Significance Statement
IgA nephropathy is the most common glomerulonephritis worldwide and a leading cause of kidney failure. The disease often progresses through episodes of flare-ups that require effective treatments to tame inflammation. We followed a rational design strategy to construct a recombinant fusion IgA protease derived from commensal gut microbiota Clostridium ramosum. The fusion protease, referred to as Fc-AK183, showed week-long activity in mice to completely obliterate IgA in circulation and clear pathologic deposits in the kidney. Therefore, the recombinant enzyme is a promising drug candidate for future treatment of IgA nephropathy.
Keywords: IgA nephropathy, IgA protease, AK183, Clostridium ramosum, Fc-fusion protein, neonatal Fc receptor/FcRn, recombinant biologics
Visual Abstract
Abstract
Background
IgA nephropathy is a common primary glomerulonephritis caused by mesangial deposition of poly-IgA complexes. The disease follows a variable course of clinical progression, with a high risk of kidney failure. Although no specific therapy is available, enzymatic strategies to clear IgA deposits are being considered for the treatment of rapidly progressive IgA nephropathy.
Methods
We chose an IgA protease of commensal bacterium Clostridium ramosum, termed AK183, as the template for constructing a recombinant biologic. To extend the t1/2 in blood, we fused AK183 to the Fc segment of human IgG1. Activities of this Fc-AK183 fusion protein toward the cleavage and subsequent clearance of IgA were tested in mouse models.
Results
First, we discovered an autocleavage activity of AK183 that separates the N-terminal protease from its C-terminal autotransporter β domain. Therefore, we grafted Fc to the N terminus of AK183 and demonstrated its week-long enzymatic activity in mice. In addition, the proteolytic fragments of IgA generated in the reaction with Fc-AK183 were effectively removed from circulation via kidney filtration. The combined actions of Fc-AK183-mediated cleavage and subsequent renal clearance of IgA resulted in a lasting obliteration of blood IgA, as demonstrated in a human IgA-injection model and in a humanized α1KI transgenic model. Fc-AK183 was also able to remove chronic IgA and associated complement C3 deposits in the glomerulus.
Conclusion
We constructed a chimeric fusion of IgA protease with Fc and demonstrated its long-lasting efficacy as a promising targeted therapy for IgA nephropathy in mouse models.
IgA nephropathy (IgAN) is the most common type of primary glomerulonephritis worldwide and a leading cause of ESKD.1,2 Current therapies are mainly supportive treatments for blood pressure control with RAAS blockade.3,4 Steroid usage remains controversial and is strictly for those having high risk for kidney progression5 because steroids and/or immunosuppressive therapies are associated with serious adverse effects.6,7 Recurrence of IgAN is also common. In the absence of specific and effective treatment, between 20% and 40% of patients will progress to ESKD in 20–30 years.
Glomerular mesangial deposition of IgA1 immune complexes is the hallmark of IgAN and circulating poly-IgA complexes are considered the source of the deposits.8–11 Although most patients with IgAN have long indolent courses, rapidly progressing IgAN is seen in approximately 5% of patients12 who display >50% crescents in the glomerulus. These patients generally have poor prognoses and are often treated with immunosuppressive drugs, and/or plasma exchange therapy to remove the injurious IgA complexes from blood.12,13 Recently, alternative therapies for lowering poly-IgA levels are being considered.14–17
Certain bacterial pathogens produce antibody-degrading proteases for counteracting host immunity. Drugs based on these bacterial proteases have been developed. For instance, IgG endopeptidase from Streptococcus pyogenes (IdeS) has been used in clinic to treat autoimmune diseases and transplant rejection.18,19 There are also bacteria that possess IgA proteases (IgA-Ps).20 These IgA-Ps cleave the hinge segment of IgA1 that is unique in humans and in higher primates such as chimpanzees and gorillas. Preclinical study on exploring the therapeutic potentials of IgA-Ps has been carried out. Lechner and colleagues performed proof-of-concept experiments with recombinant Haemophilus influenzae IgA1-P and showed complete obliteration of IgA1 in humanized α1K1-CD89Tg mouse.17 However, it was also discovered that, unlike IdeS acting on IgG, the IgA1-P only achieved a short period of IgA1 suppression and repeated daily doses were needed for sustained treatment effects. This shortcoming is mainly due to the fast turnover of IgA of 2–3 days21 as compared with IgG, which typically lasts for 3 weeks.22 Moreover, it is also recognized that bacteria-derived biologics such as IdeS and IgA-P are potentially antigenic, and therefore repeated dosing should be avoided.
Our overall goal toward constructing a therapeutic IgA-P is two-fold: the biologic has a long t1/2, while having a relatively low risk of antigenicity. We previously followed a fusion strategy to construct a long-acting protease of angiotensin-converting enzyme 2 (ACE2) in a fusion with Fc of human IgG1.23 The Fc-fusion tag extended the in vivo t1/2 of ACE2-Fc to about 1 week, matching that of IgG in mice. Fc-fusion strategy is broadly used in drug design to improve pharmacokinetics24,25 through neonatal Fc receptor (FcRn)-mediated protection from blood catabolism.26,27 Concerning the risk of immunogenicity, we noted that most IgA-P-producing bacteria are human pathogens. As an exception, Clostridium ramosum constitutes normal gut flora. Its IgA-P, known as AK183, is expected to be part of the resistance mechanism of the bacteria to overcome human immune recognition in the intestinal tract. Conversely, it is plausible that the commensalism between C. ramosum and humans through the history of evolution suggests a possibility of the bacterial enzyme being more compatible with, and possibly tolerated by, the human immune system than other IgA-Ps produced by pathogenic bacteria. Therefore, we chose AK183 as the template to construct a therapeutic enzyme and also had it fused to human Fc to extend the t1/2.
Methods
Construction of Recombinant Fc-AK183 Fusion Protein
Considering the excessive molecular size of AK183 (approximately 250 kDa) that would be difficult to produce in Escherichia coli, we first designed two constructions of Fc-AK183 fusion for a mammalian expression system, which we used successfully in the past to produce an unrelated fusion protein.23 Full-length AK183 (GenBank Accession AY028440) minus the signal peptide sequence was in-frame fused with an IL-2 signal peptide and a sequence encoding the Fc segment (HR-CH2-CH3) of human IgG1 at the N- and C-terminal ends, respectively. The fusion cDNA was then cloned into expression vector pcDNA3 (Invitrogen) for transfection of HEK293 cells (American Type Culture Collection). The recombinant proteins were collected from culture medium and subsequently purified using fast protein liquid chromatography.23 Bacterial expression of recombinant Fc-AK183 fusion protein was assisted by pET30a vector (EMD Millipore). DNA sequence encoding AK183 without signal peptides was fused to an N-terminal human IgG1 Fc sequence (codon optimized for E. coli). The fusion was expressed with the addition of a 6xHis purification tag from the vector. Recombinant AK183 (AK183) without the Fc-fusion tag was expressed from PUC57 plasmid in E. coli.
Expression and Purification of Fc-AK183
Recombinant protein production was in 200 ml culture of BL21 (DE3) strain of E. coli transformed with PET30a Fc-AK183 or PUC57 AK183. Expression was induced with 0.3 mM isopropyl-β-d-thiogalactoside when the bacterial culture reached OD600 of 0.6–0.8. The culture continued for 24 hours at 16°C. Culture medium was removed by centrifugation and the bacterial pellet was stored at −20°C. On the day of purification, the bacterial pellet was resuspended in lysis buffer containing 0.5 M NaCl, 20 mM Na2HPO4, 0.8 Mm EDTA, pH 7.4. Lysis was supplemented with 0.5 mg/ml lysozyme (Sigma-Aldrich) for 30 minutes on ice. Sonication was applied to the mixture followed by 25,000 rpm centrifugation. The clear supernatant that contained Fc-AK183 was then loaded to a HisTrap column (GE Healthcare), and the recombinant protein was collected with elution buffer containing 250 mM imidazole. The elution was then resolved by Superdex S200 Increase 10/300 column (GE Healthcare). Fractions were collected and subsequently analyzed for Fc-AK183 contents by Western blotting and for IgA protease activity. Final protein concentration was measured using BCA kit (Pierce).
Fc-AK183 Activity Assay In Vitro and In Vivo
Sera of IgAN and Henoch-Schönlein purpura nephritis (HSPN) were collected from patients of Peking University First Hospital. All participants provided written informed consent before study inclusion and their diagnoses were based on kidney biopsy. The research was conducted in accordance with the principles of the Declaration of Helsinki and approved by the local ethics committees. Animal studies were approved separately by Northwestern University Institutional Animal Care and Use Committee (Protocol IS00009990) and by Peking University First Hospital.
In vitro protease activities of Fc-AK183 were conducted with human IgA1 as substrate, either in the form of purified poly-IgA1 or as total IgA mixed in human sera (Sigma-Aldrich). Poly-IgA contents in IgAN patient sera were purified by jacalin (Thermo Scientific, USA) affinity chromatography followed by an S300 gel filtration molecular sieve (GE Biosciences) on AKTA protein purification system (GE Biosciences).28 Different doses of Fc-AK183 were added to purified poly-IgA or whole serum samples and the reactions were carried out at 37°C for the indicated length of time. To analyze IgA cleavage in whole serum, uncleaved human IgA1 and production IgA1 heavy chain fragments from reactions with Fc-AK183 were purified using jacalin beads. Then Western blotting was performed on the samples to determine the proportion of cleaved Fd fragment. IgA1 fragments were probed with horseradish peroxidase (HRP)-conjugated goat anti-human IgA α chain antibody (SouthernBiotech). In vivo test of Fc-AK183 efficacy was performed via the detection of the levels of intact IgA1 (uncleaved) in serum samples collected at different time points after treatment with Fc-AK183. Detection was performed using ELISA or Western blotting. Goat anti-human IgA α chain “capturing” antibody (SouthernBiotech) at 2.5 μg/ml was coated onto 96-well plates (Nunc MaxiSorp) at 4°C for overnight. The wells were then washed three times with 0.01 M PBS supplemented with 0.1% Tween 20 (PBST), and then blocked with PBST containing 1% BSA (Sigma-Aldrich) for 60 minutes at 37°C. To detect intact IgA1 in plasma, samples were diluted 1:120,000 in blocking buffer and added to coated wells for 60 minutes at 37°C. After washing in PBS, HRP-conjugated goat anti-Ig Fab antibody (SouthernBiotech) in 1:2000 dilution with blocking buffer was added as the detection antibody. The reactions were developed with 3,3′,5,5′-tetramethylbenzidine (TMB) liquid substrate, and results were recorded as the net optical absorbance at 450 nm in an ELISA reader. Plasma samples of mice collected at different time points were used to perform Western blotting under nonreducing condition using anti-human IgA antibody (SouthernBiotech).
