Abstract
Pediatric patients with pulmonary arterial hypertension (PAH) are considered to be at risk for pulmonary hypertensive crisis (PHC) or even death during right heart catheterization (RHC). This retrospective study was designed to identify the risks and clinical characteristics associated with PHC in pediatric PAH patients. We included 163 consecutive procedures from 147 pediatric patients diagnosed with PAH who underwent diagnostic RHC in Beijing Anzhen Hospital between January 2007 and December 2020. The average patient age was 9.0 ± 4.7 years and 84 (51.5%) were females. Before RHC, over 20% of patients were in New York Heart Association (NYHA) class III–IV. Sedation or general intravenous anesthesia was used in 103 procedures (63.2%), with spontaneous breathing in 93.2%. PHC occurred in 19 patients (11.7%), 5 (3.1%) required cardiac compression, and 1 died (0.6%). Compared to patients without PHC, those who experienced PHC were more likely to be in NYHA class III–IV (p = 0.012) before RHC, require sedation (p = 0.011), had echocardiographic indices of higher peak tricuspid regurgitation velocity (p = 0.018), and right ventricle (RV) to left ventricle (LV) ratio (p < 0.001). Multivariate logistic regression for PHC identified the need for sedation and a higher RV/LV ratio as independent predictors. In conclusion, the risk of RHC remains significant in children with PAH, particularly in those with severe RV dilation who require sedation during cardiac catheterization. Comprehensive evaluation, close monitoring, and appropriate treatment before and during the procedure are essential for reducing mortality.
Keywords: cardiac catheterization, complications, pediatric, pulmonary arterial hypertension
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a progressive pulmonary vascular disease that presents as elevated mean pulmonary arterial pressure (PAP) and pulmonary vascular resistance (PVR), eventually leading to right heart failure and death. Compared to adults, children with PAH have a different distribution of etiologies, including a greater predominance of idiopathic PAH and PAH associated with congenital heart disease. Because of accurate diagnosis and advanced PAH therapies, the prognosis of pediatric PAH patients has improved over the past decades. 1 Nevertheless, children with PAH still face great challenges in early diagnosis, risk stratification, and efficient management. Right heart catheterization (RHC) plays an essential role in establishing the diagnosis of PAH and in assessing the severity of hemodynamic impairment and the response to medical management. 1 Due to the requirement for general anesthesia, pediatric patients with pulmonary hypertension are considered to be at significant risk of pulmonary hypertensive crisis (PHC), cardiac arrest, and mortality during RHC. 2 , 3 , 4 PHC consists of acute pulmonary vasoconstriction and secondary cardiopulmonary collapse triggered by multiple causes, which was often observed in patients after congenital heart surgery, or those undergoing cardiac or noncardiac procedures. 5 , 6 , 7 Previous investigations on PHC help to understand the hemodynamics and possible mechanism; however, less is known about the prevalence and severity of PHC in pediatric PAH patients undergoing RHC. This single‐center, retrospective study reviewed the clinical data of children diagnosed with PAH who underwent RHC to identify the PHC incidence and associated risk factors and management in pediatric PAH patients.
METHODS
Study population
This study retrospectively included pediatric patients younger than 18 years and diagnosed with PAH admitted for diagnostic cardiac catheterization in Beijing Anzhen Hospital between January 2007 and December 2020. PAH was defined as a mean PAP ≥ 25 mmHg, pulmonary artery wedge pressure ≤15 mmHg, and PVR index >3 wood units⋅m2 by RHC. 1 We excluded patients with significant cardiac shunts or other complex congenital heart diseases. Patients with clinical or imaging evidence of left heart disease, lung disease, and other types of pulmonary hypertension were also excluded from this study. This study protocol was approved by the institutional research committee of our hospital (2021080X). Informed consent for this retrospective study was not required.
