Abstract
Over the past 20 years, despite significant advancements in pulmonary arterial hypertension (PAH) medical therapy, many patients require admission to the hospital and are at risk for in‐hospital cardiac arrest (IHCA). Prior data found poor survival in PAH patients after cardiac arrest. The purpose of this study was to explore post‐IHCA outcomes in PAH patients receiving advanced medical therapies. This is a single‐center retrospective study of PAH patients who underwent cardiopulmonary resuscitation for IHCA between July 2005 and May 2021. Patients were identified through an internal cardiac arrest database. Twenty six patients were included. Half of the cohort had idiopathic PAH, with 54% of patients on combination therapy, 27% on monotherapy, and 19% of patients on no therapy. Mean right atrial pressure, mean pulmonary artery pressure, cardiac index, and pulmonary vascular resistance were 13 ± 6 mmHg, 57 ± 13 mmHg, 2.0 ± 0.7 L/min/m2, and 14.5 ± 7.6 Wood units, respectively. Most common etiology of cardiac arrest was circulatory collapse. Initial arrest rhythm in all but one patient was pulseless electrical activity. Six patients (23%) achieved return of spontaneous circulation (ROSC) and one patient (4%) survived to hospital discharge. Rates of ROSC and survival to discharge after IHCA are poor in patients with PAH. Even patients with mild hemodynamics had low likelihood of survival. In patients who are lung transplant candidates, there should be early consideration of extracorporeal support before cardiac arrest.
Keywords: CPR, in‐hospital cardiac arrest, pulmonary hypertension, pulseless electrical activity
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a rare progressive and incurable disease, with an estimated incidence of 5–15 cases per 1 million adults. 1 , 2 , 3 The underlying pathophysiology of PAH includes vasoconstriction, remodeling, and obliteration of the pulmonary vasculature. This leads to elevated pulmonary arterial pressures, increased pulmonary vascular resistance (PVR) and ultimately results in right ventricular (RV) failure and death. Patients with PAH are vulnerable to decompensated right heart failure resulting in hospital admission, with over 40% requiring the intensive care unit (ICU). 4 Furthermore, mortality is high, with 30% dying during the ICU stay and only 60% surviving at 6 months. 5 Death usually results from progressive right heart failure, hypoxemia, arrhythmias, and multiorgan failure.
There are ~300,000 in‐hospital cardiac arrests (IHCA) in the United States annually, 6 with a median survival to discharge that has slowly increased over the last two decades from 15% to 25%. 7 Survival for patients with PAH, who suffer IHCA, however, is very poor with a 90‐day survival rate of only 6% based on data published in 2002. 8 Since then, there have been considerable developments in PAH‐targeted therapies. Therefore, we aim to examine survival of PAH patients after IHCA in our institution in the era of advanced PAH‐targeted medical therapies.
METHODS
Study population
This was a single‐center retrospective analysis of PAH patients who had an IHCA. Our institution has maintained an institutional review board (IRB)‐approved (UC San Diego Human Protections Program, IRB 161121) quality improvement database of all cardiac arrests since 2005. All patients admitted or evaluated in the emergency department at the University of California (UC) San Diego Health System between July 2005 and May 2021, who had a diagnosis of “pulmonary hypertension (PH)” or “PAH,” and experienced a cardiac arrest, were reviewed. Patients were identified by searching the cardiac arrest database for “pulmonary hypertension” or “pulmonary arterial hypertension,” and then confirmed by review of the electronic health record. Patients with an active do not resuscitate (DNR) order, non‐World Health Organization (WHO) Group 1 PH, PH diagnosis by transthoracic echocardiography alone without confirmation by the pulmonary vascular medicine (PVM) service, and those who were status postlung transplantation for PAH were excluded. All out‐of‐hospital cardiac arrest patients were also excluded. Only index cardiac arrest episodes were included in the analysis.
Demographic variables, comorbidities, most recent right heart catheterization results, and PAH‐targeted therapies were abstracted from the electronic health record. Detailed cardiopulmonary resuscitation (CPR) data are recorded on Zoll monitors (Zoll E Series, Zoll Medical Inc.), including depth and rate of compressions, underlying rhythm, capnography, and timing of defibrillations when applicable. This CPR data and outcomes were extracted from the internal IHCA database and confirmed by chart review.
Setting
The UC San Diego Health System includes two urban academic hospitals. The primary hospital is a quaternary care referral center, with 418 beds, and includes a cardiovascular institute, transplant center, and comprehensive cancer care center. The sister campus is a 390‐bed facility that is a Level 1 trauma center and serves as the regional county hospital.
