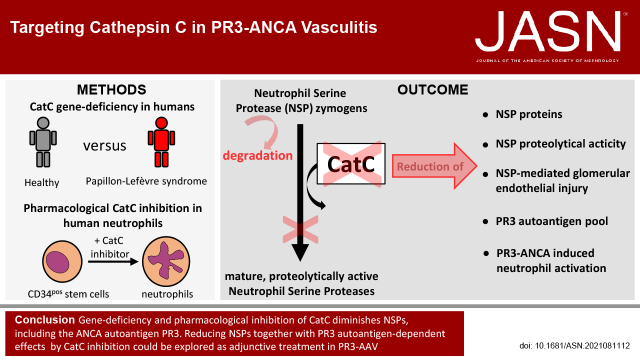
Significance Statement
In ANCA–associated vasculitis (AAV), the autoantigens proteinase 3 (PR3) and myeloperoxidase (MPO) are exclusively expressed by neutrophils and monocytes. Cathepsin C (CatC), which activates the proteolytic function of neutrophil serine proteases (NSPs), provides a potential novel treatment target by controlling NSPs in both human neutrophils and monocytes. The authors show that genetic deficiency of CatC in humans and pharmacologic inhibition of CatC in a human stem cell model effectively downregulate NSPs, including PR3. Consequently, these neutrophils showed a diminished activation response to PR3-ANCA but not to MPO-ANCA. Moreover, both genetic and pharmacologic NSP depletion resulted in less neutrophil-induced injury of glomerular microvascular endothelial cells. These findings may offer encouragement for pursuing clinical studies with adjunctive CatC inhibitor administration in patients with PR3-AAV.
Keywords: ANCA, endothelial cells, immunology, cathepsin C, vasculitis
Visual Abstract
Abstract
Background
The ANCA autoantigens proteinase 3 (PR3) and myeloperoxidase (MPO) are exclusively expressed by neutrophils and monocytes. ANCA-mediated activation of these cells is the key driver of the vascular injury process in ANCA-associated vasculitis (AAV), and neutrophil serine proteases (NSPs) are disease mediators. Cathepsin C (CatC) from zymogens activates the proteolytic function of NSPs, including PR3. Lack of NSP zymogen activation results in neutrophils with strongly reduced NSP proteins.
Methods
To explore AAV-relevant consequences of blocking NSP zymogen activation by CatC, we used myeloid cells from patients with Papillon-Lefèvre syndrome, a genetic deficiency of CatC, to assess NSPs and NSP-mediated endothelial cell injury. We also examined pharmacologic CatC inhibition in neutrophil-differentiated human hematopoietic stem cells, primary human umbilical vein cells, and primary glomerular microvascular endothelial cells.
Results
Patients with Papillon-Lefèvre syndrome showed strongly reduced NSPs in neutrophils and monocytes. Neutrophils from these patients produced a negative PR3-ANCA test, presented less PR3 on the surface of viable and apoptotic cells, and caused significantly less damage in human umbilical vein cells. These findings were recapitulated in human stem cells, in which a highly specific CatC inhibitor, but not prednisolone, reduced NSPs without affecting neutrophil differentiation, reduced membrane PR3, and diminished neutrophil activation upon PR3-ANCA but not MPO-ANCA stimulation. Compared with healthy controls, neutrophils from patients with Papillon-Lefèvre syndrome transferred less proteolytically active NSPs to glomerular microvascular endothelial cells, the cell type targeted in ANCA-induced necrotizing crescentic glomerulonephritis. Finally, both genetic CatC deficiency and pharmacologic inhibition, but not prednisolone, reduced neutrophil-induced glomerular microvascular endothelial cell damage.
Conclusions
These findings may offer encouragement for clinical studies of adjunctive CatC inhibitor in patients with PR3-AAV.
ANCA-associated vasculitis (AAV) is a systemic autoimmune disease that frequently affects the kidneys, manifesting as necrotizing crescentic GN (NCGN).1,2 The major ANCA autoantigens proteinase 3 (PR3) and myeloperoxidase (MPO) are exclusively expressed by neutrophils and monocytes.2–5 ANCA binding to their target antigens on the cell surface initiates cell activation, subsequently resulting in endothelial injury and necrotizing vasculitis.6 Current immunosuppressive treatments improved clinical outcomes, but are associated with significant side effects.7,8 Characterization and pharmacologic targeting of AAV disease mediators may lead to more disease-specific treatments. The neutrophil serine protease (NSP) family, consisting of PR3, neutrophil elastase (NE), cathepsin G (CatG), and low-abundant NSP4, provides a potential candidate for a druggable disease mediator.9 Several AAV-relevant NSP actions have been identified, including NSP transfer to and injury of endothelial cells (EC),10 promotion of receptor-interacting protein kinase–dependent neutrophil extracellular trap formation11–13 and monocyte and neutrophil IL-1β generation.14 PR3 is a unique NSP because it acts not only as a serine protease, but also as a disease-driving autoantigen in PR3-AAV. PR3 on the neutrophil and monocyte membrane (mPR3) binds PR3-ANCA, thereby initiating cell activation and PR3 on the surface of apoptotic neutrophils promotes proinflammatory efferocytosis and PR3 autoimmunity with Th17 polarization in the presence of PR3-ANCA.15 Thus, some NSP-mediated disease mechanisms pertain to both PR3- and MPO-AAV, whereas others have additional implications specifically in PR3-AAV.
CatC is a highly conserved lysosomal cysteine dipeptidyl aminopeptidase that proteolytically activates NSPs zymogens during hematopoietic stem cell (HSC) differentiation in the bone marrow (BM).16 We observed previously that CatC gene–deficient mice were protected from AAV.14 CatC loss-of-function mutations in humans cause rare autosomal-recessive Papillon-Lefèvre syndrome (PLS, OMIM:245000) affecting between one and four people per million.17,18 Patients with PLS feature periodontitis, plantar, and palmar hyperkeratosis, but do not show marked immunodeficiency despite NSP depletion. Moreover, blood neutrophils from patients PLS were found to be fully capable of bactericidal activity against Staphylococcus aureus and Klebsiella pneumoniae in vitro, indicating NSPs are not the only tool to clear common bacterial pathogens.12 Pharmacologic CatC inhibition is tested in patients with NSP-mediated diseases, such as bronchiectasis.19 We explored AAV-relevant consequences of CatC deficiency in humans using cells from patients with PLS and developed and tested a highly specific pharmacologic CatC inhibitor in a human stem cell model.
