Abstract
A novel asymmetric photodimerization reaction of coumarin derivatives bearing the (S)-4-benzyl-2-oxazolidinone auxiliary provides only the syn-head-to-tail (syn-HT) dimer with moderate diastereoselectivity (up to 75 : 25). The mechanism of complete syn-HT selectivity and moderate diastereoselectivity is proposed based on the result of density functional theory (DFT) calculation. The benzyl group of the (S)-4-benzyl-2-oxazolidinone auxiliary in combination with a Lewis acid exerts effective diastereofacial shielding of the reaction site.
Photodimerization of chiral coumarin-3-carboxamide affords the syn-HT dimer with moderate diastereoselectivity and we propose a mechanism on the basis of DFT calculations.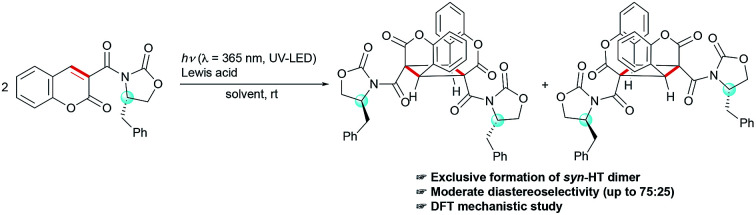
The photodimerization reactions of coumarin derivatives have been recognized as attractive photochemical transformation reactions in terms of their photophysical properties as well as their photochemical properties, from the theoretical point of view.1 Recently, photodimerization reactions of coumarins have become increasingly important in several progressive research areas, e.g., drug delivery by the use of coumarin-modified mesoporous silica MCM-412a and the use of coumarin-modified polymeric nanoparticles,2b,2c chemical biology,2d 3D cell culture,2e photopatterning of ion gels,2f reversible two-photon optical data storage,2g nanolithography,2h polyoxometalate-containing materials,2i single-chain nanoparticles,2j gelators,2k and optically active polymers.2l Although [2 + 2] photodimerization reactions of coumarin derivatives are useful photochemical reactions, difficulties in controlling head-to-head (HH)/head-to-tail (HT), syn/anti, enantio- and diastereoselectivity of the reactions in solution have prevented further improvement of the usefulness and understanding of the reaction. To overcome these issues, several methodologies for syn/anti and HH/HT selective [2 + 2] photodimerization reactions of coumarins in solution have been developed, e.g., Lewis acid catalysis,3 supramolecular photocatalysis accomplished by the use of cyclodextrins,4a hydrazine derivatives,4b bisurea macrocycles,4c cucurbit[8]uril,4d taco-type host–guest complexes4e and self-assembled monolayers of coumarin derivatives on gold.4f For an enantioselective reaction, Tanaka and Fujiwara reported outstanding results of an asymmetric photodimerization of simple coumarin by the use of TADDOL derivatives which gave an anti-HT dimer with excellent enantiomeric excess (up to 96% ee).5 However, to the best of our knowledge, the methodology to synthesis a syn-HT dimer in a diastereoselective manner has yet to be demonstrated.6 Herein, we report a novel asymmetric [2 + 2] photodimerization of chiral coumarin-3-carboxamide which gives only syn-HT dimers along with moderate diastereoselectivity.
