Abstract
Inhaled treprostinil is an approved therapy for pulmonary arterial hypertension (PAH) and pulmonary hypertension associated with interstitial lung disease in the United States. Studies have confirmed the robust benefits and safety of nebulized inhaled treprostinil, but it requires a time investment for nebulizer preparation, maintenance, and treatment. A small, portable treprostinil dry powder inhaler has been developed for the treatment of PAH. The primary objective of this study was to evaluate the safety and tolerability of treprostinil inhalation powder (TreT) in patients currently treated with treprostinil inhalation solution. Fifty‐one patients on a stable dose of treprostinil inhalation solution enrolled and transitioned to TreT at a corresponding dose. Six‐minute walk distance (6MWD), device preference and satisfaction (Preference Questionnaire for Inhaled Treprostinil Devices [PQ‐ITD]), PAH Symptoms and Impact (PAH‐SYMPACT®) questionnaire, and systemic exposure and pharmacokinetics for up to 5 h were assessed at baseline for treprostinil inhalation solution and at Week 3 for TreT. Adverse events (AEs) were consistent with studies of inhaled treprostinil in patients with PAH, and there were no study drug‐related serious AEs. Statistically significant improvements occurred in 6MWD, PQ‐ITD, and PAH‐SYMPACT. Forty‐nine patients completed the 3‐week treatment phase and all elected to participate in an optional extension phase. These results demonstrate that, in patients with PAH, transition from treprostinil inhalation solution to TreT is safe, well‐tolerated, and accompanied by statistically significant improvements in key clinical assessments and patient‐reported outcomes with comparable systemic exposure between the two formulations at evaluated doses (trial registration: clinicaltrials.gov identifier: NCT03950739).
Keywords: dry powder inhaler, PAH‐SYMPACT, pharmacokinetics, quality of life, treprostinil
INTRODUCTION
Pulmonary arterial hypertension (PAH) is defined as an elevation in mean pulmonary arterial pressure (>20 mmHg) and pulmonary vascular resistance (>3.0 WU) with normal pulmonary artery wedge pressure (<15 mmHg). 1 Elevation in pulmonary arterial pressure causes an increase in right ventricular afterload, impairing right ventricular function and ultimately leading to failure and premature death. 2 Endogenous prostaglandins, including prostacyclin (prostaglandin I2), are potent vasodilators and inhibitors of platelet aggregation produced by the vascular endothelium. 3 , 4 Synthetic prostacyclin analogs are used to treat PAH and have been shown to improve hemodynamics, exercise tolerance, and overall survival. 5
Treprostinil is a chemically stable, longer‐acting prostaglandin I2 analog 6 that was initially approved as a parenteral formulation for the treatment of PAH. 7 Treprostinil has been shown to be both safe and effective when administered parenterally, 8 , 9 but an oral inhalation solution was developed to deliver the drug directly to the site of action, avoiding the most common adverse events (AEs) of infusion‐site pain and reaction seen with subcutaneous treprostinil administration. 7 , 10 Treprostinil inhalation solution was approved in 2009 for the treatment of PAH to improve exercise ability based on the results of the TRIUMPH study, which demonstrated a placebo‐corrected median change from baseline in 6‐minute walk distance (6MWD) of 20 m (p < 0.001) after 12 weeks of treatment. 10 , 11
Treprostinil inhalation solution is delivered via a handheld ultrasonic nebulizer (Tyvaso® Inhalation System, United Therapeutics Corporation). Inhaled therapies such as treprostinil inhalation solution can be time‐consuming due to the need for both prolonged device preparation and maintenance as well as the duration of treatment. 12 Nebulized treprostinil should be administered four times per day, with each inhalation requiring up to 3 min per treatment session. To improve ease of use, a dry powder formulation of treprostinil is in development together with a reusable, breath‐powered dry powder inhaler (DPI; Tyvaso DPI™, United Therapeutics Corporation). Treprostinil inhalation powder (TreT) is supplied in single‐use cartridges. A single‐use cartridge is manually inserted into the inhaler and powder is discharged when the patient inhales. TreT contains fumaryl diketopiperazine, an inert excipient present in the US Food and Drug Administration's Inactive Ingredient Database that is approved as a carrier for inhaled formulations. 13 Fumaryl diketopiperazine can self‐assemble to form microparticles at a pH < 5 and rapidly dissolves in the neutral pH of the lungs. A Phase 1, single‐dose, ascending‐dose study (30–180 μg in 30‐μg increments) in six dose cohorts of healthy subjects confirmed that the treprostinil plasma concentrations and exposure achieved with TreT were clinically relevant and comparable to those observed with treprostinil inhalation solution in historical clinical studies; 150 μg was the maximally tolerated dose. 14
We conducted a study to assess the safety and tolerability of TreT as Tyvaso DPI™ in patients with PAH.
METHODS
BREEZE (NCT03950739) was a single‐sequence study in which patients with PAH on a stable regimen of treprostinil inhalation solution switched to a corresponding dose of TreT. The corresponding dose was based on pharmacokinetic (PK) modeling from single‐dose studies in healthy volunteers with the DPI and nebulized formulations. 14 The primary objective was to evaluate the safety and tolerability of TreT. AEs were captured if the event was new‐onset or worsened after the transition to TreT. Secondary objectives included evaluation of the systemic exposure and PK of treprostinil inhalation solution and TreT, 6MWD, device satisfaction and preference with the Preference Questionnaire for Inhaled Treprostinil Devices (PQ‐ITD), and the Pulmonary Arterial Hypertension‐Symptoms and Impact (PAH‐SYMPACT®) questionnaire. The PQ‐ITD is a questionnaire given to evaluate subject satisfaction with and preference for inhaled treprostinil devices. The questionnaire provides 12 different statements around inhaled device satisfaction and allows for 5 response options: strongly disagree, disagree, neutral, agree, and strongly agree. The PAH‐SYMPACT is a patient‐reported questionnaire given to assess PAH symptoms and impact on quality of life. The questionnaire includes four domains: cardiopulmonary symptoms, cardiovascular symptoms, physical impacts, and cognitive/emotional impacts. 15 , 16
After completing the 3‐week treatment phase of the BREEZE study, patients could participate in an optional extension phase (OEP) to assess the long‐term safety and tolerability of TreT.
