Abstract
The gene coding for aminoglycoside 2′-N-acetyltransferase Ia [AAC(2′)-Ia] from Providencia stuartii was amplified by PCR and cloned. The resulting construct, pACKF2, was transferred into Escherichia coli for overexpression of AAC(2′)-Ia as a fusion protein with an N-terminal hexa-His tag. The fusion protein was isolated and purified by affinity chromatography on Ni2+-nitrilotriacetic acid agarose and gel permeation chromatography on Superdex 75. Comparison of the specific activity of this enzyme with that of its enterokinase-digested derivative lacking the His tag indicated that the presence of the extra N-terminal peptide does not affect activity. The temperature and pH optima for activity of both forms of the 2′-N-acetyltransferase were 20°C and pH 6.0, respectively, while the enzymes were most stable at 15°C and pH 8.1. The Michaelis-Menten kinetic parameters for AAC(2′)-Ia at 20°C and pH 6.0 were determined using a series of aminoglycoside antibiotics possessing a 2′-amino group and a concentration of acetyl coenzyme A fixed at 10 times its Km value of 8.75 μM. Under these conditions, gentamicin was determined to be the best substrate for the enzyme in terms of both Km and kcat/Km values, whereas neomycin was the poorest. Comparison of the kinetic parameters obtained with the different aminoglycosides indicated that their hexopyranosyl residues provided the most important binding sites for AAC(2′)-Ia activity, while the enzyme exhibits greater tolerance further from these sites. No correlation was found between these kinetic parameters and MICs determined for P. stuartii PR50 expressing the 2′-N-acetyltransferase, suggesting that its true in vivo function is not as a resistance factor.
The gram-negative bacterium Providencia stuartii and other species of Providencia, Proteus, and Morganella comprise the Proteeae, and all members have been shown to O acetylate peptidoglycan (4). Peptidoglycan is a rigid cell wall heteropolymer comprised of repeating N-acetylglucosaminyl and N-acetylmuramyl residues with an attached tetrapeptide. The O acetylation of peptidoglycan occurs at the C-6 hydroxyl group of muramyl residues, at levels ranging from 20 to 70% (recently reviewed in reference 5). This modification to peptidoglycan confers resistance to muramidases and has also been proposed to modulate the activity of endogenous peptidoglycan hydrolases, including the autolysins (11). Other important pathogens that modify their peptidoglycan in this manner include Staphylococcus aureus and Neisseria gonorrhoeae (11). Little is known about the pathway for the O acetylation of peptidoglycan, but studies with P. stuartii suggest that a previously identified aminoglycoside acetyltransferase may contribute to the process (9–11).
The aminoglycoside acetyltransferases are comprised of four classes of enzymes, designated AAC(1), AAC(2′), AAC(3), and AAC(6′), according to the site of acetylation of the deoxystreptamine core of the aminoglycoside antibiotic (16). These enzymes are common among both gram-negative and gram-positive bacteria. Most of the genes encoding these enzymes are plasmid borne, but an exception to this general rule is the gentamicin 2′-N-acetyltransferase [AAC(2′)-Ia] from P. stuartii, which is chromosomally encoded. In wild-type P. stuartii, the aac(2′)-Ia gene is normally expressed at low levels (15), and it is regulated in part by a small transcriptional activator, AarP, and at least two other trans-acting negative regulators (14, 15).
Mutant strains of P. stuartii that either under- or overexpress AAC(2′)-Ia have correspondingly lower or higher levels of peptidoglycan O acetylation, in addition to altered MICs of aminoglycoside antibiotics (10, 11). These changes to the levels of O acetylation have been shown to result in altered cell morphologies. Further in vitro characterization of the enzyme suggested that it could use peptidoglycan metabolites, in addition to acetyl coenzyme A (acetyl-CoA), for the acetylation of both aminoglycosides and peptidoglycan (9). We have recently proposed that AAC(2′)-Ia transfers acetate to peptidoglycan from an integral membrane protein which serves to translocate it from cytoplasmic pools of acetyl-CoA to the outer surface of the cytoplasmic membrane (5).
To provide sufficient quantities of AAC(2′)-Ia for detailed enzymatic studies and delineation of its role in the pathway for peptidoglycan O acetylation, we have cloned its gene into a T7-based overexpression vector. The enzyme was purified to homogeneity, and its Michaelis-Menten parameters with different aminoglycoside substrates and pH and temperature optima were determined. Analysis of these kinetic parameters provided further support for an additional function of AAC(2′)-Ia in P. stuartii besides conferring aminoglycoside resistance.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains and plasmids used in this study are listed in Table 1. Escherichia coli DH5α and E. coli BL21 were maintained either in Luria-Bertani (LB) broth or on LB agar, which was supplemented with 30 μg of kanamycin sulfate per ml in the case of pET30a(+)-containing strains (Novagen). All cultures were grown at 37°C with aeration, unless otherwise stated.
TABLE 1.