UC San Diego Health is a worldwide referral center for chronic thromboembolic PH (CTEPH) and PAH. Patients with CTEPH and PAH are primarily managed by the PVM service, which consists of a core group of pulmonologists who specialize in pulmonary vascular disease. This includes both inpatient and outpatient management.
Cardiac arrest protocol
A “code blue” is called for a medical emergency, most commonly due to cardiopulmonary arrest. The responding code blue team consists of a critical care physician (ICU attending, ICU fellow, and/or senior internal medicine resident), anesthesiologist, critical care nurse, pharmacist, and respiratory therapist.
A cardiac arrest is defined by the absence of a palpable pulse, the performance of chest compressions, and/or a defibrillation attempt. As per Utstein criteria, sustained return of spontaneous circulation (ROSC) is defined as the return of a perfusing rhythm for at least 20 min. 9 If the pulse was lost before 20 min passed, the ensuing CPR would be included as part of the initial index event. If ROSC was sustained for more than 20 min, a subsequent cardiac arrest was recorded as a second or separate arrest.
All UC San Diego critical care providers are trained in Advanced Resuscitation Training (ART). 10 ART is an institutional treatment algorithm derived from continuous data review for cardiac arrest resuscitation, which incorporates real‐time resuscitation feedback to guide continued efforts. It utilizes quantitative capnography, electrocardiography filtering during compressions, and measures of CPR quality (e.g., chest compression rate and recoil and depth), to facilitate high‐quality CPR performance. Filtered rhythm technology (e.g., “See‐through CPR”) is used to filter out chest compression artifact during CPR and therefore minimize time without chest compressions for rhythm analysis.
Data collection
Following a code blue or CPR event, the responding ICU nurse submits an electronic report of the event, which contains demographic and clinical data, code blue etiology, interventions, outcomes, and a brief narrative of events. Compression and capnography data are exported from the defibrillators when available. A multidisciplinary committee of critical care providers reviews all resuscitation events for accuracy and process issues.
The UC San Diego Health internal IHCA database is a prospectively collected, quality improvement database, which contains demographic, clinical, and outcomes data from all in‐hospital arrests since 2005. All cardiac arrest resuscitation events are categorized by presenting rhythm (shockable or nonshockable) and by etiology of arrest using a priori criteria (detailed definitions in supplement; Figure S1). The arrests are categorized into four basic pathophysiological processes as follows: (1) circulatory, (2) respiratory, (3) dysrhythmic, and (4) neurological. This database is reviewed for accuracy and tracked through the institutional Code Blue committee.
RESULTS
Study population
From June 1, 2005, through May 31, 2021, there were a total of 96 IHCA in patients with a diagnosis of PH. Five CPR events were a second or subsequent arrest during the hospital stay and were excluded, leaving 91 patients with an index IHCA to review (Figure 1). We excluded 19 patients with a diagnosis of PH by transthoracic echocardiogram only (e.g., without invasive hemodynamic measurements), 25 patients with CTEPH, and 19 patients with WHO Group 2, 3, or 5 PH. The remaining 28 patients had a diagnosis of PAH by right heart catheterization or confirmation by the PVM service after reviewing outside records. Of the 28 patients with PAH, two were excluded. One patient had received a double lung transplant for PAH at the time of cardiac arrest and one patient did not undergo CPR, because a DNR order was entered just before the event. Twenty‐six patients were ultimately included in this study for analysis.
Figure 1.

Flowchart of patients who were screened, excluded, and included in the final analysis. CTEPH, chronic thromboembolic pulmonary hypertension; DNR/DNI, do not resuscitate/do not intubate; IHCA, in‐hospital cardiac arrest; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; TTE, transthoracic echocardiography.
Patient characteristics
Baseline demographic information is shown in Table 1. Twenty patients (77%) were female with a mean age of 48.2 ± 15.7 years and mean body mass index of 27.0 ± 5.8 kg/m2. The most common reason for admission was volume overload and/or cardiogenic shock with RV failure (65%). There were no admissions for sepsis and three admissions were for elective procedures. Of the three admissions for planned procedures, one patient had a cardiac arrest within 24 h after surgery, one patient was admitted for lung transplant but the lungs were declined after the patient was intubated in the operating room and cardiac arrest ensued within 24 h after extubation, and the final patient was admitted for tunneled central line removal with interventional radiology (IR) and cardiac arrest occurred immediately postprocedure in the IR suite.
