Abstract
Dual combination therapy with a phosphodiesterase‐5 inhibitor (PDE5i) and endothelin receptor antagonist is recommended for most patients with intermediate‐risk pulmonary arterial hypertension (PAH). The RESPITE and REPLACE studies suggest that switching from a PDE5i to a soluble guanylate cyclase (sGC) activator may provide clinical improvement in this situation. The optimal approach to escalation or transition of therapy in this or other scenarios is not well defined. We developed an expert consensus statement on the transition to sGC and other treatment escalations and transitions in PAH using a modified Delphi process. The Delphi process used a panel of 20 physicians with expertise in PAH. Panelists answered three questionnaires on the management of treatment escalations and transitions in PAH. The initial questionnaire included open‐ended questions. Later questionnaires consolidated the responses into statements that panelists rated on a Likert scale from −5 (strongly disagree) to +5 (strongly agree) to determine consensus. The Delphi process produced several consensus recommendations. Escalation should be considered for patients who are at high risk or not achieving treatment goals, by adding an agent from a new class, switching from oral to parenteral prostacyclins, or increasing the dose. Switching to a new class or within a class should be considered if tolerability or other considerations unrelated to efficacy are affecting adherence. Switching from a PDE5i to an SGC activator may benefit patients with intermediate risk who are not improving on their present therapy. These consensus‐based recommendations may be helpful to clinicians and beneficial for patients when evidence‐based guidance is unavailable.
Keywords: adherence, goal directed, tolerability, treatment escalation, treat to target
INTRODUCTION
Pulmonary arterial hypertension (PAH) causes significant morbidity and mortality despite important improvements in understanding the pathophysiology and therapy of the disease over the past two decades. Treatment can prolong survival, delay progression, and improve function and quality of life.
Current guidelines recommend a treat‐to‐target strategy aimed at achieving, as quickly as possible, clinical and laboratory findings that are associated with a low risk of 1‐year mortality as defined by clinical and hemodynamic criteria. 1 , 2 Patients at initially low or intermediate risk typically receive dual combination therapy, including a phosphodiesterase‐5 inhibitor (PDE5i) and an endothelin receptor antagonist (ERA) to target the nitric oxide/cyclic GMP and endothelin pathways, respectively. An oral or infusion (SQ/IV) prostacyclin analog (PCA) is added for patients initially at high risk and to escalate therapy in patients who do not achieve low‐risk status after 3–6 months of treatment. 2
Soluble guanylate cyclase (sGC) activators target the nitric oxide/cyclic GMP pathway by enhancing cGMP production. This contrasts with PDE5i agents, which slows cGMP degradation. The RESPITE and REPLACE studies examined the impact of switching from a PDE5i to riociguat, an sGC activator, in patients at intermediate risk who had not reached low‐risk status on a PDE5i alone or in combination with an ERA. The trials found that switching to an sGC activator is a therapeutic option for escalation because significantly more patients improved clinically after 24 weeks of therapy. 3 , 4 REPLACE used a prospective, randomized, open‐label, blinded endpoints design, so the strength of the resulting evidence does not reach that of a randomized double‐blind trial. Thus, careful clinical judgment is needed in applying these results to clinical practice. 5 Because the REPLACE trial was published recently, the guidelines for the treatment of PAH have not yet incorporated these findings. In the absence of robust evidence or guideline recommendations, expert advice can provide considerable benefit to clinicians and patients by providing informed guidance. This Delphi process aimed to develop an expert consensus on escalations and transitions of therapy in patients with PAH, with consideration of when sGC activators might be used to replace PDE5 inhibitors.
METHODS
This study used a modified Delphi process to develop and define expert consensus recommendations on treatment selection for escalation and transitions of therapy in PAH. This Delphi process is a structured method for group decision making that was developed by Delbecq et al. in 1975 and is now widely used in pulmonology and other medical settings. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14
The study was conceived by the lead and senior authors, who oversaw the recruitment of the Delphi panelists, developed the three scenarios used in the surveys, moderated the questionnaires, and managed the conduct of the study. The panelists were selected from physicians with particular expertise and interest in PAH, based on their specialties and experience in treating PAH. Panelists were included as coauthors if they completed one or both of the first 2 surveys and the third survey, and reviewed and approved each stage of the study and the final manuscript. All coauthors met the International Committee of Medical Journal Editors' criteria for authorship.
Figure 1 summarizes the modified Delphi process used in the study. The process is based on three surveys. Survey 1 included primarily open‐ended questions about panelists’ general approach to treatment selection, and whether and how they would escalate therapy in three common scenarios: a patient on dual therapy, a patient on triple oral therapy, and a patient on triple therapy including an IV PCA. Copies of the 2015 guidelines for the diagnosis and treatment of pulmonary hypertension developed by the European Society for Cardiology and European Respiratory Society and the 2019 update based on the World Symposium on Pulmonary Hypertension (WSPH) held in 2018 were circulated to panelists with Survey 1. 1 , 2 Survey 2 consisted primarily of a series of statements based on panelist's responses to Survey 1. The statements were developed by consolidating and clarifying the management options described in panelists’ answers to the questions in Survey 1. Panelists were asked to rate their agreement with each statement using a Likert scale ranging from −5 (strongly disagree) to +5 (strongly agree). Survey 3 was identical to Survey 2, except that panelists were provided with their own answers to Survey 2, the mean and standard deviation of the group's answers, and whether consensus was achieved for each answer. This additional information is intended to promote consensus by making participants aware of the overall group opinion. The final aggregate results were circulated to all participants for review and comment. Panelist anonymity was maintained throughout the process, and there was no group meeting (as used in some Delphi processes). Full results for Survey 3 are provided in the Supporting Information Appendix.