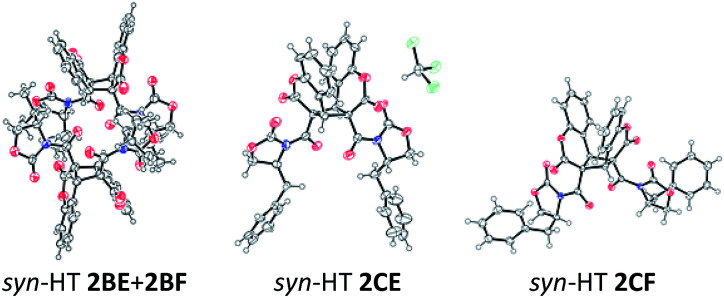
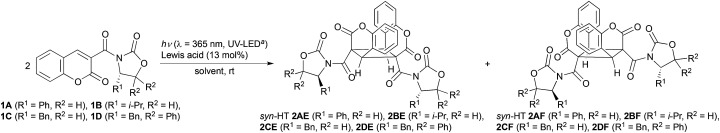
We initially conducted [2 + 2] photodimerization reactions of (S)-4-phenyl-3-(2-oxo-2H-chromene-3-carbonyl)-2-oxazolidinone (1A) in acetone, resulting in recovery of 1A without any desired dimers, which could be caused by the incredibly poor solubility of 1A (Table 1). Next, we tried to use (S)-4-isopropyl-3-(2-oxo-2H-chromene-3-carbonyl)-2-oxazolidinone (1B), which gave 2BE and 2BF as an inseparable mixture with good yield and the ratio of 2BE to 2BF as 55 : 45. X-ray crystallographic analysis provided a structure of 50 : 50 diastereomeric mixture of 2BE and 2BF, which revealed that the reaction had progressed in a syn- and HT-selective manner (Fig. 1). We also used (S)-4-benzyl-3-(2-oxo-2H-chromene-3-carbonyl)-2-oxazolidinone (1C) (entry 3) instead of 1B.
[2 + 2] Photodimerization reactions of chiral coumarin-3-carboxamides.
| ||||||
|---|---|---|---|---|---|---|
| Entry | Coumarin | Solvent | Additive | Time/h | Yieldb/% | Diastereomeric ratioc |
| 1 | 1A | Acetone | No | 48 | 0 | — (2AE) : — (2AF) |
| 2 | 1B | Acetone | No | 48 | 83 | 55 (2BE) : 45 (2BF) |
| 3 | 1C | Acetone | No | 48 | 99 | 63 (2CE) : 37 (2CF) |
| 4 | 1C | Toluene | No | 48 | 90 | 63 (2CE) : 7 (2CF) |
| 5 | 1C | CF3C6H5 | No | 48 | 56 | 59 (2CE) : 41 (2CF) |
| 6 | 1C | CH2Cl2 | No | 24 | 95 | 61 (2CE) : 39 (2CF) |
| 7 | 1C | CHCl3 | No | 24 | 96 | 58 (2CE) : 42 (2CF) |
| 8 | 1C | CH3CN | No | 24 | 92 | 53 (2CE) : 47 (2CF) |
| 9 | 1B | Toluene | Zn(ClO4)2·6H2O | 96 | 24d | 70 (2BE) : 30 (2BF) |
| 10 | 1C | Toluene | Zn(ClO4)2·6H2O | 48 | 92 | 75 (2CE) : 25 (2CF) |
| 11 | 1C | Acetone | Zn(ClO4)2·6H2O | 48 | 72 | 75 (2CE) : 25 (2CF) |
| 12 | 1C | Toluene | BF3·OEt2 | 48 | 90 | 70 (2CE) : 30 (2CF) |
| 13 | 1C | Toluene | Mg(ClO4)2 | 48 | 72 | 69 (2CE) : 31 (2CF) |
| 14 | 1C | Toluene | Ni(ClO4)2·6H2O | 48 | 75 | 67 (2CE) : 33 (2CF) |
| 15 | 1C | Toluene | Co(ClO4)2·6H2O | 48 | 51 | 70 (2CE) : 30 (2CF) |
| 16 | 1C | Toluene | Cu(ClO4)2·6H2O | 48 | 42 | 71 (2CE) : 29 (2CF) |
| 17 | 1C | Toluene | Fe(ClO4)2·xH2O | 48 | 77 | 72 (2CE) : 28 (2CF) |
| 18 | 1D | Toluene | Zn(ClO4)2·6H2O | 96 | 0 | — (2DE) : — (2CF) |
The external irradiation was directed toward the Pyrex test tube with a working distance of 1 cm. All reactions were degassed by argon bubbling for 15 min prior to irradiation.