Table 1.
Demographic characteristics
| All patients | ROSC | |
|---|---|---|
| Total number of patients, n | 26 | 6 |
| Age (years) | 48.2 ± 15.7 | 53.0 ± 16.9 |
| Female, n (%) | 20 (77%) | 5 (83%) |
| BMI (kg/m2) | 27.0 ± 5.9 | 25.7 ± 2.2 |
| Race/ethnicity, n (%) | ||
| White | 16 (61%) | 2 (33%) |
| Hispanic | 7 (27%) | 4 (67%) |
| Asian/Pacific Islander | 2 (8%) | 0 |
| Black | 1 (4%) | 0 |
| PAH diagnosis, n (%) | ||
| Idiopathic PAH | 13 (50%) | 3 (50%) |
| PAH associated with connective tissue disease | 6 (23%) | 3 (50%) |
| PAH associated with drug/toxin | 3 (11%) | 0 |
| PAH associated with congenital heart disease | 1 (4%) | 0 |
| PAH associated with HIV | 1 (4%) | 0 |
| PAH associated with cirrhosis | 1 (4%) | 0 |
| Pulmonary veno‐occlusive disease | 1 (4%) | 0 |
| Medical PAH therapy, n (%) | ||
| Triple therapy | 9 (35%) | 3 (50%) |
| Double therapy | 5 (19%) | 2 (33%) |
| Monotherapy | 7 (27%) | 0 |
| None | 5 (19%) | 1 (17%) |
| Breakdown of PAH‐targeted therapies, n | ||
| PDE5 inhibitors | 14 | 3 |
| ERA | 17 | 5 |
| sGC stimulator | 1 | 0 |
| Oral prostacyclin | 2 | 1 |
| IV prostacyclin | 5 | 2 |
| Subcutaneous prostacyclin | 1 | 1 |
| Inhaled prostacyclin | 3 | 1 |
Note: Baseline demographics and PAH characteristics of the cohort.
Abbreviations: BMI, body mass index; ERA, endothelin receptor antagonist; HIV, human immunodeficiency virus; IV, intravenous; PAH, pulmonary arterial hypertension; PDE5, phosphodiesterase type 5; ROSC, return of spontaneous circulation; sGC, soluble guanylate cyclase.
PAH characteristics
Etiology of PAH was idiopathic in 13 patients (50%; Table 1). Six patients (23%) had connective tissue disease‐associated PAH, three patients (11%) had drug or toxin‐associated PAH, and one patient (4%) each had PAH associated with congenital heart disease, human immunodeficiency virus, porto‐PH, and pulmonary veno‐occlusive disease.
Nine patients (35%) were on triple therapy, five (19%) were on double therapy, and seven (27%) on monotherapy for PAH (Table 1). Most patients were on phosphodiesterase type 5 (PDE5) inhibitors and/or endothelin receptor antagonists (ERAs). The most common PDE5 inhibitor was sildenafil (n = 11) and the most common ERAs were bosentan and macitentan (n = 15). Five patients were on intravenous (IV) prostacyclin therapy, with three of those patients on IV epoprostenol and two on IV Treprostinil.
Five patients (19%) were not on any PAH‐targeted therapies before admission. Of those five, four patients were admitted with a new diagnosis of PAH and underwent right heart catheterization during the admission. Three patients started on IV epoprostenol urgently as an inpatient and one died in the cardiac catheterization lab immediately after the right heart catheterization before initiation of IV epoprostenol. An additional patient with newly diagnosed PAH was started on sildenafil at an outside hospital before transfer and rescue IV epoprostenol was initiated on arrival. The remaining patient had PAH associated with methamphetamine use and was not on therapy due to difficulties with adherence.
Hemodynamics were available in 23 patients. Although three patients did not have a documented right heart catheterization in the electronic health record, they were included in the final analysis, because they were admitted to the PVM service and confirmed as having PAH by PVM faculty based on outside record review. Available hemodynamics are shown in Table 2. Mean values for the group for right atrial pressure was 13 ± 6 mmHg, mean pulmonary artery pressure was 57 ± 13 mmHg, cardiac index was 2.0 ± 0.7 L/min/m2, and PVR was 14.5 ± 7.6 Wood units (WU). Twelve of the right heart catheterizations were done within 3 months of the cardiac arrest. Ten of the right heart catheterizations were done at the time of PAH diagnosis, so those hemodynamics were obtained without therapy; the remaining 13 right heart catheterizations were done on PAH therapy, between 2 and 14 years after initial diagnosis (Table S2).