Data collection
Demographic and clinical data were retrieved from each patient's electronic medical record. Past medical history, percutaneous oxygen saturation (SpO2), and laboratory values for brain natriuretic peptide (BNP) were collected before each RHC, as well as echocardiographic data including peak tricuspid regurgitation velocity (TRV), maximal main pulmonary artery and aortic diameter, biventricular size and function, and the presence of pericardial effusion. Invasive hemodynamic data were obtained by RHC in the cardiac catheterization laboratory, including PAP and invasive systemic blood pressure (SBP), right atrial pressure, cardiac output (estimated using the indirect Fick method), PVR, and PVR index and acute pulmonary vasoreactivity testing result (iloprost inhalation or supplemental oxygen at flow rate of 5 L/min with oxygen mask) if available; inhaled iloprost was administered at a dose of 20 µg, with the exception of children aged <3 years or those weighing <15 kg who received 10 µg. Arterial blood gas analysis was performed following femoral artery catheter insertion. Continuous percutaneous oxygen saturations and SBP by femoral arterial line during the procedure were recorded. All baseline hemodynamic data were acquired in room air and supplemental oxygen was used only to treat acute desaturation. Procedural data included procedure duration, types of anesthetics administered, airway management, and adverse events occurring during the procedure or before discharge home.
PHC was defined as a rapid decrease both in SBP (systolic pressure lower than 80 mmHg or an absolute decrease >20%) and SpO2 (lower than 90%), and acute increase to supra‐systemic PAP during RHC. Other complications such as hypotensive episodes related to vagal reactions or acute vasoreactivity testing were excluded if oxygen saturation and hemodynamics were unchanged. Transient desaturation due to respiratory insufficiency was also not considered as PHC. We obtained follow‐up data from in/out‐patient medical records or telephone interviews.
Statistical analysis
Statistical analyses were performed using SPSS Statistics (IBM Corporation) and R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria, 2016). Continuous variables were described as mean ± standard deviation or median (interquartile range) and categorical variables as number (percent). The Kolmogorov–Smirnov test was used to determine data distribution. Categorical variables were compared using the χ 2 test or Fisher's exact test and continuous variables using the Student's t test or Mann–Whitney U test. Univariable logistic regression was used to identify predictors of the PHC. Parameters associated with PHC on univariate analysis were included in a multivariate logistic regression model. Survival analysis was performed using the Kaplan–Meier method. A p value less than 0.05 was considered statistically significant.
RESULTS
Study population and clinical characteristic
We collected data from 163 consecutive procedural records in 147 patients, including initial diagnostic cardiac catheterization in 147 patients and 16 follow‐up assessments in 12 patients. Out of 147 patients, 91 (61.9%) patients were diagnosed with idiopathic/hereditary PAH, 51 (34.7%) had PAH related to congenital heart disease (closed defect in 40, small defect in 11), 3 patients were suspected of developing PAH secondary to chemotherapeutic agents or immunosuppression following bone marrow transplantation, and 2 patients had PAH associated with connective tissue disease. One patient had Down syndrome and one had Noonan syndrome. Clinical data before each RHC were obtained and presented (Table 1). The average patient age at RHC was 9.0 ± 4.7 years and 84 (51.5%) were females. At admission, 33 (20.2%) patients were highly symptomatic (New York Heart Association [NYHA] functional class III or IV). BNP was available in 149 patients and was significantly increased (>300 pg/ml; upper limit of normal 100 pg/ml in 29.5% and mildly increased 100–300 pg/ml in 20.8%). Before catheterization, intravenous milrinone was given continuously in 56 (34.4%) patients, 28.6% of whom were in NYHA class III or IV. Ninety‐two patients (56.4%) received PAH therapies including oral calcium channel blocker (n = 8), phosphodiesterase type 5 (PDE‐5) inhibitor (n = 13), endothelin receptor antagonist (ERA) (n = 40), oral beraprost sodium (n = 3), and combination of ERA, PDE‐5 inhibitor or beraprost sodium (n = 24), combined with continuous intravenous treprostinil (n = 4).
Table 1.