Figure 1.
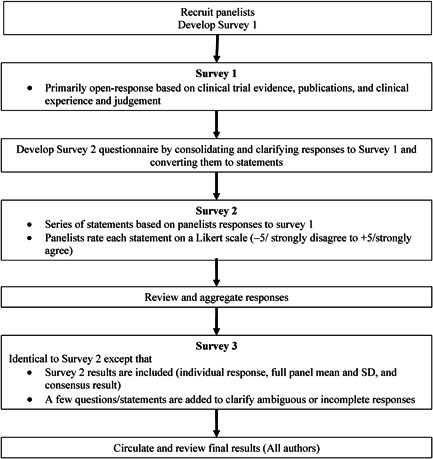
The modified Delphi process used in the study
The extent of consensus was evaluated using Likert scale questions that asked panelists to rate their agreement with statements using a scale from −5 (strongly disagree) to 5 (strongly agree). Panelists were considered to have reached a consensus if the mean Likert scale score was ≤−2.5 or ≥2.5, with an SD that did not cross 0.
RESULTS
The Delphi panel initially comprised 22 panelists. Of these, 20 panelists completed the third survey and met the other requirements for authorship. The final survey included 232 statements, of which consensus was reached on 129 (56%).
General approach to treatment selection
Panelists reached a consensus that multiple factors are important in selecting a treatment regimen for patients with PAH (Figure 2), with the strongest consensus for considering the severity of disease and the risk profile, clinical trial evidence of efficacy and safety, and the physician's clinical experience. However, panelists considered all of the factors important and that their importance varies between patients. The only factors that did not reach consensus were cost and geography (e.g., distance from the treatment center).
Figure 2.
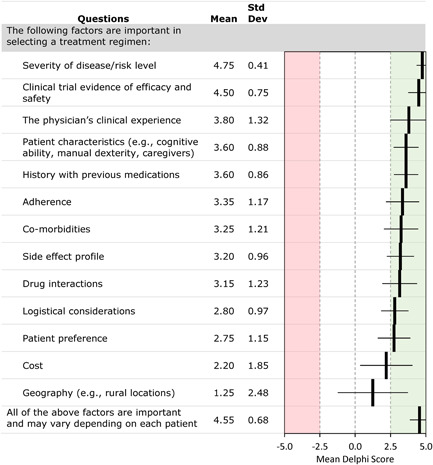
Delphi consensus results: factors important in selecting a treatment regimen (ranked by the mean consensus score)
Panelists reached a consensus that patient‐centered factors (e.g., tolerability, patient preference, and financial considerations) should be considered in a shared decision‐making process that integrates those patient factors, clinical data, and clinician experience. Panelists agreed that most newly diagnosed patients should be treated with combination oral therapy, with a Likert scale score (mean ± SD) of 4.30 ± 0.96. Most panelists agreed with the treatment algorithm presented at the 6th WSPH. Panelists reached a consensus that expert advice and clinical experience should guide therapeutic recommendations when no clinical evidence is available (3.38 ± 1.03).
Approach to deciding whether to escalate or switch therapy
Panelists reached a consensus that risk level is an important factor in deciding whether to escalate or switch therapy (4.37 ± 0.82), that escalation of therapy is needed for patients whose REVEAL risk score is greater than low risk and does not improve (3.53 ± 1.27), and that symptoms, World Health Organization functional class (FC), echocardiography characteristics, and serum brain natriuretic peptide levels are key parameters for building a clinical gestalt that will drive decision‐making. Factors considered by panelists to have important roles in the decision to switch or escalate are listed in Figure 3. Similar to the selection of an initial therapy, multiple factors should be considered, including patient‐related factors (such as the severity of disease, rate of progression), exercise capacity (but only 6‐min walk testing), right ventricular function, and pulmonary hemodynamics, which was considered most important. Interestingly, there was no consensus regarding patient age. There was a strong consensus that patient buy‐in (the patient's agreement with the physician's recommendation) is a key factor in treatment selection, with a weaker consensus that patient preference (allowing the patient to select therapy) is a key factor.
Figure 3.
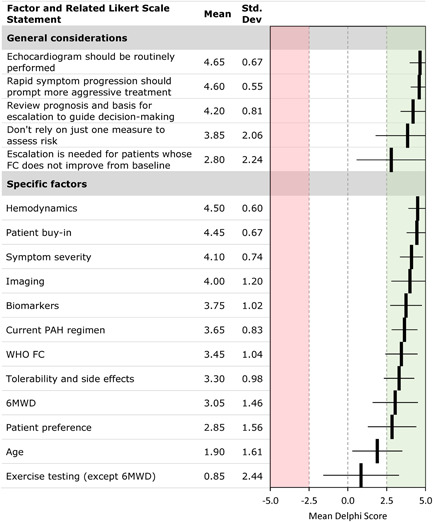
Delphi consensus results: role of key factors considered in decisions on escalation or switching. FC, functional class; 6MWD, 6‐min walk distance; PAH, pulmonary arterial hypertension; WHO, World Health Organization
Clinical scenario: A patient on dual therapy who requires escalation
Panelists considered several clinical scenarios involving a decision to escalate or switch PAH therapy to identify key considerations in the decision.