Table 2.
Hemodynamics
| All patients, n = 23 | ROSC, n = 6 | |||
|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | |
| RA (mmHg) | 13.2 ± 6.0 | 6–25 | 13.8 ± 7.3 | 7–24 |
| mPAP (mmHg) | 57.2 ± 13.0 | 33–86 | 51.2 ± 12.5 | 37–70 |
| PAWP (mmHg) | 10.3 ± 4.0 | 3–18 | 7.8 ± 3.4 | 4–12 |
| CO (L/min) | 3.7 ± 1.4 | 2.0–6.9 | 3.9 ± 1.8 | 2.5–6.9 |
| CI (L/min/m2) | 2.0 ± 0.7 | 1.1–3.7 | 2.2 ± 0.9 | 1.4–3.7 |
| PVR (WU) | 14.5 ± 7.6 | 5.7–33.6 | 11.6 ± 3.6 | 7.4–15.6 |
| TPR (WU) | 17.7 ± 9.0 | 6.4–38.6 | 13.7 ± 3.3 | 10.2–17.5 |
Note: Available hemodynamic measurements of the cohort before IHCA. Twelve of the right heart catheterizations were done within 3 months of the IHCA.
Abbreviations: CI, cardiac index; CO, cardiac output; IHCA, in‐hospital cardiac arrest; mPAP, mean pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RA, right atrial pressure; ROSC, return of spontaneous circulation; TPR, total pulmonary resistance; WU, Wood units.
Outcomes
The most common etiology of arrest was circulatory collapse, followed by vagal events and respiratory causes (Table 3). Circulatory collapse was triggered by cardiogenic shock, hemorrhage, and anaphylaxis. All cardiac arrests from a respiratory etiology were associated with preceding hypoxemia that led to pulseless electrical activity (PEA). Six patients had cardiac arrests triggered by a vasovagal event and bradyarrhythmia. Of these six arrests, two were associated with being on the commode, two were associated with central line placement/removal, one was associated with nausea and vomiting, and one was immediately after a right heart catheterization.
Table 3.
Cardiopulmonary resuscitation data
| All patients, n = 26 | ROSC, n = 6 | |
|---|---|---|
| Etiology of arrest | ||
| Circulatory | 14 (54%) | 2 (33%) |
| Vasovagal | 6 (23%) | 2 (33%) |
| Respiratory | 5 (19%) | 1 (17%) |
| Arrhythmia | 1 (4%) | 1 (17%) |
| Initial rhythm | ||
| PEA | 25 (96%) | 5 (83%) |
| VF/VT | 1 (4%) | 1 (17%) |
| Location of arrest | ||
| Emergency dept | 3 (12%) | 0 |
| General ward | 4 (15%) | 1 (17%) |
| ICU | 16 (61%) | 4 (67%) |
| Cath lab/IR suite | 3 (12%) | 1 (17%) |
| ROSC | 6 (23%) | |
| Survival to discharge | 1 (4%) | |
Note: Characteristics of cardiopulmonary resuscitation efforts in the cohort.
Abbreviations: ICU, intensive care unit; IR, interventional radiology; PEA, pulseless electrical activity; ROSC, return of spontaneous circulation; VF/VT, ventricular fibrillation/ventricular tachycardia.
The initial arrest rhythm was PEA in 25 patients and ventricular tachycardia (VT) in one patient. Sixteen of the cardiac arrests occurred in the ICU (61%), whereas the other 10 cardiac arrests occurred in the emergency department, ward unit, or the cardiac catheterization lab (Table 3). CPR parameter data was available for three of the patients with an average chest compression fraction, rate, and depth of 91%, 121 compressions/min, and 2.59 inches, respectively, consistent with institutional averages.
Emergent veno‐arterial extracorporeal membrane oxygenation (VA‐ECMO) is done selectively during CPR at our institution. It was considered for one patient with PAH and IHCA, but her vascular access issues prevented successful cannulation.
Six patients achieved ROSC (23%) and only one patient survived to hospital discharge (4%). The patient who survived had an initial arrest rhythm of VT and she was discharged with a cerebral performance score of 1. She received chest compressions and defibrillation but did not require emergent endotracheal intubation and was awake and alert post‐ROSC.