Methods
Preparation of Human Neutrophils and Monocytes
Blood from healthy human controls (HC), patients with PLS, and patients with active PR3-AAV were obtained after approval by the Charité University, after written informed consent (Ethics vote EA4/025/18). The two patients PLS were treated by P.E. and K.N., and showed the typical palmoplantar hyperkeratosis and aggressive periodontitis resulting in premature tooth loss. Clinical characteristics of patient 1 with PLS (PLS1) were described20 and the compound heterozygous CatC gene mutations in exon 7 (c.947T>G and c.1268G>C, family 2).21 Clinical symptoms of PLS2 were reported,22 and a frame shift mutation was found in exon 4 of the CatC gene (c.566–572Del, family 9).21
Neutrophils were isolated from heparinized whole blood using dextran sedimentation and density gradient centrifugation, as described previously.23 Cells were >95% neutrophils as determined by morphologic analysis and >99% viable by Trypan blue dye exclusion. For the preparation of a monocyte-enriched cell population, monocytes from the interphase were positively sorted with anti-CD14 magnetic beads on LD columns according to the manufacturer's instructions (Miltenyi, Bergisch-Gladbach, Germany).
Sorting Algorithm for Neutrophils and Monocytes from HC
After density gradient centrifugation and red blood cell lysis, neutrophils were negatively MACS sorted using a cocktail of biotinylated antibodies against CD3, CD19, CD36, CD49d, CD56, and CD235a, followed by incubation with antibiotin magnetic beads (all from Miltenyi). The resulting population contained >99% pure CD16pos neutrophils. After density gradient centrifugation, monocytes from the interphase were FACS sorted (FACSAria II cell sorter, BD Biosciences, San Diego, CA) using light scatter characteristics and cell staining with antibodies to CD11b (Biolegend, San Jose, CA), CD15 and CD14 (both BD Biosciences), respectively. The resulting population contained approximately 99% pure CD14hi monocytes.
Preparation of Human IgG
Normal- and ANCA-IgG were prepared from HC and patients with active MPO- and PR3-ANCA disease using a High-Trap-protein-G column in an Äkta-FPLC system (Cytiva Europe GmbH, Freiburg, Germany).
Cathepsin C Inhibitor
The small molecule cathepsin C inhibitor BI-9740 was obtained via Boehringer Ingelheim’s open innovation platform OpnMe (https://opnme.com). BI-9740 inhibits human CatC with an IC50 of 2 nM and mouse CatC with an IC50 of 0.8 nM. It is highly selective (>1000 fold) for CatC versus the other Cathepsins (B, F, H, K, L, S) and highly potent in reducing NSP activity in vivo. Detailed data on structure potency, selectivity, and PK profile and in vivo activity data can be found under https://opnme.com/sites/default/files/opnMe_M2O_profile_BI-9740.pdf.
Differentiation of Human Neutrophils from HSCs
Umbilical cord blood was collected (Ethics vote EA4/025/18). Cells were washed and stained using CD34pos progenitor isolation kit (Miltenyi) and sorted on LS column according to the manufacturer's instructions. Cells were expanded in stem span serum-free medium (Stem Cell Technologies, Vancouver, Canada) supplemented with penicillin/streptomycin, 100 ng/ml stem cell factor, 20 ng/ml Thrombopoietin, and 50 ng/ml Fms-related tyrosine kinase 3 ligand (PeproTech, London, United Kingdom). Neutrophil differentiation was in RPMI with 10% FCS and 10 ng/ml G-CSF (PeproTech), and either buffer control, 1 µM CatC inhibitor BI-9740 (BI-I), or 100 µM prednisolone for 10 days.
Assessment of EC Damage by Phalloidin Staining and Microscopy
Confluent human umbilical vein EC (HUVECs) or primary glomerular microvascular EC (Cell Systems, Kirkland, WA) were grown on glass coverslips. Cells were washed and incubated with cell-free supernatants (cf-SN) from resting or 2.5 µM ionophore A23187 (Merck Millipore, Calbiochem, Darmstadt, Germany) stimulated neutrophils, and 10 µM α1-AT (Sigma-Aldrich) for 8 hours, as indicated. The cf-SN from blood neutrophils was used at a 1:4, and from stem cell–differentiated neutrophils at a 1:2 dilution in basal medium. EC cells were fixed in 4% paraformaldehyde (15 minutes, room temperature [RT]) and permeabilized in 0.5% Tx-100 (2 minutes, RT) and actin filaments were stained with phalloidin-Alexa488 and nuclei with DAPI (both Invitrogen). Fluorescence images were acquired using a Leica DMI6000 B Microscope with 40× objective. EC damage was assessed by quantification of at least five black pixel areas with ImageJ 1.48v software (https://imagej.nih.gov/ij).
Apoptosis Assessment in Neutrophils and Glomerular Microvascular EC-by Annexin V and 7-AAD Staining
Next, 2 × 106 human neutrophils were kept in RPMI medium supplemented with 10% FCS for 20 hours for constitutive apoptosis. For induced apoptosis, neutrophils from differentiated HSC were treated with 25 µM MG-132 (Merck, Darmstadt, Germany) for 20 hours. Phosphatidylserine exposure on neutrophils or glomerular microvascular EC that were treated for 16 hours with buffer or cf-SN from neutrophils was determined by Annexin V-FITC (BD Biosciences, San Jose, CA) and necrosis by 7-AAD (Merck, Darmstadt, Germany) staining for 15 minutes at RT.
SDS‐PAGE and Immunoblotting Analysis
Cell lysis, SDS‐PAGE, and immunoblot were performed as described previously.14 In brief, neutrophils or glomerular microvascular EC treated with buffer or cf-SN from neutrophils for 1 hour were lysed, incubated in loading buffer, separated on a 12% SDS‐PAGE, blotted onto polyvinylidene difluoride membrane, developed with the indicated antibodies, and visualized by an ECL detection system (Super Signal West Dura Extended Duration substrate, Thermo Fisher Scientific, Schwerte, Germany) on a Chemi Only Imager (VWR International, Darmstadt, Germany). Antibodies used for immunoblotting were mAb rabbit antihuman PR3 (1:4000, Abcam, Cambridge, UK), polyclonal rabbit anti-NE (1:1000, Abcam), polyclonal goat anti-CatG (1:500), and monoclonal mouse anti-CatC (1:1000, both Santa Cruz Biotechnology, Dallas, TX, USA), polyclonal rabbit anti-MPO (1:1000, Merck, Darmstadt, Germany), polyclonal rabbit antiactin (1:2000, Cell Signaling Europe, Frankfurt/Main, Germany). Corresponding secondary horseradish peroxidase–conjugated antibodies (1:1000) were used.
