Abstract
Three clinical strains (Escherichia coli Rio-6, E. coli Rio-7, and Enterobacter cloacae Rio-9) collected in 1996 and 1999 from hospitals in Rio de Janeiro (Brazil) were resistant to broad-spectrum cephalosporins and gave a positive double-disk synergy test. Two blaCTX-M genes encoding β-lactamases of pl 7.9 and 8.2 were implicated in this resistance: the blaCTX-M-9 gene observed in E. coli Rio-7 and E. cloacae Rio-9 and a novel CTX-M-encoding gene, designated blaCTX-M-16, observed in E. coli strain Rio-6. The deduced amino acid sequence of CTX-M-16 differed from CTX-M-9 only by the substitution Asp-240→Gly. The CTX-M-16-producing E. coli transformant exhibited the same level of resistance to cefotaxime (MIC, 16 μg/ml) but had a higher MIC of ceftazidime (MIC, 8 versus 1 μg/ml) than the CTX-M-9-producing transformant. Enzymatic studies revealed that CTX-M-16 had a 13-fold higher affinity for aztreonam and a 7.5-fold higher kcat for ceftazidime than CTX-M-9, thereby showing that the residue in position 240 can modulate the enzymatic properties of CTX-M enzymes. The two blaCTX-M-9 genes and the blaCTX-M-16 gene were located on different plasmids, suggesting the presence of mobile elements associated with CTX-M-encoding genes. CTX-M-2 and CTX-M-8 enzymes were found in Brazil in 1996, and two other CTX-M β-lactamases, CTX-M-9 and CTX-M-16, were subsequently observed. These reports are evidence of the diversity of CTX-M-type extended-spectrum β-lactamases in Brazil.
The first extended-spectrum β-lactamase (ESBL) of the CTX-M type (MEN-1, CTX-M-1) was reported at the beginning of the 1990s (3, 5). Initially found in Europe, CTX-M-producing strains have now been observed over a wide geographic area including the Near East (6), Far East (20, 25, 37), South America (4, 6; M. Galas, F. Pasteran, R. Melano, A. Petroni, G. Lopez, A. Corso, A. Rossi, and WHONET Collaborative Group, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-109, p. 201, 1998), and Europe (3, 5, 14–17, 33). CTX-M enzymes have been observed in different species of the family Enterobacteriaceae: Escherichia coli (3, 5, 20, 25, 37), Salmonella enterica serovar Typhimurium (4, 15, 16), Klebsiella pneumoniae (6), Proteus mirabilis (6, 9), Citrobacter freundii (6, 17), Citrobacter amalonaticus (9), Enterobacter aerogenes (9), and Enterobacter cloacae (9), and in the species Vibrio cholerae E1 Tor (M. Galas, A. Petroni, R. Melano, A. Corso, M. Rodriguez, M. L. Cacace, A. Bru, and A. Rossi, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother. abstr. C-174, p. 119, 1998).
The CTX-M enzymes form a rapidly growing family that comprises 11 enzymes, of which 8 have been described in the last 3 years. The CTX-M enzymes have been subclassified by amino acid sequence similarities into four groups (9). The first group contains enzymes CTX-M-1 (MEN-1) (3, 5) and CTX-M-3 (17); the second group contains the enzymes CTX-M-2 (6), Toho-1 (20), CTX-M-4 (14, 16), CTX-M-5 (11), CTX-M-6 (15), and CTX-M-7 (15) (previously designated CTX-M-5); and the third and fourth groups contain CTX-M-8 (9) and Toho-2, respectively (25). E. coli strains producing CTX-M-9 and CTX-M-10, which are related to Toho-2 and CTX-M-3, respectively, were recently reported in Spain (33; A. Oliver, J. C. Pérez-Díaz, T. M. Coque, F. Baquero, and R. Cantón, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1480, p. 99, 2000).
The CTX-M-type enzymes are much more active against cefotaxime than against ceftazidime and aztreonam. The amino acid residues critical for their extended-spectrum activity are partially known. Ser-237 and Arg-276 seem to be involved in the cefotaxime-hydrolyzing activity of CTX-M enzymes, but mutagenesis experiments of these residues led to only slight changes in their catalytic activities (14, 16). The extended-spectrum activity of CTX-M-type β-lactamases seems therefore to be an “intrinsic” enzymatic property of these ESBLs and not the result of a few point mutations (14–16, 19).
To estimate the diversity of ESBLs in Brazil, clinical strains that exhibited ESBL phenotypes in different species were collected from hospitals in Rio de Janeiro in 1996 and 1999. We report three CTX-M-producing strains isolated in Brazil: two producing CTX-M-9 and one producing a novel Asp-240→Gly variant of CTX-M-9, designated CTX-M-16.
MATERIALS AND METHODS
Clinical strains.
Table 1 shows the clinical strains and the plasmids used in this study. Three clinical strains were isolated from three different patients hospitalized in 1996 (Rio-6 and Rio-7) and in 1999 (Rio-9) of distinct private hospitals of Rio de Janeiro, Brazil. E. coli strain MEN producing CTX-M-1 (3) was used to synthesize the CTX-M probe.
TABLE 1.
Clinical strains from Rio de Janeiro (Brazil) and recombinant plasmids used in the study
| Strain or plasmid | Relevant genotype or phenotype | β-Lactamase pI(s) |
|---|---|---|
| Strains | ||
| E. coli Rio-6 | Clinical strain harboring ESBL resistance phenotype (1996)a | 5.4, 8.2 |
| E. coli Rio-7 | Clinical strain harboring ESBL resistance phenotype (1996)a | 5.4, 6.3, 7.9 |
| E. cloacae Rio-9 | Clinical strain harboring ESBL resistance phenotype (1999)a | 5.4, 6.3, 7.9, >8 |
| E. coli DH5α | supE44 hsdS20(rB− mB−) recA13 ara-14 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1 | |
| E. coli HB101 | supE44 ΔlacU169(φ80 lacZDM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | |
| Plasmids | ||
| pRio-6 | Natural plasmid of 70 kb from E. coli Rio-6 containing blaCTX-M-16 | 5.4, 8.2 |
| pRio-7 | Natural plasmid of 98 kb from E. coli Rio-7 containing blaCTX-M-9 | Not obtained |
| pRio-9 | Natural plasmid of 180 kb from E. cloacae Rio-9 containing blaCTX-M-9 | 5.4, 7.9 |
| pCPRio-6 | Recombinant pBK-CMV plasmid containing 0.9-kb fragment with blaCTX-M-16 | 8.2 |
| pCPRio-7 | Recombinant pBK-CMV plasmid containing 0.9-kb fragment with blaCTX-M-9 | 7.9 |
| pBK-CMV | Phagemid vector kanamycin resistance phenotype |
Year isolated is shown in parentheses.
