Abstract
Despite strong evidence of effectiveness, colorectal cancer (CRC) screening remains underused. Currently, there are several options for CRC screening, each with its own performance characteristics and considerations for practice. This Review aims to cover current CRC screening guidelines and highlight future blood-based and imaging-based options for screening. In current practice, the leading non-invasive option is the faecal immunochemical test (FIT) based on its high specificity, good sensitivity, low cost and ease of use in mailed outreach programmes. There are currently five blood-based CRC screening tests in varying stages of evaluation, including one that is currently sold in the USA as a laboratory-developed test. There are ongoing studies on the diagnostic accuracy and longitudinal performance of blood tests and they have the potential to disrupt the CRC screening landscape. Imaging-based options, including the colon capsule, MR colonography and the CT capsule, are also being tested in active studies. As the world attempts to recover from the COVID-19 pandemic and adapts to the start of CRC screening among people at average risk starting at age 45 years, non-invasive options will become increasingly important.
Subject terms: Colonoscopy, Disease prevention, Diagnostic markers
Colorectal cancer (CRC) is a leading cause of cancer-related death worldwide and screening is useful for early diagnosis. This Review outlines currently available CRC screening options worldwide (including colonoscopy and stool-based tests). Key features of each modality and new screening tests under development are described.
Key points
Throughout the world, the most used approach for colorectal cancer screening is the faecal immunochemical test (FIT).
Colonoscopy is the most common screening modality in the USA and is also used in many other regions, including, for example, parts of Germany and Poland.
Offering non-invasive tests as a screening option effectively improves adherence to screening.
There are five blood tests currently being studied as a potential alternative to FIT and colonoscopy for CRC screening.
Novel imaging tests, such as the colon capsule, MR colonography and the CT capsule, are also undergoing active investigation.
Recovery from deferred screening due to the COVID-19 pandemic will require the use of non-invasive screening options to help select patients for colonoscopy.
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related death in the world1. Most CRCs are slow growing, arising from precursor lesions such as adenomatous polyps or sessile serrated lesions. This slow growth enables a window of time to screen for both early cancer and precursor lesions2. However, adherence to CRC screening remains low in most developed countries, ranging from 19% in Croatia and the Czech Republic to 69% in the Basque region of Spain3,4. There are multiple CRC screening modalities available, including stool-based tests such as faecal immunochemical testing (FIT) and multitarget stool DNA (mtsDNA) test; blood-based tests such as septin 9; and imaging-based tests such as CT colonography (CTC), colon capsule, flexible sigmoidoscopy and colonoscopy2. The guidelines for CRC screening differ worldwide based on population risk, resources, and patient and society values. Our Review aims to summarize evidence on each screening modality and guidelines by various regions of the world and to highlight new screening tests that have the potential to disrupt this field. Our Review does not address post-polypectomy surveillance colonoscopy.
Stool-based tests
Faecal immunochemical test
Most screening programmes throughout the world rely on faecal occult blood tests5–7 (Box 1). Faecal occult blood tests have been demonstrated to reduce CRC incidence and mortality in randomized trials enrolling between 46,000 and 152,000 individuals at average risk when used annually or biennially8,9. FITs are an improvement on the guaiac method of detecting haemoglobin in the stool, using antibodies specific for human haemoglobin rather than the more non-specific peroxidase reaction10. FIT is also a one-sample test (compared to guaiac faecal occult blood tests, which require three samples) and is easy to complete in the patient’s home, leading to improved uptake compared to guaiac-based faecal occult blood tests11. FITs are not affected by diet or medications and, if using a quantitative test, the sensitivity and specificity can be varied by adjusting the cut-off for a positive test12. The FDA-approved threshold for a positive FIT is 20 μg/g (micrograms of haemoglobin per gram of stool). The threshold for a positive test can be varied to better match colonoscopy demand with available supply7. In a meta-analysis, the pooled sensitivity of FIT at a threshold of 20 μg/g for CRC was 0.79, with a specificity of 0.94 (ref.13). In an analysis that only included studies with colonoscopy follow-up of all participants, the one-sample FIT sensitivity for CRC was 0.75 at 20 μg/g and 0.91 at 10 μg/g (ref.14) and the specificity was 0.95 at 20 μg/g and 0.90 at 10 μg/g (ref.14). FIT sensitivity for advanced adenomas was 0.40 at 10 μg/g and 0.25 at 20 μg/g (ref.14). With the latest recommendations to lower the screening age to 45 years9,15–17, it is unclear if the current or different FIT threshold for positivity should be used, pointing out a need for future research.
Multiple randomized trials in the USA and Europe have evaluated screening participation when FIT and colonoscopy are compared either in head-to-head comparisons, as a sequential choice or as a direct choice between the two approaches18–22. In every setting, more people participate in screening when FIT is offered compared to colonoscopy alone. Even though a one-time FIT is less sensitive than colonoscopy, the higher participation with FIT can lead to nearly equivalent detection of CRC with a single FIT compared to an offer of colonoscopy only20,22. In addition to increased participation, FIT performed annually or biennially has a higher cumulative rate of detecting CRC and precursor neoplasia than a single FIT, making it comparable to the yield of a colonoscopy performed once every 10 years23.
Several randomized trials of FIT versus colonoscopy for CRC screening are ongoing (NCT02078804, NCT01239082, NCT00906997)22,24–27. In the absence of randomized trial evidence, modelling studies can be used to evaluate the comparative effectiveness of alternative screening strategies. The United States Preventive Services Task Force (USPSTF) CISNET program, which includes three models, does not explicitly model costs but did report that FIT is slightly less effective than colonoscopy when participation is 100%28. However, a colonoscopy-only strategy requires substantially more colonoscopies and carries a higher risk of complications (complication rate of 11 per 1,000 screened for FIT and 16 per 1,000 screened for colonoscopy)28. One model found that both colonoscopy and FIT were cost-effective, but that FIT was the most cost-effective option for those aged 45–49 years29. The American Cancer Society also included a cost-effectiveness model as part of their most recent guideline published in 2018 and demonstrated that, if screening were to start at 45 years, FIT was the most efficient CRC screening option17.
Box 1 Stool-based CRC screening tests.
Faecal immunochemical testing
Detects human haemoglobin in stool: FDA-approved cut-off 20 μg of haemoglobin per 1 g of stool
Higher specificity than multitarget stool DNA testing; 96.4% vs 89.8%30
Reduced cost (US$25)
Can be sent through mail for mass screening
Covered by all guidelines
Reducing cut-off to 10 μg/g increases sensitivity to 91%, reducing specificity to 90%12
Multitarget stool DNA testing
Detects methylated DNA markers plus faecal haemoglobin via faecal immunochemical testing: aberrantly methylated NDRG4 and BMP3; any of seven KRAS point mutations; proprietary mathematical algorithm for test positivity
Higher sensitivity than faecal immunochemical testing; 92% vs 73.7%22
High cost (US$600)
3-year interval
Not available outside the USA
Requires UPS shipment within USA
Patient navigator system included
Approved in the USA (by FDA, US Centers for Medicaid and Medicare Services and covered by many private insurers)
Multitarget stool DNA test
The detection of methylated and tumour DNA in stool in conjunction with occult blood detection is a promising strategy to enhance the sensitivity of FIT (Box 1). The mtsDNA test (Cologuard, Exact Sciences) includes FIT to detect haemoglobin as well as tests to detect mutations associated with CRC in the DNA of cells shed by advanced adenomas or CRC30. In a prospective study of 10,023 individuals undergoing screening colonoscopy, a one-time mtsDNA test showed 92% sensitivity for CRC and 42% sensitivity for advanced adenomas. In comparison, a commercially available FIT (threshold of detection 20 μg/g) (OC FIT-CHEK, Polymedco) had 74% sensitivity for CRC and 24% sensitivity for advanced adenomas30. However, the mtsDNA test had lower specificity (87%) for CRC or advanced adenomas than the OC-Sensor with 95% specificity. The Cologuard test was approved by the FDA in 2014 and is covered by the US Centers for Medicare and Medicaid Services (CMS) since 2014. Initial barriers to the adoption of mtsDNA testing are the high cost, at ~US$600 per test. A cost-effectiveness analysis comparing the mtsDNA test, colonoscopy and FIT concluded that both FIT and colonoscopy were more cost-effective than the mtsDNA test31. In addition, participation would need to increase 1.7-fold due to the patient support function, or the combination of FIT and mtsDNA would need to cost 60% less than the current price (US$197–260)31. The mtsDNA test is not currently marketed outside the USA.