Fluorescence Resonance Energy Transfer and NSP-specific proteolytic activity
NSP-specific proteolytic activity was measured as described previously.24 Briefly, neutrophils or glomerular microvascular EC treated with buffer or cf-SN from neutrophils for 1 hour were lysed on ice, soluble fractions were separated from cell debris by centrifugation, and 5–30 µg cell lysates in 150 μl HEPES buffer containing 0.02% lauryl maltoside were incubated with fluorescence resonance energy transfer (FRET) substrate (20 μM final). Fluorescence was measured by plate reader (excitation 320 nm, emission 420 nm, SpectraMax M5, Molecular Devices, CA), and the corresponding Vmax is reported. For the calculation of the assay baseline (auto- and unspecific hydrolysis of FRET substrate) 20 µM α1-antitrypsin (Merck) was added and the corresponding Vmax was subtracted from sample values. The selective FRET substrates for human NSPs were: PR3: 2-Abz-VAD-(nor)V-ADYQ-EDA-Dnp; NE: 2-Abz-APEEIMRRQ-EDA-Dnp; and CatG: 2-Abz-EPFWEDQ-EDA-Dnp.
Flow Cytometry to Assess Neutrophil mPR3 and mCD177
For double staining of mPR3 and mCD177, cells were incubated first with 5 µg/ml Alexa488-conjugated anti-PR3 (clone 43–8-3) or corresponding Alexa488-isotype for 15 minutes on ice. After washing CD177 was assessed with anti-CD177PE (15 minutes, ice), mAb mouse IgGPE were used as isotype control (both Beckman Coulter, Munich, Germany). For MPO, cells were stained with 5 µg/ml polyclonal rabbit anti-MPO (Merck) or normal rabbit IgG (Santa Cruz Biotechnology) followed by Alexa488-conjugated secondary Abs. Then 10,000 events per sample were collected using a BD FACS Calibur or a BD FACS CANTO II and analyzed with FlowJo software (TreeStar, Ashland, OR).
ANCA Staining by Indirect Immunofluorescence
Neutrophils were centrifuged on glass slides using a Cytospin Hettich Universal device (Hettich GmbH, Tuttlingen, Germany), and permeabilized in ice-cold 99% ethanol. Slides were stored at −20°C until use. Slides were incubated with sera from patients with PR3- or MPO-ANCA (1:10 diluted in PBS) for 60 minutes at RT. After washing, cells were stained with Alexa488-conjugated antihuman IgG (1:250, Molecular Probes, Eugene, Oregon, USA) for 60 minutes at RT in the dark, washed with PBS, and covered with fluoromount (Southern Biotech, Birmingham, AL, USA). Fluorescence images were acquired using a Leica Microscope (Leica DMI6000 B, Wetzlar, Germany) with a 40× objective.
Measurement of Superoxide Release
Superoxide was measured using superoxide dismutase–inhibitable ferricytochrome c (final concentration 50 µM) reduction as described previously.25 Briefly, 2.5× 105 cells/ml were pretreated with cytochalasin B (5 µg/ml, 15 minutes), primed with TNFα (2 ng/ml, 15 minutes) before stimulating antibodies (mAb 5 µg/ml, or 75 µg/ml purified IgGs) were added. Assay was performed in 96-well plates at 37°C, and the absorption of samples with and without superoxide dismutase (300 U/ml) was scanned repetitively at 550 nm using a Microplate Reader (Molecular Devices). The 45-minute results are reported.
Statistics
Results are given as mean±SEM. Comparisons were made using ANOVA with post-hoc analysis, comparisons between two groups were carried out by unpaired t test using GraphPad Prism8 software. Differences were considered significant at P<0.05.
Results
Reduced NSPs in Neutrophils and Monocytes, and Less Cell-surface PR3 in Patients with PLS
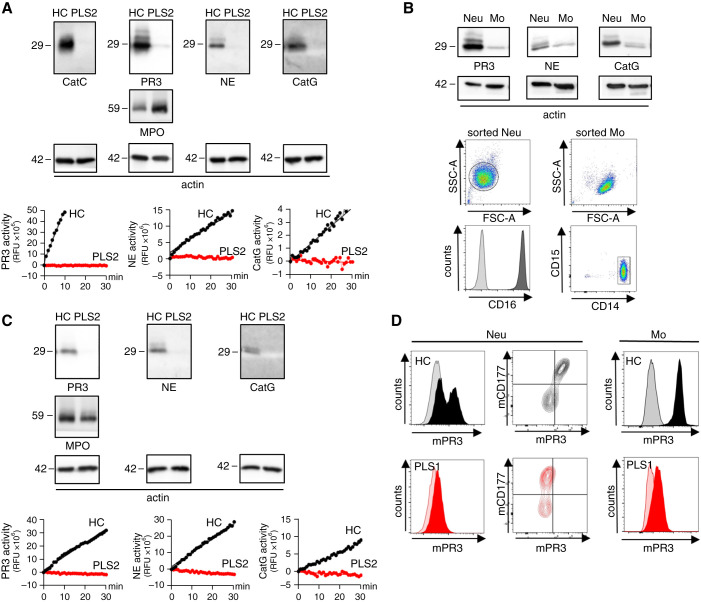
We first characterized NSPs in neutrophils and monocytes from two patients with PLS. Compared with HC neutrophils, PLS neutrophils lacked CatC protein and showed strongly reduced PR3, NE, and CatG protein and proteolytic activity (Figure 1A). MPO was not affected by CatC gene deficiency. We extended our observations to monocytes because these cells also contribute to ANCA-induced inflammation and injury26,27 and have not been studied in PLS. We first assessed NSPs in highly pure sorted human HC monocytes and neutrophils (Figure 1B). Both cell types contained PR3, NE, and CatG protein, although monocytes harbored less NSPs than neutrophils. Because this sorting approach was not feasible with the limited material from patients with PLS, we prepared a monocyte-enriched cell population from HC and patients with PLS with 80%±5 purity using CD14 magnetic beads. The preparation from the patients with PLS showed strongly reduced NSP proteins and proteolytic activity compared with HC cells (Figure 1C).
Figure 1.
NSPs are strongly decreased in and on the surface of neutrophils and monocytes from patients with CatC gene-deficient PLS. (A) CatC, NE, PR3, and CatG in isolated blood neutrophils from an HC and a patient with PLS (PLS) by immunoblotting and by protease-specific FRET assays. MPO served as control and 42 kDa actin indicates equal sample loading. (B) NSP proteins in highly pure sorted HC monocytes (Mo) and neutrophils (Neu) by immunoblotting. Flow cytometry documents the purity of the sorted neutrophils and monocytes. (C) NSP protein expression and proteolytic activity in CD14hi sorted HC and PLS monocytes. (D) Flow cytometry of neutrophils stained for mPR3 and CD177. mPR3 expression on CD11bpos/CD14hi/CD15neg blood monocytes from an HC and a patient with PLS by flow cytometry.