Strains and plasmids used for this study
| Plasmid or strain | Description | Reference or source |
|---|---|---|
| Plasmids | ||
| pSCH4500 | pBluescript II SK(−) containing 1.2-kb fragment encoding AAC(2′)-Ia | 14 |
| pACKF1 | pBAD24 + aac(2′)-Ia | This study |
| pACKF2 | pET30a(+) + aac(2′)-Ia | This study |
| pACKF3 | pUCP26 + aac(2′)-Ia | This study |
| P. stuartii PR50 | 14 | |
| E. coli | ||
| DH5α | F−recA1 endA1 hsdR17 (rK− mK+) supE44 λ−thi-1 gyrA relA1 φ80d lacZΔM15Δ (lacZYA/argF)U169 | Life Technologies (Burlington, Ontario, Canada) |
| BL21(DE3) | F−ompT gal dcm lon hsdSB (rB− mB−; an E. coli B strain) with DE3, a λ prophage carrying the T7 RNA polymerase gene | Stratagene (La Jolla, Calif.) |
| AJC102 | E. coli DH5α + pACKF1 | This study |
| AJC103 | E. coli BL21(λDE3) + pACKF1 | This study |
| AJC104 | E. coli DH5α + pACKF2 | This study |
| AJC105 | E. coli BL21(λDE3) + pACKF2 | This study |
Enzyme and biochemicals.
Tryptone, LB broth and agar, yeast extract, and Bacto agar were purchased from Difco Laboratories (Detroit, Mich.). Tobramycin was purchased from ICN Biomedical (Costa Mesa, Calif.), while all other aminoglycosides, enterokinase, and reagents were purchased from Sigma (St. Louis, Mo.). Gentamicin was supplied as a mixture of gentamicins C1 (<45%), C1a (<35%), and C2 (<30%). Ni2+-nitrilotriacetic acid (Ni2+-NTA) agarose was obtained from Qiagen (Mississauga, Ontario, Canada), and Superdex 75 was from Amersham Pharmacia Biotech (Baie d'Urfé, Quebec, Canada). Restriction enzymes were purchased from New England Biolabs (Mississauga, Ontario, Canada), while all other DNA-modifying enzymes were supplied by Roche Diagnostics (Laval, Quebec, Canada).
Construction of AAC(2′)-Ia overexpression plasmid pACKF2.
The aac(2′)-Ia gene, originally obtained from pSCH4500 (15) (kindly provided by P. Rather), was subcloned from pUCP26 (provided by L. Burrows) into pBAD24 (pACKF1). Induction trials with pACKF1 revealed poor protein expression, and hence an alternative clone was constructed. Using pACKF1 as a template, aac(2′)-Ia was amplified using primers 5′-GAGGAATTCACCATGGGCATAGAATAC-3′ and 5′-TAGAGGATCGAATTCTGATTACCACTG-3′, which introduced unique NcoI and EcoRI restriction sites (underlined), respectively, to facilitate cloning. The gene was amplified with Pwo DNA polymerase (Roche) by 30 cycles consisting of 3 min at 94°C, 45 s at 94°C, 30 s at 60°C, 45 s at 72°C, and 7 min at 72°C. The 512-bp fragment was isolated using the QIAquick Gel Extraction Kit (Qiagen), digested with NcoI and EcoRI (New England Biolabs) according to the manufacturer's instructions, and then ligated into plasmid pET30a(+) to generate pACKF2, encoding an N-terminal poly-His-tagged AAC(2′)-Ia. Potential clones were initially screened in transformed E. coli DH5α, and then pACKF2 was transferred into E. coli BL21(λDE3) (AJC105) for subsequent expression. Confirmation of the presence of the complete aac(2′)-Ia insert in the construct was obtained by DNA sequencing (Laboratory Services Division, University of Guelph).
Overexpression of AAC(2′).
LB broth (500 ml) supplemented with 30 μg of kanamycin per ml was inoculated with a sample of a 16-h culture of freshly transformed AJC105 and grown at 37°C in a rotary shaker at 250 rpm until the optical density at 600 nm reached 0.6. Sterile isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 1 mM. Cells were allowed to grow for an additional 2 h prior to harvesting by centrifugation at 5,000 × g for 15 min.
Purification of AAC(2′)-Ia.
The cell pellet was resuspended in 20 ml of lysis buffer (50 mM NaH2PO4, 300 mM NaCl, and 10 mM imidazole) in the presence of complete EDTA-free protease inhibitor cocktail tablets (Roche), 10 μg of RNase per ml, and 5 μg of DNase per ml. Cells were disrupted using a sonicator ultrasonic liquid processor (Heat Systems Inc., Toronto, Ontario, Canada) set at 50% maximal output (5 s on, 5 s off) for 5 min (total time). The lysate was subjected to centrifugation (5,000 × g at 4°C) for 15 min to remove cellular debris, and the cleared supernatants containing the hexa-His-tagged AAC(2′)-Ia [His-AAC(2′)-Ia] were mixed with Ni2+-NTA agarose and incubated with shaking for 1 h at 4°C. The slurry was then poured into a 10- by 1-cm disposable column and washed with 8 column volumes of the wash buffer (50 mM NaH2PO4, 300 mM NaCl, and 20 mM imidazole). The enzyme was eluted with the addition of 250 mM imidazole to the wash buffer.
