Abstract
Pseudomonas aeruginosa (P. aeruginosa) was identified among the most relevant antimicrobial‐resistant (AMR) bacteria in the EU for dogs and cats in a previous scientific opinion. Thus, it has been assessed according to the criteria of the Animal Health Law (AHL), in particular criteria of Article 7 on disease profile and impacts, Article 5 on its eligibility to be listed, Annex IV for its categorisation according to disease prevention and control rules as in Article 9, and Article 8 for listing animal species related to the bacterium. The assessment has been performed following a methodology previously published. The outcome is the median of the probability ranges provided by the experts, which indicates whether each criterion is fulfilled (lower bound ≥ 66%) or not (upper bound ≤ 33%), or whether there is uncertainty about fulfilment. Reasoning points are reported for criteria with uncertain outcome. According to the assessment here performed, it is uncertain whether AMR P. aeruginosa can be considered eligible to be listed for Union intervention according to Article 5 of the AHL (33–90% probability). According to the criteria in Annex IV, for the purpose of categorisation related to the level of prevention and control as in Article 9 of the AHL, the AHAW Panel concluded that the bacterium does not meet the criteria in Sections 1, 2, 3 and 4 (Categories A, B, C and D; 0–5%, 1–5%, 5–33% and 5–33% probability of meeting the criteria, respectively) and the AHAW Panel was uncertain whether it meets the criteria in Section 5 (Category E, 33–90% probability of meeting the criteria). The animal species to be listed for AMR P. aeruginosa according to Article 8 criteria are mainly dogs and cats.
Keywords: antimicrobial resistance, Pseudomonas aeruginosa, Animal Health Law, listing, categorisation, impact
1. Introduction
The European Food Safety Authority (EFSA) received a mandate from the European Commission to investigate the global state of play as regards antimicrobial‐resistant (AMR) animal pathogens that cause transmissible animal diseases (Term of Reference (ToR) 1), to identify the most relevant AMR bacteria in the European Union (EU) (first part of ToR 2), to summarise the existing or potential animal health impact of those identified bacteria in the EU (second part of ToR 2) and to perform the assessment of those bacteria to be listed and categorised according to the criteria in Article 5, Annex IV according to Article 9, and Article 8 within the Regulation (EU) No 2016/429 1 on transmissible animal diseases (‘Animal Health Law’) (ToR 3).
The global state of play for AMR animal pathogens that cause transmissible animal diseases (ToR 1) and the results of the assessment of the most relevant AMR bacteria in the EU (first part of ToR 2) for dogs and cats were published in a separate EFSA scientific opinion (EFSA AHAW Panel, 2021a).
According to the results of the assessment already conducted, Pseudomonas aeruginosa (P. aeruginosa) was identified among the most relevant AMR bacteria in the EU for dogs and cats due to its difficulty to treat and the severity of infections caused.
This scientific opinion presents the results of the assessment on AMR P. aeruginosa in dogs and cats on its eligibility to be listed and categorised within the AHL framework. Special focus is placed on the animal health impact of AMR P. aeruginosa in dogs and cats in the EU, which is also summarised here as part of the assessment conducted according to the profile of the infection and its impact on animal welfare (Article 7).
1.1. Background and Terms of Reference as provided by the requestor
The background and ToRs as provided by the European Commission for the present document are reported in Sections 1.1 and 1.2 of the scientific opinion on the ad hoc method to be followed for the assessment of animal diseases caused by bacteria resistant to antimicrobials within the AHL framework (EFSA AHAW Panel, 2021b).
1.2. Interpretation of the Terms of Reference
The interpretation of the ToRs is as in Sections 1.2.3 and 1.3.3 of the scientific opinion on the ad hoc method to be followed for the assessment of animal diseases caused by bacteria resistant to antimicrobials within the AHL framework (EFSA AHAW Panel, 2021b).
The present document reports the results of the assessment on AMR P. aeruginosa in dogs and cats according to the criteria of the AHL articles as follows:
Article 7: AMR P. aeruginosa infection profile and impacts;
Article 5: eligibility of AMR P. aeruginosa infection to be listed;
Article 9: categorisation of AMR P. aeruginosa infection according to disease prevention and control rules as in Annex IV;
Article 8: list of animal species (also apart from dogs and cats) related to AMR P. aeruginosa infection.
2. Data and methodologies
The methodology applied in this opinion is described in detail in a dedicated document about the ad hoc method developed for assessing any animal disease for listing and categorisation of animal diseases within the AHL framework (EFSA AHAW Panel, 2017).
In order to take into account the specifics related to animal diseases caused by bacteria resistant to antimicrobials, the term ‘disease’ as in the AHL was interpreted in a broader sense, referring also to colonisation by commensal and potentially opportunistic bacteria, and the general presence of the identified AMR bacteria in the EU, depending on each criterion.
The following assessment was performed by the EFSA Panel on Animal Health and Welfare (AHAW) based on the information collected and compiled in form of a fact sheet as in Section 3.1 of the present document. The outcome is the median of the probability ranges provided by the experts, which are accompanied by verbal interpretations only when they fall within the ranges as spelled out in Table 1.
Table 1.
Approximate probability scale recommended for harmonised use in EFSA (EFSA Scientific Committee, 2018)
| Probability term | Subjective probability range |
|---|---|
| Almost certain | 99–100% |
| Extremely likely | 95–99% |
| Very likely | 90–95% |
| Likely | 66–90% |
| About as likely as not | 33–66% |
| Unlikely | 10–33% |
| Very unlikely | 5–10% |
| Extremely unlikely | 1–5% |
| Almost impossible | 0–1% |
3. Assessment
3.1. Assessment of AMR Pseudomonas aeruginosa according to Article 7 criteria of the AHL
3.1.1. Article 7(a) Disease profile
This fact sheet concerns the opportunistic pathogen P. aeruginosa. It is a Gram‐negative bacterium from the genus Pseudomonas. Species within this genus can inhabit a wide variety of environments and include environmental organisms, plant pathogens as well as P. aeruginosa, a known opportunistic human and animal pathogen. P. aeruginosa has by the World Health Organization (WHO) been deemed a Priority 1 organism, for which there is an urgent need for new antimicrobials. This is due to high levels of antimicrobial resistance. When disease occurs in dogs, P. aeruginosa most commonly causes otitis, but due to its opportunistic nature can also cause a wide variety of other types of infections. P. aeruginosa can also cause infections in cats although this is less common.
Antimicrobial resistance in P. aeruginosa can be attributed to a variety of different mechanisms and is often combinatorial whereby different mechanisms can contribute to resistance to a single antimicrobial (Langendonk et al., 2021). The outer membrane of P. aeruginosa is less permeable to antibiotics than other Gram‐negative bacteria such as Escherichia coli (about 100 times less permeable) (Lister et al., 2009). This low permeability results in intrinsic resistance to many antimicrobials. The outer membrane contains a variety of porins that affect movement in and out of the cell and efflux pumps that can actively pump antimicrobials out. Acquired resistance can occur through the acquisition of new genetic material, such as plasmids, and due to the development of mutations within existing genes which result in altered function. Furthermore, additional resistance can develop through lifestyle/expression changes such as biofilm formation or tolerance (Langendonk et al., 2021).
The fact sheet will discuss antimicrobial resistance in P. aeruginosa in companion animals, with the majority of studies in dogs and to a lesser extent in cats. This has been recently reviewed by EFSA (EFSA AHAW Panel, 2021a). As P. aeruginosa is found in a wide number of environments, there are also many studies on environmental reservoirs, including in birds. These studies will be discussed in the wider context. The information will focus on multi‐ and pan‐resistance in P. aeruginosa. Where appropriate, key resistances, which include carbapenem resistance (as a WHO priority), polymyxin resistance (as a last‐resort antibiotic in humans) and fluoroquinolone resistance, an important first‐line treatment for many P. aeruginosa infections, will be highlighted.
In some cases, information will refer to P. aeruginosa (colonisation/prevalence or infection) only and not further elaborated in terms of resistance. This is because the information available on this does not specify antimicrobial resistance; however, the study is still considered important.
Some studies may report sequence type (ST) of P. aeruginosa. While ST is not always regarded as the best tool to resolve population structures and pathogenicity in P. aeruginosa, this will be reported when studies have linked these with antimicrobial resistance.
3.1.1.1. Article 7(a)(i) Animal species concerned by the disease
Susceptible animal species
P. aeruginosa, like in humans, is an opportunistic pathogen of many animal species. In this context, it can cause a range of infections including those in the ears, eyes, urogenital tract, wounds, respiratory system and skin. These infections often occur when normal barriers are breached, and therefore, it is not commonly the primary cause of disease in healthy individuals. However, once infection occurs, effective treatment can be challenging.
In dogs, P. aeruginosa can cause ulcerative keratitis (Hewitt et al., 2020), otitis, pyoderma, urinary tract infections, skin and wound infections and respiratory tract infections. The most common P. aeruginosa‐associated infection in dogs is otitis. P. aeruginosa infections can be associated with immunosuppression in companion animals. Pneumonia in a dog post‐kidney transplant has been reported (Park et al., 2013) along with cancer treatment‐associated infections (Curran et al., 2021). Some dog breeds are more prone to particular infections, with one example being eye infections in the St. Bernard: P. aeruginosa was the most common Gram‐negative pathogen in that niche and breed, and the most common multidrug‐resistant (MDR) pathogen with 100% of isolates displaying resistance to more than seven different antimicrobials (Nadăș et al., 2021).
P. aeruginosa is also a pathogen in cats; however, this is to a lesser extent than in dogs (Haenni et al., 2015; de Jong et al., 2020). In cats, respiratory tract infections have been reported (Mohan et al., 2008; Sharma et al., 2019) along with ulcerative keratitis and wound infections (Lin and Petersen‐Jones, 2008).
Parameter 1 – Naturally susceptible wildlife species (or family/order)
There is little information available on the susceptibility of wildlife species to P. aeruginosa. P. aeruginosa has been detected in migratory birds including swallows (Yanornis martini) (Zhang et al., 2017) and the white‐faced whistling duck (Dendrocygna viduata) (Martins et al., 2018). In these studies, no signs of disease were reported; however, the isolates were found to carry metallo‐β‐lactamase genes (bla‐VIM and SPM‐1, respectively). P. aeruginosa has been identified in Siberian (Leucogeranus leucogeranus) and Whooping cranes (Grus americana), particularly linked with keratitis (Miller et al., 1994). A P. aeruginosa isolate has also been cultured from a sea turtle (Eretmochemys imbricata), but there was no clear link to disease (Oliveira et al., 2017).
Parameter 2 – Naturally susceptible domestic species (or family/order)
Mainly dogs (Canis lupus familiaris) and to a lesser extent cats (Felis catus) are naturally susceptible domestic species. P. aeruginosa can cause otitis media, pneumonia, septicaemia, enteritis and sudden death in chinchillas (Chinchilla chinchilla). A study in healthy chinchillas identified P. aeruginosa in 42% of 67 animals tested (Hirakawa et al., 2010). Antimicrobial resistance was detected within this panel with 59% resistant to gentamicin, 27% resistant to ceftazidime, 23% resistant to ciprofloxacin and 23% resistant to imipenem (Hirakawa et al., 2010). Rabbits (von Degerfeld et al., 2020), sugar gliders (Petaurus breviceps) (Varriale et al., 2019) and snakes (Goldstein et al., 1981) have also been identified with P. aeruginosa. In rabbits, it can cause pyometra; however, sugar gliders and snakes are thought to be carriers of P. aeruginosa. It can cause mastitis in sheep (Wright et al., 2015). P. aeruginosa has also been identified in clinically healthy companion birds (Varriale et al., 2020).
Parameter 3 – Experimentally susceptible wildlife species (or family/order)
No information is available on experimentally susceptible wildlife species.
Parameter 4 – Experimentally susceptible domestic species (or family/order)
There are many species used as experimental P. aeruginosa infection models including mice (Kukavica‐Ibrulj et al., 2014), rats (Kukavica‐Ibrulj et al., 2008), pigs (Chevaleyre et al., 2016; Ten Have et al., 2019), ferrets (Keiser et al., 2015), chinchillas (Cotter et al., 1996) and zebrafish (Pont and Blanc‐Potard, 2021).
Reservoir animal species
Parameter 5 – Wild reservoir species (or family/order)
P. aeruginosa has been identified in the faeces of a number of different species of animals. A study by Ruiz‐Roldán et al. (2020) reported Pseudomonas spp. in 6.5% of 703 faecal samples. P. aeruginosa was identified in multiple different samples from wild boar (Ruiz‐Roldán et al., 2020). Wild snakes have also been found to carry P. aeruginosa in the faeces of some healthy animals (13%) (Colinon et al., 2010).
Parameter 6 – Domestic reservoir species (or family/order)
Farm animals such as sheep have been identified as a source of P. aeruginosa, as these have been cultured from the faeces from healthy animals (Ruiz‐Roldán et al., 2020). P. aeruginosa was cultured from the faeces of a high number (72/83) of captive snakes; however, prevalence was much lower in wild snakes (Colinon et al., 2010). MDR P. aeruginosa has also been identified in the faeces of dogs in shelters (Verma et al., 2021).
3.1.1.2. Article 7(a)(ii) The morbidity and mortality rates of the disease in animal populations
Morbidity
Parameter 1 – Prevalence/incidence
As an environmental, opportunistic pathogen, P. aeruginosa prevalence and incidence are difficult to determine. The bacterium can cause a wide variety of different infections and is often associated with altered underlying health. Most studies focus on the link with infection rather than studying incidence.
As P. aeruginosa is not a clear commensal in dogs or cats, longitudinal studies on carriage have not been performed. Few cross‐sectional studies on carriage are available; however, a limited number are available on dogs. A study on 228 dogs with no clinical signs of disease revealed that P. aeruginosa could be cultured from samples taken from 16.7% of dogs. Isolates were cultured from the ear (6.1%), eye (4.4%), genitalia and rectum (both 3.1%) (Park et al., 2020). Microbiome studies in healthy dogs and those with otitis reported that Pseudomonadaceae were present in both groups, but the relative abundance differed with a higher abundance detected in dogs with otitis (Borriello et al., 2020). However, other studies have found very little evidence of Pseudomonas spp. in healthy ears in dogs (Korbelik et al., 2019).
The prevalence varies significantly depending on infection type, and there is often little context or data available to estimate overall levels, particularly for cats. In dogs, Hattab et al. (2021) reported that overall P. aeruginosa caused 8% of infections in clinical cases submitted for routine veterinary diagnostics, accounting for 25.4% of otitis infections, 10% of skin infections and 1.6% of urinary tract infections. Urinary infections are significantly more prevalent in female dogs (Hall et al., 2013). P. aeruginosa is capable of causing respiratory tract infections in both cats and dogs; however, it is a minor pathogen in this niche (Moyaert et al., 2019a). Otitis is the most common P. aeruginosa infection in dogs. Studies on superficial canine infections, the most common being otitis, report a range in prevalence from 25% to 41% (Bourély et al., 2019; Dégi et al., 2021; Hattab et al., 2021). The variation in prevalence is likely due to a combination of differences in sampling and surveillance/identification of cases for study. Prevalence can also be associated with breed. The St. Bernard is more prone to eye infections, and P. aeruginosa (including MDR isolates) were the most prevalent Gram‐negative in this setting (Nadăş et al., 2021).
Studies reporting antimicrobial resistance are shown in Table 2. There is considerable variation in both the prevalence of resistance to certain antibiotics and the extent of antimicrobial susceptibility testing performed. Some studies report only resistance to gentamicin and enrofloxacin as common agents used to treat cats and dogs with P. aeruginosa infection. Gentamicin resistance shows a very wide variation from 4% to 62% (Table 2). This may also be affected by the inclusion criteria of some studies, and there is often little information regarding prior exposure to an antibiotic. Although comparisons are limited due to the small number of isolates from cats, there is little evidence for differences in resistance between isolates from cats and dogs (Werckenthin et al., 2007). Aminoglycoside resistance has been associated with mutations in efflux pumps such as MexXY (Poonsuk and Chuanchuen, 2012).
Table 2.
Published studies on P. aeruginosa from dogs and cats in Europe over the last two decades
| Animal | Country | No. of isolates | Resistance (%) | Notes | Infection | Year | Reference |
|---|---|---|---|---|---|---|---|
| Dogs | France (RESAPATH) | 46 | TIC 24%, TIM 35%, FEP 9%, ATM 7%, AMK 15%, GEN 57%, TOB 11%, FOF 48%, CIP 63% | Isolates from dogs significantly more resistant to GEN and TIM and CIP than isolates from other animals | Otitis | 2008–2011 | Haenni et al. (2015) |
| Dogs, cats | France (RESAPATH) | 24, 5 | Carbapenem‐resistant isolates studied: IMP 66% (19/29), MEM 69% (20/29) | 29/527 carbapenem‐resistant isolates chosen for inclusion in the study | Otitis and pulmonary infection | 2008–2014 | Haenni et al. (2017) |
| Dogs | Greece | 75 | ENR 44%, MAR 32%, PRA 48% | Prior treatment with FQ significantly increased resistance to FQ | Otitis | 2010–2014 | Vingopoulou et al. (2018) |
| Dogs | France (RESAPATH) | 2103 | ENR 68%, GEN 18% |
Trend to decline in FQ resistance over time |
Otitis | 2012–2016 | Bourély et al. (2019) |
| Dogs | Romania | 58 (from 142 assessed) | CAZ 47%, AZT 48%, AMK 55%, ATM 59%, GEN 62%, FEP 64%, MEM 74%, TZP 74%, IMP 78%, CIP 83%, TOB 91%, PMB 98% | 18 MDR isolates | Skin infections, otitis, perianal abscesses | 2019 | Dégi et al. (2021) |
| Dogs | Italy | 24 | CAZ 0%, GEN 0%, ATM 0%, IMP 0%, ENR 4%, TZP 8% | Intermediate resistance to ENR (42%) and IMP (29%) reported | Skin (6), otitis (15), UTIs (3) | 2019–2020 | Hattab et al., (2021) |
| Dogs, cats | Belgium, Czech Republic, France, Germany, Hungary, Italy, Netherlands, Poland, Spain, Switzerland and UK (COMPATH) | 23, 23 |
MICs reported instead of %. Dogs: FQ MIC50/90 of 0.5–2 μg mL−1 and NEO MIC50 and MIC90 of 8 and 32 μg mL−1 Cats: FQ MIC50/90 of 0.25–1 μg mL−1 and NEO MIC50 and MIC90 of 8 and 8 μg mL−1 |
Respiratory disease | 2013–2014 | Moyaert et al. (2019a,b) | |
| Dogs, cats | Iberian Peninsula | 825, 76 |
Dogs: ≥ 50% – AMC, FOX, AMP, LEX, CXM, CVN, CTX, CPD, SXT, FFC, CHL, FOF Cats: ≥ 50% – AMC, FOX, AMP, LEX, CXM, CVN, CTX, CPD, SXT, FFC, CHL, FOF. Of 28 antibiotics tested. |
Pseudomonas spp. Rather than P. aeruginosa | Otitis, wound infections, respiratory tract infections, pleuritis, dermatitis, abscesses, conjunctivitis | 2016–2018 | Li et al. (2020) |
| Dogs, cats | 12 European countries (ComPath) | 174, 12 | GEN – 10% R and 18% I | Only GEN tested | Skin, wound and ear infections | 2013–2014 | de Jong et al. (2020) |
| Dogs, cats | Germany | 36 |
MDR isolates in open wound treatment: 78% MDR isolates in follow treatment: 82% MDR isolates in bite wounds: 12% |
Limited data reported | Open wounds | 2011–2013 | Nolff et al. (2016) |
| Dogs, cats | Spain | 45, 19 |
Dogs: ≥ 50% – AMC, AMP, LEX, CEF, CXM, CTX, CVN, ENR, PRA, DOX, FOF, NIT, SXT Cats: ≥ 50% – AMC, AMP, LEX, CEF, CXM, CTX, CVN, DOX, FOF, NIT, SXT |
4% of isolates were MDR and 1 isolate from a cat was PDR | UTIs | 2016–2018 | Darwich et al. (2021) |
| Dogs, cats | Italy | 29, 1 |
10 antibiotics tested in total: ≥ 50% resistance in AMP, AMC, IMI, ENR, ERY, TET, SXT |
Increasing levels of R to PRA and MAR over the 4‐year period | Otitis, pyoderma | 2016–2019 | Nocera et al. (2021) |
| Dogs, cats | Czech Republic, France, Germany, Hungary, Italy, Netherlands, Poland, Spain, Sweden and UK (ComPath) | 160, 11 |
GEN – 19% R in dogs ENR – 18% R in cats MIC90 reported for a range of antibiotics. Similar for dogs and cats: PRA 2 μg/mL, ORB 8 μg/mL, MAR 2 μg/mL, Ibafloxacin > 8 μg/mL, ENR 8 μg/mL, GEN 8 μg/mL, CVN > 32 μg/mL, LEX > 32 μg/mL |
Pyoderma, wound infections, abscesses and otitis | 2008–2010 | Ludwig et al. (2016) | |
| Dogs, cats | Germany (BfT‐GermVet) | 78, 5 |
PRA resistance determined by MIC90: Skin and ear – 4 μg/mL, genital/urinary – 4 μg/mL |
Only focus was PRA | Skin, ear and genital infections, UTIs | 2004–2006 | Schink et al. (2013) |
| Dogs, cats | Germany (BfT‐GermVet) | 99 in total |
Dogs: GEN R‐27%, I‐29%, ENR R‐24%, I‐49%, GEN R‐11%, I‐39%, ENR R‐11%, I‐61% |
Skin, ear and mouth infections, urinary and genital tract infections | 2004–2006 | Werckenthin et al. (2007) | |
| Dogs, cats | Italy | 5, 1 | IPM MIC – 19 ug/mL, MEM MIC – 2–8 μg/mL | Isolates carried oprD mutations and extended‐spectrum β‐lactamases | Hospitalised pets | 2014–2015 | Gentilini et al. (2018) |
| Dogs, cats | UK | 20, 1 | PMB 92%, CST 54%, AMK 0%, CEF 92%, ENR 33%, GEN 4%, IPM 0%, MAR 21%, TIC 21%, TIM 4% | Isolates from a referral centre, therefore presumably difficult to treat | Otitis; skin, wound, genital and urinary infections | 2012 | Scott et al. (2019) |
| Dogs | Denmark | 39 | AMP 100%, AMC 100%, CET 100%, CLI 100%, ERY 100%, CHL 89.7%, SPT 97.4%, TET 89.7%, SXT 92.3%, KAN 95.0%, ENR 35.9%, GEN 15.4%, CST 2.6% | Otitis externa | 2000–2005 | Pedersen et al. (2007) | |
| Dogs | Croatia | 109 | FEP 31.7%, CAZ 0%, ENR 51.9%, CIP 8.7%, GEN 43.3%, TIM 10.6% | Increase in resistance to GEN and ENR since 2002 | Otitis | 2007–2009 | Mekić et al. (2011) |
AMC: amoxicillin–clavulanic acid; AMK: amikacin; AMX: amoxicillin; ATM: aztreonam; AZM: azithromycin; CAZ: ceftazidime; CEF: ceftiofur; CET: cefalotin; CHL: chloramphenicol; CIP: ciprofloxacin; CLI: clindamycin; CPD: cefpodoxime; CST: Colistin; CTX: cefotaxime; CVN: cefovecin; CXM: cefuroxime (axetil or sodium); DOX: doxycycline; ENR: enrofloxacin; ERY: erythromycin; FEP: cefepime; FFC: florfenicol; FOF: fosfomycin; FOX: cefoxitin; FQ: fluoroquinolones; GEN: gentamicin; I: intermediate; IPM: imipenem; KAN: kanamycin; LEX: cephalexin; MAR: marbofloxacin; MDR: multidrug‐resistant; MEM: meropenem; MIC: minimum inhibitory concentration; NEO: neomycin; NIT: nitrofurantoin; ORB: orbafloxacin; PDR: pandrug‐resistant; PMB: polymyxin B; PRA: pradofloxacin; R: resistant; SPT: spectinomycin; SXT: trimethoprim–sulfamethoxazole; TET: tetracycline; TIC: ticarcillin; TIM: ticarcillin–clavulanic acid; TOB: tobramycin; TZP: piperacillin–tazobactam, UTI: urinary tract infection.
Fluoroquinolones are also used in the treatment of companion animals with P. aeruginosa infections. Enrofloxacin is a first‐line veterinary fluoroquinolone with pradofloxacin and marbofloxacin also used. Ciprofloxacin is an important human fluoroquinolone. Like for gentamicin, resistance to enrofloxacin shows considerable study‐to‐study variation from 4% to 68% (Table 2). Vingopoulou et al. (2018) described similar resistance rates for enrofloxacin, pradofloxacin and marbofloxacin. Veterinary isolates have also displayed high resistance to ciprofloxacin (63–83%), therefore confirming the importance of studying resistance to human‐associated antibiotics in studies on veterinary isolates (Haenni et al., 2015; Dégi et al., 2021).