Because PR3-ANCA binding to mPR3 on the neutrophil and monocyte surface initiates cell activation, we assessed mPR3 on both cell types from HC and patients with PLS (Figure 1D). HC neutrophils showed the typical bimodal mPR3 pattern with distinct CD177neg/mPR3lo and CD177pos/mPR3hi populations that is caused by the subset-restricted expression of the PR3-presenting CD177 receptor,28 whereas PLS neutrophils revealed strongly reduced mPR3 on both CD177 subsets. In contrast with neutrophils, mPR3 staining in HC monocytes was monomodal because these cells lack the neutrophil-specific CD177 receptor. Compared with HC, PLS monocytes showed strongly reduced mPR3.
PLS Neutrophils Expose Less PR3, Cause Less Endothelial Injury, and Produce a Negative ANCA Test
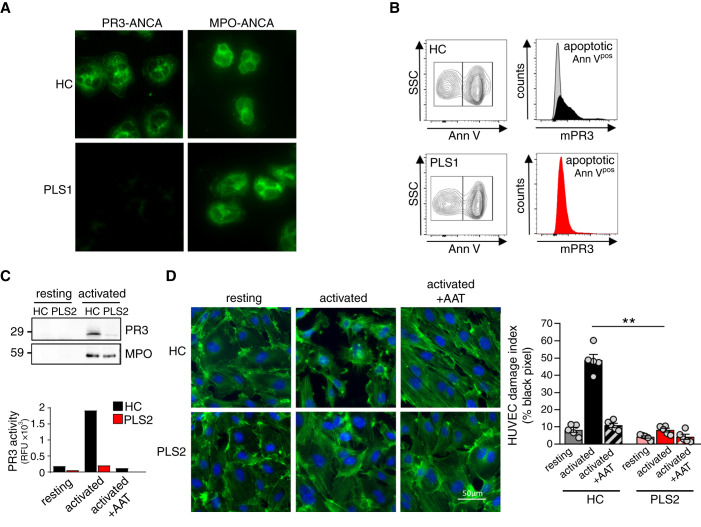
We next studied additional AAV-relevant findings in HC and PLS neutrophils. We performed an immunofluorescence ANCA test as done in clinical medicine, using serum from a patient with PR3-ANCA–positive AAV. The test produced the typical cytoplasmic (c-ANCA) staining pattern with HC neutrophils, but was negative on PLS neutrophils (Figure 2A).
Figure 2.
PLS neutrophils produce a negative clinical PR3-ANCA immunofluorescence test, expose less PR3 on apoptotic neutrophils, and cause less NSP-mediated endothelial injury. (A) Indirect immunofluorescence using HC and PLS neutrophils and sera from patients on PR3 and MPO, respectively. (B) A percentage of overnight cultured neutrophils showed constitutive apoptosis by Annexin V staining. mPR3 was analyzed after gating on apoptotic (Annpos) cells. (C) cf-SN from resting and activated (2.5 µM ionophore A23187) HC and PLS neutrophils were assessed for PR3 protein by immunoblotting and proteolytic activity by FRET assay. (D) Confluent HUVEC monolayers were incubated with cf-SN from resting and activated HC and PLS neutrophils, respectively. When indicated cf-SN from activated neutrophils were treated with α1-antitrypsin (AAT) before EC incubation. EC damage was visualized by actin staining with phalloidin-FITC (green) and nuclear DAPI staining (blue), and quantified by determining the black pixel areas using a Leica microscope (×40) and ImageJ 1.48v software. ** P<0.01.
We then investigated mPR3 on the surface of apoptotic neutrophils that was suggested to provide a do-not-eat-me signal thereby promoting inflammatory efferocytosis and PR3 autoimmunity.15,29 Culturing HC and PLS neutrophils overnight resulted in constitutive apoptosis indicated by AnnexinVpos neutrophils. The apoptotic PLS neutrophils showed strongly reduced mPR3 compared with HC cells (Figure 2B).
We next produced cf-SN from resting and activated HC and PLS neutrophils, and incubated primary HUVEC monolayers with this material. Cf-SN from activated PLS neutrophils contained significantly less proteolytically active NSPs, compared with cf-SN from HC neutrophils as exemplified by PR3 (Figure 2C). Proteolytic PR3 activity in cf-SN from HC neutrophils was strongly reduced by α1-antitrypsin, the major natural NSP inhibitor. HUVEC treatment with cf-SN from activated HC neutrophils profoundly disturbed the actin structure of the EC monolayer, and this effect was strongly reduced when cf-SN from PLS neutrophils was used or when cf-SN from HC neutrophils were treated with α1-antitrypsin (Figure 2D). Together, these data indicate human CatC gene-deficiency strongly reduced NSPs in neutrophils and monocytes. This effect included less PR3 autoantigen in cells and on their surface. Moreover, CatC gene-deficiency reduced HUVEC injury by activated neutrophils.
Pharmacologic CatC Inhibition Reduces NSPs, Neutrophil mPR3, PR3-ANCA Induced Neutrophil Activation, and NSP-dependent EC Damage
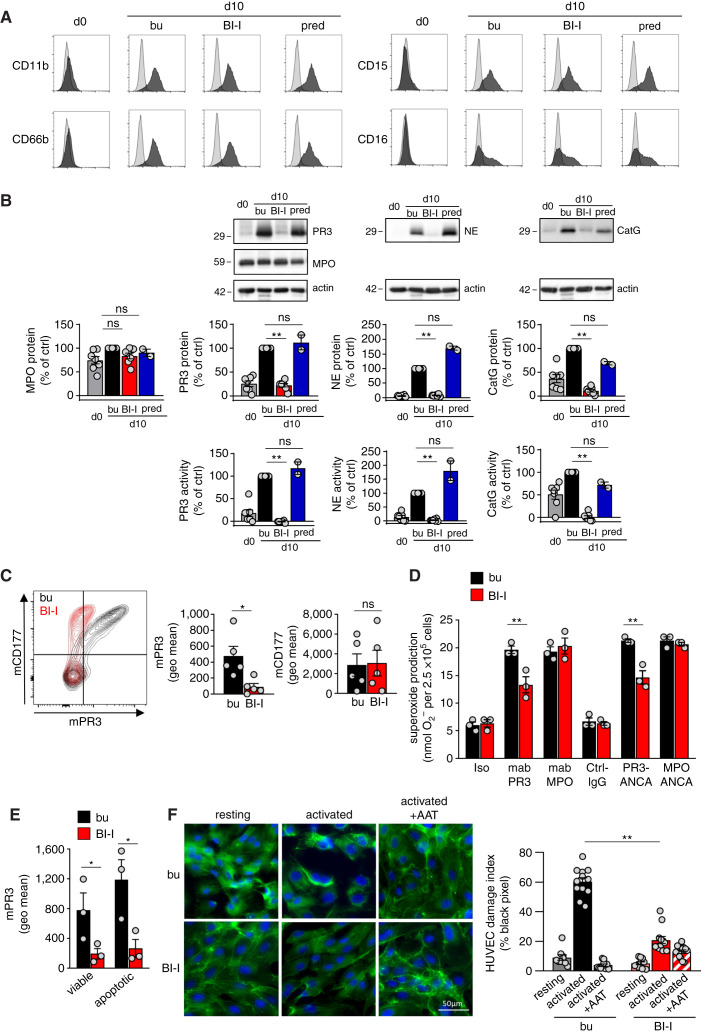
Proteolytic NSP maturation by CatC occurs in early neutrophil progenitors in the BM. We tested the potent and highly selective BI-I in a human CD34pos HSC model. BI-I treatment did not interfere with neutrophil differentiation over the 10-day differentiation period (Figure 3A), but strongly reduced NSP proteins and their proteolytic activity, whereas MPO was not affected (Figure 3B). In contrast, prednisolone that is part of the standard AAV treatment did not reduce NSPs. mPR3 on HSC-derived neutrophils was, similar to human blood neutrophils, largely presented on the CD177pos subset. BI-I treatment reduced mPR3 by 83%±5 (Figure 3C). Consequently, neutrophils differentiated in the presence of the BI-I released significantly less superoxide in response to PR3-, but not to MPO-ANCA (Figure 3D). Superoxide reduction by BI-I was approximately 50%, but did not achieve levels seen with isotype and normal IgG controls. Conceivably, incomplete reduction was caused by PR3-ANCA binding to residual mPR3. An 18-hour incubation with the proteasome inhibitor MG-132 on differentiation day 10 induced apoptosis in 16%±3 of the cells. BI-I treatment reduced mPR3 on both viable and apoptotic cells (Figure 3E). Finally, cf-SN from activated neutrophils differentiated in the presence of BI-I contained less NSP activity and caused significantly less HUVEC injury than cf-SN from control neutrophils (Figure 3F).