Pharmacokinetic Analysis
The pharmacokinetic profiles of Fc-AK183 were assessed in 3-month-old male BALB/c mice (n=3) (Charles River Laboratories). The mice received a single intravenous (iv) injection of 5 mg/kg body wt Fc-AK183. Blood samples were collected by tail bleeding either before injection or after injection at various time points of 0.08, 1.5, 4, 24, 48, 72, 96, 144, 192, and 264 hours. Collected blood samples were left undisturbed on ice, and sera were isolated by centrifugation at 6000 × g for 10 minutes at 4°C. ELISA assays for serum Fc-AK183 concentration at different times were performed with mouse anti-6xHis antibody (Thermo Fisher) as capturing antibody and HRP-conjugated goat anti-human IgG Fc antibody (SouthernBiotech) as detection antibody. The t1/2, area under the serum activity time curve, and mean residence time of Fc-AK183 were calculated using Prism 5 software (GraphPad, La Jolla, CA).
IgA Clearance in Blood and Kidney by Fc-AK183 in Passive IgA1-Injection Model
Wild-type BALB/c mice receiving iv injection of human IgA1 (25 mg/kg body wt) were established as a passive model with human IgA1 presence in circulation and in kidney deposits.16,29 Human IgA1 was purified from healthy human serum (Sigma-Aldrich) by jacalin (Thermo Scientific, USA) affinity chromatography. Five minutes after injection of human IgA1, 5 mg/kg Fc-AK183 or PBS was injected in BALB/c mice by iv for assessing in vivo enzymatic degradation of IgA1 by Fc-AK183 (n=3 in each group). A similar assay after an alternative workflow was also performed for the purpose of evaluating the in vivo activity of Fc-AK183 at a given time point after its injection. Considering Fc-AK183 has a much longer t1/2 than IgA1 in mice, at each time point of 1, 3, 5, 7, and 10 days (n=3 in each group) after a bolus dose of Fc-AK183, a fresh dose of purified IgA1 was administrated. Each round of IgA1 injection was followed by the evaluation of a “pulse-chase” series (at 0.05, 0.5, 1.5, 3, 5, and 7 hours) of blood IgA1 degradation attributable to the remaining Fc-AK183 activity. Plasma human IgA1 levels were measured by ELISA as described above. The passive renal IgA1 deposition model that resembles IgAN was created by repeated injections of human IgA1 at 25 mg/kg in mice (two injections separated by 1 hour). One hour after the last injection, mice were administrated with a therapeutic dose of 5 mg/kg Fc-AK183, or PBS as control. Another 1.5 hours later, kidneys were collected and cryosections were prepared for immunofluorescence detection of IgA1 deposits with goat anti-human IgA FITC antibody (SouthernBiotech). Rat anti-mouse CD31 (BD Pharmingen) and rabbit anti-collagen IV α1 antibody (Novus Biologicals) were used as counterstains.
Humanized α1KI-Tg Mouse Model
Humanized C57BL/6J mice were created through a contracted service at GemPharmatech (Nanjing, China). The mouse locus of IgA α1 that encodes the heavy chain constant region of CH1-HR-CH2-CH3 was targeted by CRISPR/Cas9. The region was then replaced with the corresponding human IgA1 α1 cassette of CH1-HR-CH2-CH3 via homolog repair. The transgenic model was referred to as α1KI-Tg. The expression of the human IgA1 heavy chain in conjunction with the mouse light chain as a whole IgA molecule was confirmed by the detection of this hybrid IgA1 in blood using goat anti-human IgA α chain antibody (SouthernBiotech). Fc-AK183 α1KI+/− heterozygous mice received a bolus injection of Fc-AK183 at 5 mg/kg body wt, then serial blood samples at various time points between 3 minutes and 14 days were collected. Western blotting against human IgA-α1(Abcam) was used to evaluate the levels of hybrid IgA1 in mice sera after Fc-AK183 treatment, and amount of production IgA1(Fc) fragment in the urine was tested by ELISA using goat anti-human IgA α chain (Jackson ImmunoResearch) as coated antibody and mouse anti-human IgA1 HRP (SouthernBiotech) as detecting antibody. To evaluate potential nephro- and hepatotoxicity of Fc-AK183, we longitudinally measured serum creatinine (DRI-CHEM NX700i), glutamic oxaloacetic transaminase (aspartate aminotransferase [AST], DRI-CHEM NX700i), and glutamic-pyruvic transaminase (alanine aminotransferase [ALT], DRI-CHEM NX700i) levels for up to 14 days after Fc-AK183 treatment of mice. To establish a chronic IgA1 deposition model, we raised homozygous α1KI+/+-Tg mice in nonbarrier housing until 15 weeks of age. Then each animal received three intraperitoneal (ip) doses of Freund’s complete adjuvant (FAD) between 15 and 19 weeks of age. Meanwhile, tail-bleed serum IgA1 levels (method as above) and IgA1-IgG complex levels (goat anti-human IgA α chain from Jackson ImmunoResearch as coating antibody and goat anti-mouse IgG-HRP from SouthernBiotech as detecting antibody) were monitored by ELISA at 21 weeks of age. To collect the pretreatment kidneys, unilateral nephrectomy was performed on all mice that had been induced with adjuvant. Three days after the surgery, the surviving mice were injected via the tail vein with 5 mg/kg body wt of recombinant Fc-AK183. Either 3, 5, or 7 days after Fc-AK183 administration, the contralateral kidneys were collected as post-treatment specimens. Cryosections of all pre- and post-treatment kidneys were subjected to immunofluorescence staining of FITC-labeled anti-human IgA1 (Abcam), mouse IgG (SouthernBiotech), IgM (SouthernBiotech), and C3c (MP Biomedicals). Immune signal intensities were compared with an identical camera setup applied across all specimens. For histology evaluation, periodic acid–Schiff staining of paraffin-embedded specimens was used. Mean number of mesangial nuclei in 20 randomly selected glomeruli of each mouse was calculated to evaluate the mesangial proliferation. Immunofluorescence staining was performed with anti-IgA1 FITC antibody (Abcam) to identify IgA1-secreting plasma cells in the intestine pre- and post-treatment.
Assays for Antibody Interference of Fc-AK183 Activity
Antibody titers against AK183 in mouse sera were detected by ELISA using antigen (AK183)-coated plates as described previously.30 Briefly, a MaxiSorp (Nunc) plate for ELISA was coated overnight at 4°C with recombinant AK183 or Fc-AK183 at 5 μg/ml in coating buffer. The plates were blocked with 1% BSA and incubated for 1 hour at 37°C with diluted (1:10, 1:100, 1:500, 1:1000, and 1:10,000) sera from three mice that received Fc-AK183 treatment 30 days earlier. Antibodies bound to AK183 were then detected by incubation with a goat anti-mouse IgG-HRP secondary antibody for 1 hour followed by TMB substrate. Additionally, we performed ex vivo tests of antibody interference. Antisera were collected on the 30th day from three mice previously treated with Fc-AK183 and one control mouse. The antisera were then incubated with Fc-AK183 (20 μl, 10 μl, and 2 μl serum to 5 μg Fc-AK173, respectively) for 1 hour at 37°C. Then purified human IgA1 was added as substrate and the reaction was allowed to proceed overnight at 37°C. The enzymatic activity of Fc-AK183 was determined by the efficiency of IgA1 cleavage.
In order to test the effect of anti-AK183 antisera on Fc-AK183 activity, BALB/c mice received a first iv dose of Fc-AK183, and having developed antibodies, were injected again with 5 mg/kg Fc-AK183 23 days later. After 1 hour, a pulse-chase dose of purified human IgA1 at 25 mg/kg was injected and EDTA-anticoagulated blood was collected by tail bleeding at 0.05, 0.5, 1.5, and 3 hours. α1KI-Tg mice also received a second round of 5 mg/kg Fc-AK183 after 30 days of initial treatment. Intact human IgA1 levels in plasma were measured by ELISA or Western blotting to evaluate the possible effect on Fc-AK183’s activity (in terms of loss of activity) due to antibody interference.
Statistical Analyses
Normally distributed and non-normally distributed quantitative parameters were expressed as means ±SEM and medians with interquartile ranges, respectively. Statistical differences between two groups in normal distribution were analyzed using a two-tailed t test. The difference in IgA1 levels at each time point among different Fc-AK183 concentration groups and the changes of serum IgA1 and IgA1-IgG complexes after priming with adjuvant were analyzed by the ANOVA trend test. A two-tailed P value <0.05 was considered statistically significant. All other statistical analyses were performed using SPSS version 16.0.
