Abstract
Pulmonary arterial hypertension (PAH) is a chronically progressive fatal disease. A goal‐oriented approach to achieve low risk status has been associated with improved survival. A variety of risk stratification tools are available, but use is low. We conducted a survey to assess potential reasons for under‐utilization. We conducted a survey‐based study of global PAH disease specialists with a goal of assessing risk assessment utilization and identifying modifiable barriers to use. The survey was designed by the American College of Chest Physicians’ Pulmonary Vascular Diseases (PVD) NetWork. Respondents were global members of the PVD NetWork and Pulmonary Hypertension Association. Survey invitations were sent electronically to all members. Participation was anonymous and no provider or patient level data was collected. Participants from four countries responded with the majority (84%) being from the United States. Our survey found suboptimal use of any risk stratification tool with 71/112 (63%) reporting use. A total of 85% of the respondents had more than 5 years of experience in managing PAH. REVEAL 2.0 and European Society of Cardiology/European Respiratory Society risk tools were the most commonly used. A total of 44 (65%) surveyed felt that use of risk tools led to change in PAH therapies. Only 6 (9%) felt they prompted additional testing or changed the frequency of follow‐up. A total of 5 (7%) reported they prompted goals of care/palliative care discussions and 2 (3%) that they triggered lung transplant referral. The vast majority indicated that incorporation of risk tools into electronic medical records (EMR) would improve utilization. PAH risk assessment tools remain under‐utilized. Most respondents were experienced PAH clinicians. More than one‐third were not routinely using risk tools. Most felt that risk tools led to PAH therapy changes but few reported impacts on other aspects of care. The most commonly identified barriers to use were time constraints and lack of integration with EMR.
Keywords: pulmonary arterial hypertension, quality improvement, REVEAL 2.0., risk assessment, survival
Abbreviations
- ACCP
American College of Chest Physicians
- CCC
Comprehensive Care Center
- COMPERA
Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension
- EMR
Electronic Medical Records
- ERS
European Respiratory Society
- ESC
European Society of Cardiology
- IRB
Institutional Review Board
- PAH
Pulmonary Arterial Hypertension
- PHA
Pulmonary Hypertension Association
- PVD
Pulmonary Vascular diseases
- PVR
Pulmonary Vascular Resistance
- RCP
Regional Clinical Program
- REVEAL
The Registry to Evaluate Early and Long‐Term PAH Disease Management
- RV
Right ventricle
- SPAHR
Swedish PAH Registry
- WSPH
World Symposium on Pulmonary Hypertension
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a chronic, progressive disease that is characterized by extensive narrowing of the pulmonary vasculature leading to increase in pulmonary vascular resistance, subsequent right ventricular dysfunction, and eventual death. 1 Currently approved therapies can be used in combination or as monotherapy to improve functional capacity and outcomes in PAH. 2 However, despite advances in the treatment, PAH has remained an incurable disease with a median survival of 7 years. 3 Physician's ability to comprehensively assess PAH patients, determine prognosis, monitor disease progression and response to treatment remains critical in optimizing outcomes. There is no single variable that predicts outcomes in PAH patients. In 2016 1 European guidelines for the first‐time recommended use of risk stratification in management of PAH. Risk assessment in PAH patients should include a range of clinical, hemodynamic and exercise parameters, performed in a serial fashion to reflect a patient's course during the disease. 1 , 4 A goal‐oriented treatment approach may help in achieving a low risk profile in PAH patients. 5
Routinely, clinicians assess patients during clinical visits based on their gestalt which may or may not incorporate patient's history, laboratory and ambulatory tests, right heart catheterization hemodynamics, and imaging studies into decision making. 6 Several risk assessment tools have been developed and validated from large PAH registry populations to facilitate more formal evaluation of risk. The Registry to Evaluate Early and Long‐Term PAH Disease Management (REVEAL) risk calculator was developed in 2010 to estimate PAH mortality risk based on up to 12 variables. 3 , 7 This calculator was further updated (REVEAL 2.0) to include an additional variable and to revise cutoffs for seven variables. 8 REVEAL 2.0 lite, an abridged version of REVEAL 2.0 was recently published using less number of variables. 9 Three additional risk assessment methods, the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA) method, 10 the French Pulmonary Hypertension Registry (FPHR) 11 method and the Swedish PAH Registry (SPAHR) 12 are also available and incorporate data from up to six variables, using thresholds suggested by the European Society of Cardiology/European Respiratory Society (ESC/ERS) Pulmonary Hypertension Guidelines. 1 Most of these risk scoring methods have shown that achieving the low‐risk status is associated with better survival. 13 However, utilization of these risk stratification methods in clinical practice is low and clinicians often rely on their gestalt to make treatment decisions. 14 Recent studies have clearly shown a marked discrepancy between these objective risk scoring methods and physician gestalt. 6 This observation raised concerns regarding possible barriers to use and the implementation of these validated tools in clinical practice. To better understand these barriers, we conducted a survey study focused on identifying factors associated with underutilization of PAH risk tools. This survey primarily targeted clinicians who routinely care for PAH patients.