Figure 3.
Pharmacologic CatC inhibition strongly reduces NSPs in neutrophils differentiated from HSC and subsequently reduces neutrophil activation by PR3-ANCA and EC injury. Neutrophils were differentiated from human CD34pos HSC for 10 days in the presence of buffer control (bu), BI-I, and prednisolone (pred) as indicated. (A) Neutrophil differentiation was assessed using the indicated surface markers. (B) NSP proteins were assessed by immunoblotting and the optical densities (OD) of the NSP bands were quantified. Proteolytic activity was measured by FRET assay. MPO served as a control. (C) Neutrophils differentiated for 10 days were double stained for mPR3 and CD177 and analyzed by flow cytometry. A typical experiment together with the mPR3 and CD177 mean fluorescence intensity are depicted. (D) Superoxide release by neutrophils differentiated for 10 days was assessed using mAbs to PR3 and MPO, human PR3- and MPO-ANCA IgG, and appropriate controls as indicated. (E) mPR3 on viable (AnnVneg) and apoptotic (Annpos) neutrophils differentiated for 10 days was assessed by flow cytometry. (F) The effect of cf-SN from resting and activated neutrophils differentiated for 10 days on EC injury was assessed by phalloidin-FITC (green) and nuclear DAPI staining (blue) and microscopy. *P<0.05 and **P<0.01.
Thus, pharmacologic CatC inhibition in a human neutrophil differentiation model recapitulated key findings from patients with CatC gene-deficient PLS without affecting the differentiation process. NSPs were strongly reduced resulting in less neutrophil-mediated endothelial damage. Diminished mPR3 had a functional consequence, namely less neutrophil activation by PR3-, but not MPO-ANCA.
HC but not PLS Neutrophils Transfer NSPs to Glomerular Microvascular EC and both CatC Gene-deficiency and Pharmacologic CatC Inhibition Lead to Less Neutrophil-induced Glomerular Microvascular EC Injury
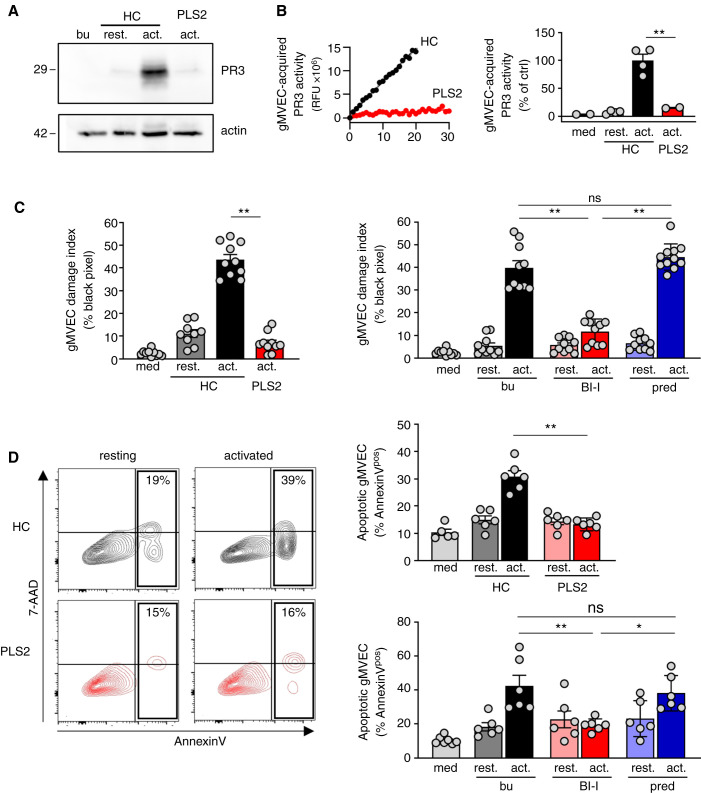
HUVECs provide a robust primary cell type to study NSP effects, but glomerular microvascular EC are more relevant for ANCA-induced NCGN. We assessed the NSP transfer from neutrophils to glomerular microvascular EC and the subsequent endothelial injury. Incubation of glomerular microvascular EC monolayers with cf-SN from activated HC but not PLS neutrophils led to NSP acquisition by glomerular microvascular EC, exemplified by immunoblotting using a PR3-specific mAb (Figure 4A). Importantly, acquired PR3 preserved its proteolytic activity by FRET assay (Figure 4B). Finally, glomerular microvascular EC-treatment with cf-SN from activated HC or HSC-derived neutrophils caused glomerular microvascular EC-injury by phalloidin (Figure 4C) and Annexin V/7–AAD (Figure 4D) staining. In contrast, cell injury was strongly reduced with cf-SN from activated PLS neutrophils or from HSC-derived neutrophils differentiated in the presence of the BI-I. Note that glomerular microvascular EC-injury was not diminished when cf-SN from neutrophils differentiated in the presence of prednisolone was used.
Figure 4.
