Abstract
Acoustic information transduced by cochlear hair cells is continuously relayed from the auditory pathway to other sensory, motor, emotional and cognitive centers in the central nervous system. Human epidemiological studies have suggested that hearing loss is a risk factor for dementia and cognitive decline, but the mechanisms contributing to these memory and cognitive impairments are poorly understood. To explore these issues in a controlled experimental setting, we exposed adult rats to a series of intense blast wave exposures that significantly reduced the neural output of the cochlea. Several weeks later, we used the Morris Water Maze test, a hippocampal-dependent memory task, to assess the ability of Blast Wave and Control rats to learn a spatial navigation task (memory acquisition) and to remember what they had learned (spatial memory retention) several weeks earlier. The elevated plus maze and open field arena were used to test for anxiety-like behaviors. Afterwards, hippocampal cell proliferation and neurogenesis were evaluated using bromodeoxyuridine (BrdU), doublecortin (DCX), and Neuronal Nuclei (NeuN) immunolabeling. The Blast Wave and Control rats learned the spatial navigation task equally well and showed no differences on tests of anxiety. However, the Blast Wave rats performed significantly worse on the spatial memory retention task, i.e., remembering where they had been two weeks earlier. Deficits on the spatial memory retention task were associated with significant decreases in hippocampal cell proliferation and neurogenesis. Our blast wave results are consistent with other experimental manipulations that link spatial memory retention deficits (long term memory) with decreased cell proliferation and neurogenesis in the hippocampus. These results add to the growing body of knowledge linking blast-induced cochlear hearing loss with the cognitive deficits often seen in combat personnel and provide mechanistic insights into these extra auditory disorders that could lead to therapeutic interventions.
Keywords: neurogenesis, memory, blast wave, hippocampus, doublecortin
1.0. Introduction
Exposure to intense noise has long been known to damage the sensory hair cells and neurons in the cochlea resulting in permanent hearing loss (Hamernik et al., 1984; Lin et al., 2011b). Although the primary site of damage is in the cochlea, there is growing recognition that noise-induced hearing loss causes structural and functional changes in the central auditory pathway (Baizer et al., 2015; Calford et al., 1993; Feng et al., 2012; Michler and Illing, 2002; Morest and Bohne, 1983; Robertson et al., 2013). These noise-induced disturbances in the central auditory system are believed to contribute to debilitating disorders such as tinnitus and hyperacusis (Eggermont, 2015; Mohan et al., 2018; Xu et al., 2016). The auditory system, however, does not function in isolation, but is part of a much larger neural connectome in which information is exchanged with other sensory, motor, emotional and cognitive centers in the brain (Chen et al., 2015; Kraus and Canlon, 2012; Lesicko et al., 2016; McIntosh and Gonzalez-Lima, 1998; Pascual et al., 2015; Xu et al., 2016; Yang et al., 2018). When viewed in this broader context, it may come as no surprise to learn that hearing loss is a risk factor for dementia and cognitive decline (Lin et al., 2011a; Liu and Lee, 2019). The link between hearing loss and cognitive decline is reinforced by intervention studies showing that hearing remediation with a cochlear implant leads to a significant improvement in cognitive function (Mosnier et al., 2015).
The hippocampus, which plays a critical role in memory and cognitive processing (Munoz-Lopez et al., 2010; Sweatt, 2004), is one of two regions in the adult brain where neurogenesis occurs (Altman and Das, 1965; Shors et al., 2001). Recent evidence suggests that neurogenesis in the granule cell layer of dentate gyrus plays a pivotal role in pattern separation and spatial navigation (Clelland et al., 2009; Goodman et al., 2010; Winocur et al., 2006). New granule cells in the dentate gyrus are integrated into hippocampal-cortical networks involved in the retrieval of remote spatial memories (Goodman et al., 2010). Granule cell neurons are spatially tuned and possess stable place fields (Jung and McNaughton, 1993).
Information from neurons in the dentate gyrus is relayed to the CA1 area of the dorsal hippocampus (Fontana et al., 2006; Gehrmann et al., 1991; McNaughton et al., 1989). Spatially-tuned pyramidal place cells in the dorsal hippocampus (Fenton et al., 2000; Hufner et al., 2011; Ravassard et al., 2013; Tamura et al., 1992a; Tamura et al., 1992b) receive inputs from grid cells in the entorhinal cortex (Moser et al., 2014). This hippocampal-entorhinal circuit has been implicated in memory-guided behaviors (Aronov et al., 2017). The spatial tuning of hippocampal place cells is not static, but can be altered by intense noise exposures that cause temporary hearing loss (Goble et al., 2009; Szczepaniak and Moller, 1996). These results suggest that the functional properties of hippocampal place cells might be permanently altered by hearing loss which could disrupt the formation or consolidation of long-term memory traces relevant to spatial recognition memory.
We previously reported that severe unilateral noise-induced hearing loss significantly reduced cell proliferation and neurogenesis in the hippocampus (Kraus et al., 2010). Subsequently, others reported that bilateral noise-induced hearing loss also suppresses cell proliferation and neurogenesis in the hippocampus (Liu et al., 2018; Shukla et al., 2019). Additionally, when their subjects were tested on the Morris Water Maze (MWM), rat and mice with noise-induced hearing loss had significantly more difficulty learning the spatial navigation task (memory acquisition) and as well as remembering the task (memory consolidation). These deficits were correlated with decreased cell proliferation and neurogenesis.
Combat personnel exposed to blast waves not only suffer from hearing loss (Helfer et al., 2011), but also memory deficits (Cooper et al., 2016; Gilbertson et al., 2001; McDermott et al., 2016). Among noise-exposed combat personnel, lower memory scores were correlated with a decline in hippocampal volume (Tischler et al., 2006). However, it is unclear if the memory deficits were due to noise-induced hearing loss or other comorbid factors. To overcome these limitations, we exposed rats to a series of 185 dB peak SPL blasts which we previously have shown causes a severe hair cell loss in the basal third and a moderate hair cell lesion (<30% loss) in the apex of the cochlea (<20 kHz) plus a 35% reduction hippocampal neurogenesis (Newman et al., 2015). This intensity was used because it is similar to those experienced by military personnel operating howitzers, rocket launchers and some rifles (Garinther, 1979; Jokel et al., 2019), but below the intensity that causes the tympanic membrane to rupture in rats. Afterwards, we tested for memory deficits, anxiety and changes in hippocampal cell proliferation and neurogenesis. Among the rats with blast-induced hearing loss, there was a significant reduction of cell proliferation and neurogenesis and a significant deficit in spatial memory consolidation. However, the blast-induced hearing loss did not impair short-term spatial memory acquisition or induce anxiety.
2.0. Methods
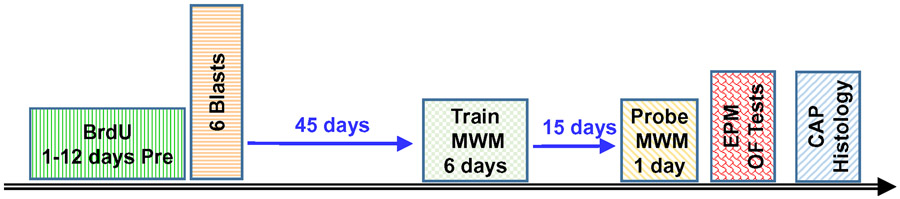
The experimental time line for conducting different components of the study are schematized in Figure 1. Bromodeoxyuridine (BrdU) was administered for 12 consecutive days prior to the blast wave exposure or sham exposure; the goal was to determine if the blast wave exposure would affect the long-term survival of new cells generated prior to the exposure. Forty-five days after the blast wave exposure, the rats were trained for 6-days on the MWM task to assess their ability to learn a spatial navigation task in which they learned the position of a hidden platform in the MWM (i.e., spatial memory acquisition). Fifteen days later, the rats were given a single probe trial with the hidden platform removed from the maze; the purpose of the probe trial was to assess spatial memory retention. A few days later, the rats were evaluated on the elevated plus maze (EPM) and open field (OF) test to assess anxiety. Several days later, the CAP was recorded to determine the degree to which the blast wave exposure suppressed the neural output of the cochlea. Immediately afterwards the anesthetized rats were perfused intracardially with fixative and the brain harvested for histological analysis of the hippocampus.
Figure 1:
Experimental time line. BrdU administered 12-days before exposure to 6-blasts of 185 dB peak SPL. Forty-five days after the blast wave exposure rats were trained for 6-days on the MWM memory acquisition task followed 15-day later by a single probe trial on the memory retention task. A few day later, rats were evaluated with the EPM and OF tests. Several days later, the CAP was measured and immediately afterwards, the brain was perfused with fixative and subsequently processed for histological analysis of the hippocampus.
2.1. Animals:
Eighteen 3-4 month old, male SASCO Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were used for this study; 14 served as sham controls and the remainder were blast wave exposed. The experimental procedures used in this study were approved by The University at Buffalo Institutional Animal Care and Use Committee.
2.2. Blast wave exposure:
The equipment and methods used for the blast wave exposures have been described in our earlier publication (Newman et al., 2015). Each rat was anesthetized with a ketamine/xylazine mixture (50 mg/kg/6 mg/kg, intraperitoneal, (i.p.)) and placed in a wire-mesh cage 5 cm in front of the opening of the blast tube with the rat facing the opening of the blast generator. Each animal was subjected to six blast waves; one blast every five-minutes. The average blast peak pressure level was 185 dB peak SPL (+/− 0.6 dB SEM; 50.4 kPa). Age-matched sham controls were treated in the identical fashion except that they were not subjected to any blast wave exposure.
2.3. Hearing assessment:
The compound action potential (CAP), which reflects the neural output of the cochlea, was used to assess the degree of hearing impairment as described in our previous reports (Chen et al., 2010; Sheppard et al., 2017). Rats were anesthetized with a ketamine/xylazine cocktail (50/6 mg/kg, i.p.) and body temperature was maintained at 37 °C using a homoeothermic blanket (Harvard Apparatus, Holliston, MA). The animal’s head was placed in a custom head holder. The ventral surface of the right bulla was surgically exposed and a small opening made in the bulla to visualize the round window membrane. A ring electrode constructed from gold Teflon coated wire was placed on the round window and a silver chloride reference electrode was inserted into the neck muscle. The output of the electrodes was amplified 1000X, (Model P15; Grass Instruments), filtered (0.1–3.0 kHz) and fed to a 16 bit A/D converter (Tucker Davis Technologies, RP2.1 real-time processor) connected to a personal computer.