For desalting and further purification, the isolated AAC(2′)-Ia was applied to a Superdex 75 column that was equilibrated in 10 mM ammonium acetate at a flow rate of 1.0 ml/min. The eluted enzyme was determined to be homogeneous by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis, and fractions were found to be stable at 4°C for approximately 2 weeks. This procedure typically yielded 31 mg of homogeneous enzyme per liter of cell culture.
Enterokinase digestion.
Samples (0.53 nmol) of purified His-AAC(2′)-Ia in 100 μl of 10 mM ammonium acetate (pH 6.0) were digested with 1 μg (16.5 U) of enterokinase for 16 h at 20°C. The released hexa-His tag was removed by adsorption to 10 μl of Ni2+-NTA agarose at 4°C, and the digested enzyme was recovered from the supernatant after centrifugation at 13,000 × g.
Determination of enzyme activity.
Routine assays for His-AAC(2′)-Ia activity were conducted using the phosphocellulose binding assay (2) with 200 μM gentamicin and 160 μM [3H]acetyl-CoA (4 μCi/μmol) as substrates. A microtiter plate assay was used for the kinetic analysis of His-AAC(2′)-Ia, monitoring in situ the time course of free CoA release from 80 μM acetyl-CoA by titration with 2 mM 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) (19) with 1 to 235 μM tobramycin as a substrate in 25 mM 2-(N-morpholino)ethanesulfonic acid (MES) (pH 6.0) buffer containing 1 mM EDTA (200-μl final volume) at 25°C. Reaction mixtures without the addition of enzyme served as controls for the slow spontaneous hydrolysis of DTNB.
pH and temperature optima.
Enzyme stability studies were performed by incubating the enzyme preparations [His-AAC(2′)-Ia and AAC(2′)-Ia following enterokinase digestion] at the desired temperature and pH for 90 min prior to determining residual activity against 64 μM tobramycin in 50 mM MES (pH 6.0) at 20°C using the microtiter plate assay. The buffers used for the pH studies were 50 mM sodium acetate, pH 3.7 to 5.7; 50 mM MES, pH 5.1 to 7.1; 50 mM MOPS (morpholinepropanesulfonic acid), pH 6.1 to 8.1; and 50 mM Tris-HCl, pH 7.1 to 9.1. The optimal temperature for activity was determined by preincubating the substrate mixture at the desired temperature for at least 10 min prior to addition of enzyme.
Enzyme kinetics.
The microtiter plate assay was used for the kinetic analysis of His-AAC(2′)-Ia. Various concentrations of kanamycin sulfate, gentamicin sulfate, tobramycin, dibekacin, neomycin sulfate, butirosin, and netilimicin (Fig. 1) were added to the DTNB assay mixture as described above, and reactions were initiated by the addition of 200 nM purified enzyme (final concentration). The concentration of acetyl-CoA was held at 80 μM for the analysis of the different aminoglycosides, while 64 μM tobramycin was used as a cosubstrate for the determination of the kinetic parameters for acetyl-CoA. DTNB was replaced by 4,4′-dithiodipyridine and monitoring was at 324 nm (extinction coefficient, 19,800 M−1 cm−1) for assays with kanamycin due to the presence of interfering contaminants in the antibiotic preparation. The Michaelis-Menten parameters were obtained by graphing the initial velocity data using Enzfitter (Biosoft, Cambridge, United Kingdom).
FIG. 1.
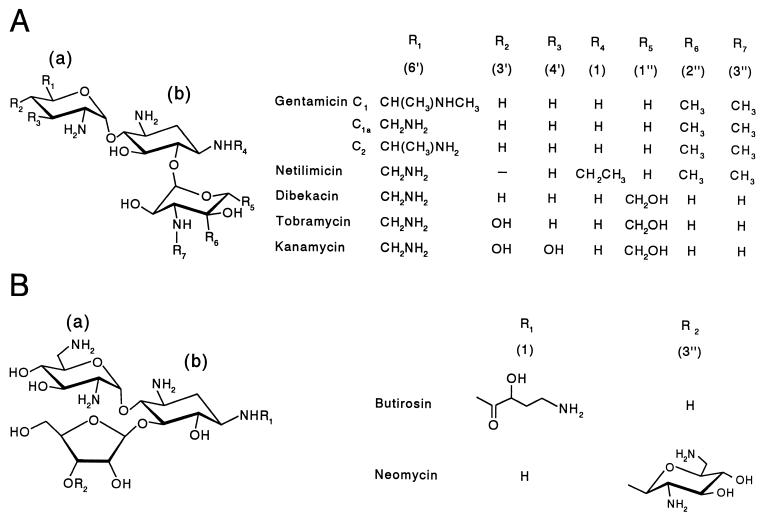
Structures of the 4,6-disubstituted (A) and 4,5-disubstituted (B) 2-deoxystreptamine-based aminoglycoside antibiotics used. (a) and (b), hexopyranosyl and 2-deoxystreptamine residues, respectively.