Inclusion/exclusion criteria
The study protocol, protocol amendments, and informed consent forms were submitted for review and approval to each site's institutional review board or independent ethics committee. Eligible patients had to be adults (≥18 years) diagnosed with PAH (6th World Symposium on Pulmonary Hypertension group 1 PAH). 1 Patients also had to have started treprostinil inhalation solution ≥3 months before the baseline visit and had to be on a stable dosing regimen (i.e., no change in dose within 30 days of baseline visit, 6–12 breaths four times daily). Additionally, patients had to have evidence of forced expiratory volume in 1 s (FEV1) ≥60% and FEV1/forced vital capacity ratio ≥60% during the 6 months before enrollment. Candidates who were pregnant or lactating, were taking any other prostacyclin analogs or agonists, or had a history of uncontrolled sleep apnea, parenchymal lung disease, or hemodynamically significant left‐sided heart disease were excluded. If receiving approved PAH background therapy (i.e., endothelin receptor antagonist, phosphodiesterase type 5 inhibitor, or soluble guanylate cyclase stimulator), candidates must have been on a stable dose with no additions or discontinuations for a minimum of 30 days before the screening visit, and patients could not newly initiate or discontinue background therapy from the screening phase through the Week 3 visit. Patients lost to follow‐up could not complete the treatment phase or the OEP. Full inclusion and exclusion criteria are in Table S1.
Study design
The study was conducted in accordance with Good Clinical Practice guidelines and included a screening phase, a treatment phase, and the OEP. The screening visit was scheduled for 14 days before the baseline visit and after informed consent was obtained. Patients who satisfied all eligibility criteria during the screening phase returned to the clinic at baseline for enrollment. The treatment phase included two study visits to the clinic approximately 3 weeks apart. At the baseline visit, patients received a single dose of treprostinil inhalation solution in the clinic and underwent PK assessments 15 min before dose and for up to 5 h after dose (5, 10, 15, 30, 45, 90, 120, 180, 240, and 300 min), safety assessments, and a 6‐minute walk test (6MWT); PK timepoints at baseline with the nebulizer were the same as at Week 3 with the DPI. Following these assessments, patients who had been treated with treprostinil inhalation solution were assigned a corresponding dose of TreT (32, 48, or 64 μg) based on their current stable treprostinil inhalation solution dose (42 μg [6–7 breaths], 54–60 μg [9–10 breaths], or 66–72 μg [11–12 breaths]; Table S2). After receiving device training and the first dose in the clinic, each patient self‐administered TreT four times daily by oral inhalation for 3 weeks. Following 3 weeks of treatment, patients returned to the clinic, received a single dose of TreT, and underwent PK assessments at the same time points used for treprostinil inhalation solution at the baseline visit, safety assessments, and a 6MWT identical to that performed at the baseline visit. PQ‐ITD and PAH‐SYMPACT questionnaires were also administered at both study visits. Following the Week 3 visit, patients could continue receiving TreT by participating in the OEP. In the OEP, clinic visits were scheduled every 8 weeks and dosing titration was encouraged (Figure S1). The dose of TreT could be titrated upward, as clinically tolerated, to identify a maximal stable dose in each patient. If a patient did not elect to participate in the OEP, TreT was discontinued, and treprostinil inhalation solution therapy could be resumed. These patients were required to return to the clinic 2 weeks after TreT discontinuation for an end‐of‐study visit.
Outcomes
Safety assessments were based on established definitions of AEs and serious AEs (SAEs). All AEs were identified using the standard mechanisms of physical examinations including vital signs, laboratory assessments, electrocardiograms, and safety requirements of the investigational product. Efficacy assessments included 6MWD and PQ‐ITD and PAH‐SYMPACT questionnaires.
Statistical analyses
For this study, the total sample size was estimated to be 45 patients and was not based on power calculations. The planned sample size was selected to provide adequate data to assess the safety and tolerability of TreT in patients with PAH currently treated with treprostinil inhalation solution. The safety population was defined as all patients in the study who received ≥1 dose of TreT during the treatment phase. All PK analyses were performed on patients with sufficient data in the PK population. All assessments were summarized by descriptive statistics as appropriate. The results for patients completing up to 51 weeks of the treatment phase and OEP are reported here. Changes in 6MWD were assessed by paired t‐tests. For PQ‐ITD responses, Mantel‐Haenszel mean score statistics were computed to determine whether the distribution of assessments for each device was the same. Changes in the PAH‐SYMPACT questionnaire domain scores were assessed by paired t‐tests at Week 3 in the treatment phase and again at Week 11 in the OEP. Improved satisfaction with TreT was confirmed by the Mantel‐Haenszel mean score statistics. PK parameters of treprostinil (area under the concentration‐time curve time 0–5 h [AUC0–5], maximal drug concentration [C max], half‐life [t 1/2], and time of maximal plasma concentration [T max]) were obtained from the plasma drug concentration‐time data. All analyses were conducted using SAS Version 9.4 except PK parameters, which were calculated using noncompartmental methods employing Phoenix® WinNonlin® Version 8.1 (Certara USA, Inc.). p values ≤0.05 were considered significant, and no adjustments were made for multiplicity.
RESULTS
Patient baseline characteristics
Fifty‐one patients enrolled and transitioned from nebulized treprostinil to TreT (Figure 1). Baseline characteristics are summarized in Table 1. The majority of patients (57%) received a diagnosis of idiopathic/heritable PAH. The overall median time since PAH diagnosis at baseline was 7.82 years (range: 0.49–30.88 years), and most patients (61%) were World Health Organization functional Class II at study start. Overall, 98% (50 of 51) of patients were receiving ≥1 background PAH medication; 41 (80%) patients took two background PAH medications, 9 (18%) patients took one background PAH medication, and 1 (2%) patient took no background PAH medication.
Figure 1.

Patient disposition. AE, adverse event; OEP, optional extension phase; TreT, treprostinil inhalation powder.
Table 1.