The CAP was elicited with tone bursts (6, 8, 12, 16, 24 and 40 kHz, 10 ms duration, 1 ms rise/fall time, cosine gated) generated with Tucker Davis Technologies hardware (RP2.1, System 3). The electrical signal was led to a programmable attenuator (TDT PA5) followed by a custom power amplifier and fed to an ACO ½” condenser microphone driven in reverse that served as the loudspeaker. The sound source was housed in a custom ear bar that was inserted into the rat’s ear canal to deliver the sound close to the tympanic membrane. Sound intensities generated by the speaker were calibrated with a coupler approximating the volume of the rat’s ear canal. Stimulus intensity was decreased by 10 dB SPL steps from 80 dB SPL to 0 dB SPL. The neural response from the electrodes was digitized over a 20 ms interval. Using custom software, the CAP was isolated from other components of the neural response by using a 3-kHz low-pass filter. The difference in amplitude between the N1 and P1 components of the CAP was used to quantify the CAP amplitude. CAP threshold was defined as the stimulus intensity that elicited a response of 5 μV. Between-group differences in CAP I/O functions were tested for significance using a repeated measure (intensity) two-way analysis of variance (ANOVA). Between group differences in CAP thresholds were tested for significance using a one-way ANOVA (Prism GraphPad or SigmaStat Software).
2.4. Hippocampus histology:
Brain tissue was collected 11 weeks after the blast wave exposure using procedures described in detail in our earlier publications (Manohar et al., 2012; Newman et al., 2015). Rats were anesthetized with a lethal dose of Fatal Plus (86 mg/kg; IP, Vortech Pharmaceuticals) and transcardially perfused first with 0.1 M phosphate-buffered saline (PBS) and then with 10% phosphate buffered formalin. The brains were carefully removed and post-fixed for one week in 10% formalin at 4 °C, followed by cryoprotection with 30% sucrose in Tris-buffered saline (TBS) for 24 h. Afterwards, the brain was sectioned at a thickness of 40 mM with a cryostat in the coronal plane to obtain sections along the rostral-caudal axis of the hippocampus. Sections were stored in a solution of 30% ethylene glycol and 30% glycerol in 0.1 M PBS at −20 °C.
DCX:
Brain sections were immunolabeled with doublecortin (DCX), a microtubule-associated protein (Santa Cruz Biotechnology, Dallas, TX) transiently expressed in newborn neurons for 2-3 weeks following neurogenesis (Brown et al., 2003). As described in our earlier publications (Manohar et al., 2011; Paolone et al., 2011), DCX was visualized by first incubating brain sections overnight at 4 °C with an anti-DCX primary antibody (goat, polyclonal, sc-8066, Santa Cruz, 1:500). DCX was visualized by incubation with an appropriate biotinylated secondary antibody (Vector Laboratories, Burlingame, CA). Sections were then processed with the avidin-biotin-peroxidase complex technique followed by the glucose oxidase modification of the diaminobenzidine tetrahydrochloride (DAB) method. After DAB processing, sections were mounted on slides, dehydrated in ethanol, cleared in xylene and sealed with Depex (Electron Microscopy Sciences, Hatfield, PA). Darkly labeled DCX-positive somas were counted along the length of the subgranular zone (SGZ) in each dentate gyrus using a light microscope (400X). DCX counts were obtained from both the left and right side of the hippocampus of each section. The length of the SGZ was measured utilizing ImageJ software. DCX data were quantified by dividing the number of DCX-labeled soma by the length of the SGZ (cells/mm as a standard measure) as described in our earlier publication (Kraus et al., 2010). DCX counts were obtained from every third section; 50 sections were evaluated from each rat. The mean value from the 50 sections constituted a single measure for each rat in the Control group (n=6) and the Blast Wave group (n=5).
2.5. BrdU and NeuN:
To identify cells in the hippocampus that had undergone cell division, rats were treated with BrdU, a thymidine analogue incorporated into the DNA of dividing cells during the S-phase of cell division. Blast wave (n=3) and sham control rats (n=3) were injected with 50 mg/kg of BrdU (Sigma, B5002, IP) for 12 consecutive days immediately prior to the blast or sham exposure. Brain sections obtained from these animals were double-labeled using a mouse antibody against BrdU (Developmental Studies Hybridoma Bank, #G3G4, deposited by Kaufman, S.J.) to cells that had undergone cell division and a rabbit monoclonal antibody against NeuN (Cell Signaling Technology, #D3S3I) to identify those neurons. Sections were incubated in blocking solution [Tris-buffered saline (TBS), 1% normal horse serum, 1% bovine serum albumin (BSA) and 0.1% Triton X-100 (T-X)] for 30 min. Afterwards, the sections were incubated overnight at 4 °C followed by three rinses with 0.1% TBS. To visualize the mouse BrdU primary antibody and rabbit NeuN primary antibody, the sections were incubated for 2 h at room temperature with a combination of donkey anti-mouse secondary antibody conjugated to Alexa Fluor 488 (1:1000) and donkey anti-rabbit Alexa Fluor 568 (1:1000). The secondary antibodies were prepared in a solution containing 1% normal horse serum, 1% BSA and 0.1% Triton X-100. Afterwards, the hippocampal sections were washed three time in 0.1 M PBS, mounted and cover-slipped on glass slides with ProLong Antifade reagent (Invitrogen, Carlsbad, CA) and examined with a Zeiss Laser Scanning Microscope (LSM 510/Zeiss Axio-confocal Microscope). BrdU-positive cells and BrdU/NeuN co-labeled cells were counted along the length of the SGZ of the dentate gyrus as described previously (Kraus et al., 2010). SGZ length was measured using ImageJ software and data were expressed as number of labeled cells/mm of SGZ.
Counts of BrdU-positive cells and BrdU-positive cells colocalized with NeuN-positive cells were obtained from both the left and right side of the hippocampus of each section. The length of the SGZ was measured utilizing ImageJ software. Results were quantified by dividing the number of BrdU immunoreactive cells and BrdU-positive NeuN immunoreactive cells by the length of the SGZ (cells/mm as a standard measure) as described in our earlier publication (Kraus et al., 2010). NeuN and BrdU counts were obtained from every third section; 25 sections were evaluated from each rat. The mean value from the 25 sections constituted a data point for each rat in the Control group (n=3) and the Blast Wave group (n=3).
2.6. Morris Water Maze:
The Morris Water Maze (MWM), a well-established test of spatial navigation and memory, was used to determine how effective rats in the Control group (n=6) and Blast Wave group (n=6) were at learning where the hidden platform was located (memory acquisition) and how well they could remember the location of hidden platform (memory consolidation/retention (Brandeis et al., 1989; Morris, 1984; Snyder et al., 2005). The diameter and height of the MWM were 2 m and 60 cm, respectively. The pool was filled with water to a depth of 44 cm and water temperature was maintained at 26 °C (+/− 1 °C). The MWM was bisected into four equal quadrants designated northeast (NE), southeast (SE), northwest (NW) and southwest (SW). An escape platform (12.2 cm length, 12.2 cm width and 43.2 cm height) was placed in the middle of the NE quadrant of the pool; the surface of the hidden platform was submerged 2 cm below the water surface. The MWM was located in a room with distinct objects on the walls to provide visual cues that the rat could utilize to locate hidden platform. Testing for memory acquisition began 45-days post-treatment and continued for six consecutive days. Each animal was given eight acquisition trials per day. The rat was placed in a random quadrant (NE, SE, NW, SW; each quadrant occurring with equal frequency) with the rat’s head facing the pool wall. When the rat found the platform, it was allowed to remain on the platform for 10 seconds. However, if the rodent failed to locate the platform within 60 s, it was guided towards and placed on the platform by the experimenter. Each animal was dried off with a towel and allowed to rest five minutes between acquisition trials in a cage located beneath a heat lamp. The output of the video camera above the MWM was fed to AnyMaze tracking software, which was used to record the swim pattern of the rat on each trial. The software calculated the time (latency) to reach the hidden platform, distance traveled, speed, and path efficiency. The time (latency) required to reach the hidden platform was used to quantify how quickly the rat learned the location of the hidden platform.
Two weeks after completing the memory acquisition trials, each rat was tested for its ability to remember the location of hidden platform. Memory retention was assessed on a single probe trial in which the hidden platform was removed from the MWM. The rat was placed in the SW quadrant of the MWM with its head facing the pool wall. The rat was allowed to swim in search of the missing hidden platform. After 60 s, the rat was removed from the MWM, dried off with a towel and placed in a cage under a heat lamp. The video camera and AnyMaze tracking software were used to record the swim pattern of the rat. Spatial memory retention was quantified by measuring the amount of time the animal spent in each quadrant. If an animal remembered the location of the hidden platform, it would spend significantly more time in the quadrant where it had acquired a spatial memory of the hidden platform. However, if the rat failed to remember where the hidden platform had been located, then it would swim randomly about and spend roughly equal time in all four quadrants.
2.6. EPM and OF tests:
Following MWM testing, the rats in the Control group (n=6) and Blast Wave group (n=6) were evaluated on the EPM and the OF paradigm to test for group differences in generalized anxiety (Fig. 1) (Sidor et al., 2010; Vetter et al., 2002). The EPM consisted of two open arms (length: 50 cm, width: 10 cm) and two closed arms (length: 50 cm, width: 10 cm, height: 30 cm) with the open and closed arms were oriented opposite to one another. The EPM was positioned 50 cm above the floor. On each trial, the rat was placed at the end of a closed arm with its head facing into an opening at the intersection of the four arms. The rat was allowed to remain in the EPM for 10 minutes during which time an overhead video camera connected to AnyMaze software was used to count the entries and amount of time the rat spent in the open arms or closed arms.
After completing the EPM test, the rats in the Control group and Blast Wave group were evaluated using the OF test to determine if there were differences in anxiety and motor activity (Anchan et al., 2014). The circular, black, plastic OF arena had a radius of 1.4 m and the height of the circular wall was 60 cm. The video camera and AnyMaze software were used to optically define the inner zone and outer zone. The inner zone was defined as the area within 1.14 m of the center. The outer donut-shaped ring was defined as the area outside the circular inner zone, but within the circular border of the arena. Each rat was placed in the center of the circular arena and allowed to move about freely for 10 minutes. The camera connected to AnyMaze software was used to measure time spent in the inner zone and outer zone, time spent immobile versus moving about, average speed, and the total distance traveled.
