IgA nephropathy (IgAN) is defined by the glomerular deposition of IgA immune complexes. These complexes predominantly contain polymeric IgA1 that lacks galactose within its O-glycosylated hinge region, termed galactose-deficient IgA1 (GdIgA1). Circulating levels of polymeric GdIgA1 are increased in patients with IgAN, and correlate with renal prognosis. Therefore, GdIgA1 is central to the pathogenesis of IgAN. However, the origin of GdIgA1 in IgAN and the processes that result in increased circulating levels have been unclear. The vast majority of IgA is produced at mucosal sites, and several lines of evidence now point toward the mucosal immune system as being the source.1
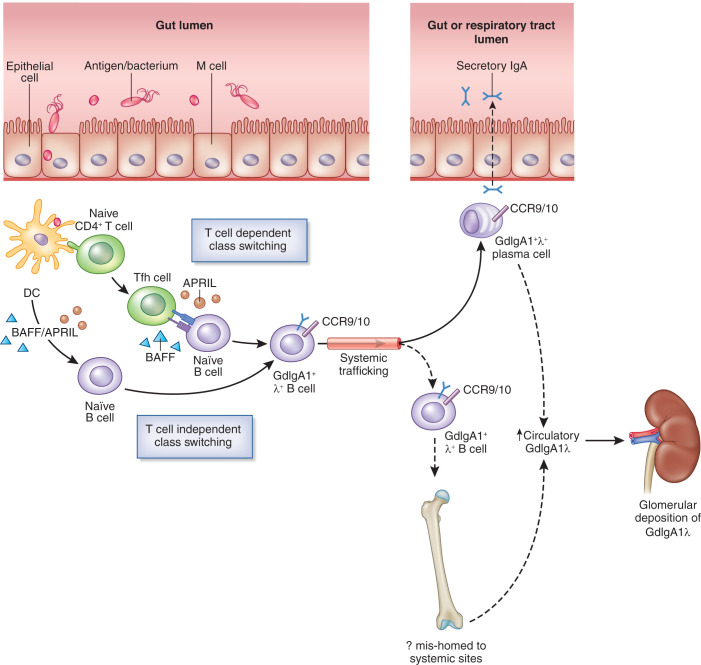
Mucosal IgA is produced within the mucosa-associated lymphoid tissue (MALT), at sites including the gut and respiratory tract, where it plays a key role in the host defense against pathogens. MALT represents specialized collections of lymphoid follicles that coordinate antigen sampling and presentation and facilitate class switching of naive B cells to IgA+ B cells via T cell–dependent and –independent mechanisms (Figure 1). Critical to these processes are B-cell activating factor (BAFF) and A PRoliferation-Inducing Ligand (APRIL) and other mediators, which include TGF-β, IL-6, and IL-10. IgA+ B cells then traffic toward effector sites within the mucosal lamina propria via the lymphatic system and systemic circulation. IgA is subsequently released and, after its transcytosis across the mucosal epithelial barrier, enters the lumen as secretory IgA.
Figure 1.
The mucosal immune system plays a key role in the production of GdIgA1 in IgA nephropathy. MALT represents key antigen sampling and inductive sites, and important interactions in IgAN are depicted here. Antigens are taken up by intestinal M cells and, subsequently, by dendritic cells (DC). After priming by DC, naive CD4+ T cells differentiate into T helper cells. T follicular helper (Tfh) cells interact with naive B cells, which undergo class switching to form IgA+ B cells. T cell–independent class switching also occurs through interaction of DC with naive B cells. These processes are promoted by factors including B-cell activating factor (BAFF) and APRIL. IgA+ cells then leave the MALT and enter into the lymphatic system and circulation, on their way to effector sites. Zachova et al.2 demonstrate increased levels of GdIgA1+ λ+ B cells in the circulation that express gut (CCR9) or respiratory tract (CCR10) homing receptors in patients with IgAN. GdIgA1+ λ+ B cells may then traffic to these sites where IgA is secreted. In IgAN, increased levels of circulating GdIgA1 are found, which may be produced by spillover, from mucosal sites or from B cells that have mishomed to systemic sites, such as the bone marrow. This leads to the formation of IgA1 immune complexes, which subsequently deposit within the glomerular mesangium. Dotted lines represent putative pathways.