In the first scenario, a hypothetical patient receiving two‐drug combination therapy has been stable with a low‐risk profile for several months, but has progressed with an increased risk level at the most recent follow‐up. Panelists were asked to consider switching to a new agent in the same class, switching one agent to a new class, or initiating triple therapy by adding a new agent. Figure 4 shows the results for statements that reached consensus in this scenario. Switching to a new agent in the same class could be considered if tolerability, adherence, or logistical reasons were impacting therapy. Switching to a new class might be considered to manage intolerance or improve adherence, particularly if the switch could reduce side effects and/or improve efficacy. The results from the RESPITE and REPLACE studies support the possibility of switching from a PDE5i to an sGC activator in this situation. 3 , 4 Adding a new class (especially a PCA) rather than changing within the class should be considered in patients who are higher risk, are progressing or not reaching targets, or have progressed to FC IV.
Figure 4.
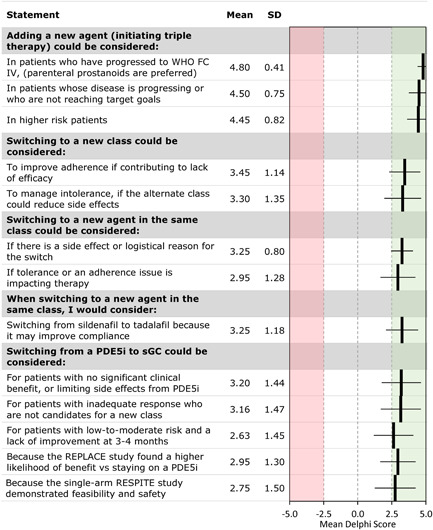
Escalation for a patient on dual therapy: Delphi consensus results for selected statements that reached consensus. FC, functional class; PDE5i, phosphodiesterase‐5 inhibitor; sGC, soluble guanylate cyclase; WHO, World Health Organization
Clinical scenario: A patient on oral triple therapy who requires escalation
In this scenario, the hypothetical patient's symptoms and FC are worsening while the patient is receiving three‐drug oral combination therapy. Panelists were asked to consider adjusting dosages, switching from a PDE5i to an sGC activator, switching from oral to parenteral PCAs, or switching one or more agents within the same class.
Panelists reached a consensus that adjusting the dosage of existing medications could be considered if the patient is not already at the maximally tolerated dose, if side effects are not significant and progression is not rapid, if the patient has benefited from prior dose increases, and if progression is mild, with no signs of right heart failure (Figure 5). However, dose adjustment is not appropriate if the patient is at higher risk or rapidly worsening; parenteral therapy is needed in this situation. Switching from a PDE5i to an sGC activator could be considered if the patient is already at the maximally tolerated dosages or if parenteral prostanoids or full‐dose PDE5i are contraindicated, intolerable, or unacceptable to the patient. It might also be considered in patients with a history of good adherence who are experiencing mild worsening and agree to close monitoring. Switching medications in other classes were only considered if there was intolerance or severe adverse effects. Panelists reached a consensus on several conditions in which escalation from oral to parenteral PCAs could be considered: FC IV, rapid progression, high‐risk hemodynamics, right heart failure, clinical worsening in moderate‐risk patients, and intolerance of higher doses or intolerable AEs of the existing medications.
Figure 5.
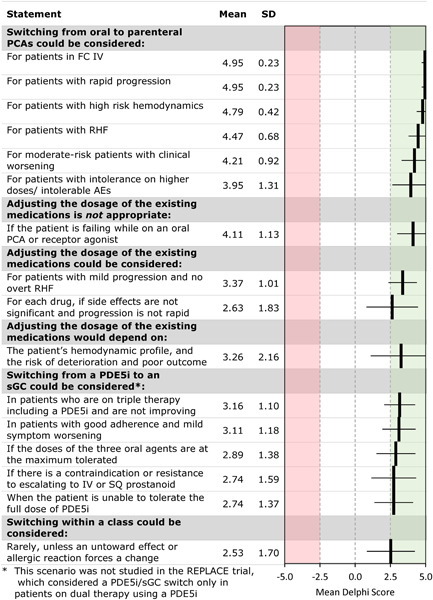
Escalation for a patient on oral triple therapy: Delphi consensus results for selected statements that reached consensus. AE, adverse event; FC, functional class; PCA, prostacyclin analog; PDE5i, phosphodiesterase‐5 inhibitor; RHF, right‐sided heart failure; sGC, soluble guanylate cyclase
Clinical scenario: A patient on triple therapy including an SQ/IV PCA who requires escalation
This scenario involves a hypothetical patient with worsening symptoms and FC while receiving three‐drug combination therapy including SQ/IV treprostinil. Panelists were asked to consider adjusting dosages, switching from a PDE5i to an sGC activator, switching other agents within a class, or other treatment approaches (Figure 6). Panelists reached a consensus that dosage adjustment could be considered if the patient is not at the maximally tolerated dose of the three drugs, particularly if invasive hemodynamic assessment by right heart catheterization (RHC) finds a low cardiac output and symptoms are worsening. Switching within a class could be considered if there is intolerance to one agent. There was a weak consensus for switching from parenteral treprostinil to epoprostenol in patients who were failing or intolerant of parenteral treprostinil. Switching from a PDE5i to an sGC activator could be considered if all three current medications are at their maximum‐tolerated doses.