3.0. Results
3.1. Hearing Impairment:
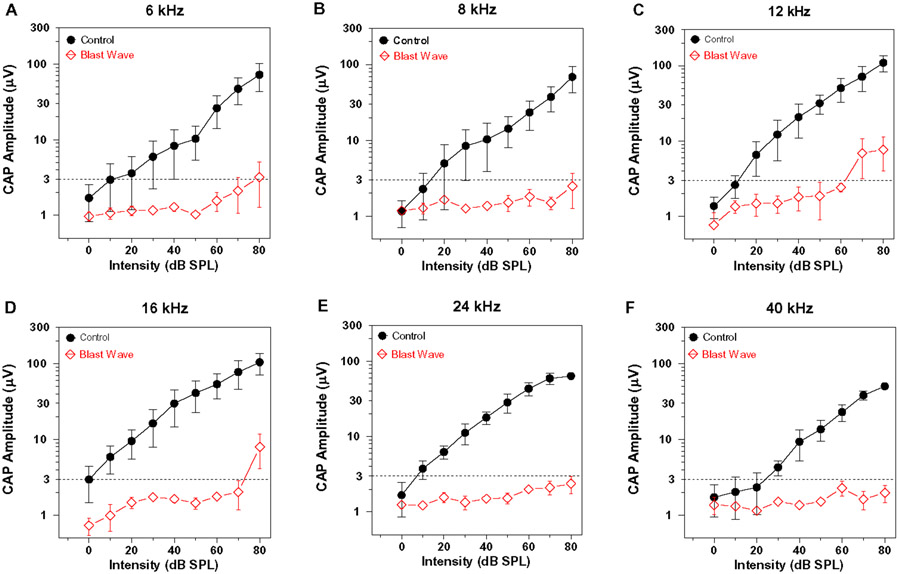
To confirm that the intense blast exposure caused a severe hearing loss similar to that observed in our earlier report (Newman et al., 2015), the CAP was evaluated in three rats in the Blast Wave group and compared to the CAP obtained from three rats in the Control group approximately 11-weeks post-exposure. The CAP input/output functions at 6, 8, 12, 16, 24 and 40 kHz are shown in Figure 2A-F. The horizontal dashed line at 3 μV was operational defined as the CAP threshold. In Controls, the thresholds ranged from 0-10 dB SPL except at 40 kHz where threshold increased to approximately 25 dB SPL. CAP amplitudes increased with stimulus intensity in the Control group; the maximum amplitude at 80 dB SPL ranged from approximately 70-100 μV. However, mean CAP amplitude in the Blast Wave group were extremely low and remained below the 3-μV threshold at 8, 24 and 40 kHz; thresholds responses of 80, 70 and 80 dB SPL were only achieved at 6, 12 and 16 kHz respectively. As expected, blast wave exposure at 185 dB peak SPL caused very severe hearing loss affecting virtually all frequencies consistent with our previous report (Newman et al., 2015).
Figure 2:
Blast wave exposure nearly abolishes the compound action potential (CAP). Mean (+/− SEM) CAP input/output functions recorded from Control group (n=3) and Blast Wave group (n=3). Data shown for (A) 6 kHz, (B) 8 kHz, (C) 12 kHz, (D) 16 kHz, (E) 24 kHz and (F) 40 kHz. Horizontal dashed line at 3 μV represents operational definition of threshold. Note very large reduction in CAP amplitudes in the Blast Wave group compared to the Control group.
3.2. Decreased DCX Immunolabeling:
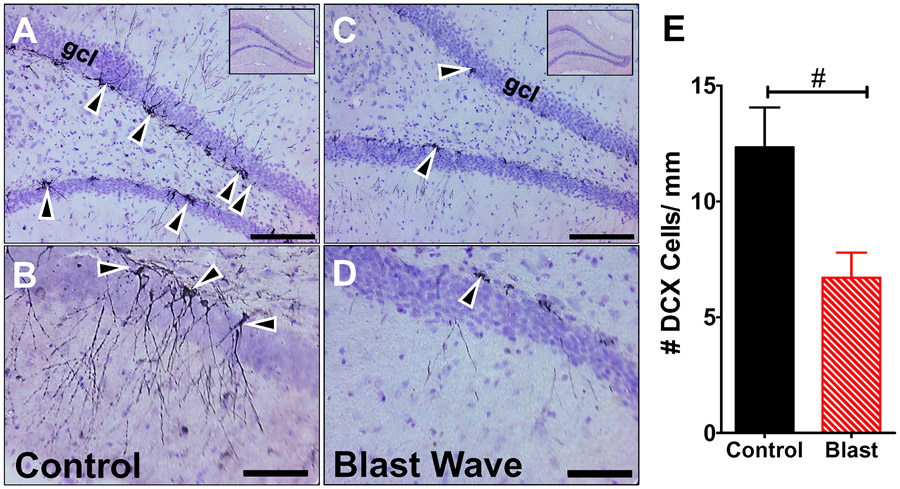
DCX, a microtubule-associated protein, is transiently expressed in newborn neurons (Brown et al., 2003). To determine if the DCX expression was affected by the blast wave exposure, we counted the numbers of DCX immunolabeled cells per mm length along the dentate gyrus in the hippocampus of rats in the Control group (n=6) and the Blast Wave group (n=5). Data were obtained approximately 11-weeks post-exposure. Figure 3A-B shows representative coronal sections from a Control rat. Many darkly-stained cells can be seen along the edge of the dentate gyrus (Fig. 3A). At a higher magnification, long, thin axonal and dendritic processes were evident extending out from the darkly-stained soma in Control animals. Figure 3C-D shows representative photomicrographs from a rat in the Blast Wave group. Few DCX-stained cells were seen along the dentate gyrus (Fig. 3C). Higher magnification views revealed a dearth of processes extending from the few DCX soma that were present (Fig. 3D); the few processes that were present were thinner and less ramified. The numbers of DCX cells per mm length of the dentate gyrus were determined bilaterally in 50 hippocampal sections from each Control rat (n=6) and Blast Wave rats (n=5). The mean (+SEM) data from the individual rats in each group are summarized in Figure 3E. Approximately 12.3 DCX cells per mm were present in Controls versus 6.7 DCX in the Blast Wave group; this difference was statistically significant (t-test, t = 2.64, 9 df, p =0.028).
Figure 3:
DCX immunolabeling is greatly reduced 11 weeks post-exposure in hippocampus of Blast Wave rats compared to Controls. (A) Representative photomicrographs of DCX immunolabeled coronal section from hippocampus of Control rats; inset shows low-magnification view of coronal sections of hippocampus. Arrowheads point to DCX labeled cells in the granule cell layer (gcl). (B) Higher magnification view of hippocampus from Control section showing long, thin, processes extending from a DCX-labeled soma. (C) Representative photomicrograph of coronal section of hippocampus from Blast Wave rats; inset shows low magnification view of hippocampus. Few DCX labeled cells (arrowheads) present in gcl layer of dentate gyrus. (D) High magnification view of dentate gyrus showing only a few DCX-labeled cells. Note the absence of processes extending from the soma (arrowhead). (Scale bars: upper row = 100 μm; lower row = 50 μm). (E) Histogram showing the mean (+SEM) number of DCX-labeled cells/mm length of the dentate gyrus in Control group (n=6) and Blast Wave group (n=5). Significantly fewer DCX cells per mm are observed in the Blast Wave group compare to the Control group. (t=2.755, 9 df, p<0.05, #).
3.3. BrdU and cell proliferation:
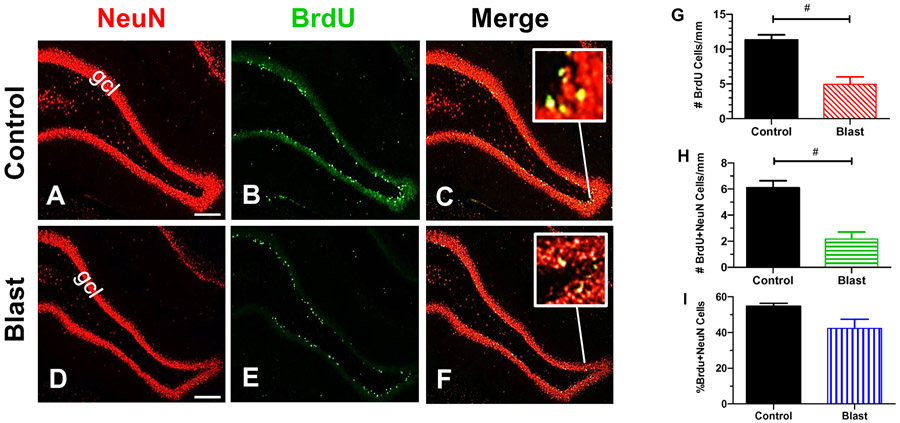
Rats in the Control group (n=3) and Blast group (n=3) were injected daily with BrdU for 12 days just prior to the blast exposure to label cells dividing around the time of the blast or sham exposure. Approximately 11-weeks post-treatment, the hippocampal sections were co-labeled with an antibody that recognizes BrdU and another recognizing NeuN, a protein expressed in neurons. Figure 4A-B show representative coronal sections from the hippocampus of Control rats. Numerous NeuN-positive cells lined the granule cell layer of the dentate gyrus (Fig. 4A). BrdU-positive cells were also present in the granule cell layer of the dentate gyrus, but as expected, there were fewer BrdU-labeled cells than NeuN-positive cells (Fig. 4B). Figure 4C shows a merge of panels A and B (yellow represents green/red overlap). The inset shows a higher magnification image of yellow labeled cells illustrating the coregistration of BrdU (green) with NeuN (red). Figure 4D-E show representative images of coronal sections from the hippocampus of a rat from the Blast Wave group. Many NeuN-positive cells were present in the granule cell layer of the dentate gyrus (Fig. 4D), but relatively few BrdU-positive cells were seen in the corresponding region (Fig. 4E). Figure 4F is a merge of panels D and E. There was a paucity of cells with yellow fluorescence because fewer cells in the Blast Wave group coexpressed BrdU and NeuN; labeling in the Blast Wave group was qualitatively less robust than in the Control group.