Miscellaneous methods.
MICs of the different aminoglycosides used in this study against P. stuartii PR50 were determined in LB broth containing twofold dilutions of the antibiotics between 0.1 and 128 μg/ml. SDS-PAGE was conducted using the method of Lamella (7), while protein concentrations were determined by the method of Smith (17) using the micro-bicinchoninic acid protein assay reagent (BCA; Pierce, Rockford, Ill.).
RESULTS AND DISCUSSION
Subcloning and expression of aac(2′)-Ia
Originally the aac(2′)-Ia gene was subcloned from pSCH4500 (15) into pBAD24 for its overexpression. However, expression trials of transformed E. coli BL21(DE3) giving E. coli AJC103 revealed that low and insufficient levels of enzyme were being produced for the purposes of this and future studies. Therefore, aac(2′)-Ia was subcloned into pET30a(+), an overexpression vector under the control of the strong IPTG-inducible T7 promoter, to give pACKF2. This construct consisted of the open reading frame encoding AAC(2′)-Ia with an N-terminal hexahistidine tag, which aided in its subsequent purification. Sequencing of pACKF2 confirmed the presence of the complete aac(2′)-Ia gene in pET30a(+) (data not shown).
E. coli BL21(λDE3) was transformed with pACKF2 to provide strain AJC105. The MIC of gentamicin against AJC105 was determined to confirm both the expression and activity of His-AAC(2′)-Ia. Strain AJC105 required an MIC of 16 μg of gentamicin per ml, which was 64 times higher than that for the parent E. coli strain harboring only pET30a(+) (0.25 μg/ml) and four times higher than that for the original E. coli transformant, strain DH5α/pSCH4500 (4 μg/ml) (15), from which we obtained the aac(2′)-Ia gene. The overexpression and activity of His-AAC(2′)-Ia were also confirmed using the phosphocellulose binding assay (data not shown).
Production and isolation of AAC(2′)-Ia.
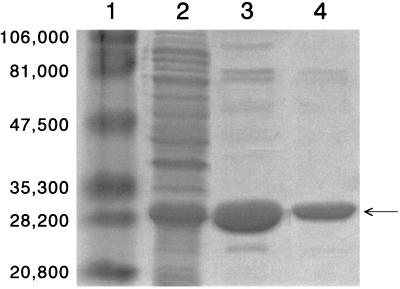
After induction of E. coli AJC105 with IPTG, 379 mg of total protein per liter of cell culture supernatant was detected (Table 2). Initial purification using Ni2+-NTA agarose to bind the His-tagged protein resulted in the isolation of a total of 41 mg of protein. Gel exclusion chromatography on Superdex was then used to further purify the enzyme of contaminating proteins which nonspecifically adsorbed to the affinity matrix and to remove the imidazole from the samples. This final gel permeation step yielded 31 mg of protein judged to be approximately 98% homogeneous by SDS-PAGE (Fig. 2). Based on the increase in specific activity using the microtiter plate assay, a ninefold purification was achieved, with a total recovery of greater than 70% of the original activity.
TABLE 2.
Purification of AAC(2′) from a 1-liter culture of E. coli AJC105
| Fraction | Total protein (mg) | Total activity (U)a | Sp act (U/mg) | Yield (%) | Purification factor |
|---|---|---|---|---|---|
| Crude extract | 379 | 1.08 × 105 | 285 | 100 | 1 |
| NTA-agarose | 40.9 | 1.07 × 105 | 2,610 | 98.7 | 9.15 |
| Superdex | 31.1 | 7.70 × 104 | 2,480 | 71.2 | 8.45 |
Units are expressed as micromoles of CoA-SH released per second from acetyl-CoA with tobramycin as a substrate in 25 mM MES buffer (pH 6.0) at 20°C.
FIG. 2.
Analysis of His-AAC(2′)-Ia purification by SDS-PAGE. Lanes: 1, high-molecular-weight markers (molecular weights are indicated on the left); 2, crude cell sonicate; 3, eluent from Ni2+-NTA agarose; 4; eluent from Superdex 75. The arrow indicates His-AAC(2′)-Ia.
The estimated molecular mass of the His-AAC(2′)-Ia was 27,900 Da (Fig. 2), which closely compares to the theoretical value of 24,944 Da calculated from its gene sequence. Whereas the partial purification of P. stuartii AAC(2′)-Ia has been previously reported (21), no specific activity was provided, making a comparison with the value obtained in the present study impossible. Nonetheless, purification of His-AAC(2′)-Ia did result in a ninefold increase in specific activity, as noted above. Moreover, the overall catalytic efficiency of the enzyme preparation acting on different substrates was significantly higher than that reported for either the AAC(2′) enzymes from Mycobacterium species (6) or the AAC(6′)-Ii from Enterococcus faecium (20), as discussed below. These data thus suggest that the presence of the N-terminal His tag does not impair the catalytic activity of His-AAC(2′)-Ia under the conditions employed. This was confirmed by obtaining the same specific activity with AAC(2′)-Ia after complete release of the N-terminal His tag by enterokinase digestion.