METHODS
The American College of Chest Physicians (ACCP) 2020–2021 Pulmonary Vascular NetWork Steering Committee designed, reviewed, and approved a quality improvement initiate via a provider survey comprised of 31 questions assessing PAH patient care. The aim of this questionnaire is to increase the understanding of the current utilization of various risk assessment tools amongst PAH care providers and to identify the potential barriers limiting implementation of risk tool use. The survey was sent electronically to PAH specialists worldwide via the clinicians’ directory in the Pulmonary Vascular Disease (PVD) NetWork of the American College of Chest Physician (ACCP) and the Clinicians and Researchers members of the Pulmonary Hypertension Association (PHA). No patient specific information was obtained. This quality improvement survey did not meet requirements for IRB submission or approval. Participation was voluntary and participating clinicians were not provided with any incentive for their time and opinion. Information was collected anonymously. Those interested in participating in the study were able to access the questionnaire using a direct email link to the online survey created in Survey Monkey.
The first eight questions were designed to collect demographic information pertinent to the responders. The subsequent questions assessed for involvement in PAH outpatient care and queried the number of patients followed, as well as PH center certification. Only those who responded in the affirmative to caring for PAH patients advanced to the remainder of the questionnaire. The remaining questions surveyed patterns of risk assessment tool use in new and established patients as well as evaluated barriers to implementation of PAH risk assessment routine use.
Statistical analysis
Simple descriptive analyses were used to interpret the survey results. Responses were calculated in percentages for each response. Risk tool use was compared amongst each demographic and detailed in Table 2. Characteristics of respondents were reported as frequencies and proportions.
Table 2.
Proportion of each demographic of respondents who used risk assessment tools versus those who did not in their clinical practice
| Proportion of respondents who used risk assessment tools | Proportion of respondents who did not use risk assessment tools | |
|---|---|---|
| (n = 71) | (n = 26) | |
| Gender | ||
| Female (31) | 24/31 (77.41%) | 7/31 (22.58%) |
| Male (65) | 46/65 (70.77%) | 19/65 (29.23%) |
| Did not specify (1) | 1/1 (100) | |
| Specialty | ||
| Pulmonary (81) | 57/81 (70.37%) | 24/81 (29.63%) |
| Cardiology (14) | 13/14 (92.86%) | 1/14 (7.14%) |
| Internal medicine (2) | 1/2 (50%) | 1/2 (50%) |
| Country of residence | ||
| USA (81) | 63/81 (77.78%) | 18/81 (22.22%) |
| Others countries (16) | 8/16 (50%) | 8/16 (50%) |
| Setting of practice | ||
| An academic center (65) | 52/65 (80%) | 13/65 (20%) |
| A community‐based hospital (22) | 12/22 (54.5%) | 10/22 (45.5%) |
| Veterans' health administration or military health system (3) | 2/3 (66.67%) | 1/3 (33.33%) |
| Others (7) | 5/7 (71.43%) | 2/7 (28.57%) |
| Community of the hospital location | ||
| Urban (70) | 54/70 (77.14%) | 16/70 (22.86%) |
| Suburban (22) | 13/22 (59.09%) | 9/22 (40.90%) |
| Rural (5) | 4/5 (80%) | 1/5 (20%) |
| Years in practice | ||
| <5 Years (9) | 7/9 (77.78%) | 2/9 (22.22%) |
| 5–10 Years (21) | 17/21 (80.95%) | 4/21 (19.05%) |
| 11–20 Years (29) | 22/29 (75.86%) | 7/29 (24.14%) |
| >20 Years (37) | 25/37 (67.57%) | 12/37 (32.43%) |
| Currently in training (1) | 1 (100%) | |
| PAH patients in their practice | ||
| <50 Patients (43) | 26/43 (60.47%) | 17/43 (39.53%) |
| 50–100 Patients (14) | 11/14 (78.57%) | 3/14 (11.54%) |
| 101–250 Patients (19) | 15/19 (21.13%) | 4/19 (21.43%) |
| >250 Patientsn (21) | 19/21 (90.48%) | 2/21 (9.52%) |
| PHA Comprehensive Care Center (CCC or not) | ||
| Yes (36) | 30/36 (83.33%) | 6/36 (16.67%) |
| No (56) | 37/56 (66.07%) | 19/56 (33.93%) |
| Unknown (5) | 4/5 (80%) | 1/5 (20%) |
| PHA Regional Clinical Program (RCP) | ||
| Yes (11) | 11/11 (100%) | 0 (0.00%) |
| No (80) | 57/80 (71.25%) | 23/80 (28.75%) |
| Unknown (6) | 3/6 (50%) | 3/6 (50%) |
RESULTS