Isolated yield.
Determined by 1H NMR.
Recovery of 1B in 52%.
Fig. 1. ORTEP drawing of photodimers: the inclusion of chloroform was observed in syn-HT 2CE.
As a result, solubility of the chiral coumarin and the diastereomeric ratio of dimers were improved to give 2CE and 2CF with the ratio of 63 : 37 in 99% yield. Photodimer 2CE and 2CF were separable. syn-HT structures of 2CE and 2CF were also successfully determined by X-ray crystallographic analyses. When the reaction was carried out in toluene (entry 4), syn-HT 2CE and syn-HT 2CF were obtained in excellent yield with the same diastereomeric ratio as the reaction in acetone (entry 3 versus entry 4). The yield of syn-HT 2CE and syn-HT 2CF was decreased by the use of α,α,α-trifluorotoluene (CF3C6H5) as a reaction solvent, which was probably due to low solubility of 1C in CF3C6H5 (entry 5). Solubility enhancement of 1C had a good impact on increasing the reaction rate by the use of CH2Cl2, CHCl3 and CH3CN as reaction solvents, but decreased the diastereoselectivity as compared to the reaction in toluene (entries 6–8 versus entries 3 and 4). As far as we know, there is only one example of a non-photochemical reaction, which is the highly diastereoselective 1,3-dipolar cycloaddition reaction, by the use of a chiral oxazolidinone-functionalized substrate in the absence of Lewis acid as reported by Sibi and co-workers.7 We were intrigued by induction of moderate diastereoselectivity in the absence of a chelating agent Lewis acid. Therefore, we tried to add Lewis acid in an effort to improve diastereoselectivity by utilizing a chelation control agent.8 Regarding a role for the Lewis acid in [2 + 2] photodimerization reactions, Lewis and co-workers reported the [2 + 2] photodimerization reaction of achiral coumarin in the presence of BF3·OEt2 to give the syn-HT dimer as a single product.3 In the search for a good chelating Lewis acid for 3-acyl-2-oxazolidinone derivatives, we tested Zn(ClO4)2·6H2O for the reaction of 1B (entry 9).9 A non-coordination solvent toluene was used for the reaction. As a result, the reaction of 1B was inhibited by the addition of Zn(ii) salt to give 2BE and 2BF with recovery of 1B (entry 9).