Another disadvantage of mtsDNA testing is the complex requirement of stool collection. The mtsDNA test is more involved than the FIT alone, requiring complex stool sample collection in a large jar with a special buffer, in addition to the FIT. There is also technical complexity requiring multistep lab analysis32. In the prospective trial, nearly 6% of participants were not able to collect or send an adequate stool specimen compared to 0.6% for FIT. Due to the lower specificity of the test, there is also a concern regarding a false-positive mtsDNA test, that is, when the mtsDNA test is positive followed by a normal colonoscopy. Given there are methylated DNA and tumour markers in the test, the question arises whether neoplasia was missed, possibly from cancer elsewhere in the gastrointestinal tract. To address this issue, Cotter et al. compared the incidence of gastrointestinal and other cancers in those with false-positive test results to those in people with negative test results and found no differences33; others have reported similar findings34. Given the harms of over-testing, over-diagnosis and the inherent cost of mtsDNA testing, current expert opinion is to follow average-risk screening intervals and repeat screening at 10 years following a negative colonoscopy after a positive mtsDNA test33. Currently, there are no studies on the performance of mtsDNA testing with repeat testing, and the currently recommended interval of 3 years is based on simulation modelling in the absence of empirical data.
Direct visualization tests
Colonoscopy
Colonoscopy is generally reserved as a follow-up procedure to a positive initial screening test in most screening programmes. In the USA in 2000, Medicare approved the use of colonoscopy as a screening modality every 10 years, which has led to a large increase in colonoscopy volume over time35. The USA is an outlier in this regard, with colonoscopy being the most common CRC screening modality36. According to the Behaviour Risk Factor Surveillance System survey, 68% of individuals sampled in 2018 aged 50–75 years were up to date on CRC screening and the most common modality reported was colonoscopy (61%), followed by FIT or faecal occult blood test (11%), flexible sigmoidoscopy (3%), mtsDNA testing (3%), and CTC (1%)37.
Multiple case–control and prospective cohort studies have estimated cancer mortality to be 29–68% lower among persons who undergo screening colonoscopy than among those who do not, and the protection is conferred for both proximal and distal CRC. Singh et al. demonstrated a 29% reduction in overall CRC mortality and 47% reduction in distal CRC mortality among 2,915 people38. Using SEER-Medicare data (n = 10,292), Baxter et al. reported a reduction in distal (OR 0.40; 95% CI 0.37–0.43) and proximal CRC mortality (OR 0.58; 95% CI 0.52–0.64) with colonoscopy39. The National Polyp Study (n = 1,418) reported a 53% reduction in CRC mortality with colonoscopy screening40. Results from the Nurses’ Health Study and the Health Professionals Follow-Up Study reported a 68% reduction in CRC-related mortality after screening colonoscopy among 88,902 individuals41. Kahi et al. reported a reduction in CRC mortality of 61% with screening colonoscopy among 24,820 US Veterans (OR 0.39; 95% CI 0.35–0.43)42. A nested case–control study on members of Kaiser Permanente reported a 67% reduction in risk of death from CRC with a 65% reduction in proximal CRC among 1,747 cases dying from CRC and 3,460 cancer-free matched controls43. Samadder et al. also reported a 67% reduction in CRC mortality in 980 residents of Utah exposed to colonoscopy compared with the general population, with a 57% reduction in mortality from proximal CRC44. Finally, a systematic review of observational studies found a 68% lower overall CRC mortality associated with colonoscopy45. Disadvantages of colonoscopy are its invasive nature, risk of complications (such as perforation and bleeding), the need for bowel preparation, and its burden on resources and associated costs. Colonoscopy detects many diminutive and small adenomatous and sessile serrated polyps. However, only a minority of such lesions have the potential to develop into cancer46. Given the lack of evidence of which polyps constitute an increased risk, individuals with polyps are placed in colonoscopy surveillance programmes at shorter intervals, leading to increased demand for colonoscopy and burden on the health-care system, all with uncertain benefit28. Due to financial and psychosocial barriers to accepting colonoscopy, when adherence has been compared in randomized trials, adherence to colonoscopy is lower than for FIT19. Thus, in programmatic screening programmes, colonoscopy is best reserved as step two of a two-stage screening cascade.
Flexible sigmoidoscopy
Flexible sigmoidoscopy is another option for direct visualization of the distal colon, with a referral for colonoscopy when polyps are detected. There are several large-scale trials comparing a one-time or repeat flexible sigmoidoscopy to no screening with outcomes of reduction in CRC incidence and mortality. Trials from the United Kingdom and Italy randomly assigned 170,432 and 34,292 individuals, respectively, aged 55–64 years to a one-time flexible sigmoidoscopy compared with no screening, reporting a reduction in CRC incidence by 23% and 18% and in CRC mortality by 31% and 22%, respectively47,48. In the USA, the PLCO trial randomly assigned 154,887 individuals aged 55–74 years to flexible sigmoidoscopy every 3–5 years versus usual care and found a reduction in both CRC incidence and mortality of 21% and 26%, respectively49. A trial in Norway (n = 98,678) also reported a reduction in CRC incidence and mortality of 20% and 27%, respectively50. However, a long-term follow-up of the Norwegian trial participants at 15 years reported no reduction in CRC incidence or mortality with flexible sigmoidoscopy screening in women51. There are also practical and logistical challenges to consider. The resources required for flexible sigmoidoscopy are similar to a colonoscopy, but colonoscopy is needed to follow up on a positive FIT and for those with polyps on flexible sigmoidoscopy. Consequently, rates of screening flexible sigmoidoscopy have declined in the USA36. The lack of sedation and fear of pain are barriers to participation with flexible sigmoidoscopy52,53. In 2021, the UK also switched their CRC screening programme from flexible sigmoidoscopy to FIT starting at age 50 years due to similar reasons of poor adherence, resources needed and programmatic effectiveness54.
CT colonography
CTC enables the detection and localization of polyps and cancers in the colon through a 3D or 4D reconstruction. Two large trials have compared the diagnostic yield of CTC with optical colonoscopy performed on the same day55,56. One study56 accrued 1,233 individuals at average risk and demonstrated test characteristics of 92% sensitivity with 96% specificity of CTC for adenomas 10 mm or larger detected by optical colonoscopy and 86% sensitivity and 80% specificity for adenomas 6 mm or larger. The National CT Colonography Trial (NCTC), sponsored by the American College of Radiology Imaging Network (ACRIN 6664)55, included 2,600 asymptomatic participants for same-day CTC and optical colonoscopy. The sensitivity for adenomas or CRC 10 mm or larger as detected by colonoscopy was 84% with a specificity of 85%. The sensitivity for adenomas 6 mm or larger was 70%, with a specificity of 86%. A critique of CTC is that lesions smaller than 6 mm were not reported, and the clinical significance of such lesions is uncertain. CTC also reports on extracolonic findings, which lead to further testing and downstream effects such as observing adrenal and renal masses, which leads to biopsies and further imaging. In the NCTC trial, extracolonic findings were observed in 66% of individuals and 16% were considered of clinical importance, requiring either additional evaluation or urgent care55.
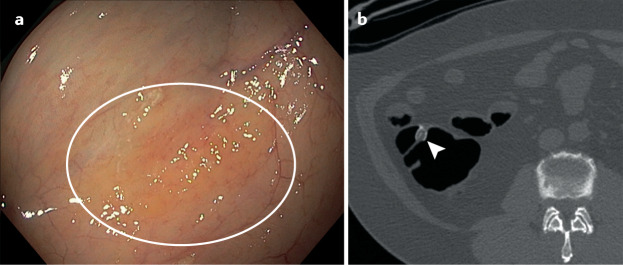
In our experience, the advantages of CTC are that it is less invasive, there is no need for procedural sedation and it has a low complication rate. Disadvantages are the requirement for bowel preparation, radiation exposure and extracolonic findings that lead to additional testing and potential overtreatment. CTC might be less sensitive for flat polyps, which are often sessile serrated adenomas, than for conventional polypoid lesions57, but contrast coating on the surface of flat polyps can enhance detection58 (Fig. 1). The use of CTC is limited due to the lack of trained radiologists and imaging centres that offer the test.
Fig. 1. Images of a sessile serrated lesion on colonoscopy and a sessile serrated lesion on CT colonography.
a | Sessile serrated adenoma on colonoscopy. b | CT colonography image of a serrated polyp; note contrast covering the surface. Note these are not the same lesion, but the images demonstrate that these lesions are indeed flat and can be detected by the adherence of mucus and contrast material on the surface of the polyp. Part b reprinted with permission from ref.136, Elsevier.