A total of 112 clinicians participated from the International CHEST PVD Network and the PHA Clinicians and Researchers Group, with respondents representing 12 countries. The majority were from the United States 95 (84%), 5 (4%) from India, 2 (2%) from Mexico and Turkey. One respondent each from Australia, Brazil, Bahrain, Canada, Lebanon, Philippines, Saudi Arabia, as well as Thailand participated in the survey. One hundred and six (95%) were physicians with a Doctor of Medicine (MD) degree, 4 (4%) were physicians with Doctor of Osteopathic Medicine (DO) degree while 2 (1%) were Nurse Practitioners or Physician Assistants. Seventy‐seven (69%) of respondents identified as males, 34 (30%) females and 1 (1%) did not disclose gender. Ninety‐two (82%) were pulmonologists, 14 (12%) were cardiologists and 6 (6%) were internal medicine trained clinicians. Type of practice information from the US clinicians is shown in Table 1. A total of 40 (37%) clinicians were more than 20 years in practice, 29 (27%) were between 11 and 20 years in practice, 23 (21%) were between 5 and 10 years, 11 (10%) were less than 5 years and 6 (5%) were currently in training. One hundred and two (94%) clinicians were involved in providing outpatient care to PAH patients. Of the 101 clinicians who responded, 45 (45%) were providing care to less than 50 patients in their practice while 56 (55%) were caring for more than 50 patients in their practice. 38/101 (38%) practiced at a PHA accredited center while 58/101 (57%) did not work at a certified center and 5/101 (5%) did not respond. Of the 38% at a PHA accredited center, 26% worked at a PHA Comprehensive Care Center (CCC) while 12% of respondents were practicing at a Regional Clinical Program (RCP). Risk assessment tools were used by 30/36 (83.33%) respondents from PH certified centers compared to 37/56 (66%) from noncertified centers in their routine clinical care. Two participants did not respond to this question.
Table 1.
Demographics and geographical distribution of the survey respondents
| (a) United States regions of the participating clinicians | ||
|---|---|---|
| United States Region | ||
| N: 93 | ||
| Northeast | 26.88% | 25 |
| Southeast | 16.13% | 15 |
| Midwest | 23.66% | 22 |
| Northwest | 4.30% | 4 |
| Southwest | 27.96% | 26 |
| Alaska | 0.00% | 0 |
| Hawaii | 1.08% | 1 |
| (b) Type of setting of clinical practice | ||
| N: 109 | ||
| An academic medical center | 65.14% | 71 |
| A community‐based hospital | 24.77% | 27 |
| Veterans' health administration or military health system | 2.75% | 3 |
| Other | 7.34% | 8 |
| (c) Type of community or location of the hospital | ||
| N: 109 | ||
| Urban | 71.56% | 78 |
| Suburban | 23.85% | 26 |
| Rural | 4.59% | 5 |
Utilization of risk assessment tools: Baseline evaluation
Of the 112 participants, 71 (63%) of clinicians reported use of PAH risk stratification tools in their practice while 26 (24%) did not use any risk tools and 15 (13%) did not provide any response. Table 2 highlights the demographic profiles of the respondents who used risk assessment tools in their practice compared with those who did not and details the proportion of each demographic of the respondents who utilized risk assessment tools. The respondents from 63/81 (77.8%) of United States and 8/16 (50%) of non‐United States practices reported using risk assessment tools. The majority 30/36 (83.33%) of participants from PHA CCCs and 11/11 (100%) from PHA RCPs reported risk tool use. Those respondents practicing in non‐CCCs reported 37/56 (66.07%) use of risk assessment. A total of 68 clinicians responded about the type of risk assessment tools used in the past year in their practice and many clinicians appear to use multiple scoring methods as outlined in Figure 1. REVEAL 2.0 was the most frequently used with 49 (72.06%) using it for risk assessment which was closely followed by the ESC/ERS tool by 43 (63.24%). A total of 66 (97%) of these clinicians used these risk assessment tools for baseline assessment and 62 (91%) believed that it impacted their patient management. A total of 44 (65%) felt that it led to change in PAH treatment medications, 6 (9%) felt that it prompted additional testing or changed the frequency of follow up visits, 5 (7%) felt that it prompted goals of care/palliative care discussions and 2 (3%) suggested it triggered lung transplant referral. In contrast, 5 (7%) felt that using risk assessment tools did not affect their treatment decisions in a new patient evaluation. An ordinal scale of responses is shown in Figure 2.