Temperature dependence of His-AAC(2′)-Ia.
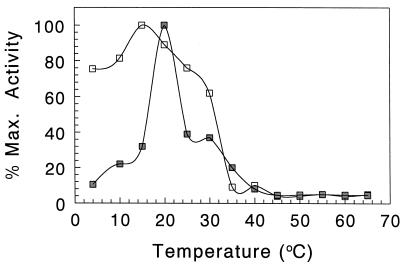
The purified enzyme was assayed for activity against tobramycin in 25 mM ammonium acetate buffer (pH 5.0) at different temperatures ranging from 5 to 65°C. Surprisingly, the enzyme appeared to be relatively temperature sensitive, as optimal activity was observed at 20°C (Fig. 3). The activity sharply declined on either side of this temperature optimum, and less than 10% of maximal activity was detected at temperatures greater than 40°C. In keeping with these results, His-AAC(2′)-Ia was found to also be thermolabile. After incubation of the enzyme at pH 5 for 1 h at different temperatures and then assaying for residual activity at 20°C, maximal activity was found to be maintained at 15°C, with only approximately 60% being retained at 30°C (Fig. 3). The activity of His-AAC(2′)-Ia dramatically declined to less than 10% at higher temperatures. These experiments were repeated with AAC(2′)-Ia, prepared by enterokinase digestion of His-AAC(2′)-Ia, and identical results were obtained (data not shown).
FIG. 3.
Temperature dependence of His-AAC(2′)-Ia on activity (▪) and stability (□). For activity determinations, enzyme (200 nM final concentration) was assayed against 64 μM tobramycin in 50 mM MES (pH 6.0) at the indicated temperatures. For stability studies, enzyme (200 nM) in the same buffer was incubated at the different temperatures for 90 min prior to being assayed against 64 μM tobramycin at 20°C.
SDS-PAGE analysis was conducted on enzyme samples following the temperature dependence experiments to determine if a contaminating protease that became increasingly active with elevated temperature was responsible for the loss of His-AAC(2′)-Ia activity. No degradation was observed in any of the samples (data not shown), and His-AAC(2′)-Ia remained intact throughout the experiment. As AAC(2′)-Ia is predicted to be a peripheral membrane protein (9), attempts were made to stabilize His-AAC(2′)-Ia by incubation in the presence of either 0.1% Triton X-100 or 10% glycerol. However, neither reagent had any effect on the temperature stability of the enzyme under the conditions employed.
Unfortunately, the effect of temperature on other purified aminoglycoside acetyltransferases in vitro has not been investigated, and so it is not known whether our findings are unique to His-AAC(2′)-Ia or reflect a general trend among these enzymes. However, a previous study on a partially purified preparation of the P. stuartii AAC(2′) indicated a higher temperature optimum of 45°C, with declining activity above 50°C (21). Hence, while there are many reasons for protein instability, it is possible that unfolding of the AAC(2′)-Ia occurs in dilute solution, as is observed with a variety of other enzymes, including β-lactamase (see, e.g., reference 8). The presence of the N-terminal His tag was not considered to be responsible for the destabilization because its complete removal by enterokinase digestion did not alter the observed temperature dependence.
pH dependence of His-AAC(2′)-Ia.
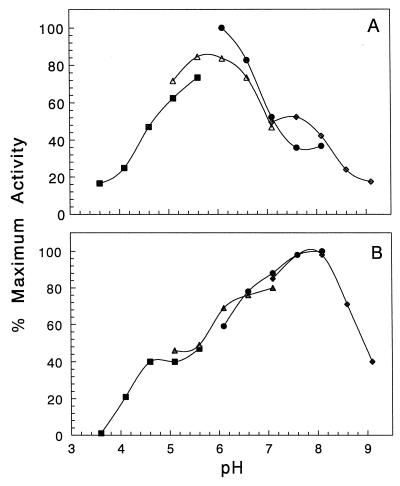
Incubation of His-AAC(2′)-Ia at various pH values showed that the enzyme was most stable at pH 8.1 (Fig. 4). The enzyme retained at least 60% of its maximal activity between pH 6.1 and 8.6, but incubation beyond these pH values rendered the enzyme virtually inactive.
FIG. 4.
pH dependence of His-AAC(2′)-Ia on activity (A) and stability (B). Buffers used were 50 mM sodium acetate, pH 3.7 to 5.7 (▪); 50 mM MES, pH 5.1 to 7.1 (▴); 50 mM MOPS, pH 6.1 to 8.1 (●); and 50 mM Tris-HCl, pH 7.1 to 9.1 (⧫). (A) Enzyme (200 nM final concentration) was assayed against 64 μM tobramycin at 20°C at the pH values indicated using the microtiter plate assay. (B) Enzyme (200 nM) was incubated in the different buffers at 20°C for 90 min prior to being assayed against 64 μM tobramycin in 50 mM MES, pH 6.0.