Figure 6.
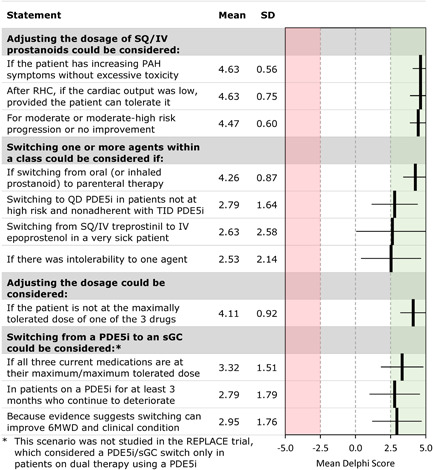
Escalation for a patient on triple therapy including SQ/IV treprostinil: Delphi consensus results for statements that reached consensus. FC, functional class; 6MWD, 6‐min walk distance; PAH, pulmonary arterial hypertension; PDE5i, phosphodiesterase‐5 inhibitor; QD, twice a day; RHC, right heart catheterization; sGC, soluble guanylate cyclase; TID, three times daily
Alternative therapies in this situation include referral for a lung transplant in appropriate candidates, palliative care and hospice, and participation in a research study. Pulmonary rehabilitation, aggressive diuretic management, and other therapies may be appropriate. Note that a repeat evaluation including echocardiography, RHC, and possibly additional imaging may be appropriate to confirm the diagnosis.
DISCUSSION
This study used a modified Delphi method to develop expert consensus recommendations on escalation and transitions of therapy for patients with PAH, focusing on the possibility of switching from a PDE5i to riociguat in patients. Panelists reached a consensus that numerous factors should be considered in selecting therapy, most notably disease severity, the patient's risk profile, clinical trial evidence, and the clinician's experience. Other factors were considered important, but a few factors did not reach consensus: cost, geography (patient's distance from the clinician's practice), exercise testing other than the 6‐min walk distance test, and patient age. Patient preference was deemed important as was patient involvement in shared decision‐making, but patient “buy‐in” was rated as even more important by panelists, suggesting that advocating for the merit of therapy while participating in shared decision‐making is paramount.
Since formal clinical guidelines aim to be evidence‐based, they are limited to making recommendations that have a strong basis in published data. Unfortunately, limitations on time and resources, ethical considerations, and challenges in enrolling sufficient numbers of patients preclude the creation of guidelines on many clinical questions. The evidence derived from studies on risk assessment calculators and other trials favors a strategy of escalating therapy to decrease patients’ risk status. 15 , 16 , 17 , 18 , 19 , 20 , 21 However, evidence to guide decisions on the escalation of therapy in PAH, particularly blinded comparative efficacy trials, is very limited. The lack of evidence heightens the relevance of the comparative RESPITE and REPLACE trials. This study is an attempt to capture the practical thought processes used by experts to make these decisions and to try to understand the reasoning behind those choices.
Panelists opined that PAH therapy should be escalated in patients who are at high risk, in WHO FC IV, are progressing/worsening, have signs of right heart failure, or are not meeting treatment goals. In these situations, patients on dual therapy should add a new agent from a different class, with parenteral PCAs preferred for patients in WHO FC IV. Patients on triple oral therapy who need treatment escalation should first maximize dosages of all medications, and, if there is no improvement, consider switching from an oral PCA to a parenteral PCA. Patients on triple therapy including an IV PCA should optimize the doses and may consider switching from SQ/IV treprostinil to IV epoprostenol.
When intolerability or nonadherence is an issue, panelists felt it reasonable to consider switching to a new agent in the same class, or (for dual therapy) switching to a new class. For select patients in an intermediate‐risk category who have not achieved low‐risk status while receiving a PDE5i, the results of the RESPITE and REPLACE studies suggest that switching from a PDE5i to an sGC activator may also provide significant clinical improvement. 3 , 4 We did not directly address dual therapy that included PCAs. In other scenarios involving PCAs, the group suggested maximizing the dose of oral/IV PCAs, and, in higher‐risk patients, switching to IV/SQ from oral or inhaled PCAs or adding a third class of medications.