Colon capsule
The colon capsule harnesses the technology of a wireless, disposable pill-sized camera capsule that is swallowed and gets activated in the terminal ileum. The capsule takes pictures of colonic mucosa without requiring radiation exposure, sedation or gas insufflation. Earlier generations of the capsule had suboptimal accuracy59. More recent advances in the technology have added enhancements to improve diagnostic yield such as an increased and adaptive capsule frame rate (increased to 35 images per second while in motion), widened angle of view, new software to estimate polyp size and improved data recording60. In an average-risk screening study of 884 individuals of whom 695 (79%) were evaluable, conventional adenomas ≥6 mm were detected with 88% sensitivity and 82% specificity61. However, 26% of false-negative tests were due to sessile serrated polyps61. Other studies have reported 79% sensitivity for polyps ≥6 mm (ref.62) and as high as 90% among patients with a positive FIT result63. In a direct comparison study of 320 individuals, the colon capsule outperformed CTC in average-risk screening64 and for incomplete colonoscopy65,66. Barriers to colon capsule testing are the requirement for colonic preparation, particularly if the colonoscopy cannot be performed on the same day. Artificial intelligence and machine learning are particularly promising for colon capsule testing, with potential for autonomous detection and its use in combination with genomic or proteomic screening67,68. Advances in technology might enable the administration of the colon capsule at the patient’s home with video uploaded to a cloud-based server that can be downloaded and read by a skilled reader remotely.
Blood-based tests
SEPT9 is a tumour suppressor gene encoding septin 9, mutated early in the CRC pathway69. The determination of plasma methylated septin 9 (mSEPT9) is the only blood-based CRC detection test and is currently approved by the FDA for CRC screening in individuals who decline or are unable to complete the higher efficacy screening tests70. However, it is not approved for reimbursement by the CMS and is not included in the most recent US Preventive Services Task Force guideline due to inadequate sensitivity15. An improved version was reported to have 68% sensitivity for CRC overall but only 64% sensitivity for stage I–III disease with 80% specificity for all stages of cancer71. In direct comparison to FIT, the mSEPT9 assay had non-inferior sensitivity but lower specificity than one-time FIT for CRC72 and lower sensitivity for CRC and precursors versus mtsDNA testing73 (Table 1; Fig. 2).
Table 1.
Summary of non-invasive CRC screening options
| Test | Evidence for efficacy | Test details and special considerations | Recommended frequency |
|---|---|---|---|
| Stool based | |||
| FIT | Sensitivity for CRC 79% and specificity 94%13; reduces CRC incidence by 22%98; sensitivity increases with repeated tests over time128,129; low sensitivity for advanced adenomas14 | Positive test needs confirmation diagnostic colonoscopy; in the USA, annual testing required; can be performed in patient’s home; no dietary or medication restrictions; conducive to programmatic screening | Annual in the USA15–17; see below for intervals elsewhere |
|
FIT-DNA (mtsDNA) Cologuard |
Sensitivity for CRC 92% and specificity 87%30 ; superior to FIT for detection of CRC and advanced precancerous lesions130,131; sensitivity for advanced polyps 42%30 ; higher false-positive rate than FIT33,34 | Positive test needs confirmation diagnostic colonoscopy; manufacturer recommends repeat testing every 3 years based on modelling studies only; single stool specimen can be collected at home; no cathartic bowel preparation or anaesthesia required; built-in patient navigation enhances adherence; 3- year interval based on modelling studies; there is limited empirical data upon which to base a recommendation; false positives can result in over-testing and harms; high cost compared to FIT31 | Every 3 years23 |
| Blood based | |||
|
Septin 9 (mSEPT9) Epi pro Colon |
Sensitivity for CRC 48.2–68% and specificity 80–91.5%71,132; direct comparison shows non-inferior sensitivity but lower specificity than one-time FIT for CRC72 ; direct comparison shows lower sensitivity for CRC and adenomas vs mtsDNA testing73 | Positive test needs confirmation diagnostic colonoscopy; screening interval not established; blood plasma assay; cannot be done with home phlebotomy; concern for false negatives | Not recommended by US guidelines or any outside the USA |
| Imaging based | |||
| Colon capsule | Sensitivity for polyps ≥6 mm 88% and specificity 82%61; sessile serrated polyps and hyperplastic polyps accounted for 26–37% of false-negative findings on capsule analysis61 | Positive test needs confirmation diagnostic colonoscopy; repeat screening interval unknown but 5 years recommended by experts; more extensive bowel preparation than traditional colonoscopy with the potential requirement of additional pro-kinetic agents on exam day; large capsule can be difficult to swallow; delayed transit can cause incomplete exam (if exceeds battery time) | 5 years16 |
| CT colonography | Sensitivity for adenomas ≥6 mm 73–98% and specificity 89–91%; pooled sensitivity of adenomas ≥10 mm 67–94%, specificity96–98%133; low detection rate for sessile and flat polyps134,135 | Positive test needs confirmation diagnostic colonoscopy; incidental extracolonic findings lead to downstream testing and treatment; lifetime risk of cumulative radiation exposure unknown | Every 5 years |
CRC, colorectal cancer; FIT, faecal immunochemical testing; mSEPT9, methylated septin 9; mtsDNA, multitarget stool DNA.
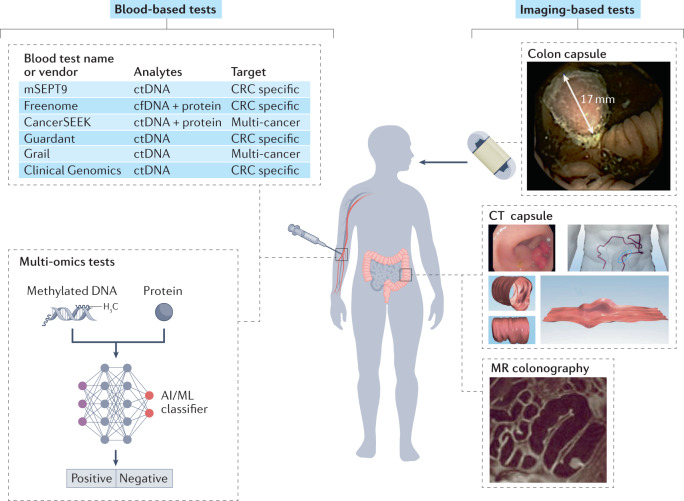
Fig. 2. CRC screening tests in development.
For blood-based tests, there are at least six blood-based colorectal cancer (CRC) screening tests in various stages of development. These tests range from CRC-specific tests to multi-cancer early-detection tests. Candidate analytical targets include cell-free DNA (cfDNA), methylated circulating tumour DNA (ctDNA), including the gene-specific methylated septin 9 (mSEPT9), and combinations of methylated DNA and proteins. Multi-omics tests use a combination of methylated DNA and protein and apply an artificial intelligence and/or machine learning (AI/ML) classifier to sort populations of individuals into ‘positive’ or ‘negative’ for cancer or advanced neoplasia. For imaging-based tests, in addition to CT colonography described in Fig. 1, other imaging-based tests in development include the colon capsule, a CT capsule of the colon and MR colonography. MR colonography image reproduced from ref.135 with permission from BMJ Publishing Group Ltd. Colon capsule image reprinted with permission from ref.61, Copyright © 2015 AGA Institute. Published by Elsevier Inc. CT capsule image reproduced from ref.118 with permission from BMJ Publishing Group Ltd.
Guidelines by worldwide region
CRC screening implementation and guidelines differ throughout the world. There are geographical differences in CRC incidence, economic resources, and health-care structure and infrastructure to support screening such as access to a cancer registry and the ability to identify the target population3. CRC incidence increases with age and was thought to have an inflexion point around age 50 years; however, with data in the past few years demonstrating an increasing incidence of CRC in individuals younger than 50 years in the USA37, four US guidelines have recommended starting screening at age 45 years for all men and women at average risk16,17,74,75. European guidelines76 continue to recommend CRC screening at age 50 years, with some variation between European countries between 50 and 60 years of age3. The UK recently changed its guidelines to drop flexible sigmoidoscopy in favour of biennial FIT with a starting age of 50 years54 (English Bowel Cancer Screening Programme). The Canadian Preventive Services Task Force recommends screening between the ages of 50 and 75 years77. In 2016, the Pan American Health Organization published a report on the results of an experts meeting of CRC screening in the Americas78. Only six countries declared having local screening guides, four declared population-based programmes in pilot experiences (Brazil, Chile, Argentina and Paraguay) and three declared opportunistic programmes (Ecuador, Trinidad and Tobago, and Uruguay)78. In the Asia-Pacific Region, Australia recommends starting FIT at age 50 years79, Japan offers screening with annual FIT at age 40 years with no upper age limit80, and Taiwan and Korea both offer biennial FIT starting at 50 years3,81,82. The testing modality is mostly biennial faecal occult blood testing or FIT for the majority for regions and countries with programmatic screening. The USA is an outlier, in that FIT and colonoscopy are treated as preferred tests, with colonoscopy being the predominant screening modality16, but Germany also recommends colonoscopy following five negative FITs83. There are no organized CRC screening programmes in African or eastern Mediterranean regions with the exception of Israel, which has implemented an annual FIT screening for individuals 50–74 years of age84, and Abu Dhabi, which recommends colonoscopy screening for individuals over the age of 40 years3. Regardless of modality, screening rates are far from ideal (often close to 20%) and efforts directed at expanding options for CRC screening, patient selection, navigation to a colonoscopy if a non-invasive test is positive and adequate follow-up are needed (Table 2).