Demographic and clinical characteristics of patients with and without pulmonary hypertensive crisis
| Characteristics | All (n = 163) | Patients with PHC (n = 19) | Patients without PHC (n = 144) | p Value |
|---|---|---|---|---|
| Age (year) | 9.0 ± 4.7 | 6.6 ± 3.7 | 9.3 ± 4.7 | 0.019 |
| Female, n (%) | 84 (51.5) | 9 (47.4) | 75 (52.1) | 0.699 |
| Weight (kg) | 27 (17,45) | 19 (16,24) | 30 (18,46) | 0.011 |
| PAH classification, n (%) | 0.108 | |||
| Idiopathic/hereditary PAH | 102 (62.6) | 16 (84.2) | 86 (59.7) | |
| PAH‐small defect | 13 (8.0) | 2 (10.5) | 11 (7.6) | |
| PAH‐closed defect | 43 (26.4) | 1 (5.3) | 42 (29.2) | |
| PAH‐CTD | 2 (1.2) | 0 (0) | 2 (1.4) | |
| PAH‐drug | 3 (1.8) | 0 (0) | 3 (2.1) | |
| Base surface area (m2) | 1.07 ± 0.39 | 0.86 ± 0.27 | 1.10 ± 0.39 | 0.002 |
| NYHA functional class III–IV, n (%) | 33 (22.2) | 8 (42.1) | 25 (17.4) | 0.012 |
| Recurrent syncope, n (%) | 46 (28.7) | 9 (47.4) | 37 (26.2) | 0.056 |
| History of RHF, n (%) | 15 (9.4) | 3 (15.8) | 12 (8.6) | 0.312 |
| PAH treatment before RHC, n (%) | 92 (56.4) | 12 (63.2) | 80 (55.6) | 0.530 |
| IV milrinone before RHC, n (%) | 56 (34.4) | 7 (36.8) | 49 (34.0) | 0.808 |
| Serum BNP (pg/ml)a | 101 (32,459) | 594 (65,1708) | 87 (27,310) | 0.010 |
| Echocardiography | ||||
| RV/LV diameter ratio | 0.76 ± 0.32 | 1.12 ± 0.36 | 0.71 ± 0.28 | <0.001 |
| PA/AO diameter ratio | 1.34 ± 0.25 | 1.51 ± 0.28 | 1.31 ± 0.24 | 0.002 |
| Peak TRV (m/s) | 4.2 ± 0.7 | 4.6 ± 0.7 | 4.2 ± 0.7 | 0.018 |
| Existence of pericardial effusion, n (%) | 20 (12.3) | 5 (26.3) | 15 (10.4) | 0.047 |
| Baseline hemodynamicsb | ||||
| Right atrial pressure (mmHg) | 8 (6,11) | 9 (7,16) | 8 (6,11) | 0.085 |
| Supra‐systemic PAP, n (%) | 57 (36.1) | 12 (85.7) | 45 (31.2) | <0.001 |
| SvO2 (%) | 68.6 ± 6.8 | 63.8 ± 6.1 | 69.0 ± 6.7 | 0.006 |
| PVR index (WU⋅m2) | 16.5 (10.3,21.8) | 24.4 (20.0,30.7) | 15.6 (10.1,20.9) | <0.001 |
| Positive response at AVT, n (%) | 35 (24.8) | 1 (10.0) | 34 (26.0) | 0.260 |
| Arterial pHc | 7.33 ± 0.05 | 7.28 ± 0.08 | 7.34 ± 0.04 | <0.001 |
| PaCO2 (mmHg) | 42 (39,44) | 44 (40,50) | 42 (39,44) | 0.008 |
| Procedure duration (min) | 80 (60,100) | 90 (60,120) | 78 (60,100) | 0.156 |
| Sedation or general anesthesia, n (%) | 103 (63.2) | 17 (89.5) | 86 (59.7) | 0.011 |
| Anesthetic drugs, n (%) | ||||
| KET | 51 (49.5) | 8 (47.1) | 43 (50.0) | 0.825 |
| Midazolam | 13 (12.6) | 3 (17.6) | 10 (11.6) | 0.495 |
| Propofol | 86 (83.5) | 14 (82.4) | 72 (83.7) | 0.890 |
| Dexmedetomidine | 29 (28.2) | 5 (29.4) | 24 (27.9) | 0.900 |
| Opioids | 25 (24.3) | 4 (23.5) | 21 (24.4) | 0.938 |
| Airway management in sedation, n (%) | ||||
| Natural unaided airway | 96 (93.2) | 14 (82.4) | 82 (95.3) | 0.052 |
| LMA | 3 (2.9) | 0 (0) | 3 (3.5) | 0.434 |
| Mechanical ventilation | 4 (3.9) | 3 (17.6) | 1 (1.2) | 0.001 |
| Unplanned intubation, n (%) | 3 (1.8) | 3 (15.8) | 0 (0) | <0.001 |
| Cardiac compressions, n (%) | 5 (3.1) | 5 (26.3) | 0 (0) | <0.001 |
| In‐hospital mortality, n (%) | 1 (0.6) | 1 (5.3) | 0 (0) | 0.006 |
Abbreviations: AO, aortic artery; AVT, acute vasoreactivity testing; BNP, B‐type natriuretic peptide; CTD, connective tissue disease; IV, intravenous; KET, ketamine; LMA, laryngeal mask airway; NYHA, New York Heart Association; PaCO2, partial pressure of carbon dioxide; PAH, pulmonary arterial hypertension; PAP, pulmonary artery pressure; PHC, pulmonary hypertensive crisis; PVR, pulmonary vascular resistance; RHC, right heart catheterization; RHF, right heart failure; RV, right ventricle; SvO2, mixed venous oxygen saturation; TRV, tricuspid regurgitation velocity.