Table 1 summarizes key consensus opinions. These are generally aligned with guidelines from the ESC/ERS, as updated in the 6th WSPH for risk stratification and medical therapy of PAH. 1 , 2 In broad outline, the WSPH update of the ESC/ERS guidelines recommends continuing existing therapy for patients at low risk, escalating to triple combination therapy for patients at intermediate risk, and escalating to maximal medical therapy for patients at intermediate or high risk in spite of triple therapy. Other transitions might be considered to improve the adverse event profile, convenience, or compliance. 1 , 2 Our consensus recommendations for specific clinical scenarios align with these guidelines, given that our hypothetical patients on dual therapy, triple therapy, and triple therapy including an SQ/IV PCA were initially at low, intermediate, and high risk, respectively. The consensus recommendations provide additional details on situations where transitions without escalation might be appropriate. The ESC/ERS guidelines state that there is insufficient evidence to recommend a transition from a PDE5i to an sGC activator based on the uncontrolled RESPITE trial. Data from the more recent prospective, randomized, open‐label, blinded endpoint REPLACE trial suggests that this transition may benefit some patients, although the open‐label design of this trial limits the strength of the evidence it provides. 4 , 5 Notably, the REPLACE trial enrolled patients receiving PDE5i alone or in combination with an ERA, but not on triple therapy. Note that the available sGC activator may cause symptomatic hypotension and that three times daily administration may be a barrier to adherence. These considerations should be weighed in decisions about switching to an sGC activator. A trial comparing an sGC activator to a PDE5i in patients on triple therapy is needed to clarify the role of sGC activators in patients at high risk. The guidelines for PAH from the American College of Chest Physicians recommend adding a third class of PAH therapy for patients with WHO FC III or IV symptoms who are deteriorating on dual therapy. 22 This recommendation aligns with our consensus for managing a patient on dual therapy, but was made before the results of the REPLACE study were known.
Table 1.
Summary of key consensus opinions on how to escalate therapy for PAH patients failing to achieve goals of therapy
| Patient on dual therapy |
|
| Patient on triple oral therapy |
|
| Patient on triple therapy including an SQ/IV PCA |
|
Abbreviations: FC, functional class; PAH, pulmonary arterial hypertension; PCA, prostacyclin analog; PDE5i, phosphodiesterase‐5 inhibitor; sGC, soluble guanylate cyclase; SQ/IV, oral or infusion; WHO, World Health Organization.
This switch has not been studied in patients on triple oral therapy.
This study has several limitations. The Delphi process is inherently based on consensus rather than evidence, and there are no generally accepted criteria for defining consensus in Delphi studies. Studies have used a variety of methods to define consensus, such as an agreement by 70% of panelists, cluster analysis, nominal group technique, and the Likert scale criteria used here. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 In addition, as with any consensus‐based process, the results may have been biased by panel selection and questionnaire development. 9 , 23 , 24 , 25 , 26 , 27 Panelists were drawn from a limited geographic area (the United States) and most are from large academic medical centers. Thus, their recommendations may not be representative of other areas or treatment centers, particularly those where cost and access to care are problematic. Panelist anonymity, which was maintained throughout the study, helps prevent individuals from dominating the process, but means that less experienced panelists are weighted equally with more experienced ones and may increase the risk that some participants responded without as much depth of knowledge. To minimize this concern, panelists were selected based on their generally recognized expertise in the subject area.
CONCLUSION
Careful consideration of multiple factors is required for effective decision‐making on escalation and transitions of therapy for patients with PAH. Broadly, switching agents can be considered as a means to address issues of lack of improvement or deterioration, tolerability, side effects, and patient preference, but adding an agent or escalating from oral to parenteral PCAs is essential when clinical improvement or risk reduction is needed in failing patients. Switching from a PDE5i to an sGC activator may also be a reasonable option for patients at low or intermediate risk who fail to improve or deteriorate on PDE5i therapy.
CONFLICTS OF INTEREST