Interestingly, the reaction of 1C in the presence of Zn(ClO4)2·6H2O in toluene proceeded to achieve an improvement of yields and diastereomeric ratio of syn-HT dimers (2CE : 2CF = 75 : 25, entry 10). The use of acetone as a reaction solvent and Zn(ClO4)2·6H2O as a Lewis acid diminished the yield of syn-HT 2CE and syn-HT 2CF (entry 11). The reaction of 1C in the presence of BF3·OEt2 in toluene showed a slightly improved diastereomeric ratio in comparison with the reaction in the absence of BF3·OEt2 (2CE : 2CF = 70 : 30, entry 12). Other metal perchlorates such as Mg, Ni, Co, Cu and Fe slightly improved the 2CE : 2CF ratio (entries 13–17). To improve solubility of the chiral coumarin, we prepared 1D having three hydrophobic phenyl groups and used this compound for the photodimerization reaction. However, undesired photodecomposition of 1D occurred (entry 18). Interestingly, no reaction was induced when the reaction of 1C was performed in the presence of a stoichiometric amount of Zn(ClO4)2·6H2O as well as BF3·OEt2. This phenomenon might suggest that the ratio of the metal complex of 1C and metal-free 1C in solution is important for the reaction to progress.3,10
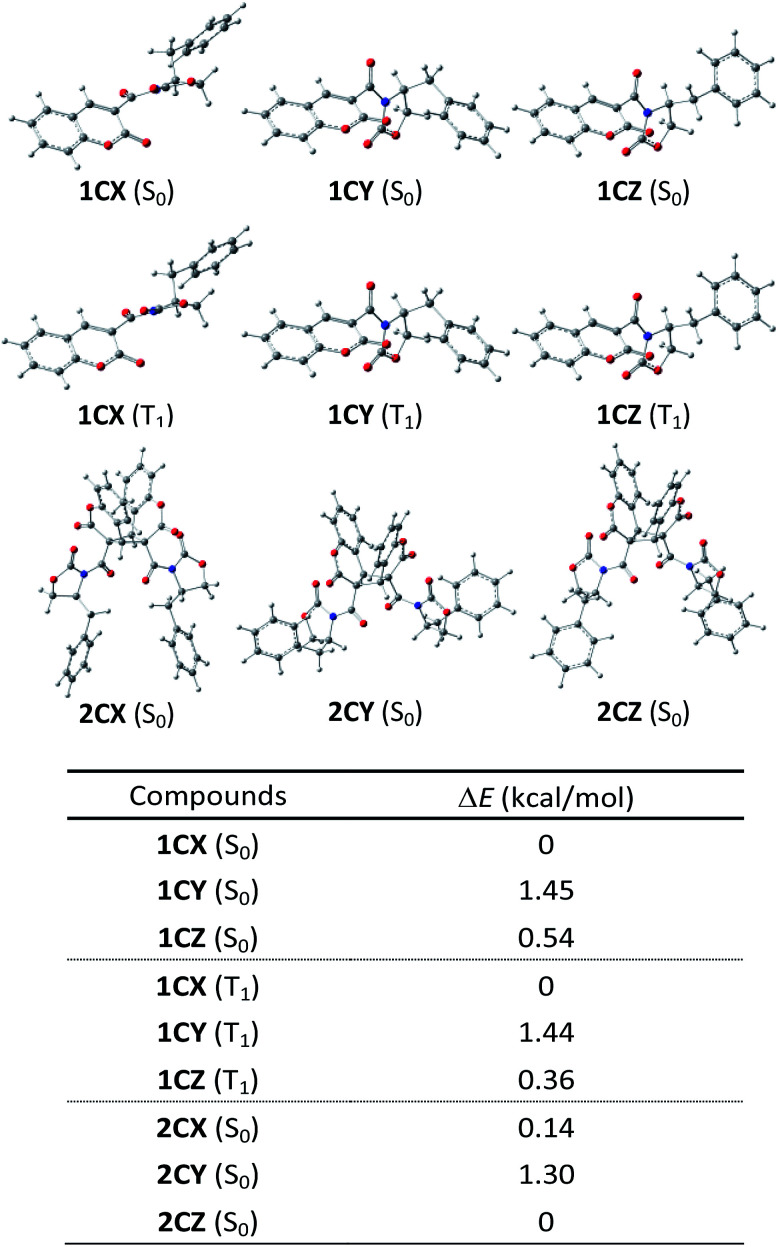
To gain insight into the mechanism of complete syn-HT selectivity and moderate diastereoselectivity, we optimized the structure of 1C in toluene using DFT calculations (Scheme 1).11 All calculations were performed using the B3LYP hybrid functional and a basis set (6-311G**) level of theory with Gaussian 16. The solvent effect of toluene was modeled via the polarizable continuum model using the integral equation formalism variant (IEFPCM). Three conformers 1CX, 1CY and 1CZ were generated which are energetically closed.12 We revealed that the order of the change in energy among three conformers was 1CX (S0) < 1CZ (S0) < 1CY (S0). The crystal structure of 1C was similar to 1CZ, which indicates that the major conformer involved in the [2 + 2] photodimerization would be changed between the solution and the crystalline states.13
Scheme 1. Optimized structures of 1C (S0 and T1) and 2C (S0).