Healthy control but not PLS neutrophils transfer proteolytically active PR3 to glomerular microvascular EC and both CatC gene deficiency and pharmacological CatC inhibition result in less neutrophil-induced glomerular microvascular EC injury. Confluent gMVEC monolayers were incubated with cf-SN from resting and activated HC or activated PLS neutrophils as indicated. After 1 hour, PR3 acquisition by glomerular microvascular EC was assessed by (A) immunoblotting using a PR3-specific mAb, and (B) FRET assay measuring proteolytic PR3 activity. Two independent experiments each with two HC and PLS2 neutrophils were performed. For assessing cell injury, glomerular microvascular EC monolayers were incubated with cf-SN from HC or PLS neutrophils, or from neutrophils that were differentiated from HSC in the presence of buffer, BI-I, or prednisolone as indicated. Each independent experiment includes neutrophils from two HC and PLS2, or two different HSC donors. Glomerular microvascular EC injury was determined (C) by phalloidin staining with the analysis of black pixel areas as in Figure 2D. The corresponding statistics is given (n=2), and (D) by Annexin V/7–AAD staining and flow cytometry (n=3). Contour plots of a typical experiment with cf-SN from resting and activated HC and PLS neutrophils are depicted together with the corresponding statistics of all experiments. *P<0.05 and **P<0.01.
Discussion
We explored CatC as a potential AAV treatment target to downregulate NSPs, including the PR3 autoantigen that drives PR3-AAV. Our study revealed several new findings. First, human CatC gene-deficiency in patients with PLS strongly reduced NSPs in both neutrophils and monocytes resulting in less neutrophil-mediated EC damage. Notably, the PR3 autoantigen on the cell surface of viable and apoptotic neutrophils was diminished. Second, pharmacologic CatC inhibition in a human HSC model, but not prednisolone, effectively reduced NSPs without affecting neutrophil differentiation. CatC inhibition recapitulated findings from PLS neutrophils and established diminished neutrophil activation by PR3- but not MPO-ANCA as a functional consequence of mPR3 reduction. Third, CatC gene deficiency and pharmacologic CatC inhibition strongly diminished neutrophil-induced injury of glomerular microvascular EC that are highly relevant to ANCA-induced NCGN.
ANCA-activated neutrophils and monocytes are central to the vascular inflammation and injury process. Activation starts with ANCA binding to its corresponding autoantigen on the cell surface resulting in cell activation and release of mediators that contribute to the vascular damage. Characterization of druggable disease mediators will possibly extend treatment options for AAV patients as recently shown for complement C5a.30–33 We explored NSPs downregulation by targeting CatC as an additional approach. The rationale was that all NSP family members have mechanistic implications in AAV as proteolytically active serine proteases. Because PR3 is a protease but also provides the autoantigen in PR3-AAV, reducing PR3 may have beneficial effects for patients with PR3-AAV. Moreover, we showed previously that PR3/NE double gene-deficient mice and CatC gene-deficient mice were protected from NCGN in an MPO-ANCA disease model.14
PLS is a rare autosomal-recessive disease characterized by CatC loss-of-function mutations (PLS, OMIM:245000). PLS affects between one and four people per million, featuring periodontitis, palmar and plantar hyperkeratosis, but no marked immunodeficiency.17,18 We show for the first time that, in addition to PLS neutrophils, PLS monocytes also harbor marginal NSP activity at best. NSP reduction in PLS resulted in less endothelial damage by activated neutrophils, a hallmark of AAV. CatC deficiency diminished PR3 on viable and apoptotic PLS neutrophils and resulted in a negative PR3-ANCA immunofluorescence test, indicating less PR3-ANCA binding to these neutrophils—an effect that reduces neutrophil activation by PR3- but not MPO-ANCA, as we showed in a neutrophil HSC model using the CatC inhibitor that we recently developed.
Potent chemical compounds inhibiting CatC in BM cells are explored for NSP downregulation in preclinical models and patients.16 We developed the highly selective CatC inhibitor BI-9740 (BI-I) that blocks human CatC activity in vitro with an IC50 of 1.8 nM, shows more than 1500× selectivity versus the related proteases CatB, F, H, K, L, and S, and has no activity against 34 unrelated proteases from different classes up to a concentration of 10 µM (https://opnme.com/molecules/cathepsin-c-inhibitor-bi-9740). We found that BI-I treatment of human CD34pos HSC did not interfere with neutrophil differentiation. This is important because PR3 was initially named myeloblastin and shown to control neutrophil differentiation.34 Most importantly, BI-I strongly reduced NSP activity in in vitro differentiated neutrophils by more than 95%. The fact that CatC controls NSPs but not neutrophil differentiation is supported by genetic and pharmacologic data from the literature. Sorensen et al. performed a pulse-chase biosynthesis study using BM cells from a patient with PLS.12 NSP production and sorting in promyelocytes was normal, whereas NSPs were degraded later, before mature neutrophil stages were reached and the cells entered blood circulation. Red blood cells, platelets, leukocyte numbers, and differential counts were normal, despite CatC deficiency. In addition, pharmacologic CatC inhibition in ex vivo cultured human BM cells reduced NSPs, but again, did not affect neutrophil differentiation.35 Importantly, NSP reduction was not seen with prednisolone that is part of AAV standard treatment protocols, suggesting CatC inhibition provides an additional therapeutic angle not covered by current strategies. Pharmacologic CatC inhibition recapitulated reduced endothelial injury by activated neutrophils seen with PLS neutrophils. mPR3 was reduced resulting in significantly less superoxide release in response to PR3-, but not to MPO-ANCA. Thus, the HSC model extends the PLS data by showing reduced neutrophil activation by PR3-ANCA as a functional consequence of mPR3 reduction. However, the reduction was incomplete, presumably limited by residual mPR3 interacting with PR3-ANCA.
Thus, our studies with human cells demonstrated that CatC controls NSPs in neutrophils and monocytes. This effect includes the PR3 autoantigen in and on the surface of viable and apoptotic neutrophils. Pharmacologic CatC inhibition effectively reduced NSPs with implications for both PR3- and MPO-AAV, such as endothelial damage. Reduced PR3 autoantigen has additional implications for PR3-AAV such as neutrophil activation by PR3-ANCA. Moreover, less PR3 on apoptotic neutrophils would possibly reduce inflammatory efferocytosis and PR3 autoimmunity.15
We reported recently that effective pharmacologic NSP abrogation needs sustained CatC inhibition in differentiating neutrophil progenitors in human BM in vitro and in a nonhuman primate model.35 Moreover, we found that NSP reduction by pharmacologic CatC inhibition was protective in murine arthritis36 and lung transplantation37 models. Unfortunately, no suitable PR3-ANCA vasculitis model is available, preventing testing CatC inhibition in an appropriate preclinical AAV model. Several attempts failed, including our own approach to generate a human transgenic PR3-AAV model.38 However, the therapeutic principle of CatC inhibition enters clinical medicine and was recently tested in patients with bronchiectasis. CatC inhibitor administration prolonged time to exacerbation without increasing the risk for severe infections.19 Additional clinical trials exploring CatC inhibition in bronchiectasis (phase 3, NCT04594369) and in patients infected with severe acute respiratory syndrome coronavirus 2 (NCT04817332) are underway. These trials will generate more data on both efficacy and potential side effects of pharmacologic CatC inhibition in inflammatory diseases.