Franck F. Rahaghi reports consultation, research, and speakership honoraria from Bayer and Janssen, consultation and speakership from United Therapeutics, and consultation fees from Acceleron. Vijay P. Balasubramanian reports a research grant from United Therapeutics and serves on a speakers bureau for Bayer. Robert C. Bourge reports research grant support to my institution from United Therapeutics and Bayer, and service on a Scientific Advisory Board at United Therapeutics. Murali M. Chakinala reports grants or contracts from Actelion/Janssen, Bayer, Medtronic, NIH, Reata, Liquidia, Phase Bio, Complexa, United Therapeutics, Altavant, Trio Health Analytics, Reata, Acceleron, Arena, and Gossamer; consulting fees from Altavant, Vaderis Therapeutics, Aerovate, Reata, VWave, and Arena; honoraria from Bayer, Gilead, Simply Speaking, WebMD, and United Therapeutics; support for attending meetings and/or travel from Actelion/Janssen, United Therapeutics, Bayer, Acceleron, Reata, and Gilead; participation on a Data Safety Monitoring Board or Advisory Board for Actelion/Janssen, Express Scripts, Phase Bio, Altavant, Gossamer, United Therapeutics, Bayer, Acceleron, and Liquidia; and leadership or a fiduciary role in the Pulmonary Hypertension Association and the Cure HHT Global Research and Medical Advisory Board. Michael S. Eggert reports research contracts with United Therapeutics (BREEZE and ADVANCE Outcomes), Acceleron, and Actelion. Jean M. Elwing reports grants from Actelion, Acceleron, Reata, United Therapeutics, Liquidia, Phase Bio, Complexa, Gossamer Bio, Bayer, Arena, Eiger, Akros, Bellerophon, and Lung LLC and consulting fees from United Therapeutics, Acceleron, Liquidia, Altavant, Bayer, Gossamer Bio, Actelion, Bayer. Jeremy Feldman reports consulting and giving talks for Bayer, United Therapeutics, and Jansen. Christopher King reports personal fees from Actelion, personal fees from Genentech, United Therapeutics, and Boehringer Ingelheim; has served on advisory boards for and is on the Speakers’ Bureau of Actelion, Boehringer‐Ingelheim Pharmaceuticals, and United Therapeutics. James R. Klinger reports that his institution receives research funding from United Technologies, service on a Steering Committee and a Clinical Outcomes Committee for Bayer, and a leadership role in the Pulmonary Hypertension Association. Stephen C. Mathai reports personal fees from United Therapeutics; participation on a data safety monitoring board or advisory board from United Therapeutics, Actelion, and Bayer, and leadership or a fiduciary role in the PCORI Rare Disease Advisory Panel and the World Symposium on Pulmonary Hypertension. John Wesley McConnell reports consulting fees from Actelion, Bayer, Gossamer, Altavant, and Liquidia; honoraria from Actelion, Bayer, Simply Speaking, Impact PH, and Reata, and participation on a data safety monitoring board or advisory board for Actelion, Liquidia, Gossamer, and Altavant. Harold I. Palevsky reports honoraria for serving on scientific advisory boards for Acceleron, Actelion/Janssen, PhaseBio and United Therapeutics, and serving on a DSMB for United Therapeutics. Ricardo Restrepo‐Jaramillo reports serving on speaker's bureau for United Therapeutics, Bayer, and Actelion. Zeenat Safdar reports serving on a speakers bureau, consultation and advisory boards for Actelion, United Therapeutics, Boehringer Ingelheim, Bayer, and Roche. Jeffrey S. Sager reports personal fees from Bayer pharmaceuticals, grants and personal fees from United Therapeutics, grants and personal fees from Janssen (J and J), and grants from Reata outside the submitted work. Namita Sood reports a speaking fee from Bayer. Roxana Sulica reports research grants from Bayer, United Therapeutics, Complexa, and Reata, and serves on advisory boards for Actelion, Bayer, United Therapeutics, and Reata. R. James White reports research grants from United Therapeutics, Reata, Bayer, Merck, and Janssen and consulting fees from Merck and Bayer. Nicholas S. Hill reports honoraria from Axon Research for this study; grants to his institution from Actelion, Bayer, Gilead, and United Therapeutics; consulting fees from United Therapeutics; and participation in Data Safety Monitoring Boards for United Therapeutics and Pfizer. All authors had access to the Delphi questionnaire analysis and data and participated in the review, revision, and approval of the content of the manuscript for submission. Charles D. Burger reports no disclosures or conflicts of interest.
ETHICS STATEMENT
The ethics statement is not available.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
Editorial assistance was provided by AXON Communications and funded by an independent grant provided by Bayer HealthCare Pharmaceuticals Inc. Edward K. Baldwin provided medical writing support.
Rahaghi FF, Balasubramanian VP, Bourge RC, Burger CD, Chakinala MM, Eggert MS, Elwing JM, Feldman J, King C, Klinger JR, Mathai SC, McConnell JW, Palevsky HI, Restrepo‐Jaramillo R, Safdar Z, Sager JS, Sood N, Sulica R, White RJ, Hill NS. Delphi consensus recommendation for optimization of pulmonary hypertension therapy focusing on switching from a phosphodiesterase 5 inhibitor to riociguat. Pulmonary Circulation. 2022;12:e12055. 10.1002/pul2.12055