Polymyxins such as colistin are important in the control of human infections and often deemed an antimicrobial of last resort. However, polymyxin B is used in veterinary medicine. Limited studies report resistance to polymyxins. Two studies have reported high levels of resistance to polymyxin B (Scott et al., 2019; Dégi et al., 2021). One of these studies also included resistance to colistin at 54% (Scott et al., 2019). However, both of these studies were on isolates from veterinary hospitals, which therefore may represent a bias towards problematic, chronic infection cases. A study from Denmark on isolates from dogs reported low levels of colistin resistance (2.6%) (Pedersen et al., 2007).
Carbapenem‐resistant P. aeruginosa has been highlighted as a major issue by the WHO. Carbapenems include meropenem, imipenem and doripenem, key antimicrobials in human medicine, although this antimicrobial class is not used for treating infections in animals. Few studies investigated resistance to carbapenems; however, high prevalence of resistance has been reported in several studies (Gentilini et al., 2018; Dégi et al., 2021; Nocera et al., 2021).
Outside of the Union, AMR P. aeruginosa has also been detected in companion animals. A study on healthy and infected dogs in South Korea reported antimicrobial resistance in strains isolated from both healthy dogs and from infection (mostly otitis externa) compared to healthy controls (Park et al., 2020). Whilst infection isolates showed higher resistance than healthy controls, this difference was not significant. Worryingly, genes encoding carbapenemases such as VIM‐2 (Hyun et al., 2018) and IMP‐45 (Wang et al., 2014) have been detected in Asia (Ekapopphan et al., 2018). These carbapenemase genes such as bla‐VIM‐2 have also been identified in other animal niches such as cattle and fowl (Argudín et al., 2017). Resistance varies between countries, as a large study in dogs from Japan revealed no imipenem resistance, but 35% of isolates showed resistance to fosfomycin (Yukawa et al., 2017). Increases in resistance have also been reported in the USA and UK (Hall et al., 2013; Hewitt et al., 2020). In the UK, a significant increase in AMR P. aeruginosa isolates was detected over a 10–year period (1999–2009) (Hall et al., 2013). Although these countries are outside of the Union, it is important to consider recent global studies for full insight.
Parameter 2 – Case‐morbidity rate (% clinically diseased animals out of infected ones)
No studies are available to measure the case‐morbidity rate for P. aeruginosa.
Mortality
Parameter 3 – Case‐fatality rate
Although P. aeruginosa is known to cause fatal conditions such as sepsis and pneumonia, there is little literature that reports case‐fatality rates. The occurrence of death associated with AMR P. aeruginosa has not been reported.
3.1.1.3. Article 7(a)(iii) The zoonotic character of the disease
Parameter 1 – Report of zoonotic human cases (anywhere)
P. aeruginosa is a known human pathogen. It is described as opportunistic and infections are normally associated with an underlying health condition or breach in a normal protective barrier. It can cause infections in the respiratory, gastrointestinal and urinary tract, otitis, keratitis, wounds and burns infections. It can also cause sepsis and in some cases meningitis. P. aeruginosa causes 10–15% of all nosocomial infections worldwide and mortality rates associated with human infections can vary from 18% to 61% (Shi et al., 2019).
There are very few studies on potential transmission between animals and humans, and a combination of the opportunistic nature of P. aeruginosa and a separation between human and animal healthcare may result in potential cases being missed. Despite this, there have been some reports. A study identified cross‐contamination of the environment and owners from dogs with otitis (Morris et al., 2017).
The pet cat of a person with cystic fibrosis (CF) developed a respiratory infection caused by a transmissible strain, the Liverpool Epidemic Strain (LES) (Mohan et al., 2008). However, there is little evidence of any risk to humans with CF. A large study of 703 people with CF studied risk factors associated with dog and cat ownership. There was no significant difference in prevalence or age of acquisition of P. aeruginosa (Morrow et al., 2014). A possible case of transmission from a dog to a young child with CF has been reported; however, similarity was by antibiogram alone and, if there was transmission, no knowledge of directionality (pet–to–human vs. human–to–pet) was known (Michl et al., 2017).
The term transmissible strain has been used to describe some unusual strains of P. aeruginosa for which there is evidence of cross‐infection between unrelated individuals (Fothergill et al., 2012). Historically, this has occurred in people with CF in either healthcare settings or holiday camps. The genetic or phenotypic cause of transmissibility has not been determined. The vast majority of P. aeruginosa infections are acquired from environmental sources rather than direct spread from another infected individual.
The P. aeruginosa population has been well characterised using a variety of molecular genomics methods. Clones with links to human infections have also been found in animal infections; however, this is not evidence of transmission in itself (Haenni et al., 2017; Scott et al., 2019). This would be expected when studying a wide variety of infections. High‐risk clones such as ST233 and ST395 have been identified in isolates from dogs. ST233 has been identified in five of six continents and contains bla‐IMP and bla‐NDM carbapenemases (Del Barrio‐Tofiño et al., 2020). An ST233 VIM‐2‐producing P. aeruginosa isolate was isolated from a dog and from the faecal sample of its owner who had recently had a long hospital stay in an intensive care unit. This case study further highlights the potential for transmission between pets and owner with particular respect to high‐risk clones (Fernandes et al., 2018).
3.1.1.4. Article 7(a)(iv) The resistance to treatments, including antimicrobial resistance
Parameter 1 – Resistant strain to any treatment, even at laboratory level
P. aeruginosa is a highly resistant bacterial species and has by the WHO been designated a Priority 1 organism for which there is an urgent need for new therapeutics. A simple search using Pubmed and the terms ‘Pseudomonas aeruginosa antibiotic resistance’ yielded over 15,000 results and over 1,000 in 2020. Certain ST types have been deemed as high‐risk clones according to a combination of the antimicrobial resistance and virulence profiles. The worldwide top 10 P. aeruginosa high‐risk clones include ST235, ST111, ST233, ST244, ST357, ST308, ST175, ST277, ST654 and ST298. These include extensively drug‐resistant (XDR) strains that are resistant to all antibiotics tested (Del Barrio‐Tofiño et al., 2020).
Fluoroquinolone resistance has been reported and associated with mutations in DNA gyrase, topoisomerase and efflux pump overexpression (Vingopoulou et al., 2018). Enrofloxacin resistance has been demonstrated at high levels (Bourély et al., 2019) and this fluoroquinolone has been used as a veterinary antibiotic for the longest. Ciprofloxacin is an important human antimicrobial. Resistance to this has been widely reported in human infections and has also been identified in companion animal isolates (Haenni et al., 2015; Dégi et al., 2021).
The main carbapenems in use are meropenem, imipenem, doripenem and ertapenem. Resistance to carbapenems can be caused by a variety of mechanisms. Resistance can occur through changes in existing genetic or the acquisition of new genetic material. Intrinsic changes include changes in outer membrane permeability through alterations to porins, efflux pump activity and existing cephalosporinase activity (Meletis et al., 2012). Mutations in genes encoding porins such as oprD and efflux pumps such as mexAB–oprM are commonly identified. Acquisition of new genetic material is often the gain of transferable carbapenemases such as metallo‐β‐lactamases (Meletis et al., 2012). These enzymes can be carried on mobile genetic elements such as plasmids and integrons. Carbapenem resistance can be multifactorial and is often associated with resistance to other antibiotics, and therefore, the therapeutics available for use are dramatically reduced. This is a major issue in human medicine; however, this has been reported in veterinary medicine, too (Haenni et al., 2017).
Polymyxin B and polymyxin E (colistin) are used in veterinary and human medicine. These polycationic compounds disrupt the cell membrane by binding lipid A of lipopolysaccharide (LPS); however, systemic use is often considered a last resort due to toxic side effects. Polymyxins have historically been widely used in the veterinary setting, particularly in livestock such as for the treatment of Enterobacteriaceae and growth promotion in pigs. Resistance to polymyxins can be associated with chromosomal alterations, largely changes in LPS structure, or through the acquisition of new genetic material such as mcr genes. LPS modification leading to polymyxin resistance has been reported due to mutations in two component systems including pmrAB and phoPQ (Khondker and Rheinstädter, 2020). Polymyxin resistance genes such as mcr‐1 have also been identified on plasmids (Wang et al., 2018). Although polymyxin resistance has been identified in isolates from companion animals (Scott et al., 2019; Dégi et al., 2021), transferable plasmid‐related mcr genes have not been reported to date. However, mcr genes were first isolated from animals and their presence in other Gram‐negative bacteria in this niche has been widely reported including in the Union, particularly from porcine origin (Liu et al., 2016; Xavier et al., 2016; Yin et al., 2017; Wang et al., 2018, 2019; Borowiak et al., 2019; Carroll et al., 2019; Gelbíčová et al., 2019).
3.1.1.5. Article 7(a)(v) The persistence of the disease in an animal population or the environment
Animal population
Parameter 1 – Duration of infectious period in animals
Infection of cats and dogs is normally from the environment and does not transmit between individuals. Animals with infections may result in high levels of the bacterium in the immediate environment (household, shelter, etc.); however, there is little data on persistence in this niche. P. aeruginosa can cause both acute and chronic infections, and therefore, the length of infection can vary from days/week to chronic infections that can potentially last years in animals and decades in humans. Duration of treatment for P. aeruginosa infections in dogs is typically 3–4 weeks but can be up to 12 weeks (Hillier et al., 2006).
A case study of a cat with severe, chronic rhinosinusitis with mucoid P. aeruginosa has been reported (Sharma et al., 2019). Mucoid P. aeruginosa is generally linked with chronic respiratory infections and in people with CF often signifies a stage at which cure is not possible. Therefore, the presence of these phenotypes in infections in cats may be associated with infections that are much harder to eliminate. In the case study, the 6‐year‐old cat had presented with sinus issues since kittenhood, but the duration of infection with P. aeruginosa is unclear.
Parameter 2 – Presence and duration of latent infection period
There are no data to estimate the duration of the latent infection period for P. aeruginosa infections.
Parameter 3 – Presence and duration of the pathogen in healthy carriers
There are limited studies on the presence and duration of P. aeruginosa in healthy dogs and no data available in cats. As stated previously, a study on 228 dogs with no clinical signs of disease revealed that P. aeruginosa could be cultured from samples taken from 16.7% of dogs. Isolates were cultured from the ear (6.1%), eye (4.4%), genitalia and rectum (both 3.1%) (Park et al., 2020). Microbiome studies in healthy dogs and those with otitis reported that Pseudomonadaceae were present in both groups, but the relative abundance differed (Borriello et al., 2020). However, other studies have found very little evidence of Pseudomonas spp. in healthy ears (Korbelik et al., 2019). In a study of bacteria in faecal samples from dogs in shelters, only a single isolate of P. aeruginosa was cultured (Verma et al., 2021).
Environment
Parameter 4 – Length of survival of the agent and/or detection of DNA in selected matrices (soil, water, air) from the environment
As P. aeruginosa is an environmental organism, it readily survives in soil and water. It can survive between 4°C and 42°C; however, virulence and growth would be reduced at lower temperatures (LaBauve and Wargo, 2012). It can also utilise a wide range of carbon sources and this flexibility is attributed to its large genome, which supports metabolic diversity.
Survival in air has been reported; however, this is normally through aerosolisation from those with respiratory infection, and the bacterium can be identified in aerosolised droplets. Aerosols from coughing have been shown to travel up to 4 m and stay in the air for around 45 min (Schelstraete et al., 2008; Knibbs et al., 2014). However, these studies are based on humans and no data exist for potential aerosolisation from animals. Environmental contamination around infected individuals has been identified although there appears to be strain–to–strain variation in survival on dry surfaces (Panagea et al., 2005). Sinks can be a significant source of P. aeruginosa contamination and good cleaning/hygiene measures around sinks and water sources is needed, particularly in the healthcare setting (Fusch et al., 2015).
3.1.1.6. Article 7(a)(vi) The routes and speed of transmission of the disease between animals, and, when relevant, between animals and humans
Routes of transmission
Parameter 1 – Types of routes of transmission from animal to animal (horizontal, vertical)
There is little published evidence of animal–to–animal transmission. The vast majority of P. aeruginosa infection cases would be infection from the environment and there are no reports of nosocomial transmission in veterinary clinics resulting in infection. However, transmission between susceptible individuals (people with CF) has been documented in humans in both healthcare and leisure settings (Fothergill et al., 2012); therefore, there may be potential for transmission between susceptible individuals in certain settings. For people with CF, P. aeruginosa causes lung infections and therefore can be aerosolised through coughing. This may contribute to transmission and potential transmission dynamics may be altered for different infection types such as otitis or pyoderma. P. aeruginosa has been previously listed as a concern for transmission in small animal clinics with challenges highlighted as lesser patient compliance and hygiene (Stull and Weese, 2015). The bacterium was repeatedly isolated from bedding and the veterinary clinical environment before and after infection control intervention at a dog shelter (Horsman et al., 2020). The presence of P. aeruginosa in the surrounding environment and the data regarding transmission of P. aeruginosa in certain settings (Fothergill et al., 2012) may mean that the bacterium could be transmitted horizontally between susceptible animals (those with a breach to normal defence barriers or underlying health issues) in certain settings, such as veterinary inpatient facilities or homes with multiple animals/animal shelters.
Parameter 2 – Types of routes of transmission between animals and humans (direct, indirect, including food‐borne)
A potential direct route of transmission from an infected human to a cat has been reported (Mohan et al., 2008); however, this involved a P. aeruginosa strain that is known for its link with person–to–person transmission (Fothergill et al., 2012). Bacterial contamination of the environment surrounding an infected individual has also been reported; therefore showing that indirect transmission may be possible (Panagea et al., 2005). The majority of cases of P. aeruginosa infection would be independently acquired from the environment. There is no evidence of food‐borne transmission.
Speed of transmission
Parameter 3 – Incidence between animals and, when relevant, between animals and humans
Although there have been isolated case reports of transmission from humans to animals, there is little population level data on this topic. There have been no clear reports of transmission of AMR P. aeruginosa from an animal to a human resulting in infection (Pomba et al., 2017). However, cases resulting in animal infection have been reported (Mohan et al., 2008) and P. aeruginosa has been isolated from the faeces of the immunosuppressed owner (Fernandes et al., 2018). Therefore, despite little information regarding the incidence, there is potential for transmission between these two groups and this may be associated with the susceptibility of the individuals/animals involved and the specific P. aeruginosa strain.
Parameter 4 – Transmission rate (β) (from R0 and infectious period) between animals and, when relevant, between animals and humans
There are no data on the rate of transmission.
3.1.1.7. Article 7(a)(vii) The absence or presence and distribution of the disease in the Union and, where the disease is not present in the Union, the risk of its introduction into the Union
Presence and distribution
Parameter 2 – Type of epidemiological occurrence (sporadic, epidemic, endemic) at MS level
The bacterial species is globally endemic and can be found in a wide variety of niches from soil and water along with contaminating the clinical environment. Infections caused by P. aeruginosa are largely considered to be sporadic and based on underlying health or genetic factors. There are examples of transmission of P. aeruginosa in humans in specific settings; however, these have not been widely reported in cats and dogs.
A comparison of the epidemiological occurrence and resistance levels in different countries is not possible due to differences in surveillance and reporting practices. This is also true for antimicrobial resistance occurrence. In particular, there is a paucity of studies that include resistance to human antibiotics in isolates in dogs and cats. Collateral resistance and cross‐resistance have been reported for P. aeruginosa whereby resistance to several antimicrobials can occur simultaneously, and therefore, resistance may be present in niches where that particular antimicrobial has not been used routinely (Barbosa et al., 2017). MDR P. aeruginosa could pose a health risk to humans and animals.
Risk of introduction
This section is not relevant due to the ubiquitous occurrence of this bacterial species; the risk of introduction is therefore not relevant to assess, as the pathogen is already present in the EU.
3.1.1.8. Article 7(a)(viii) The existence of diagnostic and disease control tools
Diagnostic tools
Parameter 1 – Existence of diagnostic tools
Routine diagnostics are heavily reliant on bacterial culture. P. aeruginosa grows readily on a wide variety of media and under a wide variety of conditions. Although selective media are readily available, the majority of diagnostic laboratories detects P. aeruginosa on standard media. Colony morphology can show wide variation in both colour (e.g. cream, yellow, green, red and translucent) and form (e.g. smooth, wrinkly, mucoid and rough), and therefore, identification could be challenging to someone inexperienced. Following culture, matrix‐assisted laser desorption ionisation–time‐of‐flight mass spectrometry (MALDI‐TOF MS) has become a reference standard for species identification in many diagnostic laboratories. Specific PCR‐based assays are available and often target porins such as the oprL gene or the 16S rRNA gene (followed by amplicon sequencing). The vast majority of these methods are reliant on an initial culture period that can take 24–48 h. Direct methods to extract DNA directly from clinical samples followed by qPCR have been developed; however, these are not widely used, particularly with regard to clinical isolates from cats and dogs. Other rapid testing combinations have also been recently described (Ulrich et al., 2020).
AMR phenotype is determined following culture through use of either disk diffusion assays or agar plates, or broth microdilution minimum inhibitory concentration assays. Published clinical breakpoints are available through the European Committee on Antimicrobial Susceptibility Testing (EUCAST) or the Clinical and Laboratory Standards Institute (CLSI), with breakpoints for some commonly used antibiotics in cats and dogs such as polymyxin B only available through CLSI. Alternatives such as E‐test strips are also used to determine resistance. For some antibiotics such as colistin and polymyxin B, liquid culture in cationic adjusted nutrient broth must be used to determine resistance due to limited diffusion through in agar. Amplification of specific resistance genes is not routinely used in the context of determining P. aeruginosa resistance as linkage between phenotype and genotype is problematic. This is due to multiple resistance mechanisms and their relative gene expression that can simultaneously contribute to increments in resistance. However, the presence of some genes has clear relevance including mcr‐1 and mcr‐2 for polymyxin resistance and genes encoding carbapenemases.
Parameter 2 – Existence of control tools
Currently, there are no licensed vaccines available for use against P. aeruginosa. However, there has been an increase in research in this area over recent years (Tümmler, 2019; Sainz‐Mejías et al., 2020). Control is performed through the use of antimicrobials and this can be systemic or local. The choice is dependent on the site of infection. Disinfectants can also be used as part of hygiene and contamination control measures.
Antibiotics are widely available and in general effective in cats and dogs (Hillier et al., 2006). Treatments are well tolerated and there are no data on treatment failure. However, the lack of data on treatment failure may be due to a lack of published studies specifically on this topic. Alternatively, the lack of data may suggest that treatment failure is not an issue, therefore highlighting a potential disparity between reported in vitro resistance and clinical resistance of infections in dogs and cats.
P. aeruginosa in dogs and cats are treated with fluoroquinolones including enrofloxacin, marbofloxacin and pradofloxacin or aminoglycosides such as gentamicin. Polymyxin B is also used topically in ear preparations for P. aeruginosa‐associated otitis externa (Pye, 2018). Resistance to these antimicrobials, particularly enrofloxacin and gentamicin, are reported in Table 2. For otitis, ear hygiene can also aid in treatment success. Preparations that include Tris‐EDTA have been shown to resolve infection and reduce MICs to some antibiotics including against biofilms (Pye, 2018).
3.1.2. Article 7(b) The impact of diseases
3.1.2.1. Article 7(b)(i) The impact of the disease on agricultural and aquaculture production and other parts of the economy
The level of presence of the disease in the Union
Parameter 1 – Number of MSs where the disease is present
Infections caused by P. aeruginosa are present in all MSs; however, the extent to which they are reported/studied varies based on internal surveillance studies. Infections of this type could be identified in any country of the world due to the ubiquitous occurrence of this bacterial species. Although P. aeruginosa is intrinsically resistant to many antibiotics, further antimicrobial resistance varies a lot between MSs (EFSA AHAW Panel, 2021a). Nevertheless, these reports can be affected by sample/isolate inclusion or study focus and wider data collection. Many manuscripts only report resistance to a narrow range of antimicrobials, thereby making detailed comparisons between MSs difficult.
The loss of production due to the disease
Parameter 2 – Proportion of production losses (%) by epidemic/endemic situation
There is little evidence of animal neonatal deaths associated with P. aeruginosa although it does have the potential to cause fatal disease. Increasing antimicrobial resistance and associated treatment challenges may have the potential to affect the breeding industry if not well controlled in the future. This is most applicable to dogs, as in cats, infections are much less common.
3.1.2.2. Article 7(b)(ii) The impact of the disease on human health
Transmissibility between animals and humans
Parameter 1 – Types of routes of transmission between animals and humans
There are few reports on routes of transmission between animals and humans. The case report of infection from a human to a cat was associated with a chronic respiratory infection in the human and led to a respiratory infection in the cat (Mohan et al., 2008). There was reported very close contact between the two, and possible routes of transmission include via droplet spread or aerosolisation. Transmission could also occur indirectly via the contaminated shared environment of animals and humans.
Parameter 2 – Incidence of zoonotic cases