DISCUSSION
In this retrospective review of patients with PAH and IHCA, ROSC occurred in just 23% and in‐hospital mortality was over 95%. For those with PEA arrest, mortality was 100%. Nationally, the reported ROSC rate is 70% for all‐comer, risk‐adjusted IHCA, 11 and the national median survival to discharge after IHCA is ~25%, 12 whereas survival to discharge after IHCA for UC San Diego is 44% (Fiscal year 2020–2021, personal data). Since the last comprehensive study of outcomes after IHCA in patients with PAH over 20 years ago, an additional 13 PAH‐targeted medications are now approved (Figure S2). Although 5‐year survival of patients with PAH remains low, it has improved compared with historical controls before initiation of PAH‐targeted therapies. 13 , 14 Moreover, in the past 7 years, most patients with PAH are started on upfront combination therapy based on newer clinical trial data, 15 representing another important treatment paradigm shift midway through our capture period. This is reflected in our cohort, with more patients in the recent past seven years (2014–2021) on combination therapy compared with 2005–2013 (82% vs. 33%; Table S1). Mean values of mean pulmonary artery pressure, cardiac index, and PVR for our cohort were mildly better compared with those reported in the earlier study 8 (57 ± 13 mmHg vs. 61 ± 16 mmHg, 2.0 ± 0.7 L/min/m2 vs. 1.7 ± 0.4 L/min/m2, and 14.5 ± 7.6 WU vs. 21.2 ± 11.8 WU, respectively), which may reflect the more available PAH‐targeted therapies. However, even patients with mild hemodynamic disturbances did not survive to hospital discharge after IHCA.
The majority of patients in our cohort had a cardiac arrest with nonshockable (PEA) rhythms due to circulatory etiologies, such as cardiogenic shock or hemorrhage. This is similar to the findings in the previous study, 8 in which the majority of patients had an initial nonshockable rhythm as well. In our cohort, there were also six patients who had a vasovagal event triggering the IHCA. Patients with PAH do not tolerate wide swings in hemodynamics. The sudden hypotension and bradycardia associated with a vasovagal response can lead to a rapid drop in cardiac preload and cardiac output, further potentiating RV ischemia and ultimately leading to cardiac arrest (Figure 2). Valsalva maneuvers, prolonged breath holds and pain can all potentiate a vagal response, and patients with PAH are more susceptible to the resulting hemodynamic changes, having a longer blood pressure recovery time and do not return to their baseline mean arterial pressures. 16 In addition, CPR is known to cause further RV dysfunction as noted on post‐ROSC echocardiography 17 and it can be difficult to achieve adequate pulmonary blood flow and left ventricular filling with chest compressions in the setting of high PVR.
Figure 2.
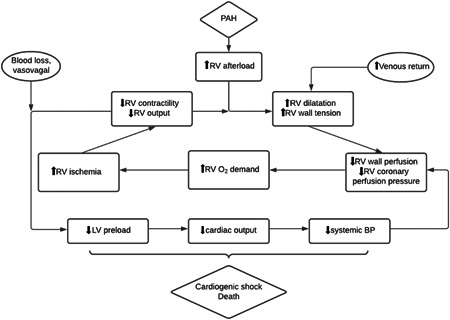
Cycle of the right ventricular failure leading to cardiovascular collapse. BP, blood pressure; LV, left ventricle; PAH, pulmonary arterial hypertension; RV, right ventricle.
In recent years, emergent cannulation for extracorporeal support during CPR (e‐CPR) has gained traction as a management strategy for refractory cardiac arrest in select patients who have a reversible etiology of arrest. Although e‐CPR remains controversial and is offered in limited centers, there are studies that suggest improved survival and neurological outcomes with e‐CPR during IHCA compared with conventional CPR. 18 , 19 However, no patients with PAH were included in these studies so applicability to our population is unclear. In addition, over the course of 10 years, survival after e‐CPR remains poor 20 and there are significant complications associated with its use. 21 In our cohort, none of the patients were cannulated for VA‐ECMO during CPR. Although VA‐ECMO may serve as a bridge to lung transplant in select patients with PAH, it usually cannot be used successfully as a bridge to recovery, unless there is a clear acute and reversible cause to decompensation. 22 In a decompensating PAH patient who is a lung transplant candidate, there should be early consideration for VA‐ECMO, as these patients are hemodynamically tenuous and, if cardiac arrest ensues, survival is poor.