Mating-out assays.
Direct transfers of plasmids harboring bla genes were performed by mating donor strains with in vitro-obtained rifampin- or nalidixic acid-resistant mutants of E. coli HB101 (34) as recipient strains, at 37°C on solid Mueller-Hinton medium. Transconjugants were selected on Mueller-Hinton agar containing rifampin (300 μg/ml) or nalidixic acid (150 μg/ml) and cefotaxime (2 μg/ml).
Susceptibility to β-lactams.
MICs were determined by a dilution method on Mueller-Hinton agar (Sanofi Diagnostics Pasteur, Marnes la Coquette, France) with an inoculum of 104 CFU per spot. Antibiotics were provided as powders by SmithKline Beecham Pharmaceuticals (clavulanate), Lederle Laboratories (tazobactam), Eli Lilly, Paris, France (cephalothin), Roussel-Uelaf (cefotaxime, cefpirome), Glaxo Wellcome Research and Development (ceftazidime), and Bristol-Myers Squibb (aztreonam).
Detection of ESBLs was performed with the standard double-disk synergy tests as described previously (21). Antibiotic disks for agar tests were obtained from Sanofi Diagnostics Pasteur.
Isoelectric focusing.
Isoelectric focusing was performed with polyacrylamide gels containing ampholines with a pH range of 3.5 to 10 as previously described (8). Visualization of β-lactamase activity was carried out with an agarose overlay containing 6% (wt/vol) potassium iodide, 0.6% (wt/vol) iodine, and 0.6% (wt/vol) β-lactam substrate: penicillin G to show overall β-lactamase content or cefotaxime to show the cefotaxime-hydrolyzing β-lactamases. β-lactamases of known pIs were used as standards: TEM-1 (pI 5.4), TEM-3 (pI 6.3), TEM-24 (pI 6.5), SHV-1 (pI 7.6), P99 (pI 7.8), and SHV-5 (pI 8.2).
β-Lactamase preparation.
CTX-M-producing E. coli DH5α (pCIRio-6 and pCIRio-7) were grown in 6 liters of brain heart infusion broth containing cefotaxime at 2 μg/ml for 18 h at 37°C. The bacteria collected by centrifugation were suspended with 20 mM morpholineethane sulfonic acid (MES)-NaOH (pH 6.0) and disrupted by ultrasonic treatment (four times for 30 s, each time at 20 W). After centrifugation (10,000 × g for 10 min at 4°C), nucleic acids were precipitated by addition of 0.2 M (7% [vol/vol]) spermine and centrifugation at 48,000 × g for 60 min at 4°C. The clarified supernatant was dialyzed overnight against 20 mM MES-NaOH (pH 6.0). The CTX-M purification was carried out as previously described (8) by ion-exchange chromatography with an SP Sepharose column (Amersham Pharmacia Biotech) and gel-filtration chromatography with a Superose 12 column (Amersham Pharmacia Biotech). The total protein concentration was estimated by the Bio-Rad protein assay (Bio-Rad, Richmond, Calif.), with bovine serum albumin (Sigma Chemical Co., St. Louis, Mo.) used as a standard.
The purity of CTX-M extracts was estimated as previously described (8) by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and staining with Coomassie blue R-250 (Sigma Chemical Co.). The renaturation of proteins and the detection of β-lactamase activity were performed as previously described (8) with renaturation buffer {Tris-HCI (100 mM), Triton X-100 (2% [vol/vol]), pH 7.0} and 0.5 mM nitrocephin (Oxoid, Paris, France) in 100 mM phosphate buffer (pH 7.0), respectively.
Determination of β-lactamase kinetic constants.
The kinetic constants Km and Kcat of the β-lactamases were obtained by computerized microacidimetric method as previously described (24). The concentrations of the inhibitors (clavulanate and tazobactam) required to inhibit enzyme activity by 50% (IC50s) were determined as described previously with penicillin G (7). The specific activities, IC50, and Ki values were determined with penicillin G (200 mM) as the reporter substrate. The kinetic constants were determined three times. The variation coefficients had a maximum of 25%, except with the CTX-M-9 enzyme for aztreonam and ceftazidime, for which the maximum was 40%.
PCR of CTX-M genes.
The detection of CTX-M-1-, CTX-M-2-, and CTX-M-9-like-encoding genes and the synthesis of CTX-M probe were performed with the primers CTX-MA (5′-CGCTTTGCGATGTGCAG-3′) and CTX-MB (5′-ACCGCGATATCGTTGGT-3′) (temperature of annealing, 54°C), which correspond to conserved regions of CTX-M-type genes. An internal fragment of 550 pb was amplified from positions 264 to 814 (blaCTX-M-1 numbering). The complete open reading frames of blaCTX-M-9-like genes were amplified with the primers CTX-M-9A (5′-CTGATGTAACACGGATTGAC-3′) and CTX-M-9C (5′-AGCGCCCCATTATTGAGAG-3′), which were located in the flanked sequences of blaCTX-M-9 (temperature of annealing, 54°C).
Plasmid extraction and hybridization.
Plasmid DNAs were extracted by the method of Kado and Liu (22). The plasmid size was determined by comparison with plasmids Rsa (39 kb), TP114 (61 kb), pCFF04 (85 kb), and pCFF14 (180 kb) (7).