Table 2.
Comparison of CRC screening guidelines worldwide
| Country/region | Age to initiate screening (years) | Age to stop screening (years) | Modality and frequency |
|---|---|---|---|
| Canada77 (variations exist across provinces) | 50 | 74 | Biennial FIT and hsFOBT |
| EU3,76 | 50 (55 or 60 in some regions) | 74 | Biennial FIT (although Germany uses colonoscopy at age 55) |
| UK54 | 50 | 74 | Biennial FIT |
| Pan American Region78 | 50 | 74 | Biennial FIT |
| USA16,17,74 | 45 | 85 | Multiple options; colonoscopy most commonly used |
| South Korea3 | 50 and older | Not specified | Annual FIT |
| Australia79 | 55 | 75 | Biennial FIT |
| Japan80 | 40 | No upper limit | Annual FIT |
| Taiwan81,82 | 55 | 75 | Biennial FIT |
| Israel84 | 50 | 74 | Annual FIT |
| Abu Dhabi3 | 40 | Not specified | Colonoscopy |
CRC, colorectal cancer; EU, The European Union; FIT, faecal immunochemical test; hsFOBT, high-sensitivity faecal occult blood test.
Organized versus opportunistic screening
The International Agency for Research on Cancer defines an organized screening programme as having an explicit policy with specified age categories, method and interval for screening; a defined target population; a management team responsible for implementation; a health-care team for decisions and care; a quality assurance structure; and a method for identifying cancer occurrence in the population85. By contrast, opportunistic screening occurs outside of an organized programme, usually during episodes of care for unrelated problems. Organized screening focuses on the quality of the screening process, including follow-up of participants86. One example of a framework of the screening process in the USA is the Population-based Research Optimizing Screening through Personalized Regimens (PROSPR) consortium model, in which screening progresses through five stages: risk assessment; performance of screening; detection of early cancer or precancerous neoplasms; performance of appropriate follow-up tests (such as colonoscopy after a positive FIT); and referral for treatment87.
Organized screening provides protection against the harms of screening, including underuse, overuse and misuse88,89. Underuse occurs when people due for screening do not complete screening. Underuse also occurs when individuals with a personal or family history of colorectal neoplasia are offered an inappropriate screening test (for example, FIT instead of colonoscopy) or are offered screening at an inappropriate age or interval (for example, offering FIT screening at age 50 years to someone with a strong family history of CRC). Overuse occurs when screening is offered to those who are not yet due, by virtue of a normal, high-quality colonoscopy less than 10 years ago, or unlikely to benefit from screening due to limited life expectancy or frailty increasing the risk of colonoscopy complications90. Overuse of screening has been reported among Medicare beneficiaries and in the VA health-care system91,92. Screening is misused when it is done in a way that achieves no benefits — for example, faecal testing done in a patient who is not due for CRC screening or repeating a FIT after it is positive rather than proceeding to colonoscopy93.
Ultimately, organized screening takes a population-health approach to improve CRC screening practice. Under-screening and over-screening can be managed using an electronic health record to capture key risk factor information and determine who is eligible for screening and who has completed screening. Individuals appropriate for CRC screening can be identified through an automated process generating screening invitations by mail and by secure electronic messages as well as through personal or automated phone calls. Clinical reminders can be generated at the time of an office visit or as part of population tracking94. By ensuring that all members of the eligible population are offered and complete screening, diagnosis, treatment and surveillance, organized screening offers a way to address disparities in CRC screening and a setting in which to test interventions to improve uptake of screening among under-screened groups95–97 such as Black, Hispanic, non-native language speaking, uninsured or underinsured, and rural populations.
Organized screening programmes using faecal tests have been implemented throughout the world, including England6, Italy98, the Netherlands7 and Taiwan5, demonstrating a reduction in CRC incidence and mortality5,98,99. In the USA, Kaiser Permanente Northern California launched an organized mailed FIT outreach programme in 2006, with screening colonoscopy available through opportunistic referral based on a physician or patient preference or for patients at increased risk. Between 2000 and 2015, CRC mortality decreased by 52% and CRC incidence decreased by 26%23.
CRC screening tests in development
Blood-based tests
Detection of circulating and cell-free tumour DNA in the blood has opened up a potential for blood-based testing for CRC and advanced neoplasia100. Two challenges of these tests are the rarity of tumour-derived DNA in the blood and the age-related accumulation of mutations associated with cancer101,102. In 2021, the CMS provided guidance that, to meet approval thresholds, a blood-based test needs to have 90% specificity and 74% sensitivity for CRC compared to an accepted standard (such as colonoscopy) and must be approved by the FDA and endorsed by at least one professional society guideline (CAG-00454N)103. There are at least five tests under development (as discussed later). Most of the tests target early detection of CRC, whereas at least two are multi-cancer tests. Tests aimed at early detection of CRC or its precursors are easier to understand in terms of the next steps and the potential reduction in CRC-related incidence and mortality from early screening. The multi-cancer tests have a complex follow-up and the diagnostic value of the detection of different cancers and the benefit of earlier detection are unclear. Other than cancers where there is evidence supporting a benefit to screening (breast, cervix, lung), detection of inconsequential cancers can lead to over-testing, over-diagnosis and harm, in addition to anxiety for the patient104. In the multi-cancer tests, diagnostic accuracy seems highest for CRC105,106. The currently accepted CRC screening tests (based on established mortality reduction) rely on the structural identification of polyps or cancer (for example, colonoscopy) or blood in the stool (FIT). Any molecular or genetic tests might detect lesions with different biology, and the effect on incidence and mortality is harder to quantify. However, the US Preventive Services Task Force and other US guideline groups have made a policy decision to include newer tests (such as the mtsDNA test) based solely on sensitivity and specificity, without regard to possible differences in tumour biology74. In general, a blood-based test has public appeal for being minimally invasive, the ability to be coupled with other routine blood tests and the potential for high adherence using home-based phlebotomy. Adler et al.107 reported in a German study that 97% of 109 individuals refusing colonoscopy screening accept a non-invasive test and 83% chose a blood test. However, in an Australian setting, home-based stool testing seemed better for increasing participation than a hypothetical blood-based test requiring a visit to a laboratory108 (Table 3; Fig. 2).
Table 3.
Novel and other non-invasive CRC screening tests under development
| Test | Details of technology | Special considerations |
|---|---|---|
| Stool and blood based | ||
| Clinical genomics | Stool-based and blood-based biomarker study for early detection of CRC and advanced neoplasia | Study plans to recruit 1,800 individuals at average risk aged 18 years and older (NCT00843375)112; expected completion is 2022 |
| Blood based | ||
| Freenome | cfDNA plus artificial intelligence test to detect CRC and advanced adenomas | PREEMPT trial (NCT04369053)109 is a prospective multicentre study that aims to recruit 25,000 individuals at average risk aged between 45 and 85 years undergoing colonoscopy screening |
| Guardant | The ctDNA LUNAR test is designed to detect cell-free tumour DNA in blood | The ECLIPSE trial (NCT04136002)110 is a prospective multicentre study that aims to recruit 10,000 individuals at average risk aged between 45 and 84 years undergoing colonoscopy screening |
| CancerSEEK | Multi-cancer detection test to identify eight common cancers, including CRC; detects circulating proteins and mutations in ctDNA using an AI/ML classifier | The primary case–control study (NCT04213326)111 has enrolled, since 2019, 6,399 individuals without cancer as well as individuals with cancer aged 50 years and older; primary end points are sensitivity and specificity for detection of invasive cancer; expected completion year is 2022 |
| GRAIL | Multi-cancer early-detection test (breast, colorectal, pancreatic, lung and haematological malignancies in blood); in validation study, specificity of 99.5%, sensitivity for cancer of 51.5%105 | The GRAIL test is now available as a laboratory-developed test; it is not covered by insurance and the list price is US$949; ongoing prospective, multicentre, validation study (the PATHFINDER study), started in 2019, that plans to recruit 6,600 individuals at average risk aged 50 years and older, in the USA; a larger study is planned including 165,000 individuals in the UK115 |
| Image based | ||
| CT capsule | X-ray imaging capsule; emits low-dose X-ray beams by a rotating miniature electric motor in the colon; bowel preparation is not required | Radiation dose equivalent to an X-ray; large capsule can be difficult to swallow; delayed transit can cause incomplete exam (if exceeds battery time) |
| MR colonography | Uses MR-based imaging of the colon; allows for the evaluation of extracolonic findings and cancer metastases; advantages over CTC are the absence of ionizing radiation | Incidentally found extracolonic findings lead to downstream testing and treatment; requires bowel preparation and colon distention with air; contraindicated in those with metallic implants; no sedation or anaesthesia required; generally safe |
AI/ML, artificial intelligence, machine learning; cfDNA, cell-free DNA; CRC, colorectal cancer; CTC, CT colonography; ctDNA, circulating tumour DNA.