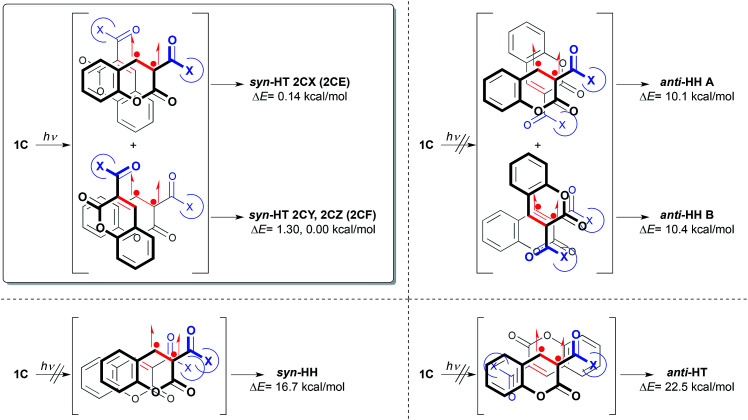
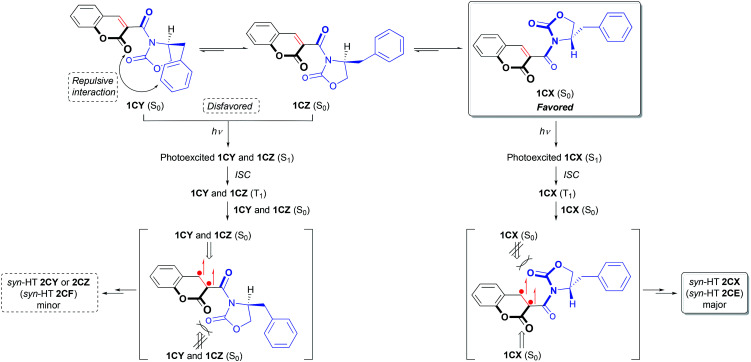
Energies of photodimers syn-HH, anti-HH and anti-HT in toluene were also optimized by DFT calculation (B3LYP/6-311G**) to explain the exclusive formation of syn-HT dimer, which would be induced predominantly by accommodating the (S)-4-benzyl-2-oxazolidinone auxiliary in a favorable direction with a special allowance in the TS (Fig. 2).14 Structures of 2CX and 2CY were found to correspond to the structures of the cycloadducts syn-HT 2CE and syn-HT 2CF, respectively. It must be noted that 2CZ is the major diastereomer with a different conformation from that of 2CY. Thus, the order of the change in energy for photodimers was 2CZ < 2CX < 2CY. There was not much difference in energy between 2CX and 2CZ. Time-dependent DFT (TD-DFT) calculations showed that HOMO → LUMO transition for each of the three conformers 1CX, 1CY and 1CZ would be best described as a triplet excited state [3(π,π)*] (T1) for the carbon–carbon double bond of the reaction site with zero oscillator strength (Scheme 2). Therefore, it is suggested that the ground state (S0) for each of the three conformers was excited to become a singlet state [1(π,π)*] (S1) followed by intersystem crossing (ISC) to generate T1. Consequently, the order of the change in energy among T1 of the three conformers was also calculated as 1CX (T1) < 1CZ (T1) < 1CY (T1). From the results mentioned above, the [2 + 2] photodimerization reaction would start from excitation of the most stable comformer 1CX (S0) which preferentially generates 1CX (T1) via ISC of a singlet excited state 1CX (S1). The resulting triplet species 1CX (T1) would be subjected to react with 1CX (S0) in syn-HT fashion (discussed below) to give 2CX. The effect of the Lewis acid is unclear at the present time. However, we postulate that the Lewis acid could stabilize 1CX by a coordination in a bidentate fashion to prevent the conformational change to 1CY and 1CZ, which would be induced by a free rotation of the carbon–carbon single bond between the coumarin ring and the 2-oxazolidinone auxiliary.3,8,15,16 The moderate diastereoselectivity could be explained by the following process (also depicted in Scheme 2). Two carbonyl groups in the (S)-3-acyl-4-benzyl-2-oxazolidinone auxiliary could be oriented opposite each other to minimize the dipole moment and the electrostatic repulsion. To minimize steric repulsion caused between the benzyl group of the 2-oxazolidinone auxiliary and the carbonyl group of the coumarin ring, the conformational bias leads more favorably toward 1CX rather than 1CY and 1CZ. The carbonyl group including the 2-oxazolidinone ring would serve as a shielding group to prevent approach from the top side, thus affording 2CX, i.e., syn-HT 2CE (Scheme 3).