DNA probes used for hybridization were PCR products obtained with the primers CTX-MA and CTX-MB. Labeling was performed by random priming with a 2,4-dinitrophenol DNA labeling kit purchased from Appligene Oncor (Illkirch, France). Hybridization and revelation were performed with a DNP-DNA chemiluminescence detection kit (Appligene Oncor) according to the manufacturer's recommendations for DNA extracts denatured and immobilized on Nytran Filters.
β-Lactamase gene cloning.
The recombinant DNA manipulations were performed as described by Sambrook et al. (34). T4 DNA ligase was purchased from Boehringer, Mannheim, Germany. The CTX-M-encoding sequence was cloned as follows. PCR products, which were obtained with proofreading Taq polymerase Tfu (Appligene Oncor) and the primers CTX-M-9A and CTX-M-9C, were ligated in the SmaI site of the phagemid pBK-CMV (Stratagene, La Jolla, Calif.). E. coli DH5α (34) was transformed by electroporation. The transformants harboring the recombinant CTX-M-encoding plasmids were selected on Mueller-Hinton agar supplemented with 2 μg of cefotaxime per ml.
DNA sequencing.
The sequences were determined by direct sequencing of PCR products, performed by the dideoxy chain termination procedure of Sanger et al. (35) on an ABI 1377 automatic sequencer using the ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction kit with AmpliTaq DNA polymerase FS (Perkin-Elmer/Applied Biosystems, Foster City, Calif.).
Computer analysis.
The nucleotide sequence and the deduced protein sequence were analyzed with the software available over the Internet at the National Center for Biotechnology Information. Hydrophobic blotting was performed using the method of Nielsen et al. (27). Multiple sequence alignment and pairwise comparisons of sequences were carried out with the help of ClustalW Software (version 1.74) (36). Twelve class A CTX-M enzymes were compared to CTX-M-16: CTX-M-1, CTX-M-2, Toho-1, CTX-M-3, CTX-M-4, CTX-M-5, CTX-M-6, CTX-M-7, CTX-M-8, CTX-M-9, CTX-M-10, and Toho-2. Phylogenetic analysis was performed by the neighbor-joining method using PHYLIP (Phylogeny Inference Package, version 3.5c) from a distance matrix, which was carried out with the Dayhoff PAM matrix (13).
Nucleotide sequence accession number.
The blaCTX-M-16nucleotide sequence data appear in the GenBank nucleotide sequence database under accession number AY029068.
RESULTS
Characterization of clinical isolates.
The clinical isolates Rio-6, Rio-7, and Rio-9 exhibited resistance to broad-spectrum cephalosporins (MICs: cefotaxime, 16 to 128 μg/ml; ceftazidime, 32 to 256 μg/ml; aztreonam, 64 to 128 μg/ml) and a positive double-disk synergy test. Isoelectric point determination with penicillin G as substrate revealed the presence of two to three different β-lactamases per strain (Table 1), but using cefotaxime as substrate, only one enzyme, of pI 7.9 in strains Rio-7 and Rio-9 or of pI 8.2 in strain Rio-6, showed a strong cefotaxime-hydrolyzing activity. The three strains exhibited a positive amplification with the primers CTX-MA and CTX-MB, which were designated from conserved sequences of blaCTX-M genes. These results suggested the presence of two distinct blaCTX-M genes which encoded enzymes of pI 8.2 in strain Rio-6 and of pI 7.9 in strains Rio-7 and Rio-9.
Plasmid content and transfer of β-lactam resistance.
Transconjugants were obtained from E. coli Rio-6 and E. cloacae Rio-9. They produced cefotaxime-hydrolyzing β-lactamases of alkaline pI (8.2 or 7.9) and associated with a β-lactamase of pI 5.4, which was identified by PCR and sequencing as the TEM-1 penicillinase.
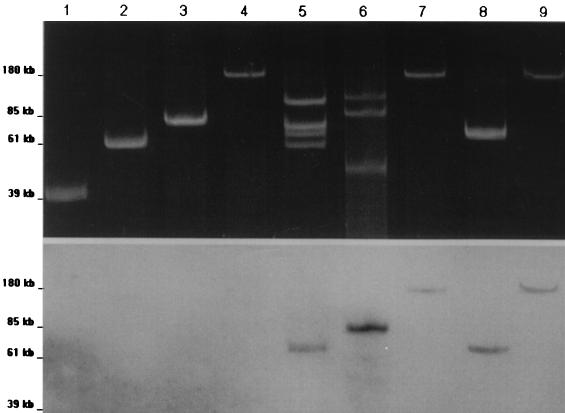
The plasmid contents of the clinical strains are shown in Fig. 1 as is their hybridization with CTX-M probe. E. coli Rio-6 contained four plasmids of 118, 78, 70, and 61 kb. Only the plasmid of 70 kb, designated pRio-6, was observed in the transconjugant of strain Rio-6 and hybridized with CTX-M probe. E. cloacae Rio-9 contained one plasmid of 180 kb, designated pRio-9, which was observed in the transconjugant of strain Rio-9 and hybridized with the CTX-M probe. E. coli Rio-7 contained three plasmids (134, 98, and 48 kb); among them the plasmid of 98 kb, designated pRio-7, hybridized with the CTX-M probe.
FIG. 1.
(A) Agarose (0.7%) electrophoresis of plasmid DNA from the three clinical strains, Rio-6, Rio-7, and Rio-9, and the E. coli transconjugants. (B) Hybridization of plasmid content with a CTX-M probe. Lanes: 1 to 4, reference plasmids RSa (39 kb), TP114 (61 kb), pCFF04 (85 kb), and pCFF14 (180 kb); 5, E. coli Rio-6; 6, E. coli Rio-7; 7, E. cloacae Rio-9; 8, E. coli DH5α(pRio-6); 9, E. coli DH5α(pRio-9).
DNA sequencing.
DNA sequencing of PCR products obtained with primers CTX-M-9A and CTX-M-9C revealed that strains Rio-7 and Rio-9 contained the gene blaCTX-M-9, previously reported in an E. coli strain isolated in Spain (33), whereas strain Rio-6 harbored a new gene, designated blaCTX-M-16, which differed from blaCTX-M-9 by the mutation A→G at position 725.