A number of blood-based tests for CRC screening are in development. The combination of cell-free methylated DNA with protein sorted by an artificial intelligence–machine learning classifier test under evaluation by Freenome is designed to identify patterns of cell-free biomarkers in the blood for the early detection of cancer. The PREEMPT trial (NCT04369053)109 is a prospective multicentre study started in 2020 that plans to recruit 25,000 individuals at average risk aged between 45 and 85 years undergoing colonoscopy screening in the USA. Primary end points are sensitivity and specificity for the detection of CRC and the expected completion year is 2022. The circulating tumour DNA (ctDNA) LUNAR test by Guardant Health is designed to detect ctDNA in blood. The ECLIPSE trial (NCT04136002)110 is a prospective multicentre study, started in 2019, that plans to recruit 10,000 individuals at average risk aged 45–84 years undergoing colonoscopy screening. Primary end points are sensitivity and specificity for detection of CRC and the expected completion year is 2024.
Cohen et al.106 reported the results of their study using a multi-omics test of ctDNA and protein with an artificial intelligence–machine learning classifier among people with known cancers (CancerSEEK) to identify eight common cancers, including CRC, by determining the levels of circulating proteins and mutations in ctDNA. The sensitivity of the test was 73% and 78% for stage II and III cancers, respectively, and 43% for stage I cancers, with the highest sensitivity for CRC (84%). The primary case–control study (NCT04213326)111 has enrolled, since 2019, 6,399 individuals without cancer as well as individuals with cancer aged 50 years and older. Primary end points are sensitivity and specificity for the detection of invasive cancer and the expected completion year is 2022. Furthermore, Clinical Genomics is conducting a prospective multicentre stool-based and blood-based biomarker study (NCT00843375)112 for early detection of CRC and advanced neoplasia; this test targets a methylated ctDNA marker113. The study plans to recruit 1,800 individuals at average risk aged 18 years and older in the USA, and the expected completion year is 2022.
A multi-cancer early-detection test by GRAIL (Galleri) seeks to detect early invasive cancers such as breast, colorectal, pancreatic, lung and haematological malignancies in blood. The Circulating Cell-free Genome Atlas (CCGA), a prospective case-controlled, observational study, published its validation sub-study in 2021 (ref.105), reporting on results across 50 cancers in 4,077 participants. Specificity was reported at 99.5% (95% CI 99.0–99.8%) and overall sensitivity for cancer was 51.5%. Sensitivity was reported as 82.0% for CRC but was only 43.3% for stage I CRC (13 of 30 cases), 85.0% for stage II (34 of 40), 87.9% for stage III (58 of 66) and 95.3% for stage IV (61 of 64). Advanced adenomas were not an end point of this study. The GRAIL test is now available as a laboratory-developed test in the USA but is not covered by insurance and the list price is US$949 (ref.114). The PATHFINDER study is a prospective multicentre study started in 2019 and plans to recruit 6,600 individuals at average risk aged 50 years and older across the USA for the detection of multiple cancers, including CRC. Primary end points are sensitivity and specificity for detection of invasive cancer, and the expected completion year is 2022. Another ongoing study is NHS-Galleri in the UK, which plans to enrol 165,000 individuals in the UK aged 40 years and older115.
MRI colonography
MR colonography is a newly developed non-invasive method for evaluating the colon for colorectal polyps and CRC116. It also enables the evaluation of extracolonic findings and cancer metastases. Advantages over CTC are the absence of ionizing radiation in MR colonography, but it does require a bowel preparation similar to CTC116. Patients need to be screened for general contraindications to MRI, including the presence of metallic implants or severe claustrophobia. During the procedure, the colon is distended using water, air or carbon dioxide, and the patient needs to change positions, similar to CTC. A systematic review summarizing 14 trials with a total of 1,305 patients reported a CRC detection rate of 98.2%, with pooled sensitivity for detection of large polyps of 82% and for any polyps of 38%. Most of the studies applied a dark lumen technique (n = 11) via rectal administration of either warm tap water (n = 5), air (n = 4), warm tap water in one group and air in another group (n = 1), or using a fat enema (n = 1) as a contrast agent. Adverse events included water spillage and/or incontinence, and one report of painful constipation after the use of highly concentrated barium sulfate116. The technology is promising but more large studies are needed.
CT capsule
A novel prepless X-ray imaging capsule, called Check-Cap, emits low-dose X-ray beams by a rotating miniature electric motor as the capsule travels through the colon117. A bowel preparation is not required but a small amount of radiopaque contrast agent is used to increase the contrast in the colon walls and differentiate polyp from stool. The X-ray images are captured by the capsule from slices of the colon as it moves and sends them via a radiofrequency link to the capsule positioning system in an external recording unit positioned on the patient’s back. In preliminary safety studies118, 49 capsules were ingested by 46 volunteers. Total patient radiation exposure was 0.03 ± 0.0007 mSv, about the dose of radiation from one chest X-ray118. Further multicentre studies to validate the performance of this capsule are under way.
The COVID-19 pandemic
The COVID-19 pandemic has caused an unprecedented decrease in CRC screening and colonoscopy procedures worldwide119,120. During the height of the pandemic, CRC screening activity was decreased by 85–95%. Care delivery was abruptly shut down and, after the resumption of full-scale operations, patients often declined colonoscopies due to fear of exposure to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and scheduling processes were more difficult due to the need for pre-procedure SARS-CoV-2 viral testing121. Delayed CRC screening and surveillance will likely result in adenomatous polyps progressing to advanced adenomas or CRC and to cancers being detected at a later, less treatable stage122. The main evidence concerning the effect of the COVID-19 pandemic has been obtained from modelling studies. A modelling study published in 2021 using CRC-specific models for Australia, Canada and the Netherlands reported that disruption in screening for up to 12 months could result in a relative increase in CRC incidence by 0.6–1.8%, and that providing immediate catch-up screening could minimize the impact of the disruption to less than 0.1% by 2050 (ref.123). Early estimates were that there would be approximately 10,000 excess deaths in the USA alone from breast cancer and CRC due to pandemic-related disruptions in care124, and models estimate that 18,800 individuals in the USA might experience delays in CRC diagnosis125. Over time, it is apparent that the rate of recovery of CRC screening will determine how large an effect deferring CRC screening will have on CRC incidence and mortality126. The implementation of organized screening using a population-based approach and leveraging non-invasive screening with FIT is an excellent way to accelerate the recovery from the COVID-19 pandemic121.
Conclusions
In the USA, only 67% of patients are up to date with CRC screening, as reported in 2021 (ref.127). Although colonoscopy is highly sensitive and specific for CRC detection and polyp removal, it is invasive, expensive and resource intensive. Hence, there is an unfulfilled need for multiple-modality CRC screening that can improve current CRC screening rates. Newer technologies might be resource-effective strategies when used to select patients for colonoscopy. Our Review highlights the complementary, often underutilized, non-invasive CRC screening methods with a focus on performance, risks, benefits and recent updates in non-invasive screening tests that will likely change the landscape of CRC screening. Implementation of these new approaches to screening and surveillance will require physicians to understand the advantages and limitations of these new technologies as well as a shared decision-making process with our patients.