Together, our study shows that genetic deficiency and pharmacologic inhibition of CatC significantly diminished NSPs, including the PR3-ANCA target autoantigen. Conceivably, reducing NSP activity together with PR3 autoantigen-dependent disease mechanisms has additive protective effects in patients with PR3-AAV. This can currently not be directly studied in animal models. However, our findings may encourage clinical studies with adjunctive CatC inhibitor administration in patients with PR3-AAV.
Disclosures
A. Schreiber reports being a consultant for Alexion, Hansa Biopharm, Otsuka, Sanofi, and Travere; reports receiving research funding from eleva GmbH; and reports other interests or relationships with Dt. Gesellschaft für Nephrologie. B. Korkmaz reports consultancy and an advisory or leadership role with Insmed Incorporated. M. Grundl reports employment and patents or royalties with Boehringer-Ingelheim Pharma. P. Eickholz reports being a consultant for Boehringer-Ingelheim, CP GABA, Kassenzahnärztliche Bundesvereinigung, and Kulzer; reports having an ownership interest in Biontec Germany, Curevac Germany, Novartis Switzerland, and Roche Switzerland; reports receiving research funding from Boehringer Ingelheim, Hain Life Science, and ITI Straumann; reports receiving honoraria from Boehringer Ingelheim, Hain Life Science, ITI Straumann, Kulzer, Sanofi Aventis Deutschland; reports having an advisory or leadership role with the Deutsche Zahnärztliche Zeitschrift, Deutscher Ärzteverlag, Journal of Clinical Periodontology, Parodontologie, PLOS One, and Quintessenz Verlag (with payment); and reports serving on a Boehringer-Ingelheim advisory board. P. Nicklin reports being employed by Boehringer-Ingelheim. R. Kettritz reports receiving research funding from Boehringer-Ingelheim; reports receiving honoraria from med update (medical education), and Vifor Pharma; and reports having an advisory or leadership role with the advisory board for Boehringer-Ingelheim and Insmed Incorporated. S. Kreideweiss reports being employed by Boehringer-Ingelheim Pharma. All remaining authors have nothing to disclose.
Funding
This work was supported by Deutsche Forschungsgemeinschaft grants KE 576/10-1 (to R. Kettritz), SCHR 771/8-1 (to A. Schreiber), and 394046635– SFB 1365 (to R. Kettritz and A. Schreiber), ECRC grants (to A. Schreiber and R. Kettritz), and the Région Centre Val de Loire (Project PIRANA, 2019-00134916 to B. Korkmaz).
Acknowledgments
We thank Susanne Rolle, Sylvia Krüger, and Tanja Filipowski for excellent technical support.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “A View on Cathepsin C as a Target for Therapy in AAV,” on pages 875–878.
Author Contributions
We thank Susanne Rolle, Sylvia Krüger, and Tanja Filipowski for excellent technical support. M. Grundl, U. Jerke, R. Kettritz, B. Korkmaz, P. Nicklin, and A. Schreiber conceptualized the study; P. Eickholz, R. Kettritz, and K. Nickles were responsible for the data curation; C. Eulenberg-Gustavus, M. Grundl, U. Jerke, R. Kettritz, S. Kreideweiss, and A. Rousselle were responsible for formal analysis; R. Kettritz was responsible for funding acquisition; C. Eulenberg-Gustavus, M. Grundl, U. Jerke, R. Kettritz, S. Kreideweiss, A. Rousselle, and A. Schreiber were responsible for investigation; P. Eickholz, B. Korkmaz, and K. Nickles were responsible for the resources; R. Kettritz wrote the original draft; and M. Grundl, S. Kreideweiss, B. Korkmaz, P. Nicklin, and A. Schreiber reviewed and edited the manuscript.
Data Sharing Statement
Detailed data on structure potency, selectivity as well as PK profile and in vivo activity data can be found under https://opnme.com/sites/default/files/opnMe_M2O_profile_BI-9740.pdf.
References
- 1.van der Woude FJ, Rasmussen N, Lobatto S, Wiik A, Permin H, van Es LA, et al. : Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener’s granulomatosis. Lancet 1: 425–429, 1985 [DOI] [PubMed] [Google Scholar]
- 2.Falk RJ, Jennette JC: Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med 318: 1651–1657, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Lüdemann J, Utecht B, Gross WL: Anti-neutrophil cytoplasm antibodies in Wegener’s granulomatosis recognize an elastinolytic enzyme. J Exp Med 171: 357–362, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldschmeding R, van der Schoot CE, ten Bokkel Huinink D, Hack CE, van den Ende ME, Kallenberg CG, et al. : Wegener’s granulomatosis autoantibodies identify a novel diisopropylfluorophosphate-binding protein in the lysosomes of normal human neutrophils. J Clin Invest 84: 1577–1587, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta SK, Niles JL, McCluskey RT, Arnaout MA: Identity of Wegener’s autoantigen (p29) with proteinase 3 and myeloblastin. Blood 76: 2162, 1990 [PubMed] [Google Scholar]
- 6.Kettritz R: How anti-neutrophil cytoplasmic autoantibodies activate neutrophils. Clin Exp Immunol 169: 220–228, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Little MA, Nightingale P, Verburgh CA, Hauser T, De Groot K, Savage C, et al. : Early mortality in systemic vasculitis: Relative contribution of adverse events and active vasculitis. Ann Rheum Dis 69: 1036–1043, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Flossmann O, Berden A, de Groot K, Hagen C, Harper L, Heijl C, et al. ; European Vasculitis Study Group : Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis 70: 488–494, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Kettritz R: Neutral serine proteases of neutrophils. Immunol Rev 273: 232–248, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Jerke U, Hernandez DP, Beaudette P, Korkmaz B, Dittmar G, Kettritz R: Neutrophil serine proteases exert proteolytic activity on endothelial cells. Kidney Int 88: 764–775, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A: Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 191: 677–691, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sørensen OE, Clemmensen SN, Dahl SL, Østergaard O, Heegaard NH, Glenthøj A, et al. : Papillon-Lefèvre syndrome patient reveals species-dependent requirements for neutrophil defenses. J Clin Invest 124: 4539–4548, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schreiber A, Rousselle A, Becker JU, von Mässenhausen A, Linkermann A, Kettritz