Polymeric GdIgA1, produced mainly from MALT, appears to enter into the circulation in increased quantities in IgAN, but the exact processes by which this happens have been not been clearly established. This may involve “spillover” from mucosal sites to the circulation or mistrafficking of mucosal-primed IgA+ B cells to systemic sites, such as the bone marrow.1 In addition, IgA glomerular deposits are enriched for λ light chains and IgA1 isoforms, but the reasons for this are unclear, especially as mucosal sites produce a combination of IgA1 and IgA2. Defining the characteristics of GdIgA1-secreting B cells to better understand these processes has been technically challenging due to their low frequency in the circulation and the lack of a suitable antibody.
In this issue of JASN, Zachova et al.2 used the novel antibody 35A12 to immunophenotype circulating B-cell subsets that express surface GdIgA1. A number of differences are reported in patients with IgAN that reveal insights into this disease. Compared with healthy subjects and a non-IgAN kidney disease control group (patients with membranous nephropathy), patients with IgAN had increased levels of circulating surface GdIgA1+ B cells that were enriched for λ light chains, consistent with previous observations of an increase in IgA-λ in the circulation and within glomerular deposits in IgAN.3 Patients with IgAN also displayed increased levels of GdIgA1+ λ+ B cells expressing the chemokine receptors CCR10 and CCR9, which home to the upper respiratory tract and gut, respectively. Finally, GdIgA1+ B cells in patients with IgAN were enriched for plasmablast and plasma cell populations, as compared with total IgA+ B cells. Total proportions of GdIgA1+ cells were low and did not differ significantly across the studied groups, indicating that, in IgAN, factors within the mucosal microenvironment are likely to play a critical role in driving GdIgA1 production from these cells once they reach their effector sites.
These findings indicate that, in IgAN, there is an increase in circulating GdIgA1+ λ+ cells that are predestined for homing to the MALT of the upper respiratory tract and gut, implying that production of GdIgA1-λ may be stimulated during upper respiratory or gut infections and is a marker of mucosal B-cell induction. These studies complement the recent findings that patients with IgAN display increased numbers of gut homing (CCR9+ β7 integrin+) IgA+ regulatory B cells, memory B cells, and plasmablasts, as compared with healthy subjects.4 The reasons for the increase in λ light chain expression in GdIgA1+ B cells are unclear, and the authors speculate that this could be a consequence of the immune system eliminating potentially autoreactive B cells in the bone marrow by light chain receptor editing.5 In addition, gut-derived memory B cells have been observed to display a marked increase in λ light chains, which is thought to be due to revision of λ chain–encoding genes to enhance the diversification of the intestinal IgA response.6 As the authors acknowledge, patients with IgAN were all recruited from a single European center, and it would be important to see if these findings are observed in other IgAN cohorts, especially as factors involved in the pathogenesis of this disease and its clinical features can vary markedly between different populations.
The findings by Zachova et al. add to our current understanding of IgAN and confirm the central role of mucosal-derived IgA1 in this disease. Further characterization of GdIgA1+ B-cell populations may lead to insights into factors that regulate GdIgA1 production and stimulate its release into the circulation in IgAN. The authors describe interesting findings of a reduction in IgA+ plasma cells in a small subgroup of patients with IgAN who were treated with prednisone, while no reduction in GdIgA1+ cells was observed. It would be fascinating to assess dynamic changes in GdIgA1+ B-cell populations in response to the mucosal-directed therapies that are being developed in this condition. Indeed, early studies of targeted-release formulation (TRF) budesonide (the first US Food and Drug Administration–approved treatment for IgAN that specifically targets gut-associated lymphoid tissue), BION-1301 (an anti-APRIL mAb), and atacicept (a recombinant fusion protein containing the binding portion of transmembrane activator and CAML interactor (TACI) that inhibits both BAFF and APRIL) have each been demonstrated to reduce GdIgA1 levels in IgAN.7–9 In contrast, depletion of peripheral CD20+ B cells with rituximab did not affect GdIgA1 levels, which is likely due to GdIgA1-secreting plasma cells not being targeted by this approach.10 Further characterization of circulating GdIgA1+ B-cell populations, as well as improving our understanding of the pathogenesis, could eventually lead to their use as a biomarker to assess response to therapy or to the identification of new therapeutic targets.