BNP with normal range <100 pg/ml.
Baseline hemodynamics was available in 158 procedures, and acute vasoreactivity testing was performed in 141 procedures (iloprost inhalation in 136, oxygen in 10).
Arterial blood gas analysis is available in 128 procedures.
Procedural sedation or general intravenous anesthesia were performed in 103 patients (63.2%) by a pediatric cardiac anesthesiologist, while other procedures were under local anesthesia. Patients who need sedation were younger than those without any sedation (5.9 years, range 0.9–14.9 vs. 14.0 years, range 6.8–18.0, p < 0.001), and 95.1% of the sedated children were under 12 years old. Intravenous or intramuscular ketamine (n = 51), intravenous propofol (n = 86), dexmedetomidine (n = 29), and midazolam (n = 13) were used for anesthesia induction and maintenance; the most common anesthetic protocols were propofol alone (n = 26, 25.2%), propofol combined with ketamine (n = 37, 35.9%), or propofol with dexmedetomidine (n = 18, 17.5%). Opioids (fentanyl or sufentanil) were administered to 25 patients (24.3%). Among them, 96 procedures (93.2%) were performed under spontaneous breathing; laryngeal mask airway was required in three procedures. The average arterial pH was 7.31 ± 0.05, partial pressure of carbon dioxide (PaCO2) was 43 (41–46) mmHg, and 27 patients (26%) had PaCO2 above 45 mmHg. Intubation was performed in four procedures including unplanned intubation in three with PHC. A complete diagnostic cardiac catheterization was completed (158/163, 96.9%) of procedures and included pulmonary artery angiography (143/163, 87.7%) and acute vasoreactivity testing (141/163, 86.5%), respectively.
Characteristic and outcome of PHC
PHC was identified in 19 patients with an incidence rate of 11.7%. Eighteen PHCs occurred in the cardiac catheterization laboratory, 4 of them occurred after anesthesia induction, 12 during catheter manipulation, and 2 after pulmonary angiography. One patient had a late‐onset PHC 3 h following the completion of the procedure. Sixteen PHC events occurred in patients who required sedation or general anesthesia during catheterization. Most commonly, a rapid drop in peripheral oxygen saturations (n = 18, 94.7%) abruptly decreased SBP below 70 mmHg (n = 15, 78.9%), and tachycardia (n = 10, 52.6%) were the first warning signs of PHC. Central venous pressure significantly increased above 20 mmHg in 8 patients (42.1%), and elevated supra‐systemic PAP was recorded in 13 patients (68.4%). PAP was not obtained in six patients at the time of onset, PHC was identified when they presented desaturation and critical hypotension (lower than 70 mmHg), as well as highly increased peak TRV (over 4.2 m/s in six patients), and overload of the right ventricle (RV) was detected by transthoracic echocardiography. Two patients (10.5%) had significant ST‐T changes, and new‐onset or deepened right branch bundle blocks were observed in four patients (21.1%). Immediate initiation of high flow oxygen therapy and iloprost inhalation prevented 21.1% of patients from deteriorating. Ten patients (52.6%) received vasoactive agents (dopamine, dobutamine or epinephrine, norepinephrine) to maintain proper SBP. Emergency use of intravenous treprostinil was attempted in four patients. Fourteen patients received acidosis correction. Five (26.3%) required external cardiac compression and 3 (15.8%) needed emergent intubation. One patient received cardiac pacing for advanced atrioventricular block. One patient with hereditary PAH died at 76 h following the procedure. This patient had been identified to have a BMPR2 mutation, had significantly elevated BNP (1774 pg/ml) before RHC, and at the time of this patient's catheterization intravenous prostacyclin was unavailable in China. The total mortality rate associated with PHC was 0.6% in this study.