REFERENCES
- 1. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015. Oct;46(4):903–75. 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 2. Galie N, Channick RN, Frantz RP, Grunig E, Jing ZC, Moiseeva O, Preston IR, Pulido T, Safdar Z, Tamura Y, McLaughlin VV. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019. Jan;53(1):1801889. 10.1183/13993003.01889-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoeper MM, Simonneau G, Corris PA, Ghofrani HA, Klinger JR, Langleben D, Naeije R, Jansa P, Rosenkranz S, Scelsi L, Grunig E, Vizza CD, Chang M, Colorado P, Meier C, Busse D, Benza RL. RESPITE: switching to riociguat in pulmonary arterial hypertension patients with inadequate response to phosphodiesterase‐5 inhibitors. Eur Respir J. 2017. Sep;50(3):1602425. 10.1183/13993003.02425-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoeper MM, Al‐Hiti H, Benza RL, Chang SA, Corris PA, Gibbs JSR, Grunig E, Jansa P, Klinger JR, Langleben D, McLaughlin VV, Meyer GMB, Ota‐Arakaki J, Peacock AJ, Pulido T, Rosenkranz S, Vizza CD, Vonk‐Noordegraaf A, White RJ, Chang M, Kleinjung F, Meier C, Paraschin K, Ghofrani HA, Simonneau G, Olschewski H, Delcroix M, Andrade‐Lima M, de Amorim Correac R, Figueiredo Campos F, Ota Arakaki J, Meyer G, De Souza R, Langleben D, Al‐Hiti H, Jansa P, Mellemkjær S, Bauer F, Montani D, Simonneau G, Dromann D, Ghofrani HA, Grunig E, Halank M, Held M, Hoeper M, Klose H, Kneidinger N, Leuchte H, Opitz C, Rosenkranz S, Wilkens H, Wirtz H, Karvounis H, Pitsiou G, Orfanos S, D'Alto M, Ghio S, Vizza C, Vitulo P, Nakayama T, Maki H, Tatebe S, de los Rios Ibarra M, Pulido T, Van Dijk A, Vonk‐Noordegraaf A, Roleder T, Castro G, Loureiro M, Robalo‐Martins S, Barbera J, Lazaro M, Perez‐Penate G, Roman A, Cheng CC, Hsu CH, Hsu HH, Atahan E, Mogulkoc Bishop N, Okumus N, Onen Z, Chang HJ, Chang SA, Lee JS, Kim HK, Coghlan J, Corris P, Church A, Condliffe R, Gibbs J, Peacock A, Wort S, Allen R, Allen S, Awdish R, Benza R, DeSouza S, Feldman J, Johri S, Klinger J, Layish D, McConnell J, McLaughlin V, Migliore C, Rahaghi F, Rischard F, Robbins I, Satterwhite L, Shah T, Sulica R, White R. Switching to riociguat versus maintenance therapy with phosphodiesterase‐5 inhibitors in patients with pulmonary arterial hypertension (REPLACE): a multicentre, open‐label, randomised controlled trial. Lancet Respir Med. 2021. Jun;9(6):573–84. 10.1016/s2213-2600(20)30532-4 [DOI] [PubMed] [Google Scholar]
- 5. Frantz RP. REPLACE and the role of riociguat in pulmonary arterial hypertension therapy. Lancet Respir Med. 2021. Jun;9(6):546–7. 10.1016/s2213-2600(20)30567-1 [DOI] [PubMed] [Google Scholar]
- 6. de Meyrick J. The Delphi method and health research. Health Educ. 2003;103(1):7–16. 10.1108/09654280310459112 [DOI] [Google Scholar]
- 7. Hsu CC, Sandford BA. The Delphi technique: making sense of consensus. Pract Assess Res Eval. 2007;12(10):1–8. [Google Scholar]
- 8. Schutt AC, Bullington WM, Judson MA. Pharmacotherapy for pulmonary sarcoidosis: a Delphi consensus study. Respir Med. 2010. May;104(5):717–23. 10.1016/j.rmed.2009.12.009 [DOI] [PubMed] [Google Scholar]
- 9. Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000. Oct;32(4):1008–15. [PubMed] [Google Scholar]
- 10. Rahaghi FF, Feldman JP, Allen RP, Tapson V, Safdar Z, Balasubramanian VP, Shapiro S, Mathier MA, Elwing JM, Chakinala MM, White RJ. Recommendations for the use of oral treprostinil in clinical practice: a Delphi consensus project. Pulm Circ. 2017;7(1):167–74. 10.1086/690109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rahaghi FF, Alnuaimat HM, Awdish RLA, Balasubramanian VP, Bourge RC, Burger CD, Butler J, Cauthen CG, Chakinala MM, deBoisblanc BP, Eggert MS, Engel P, Feldman J, McConnell JW, Park M, Sager JS, Sood N, Palevsky HI. Recommendations for the clinical management of patients receiving macitentan for pulmonary arterial hypertension (PAH): a Delphi consensus document. Pulm Circ. 2017. Jul–Sep;7(3):702–11. 10.1177/2045893217721695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saketkoo LA, Mittoo S, Huscher D, Khanna D, Dellaripa PF, Distler O, Flaherty KR, Frankel S, Oddis CV, Denton CP, Fischer A, Kowal‐Bielecka OM, LeSage D, Merkel PA, Phillips K, Pittrow D, Swigris J, Antoniou K, Baughman RP, Castelino FV, Christmann RB, Christopher‐Stine L, Collard HR, Cottin V, Danoff S, Highland KB, Hummers L, Shah AA, Kim DS, Lynch DA, Miller FW, Proudman SM, Richeldi L, Ryu JH, Sandorfi N, Sarver C, Wells AU, Strand V, Matteson EL, Brown KK, Seibold JR, CTD‐ILD Special Interest G . Connective tissue disease related interstitial lung diseases and idiopathic pulmonary fibrosis: provisional core sets of domains and instruments for use in clinical trials. Thorax. 2014. May;69(5):428–36. 10.1136/thoraxjnl-2013-204202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huscher D, Pittrow D, Distler O, Denton CP, Foeldvari I, Humbert M, Matucci‐Cerinic M, Kowal‐Bielecka O, Avouac J, Behrens F, Nash P, Opitz CF, Rubin LJ, Seibold JR, Strand V, Furst DE, EPOSS‐OMERACT G . Interactions between rheumatologists and cardio‐/pulmonologists in the assessment and use of outcome measures in pulmonary arterial hypertension related to systemic sclerosis. Clin Exp Rheumatol. 2010. Mar–Apr;28(2, Suppl 58):S47–52. [PubMed] [Google Scholar]