There are no data on the incidence of zoonotic cases of P. aeruginosa infection.
Transmissibility between humans
The most common route of P. aeruginosa infection is contamination from the environment associated with a breach in normal defences. In this way, the bacterium is described as an opportunistic pathogen. Transmissibility between humans has been identified in individuals with CF, particularly attending summer camps or shared clinics. A number of transmissible strains have been identified including the LES, DK2 from Denmark, the Prairie Epidemic Strain (PES), Australian Epidemic Strain (AES 1–3), amongst others (Fothergill et al., 2012). The genetic factors underlying transmissibility are unclear and different transmissible strains do not cluster together in terms of genotype. These strains have been associated with increased morbidity and mortality in this patient population; however, there is variation between each strain (Fothergill et al., 2012).
Parameter 3 – Human‐to‐human transmission is sufficient to sustain sporadic cases or community‐level outbreak
As P. aeruginosa is an endemic, environmental bacterium, this section is not applicable.
Parameter 4 – Sporadic, epidemic or pandemic potential
P. aeruginosa is an endemic bacterium. It generally causes sporadic cases in susceptible people although it is a significant cause of disease and a major healthcare‐associated opportunistic pathogen.
The severity of human forms of the disease
P. aeruginosa can cause significant morbidity and mortality in human infections. As stated previously, infection is normally associated with an underlying condition or breach in an existing barrier. It can cause a range of infections including those in the ears, eyes, urogenital tract, wounds, respiratory system and skin. P. aeruginosa can also cause severe and life‐threatening illnesses including sepsis and meningitis (Huang et al., 2002). It has also been reported as causing Shanghai Fever, a poorly understood condition of children (Chuang et al., 2014).
Parameter 5 – Disability‐adjusted life year (DALY)
DALY attributed to carbapenem‐resistant P. aeruginosa in the EU has been extensively reviewed (Cassini et al., 2019). In 2015, the median number of infections was 61,892 and the median number of deaths attributed was 4,155. The median number of DALYs per 100,000 population was 27.2 and the median percentage of total DALYs was 16%. Italy and Greece had a substantially higher estimated burden of AMR bacteria than other EU and European Economic Area (EEA) countries (Cassini et al., 2019).
The availability of effective prevention or medical treatment in humans
Parameter 6 – Availability of medical treatment and their effectiveness (therapeutic effect and any resistance)
P. aeruginosa is a highly resistant Gram‐negative pathogen. Treatment is through the use of antimicrobials in either single or dual combination. Breakpoints have been published by EUCAST (Matuschek et al., 2014) and antibiotics used against P. aeruginosa in humans are shown in Table 3. Antibiotic administration routes can be oral, topical, intravenous or inhaled, dependent on the infection type and antibiotic type.
Table 3.
Antibiotics with activity against P. aeruginosa with breakpoints published by EUCAST, including their suggested use in veterinary medicine according to the European Medicines Agency (EMA, 2019)
| Antibiotic class | Antibiotic | Usage in veterinary medicine |
|---|---|---|
| Penicillins | Piperacillin | Avoid |
| Piperacillin–tazobactam | Avoid | |
| Ticarcillin | Avoid | |
| Ticarcillin–clavulanic acid | Avoid | |
| Cephalosporins | Cefepime | Restrict |
| Cefiderocol | Not stated | |
| Ceftazidime | Restrict | |
| Ceftazidime–avibactam | Avoid | |
| Cefoxitin | Caution | |
| Ceftolozane–tazobactam | Avoid | |
| Carbapenems | Doripenem | Avoid |
| Imipenem | Avoid | |
| Imipenem–relebactam | Avoid | |
| Meropenem | Avoid | |
| Meropenem–vaborbactam | Avoid | |
| Monobactams | Aztreonam | Avoid |
| Fluoroquinolones | Ciprofloxacin | Restrict |
| Levofloxacin | Restrict | |
| Aminoglycosides | Amikacin | Caution |
| Tobramycin | Caution | |
| Polymyxins | Colistin | Restrict |
For Pseudomonas otitis, first‐line treatment is often topical using antibiotics such as neomycin, polymyxin B and gentamicin. Other treatment can include systemic antibiotics including tobramycin, amikacin, enrofloxacin (and other quinolones such as marbofloxacin or pradofloxacin).
Fosfomycin and mupirocin should also be avoided, but no P. aeruginosa‐specific breakpoint is currently available through EUCAST.
P. aeruginosa is an important human pathogen that can lead to death. MDR and XDR P. aeruginosa has been widely reported. Therefore, treatment failure has been reported in humans. A study on ventilator‐associated pneumonia caused by P. aeruginosa reported treatment failure in 112/314 (36%) of patients (Planquette et al., 2013). A mortality rate of 19% has been reported for patients with carbapenem‐resistant P. aeruginosa bacteraemia (Buehrle et al., 2017). In people with CF with P. aeruginosa lung infection, early eradication therapy is possible but a treatment failure of 15–19% has been reported (Høiby et al., 2005; Taccetti et al., 2005). Once established, P. aeruginosa cannot be eradicated from this niche.
There are little data on treatment failure in animals; however, a paper by Hawkins et al. (2010) on a novel therapeutic reported inclusion criteria for dogs with otitis of at least 3 months with at least three failed antibiotic treatments and therefore suggests treatment failure is an issue. Effective treatments can be challenging and reoccurrence of infection following the cessation of treatment has been reported (Barnard and Foster, 2018). A study on 20 dogs with pyoderma caused by P. aeruginosa, one dog (5%) displayed treatment failure. The remaining dogs were treated for between 3 and 12 weeks and showed resolution of infection (Barnard and Foster, 2018).
Parameter 7 – Availability of vaccines and their effectiveness (reduced morbidity)
No vaccines are currently available.
3.1.2.3. Article 7(b)(iii) The impact of the disease on animal welfare
Parameter 1 – Severity of clinical signs at case level and related level, and duration of impairment
P. aeruginosa can cause a wide variety of infections in dogs and cats, and the severity of disease will be based on the type of infection along with individual risk factors of the animal and the infecting bacterial strain. However, in dogs, P. aeruginosa most commonly causes infections that are not life‐threatening, such as otitis and pyoderma. A recent study on the cause of deaths in dogs did not reveal P. aeruginosa as a cause (Cardillo et al., 2020).
Clinical signs of otitis can include head shaking, discharge from the ears and ulceration of the ear canal. If infection penetrates deeper, this can progress to neurological involvement including hearing loss and pain when opening the mouth or swallowing (Pye, 2018). Treatment requires washing of the affected area and antibiotic treatment for 4 weeks. Following this, diagnostics are repeated to confirm the presence of the pathogen or not (Pye, 2018).
Pyoderma is a pyogenic bacterial skin infection and is a common cause of infection in dogs. Although P. aeruginosa is not the main pathogen causing pyoderma in dogs, it has been associated with deep pyoderma and is often considered challenging to treat. Studies have linked P. aeruginosa pyoderma with necrotic and ulcerative skin lesions with green discharge and haemorrhagic bullae, cellulitis and abscessation (Done, 1974; Hillier et al., 2006). Additional symptoms such as lethargy, anorexia and exercise intolerance have also been reported (Hillier et al., 2006).
3.1.2.4. Article 7(b)(iv) The impact of the disease on biodiversity and the environment
Biodiversity
Parameter 1 – Endangered wild species affected: listed species as in CITES and/or IUCN list
There are no data on this aspect. One isolate of P. aeruginosa was found in a study on sea turtles; however, there was no evidence of disease and the isolate was highly susceptible to antibiotics (Oliveira et al., 2017).
Parameter 2 – Mortality in wild species
There are no data on mortality in wild species attributed to P. aeruginosa.
Environment
Parameter 3 – Capacity of the pathogen to persist in the environment and cause mortality in wildlife
P. aeruginosa is an environmental bacterium and therefore can survive in the environment. The environment could therefore be a source of sporadic infection in any susceptible species.
3.1.3. Article 7(c) Its potential to generate a crisis situation and its potential use in bioterrorism
Parameter 1 – Listed in OIE/CFSPH classification of pathogens
Not listed.
Parameter 2 – Listed in the Encyclopaedia of Bioterrorism Defence of Australia Group
Not listed.
Parameter 3 – Included in any other list of potential bio‐agro‐terrorism agents
Not listed.
3.1.4. Article 7(d) The feasibility, availability and effectiveness of the following disease prevention and control measures
3.1.4.1. Article 7(d)(i) Diagnostic tools and capacities
Availability
Parameter 1 – Officially/internationally recognised diagnostic tools, OIE‐certified
There are no officially/internationally recognised diagnostic tests that are certified or recommended by the OIE.
Diagnosis of P. aeruginosa is based on a combination of clinical signs to identify the disease and standard bacterial culture to identify the causative pathogen. If available, this can be followed up by the use of MALDI‐TOF MS. Detection of resistance is based on the previously mentioned tools, namely MIC testing and disk diffusion. PCR for detection of resistance genes can be performed, but this is not a routine practice for P. aeruginosa.
Effectiveness
Parameter 2 – Sensitivity and specificity of diagnostic tests
There are no officially/internationally recognised diagnostic tests.
MALDI‐TOF MS has been used to identify high‐risk clones of P. aeruginosa with a sensitivity and specificity of 97.1% and 99.4%, respectively (Mulet et al., 2021). Culture using Pseudomonas‐selective media has shown a high sensitivity (98–100%) but low specificity ranging between 40% and 72% (Weiser et al., 2014). This highlights that bacteria can be misclassified using culture alone, even if selective media are used. Other confirmation such as MALDI‐TOF MS could be used to confirm species identification.
Feasibility
Parameter 3 – Type of sample matrix to be tested (blood, tissue, etc.)
The type of sample is based upon the clinical disease presented. For otitis and skin infections, swabs would be used. For UTIs, a urine sample may be obtained. For other infections such as genital, respiratory and wounds, swabs would again be the most common type of sample. Biopsies or tissue scrapes may also be applicable for some infections.
3.1.4.2. Article 7(d)(ii) Vaccination
No vaccines are currently available against P. aeruginosa.
3.1.4.3. Article 7(d)(iii) Medical treatments
Availability
Parameter 1 – Types of drugs available on the market
As stated previously, P. aeruginosa in dogs and cats are treated with fluoroquinolones including enrofloxacin, marbofloxacin and pradofloxacin or aminoglycosides such as gentamicin. Polymyxin B is also used topically in ear preparations for P. aeruginosa‐associated otitis externa (Pye, 2018).
Parameter 2 – Availability/production capacity (per year)
Antimicrobials that can be used against P. aeruginosa are available globally.
Effectiveness
Parameter 3 – Therapeutic effects in the field (effectiveness)
In dogs and cats, P. aeruginosa treatment appears to be generally effective although the bacterium has the ability to cause chronic infections due to a combination of biofilm formation ability and antimicrobial resistance. The rate of treatment failure in cats and dogs is not reported. Increasing antimicrobial resistance is likely to lead to increased treatment times and poorer outcomes. MDR bacteria carrying mobile resistance genes have been reported (Lin et al., 2012).
Feasibility
Parameter 4 – Way of administration
Systemic antimicrobials are usually administered orally. This enables owners to treat pets relatively easily and at home. Skin infections can be treated topically without the need for systemic antimicrobial therapy. Ear drops and washes are also available for some otitis infections. With increasing antimicrobial resistance, further antimicrobial options may require alternative routes of administration. This could include repeated injection or intravenous administration. However, this would likely be associated with a greater impact on animal welfare and higher treatment costs.
3.1.4.4. Article 7(d)(iv) Biosecurity measures
Availability
Parameter 1 – Available biosecurity measures
Decontamination using disinfectants is possible for P. aeruginosa, with hydrogen peroxide and sodium hypochlorite being particularly effective (Lineback et al., 2018). This is important in the surgical environment and for surgical equipment. These interventions help to prevent hospital‐acquired infections. Disinfectants are available in wipes, sprays and concentrate format.
Effectiveness
Parameter 2 – Effectiveness of biosecurity measures in preventing the pathogen introduction
Disinfection‐based biosecurity measures are effective against P. aeruginosa in the healthcare setting. However, disinfectants should not be kept for long periods as there have been reports on P. aeruginosa contaminating such products and this would ameliorate the effectiveness of this intervention. Effective disinfectants include didecyldimethylammonium chloride, hydrogen peroxide and sodium hypochlorite (Beier et al., 2015; Lineback et al., 2018). Resistance to disinfectants in veterinary‐associated isolates has been reported but is not routinely monitored. This included resistance to cetyl ammonium halides, chlorhexidine and benzyl ammonium chlorides, which are common formulations used in the veterinary setting (Beier et al., 2015).
Feasibility
Parameter 3 – Feasibility of biosecurity measures
These biosecurity measures are feasible and relatively low cost. They should be part of routine decontamination in surgical and healthcare settings.
3.1.4.5. Article 7(d)(v) Restrictions on the movement of animals and products
Availability
Parameter 1 – Available movement restriction measures
Isolation of diseased animals with infection with MDR P. aeruginosa would be possible. Such isolation could involve housing a patient in a dedicated isolation ward or using enhanced precautions in a general ward in a veterinary setting if the diseased animal needs to be admitted. Movement restrictions could prevent the contamination of the environment with MDR isolates.
Effectiveness
Parameter 2 – Effectiveness of restriction of animal movement in preventing the between‐farm spread
There is no data available on this aspect.
Feasibility
Parameter 3 – Feasibility of restriction of animal movement
Within a veterinary facility or hospital, the ability to separate and isolate animals may be limited based on structure and size. Most diseased animals would be sent home during treatment. Owners could be advised to not keep the diseased animal with susceptible animals during treatment. Treatment typically takes 1–3 months; however, otitis can become a chronic issue in some dogs. Movement restrictions could potentially prevent the contamination of the environment with MDR/XDR isolates.
3.1.4.6. Article 7(d)(vi) Killing of animals
Availability
Parameter 1 – Available methods for killing animals
Veterinarians may recommend euthanasia of diseased animals affected by severe P. aeruginosa infections that have poor prognosis and cannot be treated effectively with veterinary antimicrobials. This would be an individual decision based on the health of the animal and ultimately the agreement of the owner.
Effectiveness
Parameter 2 – Effectiveness of killing animals (at farm level or within the farm) for reducing/stopping spread of the disease
Euthanasia would usually not be considered a method for controlling spread of disease in companion animals. The ubiquitous nature of the bacterium and the opportunistic nature of infection would make this intervention inappropriate. Euthanasia would be a method for limiting suffering in individual animals.
Feasibility
Parameter 3 – Feasibility of killing animals
Euthanasia is feasible but is usually considered a last resort following a number of treatment failures. However, P. aeruginosa is ubiquitous and killing infected animals does not solve the occurrence of the agent.
3.1.4.7. Article 7(d)(vii) Disposal of carcasses and other relevant animal by‐products
Bodies of dead animals infected with P. aeruginosa do not pose any additional risks to the public or indeed animal health. Disposal options would be the same methods as other companion animal deaths, namely burial or incineration. Effectiveness and feasibility are the same as for other deaths of companion animals. This is routine in most veterinary practices.
3.1.5. Article 7(e) The impact of disease prevention and control measures
3.1.5.1. Article 7(e)(i) The direct and indirect costs for the affected sectors and the economy as a whole
Parameter 1 – Cost of control (e.g. treatment/vaccine, biosecurity)
Treatment costs would impact companion animal owners and these costs may be increased when faced with AMR strains. Intensive treatment may involve the use of prolonged treatment periods (months) and the use of multiple antimicrobial agents. If front‐line treatments fail, alternative treatments would currently be off‐license and may require repeated administration via injection (Pye, 2018). Despite intensive treatment, treatment failure is possible and can result in specialist treatment, hospitalisation, additional outpatient visits, detailed diagnostics and further therapy (both direct and supportive). However, specific costs associated with this and potential increases are not available.
Newer antimicrobials are being developed, but these would be likely reserved for human use. Other alternative such as phage therapy have been trialled in dogs with P. aeruginosa otitis (Hawkins et al., 2010).
Parameter 2 – Cost of eradication (culling, compensation)
Due to the ubiquitous nature of the bacterium, eradication is not possible. Euthanasia of individuals would be possible if clinically indicated. The cost of this will likely vary between veterinary clinics.
Parameter 3 – Cost of surveillance and monitoring
There are no specific data to estimate cost of surveillance in the Union. Current monitoring tends to be passive and often country‐specific. Surveillance systems include Resapath, Compath and BfT‐GermVet. However, there are no data on the specific costs of monitoring P. aeruginosa in dogs and cats.
Parameter 4 – Trade loss (bans, embargoes, sanctions) by animal product
There are no official embargoes or bans associated with P. aeruginosa infection in dogs and cats; however, affected animals would not be able to participate in shows if showing signs of infections. This could lead to a limited amount of trade loss; however, there is no information on this. The value of keeping, breeding and trading cats and dogs in the EU is €1.3 billion (Schrijver et al., 2015).
Parameter 5 – Importance of the disease for the affected sector (% loss or € lost compared to business amount of the sector)
As stated above, the value of keeping, breeding and trading cats and dogs in the EU is €1.3 billion (Schrijver et al., 2015). There is little information on how AMR P. aeruginosa may affect this, but it could have an impact on dog and cat shows, as animals with P. aeruginosa infection and showing clinical signs could not participate.
3.1.5.2. Article 7(e)(ii) The societal acceptance of disease prevention and control measures
Disease prevention methods are currently limited and likely revolve around hygiene at potential routes of entry or breached barriers; however, evidence of the impact of these interventions, particularly in the companion animal context are lacking. Control measures are likely to be well tolerated; however, increased antimicrobial resistance may lead to higher veterinary costs and less choice of antimicrobials. In situations of treatment failure with potentially life‐threatening consequences, the lack of range of approved veterinary antibiotics may prove more difficult for owners to tolerate. This could put pressure on the use of antimicrobials reserved for human use only.
3.1.5.3. Article 7(e)(iii) The welfare of affected subpopulations of kept and wild animals
Parameter 1 – Welfare impact of control measures on domestic animals
Increasing antimicrobial resistance has the potential to affect animal welfare due to prolonged treatment, greater side effects and poor outcomes associated with treatment failure. Resistance to antibiotics that can be administered orally would lead to greater intervention and treatments that may require repeated injections. This would have an impact on welfare.
Parameter 2 – Wildlife depopulation as control measure
Wildlife depopulation is not a measure that would be used in the control of this disease.
3.1.5.4. Article 7(e)(iv) The environment and biodiversity
Environment
Parameter 1 – Use and potential residuals of biocides or medical drugs in environmental compartments (soil, water, feed, manure)
With increasing prevalence of AMR P. aeruginosa, more antibiotics may be used to treat infections. During treatment, antibiotics are not completely absorbed and metabolised by the body and therefore can be found in excreted urine and faeces. Antimicrobials do have the potential to contaminate water; however, specific contamination directly from use of antimicrobials in companion animals has not been reported. Certain antibiotics are known to persist in the environment. Fluoroquinolones can persist in the environment for around 100 days and have good water solubility (Janecko et al., 2016). Furthermore, the breakdown of enrofloxacin, an important veterinary antibiotic, leads to the production of compounds highly similar to ciprofloxacin, an important human antibiotic. Long‐term presence in the environment could lead to altered ecosystems such as in soil or aquatic niches.
Biodiversity
Parameter 1 – Mortality in wild species
There are no reports of mortality in wild species due to specific P. aeruginosa control measures. However, fluoroquinolones have the potential to affect algal and bacterial species along with some vertebrates and invertebrates (Kümmerer, 2009).
3.2. Assessment of AMR Pseudomonas aeruginosa according to Article 5 criteria of the AHL on its eligibility to be listed
3.2.1. Detailed outcome on Article 5 criteria
In Table 4 and Figure 1, the results of the expert judgement on the Article 5 criteria of the AHL for AMR P. aeruginosa in dogs and cats are presented.
Table 4.
Outcome of the expert judgement on Article 5 criteria
| Criteria to be met by the disease: According to the AHL, a disease shall be included in the list referred to in point (b) of paragraph 1 of Article 5 if it has been assessed in accordance with Article 7 and meets all of the following criteria | Outcome | ||||
|---|---|---|---|---|---|
|
Median range (%) |
Criterion fulfilment | Number of na | Number of experts | ||
| A(i) | The disease is transmissible | 33–90 | Uncertain | 0 | 12 |
| A(ii) | Animal species are either susceptible to the disease or vectors and reservoirs thereof exist in the Union | 99–100 | Fulfilled | 0 | 14 |
| A(iii) | The disease causes negative effects on animal health or poses a risk to public health due to its zoonotic character | 90–99 | Fulfilled | 0 | 14 |
| A(iv) | Diagnostic tools are available for the disease | 95–100 | Fulfilled | 0 | 14 |
| A(v) | Risk‐mitigating measures and, where relevant, surveillance of the disease are effective and proportionate to the risks posed by the disease in the Union | 33–90 | Uncertain | 0 | 13 |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at point A(i)–A(v), the disease needs to fulfil at least one of the following criteria | |||||
| B(i) | The disease causes or could cause significant negative effects in the Union on animal health, or poses or could pose a significant risk to public health due to its zoonotic character | 33–66 | Uncertain | 0 | 13 |
| B(ii) | The disease agent has developed resistance to treatments which poses a significant danger to public and/or animal health in the Union | 75–95 | Fulfilled | 0 | 13 |
| B(iii) | The disease causes or could cause a significant negative economic impact affecting agriculture or aquaculture production in the Union | 5–33 | Not fulfilled | 0 | 13 |
| B(iv) | The disease has the potential to generate a crisis or the disease agent could be used for the purpose of bioterrorism | 1–5 | Not fulfilled | 0 | 14 |
| B(v) | The disease has or could have a significant negative impact on the environment, including biodiversity, of the Union | 5–10 | Not fulfilled | 0 | 13 |
na: not applicable.
Figure 1.