After being discharged from the hospital, 117 patients were followed up for a medium period of 23 (9–42) months from the first diagnostic RHC, and 21 patients (17.9%) died. Using the Kaplan–Meier method to evaluate survival, patients without PHC had a better survival rate (96.7% at 1 year) than patients with PHC (55.6% at 1 year, log‐rank χ 2 = 23.38, p < 0.001) (Figure 1).
Figure 1.
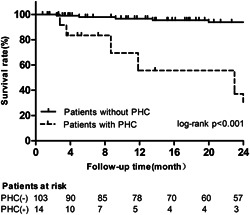
The overall survival rate in patients with and without pulmonary hypertensive crisis during follow‐up. PHC, pulmonary hypertensive crisis
Risk factors for PHC
Patients with PHC were more likely to be younger (6.6 ± 3.7 vs. 9.3 ± 4.7 years, p = 0.019) and require some form of sedation (89.5% vs. 59.7%, p = 0.011) than those without PHC. After adjusting for age, sedation remained significantly associated with PHC (odds ratio [OR]: 5.733; 95% confidence interval [CI]: 1.276–25.757; p = 0.023). There was no significant difference in the underlying diagnosis between patients with and without PHC (p = 0.108). PHC was more likely to occur in idiopathic/hereditary PAH (15.7%) compared to postoperative PAH (2.3%, p = 0.022), but idiopathic/hereditary PAH patients were younger (8.4 ± 4.5 vs. 11.5 ± 4.3, p < 0.001) and more likely to require sedation (79.8% vs. 20.2%, p = 0.002). One patient experienced PHC out of 16 follow‐up RHC procedures, which was similar to the PHC risk at initial RHC (6.25% vs. 12.2%, p = 0.696). The percentage of PAH‐specific therapy before RHC was similar between patients with and without PHC (63.2% vs. 55.6%, p = 0.530). Considering the differences in disease severity and background times, a subgroup analysis was conducted on 33 patients in NYHA functional class III–IV. In more severe patients, we discovered that patients on whom RHC was performed more recently (2016–2020) were more likely to receive PAH therapy (65.2% vs. 20.0%, p = 0.026) and had a lower incidence rate of PHC (13.0% vs. 50.0%, p = 0.036) than patients on whom RHC was performed in 2007–2015.
Patients in whom PHC occurred were more likely to be in NYHA class III and IV (42.1% vs. 17.4%, p = 0.012), have higher peak TRV (4.6 ± 0.7 vs. 4.2 ± 0.7 m/s, p = 0.018) and greater RV to left ventricle (LV) ratio (1.12 ± 0.36 vs. 0.71 ± 0.28, p < 0.001). These three factors and need for sedation were entered into a multivariate logistic regression model and the need for sedation and higher ratio of RV to LV were identified as independent predictors for PHC (Table 2).
Table 2.
Results of univariable logistic regression for pulmonary hypertensive crisis
| Predictors | Odds ratio | 95% Confidence interval | p Value |
|---|---|---|---|
| Univariate predictor | |||
| NYHA functional class III–IV | 3.462 | 1.264–9.582 | 0.016 |
| Sedation | 5.733 | 1.276–25.757 | 0.023 |
| The ratio of RV/LV | 32.031 | 6.350–161.579 | <0.001 |
| Peak TRV | 2.355 | 1.139–4.869 | 0.021 |
| Multivariate predictor | |||
| Sedation | 17.433 | 1.923–158.5037 | 0.011 |
| The ratio of RV/LV | 43.378 | 6.696–281.021 | <0.001 |
Abbreviations: LV, left ventricle; NYHA, New York Heart Association; RV, right ventricle; TRV, tricuspid regurgitation velocity.