Baseline demographics and disease characteristics
| Treprostinil inhaled powder (treatment phase) | |||||
|---|---|---|---|---|---|
| 32 μg | 48 μg | 64 μg | Overall | ||
| n = 2 | n = 27 | n = 22 | N = 51 | ||
| Age, years | |||||
| Mean (SD) | 48.0 (28.3) | 54.7 (13.1) | 58.0 (12.8) | 55.9 (13.4) | |
| Median | 48.0 | 55.0 | 59.5 | 57.0 | |
| Min, max | 28, 68 | 28, 81 | 23, 82 | 23, 82 | |
| Sex, n (%) | |||||
| Male | 0 | 5 (18.5) | 3 (13.6) | 8 (15.7) | |
| Female | 2 (100.0) | 22 (81.5) | 19 (86.4) | 43 (84.3) | |
| Ethnicity, n (%) | |||||
| Hispanic or Latino | 0 | 1 (3.7) | 3 (13.6) | 4 (7.8) | |
| Not Hispanic or Latino | 2 (100.0) | 26 (96.3) | 19 (86.4) | 47 (92.2) | |
| Race, n (%) | |||||
| White | 2 (100.0) | 23 (85.2) | 15 (68.2) | 40 (78.4) | |
| Black or African American | 0 | 4 (14.8) | 5 (22.7) | 9 (17.6) | |
| American Indian or Alaska Native | 0 | 0 | 1 (4.5) | 1 (2.0) | |
| Native Hawaiian or Other Pacific Islander | 0 | 0 | 0 | 0 | |
| Asian | 0 | 0 | 1 (4.5) | 1 (2.0) | |
| Baseline BMI, kg/m 2 | |||||
| Mean (SD) | 30.20 (11.03) | 27.89 (5.94) | 32.18 (6.91) | 29.87 (6.74) | |
| Median | 30.20 | 26.35 | 32.35 | 29.25 | |
| Min, max | 22.4, 38.0 | 18.9, 40.2 | 20.1, 47.7 | 18.9, 47.7 | |
| Time since PAH diagnosis, years | |||||
| N | 2 | 27 | 22 | 51 | |
| Mean (SD) | 5.68 (7.33) | 7.97 (7.17) | 9.83 (5.63) | 8.69 (6.51) | |
| Median | 5.68 | 6.09 | 9.31 | 7.82 | |
| Min, max | 0.49, 10.86 | 0.58, 30.88 | 2.04, 25.22 | 0.49, 30.88 | |
| Current PAH diagnosis, n (%) | |||||
| Idiopathic/familial | 1 (50.0) | 17 (63.0) | 11 (50.0) | 29 (56.9) | |
| Associated with unrepaired or repaired congenital systemic‐to‐pulmonary shunts | 0 | 2 (7.4) | 2 (9.1) | 4 (7.8) | |
| Associated with collagen vascular disease | 1 (50.0) | 6 (22.2) | 7 (31.8) | 14 (27.5) | |
| Associated with HIV | 0 | 0 | 1 (4.5) | 1 (2.0) | |
| Associated with appetite suppressant/other drug or toxin use | 0 | 2 (7.4) | 1 (4.5) | 3 (5.9) | |
| WHO functional class at screening, n (%) | |||||
| I | 1 (50.0) | 5 (18.5) | 0 | 6 (11.8) | |
| II | 1 (50.0) | 18 (66.7) | 12 (54.5) | 31 (60.8) | |
| III | 0 | 4 (14.8) | 10 (45.5) | 14 (27.5) | |
| Background PAH medications, n (%) | |||||
| Any background PAH medication | 2 (100.0) | 27 (100.0) | 21 (95.5) | 50 (98.0) | |
| ERA | 2 (100.0) | 22 (81.5) | 19 (86.4) | 43 (84.3) | |
| PDE5i | 1 (50.0) | 23 (85.2) | 17 (77.3) | 41 (80.4) | |
| sGC | 0 | 3 (11.1) | 4 (18.2) | 7 (13.7) | |
Abbreviations: BMI, body mass index; ERA, endothelin receptor antagonist; PAH, pulmonary arterial hypertension; PDE5i, phosphodiesterase‐5 inhibitor; sGC, soluble guanylate cyclase stimulator; WHO, World Health Organization.
Safety outcomes
During the treatment phase, 30 (59%) patients experienced new or worsened AEs following TreT treatment (Table 2). There were no study drug‐related SAEs. There was one event of colon cancer and one event of mechanical fall, but neither of these SAEs was considered to be treatment‐related. During the OEP, 39 (80%) patients experienced AEs following TreT treatment; 21 (43%) patients experienced ≥1 AE considered attributable to TreT. In the treatment phase, 1 of the 27 (4%) patients treated with 48 μg of TreT and 2 of the 22 (9%) patients treated with 64 μg of TreT experienced an AE leading to withdrawal. (Note: One subject completed the treatment phase but withdrew due to nausea during the OEP; the onset of nausea AE was during the treatment phase and is therefore summarized with the treatment phase data).
Table 2.