- 14. Distler O, Behrens F, Pittrow D, Huscher D, Denton CP, Foeldvari I, Humbert M, Matucci‐Cerinic M, Nash P, Opitz CF, Rubin LJ, Seibold JR, Furst DE, EPOSS‐Omeract G . Defining appropriate outcome measures in pulmonary arterial hypertension related to systemic sclerosis: a Delphi consensus study with cluster analysis. Arthritis Rheum. 2008. Jun 15;59(6):867–75. 10.1002/art.23718 [DOI] [PubMed] [Google Scholar]
- 15. Hirashiki A, Kondo T, Adachi S, Nakano Y, Kamimura Y, Shimokata S, Okumura N, Shimizu A, Washimi Y, Arai H, Murohara T. Goal‐oriented sequential combination therapy evaluated using cardiopulmonary exercise parameters for the treatment of newly diagnosed pulmonary arterial hypertension—goal‐oriented therapy evaluated by cardiopulmonary exercise testing for pulmonary arterial hypertension (GOOD EYE). Circ Rep. 2019. Jun 2;1(7):303–11. 10.1253/circrep.CR-19-0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nickel N, Golpon H, Greer M, Knudsen L, Olsson K, Westerkamp V, Welte T, Hoeper MM. The prognostic impact of follow‐up assessments in patients with idiopathic pulmonary arterial hypertension. Eur Respir J. 2012. Mar;39(3):589–96. 10.1183/09031936.00092311 [DOI] [PubMed] [Google Scholar]
- 17. Humbert M, Farber HW, Ghofrani H‐A, Benza RL, Busse D, Meier C, Hoeper MM. Risk assessment in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Eur Respir J. 2019;53(6):1802004. 10.1183/13993003.02004-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benza RL, Farber HW, Frost A, Ghofrani HA, Gómez‐Sánchez MA, Langleben D, Rosenkranz S, Busse D, Meier C, Nikkho S, Hoeper MM. REVEAL risk scores applied to riociguat‐treated patients in PATENT‐2: impact of changes in risk score on survival. J Heart Lung Transplant. 2018. Apr;37(4):513–9. 10.1016/j.healun.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 19. Kylhammar D, Kjellstrom B, Hjalmarsson C, Jansson K, Nisell M, Soderberg S, Wikstrom G, Radegran G. A comprehensive risk stratification at early follow‐up determines prognosis in pulmonary arterial hypertension. Eur Heart J. 2018. Dec;39(47):4175–81. 10.1093/eurheartj/ehx257 [DOI] [PubMed] [Google Scholar]
- 20. Hoeper MM, Markevych I, Spiekerkoetter E, Welte T, Niedermeyer J. Goal‐oriented treatment and combination therapy for pulmonary arterial hypertension. Eur Respir J. 2005. Nov;26(5):858–63. 10.1183/09031936.05.00075305 [DOI] [PubMed] [Google Scholar]
- 21. Boucly A, Cottin V, Nunes H, Jais X, Tazi A, Prevot G, Reynaud‐Gaubert M, Dromer C, Viacroze C, Horeau‐Langlard D, Pison C, Bergot E, Traclet J, Weatherald J, Simonneau G, Valeyre D, Montani D, Humbert M, Sitbon O, Savale L. Management and long‐term outcomes of sarcoidosis‐associated pulmonary hypertension. Eur Respir J. 2017;50(4):1700465. 10.1183/13993003.00465-2017 [DOI] [PubMed] [Google Scholar]
- 22. Klinger JR, Elliott CG, Levine DJ, Bossone E, Duvall L, Fagan K, Frantsve‐Hawley J, Kawut SM, Ryan JJ, Rosenzweig EB, Sederstrom N, Steen VD, Badesch DB. Therapy for pulmonary arterial hypertension in adults: update of the CHEST Guideline and Expert Panel Report. Chest. 2019;155(3):565–86. 10.1016/j.chest.2018.11.030 [DOI] [PubMed] [Google Scholar]
- 23. Phillips AC, Lewis LK, McEvoy MP, Galipeau J, Glasziou P, Hammick M, Moher D, Tilson J, Williams MT. Protocol for development of the guideline for reporting evidence based practice educational interventions and teaching (GREET) statement. BMC Med Educ. 2013;13:9. 10.1186/1472-6920-13-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Phillips AC, Lewis LK, McEvoy MP, Galipeau J, Glasziou P, Hammick M, Moher D, Tilson JK, Williams MT. A Delphi survey to determine how educational interventions for evidence‐based practice should be reported: stage 2 of the development of a reporting guideline. BMC Med Educ. 2014;14:159. 10.1186/1472-6920-14-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Powell C. The Delphi technique: myths and realities. J Adv Nurs. 2003. Feb;41(4):376–82. [DOI] [PubMed] [Google Scholar]
- 26. Li Y, Ehiri J, Hu D, Zhang Y, Wang Q, Zhang S, Cao J. Framework of behavioral indicators for outcome evaluation of TB health promotion: a Delphi study of TB suspects and TB patients. BMC Infect Dis. 2014;14:268. 10.1186/1471-2334-14-268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mansell G, Shapley M, van der Windt D, Sanders T, Little P. Critical items for assessing risk of lung and colorectal cancer in primary care: a Delphi study. Br J Gen Pract. 2014. Aug;64(625):e509–15. 10.3399/bjgp14X681001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