Figure 4:
Blast wave exposure reduce the numbers of newborn cells and neurons. (A-B) Representative photomicrograph of hippocampus from a Control rat labeled with (A) antibody against NeuN, (B) an antibody against BrdU (B) and (C) Merge of panels A and B; inset shows yellow cells that co-express NeuN plus BrdU. Numerous NeuN-positive cells in granule cell layer (gcl) of dentate gyrus (A) of Control; fewer BrdU-positive neurons in glcl layer of dentate gyrus of Control (B). (D-F) Representative photomicrographs taken from rat in Blast Wave group 11 weeks post-exposure. Many NeuN-positive cells are present in gcl layer of dentate gyrus (D), but few BrdU-positive cells are present in gcl layer of dentate gyrus (E). (F) Merge of panels D and E; inset shows yellow cells that co-express NeuN plus BrdU. Scale bar in A, D: 200 μm. (G) Mean (+SEM) numbers of BrdU-positive cells per mm length of granule layer of dentate gyrus in Control group (n=3) and Blast Wave group (n=3); significantly fewer BrdU-positive cells are present in the Blast Wave group compared to Controls (t =4.93, 4 df; p<0.01, #). (H) Mean (+SEM) numbers of cell co-labeled with BrdU and NeuN per mm length of granule cell in dentate gyrus. Significantly fewer BrdU/NeuN co-labeled cells are present in dentate gyrus of Blast Wave group compared to Controls (t=5.164, 4 df, p<0.001, #). (I) Mean (+SEM) percentage of dividing cells labeled by both BrdU and NeuN in Control group (n=3) and Blast Wave (n=3) group. Percentage of cells that coexpressed BrdU/NeuN is slightly less in Blast Wave group compared to Controls, but the difference is not significant.
The numbers of labeled BrdU cells/mm and BrdU/NeuN cells/mm of the SGZ were determined for the left and right hippocampus (25 sections/rat) in the Control (n=3) and Blast Wave (n=3) groups. The mean number of BrdU-positive cells/mm was 11.32 in the Control group versus 4.95 in the Blast Wave group (Fig. 4G). The mean number/mm of BrdU-positive cells was significantly lower in the Blast Wave group than the Control group (t=4.93, 4 df, p<0.01), results suggesting there was less cell proliferation after the blast wave exposure.
3.4. Neurogenesis:
Coexpression of NeuN with BrdU has been used to identify proliferating cells that have differentiated into neurons (Eriksson et al., 1998; Scott et al., 2000). We counted the numbers of NeuN/BrdU cells/mm in the SGZ of rats in the Control group and Blast Wave group. There were only 2.17 NeuN/BrdU cells/mm in the Blast Wave group compared to 6.10 NeuN/BrdU cells/mm (Fig. 4H). The decreased number of cells/mm in the Blast Wave group compared to Controls was statistically significant (t=5.16, 4 df, p<0.01). A subpopulation of dividing cells labeled by BrdU differentiate into NeuN-expressing neurons. In the Control group, approximately 55% of the BrdU-labeled proliferating cells differentiated into NeuN-expressing neurons whereas only 42% of BrdU-labeled cells in the Blast Wave groups expressed NeuN; although the percentage was slightly less in the Blast Wave group compared to Controls, this difference was not statistically significant. These results indicate that the percentage of dividing cells that differentiate into neurons is similar in both the Blast Wave and Control groups.
3.5. Normal spatial acquisition memory:
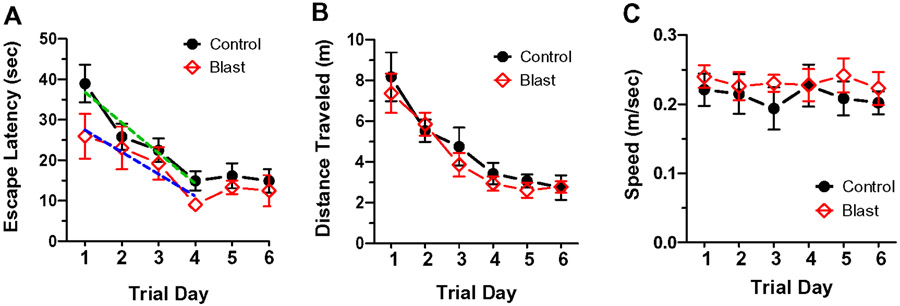
To test for deficits on spatial acquisition memory, rats in the Control group (n=8) and Blast Wave group (n=8) were evaluated on the MWM test 45-days post-treatment. Each rat was given eight memory acquisition trials per day over six consecutive days. The escape latency time required for the rat to swim around the pool and to locate and climb onto the escape platform was recorded. The mean (+/−SEM) daily escape latencies for both groups declined with test day (two-way repeated measure ANOVA, F5,70 df =12.94, p<0.001); escape latency time mainly declined over the first four test days and then plateaued at a stable value. The mean latencies were slightly longer for the Control group than the Blast Wave group across the six test days (Fig. 5A); however, these differences were not significantly different from one another (two-way repeated measure ANOVA, F1, 70 df = 2.4, p=0.14). To determine if the rate of memory acquisition was different, we computed the slopes for the escape latency values from days 1-4 when the escape latency values were declining. We found that the slopes (sec/trial day) were not significantly different from one another (F 3, 152 df = 2.05, p=0.109). We also compared the Control and Blast Wave groups on distance traveled (m) (Fig. 5B) and swim speed (m/sec) (Fig. 5C) to reach the hidden platform over test days 1-6 and found no significant difference between the groups. Taken together, these results suggest that the blast wave exposure did not disrupt the acquisition of spatial memory; rats in the Blast wave group were able to learn the task as well as the Control group consistent with previous studies of hippocampal spatial memory acquisition (Madsen et al., 2003; Raber et al., 2004; Rola et al., 2004; Shors et al., 2002; Snyder et al., 2005). Although the differences were not significantly different, the escape latencies were slightly shorter and swim speed slightly faster in the Blast Wave group compared to the Control group. While these differences were small, they raise the possibility that Blast Wave rats may be slightly more motivated to perform the task as suggested by previous studies linking shorter escape latencies to greater stress-induced motivation (Sandi and Pinelo-Nava, 2007).
Figure 5:
MWM memory acquisition test results 45-days post-blast. Mean latency (+/−SEM) to reach the hidden platform in northeast quadrant on test days 1-6 for rats in the Control group (n=8) and Blast Wave group (n=8); no significant between group differences observed. Escape latencies reached a stable plateau by day 4. Dashed lines show computed slopes for the Control group (green) and Blast Wave (blue) group for days 1-4; no significant difference in slope between the Blast Wave and Control groups. (B) Mean (+/− SEM) distance traveled to reach the hidden platform on test days 1-6 in Control group (n=8) and Blast Wave group (n=8). No significant difference in distance traveled exits between Blast Wave and Control groups. (C) Mean (+/− SEM) speed to reach the hidden platform on test days 1-6 in Control group (n=8) and Blast Wave group (n=8). No significant difference in swim speed exists between Blast Wave and Control groups.
3.6. Disrupted long-term retention memory:
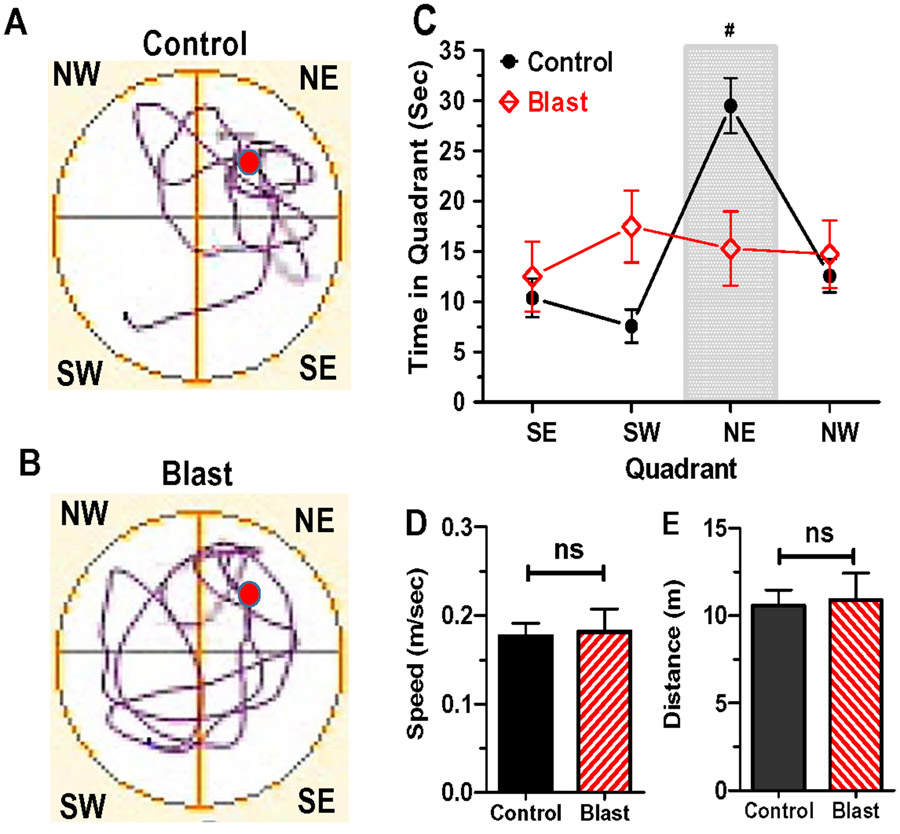
To test for long-term retention of spatial memory, the hidden platform was removed from the northeast (NE) quadrant of the MWM. Testing was performed 15-days after acquisition training. Rats were placed in the MWM and allowed to search for the missing platform during a 60 second probe trial (Snyder et al., 2005). The rat in the Control group spent considerable time swimming in the NE quadrant searching for the missing platform (Fig. 6A) whereas the rat in the Blast Wave group swam randomly about in all four quadrants (Fig. 6B). Control rats spent on average 30 seconds (+/− SEM) swimming in the NE quadrant whereas time spent swimming in the other three quadrants ranged from ~8 to 12 seconds (Fig. 6C). Rats in the Blast Wave group spent roughly the same amount of time swimming in all four quadrants (Fig. 6C). The amount of time Control rats spent in the NE quadrants was significantly greater than the other three quadrants (one-way ANOVA, F3, 20 df = 23.36, p<0.0001, Bonferroni post-hoc, p<0.05). These results suggest that the Control rats had retained their memory of the location of the hidden platform previously located in the NE quadrant. Rats in the Blast Wave groups behaved much differently than Controls; they spent nearly the same amount of time (~13-17 seconds) searching for the hidden platform in all four quadrants. There were no significant differences in the amount of time spent in the four quadrants (one-way ANOVA, F3,20 df = 0.34, p=0.80) suggesting that the Blast Wave rats had no long-term memory recall of the where the hidden platform was formerly located. We also measured the swim speed (m/sec) and distance (m) traveled in the Control group and Blast Wave group. There was no significant difference between Control and Blast Wave groups in terms of mean (+SEM) swim speed (Fig. 6D) or mean (+SEM) distance traveled (Fig. 6E).