Author contributions
The authors contributed equally to all aspects of the article.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Han-Mo Chiu, Stephen Halloran and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
A.S. has acted as a consultant for Freenome, Iterative Scopes and Medtronic. T.R.L. receives research funding from Freenome, is supported by The Permanente Medical Group, Delivery Science and Applied Research (TPMG DARE) Physician Researcher Program and is a board member of the California Colorectal Cancer Coalition.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
English Bowel Cancer Screening Programme: https://www.gov.uk/guidance/bowel-cancer-screening-programme-overview
Change history
7/4/2022
A Correction to this paper has been published: 10.1038/s41575-022-00661-3
References
- 1.Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019;16:713–732. doi: 10.1038/s41575-019-0189-8. [DOI] [PubMed] [Google Scholar]
- 2.CDC Division of Cancer Prevention and Control CfDCaP. Colorectal Cancer Statistics (CDC, 2019).
- 3.Schreuders EH, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637–1649. doi: 10.1136/gutjnl-2014-309086. [DOI] [PubMed] [Google Scholar]
- 4.Portillo I, et al. Colorectal and interval cancers of the Colorectal Cancer Screening Program in the Basque Country (Spain) World J. Gastroenterol. 2017;23:2731–2742. doi: 10.3748/wjg.v23.i15.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiu HM, et al. Effectiveness of fecal immunochemical testing in reducing colorectal cancer mortality from the one million Taiwanese screening program. Cancer. 2015;121:3221–3229. doi: 10.1002/cncr.29462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logan RF, et al. Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut. 2012;61:1439–1446. doi: 10.1136/gutjnl-2011-300843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toes-Zoutendijk E, et al. Real-time monitoring of results during first year of Dutch colorectal cancer screening program and optimization by altering fecal immunochemical test cut-off levels. Gastroenterology. 2017;152:767–775.e2. doi: 10.1053/j.gastro.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 8.Mandel JS, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N. Engl. J. Med. 2000;343:1603–1607. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 9.Shaukat A, et al. Long-term mortality after screening for colorectal cancer. N. Engl. J. Med. 2013;369:1106–1114. doi: 10.1056/NEJMoa1300720. [DOI] [PubMed] [Google Scholar]
- 10.Allison JE, et al. A comparison of fecal occult-blood tests for colorectal-cancer screening. N. Engl. J. Med. 1996;334:155–159. doi: 10.1056/NEJM199601183340304. [DOI] [PubMed] [Google Scholar]
- 11.van Rossum LG, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008;135:82–90. doi: 10.1053/j.gastro.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 12.Selby K, Levin TR, Corley DA. Influence of varying quantitative fecal immunochemical test positivity thresholds on colorectal cancer detection. Ann. Intern. Med. 2019;170:736–737. doi: 10.7326/L19-0095. [DOI] [PubMed] [Google Scholar]
- 13.Lee JK, et al. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann. Intern. Med. 2014;160:171. doi: 10.7326/M13-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imperiale TF, et al. Performance characteristics of fecal immunochemical tests for colorectal cancer and advanced adenomatous polyps: a systematic review and meta-analysis. Ann. Intern. Med. 2019;170:319–329. doi: 10.7326/M18-2390. [DOI] [PubMed] [Google Scholar]
- 15.Davidson KW, et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2021;325:1965–1977. doi: 10.1001/jama.2021.6238. [DOI] [PubMed] [Google Scholar]
- 16.Shaukat A, et al. ACG clinical guidelines: colorectal cancer screening 2021. Am. J. Gastroenterol. 2021;116:458–479. doi: 10.14309/ajg.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 17.Wolf AMD, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J. Clin. 2018;68:250–281. doi: 10.3322/caac.21457. [DOI] [PubMed] [Google Scholar]
- 18.Gupta S, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern. Med. 2013;173:1725–1732. doi: 10.1001/jamainternmed.2013.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inadomi JM, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch. Intern. Med. 2012;172:575–582. doi: 10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pilonis ND, et al. Participation in competing strategies for colorectal cancer screening: a randomized health services study (PICCOLINO Study) Gastroenterology. 2021;160:1097–1105. doi: 10.1053/j.gastro.2020.11.049. [DOI] [PubMed] [Google Scholar]
- 21.Singal AG, et al. Outreach invitations for FIT and colonoscopy improve colorectal cancer screening rates: a randomized controlled trial in a safety-net health system. Cancer. 2016;122:456–463. doi: 10.1002/cncr.29770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quintero E, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N. Engl. J. Med. 2012;366:697–706. doi: 10.1056/NEJMoa1108895. [DOI] [PubMed] [Google Scholar]
- 23.Levin TR, et al. Effects of organized colorectal cancer screening on cancer incidence and mortality in a large community-based population. Gastroenterology. 2018;155:1383–1391.e5. doi: 10.1053/j.gastro.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dominitz JA, et al. Colonoscopy vs. fecal immunochemical test in reducing mortality from colorectal cancer (CONFIRM): rationale for study design. Am. J. Gastroenterol. 2017;112:1736–1746. doi: 10.1038/ajg.2017.286. [DOI] [PubMed] [Google Scholar]
- 25.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02078804 (2021).
- 26.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT01239082 (2022).
- 27.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT00906997 (2015).
- 28.Knudsen AB, et al. Colorectal cancer screening: an updated modeling study for the US preventive services task force. JAMA. 2021;325:1998–2011. doi: 10.1001/jama.2021.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ladabaum U, et al. Cost-effectiveness and national effects of initiating colorectal cancer screening for average-risk persons at Age 45 years instead of 50 years. Gastroenterology. 2019;157:137–148. doi: 10.1053/j.gastro.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imperiale TF, et al. Multitarget stool DNA testing for colorectal-cancer screening. N. Engl. J. Med. 2014;370:1287–1297. doi: 10.1056/NEJMoa1311194. [DOI] [PubMed] [Google Scholar]
- 31.Ladabaum U, Mannalithara A. Comparative effectiveness and cost effectiveness of a multitarget stool DNA test to screen for colorectal neoplasia. Gastroenterology. 2016;151:427–439.e6. doi: 10.1053/j.gastro.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Loktionov A. Biomarkers for detecting colorectal cancer non-invasively: DNA, RNA or proteins? World J. Gastrointest. Oncol. 2020;12:124–148. doi: 10.4251/wjgo.v12.i2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cotter TG, et al. Long-term follow-up of patients having false-positive multitarget stool DNA tests after negative screening colonoscopy: the LONG-HAUL Cohort Study. Cancer Epidemiol. Biomark. Prev. 2017;26:614–621. doi: 10.1158/1055-9965.EPI-16-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooper GS, et al. Evaluation of patients with an apparent false positive stool DNA test: the role of repeat stool DNA testing. Dig. Dis. Sci. 2018;63:1449–1453. doi: 10.1007/s10620-018-5001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zapka J, et al. Screening colonoscopy in the US: attitudes and practices of primary care physicians. J. Gen. Intern. Med. 2012;27:1150–1158. doi: 10.1007/s11606-012-2051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. National Comprehensive Cancer Control Program (NCCCP)https://www.cdc.gov/cancer/ncccp/screening-test-use/ (2021).
- 37.Siegel RL, et al. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020;70:145–164. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 38.Singh H, et al. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139:1128–1137. doi: 10.1053/j.gastro.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 39.Baxter NN, et al. Association between colonoscopy and colorectal cancer mortality in a US cohort according to site of cancer and colonoscopist specialty. J. Clin. Oncol. 2012;30:2664–2669. doi: 10.1200/JCO.2011.40.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zauber AG, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N. Engl. J. Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishihara R, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N. Engl. J. Med. 2013;369:1095–1105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kahi CJ, et al. Colonoscopy and colorectal cancer mortality in the Veterans Affairs health care system: a case-control study. Ann. Intern. Med. 2018;168:481–488. doi: 10.7326/M17-0723. [DOI] [PubMed] [Google Scholar]
- 43.Doubeni CA, et al. Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer: a large community-based study. Gut. 2018;67:291–298. doi: 10.1136/gutjnl-2016-312712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samadder NJ, et al. Risk of incident colorectal cancer and death after colonoscopy: a population-based study in Utah. Clin. Gastroenterol. Hepatol. 2016;14:279–86.e1-2. doi: 10.1016/j.cgh.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ. 2014;348:g2467. doi: 10.1136/bmj.g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Itzkowitz SH. Gastrointestinal adenomatous polyps. Semin. Gastrointest. Dis. 1996;7:105–116. [PubMed] [Google Scholar]
- 47.Atkin WS, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–1633. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 48.Segnan N, et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial — SCORE. J. Natl Cancer Inst. 2011;103:1310–1322. doi: 10.1093/jnci/djr284. [DOI] [PubMed] [Google Scholar]
- 49.Schoen RE, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N. Engl. J. Med. 2012;366:2345–2357. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holme O, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA. 2014;312:606–615. doi: 10.1001/jama.2014.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holme O, et al. Long-term effectiveness of sigmoidoscopy screening on colorectal cancer incidence and mortality in women and men: a randomized trial. Ann. Intern. Med. 2018;168:775–782. doi: 10.7326/M17-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vernon SW. Participation in colorectal cancer screening: a review. J. Natl Cancer Inst. 1997;89:1406–1422. doi: 10.1093/jnci/89.19.1406. [DOI] [PubMed] [Google Scholar]
- 53.Jones RM, et al. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. Am. J. Prev. Med. 2010;38:508–516. doi: 10.1016/j.amepre.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Public Health England. Bowel Cancer Screening: Programme Overviewhttps://www.gov.uk/guidance/bowel-cancer-screening-programme-overview (2021).