Fig. 2. Exclusive formation of syn-HT dimers caused by the steric hindrance between two chiral coumarins.
Scheme 2. Proposed mechanism for diastereofacial selectivity.
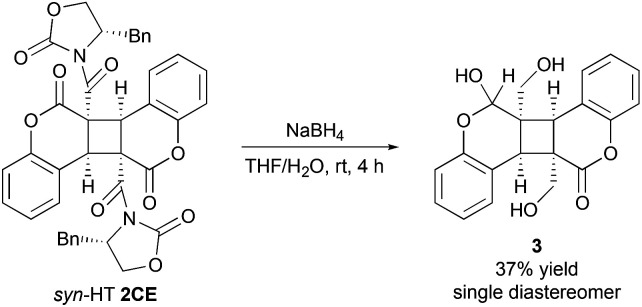
Scheme 3. Removal of the chiral auxiliary with the reduction of chroman-2-one moiety.
The reduction of the chiral oxazolidinone moiety was successfully proceeded to give triol 3.17 Interestingly, the reduction of chroman-2-one moiety also concomitantly occured with the removal of chiral auxiliary affording 3 as a single diastereomer.18
In conclusion, we have developed a novel asymmetric photodimerization reaction of chiral coumarin-3-carboxamide which affords syn-HT dimer selectively with a moderate level of diastereoselectivity. Removal of chiral auxiliary was conducted to expand the applicability of the photodimer by the reduction using NaBH4 in THF–H2O. Further studies to elucidate the reaction mechanism and for application in the area of medicinal chemistry, drug delivery, and chemical biology are ongoing.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was supported by JSPS KAKENHI Grant numbers JP17K14550 and JP18K05111. The computation was mainly carried out using the computer facilities at Research Institute for Information Technology, Kyushu University. We gratefully thank Ms. Noriko Sato (the Institute of Instrumental Analysis of Kitasato University, School of Pharmacy) for 1H NMR analyses.
Electronic supplementary information (ESI) available: Materials, experimental details, and characterization data. CCDC 1552472 (1C), 1885142 (2BE + 2BF), 1484443 (2CE) and 1552473 (2CF). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ra00822e
Notes and references
- (a) Hammond G. S. Stout C. A. Lamola A. A. J. Am. Chem. Soc. 1964;86:3103. doi: 10.1021/ja01069a026. [DOI] [Google Scholar]; (b) Ray N. K. Ahuja V. K. Photochem. Photobiol. 1973;17:347. doi: 10.1111/j.1751-1097.1973.tb06363.x. [DOI] [Google Scholar]; (c) Rao D. V. Ulrich H. Stuber F. A. Sayigh A. A. R. Chem. Ber. 1973;106:388. doi: 10.1002/cber.19731060204. [DOI] [PubMed] [Google Scholar]; (d) Turro J., Modern Molecular Photochemistry, University Science Books, 1991, p. 462 [Google Scholar]; (e) Wolff T. Görner H. Phys. Chem. Chem. Phys. 2004;6:368. doi: 10.1039/B312335A. [DOI] [Google Scholar]; (f) Trenor S. R. Shultz A. R. Love B. J. Long T. E. Chem. Rev. 2004;104:3059. doi: 10.1021/cr030037c. [DOI] [PubMed] [Google Scholar]; (g) Görner H. Wolff T. Photochem. Photobiol. 2008;84:1224–1230. doi: 10.1111/j.1751-1097.2008.00339.x. [DOI] [PubMed] [Google Scholar]; (h) Pattabiraman M. Sivaguru J. Ramamurthy V. Isr. J. Chem. 2018;58:264. doi: 10.1002/ijch.201700100. [DOI] [Google Scholar]