Outcome of the expert judgement on Article 5 criteria and overall probability of AMR P. aeruginosa on its eligibility to be listed
- Listing: the probability of the disease to be listed according to Article 5 criteria of the AHL (overall outcome).
The distribution of the individual answers (probability ranges) provided by each expert for each criterion is reported in Sections A.1 and A.2 of Appendix A.
In Figure 1, the outcome of the expert judgement is graphically shown together with the estimated overall probability of the AMR bacterium meeting the criteria of Article 5 on its eligibility to be listed.
3.2.1.1. Reasoning for uncertain outcome on Article 5 criteria
Criterion A(i) (the disease is transmissible):
P. aeruginosa is an opportunistic pathogen.
Transmission of P. aeruginosa is mainly indirect through the environment.
Direct transmission (nosocomial infections) between susceptible individuals has only been described in humans.
Direct transmission between animals may be feasible under certain conditions (e.g. veterinary inpatient facilities) and considering certain strains.
Criterion A(v) (risk‐mitigating measures and, where relevant, surveillance of the disease are effective and proportionate to the risks posed by the disease in the Union):
Antimicrobial treatment is only partly effective with increasing treatment time and antimicrobial resistance being common. Treatment is further complicated by intrinsic resistance of P. aeruginosa and its ability to produce biofilm.
Diagnostic tools and biosecurity measures are available, but there is no information about their effectiveness.
No vaccines are available.
No structured or harmonised surveillance is in place.
Risk‐mitigating measures are not proportionate to the risk posed by AMR P. aeruginosa.
Criterion B(i) (the disease causes or could cause significant negative effects in the Union on animal health, or poses or could pose a significant risk to public health due to its zoonotic character):
P. aeruginosa is an opportunistic pathogen, which occurs only sporadically in dogs and cats.
P. aeruginosa is a frequent pathogen in dogs and among the most frequently reported among clinical cases submitted for routine diagnostics (Hattab et al., 2021).
P. aeruginosa may lead to infections that are difficult to treat (e.g. otitis, UTIs, skin and wound infections).
P. aeruginosa causes frequent and serious nosocomial infections in humans. It is designated a Priority 1 organism by the WHO for which there is an urgent need for new therapeutics. However, zoonotic transmission from animals to humans is questionable.
3.2.2. Overall outcome on Article 5 criteria
As from the legal text of the AHL, a disease is considered eligible to be listed as laid down in Article 5 if it fulfils all criteria of the first set from A(i) to A(v) and at least one of the second set of criteria from B(i) to B(v). According to the assessment methodology, a criterion is considered fulfilled when the lower bound of the median range lays above 66%.
According to the results shown in Table 4, AMR P. aeruginosa complies with three criteria of the first set (A(ii)–A(iv)), but there is uncertainty (33–90% probability) on the assessment on compliance with both Criteria A(i) and A(v). Therefore, it is uncertain whether AMR P. aeruginosa can be considered eligible to be listed for Union intervention as laid down in Article 5 of the AHL. The estimated overall probability range for the AMR bacterium being eligible to be listed is 33–90% (Figure 1).
3.3. Assessment of AMR Pseudomonas aeruginosa according to criteria in Annex IV for the purpose of categorisation as in Article 9 of the AHL
In Tables 5, 6, 7, 8–9 and related graphs (Figures 2, 3–4), the results of the expert judgement on AMR P. aeruginosa in dogs and cats according to the criteria in Annex IV of the AHL, for the purpose of categorisation as in Article 9, are presented.
Table 5.
Outcome of the expert judgement related to the criteria of Section 1 of Annex IV (Category A of Article 9)
| Criteria to be met by the disease: The disease needs to fulfil all of the following criteria | Outcome | ||||
|---|---|---|---|---|---|
|
Median range (%) |
Criterion fulfilment | Number of na | Number of experts | ||
| 1 | The disease is not present in the territory of the Union or present only in exceptional cases (irregular introductions) or present in only in a very limited part of the territory of the Union | 0–5 | Not fulfilled | 0 | 13 |
| 2.1 | The disease is highly transmissible | 5–10 | Not fulfilled | 0 | 14 |
| 2.2 | There are possibilities of airborne or waterborne or vector‐borne spread | 5–10 | Not fulfilled | 0 | 13 |
| 2.3 | The disease affects multiple species of kept and wild animals or single species of kept animals of economic importance | 90–99 | Fulfilled | 0 | 13 |
| 2.4 | The disease may result in high morbidity and significant mortality rates | 5–10 | Not fulfilled | 0 | 13 |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at point 1–2.4, the disease needs to fulfil at least one of the following criteria | |||||
| 3 | The disease has a zoonotic potential with significant consequences for public health, including epidemic or pandemic potential, or possible significant threats to food safety | 5–10 | Not fulfilled | 0 | 14 |
| 4 | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | 1–10 | Not fulfilled | 0 | 13 |
| 5(a) | The disease has a significant impact on society, with in particular an impact on labour markets | 1–10 | Not fulfilled | 0 | 14 |
| 5(b) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | 25–66 | Uncertain | 0 | 13 |
| 5(c) | The disease has a significant impact on the environment, due to the direct impact of the disease or due to the measures taken to control it | 5–33 | Not fulfilled | 0 | 13 |
| 5(d) | The disease has a significant impact in the long term on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | 1–10 | Not fulfilled | 0 | 13 |
na: not applicable.
Table 6.
Outcome of the expert judgement related to the criteria of Section 2 of Annex IV (Category B of Article 9)
|
Criteria to be met by the disease: The disease needs to fulfil all of the following criteria |
Outcome | ||||
|---|---|---|---|---|---|
|
Median range (%) |
Criterion fulfilment | Number of na | Number of experts | ||
| 1 | The disease is present in the whole or part of the Union territory with an endemic character and (at the same time) several Member States or zones of the Union are free of the disease | 1–5 | Not fulfilled | 0 | 13 |
| 2.1 | The disease is moderately to highly transmissible | 5–33 | Not fulfilled | 0 | 13 |
| 2.2 | There are possibilities of airborne or waterborne or vector‐borne spread | 5–10 | Not fulfilled | 0 | 13 |
| 2.3 | The disease affects single or multiple species | – | Fulfilled | 0 | 13 |
| 2.4 | The disease may result in high morbidity with in general low mortality | 10–33 | Not fulfilled | 0 | 13 |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at point 1–2.4, the disease needs to fulfil at least one of the following criteria | |||||
| 3 | The disease has a zoonotic potential with significant consequences for public health, including epidemic potential, or possible significant threats to food safety | 5–10 | Not fulfilled | 0 | 13 |
| 4 | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | 1–10 | Not fulfilled | 0 | 13 |
| 5(a) | The disease has a significant impact on society, with in particular an impact on labour markets | 1–10 | Not fulfilled | 0 | 14 |
| 5(b) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | 25–66 | Uncertain | 0 | 13 |
| 5(c) | The disease has a significant impact on the environment, due to the direct impact of the disease or due to the measures taken to control it | 5–33 | Not fulfilled | 0 | 13 |
| 5(d) | The disease has a significant impact in the long term on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | 1–10 | Not fulfilled | 0 | 13 |
na: not applicable.
Table 7.
Outcome of the expert judgement related to the criteria of Section 3 of Annex IV (Category C of Article 9)
|
Criteria to be met by the disease: The disease needs to fulfil all of the following criteria |
Outcome | ||||
|---|---|---|---|---|---|
|
Median range (%) |
Criterion fulfilment | Number of na | Number of experts | ||
| 1 | The disease is present in the whole or part of the Union territory with an endemic character | 90–100 | Fulfilled | 0 | 13 |
| 2.1 | The disease is moderately to highly transmissible | 5–33 | Not fulfilled | 0 | 13 |
| 2.2 | The disease is transmitted mainly by direct or indirect transmission | – | Fulfilled | 0 | 13 |
| 2.3 | The disease affects single or multiple species | – | Fulfilled | 0 | 13 |
| 2.4 | The disease usually does not result in high morbidity and has negligible or no mortality and often the most observed effect of the disease is production loss | 5–33 | Not fulfilled | 0 | 13 |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at point 1–2.4, the disease needs to fulfil at least one of the following criteria | |||||
| 3 | The disease has a zoonotic potential with significant consequences for public health or possible significant threats to food safety | 10–33 | Not fulfilled | 0 | 13 |
| 4 | The disease has a significant impact on the economy of the Union, mainly related to its direct impact on certain types of animal production systems | 1–10 | Not fulfilled | 0 | 13 |
| 5(a) | The disease has a significant impact on society, with in particular an impact on labour markets | 1–10 | Not fulfilled | 0 | 14 |
| 5(b) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | 25–66 | Uncertain | 0 | 13 |
| 5(c) | The disease has a significant impact on the environment, due to the direct impact of the disease or due to the measures taken to control it | 5–33 | Not fulfilled | 0 | 13 |
| 5(d) | The disease has a significant impact in the long term on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | 1–10 | Not fulfilled | 0 | 13 |
na: not applicable.
Figure 2.