Figure 6:
MWM spatial memory retention task with missing hidden platform (red circle) removed from the northeast quadrant (NE). Representative swim pattern on probe trials for a rat in the Control group (A) and rat in Blast Wave group (B). Rat in Control group swims predominantly in the NE quadrant whereas rat in Blast Wave group swims nearly equally in all quadrants. (C) Mean (+/− SEM) time spent in southeast (SE), southwest (SW), northeast (NE) and northwest (NW) quadrants on 60-second probe trials for Control group (n=8) and Blast Wave group. Control group spent significantly (p<0.001) more time in the NE quadrant where that hidden platform had previously been located; Blast Wave group spends nearly equal amount of time in all four quadrants. (D) mean (+SEM) swim speed of rats in Control group and Blast Wave group not significantly (ns) different). (E) Mean (+SEM) distance traveled in Control group and Blast Wave group is not significantly (ns) different.
3.7. No evidence of blast-wave induced anxiety:
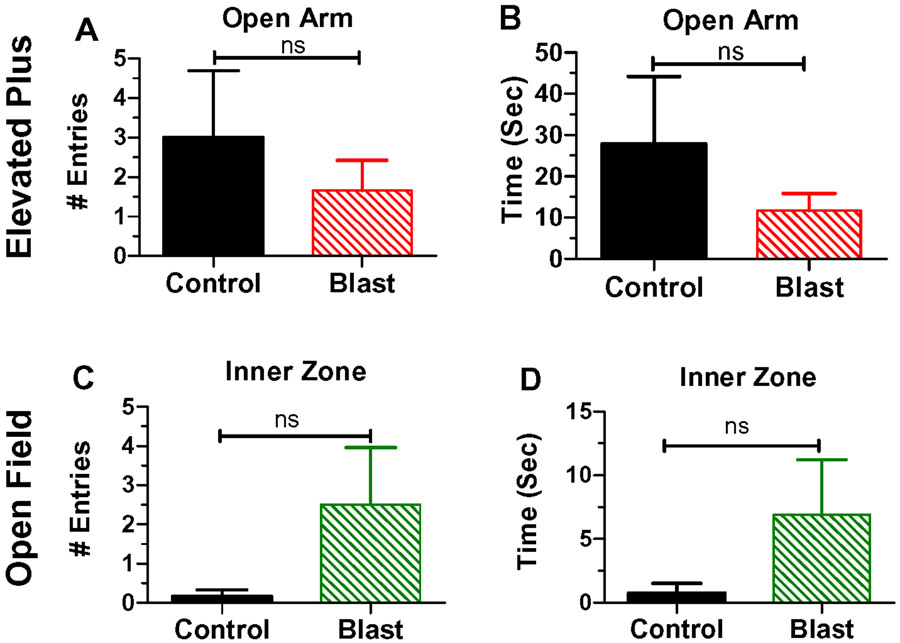
A number of human studies have noted an association between anxiety and hearing loss (Arslan et al., 2018; Contrera et al., 2017; Cosh et al., 2018) and transient increases in anxiety have been observed in rats following blast wave exposure (Awwad et al., 2015). Therefore, rats in the Control group and Blast Wave group were evaluated approximately 2-months post-exposure on the EPM and OF test, two commonly used behavioral measures of anxiety. The mean (+SEM) of entries into the open arm of the EPM maze were slightly greater in the Control group (n=6) than the Blast Wave group (n=6); however, this difference was not significantly different (Fig. 7A). The mean (+SEM) time spent in the open arm of the EPM was also slightly greater in the Control group (n=6) than the Blast Wave group (n=6); however, this difference was not significantly different (Fig. 7B). When evaluated on the OF test, rats in the Blast Wave group (n=6) made more entries into the inner zone of the OF arena than the Control group (n=6); however, this difference was not significantly different (Fig. 7C). The mean (+SEM) time spent in the inner zone of the OF arena was greater in the Blast Wave group (n=6) than the Control group (n=6); however, this difference was not significantly different (Fig. 7D). The lack of any significant difference between the Control group and Blast Wave group on these two suggest that the blast wave exposure did not lead to an increase in anxiety.
Figure 7:
Elevated Plus Maze (EPM) results in upper row. (A) Mean (+SEM) numbers of entries into the open arm of the EPM; no significant difference exits between rats in Control groups(n=6) and Blast Wave group (n=6). (B) Mean (+SEM) times in seconds spent in the open arm of EPM; no significant (ns) difference exits between rats in the Control group (n=6) and Blast Wave group (n=6). Open Field (OF) results shown in lower row. (C) Mean (+SEM) numbers of entries into the inner zone of the OF; no significant (ns) difference exists between rats in the Control group (n=6) and Blast Wave group (n=6). (D) Mean (+SEM) time in seconds spent in inner zone of OF; no significant (ns) difference exits between Control group (n=6) and Blast Wave (n=6) group.
4.0. Discussion
The 185 dB peak SPL blast wave exposure employed in this study caused a massive decrease in CAP amplitudes that reduced the neural input to the central auditory pathway. The pathophysiological changes in the rat cochlea were associated with a persistent reduction in hippocampal cell proliferation and neurogenesis. Despite the substantial decrease in cell proliferation and neurogenesis in the hippocampus, the Blast Wave rats were able to learn where the hidden platform was located in the MWM test as well as Control rats, i.e., spatial memory acquisition was normal 45 days after the blast wave exposure. However, when the rats in the Blast Wave group were evaluated for memory retention 15-days after being trained to locate the hidden platform in the MWM, they had much more difficulty remembering where the hidden platform had previously been located. Rats in the Blast Wave group were unable to remember the spatial location of what they had learned just a few weeks earlier, i.e., impaired memory retention. On the other hand, the blast-exposed rats with memory retention deficits exhibited normal ambulatory and swimming activity and showed no behavioral evidence of anxiety-like behaviors during the OF test and EPM.
4.1. Suppression of cell proliferation and neurogenesis:
The BrdU administered to rats just prior to the sham or blast wave exposure is incorporated into the DNA of hippocampal cells undergoing cell division around the time of treatment. Therefore, the cells colabeled with BrdU plus NeuN represent those that had divided around the time of the blast wave exposure and had matured into neurons that survived 11-weeks post-exposure. Compared to the Control group, there were ~60% fewer BrdU-positive cells in the Blast Wave group 11-weeks post-exposure suggesting that the Blast Wave exposure had suppressed cell proliferation shortly after the exposure and/or that the cells that had proliferated around the time of the injection had not survived for approximately 3 months following the exposure. There were also ~60% fewer BrdU/NeuN colabeled cells (i.e., neurons) in the Blast Wave group compared to the Control group. One interpretation of these results is that the reduced number of mature neurons colabeled with BrdU/NeuN was largely due to the blast-induced suppression of cell proliferation that occurred shortly after the blast exposure or alternatively that the cells which had proliferated and differentiated into neurons had not survived for 2-months post-exposure.
Increased stress hormone levels have been shown to depress hippocampal neurogenesis (Cameron and Gould, 1994; Chan et al., 2017) raising the possibility that acute biological stress associated with our blast exposure could be responsible for the decline in cell proliferation and neurogenesis shortly after the exposure (Lucassen et al., 2015; Saaltink and Vreugdenhil, 2014; Zuckerman et al., 2019). Support for this view comes from studies showing that basal corticosterone stress hormone levels are transiently upregulated by intense noise, but typically decline after the exposure (Hayes et al., 2019; Liu et al., 2016). However, others have reported a significant long-term, post-exposure increase in corticosterone which presumably lead to decreased neurogenesis in this study (Shukla et al., 2019). To determine if blast- or noise-induced increases in corticosterone are responsible for the long-term suppression of neurogenesis, corticosterone levels could be experimentally reduced prior to the exposure to see if this prevents the decline in neurogenesis (Cameron et al., 1994; Tea et al., 2019). Glucocorticoid receptors play a critical role in the feedback network in the hypothalamic-pituitary-adrenal axis that regulates an organism’s response to stress and neurogenesis. We recently reported a persistent upregulation of glucocorticoid receptors in the dentate gyrus of the hippocampus following an intense noise-exposure that caused a persistent decrease in hippocampal neurogenesis (Hayes et al., 2019). Interestingly, others have reported that mifepristone, a glucocorticoid receptor antagonist, restored hippocampal neurogenesis following chronic stress (Oomen et al., 2007) and restored cognitive function following chronic stress (Oitzl et al., 1998; Zuo et al., 2011).
Direct blast-induced compression of the brain could increase oxidative stress, cell death and chronic inflammation and contribute to the chronic decline in cell proliferation and neurogenesis in the hippocampus (Huang, 2012; Limoli et al., 2004; Liu et al., 2016; Lucassen et al., 2006; Sierra et al., 2010)(Whitney et al., 2009). We did not observe any evidence of gross structural damage to the hippocampus when comparing brain sections from Blast Wave and Control rats. Consistent with our informal observations, others have reported little, if any, signs axonal injury or hippocampal cell death using blast exposures (196-202 dB peak SPL) more intense than those employed in this study. However, the blast exposures decreased the expression of genes involved in neurogenesis and synaptic plasticity (Risling et al., 2011). In contrast, some have found that intense blast-wave exposures (~194 dB peak SPL) increased oxidative stress protein in parts of the hippocampus and corpus callosum, increased expression of amyloid precursor proteins in the hippocampus, medial geniculate body and auditory cortex and increased expression of glial fibrillary acid protein in many parts of the brain (Du et al., 2013; Du et al., 2017). Antioxidant therapy reduced many of these histopathological changes raising the possibility that this antioxidant therapy might alleviate the blast-induced decline in hippocampal neurogenesis and prevent memory deficits.