Although there are now numerous and effective advanced medical therapies, PAH remains a progressive disease with no cure. However, less than half of patients with PAH have a documented advanced directive and many PAH patients die in the hospital, with the majority in the ICU. 23 , 24 When end‐of‐life discussions are first broached in the ICU, patients and family may feel surprised or rushed into complex conversations, especially as the ICU often warrants rapid decisions. Therefore, when possible, these discussions regarding the role of palliative care and potential limitations in escalating care depending on prognosis and transplant suitability, should occur before hemodynamic deterioration. Hospice care can be prohibitive due to the high costs of PAH medications and the unwillingness to acutely stop continuous prostacyclin therapy, which could lead to rapid deterioration and RV failure. However, palliative care may have a role but is rarely involved in the care of PAH patients. 23 , 25 Although a common perception of palliative care is “giving up,” 26 it can actually be adjunctive to the comprehensive care of patients with PAH, allowing patients to identify and achieve their goals in their own care and end of life wishes.
The practice of the PVM providers at our institution is to be proactive in discussing end‐of‐life care for patients with advanced PAH. As a result, many patients choose to allow natural death and avoid non‐beneficial resuscitation attempts. We do not have data, however, on these patients who died while admitted but did not have a “code blue” activated due to an active DNR status.
We acknowledge there are limitations to this study. First, this was a retrospective, single‐center study and included a small number of patients. These patients spanned from 2005 to 2021 and PAH management has changed during this time. Upfront combination PAH‐targeted therapy and the increasing number of available PAH‐targeted therapies are reflected by the management differences in our cohort in later years (Table S1). In addition, not all right heart catheterization data were from within 3 months of the cardiac arrest and some patients were included without the right heart catheterization data. However, we limited patients to those who had been reviewed by PAH specialists, thus ensuring a high likelihood of PAH. Furthermore, our cardiac arrest teams follow ART and not advanced cardiovascular life support algorithms, which many limit generalizability to other centers. However, using ART, we have a high institutional survival to hospital discharge rate for non‐PAH patients with IHCA. Finally, we do not have data on admitted PAH patients who were not offered CPR and died without CPR attempts.
CONCLUSION
Despite advancements in PAH therapies, outcomes of CPR efforts in IHCA remain poor in patients with PAH. Our data suggests there should be ongoing goals of care discussions and early consideration for VA‐ECMO in select patients who may be lung transplant candidates. Additional studies are needed to confirm our findings.
AUTHOR CONTRIBUTIONS
All authors have reviewed, contributed, and approved this manuscript.
CONFLICTS OF INTEREST
Gabriel Wardi is supported by the National Foundation of Emergency Medicine and receives funding from the Gordon and Betty Moore Foundation (#GBMF9052) and the National Institutes of Health, although not related to this study. Demosthenes G. Papamatheakis has received honoraria from Janssen PH. Timothy M. Fernandes has served as consultant for Bayer and Janssen PH. Nick H. Kim has served as consultant/steering committee for Bayer, Gossamer Bio, Janssen, Merck, United Therapeutics; Speakers Bureau for Bayer, Janssen; and has received research support from Acceleron, Bellerophon, Eiger, Lung Biotechnology, and SoniVie. The remaining authors report no relevant disclosures or conflicts of interest.
ETHICS STATEMENT
Not applicable.
Supporting information
Supporting information.