Outcome of the expert judgement on criteria of Section 1 of Annex IV and overall probability of the AMR bacterium to be fitting in Category A of Article 9
- Category A: the probability of the disease to be categorised according to Section 1 of Annex IV of the AHL (overall outcome).
Figure 3.

Outcome of the expert judgement on criteria of Section 2 of Annex IV and overall probability of the AMR bacterium to be fitting in Category B of Article 9
- Category B: The probability of the disease to be categorised according to Section 2 of Annex IV of the AHL (overall outcome).
Figure 4.
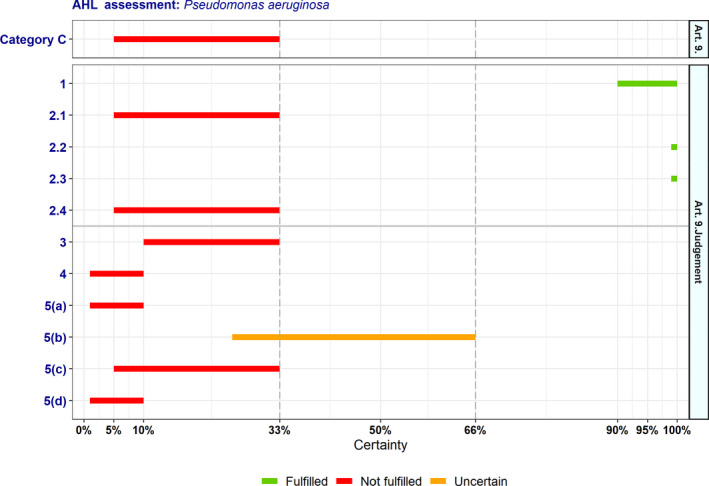
Outcome of the expert judgement on criteria of Section 3 of Annex IV and overall probability of the AMR bacterium to be fitting in Category C of Article 9
- Category C: The probability of the disease to be categorised according to Section 3 of Annex IV of the AHL (overall outcome).
The distribution of the individual answers (probability ranges) provided by each expert for each criterion are reported in Sections B.1 and B.2 of Appendix B.
3.3.1. Detailed outcome on Category A criteria
3.3.1.1. Reasoning for uncertain outcome on Category A criteria
Criterion 5(b) (the disease has a significant impact on animal welfare, by causing suffering of large numbers of animals):
Ear and skin infections (e.g. pyoderma) are frequent and may cause suffering to affected dogs and potentially cats.
P. aeruginosa infection in dogs is quite common, but it is unclear whether this relates to large numbers of animals. All affected dogs in the EU may be considered a large number, but it is unclear to which extent AMR clones contribute to the burden of disease.
In general, morbidity and mortality rates are low.
Antimicrobial treatment is usually effective, but treatment time may be increased in case of antimicrobial resistance and treatment failure.
3.3.2. Detailed outcome on Category B criteria
3.3.2.1. Reasoning for uncertain outcome on Category B criteria
Criterion 5(b) (the disease has a significant impact on animal welfare, by causing suffering of large numbers of animals): See above in Section 3.3.1.1.
3.3.3. Detailed outcome on Category C criteria
3.3.3.1. Reasoning for uncertain outcome on Category C criteria
Criterion 5(b) (the disease has a significant impact on animal welfare, by causing suffering of large numbers of animals): See above in Section 3.3.1.1.
3.3.4. Detailed outcome on Category D criteria
Table 8.
Outcome of the expert judgement related to the criteria of Section 4 of Annex IV (Category D of Article 9)
| Diseases in Category D need to fulfil criteria of Section 1, 2, 3 or 5 of Annex IV of the AHL and the following: | Outcome | ||||
|---|---|---|---|---|---|
|
Median range (%) |
Criterion fulfilment | Number of na | Number of experts | ||
| D | The risk posed by the disease can be effectively and proportionately mitigated by measures concerning movements of animals and products in order to prevent or limit its occurrence and spread | 5–33 | Not fulfilled | 0 | 13 |
na: not applicable.
3.3.5. Detailed outcome on Category E criteria
Table 9.
Outcome of the expert judgement related to the criteria of Section 5 of Annex IV (Category E of Article 9)
| Diseases in Category E need to fulfil criteria of Section 1, 2 or 3 of Annex IV of the AHL and/or the following: | Outcome | ||
|---|---|---|---|
|
Median range (%) |
Fulfilment | ||
| E |
Surveillance of the disease is necessary for reasons related to animal health, animal welfare, human health, the economy, society or the environment (If a disease fulfils the criteria as in Article 5, thus being eligible to be listed, consequently Category E would apply.) |
33–90 | Uncertain |
3.3.6. Overall outcome on criteria in Annex IV for the purpose of categorisation as in Article 9
As from the legal text of the AHL, a disease is considered fitting in a certain category (A, B, C, D or E – corresponding to points (a) to (e) of Article 9(1) of the AHL) if it fulfils all criteria of the first set from 1 to 2.4 and at least one of the second set of criteria from 3 to 5(d), as shown in Tables 5–59. According to the assessment methodology, a criterion is considered fulfilled when the lower bound of the median range lays above 66%.
The overall outcome of the assessment on criteria in Annex IV of the AHL, for the purpose of categorisation of AMR P. aeruginosa as in Article 9, is presented in Table 10 and Figure 5.
Table 10.
Outcome of the assessment on criteria in Annex IV of the AHL for the purpose of categorisation as in Article 9
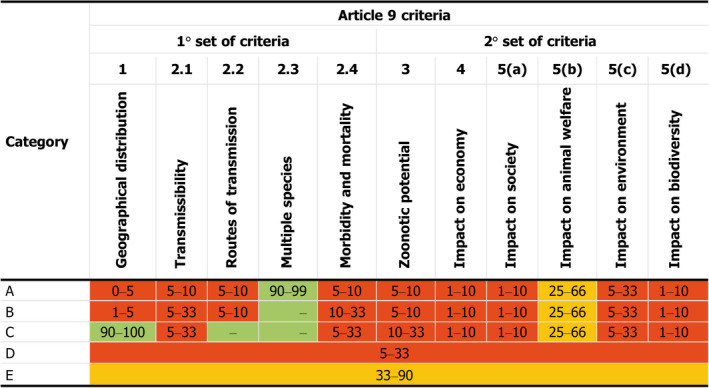
Probability ranges (% certainty; –: criterion fulfilled by default) and fulfilment of criteria (green: fulfilled; red: not fulfilled; orange: uncertain) (EFSA AHAW Panel, 2017).
Figure 5.
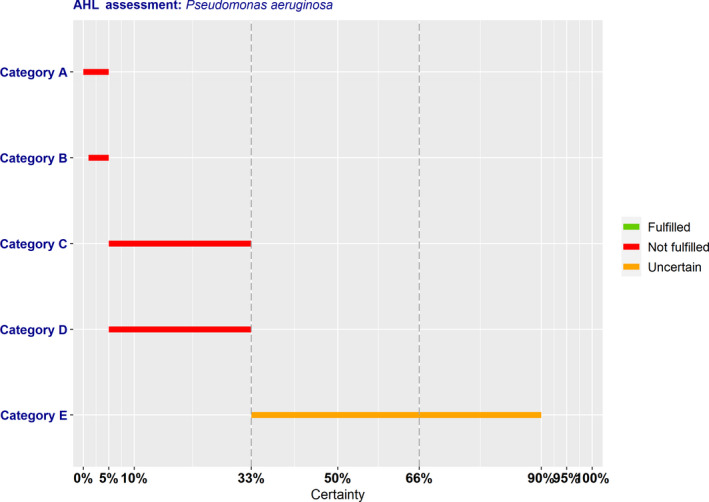
Outcome of the expert judgement on criteria in Annex IV and overall probabilities for categorisation of the AMR bacterium in accordance with Article 9
According to the assessment here performed, AMR P. aeruginosa complies with the following criteria of Sections 1–5 of Annex IV of the AHL for the application of the disease prevention and control rules referred to in points (a)–(e) of Article 9(1):
To be assigned to Category A, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and, according to the assessment, AMR P. aeruginosa complies only with Criterion 2.3 (90–99% probability). To be eligible for Category A, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5(a)–(d)) and AMR P. aeruginosa does not comply with any apart from Criterion 5(b), for which the assessment was inconclusive (25–66% probability). Overall, it was assessed with 0–5% probability that AMR P. aeruginosa may be assigned to Category A according to criteria in Section 1 of Annex IV for the purpose of categorisation as in Article 9 of the AHL.
To be assigned to Category B, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and, according to the assessment, AMR P. aeruginosa complies only with Criterion 2.3, which is fulfilled by default. To be eligible for Category B, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5(a)–(d)) and AMR P. aeruginosa does not comply with any apart from Criterion 5(b), for which the assessment was inconclusive (25–66% probability). Overall, it was assessed with 1–5% probability that AMR P. aeruginosa may be assigned to Category B according to criteria in Section 2 of Annex IV for the purpose of categorisation as in Article 9 of the AHL.
To be assigned to Category C, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and, according to the assessment, AMR P. aeruginosa complies with Criteria 1 (90–100% probability), 2.2 and 2.3 (both fulfilled by default). To be eligible for Category C, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5(a)–(d)) and AMR P. aeruginosa does not comply with any apart from Criterion 5(b), for which the assessment was inconclusive (25–66% probability). Overall, it was assessed with 5–33% probability that AMR P. aeruginosa may be assigned to Category C according to criteria in Section 3 of Annex IV for the purpose of categorisation as in Article 9 of the AHL.
To be assigned to Category D, a disease needs to comply with criteria of Section 1, 2, 3 or 5 of Annex IV of the AHL and with the specific Criterion D of Section 4, with which AMR P. aeruginosa does not comply (5–33% probability).
To be assigned to Category E, a disease needs to comply with criteria of Section 1, 2 or 3 of Annex IV of the AHL, and/or the surveillance of the disease is necessary for reasons related to animal health, animal welfare, human health, the economy, society or the environment. The latter is applicable if a disease fulfils the criteria as in Article 5, for which the assessment is inconclusive with a large uncertainty (33–90% probability of fulfilling the criteria).
3.4. Assessment of AMR Pseudomonas aeruginosa according to Article 8 criteria of the AHL
In this section, the results of the assessment on the criteria of Article 8(3) of the AHL for AMR P. aeruginosa are presented. The Article 8(3) criteria are about animal species to be listed, as it reads below:
‘3. Animal species or groups of animal species shall be added to the list if they are affected or if they pose a risk for the spread of a specific listed disease because:
they are susceptible to a specific listed disease, or scientific evidence indicates that such susceptibility is likely; or
they are vector species or reservoirs for that disease, or scientific evidence indicates that such role is likely.’
For this reason, the assessment on Article 8 criteria is based on the evidence as extrapolated from the relevant criteria of Article 7, i.e. the ones related to susceptible and reservoir species or routes of transmission, which cover also the possible role of biological or mechanical vectors. 2
According to the mapping, as presented in Table 5, Section 3.2, of the scientific opinion on the ad hoc methodology (EFSA AHAW Panel, 2017), the animal species to be listed for AMR P. aeruginosa according to the criteria of Article 8(3) of the AHL are as displayed in Table 11 (elaborated from information reported in Section 3.1.1.1 of the present document).
Table 11.
Animal species to be listed for AMR P. aeruginosa according to the criteria of Article 8
| Class/Order | Family | Genus/Species | |
|---|---|---|---|
| Susceptible | Carnivora | Canidae | Domestic dog (Canis lupus familiaris) a |
| Felidae | Domestic cat (Felis catus) a | ||
| Mustelidae | Ferret (Mustela furo) | ||
| Artiodactyla | Bovidae | Sheep (Ovis aries) | |
| Suidae | Pig (Sus domesticus) | ||
| Rodentia | Chinchillidae | Chinchilla (Chinchilla chinchilla) | |
| Muridae | House mouse (Mus musculus) | ||
| Rat (Rattus sp.) | |||
| Lagomorpha | Leporidae | Rabbits | |
| Diprotodontia | Petauridae | Sugar glider (Petaurus breviceps) | |
| Anseriformes | Anatidae | White‐faced whistling duck (Dendrocygna viduata) | |
| Gruiformes | Gruidae | Siberian crane (Leucogeranus leucogeranus) | |
| Whooping crane (Grus americana) | |||
| Testudines | Cheloniidae | Hawksbill sea turtle (Eretmochemys imbricata) | |
| Squamata | |||
| Passeriformes | Hirundinidae | Swallows | |
| Cypriniformes | Cyprinidae | Zebrafish (Danio rerio) | |
| Reservoir | Carnivora | Canidae | Domestic dog (Canis lupus familiaris) |
| Artiodactyla | Suidae | Wild boar (Sus scrofa) | |
| Squamata | |||
| Vector | None | ||
Most evidence reported in the fact sheet relates to these animal species.
The table contains all animal species in which AMR P. aeruginosa has been described, but also those animal species from which only the bacterium itself has been isolated. The latter makes susceptibility to AMR clones likely. However, most evidence reported in the fact sheet relates to dogs and cats.
4. Conclusions
The AHAW Panel emphasises that the assessment of impacts, as well as prevention and control measures, related to AMR bacteria using the criteria as laid down in Articles 5 and 9 of the AHL is particularly challenging for opportunistic pathogens that can also be found as commensal bacteria in healthy animals.
TOR 1: For each of those identified AMR bacteria considered most relevant in the EU, following the criteria laid down in Article 7 of the AHL, an assessment on its eligibility to be listed for Union intervention as laid down in Article 5(3) of the AHL;
It is uncertain (33–90% probability, from ‘as likely as not’ to ‘likely’) whether AMR P. aeruginosa can be considered eligible to be listed for Union intervention as laid down in Article 5 of the AHL.
TOR 2: For each of the AMR bacteria which was found eligible to be listed for Union intervention, an assessment on its compliance with the criteria in Annex IV for the purpose of categorisation in accordance with Article 9 of the AHL;
The AHAW Panel considered with 0–5% probability (from ‘almost impossible’ to ‘extremely unlikely’) that AMR P. aeruginosa meets the criteria as in Section 1 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in point (a) of Article 9(1) of the AHL.
The AHAW Panel considered with 1–5% probability (‘extremely unlikely’) that AMR P. aeruginosa meets the criteria as in Section 2 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in point (b) of Article 9(1) of the AHL.
The AHAW Panel considered with 5–33% probability (from ‘very unlikely’ to ‘unlikely’) that AMR P. aeruginosa meets the criteria as in Section 3 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in point (c) of Article 9(1) of the AHL.
The AHAW Panel considered with 5–33% probability (from ‘very unlikely’ to ‘unlikely’) that AMR P. aeruginosa meets the criteria as in Section 4 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in point (d) of Article 9(1) of the AHL.
The AHAW Panel was uncertain (33–90% probability, from ‘as likely as not’ to ‘likely’) whether AMR P. aeruginosa meets the criteria as in Section 5 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in point (e) of Article 9(1) of the AHL.
TOR 3: For each of the AMR bacteria which was found eligible to be listed for Union intervention, a list of animal species that should be considered candidates for listing in accordance with Article 8 of the AHL;
The animal species that can be considered to be listed for AMR P. aeruginosa according to Article 8(3) of the AHL are mainly dogs and cats, as reported in Table 11 in Section 3.4 of the present document.
The AHAW Panel highlights that monitoring of antimicrobial resistance in opportunistic bacteria could help to assess their impacts. Therefore, even though the assessment on AMR P. aeruginosa is inconclusive on its eligibility to be listed for Union intervention, specific initiatives (e.g. monitoring or applied research) into various aspects of AMR P. aeruginosa can be useful to better understand its distribution and to assess its impact on animal health and welfare in the EU.
Abbreviations
- AES
Australian Epidemic Strain
- AHAW
Animal Health and Welfare
- AHL
Animal Health Law
- AMC
Amoxicillin–clavulanic acid
- AMK
Amikacin
- AMR
Antimicrobial‐resistant
- AMX
Amoxicillin
- ATM
Aztreonam
- AZM
Azithromycin
- CAZ
Ceftazidime
- CEF
Ceftiofur
- CET
Cefalotin
- CF
Cystic fibrosis
- CFSPH
Center for Food Security and Public Health
- CHL
Chloramphenicol
- CI
Current impact
- CIP
Ciprofloxacin
- CITES
Convention on International Trade in Endangered Species
- CLI
Clindamycin
- CLSI
Clinical and Laboratory Standards Institute
- CPD
Cefpodoxime
- CST
Colistin
- CTX
Cefotaxime
- CVN
Cefovecin
- CXM
Cefuroxime
- DALY
Disability‐adjusted life year
- DIVA
Differentiation of infected from vaccinated animals
- DOX
Doxycycline
- EEA
European Economic Area
- ENR
Enrofloxacin
- ERY
Erythromycin
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- FEP
Cefepime
- FFC
Florfenicol
- FOF
Fosfomycin
- FOX
Cefoxitin
- FQ
Fluoroquinolones
- GEN
Gentamicin
- I
Intermediate
- IPM
Imipenem
- IUCN
International Union for Conservation of Nature
- KAN
Kanamycin
- LES
Liverpool Epidemic Strain
- LEX
Cephalexin
- LPS
Lipopolysaccharide
- MALDI‐TOF MS
Matrix‐assisted laser desorption ionisation–time‐of‐flight mass spectrometry
- MAR
Marbofloxacin
- MDR
Multidrug‐resistant
- MEM
Meropenem
- MIC
Minimum inhibitory concentration
- MS
Member State
- NEO
Neomycin
- NIT
Nitrofurantoin
- OIE
Office International des Épizooties (World Organisation for Animal Health)
- ORB
Orbafloxacin
- PDR
Pandrug‐resistant
- PES
Prairie Epidemic Strain
- PI
Potential impact
- PMB
Polymyxin B
- PRA
Pradofloxacin
- R
Resistant
- SPT
Spectinomycin
- ST
Sequence type
- SXT
Trimethoprim–sulfamethoxazole
- TET
Tetracycline
- TIC
Ticarcillin
- TIM
Ticarcillin–clavulanic acid
- TOB
Tobramycin
- ToR
Term of Reference
- TZP
Piperacillin–tazobactam
- UTI
Urinary tract infection
- WHO
World Health Organization
- XDR
Extensively drug‐resistant
Appendix A – Criteria with certain outcome
A.1. Article 5 criteria

