual partners. AIDS. 1993;7:1113–1118. doi: 10.1097/00002030-199308000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Doms R W, Peiper S C. Unwelcomed guests with master keys: how HIV uses chemokine receptors for cellular entry. Virology. 1997;235:179–190. doi: 10.1006/viro.1997.8703. [DOI] [PubMed] [Google Scholar]
- 16.du Guerny J, Sjoberg E. Inter-relationship between gender relations and the HIV/AIDS epidemic: some possible considerations for policies and programmes. AIDS. 1993;7:1027. doi: 10.1097/00002030-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Easterbrook M D, Levy M H, Gomez A M, Turco S J, Epand R M, Rosenthal K L. Inhibition of HIV-1-induced syncytia formation and infectivity by lipophosphoglycan from Leishmania. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:496–505. [PubMed] [Google Scholar]
- 18.Eiden L E, Lifson J D. HIV interactions with CD4: a continuum of conformations and consequences. Immunol Today. 1992;13:201–206. doi: 10.1016/0167-5699(92)90154-Y. [DOI] [PubMed] [Google Scholar]
- 19.Elias C J, Heise L L. Challenges for the development of female-controlled vaginal microbicides. AIDS. 1994;8:1–9. doi: 10.1097/00002030-199401000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Feldblum P J, Morrison C S, Roddy R E, Cates W. The effectiveness of barrier methods of contraception in preventing the spread of HIV. AIDS. 1995;9(Suppl. A):S85–S93. [PubMed] [Google Scholar]
- 21.Feldblum P J, Weir S S. The protective effect of nonoxynol-9 against HIV infection. Am J Public Health. 1994;84:1032–1034. doi: 10.2105/ajph.84.6.1032-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fortin J-F, Cantin R, Lamontagne G, Tremblay M. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J Virol. 1997;71:3588–3596. doi: 10.1128/jvi.71.5.3588-3596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunther-Ausborn S, Stegmann T. How lysophosphatidylcholine inhibits cell-cell fusion mediated by the envelope glycoprotein of human immunodeficiency virus. Virology. 1997;235:201–208. doi: 10.1006/viro.1997.8699. [DOI] [PubMed] [Google Scholar]
- 24.Hermonat P L, Daniel R W, Shah K V. The spermicide nonoxynol-9 does not inactivate papillomavirus. Sex Transm Dis. 1992;19:203–205. doi: 10.1097/00007435-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Howett M K, Neely E B, Christensen N D, Wigdahl B, Krebs F C, Malamud D, Patrick S D, Pickel M D, Welsh P A, Reed C A, Ward M G, Budgeon L R, Kreider J W. A broad-spectrum microbicide with virucidal activity against sexually transmitted viruses. Antimicrob Agents Chemother. 1999;43:314–321. doi: 10.1128/aac.43.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irwin K, Scarlett M, Moseley R. Observations from the CDC. The urgent need for new HIV/STD prevention options for women. J Women's Health. 1998;7:1081–1086. doi: 10.1089/jwh.1998.7.1081. [DOI] [PubMed] [Google Scholar]
- 27.Jennings R, Clegg A. The inhibitory effect of spermicidal agents on replication of HSV-2 and HIV-1 in vitro. J Antimicrob Chemother. 1993;32:71–82. doi: 10.1093/jac/32.1.71. [DOI] [PubMed] [Google Scholar]
- 28.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman J C, Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 29.Krebs F C, Miller S R, Malamud D, Howett M K, Wigdahl B. Inactivation of human immunodeficiency virus type 1 by nonoxynol-9, C31G, or an alkyl sulfate, sodium dodecyl sulfate. Antivir Res. 1999;43:157–173. doi: 10.1016/s0166-3542(99)00044-3. [DOI] [PubMed] [Google Scholar]
- 30.Kreiss J, Ngugi E, Holmes K, Ndinya-Achola J, Waiyaki P, Roberts P L, Ruminjo I, Sajabi R, Kimata J, Fleming T R, Anzala A, Holton D, Plummer F. Efficacy of nonoxynol-9 contraceptive sponge use in preventing heterosexual acquisition of HIV in Nairobi prostitutes. JAMA. 1992;268:477–482. [PubMed] [Google Scholar]