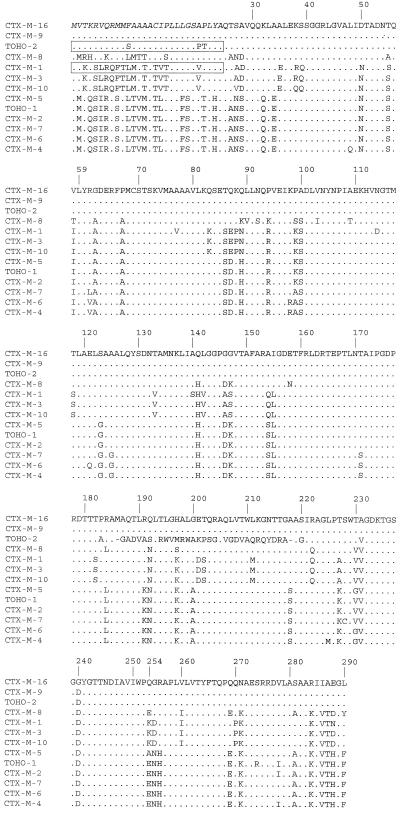
The amino acid sequence deduced for blaCTX-M-16 differed from that of CTX-M-9 by the substitution Asp→Gly in position 240 (Fig. 2). The strain Rio-6 thus produced a novel CTX-M-type enzyme, which was designated CTX-M-16.
FIG. 2.
Alignments of the CTX-M-16 amino acid sequence with those of CTX-M-1, CTX-M-2, CTX-M-3, CTX-M-4, CTX-M-5, CTX-M-6, CTX-M-7, CTX-M-8, CTX-M-9, Toho-1, and Toho-2. Dots indicate amino acids identical to those of CTX-M-16. Italic letters represent the peptide signal of CTX-M-16, as determined by hydropathy plotting. The peptide signals determined by amino acid sequencing are boxed. The amino acids are numbered according to the standard numbering scheme for the class A β-lactamases of Ambler et al. (1).
On the basis of amino acid sequence alignments (Fig. 2) with CTX-M signal peptide sequences previously determined by direct amino acid sequencing (4, 29) and from hydropathy plots, it is likely that the signal peptide of CTX-M-16 and CTX-M-9 comprise 28 amino acids. Thus, the putative mature enzymes CTX-M-9 and CTX-M-16 consist of 263 amino acid residues with calculated molecular masses of 28,945 and 27,887 Da, respectively.
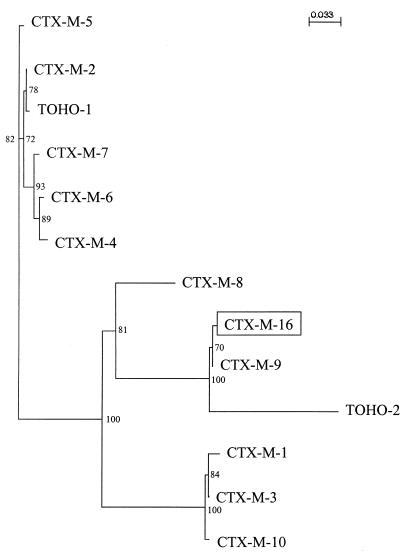
A dendrogram was constructed on the basis of peptide alignment (Fig. 3). Bootstrapping gave a high degree of resolution for internal nodes (superior to 50% majority-consensus confidence). The dendrogram showed four major branches, of which one comprised the enzymes Toho-2, CTX-M-9, and CTX-M-16.
FIG. 3.
Dendrogram of CTX-M family. Branch lengths are scaled according to the amino acid changes. The scale indicates the distance measured for protein sequences based on the Dayhoff PAM matrix (13). The percentages at the branch points refer to the number of times a particular nod was found in 100 bootstrap replications. The distance along the vertical axis has no significance.
Cloning of the β-lactamase gene.
The coding sequences of CTX-M-9 and CTX-M-16, obtained by PCR with primers CTX-M-9A and CTX-M-9B, were cloned downstream of the LacZ promoter of plasmid pBK-CMV. The obtained recombinant plasmids were designated pCPRio-6 and pCPRio-7 and contained a 0.9-kb insert encoding the enzymes CTX-M-16 and CTX-M-9, respectively.
β-Lactam susceptibility.
MICs of β-lactams for the E. coli DH5α tranformants producing CTX-M-9 (pCPRio-7) and CTX-M-16 (pCPRio-6) are listed in Table 2. These CTX-M-producing strains exhibited a high level of resistance to amino- and carboxypenicillins (MICs, >2,048 μg/ml), piperacillin (MIC, 256 μg/ml), and cephalothin and cefuroxime (MICs, 512 μg/ml). The similar level of resistance to cefotaxime (MIC, 16 μg/ml) and aztreonam (MICs, 4 to 8 μg/ml) was observed with CTX-M-16 and CTX-M-9 producers, while the MICs of ceftazidime were higher for CTX-M-16 producers than for CTX-M-9 producers (MICs, 8 versus 1 μg/ml).
TABLE 2.
β-Lactam MICs for CTX-M-9- and CTX-M-16-producing E. coli DH5α
| β-Lactam | MIC (μg/ml) for E. coli DH5α with:
|
||
|---|---|---|---|
| PCPRio-6a CTX-M-16 | pCPRio-7b CTX-M-9 | pBK-CMV | |
| Amoxicillin | >2,048 | >2,048 | 2 |
| Amoxicillin + CLAc | 8 | 8 | 2 |
| Ticarcillin | >2,048 | >2,048 | 2 |
| Ticarcillin + CLAc | 16 | 16 | 2 |
| Piperacillin | 256 | 256 | 2 |
| Piperacillin + TZBd | 2 | 2 | 2 |
| Cephalothin | 512 | 512 | 4 |
| Cefuroxime | 512 | 512 | <2 |
| Cefotaxime | 16 | 16 | 0.06 |
| Cefotaxime + CLAc | 0.06 | 0.06 | 0.06 |
| Cefotaxime + TZBc | 0.06 | 0.06 | 0.06 |
| Cefotaxime + SULc | 0.50 | 2 | 0.06 |
| Aztreonam | 8 | 4 | 0.12 |
| Aztreonam + CLAc | 0.06 | 0.06 | 0.06 |
| Aztreonam + TZBc | 0.06 | 0.06 | 0.12 |
| Aztreonam + SULc | 0.25 | 0.50 | 0.12 |
| Ceftazidime | 8 | 1 | 0.12 |
| Ceftazidime + CLAc | 0.25 | 0.25 | 0.12 |
| Ceftazidime + TZBc | 0.25 | 0.25 | 0.12 |
| Ceftazidime + SULc | 0.50 | 0.50 | 0.12 |
| Cefpirome | 2 | 2 | 0.06 |
| Cefpirome + CLA | 0.06 | 0.06 | 0.06 |
| Cefpirome + TZBc | 0.06 | 0.06 | 0.06 |
| Cefpirome + SULc | 0.25 | 0.50 | 0.06 |
E. coli DH5α harboring recombinant plasmid pCPRio-6, which encodes β-lactamase CTX-M-16.
E. coli DH5α harboring recombinant plasmid pCPRio-7, which encodes β-lactamase CTX-M-9.
CLA, clavulanate; TZB, tazobactam; SUL, sulbactam at fixed concentration of 2 μg/ml.
TZB at fixed concentration of 4 μg/ml, as in the commercially available combination with piperacillin.
Clavulanate and tazobactam restored partially or totally the activities of the β-lactams. All strains were susceptible to associations of clavulanate or tazobactam and broad-spectrum cephalosporins (MICs, 0.06 to 0.25 μg/ml).
Biochemical properties of the β-lactamase CTX-M-16.
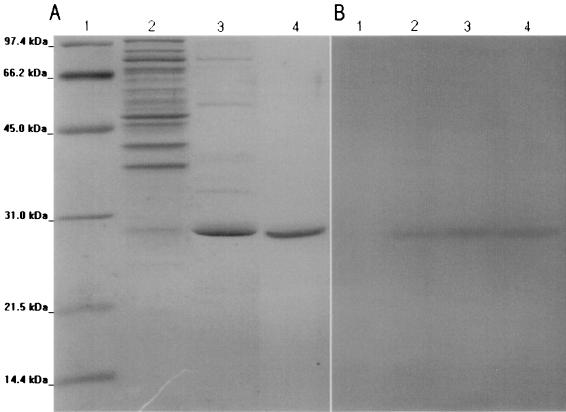
The purified proteins appeared on SDS-polyacrylamide gels as a band of 28.6 kDa for CTX-M-16 (Fig. 4) and CTX-M-9 (data not shown). The specific activities of purified (≥97% pure) CTX-M-16 and CTX-M-9 were 140 and 615 μmol · min−1 · mg of protein−1, respectively, with 200 mM penicillin G as the substrate.
FIG. 4.
Electrophoresis analysis of CTX-M-9 and CTX-M-16 purified extracts. (A) SDS-PAGE stained with Coomassie brillant blue R-250. (B) Zymogram detection of β-lactamase activity with nitrocefin after renaturation treatment and SDS-PAGE. Lanes: 1, protein molecular mass reference; 2, clarified extract of β-lactamase CTX-M-16; 3, fraction with β-lactamase activity eluted from ionic exchange column; 4, purified extract of β-lactamase CTX-M-16.
The substrates of CTX-M-9 and CTX-M-16 are shown in Table 3. Common enzymatic features included the following: better affinities were found for penicillins (Km, 6 to 35 μM) than for cephalosporins, except for CTX-M-16 and aztreonam (Km 17 μM); cephalothin was the best substrate (Kcat for cephalothin 10- to 43-fold higher than that for penicillin); and a higher kcat for cefotaxime (450 to 1400 s−1) was found than for ceftazidime (2 to 15 s−1) and aztreonam (10 to 3 s−1).
TABLE 3.
Substrate profiles of β-lactamases CTX-M-9 and CTX-M-16
| Substrate | CTX-M-9
|
CTX-M-16
|
||||
|---|---|---|---|---|---|---|
| Kcat (s−1) | Km (μM) | Kcat/Km (μM−1 · s−1) | Kcat (s−1) | Km (μM) | Kcat/Km (μM−1 · s−) | |
| Penicillin G | 295 | 2.5 | 11.8 | 65 | 6 | 10.8 |
| Amoxicillin | 90 | 20 | 4.5 | 40 | 10 | 4.0 |
| Ticarcillin | 60 | 35 | 1.7 | 10 | 13 | 0.8 |
| Piperacillin | 110 | 20 | 5.5 | 45 | 8 | 5.6 |
| Cephalothin | 3,000 | 150 | 20.0 | 2,800 | 83 | 33.7 |
| Cefuroxime | 350 | 50 | 7.0 | 500 | 45 | 11.0 |
| Cefotaxime | 450 | 120 | 3.7 | 1,400 | 150 | 9.3 |
| Cefpirome | 950 | 800 | 1.2 | 840 | 520 | 1.6 |
| Aztreonam | 10 | 220 | 0.04 | 3 | 17 | 0.2 |
| Ceftazidime | 2 | 600a | 0.003 | 15 | 350 | 0.04 |
Km values were determined as Ki by substrate competition with penicillin G.
However, CTX-M-16 had globally higher affinities (lower Km values) than CTX-M-9 for β-lactams, particularly for aztreonam, since the binding of this substrate was 13-fold higher for CTX-M-16 than for CTX-M-9. Conversely, CTX-M-9 had higher Kcat values than CTX-M-16 with all substrates except cefuroxime and particularly for cefotaxime and ceftazidime, for which kcat values were higher for CTX-M-16 than for CTX-M-9.
CTX-M-9 and CTX-M-16 were susceptible to tazobactam (IC50s, 0.036 and 0.030 μM, respectively), clavulanate (IC50s, 0.007 and 0.008 μM, respectively), and to a lesser extent to sulbactam (IC50s, 3.0 and 4.5 μM, respectively).