Results
C. Ramosum IgA Protease AK183 Is Autoproteolytically Cleaved for Its Activation
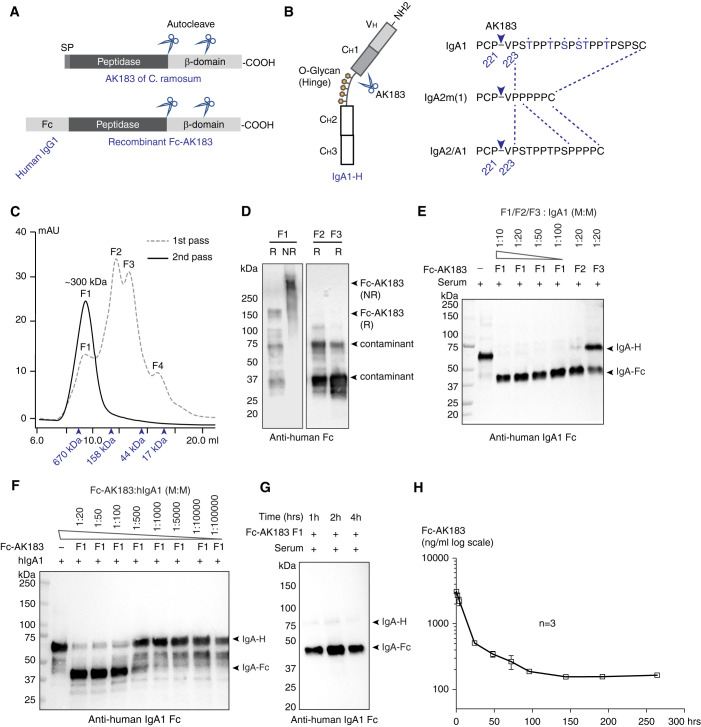
Full-length AK183 is a 1234 amino acid (aa) protein. Unlike other classes of IgA-Ps (Supplemental Figure 1A), AK183 has not been studied extensively regarding its structural characteristics. Like all other IgA-Ps, AK183 is a secreted protein comprising a signal peptide sequence, a peptidase domain, and a cell-anchoring domain (Figure 1A). Sequence analysis of AK183 C terminus revealed a CBM-X2 domain and an SPXTG motif that are likely anchored to the cell wall.31–34 AK183 was known to cleave IgA heavy chain between Pro221 and Val222 upstream of the hinge segment that is shared among all IgA isotypes of IgA1, IgA2m(1), and IgA2 (Figure 1B, Supplemental Figure 1A). Rodent IgA and other human immunoglobulins do not possess AK183 cutting site (Supplemental Figure 2).
Figure 1.
Construction, production, and activity testing of recombinant Fc-AK183. (A) The domain architecture of C. ramosum AK183 IgA protease (top). In an N- to C-terminal order, the protein is composed of signal peptide (SP), metallopeptidase domain, and cell wall anchoring β domain. Multiple autoproteolytic cleavage sites were identified within the β domain. Recombinant Fc-AK183 fusion protein was constructed by replacing a signal peptide of AK183 with an Fc segment derived from human IgG1 (bottom). (B) Human IgA1 heavy chain comprises a VH domain followed by CH1–3 domains (left), of which CH1 and CH2 are connected by a glycosylated hinge segment. AK183 specifically cleaves Pro221-Val222 peptide bond immediately upstream of the hinge sequence, which is present in all IgA isotypes (right). (C) Recombinant Fc-AK183 produced by E. coli was purified by using SEC (1st pass) after Ni-affinity chromatography. The first elution fraction (F1) of approximately 300 kDa was expected to be the dimeric form of Fc-AK183 via Fc-Fc dimerization. Molecular weight markers are indicated by arrowheads (from left to right: 670, 158, 44, and 17 kDa). Four distinct peaks (F1–F4) were separately collected. F1 was subsequently analyzed by a 2nd pass on SEC, which then formed a single peak of dimeric Fc-AK183. This repurified F1 fraction was considered Fc-AK183 that was subsequently used in all experiments. (D) Immunoblotting blotting analyses of F1, F2, and F3 on reducing (R) and nonreducing (NR) SDS-PAGE with anti-human IgG Fc antibody confirmed dimeric Fc-AK183 in F1 fraction. (E) Protease activities of elution fractions F1–F3 were evaluated using human IgA1 as substrate. Anti-IgA1-Fc antibody detected both intact and Fc-AK183-cleaved IgA1 heavy chain running at approximately 70 kDa (IgA-H) and approximately 37 kDa [IgA(Fc)], respectively. Dilution series of F1 fraction showed higher activity than F2 and F3 in terms of complete digestion of IgA1 at higher dilution (1:100 versus 1:20; W: weight). (F) F1 IgA protease activity was further confirmed by a broader dilution series of the fraction against purified IgA1 with the presence of a production fragment of IgA-Fc. (G) Fc-AK183 cleaved all IgA1 in whole human serum within 1 hour. (H) After iv injection in mice (n=3), serum levels of Fc-AK183 remained detectable beyond 10 days (over 250 hours). The blood t1/2 of recombinant Fc-AK183 was calculated as 62.5 hours based on two-phase decay model (see Methods section).
Considering that exogenous proteins usually have a short t1/2 in humans, we followed a design to incorporate an Fc-fusion tag to AK183 for extending its blood t1/2 (Figure 1A). The important aspect of nonpeptidase domains in AK183 sequence is that many, if not all, IgA-Ps have proenzyme and mature enzyme forms.35 To identify the location of the catalytic core in AK183 sequence, we constructed full-length AK183 with its N and C termini differentially tagged with FLAG and Fc sequences, respectively (Supplemental Figure 1B). Considering the aa length of recombinant AK183, we expressed the constructs using mammalian HEK293 cells. Through detecting the self-cleaved fragments in association with the tags, we discovered multiple cleavage sites between aa 680 and 800, downstream of the predicted catalytic core (Supplemental Figure 1, C and D). Unexpectedly, despite the observed autocleaving, HEK293-expressed Fc-AK183 could not hydrolyze IgA (Supplemental Figure 1E). To circumvent this problem, we recloned Fc-AK183 fusion with Fc at the N terminus of AK183 in bacterial expression vector pET30a (Figure 1A).
Chimeric Fc-AK183 Fusion Has Long In Vivo t1/2
Recombinant Fc-AK183 was expressed in E. coli (see Methods section). After purification with HisTrap column followed by Superdex S200 size exclusion chromatography (SEC), we collected four elution fractions (Figure 1C) and performed activity assays with human IgA1 as substrate. Fragment F1, running at approximately 300 kDa as a homodimer of Fc-AK183 (Figure 1D), showed the highest IgA-P activity (Figure 1E). Serial dilution of the enzyme showed that one molecule of Fc-AK183 hydrolyzed approximately 100 IgA1 molecules in 1 hour (Figure 1, F and G).
After confirming the enzymatic activity of Fc-AK183, we examined pharmacokinetics of the biologic in BALB/c mice. After a bolus 5 mg/kg body wt iv injection, blood samples were collected in a time series for measuring the serum concentration of Fc-AK183 by ELISA (Figure 1H). In keeping with the expected function of Fc-tag, the recombinant enzyme with Fc remained detectable in blood for as long as 11 days and its blood t1/2 was calculated as 62.5 hours (Figure 1H and Methods).
Fc-AK183 Cleaves Purified Poly-IgA of IgAN Patients and Total IgA in Complete Serum
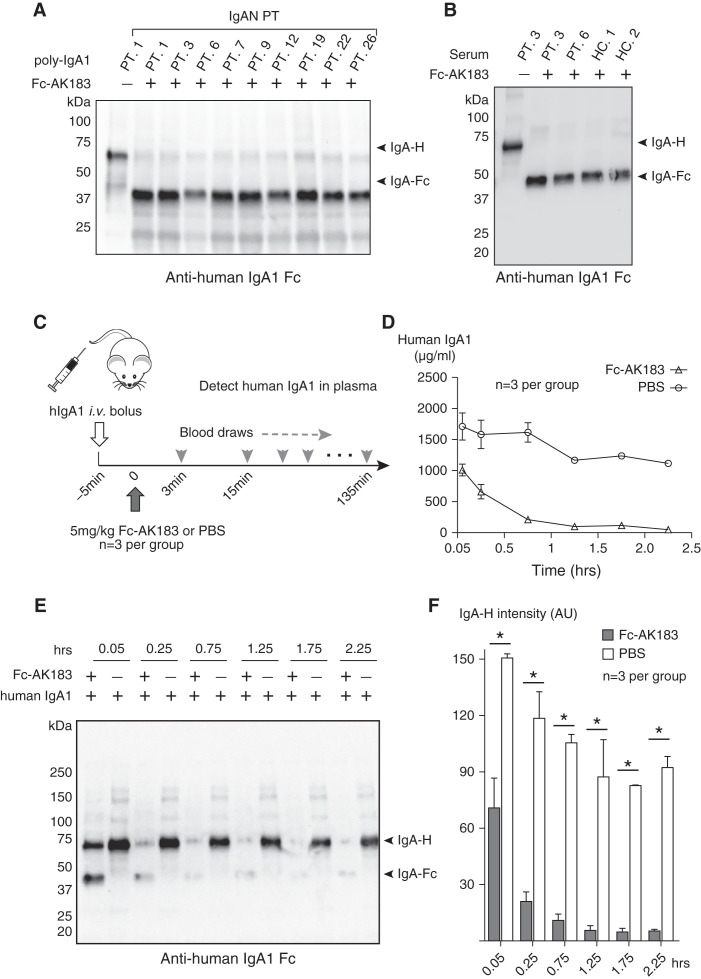
We specifically isolated poly-IgA contents from nine IgAN and four HSPN patients (Supplemental Figure 3A, Supplemental Tables 1 and 2) and subjected the samples to enzymatic reactions with Fc-AK183. Encouragingly, recombinant Fc-AK183 completely cleaved poly-IgA from all patients (Figure 2A, Supplemental Figure 3B). As expected, immunoblotting detected a production fragment of IgA(Fc) at approximately 37 kDa with the absence of the intact heavy chain of 70 kDa under reducing condition. It is conceivable that the digestion generated three separated pieces from a whole IgA molecule (Supplemental Figure 3C), namely two Fab pieces and one IgA(Fc) dimer of approximately 50 kDa and approximately 70 kDa, respectively. It is important to note that, although whole IgA is not filtered at the glomerulus due to its molecular size, these digestion pieces could possibly be removed from circulation via kidney filtration.
Figure 2.