- (a) Mal N. K. Fujiwara M. Tanaka Y. Nature. 2003;421:350. doi: 10.1038/nature01362. [DOI] [PubMed] [Google Scholar]; (b) Guardado-Alvarez T. M. Devi L. S. Russell M. M. Schwartz B. J. Zink J. I. J. Am. Chem. Soc. 2013;135:14000. doi: 10.1021/ja407331n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chung J. W. Lee K. Neikirk C. Nelson C. M. Priestley R. D. Small. 2012;8:1693. doi: 10.1002/smll.201102263. [DOI] [PubMed] [Google Scholar]; (d) Miska B. Med. Chem. 2016;6:611. [Google Scholar]; (e) Tamae R. Ueki T. Kitazawa Y. Kuzunuki M. Watanabe M. Akimoto A. M. Yoshida R. Chem. Mater. 2016;28:6401. doi: 10.1021/acs.chemmater.6b02839. [DOI] [Google Scholar]; (f) Tamae R. Ueki T. Akimoto A. M. Yoshida R. Oyama T. Kokubo H. Watanabe M. RSC Adv. 2018;8:3418. doi: 10.1039/C7RA13181J. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) lliopoulos K. Krupka O. Gindre D. Sallé M. J. Am. Chem. Soc. 2010;132:14343. doi: 10.1021/ja1047285. [DOI] [PubMed] [Google Scholar]; (h) d'Halluin M. Rull-Barrull J. Le Grognec E. Jacquemin D. Felpin F.-X. Chem. Commun. 2016;52:7672. doi: 10.1039/C6CC02915A. [DOI] [PubMed] [Google Scholar]; (i) Chen W. Tong U. Zeng T. Streb C. Song Y.-F. J. Mater. Chem. C. 2015;3:4388. doi: 10.1039/C5TC00379B. [DOI] [Google Scholar]; (j) Fan W. Tong X. Yan Q. Fu S. Zhao Y. Chem. Commun. 2014;50:13492. doi: 10.1039/C4CC06629D. [DOI] [PubMed] [Google Scholar]; (k) Draper E. M. McDonald T. O. Adams D. J. Chem. Commun. 2015;51:12837. doi: 10.1039/c5cc03817k. [DOI] [PubMed] [Google Scholar]; (l) Chen Y. Saigo K. Yonezawa N. Tachibana K. Hasegawa M. Bull. Chem. Soc. Jpn. 1987;60:3341. doi: 10.1246/bcsj.60.3341. [DOI] [Google Scholar]
- Lewis F. D. Howard D. K. Oxman J. D. J. Am. Chem. Soc. 1983;105:3344. doi: 10.1021/ja00348a069. [DOI] [Google Scholar]
- (a) Moorthy J. N. Venkatesan K. Weiss R. G. J. Org. Chem. 1992;57:3292. doi: 10.1021/jo00038a012. [DOI] [Google Scholar]; (b) Skene W. G. Couzigné E. Lehn J.-M. Chem.–Eur. J. 2003;9:5560. doi: 10.1002/chem.200305268. [DOI] [PubMed] [Google Scholar]; (c) Yang J. Dewal M. B. Shimizu L. S. J. Am. Chem. Soc. 2006;128:8122. doi: 10.1021/ja062337s. [DOI] [PubMed] [Google Scholar]; (d) Barooah N. Pemberton B. C. Sivaguru J. Org. Lett. 2008;10:3339. doi: 10.1021/ol801256r. [DOI] [PubMed] [Google Scholar]; (e) Wei P. Wang H. Jie K. Huang F. Chem. Commun. 2017;53:1688. doi: 10.1039/C6CC10089A. [DOI] [PubMed] [Google Scholar]; (f) Li W. Lynch V. Thompson H. Fox M. A. J. Am. Chem. Soc. 1997;119:7211. doi: 10.1021/ja970633m. [DOI] [Google Scholar]
- Tanaka K. Fujiwara T. Org. Lett. 2005;7:1501. doi: 10.1021/ol0501471. [DOI] [PubMed] [Google Scholar]
- Synthesis of syn-HT dimer from simple coumarin without asymmetric induction was achieved by taking advantage of Lewis acid catalysis (ref. 3), solvent effects (ref. 1e and f) and supramolecular catalysis (ref. 4c and d).