Figure A.1 Individual probability ranges reflecting fulfilment of Criterion A(ii) (animal species are either susceptible to the disease or vectors and reservoirs thereof exist in the Union) after the collective judgement
- The median range is displayed as a dashed line.
Figure A.2 Individual probability ranges reflecting fulfilment of Criterion A(iii) (the disease causes negative effects on animal health or poses a risk to public health due to its zoonotic character) after the collective judgement
- The median range is displayed as a dashed line.

Figure A.3 Individual probability ranges reflecting fulfilment of Criterion A(iv) (diagnostic tools are available for the disease) after the collective judgement
- The median range is displayed as a dashed line.

Figure A.4 Individual probability ranges reflecting fulfilment of Criterion B(ii) (the disease agent has developed resistance to treatments which poses a significant danger to public and/or animal health in the Union) after the collective judgement
- The median range is displayed as a dashed line.

Figure A.5 Individual probability ranges reflecting non‐fulfilment of Criterion B(iii) (the disease causes or could cause a significant negative economic impact affecting agriculture or aquaculture production in the Union) after the collective judgement
- The median range is displayed as a dashed line.
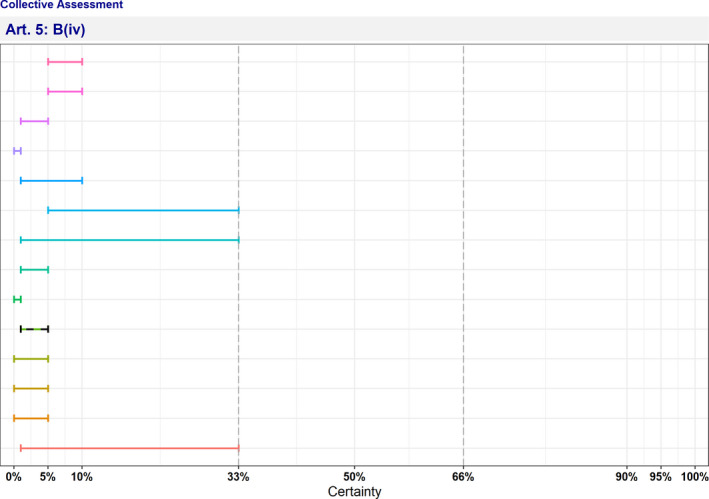
Figure A.6 Individual probability ranges reflecting non‐fulfilment of Criterion B(iv) (the disease has the potential to generate a crisis or the disease agent could be used for the purpose of bioterrorism) after the collective judgement
- The median range is displayed as a dashed line.
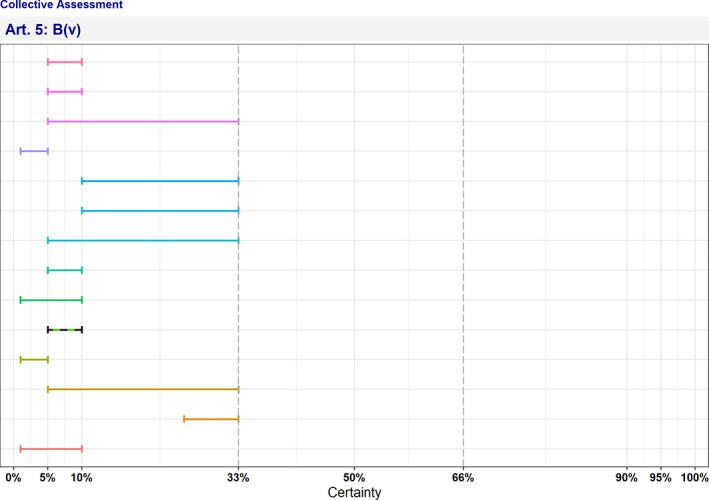
Figure A.7 Individual probability ranges reflecting non‐fulfilment of Criterion B(v) (the disease has or could have a significant negative impact on the environment, including biodiversity, of the Union) after the collective judgement
- The median range is displayed as a dashed line.
A.2. Article 9 criteria
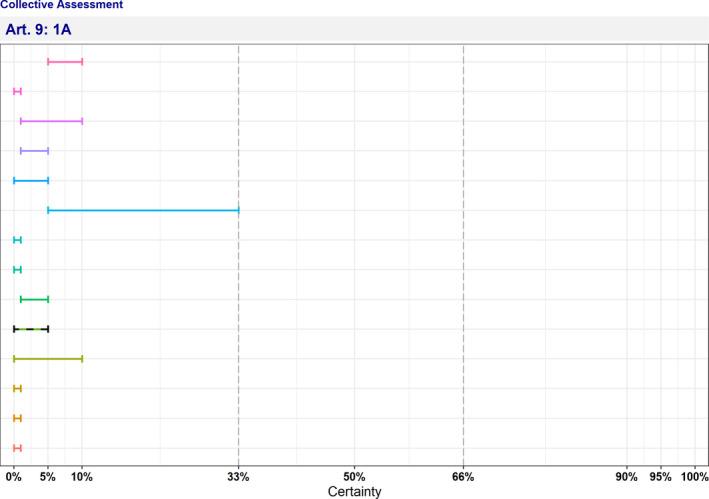
Figure A.8 Individual probability ranges reflecting non‐fulfilment of Criterion 1A (the disease is not present in the territory of the Union or present only in exceptional cases (irregular introductions) or present in only in a very limited part of the territory of the Union) after the collective judgement
- The median range is displayed as a dashed line.

Figure A.9 Individual probability ranges reflecting non‐fulfilment of Criterion 1B (the disease is present in the whole or part of the Union territory with an endemic character and (at the same time) several Member States or zones of the Union are free of the disease) after the collective judgement
- The median range is displayed as a dashed line.

Figure A.10 Individual probability ranges reflecting fulfilment of Criterion 1C (the disease is present in the whole or part of the Union territory with an endemic character) after the collective judgement
- The median range is displayed as a dashed line.

Figure A.11 Individual probability ranges reflecting non‐fulfilment of Criterion 2.1A (the disease is highly transmissible) after the collective judgement
- The median range is displayed as a dashed line.

Figure A.12 Individual probability ranges reflecting non‐fulfilment of Criterion 2.1BC (the disease is moderately to highly transmissible) after the collective judgement
- The median range is displayed as a dashed line.

Figure A.13 Individual probability ranges reflecting non‐fulfilment of Criterion 2.2AB (there are possibilities of airborne or waterborne or vector‐borne spread) after the collective judgement
- The median range is displayed as a dashed line.
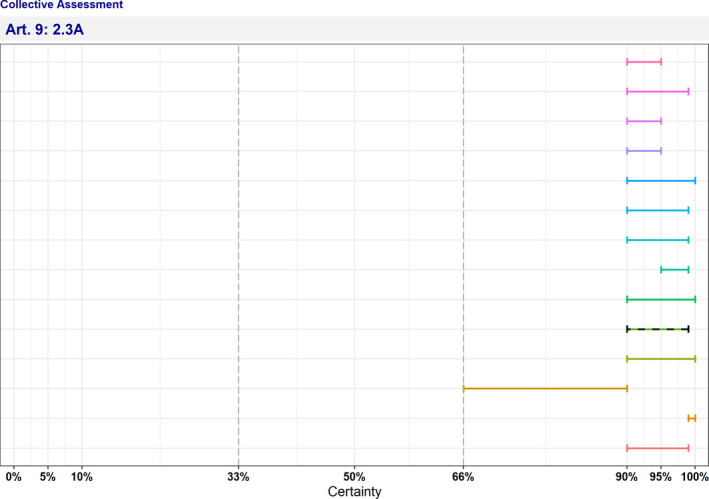
Figure A.14 Individual probability ranges reflecting fulfilment of Criterion 2.3A (the disease affects multiple species of kept and wild animals or single species of kept animals of economic importance) after the collective judgement
- The median range is displayed as a dashed line.

Figure A.15 Individual probability ranges reflecting non‐fulfilment of Criterion 2.4A (the disease may result in high morbidity and significant mortality rates) after the collective judgement
- The median range is displayed as a dashed line.

Figure A.16 Individual probability ranges reflecting non‐fulfilment of Criterion 2.4B (the disease may result in high morbidity with in general low mortality) after the collective judgement
- The median range is displayed as a dashed line.

Figure A.17 Individual probability ranges reflecting non‐fulfilment of Criterion 2.4C (the disease usually does not result in high morbidity and has negligible or no mortality and often the most observed effect of the disease is production loss) after the collective judgement
- The median range is displayed as a dashed line.
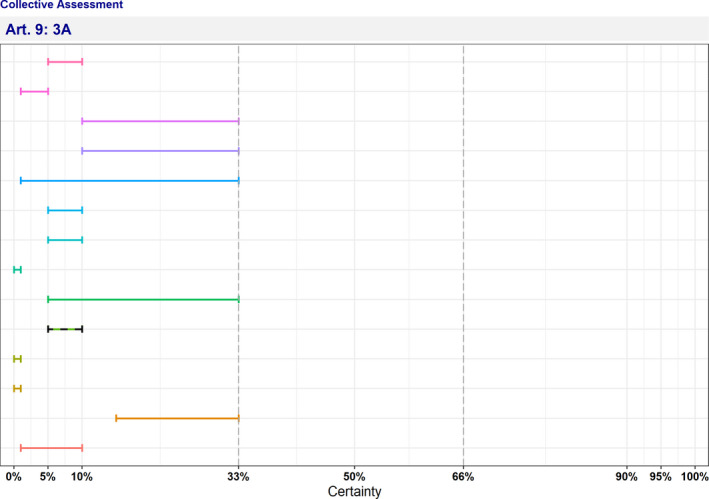
Figure A.18 Individual probability ranges reflecting non‐fulfilment of Criterion 3A (the disease has a zoonotic potential with significant consequences for public health, including epidemic or pandemic potential, or possible significant threats to food safety) after the collective judgement
- The median range is displayed as a dashed line.
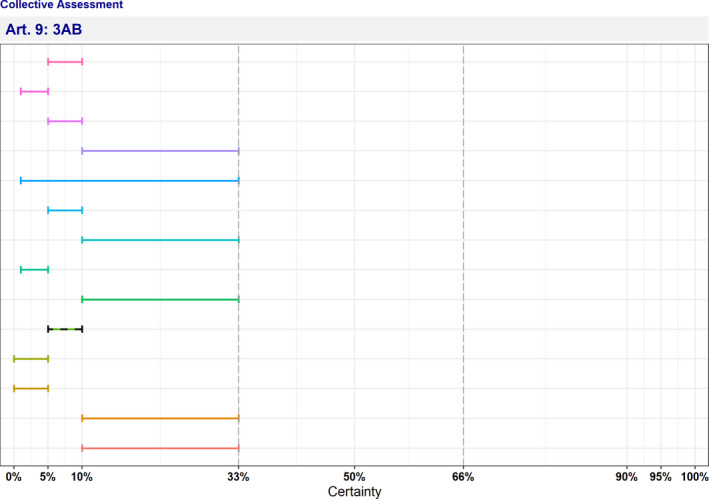
Figure A.19 Individual probability ranges reflecting non‐fulfilment of Criterion 3AB (the disease has a zoonotic potential with significant consequences for public health, including epidemic potential, or possible significant threats to food safety) after the collective judgement
- The median range is displayed as a dashed line.

Figure A.20 Individual probability ranges reflecting non‐fulfilment of Criterion 3ABC (the disease has a zoonotic potential with significant consequences for public health or possible significant threats to food safety) after the collective judgement
- The median range is displayed as a dashed line.

Figure A.21 Individual probability ranges reflecting non‐fulfilment of Criterion 4AB (current impact) (the disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals) after the collective judgement
-
CI: current impact.The median range is displayed as a dashed line.

Figure A.22 Individual probability ranges reflecting non‐fulfilment of Criterion 4AB (potential impact) (the disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals) after the collective judgement
-
PI: potential impact.The median range is displayed as a dashed line.

Figure A.23 Individual probability ranges reflecting non‐fulfilment of Criterion 4C (current impact) (the disease has a significant impact on the economy of the Union, mainly related to its direct impact on certain types of animal production systems) after the collective judgement
-
CI: current impact.The median range is displayed as a dashed line.

Figure A.24 Individual probability ranges reflecting non‐fulfilment of Criterion 4C (potential impact) (the disease has a significant impact on the economy of the Union, mainly related to its direct impact on certain types of animal production systems) after the collective judgement
-
PI: potential impact.The median range is displayed as a dashed line.

Figure A.25 Individual probability ranges reflecting non‐fulfilment of Criterion 5(a) (current impact) (the disease has a significant impact on society, with in particular an impact on labour markets) after the collective judgement
-
CI: current impact.The median range is displayed as a dashed line.
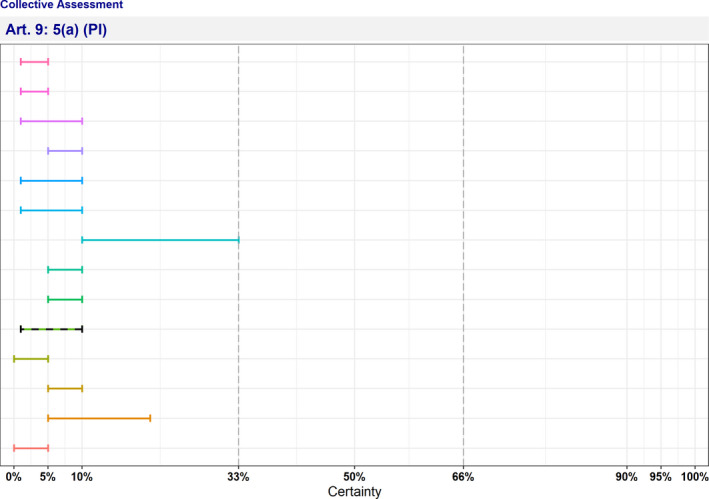
Figure A.6 Individual probability ranges reflecting non‐fulfilment of Criterion 5(a) (potential impact) (the disease has a significant impact on society, with in particular an impact on labour markets) after the collective judgement
-
PI: potential impact.The median range is displayed as a dashed line.

Figure A.27 Individual probability ranges reflecting non‐fulfilment of Criterion 5(c) (current impact) (the disease has a significant impact on the environment, due to the direct impact of the disease or due to the measures taken to control it) after the collective judgement
-
CI: current impact.The median range is displayed as a dashed line.
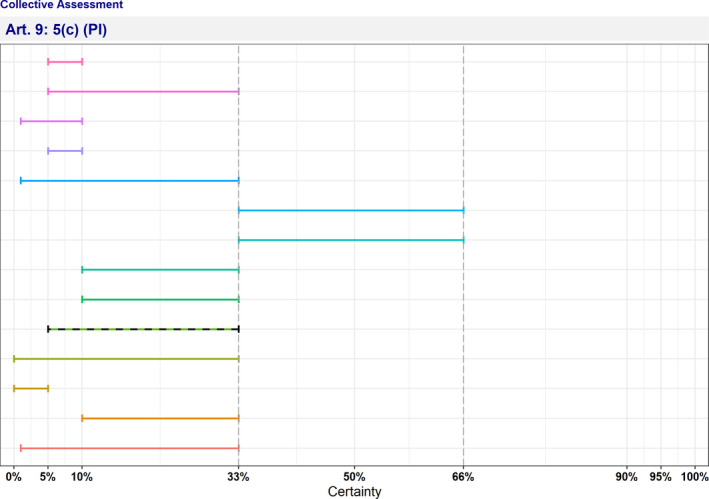
Figure A.28 Individual probability ranges reflecting non‐fulfilment of Criterion 5(c) (potential impact) (the disease has a significant impact on the environment, due to the direct impact of the disease or due to the measures taken to control it) after the collective judgement
-
PI: potential impact.The median range is displayed as a dashed line.

Figure A.29 Individual probability ranges reflecting non‐fulfilment of Criterion 5(d) (current impact) (the disease has a significant impact in the long term on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds) after the collective judgement
-
CI: current impact.The median range is displayed as a dashed line.

Figure A.30 Individual probability ranges reflecting non‐fulfilment of Criterion 5(d) (potential impact) (the disease has a significant impact in the long term on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds) after the collective judgement
-
PI: potential impact.The median range is displayed as a dashed line.

Figure A.31 Individual probability ranges reflecting non‐fulfilment of Criterion D (the risk posed by the disease can be effectively and proportionately mitigated by measures concerning movements of animals and products in order to prevent or limit its occurrence and spread) after the collective judgement
- The median range is displayed as a dashed line.
Appendix B – Criteria with uncertain outcome
B.1. Article 5 criteria

Figure B.1 Individual probability ranges reflecting uncertain outcome on Criterion A(i) (the disease is transmissible) after the collective judgement
- The median range is displayed as a dashed line.

Figure B.2 Individual probability ranges reflecting uncertain outcome on Criterion A(v) (risk‐mitigating measures and, where relevant, surveillance of the disease are effective and proportionate to the risks posed by the disease in the Union) after the collective judgement
- The median range is displayed as a dashed line.
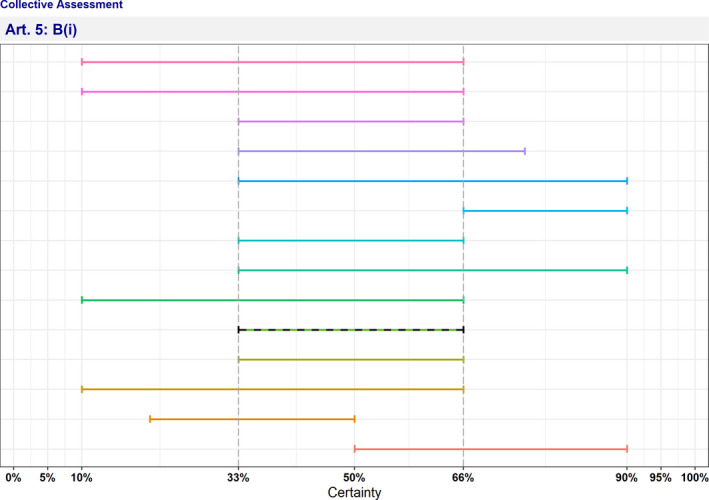
Figure B.3 Individual probability ranges reflecting uncertain outcome on Criterion B(i) (the disease causes or could cause significant negative effects in the Union on animal health, or poses or could pose a significant risk to public health due to its zoonotic character) after the collective judgement
- The median range is displayed as a dashed line.
B.2. Article 9 criteria

Figure B.4 Individual probability ranges reflecting uncertain outcome on Criterion 5(b) current impact) (the disease has a significant impact on animal welfare, by causing suffering of large numbers of animals) after the collective judgement
-
CI: current impact.The median range is displayed as a dashed line.