Acute inflammation can stimulate neurogenesis during the initial phase whereas chronic inflammation tends to suppress it particularly when inflammatory microglia are activated (Kohman and Rhodes, 2013). The ketamine used to anesthetize the rats during the blast wave and sham exposure could potentially affect results. It has been reported that neurogenesis is enhanced by chronic, but not acute ketamine treatment (Clarke et al., 2017). Therefore, it is unlikely that the single ketamine treatment used in our study would neurogenesis. On the other hand, ketamine suppresses cytokine activation (Clarke et al., 2017) and could potentially protect against blast wave damage to the cochlea and brain (Church et al., 1988; Proescholdt et al., 2001) and indirectly influence neurogenesis in the Blast Wave group..
4.2. Chronic suppression of neurogenesis:
Newborn neurons in the hippocampus express DCX for approximately 2-weeks after they are born. Therefore, the DCX-positive cells seen 11-weeks post-exposure represent neurons of recent origin. Substantially fewer DCX-positive cells were present in the Blast Wave group compared to Controls. The DCX-positive cells that were present had fewer, thinner and less ramified processes than those in the Control group. These changes are reminiscent to the changes seen in DCX-positive cells in animals that have undergone chronic stress (Oomen et al., 2007). In general, our DCX results are in line with other reports showing a persistent reduction in hippocampal neurogenesis caused by other types of noise-induced hearing loss (Kraus et al., 2010; Liu et al., 2016)(Shukla et al., 2019). We speculate that the long-term reduction of neurogenesis is caused by a persistent decline in cell proliferation in the hippocampus as previously reported after blast wave or continuous noise exposure (Kraus et al., 2010; Liu et al., 2016; Newman et al., 2015; Shukla et al., 2019). Long-term depression of neurogenesis has also been observed with the loss of sensory information from vestibular, visual and olfactory sensory systems (Scotto-Lomassese et al., 2002; Zheng et al., 2001). Similarly, hippocampal neurogenesis is chronically depressed occurs after severe social defeat. However, imipramine, a broad-acting tricyclic antidepressant, restored neurogenesis and reversed the depressive behavior induced by social defeat (Van Bokhoven et al., 2011). These results suggest that antidepressants could restore neurogenesis and potentially ameliorate the blast-induced memory deficits.
Epidemiological studies indicate that hearing loss is a risk factor for dementia and cognitive impairment (Lin et al., 2011a; Liu et al., 2019). Moreover, cognitive decline occurred more rapidly in subjects with hearing loss compared to normal hearing subjects (Alattar et al., 2020; Gurgel et al., 2014). Importantly, military personnel with a history of blast exposure and hearing loss suffer from memory deficits (Cooper et al., 2016; Gilbertson et al., 2001; Helfer et al., 2011; McDermott et al., 2016; Tischler et al., 2006). Our blast wave exposures suppressed DCX-labeling in the hippocampus by approximately 35% and resulted in memory retention deficits. The degree of DCX suppression was nearly identical to that seen in our earlier study using the same blast wave, presented three times (Newman et al., 2015). The blast wave-induced suppression of DCX immunolabeling was approximately 50% greater than that reported in three previous studies in which an intense continuous noise was used to induce hearing loss in mice and/or rats (Kraus et al., 2010; Liu et al., 2016; Shukla et al., 2019). One interpretation of these results is that intense blast wave exposures may be more effective at suppressing hippocampal neurogenesis than a less intense, but longer duration continuous noise exposures. This is a potentially significant observation given that blast-exposed combat personnel often experience cognitive and memory impairments (Karr et al., 2014; Shively and Perl, 2012). The link between hearing loss and cognitive decline is largely correlational; however, significant improvements in cognitive function have been observed in profoundly hearing impaired subjects that have receive a cochlear implant (Mosnier et al., 2015). Hearing aids, which can compensate for cochlear hearing loss, are being evaluated to determine if they can prevent or slow cognitive decline (https://clinicaltrials.gov/ct2/show/NCT03243422). Clearly, more research needs to be conducted to determine how hippocampal cell proliferation and neurogenesis are affected by factors such as the amount of hearing loss and the contribution of acoustic variables such as the intensity, frequency and duration of the noise exposure.
4.3. Spatial memory acquisition:
Forty-five days after the exposure, rats in the Blast Wave and Control groups were trained over a period of 6 days to locate the hidden platform. We found little difference between the blast and control groups in this spatial memory acquisition task in term of escape latency, rate of memory acquisition (slope), distance traveled or speed to reach the hidden platform. Our blast wave memory retention deficits are thus consistent with earlier studies showing that other manipulations that suppress adult neurogenesis result in memory retention deficits, but do not disrupt spatial memory acquisition, (Madsen et al., 2003; Raber et al., 2004; Rola et al., 2004; Shors et al., 2002; Snyder et al., 2005). In contrast to previous reports, a recent study claimed that noise-exposed mice learned the MWM spatial memory task more slowly that control mice (Liu et al., 2016). Closer inspection of their data, however, suggests that the rate (slope) at which the noise-exposed mice learned the task was similar to controls. There was a difference in mean escape latency, but this difference could have been due to other factors such as swim speed rather than rate of memory acquisition. Therefore, we conclude that prior hearing loss induced by blast wave or intense continuous noise does not disrupt spatial memory acquisition.
4.4. Spatial memory retention:
Although our rats were able to learn where the hidden platform was located, the blast wave exposure disrupted their ability to remember its location several weeks later, i.e., the memory trace needed to remember the location of the hidden platform had dissipated. Our results are consistent with many earlier studies that have shown that suppressing hippocampal neurogenesis significantly impairs long-term spatial memory retention (Madsen et al., 2003; Raber et al., 2004; Rola et al., 2004; Shors et al., 2002; Snyder et al., 2005). Our results are also consistent with an earlier study that reported spatial memory retention deficits in mice several months after they were exposed 2 h to 123 dB SPL broadband noise. This single exposure caused a significant hearing loss over a broad range of frequencies and destroyed more than 70% the outer hair cell loss in the high-frequency, basal half of the cochlea (Liu et al., 2016). A more recent study also reported spatial memory retention deficits in rats exposed to 100 dB SPL broadband noise 2 h per day for 15 days (Shukla et al., 2019). This exposure caused a relatively modest hearing loss (10-25 dB) and mild outer hair cell lesion (30-40%). However, in contrast to our results and other reports in the literature (Snyder et al., 2005), the noise-exposed rats in study had surprisingly longer escape latencies (memory acquisition deficit) and swam longer and slower than controls (see Fig. 1, (Shukla et al., 2019)). The reasons for these differences are unclear, but could be related to procedural differences or the much longer, repetitive and potentially stressful noise exposure.
4.6. Summary:
Our earlier study from 2010 (Kraus et al., 2010) as well as more recent results showed that noise-induced hearing loss induced strong extra-auditory effects that resulted in a significant and persistent suppression of cell proliferation and neurogenesis in the rat hippocampus. The blast wave-induced suppression of hippocampal proliferation and neurogenesis was associated with the inability of rats to remember a vitally important location, which they had previously learned to a criterion level. Our blast wave results and those of other using intense continuous noise show that cochlear hearing loss can contribute to deficits on a spatial memory retentions task and that suppression of hippocampal neurogenesis may contribute to these deficits. More recent results from our lab have revealed a persistent noise-induced upregulation of glucocorticoid receptors in the hippocampus (Hayes et al., 2019). Glucocorticoid receptors play a critical role in the feedback network in the hypothalamic-pituitary-adrenal axis that regulates an organism’s response to various forms of stress that could exert long-term effects on hippocampal neurogenesis.
Acknowledgements:
Research supported in part by grants from the United States of America National Institutes of Health (R01DC011808 and R01DC014452). We gratefully acknowledgement the technical assistance of Kimberly Dahar and Robert Dingman.