DISCUSSION
The starting point of this work was the observation of three clinical strains isolated in Brazil, E. coli Rio-6 (isolated from urine of a patient hospitalized in a home care unit) and E. coli Rio-7 and E. cloacae Rio-9 (isolated from tracheal aspirations of patients hospitalized in an intensive care unit), that exhibited resistance to broad-spectrum cephalosporins with a positive double-disk synergy test. Two different CTX-M-encoding genes were implicated in this resistance phenotype: the novel CTX-M-encoding gene blaCTX-M-16, which was related to blaCTX-M-9 and was observed in E. coli strain Rio-6, and the blaCTX-M-9 gene (33) in strains E. coli Rio-7 and E. cloacae Rio-9.
The blaCTX-M-16 gene is probably the result of a point mutation of the blaCTX-M-9 gene, because the two genes were characterized in the same geographic area and differed by one nucleotide only. blaCTX-M-16 and the closely related gene blaToho-2 were observed at almost the same time, in Spain and Japan, respectively (25, 33). The concurrent emergence of these blaCTX-M-9-type genes in two geographic areas widely separated could be explained by their transfer from widespread environmental strains, as previously observed with the gene blaSFO-1, which was probably transferred from Serratia fonticola to E. cloacae (26). The most closely related enzymes to CTX-M genes are the chromosome-encoded enzymes of Klebsiella oxytoca (2, 32), Serratia fonticola (28), Citrobacter diversus (30), and Proteus vulgaris (29), but there is no direct phylogenetic connection between these β-lactamases and CTX-M enzymes. In addition, blaCTX-M-2-like genes have been observed in the chromosome of K. ascorbata (A. Philippon, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr 2044a, 1999), which therefore is a potential reservoir for a CTX-M-encoding gene. However, the CTX-M-9 and CTX-M-16 enzymes are distantly related to the CTX-M-2-like β-lactamases and hence could originate from variant genes present in the same species or in related species. The two blaCTX-M-9 genes and the blaCTX-M-16 gene, like blaCTX-M-8 and blaCTX-M-3 (9, 17), were located on different plasmids. This suggests that mobile elements associated with CTX-M-encoding genes are present and that they could be involved in gene transfers.
The phylogenetic relations between the enzymes reported in the CTX-M family show four types of CTX-M enzymes-CTX-M-1 type, which includes CTX-M-1, CTX-M-3, and CTX-M-10; CTX-M-2 type, which includes CTX-M-2, Toho-1, CTX-M-4, CTX-M-5, CTX-M-6, and CTX-M-7; CTX-M-8 type, which has only one member; and CTX-M-9 (or Toho-2) type, which includes Toho-2, CTX-M-9, and the novel enzyme CTX-M-16.
The catalytic properties of CTX-M-9 and CTX-M-16 have similarities with those of previously reported CTX-M enzymes, such as higher catalytic activity against cefotaxime than against ceftazidime and aztreonam. The enzymes CTX-M-9 and CTX-M-16 include the amino acid residues Ser-237, Phe-160, Gly-232, and Arg-276, which are thought to play a part in the cefotaxime-hydrolyzing activity of CTX-M enzymes (3, 14–16, 19, 20, 25). In addition, the CTX-M-9 and CTX-M-16 β-lactamases are susceptible to inhibitors, and in particular to tazobactam, as reported elsewhere (9, 15, 16, 25).
The residue in position 240 is not conserved among β-lactamases of class A. At least three types of residue are observed at this position: (i) acid residues (Glu or Asp) in TEM and SHV-1 penicillinases, as well as in CTX-M ESBLs and their closely related enzymes; (ii) a Lys residue in some SHV- and TEM-type ESBLs; (iii) a Gly residue in carboxypenicillinases and in the ESBLs PER, VEB-1, and BES-1 (8, 31). CTX-M-16 is the first CTX-M-type ESBL which harbors the substitution Asp-240→Gly. This substitution increased the binding of aztreonam (13-fold), as well as the kcat against ceftazidime (7.5-fold) and to a lesser extent that against cefotaxime (3-fold). The cefotaximase BES-1, which harbors the same Gly residue in position 240, exhibits the same behavior against aztreonam, cefotaxime, and ceftazidime as does CTX-M-16 (8). The ESBL PER-1, which has a good catalytic efficiency against cefotaxime and ceftazidime, also contains a Gly-240 residue. Bouthors et al. (10) showed that the substitution Gly-240→Glu of PER-1 causes a reduction of affinity for aztreonam (around fourfold) and a decrease in catalytic activity against cefotaxime and ceftazidime (around twofold and threefold, respectively).
Residues Lys-240 and Arg-240 are known to play a major role in the extended-spectrum activity of TEM- and SHV-type ESBLs. They lead to an increase in hydrolyzing activities against ceftazidime and aztreonam but are not involved in cefotaxime-hydrolyzing activity. Lys and Arg are positively-charged residues which can form an electrostatic bond with the carboxylic acid group on oximino substituents of aztreonam and ceftazidime (23) and induce a rotation of the aminothiazole-oxime group (18). They could therefore remove the obstruction caused by the bulky t-carbon group of the oxime and allow hydrogen bonding of the acylamide NH to the β3-strand of the enzyme (18). Thus, Lys and Arg in position 240 favor carboxy oximino β-lactam bonding and hydrolyzing. Residue Gly-240, which has no side chain, is not able to form electrostatic interactions with β-lactams. However, the absence of a side chain in position 240 could facilitate the positioning of the aminothiazole-oxime group of cefotaxime, aztreonam, and ceftazidime in the catalytic pocket.
In contrast with cefotaxime- and ceftazidime-hydrolyzing activities, aztreonam-hydrolyzing activity was not modified by the residue Gly-240. The sulfonic acid group of aztreonam may form weaker interactions with the side chains of residues Ser-130, Lys-234, Thr-235, and Arg-276 of the CTX-M β-lactamases than does the functionally equivalent carboxylate group of cefotaxime and ceftazidime (12). Thus, the weak interaction of aztreonam with CTX-M-16 may alter its positioning in its catalytic pocket, resulting in a weak aztreonam-hydrolyzing activity. It is likely therefore that aztreonam, unlike cefotaxime and ceftazidime, requires the electrostatic interaction mediated by the positively-charged residues in position 240 for correct positioning and, hence, for hydrolysis. However, the Asp-240→Lys substitution would require two mutations in the corresponding codon and thus is a more improbable spontaneous genetic event than the single mutation observed in the CTX-M-16 enzyme.