Summary of AEs
| Treatment phase | ||||||||
|---|---|---|---|---|---|---|---|---|
| Preferred term | 32 μg | 48 μg | 64 μg | Overall | ||||
| n = 2 | n = 27 | n = 22 | N = 51 | |||||
| n (%) | No. AEs (AE rate) | n (%) | No. AEs (AE rate) | n (%) | No. AEs (AE rate) | n (%) | No. AEs (AE rate) | |
| Any AE | 0 | 0 | 16 (59) | 40 (25.91) | 14 (64) | 30 (23.17) | 30 (59) | 70 (23.63) |
| Cough | 0 | 0 | 11 (41) | 11 (7.12) | 7 (32) | 7 (5.41) | 18 (35) | 18 (6.08) |
| Headache | 0 | 0 | 4 (15) | 4 (2.59) | 4 (18) | 4 (3.09) | 8 (16) | 8 (2.70) |
| Dyspnea | 0 | 0 | 2 (7) | 2 (1.30) | 2 (9) | 2 (1.54) | 4 (8) | 4 (1.35) |
| Nausea | 0 | 0 | 2 (7) | 2 (1.30) | 1 (5) | 1 (0.77) | 3 (6) | 3 (1.01) |
| Diarrhea | 0 | 0 | 0 | 0 | 2 (9) | 2 (1.54) | 2 (4) | 2 (0.68) |
| Flushing | 0 | 0 | 1 (4) | 1 (0.65) | 1 (5) | 1 (0.77) | 2 (4) | 2 (0.68) |
| Throat irritation | 0 | 0 | 1 (4) | 1 (0.65) | 1 (5) | 1 (0.77) | 2 (4) | 2 (0.68) |
| Optional extension phase | ||||||||
|---|---|---|---|---|---|---|---|---|
| All AEs | 32 μg | 48 μg | 64 μg | Overall | ||||
| n = 2 | n = 26 | n = 21 | N = 49 | |||||
| n (%) | No. AEs (AE rate) | n (%) | No. AEs (AE rate) | n (%) | No. AEs (AE rate) | n (%) | No. AEs (AE rate) | |
| Any AE | 1 (50) | 2 (1.04) | 20 (77) | 90 (3.80) | 18 (86) | 93 (5.34) | 39 (80) | 185 (4.30) |
| Cough | 0 | 0 | 4 (15) | 4 (0.17) | 3 (14) | 4 (0.23) | 7 (14) | 8 (0.19) |
| Dyspnea | 1 (50) | 1 (0.52) | 4 (15) | 4 (0.17) | 2 (10) | 2 (0.11) | 7 (14) | 7 (0.16) |
| Diarrhea | 0 | 0 | 1 (4) | 2 (0.08) | 4 (19) | 4 (0.23) | 5 (10) | 6 (0.14) |
| Dizziness | 0 | 0 | 4 (15) | 4 (0.17) | 1 (5) | 1 (0.06) | 5 (10) | 5 (0.12) |
| Headache | 0 | 0 | 2 (8) | 2 (0.08) | 2 (10) | 3 (0.17) | 4 (8) | 5 (0.12) |
| Arthralgia | 0 | 0 | 2 (8) | 2 (0.08) | 1 (5) | 2 (0.11) | 3 (6) | 4 (0.09) |
| Fatigue | 0 | 0 | 1 (4) | 1 (0.04) | 2 (10) | 4 (0.23) | 3 (6) | 5 (0.12) |
| Hypotension | 0 | 0 | 2 (8) | 2 (0.08) | 1 (5) | 1 (0.06) | 3 (6) | 3 (0.07) |
| Pneumonia | 0 | 0 | 2 (8) | 2 (0.08) | 1 (5) | 1 (0.06) | 3 (6) | 3 (0.07) |
Note: AE rate is calculated as the number of AEs divided by the total patient years within each group.
Abbreviation: AE, adverse event.
In the OEP, 1 of the 26 (4%) patients treated with 48 μg of TreT and 2 of the 21 (9%) patients treated with 64 μg of TreT experienced an AE leading to withdrawal. Cough (35%), headache (16%), and dyspnea (8%) were the most commonly reported AEs during the treatment phase; these events, as well as low incidences (≤5 events each) of arthralgia, diarrhea, dizziness, and pneumonia, most commonly occurred during the OEP. Twenty‐seven (53%) and 6 (12%) of the 51 patients in the treatment phase and 30 (61%) and 24 (49%) of the 49 patients in the OEP experienced mild or moderate AEs, respectively. Most of these events were considered attributable to TreT. Most patients in the OEP either maintained or increased their study treatment dose from baseline. Although 15 SAEs occurred during the OEP, all events were reported for single patients with the exception of pneumonia (two patients). In addition, none of the SAEs during the OEP was considered to be treatment‐related. No clinically relevant changes in vital signs, clinical laboratory parameters, or electrocardiogram parameters were observed over the course of the study.
Efficacy outcomes
The change from baseline in 6MWD with TreT overall demonstrated a statistically significant improvement (11.5‐m increase; p = 0.0217) at Week 3 (Table 3 and Figure 2). Beyond Week 3, the sample size decreases over time due to the timing of the data cut (i.e., subjects who enrolled toward the end of the study had not reached the later visits at the time of the data cut). For those patients with contributing data, improvements in 6MWD were sustained for patients in the OEP up to Week 51 (7.9 m at Week 11 and 26.4 m at Week 43; mean change from baseline: 24.4 m; range: 7.9–26.4 m).
Table 3.
Summary and analysis of 6MWD
| Visit week | 6MWD, mean (SD), m | Change from baseline, mean (SD) | p | Dose, median (range), μg |
|---|---|---|---|---|
| Baseline | 418.9 (109.4) | 48 (32, 64) | ||
| (n = 51) | (n = 51)a | |||
| 3 | 438.9 (110.5) | 11.5 (32.9) | 0.0217 | 48 (32, 80) |
| (n = 46) | (n = 49) | |||
| 11 | 416.1 (125.2) | 7.9 (45.5) | 0.3354 | 64 (0, 96) |
| (n = 32) | (n = 46) | |||
| 19 | 439.1 (112.3) | 7.8 (43.0) | 0.3036 | 64 (32, 112) |
| (n = 33) | (n = 43) | |||
| 27 | 446.5 (122.3) | 13.1 (54.7) | 0.1702 | 64 (32, 144) |
| (n = 34) | (n = 41) | |||
| 35 | 462.4 (122.6) | 17.3 (40.3) | 0.0518 | 64 (32, 160) |
| (n = 23) | (n = 28) | |||
| 43 | 476.7 (101.9) | 26.4 (53.7) | 0.0522 | 64 (48, 176) |
| (n = 18) | (n = 22) | |||
| 51 | 467.6 (120.5) | 30.1 (60.2) | 0.0563 | 64 (0, 176) |
| (n = 17) | (n = 21) |
Note: p value is from a paired t‐test to assess change from baseline in 6MWD.
Abbreviation: 6MWD, 6‐minute walk distance.
Note: “n” different from “n” for 6MWD except for baseline.
Figure 2.

Mean change from baseline in 6MWD (m) by visit: TreT overall. aRepresentative sample data were captured from study start through July 2021. Duration on therapy is dependent on the TreT start date, and the decreasing sample size over time reflects the results for those patients who completed 51 weeks of the treatment phase and OEP; it does not represent dropouts. Not all patients have had the opportunity to reach later time points out to 51 weeks at the time of data cut. The OEP is currently ongoing. 6MWD, 6‐minute walk distance; OEP, optional extension phase; TreT, treprostinil inhalation powder.
Overall, patient‐reported satisfaction with TreT was significantly improved at Week 3 compared with satisfaction with the treprostinil nebulizer at baseline (Table 4 and Figure 3). With the nebulizer at baseline, 31% of patients agreed/strongly agreed that they were satisfied and 45% of patients provided a neutral response. At Week 3, 96% (p < 0.0001) of patients agreed/strongly agreed that they were satisfied with the TreT inhaler. In addition, a notable shift from disagreement/strong disagreement to agreement/strong agreement for overall satisfaction with the TreT inhaler was observed from baseline to Week 3.