DISCUSSION
In this retrospective, single‐center study, the incidence of PHC (11.7%), need for cardiac compression (3.1%), and mortality (0.6%) highlight the clinical vulnerability of the pediatric PAH population receiving RHC. The risk of cardiac arrest during RHC in children with pulmonary hypertension has been reported with estimates between 0.8% and 5.7%. 3 , 4 , 8 , 9 Despite the progress in modern PAH management and monitoring, the risks of RHC remain high in children with PAH, which suggested a careful preprocedural evaluation at an experienced center.
In this study, over 22% of patients were in NYHA class III and IV, and 36% had a baseline supra‐systemic PAP, which has been reported as a significant predictor of cardiac arrest and PHC. 4 A total of 56.4% of patients were receiving baseline PAH therapies. It is generally accepted that the diagnosis of PAH should be established by RHC before initiating advanced PAH therapies. 1 In clinical practice, the initiation of PAH therapies before RHC is considered feasible and effective, especially in high‐risk patients. According to our experience, patients with NYHA class III and IV and elevated BNP over 500 pg/ml are likely to have a greater risk during RHC and should receive PAH treatment before RHC with careful observation. We observed a decreased incidence of PHC in patients with NYHA class III and IV over past 5 years, which is likely due to the increasing use of advanced PAH therapies in this patient population. Before RHC, over one‐third of patients in this study received continuous use of milrinone (87.5% were performed after 2018 for patients in NYHA class III and IV). Milrinone has been reported to have potential benefits in improving pulmonary hemodynamics and right ventricular dysfunction in persistent pulmonary hypertension of newborns and children undergoing congenital heart surgery, but its efficacy in PAH needs more study. 10
PHC is more frequent in patients who require sedation. In a retrospective study of PAH patients who underwent anesthesia for cardiac catheterizations, Carmosino et al. 4 reported an incidence rate of 2.1% for PHC and a mortality rate of 1.4%. In comparison, 17 PHCs (16.5%) and 1 death (1.0%) occurred in our patients who need sedation, representing a higher incidence of PHC and a similar mortality rate. Specifically, 93% of patients in our group were under spontaneous breathing, and significant increases in PaCO2 (>45 mmHg) were observed in about one‐fourth of the sedated patients. Insufficient respiratory effort or ventilation might be a trigger for the onset of PHC. 11 Elective intubation would be a more favorable option in patients anticipated to undergo procedures with longer duration or high‐risk interventions albeit positive pressure ventilation might impair cardiac filling and output, especially in patients with RV dysfunction. 2 More experience about anesthetic goals and airway management requires further investigation.
Previous authors have reported that RV to LV diameter ratio, which incorporates both pathological septal shift and RV dilation, is associated with the adverse events in pediatric PAH. 12 , 13 In our study, a higher RV/LV was a statistically significant risk factor for PHC. Severe RV dilation and secondary LV impairment play a fundamental role in the onset and progression stages of PHC (Figure 2), while transthoracic echocardiography can be used to evaluate the effectiveness of emergency treatment.
Figure 2.