R: Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proc Natl Acad Sci U S A 114: E9618–E9625, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schreiber A, Pham CT, Hu Y, Schneider W, Luft FC, Kettritz R: Neutrophil serine proteases promote IL-1β generation and injury in necrotizing crescentic glomerulonephritis. J Am Soc Nephrol 23: 470–482, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Millet A, Martin KR, Bonnefoy F, Saas P, Mocek J, Alkan M, et al. : Proteinase 3 on apoptotic cells disrupts immune silencing in autoimmune vasculitis. J Clin Invest 125: 4107–4121, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korkmaz B, Caughey GH, Chapple I, Gauthier F, Hirschfeld J, Jenne DE, et al. : Therapeutic targeting of cathepsin C: From pathophysiology to treatment. Pharmacol Ther 190: 202–236, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Hart TC, Hart PS, Bowden DW, Michalec MD, Callison SA, Walker SJ, et al. : Mutations of the cathepsin C gene are responsible for Papillon-Lefèvre syndrome. J Med Genet 36: 881–887, 1999 [PMC free article] [PubMed] [Google Scholar]
- 18.Toomes C, James J, Wood AJ, Wu CL, McCormick D, Lench N, et al. : Loss-of-function mutations in the cathepsin C gene result in periodontal disease and palmoplantar keratosis. Nat Genet 23: 421–424, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Chalmers JD, Haworth CS, Metersky ML, Loebinger MR, Blasi F, Sibila O, et al. ; WILLOW Investigators : Phase 2 trial of the DPP-1 inhibitor brensocatib in bronchiectasis. N Engl J Med 383: 2127–2137, 2020 [DOI] [PubMed] [Google Scholar]
- 20.Eickholz P, Kugel B, Pohl S, Näher H, Staehle HJ: Combined mechanical and antibiotic periodontal therapy in a case of Papillon-Lefèvre syndrome. J Periodontol 72: 542–549, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Noack B, Görgens H, Schacher B, Puklo M, Eickholz P, Hoffmann T, et al. : Functional cathepsin C mutations cause different Papillon-Lefèvre syndrome phenotypes. J Clin Periodontol 35: 311–316, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Rüdiger S, Petersilka G, Flemmig TF: Combined systemic and local antimicrobial therapy of periodontal disease in Papillon-Lefèvre syndrome. A report of 4 cases. J Clin Periodontol 26: 847–854, 1999 [PubMed] [Google Scholar]
- 23.von Vietinghoff S, Tunnemann G, Eulenberg C, Wellner M, Cristina Cardoso M, Luft FC, et al. : NB1 mediates surface expression of the ANCA antigen proteinase 3 on human neutrophils. Blood 109: 4487–4493, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Korkmaz B, Attucci S, Juliano MA, Kalupov T, Jourdan ML, Juliano L, et al. : Measuring elastase, proteinase 3 and cathepsin G activities at the surface of human neutrophils with fluorescence resonance energy transfer substrates. Nat Protoc 3: 991–1000, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Schreiber A, Luft FC, Kettritz R: Membrane proteinase 3 expression and ANCA-induced neutrophil activation. Kidney Int 65: 2172–2183, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Popat RJ, Hakki S, Thakker A, Coughlan AM, Watson J, Little MA, et al. : Anti-myeloperoxidase antibodies attenuate the monocyte response to LPS and shape macrophage development. JCI Insight 2: e87379, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rousselle A, Kettritz R, Schreiber A: Monocytes Promote Crescent Formation in Anti-Myeloperoxidase Antibody-Induced Glomerulonephritis. Am J Pathol 187: 1908–1915, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Eulenberg-Gustavus C, Bähring S, Maass PG, Luft FC, Kettritz R: Gene silencing and a novel monoallelic expression pattern in distinct CD177 neutrophil subsets. J Exp Med 214: 2089–2101, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kantari C, Pederzoli-Ribeil M, Amir-Moazami O, Gausson-Dorey V, Moura IC, Lecomte MC, et al. : Proteinase 3, the Wegener autoantigen, is externalized during neutrophil apoptosis: evidence for a functional association with phospholipid scramblase 1 and interference with macrophage phagocytosis. Blood 110: 4086–4095, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Huugen D, van Esch A, Xiao H, Peutz-Kootstra CJ, Buurman WA, Tervaert JW, et al. : Inhibition of complement factor C5 protects against anti-myeloperoxidase antibody-mediated glomerulonephritis in mice. Kidney Int 71: 646–654, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Schreiber A, Xiao H, Jennette JC, Schneider W, Luft FC, Kettritz R: C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol 20: 289–298, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao H, Dairaghi DJ, Powers JP, Ertl LS, Baumgart T, Wang Y, et al. : C5a Receptor (CD88) blockade protects against MPO-ANCA GN. J Am Soc Nephrol 25: 225–231, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jayne DRW, Merkel PA, Schall TJ, Bekker P; ADVOCATE Study Group : Avacopan for the treatment of ANCA-associated vasculitis. N Engl J Med 384: 599–609, 2021 [DOI] [PubMed] [Google Scholar]
- 34.Bories D, Raynal MC, Solomon DH, Darzynkiewicz Z, Cayre YE: Down-regulation of a serine protease, myeloblastin, causes growth arrest and differentiation of promyelocytic leukemia cells. Cell 59: 959–968, 1989 [DOI] [PubMed] [Google Scholar]
- 35.Guarino C, Hamon Y, Croix C, Lamort AS, Dallet-Choisy S, Marchand-Adam S, et al. : Prolonged pharmacological inhibition of cathepsin C results in elimination of neutrophil serine proteases. Biochem Pharmacol 131: 52–67, 2017 [DOI] [PubMed] [Google Scholar]
- 36.Korkmaz B, Lesner A, Wysocka M, Gieldon A, Håkansson M, Gauthier F, et al. : Structure-based design and in vivo anti-arthritic activity evaluation of a potent dipeptidyl cyclopropyl nitrile inhibitor of cathepsin C. Biochem Pharmacol 164: 349–367, 2019 [DOI] [PubMed] [Google Scholar]
- 37.Rehm SRT, Smirnova NF, Morrone C, Götzfried J, Feuchtinger A, Pedersen J, et al. : Premedication with a cathepsin C inhibitor alleviates early primary graft dysfunction in mouse recipients after lung transplantation. Sci Rep 9: 9925, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schreiber A, Eulenberg-Gustavus C, Bergmann A, Jerke U, Kettritz R: Lessons from a double-transgenic neutrophil approach to induce antiproteinase 3 antibody-mediated vasculitis in mice. J Leukoc Biol 100: 1443–1452, 2016 [DOI] [PubMed] [Google Scholar]