Table 4.
Summary of overall satisfaction with inhalation devices
| Question and response | Baseline: treprostinil nebulizer | Week 3: treprostinil dry powder inhaler | p |
|---|---|---|---|
| (N = 51) | (N = 46) | ||
| n (%) | n (%) | ||
| I like the size of the inhaler | <0.0001 | ||
| Strongly disagree | 20 (39.2) | 1 (2.2) | |
| Disagree | 12 (23.5) | 0 | |
| Neutral | 11 (21.6) | 0 | |
| Agree | 7 (13.7) | 5 (10.9) | |
| Strongly agree | 1 (2.0) | 40 (87.0) | |
| The inhaler is easy to travel with | <0.0001 | ||
| Strongly disagree | 20 (39.2) | 1 (2.2) | |
| Disagree | 14 (27.5) | 0 | |
| Neutral | 8 (15.7) | 1 (2.2) | |
| Agree | 8 (15.7) | 0 | |
| Strongly agree | 1 (2.0) | 44 (95.7) | |
| The inhaler is easy to hold | <0.0001 | ||
| Strongly disagree | 4 (7.8) | 0 | |
| Disagree | 5 (9.8) | 0 | |
| Neutral | 18 (35.3) | 1 (2.2) | |
| Agree | 20 (39.2) | 4 (8.7) | |
| Strongly agree | 4 (7.8) | 41 (89.1) | |
| The inhaler instructions are easy to follow | <0.0001 | ||
| Strongly disagree | 0 | 0 | |
| Disagree | 0 | 0 | |
| Neutral | 7 (13.7) | 1 (2.2) | |
| Agree | 30 (58.8) | 4 (8.7) | |
| Strongly agree | 14 (27.5) | 41 (89.1) | |
| The inhaler is easy to set up and prepare for use | <0.0001 | ||
| Strongly disagree | 0 | 0 | |
| Disagree | 7 (13.7) | 0 | |
| Neutral | 10 (19.6) | 1 (2.2) | |
| Agree | 27 (52.9) | 3 (6.5) | |
| Strongly agree | 7 (13.7) | 42 (91.3) | |
| The inhaler is easy to use | <0.0001 | ||
| Strongly disagree | 1 (2.0) | 0 | |
| Disagree | 1 (2.0) | 0 | |
| Neutral | 9 (17.6) | 0 | |
| Agree | 34 (66.7) | 4 (8.7) | |
| Strongly agree | 6 (11.8) | 42 (91.3) | |
| The inhaler cartridge is easy to load | <0.0001 | ||
| Strongly disagree | – | 0 | |
| Disagree | – | 0 | |
| Neutral | – | 1 (2.2) | |
| Agree | – | 7 (15.2) | |
| Strongly agree | – | 38 (82.6) | |
| The inhaler cartridge is easy to remove | <0.0001 | ||
| Strongly disagree | – | 0 | |
| Disagree | – | 0 | |
| Neutral | – | 1 (2.2) | |
| Agree | – | 2 (4.3) | |
| Strongly agree | – | 43 (93.5) | |
| I am satisfied with the number of daily breaths required | <0.0001 | ||
| Strongly disagree | 4 (7.8) | 0 | |
| Disagree | 15 (29.4) | 0 | |
| Neutral | 12 (23.5) | 2 (4.3) | |
| Agree | 14 (27.5) | 8 (17.4) | |
| Strongly agree | 6 (11.8) | 36 (78.3) | |
| I would recommend the inhaler to others | <0.0001 | ||
| Strongly disagree | 1 (2.0) | 0 | |
| Disagree | 6 (11.8) | 0 | |
| Neutral | 21 (41.2) | 1 (2.2) | |
| Agree | 19 (37.3) | 6 (13.0) | |
| Strongly agree | 4 (7.8) | 39 (84.8) | |
| Overall, I am satisfied with the inhaler | <0.0001 | ||
| Strongly disagree | 0 | 0 | |
| Disagree | 12 (23.5) | 0 | |
| Neutral | 23 (45.1) | 1 (2.2) | |
| Agree | 13 (25.5) | 5 (10.9) | |
| Strongly agree | 3 (5.9) | 40 (87.0) | |
| The inhaler stays clean | <0.0001 | ||
| Strongly disagree | – | 2 (4.3) | |
| Disagree | – | 0 | |
| Neutral | – | 4 (8.7) | |
| Agree | – | 5 (10.9) | |
| Strongly agree | – | 35 (76.1) | |
Note: –, not evaluated.
Figure 3.
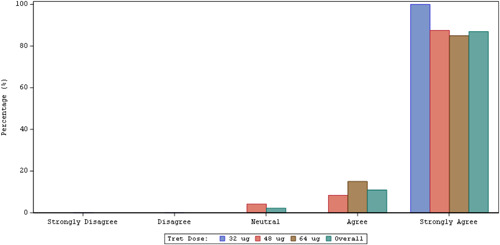
Summary of overall satisfaction with the TreT inhaler at Week 3.a aResponse to PQ‐ITD question “Overall, I am satisfied with the inhaler.” PQ‐ITD, Preference Questionnaire for Inhaled Treprostinil Devices; TreT, treprostinil inhalation powder.
The distribution of responses to all components of the PQ‐ITD showed a significant (p ≤ 0.0001) shift toward increased satisfaction with the TreT inhaler at Week 3 compared with the nebulizer at baseline.
Analysis of patient‐reported PAH‐SYMPACT data revealed that mean changes from baseline to Week 3 and to Week 11 were improved for all domain scores across all weeks (range: −0.04 to −0.21; Table 5). Significant improvements for physical impacts were observed at Week 3 (mean change from baseline: −0.14 at Week 3, p = 0.0438, and −0.21 at Week 11, p = 0.0429, and cognitive/emotional impacts (significant mean change from baseline: −0.17 at Week 3, p = 0.0048) were observed.
Table 5.