Since the first report of MEN-1 (CTX-M-1) a decade ago (3, 5), a great variety of CTX-M enzymes have been observed. In a previous study, we reported CTX-M-2- and CTX-M-8-producing Enterobacteriaceae isolated in Brazil (9), and in this work we report CTX-M-9- and CTX-M-16-producing Enterobacteriaceae. These findings show the spread, diversity, and implantation of CTX-M enzymes in Brazil. CTX-M-2 was first characterized in Argentina, and Galas et al. reported that the predominant ESBL in the country is CTX-M-2 (M. Galas et al., 38th ICAAC, abstr. E-109). Like Eastern Europe and Japan (14–17, 37), South America is an important source of CTX-M-producing bacteria.
ACKNOWLEDGMENTS
We thank Rolande Perroux, Marlène Jan, and Dominique Rubio for technical assistance. We are also grateful to Ferran Navarro, Department de Microbiologia, Hospital de la Santa Creu i Sant Pau, Universitat Autonoma Barcelona (Barcelona, Spain), for the flanked sequences of blaCTX-M-9 and to Bio-Merieux for the transport of strains from Brazil.
This work was supported in part by a grant from Ministère de l'Education Nationale, de la Recherche et de la Technologie.
REFERENCES
- 1.Ambler R P, Coulson A F W, Frére J-M, Ghuysen J M, Joris B, Forsman M, Lévesque R C, Tiraby G, Waley S G. A standard numbering scheme for the class A β-lactamases. Biochem J. 1991;276:269–272. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakawa Y, Ohta M, Kido N, Mori M, Ito H, Komatsu T, Fujii Y, Kato N. Chromosomal β-lactamase of Klebsiella oxytoca, a new class A enzyme that hydrolyzes broad-spectrum β-lactam antibiotics. Antimicrob Agents Chemother. 1989;33:63–70. doi: 10.1128/aac.33.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barthélémy M, Péduzzi J, Bernard H, Tancrede C, Labia R. Close amino acid sequence relationship between the new plasmid-mediated extended-spectrum β-lactamase MEN-1 and chromosomally encoded enzymes of Klebsiella oxytoca. Biochim Biophys Acta. 1992;1122:15–22. doi: 10.1016/0167-4838(92)90121-s. [DOI] [PubMed] [Google Scholar]
- 4.Bauernfeind A, Casellas J M, Goldberg M, Holley M, Jungwirth R, Mangold P, Rohnisch T, Schweighart S, Wilhelm R. A new plasmidic cefotaximase from patients infected with Salmonella typhimurium. Infection. 1992;20:158–163. doi: 10.1007/BF01704610. [DOI] [PubMed] [Google Scholar]
- 5.Bauernfeind A, Grimm H, Schweighart S. A new plasmidic cefotaximase in a clinical isolate of Escherichia coli. Infection. 1990;18:294–298. doi: 10.1007/BF01647010. [DOI] [PubMed] [Google Scholar]
- 6.Bauernfeind A, Stemplinger I, Jungwirth R, Ernst S, Casellas J M. Sequences of β-lactamase genes encoding CTX-M-1 (MEN-1) and CTX-M-2 and relationship of their amino acid sequences with those of other β-lactamases. Antimicrob Agents Chemother. 1996;40:509–513. doi: 10.1128/aac.40.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonnet R, De Champs C, Sirot D, Chanal C, Labia R, Sirot J. Diversity of TEM mutants in Proteus mirabilis. Antimicrob Agents Chemother. 1999;43:2671–2677. doi: 10.1128/aac.43.11.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnet R, Sampaio J L M, Chanal C, Sirot D, De Champs C, Viallard J L, Labia R, Sirot J. A novel class A extended-spectrum β-lactamase (BES-1) in Serratia marcescens isolated in Brazil. Antimicrob Agents Chemother. 2000;44:3061–3068. doi: 10.1128/aac.44.11.3061-3068.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonnet R, Sampaio J L M, Labia R, De Champs C, Sirot D, Chanal C, Sirot J. A novel CTX-M β-lactamase (CTX-M-8) in cefotaxime-resistant Enterobacteriaceae isolated in Brazil. Antimicrob Agents Chemother. 2000;44:1936–1942. doi: 10.1128/aac.44.7.1936-1942.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouthors A T, Dagoneau-Blanchard N, Naas T, Nordmann P, Jarlier V, Sougakoff W. Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 β-lactamase hydrolysing third-generation cephalosporins. Biochem J. 1998;330:1443–1449. doi: 10.1042/bj3301443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradford P A, Yang Y, Sahm D, Grope I, Gardovska D, Storch G. CTX-M-5, a novel cefotaxime-hydrolyzing β-lactamase from an outbreak of Salmonella typhimurium in Latvia. Antimicrob Agents Chemother. 1998;42:1980–1984. doi: 10.1128/aac.42.8.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantu C, Huang W, Palzkill T. Selection and characterization of amino acid substitutions at residues 237–240 of TEM-1 β-lactamase with altered substrate specificity for aztreonam and ceftazidime. J Biol Chem. 1996;271:22538–22545. doi: 10.1074/jbc.271.37.22538. [DOI] [PubMed] [Google Scholar]
- 13.Felsenstein J. PHYLIP, phylogeny inference package, (version 3.2) Cladistics. 1989;5:164–166. [Google Scholar]
- 14.Gazouli M, Legakis N J, Tzouvelekis L S. Effect of substitution of Asn for Arg-276 in the cefotaxime-hydrolyzing class A β-lactamase CTX-M-4. FEMS Microbiol Lett. 1998;169:289–293. doi: 10.1111/j.1574-6968.1998.tb13331.x. [DOI] [PubMed] [Google Scholar]
- 15.Gazouli M, Tzelepi E, Markogiannakis A, Legakis N J, Tzouvelekis L S. Two novel plasmid-mediated cefotaxime-hydrolyzing β-lactamases (CTX-M-5 and CTX-M-6) from Salmonella typhimurium. FEMS Microbiol Lett. 1998;165:289–293. doi: 10.1111/j.1574-6968.1998.tb13159.x. [DOI] [PubMed] [Google Scholar]