- 55.Johnson CD, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N. Engl. J. Med. 2008;359:1207–1217. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pickhardt PJ, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N. Engl. J. Med. 2003;349:2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 57.Sakamoto T, et al. Detection of flat colorectal polyps at screening CT colonography in comparison with conventional polypoid lesions. Acta Radiol. 2012;53:714–719. doi: 10.1258/ar.2012.110685. [DOI] [PubMed] [Google Scholar]
- 58.Kim DH, et al. Flat serrated polyps at CT colonography: relevance, appearance, and optimizing interpretation. Radiographics. 2018;38:60–74. doi: 10.1148/rg.2018170110. [DOI] [PubMed] [Google Scholar]
- 59.Eliakim R, et al. Evaluation of the PillCam colon capsule in the detection of colonic pathology: results of the first multicenter, prospective, comparative study. Endoscopy. 2006;38:963–970. doi: 10.1055/s-2006-944832. [DOI] [PubMed] [Google Scholar]
- 60.Spada C, et al. Accuracy and safety of second-generation PillCam COLON capsule for colorectal polyp detection. Ther. Adv. Gastroenterol. 2012;5:173–178. doi: 10.1177/1756283X12438054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rex DK, et al. Accuracy of capsule colonoscopy in detecting colorectal polyps in a screening population. Gastroenterology. 2015;148:948–957.e2. doi: 10.1053/j.gastro.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 62.Voska M, et al. Accuracy of colon capsule endoscopy for colorectal neoplasia detection in individuals referred for a screening colonoscopy. Gastroenterol. Res. Pract. 2019;2019:5975438. doi: 10.1155/2019/5975438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pecere S, et al. Accuracy of colon capsule endoscopy for advanced neoplasia. Gastrointest. Endosc. 2020;91:406–414.e1. doi: 10.1016/j.gie.2019.09.041. [DOI] [PubMed] [Google Scholar]
- 64.Cash BD, et al. Multicentre, prospective, randomised study comparing the diagnostic yield of colon capsule endoscopy versus CT colonography in a screening population (the TOPAZ study) Gut. 2020;70:2115–2122. doi: 10.1136/gutjnl-2020-322578. [DOI] [PubMed] [Google Scholar]
- 65.Spada C, et al. Colon capsule endoscopy: What we know and what we would like to know. World J. Gastroenterol. 2014;20:16948–16955. doi: 10.3748/wjg.v20.i45.16948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Milluzzo SM, et al. Colon capsule endoscopy and its effectiveness in the diagnosis and management of colorectal neoplastic lesions. Expert Rev. Anticancer. Ther. 2019;19:71–80. doi: 10.1080/14737140.2019.1538798. [DOI] [PubMed] [Google Scholar]
- 67.Blanes-Vidal V, et al. Capsule endoscopy vs. colonoscopy vs. histopathology in colorectal cancer screening: matched analyses of polyp size, morphology, and location estimates. Int. J. Colorectal Dis. 2018;33:1309–1312. doi: 10.1007/s00384-018-3064-0. [DOI] [PubMed] [Google Scholar]
- 68.Meklin J, Syrjänen K, Eskelinen M. Colorectal cancer screening with traditional and new-generation fecal immunochemical tests: a critical review of fecal occult blood tests. Anticancer. Res. 2020;40:575–581. doi: 10.21873/anticanres.13987. [DOI] [PubMed] [Google Scholar]
- 69.Molnár B, et al. Plasma methylated septin 9: a colorectal cancer screening marker. Expert Rev. Mol. Diagn. 2015;15:171–184. doi: 10.1586/14737159.2015.975212. [DOI] [PubMed] [Google Scholar]
- 70.US Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 71.Potter NT, et al. Validation of a real-time PCR-based qualitative assay for the detection of methylated SEPT9 DNA in human plasma. Clin. Chem. 2014;60:1183–1191. doi: 10.1373/clinchem.2013.221044. [DOI] [PubMed] [Google Scholar]
- 72.Johnson DA, et al. Plasma Septin9 versus fecal immunochemical testing for colorectal cancer screening: a prospective multicenter study. PLoS One. 2014;9:e98238. doi: 10.1371/journal.pone.0098238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ahlquist DA, et al. The stool DNA test is more accurate than the plasma septin 9 test in detecting colorectal neoplasia. Clin. Gastroenterol. Hepatol. 2012;10:272–7.e1. doi: 10.1016/j.cgh.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.US Preventive Services Task Force et al. Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:1965–1977. doi: 10.1001/jama.2021.6238. [DOI] [PubMed] [Google Scholar]
- 75.Patel SG, et al. Updates on age to start and stop colorectal cancer screening: recommendations from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2021 doi: 10.1053/j.gastro.2021.10.007. [DOI] [PubMed] [Google Scholar]
- 76.von Karsa L, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis: overview and introduction to the full supplement publication. Endoscopy. 2013;45:51–59. doi: 10.1055/s-0032-1325969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Canadian Task Force on Preventive Health Care. Recommendations on screening for colorectal cancer in primary care. CMAJ. 2016;188:340–348. doi: 10.1503/cmaj.151125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pan American Health Organization. Expert Consultation on Colorectal Cancer Screening in Latin America and the Caribbean. Meeting Report (Pan American Health Organization Washington, DC, 2016).
- 79.Jenkins MA, et al. Revised Australian national guidelines for colorectal cancer screening: family history. Med. J. Aust. 2018;209:455–460. doi: 10.5694/mja18.00142. [DOI] [PubMed] [Google Scholar]
- 80.Saito Y, et al. Colonoscopy screening and surveillance guidelines. Dig. Endosc. 2021;33:486–519. doi: 10.1111/den.13972. [DOI] [PubMed] [Google Scholar]
- 81.Wang YW, et al. Current status and future challenge of population-based organized colorectal cancer screening: Lesson from the first decade of Taiwanese program. J. Formos. Med. Assoc. 2018;117:358–364. doi: 10.1016/j.jfma.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 82.Chiu HM, et al. Long-term effectiveness of faecal immunochemical test screening for proximal and distal colorectal cancers. Gut. 2021;70:2321–2329. doi: 10.1136/gutjnl-2020-322545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heisser T, et al. Age-specific sequence of colorectal cancer screening options in Germany: a model-based critical evaluation. PLoS Med. 2020;17:e1003194. doi: 10.1371/journal.pmed.1003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Paltiel O, et al. Adherence to national guidelines for colorectal cancer screening in Israel: comprehensive multi-year assessment based on electronic medical records. J. Med. Screen. 2021;28:25–33. doi: 10.1177/0969141320919152. [DOI] [PubMed] [Google Scholar]
- 85.International Agency for Research on Cancer. Cervix cancer screening. IARC Handb. Cancer Prev. 2005;10:117–162. [Google Scholar]
- 86.Miles A, et al. A perspective from countries using organized screening programs. Cancer. 2004;101:1201–1213. doi: 10.1002/cncr.20505. [DOI] [PubMed] [Google Scholar]
- 87.Tiro JA, et al. The colorectal cancer screening process in community settings: a conceptual model for the population-based research optimizing screening through personalized regimens consortium. Cancer Epidemiol. Biomark. Prev. 2014;23:1147–1158. doi: 10.1158/1055-9965.EPI-13-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dubé C. Organized screening is better than opportunistic screening at decreasing the burden of colorectal cancer in the United States. Gastroenterology. 2018;155:1302–1304. doi: 10.1053/j.gastro.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 89.Senore C, et al. Optimising colorectal cancer screening acceptance: a review. Gut. 2015;64:1158–1177. doi: 10.1136/gutjnl-2014-308081. [DOI] [PubMed] [Google Scholar]
- 90.Walter LC, et al. Impact of age and comorbidity on colorectal cancer screening among older veterans. Ann. Intern. Med. 2009;150:465–473. doi: 10.7326/0003-4819-150-7-200904070-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goodwin JS, et al. Overuse of screening colonoscopy in the Medicare population. Arch. Intern. Med. 2011;171:1335–1343. doi: 10.1001/archinternmed.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Powell AA, et al. Rates and correlates of potentially inappropriate colorectal cancer screening in the Veterans Health Administration. J. Gen. Intern. Med. 2015;30:732–741. doi: 10.1007/s11606-014-3163-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Steinwachs D, et al. National Institutes of Health state-of-the-science conference statement: enhancing use and quality of colorectal cancer screening. Ann. Intern. Med. 2010;152:663–667. doi: 10.7326/0003-4819-152-10-201005180-00237. [DOI] [PubMed] [Google Scholar]