Summary and analysis of PAH‐SYMPACT questionnaire
| Visit week | No. of patients | Score, mean (SD) | Change from baseline, mean (SD) | p |
|---|---|---|---|---|
| Cardiopulmonary symptoms domain score | ||||
| Baseline | 51 | 0.81 (0.49) | ||
| 3 | 46 | 0.76 (0.45) | −0.05 (0.27) | 0.2451 |
| 11 | 37 | 0.82 (0.55) | −0.04 (0.36) | 0.4989 |
| Cardiovascular symptoms domain score | ||||
| Baseline | 51 | 0.32 (0.39) | ||
| 3 | 46 | 0.29 (0.36) | −0.06 (0.33) | 0.2492 |
| 11 | 37 | 0.30 (0.43) | −0.05 (0.40) | 0.4685 |
| Physical impacts domain score | ||||
| Baseline | 51 | 0.87 (0.64) | ||
| 3 | 46 | 0.75 (0.63) | −0.14 (0.46) | 0.0438 |
| 11 | 36 | 0.73 (0.59) | −0.21 (0.59) | 0.0429 |
| Cognitive/emotional impacts domain score | ||||
| Baseline | 51 | 0.66 (0.71) | ||
| 3 | 46 | 0.47 (0.56) | −0.17 (0.40) | 0.0048 |
| 11 | 36 | 0.49 (0.46) | −0.13 (0.51) | 0.1287 |
Note: p value is from a paired t‐test to assess change from baseline in domain scores.
Abbreviation: PAH‐SYMPACT, Pulmonary Arterial Hypertension‐Symptoms and Impact.
The mean treprostinil concentration versus time plot by dose‐matched treatment is shown in Figure 4. Both TreT and treprostinil inhalation solutions were absorbed rapidly, with median T max occurring ≤10min post‐dose for both the mid‐ (48 μg) and high‐dose (64 μg) treatments. Between‐subject variability for AUC and C max parameters were similar across dose levels within treatment (TreT or treprostinil inhalation solution); variability of these parameters was approximately two‐ to threefold lower for TreT compared with treprostinil inhalation solution.
Figure 4.

Mean treprostinil plasma concentration versus time plots by treatment (dose pooled). Mean plasma concentrations may be less than the lower limit of quantification due to imputation of below‐the‐limit‐of‐quantification samples to 0. Each breath of treprostinil inhaled solution is equivalent to 6 μg of treprostinil. Mean plots include patients who received both treprostinil inhaled solution 72 μg (n = 18) or treprostinil inhaled solution 66 μg (n = 1) and treprostinil inhaled powder 64 μg (n = 19).
When all treprostinil inhalation solution doses were pooled, geometric mean (geometric coefficient of variation [CV] %) for C max and AUC0–5 were 0.901 ng/ml (88%) and 0.833 h*ng/ml (78%), respectively. Median T max for the pooled treprostinil inhalation solution dose groups was 0.17 h (range: 0.08–0.50 h). Geometric CV% t 1/2 was 0.971 h (45%).
DISCUSSION
This open‐label, single‐sequence, multicenter study was designed to evaluate the safety and tolerability of TreT in patients with PAH who were being treated with treprostinil inhalation solution for ≥30 days before enrollment. Most AEs were mild to moderate in severity and occurred at severities and frequencies consistent with those seen in other studies of inhaled treprostinil. 10 , 11 , 17 , 18 The administration of a new formulation could explain patients experiencing new or worsened AEs; however, tolerability seemingly improves with time. 6MWD changes were captured to monitor for acute clinical deterioration. Following 3 weeks of TreT administration, patients who switched from treprostinil inhalation solution demonstrated improvements in 6MWD, significant satisfaction with and preference for the use of TreT, significant improvement in PAH impact scores, and a trend toward improvement in PAH symptom scores. Improvement in 6MWD was sustained through Week 51 of the long‐term OEP.
Prostanoid therapy has been a mainstay for the treatment of PAH for many years, and synthetic prostacyclin analogs are available for administration by intravenous, subcutaneous, oral, and inhaled routes. However, with the exception of the oral route, these routes of delivery and/or the associated delivery systems can be cumbersome for patients, creating an opportunity to enhance the usability of these delivery devices.
The effectiveness of inhaled drugs such as treprostinil for PAH depends primarily on the delivery system and the particle size. 19 In this study, pulmonary exposure to treprostinil dry powder administered via DPI was attained with fewer inhalations than required with treprostinil inhalation solution administered via nebulizer without any unexpected safety issues. Accordingly, the dry powder formulation may enhance the effectiveness of treprostinil in patients with PAH by increasing alveolar delivery. 20 , 21 , 22 , 23 This results in higher drug levels in the lungs for an administered dose compared with current inhaled therapies without some of the limitations of treprostinil delivered by other parenteral routes.
This innovative study confirmed the safety of TreT using a delivery device that is much more convenient, more portable, and easier to use for self‐administration. These features may have been responsible for improved compliance, better device use, and inhalation of the full dose due to more consistent breaths, resulting in 6MWD improvement and further improvement in patient satisfaction. In addition, these features might facilitate the introduction of inhaled treprostinil earlier in the clinical course of PAH for selected patients, which potentially could slow the characteristically progressive course of the disease.
The study also demonstrated the safety of increasing the overall dose of TreT beyond the current recommended dose of 9–12 breaths four times daily, potentially allowing for titration to higher dose levels (12 breaths) without resulting in prolonged treatment sessions. An analysis of specialty pharmacy data by Mandras et al. 24 identified significantly higher rates of drug persistence and survival over 3 years in patients who received higher doses of inhaled treprostinil. Data suggest that higher doses result in better outcomes, 24 , 25 , 26 , 27 with the potential to prolong the time on prostanoid inhalation therapy and reduce the need to transition to more complex treprostinil regimens. In addition, easier device storage and accessibility in hospital and clinic settings or during more strenuous activity may enhance compliance. The potential utility and impact of inhaled treprostinil powder in these settings should be studied in future clinical trials.
The time required for device preparation and maintenance as well as the need for multiple daily prolonged inhalations may adversely affect not only adherence and compliance but also quality of life. Quality of life among those with a chronic illness such as PAH is critically important to both patients and their healthcare providers. The patients who participated in this study already had PAH‐SYMPACT scores indicating that they were “doing well” with respect to their PAH care before transitioning from treprostinil inhalation solution to TreT. Accordingly, it is particularly notable that these scores improved in patients once the transition occurred, indicating that this device and the simplified dosing regimen could potentially increase compliance and create a pathway for prostanoid therapy earlier in the disease process.