- 16.Gazouli M, Tzelepi E, Sidorenko S V, Tzouvelekis L S. Sequence of the gene encoding a plasmid-mediated cefotaxime-hydrolyzing class A β-lactamase (CTX-M-4): involvement of serine 237 in cephalosporin hydrolysis. Antimicrob Agents Chemother. 1998;42:1259–1262. doi: 10.1128/aac.42.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gniadkowski M, Schneider I, Palucha A, Jungwirth R, Mikiewicz B, Bauernfeind A. Cefotaxime-resistant Enterobacteriaceae isolates from a hospital in Warsaw, Poland: identification of a new CTX-M-3 cefotaxime-hydrolyzing β-lactamase that is closely related to the CTX-M-1/MEN-1 enzyme. Antimicrob Agents Chemother. 1998;42:827–832. doi: 10.1128/aac.42.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huletsky A, Knox J R, Levesque R C. Role of Ser-238 and Lys-240 in the hydrolysis of third-generation cephalosporins by SHV-type β-lactamases probed by site-directed mutagenesis and three-dimensional modeling. J Biol Chem. 1993;268:3690–3697. [PubMed] [Google Scholar]
- 19.Ibuka A, Taguchi A, Ishiguro M, Fushinobu S, Ishii Y, Kamitori S, Okuyama K, Yamaguchi K, Konno M, Matsuzawa H. Crystal structure of the E166A mutant of extended-spectrum β-lactamase Toho-1 at 1.8 A resolution. J Mol Biol. 1999;285:2079–2087. doi: 10.1006/jmbi.1998.2432. [DOI] [PubMed] [Google Scholar]
- 20.Ishii Y, Ohno A, Taguchi H, Imajo S, Ishiguro M, Matsuzawa H. Cloning and sequence of the gene encoding a cefotaxime-hydrolyzing class A β-lactamase isolated from Escherichia coli. Antimicrob Agents Chemother. 1995;39:2269–2275. doi: 10.1128/aac.39.10.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarlier V, Nicolas M H, Fournier G, Philippon A. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988;10:867–878. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- 22.Kado C I, Liu L. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knox J R. Extended-spectrum and inhibitor-resistant TEM-type β-lactamases: mutations, specificity, and three-dimensional structure. Antimicrob Agents Chemother. 1995;39:2593–2601. doi: 10.1128/aac.39.12.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labia R, Andrillon J, Le Goffic F. Computerized microacidimetric determination of β-lactamase Michaelis-Menten constants. FEBS Lett. 1973;33:42–44. doi: 10.1016/0014-5793(73)80154-1. [DOI] [PubMed] [Google Scholar]
- 25.Ma L, Ishii Y, Ishiguro M, Matsuzawa H, Yamaguchi K. Cloning and sequencing of the gene encoding Toho-2, a class A β-lactamase preferentially inhibited by tazobactam. Antimicrob Agents Chemother. 1998;42:1181–1186. doi: 10.1128/aac.42.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumoto Y, Inoue M. Characterization of SFO-1, a plasmid-mediated inducible class A beta-lactamase from Enterobacter cloacae. Antimicrob Agents Chemother. 1999;43:307–313. doi: 10.1128/aac.43.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nielsen H, Engelbrecht J, Brunak S, Von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Péduzzi J, Farzaneh S, Reynaud A, Barthélémy M, Labia R. Characterization and amino acid sequence analysis of a new oxyimino cephalosporin-hydrolyzing class A beta-lactamase from Serratia fonticola CUV. Biochim Biophys Acta. 1997;1341:58–70. doi: 10.1016/s0167-4838(97)00020-4. [DOI] [PubMed] [Google Scholar]
- 29.Péduzzi J, Reynaud A, Baron P, Barthélémy M, Labia R. Chromosomally encoded cephalosporin-hydrolyzing β-lactamase of Proteus vulgaris R0104 belongs to Ambler's class A. Biochim Biophys Acta. 1994;1207:31–39. doi: 10.1016/0167-4838(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 30.Perilli M G, Franceschini N, Segatore B, Amicosante G, Oratore A, duez C, Joris B, Frére J M. Cloning and nucleotide sequencing of the gene encoding the beta-lactamase from Citrobacter diversus. FEMS Microbiol Lett. 1991;67:79–84. doi: 10.1016/0378-1097(91)90448-j. [DOI] [PubMed] [Google Scholar]
- 31.Poirel L, Naas T, Guibert M, Chaibi E B, Labia R, Nordmann P. Molecular and biochemical characterization of VEB-1, a novel class A extended-spectrum β-lactamase encoded by an Escherichia coli integron gene. Antimicrob Agents Chemother. 1999;43:573–581. doi: 10.1128/aac.43.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynaud A, Péduzzi J, Barthélémy M, Labia R. Cefotaxime-hydrolyzing activity of the β-lactamase of Klebsiella oxytoca D488 could be related to a threonine residue at position 140. FEMS Microbiol Lett. 1991;81:185–192. doi: 10.1016/0378-1097(91)90301-p. [DOI] [PubMed] [Google Scholar]
- 33.Sadate M, Tarrago R, Navarro F, Miro E, Verges C, Barbe J, Prats G. Cloning and sequence of the gene encoding a novel cefotaxime-hydrolyzing beta-lactamase (CTX-M-9) from Escherichia coli in Spain. Antimicrob Agents Chemother. 2000;44:1970–1973. doi: 10.1128/aac.44.7.1970-1973.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Vol. 1. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yagi T, Kurokawa H, Senda K, Ichiyama S, Ito H, Ohsuka S, Shibayama K, Shimokata K, Kato N, Ohta M, Arakawa Y. Nosocomial spread of cephem-resistant Escherichia coli strains carrying multiple Toho-1-like β-lactamase genes. Antimicrob Agents Chemother. 1997;41:2606–2611. doi: 10.1128/aac.41.12.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]