Figure 1.
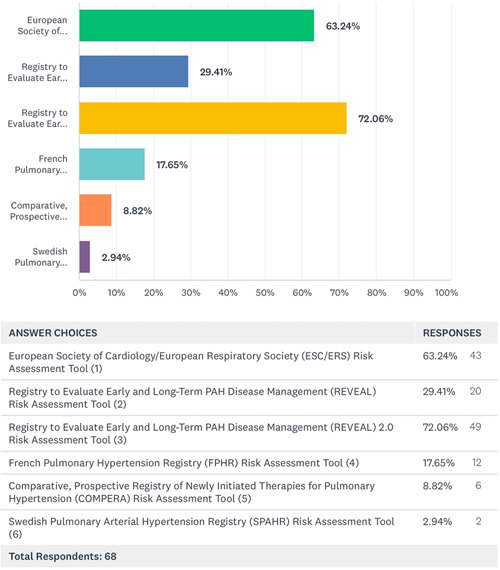
Type of risk assessment tools used by the clinicians for baseline or follow up evaluation of PAH patients in their clinics (Respondents may have chosen more than one option). PAH, pulmonary arterial hypertension
Figure 2.
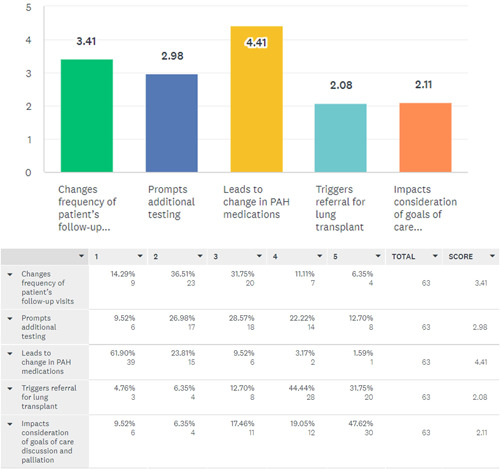
Ordinal scale showing the degree of impact of risk assessment during baseline PAH patient evaluations on the elements of patient care listed below. PAH, pulmonary arterial hypertension
Follow up evaluation
A total of 61 (54%) responded to questions regarding use of risk stratification tools during follow up visits. Of these, 57 (93%) responded favorably to using risk assessment tools during follow up visits and felt it impacted their patient management. A total of 31 (51%) used these tools on every follow up visit, 16 (26%) used them only after change in clinical status, 12 (20%) used them at a predetermined interval (6 months follow up) and 2 (3%) used it only after medication change. A total of 41 (67%) documented each visit risk assessment score in patients’ medical records, while 20 (33%) did not document it in medical records. Regarding the impact of using risk assessment tools during follow up visits, 40 (66%) felt that it led to changes in PAH medications, 6 (10%) felt that it changed frequency of follow up visits, 5 (8%) reported it prompted additional testing or transplant referral. Three (5%) clinicians initiated palliative care discussions with patients based on risk assessment. The remaining 2 respondents (3%) felt these tools did not impact their management. Figure 3 shows an ordinal scale of the responses received regarding using risk assessment tools during follow up visits.
Figure 3.