Recombinant Fc-AK183 cleaved human IgA1 ex vivo and in vivo. (A) Whole serum samples of nine IgAN patients (PT) were subjected to jacalin purification of IgA1 followed by isolation of high molecular weight poly-IgA1 (see Methods section). Poly-IgA1 samples were subsequently subjected to reactions with either buffer control (−), or Fc-AK183 (+). Production IgA(Fc) bands were present in all samples after Fc-AK183 digestion. (B) Fc-AK183 or buffer was added directly to whole sera of two IgAN patients (PT. 3 and 6) and two healthy controls (HC. 1 and 2). After the reaction, IgA1 or its reaction product of IgA1-Fc was purified by jacalin beads and then resolved by SDS-PAGE. Anti-IgA1 immunoblotting showed complete digestion of all IgA1 by Fc-AK183. (C) A total of six 12-week-old BALB/c mice each received a bolus injection of purified human IgA1. Five minutes later, three animals were injected with 5 mg/kg Fc-AK183. Plasma samples were then collected at those indicated time points for detection of intact human IgA1 by ELISA (see Methods section). (D) Intact IgA1 rapidly dissipated after Fc-AK183, as opposed to PBS treatment, as measured by ELISA. (E) Western blotting of the in vivo blood samples also showed rapid disappearance of intact IgA band after Fc-AK183 injection. The cleaved product of IgA(Fc) only appeared transiently (within 0.25 hours). (F) Quantification of intact human IgA1 amount at each time point based on Western blotting signals in (E.) The differences between Fc-AK183-and PBS-treated mice across all post-treatment time points were statistically significant (*P<0.001).
Given the endogenous presence of serpin family protease inhibitors in blood,36 we tested whether whole serum condition would affect the catalytic performance of Fc-AK183. To this end, we added the recombinant biologic directly to serum samples, including two from IgAN patients and two more from healthy individuals. Recombinant Fc-AK183 efficiently cleaved IgA in these serum conditions that closely resemble the in vivo environment for intended drug actions (Figure 2B).
In a Passive Injection Model, Fc-AK183 Degrades Human IgA1 In Vivo and Further Promotes Its Clearance from Circulation
To circumvent the in vivo limitation that murine IgA cannot be cleaved by Fc-AK183 (Supplemental Figure 2), we sought to supplement mice with human IgA1 to examine the efficacy of the recombinant enzyme. In a passive injection model, purified human IgA1 was iv injected in wild-type BALB/c mice followed by iv injection of Fc-AK183 or buffer control (Figure 2C). Blood samples were collected thereafter, and the concentration of intact human IgA1 was measured by ELISA and Western blotting (Figure 2, D–F). The control treatment group showed relatively steady levels of human IgA1 for up to 135 minutes, as expected. In contrast, the Fc-AK183 treatment group showed a rapid decline of human IgA1 levels in blood starting almost immediately (Figure 2, D–F).
Western blotting against human IgA α chain showed that the shorter IgA(Fc) fragment generated from the reaction was already at its highest level 3 minutes after Fc-AK183 administration. In the meantime, the level of intact human IgA1 heavy chain had been reduced by half within minutes after Fc-AK183 treatment (Figure 2, E and F). Meanwhile, although the level of intact heavy chain reduced further by 15 minutes to almost background, the production fragment of free IgA(Fc) did not increase further (Figure 2E). Instead, Fc-AK183 treatment of mice resulted in quick reductions of both the parent IgA1 heavy chain (approximately 70 kDa) and its production fragment (approximately 37 kDa). The most plausible explanation is that the production fragment of IgA(Fc) had been further removed by kidney filtration due to its permissive molecular size. This is an important attribute of the drug to have this dual effect of proteolytic cleavage IgA in the blood, followed by filtration clearance of IgA by the kidney.
Week-Long Action Time of Fc-AK183 in Mice
From our earlier experience with Fc-fusion of a different protease,23 we expected Fc-AK183 to last for days attributable to the longevity of Fc.24 However, the passive injection model has its limitation of a natural decline of IgA1 levels even without intervention. To circumvent the issue of Fc-AK183 to generally outlast its substrate of the injected human IgA1, we changed the injection schedule of Fc-AK183 to be ahead of human IgA1. We expected the presence of Fc-AK183, expected to be staying in circulation for days, to accelerate the elimination of IgA1 (Figure 3A).
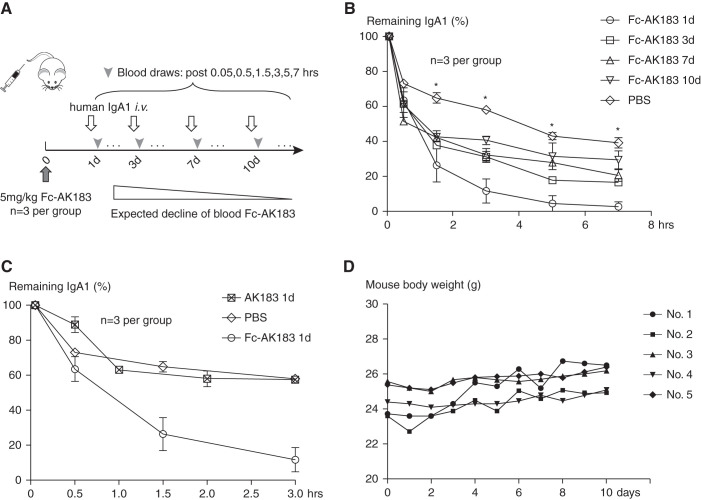
Figure 3.
Fc-AK183 maintained week-long activity in vivo. (A) After iv injection of a single dose of 5 mg/kg Fc-AK183 in BALB/c mice (day 0), the animals received a bolus injection of purified human IgA1 iv after 1, 3, 7, and 10 days (three mice for each time point). Blood was collected in a time series (from 0 to 7 hours) for detecting IgA1 levels by ELISA to assess remaining Fc-AK183 activity in circulation. Over a 10-day period, the in vivo activity of Fc-AK183 from the initial injection was expected to gradually decline. (B) Compared with the PBS group, mice receiving Fc-AK183 treatment had significantly faster decline of IgA1 levels at the 1.5-, 3-, 5-, and 7-hour time points. Although the rate of substrate (IgA1) clearance slowed down toward the end of the 10-day (10d) period, significant Fc-AK183 activities toward IgA1 clearance as compared with that by control PBS injection were observed across all IgA1 time points (*P<0.005 for remaining IgA1 percentage). (C) To directly compare the action time of Fc-AK183 versus untagged AK183 (also similarly produced from E. coli expression), we injected Fc-AK183, AK183, or PBS control to mice. One day after the injection, we administrated a separate dose of purified human IgA1 for measuring the in vivo activities of the biologics, as in (A). Untagged AK183 and PBS were indistinguishable from each other, indicating a complete loss of activity of untagged AK183 one day after its administration. In contrast, Fc-AK183 induced significantly quicker clearance of IgA1 from circulation as compared with its untagged counterpart. (D) Body weight of mice (n=5) receiving Fc-AK183 injection did not show a decline during post-treatment days.
On day 0, we injected Fc-AK183 in 12 mice that were evenly subdivided into four groups. Each group was assigned to a scheduled time point of 1, 3, 7, or 10 days for receiving a bolus dose of human IgA1 for measuring remaining Fc-AK183 activity. An additional group of three mice was used as the control without the pretreatment with Fc-AK183. By comparing the slope of decline in blood human IgA1 levels with that in the PBS group, the remaining protease activity at the time of the post-treatment day was determined. As expected, the Fc-AK183 activity gradually wore off during the 10-day period. Nevertheless, after 10 days, remaining IgA-P activity of the biologic was still detectable in circulation (Figure 3B).
In order to ascertain the long action of Fc-AK183 was due to its Fc-tag, we performed a direct comparison of activity of AK183 with or without the Fc fusion. One day after the mice received either Fc-AK183, untagged AK183, or buffer control, pulse-chase analysis of human IgA1 was performed. Evidently, native AK183 without Fc had already lost all its activity in circulation, whereas the Fc-tagged enzyme remained active (Figure 3C), in keeping with the expected t1/2 extension by Fc-fusion. Furthermore, we performed an additional toxicology study of Fc-AK183. With the injection of Fc-AK183 at 5 mg/kg body wt in mice, no weight loss was observed for 10 days (Figure 3D).
Fc-AK183 Clears Existing IgA Deposits in the Glomerulus
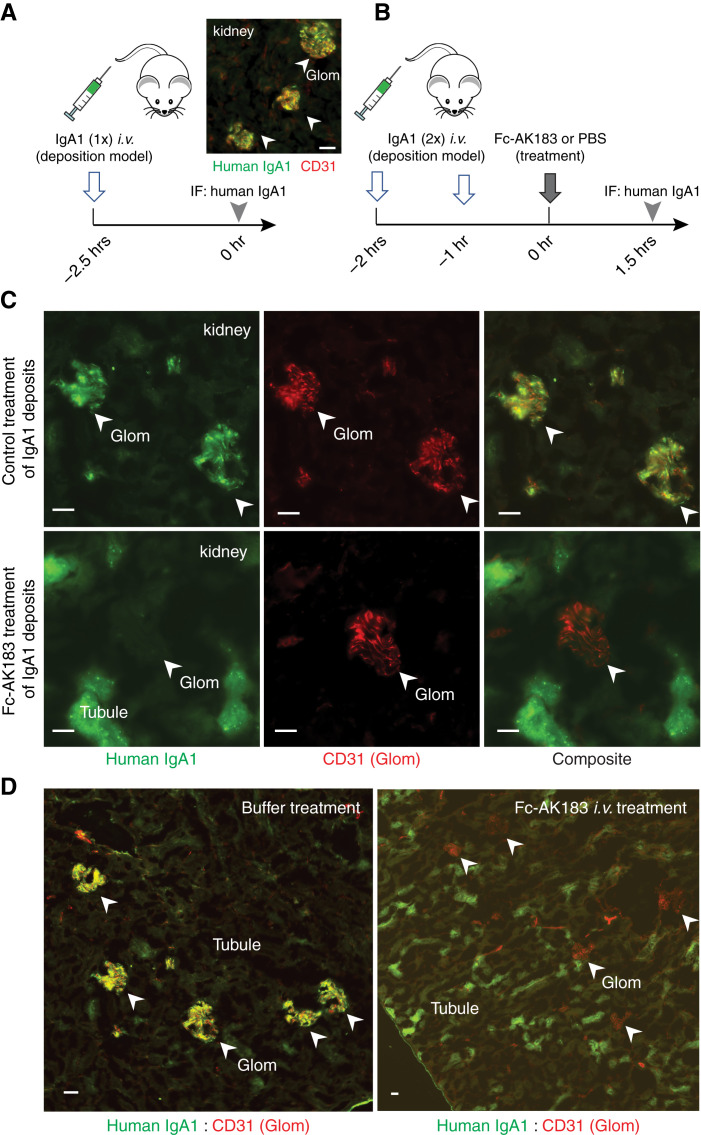
Next, we sought to determine whether AK183 is also able to directly act on IgA1 deposits in the glomerular mesangium. A kidney IgA deposition model was created by injecting purified human IgA1 in mice (Figure 4A). Two injections of purified human IgA1 resulted in kidney deposition.16,29 One hour after the last injection of IgA1, 5 mg/kg body wt Fc-AK183 or PBS control was administrated in mice (n=3 for each group) and kidneys were harvested 1.5 hours later (Figure 4B). IgAN-like deposits occurred in virtually all glomeruli in mice that received the combination of IgA1 and PBS injections (Figure 4C upper panel, Figure 4D left panel). Renal tubules had no IgA signals. In contrast, mice that received Fc-AK183 treatment after IgA1 injection showed no deposits in the glomerulus (Figure 4C lower panel, Figure 4D right panel). Instead, strong tubular signals of IgA1 were detected, suggesting IgA1 fragments from Fc-AK183 cleavage were able to be cleared by glomerular filtration. These results indicate that iv injection of soluble Fc-AK183 was capable of removing existing deposits in the kidney, highlighting important therapeutic potentials.