- 31.Laga M, Manoka A, Kivuvu M, Malele B, Tuliza M, Nzila N, Goeman J, Behets F, Batter V, Alary M, Heyward W L, Ryder R W, Piot P. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993;7:95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Lifson J D, Feinberg M B, Reyes G R, Rabin L, Banapour B, Chakrabarti S, Moss B, Wong-Staal F, Steimer K S, Engleman E G. Induction of CD4-dependent cell fusion by the HTLV-III/LAV envelope glycoprotein. Nature. 1986;323:725–728. doi: 10.1038/323725a0. [DOI] [PubMed] [Google Scholar]
- 33.Lifson J D, Reyes G R, McGrath M S, Stein B S, Engleman E G. AIDS retrovirus induced cytopathology: giant cell formation and involvement of CD4 antigen. Science. 1986;232:1123–1127. doi: 10.1126/science.3010463. [DOI] [PubMed] [Google Scholar]
- 34.Maddon P J, Dalgleish A G, McDougal J S, Clapham P R, Weiss R A, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 35.Maréchal V, Clavel F, Heard J M, Schwartz O. Cytosolic gag p24 as an index of productive entry of human immunodeficiency virus type 1. J Virol. 1998;72:2208–2212. doi: 10.1128/jvi.72.3.2208-2212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCusker J, Stoddard A M, McDonald M, Zapka J G, Mayer K H. Maintenance of behavioral change in a cohort of homosexually active men. AIDS. 1992;6:861–868. doi: 10.1097/00002030-199208000-00015. [DOI] [PubMed] [Google Scholar]
- 37.McDougal J S, Kennedy M S, Sligh J M, Cort S P, Mawle A, Nicholson J K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986;231:382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- 38.McKnight A, Wilkinson D, Simmons G, Talbot S, Picard L, Ahuja M, Marsh M, Hoxie J A, Clapham P R. Inhibition of human immunodeficiency virus fusion by a monoclonal antibody to a coreceptor (CXCR4) is both cell type and virus strain dependent. J Virol. 1997;71:1692–1696. doi: 10.1128/jvi.71.2.1692-1696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Momota K, Kaneko I, Kimura S, Mitamura K, Shimada K. Inhibition of human immunodeficiency virus type-1-induced syncytium formation and cytopathicity by complestatin. Biochem Biophys Res Commun. 1991;179:243–250. doi: 10.1016/0006-291x(91)91361-f. [DOI] [PubMed] [Google Scholar]
- 40.Moore J P, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551–562. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- 41.Okada T, Patterson B K, Ye S Q, Gurney M E. Aurothiolates inhibit HIV-1 infectivity by gold(I) ligand exchange with a component of the virion surface. Virology. 1993;192:631–642. doi: 10.1006/viro.1993.1079. [DOI] [PubMed] [Google Scholar]
- 42.Paquette J S, Fortin J F, Blanchard L, Tremblay M J. Level of ICAM-1 surface expression on virus producer cells influences both the amount of virion-bound host ICAM-1 and human immunodeficiency virus type 1 infectivity. J Virol. 1998;72:9329–9336. doi: 10.1128/jvi.72.11.9329-9336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez V L, Rowe T, Justement J S, Butera S T, June C H, Folks T M. An HIV-1-infected T cell clone defective in IL-2 production and Ca2+ mobilization after CD3 stimulation. J Immunol. 1991;147:3145–3148. [PubMed] [Google Scholar]
- 44.Piret J, Lamontagne J, Bestman-Smith J, Roy S, Gourde P, Désormeaux A, Omar R F, Juhász J, Bergeron M G. In vitro and in vivo evaluations of sodium lauryl sulfate and dextran sulfate as microbicides against herpes simplex and human immunodeficiency viruses. J Clin Microbiol. 2000;38:110–119. doi: 10.1128/jcm.38.1.110-119.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quinn T C. The epidemiology of the acquired immunodeficiency syndrome in the 1990s. Emerg Med Clin North Am. 1995;13:1–25. [PubMed] [Google Scholar]