Abbreviations:
- BrdU
bromodeoxyuridine
- BSA
bovine serum albumin
- CAP
compound action potential
- DCX
doublecortin
- EPM
elevated plus maze
- gcl
granule cell layer
- OF
open field
- PBS
phosphate buffered saline
- MWM
Morris Water Maze
- NeuN
Neuronal Nuclei
- TBS
tris-buffered saline
- SGZ
subgranular zone
References
- Alattar AA, Bergstrom J, Laughlin GA, Kritz-Silverstein D, Richard EL, Reas ET, Harris JP, Barrett-Connor E, McEvoy LK 2020. Hearing Impairment and Cognitive Decline in Older, Community-Dwelling Adults. J Gerontol A Biol Sci Med Sci 75, 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Das GD 1965. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. Journal of Comparative Neurology 124, 319–35. [DOI] [PubMed] [Google Scholar]
- Anchan D, Clark S, Pollard K, Vasudevan N 2014. GPR30 activation decreases anxiety in the open field test but not in the elevated plus maze test in female mice. Brain Behav 4, 51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronov D, Nevers R, Tank DW 2017. Mapping of a non-spatial dimension by the hippocampal-entorhinal circuit. Nature 543, 719–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan F, Aydemir E, Kaya YS, Arslan H, Durmaz A 2018. Anxiety and depression in patients with sudden one-sided hearing loss. Ear Nose Throat J 97, E7–E10. [DOI] [PubMed] [Google Scholar]
- Awwad HO, Gonzalez LP, Tompkins P, Lerner M, Brackett DJ, Awasthi V, Standifer KM 2015. Blast Overpressure Waves Induce Transient Anxiety and Regional Changes in Cerebral Glucose Metabolism and Delayed Hyperarousal in Rats. Front Neurol 6, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baizer JS, Wong KM, Manohar S, Hayes SH, Ding D, Dingman R, Salvi RJ 2015. Effects of acoustic trauma on the auditory system of the rat: The role of microglia. Neuroscience 303, 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandeis R, Brandys Y, Yehuda S 1989. The use of the Morris Water Maze in the study of memory and learning. Int J Neurosci 48, 29–69. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG 2003. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol 467, 1–10. [DOI] [PubMed] [Google Scholar]
- Calford MB, Rajan R, Irvine DRF 1993. Rapid changes in frequency tuning of neurons in cat auditory cortex resulting from pure-tone-induced temporary threshold shift. Neuroscience 55, 953–964. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E 1994. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience 61, 203–209. [DOI] [PubMed] [Google Scholar]
- Chan JN, Lee JC, Lee SS, Hui KK, Chan AH, Fung TK, Sanchez-Vidana DI, Lau BW, Ngai SP 2017. Interaction Effect of Social Isolation and High Dose Corticosteroid on Neurogenesis and Emotional Behavior. Front Behav Neurosci 11, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Kermany MH, D'Elia A, Ralli M, Tanaka C, Bielefeld EC, Ding D, Henderson D, Salvi R 2010. Too much of a good thing: long-term treatment with salicylate strengthens outer hair cell function but impairs auditory neural activity. Hear Res 265, 63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Li X, Liu L, Wang J, Lu CQ, Yang M, Jiao Y, Zang FC, Radziwon K, Chen GD, Sun W, Krishnan Muthaiah VP, Salvi R, Teng GJ 2015. Tinnitus and hyperacusis involve hyperactivity and enhanced connectivity in auditory-limbic-arousal-cerebellar network. Elife 4, e06576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church J, Zeman S, Lodge D 1988. The neuroprotective action of ketamine and MK-801 after transient cerebral ischemia in rats. Anesthesiology 69, 702–9. [DOI] [PubMed] [Google Scholar]
- Clarke M, Razmjou S, Prowse N, Dwyer Z, Litteljohn D, Pentz R, Anisman H, Hayley S 2017. Ketamine modulates hippocampal neurogenesis and pro-inflammatory cytokines but not stressor induced neurochemical changes. Neuropharmacology 112, 210–220. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD Jr., Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ 2009. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contrera KJ, Betz J, Deal J, Choi JS, Ayonayon HN, Harris T, Helzner E, Martin KR, Mehta K, Pratt S, Rubin SM, Satterfield S, Yaffe K, Simonsick EM, Lin FR, Health, A.B.C.S. 2017. Association of Hearing Impairment and Anxiety in Older Adults. J Aging Health 29, 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CM, Briggs RW, Farris EA, Bartlett J, Haley RW, Odegard TN 2016. Memory and functional brain differences in a national sample of U.S. veterans with Gulf War Illness. Psychiatry Res Neuroimaging 250, 33–41. [DOI] [PubMed] [Google Scholar]
- Cosh S, von Hanno T, Helmer C, Bertelsen G, Delcourt C, Schirmer H, Group SE-C 2018. The association amongst visual, hearing, and dual sensory loss with depression and anxiety over 6 years: The Tromso Study. International Journal of Geriatric Psychiatry 33, 598–605. [DOI] [PubMed] [Google Scholar]
- Du X, Ewert DL, Cheng W, West MB, Lu J, Li W, Floyd RA, Kopke RD 2013. Effects of antioxidant treatment on blast-induced brain injury. PLoS One 8, e80138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, West MB, Cai Q, Cheng W, Ewert DL, Li W, Floyd RA, Kopke RD 2017. Antioxidants reduce neurodegeneration and accumulation of pathologic Tau proteins in the auditory system after blast exposure. Free Radic Biol Med 108, 627–643. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ 2015. The auditory cortex and tinnitus - a review of animal and human studies. Eur J Neurosci 41, 665–76. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH 1998. Neurogenesis in the adult human hippocampus. Nat Med 4, 1313–7. [DOI] [PubMed] [Google Scholar]
- Feng J, Bendiske J, Morest DK 2012. Degeneration in the ventral cochlear nucleus after severe noise damage in mice. J Neurosci Res 90, 831–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton AA, Csizmadia G, Muller RU 2000. Conjoint control of hippocampal place cell firing by two visual stimuli. Ii. A vector-field theory that predicts modifications of the representation of the environment. Journal of General Physiology 116, 211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana X, Nacher J, Soriano E, del Rio JA 2006. Cell proliferation in the adult hippocampal formation of rodents and its modulation by entorhinal and fimbria-fornix afferents. Cereb Cortex 16, 301–12. [DOI] [PubMed] [Google Scholar]
- Garinther GR 1979. Impulse noise of army weapons. The Journal of the Acoustical Society of America 65, S29–S29. [Google Scholar]
- Gehrmann J, Schoen SW, Kreutzberg GW 1991. Lesion of the rat entorhinal cortex leads to a rapid microglial reaction in the dentate gyrus. A light and electron microscopical study. Acta Neuropathol 82, 442–55. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Gurvits TV, Lasko NB, Orr SP, Pitman RK 2001. Multivariate assessment of explicit memory function in combat veterans with posttraumatic stress disorder. Journal of Traumatic Stress 14, 413–32. [DOI] [PubMed] [Google Scholar]
- Goble TJ, Moller AR, Thompson LT 2009. Acute high-intensity sound exposure alters responses of place cells in hippocampus. Hear Res 253, 52–9. [DOI] [PubMed] [Google Scholar]
- Goodman T, Trouche S, Massou I, Verret L, Zerwas M, Roullet P, Rampon C 2010. Young hippocampal neurons are critical for recent and remote spatial memory in adult mice. Neuroscience 171, 769–78. [DOI] [PubMed] [Google Scholar]
- Gurgel RK, Ward PD, Schwartz S, Norton MC, Foster NL, Tschanz JT 2014. Relationship of hearing loss and dementia: a prospective, population-based study. Otol Neurotol 35, 775–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamernik RP, Turrentine G, Roberto M, Salvi R, Henderson D 1984. Anatomical correlates of impulse noise-induced mechanical damage in the cochlea. Hear Res 13, 229–47. [DOI] [PubMed] [Google Scholar]
- Hayes SH, Manohar S, Majumdar A, Allman BL, Salvi R 2019. Noise-induced hearing loss alters hippocampal glucocorticoid receptor expression in rats. Hear Res 379, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer TM, Jordan NN, Lee RB, Pietrusiak P, Cave K, Schairer K 2011. Noise-induced hearing injury and comorbidities among postdeployment U.S. Army soldiers: April 2003-June 2009. Am J Audiol 20, 33–41. [DOI] [PubMed] [Google Scholar]
- Huang TT 2012. Redox balance- and radiation-mediated alteration in hippocampal neurogenesis. Free Radic Res. [DOI] [PubMed] [Google Scholar]
- Hufner K, Strupp M, Smith P, Brandt T, Jahn K 2011. Spatial separation of visual and vestibular processing in the human hippocampal formation. Ann N Y Acad Sci 1233, 177–86. [DOI] [PubMed] [Google Scholar]
- Jokel C, Yankaskas K, Robinette MB 2019. Noise of military weapons, ground vehicles, planes and ships. J Acoust Soc Am 146, 3832. [DOI] [PubMed] [Google Scholar]
- Jung MW, McNaughton BL 1993. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus 3, 165–82. [DOI] [PubMed] [Google Scholar]
- Karr JE, Areshenkoff CN, Duggan EC, Garcia-Barrera MA 2014. Blast-related mild traumatic brain injury: a Bayesian random-effects meta-analysis on the cognitive outcomes of concussion among military personnel. Neuropsychology Review 24, 428–44. [DOI] [PubMed] [Google Scholar]
- Kohman RA, Rhodes JS 2013. Neurogenesis, inflammation and behavior. Brain Behav Immun 27, 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus KS, Canlon B 2012. Neuronal connectivity and interactions between the auditory and limbic systems. Effects of noise and tinnitus. Hear Res 288, 34–46. [DOI] [PubMed] [Google Scholar]
- Kraus KS, Mitra S, Jimenez Z, Hinduja S, Ding D, Jiang H, Gray L, Lobarinas E, Sun W, Salvi RJ 2010. Noise trauma impairs neurogenesis in the rat hippocampus. Neuroscience 167, 1216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesicko AM, Hristova TS, Maigler KC, Llano DA 2016. Connectional Modularity of Top-Down and Bottom-Up Multimodal Inputs to the Lateral Cortex of the Mouse Inferior Colliculus. J Neurosci 36, 11037–11050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limoli CL, Giedzinski E, Rola R, Otsuka S, Palmer TD, Fike JR 2004. Radiation response of neural precursor cells: linking cellular sensitivity to cell cycle checkpoints, apoptosis and oxidative stress. Radiat Res 161, 17–27. [DOI] [PubMed] [Google Scholar]
- Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, Resnick SM 2011a. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology 25, 763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, Liberman MC 2011b. Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol 12, 605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CM, Lee CT 2019. Association of Hearing Loss With Dementia. JAMA Netw Open 2, e198112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Xuan C, Shen P, He T, Chang Y, Shi L, Tao S, Yu Z, Brown RE, Wang J 2018. Hippocampal Mechanisms Underlying Impairment in Spatial Learning Long After Establishment of Noise-Induced Hearing Loss in CBA Mice. Front Syst Neurosci 12, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Shen P, He T, Chang Y, Shi L, Tao S, Li X, Xun Q, Guo X, Yu Z, Wang J 2016. Noise induced hearing loss impairs spatial learning/memory and hippocampal neurogenesis in mice. Sci Rep 6, 20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen PJ, Oomen CA, Naninck EF, Fitzsimons CP, van Dam AM, Czeh B, Korosi A 2015. Regulation of Adult Neurogenesis and Plasticity by (Early) Stress, Glucocorticoids, and Inflammation. Cold Spring Harb Perspect Biol 7, a021303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen PJ, Heine VM, Muller MB, van der Beek EM, Wiegant VM, De Kloet ER, Joels M, Fuchs E, Swaab DF, Czeh B 2006. Stress, depression and hippocampal apoptosis. CNS & Neurological Disorders Drug Targets 5, 531–46. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Kristjansen PE, Bolwig TG, Wortwein G 2003. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience 119, 635–42. [DOI] [PubMed] [Google Scholar]