Figure 4.
Efficacy and kinetics of glomerular clearance of IgA1 deposits after bolus Fc-AK183 injection. (A) A passive IgA deposits model was created by bolus injection of purified human IgA1 in mice (left). IgA1 deposition in the glomerular mesangium was evident (right). (B) We followed a workflow to demonstrate the efficacy of therapeutic intervention by Fc-AK183. Wild-type BALB/c mice received two consecutive doses of purified human IgA1 injections. One hour after the second dose of IgA1, the mice received a treatment dose of either 5 mg/kg body wt Fc-AK183 or PBS control, and kidneys were harvested 1.5 hours later. (C) The buffer-treated group showed extensive IgA1 deposition in the glomerulus (marked with CD31 counterstain in red) (top). In contrast, mice receiving a treatment dose of 5 mg/kg Fc-AK183 completely lacked glomerular deposits. Instead, Fc-AK183 treatment resulted in IgA1 signals in proximal tubules (bottom). (D) Additional examples of contrasting differences between PBS and Fc-AK183 treatments on IgA1 renal deposition: Fc-AK183 completely removed the glomerular deposits of IgA1 and showed broad presence of tubular IgA1 signals, indicating renal clearance of IgA1. Scale bar, 30 μm.
In Vivo Efficacy of Fc-AK183 as Being Evaluated with a Humanized IgA1 α1KI-Tg Mouse Model
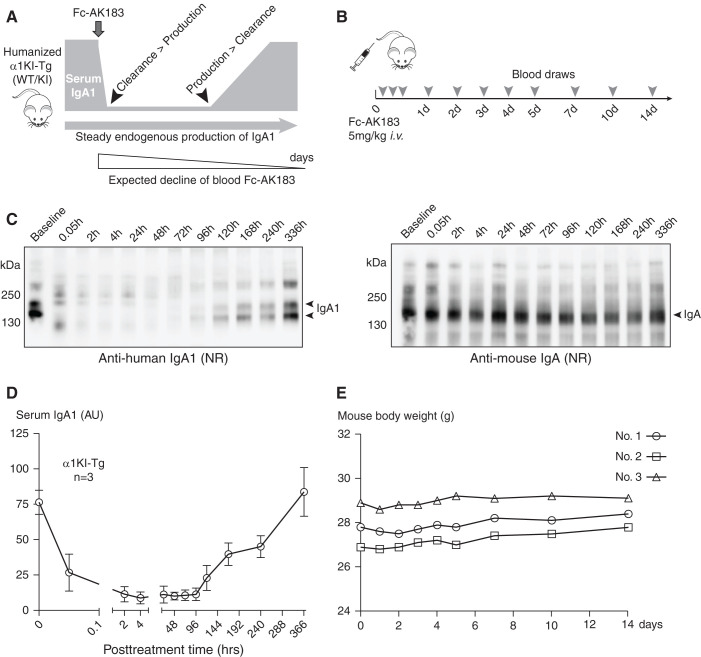
One of the first clinical objectives of IgAN therapy using IgA protease is to achieve sustained suppression of IgA levels. As long as the activity of Fc-AK183 is maintained at a level sufficient to counteract the rate of new IgA production, the drug remains efficacious despite its declining levels and activity over time. To better evaluate the dynamic balance among IgA-P drug activity, the net amount of total IgA, and the synthesis rate of new IgA (Figure 5A), we generated a humanized transgenic mouse model (α1KI-Tg; details in the Methods section) that expressed human IgA α1 chain of CH1-CH3, including the AK183 cleavage site. The expression of this human-mouse hybrid IgA1 (human heavy chain paired with mouse light chain; see Methods section) was detected in mouse blood by Western blotting (Figure 5C). We used α1KI+/− heterozygous mice, which expressed both human (hybrid) IgA1 and mouse IgA. After a bolus injection of Fc-AK183 at 5 mg/kg body wt, we collected serial blood samples at various time points between 3 minutes and 14 days (Figure 5B). Western blotting against human IgA-α1 showed rapid degradation of the heavy chain (Figure 5C left panel, Supplemental Figure 4, and a separate cohort in Supplemental Figure 5). Consistent with the earlier treatment results of the passive injection model, within 3 minutes, therapeutic Fc-AK183 completely cleaved all of human IgA1 in blood. IgA1 did not reoccur in blood until day 5, and its levels then gradually rose until reaching its pretreatment levels by day 14 (Figure 5D). As a control, mouse IgA levels in the same animals remained steady throughout the 2-week period, as expected (Figure 5C right panel, Supplemental Figure 4). It is conceivable that, at the beginning of treatment, there was an oversupply of enzymatic activity, resulting in a rapid elimination of all IgA1 within minutes. However, as the activities of Fc-AK183 declined after several days, the balance of drug-mediated IgA1 degradation versus endogenous synthesis gradually tilted in favor of IgA1 accumulation by day 5. It was not until day 14 that Fc-AK183 was no longer efficacious toward lowering IgA1 levels. Again, all three mice with Fc-AK183 treatment did not experience weight loss during that time (Figure 5E).
Figure 5.
A single injection of long-acting Fc-AK183 achieved week-long suppression of circulating IgA1 levels in humanized transgenic models of IgA1. (A) Through gene-targeting we created a transgenic mouse line with the replacement of the mouse IgA α chain locus with human α1 sequence (termed α1KI-Tg). The transgenic mice express hybrid IgA1 with human IgA1 heavy chain linked to mouse light chain. Heterozygous (WT/KI) mice were subjected to treatment with Fc-AK183 and changes in blood levels of humanized IgA1 were monitored by immunoblotting again human IgA1. The schematic showed the dynamics of IgA1 levels in response to treatment: before intervention (at regular serum level of IgA1), therapeutic Fc-AK183 degradation and clearance of IgA1, and continuous expression of the IgA1 transgene during the course of treatment. It should be noted that Fc-AK183 activity was expected to decline over time, and the therapeutic objective is to maintain low levels of blood IgA1 when the rate of clearance by Fc-AK183 is higher than endogenous production of IgA1 (between the two arrowheads). (B) The humanized mice (n=3) each received a single dose of 5 mg/kg body wt Fc-AK183. Postinjection blood draws were performed at 11 time points for up to 14 days (14d). (C) A representative example of a mouse treated with Fc-AK183 is shown with serum IgA1 levels by Western blotting (results of the other mice are in Supplemental Figure 4, and a separate cohort in Supplemental Figure 5). Within 0.05 hours after Fc-AK183 injection of the mouse, blood IgA1 in its intact form completely disappeared. The levels gradually reoccurred after 96 hours and only returned to its pretreatment level by 336 hours (14 days). In the meantime, mouse form of IgA remained stable because mouse IgA is not a substrate of AK183. (D) A summary of endogenous human IgA1 levels in response to Fc-AK183 treatment showed a U-shaped curve, with total suppression of blood IgA1 levels between 2 and 96 hours. (E) Body weight of mice (n=3) receiving Fc-AK183 injection did not show a decline during post-treatment days.
