We thank Ma et al. for their interest in our recent report, which identified mutations in RRAGD as a cause for kidney tubulopathy and dilated cardiomyopathy.1 In their letter, Ma et al. report RagD staining in the nucleus of tubular epithelial cells. As the authors correctly note, the RagD staining in our manuscript is predominantly located in the cytoplasm of epithelial cells in the thick ascending limb of the loop of Henle (TAL) and the distal convoluted tubule (DCT) segments of the kidney.1 Our findings are in line with mRNA expression data from the mouse and the rat nephron, and human single-cell RNA sequencing data which demonstrate high expression in these segments. 2–4
Our manuscript reports immunohistochemistry of mouse kidney tissue because we have good segmental markers against mouse proteins available and the tissue can be better fixated due to perfusions. However, we have also analyzed human kidney cortex samples, which confirm the mouse stainings. In these human kidney sections, RagD expression is observed predominantly in the cytoplasm (Figure 1).
Figure 1.
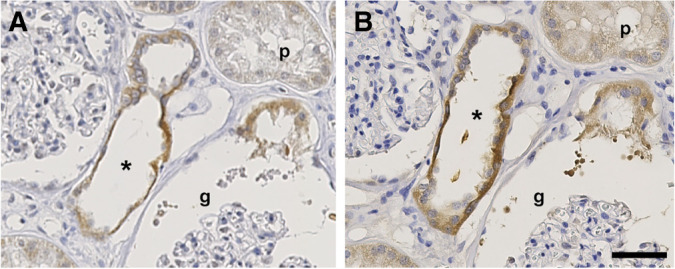
RagD is expressed in the cytoplasm of the distal convoluted tubule. Serial sections (as notified by the asterisk located in the same renal tubule) of human kidney were incubated with primary antibodies against (A) RagD or (B) uromodulin. The RagD protein was detected in uromodulin-positive the thick ascending limb of the loop of Henle (*). No significant signal was observed in the proximal tubule (p) or the glomerulus (g). Scale bar, 50 μm.
Indeed, the Rag GTPase complex is extensively described as a cytoplasmic nutrient sensor, where it resides at the lysosomal membrane and detects leucine and arginine amino acids.5 Upon amino acid binding, the Rag complex recruits mammalian target of rapamycin (mTOR) to the lysosomal membrane and activates the mTOR signaling pathway.6 Although cytoplasmic localization is, therefore, expected, there are reports of nuclear translocation of Rag proteins via the nuclear pore complex.7 In HEK293 cells, it has been demonstrated that RagC requires nuclear passage to fully activate mTOR signaling.7 Moreover, the metformin analogue phenformin could prevent this nuclear translocation, suggesting intracellular signaling pathways may determine the nuclear versus cytoplasmic expression of Rag proteins. Consequently, differences in subcellular Rag expression may be explained by the metabolic status of the assessed kidney tissue. Therefore, the interesting observation of Ma et al. spikes an interest in further investigations of metabolic regulation of the Rag complex in the kidney.
Disclosures
J. De Baaij reports receiving research funding from the Dutch Kidney Foundation and the Dutch Diabetes Research Foundation. F. Jouret reports advisory or leadership role with Belgian Society of Nephrology and French-speaking Society of Transplantation. N. Knoers reports honoraria from ErasmusMC, The Netherlands, for SEP evaluation; advisory or leadership role: research advisory committees with no financial compensation; and other interests or relationships: Chair board ERA-EDTA WGIKD, Chair Task force Molecular Diagnostics, European reference network of rare renal diseases, and ERKNET. The remaining author has nothing to disclose.
Funding
This work was supported by Nederlandse Organisatie voor Wetenschappelijk Onderzoek grant VENI 016.186.012, Diabetesfonden grant 2017.81.014, and the European Union Seventh Framework Program EURenOmics project (FP7/2007-2013) grant no. 305608. F. Jouret is a Fonds National de la Recherche Scientifique Luxembourg Fellow in Belgium.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Author Contributions
J. de Baaij wrote the original draft; J. de Baaij, F. Jouret, N. Knoers, and K.-P. Schlingmann reviewed and edited the manuscript; and F. Jouret was responsible for investigation.
References
- 1.Schlingmann KP, Jouret F, Shen K, Nigam A, Arjona FJ, Dafinger C, et al. : mTOR-activating mutations in RRAGD are causative for kidney tubulopathy and cardiomyopathy. J Am Soc Nephrol 32: 2885–2899, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L, Chou CL, Knepper MA: A comprehensive map of mRNAs and their isoforms across all 14 renal tubule segments of mouse. J Am Soc Nephrol 32: 897–912, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JW, Chou CL, Knepper MA: Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muto Y, Wilson PC, Ledru N, Wu H, Dimke H, Waikar SS, et al. : Single cell transcriptional and chromatin accessibility profiling redefine cellular heterogeneity in the adult human kidney. Nat Commun 12: 2190, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, et al. : The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320: 1496–1501, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM: Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141: 290–303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu L, Zhou B, Oshiro-Rapley N, Li M, Paulo JA, Webster CM, et al. : An ancient, unified mechanism for metformin growth inhibition in C. elegans and cancer. Cell 167: 1705–1718.e13, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]