- 46.Rieber E P, Federle C, Reiter C, Krauss S, Gurtler L, Eberle J, Deinhardt F, Riethmuller G. The monoclonal CD4 antibody M-T413 inhibits cellular infection with human immunodeficiency virus after viral attachment to the cell membrane: an approach to postexposure prophylaxis. Proc Natl Acad Sci USA. 1992;89:10792–10796. doi: 10.1073/pnas.89.22.10792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roddy R E, Zekeng L, Ryan K A, Tamoufé U, Weir S S, Wong E L. A controlled trial of nonoxynol-9 film to reduce male-to-female transmission of sexually transmitted diseases. N Engl J Med. 1998;339:504–510. doi: 10.1056/NEJM199808203390803. [DOI] [PubMed] [Google Scholar]
- 48.Roy S, Gourde P, Piret J, Désormeaux A, Lamontagne J, Haineault C, Omar R F, Bergeron M G. Thermoreversible gel formulations containing sodium lauryl sulfate or n-lauroylsarcosine as potential topical microbicides against sexually transmitted diseases. Antimicrob Agents Chemother. 2001;45:1671–1681. doi: 10.1128/AAC.45.6.1671-1681.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Short R V. Contraceptives of the future in the light of HIV infection. Aust N Z J Obstet Gynaecol. 1994;34:330–332. doi: 10.1111/j.1479-828x.1994.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 50.Sodroski J, Goh W C, Rosen C, Campbell K, Haseltine W A. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. Nature. 1986;322:470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- 51.Stafford M K, Ward H, Flanagan A, Rosenstein I J, Taylor-Robinson D, Smith J R, Weber J, Kitchen V S. Safety study of nonoxynol-9 as a vaginal microbicide: evidence of adverse effects. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:327–331. doi: 10.1097/00042560-199804010-00006. [DOI] [PubMed] [Google Scholar]
- 52.Stein Z A. HIV prevention: the need for methods women can use. Am J Public Health. 1990;80:460–462. doi: 10.2105/ajph.80.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tatsuta K, Masuda N. The first total synthesis of natural (+)-terpestacin, syncytium formation inhibitor. J Antibiot. 1998;51:602–606. doi: 10.7164/antibiotics.51.602. [DOI] [PubMed] [Google Scholar]
- 54.Tremblay M J, Fortin J F, Cantin R. The acquisition of host-encoded proteins by nascent HIV-1. Immunol Today. 1998;19:346–351. doi: 10.1016/s0167-5699(98)01286-9. [DOI] [PubMed] [Google Scholar]
- 55.Van Damme L. Advances in topical microbicides. 2000. Presented at the XIII International AIDS Conference, Durban, South Africa. [Google Scholar]
- 56.Wainberg M A. The need for vaginal microbicides with antiviral specificity. AIDS. 1998;12:4–6. [Google Scholar]
- 57.Ward R L, Ashley C S. pH modification of the effects of detergents on the stability of enteric viruses. Appl Environ Microbiol. 1979;38:314–322. doi: 10.1128/aem.38.2.314-322.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weir S S, Feldblum P J, Zekeng L, Roddy R E. The use of nonoxynol-9 for protection against cervical gonorrhea. Am J Public Health. 1994;84:910–914. doi: 10.2105/ajph.84.6.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weiss A, Imboden J, Shoback D, Strobo J. Role of T3 surface molecules in human T-cell activation: T3-dependent activation results in an increase in cytoplasmic free calcium. Proc Natl Acad Sci USA. 1984;81:4169–4173. doi: 10.1073/pnas.81.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu C S, Ikeda K, Yang J T. Ordered conformation of polypeptides and proteins in acidic dodecyl sulfate solution. Biochemistry. 1981;20:566–570. doi: 10.1021/bi00506a019. [DOI] [PubMed] [Google Scholar]
- 61.Yee J K, Friedmann T, Burns J C. Generation of high-titer pseudotyped retroviral vectors with very broad host range. Methods Cell Biol. 1994;43:99–112. doi: 10.1016/s0091-679x(08)60600-7. [DOI] [PubMed] [Google Scholar]
- 62.Zekeng L, Feldblum P J, Oliver R M, Kaptue L. Barrier contraceptive use and HIV infection among high-risk women in Cameroon. AIDS. 1993;7:725–731. doi: 10.1097/00002030-199305000-00018. [DOI] [PubMed] [Google Scholar]