Overall, this study represents a very exciting addition to the treatment paradigm for the PAH population. The ease of use, portability, and ability to titrate the dose with TreT should have a clinically significant, beneficial impact on patient compliance and persistence, quality of life, and, potentially, the disease process itself.
STUDY LIMITATIONS
This was an open‐label, unblinded study with short follow‐up and without a control group and was not designed to show improvement in efficacy. It was designed primarily to demonstrate parity, identify patients' preference and improvement in quality of life, and assess tolerability and safety. As a result, the significant improvements in 6MWD that were observed may be due to factors such as clinical trial participation and the associated increase in compliance and, therefore, should be interpreted accordingly. Moreover, changes in other clinical parameters such as changes in WHO functional class and NT‐proBNP were not assessed. In addition, there were fewer than 20 patients available for evaluation at the last two visits. Representative sample data were captured from study start through July 2021; duration of therapy is dependent on the TreT start date and reflects the results for subjects who completed up to 51 weeks of the treatment phase and OEP. The OEP is currently ongoing.
CONCLUSIONS
Transition from treprostinil inhalation solution to TreT was safe and well‐tolerated, and systemic exposure to treprostinil was comparable between the two formulations. Treatment with TreT resulted in statistically significant improvements in important clinical parameters (6MWD, PQ‐ITD, and PAH‐SYMPACT) among patients with PAH.
Prostacyclin therapy is often delayed in patients with PAH because of the complexity of administration and its associated, perceived quality‐of‐life issues. The results of this study indicate that prostacyclin in a convenient, tolerable formulation may increase its accessibility to more patients earlier in the course of their disease, thereby potentially improving long‐term outcomes.
CONFLICTS OF INTEREST
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.A.B. has received funding from Janssen, Bayer, and Actelion for research and honoraria from United Therapeutics Corporation, Bayer, Janssen, AstraZeneca, Intuitive, and Boehringer Ingelheim for lectures, presentations, or speakers' bureau. C.D.B. has no conflict of interest. C.D. is an employee of United Therapeutics Corporation. S.V.D. has no conflict of interest. M.S.E. reports ongoing industry‐sponsored research with Acceleron, Merck, and United Therapeutics Corporation. K.A.E. has received fees from United Therapeutics Corporation for serving on a speakers' bureau and advisory boards; from J&J Actelion for serving on advisory boards; and from Acceleron for consulting services; and institutional research funding from UT and J&J Actelion. M.R.F. has no conflict of interest. S.J. has received research funding from United Therapeutics Corporation and Bellerophon Therapeutics and honoraria from Actelion and Bayer for speakers programs. J.M. has received speaker fees from United Therapeutics Corporation. M.M. is an employee of United Therapeutics Corporation. J.M.J. has no conflict of interest. H.I.P. has received honoraria from Acceleron, United Therapeutics Corporation, and Actelion‐Janssen for serving on advisory boards. G.V.R. has no conflict of interest. R.R.J. has received fees from United Therapeutics Corporation, Bayer, and J&J Actelion for consulting, serving on a speakers' bureau, and research funding. S.S. has served as a consultant for United Therapeutics Corporation, Acceleron, Actelion, and Bayer Pharmaceuticals; as an advisor and speaker for United Therapeutics Corporation, Actelion, and Bayer; and as clinical trial site PI for United Therapeutics Corporation, Actelion, Merck, Liquidia Technologies, Altavant Sciences, and Gossamer Bio; and has received research grant support from ACCP CHEST and United Therapeutics Corporation. T.G.S. has served as an advisor for Bayer Pharmaceuticals and Liquidia Technologies and as clinical trial site PI for United Therapeutics Corporation, Actelion/J &J, Liquidia Technologies, Bayer Pharmaceuticals, and Regeneron Pharmaceuticals. S.M.S. has no conflict of interest. P.S. is an employee of United Therapeutics Corporation. L.A.S. has received consulting fees from Gossamer Bio and has served as clinical trial site PI for United Therapeutics Corporation, Gossamer Bio, INSMED, Actelion, Liquidia, Merck, and Altavant Sciences.
AUTHOR CONTRIBUTIONS
Abubakr A. Bajwa, Charles D. Burger, Sapna V. Desai, Michael S. Eggert, Karim A. El‐Kersh, Micah R. Fisher, Shilpa Johri, Joanna M. Joly, Jinesh Mehta, Harold I. Palevsky, Gautam V. Ramani, Ricardo Restrepo‐Jaramillo, Sandeep Sahay, Trushil G. Shah, and Leslie A. Spikes were study investigators and wrote and edited the manuscript. Chunqin Deng, Melissa Miceli, Shelley M. Shapiro, and Peter Smith reviewed the data and edited the manuscript. All authors read and approved the final article.
ETHICS STATEMENT
The ethics committees (e.g., institutional review boards) overseeing clinical research at each of the sites are responsible for ethical review of the protocol. Leslie A. Spikes, MD, accepts official responsibility for the overall integrity of the manuscript (including ethics, data handling, reporting of results, and study conduct).
Supporting information
Supporting information.
Supporting information.
Supporting information.
Supporting information.
ACKNOWLEDGMENT
Funding for this manuscript was provided by United Therapeutics Corporation.