- Manohar S, Hayes S, Salvi R, Baizer JS 2011. Unipolar brush cells express DCX in the dorsal cochlear nucleus, paraflocculus and flocculus of adult rat, ARO, Baltimore, Maryland. pp. #431. [Google Scholar]
- Manohar S, Paolone NA, Bleichfeld M, Hayes SH, Salvi RJ, Baizer JS 2012. Expression of doublecortin, a neuronal migration protein, in unipolar brush cells of the vestibulocerebellum and dorsal cochlear nucleus of the adult rat. Neuroscience 202, 169–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott TJ, Badura-Brack AS, Becker KM, Ryan TJ, Khanna MM, Heinrichs-Graham E, Wilson TW 2016. Male veterans with PTSD exhibit aberrant neural dynamics during working memory processing: an MEG study. J Psychiatry Neurosci 41, 251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F 1998. Large-scale functional connectivity in associative learning: interrelations of the rat auditory, visual, and limbic systems. Journal of Neurophysiology 80, 3148–62. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, Meltzer J, Sutherland RJ 1989. Hippocampal granule cells are necessary for normal spatial learning but not for spatially-selective pyramidal cell discharge. Exp Brain Res 76, 485–96. [DOI] [PubMed] [Google Scholar]
- Michler SA, Illing RB 2002. Acoustic trauma induces reemergence of the growth- and plasticity-associated protein GAP-43 in the rat auditory brainstem. J Comp Neurol 451, 250–66. [DOI] [PubMed] [Google Scholar]
- Mohan A, De Ridder D, Idiculla R, C DS, Vanneste S 2018. Distress-dependent temporal variability of regions encoding domain-specific and domain-general behavioral manifestations of phantom percepts. Eur J Neurosci 48, 1743–1764. [DOI] [PubMed] [Google Scholar]
- Morest DK, Bohne BA 1983. Noise-induced degeneration in the brain and representation of inner and outer hair cells. Hear Res 9, 145–51. [DOI] [PubMed] [Google Scholar]
- Morris R 1984. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11, 47–60. [DOI] [PubMed] [Google Scholar]
- Moser EI, Moser MB, Roudi Y 2014. Network mechanisms of grid cells. Philos Trans R Soc Lond B Biol Sci 369, 20120511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosnier I, Bebear JP, Marx M, Fraysse B, Truy E, Lina-Granade G, Mondain M, Sterkers-Artieres F, Bordure P, Robier A, Godey B, Meyer B, Frachet B, Poncet-Wallet C, Bouccara D, Sterkers O 2015. Improvement of cognitive function after cochlear implantation in elderly patients. JAMA Otolaryngol Head Neck Surg 141, 442–50. [DOI] [PubMed] [Google Scholar]
- Munoz-Lopez MM, Mohedano-Moriano A, Insausti R 2010. Anatomical pathways for auditory memory in primates. Frontiers in Neuroanatomy 4, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AJ, Hayes SH, Rao AS, Allman BL, Manohar S, Ding D, Stolzberg D, Lobarinas E, Mollendorf JC, Salvi R 2015. Low-cost blast wave generator for studies of hearing loss and brain injury: blast wave effects in closed spaces. J Neurosci Methods 242, 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzl MS, Fluttert M, Sutanto W, de Kloet ER 1998. Continuous blockade of brain glucocorticoid receptors facilitates spatial learning and memory in rats. Eur J Neurosci 10, 3759–66. [DOI] [PubMed] [Google Scholar]
- Oomen CA, Mayer JL, de Kloet ER, Joels M, Lucassen PJ 2007. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalizes the reduction in neurogenesis after chronic stress. Eur J Neurosci 26, 3395–401. [DOI] [PubMed] [Google Scholar]
- Paolone NA, Manohar S, S.H. H, Baizer JS, Salvi RJ 2011. Expression of a neuronal migratory protein in unipolar brush cells f the adult rat dorsal cochlear nucleus, paraflocculus, and flocculus., Society for Neuroscience, abstracts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual B, Masdeu JC, Hollenbeck M, Makris N, Insausti R, Ding SL, Dickerson BC 2015. Large-scale brain networks of the human left temporal pole: a functional connectivity MRI study. Cereb Cortex 25, 680–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proescholdt M, Heimann A, Kempski O 2001. Neuroprotection of S(+) ketamine isomer in global forebrain ischemia. Brain Res 904, 245–51. [DOI] [PubMed] [Google Scholar]
- Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR 2004. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res 162, 39–47. [DOI] [PubMed] [Google Scholar]
- Ravassard P, Kees A, Willers B, Ho D, Aharoni D, Cushman J, Aghajan ZM, Mehta MR 2013. Multisensory control of hippocampal spatiotemporal selectivity. Science 340, 1342–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risling M, Plantman S, Angeria M, Rostami E, Bellander BM, Kirkegaard M, Arborelius U, Davidsson J 2011. Mechanisms of blast induced brain injuries, experimental studies in rats. Neuroimage 54 Suppl 1, S89–97. [DOI] [PubMed] [Google Scholar]
- Robertson D, Bester C, Vogler D, Mulders WH 2013. Spontaneous hyperactivity in the auditory midbrain: relationship to afferent input. Hear Res 295, 124–9. [DOI] [PubMed] [Google Scholar]
- Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR 2004. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol 188, 316–30. [DOI] [PubMed] [Google Scholar]
- Saaltink DJ, Vreugdenhil E 2014. Stress, glucocorticoid receptors, and adult neurogenesis: a balance between excitation and inhibition? Cell Mol Life Sci 71, 2499–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C, Pinelo-Nava MT 2007. Stress and memory: behavioral effects and neurobiological mechanisms. Neural Plast 2007, 78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BW, Wojtowicz JM, Burnham WM 2000. Neurogenesis in the dentate gyrus of the rat following electroconvulsive shock seizures. Exp Neurol 165, 231–6. [DOI] [PubMed] [Google Scholar]
- Scotto-Lomassese S, Strambi C, Aouane A, Strambi A, Cayre M 2002. Sensory inputs stimulate progenitor cell proliferation in an adult insect brain. Curr Biol 12, 1001–5. [DOI] [PubMed] [Google Scholar]
- Sheppard AM, Chen GD, Manohar S, Ding D, Hu BH, Sun W, Zhao J, Salvi R 2017. Prolonged low-level noise-induced plasticity in the peripheral and central auditory system of rats. Neuroscience 359, 159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively SB, Perl DP 2012. Traumatic brain injury, shell shock, and posttraumatic stress disorder in the military--past, present, and future. Journal of Head Trauma Rehabilitation 27, 234–9. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E 2002. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus 12, 578–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E 2001. Neurogenesis in the adult is involved in the formation of trace memories. Nature 410, 372–6. [DOI] [PubMed] [Google Scholar]
- Shukla M, Roy K, Kaur C, Nayak D, Mani KV, Shukla S, Kapoor N 2019. Attenuation of adverse effects of noise induced hearing loss on adult neurogenesis and memory in rats by intervention with Adenosine A2A receptor agonist. Brain Res Bull 147, 47–57. [DOI] [PubMed] [Google Scholar]
- Sidor MM, Rilett K, Foster JA 2010. Validation of an automated system for measuring anxiety-related behaviours in the elevated plus maze. J Neurosci Methods 188, 7–13. [DOI] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M 2010. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7, 483–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM 2005. A role for adult neurogenesis in spatial long-term memory. Neuroscience 130, 843–52. [DOI] [PubMed] [Google Scholar]
- Sweatt JD 2004. Hippocampal function in cognition. Psychopharmacology (Berl) 174, 99–110. [DOI] [PubMed] [Google Scholar]
- Szczepaniak WS, Moller AR 1996. Evidence of neuronal plasticity within the inferior colliculus after noise exposure: a study of evoked potentials in the rat. Electroencephalography of Clinical Neurophysiology 100, 158–64. [DOI] [PubMed] [Google Scholar]
- Tamura R, Ono T, Fukuda M, Nishijo H 1992a. Monkey hippocampal neuron responses to complex sensory stimulation during object discrimination. Hippocampus 2, 287–306. [DOI] [PubMed] [Google Scholar]
- Tamura R, Ono T, Fukuda M, Nakamura K 1992b. Spatial responsiveness of monkey hippocampal neurons to various visual and auditory stimuli. Hippocampus 2, 307–22. [DOI] [PubMed] [Google Scholar]
- Tea J, Alderman SL, Gilmour KM 2019. Social stress increases plasma cortisol and reduces forebrain cell proliferation in subordinate male zebrafish (Danio rerio). Journal of Experimental Biology 222. [DOI] [PubMed] [Google Scholar]
- Tischler L, Brand SR, Stavitsky K, Labinsky E, Newmark R, Grossman R, Buchsbaum MS, Yehuda R 2006. The relationship between hippocampal volume and declarative memory in a population of combat veterans with and without PTSD. Ann N Y Acad Sci 1071, 405–9. [DOI] [PubMed] [Google Scholar]
- Van Bokhoven P, Oomen CA, Hoogendijk WJ, Smit AB, Lucassen PJ, Spijker S 2011. Reduction in hippocampal neurogenesis after social defeat is long-lasting and responsive to late antidepressant treatment. Eur J Neurosci 33, 1833–40. [DOI] [PubMed] [Google Scholar]
- Vetter DE, Li C, Zhao L, Contarino A, Liberman MC, Smith GW, Marchuk Y, Koob GF, Heinemann SF, Vale W, Lee KF 2002. Urocortin-deficient mice show hearing impairment and increased anxiety-like behavior. Nat Genet 31, 363–9. [DOI] [PubMed] [Google Scholar]
- Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC 2009. Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem 108, 1343–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S 2006. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus 16, 296–304. [DOI] [PubMed] [Google Scholar]
- Xu H, Fan W, Zhao X, Li J, Zhang W, Lei P, Liu Y, Wang H, Cheng H, Shi H 2016. Disrupted functional brain connectome in unilateral sudden sensorineural hearing loss. Hear Res 335, 138–48. [DOI] [PubMed] [Google Scholar]
- Yang FC, Chou KH, Hsu AL, Fuh JL, Lirng JF, Kao HW, Lin CP, Wang SJ 2018. Altered Brain Functional Connectome in Migraine with and without Restless Legs Syndrome: A Resting-State Functional MRI Study. Front Neurol 9, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Horii A, Appleton I, Darlington CL, Smith PF 2001. Damage to the vestibular inner ear causes long-term changes in neuronal nitric oxide synthase expression in the rat hippocampus. Neuroscience 105, 1–5. [DOI] [PubMed] [Google Scholar]
- Zuckerman A, Ram O, Ifergane G, Matar MA, Kaplan Z, Hoffman JR, Sadot O, Cohen H 2019. Role of Endogenous and Exogenous Corticosterone on Behavioral and Cognitive Responses to Low-Pressure Blast Wave Exposure. J Neurotrauma 36, 380–394. [DOI] [PubMed] [Google Scholar]
- Zuo ZF, Wang W, Niu L, Kou ZZ, Zhu C, Wang W, Zhao XH, Luo DS, Zhang T, Zhang FX, Liu XZ, Wu SX, Li YQ 2011. RU486 (mifepristone) ameliorates cognitive dysfunction and reverses the down-regulation of astrocytic N-myc downstream-regulated gene 2 in streptozotocin-induced type-1 diabetic rats. Neuroscience 190, 156–65. [DOI] [PubMed] [Google Scholar]