Figure B.5 Individual probability ranges reflecting uncertain outcome on Criterion 5(b) (potential impact) (the disease has a significant impact on animal welfare, by causing suffering of large numbers of animals) after the collective judgement
-
PI: potential impact.The median range is displayed as a dashed line.
Suggested citation: EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar C, Herskin M, Michel V, Miranda Chueca MÁ, Padalino B, Pasquali P, Roberts HC, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, Baldinelli F, Broglia A, Kohnle L and Alvarez J, 2022. Scientific Opinion on the assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): antimicrobial‐resistant Pseudomonas aeruginosa in dogs and cats. EFSA Journal 2022;20(5):7310, 78 pp. 10.2903/j.efsa.2022.7310
Requestor: European Commission
Question number: EFSA‐Q‐2022‐00092
Panel members: Søren Saxmose Nielsen, Julio Alvarez, Dominique Joseph Bicout, Paolo Calistri, Elisabetta Canali, Julian Ashley Drewe, Bruno Garin‐Bastuji, José Luis Gonzales Rojas, Christian Gortázar, Mette Herskin, Virginie Michel, Miguel Ángel Miranda Chueca, Barbara Padalino, Paolo Pasquali, Helen Clare Roberts, Hans Spoolder, Karl Ståhl, Antonio Velarde, Arvo Viltrop and Christoph Winckler.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgments: The AHAW Panel wishes to thank Joanne Leri Fothergill from the University of Liverpool for conducting the extensive literature review under the contract EOI/EFSA/SCIENCE/2020/01. The AHAW Panel also wishes to Verena Oswaldi from EFSA for the support provided for this scientific output.
Adopted: 30 March 2022
Notes
Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (‘Animal Health Law’). OJ L 84, 31.3.2016, p. 1–208.
A vector is a living organism that transmits an infectious agent from an infected animal to a human or another animal. Vectors are frequently arthropods. Biological vectors may carry pathogens that can multiply within their bodies and be delivered to new hosts, usually by biting. In mechanical vectors, the pathogens do not multiply within the vector, which usually remains infected for shorter time than in biological vectors.
References
- Argudín MAA, Deplano A, Meghraoui A, Dodémont M, Heinrichs A, Denis O, Nonhoff C and Roisin S, 2017. Bacteria from animals as a pool of antimicrobial resistance genes. Antibiotics (Basel), 6, 12. 10.3390/antibiotics6020012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa C, Trebosc V, Kemmer C, Rosenstiel P, Beardmore R, Schulenburg H and Jansen G, 2017. Alternative evolutionary paths to bacterial antibiotic resistance cause distinct collateral effects. Molecular Biology and Evolution, 34, 2229–2244. 10.1093/molbev/msx158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard N and Foster A, 2018. How to treat Pseudomonas otitis in dogs. Veterinary Record, 182, 109–110. 10.1136/vr.k413 [DOI] [PubMed] [Google Scholar]
- Beier RC, Foley SL, Davidson MK, White DG, McDermott PF, Bodeis‐Jones S, Zhao S, Andrews K, Crippen TL, Sheffield CL, Poole TL, Anderson RC and Nisbet DJ, 2015. Characterization of antibiotic and disinfectant susceptibility profiles among Pseudomonas aeruginosa veterinary isolates recovered during 1994–2003. Journal of Applied Microbiology, 118, 326–342. 10.1111/jam.12707 [DOI] [PubMed] [Google Scholar]
- Borowiak M, Hammerl JA, Deneke C, Fischer J, Szabo I and Malorny B, 2019. Characterization of mcr‐5‐Harboring Salmonella enterica subsp. enterica Serovar Typhimurium Isolates from Animal and Food Origin in Germany. Antimicrobial Agents and Chemotherapy, 63, e00063–e119. 10.1128/AAC.00063-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borriello G, Paradiso R, Catozzi C, Brunetti R, Roccabianca P, Riccardi MG, Cecere B, Lecchi C, Fusco G, Ceciliani F and Galiero G, 2020. Cerumen microbial community shifts between healthy and otitis affected dogs. PLoS One, 15, e0241447. 10.1371/journal.pone.0241447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourély C, Cazeau G, Jarrige N, Leblond A, Madec JY, Haenni M and Gay E, 2019. Antimicrobial resistance patterns of bacteria isolated from dogs with otitis. Epidemiology and Infection, 147, e121. 10.1017/S0950268818003278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehrle DJ, Shields RK, Clarke LG, Potoski BA, Clancy CJ and Nguyen MH, 2017. Carbapenem‐resistant Pseudomonas aeruginosa bacteremia: risk factors for mortality and microbiologic treatment failure. Antimicrobial Agents and Chemotherapy, 61, e01243–e1316. 10.1128/AAC.01243-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardillo L, Piegari G, Iovane V, Viscardi M, Alfano F, Cerrone A, Pagnini U, Montagnaro S, Galiero G, Pisanelli G and Fusco G, 2020. Lifestyle as risk factor for infectious causes of death in young dogs: a retrospective study in Southern Italy (2015–2017).". Veterinary Medicine International, 6207297. 10.1155/2020/6207297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll LM, Gaballa A, Guldimann C, Sullivan G, Henderson LO and Wiedmann M, 2019. Identification of novel mobilized colistin resistance gene mcr‐9 in a multidrug‐resistant, colistin‐susceptible salmonella enterica serotype typhimurium isolate. MBio, 10, e00853–19. 10.1128/mBio.00853-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, Colomb‐Cotinat M, Kretzschmar ME, Devleesschauwer B, Cecchini M, Ouakrim DA, Oliveira TC, Struelens MJ, Suetens C, Monnet DL, Strauss R, Mertens K, Struyf T, Catry B, Latour K, Ivanov IN, Dobreva EG, Tambic Andraševic A, Soprek S, Budimir A, Paphitou N, Žemlicková H, Schytte Olsen S, Wolff Sönksen U, Märtin P, Ivanova M, Lyytikäinen O, Jalava J, Coignard B, Eckmanns T, Abu Sin M, Haller S, Daikos GL, Gikas A, Tsiodras S, Kontopidou F, Tóth Á, Hajdu Á, Guólaugsson Ó, Kristinsson KG, Murchan S, Burns K, Pezzotti P, Gagliotti C, Dumpis U, Liuimiene A, Perrin M, Borg MA, de Greeff SC, Monen JCM, Koek MBG, Elstrøm P, Zabicka D, Deptula A, Hryniewicz W, Caniça M, Nogueira PJ, Fernandes PA, Manageiro V, Popescu GA, Serban RI, Schréterová E, Litvová S, Štefkovicová M, Kolman J, Klavs I, Korošec A, Aracil B, Asensio A, Pérez‐Vázquez M, Billström H, Larsson S, Reilly JS, Johnson A and Hopkins S, 2019. Attributable deaths and disability‐adjusted life‐years caused by infections with antibiotic‐resistant bacteria in the EU and the European Economic Area in 2015: a population‐level modelling analysis. The Lancet, Infectious Diseases, 19, 56–66. 10.1016/S1473-3099(18)30605-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre C, Riou M, Bréa D, Vandebrouck C, Barc C, Pezant J, Melo S, Olivier M, Delaunay R, Boulesteix O, Berthon P, Rossignol C, Burlaud Gaillard J, Becq F, Gauthier F, Si‐Tahar M, Meurens F, Berri M, Caballero‐Posadas I and Attucci S, 2016. The pig: a relevant model for evaluating the neutrophil serine protease activities during acute Pseudomonas aeruginosa lung infection. PLoS One, 11, e0168577. 10.1371/journal.pone.0168577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang C‐H, Wang Y‐H, Chang H‐J, Chen H‐L, Huang Y‐C, Lin T‐Y, Ozer EA, Allen JP, Hauser AR and Chiu C‐H, 2014. Shanghai fever: a distinct Pseudomonas aeruginosa enteric disease. Gut, 63, 736–743. 10.1136/gutjnl-2013-304786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colinon C, Jocktane D, Brothier E, Rossolini M, Cournoyer B and Nazaret S, 2010. Genetic analyses of Pseudomonas aeruginosa isolated from healthy captive snakes: evidence of high inter‐ and intrasite dissemination and occurrence of antibiotic resistance genes. Environmental Microbiology, 12, 716–729. 10.1111/j.1462-2920.2009.02115.x [DOI] [PubMed] [Google Scholar]
- Cotter CS, Avidano MA, Stringer SP and Schultz GS, 1996. Inhibition of proteases in Pseudomonas otitis media in chinchillas. Otolaryngology – Head and Neck Surgery, 115, 342–351 [DOI] [PubMed] [Google Scholar]
- Curran K, Leeper H, O'Reilly K, Jacob J and Bermudez LE, 2021. An analysis of the infections and determination of empiric antibiotic therapy in cats and dogs with cancer‐associated infections. Antibiotics (Basel), 10, 700. 10.3390/antibiotics10060700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwich L, Seminati C, Burballa A, Nieto A, Durán I, Tarradas N and Molina‐López RA, 2021. Antimicrobial susceptibility of bacterial isolates from urinary tract infections in companion animals in Spain. Veterinary Record, 188, e60. 10.1002/vetr.60 [DOI] [PubMed] [Google Scholar]
- de Jong A, Youala M, El Garch F, Simjee S, Rose M, Morrissey I and Moyaert H, 2020. Antimicrobial susceptibility monitoring of canine and feline skin and ear pathogens isolated from European veterinary clinics: results of the ComPath Surveillance programme. Veterinary Dermatology, 31, 431–e114. 10.1111/vde.12886 [DOI] [PubMed] [Google Scholar]
- Dégi J, Motco OA, Dégi DM, Suici T, Mares M, Imre K and Cristina RT, 2021. Antibiotic susceptibility profile of Pseudomonas aeruginosa canine isolates from a multicentric study in romania. Antibiotics (Basel), 10, 846. 10.3390/antibiotics10070846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Barrio‐Tofiño E, López‐Causapé C and Oliver A, 2020. Pseudomonas aeruginosa epidemic high‐risk clones and their association with horizontally‐acquired beta‐lactamases: 2020 update." International Journal of Antimicrobial Agents, 56. 10.1016/j.ijantimicag.2020.106196 [DOI] [PubMed] [Google Scholar]
- Done SH, 1974. Pseudomonas aeruginosa infection in the skin of a dog: a case report. British Veterinary Journal, 130, lxviii–lxix. 10.1016/S0007-1935(17)35852-9 [DOI] [PubMed] [Google Scholar]
- EFSA Ahaw Panel (EFSA Panel on Animal Health and Welfare) , More S, Bøtner A, Butterworth A, Calistri P, Depner K, Edwards S, Garin‐Bastuji B, Good M, Gortázar Schmidt C, Michel V, Miranda MA, Nielsen SS, Raj M, Sihvonen L, Spoolder H, Stegeman JA, Thulke H‐H, Velarde A, Willeberg P, Winckler C, Baldinelli F, Broglia A, Candiani D, Gervelmeyer A, Zancanaro G, Kohnle L, Morgado J and Bicout D, 2017. Scientific opinion on an ad hoc method for the assessment on listing and categorisation of animal diseases within the framework of the Animal Health Law. EFSA Journal 2017;15(7):4783, 42 pp. 10.2903/j.efsa.2017.4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar Schmidt C, Herskin M, Michel V, Miranda Chueca MA, Padalino B, Pasquali P, Roberts HC, Sihvonen LH, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, Guardabassi L, Hilbert F, Mader R, Aznar I, Baldinelli F and Alvarez J, 2021a. Scientific Opinion on the assessment of animal diseases caused by bacteria resistant to antimicrobials: Dogs and cats. EFSA Journal 2021;19(6):6680, 58 pp. 10.2903/j.efsa.2021.6680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar Schmidt C, Herskin M, Michel V, Miranda Chueca MA, Padalino B, Pasquali P, Roberts HC, Sihvonen LH, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, Guardabassi L, Hilbert F, Mader R, Smith P, Aznar I, Muñoz Guajardo I, Baldinelli F and Alvarez J, 2021b. Scientific Opinion on the ad hoc method for the assessment of animal diseases caused by bacteria resistant to antimicrobials. EFSA Journal 2021;19(6):6645, 29 pp. 10.2903/j.efsa.2021.6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Martino L, Merten C, Mosbach‐Schulz O and Hardy A, 2018. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed]
- Ekapopphan D, Srisutthakarn A, Moonarmart W, Buddhirongawatr R and Bangphoomi N, 2018. Identification and antimicrobial susceptibility of microorganisms isolated from severe corneal ulcers of dogs in Thailand. Journal of Veterinary Medical Science, 80, 1259–1265. 10.1292/jvms.18-0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMA (European Medicines Agency) , 2019. Categorisation of antibiotics in, the European Union, EMA, Amsterdam, Netherlands. 73 pp. Available online: https://www.ema.europa.eu/en/documents/report/categorisation‐antibiotics‐european‐union‐answer‐request‐european‐commission‐updating‐scientific_en.pdf [Google Scholar]
- Fernandes MR, Sellera FP, Moura Q, Carvalho MPN, Rosato PN, Cerdeira L and Lincopan N, 2018. Zooanthroponotic transmission of drug‐resistant Pseudomonas aeruginosa, Brazil. Emerging Infectious Diseases, 24, 1160–1162. 10.3201/eid2406.180335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fothergill JL, Walshaw MJ and Winstanley C, 2012. Transmissible strains of Pseudomonas aeruginosa in cystic fibrosis lung infections. European Respiratory Journal, 40, 227–238. 10.1183/09031936.00204411 [DOI] [PubMed] [Google Scholar]
- Fusch C, Pogorzelski D, Main C, Meyer C‐L, El Helou S and Mertz D, 2015. Self‐disinfecting sink drains reduce the Pseudomonas aeruginosa bioburden in a neonatal intensive care unit. Acta Paediatrica, 104, e344–349. 10.1111/apa.13005 [DOI] [PubMed] [Google Scholar]
- Gelbíčová T, Baráková A, Florianová M and Karpíšková R, 2019. Detection of colistin‐resistant Acinetobacter baumannii with the mcr‐4 gene. Klinicka Mikrobiologie a Infekcni Lekarstvi, 25, 4–6. [PubMed] [Google Scholar]
- Gentilini F, Turba ME, Pasquali F, Mion D, Romagnoli N, Zambon E, Terni D, Peirano G, Pitout JDD, Parisi A, Sambri V and Zanoni RG, 2018. Hospitalized pets as a source of carbapenem‐resistance. Frontiers in Microbiology, 9, 2872. 10.3389/fmicb.2018.02872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein EJ, Agyare EO, Vagvolgyi AE and Halpern M, 1981. Aerobic bacterial oral flora of garter snakes: development of normal flora and pathogenic potential for snakes and humans. Journal of Clinical Microbiology, 13, 954–956. 10.1128/jcm.13.5.954-956.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenni M, Bour M, Châtre P, Madec J‐Y, Plésiat P and Jeannot K, 2017. Resistance of Animal Strains of Pseudomonas aeruginosa to Carbapenems. Frontiers in Microbiology, 8, 1847. 10.3389/fmicb.2017.01847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenni M, Hocquet D, Ponsin C, Cholley P, Guyeux C, Madec J‐Y and Bertrand X, 2015. Population structure and antimicrobial susceptibility of Pseudomonas aeruginosa from animal infections in France. BMC Veterinary Research, 11. 10.1186/%2Fs12917-015-0324-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JL, Holmes MA and Baines SJ, 2013. Prevalence and antimicrobial resistance of canine urinary tract pathogens. Veterinary Record, 173, 549. 10.1136/vr.101482 [DOI] [PubMed] [Google Scholar]
- Hattab J, Mosca F, Di Francesco CE, Aste G, Marruchella G, Guardiani P and Tiscar PG, 2021. Occurrence, antimicrobial susceptibility, and pathogenic factors of Pseudomonas aeruginosa in canine clinical samples. Veterinary World, 14, 978–985. 10.14202/%2Fvetworld.2021.978-985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins C, Harper D, Burch D, Anggård E and Soothill J, 2010. Topical treatment of Pseudomonas aeruginosa otitis of dogs with a bacteriophage mixture: a before/after clinical trial. Veterinary Microbiology, 146, 309–313. 10.1016/j.vetmic.2010.05.014 [DOI] [PubMed] [Google Scholar]
- Hewitt JS, Allbaugh RA, Kenne DE and Sebbag L, 2020. Prevalence and antibiotic susceptibility of bacterial isolates from dogs with ulcerative keratitis in midwestern United States. Frontiers in Veterinary Science, 7. 10.3389/fvets.2020.583965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier A, Alcorn JR, Cole LK and Kowalski JJ, 2006. Pyoderma caused by Pseudomonas aeruginosa infection in dogs: 20 cases. Veterinary Dermatology, 17, 432–439. 10.1111/j.1365-3164.2006.00550.x [DOI] [PubMed] [Google Scholar]
- Hirakawa Y, Sasaki H, Kawamoto E, Ishikawa H, Matsumoto T, Aoyama N, Kawasumi K and Amao H, 2010. Prevalence and analysis of Pseudomonas aeruginosa in chinchillas. BMC Veterinary Research, 6, 52. 10.1186/1746-6148-6-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høiby N, Frederiksen B and Pressler T, 2005. Eradication of early Pseudomonas aeruginosa infection. Journal of Cystic Fibrosis, 4, 49–54. 10.1016/j.jcf.2005.05.018 [DOI] [PubMed] [Google Scholar]
- Horsman S, Rynhoud H, Zhou X, Soares Magalhães RJ, Gibson JS and Meler E, 2020. Environmental recovery of nosocomial bacteria in a companion animal shelter before and after infection control procedures. Frontiers in Veterinary Science, 7. 10.3389/fvets.2020.608901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y‐C, Lin T‐Y and Wang C‐H, 2002. Community‐acquired Pseudomonas aeruginosa sepsis in previously healthy infants and children: analysis of forty‐three episodes. Pediatric Infectious Disease Journal, 21, 1049–1052. 10.1097/00006454-200211000-00015 [DOI] [PubMed] [Google Scholar]
- Hyun J‐E, Chung T‐H and Hwang C‐Y, 2018. Identification of VIM‐2 metallo‐beta‐lactamase‐producing Pseudomonas aeruginosa isolated from dogs with pyoderma and otitis in Korea. Veterinary Dermatology, 29, 186–e168. 10.1111/vde.12534 [DOI] [PubMed] [Google Scholar]
- Janecko N, Pokludova L, Blahova J, Svobodova Z and Literak I, 2016. Implications of fluoroquinolone contamination for the aquatic environment – a review. Environmental Toxicology and Chemistry, 35, 2647–2656. 10.1002/etc.3552 [DOI] [PubMed] [Google Scholar]
- Keiser NW, Birket SE, Evans IA, Tyler SR, Crooke AK, Sun X, Zhou W, Nellis JR, Stroebele EK, Chu KK, Tearney GJ, Stevens MJ, Harris JK, Rowe SM and Engelhardt JF, 2015. Defective innate immunity and hyperinflammation in newborn cystic fibrosis transmembrane conductance regulator‐knockout ferret lungs. American Journal of Respiratory Cell and Molecular Biology, 52, 683–694. 10.1165/%2Frcmb.2014-0250OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khondker A and Rheinstädter MC, 2020. How do bacterial membranes resist polymyxin antibiotics? Communications Biology, 3, 77. 10.1038/s42003-020-0803-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibbs LD, Johnson GR, Kidd TJ, Cheney J, Grimwood K, Kattenbelt JA, O’Rourke PK, Ramsay KA, Sly PD, Wainwright CE, Wood CE, Morawska L and Bell SC, 2014. Viability of Pseudomonas aeruginosa in cough aerosols generated by persons with cystic fibrosis. Thorax, 69, 740–745. 10.1136/thoraxjnl-2014-205213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbelik J, Singh A, Rousseau J and Weese JS, 2019. Characterization of the otic bacterial microbiota in dogs with otitis externa compared to healthy individuals. Veterinary Dermatology, 30, 228–e270. 10.1111/vde.12734 [DOI] [PubMed] [Google Scholar]
- Kukavica‐Ibrulj I, Bragonzi A, Paroni M, Winstanley C, Sanschagrin F, O’Toole GA and Levesque RC, 2008. In vivo growth of Pseudomonas aeruginosa strains PAO1 and PA14 and the hypervirulent strain LESB58 in a rat model of chronic lung infection. Journal of Bacteriology, 190, 2804–2813. 10.1128/%2FJB.01572-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukavica‐Ibrulj I, Facchini M, Cigana C, Levesque EC and Bragonzi A, 2014. Assessing Pseudomonas aeruginosa virulence and the host response using murine models of acute and chronic lung infection. Methods in Molecular Biology, 1149, 757–771. 10.1007/978-1-4939-0473-0_58 [DOI] [PubMed] [Google Scholar]