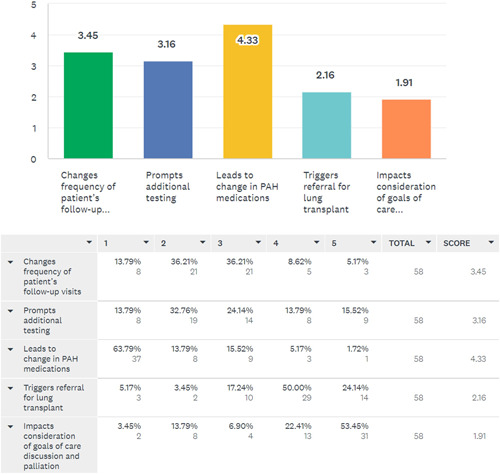
Ordinal scale showing the impact of risk assessment during follow‐up PAH patient evaluations on the elements of patient care. PAH, pulmonary arterial hypertension
Electronic medical records (EMR) utilization and risk assessment tools
A detailed description of the type of EMR system used by the respondents is shown in Table 3. A total of 60 (54%) responded about the EMR system information. A total of 48 (80%) of those respondents believed that having risk assessment tools incorporated in the EMR would improve patient care by facilitation of tracking and comparison of patients’ risk assessments during patient encounters, whereas 6 (10%) were unsure or felt it would not make a difference if risk tools were embedded in their EMR. A total of 41 (68%) felt the greatest barrier to the use of risk assessment tools on initial or follow up visits was the creation of risk assessment tools with the hospital EMR support team. A total of 12 (20%) felt that the time taken to calculate the risk score during each visit was the most significant impediment to regular use. A total of 3 (5%) believed that performing risk assessment tools has little impact on patient care and 4 (7%) did not believe there were any barriers to performing risk assessment during follow up visits. Figure 4 shows the interventions which clinicians believed may improve the utilization of risk assessment tools in PAH patient care.
Table 3.
Type of electronic medical records system utilized in the clinical settings of the participants
| N: 60 | ||
|---|---|---|
| Epic | 60.00% | 36 |
| Computerized Patient Record System (CPRS) | 3.33% | 2 |
| Cerner | 18.33% | 11 |
| Allscripts | 5.00% | 3 |
| CureMD | 0.00% | 0 |
| eClinicalWorks | 5.00% | 3 |
| GE Healthcare | 3.33% | 2 |
| Amazing Charts | 0.00% | 0 |
| Other (please specify EMR) | 5.00% | 3 |
Abbreviation: EMR, electronic medical records.
Figure 4.
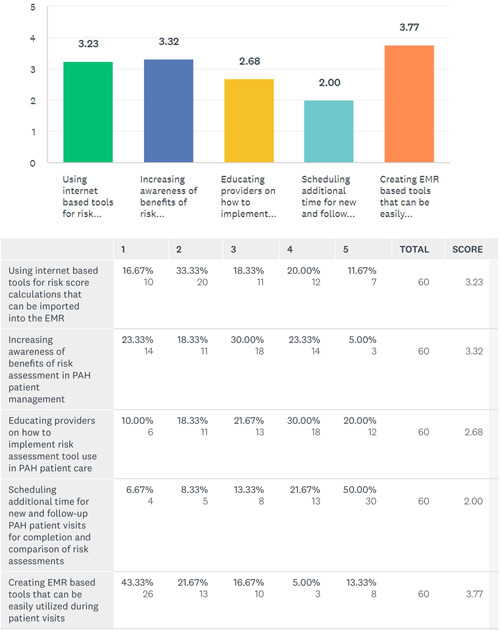
Ordinal scale of interventions which respondents believed may improve risk stratification utilization in clinical practice
DISCUSSION
Our survey is the first survey which was conducted to assess the utilization of risk assessment tools assessing impact of baseline and follow up management of patients with PAH. From a respondent pool comprised mostly of physicians in academic settings, we found suboptimal utilization of any risk assessment tool in clinical practice. However, we found that while a majority of respondents felt that risk scores influenced initial and subsequent management of medications for PAH, fewer felt that risk scores informed other aspects of care, such as frequency of follow up, recommended testing, or referrals to palliative care or lung transplantation. Importantly, most respondents felt that lack of integration of risk tool calculators in the EMR impacted their ability to use these tools in clinical practice.
In 2015, 1 the ESC/ERS Guidelines were the first to recommend routine use of objective risk assessment in clinical PAH patient care. Subsequently, proceedings of the World Symposium on Pulmonary Hypertension (WSPH) also emphasized the use of risk stratification in management of PAH. 15 Despite this, risk assessment has remained an under‐utilized tool in the management of PAH patients. Wilson et al., 14 reported in 2020 that only 59% of the respondents in their survey utilized risk assessment methods. 14 Our survey showed similar utilization of the risk assessment tools by only 63% of the participants. However, our respondent population differed from the cohort in the study from Wilson et al. 14 For example, more than 95% of the respondents to the current survey were physicians, compared to 77% in the prior survey. Sixty‐three percent of respondents in the current study had more than 10 years of experience treating PAH compared to 46%. Further, fewer respondents worked at PHA accredited centers (38% vs. 48%) and fewer cared for more than 50 PAH patients (55% vs. 82%) in our study compared to the cohort in the study by Wilson et al. 14 Whether these factors influenced the likelihood of use of risk assessment tools between these studies remains unclear.