Induction of Humanized IgA1 Deposition in α1KI-Tg Mice and Treatment with Fc-AK183
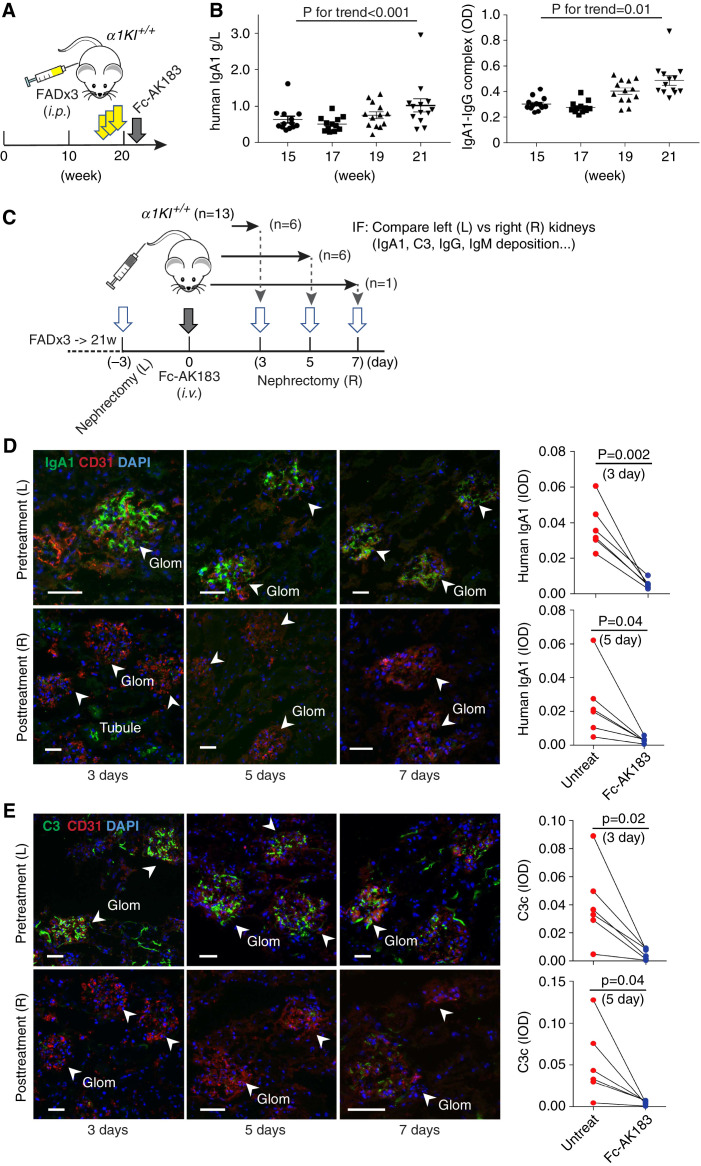
When raised in a clean environment, humanized α1KI-Tg mice aged normally without urinary abnormality and kidney deposition of IgA1 (not shown). Meanwhile, nonbarrier housing incurred low levels of IgA1 deposits in the glomerulus around 20 weeks of age, with the absence of C3 deposition (not shown). In order to create an IgAN-like condition, we subjected homozygous α1KI-Tg mice to chronic inflammation by ip injecting FAD in three doses between 15 and 19 weeks of age (Figure 6A). After induction, blood levels of IgA1 and IgA1-IgG complexes steadily increased (Figure 6B), as expected. Examination of the kidneys showed all animals having chronic IgA1 and C3 deposits in the glomerulus (Figure 6, D and E, top panels, and additional IgG and IgM staining in Supplemental Figure 6). Periodic acid–Schiff staining of the specimens also showed mesangial hypercellularity and matrix deposition (Supplemental Figure 7) that resembles clinical IgAN pathology.
Figure 6.
Recombinant Fc-AK183 removed chronic IgA1 deposits in α1KI-Tg mice. (A) Despite transgenic expression of human IgA1, α1KI mice did not develop glomerular deposition (not shown). Instead, we subjected homozygous α1KI+/+ mice to three rounds of ip injections with FAD at 15, 17, and 19 weeks of age (yellow arrows). These adjuvant-primed mice showed IgAN-like glomerular deposition of IgA1 in the following weeks, during which a single iv dose of Fc-AK183 (gray arrow) was injected into each mouse at 21 weeks to evaluate the effect on IgA1 deposits. (B) Blood levels of total IgA1 and IgA1-IgG complexes were measured by ELISA (see Methods section) between 15 and 21 weeks of age, showing trends of significant increases. (C) After adjuvant induction, α1KI+/+-Tg mice (n=13) were subjected to unilateral nephrectomy of the left (L) kidneys in week 21. Three days later, each mouse was treated with an iv injection of Fc-AK183 (denoted as day 0). Six mice were subsequently euthanized to collect the post-treatment right (R) kidneys 3 days after Fc-AK183 administration, with another six mice collected at post-treatment day 5 and one more at post-treatment day 7. Cryosections of all collected kidneys were processed together for immunofluorescence (IF) detection of IgA1 deposition in association with C3, IgG, and IgM. (D) Representative IF images of paired pretreatment (L) versus post-treatment (R) kidneys of the same animal are shown (top and bottom images for paired kidneys). Before Fc-AK183 treatment, all kidneys showed varying levels of human IgA1 deposits in the glomerulus (top images: arrowheads; scale bar, 30 μm). In contrast, few IgA1 deposits were observed 3, 5, or 7 days after treatment with Fc-AK183 (bottom images). Quantitative comparisons (same animal connected by a line) of kidneys pre- and post-treatment are shown in the right panels. (E) Similarly, the same mice (top versus bottom images) were used for detecting complement C3c deposits in the glomerulus. Coinciding with the complete removal of IgA1 deposits (as in D), post-Fc-AK183 treatment also showed clearance of IgA1-associated C3c deposition in the glomerulus (follow arrows). Quantitative comparisons of kidneys pre- and post-treatment are shown in the right panels. Scale bar, 30 μm.
To circumvent the issue of observed variability in IgA1 intensity among adjuvant-induced mice, we performed pretreatment unilateral nephrectomy and had each kidney directly compared with the corresponding contralateral kidney of the same animal collected 3, 5, or 7 days after Fc-AK183 treatment (Figure 6C). Three days after nephrectomy of the left kidney, all mice were treated with a bolus of 5 mg/kg body wt Fc-AK183. Consistent with the blood IgA1 results (Figure 5), all post-treatment right kidneys between 3 and 7 days showed disappearance of IgA1 deposits as compared with their contralateral control kidneys collected before Fc-AK183 treatment (Figure 6D). In the meantime, glomerular C3 deposits were also ameliorated (Figure 6E), indicating its tight association with IgA1 deposits, whereas the baseline glomerular IgM signals remained unchanged (Supplemental Figure 6). These staining results indicated the efficacy of Fc-AK183 in achieving week-long clearance of chronic IgA1 and C3 deposits, as intended.
Kidney and Liver Toxicity Not Observed in Fc-AK183 Treatment of α1KI+/+-Tg Mice
We should also specially note that although it is beneficial to have both cleavage and subsequent filtration clearance of IgA1 from blood and from kidney glomerulus, one also needs to be concerned about the extra protein load of IgA1 fragments that may overburden the kidney. For that we tested the amount of production IgA1(Fc) fragment in the urine by ELISA. In keeping with the expectation that the proximal tubule is capable of reuptaking a majority of the fragments for their lysosomal degradation, we detected only a slight increase of the urinary IgA1(Fc) level after Fc-AK183 treatment of α1KI+/+-Tg mice (Supplemental Figure 8). For assessing potential kidney and liver toxicity of the recombinant drug, we measured serum creatinine, ALT, and AST levels in a longitudinal series after a bolus injection of Fc-AK183 at 5 mg/kg body wt. For up to 14 days, serum creatinine, ALT, and AST levels remained at levels comparable to those before treatment (Supplemental Figure 9), indicating no toxicity of the drug to the kidney and the liver. Furthermore, IgA1-secreting plasma cells in the intestine did not decrease after Fc-AK183 treatment (Supplemental Figure 10), suggesting the drug in blood circulation did not adversely affect the activity of mucosal plasma B cells.
Fc-AK183 Elicits Antibody Response without Losing Therapeutic Efficacy
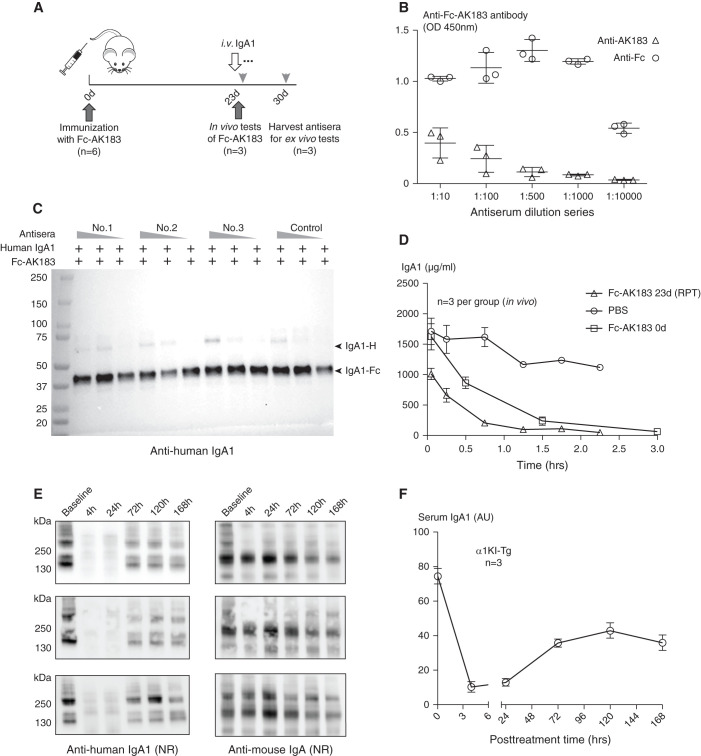
Concerning that repeated therapeutic injections of the biologic may incite antibody production, we conducted new tests with a second round of Fc-AK183 injection 23 days after the first dose (Figure 7A). Blood samples collected 30 days after the first injection of Fc-AK183 showed antibody activities against the drug. We separately measured the antibody titers against either AK183 or the Fc-tag (Figure 7B, Supplemental Figure 11). Interestingly, much higher antibody titers were in fact directed toward the Fc part of the fusion, as opposed to against the bacterial protease segment.
Figure 7.