The pH optimum of His-AAC(2′)-Ia using tobramycin as a substrate was 6.0 (Fig. 4). As previously seen with AAC(2′)-Ia and E. faecium AAC(6′)-Ii (3, 20), the plot of activity as a function of pH was bell-shaped, with greater than 50% of maximal activity between pH 4.7 and 7.0. However, at pH values below 4.7, the activity dropped to below 20%, and it was inapparent at pH 3.0 and below. Above pH values of 7.0, activity rapidly decreased, obtaining only 29% maximal velocity at pH 9.0.
Using partially purified AAC(2′)-Ia, Chevereau et al. (3) observed different pH optima with different substrates, but each was between pH 6.0 and 7.0. Such dependence on the substrate is not likely due to ionization of critical residues on the enzyme but probably reflects the ionization of the different free amines on the various aminoglycoside substrates. Indeed, Wright and Ladak (20) noted with AAC(6′) a complex relationship between the Michaelis-Menten parameters and pH, and they were unable to differentiate between ionizable groups on the enzyme and those of the kanamycin substrate. For this reason, further detailed characterization of the pH-activity relationship of His-AAC(2′)-Ia was not pursued.
Kinetic parameters of AAC(2′)-Ia.
As previously documented with crude preparations of P. stuartii AAC(2′)-Ia (15), His-AAC(2′)-Ia demonstrated a broad specificity toward substrates with an amino group at the 2′ position of aminoglycosides. Seven aminoglycosides were selected for kinetic analysis using the microtiter plate assay, based on free CoA-SH titration, of Williams and Northrop (19). The kinetic parameters of His-AAC(2′)-Ia for these different substrates were determined assuming one active site per monomer and are presented in Table 3. Using a fixed acetyl-CoA concentration of 80 μM, which was 10-fold higher than its determined Km, gentamicin was found to be the best aminoglycoside substrate with respect to catalytic efficiency (kcat/Km), whereas neomycin was the poorest. These differences appeared to result from greater differences in turnover number (kcat), which varied by almost 10-fold compared to affinity, as reflected by the Km values differing by less than 4-fold. This was somewhat surprising and is very difficult to rationalize in the absence of mechanistic details of the reaction pathway. Nonetheless, these data indicated that His-AAC(2′)-Ia was 1 and 2 orders of magnitude more efficient than the AAC(2′) enzymes from different Mycobacterium species (6) and E. faecium AAC(6′)-Ii, respectively. In general, however, none of the aminoglycosides tested were considered to be good substrates for the enzyme, as the efficiency constants for each were well below both the diffusion limit of 108 to 109 M−1 s−1.
TABLE 3.
Kinetic parameters of purified overexpressed His-AAC(2′)-Ia
| Substratea | Km (μM) | kcat (s−1) | kcat/Km (M−1 s−1) | MICb (μg/ml) |
|---|---|---|---|---|
| Gentamicin sulfate | 11.4 ± 3.02 | 23.6 ± 10.7 | 20.6 × 105 | 8 |
| Neomycin sulfate | 12.8 ± 4.40 | 2.98 ± 0.299 | 2.33 × 105 | 4 |
| Kanamycin sulfate | 17.1 ± 2.69 | 18.5 ± 5.48 | 10.9 × 105 | 32 |
| Butirosin | 19.2 ± 9.13 | 20.9 ± 3.18 | 10.9 × 105 | 64 |
| Dibekacin | 26.2 ± 13.4 | 19.1 ± 6.20 | 7.29 × 105 | 64 |
| Tobramycin | 39.3 ± 8.90 | 45.2 ± 12.6 | 11.5 × 105 | 16 |
| Netilimicin | 46.5 ± 15.9 | 26.0 ± 5.01 | 5.59 × 105 | 64 |
| Acetyl-CoA | 8.75 ± 1.26 | 4.3 ± 0.312 | 5.26 × 105 |
Acetyl-CoA concentrations were fixed at 80 μM for the analysis of the different aminoglycosides, while 65 μM tobramycin was used to determine the kinetic parameters for acetyl-CoA.
MICs determined using P. stuartii PR50.
In spite of the similar kinetic parameters obtained, some insight was gained with respect to the specificity of His-AAC(2′)-Ia to the different aminoglycosides used in this study (Fig. 1). Except for the unsaturation of the linkage between carbons 4′ and 5′ of its hex-4-enopyranosyl residue, netilimicin is similar in structure to gentamicins C1, C1a, and C2 (Fig. 1), which comprise the gentamicin preparation used in this study. This aminoglycoside proved to be one of the poorest for His-AAC(2′)-Ia, and it yielded the highest Km value. Thus, the more planar structure of the hex-4-enopyranosyl ring appears to lessen enzyme affinity as reflected by the fourfold increase in Km value compared to the gentamicins. That this difference can be attributed to the altered hexopyranose ring structure and not to the presence of an ethyl group on the 1-amino group of the 2-deoxystreptamine ring is suggested by the data provided with butirosin, which is also modified at this position (with 4-amino-2-hydroxybutyric acid) but gave a Km value similar to that of the gentamicins. The importance of the hexopyranosyl residue for His-AAC(2′)-Ia specificity is further illustrated with the data obtained for dibekacin. While having the same substitutions on both the hexopyranosyl and 2-deoxystreptamine residues, this aminoglycoside differs from the gentamicins only in the substitutions around its arabinosyl residue, and its Km value was only double that of the gentamicins. Finally, the only difference between tobramycin and dibekacin is the presence of a 3′ hydroxyl group on the hexopyranose of the former aminoglycoside, and this appears to cause a loss of apparent affinity. These data thus suggest that the hexopyranosyl residue provides the most important recognition and binding sites for His-AAC(2′)-Ia activity, while the enzyme exhibits greater tolerance further from this sugar. Indeed, compared to the other aminoglycosides, neomycin possess an additional aminosugar but far removed for the hexopyranose, and yet the Km value obtained with this antibiotic was almost the same as that for the gentamicins.