Most respondents in our survey were from an academic center in an urban setting. Respondents in our survey were well experienced clinicians with almost 85% having at least 5 years with 37% having over 20 years of experience in managing PAH. The minority (38%) of US respondents reported their institution was associated with a PHA accredited CCC. In our survey, 97 participants responded to the question pertaining to the use of risk assessment tool utilization to assess 1‐year risk, of which 71 (73%) responded affirmatively to using the risk assessment tools. The majority used either REVEAL 2.0 8 or ESC/ERS 1 risk assessment tools. In 2020 Sahay 6 and colleagues reported a significant discrepancy between the physician gestalt and objective risk assessment in functional class II patients. In this analysis, up to 46% of patients judged to be low risk by gestalt were classified as intermediate risk by formal tools, and up to 28% of these ostensibly low‐risk patients were classified as high‐risk by the formal tools. Underestimation of patient's risk has potential to lead to undertreatment of PAH and adversely affect clinical outcomes. Our survey did detect an overall underutilization of formal PAH risk assessment tools and identified several obstacles to implementation; however, the survey was not designed to assess the provider characteristics of those that employ use of gestalt versus formal tools and the degree that each of the identified obstacles individually had on this practice pattern.
Our survey also provides unique insights into how clinicians use risk tools in clinical practice. Among those who use risk tools at baseline or follow up, the risk assessment impacted their treatment decisions for almost two‐thirds of clinicians. This is an important finding as it reflects that physicians are likely to make treatment or management interventions when objective risk assessment tools are employed. However, only a minority of respondents reported that risk tools influenced other aspects of clinical management, such as frequency of follow up or additional testing. Importantly, despite the strong association between risk scores and risk of death at 1 year, few respondents reported that risk scores influenced referral patterns to either palliative care or lung transplantation. Prior studies examining physician practice patterns have demonstrated low referral rates to palliative care for patients with PAH, citing physician reluctance as a common barrier. 16 , 17 Use of objective tools such as risk assessment could provide a structured approach to facilitate referrals to palliative care and thereby address this practice gap identified by the 6th WSPH. 18 Similarly, referral for lung transplantation could be based upon risk assessment to ensure timely evaluation. The results of this survey suggest that current use of risk assessment do not influence these important aspects of care of the patient with PAH and highlights the need for further education regarding the role of risk assessment in all facets of clinical care.
Despite the benefit of standardized PAH risk assessment, several barriers exist to implementation of risk scoring. Our survey participants suggested EMR integration is the most significant hurdle to overcome in using the risk tools followed by time constraints. We believe these two are likely interrelated. Since currently these tools are not widely integrated in EMR systems, it takes more time for clinicians to perform, and document results. Additionally, if an embedded EMR risk assessment tool is not available, future comparisons are hampered, likely resulting in limiting routine risk tracking in follow‐up patient care. Furthermore, use of streamlined risk assessment tools like REVEAL lite 2.0 or the simplified French method utilize less variables and potentially can be administered in a shorter time.
Our study has limitations. This survey was intended to target global PAH clinicians to obtain a worldwide view of utilization of risk tools. We did receive responses from several countries, but our findings are largely representative of the US based care due to limited participation from the international participants. We consider this survey findings largely representative of the US clinicians. Overall, the response rate was low in this survey. Although we had 112 participants who responded to the survey, only half responded to the questions related to the utilization of risk assessment tools which was the main objective of our questionnaire. This may reflect unfamiliarity with risk scores in general amongst respondents, highlighting a knowledge gap in the evaluation of patients with PAH. Further, the vast majority of respondents in our survey were pulmonologists, reflecting the membership of the ACCP; whether practice patterns by other subspecialists who care for PAH patients differ remains unknown. Along the same lines, inviting respondents via email to take part in an online survey could potentially introduce selection bias, in which email recipients with a greater interest in and or awareness of PAH risk assessment tools are more likely to participate in the survey.