Disclosures
J. Barratt reports receiving research funding from Argenx, Calliditas, Chinook, Galapagos, GlaxoSmithKline, Novartis, Omeros, Travere Therapeutics, and Visterra; having consultancy agreements with Astellas, Alnylam, BioCryst, Calliditas, Chinook, Dimerix, Novartis, Omeros, Travere Therapeutics, Vera Therapeutics, and Visterra; and reports being a scientific advisor or member via the editorial boards of CJASN, Clinical Science, Glomerular Diseases, and Kidney International. C.K. Cheung reports having consultancy agreements with Calliditas, George Clinical, and Vifor Pharma; receiving honoraria from Calliditas and Vifor Pharma; and receiving research funding from GlaxoSmithKline and Travere Therapeutics.
Funding
None.
Acknowledgments
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendations. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or JASN. Responsibility for the information and views expressed herein lies entirely with the author(s).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related article, “Galactose-Deficient IgA1 B Cells in the Circulation of IgA Nephropathy Patients Carry Preferentially Lambda Light Chains and Mucosal Homing Receptors,” on pages 908–917.
Author Contributions
J. Barratt and C.K. Cheung conceptualized the study and reviewed and edited the manuscript; and C.K. Cheung wrote the original draft.
References
- 1.Selvaskandan H, Barratt J, Cheung CK: Immunological drivers of IgA nephropathy: Exploring the mucosa-kidney link. Int J Immunogenet 49: 8–21, 2022 [DOI] [PubMed] [Google Scholar]
- 2.Zachova K, Jemelkova J, Kosztyu P, Ohyama Y, Takahashi K, Zadrazil J, et al. : Galactose-deficient IgA1 B cells in the circulation of IgA nephropathy patients carry preferentially lambda light chains and mucosal homing receptors. J Am Soc Nephrol 33: 908–917, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai KN, Chui SH, Lai FM, Lam CW: Predominant synthesis of IgA with lambda light chain in IgA nephropathy. Kidney Int 33: 584–589, 1988 [DOI] [PubMed] [Google Scholar]
- 4.Sallustio F, Curci C, Chaoul N, Fontò G, Lauriero G, Picerno A, et al. : High levels of gut-homing immunoglobulin A+ B lymphocytes support the pathogenic role of intestinal mucosal hyperresponsiveness in immunoglobulin A nephropathy patients. Nephrol Dial Transplant 36: 1765, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wardemann H, Hammersen J, Nussenzweig MC: Human autoantibody silencing by immunoglobulin light chains. J Exp Med 200: 191–199, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su W, Gordon JN, Barone F, Boursier L, Turnbull W, Mendis S, et al. : Lambda light chain revision in the human intestinal IgA response. J Immunol 181: 1264–1271, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhachu JS, Scionti K, Muto M, Molyneux K, Barratt J: Targeted release-budesonide (NEFECON) modifies circulating IGA-IGG immune complex levels and levels of poorly O-Galactosylated IgA in IgAN. Kidney Dis 4: 121–122, 2018 [Google Scholar]
- 8.Barratt J, Hour BT, Schwartz BS, Sorensen B, Roy SE, Stromatt CL, et al. : Pharmacodynamic and clinical responses to BION-1301 in patients with IgA nephropathy: Initial results of a phase 1/2 trial. Presented at ASN Kidney Week 2021, online, November 4–7, 2021
- 9.Barratt J, Tumlin JA, Suzuki Y, Kao A, Aydemir A, Zima Y, Appel G. The 24-week interim analysis results of a randomized, double-blind, placebo-controlled phase II study of atacicept in patients with IGA nephropathy and persistent proteinuria. Nephrol Dial Transplantation 35: gfaa140.mO039, 2020 [Google Scholar]
- 10.Lafayette RA, Canetta PA, Rovin BH, Appel GB, Novak J, Nath KA, et al. : A randomized, controlled trial of rituximab in IgA nephropathy with proteinuria and renal dysfunction. J Am Soc Nephrol 28: 1306–1313, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]