Correlation between kinetic parameters and MIC.
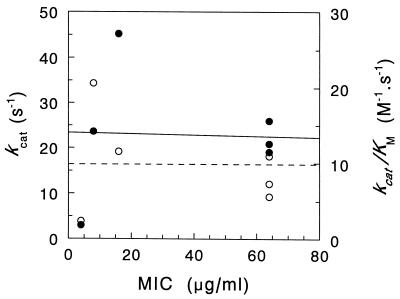
The finding that the aminoglycosides are relatively poor substrates for His-AAC(2′)-Ia is in keeping with our previous observations that AAC(2′)-Ia has a housekeeping function in P. stuartii, namely, the O acetylation of peptidoglycan (9–11), and that conferring resistance to aminoglycosides is simply a fortuitous (to the bacterium) side reaction (15). Likewise, it is suspected that AAC(6′)-Ii may play a physiological role in E. faecium, the nature of which is presently unknown (20). Both Piepersberg et al. (12) and Uduo et al. (18) had questioned over 10 years ago whether or not aminoglycoside acetyltransferase had a metabolic function, while Ho et al. (6) more recently went as far as to question whether AAC(2′) enzymes in Mycobacterium species play any significant role in conferring resistance to aminoglycosides, even though they are apparently universally present in the mycobacteria (1). This view is actually supported by an analysis of the correlation between MICs of the various aminoglycosides against P. stuartii and the kinetic parameters obtained with His-AAC(2′)-Ia. It has been argued that an efficient detoxifying enzyme should be maximally effective at low antibiotic concentrations (13), and such efficiency can be experimentally demonstrated by a positive correlation between antibiotic resistance of a bacterial stain (as reflected by MICs) and values of kcat/Km (the maximal rate at sub-Km substrate concentrations) obtained with the isolated putative resistance factor. Such was demonstrated with AAC(6′)-IV from E. coli W677 (13), but as depicted in Fig. 5, the aminoglycosides tested against P. stuartii PR50 and His-AAC(2′)-Ia did not exhibit this relationship. There appeared to be no correlation between the kinetic parameters kcat/Km and kcat and MICs of the different aminoglycosides against P. stuartii PR50. A similar situation was observed with ACC(6′)-Ii and aminoglycoside susceptibility with E. faecium (20). With the latter enzyme, however, a positive correlation was noted between MIC and kcat, the catalytic rate at saturating substrate concentrations, indicating that the cell would have to be overrun with antibiotic before an effective resistance was mounted by AAC(6′)-Ii, which would obviously be too late.
FIG. 5.
Relationship between kinetic parameters of His-AAC(2′)-Ia and MIC. MICs of the different aminoglycosides against P. stuartii PR50 were determined in LB broth and plotted as a function of kcat (●, solid line) (r = 0.1191) and kcat/Km (○, dashed line) (r = 0.009773).
Concluding remarks.
The O acetylation of peptidoglycan occurs as a maturation event, after the transglycosylation and transpeptidation of precursor subunits into the existing sacculus. This would suggest a periplasmic localization of AAC(2′)-Ia by the cell and hence its indirect use of acetyl-CoA as a cosubstrate. We have proposed that an intrinsic membrane protein serves to translocate acetate from cytoplasmic pools of acetyl-CoA to the external surface of the cytoplasmic membrane and make it available to the peptidoglycan O-acetyltransferase(s) (5). The availability of highly purified AAC(2′)-Ia will permit us to design experiments to test this hypothesis.
ACKNOWLEDGMENTS
We thank P. Rather and L. Burrows for the kind gifts of bacterial strains and plasmids.
These studies were supported by an operating grant to A.J.C. from the Medical Research Council.