Yang JZ, Odish MF, Mathers H, Pebley N, Wardi G, Papamatheakis DG, Poch DS, Kim NH, Fernandes TM, Sell RE. Outcomes of cardiopulmonary resuscitation in patients with pulmonary arterial hypertension. Pulmonary Circulation. 2022;12:e12066. 10.1002/pul2.12066
REFERENCES
- 1. McGoon MD, Benza RL, Escribano‐Subias P, Jiang X, Miller DP, Peacock AJ, Pepke‐Zaba J, Pulido T, Rich S, Rosenkranz S, Suissa S, Humbert M. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol. 2013;62:D51–9. 10.1016/j.jacc.2013.10.023 [DOI] [PubMed] [Google Scholar]
- 2. Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, Gibbs JS, Howard LS, Pepke‐Zaba J, Sheares KK, Corris PA, Fisher AJ, Lordan JL, Gaine S, Coghlan JG, Wort SJ, Gatzoulis MA, Peacock AJ. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med. 2012;186(8):790–6. 10.1164/rccm.201203-0383OC [DOI] [PubMed] [Google Scholar]
- 3. Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud‐Gaubert M, Haloun A, Laurent M, Hachulla E, Simonneau G. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173(9):1023–30. 10.1164/rccm.200510-1668OC [DOI] [PubMed] [Google Scholar]
- 4. Campo A, Mathai SC, Le Pavec J, Zaiman AL, Hummers LK, Boyce D, Housten T, Lechtzin N, Chami H, Girgis RE, Hassoun PM. Outcomes of hospitalisation for right heart failure in pulmonary arterial hypertension. Eur Respir J. 2011;38(2):359–67. 10.1183/09031936.00148310 [DOI] [PubMed] [Google Scholar]
- 5. Huynh TN, Weigt SS, Sugar CA, Shapiro S, Kleerup EC. Prognostic factors and outcomes of patients with pulmonary hypertension admitted to the intensive care unit. J Crit Care. 2012;27(6):739.e7–13. 10.1016/j.jcrc.2012.08.006 [DOI] [PubMed] [Google Scholar]
- 6. Holmberg MJ, Ross CE, Fitzmaurice GM, Chan PS, Duval‐Arnould J, Grossestreuer AV, Yankama T, Donnino MW, Andersen LW. American Heart Association's Get With The Guidelines–Resuscitation I. Annual incidence of adult and pediatric in‐hospital cardiac arrest in the United States. Circ Cardiovasc Qual Outcomes. 2019;12(7):e00558. 10.1161/CIRCOUTCOMES.119.005580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson U, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics−2018 update: a report from the American Heart Association. Circulation. 2018;137(12):E67–492. 10.1161/CIR.0000000000000558 [DOI] [PubMed] [Google Scholar]
- 8. Hoeper MM, Galié N, Murali S, Olschewski H, Rubenfire M, Robbins IM, Farber HW, McLaughlin V, Shapiro S, Pepke‐Zaba J, Winkler J, Ewert R, Opitz C, Westerkamp V, Vachiéry JL, Torbicki A, Behr J, Barst RJ. Outcome after cardiopulmonary resuscitation in patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002;165(3):341–4. 10.1164/ajrccm.165.3.200109-0130c [DOI] [PubMed] [Google Scholar]
- 9. Jacobs I, Nadkarni V, Bahr J, Berg RA, Billi JE, Bossaert L, Cassan P, Coovadia A, D'Este K, Finn J, Halperin H, Handley A, Herlitz J, Hickey R, Idris A, Kloeck W, Larkin GL, Mancini ME, Mason P, Mears G, Monsieurs K, Montgomery W, Morley P, Nichol G, Nolan J, Okada K, Perlman J, Shuster M, Steen PA, Sterz F, Tibballs J, Timerman S, Truitt T, Zideman D, International Liaison Committee on Resuscitation, American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa, ILCOR Task Force on Cardiac Arrest and Cardiopulmonary Resuscitation Outcomes. Cardiac arrest and cardiopulmonary resuscitation outcome reports. Circulation. 2004;110(21):3385–97. 10.1161/01.CIR.0000147236.85306.15 [DOI] [PubMed] [Google Scholar]
- 10. Davis DP, Graham PG, Husa RD, Lawrence B, Minokadeh A, Altieri K, Sell RE. A performance improvement‐based resuscitation programme reduces arrest incidence and increases survival from in‐hospital cardiac arrest. Resuscitation. 2015;92:63–9. 10.1016/j.resuscitation.2015.04.008 [DOI] [PubMed] [Google Scholar]
- 11. Chan PS, Tang Y. Risk‐standardizing rates of return of spontaneous circulation for in‐hospital cardiac arrest to facilitate hospital comparisons. J Am Heart Assoc. 2020;9(7):014837. 10.1161/JAHA.119.014837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andersen LW, Holmberg MJ, Berg KM, Donnino MW, Granfeldt A. In‐hospital cardiac arrest: a review. JAMA. 2019;321(12):1200–10. 10.1001/jama.2019.1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farber HW, Miller DP, Poms AD, Badesch DB, Frost AE, Muros‐Le Rouzic E, Romero AJ, Benton WW, Elliott CG, McGoon MD, Benza RL. Five‐year outcomes of patients enrolled in the REVEAL registry. Chest. 2015;148(4):1043–54. 10.1378/chest.15-0300 [DOI] [PubMed] [Google Scholar]