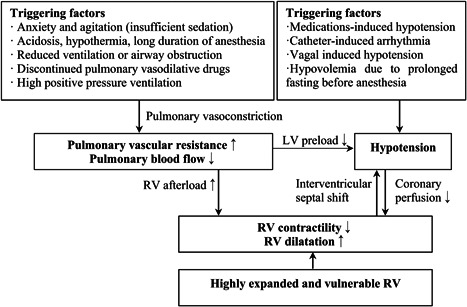
Pulmonary hypertensive crisis triggering factors and mechanisms of hemodynamic collapse. LV, left ventricle; RV, right ventricle
Most of the PHCs occurred during anesthesia induction and catheter manipulation, caused by the many triggering factors and acute pulmonary vasoconstriction (Figure 2). 14 Two PHCs occurred after pulmonary angiography, which has been considered to be dangerous in PAH. However, over 87% of patients in this study received angiography and most angiograms were well tolerated. One case occurred after returning to the ward, indicating PHC risks persist during weaning from sedation, so prolonged intensive care and monitoring might be necessary for high‐risk patients. In addition to the avoidance of triggering stimuli, the risk of cardiac arrest and death can be reduced by timely identification and treatment as PHC occurs. The drop in percutaneous oxygen saturations, low blood pressure, and tachycardia were the most common first sign; significantly increased central venous pressure was a sign of RV decompensation and highly suggests a PHC. Two patients had significant ST‐T changes and new‐onset or deepened right branch bundle blocks, which implies increased RV load and wall tension were found in four patients. On the contrary, the recovery of ST‐T changes or resolved right bundle branch block after treatment indicates remission. Vasoactive agents, such as dopamine, epinephrine, or norepinephrine, can improve coronary perfusion and cardiac function, and help to maintain a normal SBP. 15 , 16 Four patients with immediate reversal of supra‐systemic PAP by high flow oxygen therapy and iloprost inhalation were observed in our cohort. The prostacyclin analog iloprost is a widely used agent in testing pulmonary vascular reactivity during cardiac catheterization. It can be simply delivered in nonintubated patients and could replace nitric oxide in areas where nitric oxide is not available. 17 The clinical use of intravenous treprostinil or phosphodiesterase‐5 inhibitor sildenafil may be an additional emergent treatment option. 18 , 19 When PHC was difficult to treat and hemodynamics continued to deteriorate, atrial septostomy or the use of extracorporeal membrane oxygenation may be necessary.
Our study has several limitations. As a single‐center, retrospective study, there may be selection bias, and some patients who were considered to be at high risk for PHC did not undergo RHC. The long inclusion time, different anesthesia regimens, and increasing availability of multiple advanced PAH therapies also make the determination of the individual patient's risk of PHC difficult to predict. Finally, the incidence of PHC was low, limiting the number of adjustment variables possible in the multivariate analysis for independent risk factors.
CONCLUSION
Despite substantial progress in modern PAH management and monitoring and supportive techniques during cardiac catheterization, the risk of RHC remains significant in children with PAH, especially those with severe RV dilation and who require sedation. Careful preprocedural evaluation, prevention of PHC triggers, timely identification, and treatment are essential for reducing mortality. Further investigation about the impact of sedation and airway management may provide stronger appreciation and therapeutic strategies to the condition.
AUTHOR CONTRIBUTIONS
Qiangqiang Li designed the study, performed data analyses, and drafted the manuscript. Chen Zhang and Rong Wang carried out the study. Bradley B. Keller and Hong Gu contributed to data interpretation and manuscript writing. Hong Gu takes responsibility for the content of the manuscript. All authors read and approved the final manuscript.
ETHICS STATEMENT
This study has been approved by the institutional research committee, who permitted the collection of data for audit and research purposes (2021080X).
ACKNOWLEDGMENT
This study was supported by the National Natural Science Foundation of China (82070243).
Li Q, Zhang C, Wang R, Keller BB, Gu H. Pulmonary hypertensive crisis in children with pulmonary arterial hypertension undergoing cardiac catheterization. Pulm Circ. 2022;12:e12067. 10.1002/pul2.12067
DATA AVAILABILITY STATEMENT
Data are available on request from the authors.