- Sibi M. P. Soeta T. Jasperse C. P. Org. Lett. 2009;11:5366. doi: 10.1021/ol9018584. [DOI] [PubMed] [Google Scholar]
- Evans D. A. Chapman K. T. Bisaha J. J. Am. Chem. Soc. 1988;110:1238. doi: 10.1021/ja00212a037. [DOI] [Google Scholar]
- (a) Kanemasa S. Oderaotoshi Y. Yamamoto H. Tanaka J. Wada E. J. Org. Chem. 1997;62:6454. doi: 10.1021/jo970906w. [DOI] [Google Scholar]; (b) Kanemasa S. Oderaotoshi Y. Sakaguchi S. Yamamoto H. Tanaka J. Wada E. Curran D. P. J. Am. Chem. Soc. 1998;120:3074. doi: 10.1021/ja973519c. [DOI] [Google Scholar]
- Lewis and co-workers revealed that increasing the amount of Lewis acid caused a lower quantum yield (Φ) of the photodimerization. See ref. 3.
- Frisch M. J., et al., Gaussian 16, Revision A.03, Gaussian, Inc., Wallingford CT, 2016 [Google Scholar]
- It was difficult to distinguish three conformers of chiral coumarins 1CX, 1CY and 1CZ in CD3C6D5 and (CD3)2CO at room temperature by 1D 1H NMR and 2D rotating-frame Overhauser spectroscopy (ROESY) NMR measurements. While conducting variable-temperature (VT) NMR measurements, chiral coumarin 1C precipitated out under low temperature because of its low solubility.
- See the ESI† for the crystal structure of 1A.
- See the ESI† for the experimental and the computational details and results.
- A small extent of downfield-shifts for the H-4 and C-4 of the coumarin ring of 1C was observed in the presence of BF3·OEt2 and Zn(ClO4)2·6H2O (1 equiv.) by 1H and 13C NMR spectroscopic analyses. Absorption of 1C at 287 nm was enhanced slightly with an increasing amount of Zn(ClO4)2·6H2O. See the ESI† for the spectra.
- The structure of 1C ZnCl2 complex was optimized by DFT calculation (B3LYP/6-311G) to show bidentate complexation of Zn(ii) ion between the carbonyl oxygen of coumarin ring and the acyl oxygen of (S)-3-acyl-4-benzyl-2-oxazolidinone auxiliary. See the ESI† for the computational results.
- (a) Prashad M. Shieh W.-C. Liu Y. Tetrahedron. 2016;72:17. doi: 10.1016/j.tet.2015.09.076. [DOI] [Google Scholar]; (b) Hashimoto Y. Itoh K. Kakehi A. Shiro M. Suga H. J. Org. Chem. 2013;78:6182. doi: 10.1021/jo400858u. [DOI] [PubMed] [Google Scholar]
- The structure of 3 was assigned by means of NMR analyses (1H, 13C, 1H–1H COSY, HSQC and HMBC). The stereochemistry of C-2 of chroman-2-ol moiety was not determined, albeit a single diastereomer. See the ESI† for the spectra.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.