REFERENCES
- 1.Aínsa J A, Pérez E, Pelicic V, Berthet F-X, Gicquel B, Martín C. Aminoglycoside 2′-N-acetyltransferase genes are universally present in mycobacteria: characterization of the aac(2′)-Ic gene from Mycobacterium tuberculosis and the aac(2′)-Id gene from Mycobacterium smegmatis. Mol Microbiol. 1997;24:431–441. doi: 10.1046/j.1365-2958.1997.3471717.x. [DOI] [PubMed] [Google Scholar]
- 2.Benveniste R, Davies J. Enzymatic acetylation of aminoglycoside antibiotics by Escherichia coli carrying an R factor. Biochemistry. 1971;10:1787–1796. doi: 10.1021/bi00786a009. [DOI] [PubMed] [Google Scholar]
- 3.Chevereau M, Daniels P J L, Davies J, LeGoffic F. Aminoglycoside resistance in bacteria mediated by gentamicin acetyltransferase II, an enzyme modifying the 2′-amino group of aminoglycoside antibiotics. J Biol Chem. 1974;13:598–603. doi: 10.1021/bi00700a030. [DOI] [PubMed] [Google Scholar]
- 4.Clarke A J. Extent of peptidoglycan O acetylation in the tribe Proteeae. J Bacteriol. 1993;175:4550–4553. doi: 10.1128/jb.175.14.4550-4553.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke A J, Strating H, Blackburn N T. Pathways for the O-acetylation of bacterial cell wall polysaccharides. In: Doyle R J, editor. Glycomicrobiology. New York, N.Y: Plenum Publishing Co.; 2000. pp. 187–223. [Google Scholar]
- 6.Ho I I Y, Chan C Y, Cheng A F B. Aminoglycoside resistance in Mycobacterium kansasii, Mycobacterium avium-M. intracellulare, and Mycobacterium fortuitum: are aminoglycoside-modifying enzymes responsible? Antimicrob Agents Chemother. 2000;44:39–42. doi: 10.1128/aac.44.1.39-42.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 8.Laraki N, Franceschini N, Rossolini G M, Santucci P, Meunier C, de Pauw E, Amicosante G, Frère J M, Galleni M. Biochemical characterization of the Pseudomonas aeruginosa 101/1477 metallo-β-lactamase IMP-1 produced by Escherichia coli. Antimicrob Agents Chemother. 1999;43:902–906. doi: 10.1128/aac.43.4.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Payie K G, Clarke A J. Characterization of gentamicin 2′-N-acetyltransferase from Providencia stuartii: its use of peptidoglycan metabolites for acetylation of both aminoglycosides and peptidoglycan J. Bacteriol. 1997;179:4106–4114. doi: 10.1128/jb.179.13.4106-4114.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Payie K, Rather P N, Clarke A J. Contribution of gentamicin 2′-N-acetyltransferase to the O acetylation of peptidoglycan in Providencia stuartii. J Bacteriol. 1995;177:4303–4310. doi: 10.1128/jb.177.15.4303-4310.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Payie K G, Strating H, Clarke A J. The role of O-acetylation in metabolism of peptidoglycan in Providencia stuartii. Microb Drug Resist. 1996;2:135–140. doi: 10.1089/mdr.1996.2.135. [DOI] [PubMed] [Google Scholar]
- 12.Piepersberg W, Distler J, Heinzel P, Perez-Gonzalez J-A. Antibiotic resistance by modification: many resistance genes could be derived from cellular control genes in actinomycetes—a hypothesis. Actinomycetologica. 1988;2:83–98. [Google Scholar]
- 13.Radika K, Northrop D B. Correlation of antibiotic resistance with Vmax/Km ratio of enzymatic modification of aminoglycosides by kanamycin acetyltransferase. Antimicrob Agents Chemother. 1984;25:479–482. doi: 10.1128/aac.25.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rather P N, Orosz E. Characterization of aarA, a pleiotrophic negative regulator of the 2′-N-acetyltransferase gene in Providencia stuartii. J Bacteriol. 1994;176:5140–5144. doi: 10.1128/jb.176.16.5140-5144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rather P N, Orosz E, Shaw K J, Hare R, Miller G. Characterization and transcriptional regulation of the 2′-N-acetyltransferase gene from Providencia stuartii. J Bacteriol. 1993;175:6492–6498. doi: 10.1128/jb.175.20.6492-6498.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw K J, Rather P N, Hare R S, Miller G. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev. 1993;57:138–163. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith P K. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 18.Uduo T, Mizuguchi Y, Wallace R J., Jr Does aminoglycoside-acetyltransferase in rapidly growing mycobacteria have a metabolic function in addition to aminoglycoside inactivation? FEMS Microbiol Lett. 1989;57:227–230. doi: 10.1111/j.1574-6968.1989.tb03304.x. [DOI] [PubMed] [Google Scholar]
- 19.Williams J W, Northrop D. Kinetic mechanism of gentamicin acetyltransferase I. J Biol Chem. 1978;253:5902–5907. [PubMed] [Google Scholar]
- 20.Wright G D, Ladak P. Overexpression and characterization of the chromosomal aminoglycoside 6′-N-acetyltransferase from Enterococcus faecium. Antimicrob Agents Chemother. 1997;41:956–960. doi: 10.1128/aac.41.5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamaguchi M, Mitsuhashi S, Kobayashi F, Zenda H. A 2′-N-acetylating enzyme of aminoglycosides. J Antibiot. 1974;27:507–515. doi: 10.7164/antibiotics.27.507. [DOI] [PubMed] [Google Scholar]