REFERENCES
- 1. Hansmann G, Koestenberger M, Alastalo TP, Apitz C, Austin ED, Bonnet D, Budts W, D'Alto M, Gatzoulis MA, Hasan BS, Kozlik‐Feldmann R, Kumar RK, Lammers AE, Latus H, Michel‐Behnke I, Miera O, Morrell NW, Pieles G, Quandt D, Sallmon H, Schranz D, Tran‐Lundmark K, Tulloh R, Warnecke G, Wåhlander H, Weber SC, Zartner P. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. 2019;38(9):879–901. [DOI] [PubMed] [Google Scholar]
- 2. Twite MD, Friesen RH. The anesthetic management of children with pulmonary hypertension in the cardiac catheterization laboratory. Anesthesiol Clin. 2014;32(1):157–73. [DOI] [PubMed] [Google Scholar]
- 3. O'Byrne ML, Glatz AC, Hanna BD, Shinohara RT, Gillespie MJ, Dori Y, Rome JJ, Kawut SM. Predictors of catastrophic adverse outcomes in children with pulmonary hypertension undergoing cardiac catheterization: a multi‐institutional analysis from the pediatric health information systems database. J Am Coll Cardiol. 2015;66(11):1261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carmosino MJ, Friesen RH, Doran A, Ivy DD. Perioperative complications in children with pulmonary hypertension undergoing noncardiac surgery or cardiac catheterization. Anesth Analg. 2007;104(3):521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hopkins RA, Bull C, Haworth SG, de Leval MR, Stark J. Pulmonary hypertensive crises following surgery for congenital heart defects in young children. Eur J Cardiothorac Surg. 1991;5(12):628–34. [DOI] [PubMed] [Google Scholar]
- 6. Bernier ML, Romer LH, Bembea MM. Spectrum of current management of pediatric pulmonary hypertensive crisis. Crit Care Explor. 2019;1(8):e0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dean T, Kaushik N, Williams S, Zinter M, Kim P. Cardiac arrest and pulmonary hypertension in scurvy: a case report. Pulm Circ. 2019;9(1):2045894018812052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taylor CJ, Derrick G, McEwan A, Haworth SG, Sury MR. Risk of cardiac catheterization under anaesthesia in children with pulmonary hypertension. Br J Anaesth. 2007;98(5):657–61. [DOI] [PubMed] [Google Scholar]
- 9. Williams GD, Maan H, Ramamoorthy C, Kamra K, Bratton SL, Bair E, Kuan CC, Hammer GB, Feinstein JA. Perioperative complications in children with pulmonary hypertension undergoing general anesthesia with ketamine. Paediatr Anaesth. 2010;20(1):28–37. [DOI] [PubMed] [Google Scholar]
- 10. Loomba RS, Dorsey V, Villarreal EG, Flores S. The effect of milrinone on hemodynamic and gas exchange parameters in children. Cardiol Young. 2020;30(1):55–61. [DOI] [PubMed] [Google Scholar]
- 11. Friesen RH, Alswang M. Changes in carbon dioxide tension and oxygen saturation during deep sedation for paediatric cardiac catheterization. Pediatr Anesth. 1996;6(1):15–20. [DOI] [PubMed] [Google Scholar]
- 12. Jone PN, Hinzman J, Wagner BD, Ivy DD, Younoszai A. Right ventricular to left ventricular diameter ratio at end‐systole in evaluating outcomes in children with pulmonary hypertension. J Am Soc Echocardiogr. 2014;27(2):172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hopkins WE. Right ventricular performance in congenital heart disease: a physiologic and pathophysiologic perspective. Cardiol Clin. 2012;30(2):205–18. [DOI] [PubMed] [Google Scholar]
- 14. Kulik TJ. Pathophysiology of acute pulmonary vasoconstriction. Pediatr Crit Care Med. 2010;11(2, Suppl):S10–4. [DOI] [PubMed] [Google Scholar]
- 15. Salehi A. Pulmonary hypertension: a review of pathophysiology and anesthetic management. Am J Ther. 2012;19(5):377–83. [DOI] [PubMed] [Google Scholar]
- 16. Blaise G, Langleben D, Hubert B. Pulmonary arterial hypertension‐pathophysiology and anesthetic approach. Anesthesiology. 2003;99(6):1415–32. [DOI] [PubMed] [Google Scholar]
- 17. Li Q, Dimopoulos K, Zhang C, Zhu Y, Liu Q, Gu H. Acute effect of inhaled iloprost in children with pulmonary arterial hypertension associated with simple congenital heart defects. Pediatr Cardiol. 2018;39(4):757–62. [DOI] [PubMed] [Google Scholar]
- 18. Maxted AP, Hill A, Davies P. Oral sildenafil as a rescue therapy in presumed acute pulmonary hypertensive crisis. Pediatrics. 2013;131(2):e626–8. [DOI] [PubMed] [Google Scholar]
- 19. Hall K, Ogawa M, Sakarovitch C, Hopper RK, Adamson GT, Hanna B, Ivy DD, Miller‐Reed K, Yung D, McCarthy E, Siehr‐Handler SL, Feinstein JA. Subcutaneous and intravenous treprostinil pharmacokinetics in children with pulmonary vascular disease. J Cardiovasc Pharmacol. 2019;73(6):383–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request from the authors.