Antibodies against recombinant Fc-AK183 (Fc-AK183) did not interfere with enzymatic activity toward IgA1 ex vivo and in vivo. (A) A total of six mice each received an initial iv dose of 5 mg/kg body wt Fc-AK183. Then 23 days later, three mice were each injected with a second dose of Fc-AK183. After 1 hour, purified human IgA1 was injected as substrate for measuring in vivo efficacy of Fc-AK183 in clearing blood IgA1. The three remaining mice in another experimental group were sacrificed on day 30 for total blood collection. Antisera from the second group were used for ex vivo testing of blockage activities against Fc-AK183 enzymatic activity. (B) Using either recombinant AK183- or human Fc-coated ELISA plates, we measured antibody activities against either AK183 portion or Fc-portion of recombinant Fc-AK183 fusion. Evidently, much higher antibody titers were present against human Fc as compared with relatively low antibody signals against AK183. (C) Ex vivo examination of the antisera in reactions of Fc-AK183 against human IgA1 was conducted with the presence of three different dilutions of the antisera (20 μl, 10 μl, and 2 μl serum to 5 μg Fc-AK173, respectively; animal Nos. 1–3; the control animal had not been immunized with Fc-AK183). Overall, the recombinant IgA protease cleaved IgA1 substrates without being inhibited by the antisera. (D) The in vivo group of mice each received a repeated (RPT) dose of Fc-AK183 on day 26 followed by a bolus injection of IgA1. Pulse-chase analyses of IgA1 levels in response to Fc-AK183 treatment were conducted. Mice receiving a repeated dose of Fc-AK183 after 26 days did not show compromised efficacy when compared with its in vivo efficacy of the first dose Fc-AK183, suggesting no interference of therapeutic activities by antibody production. (E) In vivo effect of autoantibodies against Fc-AK183 on its activity in humanized α1KI-Tg transgenic mice. Second round of Fc-AK183 treatment 30 days after the first injection of Fc-AK183 rapidly cleared IgA1 in blood. IgA1 reappeared after 3 days, and slowly recovered to approximately 50% of its pretreatment levels after 7 days. (F) A summary of the results in (E).
Next, we performed additional ex vivo and in vivo tests to determine whether these antibodies could impair enzymatic activity. Ex vivo experiments were performed with the mixing of Fc-AK183 with mouse antisera. The substrate was nearly completed cleaved by Fc-AK183 regardless of the presence of positive antisera (Figure 7C), suggesting that the antibodies did not hamper the enzymatic activity of the IgA protease. In addition, an in vivo study was performed on BALB/c mice that had previously been treated with Fc-AK183 23 days ago (Figure 7A). These antibody-positive mice then received a second dose of Fc-AK183, followed by passive human IgA1 injection for measuring the rate of IgA1 clearance. The efficacy of Fc-AK183 remained comparable to that in mice that lacked antibodies (Figure 7D), indicating the antibodies did not compromise IgA protease activity. Similarly, in vivo study was also performed on humanized α1KI-Tg transgenic mice with a second round of therapeutic injection of Fc-AK183 30 days after the first dose. The drug remained efficacious in lowering IgA1 levels that lasted for at least 7 days (Figure 7, E and F).
Discussion
Our study focused on the construction and testing of a long-acting IgA protease for the treatment of IgAN. We selected bacterial IgA protease AK183 as the template and followed an Fc-fusion strategy to extend the in vivo t1/2 of the therapeutic enzyme. In keeping with this concept, injection of recombinant Fc-AK183 in mice achieved week-long activity attributable to the function of Fc in protecting the fusion from rapid catabolism.37 Using humanized mice with IgA gene locus replaced by IgA1 sequence of human, we showed that a single injection of Fc-AK183 completely obliterated plasma IgA1 for 5 days. Thereafter, plasma IgA1 levels remained suppressed for at least a further 5 days. No adverse effects were observed. Moreover, Fc-AK183 administration was efficacious in clearing existing renal deposits of IgA1 and associated C3 in the glomerulus.
The clinical success of IgG-protease drug imlifidase (IdeS/Idefirix) had inspired this study. Individuals treated with one dose of imlifidase maintained 85%–90% suppression of their IgG levels for 35 days.38 The impressive in vivo efficacy of imlifidase despite its short pharmacologic t1/2 of approximately 4.9 hours was attributable to the very slow turnover of IgG. Because IgA has faster turnover kinetics,39,40 it becomes more challenging to suppress its levels for a prolonged period of time that is needed for treating IgAN. Lechner et al. treated a humanized mouse model of IgAN with a recombinant IgA1-P derived from pathogenic H. influenzae.17 Although rapid degradation of IgA1 was observed as soon as 5 minutes after dosing, plasma IgA1 levels soon recovered. Therefore, our innovation in our therapeutic design was the inclusion of an Fc-tag to extend the pharmacologic t1/2. It is also suggested that the actual t1/2 of Fc-tagged drugs lasts twice as long in humans than in mice.41
A key consideration of choosing AK183 was because C. ramosum that produces the enzyme is indigenous to the human intestinal tract42 and therefore the drug could potentially be well tolerated by humans. In the lack of human test subjects, we measured neutralizing antibodies in mice after the initial treatment with Fc-AK183. Although the biologic indeed incited antibody production, we did not find inhibition of its therapeutic efficacy toward clearing IgA. Admittedly, unlike in humans, it is unlikely that C. ramosum colonizes in mice and therefore seeking validation of antibody response using animal models is not directly relevant. Nonetheless, given the length of time and extensive resource usually needed to develop a drug, choosing the right candidate with the best chance to be successful at the preclinical stage is a very important part of decision making. In this regard, IgA-P secreted from commensal bacteria of human microbiota, as opposed to disease-causing bacterial pathogens, represents a better chance of compatibility. Nevertheless, the potential risk of antigenicity with Fc-AK183 is real and it can only be properly addressed at the clinical trial stages, and on an individual basis. More importantly, adverse effects from anti-drug antibodies are challenging obstacles that are particularly relevant with bacteria-based biotherapeutics. It could be additionally noted that our transgenic IgA1 model in conjunction with systemic inflammatory induction using adjuvant only partially resembled the clinicopathologic characteristics of IgAN, such as mesangial proliferation, which is the most frequently observed glomerular alteration in early stages of the disease. IgAN tends to follow remission and relapse cycles with individuals displaying a broad range of clinical and pathologic variations. Because our biotherapeutic primarily removes blood IgA and its glomerular deposits, we anticipate its future indication in rapidly progressive IgAN.
We cannot adequately stress the urgent need for new therapies for IgAN, as millions are living with the disease worldwide with the lack of safe and effective treatments.43 Existing medications for IgAN aim at renal preservation via RAAS inhibitors as first-line treatments and via corticosteroids that are given to patients having a rapidly progressing disease.4 New immune regulatory drugs that were not specifically developed for IgAN are also being evaluated,44–46 including the recently approved corticosteroid drug budesonide in a delayed-release formulation with reduced side effects. The IgA-P class of drug candidates has a unique advantage of directly addressing the source of IgA deposits and therefore represents an attractive option, particularly for treating patients with highly progressive IgAN. In perspective, IgA-P therapies will be suitable for indications associated with rapid decline of kidney functions. The drug is expected to render instant clinical remission for a short duration of 2–3 weeks with complete suppression of injurious IgA in circulation and rapid clearance of glomerular deposits of IgA and C3. This will provide a window opportunity for the kidney to recover itself so that standard maintenance treatment thereafter with current management strategies will be more effective.
Beside IgAN, other IgA deposition diseases may also benefit from Fc-AK183 therapy. For instance, IgA vasculitis in children has rare recurrence,47 and therefore is suitable for IgA-P treatment to prevent acute-phase organ damage. Similarly, Kawasaki disease affects young children and teens with low risk of recurrence in adulthood.48 Kawasaki disease causes inflammation of medium-sized blood vessels with IgA deposition to the coronary arteries and patients may benefit from systemic IgA-P treatment. Given the excellent in vivo efficacy in lowering blood IgA levels in mouse models, Fc-AK183 with its long-action feature is an attractive approach in addressing a variety of diseases associated with IgA deposition.
Disclosures
J. Jin, J. Lv, and X. Xie filed a patent application on recombinant protease Fc-AK183. J. Jin is a cofounder of Accubit LLC; advisor to Q BioMed and Enlighten Biotechnology; owns shares in Mannin Research Delaware subsidiary; reports consultancy with Enlighten Biotechnology, Mannin Research, Alebund Pharmaceuticals, and Q BioMed; reports ownership interest with Accubit LLC; and reports advisory or leadership role with Scientific Reports. J. Lv reports consultancy with Chinook Therapeutics. H. Zhang reports consultancy with Calliditas, Janssen, Novartis, and Omeros. All remaining authors have nothing to disclose.
Funding
This study was supported by the National Natural Science Funds for Distinguished Young Scholar 81925006 (to J. Lv) and National Natural Science Foundation of China grant 81800639 (to X. Xie).
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Author Contributions
J. Jin and J. Lv were responsible for investigation and supervision; X. Xie and J. Lv were responsible for funding acquisition; L. Gao, J. Jin, P. Liu, F. Wan, and X. Xie were responsible for data curation; P. Liu, M. Wang, and X. Xie were responsible for methodology; L. Gao, J. Li, P. Liu, and X. Xie were responsible for formal analysis; J. Jin and J. Lv were responsible for validation; J. Jin and X. Xie were responsible for project administration and wrote the original draft; and L. Gao, J. Jin, P. Liu, J. Lv, and H. Zhang reviewed and edited the manuscript.
Data Sharing Statement
Data available via GenBank Accession: AY028440.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021030372/-/DCSupplemental.
Supplemental Table 1. Baseline characteristics of patients with IgAN.
Supplemental Table 2. Baseline characteristics of patients with Henoch-Schönlein purpura nephritis.
Supplemental Figure 1. AK183 has autocleavage sites in the β domain and there is no activity of Fc-AK183 expression from HEK293 cells.
Supplemental Figure 2. Alignment of CH1-CH3 sequences of murine IgA, human IgA1, IgA2, IgG, IgM, IgD, and IgE.
Supplemental Figure 3. Recombinant Fc-AK183 cleaved poly-IgA1 purified from patients with HSPN.
Supplemental Figure 4. A single injection of Fc-AK183 achieved week-long suppression of circulating IgA1 levels in humanized transgenic models of IgA1 (additional examples to Figure 5).
Supplemental Figure 5. A new cohort of three α1KI+/−-Tg mice treated with Fc-AK183 (additional cohort to Figure 5 and Supplemental Figure 4).
Supplemental Figure 6. Fc-AK183 treatment of humanized α1KI+/−-Tg mice did not affect glomerular IgG and IgM levels.
Supplemental Figure 7. Induction of α1KI+/−-Tg mice with FAD caused IgAN-like glomerular mesangial expansion.
Supplemental Figure 8. Changes of urine IgA1(Fc) levels in α1KI+/−-Tg mice treated with Fc-AK183.