- 94.Selby K, et al. Program components and results from an organized colorectal cancer screening program using annual fecal immunochemical testing. Clin. Gastroenterol. Hepatol. 2020;20:145–152. doi: 10.1016/j.cgh.2020.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dominitz JA, Levin TR. What is organized screening and what is its value? Gastrointest. Endosc. Clin. N. Am. 2020;30:393–411. doi: 10.1016/j.giec.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 96.Fiscella K, et al. Eliminating disparities in cancer screening and follow-up of abnormal results: what will it take? J. Health Care Poor Underserved. 2011;22:83–100. doi: 10.1353/hpu.2011.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wardle J, et al. Effects of evidence-based strategies to reduce the socioeconomic gradient of uptake in the English NHS Bowel Cancer Screening Programme (ASCEND): four cluster-randomised controlled trials. Lancet. 2016;387:751–759. doi: 10.1016/S0140-6736(15)01154-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zorzi M, et al. Impact on colorectal cancer mortality of screening programmes based on the faecal immunochemical test. Gut. 2015;64:784–790. doi: 10.1136/gutjnl-2014-307508. [DOI] [PubMed] [Google Scholar]
- 99.McClements PL, et al. Impact of the UK colorectal cancer screening pilot studies on incidence, stage distribution and mortality trends. Cancer Epidemiol. 2012;36:e232–e242. doi: 10.1016/j.canep.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 100.Khakoo S, et al. Circulating tumour DNA, a promising biomarker for the management of colorectal cancer. Crit. Rev. Oncol. Hematol. 2018;122:72–82. doi: 10.1016/j.critrevonc.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 101.Grady WM, Markowitz SD. The molecular pathogenesis of colorectal cancer and its potential application to colorectal cancer screening. Dig. Dis. Sci. 2015;60:762–772. doi: 10.1007/s10620-014-3444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kolenčík D, et al. Liquid biopsy in colorectal carcinoma: clinical applications and challenges. Cancers. 2020;12:1376. doi: 10.3390/cancers12061376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jensen T. S. et al. Decision Memo for Screening for Colorectal Cancer — Blood-Based Biomarker Tests (CAG-00454N) (CMS, 2021).
- 104.Parikh RB, Prasad V. Blood-based screening for colon cancer: a disruptive innovation or simply a Disruption? JAMA. 2016;315:2519–2520. doi: 10.1001/jama.2016.7914. [DOI] [PubMed] [Google Scholar]
- 105.Klein EA, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann. Oncol. 2021;32:1167–1177. doi: 10.1016/j.annonc.2021.05.806. [DOI] [PubMed] [Google Scholar]
- 106.Cohen JD, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. 2018;359:926–930. doi: 10.1126/science.aar3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Adler A, et al. Improving compliance to colorectal cancer screening using blood and stool based tests in patients refusing screening colonoscopy in Germany. BMC Gastroenterol. 2014;14:183. doi: 10.1186/1471-230X-14-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Young GP, et al. “Rescue” of nonparticipants in colorectal cancer screening: a randomized controlled trial of three non-invasive test options. Cancer Prev. Res. 2021;14:803–810. doi: 10.1158/1940-6207.CAPR-21-0080. [DOI] [PubMed] [Google Scholar]
- 109.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04369053 (2020).
- 110.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04136002 (2019).
- 111.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04213326 (2019).
- 112.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT00843375 (2009).
- 113.Symonds EL, et al. Circulating epigenetic biomarkers for detection of recurrent colorectal cancer. Cancer. 2020;126:1460–1469. doi: 10.1002/cncr.32695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grail. Grail Productshttps://grail.com/our-products/ (2015).
- 115.Russell P. NHS to Trial Galleri Cancer Blood Test (Medscape News UK, 2021).
- 116.Medical Advisory Secretariat. Magnetic Resonance (MR) colonography for colorectal cancer screening: an evidence-based analysis. Ont. Health Technol. Assess. Ser. 2009;9:1–35. [PMC free article] [PubMed] [Google Scholar]
- 117.Check-Cap. C-Scan redefining colorectal cancer screening. Check-Caphttps://check-cap.com/ (2022).
- 118.Gluck N, et al. A novel prepless X-ray imaging capsule for colon cancer screening. Gut. 2016;65:371–373. doi: 10.1136/gutjnl-2015-310893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Walker MJ, et al. Measuring the impact of the COVID-19 pandemic on organized cancer screening and diagnostic follow-up care in Ontario, Canada: a provincial, population-based study. Prev. Med. 2021;151:106586. doi: 10.1016/j.ypmed.2021.106586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kortlever TL, et al. The national FIT-based colorectal cancer screening program in the Netherlands during the COVID-19 pandemic. Prev. Med. 2021;151:106643. doi: 10.1016/j.ypmed.2021.106643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gupta S, Lieberman D. Screening and surveillance colonoscopy and COVID-19: avoiding more casualties. Gastroenterology. 2020;159:1205–1208. doi: 10.1053/j.gastro.2020.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ricciardiello L, et al. Impact of SARS-CoV-2 Pandemic on colorectal cancer screening delay: effect on stage shift and increased mortality. Clin. Gastroenterol. Hepatol. 2021;19:1410–1417.e9. doi: 10.1016/j.cgh.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.de Jonge L, et al. Impact of the COVID-19 pandemic on faecal immunochemical test-based colorectal cancer screening programmes in Australia, Canada, and the Netherlands: a comparative modelling study. Lancet Gastroenterol. Hepatol. 2021;6:304–314. doi: 10.1016/S2468-1253(21)00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sharpless NE. COVID-19 and cancer. Science. 2020;368:1290. doi: 10.1126/science.abd3377. [DOI] [PubMed] [Google Scholar]
- 125.Aitken M., Kleinrock M. Shifts in Healthcare Demand, Delivery and Care During the COVID-19 Era: Tracking the Impact in the United States (IQVIA, 2020).
- 126.Carethers JM, et al. Disparities in cancer prevention in the COVID-19 Era. Cancer Prev. Res. 2020;13:893–896. doi: 10.1158/1940-6207.CAPR-20-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sabatino SA, et al. Cancer screening test receipt — United States, 2018. MMWR Morb. Mortal Wkly Rep. 2021;70:29–35. doi: 10.15585/mmwr.mm7002a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ladabaum U. Cost-effectiveness of current colorectal cancer screening tests. Gastrointest. Endosc. Clin. N. Am. 2020;30:479–497. doi: 10.1016/j.giec.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 129.Ladabaum U, et al. Strategies for colorectal cancer screening. Gastroenterology. 2020;158:418–432. doi: 10.1053/j.gastro.2019.06.043. [DOI] [PubMed] [Google Scholar]
- 130.Bosch LJW, et al. Multitarget Stool DNA test performance in an average-risk colorectal cancer screening population. Am. J. Gastroenterol. 2019;114:1909–1918. doi: 10.14309/ajg.0000000000000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dickinson BT, et al. Molecular markers for colorectal cancer screening. Gut. 2015;64:1485–1494. doi: 10.1136/gutjnl-2014-308075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Church TR, et al. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut. 2014;63:317–325. doi: 10.1136/gutjnl-2012-304149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lin JS, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315:2576–2594. doi: 10.1001/jama.2016.3332. [DOI] [PubMed] [Google Scholar]
- 134.Rex DK, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2017;112:1016–1030. doi: 10.1038/ajg.2017.174. [DOI] [PubMed] [Google Scholar]
- 135.Debatin JF, Lauenstein TC. Virtual magnetic resonance colonography. Gut. 2003;52(Suppl. 4):iv17–iv22. doi: 10.1136/gut.52.suppl_4.iv17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim DH, Moreno CC, Pickhardt PJ. Computed tomography colonography: pearls and pitfalls. Radiol. Clin. North Am. 2018;56:719–735. doi: 10.1016/j.rcl.2018.05.004. [DOI] [PubMed] [Google Scholar]