- 14. McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation. 2002;106(12):1477–82. 10.1161/01.CIR.0000029100.82385.58 [DOI] [PubMed] [Google Scholar]
- 15. Galiè N, Barberà JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, Peacock AJ, Simonneau G, Vachiery JL, Grünig E, Oudiz RJ, Vonk‐Noordegraaf A, White RJ, Blair C, Gillies H, Miller KL, Harris JH, Langley J, Rubin LJ, AMBITION Investigators. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373(9):834–44. 10.1056/NEJMoa1413687 [DOI] [PubMed] [Google Scholar]
- 16. Mar PL, Nwazue V, Black BK, Biaggioni I, Diedrich A, Paranjape SY, Loyd JE, Hemnes AR, Robbins IM, Robertson D, Raj SR, Austin ED. Valsalva maneuver in pulmonary arterial hypertension: susceptibility to syncope and autonomic dysfunction. Chest. 2016;149(5):1252–60. 10.1016/j.chest.2015.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wardi G, Blanchard D, Dittrich T, Kaushal K, Sell R. Right ventricle dysfunction and echocardiographic parameters in the first 24 h following resuscitation in the post‐cardiac arrest patient: A retrospective cohort study. Resuscitation. 2016;103:71–4. 10.1016/j.resuscitation.2016.03.009 [DOI] [PubMed] [Google Scholar]
- 18. Chen YS, Lin JW, Yu HY, Ko WJ, Jerng JS, Chang WT, Chen WJ, Huang SC, Chi NH, Wang CH, Chen LC, Tsai PR, Wang SS, Hwang JJ, Lin FY. Cardiopulmonary resuscitation with assisted extracorporeal life‐support versus conventional cardiopulmonary resuscitation in adults with in‐hospital cardiac arrest: an observational study and propensity analysis. Lancet. 2008;372(9638):554–61. 10.1016/S0140-6736(08)60958-7 [DOI] [PubMed] [Google Scholar]
- 19. Avalli L, Maggioni E, Formica F, Redaelli G, Migliari M, Scanziani M, Celotti S, Coppo A, Caruso R, Ristagno G, Fumagalli R. Favourable survival of in‐hospital compared to out‐of‐hospital refractory cardiac arrest patients treated with extracorporeal membrane oxygenation: an Italian tertiary care centre experience. Resuscitation. 2012;83(5):579–83. 10.1016/j.resuscitation.2011.10.013 [DOI] [PubMed] [Google Scholar]
- 20. Richardson AC, Schmidt M, Bailey M, Pellegrino VA, Rycus PT, Pilcher DV. ECMO cardio‐pulmonary resuscitation (ECPR), trends in survival from an international multicentre cohort study over 12‐years. Resuscitation. 2017;112:34–40. 10.1016/j.resuscitation.2016.12.009 [DOI] [PubMed] [Google Scholar]
- 21. Cheng R, Hachamovitch R, Kittleson M, Patel J, Arabia F, Moriguchi J, Esmailian F, Azarbal B. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta‐analysis of 1,866 adult patients. Ann Thorac Surg. 2014;97(2):610–6. 10.1016/j.athoracsur.2013.09.008 [DOI] [PubMed] [Google Scholar]
- 22. Hoeper MM, Benza RL, Corris P, de Perrot M, Fadel E, Keogh AM, Kühn C, Savale L, Klepetko W. Intensive care, right ventricular support and lung transplantation in patients with pulmonary hypertension. Eur Respir J. 2018;53(1):1801906. 10.1183/13993003.01906-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grinnan DC, Swetz KM, Pinson J, Fairman P, Lyckholm LJ, Smith T. The end‐of‐life experience for a cohort of patients with pulmonary arterial hypertension. J Palliat Med. 2012;15(10):1065–70. 10.1089/jpm.2012.0085 [DOI] [PubMed] [Google Scholar]
- 24. Tonelli AR, Arelli V, Minai OA, Newman J, Bair N, Heresi GA, Dweik RA. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;188(3):365–9. 10.1164/rccm.201209-1640OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khirfan G, Tonelli AR, Ramsey J, Sahay S. Palliative care in pulmonary arterial hypertension: An underutilised treatment. Eur Respir Rev. 2018;27(150):180069. 10.1183/16000617.0069-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fenstad ER, Shanafelt TD, Sloan JA, Novotny PJ, Durst LA, Frantz RP, McGoon MD, Swetz KM. Physician attitudes toward palliative care for patients with pulmonary arterial hypertension: Results of a cross‐sectional survey. Pulm Circ. 2014;4(3):504–10. 10.1086/677365 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