Supplemental Figure 9. Fc-AK183 did not show kidney and liver toxicity in mice.
Supplemental Figure 10. Fc-AK183 did not abrogate IgA1-expressing plasma cells in the small intestine.
Supplemental Figure 11. Neutralizing antibody test against AK183 and human Fc in mice with and without Fc-AK183 injection.
References
- 1.Wyatt RJ, Julian BA: IgA nephropathy. N Engl J Med 368: 2402–2414, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Lai KN, Tang SC, Schena FP, Novak J, Tomino Y, Fogo AB, et al. : IgA nephropathy. Nat Rev Dis Primers 2: 16001, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Barratt J, Tang SCW: Treatment of IgA nephropathy: Evolution over half a century. Semin Nephrol 38: 531–540, 2018 [DOI] [PubMed] [Google Scholar]
- 4.KDIGO Clinical Practice Guideline for Glomerulonephritis. Available at: https://kdigo.org/guidelines/gd/
- 5.Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, et al. ; STOP-IgAN Investigators : Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 373: 2225–2236, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Lv J, Zhang H, Wong MG, Jardine MJ, Hladunewich M, Jha V, et al. ; TESTING Study Group : Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: The TESTING randomized clinical trial. JAMA 318: 432–442, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rauen T, Fitzner C, Eitner F, Sommerer C, Zeier M, Otte B, et al. : Effects of two immunosuppressive treatment protocols for IgA nephropathy. J Am Soc Nephrol 29: 317–325, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knoppova B, Reily C, Maillard N, Rizk DV, Moldoveanu Z, Mestecky J, et al. : The origin and activities of IgA1-containing immune complexes in IgA nephropathy. Front Immunol 7: 117, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mestecky J, Raska M, Julian BA, Gharavi AG, Renfrow MB, Moldoveanu Z, et al. : IgA nephropathy: Molecular mechanisms of the disease. Annu Rev Pathol 8: 217–240, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Novak J, Julian BA, Tomana M, Mestecky J: IgA glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Semin Nephrol 28: 78–87, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, et al. : The pathophysiology of IgA nephropathy. J Am Soc Nephrol 22: 1795–1803, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lv J, Yang Y, Zhang H, Chen W, Pan X, Guo Z, et al. : Prediction of outcomes in crescentic IgA nephropathy in a multicenter cohort study. J Am Soc Nephrol 24: 2118–2125, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie X, Lv J, Shi S, Zhu L, Liu L, Chen M, et al. : Plasma exchange as an adjunctive therapy for crescentic IgA nephropathy. Am J Nephrol 44: 141–149, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Eitner F, Floege J: Bacterial protease for the treatment of IgA nephropathy. Nephrol Dial Transplant 23: 2173–2175, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Lamm ME, Emancipator SN, Robinson JK, Yamashita M, Fujioka H, Qiu J, et al. : Microbial IgA protease removes IgA immune complexes from mouse glomeruli in vivo: Potential therapy for IgA nephropathy. Am J Pathol 172: 31–36, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Li X, Shen H, Mao N, Wang H, Cui L, et al. : Bacterial IgA protease-mediated degradation of agIgA1 and agIgA1 immune complexes as a potential therapy for IgA nephropathy. Sci Rep 6: 30964, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lechner SM, Abbad L, Boedec E, Papista C, Le Stang MB, Moal C, et al. : IgA1 Protease treatment reverses mesangial deposits and hematuria in a model of IgA nephropathy. J Am Soc Nephrol 27: 2622–2629, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan SC, Lorant T, Choi J, Kjellman C, Winstedt L, Bengtsson M, et al. : IgG endopeptidase in highly sensitized patients undergoing transplantation. N Engl J Med 377: 442–453, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Soveri I, Mölne J, Uhlin F, Nilsson T, Kjellman C, Sonesson E, et al. : The IgG-degrading enzyme of Streptococcus pyogenes causes rapid clearance of anti-glomerular basement membrane antibodies in patients with refractory anti-glomerular basement membrane disease. Kidney Int 96: 1234–1238, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Kilian M, Mestecky J, Kulhavy R, Tomana M, Butler WT: IgA1 proteases from Haemophilus influenzae, Streptococcus pneumoniae, Neisseria meningitidis, and Streptococcus sanguis: Comparative immunochemical studies. J Immunol 124: 2596–2600, 1980 [PubMed] [Google Scholar]
- 21.Launay P, Grossetête B, Arcos-Fajardo M, Gaudin E, Torres SP, Beaudoin L, et al. : Fcalpha receptor (CD89) mediates the development of immunoglobulin A (IgA) nephropathy (Berger’s disease). Evidence for pathogenic soluble receptor-Iga complexes in patients and CD89 transgenic mice. J Exp Med 191: 1999–2009, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo TT, Aveson VG: Neonatal Fc receptor and IgG-based therapeutics. MAbs 3: 422–430, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu P, Wysocki J, Souma T, Ye M, Ramirez V, Zhou B, et al. : Novel ACE2-Fc chimeric fusion provides long-lasting hypertension control and organ protection in mouse models of systemic renin angiotensin system activation. Kidney Int 94: 114–125, 2018 [DOI] [PubMed] [Google Scholar]
- 24.Czajkowsky DM, Hu J, Shao Z, Pleass RJ: Fc-fusion proteins: New developments and future perspectives. EMBO Mol Med 4: 1015–1028, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rath T, Baker K, Dumont JA, Peters RT, Jiang H, Qiao SW, et al. : Fc-fusion proteins and FcRn: Structural insights for longer-lasting and more effective therapeutics. Crit Rev Biotechnol 35: 235–254, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roopenian DC, Akilesh S: FcRn: The neonatal Fc receptor comes of age. Nat Rev Immunol 7: 715–725, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Dall’Acqua WF, Kiener PA, Wu H: Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J Biol Chem 281: 23514–23524, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Zhang X, Han W, Yu G, Ying Z, Xu X, et al. : Immune characteristics of renal allograft donors with mesangial IgA deposition. Int Immunopharmacol 91: 107282, 2021 [DOI] [PubMed] [Google Scholar]
- 29.Moldoveanu Z, Suzuki H, Reily C, Satake K, Novak L, Xu N, et al. : Experimental evidence of pathogenic role of IgG autoantibodies in IgA nephropathy. J Autoimmun 118: 102593, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wysocki J, Ye M, Rodriguez E, González-Pacheco FR, Barrios C, Evora K, et al. : Targeting the degradation of angiotensin II with recombinant angiotensin-converting enzyme 2: Prevention of angiotensin II-dependent hypertension. Hypertension 55: 90–98, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kosugi A, Amano Y, Murashima K, Doi RH: Hydrophilic domains of scaffolding protein CbpA promote glycosyl hydrolase activity and localization of cellulosomes to the cell surface of Clostridium cellulovorans. J Bacteriol 186: 6351–6359, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosbah A, Belaïch A, Bornet O, Belaïch JP, Henrissat B, Darbon H: Solution structure of the module X2 1 of unknown function of the cellulosomal scaffolding protein CipC of Clostridium cellulolyticum. J Mol Biol 304: 201–217, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Pallen MJ, Lam AC, Antonio M, Dunbar K: An embarrassment of sortases—A richness of substrates? Trends Microbiol 9: 97–102, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Poulsen K, Reinholdt J, Jespersgaard C, Boye K, Brown TA, Hauge M, et al. : A comprehensive genetic study of streptococcal immunoglobulin A1 proteases: Evidence for recombination within and between species. Infect Immun 66: 181–190, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vitovski S, Sayers JR: Relaxed cleavage specificity of an immunoglobulin A1 protease from Neisseria meningitidis. Infect Immun 75: 2875–2885, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gettins PG: Serpin structure, mechanism, and function. Chem Rev 102: 4751–4804, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Strohl WR: Fusion proteins for half-life extension of biologics as a strategy to make biobetters. BioDrugs 29: 215–239, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winstedt L, Järnum S, Nordahl EA, Olsson A, Runström A, Bockermann R, et al. : Complete removal of extracellular IgG antibodies in a randomized dose-escalation phase I study with the bacterial enzyme IdeS A novel therapeutic opportunity. PLoS One 10: e0132011, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mankarious S, Lee M, Fischer S, Pyun KH, Ochs HD, Oxelius VA, et al. : The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. J Lab Clin Med 112: 634–640, 1988 [PubMed] [Google Scholar]
- 40.Rifai A, Fadden K, Morrison SL, Chintalacharuvu KR: The N-glycans determine the differential blood clearance and hepatic uptake of human immunoglobulin (Ig)A1 and IgA2 isotypes. J Exp Med 191: 2171–2182, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strohl WR, Strohl LM: Therapeutic Antibody Engineering: Current and Future Advances Driving the Strongest Growth Area in the Pharma Industry. Cambridge: Woodhead Publishing Limited; 2012. [Google Scholar]
- 42.Forrester JD, Spain DA: Clostridium ramosum bacteremia: Case report and literature review. Surg Infect (Larchmt) 15: 343–346, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Schena FP, Nistor I: Epidemiology of IgA nephropathy: A global perspective. Semin Nephrol 38: 435–442, 2018 [DOI] [PubMed] [Google Scholar]
- 44.Fellström BC, Barratt J, Cook H, Coppo R, Feehally J, de Fijter JW, et al. ; NEFIGAN Trial Investigators : Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): A double-blind, randomised, placebo-controlled phase 2b trial. Lancet 389: 2117–2127, 2017 [DOI] [PubMed] [Google Scholar]
- 45.Hahn BH: Belimumab for systemic lupus erythematosus. N Engl J Med 368: 1528–1535, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Zipfel PF, Wiech T, Rudnick R, Afonso S, Person F, Skerka C: Complement inhibitors in clinical trials for glomerular diseases. Front Immunol 10: 2166, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davin JC, Coppo R: Henoch-Schönlein purpura nephritis in children. Nat Rev Nephrol 10: 563–573, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Kumrah R, Vignesh P, Rawat A, Singh S: Immunogenetics of Kawasaki disease. Clin Rev Allergy Immunol 59: 122–139, 2020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.