Within the limitations of our survey, we believe that it has highlighted some important aspects and challenges of the care of PAH patients. Based on our findings, we would stress the importance of including these risk tools into the EMR systems with hopes for improved provider efficiency allowing close follow up of patients and adherence to current guidelines with the ultimate goal of optimization of care of PAH patients. Many physicians often are reluctant to use risk scores due to the limited prospective data behind derivation of these risk tools; however, assessing risk tool use impact in a prospective randomized blinded fashion has not been completed due to the complexities of such an assessment. Risk tools are being incorporated into recent clinical trials which will allow further insight into their value.
CONCLUSIONS
Objective risk stratification is recommended by most recent guidelines for the evaluation and management of patients with PAH. Despite these guidelines, our survey shows suboptimal utilization of PAH risk assessment tools even by experienced PAH providers. Our survey suggests that when employed, these tools are largely used to guide medical therapies, but not routinely utilized to guide follow up or referral to palliative care or transplantation. EMR integration appears to be one of the major impediments to implementation of these tools. EMR incorporation of risk tools could positively impact the frequency and ease of use of risk assessment tools by clinicians. Additionally, educational programs to increase awareness of the clinical impact of formal risk assessment in routine PAH care represents a potential strategy for increasing the utilization of routine risk assessment by PAH providers.
CONFLICT OF INTERESTS
Sandeep Sahay: Consultant for United Therapeutics, Acceleron, Actelion, Bayer Pharmaceuticals. Advisor and speaker for United Therapeutics, Actelion and Bayer. Research grant support from ACCP CHEST and United Therapeutics. Clinical Trial site PI for United Therapeutics, Actelion, Merck, Liquidia Altavant Sciences, and Gossamer Bio. Vijay Balasubramanian: Research support—United Therapeutics, Speaker bureau—Bayer, Boehringer Ingelheim, Consultant—Bayer, United Therapeutics. Lana Melendres‐Groves: Consultant, speaker and advisor—Bayer, United Therapeutics, Janssen. Stephen C Mathai: Consultant: Actelion, Bayer, Acceleron, United Therapeutics, Funding: NHLBI, DOD. Oksana Shlobin: Speaker & Consultant for United Therapeutics, J&J, and Bayer. Jean M Elwing: Consultant/advisor for Janssen/Actelion, United Therapeutics, Acceleron, Liquidia, Altavant, Aerovate, Gossamer Bio, and participated in clinical trials with Actelion, Acceleron, Altavant, Aerovate, Reata, United Therapeutics, Liquidia, Phase Bio, Gossamer Bio, Bayer, LungLLC. All other authors declare no conflict of interests.
AUTHOR CONTRIBUTIONS
Jean M Elwing and Humna Memon: were involved in the creation of the survey. All authors were involved in review and vetting of this survey content and questions. Sandeep Sahay: prepared the initial draft of the manuscript, revised and prepared responses to the comments from reviewers along with Jean M Elwing. All authors were actively involved in the review of findings and manuscript preparation.
ETHICS STATEMENT
The information presented is the authors' own original work, which has not been previously published elsewhere. The paper is not currently being considered for publication elsewhere. The paper is presented in a truthful and complete manner.
Supporting information
Supporting information.
ACKNOWLEDGEMENTS
Michelle Kosobucki: ACCP CHEST PVD NetWork Coordinator. Elizabeth Joseloff: PHA, Vice President, Quality Care & Research. Ed Graviss PhD and Duc Nguyen MD, PhD from Houston Methodist Hospital provided statistical support.
Sahay S, Balasubramanian V, Memon H, Poms A, Bossone E, Highland K, Kay D, Levine DJ, Mullin CJ, Melendres‐Groves L, Mathai SC, Soto FJ, Shlobin O, Elwing JM. Utilization of Risk Assessment Tools in Management of PAH: a PAH Provider Survey. Pulmonary Circulation. 2022;12:e12057. 10.1002/pul2.12057
GUARANTOR AUTHORS: Jean M. Elwing and Sandeep Sahay.