- Kümmerer K, 2009. Antibiotics in the aquatic environment – a review – Part I. Chemosphere, 75, 417–434. 10.1016/j.chemosphere.2008.11.086 [DOI] [PubMed] [Google Scholar]
- LaBauve AE and Wargo MJ, 2012. Growth and laboratory maintenance of Pseudomonas aeruginosa. Current Protocols in Microbiology, Chapter 6: Unit 6E 1.8. 10.1002/9780471729259.mc06e01s25 [DOI] [PMC free article] [PubMed]
- Langendonk RF, Neill DR and Fothergill JL, 2021. The building blocks of antimicrobial resistance in Pseudomonas aeruginosa: implications for current resistance‐breaking therapies. Frontiers in Cellular and Infection Microbiology, 11. 10.3389/fcimb.2021.665759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fernández R, Durán I, Molina‐López RA and Darwich L, 2020. Antimicrobial resistance in bacteria isolated from cats and dogs from the iberian peninsula. Frontiers in Microbiology, 11. 10.3389/fmicb.2020.621597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CT and Petersen‐Jones SM, 2008. Antibiotic susceptibility of bacteria isolated from cats with ulcerative keratitis in Taiwan. Journal of Small Animal Practice, 49, 80–83. 10.1111/j.1748-5827.2007.00437.x [DOI] [PubMed] [Google Scholar]
- Lin D, Foley SL, Qi Y, Han J, Ji C, Li R, Wu C, Shen J and Wang Y, 2012. Characterization of antimicrobial resistance of Pseudomonas aeruginosa isolated from canine infections. Journal of Applied Microbiology, 113, 16–23. 10.1111/j.1365-2672.2012.05304.x [DOI] [PubMed] [Google Scholar]
- Lineback CB, Nkemngong CA, Wu ST, Li X, Teska PJ and Oliver HF, 2018. Hydrogen peroxide and sodium hypochlorite disinfectants are more effective against Staphylococcus aureus and Pseudomonas aeruginosa biofilms than quaternary ammonium compounds. Antimicrobial Resistance and Infection Control, 7, 154. 10.1186/s13756-018-0447-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister PD, Wolter DJ and Hanson ND, 2009. Antibacterial‐resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clinical Microbiology Revisions, 22, 582–610. 10.1128/cmr.00040-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y‐Y, Wang Y, Walsh TR, Yi L‐X, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu L‐F, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu J‐H and Shen J, 2016. Emergence of plasmid‐mediated colistin resistance mechanism MCR‐1 in animals and human beings in China: a microbiological and molecular biological study. The Lancet, Infectious Diseases, 16, 161–168. 10.1016/s1473-3099(15)00424-7 [DOI] [PubMed] [Google Scholar]
- Ludwig C, de Jong A, Moyaert H, El Garch F, Janes R, Klein U, Morrissey I, Thiry J and Youala M, 2016. Antimicrobial susceptibility monitoring of dermatological bacterial pathogens isolated from diseased dogs and cats across Europe (ComPath results). Journal of Applied Microbiology, 121, 1254–1267. 10.1111/jam.13287 [DOI] [PubMed] [Google Scholar]
- Martins WMBS, Narciso AC, Cayô R, Santos SV, Fehlberg LCC, Ramos PL, da Cruz JB and Gales AC, 2018. SPM‐1‐producing Pseudomonas aeruginosa ST277 clone recovered from microbiota of migratory birds. Diagnostic Microbiology and Infectious Disease, 90 3 pp. 221–227. 10.1016/j.diagmicrobio.2017.11.003 [DOI] [PubMed] [Google Scholar]
- Matuschek E, Brown DFJ and Kahlmeter G, 2014. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clinical Microbiology and Infection, 20, 255–266. 10.1111/1469-0691.12373 [DOI] [PubMed] [Google Scholar]
- Mekić S, Matanović K and Šeol B, 2011. Antimicrobial susceptibility of Pseudomonas aeruginosa isolates from dogs with otitis externa. Veterinary Record, 169, 125. 10.1136/vr.d2393 [DOI] [PubMed] [Google Scholar]
- Meletis G, Exindari M, Vavatsi N, Sofianou D and Diza E, 2012. Mechanisms responsible for the emergence of carbapenem resistance in Pseudomonas aeruginosa . Hippokratia, 16, 303–307. [PMC free article] [PubMed] [Google Scholar]
- Michl RK, Beck JF and Mainz JG, 2017. Cystic fibrosis patient’s best friend? Potential transmission of Pseudomonas aeruginosa from a dog. Klinische Pediatrie, 229, 245–246. 10.1055/s-0043-110766 [DOI] [PubMed] [Google Scholar]
- Miller PE, Langenberg JA, Baeten LA and Moore CP, 1994. Pseudomonas aeruginosa‐associated corneal ulcers in captive cranes. Journal of Zoo and Wildlife Medicine, 25, 449–454. [Google Scholar]
- Mohan K, Fothergill JL, Storrar J, Ledson MJ, Winstanley C and Walshaw MJ, 2008. Transmission of Pseudomonas aeruginosa epidemic strain from a patient with cystic fibrosis to a pet cat. Thorax, 63, 839–840. 10.1136/thx.2007.092486 [DOI] [PubMed] [Google Scholar]
- Morris DO, Davis MF, Palmeiro BS, O’Shea K and Rankin SC, 2017. Molecular and epidemiological characterization of canine Pseudomonas otitis using a prospective case‐control study design. Veterinary Dermatology, 28, 118–e125. 10.1111/vde.12347 [DOI] [PubMed] [Google Scholar]
- Morrow CB, Raraigh KS, Green DM, Blackman SM, Cutting GR and Collaco JM, 2014. Cat and dog exposure and respiratory morbidities in cystic fibrosis. The Journal of Pediatrics, 165, 830–835, e832. 10.1016/j.jpeds.2014.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyaert H, de Jong A, Simjee S, Rose M, Youala M, El Garch F, Vila T, Klein U, Rzewuska M and Morrissey I, 2019a. Erratum: survey of antimicrobial susceptibility of bacterial pathogens isolated from dogs and cats with respiratory tract infections in Europe: ComPath results. Journal of Applied Microbiology, 127, 1594. 10.1111/jam.14420 [DOI] [PubMed] [Google Scholar]
- Moyaert H, de Jong A, Simjee S, Rose M, Youala M, El Garch F, Vila T, Klein U, Rzewuska M and Morrissey I, 2019b. Survey of antimicrobial susceptibility of bacterial pathogens isolated from dogs and cats with respiratory tract infections in Europe: ComPath results. Journal of Applied Microbiology, 127, 29–46 [DOI] [PubMed] [Google Scholar]
- Mulet X, Fernández‐Esgueva M, Norte C, Zamorano L, Del Barrio‐Tofiño E and Oliver A, 2021. Validation of MALDI‐TOF for the early detection of the ST175 high‐risk clone of Pseudomonas aeruginosa in clinical isolates belonging to a Spanish nationwide multicenter study. Enfermedades Infecciosas Y Microbiología Clínica, 39, 279–282. 10.1016/j.eimce.2020.05.015 [DOI] [PubMed] [Google Scholar]
- Nadăş GC, Novac CȘ, Matei IA, Bouari CM, Gal ZM, Tamas‐Krumpe OM, Macri AM and Fiț NI, 2021. Prevalence of antimicrobial resistant bacteria from conjunctival flora in an eye infection prone breed (saint bernard). Molecules, 26, 2219. 10.3390/molecules26082219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocera FP, Ambrosio M, Fiorito F, Cortese L and De Martino L, 2021. On gram‐positive‐ and gram‐negative‐bacteria‐associated canine and feline skin infections: a 4‐year retrospective study of the university veterinary microbiology diagnostic laboratory of Naples, Italy. Animals (Basel), 11, 1603. 10.3390/ani11061603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolff MC, Reese S, Fehr M, Dening R and Meyer‐Lindenberg A, 2016. Assessment of wound bio‐burden and prevalence of multi‐drug resistant bacteria during open wound management. Journal of Small Animal Practice, 57, 255–259. 10.1111/jsap.12476 [DOI] [PubMed] [Google Scholar]
- Oliveira M, Serrano I, Santos JP, Bilocq F, Pereira N, Loureiro NS, Tavares L, Pirnay J‐P and De Vos D, 2017. Pseudomonads from wild free‐living sea turtles in Príncipe Island, Gulf of Guinea. Ecological Indicators, 81, 260–264. 10.1016/j.ecolind.2017.06.005 [DOI] [Google Scholar]
- Panagea S, Winstanley C, Walshaw MJ, Ledson MJ and Hart CA, 2005. Environmental contamination with an epidemic strain of Pseudomonas aeruginosa in a Liverpool cystic fibrosis centre, and study of its survival on dry surfaces. The Journal of Hospital Infection, 59, 102–107. 10.1016/j.jhin.2004.09.018 [DOI] [PubMed] [Google Scholar]
- Park K‐M, Nam H‐S and Woo H‐M, 2013. Successful management of multidrug‐resistant Pseudomonas aeruginosa pneumonia after kidney transplantation in a dog. Journal of Veterinary Medical Science, 75, 1529–1533. 10.1292/%2Fjvms.13-0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Oh J, Park S, Sum S, Song W, Chae J and Park H, 2020. Antimicrobial resistance and novel mutations detected in the gyrA and parC genes of Pseudomonas aeruginosa strains isolated from companion dogs. BMC Veterinary Research, 16, 111. 10.1186/s12917-020-02328-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen K, Pedersen K, Jensen H, Finster K, Jensen VF and Heuer OE, 2007. Occurrence of antimicrobial resistance in bacteria from diagnostic samples from dogs. Journal of Antimicrobial Chemotherapy, 60, 775–781. 10.1093/jac/dkm269 [DOI] [PubMed] [Google Scholar]
- Planquette B, Timsit J‐F, Misset BY, Schwebel C, Azoulay E, Adrie C, Vesin A, Jamali S, Zahar J‐R, Allaouchiche B, Souweine B, Darmon M, Dumenil A‐S, Goldgran‐Toledano D, Mourvillier BH and Bedos J‐P, 2013. Pseudomonas aeruginosa ventilator‐associated pneumonia. predictive factors of treatment failure. American Journal of Respiratory and Critical Care Medicine, 188, 69–76. 10.1164/rccm.201210-1897OC [DOI] [PubMed] [Google Scholar]
- Pomba C, Rantala M, Greko C, Baptiste KE, Catry B, van Duijkeren E, Mateus A, Moreno MA, Pyörälä S, Ruzauskas M, Sanders P, Teale C, Threlfall EJ, Kunsagi Z, Torren‐Edo J, Jukes H and Törneke K, 2017. Public health risk of antimicrobial resistance transfer from companion animals. Journal of Antimicrobial Chemotherapy, 72, 957–968. 10.1093/jac/dkw481 [DOI] [PubMed] [Google Scholar]
- Pont S and Blanc‐Potard A‐B, 2021. Zebrafish Embryo infection model to investigate Pseudomonas aeruginosa interaction with innate immunity and validate new therapeutics. Frontiers in Cellular and Infection Microbiology, 11, 745851. 10.3389/fcimb.2021.745851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poonsuk K and Chuanchuen R, 2012. Contribution of the MexXY multidrug efflux pump and other chromosomal mechanisms on aminoglycoside resistance in Pseudomonas aeruginosa isolates from canine and feline infections. Journal of Veterinary Medical Science, 74, 1575–1582. 10.1292/jvms.12-0239 [DOI] [PubMed] [Google Scholar]
- Pye C, 2018. Pseudomonas otitis externa in dogs. The Canadian Veterinary Journal, 59, 1231–1234. [PMC free article] [PubMed] [Google Scholar]
- Ruiz‐Roldán L, Rojo‐Bezares B, de Toro M, López M, Toledano P, Lozano C, Chichón G, Alvarez‐Erviti L, Torres C and Sáenz Y, 2020. Antimicrobial resistance and virulence of Pseudomonas spp. among healthy animals: concern about exolysin ExlA detection. Scientific Reports, 10, 11667. 10.1038/%2Fs41598-020-68575-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainz‐Mejías M, Jurado‐Martín I and McClean S, 2020. Understanding Pseudomonas aeruginosa‐host interactions: the ongoing quest for an efficacious vaccine. Cells, 9, 2617. 10.3390/cells9122617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelstraete P, Van Daele S, De Boeck K, Proesmans M, Lebecque P, Leclercq‐Foucart J, Malfroot A, Vaneechoutte M and De Baets F, 2008. Pseudomonas aeruginosa in the home environment of newly infected cystic fibrosis patients. European Respiratory Journal, 31, 822–829. 10.1183/09031936.00088907 [DOI] [PubMed] [Google Scholar]
- Schink A‐K, Kadlec K, Hauschild T, Brenner Michael G, Dörner JC, Ludwig C, Werckenthin C, Hehnen H‐R, Stephan B and Schwarz S, 2013. Susceptibility of canine and feline bacterial pathogens to pradofloxacin and comparison with other fluoroquinolones approved for companion animals. Veterinary Microbiology, 162, 119–126. 10.1016/j.vetmic.2012.08.001 [DOI] [PubMed] [Google Scholar]
- Schrijver R, Sikkema R, de Vries H, Dewar D, Bergevoet R, Messori S, D’Albenzio S and Barnard S, 2015. Study on the welfare of dogs and cats involved in commercial practices. Available online: https://ec.europa.eu/food/system/files/2016‐10/aw_eu‐strategy_study_dogs‐cats‐commercial‐practices_en.pdf
- Scott A, Pottenger S, Timofte D, Moore M, Wright L, Kukavica‐Ibrulj I, Jeukens J, Levesque RC, Freschi L, Pinchbeck GL, Schmidt VM, McEwan N, Radford AD and Fothergill JL, 2019. Reservoirs of resistance: polymyxin resistance in veterinary‐associated companion animal isolates of Pseudomonas aeruginosa . Veterinary Record, 185, 206. 10.1136/vr.105075 [DOI] [PubMed] [Google Scholar]
- Sharma D, Pakravan N, Pritchard JC, Hartmann FA and Young KM, 2019. Mucoid Pseudomonas aeruginosa infection in a cat with severe chronic rhinosinusitis. Veterinary Clinical Pathology, 48, 300–304. 10.1111/vcp.12749 [DOI] [PubMed] [Google Scholar]
- Shi Q, Huang C, Xiao T, Wu Z and Xiao Y, 2019. A retrospective analysis of Pseudomonas aeruginosa bloodstream infections: prevalence, risk factors, and outcome in carbapenem‐susceptible and ‐non‐susceptible infections. Antimicrobial Resistance and Infection Control, 8, 68. 10.1186/s13756-019-0520-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull JW and Weese JS, 2015. Hospital‐associated infections in small animal practice. The Veterinary Clinics of North America. Small Animal Practice, 45, 217–233. 10.1016/j.cvsm.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taccetti G, Campana S, Festini F, Mascherini M and Döring G, 2005. Early eradication therapy against Pseudomonas aeruginosa in cystic fibrosis patients. European Respiratory Journal, 26, 458–461. 10.1183/09031936.05.00009605 [DOI] [PubMed] [Google Scholar]
- Ten Have GAM, Engelen MPKJ, Wolfe RR and Deutz NEP, 2019. Inhibition of jejunal protein synthesis and breakdown in Pseudomonas aeruginosa‐induced sepsis pig model. American Journal of Physiology, Gastrointestinal and Liver Physiology, 316, G755–G762. 10.1152/ajpgi.00407.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tümmler B, 2019. Emerging therapies against infections with Pseudomonas aeruginosa. F1000Res, 8, Faculty Rev‐1371. 10.12688/f1000research.19509.1 [DOI] [PMC free article] [PubMed]
- Ulrich S, Gottschalk C, Straubinger RK, Schwaiger K and Dörfelt R, 2020. Acceleration of the identification of sepsis‐inducing bacteria in cultures of dog and cat blood. Journal of Small Animal Practice, 61, 42–45. 10.1111/jsap.13056 [DOI] [PubMed] [Google Scholar]
- von Degerfeld MM, Banchi P and Quaranta G, 2020. Successful Treatment of pyometra caused by Pseudomonas aeruginosa infection in a rabbit. Topics in Companion Animal Medicine, 41. 10.1016/j.tcam.2020.100473 [DOI] [PubMed] [Google Scholar]
- Varriale L, Dipineto L, Russo TP, Borrelli L, Romano V, D’Orazio S, Pace A, Menna LF, Fioretti A and Santaniello A, 2020. Antimicrobial Resistance of Escherichia coli and Pseudomonas aeruginosa from Companion Birds. Antibiotics (Basel), 9, 780. 10.3390/antibiotics9110780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varriale L, Russo TP, Pace A, Mediatore S, Borrelli L, Santaniello A, Menna LF, Fioretti A and Dipineto L, 2019. Microbiological survey of sugar gliders (Petaurus breviceps) kept as pets in Italy. Letters in Applied Microbiology, 69, 399–402. 10.1111/lam.13233 [DOI] [PubMed] [Google Scholar]
- Verma A, Carney K, Taylor M, Amsler K, Morgan J, Gruszynski K, Erol E, Carter C, Locke S, Callipare A and Shah DH, 2021. Occurrence of potentially zoonotic and cephalosporin resistant enteric bacteria among shelter dogs in the Central and South‐Central Appalachia. BMC Veterinary Research, 17, 313. 10.1186/s12917-021-03025-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingopoulou EI, Delis GA, Batzias GC, Kaltsogianni F, Koutinas A, Kristo I, Pournaras S, Saridomichelakis MN and Siarkou VI, 2018. Prevalence and mechanisms of resistance to fluoroquinolones in Pseudomonas aeruginosa and Escherichia coli isolates recovered from dogs suffering from otitis in Greece. Veterinary Microbiology, 213, 102–107. 10.1016/j.vetmic.2017.11.024 [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang X, Schwarz S, Zhang R, Lei L, Liu X, Lin D and Shen J, 2014. IMP‐45‐producing multidrug‐resistant Pseudomonas aeruginosa of canine origin. Journal of Antimicrobial Chemotherapy, 69, 2579–2581. 10.1093/jac/dku133 [DOI] [PubMed] [Google Scholar]
- Wang R, van Dorp L, Shaw LP, Bradley P, Wang Q, Wang X, Jin L, Zhang Q, Liu Y, Rieux A, Dorai‐Schneiders T, Weinert LA, Iqbal Z, Didelot X, Wang H and Balloux F, 2018. The global distribution and spread of the mobilized colistin resistance gene mcr‐1. Nature Communications, 9, 1179. 10.1038/s41467-018-03205-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang Y, Zhou Y, Wang Z, Wang Y, Zhang S and Shen Z, 2019. Emergence of Colistin resistance gene mcr‐8 and Its Variant in Raoultella ornithinolytica . Frontiers in Microbiology, 10, 228. 10.3389/fmicb.2019.00228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser R, Donoghue D, Weightman A and Mahenthiralingam E, 2014. Evaluation of five selective media for the detection of Pseudomonas aeruginosa using a strain panel from clinical, environmental and industrial sources. Journal of Microbiology Methods, 99, 8–14. 10.1016/j.mimet.2014.01.010 [DOI] [PubMed] [Google Scholar]
- Werckenthin C, Alesík E, Grobbel M, Lübke‐Becker A, Schwarz S, Wieler LH and Wallmann J, 2007. Antimicrobial susceptibility of Pseudomonas aeruginosa from dogs and cats as well as Arcanobacterium pyogenes from cattle and swine as determined in the BfT‐GermVet monitoring program 2004–2006. Berliner Und Münchener Tierärztliche Wochenschrift, 120, 412–422. [PubMed] [Google Scholar]
- Wright EA, Di Lorenzo V, Trappetti C, Liciardi M, Orru G, Viti C, Bronowski C, Hall AJ, Darby AC, Oggioni MR and Winstanley C, 2015. Divergence of a strain of Pseudomonas aeruginosa during an outbreak of ovine mastitis. Veterinary Microbiology, 175, 105–113. 10.1016/j.vetmic.2014.11.011 [DOI] [PubMed] [Google Scholar]
- Xavier BB, Lammens C, Ruhal R, Kumar‐Singh S, Butaye P, Goossens H and Malhotra‐Kumar S, 2016. Identification of a novel plasmid‐mediated colistin‐resistance gene, mcr‐2, in Escherichia coli, Belgium, June 2016. Euro Surveillance, 21. 10.2807/1560-7917.es.2016.21.27.30280 [DOI] [PubMed] [Google Scholar]
- Yin W, Li H, Shen Y, Liu Z, Wang S, Shen Z, Zhang R, Walsh TR, Shen J and Wang Y, 2017. Novel plasmid‐mediated colistin resistance gene mcr‐3 in Escherichia coli . MBio, 8, e00543–e617. 10.1128/mbio.00543-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukawa S, Tsuyuki Y, Sato T, Fukuda A, Usui M and Tamura Y, 2017. Antimicrobial resistance of Pseudomonas aeruginosa isolated from dogs and cats in primary veterinary hospitals in Japan. Japanese Journal of Infectious Diseases, 70, 461–463. 10.7883/yoken.jjid.2016.536 [DOI] [PubMed] [Google Scholar]
- Zhang R, Liu Z, Li J, Lei L, Yin W, Li M, Wu C, Walsh TR, Wang Y, Wang S and Wu Y, 2017. Presence of VIM‐positive Pseudomonas species in chickens and their surrounding environment. Antimicrobial Agents and Chemotherapy, 61. 10.1128/%2FAAC.00167-17 [DOI] [PMC free article] [PubMed] [Google Scholar]