Spikes LA, Bajwa AA, Burger CD, Desai SV, Eggert MS, El‐Kersh KA, Fisher MR, Johri S, Joly JM, Mehta J, Palevsky HI, Ramani GV, Restrepo‐Jaramillo R, Sahay S, Shah TG, Deng C, Miceli M, Miceli M, Smith P, Shapiro SM BREEZE: Open‐label clinical study to evaluate the safety and tolerability of treprostinil inhalation powder as Tyvaso DPI™ in patients with pulmonary arterial hypertension. Pulmonary Circulation. 2022;12:e12063. 10.1002/pul2.12063
REFERENCES
- 1. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rubin LJ. Primary pulmonary hypertension. New Engl J Med. 1997;336:111–7. [DOI] [PubMed] [Google Scholar]
- 3. Ivy DD. Prostacyclin in the intensive care setting. Pediatr Crit Care Med. 2010;11(2 Suppl):41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA. Prostanoids in health and disease. J Lipid Res. 2009;50(Suppl):423–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruan C‐H, Dixon RAF, Willerson JT, Ruan K‐H. Prostacyclin therapy for pulmonary arterial hypertension. Tex Heart Inst J. 2010;37:391–9. [PMC free article] [PubMed] [Google Scholar]
- 6. Phares KR, Weiser WE, Miller SP, Myers MA, Wade M. Stability and preservative effectiveness of treprostinil sodium after dilution in common intravenous diluents. Am J Health Syst Pharm. 2003;60:916–22. [DOI] [PubMed] [Google Scholar]
- 7. United Therapeutics Corporation . Remodulin (prescribing information). Research Triangle Park, NC: United Therapeutics Corporation; 2021. [Google Scholar]
- 8. Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Bourge RC, Keogh A, Oudiz R, Frost A, Blackburn SD, Crow JW, Rubin LJ. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double‐blind, randomized, placebo‐controlled trial. Am J Respir Crit Care Med. 2002;165:800–4. [DOI] [PubMed] [Google Scholar]
- 9. Tapson VF, Gomberg‐Maitland M, McLaughlin VV, Benza RL, Widlitz AC, Krichman A, Barst RJ. Safety and efficacy of IV treprostinil for pulmonary arterial hypertension: a prospective, multicenter, open‐label, 12‐week trial. Chest. 2006;129:683–8. [DOI] [PubMed] [Google Scholar]
- 10. United Therapeutics Corporation . Tyvaso (prescribing information). Research Triangle Park, NC: United Therapeutics Corporation; 2021. [Google Scholar]
- 11. McLaughlin VV, Benza RL, Rubin LJ, Channick RN, Voswinckel R, Tapson VF, Robbins IM, Olschewski H, Rubenfire M, Seeger W. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol. 2010;55:1915–22. [DOI] [PubMed] [Google Scholar]
- 12. Farber HW, Gin‐Sing W. Practical considerations for therapies targeting the prostacyclin pathway. Eur Respir Rev. 2016;25:418–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Q, Shen Y, Mi G, He D, Zhang Y, Xiong Y, Webster TJ, Tu J. Fumaryl diketopiperazine based effervescent microparticles to escape macrophage phagocytosis for enhanced treatment of pneumonia via pulmonary delivery. Biomaterials. 2020;228:119575. [DOI] [PubMed] [Google Scholar]
- 14. Smith P, Watkins C, Kraft K, Grant M. A phase 1, single‐center, open‐label, dose‐rising clinical trial to evaluate the pharmacokinetics, safety and tolerability of treprostinil inhalation powder (TreT) in healthy normal volunteers. Eur Respir J. 2019;54:PA4749. [Google Scholar]
- 15. Chin KM, Gomberg‐Maitland M, Channick RN, Cuttica MJ, Fischer A, Frantz RP, Hunsche E, Kleinman L, McConnell JW, McLaughlin VV, Miller CE, Zamanian RT, Zastrow MS, Badesch DB. Psychometric validation of the Pulmonary Arterial and Hypertension‐Symptoms and Impact (PAH‐SYMPACT) questionnaire. Results of the SYMPHONY trial. Chest. 2018;154:848–61. [DOI] [PubMed] [Google Scholar]
- 16. McCollister D, Shaffer S, Badesch DB, Filusch A, Hunsche E, Schüler R, Wiklund I, Peacock A, IRB information for the 5 clinical sites. Development of the Pulmonary Arterial Hypertension‐Symptoms and Impact (PAH‐SYMPACT®) questionnaire: a new patient‐reported outcome instrument for PAH. Respir Res. 2016;17:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roscigno R, Vaughn T, Anderson S, Wargin W, Hunt T, Hill NS. Pharmacokinetics and tolerability of LIQ861, a novel dry‐powder formulation of treprostinil. Pulm Circ. 2020;10:2045894020971509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Waxman A, Restrepo‐Jaramillo R, Thenappan T, Ravichandran A, Engel P, Bajwa A, Allen R, Feldman J, Argula R, Smith P, Rollins K, Deng C, Peterson L, Bell H, Tapson V, Nathan SD. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med. 2021;384:325–34. [DOI] [PubMed] [Google Scholar]
- 19. Parker DK, Shen S, Zheng J, Ivy DD, Crotwell DN, Hotz JC, DiBlasi RM. Inhaled treprostinil drug delivery during mechanical ventilation and spontaneous breathing using two different nebulizers. Pediatr Crit Care Med. 2017;18:e253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Channick RN, Voswinckel R, Rubin LJ. Inhaled treprostinil: a therapeutic review. Drug Des Devel Ther. 2012;6:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patton JS, Brain JD, Davies LA, Fiegel J, Gumbleton M, Kim KJ, Sakagami M, Vanbever R, Ehrhardt C. The particle has landed‐characterizing the fate of inhaled pharmaceuticals. J Aerosol Med Pulm Drug Deliv. 2010;23(Suppl 2):S71–87. [DOI] [PubMed] [Google Scholar]
- 22. Dolovich MB, Dhand R. Aerosol drug delivery: developments in device design and clinical use. Lancet. 2011;377:1032–45. [DOI] [PubMed] [Google Scholar]
- 23. Hill NS, Preston IR, Roberts KE. Inhaled therapies for pulmonary hypertension. Respir Care. 2015;60:794–805. [DOI] [PubMed] [Google Scholar]
- 24. Mandras S, Shapiro S, Shen E, Chen L, Rao Y, Nelsen A. Survival and drug persistence in patients with pulmonary hypertension receiving inhaled treprostinil at doses greater than 54 mcg (9 breaths) 4 times daily. Chest. 2019;156(Suppl):A283–84. [Google Scholar]
- 25. Ramani G, Cassady S, Shen E, Broderick M, Wasik A, Sui Q, Nelsen A. Novel dose–response analyses of treprostinil in pulmonary arterial hypertension and its effects on six‐minute walk distance and hospitalizations. Pulm Circ. 2020;10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benza RL, Gomberg‐Maitland M, Naeije R, Arneson CP, Lang IM. Prognostic factors associated with increased survival in patients with pulmonary arterial hypertension treated with subcutaneous treprostinil in randomized, placebo‐controlled trials. J Heart Lung Transplant. 2011;30:982–989. [DOI] [PubMed] [Google Scholar]
- 27. Preston IR, Farber HW. Impact of parenteral treprostinil dosing in pulmonary arterial hypertension. J Heart Lung Transplant. 2013;32(Suppl):64–5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Supporting information.