REFERENCES
- 1. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, ESC Scientific Document Group . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67–119. [DOI] [PubMed] [Google Scholar]
- 2. Kanwar MK, Thenappan T, Vachiery JL. Update in treatment options in pulmonary hypertension. J Heart Lung Transplant. 2016;35(6):695–703. [DOI] [PubMed] [Google Scholar]
- 3. Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long‐term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142(2):448–56. [DOI] [PubMed] [Google Scholar]
- 4. Benza RL, Farber HW, Selej M, Gomberg‐Maitland M. Assessing risk in pulmonary arterial hypertension: what we know, what we don't. Eur Respir J. 2017;50(2):1701353. [DOI] [PubMed] [Google Scholar]
- 5. Weatherald J, Boucly A, Sahay S, Humbert M, Sitbon O. The low‐risk profile in pulmonary arterial hypertension. Time for a Paradigm Shift to goal‐oriented clinical trial endpoints? Am J Respir Crit Care Med. 2018;197(7):860–868. [DOI] [PubMed] [Google Scholar]
- 6. Sahay S, Tonelli AR, Selej M, Watson Z, Benza RL. Risk assessment in patients with functional class II pulmonary arterial hypertension: comparison of physician gestalt with ESC/ERS and the REVEAL 2.0 risk score. PLoS One. 2020;15(11):e0241504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benza RL, Miller DP, Gomberg‐Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, McGoon MD. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long‐Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation. 2010;122(2):164–72. [DOI] [PubMed] [Google Scholar]
- 8. Benza RL, Gomberg‐Maitland M, Elliott CG, Farber HW, Foreman AJ, Frost AE, McGoon MD, Pasta DJ, Selej M, Burger CD, Frantz RP. Predicting survival in patients with pulmonary arterial hypertension: The REVEAL Risk Score Calculator 2.0 and comparison with ESC/ERS‐based risk assessment strategies. Chest. 2019;156(2):323–37. [DOI] [PubMed] [Google Scholar]
- 9. Benza RL, Kanwar MK, Raina A, Scott JV, Zhao CL, Selej M, Elliott CG, Farber HW. Development and Validation of an Abridged Version of the REVEAL 2.0 Risk Score Calculator, REVEAL Lite 2, for Use in Patients With Pulmonary Arterial Hypertension. Chest. 2021;159(1):337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoeper MM, Kramer T, Pan Z, Eichstaedt CA, Spiesshoefer J, Benjamin N, Olsson KM, Meyer K, Vizza CD, Vonk‐Noordegraaf A, Distler O, Opitz C, Gibbs J, Delcroix M, Ghofrani HA, Huscher D, Pittrow D, Rosenkranz S, Grünig E. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J. 2017;50(2):1700740. [DOI] [PubMed] [Google Scholar]
- 11. Boucly A, Weatherald J, Savale L, Jaïs X, Cottin V, Prevot G, Picard F, de Groote P, Jevnikar M, Bergot E, Chaouat A, Chabanne C, Bourdin A, Parent F, Montani D, Simonneau G, Humbert M, Sitbon O. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J. 2017;50(2):1700889. [DOI] [PubMed] [Google Scholar]
- 12. Kylhammar D, Kjellström B, Hjalmarsson C, Jansson K, Nisell M, Söderberg S, Wikström G, Rådegran G. A comprehensive risk stratification at early follow‐up determines prognosis in pulmonary arterial hypertension. Eur Heart J. 2018;39(47):4175–81. [DOI] [PubMed] [Google Scholar]
- 13. Humbert M, Farber HW, Ghofrani HA, Benza RL, Busse D, Meier C, Hoeper MM. Risk assessment in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Eur Respir J. 2019;53(6):1802004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilson M, Keeley J, Kingman M, Wang J, Rogers F. Current clinical utilization of risk assessment tools in pulmonary arterial hypertension: a descriptive survey of facilitation strategies, patterns, and barriers to use in the United States. Pulm Circ. 2020;10(3):2045894020950186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galiè N, Channick RN, Frantz RP, Grünig E, Jing ZC, Moiseeva O, Preston IR, Pulido T, Safdar Z, Tamura Y, McLaughlin VV. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53(1):1801889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anand V, Vallabhajosyula S, Cheungpasitporn W, Frantz RP, Cajigas HR, Strand JJ, DuBrock HM. Inpatient palliative care use in patients with pulmonary arterial hypertension: temporal trends, predictors, and outcomes. Chest. 2020;158(6):2568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fenstad ER, Shanafelt TD, Sloan JA, Novotny PJ, Durst LA, Frantz RP, McGoon MD, Swetz KM. Physician attitudes toward palliative care for patients with pulmonary arterial hypertension: results of a cross‐sectional survey. Pulm Circ. 2014;4(3):504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McGoon MD, Ferrari P, Armstrong I, Denis M, Howard LS, Lowe G, Mehta S, Murakami N, Wong BA. The importance of patient perspectives in pulmonary hypertension. Eur Respir J. 2019;53(1):1801919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


